While conventional wisdom initially postulated that PD-L1 serves as the inert ligand for PD-1, an emerging body of literature suggests that PD-L1 has cell-intrinsic functions in immune and cancer cells. In line with these studies, here we show that engagement of PD-L1 via cellular ligands or agonistic antibodies, including those used in the clinic, potently inhibits the type I interferon pathway in cancer cells. Hampered type I interferon responses in PD-L1–expressing cancer cells resulted in enhanced efficacy of oncolytic viruses in vitro and in vivo. Consistently, PD-L1 expression marked tumor explants from cancer patients that were best infected by oncolytic viruses. Mechanistically, PD-L1 promoted a metabolic shift characterized by enhanced glycolysis rate that resulted in increased lactate production. In turn, lactate inhibited type I IFN responses. In addition to adding mechanistic insight into PD-L1 intrinsic function, our results will also help guide the numerous ongoing efforts to combine PD-L1 antibodies with oncolytic virotherapy in clinical trials.
Introduction
Being expressed in many cell types, PD-L1 is a readily available ligand for PD-1 present in immune cells in different tissues (Keir et al., 2006). The current model places PD-L1 as an agonistic ligand for PD-1, whose engagement results in inhibition of T and natural killer (NK) cells (Patsoukis et al., 2020). This pathway is exploited by tumors as a mechanism of immune evasion, as evidenced by the success of antibodies blocking PD-1/PD-L1 interactions in several cancer indications (Homet Moreno and Ribas, 2015; Ribas and Wolchok, 2018). However, the implementation of these therapies outpaced mechanistic understanding of this pathway, and many questions remain open on the PD-1/PD-L1 axis, including what other possible functions PD-L1 has.
Emerging literature suggests that PD-L1, beyond its one-dimensional role as the ligand for PD-1, has cell-intrinsic signaling in cancer and immune cells (Kornepati et al., 2022; Lucas et al., 2020). PD-L1 signaling modulates many cellular processes, including the TGF-β pathway and the epithelial–mesenchymal transition (Chen et al., 2022a, 2022b; Saleh et al., 2019; Wang et al., 2015), epidermal growth factor receptor (EGFR) signaling (Li et al., 2019), MAPK activation (Chen et al., 2021; Passariello et al., 2019), apoptosis (Ghebeh et al., 2010; Kong et al., 2020), DNA damage (De et al., 2021; Tu et al., 2019; Yu et al., 2020), proliferation and metastasis (Clark et al., 2016), and cellular metabolism (Chang et al., 2015; Garige et al., 2022). In most cases, the underlying mechanisms by which PD-L1 impacts these biological processes have not been uncovered.
The type I interferon (IFN) response is a pathway regulated by PD-L1 (Cheon et al., 2021; Diskin et al., 2020; Gao et al., 2020; Gato-Cañas et al., 2017). Type I IFNs are a family of cytokines that induce a cellular antiviral state via a JAK/STAT signaling pathway that promotes the transcription of hundreds of interferon-stimulated genes (ISGs) (McNab et al., 2015). Type I IFNs have other important roles in immunity and cell death (Zitvogel et al., 2015), and it is in these contexts that their connection with PD-L1 has been established. However, the mechanism by which PD-L1 regulates type I IFN responses is unknown. Importantly, the ability of PD-L1 to control viral infection, arguably the most prominent function of type I IFNs, has not been explored. When investigating PD-L1 expression in cancer cells, this relationship becomes even more important in light of the tremendous interest in combining PD-L1 blockade with oncolytic viruses (OVs).
OVs are viruses with a natural or engineered tropism for cancer cells over normal cells, as a result of deficiencies in type I IFN signaling in cancer cells arising during transformation (Geoffroy and Bourgeois-Daigneault, 2020; Lichty et al., 2014). Preclinical and clinical studies frequently combine OVs with PD-1/PD-L1 blockade or even engineer the OV to reduce PD-L1 expression in the tumor microenvironment (Wedge et al., 2022; Zamarin et al., 2018). However, the potential for synergy or antagonism between PD-L1 blockade and OVs should be carefully assessed prior to clinical translation.
To this end, we found that by suppressing type I IFN responses, PD-L1 enhances infection with OVs in vitro and in vivo. Inhibition of type I IFNs depended on a metabolic shift promoted by PD-L1, resulting in enhanced rates of glucose uptake and glycolysis. Lactate generated from glycolysis was key to inhibiting type I IFN responses. Taken together, our data mechanistically link PD-L1 cell-intrinsic functions with susceptibility to OVs and provide a framework to further develop combinatorial therapies that better exploit the ability of PD-L1 to promote OV efficacy.
Results
PD-L1 engagement promotes oncolysis of cancer cells
To test the hypothesis that PD-L1 regulates infection and oncolysis of cancer cells, we took advantage of the murine prostate cancer cell line TRAMP-C2 (Foster et al., 1997), which is widely used in OV preclinical studies (Annels et al., 2020; Atherton et al., 2018; Varghese et al., 2006) and constitutively expresses PD-L1 in culture (Fig. S1 A). To generate PD-L1–deficient cells, we targeted Cd274 (the gene coding for murine PD-L1) by CRISPR/Cas9 and sorted cells lacking PD-L1 expression (Fig. S1 A).
PD-L1 does not regulate VSVΔ51 entry. (A) TRAMP-C2 cells (blue) were transfected with Cas9 and gRNA targeting PD-L1 to generate TRAMP-C2-Cd274−/− (red). A representative plot depicting PD-L1 expression is shown. (B) Expression of PD-1 and CD80 in TRAMP-C2 cells. Representative flow plots are shown. (C) TRAMP-C2 cells stably expressing CD80 (or empty vector [EV] control) were infected with VSVΔ51 at MOI 0.1 for 24 h prior to analysis of viral titer by plaque assay. The data depicted are representative of two experiments performed with similar results. (D) TRAMP-C2 or TRAMP-C2-Cd274−/− were infected with VSVΔ51-YFP at MOI 0.1, and RNA was collected at indicated times after infection for qPCR analysis of the viral nucleocapsid (N) transcript. n = 3 biological replicates depicted. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (E) Expression of the VSV entry receptor LDL-R in TRAMP-C2 (blue) or TRAMP-C2-Cd274−/− (red) cells. Representative histograms are shown. For all panels: ****P < 0.0001; P > 0.05 not significant (ns).
PD-L1 does not regulate VSVΔ51 entry. (A) TRAMP-C2 cells (blue) were transfected with Cas9 and gRNA targeting PD-L1 to generate TRAMP-C2-Cd274−/− (red). A representative plot depicting PD-L1 expression is shown. (B) Expression of PD-1 and CD80 in TRAMP-C2 cells. Representative flow plots are shown. (C) TRAMP-C2 cells stably expressing CD80 (or empty vector [EV] control) were infected with VSVΔ51 at MOI 0.1 for 24 h prior to analysis of viral titer by plaque assay. The data depicted are representative of two experiments performed with similar results. (D) TRAMP-C2 or TRAMP-C2-Cd274−/− were infected with VSVΔ51-YFP at MOI 0.1, and RNA was collected at indicated times after infection for qPCR analysis of the viral nucleocapsid (N) transcript. n = 3 biological replicates depicted. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (E) Expression of the VSV entry receptor LDL-R in TRAMP-C2 (blue) or TRAMP-C2-Cd274−/− (red) cells. Representative histograms are shown. For all panels: ****P < 0.0001; P > 0.05 not significant (ns).
To assess whether PD-L1 expression affects susceptibility to OVs, we infected parental and PD-L1–deficient TRAMP-C2 cells with VSV∆51, which is highly sensitive to the antiviral effects of type I IFNs (Hastie and Grdzelishvili, 2012; Stojdl et al., 2003). Strikingly, PD-L1 deletion resulted in a dramatic reduction of infection and VSV∆51-induced cell death (Fig. 1, A–D). To confirm that PD-L1 deletion, and not an experimental artifact, resulted in differences in OV infection, we complemented PD-L1 expression in TRAMP-C2-Cd274−/− cells and tested if the phenotype was rescued. As expected, PD-L1 complementation enhanced VSV∆51 infection compared with the empty vector control (Fig. 1, E and F). Increased resistance of PD-L1–deficient cells was observed not only in response to VSV∆51 but also to vaccinia virus (Fig. 1 G), an oncolytic DNA virus undergoing clinical testing (Monge et al., 2020), showing that PD-L1 controls OV infection and oncolysis of cancer cells.
PD-L1 engagement promotes infection of VSVΔ51 and vaccinia virus. (A–D) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were infected with VSVΔ51-YFP at indicated MOIs and subjected to flow cytometry to quantify the viral YFP reporter 24 h after infection (A), plaque assay to quantify viral titers 24 h after infection (B), Coomassie staining to visualize cell death 48 h after infection (C), or flow cytometry to detect cleaved caspases 3 and 7 using a fluorogenic substrate (D). The data depicted are representative of three to four experiments performed with similar results, n = 3 biological replicates for the viral titer data. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (E and F) TRAMP-C2-Cd274−/− transduced with PD-L1 or empty vector (EV) were infected with VSVΔ51-YFP at indicated MOIs for 24 h and analyzed by flow cytometry (E) or by plaque assay (F). The data depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons in E and two-tailed unpaired Student’s t test in F. (G) TRAMP-C2 cells were infected with GFP-expressing vaccinia virus (Copenhagen strain) at MOI 0.1 for 48 h, prior to fluorescence imaging. Representative of two performed with similar results. Viral titer was assessed by plaque assay and statistical analysis by two-tailed unpaired Student’s t test. Scale bar = 1,000 μm. (H) CD80+ and CD80− cells were isolated by FACS and subjected to VSVΔ51-YFP at indicated MOIs for 24 h prior to flow cytometry to quantify viral YFP reporter. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (I) TRAMP-C2 and TRAMP-C2-Cd274−/− were pretreated for 24 h with 500 ng of recombinant PD-1-Fc chimeric protein and infected with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (J) TRAMP-C2 and TRAMP-C2-Cd274−/− were pretreated for 24 h with 10 µg of PD-L1 antibodies and infected with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. For all panels: ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
PD-L1 engagement promotes infection of VSVΔ51 and vaccinia virus. (A–D) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were infected with VSVΔ51-YFP at indicated MOIs and subjected to flow cytometry to quantify the viral YFP reporter 24 h after infection (A), plaque assay to quantify viral titers 24 h after infection (B), Coomassie staining to visualize cell death 48 h after infection (C), or flow cytometry to detect cleaved caspases 3 and 7 using a fluorogenic substrate (D). The data depicted are representative of three to four experiments performed with similar results, n = 3 biological replicates for the viral titer data. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (E and F) TRAMP-C2-Cd274−/− transduced with PD-L1 or empty vector (EV) were infected with VSVΔ51-YFP at indicated MOIs for 24 h and analyzed by flow cytometry (E) or by plaque assay (F). The data depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons in E and two-tailed unpaired Student’s t test in F. (G) TRAMP-C2 cells were infected with GFP-expressing vaccinia virus (Copenhagen strain) at MOI 0.1 for 48 h, prior to fluorescence imaging. Representative of two performed with similar results. Viral titer was assessed by plaque assay and statistical analysis by two-tailed unpaired Student’s t test. Scale bar = 1,000 μm. (H) CD80+ and CD80− cells were isolated by FACS and subjected to VSVΔ51-YFP at indicated MOIs for 24 h prior to flow cytometry to quantify viral YFP reporter. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (I) TRAMP-C2 and TRAMP-C2-Cd274−/− were pretreated for 24 h with 500 ng of recombinant PD-1-Fc chimeric protein and infected with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (J) TRAMP-C2 and TRAMP-C2-Cd274−/− were pretreated for 24 h with 10 µg of PD-L1 antibodies and infected with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. For all panels: ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
Of the two known ligands for murine PD-L1 (Kornepati et al., 2022), TRAMP-C2 cells fail to express PD-1, whereas CD80 was expressed by ∼40% of cells in culture (Fig. S1 B). To determine if engagement by CD80 was needed for PD-L1 to enhance OV infection, we isolated CD80+ or CD80− cells from both TRAMP-C2 or TRAMP-C2-Cd274−/− by FACS and infected them with VSV∆51. In the cells expressing CD80, PD-L1 enhanced viral infection (Fig. 1 H, left side). In stark contrast, in the absence of CD80, PD-L1 failed to promote viral infection and there was no longer any difference in infection between parental and PD-L1–deficient TRAMP-C2 cells (Fig. 1 H, right side). On the other hand, overexpression of CD80 further increased VSV∆51 infection (Fig. S1 C). These data suggest that PD-L1 engagement is required to drive permissiveness to viral infection.
Given the abundance of PD-1 in the tumor microenvironment, we tested if PD-1 engagement of PD-L1 also resulted in an enhanced permissiveness to viral infection. Treatment with a recombinant PD-1-hIgG1 Fc chimeric protein enhanced infection in parental TRAMP-C2 cells compared with control-treated cells (Fig. 1 I), whereas PD-1-Fc treatment had no effect in PD-L1–deficient tumor cells (Fig. 1 I). These data show that both CD80 and PD-1 binding to PD-L1 promotes viral infection.
Next, we explored whether engagement of PD-L1 with antibodies promoted OV infection in cancer cells, a question of particular interest when considering the use of PD-L1 antibodies in the clinic. Reasoning that they may serve as agonists for PD-L1, we pretreated TRAMP-C2 cells with different PD-L1 antibodies that prevent interactions between PD-L1 and: PD-1 (clone 27C11), CD80 (clone 17H9), or both (clone 6E11) (Oh et al., 2020). Treatment with the two antibodies mimicking PD-1 binding to PD-L1, clones 27C11 and 6E11, significantly enhanced infection in parental TRAMP-C2, compared with isotype-treated, cells (Fig. 1 J), whereas no effect was observed with the clone mimicking CD80 interactions with PD-L1 (17H9), perhaps because CD80 was already present in our system. No effect was observed in PD-L1–deficient cells (Fig. 1 J). Taken together, this data confirms our hypothesis that PD-L1 engagement and signaling enhances susceptibility to infection. Moreover, the fact that direct engagement of PD-L1 with antibodies enhanced the susceptibility of cancer cells to infection rules out that uncharacterized signaling downstream of CD80 was responsible for the observed phenotype.
PD-L1 promotes oncolysis by inhibiting type I IFN responses
Next, we set out to determine the mechanisms underlying PD-L1–driven enhancement of OV infection. We ruled out that entry of VSV∆51 was impacted by deletion of PD-L1, as we found equal levels of viral RNA at early time points (e.g., 1–3 h after infection, Fig. S1 D), and both cell lines expressed similar levels of the vesicular stomatitis virus (VSV) entry receptor LDL-R (Fig. S1 E).
As the type I IFN pathway is paramount for antiviral defense, we examined IFN responses induced by VSV∆51 in parental and PD-L1–deficient cells. PD-L1–deficient TRAMP-C2 cells produced approximately twofold more IFN-β compared with parental cells after infection both at the protein and the transcript levels (Fig. 2, A and B) and, accordingly, had higher transcription of antiviral ISGs (Fig. 2 C), which was inhibited by PD-L1 recomplementation (Fig. 2 D and Fig. S2 A). PD-L1 regulated type I IFNs not only in response to viral infection but also to the TLR3 and RIG-I agonist poly(I:C) (Fig. S2, B and C), suggesting that PD-L1 regulates the type I IFN response triggered by diverse stimuli.
PD-L1 inhibits the type I IFN response to VSVΔ51. (A and B) IFN-β protein in culture supernatant (by ELISA, A) or transcript levels (by qPCR, B) were quantified following infection with VSVΔ51-YFP for 8 h. Statistical analysis with two-tailed unpaired Student’s t test. (C) Expression of ISGs was quantified following infection with VSVΔ51-YFP for 8 h. Statistical analysis with two-tailed unpaired Student’s t test. (D) TRAMP-C2-Cd274−/− transduced with PD-L1 or empty vector (EV) were infected with VSVΔ51-YFP at MOI 0.1 and analyzed by qPCR at indicated times after infection to quantify Ifnb1 transcripts. The data depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (E) Type I IFN in culture supernatant was quantified using the L929-ISRE reporter cell line, in CD80+ and CD80− cells isolated by FACS and infected with VSVΔ51-YFP for 8 h. The experiment depicted is representative of three performed with similar results. Statistical analysis by one-way ANOVA with Šídák’s correction for multiple comparisons. RLU, relative luciferase units. (F) TRAMP-C2 and TRAMP-C2-Cd274−/− were pre-treated for 24 h with 5 µg of PD-L1 antibodies, and infected with VSVΔ51-YFP at MOI 0.1 for 8 h prior to qPCR analysis. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (G) TRAMP-C2-Cd274−/− cells transduced with PD-L1 or empty vector were infected with VSVΔ51-YFP at MOI 0.1 and analyzed by western blotting at indicated times after infection. The images depicted are representative of three performed with similar results. Molecular weight values are indicated in kilodaltons (kD). (H) TRAMP-C2 and TRAMP-C2-Cd274−/− were pre-treated with 25 µg of anti-IFNAR1 for 24 h, followed by infection with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by Coomassie staining or flow cytometry. The experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. For all panels: ****P < 0.0001; ***P < 0.001; *P < 0.05; P > 0.05 not significant (ns). Source data are available for this figure: SourceData F2.
PD-L1 inhibits the type I IFN response to VSVΔ51. (A and B) IFN-β protein in culture supernatant (by ELISA, A) or transcript levels (by qPCR, B) were quantified following infection with VSVΔ51-YFP for 8 h. Statistical analysis with two-tailed unpaired Student’s t test. (C) Expression of ISGs was quantified following infection with VSVΔ51-YFP for 8 h. Statistical analysis with two-tailed unpaired Student’s t test. (D) TRAMP-C2-Cd274−/− transduced with PD-L1 or empty vector (EV) were infected with VSVΔ51-YFP at MOI 0.1 and analyzed by qPCR at indicated times after infection to quantify Ifnb1 transcripts. The data depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (E) Type I IFN in culture supernatant was quantified using the L929-ISRE reporter cell line, in CD80+ and CD80− cells isolated by FACS and infected with VSVΔ51-YFP for 8 h. The experiment depicted is representative of three performed with similar results. Statistical analysis by one-way ANOVA with Šídák’s correction for multiple comparisons. RLU, relative luciferase units. (F) TRAMP-C2 and TRAMP-C2-Cd274−/− were pre-treated for 24 h with 5 µg of PD-L1 antibodies, and infected with VSVΔ51-YFP at MOI 0.1 for 8 h prior to qPCR analysis. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (G) TRAMP-C2-Cd274−/− cells transduced with PD-L1 or empty vector were infected with VSVΔ51-YFP at MOI 0.1 and analyzed by western blotting at indicated times after infection. The images depicted are representative of three performed with similar results. Molecular weight values are indicated in kilodaltons (kD). (H) TRAMP-C2 and TRAMP-C2-Cd274−/− were pre-treated with 25 µg of anti-IFNAR1 for 24 h, followed by infection with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by Coomassie staining or flow cytometry. The experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. For all panels: ****P < 0.0001; ***P < 0.001; *P < 0.05; P > 0.05 not significant (ns). Source data are available for this figure: SourceData F2.
PD-L1 inhibits the type I IFN response to VSVΔ51. (A) TRAMP-C2-Cd274−/− cells transduced with PD-L1 or empty vector (EV) were infected with VSVΔ51-YFP at MOI 0.1 and analyzed by qPCR at indicated times after infection to quantify viral N transcripts. The data depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (B and C) Cells were transfected with 1 µg of poly(I:C) for 8 h prior to quantification of IFN-β (by ELISA, B) and IFN-β and MX1 transcripts (by qPCR, C). The experiments depicted are representative of three performed with similar results. Statistical analysis by two-tailed unpaired Student’s t test. (D) TRAMP-C2 cells were infected with VSVΔ51-YFP or VSV WT at MOI 0.1 for 12 h prior to analysis by ELISA to quantify IFN-β in supernatant. The experiment depicted is representative of two performed with similar results. N.D. = not detected. For all panels: ****P < 0.0001; **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
PD-L1 inhibits the type I IFN response to VSVΔ51. (A) TRAMP-C2-Cd274−/− cells transduced with PD-L1 or empty vector (EV) were infected with VSVΔ51-YFP at MOI 0.1 and analyzed by qPCR at indicated times after infection to quantify viral N transcripts. The data depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (B and C) Cells were transfected with 1 µg of poly(I:C) for 8 h prior to quantification of IFN-β (by ELISA, B) and IFN-β and MX1 transcripts (by qPCR, C). The experiments depicted are representative of three performed with similar results. Statistical analysis by two-tailed unpaired Student’s t test. (D) TRAMP-C2 cells were infected with VSVΔ51-YFP or VSV WT at MOI 0.1 for 12 h prior to analysis by ELISA to quantify IFN-β in supernatant. The experiment depicted is representative of two performed with similar results. N.D. = not detected. For all panels: ****P < 0.0001; **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
PD-L1 engagement by CD80 was required to inhibit IFN-β production in TRAMP-C2 cells (Fig. 2 E). Additionally, antibody crosslinking of PD-L1 further suppressed type I IFN responses in parental, but not PD-L1–deficient, TRAMP-C2 cells (Fig. 2 F). Taken together, these data show that PD-L1 engagement strongly dampens type I IFN responses.
We next assessed whether PD-L1, in addition to inhibiting IFN production, also controlled signaling involved in the type I IFN response. After VSV∆51 infection, PD-L1 altered almost every step of the type I IFN pathway, from phosphorylation of TBK1 and IRF-3 (which together control initial production of type I IFN), to STAT1 levels and STAT3 phosphorylation (Fig. 2 G). Therefore, the effect of PD-L1 trickles down to the signaling events of the type I IFN pathway.
To implicate type I IFNs as the pathway responsible for the PD-L1–mediated promotion of oncolysis, we pretreated tumor cells for 24 h with an antagonistic antibody against the type I IFN receptor subunit IFNAR1. Antibody treatment ablated the differences in infection between TRAMP-C2 and TRAMP-C2-Cd274−/− cells (Fig. 2 H), suggesting that the phenotype was caused by PD-L1 inhibition of the type I IFN pathway. Altogether, these data show that PD-L1 engagement promotes oncolysis via inhibition of type I IFN responses.
PD-L1 poises cancer cells to be more susceptible to OVs
The mechanistic link between PD-L1 promotion of OV infection and inhibition of type I IFN responses prompted us to hypothesize that parental and PD-L1–deficient TRAMP-C2 cells will be equally permissive to wild-type (WT) VSV infection, which, differently than VSV∆51, blocks translation of newly synthesized type I IFNs upon infection. Surprisingly, PD-L1 still enhanced WT VSV infection of parental TRAMP-C2 cells compared with PD-L1–deficient TRAMP-C2 cells (Fig. 3 A), despite there being no detectable virus-induced type I IFN response in these cells (Fig. S2 D).
PD-L1 poises TRAMP-C2 cells to be more sensitive to viral infection. (A) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were infected with VSV WT at indicated MOIs for 24 h prior to analysis by plaque assay. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (B) qPCR analysis of type I IFN and ISG transcripts expressed by TRAMP-C2 and TRAMP-C2-Cd274−/− cells prior to infection. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (C) Measurement of type I IFN in uninfected TRAMP-C2 and TRAMP-C2-Cd274−/− supernatant using the L929-ISRE reporter line. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-tailed unpaired Student’s t test. (D) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were infected with VSVΔ51-YFP at MOI 0.1 and treated with actinomycin D (or DMSO as vehicle control) at the time of infection. 24 h later, cells were analyzed by flow cytometry. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. For all panels: ****P < 0.0001; ***P < 0.001; **P < 0.01; P > 0.05 not significant (ns).
PD-L1 poises TRAMP-C2 cells to be more sensitive to viral infection. (A) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were infected with VSV WT at indicated MOIs for 24 h prior to analysis by plaque assay. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (B) qPCR analysis of type I IFN and ISG transcripts expressed by TRAMP-C2 and TRAMP-C2-Cd274−/− cells prior to infection. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (C) Measurement of type I IFN in uninfected TRAMP-C2 and TRAMP-C2-Cd274−/− supernatant using the L929-ISRE reporter line. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-tailed unpaired Student’s t test. (D) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were infected with VSVΔ51-YFP at MOI 0.1 and treated with actinomycin D (or DMSO as vehicle control) at the time of infection. 24 h later, cells were analyzed by flow cytometry. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. For all panels: ****P < 0.0001; ***P < 0.001; **P < 0.01; P > 0.05 not significant (ns).
To better understand this unexpected result, we drew on literature showing that cancer cells often exhibit a constitutive type I IFN response (Bakhoum et al., 2018; Taniguchi and Takaoka, 2001) and hypothesized that PD-L1 inhibited basal type I IFN responses. Indeed, TRAMP-C2 cells presented low but detectable expression of type I IFN and ISG transcripts even before infection, which was more pronounced in the absence of PD-L1 (Fig. 3 B). Additionally, phosphorylation of key molecules of the type I IFN pathway was readily detected in TRAMP-C2 cells prior to infection (Fig. S2 D, mock-infected lanes). Using a sensitive IFN-reporter assay, we detected type I IFN activity in uninfected TRAMP-C2 culture supernatant and more in PD-L1–deficient cells (Fig. 3 C). Therefore, TRAMP-C2 cells present a constitutive type I IFN response which is exacerbated by PD-L1 deletion.
The constitutive expression and regulation of type I IFNs, and the differential infection by VSV WT, suggested that PD-L1 poised cancer cells to a proviral state by constitutively repressing the type I IFN response and subsequent anti-viral ISG expression. To determine if this basal type I IFN response was sufficient to drive differences in OV infection, we treated TRAMP-C2 cells with actinomycin D at the time of VSV∆51 infection to block all cellular transcription, including virus-induced production of type I IFNs. In this setting, the only source of type I IFNs comes prior to infection as part of the constitutive IFN response observed in cancer cells. Despite the absence of virus-induced IFN, we still observed more VSV∆51 infection in TRAMP-C2 compared with TRAMP-C2-Cd274−/− cells (Fig. 3 D), suggesting that PD-L1 poises cancer cells to be more amenable to oncolysis.
We next investigated the source of type I IFNs in TRAMP-C2 cells prior to infection. In cancer cells, type I IFNs can be generated downstream of the DNA-sensing cyclic GMP/AMP synthase/stimulator of IFN genes (cGAS/STING) pathway, which is activated in response to cytosolic DNA (Guan et al., 2021; Lu et al., 2021). Indeed, we were able to detect cytosolic DNA in TRAMP-C2 cells without a significant difference between cells expressing or not PD-L1 (Fig. 4 A). To determine if cytosolic DNA triggered cGAS/STING activation, therefore promoting a type I IFN response, we used small-molecule inhibitors of cGAS or STING prior to analysis of type I IFN in the culture supernatant. Inhibition of either cGAS or STING ablated the constitutive type I IFN response (Fig. 4 B), suggesting that activation of this pathway in TRAMP-C2 cells results in a basal type I IFN response.
PD-L1 inhibits the IFN response induced by cGAS/STING. (A) Quantification of cytosolic DNA in uninfected TRAMP-C2 cells. N = 3 biological replicates. Statistical analysis by two-tailed unpaired Student’s t test. (B) Measurement of type I IFN in uninfected TRAMP-C2 and TRAMP-C2-Cd274−/− supernatant using the L929-ISRE reporter line following treatment with the cGAS inhibitor RU.521 (10 µM) or STING inhibitor H-151 (10 µM) for 72 h. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (C) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were infected with VSVΔ51-YFP at MOI 0.1 following treatment with the cGAS inhibitor RU.521 (10 µM) or STING inhibitor H-151 (10 µM) for 72 h. 24 h later, cells were analyzed by flow cytometry. The experiment depicted is representative of three performed with similar results. Fold-change in average infection between TRAMP-C2 and TRAMP-C2-Cd274−/− cells presented below graph. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. For all panels: **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
PD-L1 inhibits the IFN response induced by cGAS/STING. (A) Quantification of cytosolic DNA in uninfected TRAMP-C2 cells. N = 3 biological replicates. Statistical analysis by two-tailed unpaired Student’s t test. (B) Measurement of type I IFN in uninfected TRAMP-C2 and TRAMP-C2-Cd274−/− supernatant using the L929-ISRE reporter line following treatment with the cGAS inhibitor RU.521 (10 µM) or STING inhibitor H-151 (10 µM) for 72 h. The experiment depicted is representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (C) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were infected with VSVΔ51-YFP at MOI 0.1 following treatment with the cGAS inhibitor RU.521 (10 µM) or STING inhibitor H-151 (10 µM) for 72 h. 24 h later, cells were analyzed by flow cytometry. The experiment depicted is representative of three performed with similar results. Fold-change in average infection between TRAMP-C2 and TRAMP-C2-Cd274−/− cells presented below graph. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. For all panels: **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
We, therefore, hypothesized that pretreatment with cGAS/STING inhibitors would ablate PD-L1 function since the constitutive type I IFN response is normalized between TRAMP-C2 and TRAMP-C2-Cd274−/− cells. Consistent with our hypothesis, treatment with cGAS or STING inhibitors partially or fully ablated differences in VSV∆51 infection between TRAMP-C2 and TRAMP-C2-Cd274−/− cells (Fig. 4 C). In all, these data suggest that PD-L1 regulates the type I IFN response downstream of a constitutive cGAS/STING activation in these cells, without in itself regulating cytosolic DNA content or cGAS/STING activation.
PD-L1 promotes a metabolic shift in cancer cells resembling the Warburg effect
To better understand the mechanisms underlying inhibition of type I IFNs by PD-L1, we performed RNA sequencing (RNA-seq) on TRAMP-C2 and TRAMP-C2-Cd274−/− cells, both before and after infection with VSV∆51. We observed 2,690 and 5,486 differentially expressed genes in mock and infected samples respectively (false discovery rate <0.05), confirming that PD-L1 regulated cellular pathways before infection (Fig. S3 A). To investigate the involvement of these pathways in the function of PD-L1, we experimentally activated or inhibited some of them and examined oncolytic viral infection in the presence and absence of PD-L1. TGF-β, EGFR, and estrogen/androgen pathways were not found to be involved in the ability of PD-L1 to promote OV infection in cancer cells, as the phenotype was not lost upon experimental manipulation (Fig. S3, B–D).
TGF-β, EGFR, and androgen/estrogen pathways are not involved in PD-L1’s promotion of VSVΔ51 infection. (A) PROGENy pathway analysis of bulk RNA-seq performed on TRAMP-C2 and TRAMP-C2-Cd274−/− either mock-infected or infected with VSVΔ51-YFP at MOI 0.1 for 8 h (three biological replicates per condition). (B and C) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were pretreated with the TGF-βRI inhibitor SB431452 for 24 h (B) or the EGFR inhibitor afatinib for 6 h (C) at indicated concentrations (or DMSO as vehicle control), followed by infection with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry for viral YFP reporter expression. Experiments depicted are representative of two with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (D) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were cultured in phenol red-free DMEM supplemented with 10% charcoal-stripped serum (CSS) for 48 h prior to treatment with estradiol (E2) or DHT for 24 h. Following that, cells were infected with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry for viral YFP reporter expression. The experiments depicted are representative of two with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. For all panels, ****P < 0.0001; ***P < 0.001; **P < 0.01.
TGF-β, EGFR, and androgen/estrogen pathways are not involved in PD-L1’s promotion of VSVΔ51 infection. (A) PROGENy pathway analysis of bulk RNA-seq performed on TRAMP-C2 and TRAMP-C2-Cd274−/− either mock-infected or infected with VSVΔ51-YFP at MOI 0.1 for 8 h (three biological replicates per condition). (B and C) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were pretreated with the TGF-βRI inhibitor SB431452 for 24 h (B) or the EGFR inhibitor afatinib for 6 h (C) at indicated concentrations (or DMSO as vehicle control), followed by infection with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry for viral YFP reporter expression. Experiments depicted are representative of two with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (D) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were cultured in phenol red-free DMEM supplemented with 10% charcoal-stripped serum (CSS) for 48 h prior to treatment with estradiol (E2) or DHT for 24 h. Following that, cells were infected with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry for viral YFP reporter expression. The experiments depicted are representative of two with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. For all panels, ****P < 0.0001; ***P < 0.001; **P < 0.01.
Analysis of the differentially expressed genes showed that many metabolic enzymes were less abundantly expressed in PD-L1–deficient cells, resulting in a decreased glycolysis gene set score compared with parental TRAMP-C2 cells (Fig. 5 A), which was reflected in the differential Hypoxia pathway activity in the PROGENy (pathway responsive genes for activity inference) analysis (Fig. S3 A). We observed increased expression of most glycolysis enzymes in TRAMP-C2 compared with TRAMP-C2-Cd274−/− cells (Fig. S4 A), suggesting regulation of glycolysis by PD-L1. In corroboration to this hypothesis, we found that PD-L1–deficient cells had decreased glycolysis (Fig. 5 B) and increased oxidative phosphorylation (OXPHOS) (Fig. 5 C). Both phenotypes were fully rescued by re-expression of PD-L1 in TRAMP-C2-Cd274−/− cells (Fig. 5. D and E). Accordingly, bioenergetic calculations (Mookerjee et al., 2018) show that PD-L1 reduced ATP production from OXPHOS while increasing ATP production from glycolysis (Fig. 5, F and G). Many key parameters of mitochondrial respiration were reduced in PD-L1–expressing cells (Fig. S4 B), which also had reduced mitochondrial content (Fig. S4, C and D), without impacting mitochondrial ROS (Fig. S4 E). Increased glycolytic rates in parental TRAMP-C2 cells were also confirmed by untargeted metabolomics. We quantified ∼100 water-soluble metabolites on TRAMP-C2 and TRAMP-C2-Cd274−/− in the absence of infection (Table S1). The abundance of 52 metabolites was statistically different between parental and PD-L1–deficient cells (Fig. 5 H) including the glycolysis metabolites fructose-1,6-biphosphate, dihydroxyacetone phosphate, and pyruvate, which are key indicators of glycolysis rates (Tanner et al., 2018) (Fig. S4 F).
PD-L1 promotes Warburg metabolism in TRAMP-C2 cells. (A) Scoring for expression of genes included in the glycolysis gene set. RNA-seq dataset performed on TRAMP-C2 and TRAMP-C2-Cd274−/− either mock-infected or infected with VSVΔ51-YFP at MOI 0.1 for 8 h (three biological replicates per condition). (B–E) Seahorse metabolic flux analysis on TRAMP-C2, TRAMP-C2-Cd274−/−, TRAMP-C2-Cd274−/− + empty vector (EV), and TRAMP-C2-Cd274−/− + PD-L1 (tested in n = 6 technical replicates). ECAR and OCR are measures of glycolysis and mitochondrial respiration, respectively. The images depicted are representative of three experiments with similar results. (F and G) Bioenergetic calculations based on Seahorse metabolic flux assay. N = 3 biological replicates. (H) Hierarchical clustered heat map depicting the relative abundance of 50 metabolites quantified in untargeted metabolomics of TRAMP-C2 and TRAMP-C2-Cd274−/− cells. Six biological replicates per cell line are shown. (I and J) Glucose uptake was measured in TRAMP-C2 and TRAMP-C2-Cd274−/− cells (I), as well as CD80+ versus CD80− cells FACS-isolated from TRAMP-C2 or TRAMP-C2-Cd274−/− cells (J). The experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (K) Male NCG mice were implanted with subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors. The SUV of [18F]-FDG was assessed by PET imaging. The two mice shown are representative of five to six analyzed. For all panels: **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
PD-L1 promotes Warburg metabolism in TRAMP-C2 cells. (A) Scoring for expression of genes included in the glycolysis gene set. RNA-seq dataset performed on TRAMP-C2 and TRAMP-C2-Cd274−/− either mock-infected or infected with VSVΔ51-YFP at MOI 0.1 for 8 h (three biological replicates per condition). (B–E) Seahorse metabolic flux analysis on TRAMP-C2, TRAMP-C2-Cd274−/−, TRAMP-C2-Cd274−/− + empty vector (EV), and TRAMP-C2-Cd274−/− + PD-L1 (tested in n = 6 technical replicates). ECAR and OCR are measures of glycolysis and mitochondrial respiration, respectively. The images depicted are representative of three experiments with similar results. (F and G) Bioenergetic calculations based on Seahorse metabolic flux assay. N = 3 biological replicates. (H) Hierarchical clustered heat map depicting the relative abundance of 50 metabolites quantified in untargeted metabolomics of TRAMP-C2 and TRAMP-C2-Cd274−/− cells. Six biological replicates per cell line are shown. (I and J) Glucose uptake was measured in TRAMP-C2 and TRAMP-C2-Cd274−/− cells (I), as well as CD80+ versus CD80− cells FACS-isolated from TRAMP-C2 or TRAMP-C2-Cd274−/− cells (J). The experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (K) Male NCG mice were implanted with subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors. The SUV of [18F]-FDG was assessed by PET imaging. The two mice shown are representative of five to six analyzed. For all panels: **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
PD-L1 promotes Warburg metabolism in TRAMP-C2 cells. (A) Transcripts per million (TPM) for various glycolysis and glucose metabolism enzymes/transporters, from bulk RNA-seq experiment. (B) Cellular respiration parameters calculated using Seahorse mitochondrial stress test data. N = 3 biological replicates are shown. For each replicate, data were normalized to TRAMP-C2 values. Statistical analysis by two-tailed unpaired Student’s t test. (C) Relative quantification of mitochondrial DNA (mtDNA) by qPCR (normalized to nuclear DNA [nDNA] in each sample) by qPCR analysis. N = 3 biological replicates. Statistical analysis by two-tailed unpaired Student’s t test. (D and E) Mean fluorescence intensity (MFI) of MitoTracker and MitoSOX dyes by flow cytometry for TRAMP-C2, TRAMP-C2-Cd274−/−, TRAMP-C2-Cd274−/− + empty vector (EV), and TRAMP-C2-Cd274−/− + PD-L1 cells. MitoSOX MFI was corrected for total mitochondrial content by normalizing to MitoTracker MFI. Statistical analysis by one-way ANOVA with Šídák’s correction for multiple comparisons. (F) Relative abundance of glycolysis metabolites in TRAMP-C2 and TRAMP-C2-Cd274−/− cells, measured in the untargeted metabolomics study. Six biological replicates per cell line. Statistical analysis by unpaired two-tailed Student’s t test. (G) Male NCG mice were implanted with subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors. Tumor growth was assessed over time and tumor mass was measured at the endpoint. N = 5–6 per group. (H) Male NCG mice were implanted with subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors. The SUV of (18F)-FDG was assessed by PET imaging. Statistical analysis by two-tailed unpaired Student’s t test. For all panels: ***P < 0.001; **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
PD-L1 promotes Warburg metabolism in TRAMP-C2 cells. (A) Transcripts per million (TPM) for various glycolysis and glucose metabolism enzymes/transporters, from bulk RNA-seq experiment. (B) Cellular respiration parameters calculated using Seahorse mitochondrial stress test data. N = 3 biological replicates are shown. For each replicate, data were normalized to TRAMP-C2 values. Statistical analysis by two-tailed unpaired Student’s t test. (C) Relative quantification of mitochondrial DNA (mtDNA) by qPCR (normalized to nuclear DNA [nDNA] in each sample) by qPCR analysis. N = 3 biological replicates. Statistical analysis by two-tailed unpaired Student’s t test. (D and E) Mean fluorescence intensity (MFI) of MitoTracker and MitoSOX dyes by flow cytometry for TRAMP-C2, TRAMP-C2-Cd274−/−, TRAMP-C2-Cd274−/− + empty vector (EV), and TRAMP-C2-Cd274−/− + PD-L1 cells. MitoSOX MFI was corrected for total mitochondrial content by normalizing to MitoTracker MFI. Statistical analysis by one-way ANOVA with Šídák’s correction for multiple comparisons. (F) Relative abundance of glycolysis metabolites in TRAMP-C2 and TRAMP-C2-Cd274−/− cells, measured in the untargeted metabolomics study. Six biological replicates per cell line. Statistical analysis by unpaired two-tailed Student’s t test. (G) Male NCG mice were implanted with subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors. Tumor growth was assessed over time and tumor mass was measured at the endpoint. N = 5–6 per group. (H) Male NCG mice were implanted with subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors. The SUV of (18F)-FDG was assessed by PET imaging. Statistical analysis by two-tailed unpaired Student’s t test. For all panels: ***P < 0.001; **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
Not only were PD-L1–expressing cells more glycolytically active but they also presented an enhanced rate of in vitro glucose uptake (Fig. 5 I), which was dependent on PD-L1 engagement by CD80 (Fig. 5 J). The same phenotype was conserved in vivo, as determined in subcutaneous TRAMP-C2 and TRAMP-C2-Cd274−/− tumors established in immunodeficient NOD-Prkdcem26Cd52Il2rgem26Cd22/NjuCrl (NCG) mice subjected to positron emission tomography (PET) scanning with the radiolabeled glucose analog [18F]-fluorodeoxyglucose (FDG). In this model, any differences would be driven by PD-L1 activity on cancer cells, rather than immune-dependent or PD-1–dependent mechanisms. Parental and PD-L1–deficient tumors grew at similar rates in NCG mice (Fig. S4 G). Consistent with our in vitro data, parental TRAMP-C2 tumors had enhanced rates of [18F]-FDG uptake compared with PD-L1–deficient tumors (Fig. 5 K and Fig. S4 H). Higher rates of glycolysis and glucose uptake, along with increased reliance on glycolysis for ATP generation, are highly consistent with the Warburg effect, where cancer cells preferentially use glycolysis over OXPHOS to meet bioenergetic demands and generate other metabolites (DeBerardinis and Chandel, 2020).
PD-L1 promotes glycolysis and inhibits IFN responses in human cancer cells
If PD-L1 regulation of type I IFN is a well-conserved feature, we expect our findings to be replicated in other cancer cell lines with similar features, in particular the metabolic characteristics of TRAMP-C2 cells. To test this hypothesis, we made use of an RNA-seq dataset of 675 human cancer cell lines (Klijn et al., 2015) and scored each of those cell lines for their expression of PD-L1 (CD274) and expression of genes in the glycolysis gene set, which includes glycolysis and other metabolic enzymes (Fig. 6 A). We hypothesized that in cell lines with high PD-L1 expression and high score for the glycolysis gene set, PD-L1 would promote glycolysis, inhibit type I IFN responses, and make tumor cells more susceptible to OVs. From this analysis, we chose two readily available cell lines: the renal cell carcinoma line 786-0 and the gastric carcinoma line Hs746, both with high glycolysis gene set scores, but with different levels of PD-L1. In both cell lines, we deleted PD-L1 using CRISPR/Cas9 (Fig. S5 A) and subjected parental and CD274−/− cells to VSV∆51 infection. In both 786-0 and Hs746 cells, PD-L1 deletion resulted in resistance to OV infection (Fig. 6, B and C), consistent with our hypothesis and the data in the TRAMP-C2 model. Furthermore, PD-L1 inhibited the constitutive and virus-induced IFN response, as well as signaling downstream of the type I IFN receptor in both cell lines (Fig. 6, D and E; and Fig. S5, B and C).
PD-L1 inhibits type I IFN responses in human cancer cells. (A) Bioinformatic analysis of publicly available RNA-seq from 675 human cancer cell lines, scored for their expression of PD-L1 (CD274) and glycolysis gene signatures. Blue lines on the plot indicate mean + 2 SD; Pearson correlation coefficient is indicated. (B and C) 786-0 and Hs746 (WT and CD274−/−) infected with VSVΔ51-YFP at indicated MOIs for 24 h prior to analysis by flow cytometry. Experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (D and E) 786-0 and 786-0-CD274−/− cells (D) or Hs746 and Hs746-CD274−/− cells (E) infected with VSVΔ51-YFP at MOI 1 or 0.1, respectively (or mock-infected) prior to qPCR analysis for IFNB1 and MX1 transcripts. n = 3 biological replicates. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (F) Association between the expression of PD-L1 and Glycolysis-associated genes in the malignant cells of 266 tumors. Each point represents the average profile of malignant cells from scRNA-seq data sets, and dotted line represents mean PD-L1 expression of samples. Expression values reflect log-transformed gene counts per 10k transcripts and Glycolysis activity represents gene set scores from the associated MSigDB Hallmark gene set. Statistical significance assessed by Wilcoxon rank-sum test to compare glycolysis scores of tumors expressing PD-L1 above or below mean expression. NPC: nasopharyngeal cancer, PDAC: pancreatic ductal adenocarcinoma, SCC: squamous cell carcinoma. For all panels: ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
PD-L1 inhibits type I IFN responses in human cancer cells. (A) Bioinformatic analysis of publicly available RNA-seq from 675 human cancer cell lines, scored for their expression of PD-L1 (CD274) and glycolysis gene signatures. Blue lines on the plot indicate mean + 2 SD; Pearson correlation coefficient is indicated. (B and C) 786-0 and Hs746 (WT and CD274−/−) infected with VSVΔ51-YFP at indicated MOIs for 24 h prior to analysis by flow cytometry. Experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (D and E) 786-0 and 786-0-CD274−/− cells (D) or Hs746 and Hs746-CD274−/− cells (E) infected with VSVΔ51-YFP at MOI 1 or 0.1, respectively (or mock-infected) prior to qPCR analysis for IFNB1 and MX1 transcripts. n = 3 biological replicates. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (F) Association between the expression of PD-L1 and Glycolysis-associated genes in the malignant cells of 266 tumors. Each point represents the average profile of malignant cells from scRNA-seq data sets, and dotted line represents mean PD-L1 expression of samples. Expression values reflect log-transformed gene counts per 10k transcripts and Glycolysis activity represents gene set scores from the associated MSigDB Hallmark gene set. Statistical significance assessed by Wilcoxon rank-sum test to compare glycolysis scores of tumors expressing PD-L1 above or below mean expression. NPC: nasopharyngeal cancer, PDAC: pancreatic ductal adenocarcinoma, SCC: squamous cell carcinoma. For all panels: ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
PD-L1 alters type I IFN responses in human cancer cells. (A) 786-0 (left) and Hs746 (right) cells were electroporated with Cas9 and gRNA targeting PD-L1 to generate CD274−/− cells. Representative plots depicting PD-L1 expression are shown. (B) 786-0 and 786-0-CD274−/− cells infected with VSVΔ51-YFP at MOI 1 (or mock-infected) and analyzed by western blotting at indicated times after infection. Images depicted are representative of three with similar results. (C) Hs746 and Hs746-CD274−/− cells infected with VSVΔ51-YFP at MOI 0.1 (or mock-infected) for 16 h prior to western blotting analysis. Images depicted are representative of three with similar results. (D) Fluorescent imaging of tumor explant cores from patient biopsies infected with VSVΔ51-YFP for 48 h prior to imaging for viral YFP reporter. Images of poorly infected (left) and well-infected (right) tumors are depicted. Scale bar = 600 μm. (E) Patient tumor biopsies subjected to PD-L1 IHC to determine PD-L1 status of tumors. Images of PD-L1− (left) and PD-L1+ (right) tumors are depicted. Scale bar = 100 μm. (F and G) Average tumor infection plotted against viability (assessed by Alamar Blue) and necrosis (% of tumor section necrotic, assessed by histology). (H and I) Distribution of PD-L1+ and PD-L1− tumors and average tumor infection between male and female donors. Statistical analysis by two-tailed unpaired Student’s t test. P > 0.05 not significant (ns). Source data are available for this figure: SourceData FS5.
PD-L1 alters type I IFN responses in human cancer cells. (A) 786-0 (left) and Hs746 (right) cells were electroporated with Cas9 and gRNA targeting PD-L1 to generate CD274−/− cells. Representative plots depicting PD-L1 expression are shown. (B) 786-0 and 786-0-CD274−/− cells infected with VSVΔ51-YFP at MOI 1 (or mock-infected) and analyzed by western blotting at indicated times after infection. Images depicted are representative of three with similar results. (C) Hs746 and Hs746-CD274−/− cells infected with VSVΔ51-YFP at MOI 0.1 (or mock-infected) for 16 h prior to western blotting analysis. Images depicted are representative of three with similar results. (D) Fluorescent imaging of tumor explant cores from patient biopsies infected with VSVΔ51-YFP for 48 h prior to imaging for viral YFP reporter. Images of poorly infected (left) and well-infected (right) tumors are depicted. Scale bar = 600 μm. (E) Patient tumor biopsies subjected to PD-L1 IHC to determine PD-L1 status of tumors. Images of PD-L1− (left) and PD-L1+ (right) tumors are depicted. Scale bar = 100 μm. (F and G) Average tumor infection plotted against viability (assessed by Alamar Blue) and necrosis (% of tumor section necrotic, assessed by histology). (H and I) Distribution of PD-L1+ and PD-L1− tumors and average tumor infection between male and female donors. Statistical analysis by two-tailed unpaired Student’s t test. P > 0.05 not significant (ns). Source data are available for this figure: SourceData FS5.
To test if the link between PD-L1 expression and glycolysis in cancer cells held true in primary human samples, we took advantage of a single-cell RNA-seq (scRNA-seq) dataset of 266 human tumors, spanning eight types of cancer (Table S2) (Cook and Vanderhyden, 2022). We scored each tumor for expression of PD-L1 and genes included in the glycolysis gene set. When we correlated the glycolysis score to PD-L1 expression at the single-cell level in these cancers, we observed that tumors with high expression of PD-L1 had significantly higher glycolysis gene scores, in line with our hypothesis that PD-L1 drives glycolytic metabolism in cancer cells (Fig. 6 F). Therefore, the effect of PD-L1 on OV infection, type I IFN responses, and cellular metabolism is not unique to the TRAMP-C2 model and is conserved in other human cancer cell types.
PD-L1 inhibits type I IFN via lactate dynamics
Metabolic alterations are now known to control inflammatory pathways, including type I IFN (Ahmed and Cassol, 2017), e.g., glycolytic enzymes and metabolites are key regulators of inflammatory cytokines and anti-viral defenses (Burke et al., 2014; Jiang et al., 2016; Shirai et al., 2016; Wang et al., 2014). In line with this literature and considering our data, we reasoned that PD-L1 inhibition of type I IFN responses was linked with its ability to promote glycolysis. Recent research has mechanistically linked lactate to regulation of the type I IFN response (Zhang et al., 2019b). Lactate is an important metabolite generated during Warburg metabolism, whose physiological role is now beginning to be uncovered (Rabinowitz and Enerbäck, 2020). Given its emerging role as a regulator of inflammatory responses, we hypothesized that lactate was responsible for the ability of PD-L1 to inhibit type I IFNs and promote virus infection.
In corroboration to our hypothesis, lactate was more abundantly produced in parental over PD-L1–deficient cancer cell lines (Fig. 7 A). To directly test the role of lactate in PD-L1–driven inhibition of type I IFN responses, we pharmacologically perturbed lactate abundance prior to VSV∆51 infection. First, to suppress lactate production, we used sodium oxamate and GNE-140, two structurally distinct inhibitors of the enzymes responsible for conversion of pyruvate into lactate: lactate dehydrogenases. Both inhibitors blocked the ability of PD-L1 to enhance virus infection (Fig. 7, B and C). On the other hand, treatment with lactate increased the permissiveness of PD-L1–deficient cells to OV infection, phenocopying the effect of PD-L1 (Fig. 7, D and E). Further, boosting glycolysis and lactate production through treatment with the ATP synthase inhibitor oligomycin also mimicked the effect of PD-L1 on VSV∆51 infection (Fig. 7 F). We observed changes in susceptibility to OVs when tampering with lactate abundance not only in TRAMP-C2 cells (Fig. 7, A–F) but also in 786-0 and Hs746 cells (Fig. 7, G–J). Taken together, these experiments highlight the key role of lactate in promoting PD-L1–driven permissiveness to OV infection.
PD-L1 inhibits type I IFN via lactate dynamics. (A) Lactate quantification in TRAMP-C2, 786-0, or Hs746 culture supernatant. n = 3 biological replicates. Statistical analysis by two-tailed unpaired Student’s t test. (B–F) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were pre-treated with oxamate at 10 mM (B); GNE-140 at 10 µM (C); lactate at 5 mM (D); oligomycin at 0.2 µM (F) for 24 h, followed by infection with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry or Alamar Blue (E). Experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (G and H) 786-0 and 786-0-CD274−/− cells were pretreated with GNE-140 at 10 µM (G) or lactate at 5 mM (H) for 24 h, followed by infection with VSVΔ51-YFP at MOI 1 for 24 h prior to analysis by flow cytometry. The experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (I and J) Hs746 and Hs746-CD274−/− cells were pre-treated with GNE-140 at 10 µM (I) or lactate at 5 mM (J) for 24 h, followed by infection with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry. Experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (K and L) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were pretreated with lactate at 5 mM for 24 h, followed by infection with VSVΔ51-YFP at MOI 0.1 for 8 h prior to qPCR analysis for Ifnb1 and Mx1. n = 3 biological replicates. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (M) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were pretreated with lactate at 10 mM for 24 h, followed by transfection with poly(I:C) for 6 h prior to western blotting analysis. The images depicted are representative of three with similar results. For all panels: ****P < 0.0001; **P < 0.01; P > 0.05 not significant (ns). Source data are available for this figure: SourceData F7.
PD-L1 inhibits type I IFN via lactate dynamics. (A) Lactate quantification in TRAMP-C2, 786-0, or Hs746 culture supernatant. n = 3 biological replicates. Statistical analysis by two-tailed unpaired Student’s t test. (B–F) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were pre-treated with oxamate at 10 mM (B); GNE-140 at 10 µM (C); lactate at 5 mM (D); oligomycin at 0.2 µM (F) for 24 h, followed by infection with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry or Alamar Blue (E). Experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (G and H) 786-0 and 786-0-CD274−/− cells were pretreated with GNE-140 at 10 µM (G) or lactate at 5 mM (H) for 24 h, followed by infection with VSVΔ51-YFP at MOI 1 for 24 h prior to analysis by flow cytometry. The experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (I and J) Hs746 and Hs746-CD274−/− cells were pre-treated with GNE-140 at 10 µM (I) or lactate at 5 mM (J) for 24 h, followed by infection with VSVΔ51-YFP at MOI 0.1 for 24 h prior to analysis by flow cytometry. Experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (K and L) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were pretreated with lactate at 5 mM for 24 h, followed by infection with VSVΔ51-YFP at MOI 0.1 for 8 h prior to qPCR analysis for Ifnb1 and Mx1. n = 3 biological replicates. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (M) TRAMP-C2 and TRAMP-C2-Cd274−/− cells were pretreated with lactate at 10 mM for 24 h, followed by transfection with poly(I:C) for 6 h prior to western blotting analysis. The images depicted are representative of three with similar results. For all panels: ****P < 0.0001; **P < 0.01; P > 0.05 not significant (ns). Source data are available for this figure: SourceData F7.
To associate the PD-L1–mediated metabolic switch with the type I IFN response, we examined IFN-β induction and IFN receptor signaling following treatment with lactate. Lactate treatment resulted in normalization of the type I IFN response between parental and PD-L1–deficient TRAMP-C2 cells (Fig. 7, K–M), indicating that lactate is responsible for PD-L1–mediated regulation of type I IFNs.
PD-L1 promotes OV infection in vivo
To determine if PD-L1 retains its ability to enhance OV infection in the more complex tumor microenvironment, we investigated whether PD-L1 promoted cancer cell infection in vivo. We established subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors in immunodeficient NCG mice, and when tumors reached ∼750 mm3, we injected them with VSV∆51 (expressing a luciferase reporter). After 24 h we assessed viral infection by in vivo imaging and plaque assays. In corroboration of our in vitro studies, PD-L1–deficient tumors presented reduced infection compared to parental tumors (Fig. 8, A and B), indicating that PD-L1 expression on tumor cells drives increased OV infection in vivo. As expected, tumor size was unchanged 24 h after VSVΔ51 treatment when the viral titer was assessed (Fig. 8 C). Differences in viral replication translated to increased therapeutic effect in mice carrying parental TRAMP-C2 tumors where a more significant control of tumor growth following oncolytic virotherapy was observed (Fig. 8 D).
PD-L1 promotes VSVΔ51 infection in vivo. (A–C) Male NCG mice were implanted with subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− cells and injected intratumorally with 108 PFU of VSVΔ51 expressing a luciferase reporter. 24 h after infection, mice were injected subjected to bioluminescence imaging and tumors were weighed and then homogenized to quantify viral titers by plaque assay. The bioluminescence images are representative of two experiments with similar results. Statistical analysis by unpaired two-tailed Student’s t test. (D) Subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors (150 mm3) in male NCG mice were injected twice intratumorally with 108 PFU of VSVΔ51, and tumor growth was assessed over time. Data analyzed by two-way ANOVA. N = 7–8 per group; the experiment depicted is representative of two performed with similar results. (E and F) 786-0 (E) and Hs746 (F) cells were treated with 5 μg of atezolizumab for 24 h prior to VSVΔ51-YFP infection at MOI 1 or 0.1, respectively. 24 h after infection, cells were analyzed by flow cytometry to quantify the viral YFP reporter. Alternatively, type I IFN expression was analyzed by qPCR at 2 h after infection (786-0) or 16 h after infection (Hs746). Experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. RQ, relative quantity. (G) PD-L1 tumor status (where PD-L1+ tumors are defined as >1% PD-L1+ tumor/immune cells) plotted against average tumor infection (percentage of tumor explant area that is YFP+). Statistical analysis by two-tailed unpaired Student’s t test. For all panels: ****P < 0.0001; **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
PD-L1 promotes VSVΔ51 infection in vivo. (A–C) Male NCG mice were implanted with subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− cells and injected intratumorally with 108 PFU of VSVΔ51 expressing a luciferase reporter. 24 h after infection, mice were injected subjected to bioluminescence imaging and tumors were weighed and then homogenized to quantify viral titers by plaque assay. The bioluminescence images are representative of two experiments with similar results. Statistical analysis by unpaired two-tailed Student’s t test. (D) Subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors (150 mm3) in male NCG mice were injected twice intratumorally with 108 PFU of VSVΔ51, and tumor growth was assessed over time. Data analyzed by two-way ANOVA. N = 7–8 per group; the experiment depicted is representative of two performed with similar results. (E and F) 786-0 (E) and Hs746 (F) cells were treated with 5 μg of atezolizumab for 24 h prior to VSVΔ51-YFP infection at MOI 1 or 0.1, respectively. 24 h after infection, cells were analyzed by flow cytometry to quantify the viral YFP reporter. Alternatively, type I IFN expression was analyzed by qPCR at 2 h after infection (786-0) or 16 h after infection (Hs746). Experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. RQ, relative quantity. (G) PD-L1 tumor status (where PD-L1+ tumors are defined as >1% PD-L1+ tumor/immune cells) plotted against average tumor infection (percentage of tumor explant area that is YFP+). Statistical analysis by two-tailed unpaired Student’s t test. For all panels: ****P < 0.0001; **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).
We next investigated if atezolizumab, the clinically approved PD-L1 antibody used in checkpoint inhibition immunotherapy, triggered PD-L1 function and enhanced OV infection. Pretreatment of the 786-0 and Hs746 cells with atezolizumab significantly enhanced OV infection and inhibited the type I IFN response compared with the isotype control (Fig. 8, E and F); as expected, atezolizumab treatment had no impact on OV infection in PD-L1–deficient cells.
Lastly, we asked if PD-L1 favored OV infection in primary human cancer reasoning that PD-L1+ tumors should have higher rates of infection based on the totality of the data presented thus far. We obtained fresh tumor biopsies and subjected them to both (i) ex vivo VSVΔ51-YFP infection for 48 h (Fig. S5 D) and (ii) PD-L1 immunohistochemistry to determine the PD-L1 status of tumors (PD-L1+ tumors are defined as ≥1% of tumor/immune cells staining for PD-L1, in accordance with clinical protocols) (Fig. S5 E). In a cohort of 21 patient tumors (Table S3), PD-L1+ tumor explants were significantly better infected compared with PD-L1− explants (Fig. 8 G). Importantly, infection did not correlate with preinfection tumor viability (Fig. S5 F) nor degree of biopsy necrosis (Fig. S5 G), suggesting that this analysis was not confounded by tissue viability. Tumors derived from male and female patients were equally represented in terms of PD-L1 status (Fig. S5 H) and infection levels (Fig. S5 I). Therefore, in a cohort of tumors of diverse origins and treatment history, PD-L1 marked tumors that were more likely to be infected by VSVΔ51, corroborating our results in human tumors, and revealing an unappreciated role of PD-L1 as an OV infectivity biomarker.
Discussion
Here, we show that PD-L1 inhibits the type I IFN response and enhances OV infection via a proglycolytic shift in cancer cells resembling Warburg metabolism. The requirement for engagement by an extracellular binding partner strongly suggests that this function is mediated by some signaling capacity of PD-L1. While the idea of PD-L1 “reverse signaling” has quickly gained traction, there is still a lack of data toward understanding if PD-L1 functions require crosslinking or conformational changes triggered by other proteins. Our data showing that CD80, a PD-1 fusion protein, or monoclonal antibodies (including the clinically approved therapeutic atezolizumab) boost PD-L1 function suggests that PD-L1 needs to be engaged to mediate its cell-intrinsic functions.
PD-L1 has previously been shown to modulate type I IFNs with biochemical, transcriptomic, and bioinformatic approaches. Surprisingly, some of these early studies show that PD-L1 inhibits type I IFN responses, while others showed promotion of type I IFN responses (Cheon et al., 2021; Diskin et al., 2020; Gao et al., 2020). Overall, the mechanisms underlying this context specificity of PD-L1 function are unknown. It is possible that PD-L1 signals differently depending on the cell type. What may be underpinning these signaling differences is the extensive glycosylation of PD-L1, which is responsible for ∼50% of its observed molecular weight. Different cancer cell types/lines express different PD-L1 glycoforms (Lee et al., 2019), and differential glycosylation influences PD-L1 interactions (Li et al., 2016), and, potentially, its downstream signaling. Our discovery that PD-L1 promotes glycolysis creates an intriguing link with PD-L1 glycosylation, which warrants further investigation.
Mechanistically, we found that PD-L1 regulates type I IFNs by promoting Warburg metabolism. Strengthening our finding, previous work has suggested a link between PD-L1 expression and metabolism. For example, a correlation was found between tumor PD-L1 expression and PET signal in different cancer types (Kaira et al., 2021), similar to what we observed in vivo. Additionally, PD-L1 was shown to influence aerobic glycolysis and other metabolic pathways in cancer cells (Chang et al., 2015; Garige et al., 2022; Pacheco-Torres et al., 2021). The biological consequences of PD-L1–mediated metabolic shifts remain understudied, particularly given the fact that PD-L1 is expressed on cells with metabolic functions, such as pancreatic islet cells (Keir et al., 2006), warranting more work investigating the impact of metabolic regulation by PD-L1. We have linked PD-L1 regulation of type I IFN to lactate produced during Warburg metabolism. Lactate is no longer considered simply a waste product of glycolysis and is now known to be involved in the regulation of key oncogenes and tumor suppressor genes (San-Millán et al., 2020) as well as inflammation (Haas et al., 2015), and a novel posttranslational modification (lactylation) involving addition of lactate to lysine and phenylalanine residues has been described (Xin et al., 2022; Zhang et al., 2019a).
Certainly, our data shed new light on the combination of PD-L1 blockade with OVs. The rationale behind this combination lies in the fact that OV infection leads to upregulation of PD-L1 in many tumor models (Annels et al., 2020; Zamarin et al., 2018), and therefore blocking PD-L1 will unleash the full range of antitumor immunity induced by OVs. Indeed, OVs combined with anti-PD-1/PD-L1 led to improvements in tumor immune infiltrate and the activation status of immune cells (Bourgeois-Daigneault et al., 2018; Panagioti et al., 2021; Zamarin et al., 2018). At the same time, it is now reasonable to test if anti-PD-L1 triggers the ability of PD-L1 to enhance OV infection in tumors, independent of its effect on antitumor immunity. In accordance with our in vitro and in vivo data, in a small cohort of cancer patients, we found that PD-L1 expression predicted susceptibility to OV infection, with only one PD-L1− tumor well-infected ex vivo. Since PD-L1 in tumors is regularly measured in clinical settings, it will be interesting to determine if this relationship between PD-L1 and OV infection holds true in future trials.
Lastly, from a clinical perspective, the fact that PD-L1 antibodies can trigger PD-L1 activity is highly relevant. It is tempting to speculate that these novel, cell-intrinsic functions of PD-L1 are being modulated in tumors of patients undergoing anti-PD-L1 therapy, and this may contribute to antitumor efficacy (or lack thereof) of these therapeutic agents. Investigation in appropriate murine models of cancer is needed to elucidate the role of cell-intrinsic PD-L1 function on checkpoint blockade efficacy.
Materials and methods
Cell lines
Cell lines were cultured at 37°C in a humidified atmosphere containing 5% CO2. TRAMP-C2 was maintained in DMEM supplemented with 5% FBS, 5% NuSerum, 0.005 mg/ml bovine insulin, 10 nM dehydroisoandrosterone, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 μg/ml gentamicin sulfate, and 20 mM HEPES. 786-0, Hs746, HEK293T, L929-ISRE, and Vero cells were maintained in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 μg/ml gentamicin sulfate, and 20 mM HEPES. Cells were regularly tested for mycoplasma using PCR protocol adapted from Young et al. (2010). 786-0 and Vero cells were a gift from Dr. John Bell (Ottawa Hospital Research Institute [OHRI]), Hs746 was purchased from ATCC, L929-ISRE generated by Dr. Bruce Beutler (UT Southwestern) was a gift from Dr. Subash Sad (University of Ottawa, Ottawa, Ontario, Canada), and HEK293T was a gift from Dr. Ian Lorimer (OHRI, Ottawa, Ontario, Canada).
Generation of cell line variants
Single-guide RNA targeting exon 3 of the Cd274 gene (sequence: 5′-GTATGGCAGCAACGTCACGA-3′) was cloned into the Cas9-expressing lentiCRISPR v2 vector according to lentiCRISPR cloning protocol from the Zhang lab; lentiCRISPR v2 was a gift from Feng Zhang (Addgene plasmid 52961; http://n2t.net/addgene:52961; RRID: Addgene_52961). TRAMP-C2 were transiently transfected with plasmid and subsequently treated with murine IFN-γ (Peprotech) to upregulate PD-L1 in all cells, except those with deletion of PD-L1. PD-L1− cells were isolated by FACS. To generate 786-0 and Hs746 CD274−/−, cells were electroporated with a ribonucleoprotein complex of ATTO550-labeled guide RNA (gRNA) (IDT; sequences: 5′-UGGCUGCACUAAUUGUCUAUGUUUUAGAGCUAUGCU-3′; 5′-AUUUACUGUCACGGUUCCCAGUUUUAGAGCUAUGCU-3′; 5′-AGCUACUAUGCUGAACCUUCGUUUUAGAGCUAUGCU-3′; 5′-UUGAAGGACCAGCUCUCCCUGUUUUAGAGCUAUGCU-3′) and recombinant Cas9 (IDT) using protocols modified from the Alt-R CRISPR-Cas9 system (IDT), and subsequently treated with human IFN-γ (Peprotech) to upregulate PD-L1 in all cells except those with deletion of PD-L1. PD-L1− cells were isolated by FACS.
To stably express PD-L1 or control vector in TRAMP-C2-Cd274−/−, the cDNA encoding full-length murine PD-L1 was cloned into the retroviral vector pQCXIN-IRES-Thy1.1, and the resulting pQCXIN-PD-L1-IRES-Thy1.1 plasmid was transfected into HEK293T cells along with pCMV-VSV-G (a gift from Bob Weinberg; Addgene plasmid 8454; http://n2t.net/addgene:8454; RRID:Addgene_8454) and pCL-Eco (a gift from Inder Verma; Addgene plasmid 12371; http://n2t.net/addgene:12371; RRID:Addgene_12371) to generate retrovirus. TRAMP-C2-Cd274−/− were infected with this retrovirus (or retrovirus encoding pQCXIN-IRES-Thy1.1 as empty vector control) supplemented with 8 µg/ml polybrene. Cells staining positively for PD-L1 and Thy1.1 (or Thy1.1 only for empty vector control) were isolated by FACS. To stably express CD80, cells were similarly transduced with retrovirus encoding pMSCV-CD80-IRES-mCherry (or pMSCV-IRES-mCherry empty vector as a control).
OV production and infection
VSVΔ51-YFP, VSV WT, VSVΔ51-firefly luciferase, and vaccinia virus were gifts from Dr. John Bell (OHRI). The original virus stock was propagated on Vero cells (at multiplicity of infection [MOI] 0.01) and cell supernatant was isolated 16–20 h later for concentration of virus by high-speed centrifugation. All subsequent virus stocks were generated from the original stock to avoid genetic drift. VSVΔ51 titers were quantified by plaque assay using methods previously described (Diallo et al., 2012).
TRAMP-C2, 786-0, and Hs746 were infected with VSVΔ51-YFP or VSV WT by first removing and washing out culture media with PBS and adding low volume of virus diluted in cold DMEM to MOIs ranging from 0.001 to 100. After incubation at 37°C + 5% CO2, supplemented growth media was added.
Alternatively, TRAMP-C2 were infected with GFP-expressing B19R− vaccinia virus (Copenhagen strain) at MOI 0.1 for 48 h.
In vitro treatments
Cells were treated with the following reagents at concentrations and times indicated in figure legends: recombinant murine IFN-β (PBL Assay Sciences), poly(I:C) (Invivogen), actinomycin D (Sigma-Aldrich), recombinant mouse PD-1-Fc chimeric protein (R&D Systems) or human IgG1 control (R&D Systems), afatinib (Selleck Chemicals), SB431542 (Selleck Chemicals), estradiol/E2 (Sigma-Aldrich), dihydrotestosterone/DHT (Sigma-Aldrich), RU.521 (Selleck Chemicals), H-151 (Selleck Chemicals), sodium oxamate (Selleck Chemicals), (R)-GNE-140 (Selleck Chemicals), sodium lactate (Sigma-Aldrich), or oligomycin A (Selleck Chemicals).
Coomassie staining
Culture media was removed and washed out with PBS and cells were fixed with 3:1 methanol:acetic acid solution for 1–3 h. Cells were then rinsed in tap water and stained with Coomassie Blue solution for 30 min and rinsed with tap water to remove excess dye.
Flow cytometry
Cells were briefly trypsinized, washed, and resuspended in PBS for staining. The cell suspension was stained with Zombie NIR Fixable Viability Dye or Zombie Aqua Fixable Viability Dye (BioLegend) to label dead cells, followed by incubation with rat anti-mouse CD16/CD32 (clone 2.4G2; BD Biosciences) to block FcγRII/III receptors. Cells were then washed and incubated with fluorescently labeled antibodies. Cells were washed and resuspended in PBS and analyzed using an LSRFortessa (BD Biosciences) or Celesta (BD Biosciences) or isolated using MoFlo XDP (Beckman Coulter) or MA900 (SONY). Alternatively, cells were treated with the fluorogenic Caspase-3/7 Red Detection Reagent (Thermo Fisher Scientific) as per the manufacturer’s instructions prior to flow cytometry analysis. Data were analyzed using FlowJo (Tree Star, Inc.).
For experiments with murine cells, the following antibodies were used: BV421-anti-PD-L1 (clone MIH5; BD), PE-Cy5-anti-CD80 (clone 16-10A1; BioLegend), PE-Cy7-anti-PD-1 (clone 29F.1A12; BioLegend), PE-anti-LDLR (clone 263123; R&D Systems). For experiments with human cells, BV421-anti-PD-L1 (clone MIH1; BD) was used.
RNA isolation, cDNA synthesis, and quantitative PCR (qPCR)
RNA was isolated using GenElute RNA Miniprep Kit (Sigma-Aldrich) as per the manufacturer’s protocol. cDNA was synthesized using iScript Reverse Transcription Supermix (Bio-Rad) as per the manufacturer’s protocol. qPCR was run using iTaq Universal SYBR Green Supermix (Bio-Rad) using primers listed in Table 1. qPCR data normalized to murine Gapdh gene or human 18S gene using the 2-∆∆Ct method.
Primers for qPCR
| VSV N | Forward | 5′-GATAGTACCGGAGGATTGACGACTA-3′ |
| Reverse | 5′-TCAAACCATCCGAGCCATTC-3′ | |
| Ifnb1 | Forward | 5′-CGCTGCGTTCCTGCTGTG-3′ |
| Reverse | 5′-GATCTTGAAGTCCGCCCTGTAG-3′ | |
| Mx1 | Forward | 5′-GACCATAGGGGTCTTGACCAA-3′ |
| Reverse | 5′-AGACTTGCTCTTTCTGAAAAGCC-3′ | |
| Oas1b | Forward | 5′-TTCTACGCCAATCTCATCAGTG-3′ |
| Reverse | 5′-GGTCCCCCAGCTTCTCCTTAC-3′ | |
| Eif2ak | Forward | 5′-ATGCACGGAGTAGCCATTACG-3′ |
| Reverse | 5′-TGACAATCCACCTTGTTTTCGT-3′ | |
| Isg15 | Forward | 5′-TGACGCAGACTGTAGACACG-3′ |
| Reverse | 5′-TGGGGCTTTAGGCCATACTC-3′ | |
| Gapdh | Forward | 5′-CATCACCATCTTCCAGGAGCG-3′ |
| Reverse | 5′-GAGGGGCCATCCACAGTCTTC-3′ | |
| MT-ND1 | Forward | 5′-AACATACCCATGGCCAACCT-3′ |
| Reverse | 5′-AGCGAAGGGTTGTAGTAGCCC-3′ | |
| IFNB1 | Forward | 5′-TCCAAATTGCTCTCCTGTTG-3′ |
| Reverse | 5′-GCAGTATTCAAGCCTCCCAT-3′ | |
| MX1 | Forward | 5′-CTGCGAGGAGATCGGTTCTG-3′ |
| Reverse | 5′-CTGCACCTCCTTGGAATGGT-3′ | |
| 18S | Forward | 5′-GTAACCCGTTGAACCCCATT-3′ |
| Reverse | 5′-CCATCCAATCGGTAGTAGCG-3′ |
| VSV N | Forward | 5′-GATAGTACCGGAGGATTGACGACTA-3′ |
| Reverse | 5′-TCAAACCATCCGAGCCATTC-3′ | |
| Ifnb1 | Forward | 5′-CGCTGCGTTCCTGCTGTG-3′ |
| Reverse | 5′-GATCTTGAAGTCCGCCCTGTAG-3′ | |
| Mx1 | Forward | 5′-GACCATAGGGGTCTTGACCAA-3′ |
| Reverse | 5′-AGACTTGCTCTTTCTGAAAAGCC-3′ | |
| Oas1b | Forward | 5′-TTCTACGCCAATCTCATCAGTG-3′ |
| Reverse | 5′-GGTCCCCCAGCTTCTCCTTAC-3′ | |
| Eif2ak | Forward | 5′-ATGCACGGAGTAGCCATTACG-3′ |
| Reverse | 5′-TGACAATCCACCTTGTTTTCGT-3′ | |
| Isg15 | Forward | 5′-TGACGCAGACTGTAGACACG-3′ |
| Reverse | 5′-TGGGGCTTTAGGCCATACTC-3′ | |
| Gapdh | Forward | 5′-CATCACCATCTTCCAGGAGCG-3′ |
| Reverse | 5′-GAGGGGCCATCCACAGTCTTC-3′ | |
| MT-ND1 | Forward | 5′-AACATACCCATGGCCAACCT-3′ |
| Reverse | 5′-AGCGAAGGGTTGTAGTAGCCC-3′ | |
| IFNB1 | Forward | 5′-TCCAAATTGCTCTCCTGTTG-3′ |
| Reverse | 5′-GCAGTATTCAAGCCTCCCAT-3′ | |
| MX1 | Forward | 5′-CTGCGAGGAGATCGGTTCTG-3′ |
| Reverse | 5′-CTGCACCTCCTTGGAATGGT-3′ | |
| 18S | Forward | 5′-GTAACCCGTTGAACCCCATT-3′ |
| Reverse | 5′-CCATCCAATCGGTAGTAGCG-3′ |
ELISA
IFN-β was analyzed in culture supernatant following VSVΔ51-YFP/VSV WT infection or transfection with poly(I:C) (Invivogen) using Mouse IFN-β Quantikine ELISA Kit (R&D Systems), as per manufacturer’s instructions.
Western blotting
Following infection, cells were lysed in radioimmunoprecipitation assay supplemented with cOmplete protease inhibitor cocktail (Roche) and PhosSTOP phosphatase inhibitor cocktail (Roche). Protein concentration was quantified by BCA Assay using MicroBCA Protein Assay Kit (Thermo Fisher Scientific), and samples were denatured in Laemmli buffer (Bio-Rad) supplemented with 5% β-mercaptoethanol. Proteins were separated on 8–12% polyacrylamide (acrylamide/bis-acrylamide 37.5:1; Bio-Rad) gel at 60 mA and transferred to a polyvinylidene diflouride membrane for 90 min at 100 V. Membranes were probed with the following primary antibodies diluted in 5% wt/vol BSA in 1X TBS + 0.1% Tween20: anti-pIRF3 S396 (CST), anti-IRF3 (CST), anti-pTBK1 S172 (CST), anti-TBK1 (CST), anti-pSTAT1 Y701 (CST), anti-STAT1 (CST), anti-pSTAT3 Y705 (CST), anti-STAT3 (CST), anti-PD-L1 (Abcam), anti-PD-1 (CST), and anti-β-actin (CST). Membranes were further probed with appropriate species-specific HRP-conjugated secondary antibodies (CST) and developed using ECL reagent (Bio-Rad).
In vitro antibody treatment
Anti-IFNAR1 (clone MAR1-5A3) was purchased from Leinco. PD-L1 monoclonal antibody clones 6E11, 17H9, and 27C11 were gifts from Dr. Ira Mellman (Genentech, South San Francisco, CA, USA). Tecentriq (atezolizumab) was purchased from The Ottawa Hospital Pharmacy Department. TRAMP-C2, 786-0, or Hs746 cells were pretreated with these antibodies or appropriate isotype controls (Leinco) for 24 h prior to further manipulation/analysis at concentrations indicated in figure legends.
Type I IFN reporter assay
100–200 µl of cell culture supernatant was isolated from cultured cells and placed on adherent L929-ISRE cells (expressing luciferase under the control of a type I IFN sensitive ISRE promoter) for 4–6 h. Luciferase expression was assessed using Luciferase Assay System (Promega), and luminescence was measured on a plate reader (BioTek).
Cytosolic DNA isolation and quantification
Cytosolic DNA was isolated and quantified using methods detailed in Mosley and Baker (2022).
RNA-seq sample preparation
RNA was collected as described above from mock-infected TRAMP-C2 cells and TRAMP-C2-Cd274−/− or those same cells infected with VSVΔ51-YFP at MOI 0.1 for 8 h.
RNA-seq library preparation and sequencing
Total RNA was quantified using a NanoDrop Spectrophotometer ND-1000 (NanoDrop Technologies, Inc.) and its integrity was assessed on a 2100 Bioanalyzer (Agilent Technologies). Libraries were generated from 250 ng of total RNA as follows: mRNA enrichment was performed using the NEBNext Poly(A) Magnetic Isolation Module (New England BioLabs). cDNA synthesis was performed using the NEBNext RNA First Strand Synthesis and NEBNext Ultra Directional RNA Second Strand Synthesis Modules (New England BioLabs). The remaining steps of library preparation were done using the NEBNext Ultra II DNA Library Prep Kit for Illumina (New England BioLabs). Adapters and PCR primers were purchased from New England BioLabs. Libraries were quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies) and the Kapa Illumina GA with Revised Primers-SYBR Fast Universal kit (Kapa Biosystems). The average size fragment was determined using a LabChip GX (PerkinElmer) instrument. Libraries were then sequenced on a NovaSeq6000 S4 200 cycle (2 × 100 bp) flow cell to an approximate depth of 30 million reads per sample.
RNA-seq processing and differential expression
Transcript quantification for each sample was performed using Kallisto (v0.45.0) (Bray et al., 2016) with the GRCm38 transcriptome reference and the -b 50 bootstrap option. The R package Sleuth (v0.30.0) (Pimentel et al., 2017) was then used to construct general linear models for the log-transformed expression of each gene across experimental conditions. Wald’s test was used to test for differential expression between groups, and the resultant P values were adjusted to q-values using the Benjamini–Hochberg false discovery rate method.
Signaling inference and gene set scoring
Relative activities of 14 signaling pathways were inferred using the R package PROGENy (v.1.9.6) (Schubert et al., 2018), which provides a prebuilt regression model for signaling activity based on consistently responsive genes. Single-sample gene set scoring was performed using the R package singscore (v1.17.) (Foroutan et al., 2018), which computes independent scores for each sample from rank-based statics for each gene in the set.
MitoTracker/MitoSOX
TRAMP-C2 cells were briefly trypsinized and resuspended in PBS. Cells were counted and 500,000 cells were stained with 500 nM MitoTracker Deep Red FM (Thermo Fisher Scientific) and 10 µM MitoSOX Red Mitochondrial Superoxide Indicator (Thermo Fisher Scientific) for 10 min at 37°C. Dye was washed out with PBS and cells were analyzed by flow cytometry within 1 h.
Mitochondrial DNA and nuclear DNA quantification
Mitochondrial DNA and nuclear DNA were isolated using organic solvent extraction-based protocol adapted from Guo et al. (2009). qPCR was performed as described above using primers amplifying MT-ND1 (for mitochondrial DNA) and 18S rRNA (for nuclear DNA).
Seahorse extracellular flux analysis
20,000 TRAMP-C2 cells/well were seeded into a 96-well Seahorse plate 1 day prior to the Seahorse assay. On the day of the assay, cells were equilibrated for 1 h in DMEM supplemented with 4 mM glutamine, 1 mM sodium pyruvate, and 25 mM glucose, at pH 7.4. Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured by monitoring dissolved oxygen and pH using the XF96 extracellular flux analyzer (Seahorse Bioscience) above the cell monolayer under basal conditions and following treatment with oligomycin (1 μM), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (0.5 μM), and rotenone + antimycin A (1 μM each).
Glucose uptake
In vitro glucose uptake was measured following 10 min of uptake using the Glucose Uptake-Glo Assay kit (Promega), as per the manufacturer’s instructions.
Metabolomics
Levels of metabolites from TRAMP-C2 cells and culture media following 48 h of culture were quantified by liquid chromatography–mass spectrometry (LC-MS). Sample temperature was maintained on ice or dry ice where possible, and all solvents were MS grade and pre-equilibrated to −20°C.
Cell pellets and media/supernatant were collected to a prechilled 2 ml tube containing six washed ceramic beads (1.4 mm) and 230 µl of methanol:water (1:1). Samples were vortexed for 10 s and cell lysis was done by beating for 60 s at 2,000 rpm (bead beating was done twice) after adding 220 µl of acetonitrile. Samples were then incubated with a 2:1 dichloromethane:water solution on ice for 10 min. The polar and non-polar phases were separated by centrifugation at 4,000 g for 10 min at 1°C. The upper polar phase was dried using a refrigerated CentriVap Vacuum Concentrator at −4°C (LabConco Corporation). Samples were resuspended in water and run on an Agilent 6470A tandem quadruple mass spectrometer equipped with a 1290 Infinity II ultra-high-performance LC (Agilent Technologies) using the Metabolomics Dynamic MRM Database and Method (Agilent), which uses an ion-pairing reverse phase chromatography. This method was further optimized for phosphate-containing metabolites with the addition of 5 µM InfinityLab deactivator (Agilent) to mobile phases A and B, which requires decreasing the backflush acetonitrile to 90%. Multiple reaction monitoring (MRM) transitions were optimized using authentic standards and quality control samples. Metabolites were quantified by integrating the area under the curve of each compound using external standard calibration curves with Mass Hunter Quant (Agilent). No corrections for ion suppression or enhancement were performed, as such, uncorrected metabolite concentrations are presented.
Enzymatic measurement of L-lactate
L-lactate was quantified in the culture supernatant using a colorimetric assay (Abcam), as per the manufacturer’s protocol.
Mice and tumor injection
NCG mice (NOD-Prkdcem26Cd52Il2rgem26Cd22/NjuCrl, lacking functional T, B, and NK cells) were purchased from Charles River Laboratory and maintained at uOttawa. For all experiments, male mice were used to match the male origin of injected prostate cancer cell lines (TRAMP-C2). To generate subcutaneous tumors, 1–2*106 cancer cells were resuspended in Matrigel (BD Biosciences) and injected in the left flank.
In vivo VSVΔ51 infection and bioluminescence imaging
For in vivo treatments of NCG mice with VSVΔ51, subcutaneous TRAMP-C2 tumors were injected with 108 PFU of the virus by intratumoral injection of 100 µl of a 109 PFU/ml solution in sterile PBS. For bioluminescence studies, mice were injected when tumors reached 750 mm3. 24 h after infection with VSVΔ51-firefly luciferase, tumor-bearing mice were subjected to IVIS imaging. Mice were injected intraperitoneally with D-luciferin (Perkin Elmer) at a dose of 150 mg/kg for 15 min prior to isoflurane anesthesia. Mice were imaged using an IVIS Spectrum (Perkin Elmer). Investigators were not blinded during imaging.
For tumor growth studies, mice were injected with VSVΔ51 when tumors reached 150 mm3 and tumor volume was measured every 3 days until humane endpoint.
Ex vivo virion quantification of infected mouse tumors
Tumors were resected, weighed, and flash-frozen in dry ice. Later, tumors were mechanically lysed using a TissueLyser II (Qiagen) in PBS supplemented with cOmplete protease inhibitor cocktail (Roche) using glass beads. Cell debris was removed via centrifugation and 70-µm filters, and supernatant was serially diluted for plaque assay as described above. Titers were reported in PFU/mg of the tumor.
[18F]-FDG PET
Tumor-bearing mice (tumor diameter 1,000 mm3, n = 5–6 per group) fasted 5–8 h before the imaging session were anesthetized with 2% isoflurane and intravenously injected with [18F]-FDG (5.98 ± 1.92 MBq) as a bolus over 30 s via the lateral tail vein. Mice remained under isoflurane anesthesia in an induction chamber and body temperature was maintained with a heat lamp. Blood glucose measurements (mM) were taken via tail vein blood sampling before and after the PET scan using a MediSure Blood Glucose Monitoring System. 40 min after radiotracer delivery, mice were positioned in the PET scanner and a 10-min transmission scan was performed. A whole-body static scan was immediately acquired between 50 and 60 min using a Siemens DPET scanner. Emission data were corrected for attenuation and scatter, and then reconstructed using the 3D-OSEM/MAP algorithm. Volumetric regions of interest were drawn conforming to tumor margins and quantified using a threshold of 25% of SUVmax (SUV25). Uptake values obtained in Bq·cc−1 were converted to standardized uptake value (SUV) using the injected dose (Bq) and animal body weight (kg).
Bioinformatic analyses of human cell line RNA-seq
Analysis of expression levels of CD274 transcript and HALLMARK_GLYCOLYSIS gene module (Liberzon et al., 2015; Subramanian et al., 2005) in cell lines was performed using information from the RNA-seq dataset E-MTAB-2706, which contains genome-wide transcriptome profiles of 675 cancer cell lines. Briefly, we downloaded the reads per kilobase of transcript, per million file containing all sequencing reads for each cell line, along with a file containing the gene names in various formats and a metadata file describing the samples. We then converted gene IDs in the expression matrix to gene symbols and removed duplicates and missing values. We next added sample names to the columns of the expression matrix, before leveraging the R-based package singscore (Foroutan et al., 2018), which allows for rank-based statistics to score a sample’s gene expression profile according to the activities of genes provided by curated gene modules. We set a cut-off on each axis at the mean of each value plus two standard deviations (SD).
Malignant cell PD-L1 expression and gene set activity
We have previously compiled and processed a collection of scRNA-seq data from 266 epithelial tumors (Cook and Vanderhyden, 2022). Automated annotation of cell types was performed in conjunction with a copy number alteration inference to identify the malignant population of each data set. Only tumors with >200 malignant cells were retained in the cohort. Average PD-L1 expression (log-transformed counts per 10,000 transcripts) was calculated from this fraction. Gene set scores for the MSigDB Hallmark hypoxia and glycolysis gene sets was calculated for individual cells using the R package UCell, which implements a rank-based signature scoring method based on the Mann–Whitney U statistic.
Immunohistochemistry
Fresh tumor biopsies were fixed in 10% neutral-buffered formalin for 24 h prior to paraffin embedding and sectioning. Sections were rehydrated using xylene and ethanol and subjected to antigen retrieval using 10 mM citrate buffer (pH 6.0) in a pressure cooker for 10 min. Sections were blocked in 10% normal goat serum (BioLynx) and incubated with anti-PD-L1 (clone 28-8; Abcam) overnight at 4°C. Endogenous peroxidase activity was quenched using 3% hydrogen peroxide and incubated with HRP-conjugated goat anti-rabbit (Cell Signaling Technologies). Detection was performed using DAB Substrate (Vector Laboratories), followed by hematoxylin counterstaining. Sections were dehydrated and stabilized with mounting medium (Thermo Fisher Scientific). PD-L1 expression was scored by a blinded trained pathologist. The percentage of tumor cells that stained with the PD-L1 antibody was estimated on each slide. The average intensity of staining was scored as 0 (no staining), 1+ (weak intensity), 2+ (moderate intensity), and 3+ (strong intensity). The cellular compartment with positive staining was noted; this included nuclear, cytoplasmic, or membranous. The background inflammatory cells were examined for positive staining as well, and percentage of necrotic cells.
Ex vivo infection of patient tumors
Fresh tumor biopsies were cut into 2 × 2 × 2 cm cores using a biopsy punch and scalpel. Each tumor core was placed in individual wells of a 24-well plate with DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 10 µg/ml gentamicin sulfate, and 20 mM HEPES. Cores were infected in quadruplicate with MOI 100, 30, 3, 1, or 0.1 of VSVΔ51-YFP (assuming ∼1*106 cells per core) or uninfected control. Concurrently, tumor cores were analyzed by alamarBlue Assay (Thermo Fisher Scientific) as per the manufacturer’s protocol. Tumors were infected for 48 h prior to imaging YFP reporter from the virus using EVOS Imaging System (Thermo Fisher Scientific). Virus infection was quantified by quantifying the percentage of tumor area that is YFP+ using ImageJ (in a blinded fashion). The only exclusion criteria for a tumor in this study was lack of viability based on alamarBlue results.
Statistical analyses
All in vitro experiments were repeated at least three times unless otherwise stated. Mouse studies were performed twice unless otherwise stated. Statistical analyses were performed using GraphPad Prism (GraphPad). Experiments with two independent conditions were analyzed by two-tailed unpaired Student’s t test, one-way ANOVA to compare three or more conditions, and two-way ANOVA (with Šídák’s correction for multiple comparisons) to compare groups influenced by two variables. Differences between experimental groups were considered significant when P < 0.05.
Study approvals
Mouse studies were reviewed and approved by Animal Care and Veterinary Services at uOttawa in accordance with the guidelines of the Canadian Institutes of Health Research (CIHR). For human studies, informed and written consent in accordance with the Declaration of Helsinki was obtained from all patients, and approval was obtained from The Ottawa Hospital (REB, 20180221-02H).
Online supplemental material
Fig. S1 shows that PD-L1 does not regulate viral entry and that CD80 is important for PD-L1 activity. Fig. S2 shows that PD-L1 regulates the type I IFN response following poly(I:C) stimulation. Fig. S3 shows pathways regulated by PD-L1 according to bulk transcriptomic analysis, and that TGF-β, EGFR, and androgen/estrogen signaling are not required for PD-L1 activity. Fig. S4 shows metabolic alterations following genetic deletion of PD-L1. Fig. S5 shows that PD-L1 inhibits the type I IFN response in primary human biopsies and human cell lines. Table S1 shows the metadata for tumor scRNA-seq data analyzed for PD-L1 expression and glycolysis gene set score. Table S2 shows the PD-L1 remodeling of the cancer cell metabolome: Summary of unbiased metabolomics study performed on cell lysate and culture supernatant from TRAMP-C2 and TRAMP-C2-Cd274−/− after 48 h in culture. All data in units of relative concentration, measured in µM. Culture supernatant data were corrected using culture media without cells to determine the production/depletion of media metabolites. Table S3 shows the summary of the patient cohort for PD-L1 biomarker study.
Data availability
The transcriptomic data that support the findings of this study are openly available in GEO at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE210884, accession number GSE210884. The metabolomic data that support the findings of this study are available at https://doi.org/10.5061/dryad.dncjsxm77 and will be deposited in the National Metabolomics Data Repository.
Acknowledgments
\We would like to thank Dr. Tang and the uOttawa Flow Cytometry and Virometry Core Facility, Mr. Ortiz and the OHRI Flow Cytometry Core Facility, Dr. Naz and the uOttawa Metabolomics Core Facility (supported by the Terry Fox Foundation and uOttawa), Ms. Delic and the Global Tissue Consent program at The Ottawa Hospital, the Ottawa Methods Centre at OHRI, the uOttawa Pre-clinical Imaging Core (RRID:SCR_021832), the uOttawa Louise Pelletier Histology Core Facility (RRID: SCR_021737), Dr. Kristi Baker and Shayla Mosley for help with cytosolic DNA quantification, and the Animal Care and Veterinary Services staff. Thank you to all former and current Ardolino lab members for fruitful and enlightening discussions. We are also thankful to Dr. Auer for precious suggestions and to Dr. Roy for honest and insightful comments on the manuscript.
M. Ardolino is supported by Ride for Dad, CIHR, and Cancer Research Society; J.J. Hodgins, C. Fong-McMaster, and D.P. Cook are recipients of CIHR Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Awards; J. Abou-Hamad is the recipient of an Ontario Graduate Scholarship; A. Hagerman is the recipient of a BioCanRx scholarship; M. Marotel is the recipient of a Canadian Allergy, Asthma, and Immunology Foundation postdoctoral fellowship and a CIHR fellowship; A. Buchler is the recipient of OGS and a University of Ottawa Heart Institute Research Scholarship; M.M. Park is the recipient of a Schulich Leader Scholarship, a uOttawa Ontario Graduate Scholarship scholarship, and a Natural Sciences and Engineering Research Council of Canada (NSERC) Undergraduate Student Research Award; M.F. Crupi is the recipient of a Taggart-Parkes Fellowship from the Ottawa Hospital Research Institute; O. Makinson is a recipient of a CI3 scholarship from the University of Ottawa; R. Razaei is a recipient of a Vanier Canada Graduate Scholarship; J.C. Bell is supported by CIHR, Canadian Cancer Society Research Institute, Prostate Cancer Canada, and Terry Fox Research Institute; B.H. Rotstein is supported by CIHR, NSERC, and Canadian Fund for Innovation; R.C. Auer is supported by the Terry Fox Foundation; L.A. Sabourin is supported by Canadian Cancer Society Research Institute; M.-C. Bourgeois-Daigneault is supported by CIHR.
Author contributions: J.J. Hodgins: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing—original draft, Writing—review and editing, J. Abou-Hamad: Conceptualization, Data curation, C. E. O’Dwyer: Investigation, A. Hagerman: Investigation, Methodology, Validation, Writing—review and editing, E. Yakubovich: Formal analysis, Software, Visualization, C. Tanese De Souza: Investigation, M. Marotel: Investigation, A. Buchler: Data curation, Formal analysis, Investigation, Methodology, Resources, Writing—review and editing, S. Fadel: Investigation, Resources, M.M. Park: Investigation, C. Fong-McMaster: Investigation, Methodology, M.F. Crupi: Formal analysis, Investigation, Methodology, Resources, Writing—review and editing, O.J. Makinson: Investigation, Writing—review and editing, R. Kurdieh: Investigation, Writing—review and editing, R. Rezaei: Data curation, Investigation, Methodology, H.S. Dhillon: Investigation, Methodology, C.S. Ilkow: Conceptualization, Resources, Supervision, J.C. Bell: Conceptualization, Funding acquisition, Writing—review and editing, M.-E. Harper: Methodology, Resources, Supervision, B.H. Rotstein: Methodology, Project administration, Resources, Supervision, Visualization, Writing—review and editing, R.C. Craufurd Auer: Conceptualization, Methodology, Resources, Supervision, Writing—review and editing, B.C. Vanderhyden: Resources, Supervision, Writing—review and editing, L.A. Sabourin: Funding acquisition, Resources, Supervision, M.-C. Bourgeois-Daigneault: Methodology, Supervision, D.P. Cook: Formal analysis, Visualization, Writing—review and editing, M. Ardolino: Conceptualization, Formal analysis, Funding acquisition, Project administration, Supervision, Visualization, Writing—original draft, Writing—review and editing.
References
Author notes
Disclosures: R. Auer reported a patent to WO2018027316A1 pending “Turnstone Biologics.” M. Ardolino reported grants from Dragonfly Therapeutics and Actym Therapeutics outside the submitted work. No other disclosures were reported.
J.J. Hodgins’s current affiliation is Genentech, South San Francisco, CA, USA.



![PD-L1 does not regulate VSVΔ51 entry. (A) TRAMP-C2 cells (blue) were transfected with Cas9 and gRNA targeting PD-L1 to generate TRAMP-C2-Cd274−/− (red). A representative plot depicting PD-L1 expression is shown. (B) Expression of PD-1 and CD80 in TRAMP-C2 cells. Representative flow plots are shown. (C) TRAMP-C2 cells stably expressing CD80 (or empty vector [EV] control) were infected with VSVΔ51 at MOI 0.1 for 24 h prior to analysis of viral titer by plaque assay. The data depicted are representative of two experiments performed with similar results. (D) TRAMP-C2 or TRAMP-C2-Cd274−/− were infected with VSVΔ51-YFP at MOI 0.1, and RNA was collected at indicated times after infection for qPCR analysis of the viral nucleocapsid (N) transcript. n = 3 biological replicates depicted. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (E) Expression of the VSV entry receptor LDL-R in TRAMP-C2 (blue) or TRAMP-C2-Cd274−/− (red) cells. Representative histograms are shown. For all panels: ****P < 0.0001; P > 0.05 not significant (ns).](https://cdn.rupress.org/rup/content_public/journal/jem/221/7/10.1084_jem.20221721/1/m_jem_20221721_figs1.png?Expires=1773755679&Signature=Whpwt1nyHlPyADYXxlwUjzonZjaWS5PrfS~MrtILppdxXcLOXw1A-8uS~kejDBokEiINiUr5njn6chQONu8lFW7MmRhdNoeSL7NptvSvECXs1xBn~gcvkXSLtolq5BsxyLWFF-e60JFCLoUv9-AiSjv3cKdjqq2raFkeQIdUhcozjpU5hT0n78kLVKj91YOGOnFpBRxSm~JBlrW-38lwKnVelPecw52y-4TGW6l-dtpKlz32bo5lRG5PRB3YNU-VY78TW1dd0-9047bapa7ixyhLMXi8BF7T1ztRbgZOQcGkKCKwnmuH9QsCYbmm~Q~fh953r9FJ1es7bdRWTl5nBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

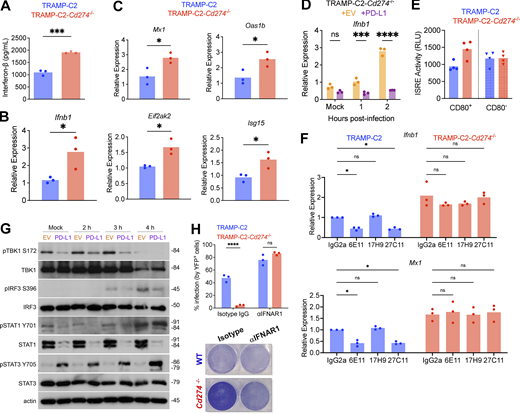




![PD-L1 promotes Warburg metabolism in TRAMP-C2 cells. (A) Scoring for expression of genes included in the glycolysis gene set. RNA-seq dataset performed on TRAMP-C2 and TRAMP-C2-Cd274−/− either mock-infected or infected with VSVΔ51-YFP at MOI 0.1 for 8 h (three biological replicates per condition). (B–E) Seahorse metabolic flux analysis on TRAMP-C2, TRAMP-C2-Cd274−/−, TRAMP-C2-Cd274−/− + empty vector (EV), and TRAMP-C2-Cd274−/− + PD-L1 (tested in n = 6 technical replicates). ECAR and OCR are measures of glycolysis and mitochondrial respiration, respectively. The images depicted are representative of three experiments with similar results. (F and G) Bioenergetic calculations based on Seahorse metabolic flux assay. N = 3 biological replicates. (H) Hierarchical clustered heat map depicting the relative abundance of 50 metabolites quantified in untargeted metabolomics of TRAMP-C2 and TRAMP-C2-Cd274−/− cells. Six biological replicates per cell line are shown. (I and J) Glucose uptake was measured in TRAMP-C2 and TRAMP-C2-Cd274−/− cells (I), as well as CD80+ versus CD80− cells FACS-isolated from TRAMP-C2 or TRAMP-C2-Cd274−/− cells (J). The experiments depicted are representative of three performed with similar results. Statistical analysis by two-way ANOVA with Šídák’s correction for multiple comparisons. (K) Male NCG mice were implanted with subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors. The SUV of [18F]-FDG was assessed by PET imaging. The two mice shown are representative of five to six analyzed. For all panels: **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).](https://cdn.rupress.org/rup/content_public/journal/jem/221/7/10.1084_jem.20221721/1/m_jem_20221721_fig5.png?Expires=1773755679&Signature=BYDiuz70yN3XLeakaaU--XxjjJHMd12jk6EF1jqd44u6daATnTcMtst7vKoc46nedEG8EdRGUGmVWgFbNtQ1DGr7KXnEgrCcpMsD6IWD3BWrJx7jNFJintPI6hiuBWDPSyDsrLIN5ZVIzudYBfHwh2DWISFs0EPpp7ZDFvhomsbewUhwNrBZitNGVZ9oBNt-~f59LT3lorXo1ybwNMhCChIWmyUan2abnwkylKSJLY0XSkMaEN3F7h~uXT6kkSquc1SncpUDyM~baP6NWaupp3-vOfpJiV0kcVGKcSyo6L8nqHD-CJUDjY~l1ue-WjduV9BYGpTgOMHJUGfTgiyIHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![PD-L1 promotes Warburg metabolism in TRAMP-C2 cells. (A) Transcripts per million (TPM) for various glycolysis and glucose metabolism enzymes/transporters, from bulk RNA-seq experiment. (B) Cellular respiration parameters calculated using Seahorse mitochondrial stress test data. N = 3 biological replicates are shown. For each replicate, data were normalized to TRAMP-C2 values. Statistical analysis by two-tailed unpaired Student’s t test. (C) Relative quantification of mitochondrial DNA (mtDNA) by qPCR (normalized to nuclear DNA [nDNA] in each sample) by qPCR analysis. N = 3 biological replicates. Statistical analysis by two-tailed unpaired Student’s t test. (D and E) Mean fluorescence intensity (MFI) of MitoTracker and MitoSOX dyes by flow cytometry for TRAMP-C2, TRAMP-C2-Cd274−/−, TRAMP-C2-Cd274−/− + empty vector (EV), and TRAMP-C2-Cd274−/− + PD-L1 cells. MitoSOX MFI was corrected for total mitochondrial content by normalizing to MitoTracker MFI. Statistical analysis by one-way ANOVA with Šídák’s correction for multiple comparisons. (F) Relative abundance of glycolysis metabolites in TRAMP-C2 and TRAMP-C2-Cd274−/− cells, measured in the untargeted metabolomics study. Six biological replicates per cell line. Statistical analysis by unpaired two-tailed Student’s t test. (G) Male NCG mice were implanted with subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors. Tumor growth was assessed over time and tumor mass was measured at the endpoint. N = 5–6 per group. (H) Male NCG mice were implanted with subcutaneous TRAMP-C2 or TRAMP-C2-Cd274−/− tumors. The SUV of (18F)-FDG was assessed by PET imaging. Statistical analysis by two-tailed unpaired Student’s t test. For all panels: ***P < 0.001; **P < 0.01; *P < 0.05; P > 0.05 not significant (ns).](https://cdn.rupress.org/rup/content_public/journal/jem/221/7/10.1084_jem.20221721/1/m_jem_20221721_figs4.png?Expires=1773755679&Signature=iGUyWY8UhgxKHTliNm4~bePmCVdWFmRea60QZkNvKWOy86vsheCvePQulO-coJj7BSSwto0YPuBxZeTyaIEl6Fx2FN2xpcjXHvzHwcvvXNXPwA9eF-n0zqXQ9x0jvXQEC2WufrRWe9ZQZF6cEevuOIiJMCvQ6RK5gpAbMEAAamO~a8vYqhgjZwVeV9b5vM6sAB4dc4rLVGvlqjIcPeXs5QIHmtS5fM0qgumi-8fS-tNhVFjo4r7CRAF0AcqDxsaex~2INu6dlq6G8wFFMqgagcziIbCUQ9P6wrap~N1PA8eQmHTsiuA9P6APdfYghqFxEqpsv36jIKTZpXCgpcrCGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)






