The nuclear translocation of YAP1 is significantly implicated in the proliferation, stemness, and metastasis of cancer cells. Although the molecular basis underlying YAP1 subcellular distribution has been extensively explored, it remains to be elucidated how the nuclear localization signal guides YAP1 to pass through the nuclear pore complex. Here, we define a globular type of nuclear localization signal composed of folded WW domains, named as WW-NLS. It directs YAP1 nuclear import through the heterodimeric nuclear transport receptors KPNA−KPNB1, bypassing the canonical nuclear localization signal that has been well documented in KPNA/KPNB1-mediated nuclear import. Strikingly, competitive interference with the function of the WW-NLS significantly attenuates YAP1 nuclear translocation and damages stemness gene activation and sphere formation in malignant breast cancer cells. Our findings elucidate a novel globular type of nuclear localization signal to facilitate nuclear entry of WW-containing proteins including YAP1.
Introduction
Yes1-associated transcriptional factor, YAP1, shuttles between the cytoplasm and the nucleus, facilitating cell proliferation, tissue regeneration, and organ development (von Gise et al., 2012; Reginensi et al., 2013; Russell and Camargo, 2022; Yu et al., 2015; Zhao et al., 2007). In particular, nuclear-localized YAP1 has been significantly implicated in the occurrence of most human solid tumors, the maintenance of cancer cell stemness, as well as metastasis (Moroishi et al., 2015; Russell and Camargo, 2022; Yu et al., 2015). The core mechanism underlying the YAP1 nucleocytoplasmic shuttling is elucidated by the inhibitory Hippo pathway composed of a kinase cascade of mammalian STE20-like protein kinase MST1/2 (STK4/3) and the large tumor suppressor 1/2 (LATS1/2). When the Hippo pathway is activated, for example, in the case of cell–cell contact, YAP1 can be phosphorylated at Ser-127 by the MST−LATS kinase cascade, resulting in its association with the cytoplasmic protein 14-3-3 (Basu et al., 2003). As a consequence, YAP1 is sequestered in the cytoplasm. After escaping from the control of the Hippo pathway, YAP1 enters the nucleus and binds to TEA domain DNA-binding factors (TEAD1-4) to facilitate gene transcription (Moroishi et al., 2015; Yu et al., 2015).
In addition to the Hippo pathway, a variety of mechanisms implicated in YAP1 subcellular localization have been intensively explored. For instance, O-GlcNAcylation, catalyzed by O-GlcNAc transferase at Ser-109, masks Ser-127 phosphorylation of YAP1, resulting in YAP1 nuclear accumulation (Peng et al., 2017). SET7-mediated monomethylation at Lys-494 sequestrates YAP1 in the cytoplasm (Oudhoff et al., 2013). Loss-of-function of FAT1 activates a CAMK2−CD44−SRC axis to promote YAP1 nuclear translocation (Pastushenko et al., 2021). K63-linked non-proteolytic ubiquitination of YAP1 enhances its interaction with TEAD family proteins, thereby inducing its nuclear retention (Yao et al., 2018). Moreover, increased stiffness of the extracellular matrix can also lead to nuclear translocation of YAP1 (Aragona et al., 2013; Dupont et al., 2011; Elosegui-Artola et al., 2017). This regulation requires RHO GTPase activity and tension of the F-actin cytoskeleton but is apparently independent of the Hippo pathway. Although the regulatory mechanism underlying YAP1 subcellular distribution has been extensively investigated in the past decades, it remains to be studied how YAP1 overcomes the barrier of the nuclear pore complex (NPC) and enters the nucleus in mammalian cells.
The NPC allows diffusion-like transport of small molecules such as ions, nucleotides, and proteins smaller than 40 kDa, while it limits the transport of macromolecules >5 nm in diameter or 40 kDa in size unless they are chaperoned by nuclear transport receptors (NTRs) (Knockenhauer and Schwartz, 2016). Most nucleocytoplasmic transports are assisted by the karyopherin β (kap-β) family NTRs, which consist of ten importins, five exportins, three biportins, and two kaps with unknown functions in humans (Wing et al., 2022). Except for β-family karyopherins, there are also α-family karyopherins (KPNA), which specifically serve as the adaptor of karyopherin β1 (kapβ1, KPNB1) to recruit the transported cargoes. An outstanding feature of kap-β–mediated nuclear transport is dependent on RAN-GTPase. Basically, the RAN-GTPase exists in two states: GTP-bound and GDP-bound. Most of RAN-GTP is localized in the nucleus, where it binds to kap-β importins in a mutually exclusive manner with the imported cargoes, leading to dissociation of the imported cargoes from importins. Subsequently, RAN-GTP–bound kap-β importins are transported out of the nucleus. In the cytoplasm, RAN-GTP is hydrolyzed to RAN-GDP releasing kap-β importins to enter a new round of nuclear import. Unlike importins, the kap-β exportins and RAN-GTP form ternary complexes with cargoes in the nucleus, thereafter crossing the NPC to the cytoplasm, where they disassemble following RAN-GTP hydrolysis (Gorlich et al., 1996; Kalita et al., 2021; Vetter et al., 1999; Wing et al., 2022).
In principle, NTRs of the kap-β family recognize and bind the nuclear localization signal (NLS) or nuclear export signal (NES) carried by the transported cargoes. Currently, four classes of NLS have been identified: the canonical NLS (cNLS) recognized by the heterodimeric KPNA−KPNB1, the proline–tyrosine NLS recognized by karyopherin β2/2b (TNPO1/2), the arginine–serine repeat NLS recognized by transportin-3 (TNPO3), and the isoleucine–lysine NLS recognized by karyopherin β3 (KAP121, IPO5) (Wing et al., 2022). Unlike NLS, only one class of NES has been identified. It is usually 8–15 residues long, contains 4–5 hydrophobic residues, and can be specifically recognized by exportin-1 (also known as CRM1) (Azmi et al., 2021). Fang et al. (2018) reported that YAP1 harbors an NES at the carboxyl-terminal transcriptional activation domain (Fang et al., 2018). Yki, a Drosophila ortholog of mammalian YAP1, had been demonstrated to harbor a non-canonical NLS at the amino terminus, but this NLS does not seem to function in mammals (Wang et al., 2016). García-García et al. (2022) reported a sequence resembling NLS7 on mammalian YAP1 to facilitate the YAP1/importin-7 complex, which is demonstrated to contribute to the mechanical-induced nuclear accumulation of both two proteins (Panagiotopoulos et al., 2021; García-García et al., 2022). Moreover, they observed that YAP1 nuclear translocation could also be implicated in the heterodimeric KPNA−KPNB1. In fact, importin-7 and the KPNA−KPNB1 axis are parallel in nuclear transport (Wing et al., 2022). Therefore, YAP1 nuclear import is actually chaperoned by varied nuclear transport pathways rather than a single transport pathway. Accordingly, YAP1 should have varied types of NLS to assist it in association with different NTRs. Leaving the guidance of its own NLS, YAP1 had also been suggested to be carried into the nucleus by its interaction partners containing the NLS (Kim et al., 2020; Yao et al., 2018). Taken together, the mechanism underlying the passage of YAP1 through the NPC may be diverse for adapting to its complex physiological or pathological roles.
WW domains are composed of 30–40 amino acid residues and featured by two highly conserved tryptophan residues separated by 20–22 amino acid residues, and their spatial structures are formed similarly by three twisted anti-parallel β-sheets (Aragón et al., 2012; Kato et al., 2004; Martinez-Rodriguez et al., 2015; Schulte et al., 2018; Sudol et al., 1995). There are two WW domains located in tandem in the middle of YAP1. Here, we identify that WW domains can function as a novel globular type of NLS (termed WW-NLS) rather than a linear type to mediate YAP1 nuclear entry and transactivation, but are dispensable for the Hippo pathway to catalyze YAP1 phosphorylation. Moreover, the WW-NLS is directly recognized and bound by KPNA bypassing the cNLS, which has been well-documented in the KPNA−KPNB1 axis. Importantly, WW domains derived from different donor proteins other than YAP1 can similarly direct nuclear import and are functionally complementary to the WW domain of YAP1. Finally, we developed a competitive binding peptide based on the WW-bound motif and confirmed that the competitive interference with the WW domain is sufficient to interfere with YAP1 nuclear translocation as well as YAP1-dependent cancer stemness. Therefore, these findings may provide a novel target to combat YAP1-dependent malignancies.
Results
WW domains are dispensable to YAP1 phosphorylation catalyzed by LATS1/2
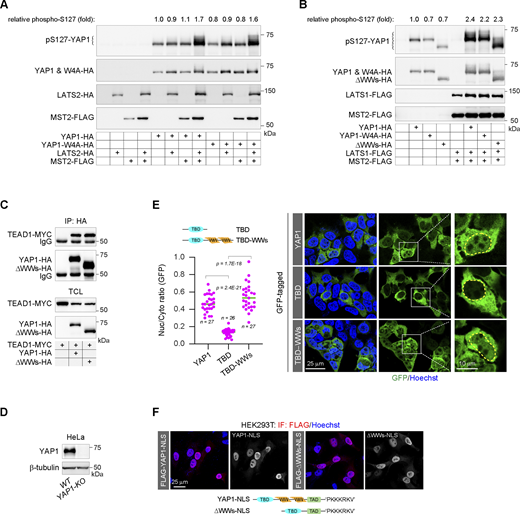
Structurally, YAP1 encompasses an amino-terminal TEAD-binding domain (TBD), a carboxyl-terminal transcriptional activation domain, and a middle tandem WW domain region (Fig. 1 A). The WW domain was first identified on YAP1 (Sudol et al., 1995), but the role of these WW domains in YAP1 activation has not been fully understood. As one of the smallest domains, the WW domain maintains a spatial conformation consisting of three β-sheets through two conserved tryptophan residues and recognizes some specific proline-rich motifs (Fig. 1 A). The MST−LATS kinase cascade catalyzes YAP1 phosphorylation at Ser-127, resulting in YAP1 cytoplasmic retention and ultimately inactivation (Basu et al., 2003; Moroishi et al., 2015; Russell and Camargo, 2022; Yu et al., 2015). Since LATS1/2 harbor the typical proline-rich motif recognized by the WW domain, the role of WW-mediated LATS/YAP1 interaction in YAP1 phosphorylation is explored. We prove that either the substitutions of the conserved tryptophan residues of WW domains to alanine residues (W4A mutant) or the deletion of WW domains (ΔWWs mutant) can result in complete disruption of the interactions of YAP1 with LATS1/2 (Fig. 1, B and C). At the same time, the tandem WW domains (tdWWs)−ΔWW chimera that ectopically restores tandem WW domains to the amino terminus of the ΔWW mutant completely restores their interaction disrupted by WW domain deletion (Fig. 1 D). The results indicate that the WW domains do mediate the interaction of YAP1 with LATS1/2. However, YAP1 phosphorylation mediated by MST−LATS kinase cascade cannot be attenuated by the W4A substitution or the deletion of WW domains (Fig. S1, A and B), indicating that the WW domains, as well as their mediated LATS1/2−YAP1 interaction, seem not to be essential for the YAP1 phosphorylation catalyzed by this kinase cascade. Notably, the tdWWs−ΔWW chimera recruits LATS1/2 kinase to the amino terminus of YAP1, leading to the conformational mismatch between the kinase and the phosphorylation site, but the phosphorylation on the chimera cannot be affected (Fig. 1 E), further supporting that the phosphorylation is catalyzed by the free LATS1/2 kinases rather than the WW-bound kinases. Therefore, we demonstrate that such two events, i.e., LATS1/2 catalyzing YAP1 phosphorylation and LATS1/2 interacting with the WW domains of YAP1, are uncoupled. Consistently, the deletion of the WW domains does not affect the interaction between YAP1 and 14-3-3 protein facilitated by the MST−LATS cascade (Fig. 1 F). to explore the effect of WW domains on endogenous YAP1 phosphorylation, CRISPR/Cas9-mediated gene knockout is carried out to deplete YAP1 expression in HEK293T cells (Plouffe et al., 2016), and then the knockout cells are stably restored to the expression of the wild-type YAP1, the W4A mutant, the ΔWWs mutant, or the S127A substituted mutant, respectively. After 12-h culture in the confluence, Ser-127 phosphorylation is analyzed (Fig. 1, G and H). We show that deficiencies of WW domains on YAP1 almost do not affect its Ser-127 phosphorylation (Fig. 1 H). As the control, S127A substitution on YAP1 completely diminishes the phosphorylation (Fig. 1 H). Taken together, the results indicate that the WW domains may not be essential for YAP1 phosphorylation catalyzed by the MST−LATS kinase cascade.
WW domains are dispensable to YAP1 phosphorylation catalyzed by LATS1/2. (A) The schematic diagram illustrates the functional modules of YAP1, the linear feature of the WW domain, and the three-dimensional structures of the WW domains of YAP1 (Protein Data Bank [PDB]: 6JK1). TAD, transcriptional activity domain. (B) The co-IP experiment shows the interaction of FLAG-tagged LATS2 with HA-tagged YAP1 or W4A mutant in HEK293T cells. W4A, alanine substitutions of four conserved tryptophan residues (W177, W199, W236, and W258) in the WW domains of YAP1; TCL, total cell lysates. (C) The co-IP experiment shows the interaction of HA-tagged LATS1 with MYC-tagged YAP1 or YAP1-ΔWWs mutant, which lacks all WW domains, in HEK293T cells. (D) The co-IP experiment shows that the ectopic restoration of the WW domains at the amino terminus of the YAP1-ΔWWs mutant rescues the interaction of LATS2 with YAP1-ΔWWs mutant in HEK293T cells. (E) The representative western blotting shows MST2–LATS2 cascade-mediated phosphorylation of Ser-127 of the wild-type YAP1, the ΔWWs mutant, and the tdWW−ΔWWs chimera in HEK293T cells, and the column chart shows normalized quantitative Ser-127 phosphorylation (quantitative data are obtained from three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). (F) The co-IP experiment shows that deletion of the WW domains does not affect phosphorylation-mediated interaction of YAP1 and 14-3-3 in HEK293T cells. (G) The CRISPR/Cas9-mediated depletion of YAP1 gene expression in HEK293T cells. The representative western blotting shows YAP1 protein content in the wild-type and the knockout (KO) cells. (H) The representative western blotting shows endogenous Ser-127 phosphorylation of YAP1 in HEK293T/YAP1-KO cells, which are stably restored to the expression of the wild-type YAP1, the W4A mutant, the ΔWWs mutant, or the S127A substitution mutant, respectively. Source data are available for this figure: SourceData F1.
WW domains are dispensable to YAP1 phosphorylation catalyzed by LATS1/2. (A) The schematic diagram illustrates the functional modules of YAP1, the linear feature of the WW domain, and the three-dimensional structures of the WW domains of YAP1 (Protein Data Bank [PDB]: 6JK1). TAD, transcriptional activity domain. (B) The co-IP experiment shows the interaction of FLAG-tagged LATS2 with HA-tagged YAP1 or W4A mutant in HEK293T cells. W4A, alanine substitutions of four conserved tryptophan residues (W177, W199, W236, and W258) in the WW domains of YAP1; TCL, total cell lysates. (C) The co-IP experiment shows the interaction of HA-tagged LATS1 with MYC-tagged YAP1 or YAP1-ΔWWs mutant, which lacks all WW domains, in HEK293T cells. (D) The co-IP experiment shows that the ectopic restoration of the WW domains at the amino terminus of the YAP1-ΔWWs mutant rescues the interaction of LATS2 with YAP1-ΔWWs mutant in HEK293T cells. (E) The representative western blotting shows MST2–LATS2 cascade-mediated phosphorylation of Ser-127 of the wild-type YAP1, the ΔWWs mutant, and the tdWW−ΔWWs chimera in HEK293T cells, and the column chart shows normalized quantitative Ser-127 phosphorylation (quantitative data are obtained from three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). (F) The co-IP experiment shows that deletion of the WW domains does not affect phosphorylation-mediated interaction of YAP1 and 14-3-3 in HEK293T cells. (G) The CRISPR/Cas9-mediated depletion of YAP1 gene expression in HEK293T cells. The representative western blotting shows YAP1 protein content in the wild-type and the knockout (KO) cells. (H) The representative western blotting shows endogenous Ser-127 phosphorylation of YAP1 in HEK293T/YAP1-KO cells, which are stably restored to the expression of the wild-type YAP1, the W4A mutant, the ΔWWs mutant, or the S127A substitution mutant, respectively. Source data are available for this figure: SourceData F1.
WW domains are dispensable to YAP1 phosphorylation catalyzed by LATS1/2. (A) The representative western blotting shows the Ser-127 phosphorylation catalyzed by the MST2−LATS2 kinase cascade on YAP1 and the W4A mutant in HEK293T cells. The inserted numbers show the relative Ser-127 phosphorylation levels normalized by the YAP1 content. (B) The representative western blotting shows the Ser-127 phosphorylation catalyzed by the MST2−LATS1 kinase cascade on YAP1, the W4A mutant, and the ΔWWs mutant in HEK293T cells. The inserted numbers show the relative Ser-127 phosphorylation levels normalized by the YAP1 content. (C) The co-IP experiment shows the interaction of MYC-tagged TEAD1 with HA-tagged YAP1 or the ΔWWs mutant in HEK293T cells. TCL, total cell lysates. (D) The representative western blotting shows the depletion of YAP1 expression in YAP1-knockout (KO) HeLa cells. (E) Representative images show subcellular distributions of the full-length YAP1, the shorter amino terminus that contains TBD, and the extended amino terminus that contains TBD and the tandem WW domains (TBD−WWs) in HEK293T cells (the yellow line indicates the nuclear outline). The scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (F) Representative images show subcellular distributions of the cNLS fused YAP1 and the ΔWWs mutant in HEK293T cells. The cNLS is derived from the SV40 virus. TAD, transcriptional activity domain. Source data are available for this figure: SourceData FS1.
WW domains are dispensable to YAP1 phosphorylation catalyzed by LATS1/2. (A) The representative western blotting shows the Ser-127 phosphorylation catalyzed by the MST2−LATS2 kinase cascade on YAP1 and the W4A mutant in HEK293T cells. The inserted numbers show the relative Ser-127 phosphorylation levels normalized by the YAP1 content. (B) The representative western blotting shows the Ser-127 phosphorylation catalyzed by the MST2−LATS1 kinase cascade on YAP1, the W4A mutant, and the ΔWWs mutant in HEK293T cells. The inserted numbers show the relative Ser-127 phosphorylation levels normalized by the YAP1 content. (C) The co-IP experiment shows the interaction of MYC-tagged TEAD1 with HA-tagged YAP1 or the ΔWWs mutant in HEK293T cells. TCL, total cell lysates. (D) The representative western blotting shows the depletion of YAP1 expression in YAP1-knockout (KO) HeLa cells. (E) Representative images show subcellular distributions of the full-length YAP1, the shorter amino terminus that contains TBD, and the extended amino terminus that contains TBD and the tandem WW domains (TBD−WWs) in HEK293T cells (the yellow line indicates the nuclear outline). The scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (F) Representative images show subcellular distributions of the cNLS fused YAP1 and the ΔWWs mutant in HEK293T cells. The cNLS is derived from the SV40 virus. TAD, transcriptional activity domain. Source data are available for this figure: SourceData FS1.
WW domains are required for YAP1 transactivation and nuclear translocation
Escaping from the cytoplasmic sequestration, YAP1 enters the nucleus and binds to TEAD1-4, resulting in gene transcription (Moroishi et al., 2015; Yu et al., 2015). Loss-of-function of WW domains, either the W4A substitution mutant or the ΔWWs truncated mutant, does not interfere with the interaction of YAP1 with TEAD1 (Fig. 2 A and Fig. S1 C) but causes significant insufficiency in TEAD-dependent transcriptional activity (Fig. 2 B). The results suggest that WW domains are required for YAP1 transactivation. At the same time, the replacement of the connecting sequence between WW domains by a flexible linker (5 x GGGGS) cannot perturb the YAP1 transcriptional activity (Fig. 2 C), suggesting that two WW domains may function individually rather than as a superstructure integrated by the connecting sequence. The result is consistent with a previous report that two WW domains of YAP1 are functionally independent (Lin et al., 2019). More interestingly, the tdWWs−ΔWWs chimera, which transfers tandem WW domains from the middle to the amino terminus, exhibits transcriptional activity similar to the wild-type YAP1 (Fig. 2 D), indicating that the regulation of WW domains manifests a unique characteristic independent of other structural elements. To further confirm this characteristic, the wild-type YAP1, the ΔWWs truncated mutant, or the tdWWs−ΔWWs chimera is expressed to restore the activation of YAP1 target genes in YAP1-KO HeLa cells, respectively (Fig. 2 E and Fig. S1 D). Similarly, the expressions of YAP1 target genes CYR61 and CTGF can be efficiently recovered by the wild-type YAP1 and the tdWWs−ΔWWs chimera instead of the ΔWWs truncated mutant (Fig. 2 E).
WW domains are required for YAP1 transactivation and nuclear translocation. (A) The co-IP experiment shows the interaction of MYC-tagged TEAD1 with HA-tagged YAP1 or the YAP1-W4A mutant in HEK293T cells. TCL, total cell lysates. (B) The W4A mutant loses the transcriptional activity in a TEAD-dependent luciferase reporter (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows expressions of YAP1 and the mutant in reporter assays. The inserted diagram illustrates the construct of the TEAD-dependent luciferase reporter. TBE, the TEAD binding element. (C) Substitution of the disordered sequence between the WW domains by five tandem flexible linkers (Gly-Gly-Gly-Gly-Ser) does not affect YAP1 transcriptional activity in HEK293T cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows expressions of YAP1 and the mutant. (D) tdWWs facilitate YAP1 transcriptional activity in a conformational position-independent manner (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows expressions of YAP1 and the mutants. (E) The mRNA levels of CYR61 and CTGF genes in YAP1-knockout (KO) HeLa cells are rescued by the wild-type YAP1 and the tdWWs−ΔWWs chimera, but not by the ΔWWs mutant (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows the expression of YAP1 and the mutants in YAP1-KO HeLa cells. (F) Representative images show the subcellular distributions of YAP1 and YAP1 mutants in YAP1-KO HeLa cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). The nucleocytoplasmic (N/C) ratios of the subcellular distributions of YAP1 as well as the mutants are measured as in the inserted diagram. Briefly, three areas in the nucleus or the cytoplasm of each cell are randomly selected and then the mean fluorescent intensities in these areas are obtained by ImageJ software. The mean values of fluorescent intensities in three areas are representative of nuclear or cytoplasmic YAP1 levels, respectively. The relative subcellular distribution of YAP1 proteins in each cell is indicated by the ratio of the nuclear and the cytoplasmic YAP1 levels. To plot the scatter chart, the ratios of each mutant are collected from at least three independent images. (G) Representative images show the subcellular localization of 3xGFP and tdWWs-fused 3xGFP in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). The inserted schematic diagram illustrates the coding sequence (cds) of the artificial 3xGFP. Source data are available for this figure: SourceData F2.
WW domains are required for YAP1 transactivation and nuclear translocation. (A) The co-IP experiment shows the interaction of MYC-tagged TEAD1 with HA-tagged YAP1 or the YAP1-W4A mutant in HEK293T cells. TCL, total cell lysates. (B) The W4A mutant loses the transcriptional activity in a TEAD-dependent luciferase reporter (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows expressions of YAP1 and the mutant in reporter assays. The inserted diagram illustrates the construct of the TEAD-dependent luciferase reporter. TBE, the TEAD binding element. (C) Substitution of the disordered sequence between the WW domains by five tandem flexible linkers (Gly-Gly-Gly-Gly-Ser) does not affect YAP1 transcriptional activity in HEK293T cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows expressions of YAP1 and the mutant. (D) tdWWs facilitate YAP1 transcriptional activity in a conformational position-independent manner (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows expressions of YAP1 and the mutants. (E) The mRNA levels of CYR61 and CTGF genes in YAP1-knockout (KO) HeLa cells are rescued by the wild-type YAP1 and the tdWWs−ΔWWs chimera, but not by the ΔWWs mutant (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows the expression of YAP1 and the mutants in YAP1-KO HeLa cells. (F) Representative images show the subcellular distributions of YAP1 and YAP1 mutants in YAP1-KO HeLa cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). The nucleocytoplasmic (N/C) ratios of the subcellular distributions of YAP1 as well as the mutants are measured as in the inserted diagram. Briefly, three areas in the nucleus or the cytoplasm of each cell are randomly selected and then the mean fluorescent intensities in these areas are obtained by ImageJ software. The mean values of fluorescent intensities in three areas are representative of nuclear or cytoplasmic YAP1 levels, respectively. The relative subcellular distribution of YAP1 proteins in each cell is indicated by the ratio of the nuclear and the cytoplasmic YAP1 levels. To plot the scatter chart, the ratios of each mutant are collected from at least three independent images. (G) Representative images show the subcellular localization of 3xGFP and tdWWs-fused 3xGFP in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). The inserted schematic diagram illustrates the coding sequence (cds) of the artificial 3xGFP. Source data are available for this figure: SourceData F2.
Next, we seek to explore the molecular basis underlying the regulation of WW domains. Translocation of YAP1 from the cytoplasm to the nucleus is pivotal for its activation. Loss-of-function of the WW domains, either the W4A substitution or the WW domain deletion, can cause cytoplasmic retention of YAP1, while the tdWWs−ΔWWs chimera can salvage the nuclear localization defect caused by the deletion of the WW domain region (Fig. 2 F), indicating that the WW domains are involved in YAP1 nuclear translocation. We then speculate that the WW domains may harbor an NLS critical for YAP1 nuclear localization. To validate this speculation, artificial 3xGFP (a fusion of three copies of the GFP) is employed as its molecular weight is much larger than 40 kDa and thus can no longer diffuse freely through the nuclear pore (Fig. 2 G). As expected, the tandem WW domain region of YAP1 fused as a tag to the amino terminus of 3xGFP is able to force efficient nuclear translocation of 3xGFP (Fig. 2 G). Therefore, the results strongly support that there is an NLS contained within WW domains.
The amino terminus of YAP1 had been reported to direct its nuclear localization in Drosophila (Wang et al., 2016). Here, we confirm that the individual amino terminus of human YAP1 containing the TBD seems to be more distributed in the cytoplasm rather than in the nucleus, as compared with the full-length protein, while the amino terminus extending to the tandem WW domain region can be redistributed into the nucleus (Fig. S1 E). Therefore, the result is consistent with the previous report that the NLS in the Drosophila ortholog may not be conserved in mammalian YAP1 (Wang et al., 2016). Importantly, when YAP1 is fused with a canonical NLS derived from the SV40 virus, deletion of the WW domain region no longer affects the nuclear localization of YAP1 (Fig. S1 F), suggestive of a functional redundancy between WW domains and the canonical NLS.
Collectively, the results demonstrate that the central WW domains may encompass the NLS to facilitate YAP1 nuclear translocation and transactivation.
The WW domain is identified as a novel class of folded NLS
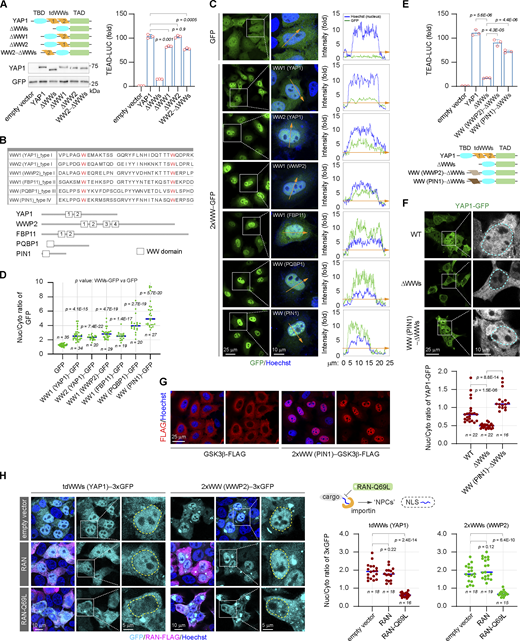
The NLS is essential for the nuclear import of nuclear-localized or nucleocytoplasmic shuttling proteins. There are at least four classes of NLS composed of linear consensus sequences that have been identified, i.e., the canonical NLS composed of highly basic amino acids, the proline–tyrosine NLS, the arginine–serine repeat NLS, and the isoleucine–lysine NLS (Wing et al., 2022). Each of these NLSs recognizes and binds to a unique NTR, leading to distinct nuclear import. However, the amino acid sequences of the two WW domains of YAP1 do not contain any of the linear NLS mentioned above. In addition to the linear NLS, there are folded domains identified as a globular type of NLS to guide specific nuclear import (Wing et al., 2022). Individual deletion of the WW domains just slightly affects YAP1 transactivation as compared with double-deletion of the tandem WW domains, while ectopic restoration of one WW domain is sufficient to rescue the transcriptional activity of the ΔWWs truncated mutant (Fig. 3 A). The result indicates that two WW domains regulate YAP1 activation in a redundant manner. We noted that the linear sequence is not conserved in the two WW domains of YAP1 (Fig. 3 B) and then speculated that the two WW domains may independently form globular rather than linear NLS. Indeed, either WW domain of YAP1 can promote the accumulation of GFP in the nucleus when they are fused to GFP (Fig. 3, C and D).
The WW domain is identified as a novel class of folded NLS. (A) Analysis of the transcriptional activities of YAP1 and YAP1 truncations in HEK293T cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows expressions of YAP1 and the truncations used in reporter assays. (B) The table and schematic diagram show the amino acid sequences of WW domains derived from different proteins. (C and D) Representative images show subcellular distributions of GFP fused with tdWWs derived from different donor proteins in HeLa cells. The inserted curve charts show relative fluorescent intensity of GFP and Hoechst staining along the orange indicator lines with the arrow, which across the cytoplasm and nucleus (collected using LAS AF Lite software) (C). The scatter chart shows nucleocytoplasmic ratios of GFP fused with different WW domains (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test) (D). (E) The transcriptional activity of the YAP1-ΔWWs truncated mutant is rescued by the heterologous WW domains derived from WWP2 or PIN1 in HEK293T cells, respectively (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). (F) The nuclear localization of the YAP1-ΔWWs mutant is rescued by the heterologous WW domains derived from PIN1 in HEK293T cells (the cyan line indicates the nuclear outline). The scatter chart shows nucleocytoplasmic ratios of YAP1 and the mutants (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (G) Representative immunostaining images show subcellular distributions of GSK3β-FLAG and the 2xWW (PIN1)−GSK3β-FLAG in HeLa cells. (H) Representative images show subcellular distributions of the tdWWs (YAP1)−3xGFP and 2xWW (WWP2)−3xGFP in the absence or presence of ectopic RAN-Q69L mutant in HEK293T cells (the yellow line indicates the outline of the nucleus), and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). Source data are available for this figure: SourceData F3.
The WW domain is identified as a novel class of folded NLS. (A) Analysis of the transcriptional activities of YAP1 and YAP1 truncations in HEK293T cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows expressions of YAP1 and the truncations used in reporter assays. (B) The table and schematic diagram show the amino acid sequences of WW domains derived from different proteins. (C and D) Representative images show subcellular distributions of GFP fused with tdWWs derived from different donor proteins in HeLa cells. The inserted curve charts show relative fluorescent intensity of GFP and Hoechst staining along the orange indicator lines with the arrow, which across the cytoplasm and nucleus (collected using LAS AF Lite software) (C). The scatter chart shows nucleocytoplasmic ratios of GFP fused with different WW domains (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test) (D). (E) The transcriptional activity of the YAP1-ΔWWs truncated mutant is rescued by the heterologous WW domains derived from WWP2 or PIN1 in HEK293T cells, respectively (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). (F) The nuclear localization of the YAP1-ΔWWs mutant is rescued by the heterologous WW domains derived from PIN1 in HEK293T cells (the cyan line indicates the nuclear outline). The scatter chart shows nucleocytoplasmic ratios of YAP1 and the mutants (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (G) Representative immunostaining images show subcellular distributions of GSK3β-FLAG and the 2xWW (PIN1)−GSK3β-FLAG in HeLa cells. (H) Representative images show subcellular distributions of the tdWWs (YAP1)−3xGFP and 2xWW (WWP2)−3xGFP in the absence or presence of ectopic RAN-Q69L mutant in HEK293T cells (the yellow line indicates the outline of the nucleus), and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). Source data are available for this figure: SourceData F3.
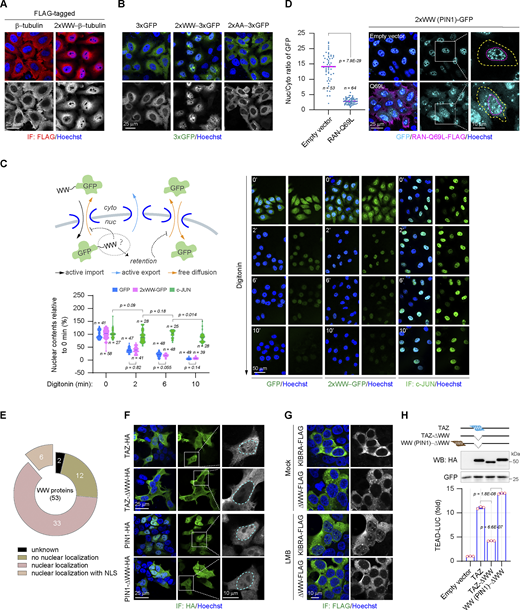
In addition to YAP1, a large number of proteins contain WW domains. Although the linear sequences of these WW domains are less conservative except two tryptophan residues separated by 20–22 amino acids, they share a similar structure composed of three anti-parallel β-sheets (Aragón et al., 2012; Kato et al., 2004; Martinez-Rodriguez et al., 2015; Schulte et al., 2018; Sudol et al., 1995). Hence, a question is raised whether the NLS-like function is unique to the WW domains of YAP1 or common to the WW domain family. To address this, four different types of WW domains from different donor proteins other than YAP1 are fused to the GFP, respectively (Fig. 3 B). Interestingly, all of these WW domains are able to promote the nuclear accumulation of GFP (Fig. 3, C and D). Therefore, the result suggests the NLS-like function is a trait of the WW domain family. Consistently, the heterologous WW domains can effectively rescue the transcriptional activity and the nuclear localization of the YAP1-ΔWWs truncated mutant (Fig. 3, E and F), indicating that the NLS function of WW domains is complementary. At the same time, cytoplasmic proteins lacking the NLS, such as GSK3β and β-tubulin, can also be forced to undergo nuclear translocation by the fusion of the WW domain (Fig. 3 G and Fig. S2 A). In addition, just the substitution of the conserved tryptophan residues essential for WW domain conformation is sufficient to eliminate its NLS function, indicating that the WW domain does perform as the globular rather than the linear NLS (Fig. S2 B).
The WW domain is identified as a novel class of folded NLS. (A) Representative immunostaining images show subcellular distributions of β-tubulin-FLAG and the 2xWW (PIN1)−β-tubulin-FLAG in HeLa cells. (B) Representative images show subcellular distributions of 3xGFP proteins fused with the wild-type WW domain or the mutant WW domain bearing the substitutions of two conserved tryptophan residues (AA) in HeLa cells. The WW domain is derived from WWP2. (C) Examination of the efflux rates of GFP and 2xWW (YAP1)−GFP from the nucleus after digitonin (40 μg/ml) permeabilization, the nuclear content of c-JUN as a control for the integrity of the nuclear envelope. The monoclonal HeLa cells with the uniform expression of GFP or 2xWW (YAP1)−GFP are employed to carry out the experiment. The WW domain is derived from the second WW domain of human YAP1. The relative nuclear contents of GFP or 2xWW (YAP1)−GFP at 0, 2, 6, and 10 min after permeabilization are plotted in a violin chart (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (D) Representative images show the subcellular localization of 2xWW (PIN1)−GFP in the absence or presence of RAN-Q69L (the yellow line indicates the cell boundary and the magenta line indicates the outline of the nucleus), and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (E) Data from Uniprot, Simple Modular Architecture Research Tool, and the Human Protein Atlas shows subcellular localization of human WW-containing proteins. Number, the number of proteins in each category. (F) Representative immunostaining images show subcellular distributions of the wild-type TAZ, the TAZ-ΔWW mutant, the wild-type PIN1, and the PIN1-ΔWW mutant in HEK293T cells (the cyan line indicates the nuclear outline). (G) Representative immunostaining images show subcellular distributions of the wild-type KIBRA and the KIBRA-ΔWW mutant in HEK293T cells. LMB (0.5 μM) is applied for 2 h. (H) The WW domain regulates the transcriptional activity of TAZ in HEK293T cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows expressions of TAZ and the mutant. Source data are available for this figure: SourceData FS2.
The WW domain is identified as a novel class of folded NLS. (A) Representative immunostaining images show subcellular distributions of β-tubulin-FLAG and the 2xWW (PIN1)−β-tubulin-FLAG in HeLa cells. (B) Representative images show subcellular distributions of 3xGFP proteins fused with the wild-type WW domain or the mutant WW domain bearing the substitutions of two conserved tryptophan residues (AA) in HeLa cells. The WW domain is derived from WWP2. (C) Examination of the efflux rates of GFP and 2xWW (YAP1)−GFP from the nucleus after digitonin (40 μg/ml) permeabilization, the nuclear content of c-JUN as a control for the integrity of the nuclear envelope. The monoclonal HeLa cells with the uniform expression of GFP or 2xWW (YAP1)−GFP are employed to carry out the experiment. The WW domain is derived from the second WW domain of human YAP1. The relative nuclear contents of GFP or 2xWW (YAP1)−GFP at 0, 2, 6, and 10 min after permeabilization are plotted in a violin chart (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (D) Representative images show the subcellular localization of 2xWW (PIN1)−GFP in the absence or presence of RAN-Q69L (the yellow line indicates the cell boundary and the magenta line indicates the outline of the nucleus), and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (E) Data from Uniprot, Simple Modular Architecture Research Tool, and the Human Protein Atlas shows subcellular localization of human WW-containing proteins. Number, the number of proteins in each category. (F) Representative immunostaining images show subcellular distributions of the wild-type TAZ, the TAZ-ΔWW mutant, the wild-type PIN1, and the PIN1-ΔWW mutant in HEK293T cells (the cyan line indicates the nuclear outline). (G) Representative immunostaining images show subcellular distributions of the wild-type KIBRA and the KIBRA-ΔWW mutant in HEK293T cells. LMB (0.5 μM) is applied for 2 h. (H) The WW domain regulates the transcriptional activity of TAZ in HEK293T cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows expressions of TAZ and the mutant. Source data are available for this figure: SourceData FS2.
Next, we explore whether the WW domain regulates protein nuclear export or nuclear retention to perturb protein nuclear localization. Digitonin treatment specifically penetrates the plasma membrane but remains the integrity of the nuclear envelope including the NPC (Miyamoto et al., 2002). The WW-fused GFP flows out of nuclei at the same rate as the non-fused GFP following digitonin treatment (Fig. S2 C). As a control, the nuclear protein c-JUN is largely retained in the nucleus, indicating the integrity of the nuclear envelope after permeabilization (Fig. S2 C). Therefore, the results support that the WW domain mainly performs as the NLS to facilitate protein nuclear translocation. Finally, most NLS-mediated nuclear imports are assisted by the RAN-dependent karyopherin family importins (Wing et al., 2022). A mutant RAN GTPase, RAN-Q69L, cannot be hydrolyzed into RAN-GDP, thereby specifically inhibiting the interaction of importins with the NLS of cargoes (Palacios et al., 1996). We observe that ectopic expression of the RAN-Q69L mutant instead of the wild-type RAN significantly blocks the WW-mediated nuclear accumulation of 3xGFP (Fig. 3 H and Fig. S2 D). Therefore, the NLS function of WW domains is accomplished by the conventional kap-β family importins.
Notably, 39 out of 53 human WW-containing proteins have been reported to be located in the nucleus, but the NLSs have been determined only in six nuclear-localized WW proteins (Fig. S2 E). Based on the present work, WW domains may be strong candidates for NLS in these proteins. At the same time, we validate that lack of the WW domain can obviously compromise the nuclear localizations of other WW-containing proteins such as PIN1, TAZ, as well as the nuclear accumulation of KIBRA, induced by the nuclear export inhibitor Leptomycin B (LMB) (Fig. S2, F and G). Moreover, as the YAP1 ortholog, the transcriptional activity of TAZ is regulated by the WW domain in a manner similar to YAP1 (Fig. S2 H). Collectively, these results clearly demonstrate that the WW domain can serve as a novel class of globular NLS, defined here as WW-NLS.
α-Family karyopherins recognize and bind to the WW-NLS
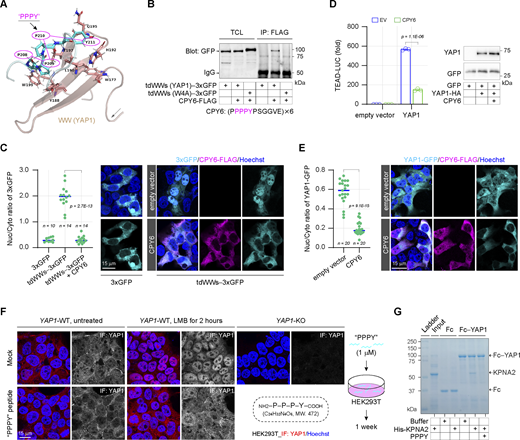
During nuclear import, most of kap-β importins directly bind the NLS carried by cargoes except for KPNB1, which recognizes cargoes through KPNA (Fig. 4 A). Analysis of these karyopherins shows that the WW-bound motifs are impressively conserved in KPNAs but not in kap-β importins. Six out of the seven KPNAs encompass two kinds of motifs, “PPx(Y/F)” and “xPxPP,” and more importantly, the positions of these motifs are conservatively adjacent to the binding region of the canonical NLS (Fig. 4 A). Structural analysis indicates that KPNA family proteins are mostly α-helices linked by short loops. These WW-bound motifs are located on the short loops but not in α-helices and are exposed on the surface of KPNAs (Fig. 4 B and Fig. S3, A–F). Notably, the self-inhibitory state of KPNA1 caused by the intramolecular IBB (importin β1 binding domain; KPNB1 binding domain) binding seems to tighten the conformation surrounding these WW-bound motifs, as compared with the open state upon the cargo engagement (Fig. 4 B).
α-Family karyopherins recognize and bind to the WW-NLS. (A) The schematic diagram illustrates the karyopherin-mediated nuclear import and highlights the cNLS-binding region and the WW-binding motifs on KPNA1–7. Basically, KPNB1 is responsible for the nuclear import of cargoes containing the cNLS under scaffold by KPNAs, while the other importins as well as biportins directly recognize the NLS other than cNLS. (B) The structures exhibit the positions of PPxY/F and xPxPP motifs on KPNA1 in a self-inhibitory state (the intramolecular IBB-bound state) and an open state (the bipartite cNLSs-bound state), respectively. (C) The WW domains from different proteins are pulled down by the PPxF motif fused to the Fc fragment of IgG in HEK293T cells. (D) The WW domain from different proteins is pulled down by the xPxPP motif fused to the Fc fragment of IgG in HEK293T cells. (E) The co-IP experiment shows the interaction of FLAG-tagged KPNA1-3 and KPNB1 with GFP-tagged tandem WW region of YAP1 in HEK293T cells. (F) The co-IP experiment shows the interaction of FLAG-tagged KPNA1-3 with GFP-tagged WW domain of PQBP1 in HEK293T cells. (G) The co-IP experiment shows the interaction of FLAG-tagged KPNA1-3 with GFP-tagged WW domain of PIN1 in HEK293T cells. (H) The co-IP experiment shows the interaction of GFP-tagged WW domain with FLAG-tagged KPNA1 and the P5A mutant bearing mutations of the WW-binding motifs in HEK293T cells. TCL, total cell lysates. Source data are available for this figure: SourceData F4.
α-Family karyopherins recognize and bind to the WW-NLS. (A) The schematic diagram illustrates the karyopherin-mediated nuclear import and highlights the cNLS-binding region and the WW-binding motifs on KPNA1–7. Basically, KPNB1 is responsible for the nuclear import of cargoes containing the cNLS under scaffold by KPNAs, while the other importins as well as biportins directly recognize the NLS other than cNLS. (B) The structures exhibit the positions of PPxY/F and xPxPP motifs on KPNA1 in a self-inhibitory state (the intramolecular IBB-bound state) and an open state (the bipartite cNLSs-bound state), respectively. (C) The WW domains from different proteins are pulled down by the PPxF motif fused to the Fc fragment of IgG in HEK293T cells. (D) The WW domain from different proteins is pulled down by the xPxPP motif fused to the Fc fragment of IgG in HEK293T cells. (E) The co-IP experiment shows the interaction of FLAG-tagged KPNA1-3 and KPNB1 with GFP-tagged tandem WW region of YAP1 in HEK293T cells. (F) The co-IP experiment shows the interaction of FLAG-tagged KPNA1-3 with GFP-tagged WW domain of PQBP1 in HEK293T cells. (G) The co-IP experiment shows the interaction of FLAG-tagged KPNA1-3 with GFP-tagged WW domain of PIN1 in HEK293T cells. (H) The co-IP experiment shows the interaction of GFP-tagged WW domain with FLAG-tagged KPNA1 and the P5A mutant bearing mutations of the WW-binding motifs in HEK293T cells. TCL, total cell lysates. Source data are available for this figure: SourceData F4.
α-Family karyopherins recognize and bind to the WW-NLS. (A–F) The structures from PDB or AlphaFold DB exhibit the positions of PPxY/F and xPxPP motifs on KPNA2 (A), KPNA3 (B), KPNA4 (C), KPNA5 (D), KPNA6 (E), and KPNA7 (F) in an open state, respectively.
α-Family karyopherins recognize and bind to the WW-NLS. (A–F) The structures from PDB or AlphaFold DB exhibit the positions of PPxY/F and xPxPP motifs on KPNA2 (A), KPNA3 (B), KPNA4 (C), KPNA5 (D), KPNA6 (E), and KPNA7 (F) in an open state, respectively.
Next, we examined whether these proline-rich motifs contribute to the association of KPNAs with the WW domain. Coimmunoprecipitation (co-IP) assays are carried out firstly to confirm that both kinds of motifs are able to be recognized by WW domains from YAP1 and other donor proteins (Fig. 4, C and D). Interestingly, although these WW domains belong to different types, they can bind both kinds of motif well, indicating that WW domains may be more flexible in motif selection than known. We further confirm that KPNAs can be bound by tandem WW domains of YAP1 as well as WW domains from other proteins (Fig. 4, E, F, and G). In contrast, KPNB1 does not bind to WW domains (Fig. 4 E). At the same time, mutation of WW-binding motifs on KPNA1 significantly impairs the interaction of KPNA with the WW domain (Fig. 4 H). Taken together, we demonstrate that the specific proline-rich motifs on KPNA are required for recognition by the WW-NLS.
KPNA−KPNB1 axis directs YAP1 nuclear import
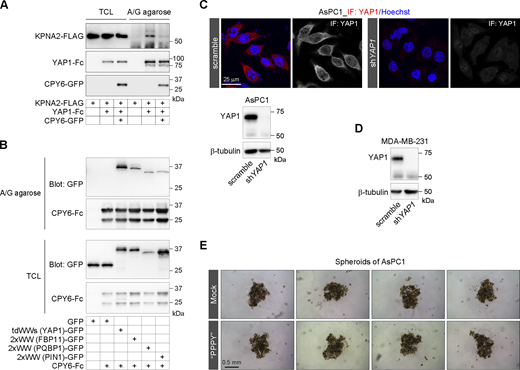
Next, we set out to explore whether the WW domain mediates the direct interaction of YAP1 with KPNA. To get soluble YAP1 proteins, the Fc fragment of IgG is fused to the carboxyl terminus of YAP1, and the YAP1-Fc recombinant proteins expressed in HEK293T cells are pulled down by protein A/G plus agarose. The in vitro binding assays are carried out by incubation of the recombinant KPNA2-His proteins with the A/G agarose-engaging Fc fragment or YAP1-Fc proteins, respectively (Fig. 5 A). We observe that the recombinant KPNA2-His can be efficiently pulled down by YAP-Fc/agarose instead of Fc/agarose (Fig. 5 B). Meanwhile, the W4A mutation on tandem WW domains of YAP1 or xPxAA mutation destroying the xPxPP motif on KPNA2 completely disrupts the interaction of YAP1 with KPNA2 (Fig. 5 B). Consistently, the WW domain derived from YAP1 does directly interact with the recombinant KPNA2-His proteins in vitro similar to the full-length YAP1 (Fig. 5 C). Taken together, the results demonstrate that the WW domain mediates the direct interaction of YAP1 with KPNA2 through the conserved proline-rich motif on KPNA2. Accordingly, transient knockdown of KPNB1 expression disrupting the KPNA−KPNB1 axis significantly attenuates the basal and LMB-induced nuclear localization of YAP1 (Fig. 5 D).
KPNA−KPNB1 axis directs YAP1 nuclear import. (A) The schematic diagram illustrates the in vitro binding assays of the YAP1/KPNA2 complex. Fc-fused YAP1 is overexpressed in HEK293T cells and then collected by protein A/G plus agarose. For binding assays, the recombinant His-KPNA2 proteins (3.3 μM) obtained from E. coli are incubated with A/G agarose engaging Fc−YAP1 for 1 h at 4°C, and the Fc fragment as the control. After washing, the pulled-down protein complexes by A/G agarose were analyzed using SDS PAGE. (B) In vitro binding assays of recombinant His-KPNA2 and His-xPxAA mutant of KPNA2 with Fc−YAP1 and Fc−W4A (YAP1), respectively. (C) In vitro binding assays of recombinant His-KPNA2 with Fc−WW (the second WW domain of YAP1). (D) Representative images show subcellular distributions of YAP1-GFP following 2-h LMB (0.5 μM) treatment in HEK293T cells bearing transient knockdown of KPNB1 expression. The scatter chart shows YAP1-GFP nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test), and the representative western blotting shows KPNB1 contents in scramble control and KPNB1-knockdown HEK293T cells. (E) In vitro binding assays of recombinant His-KPNA2 and the IBB deletion mutant ΔIBB with Fc−YAP1, respectively. The inserted schematic diagrams illustrate binding sites for KPNB1, cNLS, and WW domain on KPNA (lower panel) and the hypothesis of IBB-facilitated release of WW cargoes from KPNAs (upper panel). Source data are available for this figure: SourceData F5.
KPNA−KPNB1 axis directs YAP1 nuclear import. (A) The schematic diagram illustrates the in vitro binding assays of the YAP1/KPNA2 complex. Fc-fused YAP1 is overexpressed in HEK293T cells and then collected by protein A/G plus agarose. For binding assays, the recombinant His-KPNA2 proteins (3.3 μM) obtained from E. coli are incubated with A/G agarose engaging Fc−YAP1 for 1 h at 4°C, and the Fc fragment as the control. After washing, the pulled-down protein complexes by A/G agarose were analyzed using SDS PAGE. (B) In vitro binding assays of recombinant His-KPNA2 and His-xPxAA mutant of KPNA2 with Fc−YAP1 and Fc−W4A (YAP1), respectively. (C) In vitro binding assays of recombinant His-KPNA2 with Fc−WW (the second WW domain of YAP1). (D) Representative images show subcellular distributions of YAP1-GFP following 2-h LMB (0.5 μM) treatment in HEK293T cells bearing transient knockdown of KPNB1 expression. The scatter chart shows YAP1-GFP nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test), and the representative western blotting shows KPNB1 contents in scramble control and KPNB1-knockdown HEK293T cells. (E) In vitro binding assays of recombinant His-KPNA2 and the IBB deletion mutant ΔIBB with Fc−YAP1, respectively. The inserted schematic diagrams illustrate binding sites for KPNB1, cNLS, and WW domain on KPNA (lower panel) and the hypothesis of IBB-facilitated release of WW cargoes from KPNAs (upper panel). Source data are available for this figure: SourceData F5.
It is speculated that the amino-terminal IBB domain of KPNA regulates the release of cargoes from KPNA upon arrival at the nucleus (Wing et al., 2022). The WW-bound motifs are located in the spacer regions between IBB and two cNLS-binding regions and are very close to the cNLS-binding region (Fig. 4 A). We observed that the intramolecular IBB-bound self-inhibitory state can twist the spatial conformation surrounding these motifs (Fig. 4 B) and then hypothesized that such conformational changes may contribute to the disassociation of the WW-NLS from KPNAs once IBB is released by RAN-GTP in the nucleus and competitively binds to the cNLS binding region, leading to disassembly of the WW-cargoes/KPNA complex (Fig. 5 E). To verify this possibility, the binding assays for full-length KPNA2 and the ΔIBB mutant with YAP1 are carried out. As compared with the full-length proteins, IBB deletion results in an obvious increase in the interaction of KPNA2 with YAP1 (Fig. 5 E). Therefore, the release of WW-NLS from KPNA in the nucleus may also be mediated, at least partly, by the IBB domain.
The WW-NLS bypasses the mediation of cNLS
The cNLS composed of highly basic amino acids has been well-documented in the KPNA−KPNB1 axis (Wing et al., 2022). YAP1 had been proposed to be recruited into the nucleus through some interaction partners containing the cNLS (Kim et al., 2020; Yao et al., 2018). Since both the WW-NLS and the cNLS employ the KPNA−KPNB1 axis, it is necessary to clarify whether the WW-NLS in YAP1 nuclear import can bypass the cNLS involved in a mechanism known as hitchhiking (Fig. 6 A). Bimax2, a peptide composed of highly basic amino acids, can competitively bind to the cNLS binding region on KPNAs, thereby inhibiting the cNLS-directed nuclear import (Kosugi et al., 2008). To confirm the inhibitory effect of Bimax2 on cNLS-mediated nuclear import, the cNLS derived from SV40 is fused to 3xGFP (Fig. S4 A). As expected, ectopic expression Bimax2 significantly suppresses nuclear accumulation of 2xcNLS−3xGFP (Fig. 6 B). However, Bimax2 cannot interfere with the nuclear accumulation of 3xGFP fused with the WW domains of YAP1 (Fig. 6 B) nor can it affect the nuclear accumulation of YAP1 induced by LMB treatment (Fig. 6 C and Fig. S4 B). On the contrary, when the cNLS of SV40 is fused to the ΔWWs truncated mutant of YAP1 to replace the WW domain during YAP1 nuclear import, the nuclear accumulation of the cNLS−ΔWWs fusion can be significantly suppressed by Bimax2 (Fig. 6 D). Mastermind-like protein 1/2 (MAML1/2) harbor a defined cNLS and had been demonstrated to regulate YAP1 nuclear accumulation through the WW-mediated protein interaction (Kim et al., 2020). Unlike YAP1, the nuclear localization of MAML1 can be significantly inhibited by ectopic expression of Bimax2 (Fig. S4 C). According to these results, we demonstrate that the WW-NLS is parallel to the cNLS, bypassing the hitchhiking mechanism during YAP1 nuclear translocation.
The WW-NLS bypasses the mediation of cNLS. (A) The schematic diagram illustrates the potential hitchhike mechanism for YAP1 nuclear import, and the structure (PDB: 3UKX) illustrates the competitive binding of Bimax2 to KPNA2. (B) Representative images show the subcellular localization of 2xcNLS−3xGFP and tdWWs (YAP1)−3xGFP upon ectopic expression of Bimax2 in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (C) Representative images show LMB-induced subcellular localization of YAP1-GFP upon ectopic expression of Bimax2 in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). LMB, 0.5 μM, 2 h. (D) Representative images show the subcellular localization of cNLS-YAP1-ΔWWs-GFP upon ectopic expression of Bimax2 in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test).
The WW-NLS bypasses the mediation of cNLS. (A) The schematic diagram illustrates the potential hitchhike mechanism for YAP1 nuclear import, and the structure (PDB: 3UKX) illustrates the competitive binding of Bimax2 to KPNA2. (B) Representative images show the subcellular localization of 2xcNLS−3xGFP and tdWWs (YAP1)−3xGFP upon ectopic expression of Bimax2 in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (C) Representative images show LMB-induced subcellular localization of YAP1-GFP upon ectopic expression of Bimax2 in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). LMB, 0.5 μM, 2 h. (D) Representative images show the subcellular localization of cNLS-YAP1-ΔWWs-GFP upon ectopic expression of Bimax2 in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test).
The WW-NLS bypasses the mediation of cNLS. (A) Representative images show cNLS-mediated nuclear import of 3xGFP in HEK293T cells. (B) Representative images show the basal subcellular localization of YAP1-GFP upon ectopic expression of Bimax2 in HEK293T cells. (C) Representative images show the subcellular localization of MAML1-GFP upon ectopic expression of Bimax2 in HEK293T cells.
The WW-NLS bypasses the mediation of cNLS. (A) Representative images show cNLS-mediated nuclear import of 3xGFP in HEK293T cells. (B) Representative images show the basal subcellular localization of YAP1-GFP upon ectopic expression of Bimax2 in HEK293T cells. (C) Representative images show the subcellular localization of MAML1-GFP upon ectopic expression of Bimax2 in HEK293T cells.
Develop inhibitory peptides against the WW-NLS in YAP1 nuclear import
Specific proline-rich motifs can be recognized and bound by the WW domain (Aragón et al., 2012; Kato et al., 2004; Schulte et al., 2018). We hypothesize that peptides can be designed based on these motifs to competitively bind the WW domain, resulting in interference with the WW-mediated nuclear import (Fig. 7 A). CPY6, a peptide containing six repeats of the PPPY motif with flanking sequences, is designed to bind to the wild-type WW domains of YAP1 but not the tryptophan-mutated WW domains (Fig. 7 B). We confirmed that CPY6 can bind to YAP1 and significantly suppress the interaction of YAP1 with KPNA2, supporting the potential of CPY6 in disruption of WW-NLS–mediated nuclear import (Fig. S5 A). Notably, CPY6 is also bound by other WW domains in addition to WW domains derived from YAP1 (Fig. S5 B). As designed, ectopic expression of CPY6 peptide significantly suppresses the nuclear accumulation of 3xGFP forced by the WW domains of YAP1 (Fig. 7 C). More importantly, the YAP1-dependent gene transcription, as well as YAP1 nuclear localization, can also be suppressed by the CPY6 peptide (Fig. 7 D and E). Next, a peptide composed of a single P-P-P-Y motif is synthesized to verify whether it can also interfere with YAP1 nuclear localization. HEK293T cells are cultured with the PPPY peptide for 1 wk followed by 2-h LMB treatment, and the subcellular localization of endogenous YAP1 is examined. As shown in Fig. 7 F, the acute nuclear accumulation of YAP1 induced by LMB is significantly suppressed in the presence of this tetrapeptide. At the same time, the PPPY peptide blocks the interaction of YAP1 and KPNA2 (Fig. 7 G). Therefore, we suggest that competitive binding peptides of WW domains have great potential to weaken the nuclear translocation of YAP1.
Develop inhibitory peptides against the WW-NLS in YAP1 nuclear import. (A) The structure (PDB: 2LTW) illustrates the bound state of the PPPY peptide and the first WW domain derived from YAP1. (B) The competitive binding peptide (CPY6) is developed according to the PPPY motif, and the co-IP experiment shows the interaction of the CPY6 with the wild-type or the W4A substituted WW domains of YAP1 in HEK293T cells. TCL, total cell lysates. (C) Representative images show the subcellular localization of tdWWs−3xGFP upon ectopic expression of CPY6 peptide in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (D) The CPY6 peptide suppresses YAP1 transcriptional activity in HEK293T cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). Representative western blotting shows YAP1 expression in luciferase reporter assays. (E) Representative images show the subcellular localization of YAP1-GFP upon ectopic expression of CPY6 peptide in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (F) Representative immunostaining images show the subcellular localization of endogenous YAP1 proteins upon LMB treatment (0.5 μM) in HEK293T cells following 1-wk culture with 1 μM of synthesized PPPY peptide. (G) The synthesized PPPY peptide blocks the interaction of Fc−YAP1 and His-KPNA2 in vitro. In the absence or presence of PPPY peptides (30 μM), the recombinant His-KPNA2 (3.3 μM) is incubated with Fc- or Fc-YAP1-engaged protein A/G plus agarose at room temperature for 1 h, then the pulled-down proteins by protein A/G plus agarose are analyzed by SDS PAGE. Source data are available for this figure: SourceData F7.
Develop inhibitory peptides against the WW-NLS in YAP1 nuclear import. (A) The structure (PDB: 2LTW) illustrates the bound state of the PPPY peptide and the first WW domain derived from YAP1. (B) The competitive binding peptide (CPY6) is developed according to the PPPY motif, and the co-IP experiment shows the interaction of the CPY6 with the wild-type or the W4A substituted WW domains of YAP1 in HEK293T cells. TCL, total cell lysates. (C) Representative images show the subcellular localization of tdWWs−3xGFP upon ectopic expression of CPY6 peptide in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (D) The CPY6 peptide suppresses YAP1 transcriptional activity in HEK293T cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). Representative western blotting shows YAP1 expression in luciferase reporter assays. (E) Representative images show the subcellular localization of YAP1-GFP upon ectopic expression of CPY6 peptide in HEK293T cells, and the scatter chart shows their nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (F) Representative immunostaining images show the subcellular localization of endogenous YAP1 proteins upon LMB treatment (0.5 μM) in HEK293T cells following 1-wk culture with 1 μM of synthesized PPPY peptide. (G) The synthesized PPPY peptide blocks the interaction of Fc−YAP1 and His-KPNA2 in vitro. In the absence or presence of PPPY peptides (30 μM), the recombinant His-KPNA2 (3.3 μM) is incubated with Fc- or Fc-YAP1-engaged protein A/G plus agarose at room temperature for 1 h, then the pulled-down proteins by protein A/G plus agarose are analyzed by SDS PAGE. Source data are available for this figure: SourceData F7.
Develop inhibitory peptides to antagonize the WW-NLS in YAP1 nuclear import. (A) The pull-down experiment shows that the CPY6 peptide blocks the interaction of FLAG-tagged KPNA2 with Fc-fused YAP1 in HEK293T cells. (B) The WW domains from different proteins are pulled down by the CPY6 peptide fused to Fc fragment of IgG in HEK293T cells. TCL, total cell lysates. (C) Representative immunostaining images show the subcellular localization of YAP1 in AsPC1 cells, and representative western blotting shows the depletion of YAP1 expression in YAP1-KD AsPC1 cells. (D) Representative western blotting shows the depletion of YAP1 expression in YAP1-KD MDA-MB-231 cells. (E) Representative images show spheroids of AsPC1 cells following 1-wk culture with 1 μM of synthesized PPPY peptide. Source data are available for this figure: SourceData FS5.
Develop inhibitory peptides to antagonize the WW-NLS in YAP1 nuclear import. (A) The pull-down experiment shows that the CPY6 peptide blocks the interaction of FLAG-tagged KPNA2 with Fc-fused YAP1 in HEK293T cells. (B) The WW domains from different proteins are pulled down by the CPY6 peptide fused to Fc fragment of IgG in HEK293T cells. TCL, total cell lysates. (C) Representative immunostaining images show the subcellular localization of YAP1 in AsPC1 cells, and representative western blotting shows the depletion of YAP1 expression in YAP1-KD AsPC1 cells. (D) Representative western blotting shows the depletion of YAP1 expression in YAP1-KD MDA-MB-231 cells. (E) Representative images show spheroids of AsPC1 cells following 1-wk culture with 1 μM of synthesized PPPY peptide. Source data are available for this figure: SourceData FS5.
The WW-NLS regulates YAP1-dependent cancer cell stemness
Nuclear-localized YAP1 endows cells with various oncogenic traits such as sustaining proliferation, maintenance of stemness, and metastasis (Bora-Singhal et al., 2015; Lu et al., 2018; Moroishi et al., 2015). Thus, control of YAP1 nuclear translocation is a promising cancer therapeutic strategy. So the synthesized PPPY tetrapeptide is applied to the MDA-MB-231 cell, a malignant mammary cancer cell with positive nuclear accumulation of YAP1. We observe that the subcellular distribution of YAP1 in MDA-MB-231 cells is reversed from the nuclear accumulation to more cytoplasmic localization following 1-wk culture in the presence of PPPY (Fig. 8 A). More importantly, the application of PPPY tetrapeptide not only significantly suppresses the proliferation gene CYR61 but also suppresses the stemness genes OCT4 and NANOG (Fig. 8, B, C, and D). Unlike MDA-MB-231 cells, pancreatic ductal adenocarcinoma AsPC1 cells show a predominant cytoplasmic localization of YAP1 (Fig. S5 C). Accordingly, these proliferation and stemness genes in AsPC1 cells cannot be perturbed by the application of PPPY tetrapeptide (Fig. 8, B, C, and D). We confirm that the luciferase reporter gene driven by the promoter DNA of the OCT4 gene can be activated by YAP1 but is further perturbed by the coexpression of CPY6 peptide (Fig. 8 E) and that YAP1 knockdown in MDA-MB-231 cells significantly inhibits OCT4 and NANOG gene expressions (Fig. 8 F and Fig. S5 D). Therefore, the results indicate that the WW-NLS–mediated YAP1 nuclear translocation may be profoundly implicated in cancer stemness. To further confirm this, sphere formation is performed to evaluate the stemness of MDA-MB-231 cells, which displays an obvious decrease in the size of spheroids in the presence of the PPPY tetrapeptide (Fig. 8 G) or in the case of YAP1 knockdown (Fig. 8 H). As compared with MDA-MB-231, spheroids of AsPC1 cells appear much looser and are unaffected by the presence of the PPPY peptide (Fig. S5 E).
The WW-NLS regulates YAP1-dependent cancer cell stemness. (A) Representative immunostaining images show the subcellular localization of YAP1 in MDA-MB-231 cells following 1-wk culture with 1 μM of synthesized PPPY peptide, and the scatter chart shows YAP1 nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (B) The expression of CYR61 in MDA-MB-231 and AsPC1 cells following 1-wk culture with 1 μM of PPPY peptide (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows YAP1 expression in both cells. YAP1nuc, YAP1 nuclear localization; YAP1cyto, YAP1 cytoplasmic localization. (C and D) The expressions of OCT4 (C) and NANOG (D) in MDA-MB-231 and AsPC1 cells following 1-wk culture with 1 μM of PPPY peptide (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). (E) Ectopic expression of CPY6 peptide suppresses YAP1-activated OCT4 gene promoter activity in HEK293T cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). EV, empty vector. (F) Depletion of YAP1 suppresses the expressions of OCT4 and NANOG in MDA-MB-231 cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). Scr, scramble. (G) Representative images show spheroids of MDA-MB-231 cells following 1-wk culture with 1 μM of synthesized PPPY peptide, and the column chart shows diameters of spheroids (representative of three independent experiments, n = 8 spheroids, all data shown as the average values ± SD, two-tailed ANOVA test). (H) Representative images show spheroids of MDA-MB-231 cells following YAP1 knockdown, and the column chart shows diameters of spheroids (representative of three independent experiments, n = 8 spheroids, all data shown as the average values ± SD, two-tailed ANOVA test). (I) The schematic diagram illustrates that the WW domain regulates YAP1 nuclear import as an NLS, bypassing the hitchhiking mechanism. Source data are available for this figure: SourceData F8.
The WW-NLS regulates YAP1-dependent cancer cell stemness. (A) Representative immunostaining images show the subcellular localization of YAP1 in MDA-MB-231 cells following 1-wk culture with 1 μM of synthesized PPPY peptide, and the scatter chart shows YAP1 nucleocytoplasmic ratios (representative of three independent experiments, n represents the total number of the measured cells, two-tailed ANOVA test). (B) The expression of CYR61 in MDA-MB-231 and AsPC1 cells following 1-wk culture with 1 μM of PPPY peptide (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). The representative western blotting shows YAP1 expression in both cells. YAP1nuc, YAP1 nuclear localization; YAP1cyto, YAP1 cytoplasmic localization. (C and D) The expressions of OCT4 (C) and NANOG (D) in MDA-MB-231 and AsPC1 cells following 1-wk culture with 1 μM of PPPY peptide (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). (E) Ectopic expression of CPY6 peptide suppresses YAP1-activated OCT4 gene promoter activity in HEK293T cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). EV, empty vector. (F) Depletion of YAP1 suppresses the expressions of OCT4 and NANOG in MDA-MB-231 cells (representative of three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). Scr, scramble. (G) Representative images show spheroids of MDA-MB-231 cells following 1-wk culture with 1 μM of synthesized PPPY peptide, and the column chart shows diameters of spheroids (representative of three independent experiments, n = 8 spheroids, all data shown as the average values ± SD, two-tailed ANOVA test). (H) Representative images show spheroids of MDA-MB-231 cells following YAP1 knockdown, and the column chart shows diameters of spheroids (representative of three independent experiments, n = 8 spheroids, all data shown as the average values ± SD, two-tailed ANOVA test). (I) The schematic diagram illustrates that the WW domain regulates YAP1 nuclear import as an NLS, bypassing the hitchhiking mechanism. Source data are available for this figure: SourceData F8.
In summary, we demonstrate that the WW-NLS is required for YAP1 nuclear import in cancer cells and is therefore a promising therapeutic target for YAP1-dependent malignancies (Fig. 8 I).
Discussion
Since nuclear localized YAP1 has been profoundly implicated in various physiological and pathological processes, such as organ development, tissue regeneration, and tumorigenesis, the molecular basis underlying its nucleocytoplasmic shuttling has been intensively explored. The NLS, as well as the NES, is critical for a nucleocytoplasmic shuttle protein larger than 40 kDa to pass through the NPC (Knockenhauer and Schwartz, 2016). A nuclear export signal had been identified in the carboxyl-terminal transcriptional activation domain of YAP1 (Fang et al., 2018). But for nuclear import, YAP1 may encompass varied types of NLS, and there seem to be diverse mechanisms underlying YAP1 nuclear localization (Wang et al., 2016; Yao et al., 2018; Kim et al., 2020; García-García et al., 2022). In the present work, we defined a novel class of globular NLS formed by the folded WW domain, termed WW-NLS. The WW-NLS is specifically recognized by KPNAs, thereby directing nuclear imports of the WW-containing cargoes including YAP1 through the KPNA−KPNB1 axis. Moreover, we prove that the interference with the function of the WW-NLS is able to attenuate YAP1 nuclear import as well as YAP1-dependent cancer stemness. Thus, the findings provide a novel target for the development of anticancer drugs.
Basically, a linear sequence or a folded domain serving as a common NLS should have the following characteristics: (1) universality, i.e., it can guide the nuclear import of any protein containing the linear sequence or domain; (2) able to be directly recognized and bound by the NTR (Wing et al., 2022). WW domains derived from different donor proteins not only regulate nuclear translocation of donor proteins themselves but also can force nuclear accumulation of various cargoes lacking NLS. These cargoes include the diffusion-like molecule GFP, the natural cytoplasmic proteins GSK3β and β-tubulin, and the artificial 3xGFP. Therefore, the WW domain clearly exhibits a universal characteristic in directing nuclear import. Meanwhile, the WW-bound motifs are found to be highly conserved in KPNAs, which are specific adaptors for NTR KPNB1. These motifs can efficiently bind to WW domains including YAP1, and the co-IP and the in vitro binding assay verify the direct interaction between KPNAs and the WW domain as well as YAP1. Notably, importin-chaperoned nuclear transport is regulated by RAN. The WW-mediated nuclear import can be significantly blocked by the ectopic RAN-Q69L, and meanwhile, the WW domain cannot regulate the nuclear retention or nuclear export of proteins, indicating that the WW domain specifically regulates nuclear entry. Taken together, we demonstrate that the family of WW domains serves as a novel type of common NLS termed WW-NLS, which employs the KPNA−KPNB1 axis to regulate nuclear import. The family of WW domains is less conserved in their linear sequences except for two conserved tryptophan residues crucial for the WW domain conformation, while substitutions of the two conserved tryptophan residues are sufficient to eliminate their NLS function. Hence, the WW-NLS performs as a globular rather than linear NLS. Given WW domains exist in a large number of proteins and regulate various physiological and pathological processes, the discovery of their NLS function may provide novel insight into the roles of WW domains. Furthermore, a bioinformatics study showed that only 60% of nuclear-localized proteins contain the predictable NLS (Brameier et al., 2007). Thus, the WW-NLS expands our understanding of NLSs on proteins.
Consistent with the NLS function of WW domains, the loss-of-function of WW domains significantly damages the nuclear translocation and transactivation of YAP1. Meanwhile, the regulation of WW domains in YAP1 activation can be compensated by other WW domains. Mutation of WW domains diminishes the direct interaction of YAP1 with KPNA, and the knockdown of KPNB1, disrupting the KPNA–KPNB1 axis, attenuates YAP1 nuclear localization. Therefore, we demonstrate that the WW domain facilitates YAP1 activation in an NLS manner. Interestingly, the IBB domain of KPNA may mediate the release of WW cargoes from the nuclear importin machinery in the nucleus. A mechanism is proposed that the intramolecular interaction of IBB with the cNLS-binding region, which is very adjacent to the WW-NLS–binding sites, may perturb the WW-NLS/KPNA interaction due to the bent conformation of the amino terminus of KPNA following this intramolecular interaction. The MST−LATS axis is the core signaling mechanism to inhibit YAP1 nuclear translocation (Zhao et al., 2007). Although the WW domain does mediate the interaction of YAP1 and LATS1/2, the WW-mediated interaction does not seem to be involved in YAP1 phosphorylation. In particular, loss of the WW domain leads to attenuation rather than enhancement in YAP1 transcriptional activity and nuclear localization. Therefore, the regulation of the WW domain in YAP1 nuclear translocation is parallel to the Hippo pathway.
In addition, it was proposed that the WW-mediated protein interaction might be involved in a hitchhiking mechanism for YAP1 nuclear import (Kim et al., 2020). MAML1/2 encompass a definitive cNLS and the WW-bound motifs. In the hitchhiking mechanism, YAP1 was speculated to be cotransported along with MAML1/2 into the nucleus through the cNLS on MAML1/2. However, YAP1 and the artificial WW-contained cargoes can still be efficiently transported into the nucleus in the case of ectopic expression of Bimax2, which is a competitive molecule to specifically suppress cNLS-guided nuclear import of cargoes including MAML1 (Kosugi et al., 2008). Hence, the WW-mediated YAP1 nuclear import can bypass the cNLS-involved hitchhiking mechanism. In fact, the finding that YAP1 nuclear accumulation can be induced by LMB, which blocks the CRM1-dependent nuclear export, clearly indicates that the hitchhiking mechanism is not sufficient to interpret the dynamic nuclear import of YAP1 unless the hitchhike molecule can still export out the nucleus in case of LMB treatment. More importantly, LMB-induced YAP1 nuclear accumulation can be blocked by competitive interference with the WW-NLS instead of the cNLS. Nonetheless, the present work does not negate that the interaction with the nuclear-localized copartner can enhance the static nuclear accumulation of YAP1, as the interaction can serve as an anchor of YAP1 in the nucleus.
Targeting YAP1 is an increasingly promising strategy for the development of anticancer drugs (Pobbati and Hong, 2020). Generally, there are two distinct routes to inactivate YAP1: targeting the upstream signaling components to culminate in the enhancement of LATS1/2-dependent phosphorylation or targeting the YAP1−TEAD interaction to terminate gene transcription in the nucleus. However, loss-of-function of the Hippo pathway frequently emerges in malignancies, such as in the case of the destruction of cell–cell contact (Zhao et al., 2007). At the same time, a growing body of evidence indicates that YAP1 can facilitate cancer cell progression independently of the TEAD family transcriptional factors (Bora-Singhal et al., 2015; Croci et al., 2017; Kim et al., 2015; Totaro et al., 2018). We demonstrate here that interference with the WW-NLS using the competitive binding peptide can prevent YAP1 nuclear translocation as well as YAP1-dependent cancer stemness in malignant mammary cancer cells. Therefore, the present work not only interprets the pending nuclear import mechanism underlying YAP1 nucleocytoplasmic shuttling but also provides a novel perspective for targeting YAP1 in treating malignancies, independent of the Hippo pathway or the YAP1−TEAD axis.
Materials and methods
Cell culture and reagents
HEK293T (Cat# CRL-3216, RRID:CVCL_0063; ATCC), HeLa (Cat# CCL-2, RRID:CVCL_0030; ATCC), MDA-MB-231 (Cat# HTB-26, RRID:CVCL_0062; ATCC), and AsPC1 (Cat# CRL-1682, RRID:CVCL_0152; ATCC) were maintained in DMEM (Thermo Fisher Scientific) with 10% FBS (Thermo Fisher Scientific), 1x GlutaMAX (Thermo Fisher Scientific), and 1 × penicillin–streptomycin solution (Thermo Fisher Scientific), 37°C, 5% CO2.
Primary antibodies were: c-JUN (Cat# 9165, RRID: AB_2130165; Cell Signaling Technology); FLAG M2 (Cat# F3165, RRID:AB_259529; Sigma-Aldrich); GFP (Cat# sc-9996, RRID:AB_627695; Santa Cruz Biotechnology); HA.11 (Cat# MMS-101P-500, RRID:AB_291261; Covance); KPNB1 (Cat# sc-137016, RRID:AB_2133993; Santa Cruz Biotechnology); MYC (Cat# MMS-150P-1000, RRID:AB_291322; Covance); β-tubulin (Cat# sc-5274, RRID:AB_2288090; Santa Cruz Biotechnology); Phospho-S127-YAP1 (Cat# ab76252, RRID:AB_1524578; Abcam); and YAP1 (Cat# sc-101199, RRID: AB_1131430; Santa Cruz Biotechnology).
Corresponding secondary antibodies were: Goat anti-Mouse IgG (H+L) Secondary Antibody, HRP (Cat# 31430, RRID:AB_228307; Thermo Fisher Scientific); Goat anti-Rabbit IgG (H+L) Secondary Antibody, HRP (Cat# 31460, RRID:AB_228341; Thermo Fisher Scientific); Cy3-AffiniPure Goat anti-mouse IgG (H+L) (Cat# 115-165-003, RRID:AB_2338680; Jackson ImmunoResearch); and Cy3-AffiniPure Goat anti-rabbit IgG (H+L) (Cat#111-165-003, RRID: AB_2338000; Jackson ImmunoResearch).
The tetrapeptide PPPY was synthesized by Sangon Biotech.
Constructs and transfections
Human cDNAs for YAP1, LATS2, LATS1, MST1, 14-3-3, TEAD1, WWP2, FBP11, PQBP1, PIN1, GSK3β, RAN, KPNA1, KPNA2, KPNA3, KPNB1, β-tubulin (TUBB1), TAZ, KIBRA, and MAML1 were amplified from total mRNA of HEK293T cells. All constructs were cloned into pLenti-puro vectors (RRID:Addgene_39481) containing different tags or monomeric EGFP for overexpression in mammalian cells. Transient transfections were carried out with Lipofectamine LTX (Thermo Fisher Scientific). For a 24-well of HEK293T (Cat# CRL-3216, RRID:CVCL_0063; ATCC) and HeLa (Cat# CCL-2, RRID:CVCL_0030; ATCC) cells with 80% confluence, 500 ng of total DNA in 25 μl of Opti-MEM (Thermo Fisher Scientific) was preincubated with 0.5 μl of the PLUS reagent for 5 min at room temperature, and then the DNA/PLUS solution was mixed with 25 μl of Opti-MEM containing 1 μl of Lipofectamine LTX and incubated for additional 5 min. Finally, the transfection mixture was added to cells cultured in 200 μl of Opti-MEM. After 4 h, the transfection was terminated by 500 μl of DMEM supplemented with 10% FBS, and cells continued to be cultured for 24 h.
Reporter gene assays
For construction of luciferase reporter gene containing the TEAD binding site, the DNA fragment containing four TEAD consensus sequences, 5′-TGGAATGTGTGGAATGTGTCTCAAGCTTGCAGTGGAATGTGTGGAATGT-3′ was cloned into the pGL4.20 vector (RRID:Addgene_40173) (Dupont et al., 2011). Luciferase reporter gene for the OCT4 gene promoter was constructed by inserting the chromosomal DNA 2,000 base pairs upstream of the transcriptional start site of the OCT4 gene into the pGL4.20 vector. The reporter assays were carried out 24 h after transfection using Luciferase Reporter Gene Assay, high sensitivity (11814036001; Roche). The normalized luciferase activities were standardized by the fluorescence value of cotransfected GFP. All data were collected by SpectraMax i3 (Molecular Devices).
Western blotting
The protein samples were transferred to Immuobilon-NC Transfer Membrane (Millipore) following SDS PAGE. Membranes were blocked with 5% dry-fat milk in tris-buffered saline plus 0.1% Tween-20, incubated with primary antibodies overnight followed by incubation with HRP-conjugated secondary antibodies for 1 h. Finally, immunoreactive bands on the membranes were acquired by Tanon-5200 Multi image analyzer following reaction with SuperSignal West Pico Plus Chemiluminescent Substrate (Thermo Fisher Scientific). Quantification of the immunoreactive bands was analyzed by ImageJ software (National Institutes of Health).
CRISPR/Cas9 genome knockout
sgDNA targeting the YAP1 gene, 5′-CATCAGATCGTGCACGTCCG-3′ (Plouffe et al., 2016), was cloned into the pSpCas9 (BB)-2A-Puro (PX459) vector (RRID: Addgene_48139). 80% confluent 24-well of HEK293T (Cat# CRL-3216, RRID:CVCL_0063; ATCC) and HeLa (Cat# CCL-2, RRID:CVCL_0030; ATCC ) cells were transfected with 250 ng of plasmid. After 24 h, cells were treated with puromycin (2 μg/ml) for an additional 36 h to enrich for successfully transfected cells, and then cells were digested by trypsin and re-seeded as 150 cells per 10-cm plate. After expansion, single clones were examined by western blotting.
Stable and transient gene knockdown by lentiviral shRNA
The targeted sequences of shRNA were designed as follows: scramble control, 5′-CCTAAGGTTAAGTCGCCCTCG-3′; KPNB1, 5′-AACCAGCTACTTACTGCTTAT-3′; YAP1, 5′-AAGGAGATGGAATGAACATAG-3′. Lentiviral shRNAs were constructed with pLKO.1-TRC vector (RRID:Addgene_10878), packaged with helper plasmids p8.91 (RRID:Addgene_187441) and pCMV-VSV-G (RRID: Addgene_8454), and purified by ultracentrifugation. After infection, cells were treated with puromycin (2 μg/ml) to establish stable expression of lentiviral shRNA. For transient gene knockdown, 375 ng of lentiviral shRNA plasmid and 125 ng of the indicated plasmids were cotransfected into a 24-well of cells with 80% confluence. After 24 h, cells were treated with puromycin (2 μg/ml) for 48–60 h to maintain the transfected cells.
Gene transcription analysis
Total RNAs were isolated from cultured cells by the TRIzol reagent (Thermo Fisher Scientific). cDNAs were synthesized using the ProtoScript II, First Strand cDNA Synthesis Kit (New England Biolabs). Quantitative RT-PCR analysis was performed using a Power SYBR Green PCR Master Mix (Thermo Fisher Scientific). The primers for the quantitative RT-PCR were as follows: ACTB, F-5′-GACATTAAGGAGAAGCTGTGCTACGT-3′, R-5′-AGGAAGGAAGGCTGGAAGAGTGCCT-3′; CTGF, F-5′-TTTGAGCTTTCTGGCTGCACCA-3′, R-5′-TAATGGCAGGCACAGGTCTTGA-3′; CYR61, F-5′-CTTCATGGTCCCAGTGCTCAAA-3′, R-5′-CGGGGGATTTCTTGGTCTTGCT-3′; NANOG, F-5′-GAACATCCAGTCCTGGAGCA-3′, R-5′-AATACCTAGTGGTCTGCTGT; and OCT4, F-5′-GTGAGAGGCAACCTGGAGAA, R-5′-ATCCTCTCGTTGTGCATAGT-3′.
Co-IP
For general co-IP experiments, HEK293T (Cat# CRL-3216, RRID:CVCL_0063; ATCC) cells were transfected with indicated constructs for 24 h and then lysed in a common lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM dithiothreitol [DTT], protease inhibitor cocktail, and phosphatase inhibitor cocktail [Roche]). After centrifugation, the supernatants were mixed with indicated antibodies for 2 h at 4°C followed by the affinity adsorption of protein A/G plus agarose (Santa Cruz) for an additional 2 h. The beads were washed three times and resuspended in 30 μl of SDS loading buffer, then analyzed by western blotting. For analysis of interactions between cargoes and nuclear transport receptors, cells were lysed by an NTR lysis buffer (10 mM Hepes, pH 7.5, 100 mM KAc, 50 mM NaCl, 1% Triton X-100, 1 mM DTT, protease inhibitor cocktail, and phosphatase inhibitor cocktail) on ice, and then IP was carried out as described above.
Recombinant protein production and in vitro protein interactions
Fc-YAP1, Fc-YAP1 (W4A), and Fc-WW (second WW of YAP1) were expressed in HEK293T (Cat# CRL-3216, RRID:CVCL_0063; ATCC) cells for 24 h and then Fc-fused proteins were collected by protein A/G plus agarose. His-KPNA2, His-KPNA2 (xPxAA), and His-KPNA2-ΔIBB (deletion of aa 1–70) were constructed into pET21b vector (RRID:Addgene_111287) and expressed in Escherichia coli BL21 (DE3) at 22°C for 24 h. Recombinant proteins were extracted from E. coli with 0.5 mg/ml lysozyme in a buffer containing 20 mM Tris-HCl, pH 7.5, 300 mM NaCl, 0.1% Triton X-100, and 1 mM phenylmethylsulphonyl fluoride, and then purified by Ni-NTA Agarose (Qiagen), respectively. Finally, the purified proteins were dialyzed in a buffer containing 20 mM Tris-HCl, pH 7.5, 100 mM KAc, 50 mM NaCl, 1 mM DTT, and 0.5% Triton X-100.
For in vitro binding assays, 100 μl of recombinant proteins (200 ng/μl) in a binding solution (20 mM Tris-HCl, pH 7.5, 100 mM KAc, 50 mM NaCl, 1 mM DTT, 1% Triton X-100) were incubated with A/G agarose containing 4 μg of Fc-fused proteins for 1 h at 4°C, followed by three times of wash with the binding solution. Finally, the engaged protein complexes were eluted in 30 μl of SDS loading buffer and then 20% of the elution was analyzed by SDS-PAGE and Coomassie blue staining.
Digitonin permeabilization and nuclear export assays
Monoclonal HeLa (Cat# CCL-2, RRID:CVCL_0030; ATCC) cells stably expressing GFP or WW-fused GFP grew on coverslips. When cells grew to 70% confluence, they were rinsed with Dulbecco's Phosphate-Buffered Saline (DPBS) once and then permeabilized with digitonin (40 μg/ml) in a transport buffer (10 mM Hepes, pH 7.5, 100 mM KAc, 1 mM DTT, protease inhibitor cocktail) on ice for 0, 2, 4, 6, and 10 min. The permeabilized cells were immediately fixed with 1/10 volume of 37% formaldehyde for 20 min at room temperature, counterstained with Hoechst 33342 (Thermo Fisher Scientific), and mounted. Fluorescence images were captured using Leica SP8 confocal microscopy system under fixed parameters, and fluorescent intensities of nuclear GFP in each image were collected through ImageJ software (National Institutes of Health). The percentage of fluorescence intensity relative to 0 min was inversely proportional to the nuclear export rate of GFP.
Immunofluorescence and microscopy
Cells cultured on coverslips were washed once with DPBS and then fixed for 20 min in DPBS containing 4% paraformaldehyde at room temperature. For the detection of GFP-fused proteins, fixed cells were washed three times and then stained with Hoechst 33342 for 5 min. For the detection of immunostained proteins, fixed cells were permeabilized by 0.1% Triton X-100 for 5 min and then blocked by 2% BSA for 30 min. Finally, cells were stained with first antibodies followed by Cy3-conjugated second antibodies and Hoechst counterstaining. To induce YAP1 nuclear accumulation, cells were treated with LMB (0.5 μM; Santa Cruz) for 2 h. All fluorescence images were captured using Leica SP8 confocal microscopy system at room temperature and analyzed using LAS AF Lite (Leica) and ImageJ software (National Institutes of Health). Objective, 63×/1.40, oil CS2 (cedar oil).
Sphere formation assays
Before sphere formation, cells were cultured with 1 μM of synthesized PPPY peptide for 1 wk and then seeded into Nunclon Sphera 96-well plate (Thermo Fisher Scientific) at a density of 2,000 cells/well in the culture medium supplemented with 1 μM of synthesized PPPY peptide. The plate was centrifuged at 290 g for 3 min and then incubated at 37°C, 5% CO2. Cells cultured in the normal medium served as controls. After 1 wk, spheroids were captured using Leica DMi8 microscope and the sizes of spheroids were analyzed using LAS AF Lite (Leica) and ImageJ software (National Institutes of Health).
Statistical analysis
Two-tailed Student’s t test and ANOVA analyses of variance were used to evaluate statistical significance and P value < 0.05 to declare a statistical change. All values were presented as the means ± SD.
Online supplemental material
Fig. S1 shows that WW domains are dispensable to YAP1 phosphorylation catalyzed by LATS1/2. Fig. S2 shows that the WW domain is identified as a novel class of folded NLS. Fig. S3 shows that α-family karyopherins recognize and bind to the WW-NLS. Fig. S4 shows that the WW-NLS bypasses the mediation of canonical NLS. Fig. S5 shows they develop inhibitory peptides against the WW-NLS in YAP1 nuclear import.
Data availability
Data in this study are available within the article and its supplementary data files, or from the corresponding author upon reasonable request.
Acknowledgments
We thank Yalin Huang and Jin Li from the technology platform of the Institute of Biomedical Science of Fudan University for their help with confocal microscopies.
This work was sponsored by the National Natural Science Foundation of China 31872829 (to X. Gan).
Author contributions: Conceptualization: X. Gan and J. Wang; Formal analysis: X. Gan, J. Wang, and Y. Yang; Investigation: J. Wang, Y. Yang, M. Wu, and Y. Pan; Methodology: J. Wang, Y. Yang, M. Wu, and Y. Pan; Resources: Y. Hua, X. He, and X. Li; Validation: Y. Yang, M. Wu, Y. Pan, Y. Hua, X. He, and X. Li; Writing—original draft: X. Gan, J. Wang, Y. Yang, M. Wu, and Y. Pan; Writing—review and editing: X. Gan and J. Wang; Supervision: X. Gan and J. Wang; Project administration: X. Gan and J. Wang; Funding acquisition: X. Gan. All authors read and approved the final manuscript.
References
Author notes
Y. Yang, M. Wu, and Y. Pan contributed equally to this paper.
Disclosures: The authors declare no competing interests exist.


![WW domains are dispensable to YAP1 phosphorylation catalyzed by LATS1/2. (A) The schematic diagram illustrates the functional modules of YAP1, the linear feature of the WW domain, and the three-dimensional structures of the WW domains of YAP1 (Protein Data Bank [PDB]: 6JK1). TAD, transcriptional activity domain. (B) The co-IP experiment shows the interaction of FLAG-tagged LATS2 with HA-tagged YAP1 or W4A mutant in HEK293T cells. W4A, alanine substitutions of four conserved tryptophan residues (W177, W199, W236, and W258) in the WW domains of YAP1; TCL, total cell lysates. (C) The co-IP experiment shows the interaction of HA-tagged LATS1 with MYC-tagged YAP1 or YAP1-ΔWWs mutant, which lacks all WW domains, in HEK293T cells. (D) The co-IP experiment shows that the ectopic restoration of the WW domains at the amino terminus of the YAP1-ΔWWs mutant rescues the interaction of LATS2 with YAP1-ΔWWs mutant in HEK293T cells. (E) The representative western blotting shows MST2–LATS2 cascade-mediated phosphorylation of Ser-127 of the wild-type YAP1, the ΔWWs mutant, and the tdWW−ΔWWs chimera in HEK293T cells, and the column chart shows normalized quantitative Ser-127 phosphorylation (quantitative data are obtained from three independent experiments, N = 3, all data shown as the average values ± SD, two-tailed Student’s t test). (F) The co-IP experiment shows that deletion of the WW domains does not affect phosphorylation-mediated interaction of YAP1 and 14-3-3 in HEK293T cells. (G) The CRISPR/Cas9-mediated depletion of YAP1 gene expression in HEK293T cells. The representative western blotting shows YAP1 protein content in the wild-type and the knockout (KO) cells. (H) The representative western blotting shows endogenous Ser-127 phosphorylation of YAP1 in HEK293T/YAP1-KO cells, which are stably restored to the expression of the wild-type YAP1, the W4A mutant, the ΔWWs mutant, or the S127A substitution mutant, respectively. Source data are available for this figure: SourceData F1.](https://cdn.rupress.org/rup/content_public/journal/jcb/223/6/10.1083_jcb.202308013/1/m_jcb_202308013_fig1.png?Expires=1773764341&Signature=HiKL3ahsWeK0WU1wBG60bD8vB6MdDrU1tDOApYjeSEM7dQMrWTI4sKAgXKlRYE4jWgNQzRwuv0sRN7XtIE9B2tojewGDaJNDw~Q1lEK3tRYSD5Sq2SmtICatYmpF5Ti63mJ77IMlsd6FNiHLLtuAS2JVa1Pvnb9T7PEy75CMoPCmFr~hnX3kkjNHreSPWtolnHsu-ovAOYwagL0FwoSN8-FPgDZf-R7TBGOcsi3D5HGjQCedGmblBuYMhRS19FGcdq32PEkEYV479F--RS27xB7Ui8kuV9aUR9MjiTJPfOsCNovvzq1MjTUcKj8Hi4V1LzuUFdkjZECCIUesmz5Wdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)