Protein condensates can evade autophagic degradation under stress or pathological conditions. However, the underlying mechanisms are unclear. Here, we demonstrate that RNAs switch the fate of condensates in Caenorhabditis elegans. PGL granules undergo autophagic degradation in embryos laid under normal conditions and accumulate in embryos laid under heat stress conditions to confer stress adaptation. In heat-stressed embryos, mRNAs and RNA control factors partition into PGL granules. Depleting proteins involved in mRNA biogenesis and stability suppresses PGL granule accumulation and triggers their autophagic degradation, while loss of activity of proteins involved in RNA turnover facilitates accumulation. RNAs facilitate LLPS of PGL granules, enhance their liquidity, and also inhibit recruitment of the gelation-promoting scaffold protein EPG-2 to PGL granules. Thus, RNAs are important for controlling the susceptibility of phase-separated protein condensates to autophagic degradation. Our work provides insights into the accumulation of ribonucleoprotein aggregates associated with the pathogenesis of various diseases.
Introduction
Cells contain myriad membrane-less protein-RNA structures, including nucleoli, Cajal bodies, and paraspeckles in the nucleus, and stress granules (SGs) and processing (P) bodies in the cytosol (Banani et al., 2017; Boeynaems et al., 2018). Collectively, these structures are known as ribonucleoprotein (RNP) condensates and they exhibit distinct protein and RNA compositions, as well as possess different dynamic properties and functions (Corbet and Parker, 2019; Gallo et al., 2008; Parker and Sheth, 2007; Tauber et al., 2020; Updike and Strome, 2010). For example, P bodies are constitutive structures that are enriched in non-translating mRNAs and factors involved in mRNA decapping, miRNA-mediated gene silencing, and nonsense-mediated decay (Corbet and Parker, 2019; Gallo et al., 2008; Parker and Sheth, 2007). SGs are assembled in response to various stress conditions such as heat shock, starvation, oxidative stress, and viral infection, and rapidly dissipate following the removal of the stressor (Corbet and Parker, 2019; Riback et al., 2017; Zhang et al., 2019). SGs contain translationally stalled mRNAs and translation factors and serve as sites for regulating translation, RNA stability, and cell signaling to combat stress (Corbet and Parker, 2019). Increasing evidence shows that RNP condensates are assembled via liquid–liquid phase separation (LLPS), a process involving the concentration of biomolecules into liquid-like condensates that possess defined boundaries while maintaining dynamic exchange with the surrounding milieu (Banani et al., 2017; Shin and Brangwynne, 2017; Wang and Zhang, 2019). LLPS of RNP condensates is triggered by multivalent interactions among constituent proteins and RNAs (Corbet and Parker, 2019; Shin and Brangwynne, 2017; Tauber et al., 2020; Wang and Zhang, 2019). Phase-separated RNPs can further transition into more stable states such as gels and solid fibers to fulfill distinct functions, or they can undergo abnormal transition under pathological and stress conditions (Shin and Brangwynne, 2017; Wang and Zhang, 2019).
RNAs play a key role in modulating phase separation and transition of RNP condensates by affecting the valency and strength of the interactions among components (Banani et al., 2017; Corbet and Parker, 2019; Lin et al., 2015; Molliex et al., 2015; Shin and Brangwynne, 2017; Snead and Gladfelter, 2019). Low RNA/protein ratios facilitate, while high RNA/protein ratios inhibit, LLPS of prion-like RNA-binding proteins (RBPs; e.g., TDP-43, FUS, and hnRNPA1; Lin et al., 2015; Molliex et al., 2015; Maharana et al., 2018; Tauber et al., 2020). RNAs also maintain the liquidity of RNP condensates (e.g., LAF-1 condensates and FUS condensates), and prevent them from converting into solid structures (Elbaum-Garfinkle et al., 2015; Maharana et al., 2018). The pathological protein aggregates associated with various neurodegenerative diseases abnormally sequestrate RNAs. In the brains of patients with Alzheimer’s disease (AD), Down’s syndrome (DS), amyotrophic lateral sclerosis (ALS), corticobasal degeneration (CBD), Pick’s disease (PiD), and progressive supranuclear palsy (PSP), the intracellular neurofibrillary tangles sequestrate RNAs (Ginsberg et al., 1997; Ginsberg et al., 1998). Pathological Tau aggregates in mouse and cellular models of Tau pathologies also stain strongly for RNAs (Lester et al., 2021). The mechanism by which RNAs participate in pathological accumulation of protein condensates remains largely unknown.
LLPS has been shown to triage misfolded proteins or unwanted proteins for autophagic degradation, a process involving selective encapsulation of the proteins by a double-membrane autophagosome and subsequent delivery to lysosomes (Lu et al., 2013; Stolz et al., 2014). The gel-like material properties of protein condensates appear to be essential for degradation (Wang and Zhang, 2019; Noda et al., 2020). For example, in Caenorhabditis elegans, components of the specialized P granules in the oocyte, PGL-1 and PGL-3 (collectively called PGL proteins), are degraded by autophagy when they are partitioned into somatic cells during embryonic divisions (Zhang et al., 2009). Degradation of PGL proteins requires the receptor protein SEPA-1 (Zhang et al., 2009) and the scaffold protein EPG-2 (Tian et al., 2010). SEPA-1 promotes LLPS of PGL proteins while EPG-2 coats the surface of the condensates and leads to gelation of PGL-1/-3/SEPA-1 condensates (Zhang et al., 2018). In embryos laid by animals grown under mild heat stress conditions (26°C, compared with the normal temperature range of 15–25°C), PGL granules (PGL proteins and SEPA-1) escape autophagic degradation and accumulate in large numbers to confer stress resistance (Zhang et al., 2018). Very little is known about the switch controlling the distinct fates of protein condensates—autophagic degradation vs. accumulation—under different conditions.
In this study, we reveal that mRNAs and RNA control factors are partitioned into PGL granules under mild heat stress conditions, forming RNPs distinct from SGs and P bodies. RNAs modulate LLPS and transition of PGL granules. Depleting factors involved in RNA biogenesis or turnover affects whether PGL granules undergo autophagic degradation or accumulation. Our study uncovers that RNAs act as a switch to allow protein condensates to escape autophagic degradation. Our work also provides insights into the pathological accumulation of RNPs in various diseases.
Results
PGL granules formed under heat stress conditions contain proteins involved in mRNA metabolism
PGL-1/-3 proteins derived from the oocyte escape autophagic degradation in embryos laid under mild heat stress conditions (26°C; Zhang et al., 2018). We carried out immunoprecipitation-mass spectrometry (IP-MS) analysis to identify the protein components of PGL granules in heat-stressed embryos. GFP::PGL-3 proteins were immunoprecipitated from extracts of embryos grown at 26°C and the interacting proteins were identified by LC-MS/MS. Proteins involved in translation and RNA control factors (e.g., RNA binding proteins, ribonucleases, RNA helicases, and proteins involved in siRNA-mediated mRNA turnover) were enriched in the GFP::PGL-3 coimmunoprecipitants (Fig. S1, A–C). IFE-1, one of the five isoforms of eIF4E, was the most enriched translational machinery protein in our IP-MS analysis (Fig. S1, A and B). IFE-1 is a component of germ line P granules that directly interacts with PGL-1 (Amiri et al., 2001). IFE-1 binds the 7-methylguanosine (m7G) mRNA cap and exerts positive translational control on targeted mRNAs (Friday et al., 2015). We constructed an endogenous knock-in IFE-1::GFP reporter and found that the fluorescent signal was strongly localized to P granules in germline lineages in embryos. In somatic cells, IFE-1::GFP was diffusely localized in the cytoplasm and the signal persisted even at late embryonic stages (Fig. 1, A and B). In embryos laid at 26°C, no IFE-1::GFP granules were detected in somatic cells prior to the ∼100-cell stage. IFE-1::GFP started to form a few tiny granules at the ∼100-cell stage, and the number of granules gradually increased as development proceeded (Fig. 1, C, D, and G). PGL granules were evident at the ∼20-cell stage and then gradually increased in number at 26°C (Zhang et al., 2018). IFE-1::GFP granules were almost completely colocalized with TagRFP::SEPA-1-labeled PGL granules, and ∼68% of PGL granules were positive for IFE-1 (Fig. 1, H and I). Accumulation of IFE-1::GFP granules under heat-stress conditions was abolished in embryos depleted of SEPA-1 or PGL-1 (Fig. 1, E–G). These results indicate that in embryos laid under heat stress conditions, IFE-1::GFP is recruited to preformed PGL granules at a late embryonic stage.
PGL granules formed under heat stress conditions contain components of the translation machinery, related to Fig. 1 . (A) Proteomics data from three independent replicates were analyzed by volcano plot. Blue dots indicate proteins significantly enriched in the GFP::PGL-3 IP-MS compared to GFP control (log2 fold change ≥1 and two-tailed, unpaired t test results P < 0.05). The top 10 enriched proteins are highlighted. Gray dots indicate proteins with no significant enrichment. (B) Brief description of the top 10 enriched proteins identified in the GFP::PGL-3 IP-MS compared the GFP control. GLH-1 and GLH-2 are the known components of germline P granules. (C) The 574 proteins that are significantly enriched in the GFP::PGL-3 IP-MS compared with GFP control were analyzed by DAVID. The top GO enrichment terms for molecular function are listed according to the number of proteins with P < 0.05 (automatically generated by DAVID). Black bars indicate proteins involved in RNA-related processes. (D and E) In atg-3(bp412) mutant embryos, IFE-1::GFP forms a large number of granules in somatic cells. Compared with embryos grown at 20°C (D), the number and intensity of IFE-1::GFP granules are increased, and the level of diffuse IFE-1::GFP is decreased, in embryos grown at 26°C (E). (F and G) The number and intensity of IFE-1::GFP granules are reduced in sepa-1(bp1726); atg-3(bp412) double mutant embryos at both 20°C (F) and 26°C (G). Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in D–G. (H) Quantification of the number of IFE-1::GFP granules in somatic cells. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: *P < 0.05. (I and J) Somatic IFE-1::GFP granules are partially colocalized with PGL granules labeled by TagRFP::SEPA-1 in atg-3(bp412) mutant embryos at 20°C (I) and 26°C (J). Some of the IFE-1::GFP granules are negative for SEPA-1. Comma-stage embryos are shown in I and J. (K and L) 3 µM purified TagRFP::IFE-1 protein fails to undergo LLPS during the observation time of 5 min. The reaction contained 150 mM NaCl. K is the DIC image of L. (M and N) TagRFP::IFE-1 is not evidently partitioned into droplets formed by 3 µM PGL-3/SEPA-1 proteins in an in vitro LLPS assay. M is the DIC image of N. Scale bars: 5 µm for D–G, I, and J; 2 µm for enlarged images in I and J; 20 µm for K–N.
PGL granules formed under heat stress conditions contain components of the translation machinery, related to Fig. 1 . (A) Proteomics data from three independent replicates were analyzed by volcano plot. Blue dots indicate proteins significantly enriched in the GFP::PGL-3 IP-MS compared to GFP control (log2 fold change ≥1 and two-tailed, unpaired t test results P < 0.05). The top 10 enriched proteins are highlighted. Gray dots indicate proteins with no significant enrichment. (B) Brief description of the top 10 enriched proteins identified in the GFP::PGL-3 IP-MS compared the GFP control. GLH-1 and GLH-2 are the known components of germline P granules. (C) The 574 proteins that are significantly enriched in the GFP::PGL-3 IP-MS compared with GFP control were analyzed by DAVID. The top GO enrichment terms for molecular function are listed according to the number of proteins with P < 0.05 (automatically generated by DAVID). Black bars indicate proteins involved in RNA-related processes. (D and E) In atg-3(bp412) mutant embryos, IFE-1::GFP forms a large number of granules in somatic cells. Compared with embryos grown at 20°C (D), the number and intensity of IFE-1::GFP granules are increased, and the level of diffuse IFE-1::GFP is decreased, in embryos grown at 26°C (E). (F and G) The number and intensity of IFE-1::GFP granules are reduced in sepa-1(bp1726); atg-3(bp412) double mutant embryos at both 20°C (F) and 26°C (G). Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in D–G. (H) Quantification of the number of IFE-1::GFP granules in somatic cells. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: *P < 0.05. (I and J) Somatic IFE-1::GFP granules are partially colocalized with PGL granules labeled by TagRFP::SEPA-1 in atg-3(bp412) mutant embryos at 20°C (I) and 26°C (J). Some of the IFE-1::GFP granules are negative for SEPA-1. Comma-stage embryos are shown in I and J. (K and L) 3 µM purified TagRFP::IFE-1 protein fails to undergo LLPS during the observation time of 5 min. The reaction contained 150 mM NaCl. K is the DIC image of L. (M and N) TagRFP::IFE-1 is not evidently partitioned into droplets formed by 3 µM PGL-3/SEPA-1 proteins in an in vitro LLPS assay. M is the DIC image of N. Scale bars: 5 µm for D–G, I, and J; 2 µm for enlarged images in I and J; 20 µm for K–N.
PGL granules formed under heat-stress conditions contain proteins involved in translation. (A and B) IFE-1::GFP forms a few granules in the germ precursor cells (Z2 and Z3, highlighted in dashed circles), and is diffusely localized in the cytoplasm of somatic cells in wild-type (WT) embryos at 20°C. Embryos at the ∼100-cell stage and the comma stage are shown in A and B, respectively. (C and D) Temporal accumulation of IFE-1::GFP granules in somatic cells in WT embryos laid at 26°C. IFE-1::GFP forms a few tiny granules at the ∼100-cell stage (C), and forms a large number of granules at the comma stage (D). (E and F) IFE-1::GFP granules are absent from somatic cells in sepa-1(bp1726) (E) and pgl-1(RNAi) (F) embryos at the comma stage at 26°C. In pgl-1(RNAi) embryos, the number of IFE-1::GFP granules is also reduced in two-germ precursor cells (highlighted in dashed circles). Maximum-intensity projections of Z-stack confocal images are shown in A–F. (G) Quantification of the number of IFE-1::GFP granules in somatic cells per embryo at the ∼100-cell and comma stage at 20 and 26°C. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t-test results: ***P < 0.001. (H) IFE-1::GFP granules are colocalized with PGL granules, labeled by TagRFP::SEPA-1, in WT embryos laid at 26°C. PGL granules contain oocyte-derived PGL-1/-3 and the zygotically synthesized SEPA-1. PGL-1/-3 are present in both P granules and PGL granules, while SEPA-1 is present only in PGL granules. (I) Quantification of the percentage (%) of PGL granules positive for IFE-1::GFP in WT and atg-3 mutant embryos. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype or condition). Two-tailed, unpaired t-test results: **P < 0.01, ***P < 0.001. (J and K) Purified TagRFP::IFE-1 protein is partitioned into PGL-1/-3/SEPA-1 condensates (3 µM for each protein) in in vitro LLPS assays. J is the DIC image of K. His-tagged PGL-1 and PGL-3, His- and TagRFP-tagged IFE-1 isoform b, and His- and MBP-tagged SEPA-1 were used for LLPS in this study unless otherwise noted. Scale bars: 5 µm for A–F and H; 2 µm for enlarged images in H; 20 µm for J and K.
PGL granules formed under heat-stress conditions contain proteins involved in translation. (A and B) IFE-1::GFP forms a few granules in the germ precursor cells (Z2 and Z3, highlighted in dashed circles), and is diffusely localized in the cytoplasm of somatic cells in wild-type (WT) embryos at 20°C. Embryos at the ∼100-cell stage and the comma stage are shown in A and B, respectively. (C and D) Temporal accumulation of IFE-1::GFP granules in somatic cells in WT embryos laid at 26°C. IFE-1::GFP forms a few tiny granules at the ∼100-cell stage (C), and forms a large number of granules at the comma stage (D). (E and F) IFE-1::GFP granules are absent from somatic cells in sepa-1(bp1726) (E) and pgl-1(RNAi) (F) embryos at the comma stage at 26°C. In pgl-1(RNAi) embryos, the number of IFE-1::GFP granules is also reduced in two-germ precursor cells (highlighted in dashed circles). Maximum-intensity projections of Z-stack confocal images are shown in A–F. (G) Quantification of the number of IFE-1::GFP granules in somatic cells per embryo at the ∼100-cell and comma stage at 20 and 26°C. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t-test results: ***P < 0.001. (H) IFE-1::GFP granules are colocalized with PGL granules, labeled by TagRFP::SEPA-1, in WT embryos laid at 26°C. PGL granules contain oocyte-derived PGL-1/-3 and the zygotically synthesized SEPA-1. PGL-1/-3 are present in both P granules and PGL granules, while SEPA-1 is present only in PGL granules. (I) Quantification of the percentage (%) of PGL granules positive for IFE-1::GFP in WT and atg-3 mutant embryos. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype or condition). Two-tailed, unpaired t-test results: **P < 0.01, ***P < 0.001. (J and K) Purified TagRFP::IFE-1 protein is partitioned into PGL-1/-3/SEPA-1 condensates (3 µM for each protein) in in vitro LLPS assays. J is the DIC image of K. His-tagged PGL-1 and PGL-3, His- and TagRFP-tagged IFE-1 isoform b, and His- and MBP-tagged SEPA-1 were used for LLPS in this study unless otherwise noted. Scale bars: 5 µm for A–F and H; 2 µm for enlarged images in H; 20 µm for J and K.
In atg-3(bp412) mutants, numerous IFE-1::GFP granules accumulated in embryos under both normal conditions and heat stress conditions (Fig. S1, D and E). The IFE-1::GFP granules were more abundant and brighter in heat-stressed atg-3(bp412) embryos than in heat-stressed wild-type embryos (Fig. 1 D and Fig. S1 E). IFE-1::GFP granules were still formed in sepa-1(bp1726); atg-3(bp412) mutant embryos, although the number was less than in atg-3 mutants (Fig. S1, D–H). IFE-1::GFP granules were only partially colocalized with PGL granules in atg-3 mutants (Fig. 1 I; and Fig. S1, I and J), which indicates that IFE-1::GFP can be recruited to other accumulated protein condensates in autophagy mutants.
We next determined whether IFE-1 is recruited to PGL condensates formed in in vitro LLPS assays. Purified TagRFP::IFE-1 proteins failed to undergo LLPS (Fig. S1, K and L). IFE-1 interacts with PGL-1 (Amiri et al., 2001). TagRFP::IFE-1 was partitioned into PGL-1/-3/SEPA-1 condensates but was excluded from PGL-3/SEPA-1 condensates (Fig. 1, J and K; and Fig. S1, M and N). This indicates that the interaction between IFE-1 and PGL-1 mediates the recruitment of IFE-1 to PGL condensates.
RNAs are sorted into PGL granules in heat-stressed embryos
We next performed RNA-FISH experiments to determine whether PGL granules in embryos laid at 26°C contain mRNAs. Distinct poly(A) mRNA puncta, detected by the oligomeric dT30-Alexa 488 probe, were colocalized with P granules in the germline lineage in embryos (sequentially P1, P2, P3, P4, and eventually two germ precursor cells Z2 and Z3; Fig. 2, A–D). Distinct poly(A) mRNA puncta were absent from somatic cells of wild-type and atg-3 mutant embryos at 20°C (Fig. 2, A, C, F, and G). We found that a large number of poly(A) mRNA puncta accumulated in somatic cells in wild-type embryos and also in atg-3 mutant embryos laid at 26°C, and the mRNA puncta were colocalized with PGL granules (Fig. 2, B and D–G). These results indicate that PGL granules in heat-stressed embryos are enriched in mRNAs.
PGL granules act as sites for mRNA metabolism that are distinct from stress granules. (A) Distinct poly(A) mRNA puncta are absent from somatic cells of WT embryos at 20°C, but are detected in germ precursor cells (highlighted in dashed circles) where they colocalize with P granules. PGL proteins are quickly degraded by autophagy in WT embryos under normal growth conditions. Thus, PGL granules are largely absent in embryos at late developmental stages. A few small granules are detected at early embryonic stages. One focal plane in a ∼100-cell-stage embryo is shown in A. (B) A large number of puncta containing poly(A) mRNAs accumulate in WT embryos at 26°C. These puncta are colocalized with P granules in germ precursor cells (Z2 and Z3, highlighted in dashed circles) and PGL granules in somatic cells labeled by PGL-1::TagRFP. (C) Poly(A) mRNA puncta are largely absent from PGL granules in somatic cells in atg-3 mutant embryos at 20°C but are detected in germ precursor cells (highlighted in dashed circles). (D) In atg-3 mutant embryos at 26°C, multiple poly(A) mRNA puncta accumulate, which are colocalized with P granules in germ precursor cells (highlighted in dashed circles) and PGL granules in somatic cells. One focal plane in a ∼100–200 cell-stage embryo is shown in B–D. The poly(A) mRNA puncta in atg-3 mutants at 20°C are much weaker in intensity. (E and F) Quantification of the percentage (%) of PGL granules positive for poly(A) mRNA (E), and the number of poly(A) mRNA puncta (F) in somatic cells per focal plane. Data are shown as mean ± SEM (n = 5; n refers to five images from the corresponding five embryos analyzed for each genotype or condition). Two-tailed, unpaired t test results: *P < 0.05, **P < 0.01, ***P < 0.001. (G) Quantification of the mean fluorescence intensity of somatic poly(A) mRNA puncta. Data are shown as mean ± SEM (n = 23, 14, and 37 puncta for WT embryos at 26°C, atg-3 mutant embryos at 20, and 26°C, respectively; n refers to the number of puncta analyzed for each genotype or condition). Two-tailed, unpaired t test results: ***P < 0.001. (H) GTBP-1::GFP forms no distinct granules, while PGL granules accumulate in WT embryos at the ∼100–200 cell stage at 26°C. (I and J) P bodies, detected by anti-DCAP-1, are separate from PGL granules in WT embryos at the ∼100–200 cell stage at 26°C. The germ precursor cells (Z2 and Z3) are highlighted in dashed circles. J shows quantification of the colocalization of DCAP-1 bodies with PGL granules. Data are shown as mean ± SEM (n = 3; n refers to three images from the corresponding three embryos analyzed). Scale bars: 5 µm for A–D, H, and I; 2 µm for enlarged images in A–D, H, and I.
PGL granules act as sites for mRNA metabolism that are distinct from stress granules. (A) Distinct poly(A) mRNA puncta are absent from somatic cells of WT embryos at 20°C, but are detected in germ precursor cells (highlighted in dashed circles) where they colocalize with P granules. PGL proteins are quickly degraded by autophagy in WT embryos under normal growth conditions. Thus, PGL granules are largely absent in embryos at late developmental stages. A few small granules are detected at early embryonic stages. One focal plane in a ∼100-cell-stage embryo is shown in A. (B) A large number of puncta containing poly(A) mRNAs accumulate in WT embryos at 26°C. These puncta are colocalized with P granules in germ precursor cells (Z2 and Z3, highlighted in dashed circles) and PGL granules in somatic cells labeled by PGL-1::TagRFP. (C) Poly(A) mRNA puncta are largely absent from PGL granules in somatic cells in atg-3 mutant embryos at 20°C but are detected in germ precursor cells (highlighted in dashed circles). (D) In atg-3 mutant embryos at 26°C, multiple poly(A) mRNA puncta accumulate, which are colocalized with P granules in germ precursor cells (highlighted in dashed circles) and PGL granules in somatic cells. One focal plane in a ∼100–200 cell-stage embryo is shown in B–D. The poly(A) mRNA puncta in atg-3 mutants at 20°C are much weaker in intensity. (E and F) Quantification of the percentage (%) of PGL granules positive for poly(A) mRNA (E), and the number of poly(A) mRNA puncta (F) in somatic cells per focal plane. Data are shown as mean ± SEM (n = 5; n refers to five images from the corresponding five embryos analyzed for each genotype or condition). Two-tailed, unpaired t test results: *P < 0.05, **P < 0.01, ***P < 0.001. (G) Quantification of the mean fluorescence intensity of somatic poly(A) mRNA puncta. Data are shown as mean ± SEM (n = 23, 14, and 37 puncta for WT embryos at 26°C, atg-3 mutant embryos at 20, and 26°C, respectively; n refers to the number of puncta analyzed for each genotype or condition). Two-tailed, unpaired t test results: ***P < 0.001. (H) GTBP-1::GFP forms no distinct granules, while PGL granules accumulate in WT embryos at the ∼100–200 cell stage at 26°C. (I and J) P bodies, detected by anti-DCAP-1, are separate from PGL granules in WT embryos at the ∼100–200 cell stage at 26°C. The germ precursor cells (Z2 and Z3) are highlighted in dashed circles. J shows quantification of the colocalization of DCAP-1 bodies with PGL granules. Data are shown as mean ± SEM (n = 3; n refers to three images from the corresponding three embryos analyzed). Scale bars: 5 µm for A–D, H, and I; 2 µm for enlarged images in A–D, H, and I.
PGL granules in heat-stressed embryos are distinct from SGs and P bodies
The presence of mRNAs and the translation initiation factor IFE-1 in PGL granules in embryos laid at 26°C prompted us to investigate whether the PGL granules exhibit characteristics of stress granules (SGs). In wild-type and autophagy mutants, the SG marker GTBP-1::GFP (C. elegans G3BP1/2 homolog) displayed a diffuse pattern in embryos laid at 26°C (Fig. 2 H and Fig. S2 A). After wild-type embryos were heat-shocked at 33°C for 1 h, a large number of GTBP-1::GFP granules accumulated, while PGL granules were absent (Fig. S2 B). Of note, PGL proteins are quickly degraded by autophagy in wild-type embryos under normal conditions and thus are largely absent in embryos at the ∼100-cell stage and afterward. In atg-3 mutant embryos that were heat-shocked at 33°C for 1 h, the majority of GTBP-1::GFP granules did not colocalize with PGL granules (Fig. S2 C). After shifting the embryos back to 20°C, the number of PGL granules gradually decreased in wild-type embryos but persisted in atg-3 mutants (Fig. S2, D–J). GTBP-1::GFP granules were quickly disassembled in wild-type embryos after shifting to 20°C. In atg-3 mutants, a fraction of GTBP-1::GFP granules were still detected after ∼0.5 h recovery and the granules completely disappeared after ∼1 h recovery (Fig. S2, K–P and S). This indicates that autophagy deficiency delays SG disassembly. RNAi inactivation of pgl-3 had no effect on the assembly and disassembly of GTBP-1::GFP granules in embryos (Fig. S2, L, M, and Q–S). PGL granules were still formed in embryos depleted of GTBP-1, a key factor for SG formation in C. elegans (Fig. S2 T; Kuo et al., 2020).
PGL granules act as sites for mRNA metabolism that are distinct from stress granules, related to Fig. 2 . (A) The SG marker GTBP-1::GFP is diffuse in the cytoplasm in somatic cells in atg-3 mutant embryos at 26°C. (B) Numerous GTBP-1::GFP stress granules accumulate, while no PGL granules accumulate in WT embryos after heat shock treatment (33°C for 1 h). (C) GTBP-1::GFP forms numerous granules, which are separate from PGL granules in atg-3 mutant embryos after heat shock treatment (33°C for 1 h). Embryos at the ∼100–200 cell stage are shown in A–C. (D–J) The dynamics of PGL granules, shown by GFP::PGL-3, at the indicated time points after WT or atg-3 mutant embryos were shifted from 26 to 20°C. Quantification of the number of PGL granules in D–I is shown in J. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: n.s.: no significant difference, *P < 0.05, **P < 0.01, ***P < 0.001. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in D–I. (K–S) The dynamics of GTBP-1::GFP granules at the indicated time points in WT embryos, atg-3 mutant embryos and pgl-3(RNAi) embryos after the embryos were shifted from 33 to 20°C. S shows the quantification of the number of GTBP-1::GFP granules in K–R. Comma-stage embryos are shown in K–R. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype or condition). Two-tailed, unpaired t test results: n.s.: no significant difference, **P < 0.01. (T)gtbp-1(RNAi) has no effect on the accumulation of PGL granules in embryos laid at 26°C. Maximum-intensity projection of Z-stack confocal images of a comma-stage embryo is shown. Scale bars: 5 µm for A–C, D–I, K–R, and T; 2 µm for enlarged images in A–C.
PGL granules act as sites for mRNA metabolism that are distinct from stress granules, related to Fig. 2 . (A) The SG marker GTBP-1::GFP is diffuse in the cytoplasm in somatic cells in atg-3 mutant embryos at 26°C. (B) Numerous GTBP-1::GFP stress granules accumulate, while no PGL granules accumulate in WT embryos after heat shock treatment (33°C for 1 h). (C) GTBP-1::GFP forms numerous granules, which are separate from PGL granules in atg-3 mutant embryos after heat shock treatment (33°C for 1 h). Embryos at the ∼100–200 cell stage are shown in A–C. (D–J) The dynamics of PGL granules, shown by GFP::PGL-3, at the indicated time points after WT or atg-3 mutant embryos were shifted from 26 to 20°C. Quantification of the number of PGL granules in D–I is shown in J. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: n.s.: no significant difference, *P < 0.05, **P < 0.01, ***P < 0.001. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in D–I. (K–S) The dynamics of GTBP-1::GFP granules at the indicated time points in WT embryos, atg-3 mutant embryos and pgl-3(RNAi) embryos after the embryos were shifted from 33 to 20°C. S shows the quantification of the number of GTBP-1::GFP granules in K–R. Comma-stage embryos are shown in K–R. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype or condition). Two-tailed, unpaired t test results: n.s.: no significant difference, **P < 0.01. (T)gtbp-1(RNAi) has no effect on the accumulation of PGL granules in embryos laid at 26°C. Maximum-intensity projection of Z-stack confocal images of a comma-stage embryo is shown. Scale bars: 5 µm for A–C, D–I, K–R, and T; 2 µm for enlarged images in A–C.
Next, we detected P bodies using an antibody against the P body component DCAP-1 (mRNA-decapping enzyme 1). DCAP-1-positive P bodies were colocalized with P granules in germline blastomeres (P1 to P4) but were largely separate from P granules in the germ precursor cells Z2 and Z3 (Fig. 2 I; Zhang et al., 2009). In somatic cells, P bodies were separate from PGL granules in embryos at 26°C (Fig. 2, I and J). Therefore, PGL granules formed under mild stress conditions (26°C) are distinct from SGs formed under harsh conditions (33°C) and constitutively formed P bodies.
Depleting factors involved in mRNA processing, transport, and translation promotes autophagic degradation of PGL granules under heat stress conditions
We next performed RNAi screening to identify factors involved in mRNA biogenesis, processing, nucleus-to-cytoplasm transport, and stability that modulate the accumulation of PGL granules in heat-stressed embryos (Arribere et al., 2020; Billi et al., 2014). We found that RNAi clones targeting multiple mRNA metabolism factors reduced the number of PGL granules in embryos laid at 26°C (Fig. 3, A–F). The factors encoded by the identified genes can be classified into five groups based on function: (1) mRNA processing (e.g., snr-1, snr-2, snr-3, snr-4, snr-5, lsm-5, lsm-7, prp-3, prp-19, prp-38, and prp-39); (2) mRNA translation (e.g., aars-2, mars-1, rars-1, qars-1, dars-1, and ruvb-1); (3) poly(A)-binding proteins (e.g., pab-1 and pab-2); (4) mRNA nuclear transport (e.g., npp-6 and npp-9); and (5) ribonucleotide reductase (rnr-1; Fig. 3 A). RNAi inactivation of the identified genes snr-4, prp-19, pab-1, ruvb-1, and npp-9 failed to suppress the accumulation of PGL granules in heat-stressed atg-3 mutant embryos (Fig. 3 G and Fig. S3, A–D). This suggests that PGL granules in heat-stressed embryos with depletion of the above-identified factors are degraded by autophagy. Degradation of SQST-1 aggregates in bec-1(bp613) hypomorphic mutants, which can be promoted by increased autophagy activity (Chen et al., 2020), was not affected by RNAi inactivation of snr-4, prp-19, pab-1, and ruvb-1 (Fig. S3, E–G). Therefore, autophagy activity is not evidently elevated by RNAi inactivation of snr-4, prp-19, pab-1, and ruvb-1.
Loss of function of factors involved in mRNA metabolism suppresses the accumulation of PGL granules in embryos laid under heat stress conditions. (A) Summary of RNAi inactivations that suppress PGL granule accumulation in embryos under heat stress. (B–E) Compared to control(RNAi) (B), the number of PGL granules, labeled by GFP::PGL-3, is decreased in snr-4(RNAi) (C), ruvb-1(RNAi) (D), and pab-1(RNAi) (E) embryos at 26°C. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in B–E. (F and G) Quantification of PGL granules per embryo in the indicated genetic backgrounds at 26°C. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: n.s.: no significant difference, **P < 0.01, ***P < 0.001. Scale bars: 5 µm for B–E.
Loss of function of factors involved in mRNA metabolism suppresses the accumulation of PGL granules in embryos laid under heat stress conditions. (A) Summary of RNAi inactivations that suppress PGL granule accumulation in embryos under heat stress. (B–E) Compared to control(RNAi) (B), the number of PGL granules, labeled by GFP::PGL-3, is decreased in snr-4(RNAi) (C), ruvb-1(RNAi) (D), and pab-1(RNAi) (E) embryos at 26°C. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in B–E. (F and G) Quantification of PGL granules per embryo in the indicated genetic backgrounds at 26°C. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: n.s.: no significant difference, **P < 0.01, ***P < 0.001. Scale bars: 5 µm for B–E.
mRNA metabolism factors modulate autophagic degradation and mRNA recruitment of PGL granules in embryos laid under heat stress conditions, related to Figs. 3, 4, and 5,. (A–D) Compared with control(RNAi)-treated atg-3 mutant embryos (A), snr-4(RNAi) (B), prp-19(RNAi) (C), or ruvb-1(RNAi) (D) fails to reduce the accumulation of GFP::PGL-3 granules in atg-3(bp412) mutant embryos at 26°C. (E and F) Compared with control(RNAi)-treated bec-1(bp613) mutant embryos (E), snr-4(RNAi) fails to suppress the accumulation of SQST-1::GFP aggregates in bec-1(bp613) hypomorphic mutant embryos at 26°C (F). Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in A–F. (G) Quantification of the number of SQST-1::GFP aggregates per embryo in the indicated genetic background. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: n.s.: no significant difference. (H–K) Compared with control(RNAi)-treated atg-3 mutant embryos at 26°C (H), much fewer poly(A) mRNA puncta are formed in atg-3 embryos with simultaneous snr-4(RNAi) (I), prp-19(RNAi) (J), and npp-9(RNAi) (K). The nuclear signal of poly(A) mRNA is dramatically increased in prp-19(RNAi) and npp-9(RNAi) embryos. The germ precursor cells Z2 and Z3 are highlighted in dashed circles. Embryos at the ∼100–200 cell stage are shown in H–K. The exposure time for poly(A) mRNA puncta in atg-3 mutants shown here was shorter than that shown in Fig. 2 C and Fig. 4 A to avoid overexposure in atg-3; snr-4(RNAi), prp-19(RNAi); atg-3 and npp-9(RNAi); atg-3 embryos. (L and M) Quantification of the number of somatic poly(A) mRNA puncta (L) and % of PGL granules positive for poly(A) mRNA per focal plane in the indicated genotypes at 26°C (M). Data are shown as mean ± SEM (n = 5; n refers to five images from the corresponding five embryos analyzed for each genotype). Two-tailed, unpaired t test results: **P < 0.01, ***P < 0.001. (N) sfGFP::PAB-1 is diffusely localized in the cytoplasm of C. elegans embryos at 26°C. An embryo at the ∼200 cell stage is shown. (O and P) A large number of poly(A) mRNA puncta accumulate in dcr-1(bp132) mutant embryos at 26°C. The puncta are colocalized with P granules in germ precursor cells (highlighted in dashed circles) and PGL granules in somatic cells. Quantification of the fluorescence intensity of poly(A) puncta in somatic cells in WT and dcr-1(bp132) mutant embryos at 26°C is shown in P. Data are shown as mean ± SEM (n = 24 for WT and n = 35 for dcr-1; n refers to the number of poly(A) mRNA puncta analyzed for each genotype). Two-tailed, unpaired t test result: **P < 0.01. An embryo at the ∼100–200 cell stage is shown in O. (Q–S)dcr-1(bp132) mutant embryos show accumulation of PGL granules at 26°C, detected by anti-SEPA-1, as in WT embryos. S shows the quantification of the number of PGL granules in WT and dcr-1(bp132) mutants at 26°C. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test result: n.s.: no significant difference. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in Q and R. Scale bars: 5 µm for A–F, H–K, N, O, Q, and R; 2 µm for enlarged images in H–K and O.
mRNA metabolism factors modulate autophagic degradation and mRNA recruitment of PGL granules in embryos laid under heat stress conditions, related to Figs. 3, 4, and 5,. (A–D) Compared with control(RNAi)-treated atg-3 mutant embryos (A), snr-4(RNAi) (B), prp-19(RNAi) (C), or ruvb-1(RNAi) (D) fails to reduce the accumulation of GFP::PGL-3 granules in atg-3(bp412) mutant embryos at 26°C. (E and F) Compared with control(RNAi)-treated bec-1(bp613) mutant embryos (E), snr-4(RNAi) fails to suppress the accumulation of SQST-1::GFP aggregates in bec-1(bp613) hypomorphic mutant embryos at 26°C (F). Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in A–F. (G) Quantification of the number of SQST-1::GFP aggregates per embryo in the indicated genetic background. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: n.s.: no significant difference. (H–K) Compared with control(RNAi)-treated atg-3 mutant embryos at 26°C (H), much fewer poly(A) mRNA puncta are formed in atg-3 embryos with simultaneous snr-4(RNAi) (I), prp-19(RNAi) (J), and npp-9(RNAi) (K). The nuclear signal of poly(A) mRNA is dramatically increased in prp-19(RNAi) and npp-9(RNAi) embryos. The germ precursor cells Z2 and Z3 are highlighted in dashed circles. Embryos at the ∼100–200 cell stage are shown in H–K. The exposure time for poly(A) mRNA puncta in atg-3 mutants shown here was shorter than that shown in Fig. 2 C and Fig. 4 A to avoid overexposure in atg-3; snr-4(RNAi), prp-19(RNAi); atg-3 and npp-9(RNAi); atg-3 embryos. (L and M) Quantification of the number of somatic poly(A) mRNA puncta (L) and % of PGL granules positive for poly(A) mRNA per focal plane in the indicated genotypes at 26°C (M). Data are shown as mean ± SEM (n = 5; n refers to five images from the corresponding five embryos analyzed for each genotype). Two-tailed, unpaired t test results: **P < 0.01, ***P < 0.001. (N) sfGFP::PAB-1 is diffusely localized in the cytoplasm of C. elegans embryos at 26°C. An embryo at the ∼200 cell stage is shown. (O and P) A large number of poly(A) mRNA puncta accumulate in dcr-1(bp132) mutant embryos at 26°C. The puncta are colocalized with P granules in germ precursor cells (highlighted in dashed circles) and PGL granules in somatic cells. Quantification of the fluorescence intensity of poly(A) puncta in somatic cells in WT and dcr-1(bp132) mutant embryos at 26°C is shown in P. Data are shown as mean ± SEM (n = 24 for WT and n = 35 for dcr-1; n refers to the number of poly(A) mRNA puncta analyzed for each genotype). Two-tailed, unpaired t test result: **P < 0.01. An embryo at the ∼100–200 cell stage is shown in O. (Q–S)dcr-1(bp132) mutant embryos show accumulation of PGL granules at 26°C, detected by anti-SEPA-1, as in WT embryos. S shows the quantification of the number of PGL granules in WT and dcr-1(bp132) mutants at 26°C. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test result: n.s.: no significant difference. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in Q and R. Scale bars: 5 µm for A–F, H–K, N, O, Q, and R; 2 µm for enlarged images in H–K and O.
We examined whether the sorting of RNAs into PGL granules in heat-stressed embryos is affected by depleting the identified factors. mRNA-FISH assays showed that mRNA levels localized in PGL granules were reduced in atg-3 mutants by simultaneous ruvb-1(RNAi), pab-1(RNAi), snr-4(RNAi), prp-19(RNAi), and npp-9(RNAi) (Fig. 4, A–E and Fig. S3, H–M). Consistent with the function of SNR-4 and PRP-19 in pre-mRNA processing and NPP-9 in mRNA nucleus-to-cytoplasm transport, the intensity of poly(A) mRNA, especially in the nucleus, was increased in snr-4(RNAi), prp-19(RNAi), and npp-9(RNAi) embryos (Fig. S3, I–K). The identified factors were not enriched in our LC-MS analysis of PGL-3-interacting proteins. The transgenic reporter for the poly(A)-binding protein PAB-1, sfGFP::PAB-1, exhibited a diffuse signal in somatic cells and formed no punctate structures in embryos laid at 26°C (Fig. S3 N). Therefore, the identified factors, at least in part, modulate PGL granule accumulation by controlling the mRNA level but are not themselves components of PGL granules.
Loss of function of factors involved in mRNA processing, transport, and translation reduces the levels of mRNA and IFE-1 partitioning into PGL granules. (A–D) Compared with control(RNAi)-treated atg-3 mutant embryos (A), the number of poly(A) mRNA-positive puncta and also the ratio of PGL granules positive for poly(A) mRNA in somatic cells is significantly decreased in atg-3 mutant embryos with simultaneous ruvb-1(RNAi) (B) and pab-1(RNAi) (C) at 26°C. Embryos at the ∼100–200 cell stage are shown in A–C. The germ precursor cells (Z2, Z3) are highlighted in dashed circles. (D and E) Quantification of the number of somatic poly(A) mRNA puncta (D) and % of PGL granules positive for poly(A) mRNA per focal plane in the indicated genotypes at 26°C (E). Data are shown as mean ± SEM (n = 5; n refers to five images from the corresponding five embryos analyzed for each genotype). Two-tailed, unpaired t test results: ***P < 0.001. (F–J) IFE-1::GFP granules are less in number and weaker in intensity in pab-1(RNAi) (G) and pab-1(RNAi); atg-3(bp412) (I) embryos at 26°C, compared with control(RNAi)-treated WT embryos (F) and atg-3(bp412) embryos (H), respectively. J shows the quantification of the number of IFE-1::GFP granules in the indicated genotypes. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: **P < 0.01, ***P < 0.001. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in F–I. Scale bars: 5 µm for A–C and F–I; 2 µm for enlarged images in A–C.
Loss of function of factors involved in mRNA processing, transport, and translation reduces the levels of mRNA and IFE-1 partitioning into PGL granules. (A–D) Compared with control(RNAi)-treated atg-3 mutant embryos (A), the number of poly(A) mRNA-positive puncta and also the ratio of PGL granules positive for poly(A) mRNA in somatic cells is significantly decreased in atg-3 mutant embryos with simultaneous ruvb-1(RNAi) (B) and pab-1(RNAi) (C) at 26°C. Embryos at the ∼100–200 cell stage are shown in A–C. The germ precursor cells (Z2, Z3) are highlighted in dashed circles. (D and E) Quantification of the number of somatic poly(A) mRNA puncta (D) and % of PGL granules positive for poly(A) mRNA per focal plane in the indicated genotypes at 26°C (E). Data are shown as mean ± SEM (n = 5; n refers to five images from the corresponding five embryos analyzed for each genotype). Two-tailed, unpaired t test results: ***P < 0.001. (F–J) IFE-1::GFP granules are less in number and weaker in intensity in pab-1(RNAi) (G) and pab-1(RNAi); atg-3(bp412) (I) embryos at 26°C, compared with control(RNAi)-treated WT embryos (F) and atg-3(bp412) embryos (H), respectively. J shows the quantification of the number of IFE-1::GFP granules in the indicated genotypes. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: **P < 0.01, ***P < 0.001. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in F–I. Scale bars: 5 µm for A–C and F–I; 2 µm for enlarged images in A–C.
We further investigated whether the partitioning of IFE-1 into PGL granules depends on mRNAs. The numbers of IFE-1::GFP granules were dramatically decreased in pab-1(RNAi), prp-19(RNAi), snr-4(RNAi), and ruvb-1(RNAi) embryos laid at 26°C (Fig. 4, F, G, and J; and data not shown). Moreover, compared to atg-3 mutants, the number and intensity of IFE-1::GFP granules were also dramatically decreased in pab-1(RNAi); atg-3 mutant embryos laid at 26°C (Fig. 4, H–J). Thus, mRNAs promote the recruitment of IFE-1 to PGL granules under heat-stress conditions.
Accumulation of mRNAs in PGL granules promotes the accumulation of PGL granules in embryos under heat stress conditions
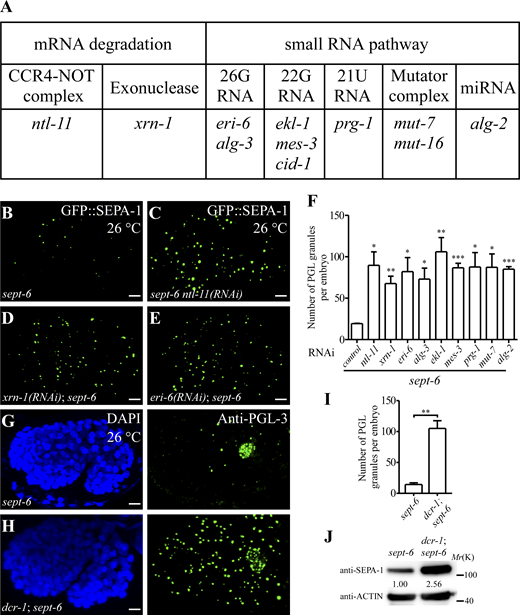
Loss of mTORC1 signaling, such as through mutation of sept-6, which encodes the ortholog of human FNIP2, suppresses the accumulation of PGL granules in heat-stressed embryos (Zhang et al., 2018). We determined whether impaired mRNA degradation restored the accumulation of PGL granules in sept-6 mutant embryos. The CCR4-NOT complex is responsible for most of the poly(A) mRNA turnover activity in C. elegans (Nousch et al., 2013). Exonuclease XRN-1 mediates mRNA degradation (Brook and Gray, 2012). Numerous small RNAs, including 26G sRNA, 22G sRNA, piRNA (21U RNA), and miRNA, are also involved in mRNA degradation in C. elegans embryos (Billi et al., 2014; Valencia-Sanchez et al., 2006). We found that RNAi inactivation of ntl-11 (encoding a component of the CCR4-NOT deadenylase complex), xrn-1, genes encoding components involved in the biogenesis of 26G and 22G sRNA (eri-6, alg-3, ekl-1, mes-3, cid-1, mut-7, mut-15, and mut-16), piRNA (prg-1), and miRNA (alg-2) restored the accumulation of PGL granules in sept-6 mutants at 26°C (Fig. 5, A–F). DCR-1 is involved in the generation of small RNAs (Billi et al., 2014). The formation of PGL granules was also restored in dcr-1(bp132); sept-6 mutants at 26°C (Fig. 5, G–I). The protein level of SEPA-1 was significantly restored in dcr-1; sept-6 mutants compared with sept-6 single mutants at 26°C (Fig. 5 J), which indicates that PGL granules evade autophagic degradation in the double mutants. The RNA FISH assay revealed that more mRNAs accumulated in PGL granules in dcr-1 mutants than in wild type at 26°C (Fig. S3, O and P). Loss of activity of dcr-1 or genes involved in small RNA biogenesis had no effect on the number of PGL granules in embryos at 26°C (Fig. S3, Q–S; and data not shown) or on the accumulation of PGL granules at 20°C (data not shown). Thus, the effect of factors involved in small RNA biogenesis on PGL granule accumulation is evident in sensitive genetic backgrounds.
Loss of function of factors involved in mRNA turnover restores PGL granule accumulation in sept-6 mutants. (A) Summary of identified RNAi inactivations that restore PGL granule accumulation in sept-6 mutants under heat stress conditions. (B–E) Compared with control(RNAi)-treated sept-6(tm6608) mutant embryos at 26°C (B), many more PGL granules, labeled by GFP::SEPA-1, are formed in sept-6 mutant embryos treated with ntl-11(RNAi) (C), xrn-1(RNAi) (D), and eri-6(RNAi) (E). (F) Quantification of the number of PGL granules per embryo in the indicated genetic backgrounds at 26°C. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: *P < 0.05, **P < 0.01, ***P < 0.001. (G–I) Compared to sept-6(tm6608) mutant embryos (G), many more PGL granules, detected by anti-PGL-3, are formed in dcr-1(bp132); sept-6(tm6608) mutant embryos (H) at 26°C. I shows the quantitative data (mean ± SEM, n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test result: **P < 0.01. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in B–E, G, and H. (J) The protein level of SEPA-1 is increased in extracts of dcr-1(bp132); sept-6(tm6608) mutant embryos compared with sept-6(tm6608) embryos at 26°C. Levels of SEPA-1 are normalized with the corresponding ACTIN level. Scale bars: 5 µm for B–E, G, and H. Source data are available for this figure: SourceData F5.
Loss of function of factors involved in mRNA turnover restores PGL granule accumulation in sept-6 mutants. (A) Summary of identified RNAi inactivations that restore PGL granule accumulation in sept-6 mutants under heat stress conditions. (B–E) Compared with control(RNAi)-treated sept-6(tm6608) mutant embryos at 26°C (B), many more PGL granules, labeled by GFP::SEPA-1, are formed in sept-6 mutant embryos treated with ntl-11(RNAi) (C), xrn-1(RNAi) (D), and eri-6(RNAi) (E). (F) Quantification of the number of PGL granules per embryo in the indicated genetic backgrounds at 26°C. Data are shown as mean ± SEM (n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test results: *P < 0.05, **P < 0.01, ***P < 0.001. (G–I) Compared to sept-6(tm6608) mutant embryos (G), many more PGL granules, detected by anti-PGL-3, are formed in dcr-1(bp132); sept-6(tm6608) mutant embryos (H) at 26°C. I shows the quantitative data (mean ± SEM, n = 3; n refers to the number of embryos analyzed for each genotype). Two-tailed, unpaired t test result: **P < 0.01. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in B–E, G, and H. (J) The protein level of SEPA-1 is increased in extracts of dcr-1(bp132); sept-6(tm6608) mutant embryos compared with sept-6(tm6608) embryos at 26°C. Levels of SEPA-1 are normalized with the corresponding ACTIN level. Scale bars: 5 µm for B–E, G, and H. Source data are available for this figure: SourceData F5.
mRNAs modulate the biophysical material properties of PGL condensates
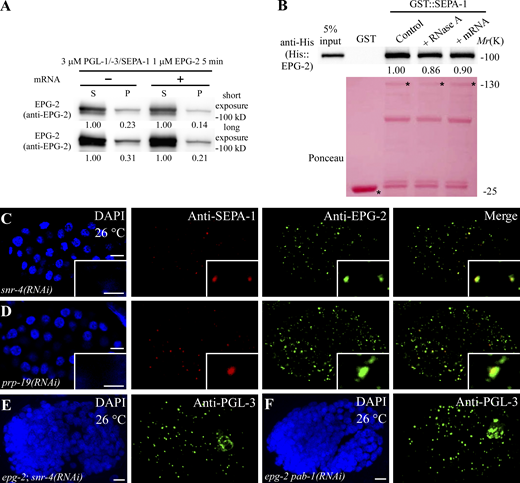
We next determined the effect of RNAs on PGL condensates in in vitro LLPS assays. In in vitro LLPS assays, mRNA-Alexa 488 (C. elegans total mRNAs labeled by Alexa Fluor 488 dye) was partitioned into PGL-1/PGL-3::mCherry/SEPA-1 condensates (3 µM for each protein; Fig. 6, A and B). The partitioning was more evident when the mRNA concentration was increased from 10 to 30 ng/μl (Fig. 6, A and B). The mRNA concentration used here was lower than the estimated concentration of mRNAs in early C. elegans embryos, which is ∼50 ng/μl (Saha et al., 2016). At a concentration of 0.2 µM for each protein, PGL-1/-3/SEPA-1 failed to undergo LLPS; however, a few small condensates were formed in the presence of 10 ng/μl mRNA (Fig. S4, A–C). PGL-1/-3/SEPA-1 condensates were enlarged by adding 10 or 30 ng/μl mRNA while adding mRNA pretreated with RNase A had no effect (Fig. 6, C–F and Fig. S4, D–F). In the sedimentation assay, we found that the addition of 30 ng/μl mRNA resulted in more PGL-1, PGL-3, and SEPA-1 proteins separated into the pellet fraction (Fig. 6 G). FRAP analysis showed that after the PGL-1::GFP signal was bleached in a small region of a PGL condensate, the recovery rate was faster and the fractional recovery of fluorescence signal was higher in condensates formed in reactions containing mRNAs (Fig. 6, H and I). The time for two fusing PGL-1/PGL-3::mCherry/SEPA-1 condensates to relax into a spherical shape was ∼10 s, which was reduced to ∼6 s for condensates containing mRNAs (Fig. 6 J). The recruitment of TagRFP::IFE-1 was slightly increased in the presence of mRNAs in the in vitro LLPS assay (Fig. S4, G–I). Taken together, these results provide evidence that mRNAs promote LLPS and also the liquidity of PGL condensates.
mRNA promotes LLPS and modulates the material properties of PGL condensates. (A and B) Alexa 488-labeled C. elegans total mRNAs (mRNA-Alexa 488) are partitioned into PGL condensates formed by purified PGL-1, PGL-3::mCherry, and SEPA-1 proteins (3 µM for each). More RNAs are detected in condensates when 30 ng/μl mRNA is added into the reaction compared with 10 ng/μl mRNA. (C–E) Adding mRNA enlarges the size of PGL-1/-3/SEPA-1 condensates. DIC images show condensates formed by 3 µM PGL-1/-3/SEPA-1 with no mRNA (C), 30 ng/μl mRNA (D), or 30 ng/μl mRNA pretreated with 0.5 µg/ml RNase A (E). (F) Column scatter charts of the diameters of condensates shown in C–E. Data are shown as mean ± SEM of condensates combined from three fields (220 × 166 µm) for each reaction (n = 142 for no mRNA, 121 for 10 ng/μl mRNA, and 130 for 30 ng/μl mRNA pretreated with 0.5 µg/ml RNase A; n refers to the total number of condensates analyzed for each reaction). Two-tailed, unpaired t test results: n.s.: no significant difference, ***P < 0.001. (G) Sedimentation assays showing that mRNA promotes the partitioning of PGL-1, PGL-3, and SEPA-1 proteins into condensates. S: supernatant, P: pellet. Protein levels in the pellet are normalized by the levels of the proteins in the corresponding supernatants, which is set to 1.00. (H and I) FRAP analysis of PGL-1::GFP/PGL-3/SEPA-1 condensates and PGL-1::GFP/PGL-3/SEPA-1/mRNA condensates. (I) shows quantitative FRAP data presented as mean ± SEM (n = 5; n refers to the number of bleached condensates for each reaction). (J) Adding 30 ng/μl mRNA decreases the time for two PGL-1/PGL-3::mCherry/SEPA-1 condensates to relax into a larger spherical one. The time refers to imaging time. The time for fusion (from the encounter of two condensates until complete relaxation into a larger spherical condensate) is shown as mean ± SEM (n = 10; n refers to the number of fusion events analyzed for each reaction). Two-tailed, unpaired t test results: ***P < 0.001. Scale bars: 5 µm for A and B and enlarged images in C–E; 2 µm for H and J and enlarged images in A and B; 20 µm for C–E. Source data are available for this figure: SourceData F6.
mRNA promotes LLPS and modulates the material properties of PGL condensates. (A and B) Alexa 488-labeled C. elegans total mRNAs (mRNA-Alexa 488) are partitioned into PGL condensates formed by purified PGL-1, PGL-3::mCherry, and SEPA-1 proteins (3 µM for each). More RNAs are detected in condensates when 30 ng/μl mRNA is added into the reaction compared with 10 ng/μl mRNA. (C–E) Adding mRNA enlarges the size of PGL-1/-3/SEPA-1 condensates. DIC images show condensates formed by 3 µM PGL-1/-3/SEPA-1 with no mRNA (C), 30 ng/μl mRNA (D), or 30 ng/μl mRNA pretreated with 0.5 µg/ml RNase A (E). (F) Column scatter charts of the diameters of condensates shown in C–E. Data are shown as mean ± SEM of condensates combined from three fields (220 × 166 µm) for each reaction (n = 142 for no mRNA, 121 for 10 ng/μl mRNA, and 130 for 30 ng/μl mRNA pretreated with 0.5 µg/ml RNase A; n refers to the total number of condensates analyzed for each reaction). Two-tailed, unpaired t test results: n.s.: no significant difference, ***P < 0.001. (G) Sedimentation assays showing that mRNA promotes the partitioning of PGL-1, PGL-3, and SEPA-1 proteins into condensates. S: supernatant, P: pellet. Protein levels in the pellet are normalized by the levels of the proteins in the corresponding supernatants, which is set to 1.00. (H and I) FRAP analysis of PGL-1::GFP/PGL-3/SEPA-1 condensates and PGL-1::GFP/PGL-3/SEPA-1/mRNA condensates. (I) shows quantitative FRAP data presented as mean ± SEM (n = 5; n refers to the number of bleached condensates for each reaction). (J) Adding 30 ng/μl mRNA decreases the time for two PGL-1/PGL-3::mCherry/SEPA-1 condensates to relax into a larger spherical one. The time refers to imaging time. The time for fusion (from the encounter of two condensates until complete relaxation into a larger spherical condensate) is shown as mean ± SEM (n = 10; n refers to the number of fusion events analyzed for each reaction). Two-tailed, unpaired t test results: ***P < 0.001. Scale bars: 5 µm for A and B and enlarged images in C–E; 2 µm for H and J and enlarged images in A and B; 20 µm for C–E. Source data are available for this figure: SourceData F6.
mRNA promotes LLPS and modulates the material properties of PGL condensates, related toFig. 6 . (A–C) Purified PGL-1/-3/SEPA-1 proteins at 0.2 μM for each fail to undergo LLPS, while a few small condensates are formed in the presence of 10 ng/μl mRNA. C shows the average number of condensates in A and B. Data are shown as mean ± SEM (n = 3; n refers to the number of randomly selected fields (220 × 166 µm) for each reaction). (D–F) Adding mRNA enlarges the size of condensates formed by purified PGL-1/-3/SEPA-1 proteins (1 µM for each protein). (F) shows column scatter charts of the diameters of condensates in D and E. Data are shown as mean ± SEM of condensates combined from three fields (220 × 166 µm) for each reaction (n = 106 for no mRNA and n = 154 for 10 ng/μl mRNA; n refers to the total number of condensates analyzed for each reaction). Two-tailed, unpaired t test result: ***P < 0.001. (G–I) Adding mRNA slightly increases the partitioning of TagRFP::IFE-1 into PGL-1/-3/SEPA-1 condensates (3 µM for each protein). (I) shows the mean TagRFP::IFE-1 fluorescence intensity per AU in condensates formed by 3 µM PGL-1/-3/SEPA-1/TagRFP::IFE-1 with or without the addition of 30 ng/μl mRNA. Data are shown as mean ± SEM (n = 27; n refers to the number of condensates analyzed for each reaction). Two-tailed, unpaired t test result: ***P < 0.001. (J–L) DIC images showing that addition of mRNA enlarges the size of condensates formed by PGL-1/-3/SEPA-1/GFP::EPG-2 (3 µM for each). (L) shows column scatter charts of the diameters of condensates in J and K. Data are shown as mean ± SEM of condensates combined from three fields (128 × 90 µm) for each reaction (n = 160 for no mRNA and n = 139 for 30 ng/μl mRNA; n refers to the total number of condensates analyzed for each reaction). Two-tailed, unpaired t test result: ***P < 0.001. (M) Protein gels for the inputs used in the in vitro LLPS assay (asterisks indicate the corresponding bands). Scale bars: 20 µm for A, B, D, E, G, H, J, and K; 5 µm for enlarged images in A, B, D, E, J, and K. Source data are available for this figure: SourceData FS4.
mRNA promotes LLPS and modulates the material properties of PGL condensates, related toFig. 6 . (A–C) Purified PGL-1/-3/SEPA-1 proteins at 0.2 μM for each fail to undergo LLPS, while a few small condensates are formed in the presence of 10 ng/μl mRNA. C shows the average number of condensates in A and B. Data are shown as mean ± SEM (n = 3; n refers to the number of randomly selected fields (220 × 166 µm) for each reaction). (D–F) Adding mRNA enlarges the size of condensates formed by purified PGL-1/-3/SEPA-1 proteins (1 µM for each protein). (F) shows column scatter charts of the diameters of condensates in D and E. Data are shown as mean ± SEM of condensates combined from three fields (220 × 166 µm) for each reaction (n = 106 for no mRNA and n = 154 for 10 ng/μl mRNA; n refers to the total number of condensates analyzed for each reaction). Two-tailed, unpaired t test result: ***P < 0.001. (G–I) Adding mRNA slightly increases the partitioning of TagRFP::IFE-1 into PGL-1/-3/SEPA-1 condensates (3 µM for each protein). (I) shows the mean TagRFP::IFE-1 fluorescence intensity per AU in condensates formed by 3 µM PGL-1/-3/SEPA-1/TagRFP::IFE-1 with or without the addition of 30 ng/μl mRNA. Data are shown as mean ± SEM (n = 27; n refers to the number of condensates analyzed for each reaction). Two-tailed, unpaired t test result: ***P < 0.001. (J–L) DIC images showing that addition of mRNA enlarges the size of condensates formed by PGL-1/-3/SEPA-1/GFP::EPG-2 (3 µM for each). (L) shows column scatter charts of the diameters of condensates in J and K. Data are shown as mean ± SEM of condensates combined from three fields (128 × 90 µm) for each reaction (n = 160 for no mRNA and n = 139 for 30 ng/μl mRNA; n refers to the total number of condensates analyzed for each reaction). Two-tailed, unpaired t test result: ***P < 0.001. (M) Protein gels for the inputs used in the in vitro LLPS assay (asterisks indicate the corresponding bands). Scale bars: 20 µm for A, B, D, E, G, H, J, and K; 5 µm for enlarged images in A, B, D, E, J, and K. Source data are available for this figure: SourceData FS4.
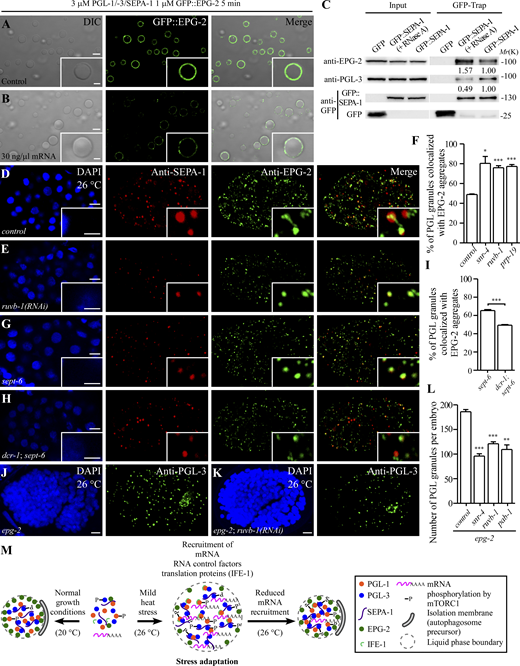
In embryos laid under heat stress conditions, the association of EPG-2 with PGL granules is decreased, while autophagic degradation of EPG-2 still proceeds (Zhang et al., 2018). EPG-2 coats the surface of PGL-1/-3/SEPA-1 condensates in vitro and decreases the size of PGL condensates by promoting a liquid-to-gel-like transition (Zhang et al., 2018). Addition of mRNAs enlarged PGL-1/-3/SEPA-1/GFP::EPG-2 condensates (Fig. S4, J–M). The recruitment of GFP::EPG-2 to PGL-1/-3/SEPA-1 condensates was decreased in reactions containing mRNAs (Fig. 7, A and B; and Fig. S5 A). Therefore, mRNAs in PGL condensates impair the recruitment of EPG-2.
mRNA regulates the recruitment of EPG-2 to PGL granules. (A and B) Purified GFP::EPG-2 (1 µM) protein coats the surface of PGL-1/-3/SEPA-1 condensates (3 µM for each; A). Adding 30 ng/μl mRNA greatly decreases the level of GFP::EPG-2 coated on the surface of PGL-1/-3/SEPA-1 condensates (B). (C) In GFP-Trap assays, the level of endogenous PGL-3 coimmunoprecipitated by GFP::SEPA-1 is decreased, while the level of endogenous EPG-2 precipitated by GFP::SEPA-1 is increased, in embryonic extracts pretreated with RNase A compared with control embryonic extracts. The level of endogenous EPG-2 and PGL-3 precipitated by GFP::SEPA-1 was normalized by GFP::SEPA-1 and set to 1.0 under normal conditions. (D and E) In control embryos at 26°C (D), EPG-2 aggregates are partially colocalized with PGL granules labeled by anti-SEPA-1. The colocalization is increased in ruvb-1(RNAi) embryos (E). (F) Quantification of the colocalization ratio of EPG-2 aggregates and PGL granules in control, snr-4(RNAi), ruvb-1(RNAi), and prp-19(RNAi) embryos at 26°C. Data are shown as mean ± SEM (n = 3; n refers to three images from the corresponding three embryos analyzed for each genotype). Two-tailed, unpaired t test results: *P < 0.05, ***P < 0.001. (G–I) Compared with sept-6(tm6608) mutant embryos at 26°C (G), the % of EPG-2 aggregates that are colocalized with PGL granules is decreased in dcr-1(bp132); sept-6(tm6608) mutant embryos (H). (I) shows quantification. Data are shown as mean ± SEM (n = 3; n refers to three images from the corresponding three embryos analyzed for each genotype). Two-tailed, unpaired t test results: ***P < 0.001. Embryos at the ∼100-cell stage are shown in D, E, G, and H. (J and K) Compared to epg-2(bp287) mutant embryos at 26°C (J), the number of PGL granules is reduced by ruvb-1(RNAi) (K). Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in J and K. (L) Quantification of the number of PGL granules per embryo in the indicated genetic backgrounds. Data are shown as mean ± SEM (n = 3; n refers to three images from the corresponding three embryos analyzed for each genotype). Two-tailed, unpaired t test results: **P < 0.01, ***P < 0.001. (M) Model for the role of RNA in regulating the fates of PGL granules under normal growth conditions (20°C) and mild heat stress conditions (26°C). At 20°C, mRNAs and translation proteins (e.g., IFE-1) are excluded from PGL granules, which are efficiently degraded by autophagy. At 26°C, mRNAs, RNA control factors, and translation proteins (e.g., IFE-1) are targeted to PGL granules, protecting them from degradation to facilitate stress adaptation. mRNAs promote LLPS of PGL granules, maintain their liquidity, and reduce the recruitment of EPG-2. Scale bars: 5 µm for A, B, D, E, G, H, J, and K; 2 µm for enlarged images in A, B, D, E, G, and H. Source data are available for this figure: SourceData F7.
mRNA regulates the recruitment of EPG-2 to PGL granules. (A and B) Purified GFP::EPG-2 (1 µM) protein coats the surface of PGL-1/-3/SEPA-1 condensates (3 µM for each; A). Adding 30 ng/μl mRNA greatly decreases the level of GFP::EPG-2 coated on the surface of PGL-1/-3/SEPA-1 condensates (B). (C) In GFP-Trap assays, the level of endogenous PGL-3 coimmunoprecipitated by GFP::SEPA-1 is decreased, while the level of endogenous EPG-2 precipitated by GFP::SEPA-1 is increased, in embryonic extracts pretreated with RNase A compared with control embryonic extracts. The level of endogenous EPG-2 and PGL-3 precipitated by GFP::SEPA-1 was normalized by GFP::SEPA-1 and set to 1.0 under normal conditions. (D and E) In control embryos at 26°C (D), EPG-2 aggregates are partially colocalized with PGL granules labeled by anti-SEPA-1. The colocalization is increased in ruvb-1(RNAi) embryos (E). (F) Quantification of the colocalization ratio of EPG-2 aggregates and PGL granules in control, snr-4(RNAi), ruvb-1(RNAi), and prp-19(RNAi) embryos at 26°C. Data are shown as mean ± SEM (n = 3; n refers to three images from the corresponding three embryos analyzed for each genotype). Two-tailed, unpaired t test results: *P < 0.05, ***P < 0.001. (G–I) Compared with sept-6(tm6608) mutant embryos at 26°C (G), the % of EPG-2 aggregates that are colocalized with PGL granules is decreased in dcr-1(bp132); sept-6(tm6608) mutant embryos (H). (I) shows quantification. Data are shown as mean ± SEM (n = 3; n refers to three images from the corresponding three embryos analyzed for each genotype). Two-tailed, unpaired t test results: ***P < 0.001. Embryos at the ∼100-cell stage are shown in D, E, G, and H. (J and K) Compared to epg-2(bp287) mutant embryos at 26°C (J), the number of PGL granules is reduced by ruvb-1(RNAi) (K). Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in J and K. (L) Quantification of the number of PGL granules per embryo in the indicated genetic backgrounds. Data are shown as mean ± SEM (n = 3; n refers to three images from the corresponding three embryos analyzed for each genotype). Two-tailed, unpaired t test results: **P < 0.01, ***P < 0.001. (M) Model for the role of RNA in regulating the fates of PGL granules under normal growth conditions (20°C) and mild heat stress conditions (26°C). At 20°C, mRNAs and translation proteins (e.g., IFE-1) are excluded from PGL granules, which are efficiently degraded by autophagy. At 26°C, mRNAs, RNA control factors, and translation proteins (e.g., IFE-1) are targeted to PGL granules, protecting them from degradation to facilitate stress adaptation. mRNAs promote LLPS of PGL granules, maintain their liquidity, and reduce the recruitment of EPG-2. Scale bars: 5 µm for A, B, D, E, G, H, J, and K; 2 µm for enlarged images in A, B, D, E, G, and H. Source data are available for this figure: SourceData F7.
mRNA regulates the recruitment of EPG-2 to PGL granules, related toFig. 7,. (A) Sedimentation assays showing that mRNA reduces the level of GFP::EPG-2 that partitions into condensates. (B) The direct interaction between SEPA-1 and EPG-2 is not evidently affected by RNase A treatment or the addition of 30 ng/μl mRNA in the reactions in the in vitro GST pull-down assay. Asterisks indicate bands with the expected molecular mass. The level of EPG-2 pulled down by GST::SEPA-1 was set to 1.0 in control conditions. (C and D) Compared with control embryos shown in (Fig. 7 D), the colocalization ratio of EPG-2 aggregates and PGL granules, detected by anti-SEPA-1, is increased in snr-4(RNAi) (C) and prp-19(RNAi) (D) embryos at 26°C. Embryos at the ∼100-cell stage are shown in C and D. (E and F) Compared to epg-2 mutant embryos shown in (Fig. 7 J), the number of PGL granules, detected by PGL-3 antibody, is reduced by simultaneous snr-4(RNAi) (E) and pab-1(RNAi) (F) at 26°C. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in E and F. Scale bars: 5 µm for C–F; 2 µm for enlarged images in C and D. Source data are available for this figure: SourceData FS5.
mRNA regulates the recruitment of EPG-2 to PGL granules, related toFig. 7,. (A) Sedimentation assays showing that mRNA reduces the level of GFP::EPG-2 that partitions into condensates. (B) The direct interaction between SEPA-1 and EPG-2 is not evidently affected by RNase A treatment or the addition of 30 ng/μl mRNA in the reactions in the in vitro GST pull-down assay. Asterisks indicate bands with the expected molecular mass. The level of EPG-2 pulled down by GST::SEPA-1 was set to 1.0 in control conditions. (C and D) Compared with control embryos shown in (Fig. 7 D), the colocalization ratio of EPG-2 aggregates and PGL granules, detected by anti-SEPA-1, is increased in snr-4(RNAi) (C) and prp-19(RNAi) (D) embryos at 26°C. Embryos at the ∼100-cell stage are shown in C and D. (E and F) Compared to epg-2 mutant embryos shown in (Fig. 7 J), the number of PGL granules, detected by PGL-3 antibody, is reduced by simultaneous snr-4(RNAi) (E) and pab-1(RNAi) (F) at 26°C. Maximum-intensity projections of Z-stack confocal images of comma-stage embryos are shown in E and F. Scale bars: 5 µm for C–F; 2 µm for enlarged images in C and D. Source data are available for this figure: SourceData FS5.
mRNAs modulate the recruitment of EPG-2 to PGL granules and autophagic degradation of PGL granules in heat-stressed epg-2 mutant embryos
We next determined whether mRNAs also have an effect on the recruitment of EPG-2 to PGL granules in embryos. We prepared extracts of embryos grown at 26°C, then pretreated the extracts with RNase A before performing the GFP-trap assay. RNase A treatment reduced the association of PGL-3 and SEPA-1 while increasing the association of SEPA-1 and EPG-2 (Fig. 7 C). RNase A treatment or adding 30 ng/μl mRNA in the reaction had no effect on the binding of EPG-2 and SEPA-1 in the in vitro GST pull-down assay (Fig. S5 B). SEPA-1 directly interacts with PGL-3 and EPG-2 (Zhang et al., 2018). These results indicate that mRNA may indirectly modulate the association of SEPA-1 and EPG-2, at least in part through affecting the interaction between SEPA-1 and PGL-3, in embryos grown at 26°C.
We further examined whether factors affecting the sorting of mRNAs into PGL granules have an effect on the recruitment of EPG-2 to PGL granules in embryos. We found that prp-19(RNAi), snr-4(RNAi), and ruvb-1(RNAi) increased the colocalization of EPG-2 aggregates with PGL granules labeled by anti-SEPA-1 in ∼100-cell-stage embryos under heat stress conditions (Fig. 7, D–F; and Fig. S5, C and D). The colocalization of EPG-2 aggregates with PGL granules was also increased in sept-6 mutants compared with wild-type embryos at 26°C. In dcr-1; sept-6 mutants, the colocalization rate was reduced compared with sept-6 mutants (Fig. 7, G–I).
EPG-2-mediated transitioning of PGL granules to a gel-like state facilitates their autophagic degradation (Zhang et al., 2018). Mutant PGL proteins, which autonomously induce gelation, render the autophagic degradation of PGL granules independent of EPG-2 (Zhang et al., 2018). We determined whether factors affecting the sorting of mRNAs affect the autophagic degradation of PGL granules in epg-2 mutants. We found that pab-1(RNAi), ruvb-1(RNAi), or snr-4 (RNAi) partially suppressed PGL granule accumulation in epg-2 mutants at 26°C (Fig. 7, J–L; and Fig. S5, E and F). This suggests that PGL granules in these mutants can undergo both EPG-2-dependent and -independent autophagic degradation. Taken together, these results indicate that the sorting of mRNAs into PGL granules in heat-stressed embryos inhibits the recruitment of EPG-2 and also the gelation of PGL granules.
Discussion
PGL granules are RNPs that confer stress resistance
Here, we found that PGL granules in embryos laid by animals experiencing mild heat stress contain mRNAs and translation machinery. These PGL granules serve to store mRNAs and translation factors, and/or as sites for the regulation of mRNA stability and translation to confer stress adaptation (Fig. 7 M). PGL granules are distinct from SGs in composition and assembly/disassembly dynamics. PGL granules contain oocyte-derived components (e.g., PGL-1/-3 and IFE-1) and the zygotically synthesized SEPA-1, while SGs contain GTBP-1, PQN-59, TIAR-1, PAB-1, and IFE-2 (Abbatemarco et al., 2021; Gallo et al., 2008; Rousakis et al., 2014; Sfakianos et al., 2018). PGL granules are formed in embryos laid by animals experiencing mild heat stress (26°C), while SGs are induced in embryos subject to harsh heat stress (30°C; Abbatemarco et al., 2021). When the animals return to normal growth conditions, PGL granules persist until they are degraded by autophagy, while SGs quickly disassemble. Loss of autophagy activity slows the disassembly of a subset of SGs, probably due to the partitioning of misfolded proteins that are normally removed by autophagy or the reduced availability of chaperones for facilitating SG disassembly.
In addition to PGL granules and SGs formed under heat stress, a variety of RNPs are also formed in different cells and at different stages during C. elegans development. Liquid-like germline P granules, which contain PGL-1/-3 and other RNA-binding proteins, function in germ cell differentiation, transcriptome surveillance, and RNA memory (Seydoux, 2018). In the oocyte, translation repressors trigger the formation of RNPs called grP-bodies (grPBs). grPBs, which contain P body proteins, mRNA control factors, and repressed mRNAs, are critical for early development. grPBs can transition between semiliquids and solid lattices, which is regulated by translation repressors and the RNA helicase CGH-1 (Hubstenberger et al., 2013, 2015). The different compositions and biophysical properties of RNP granules may correlate with their distinct functions.
mRNAs control autophagic degradation of protein condensates
RNPs serve as sites for RNA storage, stability, turnover, and translation. RNAs modulate the assembly and material states of RNPs. Low RNA/protein ratios facilitate and maintain the liquidity of RNPs, while high RNA/protein ratios inhibit LLPS of RBPs (Lin et al., 2015; Molliex et al., 2015; Maharana et al., 2018; Tauber et al., 2020). RNA control factors also modulate the phase transition of RNPs. The RNA helicase CGH-1 prevents grPBs from transitioning into nondynamic solids in C. elegans oocytes (Hubstenberger et al., 2013). Depletion of PUF-3/-5/-11, which represses the translation of mRNAs during late oogenesis, inhibits condensation and activates the mobility of GFP::CAR-1 in grPBs (Hubstenberger et al., 2013, 2015). Here, we provide evidence to show that mRNAs themselves act as a switch for changing the fate of resident granules from autophagic degradation to accumulation. Efficient degradation of PGL granules requires the concerted actions of SEPA-1, EPG-2, and posttranslation modifications of PGL-1/-3 to control the assembly rate and size and also to adopt a gel-like state (Noda et al., 2020; Zhang et al., 2018). In heat-stressed embryos, targeting of EPG-2 to PGL granules is impaired, resulting in their escape from autophagic degradation (Zhang et al., 2018). mRNAs in PGL granules modulate several properties of PGL granules to prevent them from degradation. mRNAs facilitate LLPS and maintain the liquidity of PGL condensates and also inhibit EPG-2 recruitment. Loss of function of components that reduce the partitioning of RNAs into PGL granules renders the degradation of PGL granules partially independent of EPG-2. Factors affecting the recruitment of mRNAs into PGL granules also modulate the colocalization of EPG-2 and PGL granules in heat-stressed embryos.
The assembly of RNP condensates is driven by multivalent interactions among proteins and RNAs. PGL-1/-3 are RGG-domain-containing proteins and directly bind to RNAs (Hanazawa et al., 2011). PGL-3 associates with PGL-1 as well as SEPA-1, while SEPA-1 interacts with EPG-2 (Zhang et al., 2009; Tian et al., 2010). In heat-stressed embryos, mRNA translation is inhibited, resulting in the accumulation of translationally stalled mRNAs, which are recruited into PGL granules. RNAs affect the interaction network among components of PGL granules. The RNA control factors identified in our screen may affect the levels of RNAs sorted into PGL granules and/or they may directly modulate RNA–RNA or RNA–protein interactions. Inhibiting mRNA biogenesis and stability decreases, while impairing mRNA degradation increases, the recruitment of mRNAs into PGL granules. Thus, the availability of mRNAs is a determinant for the level of mRNAs sorted into PGL granules under mild heat stress conditions. The identified factors may also facilitate mRNA trafficking into PGL granules. For example, NPP-9 associates with germline P granules and facilitates mRNA localization to P granules (Sheth et al., 2010). NPP-6/-9 may also promote the association of mRNAs with PGL granules in heat-stressed embryos. The mTORC1/LET-363 complex directly phosphorylates PGL-1/-3, promoting LLPS of PGL-1/-3/SEPA-1 (Zhang et al., 2018). mTORC1/LET-363 signaling, which acts as a stress sensor, may also control the sorting of RNAs into PGL granules by affecting the binding of PGL-1/-3 and/or other RBPs to RNAs, or by modulating the levels of translationally stalled mRNAs. Thus, multiple signaling and RNA metabolic pathways control PGL granule accumulation under heat stress by modulating RNA levels.
RNAs induce accumulation of pathological protein aggregates
Accumulation of pathological protein aggregates is a common feature of degenerative diseases (Menzies et al., 2017; Stolz et al., 2014). Protein aggregates formed by Tau or expanded-polyQ Huntingtin, and a subset of SGs with slow or no disassembly are degraded by autophagy (Buchan et al., 2013; Chitiprolu et al., 2018; Ganassi et al., 2016; Mateju et al., 2017; Rubinsztein, 2006). Abnormal posttranslational modifications such as hyperphosphorylation of Tau (Snead and Gladfelter, 2019; Wegmann et al., 2018) or mutations in disease proteins such as in SG-resident prion-like RBPs (e.g., TDP-43, FUS, hnRNPA1, or hnRNPA2B1) can elicit abnormal phase transition. This in turn renders the protein condensates less susceptible to disassembly and/or autophagic degradation, and consequently leads to pathological accumulation (Ganassi et al., 2016; Lin et al., 2015; Molliex et al., 2015; Murakami et al., 2015; Niaki et al., 2020; Noda et al., 2020; Patel et al., 2015; Ryan et al., 2018; Wang and Zhang, 2019).
Genetic etiology has been confirmed in only a small percentage of neurodegenerative diseases. For example, familial AD, caused by mutations in the genes encoding presenilin 1 (PS1), PS2, or amyloid precursor protein (APP), accounts for only 1–5% of AD cases. Only 5–10% of ALS cases are familial (Schrank et al., 2020; Talbott et al., 2016). However, accumulation of intracellular neurofibrillary tangles (NFTs) composed of Tau and TDP-43 is the hallmark of most cases of AD and ALS, respectively (Schrank et al., 2020; Talbott et al., 2016; Taylor et al., 2016). Abnormal sequestration of RNAs has been detected in various pathological protein aggregates, including in NFTs in the brains of patients with various neurodegenerative diseases. For example, ∼80% of thioflavine S (TS)-positive NFTs contain RNA in AD brains (Ginsberg et al., 1997; Ginsberg et al., 1998). RNAs are also detected in Tau aggregates in mouse and cellular models of Tau pathologies (Lester et al., 2021). The polyQ inclusions formed by mutant Huntington exon 1 also sequestrate RNAs and machinery involved in ribosome quality control (Ormsby et al., 2020). In ALS-affected cells, RNA foci are detected in the nucleus and cytoplasm (Taylor et al., 2016). Alterations of RNA dynamics in protein condensates also cause abnormal transitions of RNP granules. Mutations in FUS causing static protein-RNA binding (e.g., R244C mutation in FUS) promote phase transition (Niaki et al., 2020; Patel et al., 2015). ALS-linked mutations in UBQLN2 impair its ability to regulate the dynamics of FUS-RNA complexes (Alexander et al., 2018). Abnormal sequestration of RNAs or alterations of RNA dynamics may prevent the removal of FUS-RNA complexes by autophagy. Autophagic degradation of SGs in yeast has been shown to be affected by the mRNA decay pathway (Buchan et al., 2013). Our results provide insights into how RNA accumulation in RNP granules under stress or pathological conditions leads to their escape from autophagic degradation.
Materials and methods
C. elegans strains
All strains were cultured at 20°C on King agar plates seeded with OP50 Escherichia coli. King agar medium: 20.0 g agar, 2.0 g NaCl, 0.55 g Tris.Base, 0.27 g Tris.HCl, 2.7 g peptone, and 3.2 ml cholesterol (5 mg/ml) in 1 L. For mild heat stress (26°C) treatment, L4 and/or young adult hermaphrodites were shifted to 26°C for 16 h (hour) and the laid embryos were used for phenotypic analysis (Zhang et al., 2018). For severe heat stress treatment, worms and embryos were shifted to 33°C for 1 h and recovered for the indicated time.
The following strains were used in this study: N2 Bristol (wild type). LGI: sepa-1(bp1726), sepa-1(bp1365[GFP::sepa-1]), sepa-1(bp1687[TagRFP::sepa-1]), epg-2(bp287), LGIII: dcr-1(bp132), ife-1(bp1725[ife-1::GFP]), LGIV: atg-3(bp412), sept-6(tm6608), gtbp-1(ax2053[gtbp-1::GFP]), pgl-1(gg547[pgl-1::3xflag::tagRFP]), LGV: him-5(e1490), and axIs1464(Ppie-1::gfp::pgl-3, unc-119). Transgenic reporter: bpEx363(Ppab-1::sfGFP::pab-1+unc-76).
CRISPR/Cas9 genome editing in C. elegans
To insert IFE-1::GFP, GFP::SEPA-1, and TagRFP::SEPA-1 at the corresponding endogenous loci, the genomic sequence of GFP or TagRFP and a linker sequence (5′-GGAGGTGGAGGTGGAGCT-3′; Zeng et al., 2019) were inserted at the endogenous locus before the stop codon of ife-1 or after the initiation codon of sepa-1 by CRISPR/Cas9. The corresponding PCR products of repair templates (50 ng/μl) and sgRNAs (20 ng/μl) were coinjected with dpy-10 sgRNA (20 ng/μl) and dpy-10(cn64) sgRNA ssODN repair template (50 nM/μl; Arribere et al., 2014). The F1 Dpy and Rol animals were isolated and screened for proper insertion by fluorescence observation. All insertions were confirmed by sequencing and phenotype analysis. ife-1(bp1725[ife-1::GFP]) are superficially wild type at both 20 and 25°C, and sepa-1(bp1365[GFP::sepa-1]) and sepa-1(bp1687[TagRFP::sepa-1]) have no effect on the formation and autophagic degradation of PGL granules, indicating that the knock-in alleles are functional.
Plasmid construction
To generate IFE-1::GFP, GFP::SEPA-1, and TagRFP::SEPA-1 CRISPR/Cas9 knock-in strains, corresponding repair templates contained 500–1,000 bp homologous arms, GFP, or TagRFP, and the linker sequence were cloned into pDD49.26 vector (1686; Addgene) by ClonExpress MultiS One Step Cloning Kit (C113-02; Vazyme) through KpnI-SacI sites, then the PAM sites of sgRNAs were mutated by PCR based-mutation. sgRNAs were inserted into the pDD162 vector (47549; Addgene) by PCR. To generate sfGFP::PAB-1, 1,971 bp pab-1 promoter sequence, sfGFP cDNA, and the genomic sequence and 548 bp 3′ UTR sequence of pab-1 were amplified and cloned into pDD49.26 vector (1686; Addgene) using a ClonExpress MultiS One Step Cloning Kit (C113-02; Vazyme) through KpnI-SacI sites.
To generate TagRFP::His6::IFE-1b, a cDNA fragment of IFE-1b was amplified and cloned into pET.TagRFP.3C vector. To generate GFP::His6::EPG-2, a cDNA fragment of EPG-2 was amplified and cloned into pET.GFP.3C vector. To generate MBP::His6::SEPA-1, a cDNA fragment of SEPA-1 was amplified and cloned into pET.MBP.3C vector. To generate His6::PGL-1, a cDNA fragment of PGL-1 was amplified and cloned into pET.MEKTR.3C vector. To generate His6::PGL-3, a cDNA fragment of PGL-3 was amplified and cloned into pET.MEKTR.3C vector. His6::EPG-2, His6::PGL-1::GFP, and His6::PGL-3::mCherry were generated by inserting the corresponding sequence into pET28a (69864-3; Addgene). GST::SEPA-1 was generated by inserting SEPA-1 cDNA into pGEX-6p-1 (27-4597-01; Addgene). All the plasmids are confirmed by sequencing. The primers used for plasmid construction are listed in Table S1.
RNAi inactivation experiments in C. elegans
RNAi feeding and RNAi injection were used for RNAi inactivation. For RNAi feeding, RNAi bacterial clones were cultured in Lysogeny broth (containing 100 mg/ml ampicillin and 50 mg/ml tetracycline) for 6–8 h at 37°C and then seeded on NGM agar plates (containing 100 mg/ml ampicillin, 50 mg/ml tetracycline, and 1 mM Isopropyl β-D-1-thiogalactopyranoside) overnight. The embryos/L1 larval animals were plated onto RNAi feeding plates for 48–60 h at 20°C and then shifted to 26°C for 16 h, and the laid embryos were analyzed in initial experiments. If the RNAi clones caused the larval arrest and/or embryonic lethality, mixed-stage animals were fed for 12–24 h and then shifted to 26°C for 16 h, and their embryos were analyzed. To screen for modifiers of GFP::PGL-3 granules under heat stress conditions, 132 RNAi clones targeting various aspects of RNA metabolism, including RNA translation, RNA processing, nucleus-to-cytoplasm transport, P granules, and the endogenous RNAi pathway, were analyzed (Arribere et al., 2020; Billi et al., 2014; Updike and Strome, 2010). For RNAi injection, double-stranded RNA (dsRNA) was generated by annealing the single-stranded RNA (ssRNA) transcribed from T7- and SP6-flanked PCR templates and injected into animals (see Table S1 for the list of primers used for dsRNA synthesis). Injected animals were placed on fresh plates and recovered for 4–6 h at 20°C before shifting to 26°C for 16 h and then the phenotype of the laid embryos was analyzed.
Indirect immunofluorescence assay
Embryos of 40–50 gravid hermaphrodites were extruded on polylysine slides (1%) and then freeze-cracked in liquid nitrogen, methanol (−20°C, 20 min), and acetone (−20°C, 15 min). The embryos were then blocked with PBS (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4) containing 1% BSA for 1 h at room temperature. The embryos were incubated with primary antibodies against SEPA-1 (rabbit; 1:10,000; Zhang et al., 2009), PGL-1 (rabbit; 1:1,000; Zhang et al., 2009), PGL-3 (rat; 1:1,000; Zhang et al., 2009), EPG-2 (rat; 1:1,000; Tian et al., 2010), or DCAP-1 (rat; 1:1,000; Zhang et al., 2009) overnight at 4°C. The embryos were washed in PBS + 0.2% Tween 20 three times (each for 10 min) and incubated with secondary antibodies for 1–2 h at room temperature. The FITC- or TRITC-conjugated secondary antibodies used were as follows: FITC AffiniPure Goat Anti-Rat IgG (1:200; 112-095-003; Jackson ImmunoResearch), TRITC AffiniPure Goat Anti-Rat IgG (1:200; 112-025-003; Jackson ImmunoResearch), and TRITC AffiniPure Goat Anti-Rabbit IgG (1:200; 111-025-003; Jackson ImmunoResearch). After washing three times (each for 10 min) with PBS + 0.2% Tween 20, adding DAPI, covering with coverslips, and sealing the slides with nail polish, the embryos were imaged and analyzed.
Single-molecule fluorescence in situ hybridization (smFISH) in C. elegans embryos
The smFISH probe oligo(dT30)-Alexa Fluor 488 (dT30-488) was synthesized by Invitrogen. Embryos of 40–50 gravid hermaphrodites were extruded on polylysine slides (1%), followed by freeze-cracking in liquid nitrogen, methanol fixation (−20°C, 20 min), five times washing (5 min for each) in PBS + 0.1% Tween 20 and fixation in PBS + 4% PFA at room temperature for 1 h. The embryos were then washed four times (5 min for each) in PBS + 0.1% Tween 20, two times (5 min for each) in 2 × SCC, and one time for 5 min in wash buffer (10% formamide, 2 × SCC). The embryos were then blocked with hybridization buffer (10% formamide, 2 × SCC, 200 µg/ml BSA, 2 mM Ribonucleoside Vanadyl Complex, 0.2 mg/ml yeast total RNA, 10% dextran sulfate) at 37°C for 30 min and incubated with 100 nM dT30-488 probe at 37°C for 16–20 h. Following hybridization, embryos were washed two times (30 min for each) in wash buffer at 37°C, two times (5 min for each) in 2 × SCC, and two times (5 min for each) in PBS-Tween 20 (0.1%). DAPI was added to the embryos, then they were covered with a coverslip and sealed for the indirect immunofluorescence assay.
Protein expression and purification
Recombinant proteins were expressed in E. coli BL21-CodonPlus (DE3) in Lysogeny broth medium at 37°C. The expression of recombinant proteins was induced by adding 300 µM Isopropyl β-D-1-thiogalactopyranoside into the cultured E. coli bacteria (carrying the corresponding expression plasmids) when OD600 reached 0.6–0.8. The bacteria were further cultured for 16 h at 18°C in a shaker at 220 rpm. The bacteria were collected by sedimentation at 5,000 rpm for 15 min, resuspended in binding buffer (50 mM Tris-Cl pH 7.9, 500 mM NaCl, 10 mM imidazole) containing 1 × protease inhibitor cocktail (B14003; Bimake), lysed with a high-pressure homogenizer in binding buffer and sedimented at 18,000 rpm for 45 min at 4°C. The supernatant lysates were affinity-purified by Ni-NTA agarose beads (30210; Qiagen). After extensive washing with binding buffer, the bound proteins were eluted with elution buffer (50 mM Tris-Cl pH 7.9, 500 mM NaCl, and 500 mM imidazole). The eluted proteins were concentrated by centrifugal filtrations and loaded onto desalting columns (17-0851-01; GE Healthcare) and then eluted with a buffer containing 25 mM HEPES pH 7.5 and different concentrations of NaCl (1 M for His::PGL-1, His::PGL-3, His::PGL-3::mCherry, His::PGL-1::GFP, and TagRFP::His::IFE-1b; 500 mM for MBP::His::SEPA-1, GFP::His::EPG-2, and His::EPG-2). The concentrations of purified proteins were measured by Bradford Protein Assay Kit and stored in aliquots at −80°C.
In vitro phase separation assays
Proteins were centrifuged for 10 min at 13,000 g to remove aggregated proteins and then were mixed at a desired ratio and concentration. The concentration of NaCl was adjusted to physiological conditions (150 mM) with buffer (25 mM HEPES pH 7.5) to induce LLPS in a tube. For LLPS assays containing mRNA, LLPS was induced with the indicated concentrations of C. elegans total mRNA extracts, mRNA extracts pretreated with RNase A, or an equal volume of buffer (control, no mRNA). After LLPS induction for 5 min, images were captured on a glass slide using ZEN (blue edition) software (Carl Zeiss) under a fluorescence microscope (Imager M2; Carl Zeiss) with a 40×/0.75 objective lens (E C Plan-NEOFLUAR; Carl Zeiss) and a camera (Axiocam HRm; Carl Zeiss), or in a glass-bottom cell culture dish using ZEN 2.1 SP3 software (Carl Zeiss) under an inverted confocal microscope (LSM 880 or LSM710; Carl Zeiss) with 488 and 561 lasers, a 63×/1.40 oil-immersion objective lens (Plan-Apochromat; Carl Zeiss), and a camera (Axiocam HRm; Carl Zeiss). The images were acquired at room temperature (20–25°C). Images were processed and viewed by ZEN (blue edition) software (Carl Zeiss). The fluorochromes used were Alexa Fluor 488, mCherry, and TagRFP. For the sedimentation assay, after LLPS induction for 5 min, samples were centrifuged at 13,000 g for 5 min. The supernatant and pellet were immediately separated into two tubes and adjusted to equal volumes with LLPS buffer. After adding 5 × SDS loading buffer, the samples were boiled for 10 min and subjected to Western blotting assay.
Fluorescence recovery after photobleaching (FRAP) analysis and condensate fusion analysis
Condensates containing PGL-1::GFP were used for in vitro FRAP analysis. Selected regions were photobleached with a 488 laser, and the fluorescence intensities were collected every 2 s by mean ROI. The signal was normalized to the initial intensity before photobleaching. FRAP recovery curves were generated by GraphPad Prism software. Condensates containing PGL-3::mCherry were used for in vitro analysis of condensate fusion and time series images were collected every 5 s with a 561 laser. The FRAP and condensate fusion experiments were performed in a glass-bottom cell culture dish and imaged by confocal microscopy for the in vitro phase separation assays. Images were processed and viewed by ZEN 2.1 SP3 software (Carl Zeiss). The fluorochromes used were GFP and mCherry.
Imaging of C. elegans embryos
DIC (differential interference contrast) and fluorescence images were captured on a glass slide using ZEN 2.1 SP3 software (Carl Zeiss) under an inverted confocal microscope (LSM 880 or LSM710; Carl Zeiss) with 405, 488, and 561 lasers, a 63×/1.40 oil-immersion objective lens (Plan-Apochromat; Zeiss) and a camera (Axiocam HRm; Zeiss) at room temperature (20–25°C) unless otherwise noted. In figures showing 2D images of embryos, an optimal Z-section plane is presented. In figures showing 3D images of embryos, multiple Z-sections with one interval were taken and displayed as the maximum intensity projection. For sfGFP::PAB-1, images were captured on a glass slide using ZEN (blue edition) software (Carl Zeiss) under a fluorescence microscope (Imager M2; Carl Zeiss) with a 100×/1.4 oil-immersion objective lens (Plan-APOCHROMAT; Carl Zeiss) and a camera (Axiocam HRm; Carl Zeiss). Images were processed and viewed by ZEN (blue edition) software (Carl Zeiss). The fluorochromes used were FITC, TRITIC, Alexa Fluor 488, DAPI, GFP, sfGFP, and TagRFP.
Western blotting
The samples were subjected to SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Signals from target proteins were detected by corresponding primary and secondary antibodies with an imaging system (ChemiScope 6000 Touch, ClinX). The following primary antibodies were used: anti-SEPA-1 (rabbit; 1:10,000; Zhang et al., 2009), anti-PGL-1 (rabbit; 1:1,000; Zhang et al., 2009), anti-PGL-3 (rat; 1:1,000; Zhang et al., 2009), anti-EPG-2 (rat; 1:1,000; Tian et al., 2010), anti-GFP (mouse; 1:2,000; 1814460001; Roche), and anti-His (mouse; 1:2,000; KM8001; Tianjin Sungene Biotech). Actin detected by anti-Actin (mouse; 1:5,000; A3853; Sigma-Aldrich) was used as a loading control. The following secondary antibodies were used: Peroxidase AffiniPure Goat Anti-Rat IgG (1:8,000; 112-035-003; Jackson ImmunoResearch), Peroxidase AffiniPure Goat Anti-Rabbit IgG (1:8,000; 111-035-003; Jackson ImmunoResearch), and Peroxidase AffiniPure Goat Anti-Mouse IgG (1:8,000; 115-035-003; Jackson ImmunoResearch).
In vitro GST pull-down assays
GST or GST::SEPA-1 recombinant proteins were immobilized on glutathione sepharose beads and incubated with His::EPG-2 protein in binding buffer (25 mM Tris-Cl pH 7.6, 1 mM DTT, 1% Triton X-100, 10% glycerol, and 150 mM NaCl), or a binding buffer with 10 µg/ml RNase A, or a binding buffer with 30 ng/μl mRNA. Incubation was carried out with gentle rotation at 4°C for 4 h. After four washes with 1 ml binding buffer, the beads were boiled with 2 × SDS loading buffer for 10 min at 100°C and subjected to Western blot analysis.
Immunoprecipitation assays
Extracts (∼500 μl) of embryos expressing gfp, gfp::pgl-3, or gfp::sepa-1 were incubated with 30 μl GFP-Trap beads (GNA-50-1,000; Lablead) in lysis buffer (25 mM HEPES pH 7.5, 150 mM NaCl, 1 mM DTT, 1 mM EDTA, 0.5% NP40, and 10% glycerol) with protease inhibitor cocktail (B14003; Bimake) for 1 h at 4°C. For RNase A treatment, the extracts were treated with 10 μg/ml RNase A (GE101-01; Transgen Biotech) for 4 h at 4°C before precipitation. After extensive washes in washing buffer (25 mM HEPES pH 7.5, 300 mM NaCl, 1 mM EDTA, 0.5% NP40, and 10% glycerol), the immunoprecipitates were boiled with 2 × SDS loading buffer for 10 min at 100°C. The samples are subjected to Western blot analysis or separated by SDS-PAGE followed by LC-MS/MS analysis.
LC-MS/MS analysis and identification and quantification of proteins
An Orbitrap Exploris 480 (Thermo Fisher Scientific) equipped with an Easy n-LC 1200 HPLC system (Thermo Fisher Scientific) was used to perform all the nanoLC-MS/MS experiments. The digested peptides were loaded onto a 100 μm id×2 cm fused silica trap column packed in-house with reversed phase silica (Reprosil-Pur C18 AQ, 5 μm, Dr. Maisch GmbH) and then separated on a 75 μm id × 25 cm C18 column packed with reversed phase silica (Reprosil-Pur C18 AQ, 1.9 μm, Dr. Maisch GmbH). A 73-min linear gradient was used to elute the column-bound peptides at a flow rate of 300 nl/min. The segmented gradient was 4–9% B, 3 min; 9–20% B, 22 min; 20–30% B, 20 min; 30–40% B, 15 min; 40–95% B, 3 min; and 95% B, 10 min. Solvent A consisted of 0.1% formic acid in water. Solvent B consisted of 0.1% formic acid and 80% acetonitrile.
An Orbitrap Exploris 480 mass spectrometer with the FAIMS Pro interface (Thermo Fisher Scientific) was used to perform the MS analysis. FAIMS separations were performed with two compensation voltages (−45 and −65). For the data-dependent acquisition, the MS data were acquired at a high resolution of 60,000 (m/z 200) across the mass range of 350–1,500 m/z. The target value was 3.00E+06 with a maximum injection time of 22 ms. Data-dependent mode was selected as cycle time mode, which was set as 2 s. The precursor ions were selected from each MS full scan with isolation width of 1.6 m/z for fragmentation in the Ion Routing Multipole with normalized collision energy of 28%. Subsequently, MS/MS spectra were acquired at a resolution 15,000 at m/z 200. The target value was 7.50E+04 with a maximum injection time of 22 ms. The dynamic exclusion time was 40 s. For nanoelectrospray ion source setting, the spray voltage was 2.0 kV. There was no sheath gas flow and the heated capillary temperature was 320°C.
The raw data were processed using the Sequest HT search engine with Proteome Discoverer software (version 2.4.1.15) for protein identification. The C. elegans protein database in Uniprot (updated on 08/2022) was used for searching the data from samples. Searching parameters were set as follows: trypsin for enzyme and two missed cleavages were allowed for searching; 10 ppm for the mass tolerance of precursor; 0.02 D for the product ions tolerance; cysteine carbamidomethylation was specified as fixed modifications; and methionine oxidation was chosen as variable modifications. The false discovery rate (FDR) was set to <1% for protein identification. FDR values were calculated using Percolator. Unique and razor peptides of proteins were used to quantify the relative amounts of proteins. Quantification was carried out with Proteome Discoverer software (version 2.4.1.15) using the areas of identified peptides. Significantly enriched proteins immunoprecipitated by GFP::PGL-3 compared with GFP control were selected with unique peptides >5, log2 fold change ≥1, and P < 0.05. The total peptide amount to corrected experimental bias was selected as the normalization mode. The mass spectrometry proteomics data have been submitted to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al., 2022) partner repository with the dataset identifier PXD040246.
Gene ontology
Significantly enriched proteins in GFP::PGL-3 IP-MS were analyzed by Database for Annotation, Visualization, and Integrated Discovery (DAVID) Bioinformatics Resources for gene ontology (Huang et al., 2009).
Preparation of C. elegans mRNA
C. elegans total RNA was isolated from unsynchronized C. elegans culture using TRIzol (Thermo Fisher Scientific) and dissolved in RNase/DNase-free H2O. Total C. elegans mRNA was isolated using GenElute mRNA Miniprep Kit (Sigma-Aldrich) and labeled using Ulysis Alexa Fluor 488 (Thermo Fisher Scientific). The concentration of RNA was measured by Nanodrop. All experimental procedures followed the standard protocols.
Quantification and statistical analysis
For quantification of the number of PGL granules (GFP::PGL-3, anti-PGL-3, anti-SEPA-1, and GFP::SEPA-1), IFE-1::GFP granules and SQST-1::GFP aggregates, multiple Z-section images were taken with one interval and displayed as the maximum intensity projection; n refers to the number of embryos for each genotype or condition. For quantification of GTBP-1::GFP granules, poly(A) puncta, and colocalization analysis, n refers to the number of images from the corresponding embryos (3–5 embryos were used) for each genotype or condition. For the mean fluorescence intensity of poly(A) puncta and TagRFP::IFE-1 in PGL condensates, n refers to the total number of puncta/condensates measured for each genotype or each reaction. For determining the size of condensates, n refers to the total number of condensates measured from three randomly selected fields (220 × 166 µm or 128 × 90 µm) for each reaction. For FRAP assays, n refers to the number of bleached condensates for each reaction. For fusion assays, n refers to the number of fusion events analyzed for each reaction. For determining the average number of condensates, n refers to the number of randomly selected fields (220 × 166 µm) for each reaction. For MS analysis, n refers to the number of independent replicates. Graphs and P values were generated by GraphPad Prism 5 unless otherwise noted. Statistical analyses were performed by two-tailed, unpaired Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001, and n.s. represents “no significant difference.” For MS analysis, P values were generated in Excel with the two-tailed, unpaired Student’s t test. For GO-molecular function analysis by DAVID, P values were automatically generated by DAVID. Data distribution was assumed to be normal, but this was not formally tested.
Online supplemental material
Fig. S1 shows the volcano plot and GO-molecular function of the proteomics data and the formation of IFE-1 granules under various conditions. Fig. S2 shows that PGL granules formed under heat stress conditions are distinct from stress granules. Fig. S3 shows that mRNA metabolism factors modulate autophagic degradation and mRNA recruitment of PGL granules in embryos laid under heat-stress conditions. Fig. S4 shows that mRNA promotes LLPS of PGL condensates. Fig. S5 shows that mRNA regulates the recruitment of EPG-2 to PGL granules under mild heat stress conditions. Table S1 lists primers.
Data availability
The reagents are available from the corresponding author upon request. The MS data shown in Fig. S1, A–C are available via ProteomeXchange with the identifier PXD040246.
Acknowledgments
We are grateful to Dr. Isabel Hanson for editing work and Mr. Jifeng Wang from the Laboratory of Proteomics, IBP, for MS analysis.
This work was supported by the following grants to H. Zheng: 82188101 and 31790403 from the National Natural Science Foundation of China and 2017YFA0503401 from the Chinese Ministry of Science and Technology.
Author contributions: H. Zheng and H. Zhang designed the experiments. H. Zheng, K.F. Peng, X.M. Gou, and C. Ju performed experiments. H. Zheng and H. Zhang wrote the manuscript.
References
Author notes
Disclosures: The authors declare no competing interests exist.