The IL-17 receptor adaptor molecule Act1, an RNA-binding protein, plays a critical role in IL-17–mediated cancer progression. Here, we report a novel mechanism of how IL-17/Act1 induces chemoresistance by modulating redox homeostasis through epitranscriptomic regulation of antioxidant RNA metabolism. Transcriptome-wide mapping of direct Act1–RNA interactions revealed that Act1 binds to the 5′UTR of antioxidant mRNAs and Wilms’ tumor 1–associating protein (WTAP), a key regulator in m6A methyltransferase complex. Strikingly, Act1’s binding sites are located in proximity to m6A modification sites, which allows Act1 to promote the recruitment of elF3G for cap-independent translation. Loss of Act1’s RNA binding activity or Wtap knockdown abolished IL-17–induced m6A modification and translation of Wtap and antioxidant mRNAs, indicating a feedforward mechanism of the Act1–WTAP loop. We then developed antisense oligonucleotides (Wtap ASO) that specifically disrupt Act1’s binding to Wtap mRNA, abolishing IL-17/Act1-WTAP-mediated antioxidant protein production during chemotherapy. Wtap ASO substantially increased the antitumor efficacy of cisplatin, demonstrating a potential therapeutic strategy for chemoresistance.
Introduction
Emerging evidences indicate that IL-17 signaling plays a critical role in cancer progression and resistance to anticancer therapies for a variety of solid tumors in humans, especially those of mucosal origin such as cutaneous squamous cell carcinoma (cSCC) and head and neck SCC (Chen and Chen, 2014; Gopalakrishnan et al., 2018; Gu et al., 2012; He et al., 2011; Huang et al., 2014; Punt et al., 2016; Punt et al., 2015; Tosolini et al., 2011; Wu et al., 2012; Xu et al., 2014a; Yan et al., 2014; Zhang et al., 2012; Zhang et al., 2013; Zhang et al., 2018a). Preclinical studies have focused on the mechanisms for IL-17–mediated early tumorigenesis in various organs. However, the urgent medical need lies in understanding the different mechanisms of cancer drug resistance, which is the key to combatting cancer progression for late-stage cancer patients. The mechanism for IL-17–mediated therapeutic resistance is poorly understood.
IL-17 binds the IL-17 receptor (IL-17R) to trigger the production of proinflammatory cytokines and chemokines in tissue cells (McGeachy et al., 2019; Monin and Gaffen, 2018). This is achieved through a combination of weak transcriptional changes (activation of NF-κB [Chang et al., 2006; Garg et al., 2013; Qian et al., 2007; Sønder et al., 2011; Zhong et al., 2012] and C/EBP [Maitra et al., 2007; Ruddy et al., 2004; Shen et al., 2006, 2009; Sønder et al., 2012]) and less well-defined, but more robust posttranscriptional changes that include stabilization and translational control of specific mRNAs (Amatya et al., 2017; Bulek et al., 2011; Hartupee et al., 2007; Herjan et al., 2013; McGeachy et al., 2019; Zhong et al., 2012). Multiple mRNA destabilizing mechanisms have been discovered for cytokine and chemokine mRNAs, which provide the basis for the critical role of IL-17 signaling in promoting posttranscriptional regulation of the proinflammatory genes. Cytokine and chemokine mRNAs have short half-lives because of conserved cis-elements within the 3′ untranslated regions (UTRs) that can be recognized by RNA-binding proteins (including TTP [Datta et al., 2010; Tiedje et al., 2016], AUF1 [Abbadi et al., 2019; Sun et al., 2016], KSRP [Bollmann et al., 2014; Schmidtke et al., 2019], SF2 [Delestienne et al., 2010; Sun et al., 2011], and Regnase-1 [Iwasaki et al., 2011; Mino et al., 2015]) and mediate the sequential deadenylation, decapping, and ultimately exonucleolytic degradation of the RNA (Bulek et al., 2011; Herjan et al., 2013; Schoenberg and Maquat, 2012; Somma et al., 2015; Stumpo et al., 2010; Sun et al., 2011). Act1 is the key adaptor molecule directly recruited to IL-17R and is required for both the transcriptional and posttranscriptional changes of proinflammatory genes induced by IL-17 (Bulek et al., 2011; Herjan et al., 2018; Li et al., 2000, 2019). Significant progress has been made in understanding how specific mRNAs are targeted for regulation in response to IL-17 signaling. A key breakthrough was the unanticipated discovery that Act1 directly binds inflammatory mRNAs (Herjan et al., 2018). Upon IL-17 stimulation, Act1 is recruited to IL-17R through a SEFIR-dependent interaction (Chang et al., 2006; Li et al., 2000; Qian et al., 2007). We discovered that a specific region in the SEFIR domain of Act1 directly binds stem-loop RNA structures at the 3′UTR of inflammatory mRNAs (e.g., Cxcl1) to stabilize them and promote their translation in response to IL-17 stimulation (Herjan et al., 2018).
Intriguingly, recent studies suggest that IL-17–induced targets other than inflammatory genes play a critical role in cancer progression and resistance to anticancer therapies (Chen et al., 2022; Liao et al., 2020). Therefore, we hypothesize that transcriptome-wide mapping of direct Act1–RNA interactions may yield novel effector molecules for the molecular pathogenesis of IL-17–mediated cancer progression, implicating new therapeutic targets for anticancer therapies. We report here that transcriptome-wide mapping of direct Act1–RNA interactions in vivo revealed that Act1 binds in high density to the 5′UTR of a set of transcripts including antioxidant mRNAs and Wilms’ tumor 1–associating protein (WTAP), a key regulator in m6A methyltransferase complex. IL-17 stimulation induced the expression of these 5′UTR Act1-targets at the protein levels without detectable impact on the mRNA levels. Previous studies have reported that regulatory elements in the 5′UTR may modulate protein translation both in a cap-dependent or cap-independent manner (Hinnebusch et al., 2016; Leppek et al., 2018). Importantly, while cap-independent translation is initiated under aberrant stress conditions such as cancer, 5′UTR m6A-methylation has been shown to promote cap-independent translation during cancer progression (Coots et al., 2017; Meyer et al., 2015; Zhou et al., 2015). Interestingly, we found that Act1’s binding sites are located in proximity to m6A modification sites on the 5′UTRs of Act1 targets, including Wtap and antioxidant mRNAs. This allows Act1 to promote the recruitment of elF3G’s binding to m6A sites of Wtap and antioxidant mRNAs to drive their cap-independent translation in cancer cells. Loss of Act1’s RNA binding activity or Wtap knockdown abolished IL-17–induced m6A modification and translation of Wtap and antioxidant mRNAs, indicating a feedforward mechanism of the Act1–WTAP loop. We then developed antisense oligonucleotides (Wtap ASO) that specifically disrupt Act1’s binding to Wtap mRNA, abolishing IL-17/Act1-WTAP–mediated antioxidant protein production during chemotherapy. Furthermore, WTAP ASO was efficacious in promoting cisplatin-mediated cancer cell killing with enhanced ROS. The results unravel a novel mechanism by which IL-17 induces chemoresistance by modulating redox homeostasis through epitranscriptomic regulation of antioxidant RNA metabolism and their cap-independent translation. Importantly, WTAP ASO was indeed able to robustly enhance the antitumor efficacy of cisplatin in mice, demonstrating a novel therapeutic strategy to sensitize cancer cells to chemotherapy.
Results
Transcriptome-wide mapping of Act1–RNA interactions defines direct targets and binding sites of Act1
We previously discovered that the SEFIR domain of Act1, an IL-17R complex adaptor, directly binds stem-loop RNA structures at the 3′UTR of inflammatory mRNAs (Cxcl1) to stabilize them and promote their translation in response to IL-17 stimulation (Herjan et al., 2018). To identify Act1-binding sites at high resolution in a transcriptome-wide manner, we performed crosslinking and immunoprecipitation (CLIP) in combination with next-generation sequencing (CLIP-seq). Mouse embryonic fibroblasts (MEF) stably expressing FLAG-tagged Act1 were UV crosslinked. Immunoprecipitated radiolabeled Act1–RNA complexes were ribonuclease-treated and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. S1 A). The purified Act1–RNA complexes were then subjected to protease digestion, from which peptide remnants crosslinked to the RNA cause the reverse transcriptase to pause in the library preparation step, thereby allowing for the identification of crosslink sites at single-nucleotide resolution. We identified 75,609 genomic coordinates with overlapping CLIP reads from at least two out of three biological replicates (Fig. 1 A and Table S1). Importantly, Act1 CLIP reads in the 3′UTR of Cxcl1, Cebpb, and Hif1a indeed overlap with the Act1 binding sites previously identified by in vitro RNA-binding assays and mutagenesis (Chen et al., 2022; Herjan et al., 2018; Hong et al., 2022; and Fig. S1 B).
Act1 binds to the 5′UTR of Wtap and antioxidant mRNAs to promote their translation. (A) Autoradiograph of radiolabeled cross-linked Act1–RNA complexes immunopurified from IL-17A–stimulated WT Act1 or Act1 ΔSEF1 MEFs lysates treated with either a low (1:20,000) or high (1:1,000) concentration of RNaseA. No UV crosslinking and IgG were used as negative controls. Arrow denotes position of Act1. Open bracket indicates the region of membrane excised for library preparation. (B) Genome browser views of Act1 CLIP binding sites on the 3′UTR of Cxcl1, Cebpb, and Hif1a overlap with the previously reported SBE sites. Scaling was performed for visualization. (C) Pathway analysis of the genes with identified Act1 binding sites on the 3′UTR of their transcripts. (D) Genome browser views of Act1 CLIP binding sites on the 5′UTR of Prdx2 and Txn1. (E) WT Act1 or Act1 ΔSEF1 MEFs were either left untreated or treated with IL-17A, followed by RNA immunoprecipitation with anti-Act1 and RT-PCR. Relative values normalized against IgG control are shown (mean and SD of three independent plates of cells). One-way ANOVA was performed, followed by Tukey’s multiple-comparisons test. (F) MOC1 cells and PDVC57 cells were treated with IL-17A, followed by western blot analysis. (G) WT Act1 MEFs, MOC1 cells, and PDVC57 cells were treated with IL-17A, followed by RT-PCR analyses (n = 3 independent plates of cells). (H) MOC1 cells and PDVC57 cells were treated with IL-17A and subjected to RNA immunoprecipitation with anti-Act1 followed by RT-PCR analyses (n = 3 independent plates of cells). Relative values normalized against IgG control are shown. All data are representative of three independent experiments. Two-tailed t test was performed. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; NS, not significant. Source data are available for this figure: SourceData FS1.
Act1 binds to the 5′UTR of Wtap and antioxidant mRNAs to promote their translation. (A) Autoradiograph of radiolabeled cross-linked Act1–RNA complexes immunopurified from IL-17A–stimulated WT Act1 or Act1 ΔSEF1 MEFs lysates treated with either a low (1:20,000) or high (1:1,000) concentration of RNaseA. No UV crosslinking and IgG were used as negative controls. Arrow denotes position of Act1. Open bracket indicates the region of membrane excised for library preparation. (B) Genome browser views of Act1 CLIP binding sites on the 3′UTR of Cxcl1, Cebpb, and Hif1a overlap with the previously reported SBE sites. Scaling was performed for visualization. (C) Pathway analysis of the genes with identified Act1 binding sites on the 3′UTR of their transcripts. (D) Genome browser views of Act1 CLIP binding sites on the 5′UTR of Prdx2 and Txn1. (E) WT Act1 or Act1 ΔSEF1 MEFs were either left untreated or treated with IL-17A, followed by RNA immunoprecipitation with anti-Act1 and RT-PCR. Relative values normalized against IgG control are shown (mean and SD of three independent plates of cells). One-way ANOVA was performed, followed by Tukey’s multiple-comparisons test. (F) MOC1 cells and PDVC57 cells were treated with IL-17A, followed by western blot analysis. (G) WT Act1 MEFs, MOC1 cells, and PDVC57 cells were treated with IL-17A, followed by RT-PCR analyses (n = 3 independent plates of cells). (H) MOC1 cells and PDVC57 cells were treated with IL-17A and subjected to RNA immunoprecipitation with anti-Act1 followed by RT-PCR analyses (n = 3 independent plates of cells). Relative values normalized against IgG control are shown. All data are representative of three independent experiments. Two-tailed t test was performed. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; NS, not significant. Source data are available for this figure: SourceData FS1.
CLIP analysis of Act1–RNA interactions. (A) Percentage of various RNA species bound by Act1 based on CLIP analyses. Mouse mm10 Gencode M25(GRCm38.p6) was used to determine the gene annotation of clusters. The majority of Act1 binding sites were located in protein-coding genes. (B) Metagene profile showing the distribution of Act1 binding clusters across the mouse transcriptome. 5′UTRs, CDSs, and 3′UTRs of mRNAs were individually binned into regions spanning 1% of their longest transcript length, and the percentage of Act1 binding clusters that fall within each bin was determined. (C) Boxplot showing the density of Act1 binding clusters on 5′UTR and 3′UTR of target genes. A two-tailed t test was performed. (****P < 0.0001) (D) Scatter plot summarizing the enrichment of all possible hexamers (4,096 in total) in the 5′UTR (x-axis) and 3′UTR CLIP sites (y-axis). Hexamer enrichment analyses were performed using the EMBOSS tools. (E) Pathway analysis (ranked by significance) of the genes with identified Act1 binding sites on the 5′UTR of their transcripts.
CLIP analysis of Act1–RNA interactions. (A) Percentage of various RNA species bound by Act1 based on CLIP analyses. Mouse mm10 Gencode M25(GRCm38.p6) was used to determine the gene annotation of clusters. The majority of Act1 binding sites were located in protein-coding genes. (B) Metagene profile showing the distribution of Act1 binding clusters across the mouse transcriptome. 5′UTRs, CDSs, and 3′UTRs of mRNAs were individually binned into regions spanning 1% of their longest transcript length, and the percentage of Act1 binding clusters that fall within each bin was determined. (C) Boxplot showing the density of Act1 binding clusters on 5′UTR and 3′UTR of target genes. A two-tailed t test was performed. (****P < 0.0001) (D) Scatter plot summarizing the enrichment of all possible hexamers (4,096 in total) in the 5′UTR (x-axis) and 3′UTR CLIP sites (y-axis). Hexamer enrichment analyses were performed using the EMBOSS tools. (E) Pathway analysis (ranked by significance) of the genes with identified Act1 binding sites on the 5′UTR of their transcripts.
While it has been well documented that IL-17–Act1 modulates mRNA metabolism via the regulatory sequences at the 3′UTR, it is interesting to note that there is a substantial enrichment of Act1-binding sites in the 5′UTR of its target transcripts (Fig. 1 B). Despite the fact that the average length of 5′UTR is much shorter compared with the 3′UTR, Act1 has a higher binding density on the 5′UTR than that on 3′UTR (Fig. 1 C). We performed hexamer enrichment analysis against all possible hexamers (4,096 in total) and found that the top enriched hexamers in the Act1 binding sites are distinct for 3′UTR (AU-rich) versus 5′UTR (GC-rich) (Fig. 1 D). In most cases, Act1 binds dominantly to either the 5′UTR or 3′UTR of the transcripts. Strikingly, whereas inflammation-related GO terms are enriched for 3′UTR targets of Act1, the 5′UTR targets are enriched for mRNAs encoding proteins important for mRNA processing (such as Wtap, a key regulator in m6A methyltransferase complex), redox homeostasis (including antioxidant proteins Prdx2, Txn1, and Sod1), cellular stress, and DNA damage repair (Fig. 1 E, Fig. S1 C, and Table S2). These results suggest that functionally different cohorts of mRNAs might be subject to specific modes of Act1 binding and regulation.
Act1’s binding to the 5′UTR of Wtap and antioxidant mRNAs promotes their cap-independent translation
Analysis of CLIP in combination with RNA sequencing (RNA-seq) data indicated that a group of 5′UTR targets with robust binding of Act1 in CLIP were barely induced by IL-17 at the mRNA levels (Table S3), including important genes for mRNA processing (such as Wtap) and antioxidants (Prdx2, Txn1, and Sod1) (Fig. 2, A and B; and Fig. S1 D). Act1’s binding to these 5′UTR targets was validated by RNA electrophoretic mobility shift assay (REMSA) and RNA immunoprecipitation (Fig. 2 C and Fig. S1 E). Several critical questions arose from these observations: does the binding of Act1 to the 5′UTR targets play a role in the protein translation of these mRNAs? If yes, what is the mechanism of regulation? What is the functional impact of such regulation?
Act1’s binding to the 5′UTR of transcripts encoding Wtap and antioxidant proteins promotes their translation. (A) Normalized count of indicated genes in untreated and IL-17A–treated MEFs were measured by RNA-seq and analyzed using DEseq2. ****Padj < 0.0001. (B) Genome browser views of Act1 binding clusters on Wtap and Sod1 genes using the Integrative Genomics Viewer (IGV). Scaling was performed for visualization. (C) Binding of purified recombinant His-MBP-Act1 SEFIR domain (Act1 WT) and SEFIR1 deletion mutant (Act1 ΔSEF1) to the 5′UTR of Wtap, Prdx2, Sod1, and Txn1 were examined by REMSA. (D) Act1−/− MEFs reconstituted with FLAG-tagged wild-type (WT) Act1 by retroviral infection were treated with IL-17A. The cell lysates were then subjected to western blot analysis. (E) Act1−/− MEFs cells reconstituted retrovirally with either WT Act1 or Act1 ΔSEF1 were treated with IL-17A for 0 or 24 h, followed by fractionation. Indicated mRNAs from translation-active pools and translation-inactive pools were analyzed by RT-PCR and normalized to Gapdh. The graph shows the ratios of mRNAs from translation-active/inactive pools (mean and SD of three independent plates of cells). Two-tailed t test was performed. *P < 0.05, **P < 0.01, and ****P < 0.0001; NS, not significant. All data are representative of three independent experiments. Source data are available for this figure: SourceData F2.
Act1’s binding to the 5′UTR of transcripts encoding Wtap and antioxidant proteins promotes their translation. (A) Normalized count of indicated genes in untreated and IL-17A–treated MEFs were measured by RNA-seq and analyzed using DEseq2. ****Padj < 0.0001. (B) Genome browser views of Act1 binding clusters on Wtap and Sod1 genes using the Integrative Genomics Viewer (IGV). Scaling was performed for visualization. (C) Binding of purified recombinant His-MBP-Act1 SEFIR domain (Act1 WT) and SEFIR1 deletion mutant (Act1 ΔSEF1) to the 5′UTR of Wtap, Prdx2, Sod1, and Txn1 were examined by REMSA. (D) Act1−/− MEFs reconstituted with FLAG-tagged wild-type (WT) Act1 by retroviral infection were treated with IL-17A. The cell lysates were then subjected to western blot analysis. (E) Act1−/− MEFs cells reconstituted retrovirally with either WT Act1 or Act1 ΔSEF1 were treated with IL-17A for 0 or 24 h, followed by fractionation. Indicated mRNAs from translation-active pools and translation-inactive pools were analyzed by RT-PCR and normalized to Gapdh. The graph shows the ratios of mRNAs from translation-active/inactive pools (mean and SD of three independent plates of cells). Two-tailed t test was performed. *P < 0.05, **P < 0.01, and ****P < 0.0001; NS, not significant. All data are representative of three independent experiments. Source data are available for this figure: SourceData F2.
Previous studies have implicated that 5′UTR of mRNA can impact translation initiation, and therefore, the amount of protein produced from each mRNA (Leppek et al., 2018). IL-17 treatment markedly induced the expression of Act1’s 5′UTR targets (Wtap, Prdx2, Txn1, and Sod1) at the protein levels, but not at the RNA levels (Fig. 2 D and Fig. S1 G). Ribosomal fractionation of Act1−/− cells reconstituted with Act1 WT or Act1 ΔSEF1 (RNA-binding mutant) indicated that Act1 RNA binding activity is necessary for IL-17–induced shift of the transcripts of WTAP and antioxidants to the actively translating fractions (Fig. 2 E). Taken together, these results suggest that the binding of Act1 to the 5′UTR targets plays a critical role in driving the translation of these target mRNAs. Notably, IL-17 induced the expression of these Act1’s 5′UTR targets at the protein levels (without altering RNA levels) in various cancer cell lines including PDVC57 (mouse skin SCC cell line) and MOC1 (mouse oral cancer 1) cell lines derived from squamous cell carcinoma of the mouse oral cavity and human A431 epidermoid carcinoma cell line (Fig. S1, F and G; and Fig. S4, F and G). By performing Act1 RNA immunoprecipitation, we indeed detected IL-17–induced robust binding of Act1 to these 5′UTR targets in multiple cancer cell lines (Fig. S1 H). Since IL-17 signaling plays a critical role in tumorigenesis and resistance to anticancer therapies, the findings here implicate a potential functional impact of this newly discovered IL-17/Act1’s regulated mRNA cohort in cancer. In support of this, WTAP expression was significantly elevated in human skin tumor tissue containing a high number of IL-17A–producing cells (IL-17A high) compared with human skin tumor tissue containing a low number of IL-17A–producing cells (IL-17A low) (Fig. S2, A and B).
IL-17 enhances cap-independent translation of WTAP and antioxidants, consistent with elevated IL-17A-WTAP axis in human skin tumor tissues. (A) Green boxes indicate the stained WTAP areas that were subjected to ImageJ analysis. Arrows indicate the samples that belong to IL-17A high group. Bar graph comparing the quantification of WTAP staining in IL-17A-low and IL-17A-high groups. P = 0.0037 by t test. ±SEM. (B) Images of WTAP staining and IL-17A staining from two samples (IL-17A high: F7; IL-17A low: B7, as shown in panel A). Scale bar, 500 μm. (C) WT Act1 or Act1 ΔSEF1 MEFs transfected with siCTRL or si-eIF4E were treated with IL-17A. The cell lysates were subjected to western blot analysis. (D) MOC1 cells pretreated with rapamycin were either left untreated or treated with IL-17A, followed by fractionation. Indicated mRNAs from translation-active pools and translation-inactive pools were analyzed by RT-PCR and normalized to β-actin. Graph shows the ratios of mRNAs from translation-active/inactive pools (n = 3 independent plates of cells). Two-tailed t test was performed. *P < 0.05, **P < 0.01, and ***P < 0.001; NS, not significant. Source data are available for this figure: SourceData FS2.
IL-17 enhances cap-independent translation of WTAP and antioxidants, consistent with elevated IL-17A-WTAP axis in human skin tumor tissues. (A) Green boxes indicate the stained WTAP areas that were subjected to ImageJ analysis. Arrows indicate the samples that belong to IL-17A high group. Bar graph comparing the quantification of WTAP staining in IL-17A-low and IL-17A-high groups. P = 0.0037 by t test. ±SEM. (B) Images of WTAP staining and IL-17A staining from two samples (IL-17A high: F7; IL-17A low: B7, as shown in panel A). Scale bar, 500 μm. (C) WT Act1 or Act1 ΔSEF1 MEFs transfected with siCTRL or si-eIF4E were treated with IL-17A. The cell lysates were subjected to western blot analysis. (D) MOC1 cells pretreated with rapamycin were either left untreated or treated with IL-17A, followed by fractionation. Indicated mRNAs from translation-active pools and translation-inactive pools were analyzed by RT-PCR and normalized to β-actin. Graph shows the ratios of mRNAs from translation-active/inactive pools (n = 3 independent plates of cells). Two-tailed t test was performed. *P < 0.05, **P < 0.01, and ***P < 0.001; NS, not significant. Source data are available for this figure: SourceData FS2.
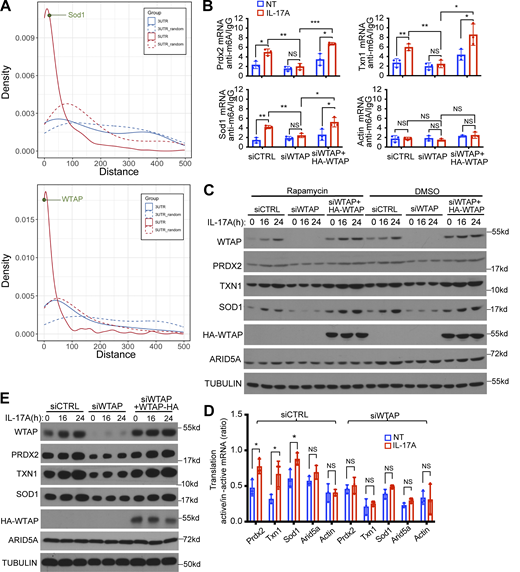
Regulatory elements in the 5′UTR may modulate protein translation both in a cap-dependent or cap-independent manner (Hinnebusch et al., 2016; Leppek et al., 2018; Meyer et al., 2015). To study the mechanism of IL-17–induced translation of Act1 5′UTR targets, we used rapamycin to block cap-dependent translation (Beretta et al., 1996; Sonenberg and Hinnebusch, 2009) in MEFs containing either Act1 WT or Act1 ΔSEF1 mutant (loss of Act1–RNA binding). We found that IL-17–induced expression of WTAP and antioxidants (Prdx2, Txn1, and Sod1) was not inhibited by rapamycin in Act1 WT cells (Fig. 3 A), implicating a potential role of cap-independent translation for the induction of these proteins. On the other hand, IL-17–induced expression of WTAP and antioxidants was greatly diminished in Act1-ΔSEF1 cells (Fig. 3 A), indicating the importance of Act1’s RNA binding activity for IL-17–induced translation of these Act1’s 5′UTR targets. As a control, we showed that rapamycin substantially inhibited IL-17–induced Arid5a translation, supporting that IL-17–induced Arid5a expression is cap-dependent as previously reported (Fig. 3 A) (Amatya et al., 2018). Moreover, knocking down eIF4E (required for Cap-dependent translation) substantially inhibited IL-17–induced Arid5a translation, but had no impact on IL-17–induced translation of these Act1’s 5′UTR targets (Fig. S2 C).
Act1 promotes cap-independent translation of transcripts encoding Wtap and antioxidant proteins. (A)Act1−/− MEFs reconstituted retrovirally with either FLAG-tagged WT Act1 or Act1 ΔSEF1 were pretreated with rapamycin or DMSO for 24 h, followed by IL-17A treatment and western blot analysis. (B) WT Act1 or Act1 ΔSEF1 MEFs were transfected with a plasmid encoding a bicistronic RNA expressing renilla luciferase (via cap-dependent translation) and firefly luciferase (via cap-independent translation driven by the inserted 5′UTR sequence, including Prdx2, Txn1, Wtap, Sod1, as indicated on diagram [top panel]), followed by IL-17A treatment for 24 h and dual luciferase reporter assay. The graph shows the ratio of firefly to renilla luciferase activity determined by luminescence (mean and SD of three independent plates of cells). (C) WT Act1 or Act1 ΔSEF1 MEFs were pretreated either with rapamycin or DMSO for 24 h and then treated with IL-17A for 24 h, followed by fractionation and RT-PCR normalized to Gapdh. The graph shows the ratios of mRNAs from translation-active/inactive pools (mean and SD of three independent plates of cells). Two-tailed t test was performed. *P < 0.05, **P < 0.01; NS, not significant. All data are representative of three independent experiments. Source data are available for this figure: SourceData F3.
Act1 promotes cap-independent translation of transcripts encoding Wtap and antioxidant proteins. (A)Act1−/− MEFs reconstituted retrovirally with either FLAG-tagged WT Act1 or Act1 ΔSEF1 were pretreated with rapamycin or DMSO for 24 h, followed by IL-17A treatment and western blot analysis. (B) WT Act1 or Act1 ΔSEF1 MEFs were transfected with a plasmid encoding a bicistronic RNA expressing renilla luciferase (via cap-dependent translation) and firefly luciferase (via cap-independent translation driven by the inserted 5′UTR sequence, including Prdx2, Txn1, Wtap, Sod1, as indicated on diagram [top panel]), followed by IL-17A treatment for 24 h and dual luciferase reporter assay. The graph shows the ratio of firefly to renilla luciferase activity determined by luminescence (mean and SD of three independent plates of cells). (C) WT Act1 or Act1 ΔSEF1 MEFs were pretreated either with rapamycin or DMSO for 24 h and then treated with IL-17A for 24 h, followed by fractionation and RT-PCR normalized to Gapdh. The graph shows the ratios of mRNAs from translation-active/inactive pools (mean and SD of three independent plates of cells). Two-tailed t test was performed. *P < 0.05, **P < 0.01; NS, not significant. All data are representative of three independent experiments. Source data are available for this figure: SourceData F3.
The bicistronic reporter system (including Renilla-Luc and Firefly-Luc reporters) is designed to measure cap-independent translation. In this system, Renilla-Luc is translated via a cap-dependent mechanism, but the downstream Firefly-Luc relies solely on the intergenic region for translation (cap-independent translation). To study IL-17–induced cap-independent translation, we cloned 5′UTRs of Prdx2, Txn1, SOD1, Arid5a, and WTAP into the intergenic region of the bicistronic reporter system (Fig. 3 B). We found that IL-17 stimulation increased the ratio of Firefly (cap-independent) over Renilla (cap-dependent) luciferase activity, indicating that these 5′UTR regions have the ability to drive IL-17–induced cap-independent translation (Fig. 3 B). Moreover, IL-17–induced cap-independent translation was abolished by the loss of Act1 RNA binding activity (Act1 ΔSEF1) (Fig. 3 B). By ribosomal fractionation experiment, we show that IL-17 induces the shift of antioxidant mRNAs to actively translating fractions in Act1 WT cells, but not Act1 ΔSEF1 cells (Fig. 3 C). Consistently, rapamycin failed to block IL-17–induced shift of Wtap and antioxidant mRNAs to actively translate fractions in MOC1 cell line (Fig. S2 D). Taken together, these results suggest that Act1’s RNA binding activity is required for IL-17–induced cap-independent translation of WTAP and antioxidant proteins mRNAs.
IL-17 induces m6A methylation and translation of Act1 5′UTR targets in a Wtap-dependent manner
Cap-independent translation is initiated under aberrant stress conditions such as cancer under which cap-dependent translation is often downregulated (El-Naggar and Sorensen, 2018; Liu and Qian, 2014; Spriggs et al., 2010; Walters and Thompson, 2016). It is plausible that IL-17 signaling, known to play a critical role in cancer progression, induces the expression of WTAP and antioxidants via cap-independent translation for cancer cells to cope with stress thereby promoting cell survival. Therefore, it is important to investigate the molecular mechanism for how IL-17 signaling turns on cap-independent translation of these Act1’s 5′UTR targets with antioxidation function. The initiation of cap-independent translation requires functional intrinsic elements such as methylated A residues located within 5′UTR (Meyer et al., 2015; Zhou et al., 2015). By comparing our CLIP data with several published MeRIP-seq database (Geula et al., 2015; Xi et al., 2020; Zhao et al., 2014), we found that Act1 binding sites are located in proximity to m6A sites on Act1’s 5′UTR targets, but not on 3′UTR targets (Fig. 4 A and Fig. S3 A). Strikingly, m6A sites in the 5′UTRs of Wtap, Sod1, Prdx2, and Txn1 transcripts are all located in regions that are within 10nt of CLIP-identified Act1 binding sites (Fig. 4 A and Fig. S3 A). Furthermore, mRNA encoding Wtap, a major component of m6A methyltransferase complex, is robustly bound by Act1 in the 5′UTR (Figs. 1 and 2 B); and Wtap is upregulated by IL-17/Act1 axis via a cap-independent manner (Figs. 2 and 3). These observations led us to propose the potential impact of the IL-17/Act1–WTAP axis on m6A-methylation of Act1’s 5′UTR targets to trigger their cap-independent translation.
Act1 RNA binding activity is required for IL-17–induced m6A methylation of Act1 5′UTR targets and cap-independent translation. (A) Density plots corresponding to distances of Act1 binding sites on 5′UTR (red) and 3′UTR regions (blue) to the closest annotated m6A sites from the published database (GEO accession no. GSE147489) on gene transcripts. Random distances on each transcript region were used as control (dashed lines). (B) MOC1 cells, Act1 WT, or Act1 ΔSEF1 MEFs were treated with IL-17A, followed by methylated (m6A) RNA immunoprecipitation (MeRIP) and RT-PCR as described in the Materials and methods section. (C) WT Act1 or Act1 ΔSEF1 MEFs with and without IL-17A stimulation were subjected to methylated (m6A) RNA immunoprecipitation (MeRIP) followed by RT-PCR. Graphs show relative levels of indicated mRNAs normalized to Gapdh (mean and SD of three independent plates of cells). (D) WT Act1 or Act1 ΔSEF1 MEFs were transfected with siCTRL, siWTAP, or siWTAP+HA-WTAP. The transfected cells pretreated with rapamycin were treated with IL-17A, followed by western blot analysis. Vehicle control (DMSO) for rapamycin was shown in Fig. S3 C. (E) Act1 WT MEFs were transfected with siCTRL or siWTAP. The transfections of cells pretreated with rapamycin were either left untreated or IL-17A, followed by fractionation and RT-PCR normalized to Gapdh. Graph shows the ratios of mRNAs from translation-active/inactive pools (mean and SD of three independent plates of cells). (F) Wtap-knockdown MEFs with or without restoration of HA-WTAP (HA-WTAP) were analyzed using a bicistronic reporter system as described in Fig. 3 B. The transfected cells pretreated with rapamycin were treated with IL-17 for 24 h, followed by dual luciferase reporter assay. Graphs show firefly and renilla luciferase activity determined by luminescence (mean and SD of three independent plates of cells). Two-tailed t test was performed. All data are representative of three independent experiments. *P < 0.05, **P < 0.01; NS, not significant. Source data are available for this figure: SourceData F4.
Act1 RNA binding activity is required for IL-17–induced m6A methylation of Act1 5′UTR targets and cap-independent translation. (A) Density plots corresponding to distances of Act1 binding sites on 5′UTR (red) and 3′UTR regions (blue) to the closest annotated m6A sites from the published database (GEO accession no. GSE147489) on gene transcripts. Random distances on each transcript region were used as control (dashed lines). (B) MOC1 cells, Act1 WT, or Act1 ΔSEF1 MEFs were treated with IL-17A, followed by methylated (m6A) RNA immunoprecipitation (MeRIP) and RT-PCR as described in the Materials and methods section. (C) WT Act1 or Act1 ΔSEF1 MEFs with and without IL-17A stimulation were subjected to methylated (m6A) RNA immunoprecipitation (MeRIP) followed by RT-PCR. Graphs show relative levels of indicated mRNAs normalized to Gapdh (mean and SD of three independent plates of cells). (D) WT Act1 or Act1 ΔSEF1 MEFs were transfected with siCTRL, siWTAP, or siWTAP+HA-WTAP. The transfected cells pretreated with rapamycin were treated with IL-17A, followed by western blot analysis. Vehicle control (DMSO) for rapamycin was shown in Fig. S3 C. (E) Act1 WT MEFs were transfected with siCTRL or siWTAP. The transfections of cells pretreated with rapamycin were either left untreated or IL-17A, followed by fractionation and RT-PCR normalized to Gapdh. Graph shows the ratios of mRNAs from translation-active/inactive pools (mean and SD of three independent plates of cells). (F) Wtap-knockdown MEFs with or without restoration of HA-WTAP (HA-WTAP) were analyzed using a bicistronic reporter system as described in Fig. 3 B. The transfected cells pretreated with rapamycin were treated with IL-17 for 24 h, followed by dual luciferase reporter assay. Graphs show firefly and renilla luciferase activity determined by luminescence (mean and SD of three independent plates of cells). Two-tailed t test was performed. All data are representative of three independent experiments. *P < 0.05, **P < 0.01; NS, not significant. Source data are available for this figure: SourceData F4.
IL-17 induces WTAP-dependent m6A methylation of Act1 5′UTR targets, facilitating their cap-independent translation. (A) Density plots corresponding to distances of Act1 binding sites on the 5′UTR (red) and 3′UTR regions (blue) mRNA transcript region to the closest annotated m6A binding sites using additional published datasets (GEO accession nos. GSE53244, GSE61995). Random distances on each transcript region were used as control (dashed lines). (B) MOC1 cells transfected with either siCTRL, siWTAP, or siWTAP+HA-WTAP were either left untreated or treated with IL-17A, followed by methylated (m6A) RNA immunoprecipitation (MeRIP) and RT-PCR. Graphs show relative levels of indicated mRNAs normalized to Gapdh (mean and SD of three independent plates of cells). (C) WT Act1 MEFs were transfected with siCTRL, siWTAP, or siWTAP+HA-WTAP. The transfected cells pretreated with rapamycin or vehicle control (DMSO) were treated with IL-17A, followed by western blot analysis. (D) MOC1 cells transfected with siCTRL or siWTAP were pretreated with rapamycin, followed by IL-17A treatment and fractionation. Indicated mRNAs from translation-active pools and translation-inactive pools were analyzed by RT-PCR and normalized to Gapdh. The graph shows the ratios of mRNAs from translation-active/inactive pools (n = 3 independent plates of cells). (E) MOC1 cells transfected with siCTRL, siWTAP, or siWTAP+HA-WTAP were pretreated with rapamycin, followed by IL-17A. The cell lysates were subjected to western blot analysis. Two-tailed t test was performed. *P < 0.05, **P < 0.01, and ***P < 0.001; NS, not significant. Source data are available for this figure: SourceData FS3.
IL-17 induces WTAP-dependent m6A methylation of Act1 5′UTR targets, facilitating their cap-independent translation. (A) Density plots corresponding to distances of Act1 binding sites on the 5′UTR (red) and 3′UTR regions (blue) mRNA transcript region to the closest annotated m6A binding sites using additional published datasets (GEO accession nos. GSE53244, GSE61995). Random distances on each transcript region were used as control (dashed lines). (B) MOC1 cells transfected with either siCTRL, siWTAP, or siWTAP+HA-WTAP were either left untreated or treated with IL-17A, followed by methylated (m6A) RNA immunoprecipitation (MeRIP) and RT-PCR. Graphs show relative levels of indicated mRNAs normalized to Gapdh (mean and SD of three independent plates of cells). (C) WT Act1 MEFs were transfected with siCTRL, siWTAP, or siWTAP+HA-WTAP. The transfected cells pretreated with rapamycin or vehicle control (DMSO) were treated with IL-17A, followed by western blot analysis. (D) MOC1 cells transfected with siCTRL or siWTAP were pretreated with rapamycin, followed by IL-17A treatment and fractionation. Indicated mRNAs from translation-active pools and translation-inactive pools were analyzed by RT-PCR and normalized to Gapdh. The graph shows the ratios of mRNAs from translation-active/inactive pools (n = 3 independent plates of cells). (E) MOC1 cells transfected with siCTRL, siWTAP, or siWTAP+HA-WTAP were pretreated with rapamycin, followed by IL-17A. The cell lysates were subjected to western blot analysis. Two-tailed t test was performed. *P < 0.05, **P < 0.01, and ***P < 0.001; NS, not significant. Source data are available for this figure: SourceData FS3.
By methylated (m6A) RNA immunoprecipitation (MeRIP), we found that IL-17 stimulation was able to induce m6A methylation in the 5′UTR of Wtap (Fig. 4 B) and antioxidant mRNAs in MOC1 cancer cells (Fig. 4 C), which can be impaired by Wtap depletion (Fig. S3 B). Consistently, Wtap depletion diminished IL-17–induced shift of antioxidant mRNAs to actively translating ribosomes as well as their IL-17–stimulated protein expression (Fig. 4, D and E; and Fig. S3, C–E). Taken together, these results indicate that Wtap is required for IL-17–induced m6A methylation of antioxidant mRNAs and their translation. In support of this, the bicistronic assay showed that Wtap depletion inhibited IL-17–induced cap-independent translation of antioxidants (Fig. 4 F).
We next examined the potential impact of Act1’s RNA binding activity on IL-17–induced m6A methylation. Interestingly, we found that loss of Act1 RNA biding (ΔSEF1) activity also diminished m6A methylation of Wtap and antioxidant mRNAs (Fig. 4, B and C). Consistently, Act1’s RNA binding activity is indeed required for their IL-17–induced cap-independent translation of Wtap and antioxidant mRNAs (Figs. 2 and 3). Taken together, either loss of Act1’s RNA binding activity or Wtap knockdown abolished IL-17–induced m6A modification (Fig. 4, B and C; and S3 B) and translation of Wtap and antioxidant mRNAs (Fig. 4, D and E), indicating a feedforward mechanism of Act1-WTAP loop.
Wtap ASO inhibits Act1 binding to Wtap mRNA and diminishes IL-17–induced m6A methylation of the Act1 5′UTR targets
Our results implicate a critical feedforward loop of IL-17–Act1–WTAP in driving IL-17–induced m6A methylation of Act1’s 5′UTR targets and their cap-independent translation. To disrupt this Act1–WTAP feedforward loop, we developed chemically modified RNA antisense oligonucleotides (ASO) to effectively block Act1 binding to Wtap mRNA. We designed four ASOs with sequences complementary to the Act1 binding region in the WTAP 5′UTR identified in the CLIP experiment as well as a control ASO. Act1–Wtap complex was efficiently disrupted with increasing amounts of W3 and W4 ASO added, whereas W1, W2, and control ASO failed to inhibit Act1 binding to Wtap 5′UTR (Fig. 5 A and Fig. S4 A). Since W4 ASO represents a unique sequence through blasting without forming any secondary structure, W4 ASO was selected for further validation. We performed a footprinting experiment to further map the binding site of Act1 on Wtap 5′UTR and to determine how the Wtap ASO disrupts this interaction. The 5′-end-labeled WTAP probe alone or together with ASO was incubated in the absence or presence of purified Act1 (Fig. 5 B). The reactions were then partially digested with RNases T1 or A as indicated. The GC-rich Wtap 5′UTR forms a two-stem loop structure. The addition of purified Act1 protein protected the top stem-loop of Wtap probe from RNases T1 or A digestion indicating this region is bound by Act1 (marked in green line in the graph). Importantly, Wtap ASO sensitized RNases T1 or A digestion of the Act1’s binding site even in the presence of purified Act1 protein, while ASO’s complementary sequence on Wtap probe was protected from RNases T1 or A digestion (marked in red line in the graph). These results unequivocally indicated that Wtap ASO was able to bind to its complementary sequence, which may result in the structural change of Wtap 5′UTR thereby blocking the binding of Act1 to Wtap RNA (Fig. S4 B).
Wtap ASO inhibits Act1 binding to Wtap mRNA and diminishes IL-17–induced m6A methylation of the Act1 5′UTR targets and their translation. (A) Top panel: The Wtap ASO candidates (W1–4) were used to compete with Wtap 5′UTR probe for binding to Act1 SEFIR. Data are representative of three independent experiments. Bottom panel: Wtap-ASO and Ctrl-ASO were modified with 2′-MOE and phosphonothioate linkage. (B) Footprinting analysis of Act1–Wtap interaction. The 5′-end-labeled Wtap probe alone or together with Wtap ASO was incubated in the absence or presence of Act1 SEFIR. The reactions were then partially digested with RNases T1 or A. The alkali and sequencing (G and C + U) ladders are shown in the left lanes. The numbers to the left of the gels indicate the positions of nucleotides according to the numbering in the RNA structure graph. While Act1-SEFIR protected area is marked in green line, the ASO binding site is labeled in red line. (C) MOC1 cells were transfected with Wtap ASO with or without restoration of HA-tagged WTAP. The rapamycin pretreated transfected cells were treated with IL-17A, followed by western blot analysis. Vehicle control (DMSO) for rapamycin was shown in Fig. S4 D. (D) MOC1 cells transfected with Wtap or control ASO were pretreated with rapamycin and then treated with IL-17A, followed by RNA immunoprecipitation with anti-Act1 and RT-PCR. Relative values normalized against IgG control are shown (mean and SD of three independent plates of cells). (E) MOC1 cells were transfected with Wtap ASO with or without restoration of HA-tagged WTAP. The rapamycin pretreated transfected cells were treated with IL-17A, followed by methylated (m6A) RNA immunoprecipitation (MeRIP) and RT-PCR. Graphs show relative levels of indicated mRNAs normalized to Gapdh (mean and SD of three independent plates of cells). (F) MOC1 cells transfected with Wtap or control ASO were pretreated with rapamycin and then treated with IL-17A, followed by immunoprecipitation with anti-Act1 and western blot analysis. WCL, whole cell lysate. (G) MOC1 cells transfected with either Wtap or control ASO were pretreated with rapamycin and then treated with IL-17A, followed by RNA immunoprecipitation with anti-eIF3G and RT-PCR. Relative values normalized against IgG control are shown (mean and SD of three independent plates of cells). (H)Act1−/− MEFs reconstituted retrovirally with Act1 WT or ΔSEF1 were pretreated with rapamycin and then treated with IL-17A, followed by RNA immunoprecipitation with anti-eIF3G and RT-PCR. Relative values normalized against IgG control are shown (mean and SD of three independent plates of cells). (I) Schematic model for IL-17–induced chemoresistance. During cisplatin treatment, IL-17 induces cap-independent translation of Wtap in cancer cells through upregulating its methylation. Increased expression of Wtap leads to increased m6A level of antioxidant genes including Prdx2, Txn1, and Sod1. Act1 binds in proximity to m6A sites on the 5′UTR and promotes cap-independent translation of antioxidant genes through the recruitment of EIF3G. All data are representative of three independent experiments. Two-tailed t test was performed. *P < 0.05, **P < 0.01, and ***P < 0.001; NS, not significant. Source data are available for this figure: SourceData F5.
Wtap ASO inhibits Act1 binding to Wtap mRNA and diminishes IL-17–induced m6A methylation of the Act1 5′UTR targets and their translation. (A) Top panel: The Wtap ASO candidates (W1–4) were used to compete with Wtap 5′UTR probe for binding to Act1 SEFIR. Data are representative of three independent experiments. Bottom panel: Wtap-ASO and Ctrl-ASO were modified with 2′-MOE and phosphonothioate linkage. (B) Footprinting analysis of Act1–Wtap interaction. The 5′-end-labeled Wtap probe alone or together with Wtap ASO was incubated in the absence or presence of Act1 SEFIR. The reactions were then partially digested with RNases T1 or A. The alkali and sequencing (G and C + U) ladders are shown in the left lanes. The numbers to the left of the gels indicate the positions of nucleotides according to the numbering in the RNA structure graph. While Act1-SEFIR protected area is marked in green line, the ASO binding site is labeled in red line. (C) MOC1 cells were transfected with Wtap ASO with or without restoration of HA-tagged WTAP. The rapamycin pretreated transfected cells were treated with IL-17A, followed by western blot analysis. Vehicle control (DMSO) for rapamycin was shown in Fig. S4 D. (D) MOC1 cells transfected with Wtap or control ASO were pretreated with rapamycin and then treated with IL-17A, followed by RNA immunoprecipitation with anti-Act1 and RT-PCR. Relative values normalized against IgG control are shown (mean and SD of three independent plates of cells). (E) MOC1 cells were transfected with Wtap ASO with or without restoration of HA-tagged WTAP. The rapamycin pretreated transfected cells were treated with IL-17A, followed by methylated (m6A) RNA immunoprecipitation (MeRIP) and RT-PCR. Graphs show relative levels of indicated mRNAs normalized to Gapdh (mean and SD of three independent plates of cells). (F) MOC1 cells transfected with Wtap or control ASO were pretreated with rapamycin and then treated with IL-17A, followed by immunoprecipitation with anti-Act1 and western blot analysis. WCL, whole cell lysate. (G) MOC1 cells transfected with either Wtap or control ASO were pretreated with rapamycin and then treated with IL-17A, followed by RNA immunoprecipitation with anti-eIF3G and RT-PCR. Relative values normalized against IgG control are shown (mean and SD of three independent plates of cells). (H)Act1−/− MEFs reconstituted retrovirally with Act1 WT or ΔSEF1 were pretreated with rapamycin and then treated with IL-17A, followed by RNA immunoprecipitation with anti-eIF3G and RT-PCR. Relative values normalized against IgG control are shown (mean and SD of three independent plates of cells). (I) Schematic model for IL-17–induced chemoresistance. During cisplatin treatment, IL-17 induces cap-independent translation of Wtap in cancer cells through upregulating its methylation. Increased expression of Wtap leads to increased m6A level of antioxidant genes including Prdx2, Txn1, and Sod1. Act1 binds in proximity to m6A sites on the 5′UTR and promotes cap-independent translation of antioxidant genes through the recruitment of EIF3G. All data are representative of three independent experiments. Two-tailed t test was performed. *P < 0.05, **P < 0.01, and ***P < 0.001; NS, not significant. Source data are available for this figure: SourceData F5.
Wtap ASO blocks Act1 binding to Wtap mRNA, attenuating IL-17–induced m6A methylation and translation of Act1's 5′UTR targets. (A) Schematic representation of the Wtap 5′UTR. Act1 probe, W1-W4 ASO, and MeRIP primers corresponding sites are indicated. W1–W4 and Ctrl ASO sequences are listed in the table. (B) Graph shows the structure of Wtap ASO bound Wtap 5′UTR RNA probe. (C) REMSA competition assay using Ctrl-ASO or Wtap-ASO to compete with indicated 5′UTR probes for binding to Act1 SEFIR. (D) MOC1 cells transfected with Ctrl-ASO or WTAP-ASO were pretreated with rapamycin or vehicle control (DMSO) and then either left untreated or treated with IL-17A. The cell lysates were subjected to western blot analysis. (E) A-431 cells transfected with h_Ctrl-ASO or h_WTAP-ASO were pretreated with rapamycin and then either left untreated or treated with IL-17A, followed by RNA immunoprecipitation with anti-Act1 and RT-PCR. Relative values normalized against IgG control are shown (mean and SD of three independent plates of cells). (F) A-431 cells transfected with h_Ctrl-ASO or h_WTAP-ASO, were pretreated with rapamycin and then either left untreated or treated with IL-17A. The cell lysates were subjected to western blot analysis. (G) A-431 cells were treated with IL-17A, followed by RT-PCR analyses (n = 3 independent plates of cells). One-way ANOVA was performed, followed by Tukey’s multiple-comparisons test. (H) MOC1 cells were stimulated with IL-17A. Cell lysates either left untreated or pretreated with RNaseA were immunoprecipitated with anti-Act1 and subjected to western blot analysis. WCL, whole cell lysate. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; NS, not significant. Source data are available for this figure: SourceData FS4.
Wtap ASO blocks Act1 binding to Wtap mRNA, attenuating IL-17–induced m6A methylation and translation of Act1's 5′UTR targets. (A) Schematic representation of the Wtap 5′UTR. Act1 probe, W1-W4 ASO, and MeRIP primers corresponding sites are indicated. W1–W4 and Ctrl ASO sequences are listed in the table. (B) Graph shows the structure of Wtap ASO bound Wtap 5′UTR RNA probe. (C) REMSA competition assay using Ctrl-ASO or Wtap-ASO to compete with indicated 5′UTR probes for binding to Act1 SEFIR. (D) MOC1 cells transfected with Ctrl-ASO or WTAP-ASO were pretreated with rapamycin or vehicle control (DMSO) and then either left untreated or treated with IL-17A. The cell lysates were subjected to western blot analysis. (E) A-431 cells transfected with h_Ctrl-ASO or h_WTAP-ASO were pretreated with rapamycin and then either left untreated or treated with IL-17A, followed by RNA immunoprecipitation with anti-Act1 and RT-PCR. Relative values normalized against IgG control are shown (mean and SD of three independent plates of cells). (F) A-431 cells transfected with h_Ctrl-ASO or h_WTAP-ASO, were pretreated with rapamycin and then either left untreated or treated with IL-17A. The cell lysates were subjected to western blot analysis. (G) A-431 cells were treated with IL-17A, followed by RT-PCR analyses (n = 3 independent plates of cells). One-way ANOVA was performed, followed by Tukey’s multiple-comparisons test. (H) MOC1 cells were stimulated with IL-17A. Cell lysates either left untreated or pretreated with RNaseA were immunoprecipitated with anti-Act1 and subjected to western blot analysis. WCL, whole cell lysate. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; NS, not significant. Source data are available for this figure: SourceData FS4.
To examine the impact of Wtap ASO on IL-17–induced Wtap and antioxidant protein expression, Wtap ASO and control ASO were modified with 2′-MOE and phosphonothioate linkage to enhance their nuclease resistance (Fig. 5 A) and transfected into MOC1 cells. We found that Wtap ASO substantially diminished IL-17–induced m6A methylation (Fig. 5 E) and expression (Fig. 5 C and Fig. S4 D) of WTAP and antioxidant mRNAs, which were restored by overexpression of WTAP. As a control, we showed that IL-17–induced Arid5a protein expression was not altered by Wtap ASO (Fig. 5 C and Fig. S4 D). It is important to note that both REMSA and Act1 RNA immunoprecipitation showed that WTAP ASO does not block the binding of Act1 to antioxidant mRNAs, indicating the specific inhibition of Wtap ASO on Act1’s binding to Wtap mRNA (Fig. 5 D and Fig. S4 C). Of note, the 5′UTR of WTAP is highly conserved between human and mouse. We found that human WTAP-ASO (h_WTAP-ASO) was indeed able to block Act1 binding to human Wtap mRNA (Fig. S4 E) and diminish IL-17–induced expression of WTAP and antioxidant proteins (Fig. S4, F and G) in A-431 (a human epidermoid carcinoma cell line). Together, these results suggest that the specific disruption of Act1’s binding to Wtap mRNA using Wtap ASO diminished IL-17–induced expression of Wtap, thereby abating IL-17–induced m6A modification and translation of antioxidant mRNAs in cancer cells.
Wtap ASO attenuates the recruitment of eIF3G to the Act1 5′UTR targets for cap-independent translation
The biological functions of m6A sites are mediated by different RNA-binding proteins called m6A “readers” which recognize the methylated adenosine. Previous studies have demonstrated that m6A sites in 5′UTR can promote cap-independent translation via recruitment of the eukaryotic initiation factor 3 (eIF3) which acts as an m6A reader (Meyer et al., 2015; Wolf et al., 2020). Our mass spectrometry analysis of Act1-immunoprecipitates (Wang et al., 2013; Zhang et al., 2018b) showed that IL-17 stimulation induced Act1’s association with several translation initiation factors, including eIF3G. Consistently, Act1 coimmunoprecipitation in MOC1 cells with and without RNAse treatment showed that IL-17–induced Act1’s interaction with eIF3G was RNA-dependent (Fig. S4 H). WTAP ASO was able to effectively abolish IL-17–induced Act1’s interaction with eIF3G (Fig. 5 F), implicating the potential role of m6A site for this interaction. By performing eIF3G RNA immunoprecipitation (RIP), we found that IL-17 stimulation indeed induced the association of eIF3G with the mRNAs of Wtap and antioxidants (Prdx2, Txn1, SOD1) (Fig. 5 G). Importantly, both the loss of Act1’s RNA binding activity and WTAP ASO abolished the recruitment of eIF3G to the Act1 5′UTR targets (Fig. 5, G and H). These results suggest that IL-17–induced Act1’s binding to the 5′UTR targets in the proximity of m6A sites may promote the recruitment of eIF3G to these sites, thereby driving cap-independent translation of the Act1 5′UTR target mRNAs (Fig. 5 I).
IL-17/Act1–Wtap axis renders MOC1 resistance to cisplatin through m6A methylation and cap-independent translation of antioxidation proteins
IL-17 signaling plays a critical role in cancer progression and resistance to anticancer therapies for a variety of solid tumors in human (Chang et al., 2014; Chen et al., 2019; Ma et al., 2014; McAllister et al., 2014; Wang et al., 2014a; Wu et al., 2015). However, the molecular and cellular mechanisms for IL-17–driven cancer progression remain unclear. Our results here showed that IL-17 stimulation induces m6A methylation of Wtap and antioxidant mRNAs, driving their active translation in cancer cells. While accumulating evidence suggests that m6A RNA methylation plays a critical role in cancer progression (Lan et al., 2019; Lin et al., 2016; Panneerdoss et al., 2018), antioxidant proteins, including Prdx2, Txn1, and Sod1, are known to contribute to tumor chemo-resistance, including cisplatin-based neoadjuvant chemotherapy (Brown et al., 2009; Zhu et al., 2019). Cisplatin is a platinum-based chemotherapy drug, commonly utilized in the treatment of solid tumors. Besides inducing DNA damage, the cisplatin mechanism of action involves the generation of oxidative stress (Yu et al., 2018) and inhibits cellular translation (Pietras et al., 2022). Notably, canonical, cap-dependent initiation of translation can often be inhibited by oxidative stress and ROS (Yang and Chen, 2021). On the other hand, non-canonical, cap-independent regulation of translation initiation often continues to operate under oxidative stress (Yang and Chen, 2021). Based on these findings, we hypothesize that IL-17 signaling may render resistance of cancer cells to cisplatin-based chemotherapy via m6A methylation of Wtap and antioxidant mRNAs and their consequent cap-independent protein translation in cancer cells.
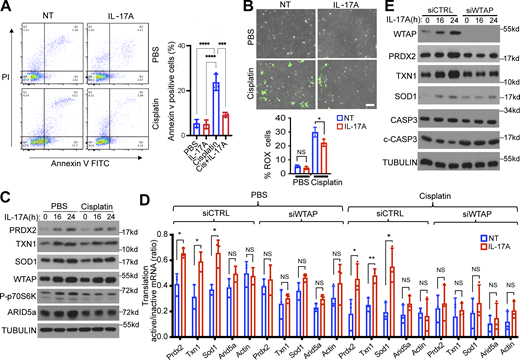
To test this hypothesis, we examined the impact of the IL-17/Act1–Wtap axis on the responsiveness of MOC1 cells to cisplatin treatment. While cisplatin efficiently induced cell death in MOC1 cells, IL-17 stimulation induced resistance of MOC1 cells to cisplatin-mediated cell killing (Fig. 6 A). Moreover, cisplatin-induced ROS levels were substantially reduced in MOC1 cells treated with IL-17, which is consistent with IL-17–induced expression of antioxidant proteins in cancer cells (Figs. S1 F and 6 B). Furthermore, whereas cisplatin substantially diminished IL-17–induced ARID5a expression (cap-dependent translation), cisplatin did not affect IL-17–induced m6A methylation and protein induction of Wtap and antioxidants (Prdx2, Txn1, and Sod1) (cap-independent translation) (Fig. 6, C and D). Moreover, Wtap depletion was able to diminish IL-17–induced expression of antioxidant proteins (Prdx2, Txn1, and Sod1) in cisplatin-pretreated MOC1 cells, which was accompanied by increased cisplatin-mediated cell death (Fig. 6 E; and Fig. S5, A and B). Taken together, these results suggest the importance of WTAP-mediated m6A methylation in cap-independent translation of antioxidant proteins in conferring IL-17–induced resistance to cisplatin-mediated cell killing.
IL-17A renders MOC1 cancer cells resistant to cisplatin-mediated cell killing through WTAP-dependent induction of antioxidants. (A) MOC1 cells were pretreated with cisplatin, followed by IL-17A treatment. Cells were then stained with annexin V/PI and analyzed by flow cytometry. Shown is the percentage of apoptotic cells (annexin V positive). The data are presented as the mean ± SD (n = 3). One-way ANOVA was performed, followed by Tukey’s multiple-comparisons test. (B) MOC1 cells were pretreated with cisplatin, followed by IL-17A treatment and CellROX Green staining assay. Representative fluorescence images show intracellular levels of ROS (green fluorescence). Scale bar, 100 μm. The graph shows the percentage of CellROX stained cells and SD (n = 3 independent plates). Three independent fields per plate were analyzed for quantification. (C) MOC1 cells were pretreated with cisplatin, followed by IL-17A treatment and western blot analysis. (D) MOC1 cells transfected with siCTRL or siWTAP were pretreated with cisplatin, followed by IL-17A treatment. Cytoplasmic extracts of these cells were fractionated, followed by RT-PCR and normalized to Gapdh. The graph shows the ratios of mRNAs from translation-active/inactive pools (mean and SD of three independent plates of cells). (E) MOC1 cells were transfected with siCTRL or siWTAP. The transfected cells were pretreated with cisplatin followed by treatment with IL-17A. The cell lysates were subjected to western blot analysis. Vehicle control (DMSO) for cisplatin is shown in Fig. S5 B. All data are representative of three independent experiments. Two-tailed t test was performed. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; NS, not significant. Source data are available for this figure: SourceData F6.
IL-17A renders MOC1 cancer cells resistant to cisplatin-mediated cell killing through WTAP-dependent induction of antioxidants. (A) MOC1 cells were pretreated with cisplatin, followed by IL-17A treatment. Cells were then stained with annexin V/PI and analyzed by flow cytometry. Shown is the percentage of apoptotic cells (annexin V positive). The data are presented as the mean ± SD (n = 3). One-way ANOVA was performed, followed by Tukey’s multiple-comparisons test. (B) MOC1 cells were pretreated with cisplatin, followed by IL-17A treatment and CellROX Green staining assay. Representative fluorescence images show intracellular levels of ROS (green fluorescence). Scale bar, 100 μm. The graph shows the percentage of CellROX stained cells and SD (n = 3 independent plates). Three independent fields per plate were analyzed for quantification. (C) MOC1 cells were pretreated with cisplatin, followed by IL-17A treatment and western blot analysis. (D) MOC1 cells transfected with siCTRL or siWTAP were pretreated with cisplatin, followed by IL-17A treatment. Cytoplasmic extracts of these cells were fractionated, followed by RT-PCR and normalized to Gapdh. The graph shows the ratios of mRNAs from translation-active/inactive pools (mean and SD of three independent plates of cells). (E) MOC1 cells were transfected with siCTRL or siWTAP. The transfected cells were pretreated with cisplatin followed by treatment with IL-17A. The cell lysates were subjected to western blot analysis. Vehicle control (DMSO) for cisplatin is shown in Fig. S5 B. All data are representative of three independent experiments. Two-tailed t test was performed. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; NS, not significant. Source data are available for this figure: SourceData F6.
The IL-17/Act1–WTAP axis confers resistance to cisplatin treatment in tumor cells. (A) MOC1 cells transfected with siCTRL or siWTAP were pretreated with cisplatin or vehicle control (PBS), followed by IL-17A treatment. Cells were then stained with annexin V/PI and analyzed by flow cytometry. Shown is the percentage of apoptotic cells (annexin V positive). The data are presented as the mean ± SD (n = 3). (B) MOC1 cells were transfected with siCTRL or siWTAP. The transfected cells were pretreated with cisplatin or PBS followed by treatment with IL-17A. The cell lysates were subjected to western blot analysis. (C) MOC1 cells transfected with a control shRNA vector or Act1 shRNA constructs were selected by puromycin treatment. Cell lysates were analyzed by western blot. (D) Treatment timeline for MOC1 tumor model–based experiments in E and F: C57BL/6 mice (n = 5/group) were injected in the right flank with MOC1 cells transfected with either shRNA Act1 or scramble shRNA as described in D (0.5 × 106, in Matrigel), and 10 days later randomized into treatment groups. Mice were then treated with cisplatin (5 mg/kg) every 4 days up to day 21, after which mice were sacrificed and tumor tissue was collected. (E) Tumors from mice treated as described in D were measured. Graph shows total tumor volume, represented as mean ± SEM, n = 5/group. Two-tailed t test was performed. *P < 0.05 and ***P < 0.001; NS, not significant. (F) Immunofluorescence of either Wtap-ASO-FAM, Ctrl-ASO-FAM (green channel) or Ep-CAM (red channel) in sections of MOC1 tumors from mice described in Fig. 7 D. Blue, DAPI nuclear staining. Scale bar, 50 μm. Source data are available for this figure: SourceData FS5.
The IL-17/Act1–WTAP axis confers resistance to cisplatin treatment in tumor cells. (A) MOC1 cells transfected with siCTRL or siWTAP were pretreated with cisplatin or vehicle control (PBS), followed by IL-17A treatment. Cells were then stained with annexin V/PI and analyzed by flow cytometry. Shown is the percentage of apoptotic cells (annexin V positive). The data are presented as the mean ± SD (n = 3). (B) MOC1 cells were transfected with siCTRL or siWTAP. The transfected cells were pretreated with cisplatin or PBS followed by treatment with IL-17A. The cell lysates were subjected to western blot analysis. (C) MOC1 cells transfected with a control shRNA vector or Act1 shRNA constructs were selected by puromycin treatment. Cell lysates were analyzed by western blot. (D) Treatment timeline for MOC1 tumor model–based experiments in E and F: C57BL/6 mice (n = 5/group) were injected in the right flank with MOC1 cells transfected with either shRNA Act1 or scramble shRNA as described in D (0.5 × 106, in Matrigel), and 10 days later randomized into treatment groups. Mice were then treated with cisplatin (5 mg/kg) every 4 days up to day 21, after which mice were sacrificed and tumor tissue was collected. (E) Tumors from mice treated as described in D were measured. Graph shows total tumor volume, represented as mean ± SEM, n = 5/group. Two-tailed t test was performed. *P < 0.05 and ***P < 0.001; NS, not significant. (F) Immunofluorescence of either Wtap-ASO-FAM, Ctrl-ASO-FAM (green channel) or Ep-CAM (red channel) in sections of MOC1 tumors from mice described in Fig. 7 D. Blue, DAPI nuclear staining. Scale bar, 50 μm. Source data are available for this figure: SourceData FS5.
To disrupt this Act1–WTAP feedforward loop, we developed Wtap ASO to effectively block Act1 binding to Wtap mRNA and Wtap translation, resulting in the attenuation of Wtap-mediated m6A methylation and cap-independent translation of antioxidant proteins. We then examined the efficacy of Wtap ASO in sensitizing IL-17–treated MOC1 cells to cisplatin treatment. Wtap ASO was indeed able to effectively enhance cisplatin-mediated cell death and ROS levels in IL-17–treated MOC1 cells compared with control ASO (Fig. 7, A and B). Consistently, Wtap ASO substantially decreased IL-17–induced expression of antioxidant proteins in cisplatin-treated MOC1cells, which was accompanied by increased caspase 3 cleavage (cell apoptosis) (Fig. 7 C).
Wtap ASO renders MOC1 tumors sensitive to cisplatin treatment. (A) MOC1 cells pretreated with cisplatin or vehicle control (PBS) were transfected with Wtap or control ASO, followed by IL-17A stimulation. Cells were then stained with annexin V/PI and analyzed by flow cytometry. Shown is the percentage of the apoptotic population (annexin V positive cells). The data are presented as the mean ± SD (n = 3). (B) MOC1 cells transfected with Wtap or control ASO were treated with cisplatin or vehicle control (PBS), followed by IL-17A treatment and CellROX Green staining assay. Representative fluorescence images show intracellular levels of ROS (green fluorescence). Scale bar, 100 μm. Graph shows the percentage of CellROX stained cells and SD (n = 3 independent plates). Three independent fields per plate were analyzed for quantification. (C) MOC1 cells transfected with Wtap or control ASO were treated with cisplatin, followed by IL-17A treatment and western blot analysis. (D) Treatment timeline for MOC1 tumor model–based experiments in D–H: C57BL/6 mice (n = 5/group) were injected in the right flank with MOC1 cells (0.5 × 106, in matrigel), and 10 days later randomized into treatment groups. Mice were then treated with cisplatin (5 mg/kg) and simultaneously subjected to intra-tumor injections of WTAP or control ASO (1 nmol/mouse). Treatment was repeated every 4 days up to day 21, after which mice were sacrificed and tumor tissue was collected. (E and F) Tumors from mice in D were measured. Graph shows total tumor volume (E) and fold increase in tumor size (F). Represented as mean ± SEM, n = 5/group. (G) IL-17A levels in tumor homogenates from mice in D were determined by ELISA (n = 5 biological samples/group ± SD). (H) Representative images of immunofluorescence staining for cleaved caspase-3 in sections of MOC1 tumors from mice in D. Blue, DAPI nuclear staining. Graph shows the percentage of cleaved caspase-3 positive tumor cells over EpCam positive cells. Five random regions were analyzed for each group; bar graphs show the mean and SD of biological replicates. (I) Representative images of immunohistochemical analysis of cleaved caspase-3 in MOC1 tumor sections from mice in D, counterstained with hematoxylin. The graph shows the percentage of cleaved caspase-3 positive cells. Five random regions were analyzed for each group; bar graphs show mean and SD of biological replicates. Scale bar, 50 μm. (J) Left panel: Representative images of TUNEL assay in MOC1 tumor sections from mice in D, counterstained with methyl-green. Graph shows the percentage of cleaved caspase-3 positive cells. Five random regions were analyzed for each group; bar graphs show mean and SD of biological replicates. Scale bar, 50 μm. (K) MOC1 tumor lysates from mice in D were subjected to western blot analysis. (L) Real-time PCR analysis of Wtap mRNA in the MOC1 tumor tissue from mice in D. (M) Representative images of immunohistochemical analysis of 4-hydroxynonenal levels in MOC1 tumor tissue lysates from mice in D. Graph shows the percentage of 4-hydroxynonenal positive cells. Five random regions were analyzed for each group; bar graphs show mean and SD of biological replicates. Scale bar, 100 μm. Two-tailed t test was performed. *P < 0.05, **P < 0.01, and ***P < 0.001; NS, not significant. All data are representative of three independent experiments. Source data are available for this figure: SourceData F7.
Wtap ASO renders MOC1 tumors sensitive to cisplatin treatment. (A) MOC1 cells pretreated with cisplatin or vehicle control (PBS) were transfected with Wtap or control ASO, followed by IL-17A stimulation. Cells were then stained with annexin V/PI and analyzed by flow cytometry. Shown is the percentage of the apoptotic population (annexin V positive cells). The data are presented as the mean ± SD (n = 3). (B) MOC1 cells transfected with Wtap or control ASO were treated with cisplatin or vehicle control (PBS), followed by IL-17A treatment and CellROX Green staining assay. Representative fluorescence images show intracellular levels of ROS (green fluorescence). Scale bar, 100 μm. Graph shows the percentage of CellROX stained cells and SD (n = 3 independent plates). Three independent fields per plate were analyzed for quantification. (C) MOC1 cells transfected with Wtap or control ASO were treated with cisplatin, followed by IL-17A treatment and western blot analysis. (D) Treatment timeline for MOC1 tumor model–based experiments in D–H: C57BL/6 mice (n = 5/group) were injected in the right flank with MOC1 cells (0.5 × 106, in matrigel), and 10 days later randomized into treatment groups. Mice were then treated with cisplatin (5 mg/kg) and simultaneously subjected to intra-tumor injections of WTAP or control ASO (1 nmol/mouse). Treatment was repeated every 4 days up to day 21, after which mice were sacrificed and tumor tissue was collected. (E and F) Tumors from mice in D were measured. Graph shows total tumor volume (E) and fold increase in tumor size (F). Represented as mean ± SEM, n = 5/group. (G) IL-17A levels in tumor homogenates from mice in D were determined by ELISA (n = 5 biological samples/group ± SD). (H) Representative images of immunofluorescence staining for cleaved caspase-3 in sections of MOC1 tumors from mice in D. Blue, DAPI nuclear staining. Graph shows the percentage of cleaved caspase-3 positive tumor cells over EpCam positive cells. Five random regions were analyzed for each group; bar graphs show the mean and SD of biological replicates. (I) Representative images of immunohistochemical analysis of cleaved caspase-3 in MOC1 tumor sections from mice in D, counterstained with hematoxylin. The graph shows the percentage of cleaved caspase-3 positive cells. Five random regions were analyzed for each group; bar graphs show mean and SD of biological replicates. Scale bar, 50 μm. (J) Left panel: Representative images of TUNEL assay in MOC1 tumor sections from mice in D, counterstained with methyl-green. Graph shows the percentage of cleaved caspase-3 positive cells. Five random regions were analyzed for each group; bar graphs show mean and SD of biological replicates. Scale bar, 50 μm. (K) MOC1 tumor lysates from mice in D were subjected to western blot analysis. (L) Real-time PCR analysis of Wtap mRNA in the MOC1 tumor tissue from mice in D. (M) Representative images of immunohistochemical analysis of 4-hydroxynonenal levels in MOC1 tumor tissue lysates from mice in D. Graph shows the percentage of 4-hydroxynonenal positive cells. Five random regions were analyzed for each group; bar graphs show mean and SD of biological replicates. Scale bar, 100 μm. Two-tailed t test was performed. *P < 0.05, **P < 0.01, and ***P < 0.001; NS, not significant. All data are representative of three independent experiments. Source data are available for this figure: SourceData F7.
Based on the potent efficacy of Wtap ASO in the cell culture model, we then tested the effect of Wtap ASO on tumor growth in cisplatin-treated mice bearing MOC1 tumors (Fig. 7, D–J). MOC1 cells were injected into flanks of wild type C57BL/6 mice; 10 days later, mice were randomized into treatment groups and subjected to cisplatin treatments in combination with either WTAP ASO or control ASO for 21 days (Fig. 7 D). Our control groups showed that cisplatin treatment increased IL-17 expression in the tumor tissue (Fig. 7 G). Knockdown of Act1 in MOC1 cells sensitized them to cisplatin-mediated suppression of tumor growth, confirming the critical role of the IL-17/Act1 axis in rendering tumor cells resistant to cisplatin treatment (Fig. S5, C–E). Importantly, intratumoral injection of Wtap ASO substantially enhanced cisplatin-mediated suppression of tumor growth (Fig. 7, E and F; and Fig. S5 F). Immunofluorescence and immunohistochemistry staining showed increased cleaved caspase-3 in tumors treated with Wtap ASO (Fig. 7, H and I), indicative of increased cell apoptosis. The observed increased cell apoptosis in tumors treated with Wtap ASO was confirmed by in situ Tunel Assay (Fig. 7 J). Western analysis confirmed elevated cleaved caspase-3 and decreased expression of WTAP and antioxidants in Wtap ASO-treated tumors (Fig. 7 K), which was accompanied by increased 4-hydroxynonenal levels (indicative of oxidative stress) in these cisplatin-sensitive tumors (Fig. 7 M). Notably, Wtap RNA level in tumors was not altered by Wtap ASO treatment, indicating that Wtap ASO mainly inhibited Wtap mRNA translation (Fig. 7 L). Taken together, these results demonstrated the specific inhibitory effect of Wtap ASO on protein expression of WTAP and antioxidants thereby rendering sensitivity of tumor cells to cisplatin-mediated tumor suppression.
Discussion
Compelling epidemiological evidence presented the association of higher levels of IL-17 with worse prognoses for a wide range of malignancies, with chemotherapy as standard of care (Cai et al., 2011; Chen and Chen, 2014; Gu et al., 2012; Huang et al., 2014; Lee et al., 2018; Li et al., 2011; Punt et al., 2016; Punt et al., 2015; Tosolini et al., 2011; Wu et al., 2012; Xu et al., 2014a; Yan et al., 2014; Zhang et al., 2012; Zhang et al., 2013; Zhang et al., 2018a), including head neck SCC (Lee et al., 2018; Li et al., 2011; Punt et al., 2016), colorectal cancer (Al Obeed et al., 2018; Bedoui et al., 2018; Lotti et al., 2013; Tosolini et al., 2011), and liver cancer (Huang et al., 2014; Liao et al., 2013; Yan et al., 2014; Zhang et al., 2009). Several studies have implicated IL-17 in the development of chemoresistance (Bi et al., 2016; Cochaud et al., 2013; Lotti et al., 2013; Sui et al., 2019), although the mechanism remains unclear. Here, we report a novel mechanism of how IL-17 induces chemoresistance by modulating redox homeostasis through epitranscriptomic regulation of antioxidant RNA metabolism and their cap-independent translation. This process is driven by Act1 (the adaptor protein of IL-17R) which has specific and select RNA binding to the 5′UTR of a set of transcripts including antioxidant mRNAs and WTAP (a modulator of m6A methyltransferase complex). Loss of Act1’s RNA binding activity or Wtap knockdown abolished IL-17–induced m6A modification and translation of Wtap and antioxidant mRNAs, suggesting a functional Act1–WTAP loop. We developed chemically modified RNA ASO complementary to the Act1 binding region in the WTAP 5′UTR. REMSA, footprinting, and RNA immunoprecipitation analyses unequivocally indicated the specific inhibition of Wtap ASO on Act1’s binding to Wtap mRNA. The specific disruption of Act1’s binding to Wtap mRNA using Wtap ASO reduced antioxidant protein production during cisplatin-mediated cancer cell killing, which robustly enhanced the antitumor efficacy of cisplatin in mice, indicating a novel therapeutic strategy for chemoresistance.
IL-17 signaling pathway plays a critical role in the pathogenesis of autoimmune disorders and cancer progression. We previously reported that the SEFIR domain of Act1, an IL-17R complex adaptor, directly binds stem-loop RNA structures at the 3′UTR of inflammatory mRNAs, including Cxcl1, to stabilize them and promote their translation in response to IL-17 stimulation. In this study, we performed transcriptome-wide mapping of direct Act1–RNA interactions via CLIP, revealing that Act1 binds in high density to the 5′UTR of a cohort of transcripts distinct from Act1’s 3′UTR targets. Whereas inflammation-related GO terms were enriched for 3′UTR targets of Act1, the 5′UTR targets were enriched for mRNAs encoding proteins important for cellular stress, protein translation regulation, and redox homeostasis. Hexamer enrichment analysis indicated that the Act1 binding sequences are distinct (AU-rich versus GC-rich) for the 3′UTR versus 5′UTR targets, implicating differential regulatory mechanism of Act1 for 3′UTR and 5′UTR targets. Strikingly, Act1’s binding sites are located in proximity to m6A modification sites on the 5′UTRs of Act1 targets (but not on 3′UTR targets), which may play a critical role in allowing Act1 to promote the recruitment of elF3G’s binding to m6A sites for cap-independent translation of the Act1 5′UTR targets.
Robust Act1 CLIP reads at the 5′UTR targets, including antioxidant mRNAs and WTAP, were barely induced by IL-17 at the mRNA levels, suggesting potential protein translational control. IL-17 treatment indeed strongly induced translation of these Act1’s 5′UTR targets in various cancer cell lines, including PDVC57, MOC1, and human A431. Consistently, Wtap expression was significantly elevated in human skin tumor tissue containing a high number of IL-17A–producing cells compared with IL-17A low tumor tissue. By methylated (m6A) RNA immunoprecipitation (MeRIP), we found that IL-17 stimulation was able to induce m6A methylation of Wtap and antioxidant mRNAs in MOC1 cancer cells, which was diminished by Wtap depletion. Importantly, the inactivation of Act1’s RNA binding activity or Wtap knockdown abolished IL-17–induced m6A modification and translation of Wtap and antioxidant mRNAs, revealing a potential feedforward mechanism of Act1–WTAP loop. M6A modification is catalyzed by RNA methyltransferases complex, composed of several proteins, including methyltransferases METTL3 and METTL14 as well as regulatory protein Wtap. Mechanistically, Wtap has been shown to bind RNA and promote the recruitment of methyltransferases to the RNA targets (Ping et al., 2014). Considering the critical role of Wtap in m6A methyltransferase complex, we examined the potential impact of IL-17/Act1-mediated WTAP induction on m6A methylation of Act1’s 5′UTR targets. We used chemically modified RNA ASO to effectively block Act1 binding to Wtap mRNA. WTAP ASO was indeed able to diminish IL-17–induced m6A methylation of Wtap and abolished IL-17–induced Wtap translation. Taken together, these results support a positive feedback mechanism by which the IL-17/Act1 axis upregulates m6A mRNA modifications by augmenting Wtap expression, followed by cap-independent translation of specific, methylated 5′UTR targets via the formation of Act1/eIF3G RNP.
While m6A is known to function by recruiting eIF3 complex to promote protein translation, m6A also exerts its effect through interaction with other “readers” such as YTH domain-containing proteins to influence RNA metabolism (Wang et al., 2014b; Xu et al., 2014b). Furthermore, noncanonical m6A readers, the IGF2 mRNA binding protein (IGF2BP) family (known as IMPs), have recently been demonstrated to stabilize target transcripts (Bechara et al., 2021; Huang et al., 2018, 2020). Interestingly, IMP2 was shown to promote IL-17–mediated Cebpb/d mRNA stabilization and translation, although m6A was found to be localized at the 3′UTR of Cebpb/d mRNAs. Taken together, it is possible that additional canonical and/or non-canonical readers might be recruited into the IL-17–WTAP axis to participate in IL-17–modulated posttranscriptional regulation of Act1-binding RNA targets.
While cap-independent translation is initiated under aberrant stress conditions such as cancer, 5′UTR m6A-methylation has been shown to promote cap-independent translation during cancer progression. The m6A writer complex METTL3/14–WTAP has indeed been implicated in cancer progression of multiple hematomas and solid malignancies, including chemotherapy resistance. A number of small molecule inhibitors targeting methyltransferases Mettl3 or Mettl14 have been identified for preclinical or clinical evaluation (Cully, 2019; Yankova et al., 2021). Our results here showed that IL-17 stimulation induces m6A methylation of Wtap and antioxidant mRNAs, driving their active cap-independent translation in cancer cells. Notably, antioxidant proteins, including Prdx2, Txn1, and Sod1, have also been shown to play critical roles in cancer chemoresistance, including cisplatin-based chemotherapy. Cisplatin is a platinum-based chemotherapy drug, a first-line treatment for a variety of solid tumors (Yu et al., 2018). In addition to DNA damage, cisplatin is known to generate oxidative stress and ROS, which can often inhibit canonical, cap-dependent initiation of cellular protein translation. Our results showed that IL-17 signaling renders resistance of cancer cells to cisplatin-based chemotherapy via m6A methylation of Wtap and antioxidant mRNAs and their consequent cap-independent protein translation in cancer cells. Indeed, we found that WTAP ASO effectively enhanced cisplatin-mediated cancer cell killing accompanied by a reduction of antioxidant protein production and dramatically enhanced ROS. Consistently, WTAP ASO inhibited MOC1 tumor growth in cisplatin-treated mice.
One important takeaway from this study is the therapeutic potential for treating IL-17–mediated pathologies by disrupting the interaction between Act1 and the disease-driven target mRNAs. ASOs are single-stranded oligonucleotides that can be designed as therapeutic agents to disrupt protein–RNA interaction. We here designed Wtap ASO to have a sequence complementary to the Act1 binding site at the 5′UTR of Wtap mRNA, which inhibited Act1’s binding to the 5′UTR of Wtap mRNA. This inhibition was specific to Wtap mRNA since Wtap ASO did not block the binding of Act1 to the antioxidant mRNAs. Importantly, the 5′UTR of WTAP is highly conserved between human and mouse. Human WTAP-ASO interfered with Act1 binding to human Wtap mRNA and reduced IL-17–induced expression of WTAP and antioxidant proteins in human epidermoid carcinoma cells, implicating the potential application of WTAP-ASO in human cancer treatment. The success of Wtap ASO in inhibiting chemoresistance serves as a proof of concept for this extremely novel therapeutic strategy, which can be applied to other disease-driven Act1 binding target mRNAs. CLIP-seq has allowed us to comprehensively characterize Act1’s RNA binding targets and precisely define Act1 binding sites. Notably, 3′UTR and 5′UTR targets were not only functionally different but also distinct sequences were enriched in Act1’s binding sites in 3′UTR versus 5′UTR targets. These results suggest that it is plausible to selectively disrupt the interaction of Act1 with specific targets. Notably, while inflammatory mRNAs were enriched in Act1 3′UTRs, genes regulating RNA metabolism and redox homeostasis were enriched among Act1 5′UTR targets, both of which are important for tumor development and progression. Consistently, in addition to cancer progression, IL-17 has a well-established role in the pathology of many autoimmune inflammatory diseases, including psoriasis, psoriatic arthritis, and ankylosing spondylitis. Therefore, the success of Wtap ASO in ameliorating chemoresistance not only demonstrated a promising novel anticancer strategy but also the potential to develop a new class of drugs for autoimmune and inflammatory diseases by selectively disrupting the interaction of Act1 with specific disease-driven 3′UTR and/or 5′UTR targets.
Materials and methods
Animals
All experiments were conducted in accordance with the guidelines of Animal Care and Use Committee of the Cleveland Clinic (IACUC). C57BL/6 mice were purchased from Jackson Laboratory.
Cell culture and reagents
The following antibodies were used: Santa Cruz Biotechnology: anti-Act1 (sc-11444, polyclonal), anti-GAPDH (sc-47724, mouse monoclonal), and anti-β-actin (sc-8432, mouse monoclonal). Sigma anti-HA (H-9658, mouse monoclonal). Cell Signaling Technology: anti-Wtap, anti-Prdx2, anti-Txn1, anti-SOD1, anti-Arid5a, anti-Cleaved Caspase-3, anti-Caspase 3, anti P-p70S6K.
TUNEL assay was performed using TUNEL Assay Kit—HRP-DAB (ab206386; Abcam) following the manufacturer’s instructions.
Primary MEFs were isolated from wild-type and Act1-deficient embryos at embryonic day 14. Cell culture of mouse embryonic fibroblasts (MEFs) was performed as previously described (Liu et al., 2011).
The MOC1 cell line was obtained from Kerafast (EWL001-FP) (Judd et al., 2012a, 2012b) and cultured according to the manufacturer’s instructions.
PDVC57 cell line was kindly provided by Dr. Allan Balmain at University of California, San Francisco, San Francisco, CA, USA and cultured as previously described (Buchmann et al., 1991; Chen et al., 2022; Quintanilla et al., 1991).
A-431 cell line was obtained from ATCC, CRL-1555 and cultured according to the manufacturer’s instructions.
When stimulated ex vivo, cells were stimulated with mouse IL-17A (50 ng/ml, 421-ML; R&D Systems), rapamycin (25 ng/ml, R8781; Sigma-Aldrich), or cisplatin (1 μg/ml, 15663-27-1; Sigma-Aldrich). 24 h before treatments, fetal bovine serum concentration was reduced to 0.5%. Cells were maintained in culture for no more than 20 passages.
Cellular ROS assay
ROS in cells was analyzed by use of the DCFDA/H2DCFDA—Cellular ROS Assay Kit (ab113851; Abcam) according to the instructions of the manufacturer. 3 × 106 cells were treated with the indicated drugs. Upon treatment, DCFDA reagent was added to a final concentration of 20 µM and cells were incubated for 45 min at 37°C. Microscopy images were taken BZ-X710 microscope (Keyence).
Annexin V-FITC/PI-staining and flow cytometry
Cells were stained using the Biolegend FITC Annexin V Apoptosis Detection Kit with PI according to the manufacturer’s instructions, followed by flow cytometry (BD FACSymphonyA1 flow cytometer). Data were analyzed using FlowJo 10.4 software.
Constructs
HA-Wtap was constructed by cloning Wtap cDNA with HA-tag into pcDNA3.1 vector. Wild-type (FLAG-Act1) and Act1 internal deletion mutant of the SEFIR domain ΔSEF1 constructs were previously described (Herjan et al., 2018).
Bicistronic reporter system
Bicistronic reporter constructs were prepared using the Renilla/Firefly expression plasmid pcDNA3 Rluc POLIRES Fluc (Plasmid #45642; Addgene). After removing poliovirus internal ribosome entry site, 5′UTR of PRDX2 (nucleotides 1–143), TXN1 (nucleotides 1–201), SOD1 (nucleotides 1–96), or Wtap (nucleotides 1–191) were cloned into the PmlI and NotI sites; plasmid without 5′UTR insert was used as a control.
Transfection and retroviral infection
Transfections of cells were conducted either with Lipofectamine 3000 (Invitrogen) or using Amaxa nucleofector apparatus (Amaxa, GmbH) according to the manufacturer’s instructions. For Act1 reconstitution into Act1−/− MEFs, cells were infected by retroviral particles containing Act1 or ΔSEF1 constructs as described previously (Qian et al., 2007).
ShRNA and siRNAi
For WTAP siRNA knock-down, siRNA duplexes (Qiagen) to the target sequence 5′-AAGCTTTGGAGGGCAAGTACA-3′ or control siRNA were transfected overnight with lipofectamine 3000 (Invitrogen) according to the manufacturer’s protocol. Both shRNA Act1 (clone TRCN0000105992) as well as non-target shRNA Control Plasmid DNA were purchased from Sigma-Aldrich.
MeRIP
Total RNA extraction was performed using TRIzol reagent (Invitrogen). rRNA was removed from 300 μg total RNA using the rRNA removal kit (Arraystar). Purified mRNA was fragmented using the NEBNext RNA Fragmentation Kit (NEB) for 5 min at 94°C, followed by RNA purification using Dynabeads MyOne Silanebeads (Thermo Fisher Scientific). The fragmented RNA was subjected to methylated (m6A) RNA immunoprecipitation (MeRIP) by using EpiMark N6-methyladenosine Enrichment kit (NEB) according to the manufacturer’s instructions. Briefly, 25 μl protein G magnetic beads per sample were washed twice and resuspended completely in 250 μl reaction buffer (150 mM NaCl, 10 mM Tris-HCl, pH 7.5, and 0.1% NP-40). 1 μl of anti-N6-methyladenosine antibody was attached to the beads. Antibody-conjugated beads were washed two more times before resuspending completely in 250 μl of reaction buffer supplemented with 1 μl RNAse inhibitor. Next, purified RNA (200 μg) was added to the resuspended beads. Samples were incubated with orbital rotation for 4 h at 4°C. Beads were then washed twice with 500 μl reaction buffer, two times with low salt wash buffer (50 mM NaCl, 10 mM Tris-HCl, pH 7.5, and 0.1% NP-40), then two times with high salt buffer (500 mM NaCl, 10 mM Tris-HCl, pH 7.5, and 0.1% NP-40), and with reaction buffer again. m6A-modified RNA was eluted twice in 100 μl reaction buffer containing 5 mM m6A salt (Santa Cruz Biotechnology) for 30 min at 4°C with rotation and concentrated by ethanol precipitation. The supernatant was discarded and beads were incubated for 1 min at room temperature in 150 μl of Monarch RNA Cleanup Binding Buffer, and RNA (in the eluent) was further cleaned up using Monarch RNA Cleanup kit (NEB), following the manufacturer’s instruction. Purified RNA was used for quantitative real-time PCR analysis using primers listed in Table S4. For Prdx2, Txn1, Sod1, and Wtap primers were designed to surround the reported 5′UTR methylation site. (Table S4, primers indicated as MeRIP).
Quantitative real-time PCR
Total RNA was isolated with TRIzol reagent (Invitrogen). The cDNA was synthesized with random hexamers (Invitrogen) and M-MLV reverse transcriptase (Promega). Real-time PCR was performed with a SYBR Green PCR Master Mix kit (Applied Biosystems). Relative expression of target genes as well as RNA enrichment in immunoprecipitation were calculated using the 2−ΔΔCt method. The primers used for qPCR are listed in Table S4.
MOC1 tumor model
C57BL/6 mice at 6–8 wk were obtained from Jackson Laboratories. Mice (n = 5/group) were injected subcutaneously in the right flank with MOC1 cells (0.5 × 106, in Matrigel) and allowed to grow for 10 days, and then randomized into treatment groups. Mice were then treated with cisplatin (5 mg/kg), simultaneously with each cisplatin treatment, and intratumor injection of WTAP or control ASO was performed at 1 nmol/mouse (∼20 μl PBS per injection). Treatment was repeated every 4 days up to day 21 after which mice were sacrificed and tumor tissue collected. Tumors were measured for size and volume using the following formula: .
Immunohistochemistry and immunofluorescence
For paraffin sections, tissues were fixed (10% formalin overnight) and then stored in 70% ethanol at 4°C before processing into paraffin blocks at Cleveland Clinic Imaging Core. Paraffin sections were deparaffinized and subjected to epitope retrieval recommended by the antibody manufacturer. Subsequently, sections were blocked for 2 h (PBS containing 2% donkey serum, 0.5% BSA, 0.5% fish skin gelatin, 0.05% Tween 20, and 0.1% Triton X-100, pH 7.2) and incubated with primary antibody overnight. Then slides were treated with 0.3% H2O2 and incubated with biotinylated secondary antibodies and peroxidase streptavidin (Vector Laboratories). For chromogenic detection of horseradish peroxidase (HRP) activity, DAB substrate kit from BD Pharmingen was used. Immunohistochemistry (IHC) staining was captured with a Keyence BZ-X700 microscope. For frozen sections, tissues were embedded in OCT (Tissue-Tek) and snap-frozen in liquid nitrogen. Frozen tissue sections (5 μm) or cells grown on a glass coverslip were fixed and permeabilized with 4% paraformaldehyde solution containing 0.2% Triton X-100 for 10 min. Subsequently, samples were incubated with primary antibody (1:100) overnight and then antigens were visualized by incubation with fluorescence-conjugated secondary Abs (Invitrogen).
Tissue array staining and analysis
Tissue array slides (with a collection of skin squamous cell carcinoma, Cat#SK802b; Tissuearray) were subjected to IHC staining for WTAP (slide 197; 1:100, ab195380; Abcam) and IL-17A (slide 196; 1:50, AF-317; R&D), and then scanned with Hamamatsu Nanozoomer S60. For WTAP, data were reviewed and exported using Aperio ImageScope. For each sample, a random rectangle region (cropped with the Rectangle tool) was selected for further analysis in ImageJ. The following criteria were applied for the regions to be analyzed: (1) Three samples were omitted due to complete poor quality or missing tissue (A2, A3, and G3). (2) Normal skin H7–H10 or irrelevant tumor H11 were not analyzed. (3) Large broken areas and the background area of the slides were avoided; and staining background areas were avoided (e.g., B8 and F2). ImageJ plugin “IHC Profiler” was used for WTAP intensity quantification (Varghese et al., 2014). The percentage of “High positive + Positive” signal was considered significant WTAP staining signals at the threshold of 85 for the DAB channel. For IL-17A, samples were reviewed and grouped into IL-17A low and IL-17A high by blindly estimating the number of infiltrating IL-17A–producing cells in each sample.
Microscopy
Both IHC staining and fluorescent images were captured with BZ-X710 microscope (Keyence).
RNA electrophoretic mobility shift assay (REMSA)
Increasing amounts of purified protein and labeled probes (10 fmol, see in vitro transcription) were combined in the binding buffer for 30 min. The final REMSA binding buffer concentrations were 140 mM KCl, 10 mM HEPES, pH 7.9, 5% glycerol, 1 mM DTT, and 0.33 mg/ml tRNA. The reaction was further supplemented with 15 μg salmon sperm DNA to reduce non-specific interactions from the lysate. Complexes were resolved on either 4% or 6% non-denaturing polyacrylamide gels. The gels were dried and the appearance of complexes was visualized by exposure to BioMax MR film.
In vitro transcription
Fragments containing the 5′UTR of Wtap (nt81–150), Sod1(nt9–126), Prdx2(nt51–143), and Txn1(nt81–201) were generated by PCR and cloned into the pGEM-3ZF (+) vector (Promega) through the EcoRI and BamHI sites. REMSA radiolabeled 5′UTR RNA probes were synthesized from BamHI linearized plasmids templates with T7 RNA polymerase using 1 mM GTP, 1 mM ATP, 1 mM CTP, 0.005 mM UTP, and 25 μCi of 32P-labeled UTP for 3 h at 37°C. Probes were DNAse I-treated for 20 min and then phenol: chloroform extracted. The aqueous phase was passed through a Micro Bio-Spin P30 column according to the manufacturer’s instructions (BioRad).
For RNase footprinting experiments, cold synthetic transcripts were dephosphorylated with SuperSAP (Affymetrix), purified, and resuspended in nuclease-free water. Dephosphorylated transcripts were end-labeled with [γ-32P] ATP (3,000 Ci/mmol; Perkin Elmer Easy Tides) and T4 PNK (NEB) using 20 units/pmol RNA. The transcripts were gel purified on 8% acrylamide (19:1)/7 M urea gels and eluted in 10 mM Tris-HCl, pH 7.5, 1 mM EDTA, pH 8, and 300 mM NaOAc, pH 5.5, at 4°C overnight. Purified RNA was stored in 10 mM Tris-HCl, pH 7.5, at −20°C.
RNase footprinting
32P-end-labeled RNA with or without cold ASO was heated to 95°C and cooled to room temperature. The RNA (2.5 nM) was incubated in REMSA binding buffer with or without Act1 SEFIR protein (1.5 μM) at 30°C for 10 min. Reactions were cooled to room temperature over a 2-min period and then placed at 22°C for 2–5 min. The indicated amounts of RNase T1 or A (Ambion) were added to the appropriate samples and incubated at 22°C for 5 min. Enzymatic reactions were quenched with 30 μl inactivation/precipitation buffer (Ambion) and purified according to the manufacturer’s directions. Samples were resuspended in 10 μl of loading buffer (Ambion), heat-denatured at 95°C for 5 min, and separated in a denaturing 8% (19:1) polyacrylamide/7 M urea gel. The dried gels were visualized on BioMax BXRfilm.
Sequencing ladders were prepared by incubating 32P-end-labeled RNA (2.5 nM) in 1× Sequencing Buffer (Ambion) supplemented with 50 ng/μl yeast tRNA. The RNAs were incubated at 50°C for 5 min and cooled to 22°C, and the indicated amounts of RNases T1 or A were then added. The samples were incubated, quenched, and purified as described above. Alkali ladders were prepared by incubating 32P-end-labeled RNA (2.5 nM) in 100 mM NaOH, 2 mM EDTA, pH 8.0, and 2 μg/μl yeast tRNA at 37°C for 3 min, to which 2 µl 1 M Tris-HCl, pH 8.0 was added. The samples were frozen on dry ice and combined with an equal volume of loading buffer.
ASO design
Fully modified 2′-O-methoxy ethyl/phosphonothioate oligonucleotides containing sequences (W1: 5′-UCACACAGGCCGAGGCCGCG-3′; W2: 5′-CGAGGCCGCGCCGCCGCCGG-3′; W3: 5′-CCGCCGCCGGCCCCCCCGCG-3′; W4: 5′-CCCCCCCGCGCUCCUCCCG-3′; Ctrl: 5′-GCGACUAUACGCGCAAUAUG-3′; h_WTAP-ASO: 5′-CCCCGCCGCGCUCCUAGUCCCG-3′; h_Ctrl-ASO: 5′-CCUAUAGGACUAUCCAGGAA-3′) were ordered form Integrated DNA Technologies. For fluorescence detection, ASOs were further modified at 5′ end with 6-FAM dyes.
Immunoblot and immunoprecipitation
Cells were harvested and lysed on ice in a lysis buffer containing 0.5% Triton X-100, 20 mM Hepes, pH 7.4, 150 mM NaCl, 12.5 mM glycerophosphate, 1.5 mM MgCl2, 10 mM NaF, 2 mM dithiothreitol, 1 mM sodium orthovanadate, 2 mM EGTA, 20 mM aprotinin, and 1 mM phenylmethylsulfonyl fluoride for 20 min, followed by centrifuging at 12,000 rpm for 15 min to extract clear lysates. For immunoprecipitation, cell lysates were incubated with 1 μg of antibody and A-sepharose beads at 4°C overnight. After incubation, the beads were washed four times with lysis buffer and the precipitates were eluted with 2× sample buffer. Elutes and whole-cell extracts were resolved on SDS-PAGE followed by immunoblotting with antibodies. Protein expression levels were quantified by measuring band intensity using ImageJ (NIH). Arbitrary densitometric units, normalized against tubulin, were calculated as the fold change versus the first sample for each blot and shown under each protein signal.
Polysomal fractionation analysis
A total of 2 × 108 cells was left untreated or stimulated with IL-17A (50 ng/ml) for 24 h. Cytoplasmic extracts were carefully layered over 10–50% linear sucrose gradients in polysome buffer (10 mM HEPES [pH 7.5], 100 mM KCl, 2.5 mM MgCl2, 1 mM DTT, 50 U recombinant RNasin (Promega), and 0.1% IGEPAL CA-630 (Sigma-Aldrich)) and centrifuged at 17,000 rpm in a Beckman SW32.1 Ti rotor for 4 h at 4°C. Gradients were fractioned using an ISCO gradient fractionation system equipped with a UA-6 detector. Light ribonucleoprotein (RNP) fractions, 40S, 60S, and 80S, as well as light and heavy polysome fractions, were monitored by the continuous UV absorption profile at A254, and fractions of 750 ml were collected. The fractions representing light RNP, free ribosomes, and light polysomes were used to prepare the translation-inactive and poorly translated pool of mRNAs, and the fractions representing heavy polysomes were used to isolate the translation-active mRNAs. One-fifth of each fraction was used for RNA isolation by extraction with TRIzol.
Statistical analysis
Statistical analysis was applied to biologically independent samples (separate plates of cells or mice) from every single experiment and data were not pooled from independent experiments for statistical analysis. Experiments were repeated at least twice and the exact number of repetitions is indicated in the figure legend for each panel. For all RT-PCR and ELISA analyses, at least three biological replicates (separate plates of cells) were used. Unless otherwise indicated, comparisons between two groups were analyzed by two-tailed Student’s t test. Comparisons between multiple groups were analyzed using one-way ANOVA, followed by Tukey’s multiple-comparisons test. All bar graphs show mean and standard deviation or standard error, which is indicated for each panel in the figure legend. GraphPad Prism 9 or R (4.2.2) was used for data analysis and representation.
CLIP-seq library construction and analysis
Three 15-cm plates of flag-Act1KO/WT MEF cells were treated with IL-17A (50 ng/ml) for 1 h, followed by irradiating with 365 nm UV light to induce crosslinking as described previously (Licatalosi et al., 2008; Zagore et al., 2018). Immunoprecipitated protein–RNA complexes were separated by SDS-PAGE and then transferred to the PVDF membrane; RNA–protein complexes were cut out from the membrane corresponding to the size of flag-Act1. Purified RNAs from RNA–protein complex were subjected to the small RNA library construction as described previously (Zagore et al., 2018) and then sequenced with the HiSeq 2000 system (Illumina, Inc.) at the Cleveland Clinic Genomic Core.
Reads with length >15 bp were mapped to the mm10 genome using Gencode M25 (GRCm38.p6) with Bowtie2 (2.4.4) (Langmead and Salzberg, 2012). The uniquely mapped reads were used to identify Act1 CLIP clusters that are present in at least two biological replicates. Reads were normalized based on the size of uniquely mapped reads of each library. CLIP clusters were identified using SAMTools (1.14) (Danecek et al., 2021) and BEDTools (2.30.0) (Quinlan and Hall, 2010). The gene annotation of CLIP clusters was assessed by intersection with gene regions retrieved from the Gencode M25 (GRCm38.p6). The transcriptome-wide distribution of the Act1 CLIP clusters was analyzed and visualized using MetaPlotR (Olarerin-George and Jaffrey, 2017). Integrative Genomics Viewer was used to visualize the Act1 CLIP cluster (Robinson et al., 2017). The distribution of CLIP clusters on gene transcript was assessed by intersection with 5′UTR, 3′UTRs, and coding sequence (CDS) regions retrieved from the Gencode M25 (GRCm38.p6). To define 5′UTR or 3′UTR binding target, we calculated the sum of normalized reads on 5′UTR and -3′′UTR regions. Genes with higher sum reads on 5′UTR/3UTR were defined as Act1 5′UTR/3′UTR dominant binding targets. Only genes with max read >1 and sum read >3 were used for further analysis. The length of 5′UTR/3′UTR was extracted using the R package GenomicFeatures (1.50.4) (Lawrence et al., 2013). The longest transcript per gene was selected for the calculation of UTR density. Hexamer enrichment analysis was performed on a 20-nucleotide window surrounding clusters using the EMBOSS tools Compseq (Rice et al., 2000). GO analyses of CLIP targets were performed using DAVID (Sherman et al., 2022). To calculate the distance between the Act1 CLIP cluster and m6A sites from published miCLIP dataset (NCBI accession no. GSE147489) or MeRIP-seq datasets (GEO accession nos. GSE53244 and GSE61995), the genomic location of clusters was converted to transcript location using R package ensembldb (2.22.0) (Rainer et al., 2019). The smallest distance between Act1 CLIP clusters and miCLIP sites or the middle sites of MeRIP-seq peaks on each gene was used for the density plot. Plots were produced with R (4.2.2) (R Core Team, 2013) ggplot2 (3.4.1) (Villanueva and Chen, 2019) with measurements indicated in the figure legends.
RNA-seq library construction and analysis
Four 10-cm plates of flag-Act1KO/WT MEF cells were untreated or treated with IL-17A (50 ng/ml) for 1 h. Total RNA was extracted using Trizol (15596026; Invitrogen) according to the manufacturer’s instructions followed by isopropanol precipitation. Total RNAs were further purified using Qiagen’s miRNeasy micro kit (217084; Qiagen) plus Qiagen’s DNase set (79254; Qiagen) for the on-column DNase digestion option. The libraries were sequenced using the 150-nt paired-end kit on Illumina NovaSeq 6000 (Novogene). RNA-seq reads were aligned against the mm10 using STAR (2.7.0e) (Dobin et al., 2013). The number of reads mapped to mouse genome mm10 was counted using RSEM (1.3.3) (Li and Dewey, 2011). Differential gene expression was determined using the R-package DEseq2 (1.38.3) (Love et al., 2014).
Online supplemental material
Data availability
Acknowledgments
The cell line PDVC57 was originally from the University of California, San Francisco (kindly provided by Dr. Allan Balmain at the University of California, San Francisco, San Francisco, CA).
This study is supported by the US National Institutes of Health (5 P01 CA 062220-24) awarded to X. Li and PO1CA272161 awarded to George Stark. This work was also supported by Case Western Reserve University through departmental Start-up funding and the High-Performance Computing Award, both awarded to Xiao Li. The Hamamatsu Nanozoomer S60 slide scanner is supported by the National Institutes of Health (NIH) Shared Instrumentation Grant NIH ORIP S10OD024981 to CWRU Light Microscopy Imaging Core.
Author contributions: L. Hong: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing, T. Herjan: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing—original draft, Writing—review & editing, X. Chen: Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, L.L. Zagore: Investigation, K. Bulek: Investigation, Project administration, Resources, H. Wang: Data curation, Methodology, C.-F.J. Yang: Investigation, Supervision, Writing—review & editing, D.D. Licatalosi: Supervision, X. Li: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing—original draft, Writing—review & editing, X. Li: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing.
References
Author notes
L. Hong and T. Herjan contributed equally to this paper.
Disclosures: L. Hong reported a patent to 63/637,516 pending. T. Herjan reported a patent to 63/637,516 pending. X. Chen reported being listed as a co-inventor on Cleveland Clinic Provisional Patent Application #63/637,516 (related to an earlier disclosure form, as a former Cleveland Clinic employee). C. Yang is a member of the advisory board for AstraZeneca and Genentech, and has received honorarium from AstraZeneca and Genentech. Xiaoxia Li reported a patent to 63/637,516 pending. No other disclosures were reported.




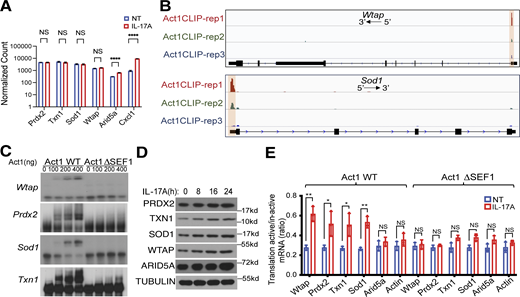

![Act1 promotes cap-independent translation of transcripts encoding Wtap and antioxidant proteins. (A)Act1−/− MEFs reconstituted retrovirally with either FLAG-tagged WT Act1 or Act1 ΔSEF1 were pretreated with rapamycin or DMSO for 24 h, followed by IL-17A treatment and western blot analysis. (B) WT Act1 or Act1 ΔSEF1 MEFs were transfected with a plasmid encoding a bicistronic RNA expressing renilla luciferase (via cap-dependent translation) and firefly luciferase (via cap-independent translation driven by the inserted 5′UTR sequence, including Prdx2, Txn1, Wtap, Sod1, as indicated on diagram [top panel]), followed by IL-17A treatment for 24 h and dual luciferase reporter assay. The graph shows the ratio of firefly to renilla luciferase activity determined by luminescence (mean and SD of three independent plates of cells). (C) WT Act1 or Act1 ΔSEF1 MEFs were pretreated either with rapamycin or DMSO for 24 h and then treated with IL-17A for 24 h, followed by fractionation and RT-PCR normalized to Gapdh. The graph shows the ratios of mRNAs from translation-active/inactive pools (mean and SD of three independent plates of cells). Two-tailed t test was performed. *P < 0.05, **P < 0.01; NS, not significant. All data are representative of three independent experiments. Source data are available for this figure: SourceData F3.](https://cdn.rupress.org/rup/content_public/journal/jem/221/7/10.1084_jem.20231442/1/m_jem_20231442_fig3.png?Expires=1773853603&Signature=SJ9iHIsmM3MhO0URl2vZrHhVCN4UWhfVLEMMCI2iZAakcyNnMqCjALWTrA1kDyLk4NjequhYlPqtSVzBgZtsKbyxHIX1LSKo1CIA85ekLzubuPMD7Q~PO32QI5374EJe8E2qHJ44Z5XlRwwcv7FGwF-yxWVQc6pJ0wRfACWZvR3unCqIs~dHRlX2LrOhLJ3i16fTrflI-~Z9nNrgSJFPNLGUL2RGDaJpx6H6b4UjUh1u9x-ThJhLYi7yUxg9rnn2Kstby4BKrK-sHd2xW4TFQHcnfj0YqrokbfqaM2eVnIApiAQIptekz5w0Fyo84ERLjh4rKMA8o-LMaAFjbAd8cQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)