VPS13B/COH1 is the only known causative factor for Cohen syndrome, an early-onset autosomal recessive developmental disorder with intellectual inability, developmental delay, joint hypermobility, myopia, and facial dysmorphism as common features, but the molecular basis of VPS13B/COH1 in pathogenesis remains largely unclear. Here, we identify Sec23 interacting protein (Sec23IP) at the ER exit site (ERES) as a VPS13B adaptor that recruits VPS13B to ERES–Golgi interfaces. VPS13B interacts directly with Sec23IP via the VPS13 adaptor binding domain (VAB), and the interaction promotes the association between ERES and the Golgi. Disease-associated missense mutations of VPS13B–VAB impair the interaction with Sec23IP. Knockout of VPS13B or Sec23IP blocks the formation of tubular ERGIC, an unconventional cargo carrier that expedites ER-to-Golgi transport. In addition, depletion of VPS13B or Sec23IP delays ER export of procollagen, suggesting a link between procollagen secretion and joint laxity in patients with Cohen disease. Together, our study reveals a crucial role of VPS13B–Sec23IP interaction at the ERES–Golgi interface in the pathogenesis of Cohen syndrome.
Introduction
The early secretory pathway, which consists of vesicular traffic between the endoplasmic reticulum (ER) and the Golgi apparatus, occurs constitutively in mammalian cells. The pathway is crucial for the constant supply of secretory and plasma membrane lipids/proteins and is considered essential for general cell function and survival (Barlowe and Helenius, 2016). Neurons exhibit a high intensity of membrane dynamics and protein/lipid transport with differential and polarized transport toward the somato-dendritic and axonal plasma membrane domains (Ye et al., 2006). Mutations in genes encoding components of the early secretory pathway are known to cause neurological or developmental disorders that manifest early in life (Tang and Ginsburg, 2023). These rare disorders are associated with autosomal recessive mutations in coat proteins, membrane tethering, and fusion complexes (Wang et al., 2020), such as subunits of coat protein complex I and II (COPI and COPII) (Dell’Angelica and Bonifacino, 2019; Russo et al., 2013), subunits of the transport protein particle complex (TRAPP) (Sacher et al., 2019), members of the YIP1 domain family (YIPF) (Shaik et al., 2019), and a member of the SNAP receptor family (SNARE) (Tang, 2021).
Cohen syndrome (MIM 216550), a rare recessive developmental disorder, was first described by Cohen and co-workers (Carey and Hall, 1978; Cohen et al., 1973), with a variable clinical presentation characterized mainly by developmental delay, mental retardation, joint laxity, microcephaly, typical facial dysmorphism, progressive pigmentary retinopathy, severe myopia, and intermittent neutropenia. Among these features, developmental delay, early-onset myopia, joint laxity, and facial dysmorphism were the cardinal clinical phenotypes present in all patients with Cohen syndrome (Pirgon, 2013).
All patients with Cohen disease were homozygous or compound heterozygous for mutations in a gene encoding vacuolar protein sorting-associated protein 13B (VPS13B, also known as COH1) (Pirgon, 2013). VPS13B is a member of the bridge-like repeating β-groove (RBG) lipid transfer protein family (Hanna et al., 2023). The human genome contains four VPS13 genes (VPS13A, VPS13B, VPS13C, and VPS13D genes) (Velayos-Baeza et al., 2004). Each of them is directly associated with certain human diseases, and therefore these proteins are of great biomedical interest (Ugur et al., 2020). Previous studies have shown that VPS13A and VPS13C are lipid transporters at ER-associated membrane contact sites (MCSs), including ER–mitochondria/plasma membrane (VPS13A) and ER–late endosome/lysosome MCSs (VPS13C) (Guillen-Samander et al., 2022; Kumar et al., 2018; Park et al., 2022). VPS13D has been reported to play a role in mitophagy in Drosophila (Anding et al., 2018; Shen et al., 2021), is localized at ER–mitochondrial/peroxisomal contacts (Guillen-Samander et al., 2021), and promotes peroxisome biogenesis (Baldwin et al., 2021). Our previous results have shown that VPS13D plays a regulatory role in ER–mitochondrial MCSs (Du et al., 2021) and facilitates LD remodeling under starvation (Wang et al., 2021). VPS13B has been shown to play essential roles in several cellular processes, such as Golgi integrity and neurite outgrowth (Seifert et al., 2011, 2015), cargo recycling transport (Koike and Jahn, 2019), acrosome biogenesis (Da Costa et al., 2020), and LD dynamics (Du et al., 2023b). However, to date, the relationship between VPS13B and MCSs is not fully understood.
In this study, we identified Sec23 interacting protein (Sec23IP) at the ER exit site (ERES) as a VPS13B adaptor that recruits VPS13B to ERES–Golgi interfaces. VPS13B binds to Sec23IP via the VPS13 adaptor binding domain (VAB), and the interaction promotes the association between ERES and the Golgi. Disease-associated missense mutations of VPS13B–VAB impair the interaction with Sec23IP. Knockout of VPS13B or Sec23IP blocks the formation of tubular ERGIC, an unconventional cargo carrier that expedites ER-to-Golgi transport. In addition, depletion of VPS13B or Sec23IP delays ER export of procollagen, suggesting a link between procollagen secretion and joint laxity in patients with Cohen disease. Collectively, our study reveals an important role of VPS13B–Sec23IP interaction at the ERES–Golgi interface in the pathogenesis of Cohen syndrome.
Results
PI4P is required for the association of VPS13B with the Golgi
First, we investigated the cellular localization of VPS13B using immunofluorescence (IF). Endogenous VPS13B was colocalized with GM130, a cis-/medial Golgi marker (Fig. S1 A). The VPS13B fluorescence was completely lost in CRISPR-Cas9-mediated VPS13B knockout HeLa cells (VPS13B KO; Fig. S1, B and C), confirming the specificity of the VPS13B antibody utilized in IF. Consistently, GFP-tagged VPS13B (VPS13B^sfGFP [Du et al., 2023b]) was well colocalized with the Golgi (Fig. S1 D).
Supplementary data toFig. 1 . (A) Representative images of a fixed control (top) or VPS13B KO HeLa cell (bottom) stained with VPS13B antibody (green) and GM130 antibody (magenta) with insets. (B) CRISPR knock-out of VPS13B in HeLa cells (VPS13B-KO). Two sgRNAs are used with the underlined letters, indicating the PAMs (AGG for sgRNA1 and TGG for sgRNA2) for spCas9. (C) Western blots of two VPS13B-KO clones from (B). (D) Representative images of fixed HeLa cells transiently expressing VPS13B^sfGFP (green) stained with GM130 antibody (magenta) with an inset on the bottom. (E) Representative images of live HeLa cells transiently expressing a PI4P probe (GFP-OSBP-PH) upon control (DMSO; top), PAO (middle; 10 μM), and LY294002 (bottom; 300 μM) treatments with three timepoints (0, 10 min, 30 min). (F) Representative images of live HeLa cells transiently expressing VPS13B^sfGFP (green) upon either PI4KA, PI4KB, or PI4KA+B siRNA treatments with insets. (G) Fluorescent intensity of VPS13B^sfGFP in Golgi area as shown in F based on at least 10 cells for each group from three independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (H) qPCR assays indicated the efficiency of siRNA-mediated suppression of PI4KA and PI4KB from three independent experiments as in F. Mean ± SD. Two-tailed unpaired Student’s t test. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in A and D–F. Source data are available for this figure: SourceData FS1.
Supplementary data toFig. 1 . (A) Representative images of a fixed control (top) or VPS13B KO HeLa cell (bottom) stained with VPS13B antibody (green) and GM130 antibody (magenta) with insets. (B) CRISPR knock-out of VPS13B in HeLa cells (VPS13B-KO). Two sgRNAs are used with the underlined letters, indicating the PAMs (AGG for sgRNA1 and TGG for sgRNA2) for spCas9. (C) Western blots of two VPS13B-KO clones from (B). (D) Representative images of fixed HeLa cells transiently expressing VPS13B^sfGFP (green) stained with GM130 antibody (magenta) with an inset on the bottom. (E) Representative images of live HeLa cells transiently expressing a PI4P probe (GFP-OSBP-PH) upon control (DMSO; top), PAO (middle; 10 μM), and LY294002 (bottom; 300 μM) treatments with three timepoints (0, 10 min, 30 min). (F) Representative images of live HeLa cells transiently expressing VPS13B^sfGFP (green) upon either PI4KA, PI4KB, or PI4KA+B siRNA treatments with insets. (G) Fluorescent intensity of VPS13B^sfGFP in Golgi area as shown in F based on at least 10 cells for each group from three independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (H) qPCR assays indicated the efficiency of siRNA-mediated suppression of PI4KA and PI4KB from three independent experiments as in F. Mean ± SD. Two-tailed unpaired Student’s t test. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in A and D–F. Source data are available for this figure: SourceData FS1.
The Golgi apparatus is a highly polarized organelle consisting of cis-/medial, trans-, and trans-Golgi network. We further investigated which compartment of the Golgi VPS13B was mainly localized in nocodazole-treated cells. Both VPS13B^sfGFP (Fig. 1, A and B) and endogenous VPS13B (Fig. 1, C and D) were better colocalized with the cis-/medial Golgi marked by GM130 than trans-Golgi labeled by TGN-46 or a TM domain of B4GALT1, as shown by colocalization analyses, suggesting that VPS13B is preferentially localized to the cis-/medial Golgi.
PI4P is required for the association of VPS13B with the Golgi. (A) Representative images of a fixed HeLa cell transiently expressing VPS13B^sfGFP (green) and stained with GM130 antibody (magenta) and TGN46 antibody (blue) upon nocodazole treatment (5 μg/ml; 60 min) with two insets on the right. (B) Pearson’s correlation coefficient of VPS13B^sfGFP versus the cis-/medial Golgi (GM130) or the trans-Golgi (TGN46) in three independent experiments (n = 10 cells). Mean ± SD. Two-tailed unpaired Student’s t test. (C) Representative images of a fixed HeLa cell transiently expressing GFP-B4GALT1-TM (blue) stained with VPS13B antibody (green) and GM130 antibody (magenta) upon nocodazole treatment (5 μg/ml; 60 min) with two insets on the right. (D) Pearson’s correlation coefficient of endogenous VPS13B versus the cis-/medial Golgi (GM130; 19 cells) or the trans-Golgi (B4GALT1-TM; 18 cells) in three independent experiments. Mean ± SD. Two-tailed unpaired Student’s t test. (E) Representative EM micrograph of a cryosection of a fixed HeLa cell with endogenous VPS13B labeled by immunogold beads with an inset shown on the right. Yellow arrowheads denote VPS13B signals on the cis-/medial Golgi. (F) Number of immunogold particles in the Golgi and other cellular compartments (mitochondria, LDs, and lysosomes). Total 168 immunogold particles from six ROIs from six cells were quantified. (G) Coomassie blue staining of purified His tagged VPS13B PH domain. (H) The PIP Strip assays using purified His-VPS13B-PH as in G. (I) Representative images of a fixed HeLa cell stained with VPS13B antibody (green) and GM130 antibody (magenta) upon control (DMSO), PAO (10 μM; 30 min), LY294002 (300 μM; 30 min) treatments with insets. (J) Pearson’s correlation coefficient of endogenous VPS13B versus the Golgi upon treatment with DMSO (10 cells), PAO (11 cells), and LY294002 (11 cells) in three independent experiments as in I. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (K) Representative images of a fixed HeLa cell stained with VPS13B antibody (green) and GM130 antibody (magenta) upon control (DMSO; top), VPS34in (1 μM; 6 h), and Wortmannin (10 μM; 6 h) treatments with insets. (L) Pearson’s correlation coefficient of endogenous VPS13B versus the Golgi upon treated with DMSO (10 cells), VPS34-IN (11 cells), Wortmannin (9 cells) in 3 independent experiments as in K. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (M) Dot blot assay showing cellular PI4P levels upon treatments of DMSO, VPS34-IN, Wortmannin, LY294002, PAO, scrambled siRNA, siPI4KA, siPI4KB, and siPI4KA+B. Tubulin is used as the load control shown on the bottom. (N) Representative images of fixed HeLa cells transiently expressing GFP-VPS13B-PH (magenta) stained by VPS13B antibody (green) and GM130 antibody (blue) with insets on the right. The yellow arrow denotes an endogenous VPS13B punctum on the Golgi. (O) Line-scan analysis for N with red arrows indicating GFP-VPS13B-PH foci while a green arrow denoting anti-VPS13B foci on a Golgi ribbon. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in A, C, I, K, and N. 2 μm in E. Source data are available for this figure: SourceData F1.
PI4P is required for the association of VPS13B with the Golgi. (A) Representative images of a fixed HeLa cell transiently expressing VPS13B^sfGFP (green) and stained with GM130 antibody (magenta) and TGN46 antibody (blue) upon nocodazole treatment (5 μg/ml; 60 min) with two insets on the right. (B) Pearson’s correlation coefficient of VPS13B^sfGFP versus the cis-/medial Golgi (GM130) or the trans-Golgi (TGN46) in three independent experiments (n = 10 cells). Mean ± SD. Two-tailed unpaired Student’s t test. (C) Representative images of a fixed HeLa cell transiently expressing GFP-B4GALT1-TM (blue) stained with VPS13B antibody (green) and GM130 antibody (magenta) upon nocodazole treatment (5 μg/ml; 60 min) with two insets on the right. (D) Pearson’s correlation coefficient of endogenous VPS13B versus the cis-/medial Golgi (GM130; 19 cells) or the trans-Golgi (B4GALT1-TM; 18 cells) in three independent experiments. Mean ± SD. Two-tailed unpaired Student’s t test. (E) Representative EM micrograph of a cryosection of a fixed HeLa cell with endogenous VPS13B labeled by immunogold beads with an inset shown on the right. Yellow arrowheads denote VPS13B signals on the cis-/medial Golgi. (F) Number of immunogold particles in the Golgi and other cellular compartments (mitochondria, LDs, and lysosomes). Total 168 immunogold particles from six ROIs from six cells were quantified. (G) Coomassie blue staining of purified His tagged VPS13B PH domain. (H) The PIP Strip assays using purified His-VPS13B-PH as in G. (I) Representative images of a fixed HeLa cell stained with VPS13B antibody (green) and GM130 antibody (magenta) upon control (DMSO), PAO (10 μM; 30 min), LY294002 (300 μM; 30 min) treatments with insets. (J) Pearson’s correlation coefficient of endogenous VPS13B versus the Golgi upon treatment with DMSO (10 cells), PAO (11 cells), and LY294002 (11 cells) in three independent experiments as in I. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (K) Representative images of a fixed HeLa cell stained with VPS13B antibody (green) and GM130 antibody (magenta) upon control (DMSO; top), VPS34in (1 μM; 6 h), and Wortmannin (10 μM; 6 h) treatments with insets. (L) Pearson’s correlation coefficient of endogenous VPS13B versus the Golgi upon treated with DMSO (10 cells), VPS34-IN (11 cells), Wortmannin (9 cells) in 3 independent experiments as in K. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (M) Dot blot assay showing cellular PI4P levels upon treatments of DMSO, VPS34-IN, Wortmannin, LY294002, PAO, scrambled siRNA, siPI4KA, siPI4KB, and siPI4KA+B. Tubulin is used as the load control shown on the bottom. (N) Representative images of fixed HeLa cells transiently expressing GFP-VPS13B-PH (magenta) stained by VPS13B antibody (green) and GM130 antibody (blue) with insets on the right. The yellow arrow denotes an endogenous VPS13B punctum on the Golgi. (O) Line-scan analysis for N with red arrows indicating GFP-VPS13B-PH foci while a green arrow denoting anti-VPS13B foci on a Golgi ribbon. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in A, C, I, K, and N. 2 μm in E. Source data are available for this figure: SourceData F1.
To confirm this hypothesis, we performed ultrastructural studies using immuno-electron microscopy (EM). While the level of endogenous VPS13B was very low, we could still observe that VPS13B signals (yellow arrowheads) were preferentially present on the cis- or medial Golgi (Fig. 1 E), but not on other organelles (Fig. 1 F).
We next asked how VPS13B was associated with the Golgi. VPS13B contains a lipid-transfer groove along its entire length, with a VPS13 adaptor binding domain (VAB), an ATG2-C domain, and a PH domain at the C-terminus (CT) (Bean et al., 2018; Guillen-Samander and De Camilli, 2023). We hypothesized that the PH domain of VPS13B might be responsible for targeting VPS13B to the Golgi by binding to phosphatidylinositol 4-phosphate (PI4P) on Golgi membranes. To test this hypothesis, we purified the PH domain (Fig. 1 G) and investigated whether it bound to PI4P. In vitro PIPs strip assays showed that the purified VPS13B–PH bound to PI3P, PI4P, and PI5P (Fig. 1 H), partially consistent with a previous study using cell lysate containing overexpressed VPS13B–GFP (Koike and Jahn, 2019).
Next, we investigated whether PI4P was required for VPS13B localization. Treatment with phenylarsine oxide (PAO), a PI4K inhibitor, strongly reduced the association of endogenous VPS13B with the Golgi (Fig. 1 I, middle panel; Fig. 1 J). In addition, after treatment with LY249002, a PI3K inhibitor that could also reduce the PI4P level when used at high concentrations (Hammond et al., 2012), the association was also greatly reduced (Fig. 1 I, right panel; Fig. 1 J). However, two PI3K inhibitors, wortmannin and VPS34in, were unable to reduce the association (Fig. 1, K and L), suggesting that PI4P, but not PI3P, is necessary for the targeting of VPS13B to the Golgi. Dot blot assays using PI4P antibody confirmed that treatments with PAO or LY294002, but not wortmannin or VPS34in, substantially reduced cellular PI4P (Fig. 1 M). In addition, the effect of PAO or LY249002 on Golgi-resident PI4P was validated by a substantial reduction in the fluorescence intensity of a PI4P probe GFP-OSBP-PH (PH domain of oxysterol-binding protein) (Fig. S1 E).
In addition, partial depletion of the Golgi-resident PI4KB by small interfering RNAs (siRNAs) did not cause a strong reduction in the association of VPS13B^sfGFP with the Golgi, and depletion of PI4KA did not affect the association as well (Fig. S1, F and G). However, we did observe a moderate reduction in the level of Golgi-associated VPS13B^sfGFP upon double depletion of PI4KA and PI4KB (Fig. S1, F and G). Consistently, the dot blot assay showed that suppression of either PI4KA or PI4KB did not dramatically reduce cellular PI4P, whereas depletion of both evidently reduced PI4P levels in cells. These data suggest a redundant role of PI4KA and PI4KB in regulating the association of VPS13B with the Golgi.
Of note, the PH domain of VPS13B alone was able to target the Golgi (Fig. 1 N). Both endogenous full-length (FL) VPS13B and the PH domain were localized on the cis-/medial Golgi but appeared to be mutually excluded (Fig. 1 N), as shown by line-scan analyses (Fig. 1 O). This suggests that FL–VPS13B and the PH domain may compete with each other for binding to PI4P. Accordingly, VPS13B with a deletion of the PH domain lost the ability to target the Golgi (Fig. S2 A), confirming the role of the PH domain in the targeting of VPS13B to the Golgi.
The Golgi localization of VPS13B appears not to be independent of Rab2A or Rab6A. (A) Representative images of live HEK293 cells transiently expressing VPS13B-ΔPH^sfGFP (green) and MGAT2-Halo (magenta) with an inset on the bottom. (B) Representative images of live HeLa cells transiently expressing GFP-VPS13B-PH (green) upon either Rab6A siRNA (top panel) or Rab2 siRNA (bottom panel) treatments with insets. (C) Pearson’s correlation coefficient of VPS13B-H versus GM130 as shown in B based on at least 20 cells from the three groups in three independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (D) Western blots of Rab2A (left panel) and Rab6A (right panel) from B. (E) Representative images of live HeLa cells transiently expressing GFP-VPS13B-PH (green) along with either Halo-Rab6A (top panel) or Halo-Rab6A T27N (bottom panel) with an inset on the bottom. (F) Representative images of fixed HeLa cells stained with VPS13B antibody (magenta) and GM130 antibody (green) upon either scrambled (top panel) or Rab6A siRNA (bottom panel) treatments with insets. (G) Representative images of fixed HeLa cells expressing Rab6A T27N mutants stained with VPS13B antibody (magenta) and GM130 antibody (green). Two insets were shown on the bottom with or without Rab6A-T27N, respectively. (H) Pearson’s correlation coefficient of anti-VPS13B versus the Golgi as shown in F and G based on at least 10 cells from the three groups in 3 independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in A, B, and E–G. Source data are available for this figure: SourceData FS2.
The Golgi localization of VPS13B appears not to be independent of Rab2A or Rab6A. (A) Representative images of live HEK293 cells transiently expressing VPS13B-ΔPH^sfGFP (green) and MGAT2-Halo (magenta) with an inset on the bottom. (B) Representative images of live HeLa cells transiently expressing GFP-VPS13B-PH (green) upon either Rab6A siRNA (top panel) or Rab2 siRNA (bottom panel) treatments with insets. (C) Pearson’s correlation coefficient of VPS13B-H versus GM130 as shown in B based on at least 20 cells from the three groups in three independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (D) Western blots of Rab2A (left panel) and Rab6A (right panel) from B. (E) Representative images of live HeLa cells transiently expressing GFP-VPS13B-PH (green) along with either Halo-Rab6A (top panel) or Halo-Rab6A T27N (bottom panel) with an inset on the bottom. (F) Representative images of fixed HeLa cells stained with VPS13B antibody (magenta) and GM130 antibody (green) upon either scrambled (top panel) or Rab6A siRNA (bottom panel) treatments with insets. (G) Representative images of fixed HeLa cells expressing Rab6A T27N mutants stained with VPS13B antibody (magenta) and GM130 antibody (green). Two insets were shown on the bottom with or without Rab6A-T27N, respectively. (H) Pearson’s correlation coefficient of anti-VPS13B versus the Golgi as shown in F and G based on at least 10 cells from the three groups in 3 independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in A, B, and E–G. Source data are available for this figure: SourceData FS2.
The PH domain of VPS13B was shown to interact with Rab6 (Seifert et al., 2015), a small GTPase residing on the Golgi. We thus investigated whether Rab6 was required for VPS13B localization on the Golgi. We found that siRNA-mediated suppression of Rab6A or Rab2A, another Golgi-resident small GTPase, did not block the association of GFP-VPS13B-PH with the Golgi (Fig. S2, B–D). Accordingly, overexpression of dominant-negative mutants of Rab6A (Rab6A-T27N) appeared not significantly to affect the Golgi localization of VPS13B-PH (Fig. S2 E). Consistent with this, Rab6 activity was not required for the association of endogenous VPS13B with the Golgi, as depletion of Rab6A (Fig. S2 F) or overexpression of Rab6A-T27N (Fig. S2 G) did not profoundly reduce colocalization between VPS13B and GM130 (Fig. S2 H). Taken together, these results suggest that the PH domain is required for VPS13B targeting the Golgi via PI4P but likely independent of Rab6 or Rab2. Nevertheless, we speculated that PI4P may not be the only determinant factor for VPS13B localization on the Golgi, and other unknown proteins may collaborate with PI4P to mediate the recruitment, reminiscent of Vps13 in yeast, where the Ypt35 protein binds to both PI3P and to Vps13 to recruit Vps13 to endosomes (Bean et al., 2018).
The association of VPS13B with the ER via VAPs
Bridge lipid transporters are thought to function at ER-associated contacts with an FFAT or phospho-FFAT motif at the N terminal (NT), recognizing the ER in a VAP-dependent manner (Guillen-Samander and De Camilli, 2023). Therefore, we investigated whether VPS13B is associated with the ER via this mechanism. We have previously shown that FFAT motifs at the NT of VPS13B (residues 1–1,500) were not sufficient for a stable association with the ER (Du et al., 2023b). Next, we tested whether VPS13B targeted the ER via a phospho-FFAT motif (Di Mattia et al., 2020). A phospho-FFAT motif was found at the NT of VPS13B (551-GSTNQQDFSSGKSEDLGTV; Fig. S3 A). The structure and position of this phospho-FFAT motif were similar to its paralog VPS13D (Guillen-Samander et al., 2021) (Fig. S3 B). Coimmunoprecipitation (coIP) assays showed that the VPS13B–NT interacted with VAPB but not VAPA or MOSPD3 (Fig. S3 C). A phosphomimetic S560D mutation moderately increases the interaction between VPS13B–NT and VAPB. However, our imaging data showed that neither the VPS13B-NT nor phosphomimetic mutants (S560D, S1403D, and S1433D) were able to target the ER (Fig. S3, D–G). These results suggest that VPS13B may associate with the ER in a transient and dynamic manner via binding to VAPB. To investigate whether VPS13B targets the ER via other unknown adaptors, we performed mass spectrometry to identify proteins interacting with VPS13B on the ER.
The association between VPS13B and VAPs. (A) Three predicted phospho-FFAT motifs in VPS13B. (B) Alphafold-predicted structures of VPS13B with the predicted phospho-FFAT motif (551-GSTNQQDFSSGKSEDLGTV) are highlighted in a red box. (C) coIP assays showed interactions between GFP-VPS13B-NT (1–1500aa) containing different point mutations in phospho-FFAT motifs and endogenous VAPs in HEK293 cells. (D–G) Representative images of HeLa cell expressing either WT or phosphomimic mutants of GFP-VPS13B-NT (green) along with Halo-VAPs (magenta). Scale bar, 10 μm in whole and 2 μm in insets in D–G. Source data are available for this figure: SourceData FS3.
The association between VPS13B and VAPs. (A) Three predicted phospho-FFAT motifs in VPS13B. (B) Alphafold-predicted structures of VPS13B with the predicted phospho-FFAT motif (551-GSTNQQDFSSGKSEDLGTV) are highlighted in a red box. (C) coIP assays showed interactions between GFP-VPS13B-NT (1–1500aa) containing different point mutations in phospho-FFAT motifs and endogenous VAPs in HEK293 cells. (D–G) Representative images of HeLa cell expressing either WT or phosphomimic mutants of GFP-VPS13B-NT (green) along with Halo-VAPs (magenta). Scale bar, 10 μm in whole and 2 μm in insets in D–G. Source data are available for this figure: SourceData FS3.
VPS13B interacted with Sec23IP at the ERES–Golgi interface
Using coimmunoprecipitation (co-IP) followed by mass spectrometry, we identified a protein called Sec23 interacting protein (Sec23IP/p125) as a protein interacting with VPS13B (Fig. 2 A). Interestingly, both VPS13B and Sec23IP are present in vertebrates and have no homolog in other organisms, such as yeast and Caenorhabditis elegans. The direct link between Sec23IP and diseases was currently lacking, but an orthologous Sec23IP gene in frogs was involved in the development of neural crest cells. Intriguingly, both VPS13B and Sec23IP were reported to be crucial in acrosome biogenesis during spermiogenesis in mice (Arimitsu et al., 2011). Importantly, CRISPR-Cas9-mediated KO of Sec23IP (Fig. S4, A and B) resulted in fragmentation of the Golgi (Fig. S4 C) that phenocopied the KO of VPS13B (Du et al., 2023b). These results strongly suggest a functional link between VPS13B and Sec23IP.
VPS13B interacts with Sec23IP at ERES-Golgi interfaces. (A) Volcano plot of protein candidates coIPed with VPS13B^sfGFP in HEK293 cells compared with protein candidates coIPed with GFP tag only. After the removal of proteins that coIPed with the GFP tag, candidates that were considered significant (−log [P value] >1.3; P < 0.05) were labeled in orange (Log2 [fold change] >1.2; increased in abundance) or blue (Log2 [fold change] less than −1.2; decreased in abundance). (B) coIP assay showed an interaction between endogenous VPS13B and endogenous Sec23IP in HEK293 cells. Blue arrows denote VPS13B while red arrows indicate Sec23IP in the blots. (C) Representative images of a fixed HeLa cell expressing MGAT2-Halo (blue; a cis-/medial Golgi marker) stained with Sec23IP antibody (magenta) and Sec31A antibody (green) with two insets. Inset 1: Golgi area; inset 2: periphery area. (D) Representative images of a live HeLa cell co-expressing Halo-Sec23IP (magenta) and VPS13B^sfGFP (green) with two insets. (E) Representative images of a fixed HeLa cell co-expressing Halo-Sec23IP (magenta) and VPS13B^sfGFP (green) stained with Sec31A antibody (blue) with two insets on the bottom. (F) Percentage of Sec23IP puncta associated with the Golgi in either control (20 cells) or cells co-expressing Halo-Sec23IP and VPS13B^sfGFP (20 cells) as in E. Mean ± SD. Two-tailed unpaired Student’s t test. (G) Representative images of fixed HeLa cells expressing VPS13B^sfGFP (green) stained with GM130 antibody (blue) and Sec23IP antibody (magenta) with two insets on the bottom. Red arrows indicated a cell with the expression of VPS13B^sfGFP while blue arrows denoted a cell without VPS13B^sfGFP expression. (H) Percentage of Sec31A puncta associated with the Golgi in either control (n = 20), WT cells (n = 21) expressing VPS13B^sfGFP as in G or Sec23IP KO cells expressing VPS13B^sfGFP (n = 11; Fig. S4 G). Mean ± SD. Two-tailed unpaired Student’s t test. (I) Representative images of a fixed HeLa cell stained with VPS13B antibody (green) and Sec23IP antibody (magenta). One inset from the Golgi region was shown on the bottom while the other inset was shown to the right. (J) Pearson’s correlation coefficient of endogenous VPS13B versus endogenous Sec23IP at the Golgi or cell periphery (20 cells) in 3 independent experiments. Mean ± SD. Two tailed unpaired Student’s t test. (K) Representative images of fixed control, VPS13B KO, or siRNA-mediated Sec23IP knockdown in VPS13B KO HeLa cells stained with GM130 antibody (magenta) and Sec31A antibody (green) with insets. (L) Percentage of Sec31A puncta associated with the Golgi in either control (13 cells), VPS13B KO (20 cells), or VPS13B/Sec23IP double depleted cells (25 cells) as in K. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (M) Representative images of fixed HeLa cells co-expressing VPS13B^sfGFP (green) and ERES protein Sec16A-flag (magenta; top panel) or halo-Sec23A (magenta; bottom panel) stained with Sec31A antibody (blue) with two insets. (N) Percentage of Sec31A puncta associated with the Golgi in either control (n = 20), cells (n = 20) co-expressing Halo-Sec23IP and VPS13B^sfGFP as in E, cells (n = 13) co-expressing Halo-Sec23A and VPS13B^sfGFP or cells (n = 15) co-expressing sec16A-flag and VPS13B^sfGFP (20 cells) as in M. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (O) coIP assay showing Sec23IP, but not Sec16 or Sec23A, interacted with VPS13B^sfGFP. The blue arrow denoted VPS13B^sfGFP while the red arrows indicated Halo-Sec23IP in the blots. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in C–E, G, I, K, and M. Source data are available for this figure: SourceData F2.
VPS13B interacts with Sec23IP at ERES-Golgi interfaces. (A) Volcano plot of protein candidates coIPed with VPS13B^sfGFP in HEK293 cells compared with protein candidates coIPed with GFP tag only. After the removal of proteins that coIPed with the GFP tag, candidates that were considered significant (−log [P value] >1.3; P < 0.05) were labeled in orange (Log2 [fold change] >1.2; increased in abundance) or blue (Log2 [fold change] less than −1.2; decreased in abundance). (B) coIP assay showed an interaction between endogenous VPS13B and endogenous Sec23IP in HEK293 cells. Blue arrows denote VPS13B while red arrows indicate Sec23IP in the blots. (C) Representative images of a fixed HeLa cell expressing MGAT2-Halo (blue; a cis-/medial Golgi marker) stained with Sec23IP antibody (magenta) and Sec31A antibody (green) with two insets. Inset 1: Golgi area; inset 2: periphery area. (D) Representative images of a live HeLa cell co-expressing Halo-Sec23IP (magenta) and VPS13B^sfGFP (green) with two insets. (E) Representative images of a fixed HeLa cell co-expressing Halo-Sec23IP (magenta) and VPS13B^sfGFP (green) stained with Sec31A antibody (blue) with two insets on the bottom. (F) Percentage of Sec23IP puncta associated with the Golgi in either control (20 cells) or cells co-expressing Halo-Sec23IP and VPS13B^sfGFP (20 cells) as in E. Mean ± SD. Two-tailed unpaired Student’s t test. (G) Representative images of fixed HeLa cells expressing VPS13B^sfGFP (green) stained with GM130 antibody (blue) and Sec23IP antibody (magenta) with two insets on the bottom. Red arrows indicated a cell with the expression of VPS13B^sfGFP while blue arrows denoted a cell without VPS13B^sfGFP expression. (H) Percentage of Sec31A puncta associated with the Golgi in either control (n = 20), WT cells (n = 21) expressing VPS13B^sfGFP as in G or Sec23IP KO cells expressing VPS13B^sfGFP (n = 11; Fig. S4 G). Mean ± SD. Two-tailed unpaired Student’s t test. (I) Representative images of a fixed HeLa cell stained with VPS13B antibody (green) and Sec23IP antibody (magenta). One inset from the Golgi region was shown on the bottom while the other inset was shown to the right. (J) Pearson’s correlation coefficient of endogenous VPS13B versus endogenous Sec23IP at the Golgi or cell periphery (20 cells) in 3 independent experiments. Mean ± SD. Two tailed unpaired Student’s t test. (K) Representative images of fixed control, VPS13B KO, or siRNA-mediated Sec23IP knockdown in VPS13B KO HeLa cells stained with GM130 antibody (magenta) and Sec31A antibody (green) with insets. (L) Percentage of Sec31A puncta associated with the Golgi in either control (13 cells), VPS13B KO (20 cells), or VPS13B/Sec23IP double depleted cells (25 cells) as in K. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (M) Representative images of fixed HeLa cells co-expressing VPS13B^sfGFP (green) and ERES protein Sec16A-flag (magenta; top panel) or halo-Sec23A (magenta; bottom panel) stained with Sec31A antibody (blue) with two insets. (N) Percentage of Sec31A puncta associated with the Golgi in either control (n = 20), cells (n = 20) co-expressing Halo-Sec23IP and VPS13B^sfGFP as in E, cells (n = 13) co-expressing Halo-Sec23A and VPS13B^sfGFP or cells (n = 15) co-expressing sec16A-flag and VPS13B^sfGFP (20 cells) as in M. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (O) coIP assay showing Sec23IP, but not Sec16 or Sec23A, interacted with VPS13B^sfGFP. The blue arrow denoted VPS13B^sfGFP while the red arrows indicated Halo-Sec23IP in the blots. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in C–E, G, I, K, and M. Source data are available for this figure: SourceData F2.
Sec23IP KO results in Golgi fragmentation phenocopying the VPS13B KO in HeLa. (A) CRISPR knock-out of Sec23IP in HeLa cells (Sec23IP-KO). Two sgRNAs are used, indicating the PAMs (GGG for sgRNA1 and sgRNA2) for spCas9. (B) Western blots of six Sec23IP-KO clones in A. Sec23IP-KO clone-1 and -2 were used in the study. (C) Representative images of fixed control or two Sec23IP-KO clones as in B stained with anti-GM130 with two insets on the right. (D) Representative images of a live HeLa cell expressing GFP-Sec23IP (magenta) and MGAT2-Halo (green) with an inset on the bottom. (E) Representative images of fixed HeLa cells expressing Halo-Sec23IP (green) stained with GM130 antibody (blue) and Sec31A antibody (magenta) with two insets at the bottom. Red arrows indicated a cell with the expression of Halo-Sec23IP while blue arrows denoted a cell without Halo-Sec23IP expression. (F) Percentage of Sec31A puncta associated with the Golgi in either control (12 cells), WT cells expressing Halo-Sec23IP (10 cells), or VPS13B KO-1 cells expressing Halo-Sec23IP (12 cells) from three independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (G) Top: representative images of Sec23IP KO HeLa cell transiently expressing VPS13B^sfGFP (magenta) and stained for Sec31A (green) with two insets on the right. Bottom: representative images of VPS13B KO HeLa cell transiently expressing Halo-Sec23IP (green) and stained for GM130 (magenta) with two insets on the right. (H) HeLa cells expressing VPS13B^sfGFP, Halo-Sec23IP, and stained by DAPI, were fixed and imaged by 3D microscopy (top left panel) and processed for TEM. Fluorescence and TEM images were correlated (top middle panel) with insets showing associations between the cis-medial Golgi marked by VPS13B^sfGFP (green) and ERES labeled by Halo-Sec23IP (magenta). (I) Western blots showing the efficiency of siRNA-mediated Sec23IP depletion in VPS13B KO HeLa cells. (J) Representative images of a live HeLa cell transiently expressing GFP-VPS13B-VAB (green), Halo-Sec23IP (magenta), and mCh-Sec23A (blue) with an inset on the bottom. (K) Pearson’s correlation coefficient of Halo-Sec23IP versus mCh-Sec23A as shown in D in the absence (10 cells) or presence of GFP-VPS13B-VAB (9 cells) in three independent experiments. Mean ± SD. Two tailed unpaired Student’s t test. Scale bar, 10 μm in whole and 2 μm in insets in C–E, G, and J, and 0.2 μm in the insets in H. Source data are available for this figure: SourceData FS4.
Sec23IP KO results in Golgi fragmentation phenocopying the VPS13B KO in HeLa. (A) CRISPR knock-out of Sec23IP in HeLa cells (Sec23IP-KO). Two sgRNAs are used, indicating the PAMs (GGG for sgRNA1 and sgRNA2) for spCas9. (B) Western blots of six Sec23IP-KO clones in A. Sec23IP-KO clone-1 and -2 were used in the study. (C) Representative images of fixed control or two Sec23IP-KO clones as in B stained with anti-GM130 with two insets on the right. (D) Representative images of a live HeLa cell expressing GFP-Sec23IP (magenta) and MGAT2-Halo (green) with an inset on the bottom. (E) Representative images of fixed HeLa cells expressing Halo-Sec23IP (green) stained with GM130 antibody (blue) and Sec31A antibody (magenta) with two insets at the bottom. Red arrows indicated a cell with the expression of Halo-Sec23IP while blue arrows denoted a cell without Halo-Sec23IP expression. (F) Percentage of Sec31A puncta associated with the Golgi in either control (12 cells), WT cells expressing Halo-Sec23IP (10 cells), or VPS13B KO-1 cells expressing Halo-Sec23IP (12 cells) from three independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (G) Top: representative images of Sec23IP KO HeLa cell transiently expressing VPS13B^sfGFP (magenta) and stained for Sec31A (green) with two insets on the right. Bottom: representative images of VPS13B KO HeLa cell transiently expressing Halo-Sec23IP (green) and stained for GM130 (magenta) with two insets on the right. (H) HeLa cells expressing VPS13B^sfGFP, Halo-Sec23IP, and stained by DAPI, were fixed and imaged by 3D microscopy (top left panel) and processed for TEM. Fluorescence and TEM images were correlated (top middle panel) with insets showing associations between the cis-medial Golgi marked by VPS13B^sfGFP (green) and ERES labeled by Halo-Sec23IP (magenta). (I) Western blots showing the efficiency of siRNA-mediated Sec23IP depletion in VPS13B KO HeLa cells. (J) Representative images of a live HeLa cell transiently expressing GFP-VPS13B-VAB (green), Halo-Sec23IP (magenta), and mCh-Sec23A (blue) with an inset on the bottom. (K) Pearson’s correlation coefficient of Halo-Sec23IP versus mCh-Sec23A as shown in D in the absence (10 cells) or presence of GFP-VPS13B-VAB (9 cells) in three independent experiments. Mean ± SD. Two tailed unpaired Student’s t test. Scale bar, 10 μm in whole and 2 μm in insets in C–E, G, and J, and 0.2 μm in the insets in H. Source data are available for this figure: SourceData FS4.
To confirm that VPS13B interacts with Sec23IP at the endogenous level, we performed co-IP assays. Endogenous Sec23IP could be copelleted by endogenous VPS13B (Fig. 2 B). Of note, there was a “cross-reacting” band highly concentrated in the anti-VPS13B precipitates, and further study is needed to investigate whether it is a modified form of Sec23IP.
Next, we examined whether the interaction between Sec23IP and VPS13B was strong enough to mediate recruitment. Consistent with previous studies (Ong et al., 2010; Shimoi et al., 2005), both endogenous (Fig. 2 C) and exogenous Sec23IP (Fig. S4 D) formed puncta over the cytosol that colocalized with Sec31A, an ER exit site (ERES) marker, with a substantial fraction of Sec23IP puncta tightly associated with the cis-/medial Golgi (inset 1) and a portion at cell periphery (inset 2). Strikingly, a much larger proportion of Halo-Sec23IP puncta was recruited to the Golgi when VPS13B^sfGFP was coexpressed (Fig. 2 D). This suggests that ERES is strongly recruited to the Golgi via VPS13B–Sec23IP interaction.
To test this hypothesis, we examined the spatial relationship between ERES (anti-Sec31A) and the cis-/medial Golgi (anti-GM130) upon coexpression of VPS13B^sfGFP and Halo-Sec23IP. IF images showed that ERES was indeed greatly recruited to the cis-/medial Golgi and formed an extensive ERES-Golgi interface (Fig. 2 E). The association was so evident that most ERES were tightly associated with the cis-Golgi, resulting in much fewer ERES in the periphery of cells (Fig. 2 F).
Next, we investigated whether the recruitment between VPS13B and Sec23IP can occur at the endogenous level. Indeed, we found that endogenous Sec23IP was recruited to the Golgi positive for VPS13B^sfGFP (Fig. 2, G and H), as indicated by a significant increase in the percentage of ERES (anti-Sec31A) around the cis-Golgi (anti-GM130), compared to cells without plasmid transfection in the same field of view (Fig. 2 G; middle versus bottom panel). In addition, the expression of Halo-Sec23IP also increased the association between ERES (anti-Sec31A) and the Golgi (anti-GM130) (Fig. S4, E and F).
In addition, we asked whether the effect of overexpression of VPS13B on ERES clustering around the Golgi was dependent on Sec23IP and vice versa. Neither VPS13B^sfGFP nor Halo-Sec23IP alone could significantly increase the association in Sec23IP KO or VPS13B KO cells (Fig. S4 G, Fig. 2 H, and Fig. S4 F), respectively. Together, these results suggest that VPS13B and Sec23IP interact with each other to mediate the ERES–Golgi association.
Since VPS13B was mainly associated with the Golgi in the perinuclear region of cells, the co-staining for these two proteins at the endogenous level was mainly found near the Golgi but not at the cell periphery (Fig. 2, I and J). This suggests that the interaction between VPS13B and Sec23IP specifically occurred at ERES–Golgi interfaces.
To obtain ultrastructural details about the extensive ERES–Golgi interface mediated by the coexpression of VPS13B and Sec23IP, we performed correlative light and electron microscopy (CLEM). We observed that the Golgi appeared to be compacted and fragmented upon the overexpression of Sec23IP (Shimoi et al., 2005), the membranes positive for Halo-Sec23IP appeared to be in the form of small vesicle clusters that were adjacent to the Golgi marked by VPS13B^sfGFP (Fig. S4 H), and the cluster of small vesicles may result from a trafficking block of cargo carriers between ERES and the Golgi due to the effects of overexpression. In addition, we also observed that several lipid droplets (dark-staining structures; yellow arrows) were present near the Golgi in the cell (Fig. S4 H), consistent with a possible role of VPS13B in the regulation of Golgi-LD association (Du et al., 2023b), suggesting that VPS13B may be involved in two types of Golgi-associated contacts, ERES–Golgi interface, and Golgi–LD contacts.
Next, we asked whether VPS13B or Sec23IP was required for the formation or maintenance of ERES–Golgi interfaces. The association between the cis-Golgi and ERES was not completely abolished, but resulted in a significant reduction in two independent VPS13B KO clones (Fig. 2 K), as shown by the colocalization analyses (Fig. 2 L). In addition, colocalization analysis showed that siRNA-mediated suppression of Sec23IP in VPS13B KO cells reduced the ERES-Golgi associations to a higher extent compared with the VPS13B KO (Fig. 2, K and L; and Fig. S4 I). These results suggest that VPS13B was necessary for the association between ERES and the Golgi, and other factors besides VPS13B might be also involved. Nevertheless, it should be noted that the depletion of VPS13B or Sec23IP resulted in Golgi fragmentation, which would consequently affect the ERES-Golgi association measured by colocalization. Further investigations are required to validate the direct role of VPS13B and Sec23IP in promoting the ERES–Golgi association.
We then investigated whether the function of VPS13B in promoting ERES–Golgi association was specific to Sec23IP. We found that coexpression of either Sec16A (Watson et al., 2006) or Sec23A, both of which were ERES proteins, with VPS13B^sfGFP could not promote the association as strongly as the coexpression of Sec23IP and VPS13B (Fig. 2, M and N). Importantly, our coIP assays showed that VPS13B^sfGFP specifically interacted with Sec23IP but not Sec16A and Sec23A (Fig. 2 O). Collectively, our results suggest that Sec23IP acts as an adaptor to recruit VPS13B to ERES-Golgi interface.
VPS13B bound to the NT of Sec23IP via the VAB domain
Next, we investigated the mechanism by which VPS13B interacted with Sec23IP. The VAB domain of VPS13B alone was diffused in the cytosol (Fig. 3 A). However, a significant portion of the VPS13B-VAB was recruited to ERES by Halo-Sec23IP (Fig. 3 B). Accordingly, GFP-trap assays confirmed the interaction of GFP-VPS13B-VAB with Halo-Sec23IP (Fig. 3 C). In addition, we found that neither the VPS13B-NT nor the PH domain were recruited by Halo-Sec23IP (Fig. 3 D; left panels). These results suggest that VPS13B interacts with Sec23IP via the VAB domain.
The VAB domain of VPS13B is responsible for the interaction with Sec23IP. (A and B) Representative images of live HeLa cells expressing GFP-VPS13B-VAB (green) alone (A) or coexpression of GFP-VPS13B-VAB (green) and Halo-Sec23IP (magenta) (B) with insets. (C) coIP assays showed an interaction between GFP-VPS13B-VAB and Halo-Sec23IP in HEK293 cells. (D) Representative images of live HeLa cells expressing indicated GFP-VPS13B-VAB truncations (green) and Halo-Sec23IP (magenta) with insets. (E) The schematic diagram and AlphaFold-predicted structure of six repeats in the VAB domain of VPS13B. (F) coIP assays showing interactions between indicated GFP-VPS13B-VAB truncations and Halo-Sec23IP in HEK293 cells. (G) Pulldown assays showing direct interactions between purified GFP-VPS13B-VAB, GFP-VAB-R1-4, GFP-R1-5, or two disease-associated missense mutants (N2993S and R3223W) and purified GST-Sec23IP. (H) Top: domain organization of Sec23IP. Bottom: representative images of live HeLa cells expressing indicated Halo-Sec23IP truncations (magenta) and GFP-VPS13B-VAB (green) with insets. (I) coIP assays showing interactions between indicated Halo-Sec23IP truncations and GFP-VPS13B-VAB in HEK293 cells. (J) AlphaFold predicted structures of Sec23IP-NT showing the locations of potential PxP Vps13-interaction motifs with partial matches to the consensus ϕxx ϕxPxPϕxϕ, where ϕ is a hydrophobic residue. (K) coIP assays showing interactions between indicated Halo-Sec23IP mutations and GFP-VPS13B-VAB in HEK293 cells. (L) Pulldown assays showing direct interactions between GFP-VPS13B-VAB and purified indicated GST-Sec23IP truncations. Red stars denoted purified proteins. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in A, B, D, and H. Source data are available for this figure: SourceData F3.
The VAB domain of VPS13B is responsible for the interaction with Sec23IP. (A and B) Representative images of live HeLa cells expressing GFP-VPS13B-VAB (green) alone (A) or coexpression of GFP-VPS13B-VAB (green) and Halo-Sec23IP (magenta) (B) with insets. (C) coIP assays showed an interaction between GFP-VPS13B-VAB and Halo-Sec23IP in HEK293 cells. (D) Representative images of live HeLa cells expressing indicated GFP-VPS13B-VAB truncations (green) and Halo-Sec23IP (magenta) with insets. (E) The schematic diagram and AlphaFold-predicted structure of six repeats in the VAB domain of VPS13B. (F) coIP assays showing interactions between indicated GFP-VPS13B-VAB truncations and Halo-Sec23IP in HEK293 cells. (G) Pulldown assays showing direct interactions between purified GFP-VPS13B-VAB, GFP-VAB-R1-4, GFP-R1-5, or two disease-associated missense mutants (N2993S and R3223W) and purified GST-Sec23IP. (H) Top: domain organization of Sec23IP. Bottom: representative images of live HeLa cells expressing indicated Halo-Sec23IP truncations (magenta) and GFP-VPS13B-VAB (green) with insets. (I) coIP assays showing interactions between indicated Halo-Sec23IP truncations and GFP-VPS13B-VAB in HEK293 cells. (J) AlphaFold predicted structures of Sec23IP-NT showing the locations of potential PxP Vps13-interaction motifs with partial matches to the consensus ϕxx ϕxPxPϕxϕ, where ϕ is a hydrophobic residue. (K) coIP assays showing interactions between indicated Halo-Sec23IP mutations and GFP-VPS13B-VAB in HEK293 cells. (L) Pulldown assays showing direct interactions between GFP-VPS13B-VAB and purified indicated GST-Sec23IP truncations. Red stars denoted purified proteins. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in A, B, D, and H. Source data are available for this figure: SourceData F3.
The VAB domain of mammalian VPS13 proteins contained six repeats (R1–R6) (Fig. 3 E, top panel) (Bean et al., 2018), the structure of which was further predicted by AlphaFold (Fig. 3 E, bottom panel). Our coIP results showed that R2, R3, R4, and R5, other than R1 or R6, could interact with Sec23IP, but to a lesser extent compared to the intact VAB domain (Fig. 3 F), suggesting R2–5 may act as a core complex for binding to Sec23IP while R1 and R6 may be supplementary to the interaction. In addition, imaging results showed that each of these six repeats could not be recruited by Sec23IP, and deletion of the R1 but not R6 abolished the recruitment (Fig. 3 D; right panels), indicating that R1, other than R6, is required for the VPS13B–Sec23IP interaction.
We further asked whether the VPS13B–VAB directly interacts with Sec23IP by performing in vitro pulldown assays. In this assay, we used GFP-trap to pellet GFP-VPS13B-VAB from HEK293 cells transiently expressing GFP–VPS13B–VAB by using a high-salt (500 mM NaCl) lysis buffer, as described previously (Du et al., 2023a). After rigorous washing to remove proteins that could copellet with GFP–VPS13B–VAB under high-salt conditions, GFP–VPS13B–VAB beads were incubated with purified glutathione S-transferase (GST) tag alone or with GST-Sec23IP, respectively. Indeed, GFP–VPS13B–VAB was bound to GST-Sec23IP but not to the GST tag (Fig. 3 G), indicating that the VAB domain of VPS13B was bound to Sec23IP. Consistently, the in vitro pulldown assays also demonstrated that the binding of a region containing R1–4 to GST-Sec23IP was significantly weaker than either a region containing R1–5 or the intact VPS13B-VAB (Fig. 3 G). Collectively, these results suggest that VPS13B–VAB binds to Sec23IP likely via R2–5.
Next, we asked how Sec23IP interacted with VPS13B. Sec23IP harbored an NT region, a sterile alpha motif (SAM), and a DDHD domain (Fig. 3 H, top panel). We found that deletion of the NT region, but not other domains, abolished the colocalization with the VPS13B-VAB (Fig. 3 H, bottom panel), suggesting that the NT of Sec23IP was necessary for the interaction with VPS13B–VAB. This result was further confirmed by GFP-trap assays (Fig. 3 I).
A motif including the consensus sequence Pro-X-Pro has been shown to mediate the binding of Vps13 partner proteins to the VAB domain in both yeast (Bean et al., 2018) and other human Vps13 family proteins (Guillen-Samander et al., 2021; Hancock-Cerutti et al., 2022; Kumar et al., 2018). Interestingly, we found that the Sec23IP-NT contained six putative PxP motifs (Fig. 3 J), and our biochemical results showed that deletion of the Pro-X-Pro sequence in two of these motifs individually (resides 176–178 or residues 213–215) reduced the interaction with VPS13B–VAB (Fig. 3 K).
In addition, in vitro pulldown assays showed that purified GST-Sec23IP-NT, but not GST-Sec23IP- ΔNT, bound to GFP–VPS13B–VAB, and the binding of GST–Sec23IP–NT to GFP–VPS13B–VAB appears to be stronger than that of GST-Sec23IP (Fig. 3 L), confirming that Sec23IP-NT is sufficient for the binding to VPS13B–VAB. Further, the pulldown assay also showed that deletion of the PxP motif (resides 176–178) reduced the binding of Sec23IP–NT to VPS13B–VAB (Fig. 3 L). Of note, the deletion of each of these PxP motifs did not completely abolish the interaction, suggesting multivalent interactions between multiple PxP motifs of Sec23IP and repeats of VPS13B-VAB.
Considering that Sec23IP also interacted with Sec31A via its NT (Ong et al., 2010), we tested whether VPS13B and Sec31A competed with each other for binding to Sec23IP at ERES. Sec23IP was able to colocalize with VPS13B-VAB and Sec31A (Fig. S4, J and K), suggesting that Sec23IP could simultaneously bind to VPS13B and Sec31A at ERES.
Cohen syndrome–associated missense mutations in VPS13B–VAB impaired the interaction with Sec23IP
Importantly, several missense mutants from Cohen syndrome patients were found in the VAB domain of VPS13B (Seifert et al., 2009; Zorn et al., 2022). We therefore systematically examined whether these pathogenic mutations in the VAB domain had impacts on the interaction between VPS13B and Sec23IP (Fig. 4 A). Our colocalization analyses showed that most of these VAB mutants interacted with Sec23IP to a lesser extent than the WT VAB (Fig. 4, B–M). Among these mutants, G2729R and N2993S most significantly impaired the interaction with Sec23IP, as shown by the colocalization analyses (Fig. 4 N).
Cohen disease–associated point mutants on VPS13B-VAB domain impaired the interaction with Sec23IP. (A) The disease-associated missense mutants indicated in the AlphaFold-predicted structures of VPS13B-CT. (B–M) Representative images of live HeLa cells expressing GFP-VPS13B-VAB containing these disease-associated mutants (green) and Halo-Sec23IP (magenta) with two insets on the bottom. (N) Pearson’s correlation coefficient of disease mutants of GFP-VPS13B-VAB versus Halo-Sec23IP as shown in B–M in three independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (O) coIP assays showed interactions between these disease mutants of GFP-VPS13B-VAB and Halo-Sec23IP as in B–M in HEK293 cells. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in B–M. Source data are available for this figure: SourceData F4.
Cohen disease–associated point mutants on VPS13B-VAB domain impaired the interaction with Sec23IP. (A) The disease-associated missense mutants indicated in the AlphaFold-predicted structures of VPS13B-CT. (B–M) Representative images of live HeLa cells expressing GFP-VPS13B-VAB containing these disease-associated mutants (green) and Halo-Sec23IP (magenta) with two insets on the bottom. (N) Pearson’s correlation coefficient of disease mutants of GFP-VPS13B-VAB versus Halo-Sec23IP as shown in B–M in three independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. (O) coIP assays showed interactions between these disease mutants of GFP-VPS13B-VAB and Halo-Sec23IP as in B–M in HEK293 cells. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in B–M. Source data are available for this figure: SourceData F4.
Accordingly, GFP-trap assays demonstrated that these pathogenic point mutations inhibited the interaction with Sec23IP (Fig. 4 O). Among these mutants, G2729R, N2993S, S3142R, and S3328R almost completely inhibited the interaction with Sec23IP (Fig. 4 O). In addition, in vitro pulldown assays showed that N2993S greatly reduced the binding with purified GST-Sec23IP while the other mutant R3323W only had a moderate effect (Fig. 3 G), in line with our imaging and coIP results.
Importantly, most pathogenic mutations of VPS13B were nonsense mutants that resulted in premature termination and the absence of the VAB domain (Pirgon, 2013; Seifert et al., 2009), eventually leading to the loss of the ability to interact with Sec23IP. Together, our results suggest a link between the VPS13B–Sec23IP interaction and Cohen disease, and a defect in the interaction may contribute to the pathogenesis of this disease.
VPS13B was required for the biogenesis of tubular ERGIC
Bridge-like RGB repeating lipid transporters, including Vps13 proteins and Atg2, were thought to play a key role in de novo biogenesis of organelles by providing membrane lipids to meet requirements during membrane expansion and growth (Baldwin et al., 2021; Enyenihi and Saunders, 2003; Osawa et al., 2019; Park and Neiman, 2012; Park et al., 2013; Valverde et al., 2019). Since our results showed that VPS13B interacted with Sec23IP at ERES-Golgi interfaces, we wondered whether VPS13B and Sec23IP were required for the biogenesis of membrane structures in the early secretory pathway. The formation of ERES, marked by either endogenous Sec31A (outer coat; Fig. S5 A) or Sec23IP (Fig. S5 B) in IF, appeared not to be greatly affected upon VPS13B KO. COPI vesicles labeled by the coatomer subunits COPA were not significantly affected as well (Fig. S5 C). Furthermore, the number or size of the conventional tubulo-vesicular ER–Golgi Intermediate Compartment (ERGIC), marked by ERGIC53, appeared not to be strongly altered in VPS13B KO (Fig. S5, D–F) except for a moderate increase in the number of ERGIC53 puncta. The specificity of the ERGIC53 antibody in IF was validated by siRNA-mediated depletion (Fig. S5 G).
Supplementary data toFig. 5 . (A–D) Representative images of fixed control (left panel) or VPS13B KO (right panel) HeLa cells stained with antibodies against Sec31A (A), Sec23IP (B), COPA (C), and ERGIC53 (D) with two insets to the right. (E and F) The number (E) and length (F) of ERGIC53 as in D. More than 20 cells were quantified for each condition from three independent experiments. Mean ± SD. Two-tailed unpaired Student’s t test. (G) Representative images of ERGIC53 siRNA-treated HeLa cells stained with antibodies against ERGIC53 (green) and GM130 (magenta). (H) Representative images of live HeLa cells transiently expressing VPS13B^sfGFP (green) and Halo-Rab1B (magenta) with an inset on the right. (I) Pearson’s correlation coefficient of VPS13B^sfGFP versus Halo-Rab1B as shown in (H; n = 11). (J) CRISPR knock-out of VPS13B in HEK293 cells. Two sgRNAs are used, indicating the PAMs (GGA for sgRNA1 and GGT for sgRNA2) for spCas9. (K) DNA gel of a VPS13B-KO HEK293 pool in A. (L) AlphaFold predicted structures of a VPS13B lipid transfer-defective mutant (VPS13B-LTPmut^GFP) with mutated hydrophobic residues in the midway of the hydrophobic groove. (M) Strategy for generation of VPS13B KO C57BL/6J mouse strain by CRISPR-Cas9, in which 51,822 bp between exon 1 and exon 7 of the VPS13B gene is deleted in VPS13B−/− mice. (N) DNA gel showing genotypes of WT, heterozygous (HE), and homozygous (HO) VPS13B KO mice. Scale bar, 10 μm in whole and 2 μm in insets in A–D, G, and H. Source data are available for this figure: SourceData FS5.
Supplementary data toFig. 5 . (A–D) Representative images of fixed control (left panel) or VPS13B KO (right panel) HeLa cells stained with antibodies against Sec31A (A), Sec23IP (B), COPA (C), and ERGIC53 (D) with two insets to the right. (E and F) The number (E) and length (F) of ERGIC53 as in D. More than 20 cells were quantified for each condition from three independent experiments. Mean ± SD. Two-tailed unpaired Student’s t test. (G) Representative images of ERGIC53 siRNA-treated HeLa cells stained with antibodies against ERGIC53 (green) and GM130 (magenta). (H) Representative images of live HeLa cells transiently expressing VPS13B^sfGFP (green) and Halo-Rab1B (magenta) with an inset on the right. (I) Pearson’s correlation coefficient of VPS13B^sfGFP versus Halo-Rab1B as shown in (H; n = 11). (J) CRISPR knock-out of VPS13B in HEK293 cells. Two sgRNAs are used, indicating the PAMs (GGA for sgRNA1 and GGT for sgRNA2) for spCas9. (K) DNA gel of a VPS13B-KO HEK293 pool in A. (L) AlphaFold predicted structures of a VPS13B lipid transfer-defective mutant (VPS13B-LTPmut^GFP) with mutated hydrophobic residues in the midway of the hydrophobic groove. (M) Strategy for generation of VPS13B KO C57BL/6J mouse strain by CRISPR-Cas9, in which 51,822 bp between exon 1 and exon 7 of the VPS13B gene is deleted in VPS13B−/− mice. (N) DNA gel showing genotypes of WT, heterozygous (HE), and homozygous (HO) VPS13B KO mice. Scale bar, 10 μm in whole and 2 μm in insets in A–D, G, and H. Source data are available for this figure: SourceData FS5.
Importantly, we found that VPS13B KO dramatically impaired the formation of an unconventional tubular ERGIC (tERGIC) (Fig. 5, A and B), which specifically accelerated ER-to-Golgi trafficking of certain soluble cargoes (Yan et al., 2022). In control cells, expression of GFP-Rab1B induced the formation of tERIGC, which was highly elongated (Fig. 5 A). In contrast, VPS13B KO greatly impaired the formation of Rab1B-induced tERGIC (Fig. 5 B), resulting in a strong reduction in both the length (Fig. 5 D) and number (Fig. 5 E) compared with control. Consistent with the role of VPS13B in tERGIC formation, we found that VPS13B^sfGFP was associated with the tubular membrane structures labeled by Halo-Rab1B (Fig. S5, H and I). In addition, we found that the formation of tERGIC was also significantly impaired in two independent Sec23IP KO clones (Fig. 5, C–E). Together, these results indicated that VPS13B and its binding partner Sec23IP were indispensable for tERGIC formation.
VPS13B KO blocks the formation of tubular ERGIC. (A–C) Representative images of live control HeLa (A), two VPS13B KO clones (B), or two Sec23IP KO clones (C) expressing GFP-Rab1B with two insets on the bottom. The left inset was from the Golgi region and the right inset was from the cell periphery. (D and E) The length (D) or number (E) of tERGIC labeled by GFP-Rab1B. N = 257 from 23 control cells, N = 120 from 35 VPS13B KO cells, and N = 20 from 19 Sec23IP KO cells in three independent experiments. Mean ± SD. Two-tailed unpaired Student’s t test. (F) Top: Representative images of WT and VPS13B-KO HEK293 pool transiently expressing GFP-Rab1B along with either WT VPS13B^sfGFP, VPS13B-LTPmut-GFP, or VPS13B- ΔR1-6^sfGFP with two insets on the bottom. Bottom: VPS13B-KO HEK293 pool transiently expressing disease mutants (VPS13B^sfGFP-N2993S, VPS13B^sfGFP-S3142R, VPS13B^sfGFP-S3328R) or VPS13B-VAB-PH-GFP along with GFP-Rab1B with two insets on the bottom. (G and H) The length (G) and number (H) of GFP-Rab1B tERGIC in F. More than 20 cells were quantified for each condition from 3 independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in A–C and F.
VPS13B KO blocks the formation of tubular ERGIC. (A–C) Representative images of live control HeLa (A), two VPS13B KO clones (B), or two Sec23IP KO clones (C) expressing GFP-Rab1B with two insets on the bottom. The left inset was from the Golgi region and the right inset was from the cell periphery. (D and E) The length (D) or number (E) of tERGIC labeled by GFP-Rab1B. N = 257 from 23 control cells, N = 120 from 35 VPS13B KO cells, and N = 20 from 19 Sec23IP KO cells in three independent experiments. Mean ± SD. Two-tailed unpaired Student’s t test. (F) Top: Representative images of WT and VPS13B-KO HEK293 pool transiently expressing GFP-Rab1B along with either WT VPS13B^sfGFP, VPS13B-LTPmut-GFP, or VPS13B- ΔR1-6^sfGFP with two insets on the bottom. Bottom: VPS13B-KO HEK293 pool transiently expressing disease mutants (VPS13B^sfGFP-N2993S, VPS13B^sfGFP-S3142R, VPS13B^sfGFP-S3328R) or VPS13B-VAB-PH-GFP along with GFP-Rab1B with two insets on the bottom. (G and H) The length (G) and number (H) of GFP-Rab1B tERGIC in F. More than 20 cells were quantified for each condition from 3 independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Mean ± SD. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in A–C and F.
Next, we investigated the mechanisms underlying the role of VPS13B in tERGIC formation by performing rescue experiments. The transfection efficiency of the VPS13B construct and its mutants in VPS13B KO HeLa cells was low and unsuitable for the rescue experiments. Therefore, we generated a pool of VPS13B-KO HEK293 cells using CRISPR-Cas9 (Fig. S5, J and K). The tERGIC defect was robustly observed in the VPS13B-KO HEK293 cell pool (Fig. 5 F). Remarkably, the introduction of WT VPS13B^sfGFP significantly rescued the tERGIC phenotype (Fig. 5, F–H), indicating that the defect in the tERGIC formation was specific to VPS13B.
Next, we investigated whether and to what extent the formation of tERGIC was dependent on the lipid transfer activity of VPS13B. First, we made a putative lipid transfer-deficient mutant (VPS13B-LTPmut-GFP), in which a few hydrophobic residues in the midway of the hydrophobic groove of VPS13B were mutated to hydrophilic residues (Fig. S5 L) to block lipid transport according to a recent study on Vps13 (Li et al., 2020). Importantly, the lipid-transfer-deficient mutant was unable to rescue the defect in tERGIC formation resulting from VPS13B KO (Fig. 5, F–H), suggesting that lipid transfer of VPS13B is indispensable for tERGIC formation.
In addition, we found that the introduction of a Sec23IP binding defective VPS13B mutant (VPS13B-∆R1-6) into VPS13B KO HEK293 cells was unable to fully rescue the phenotype (Fig. 5, F–H). Consistently, the disease-related missense mutants defective in binding to Sec23IP, N2993S, S3142R, and S3328R did not significantly restore the tERGIC defect (Fig. 5, F–H). A truncated mutant only containing the VAB and PH domain was also unable to restore the defect as well (Fig. 5, F–H). Therefore, our findings suggest that both the VAB domain and the lipid transfer activity are indispensable for the process.
Depletion of VPS13B or Sec23IP delayed the ER export of procollagen
One of the cardinal features in patients with Cohen syndrome was joint hypermobility, which was linked to defects in collagen biogenesis and/or secretion (Malfait et al., 2006). Therefore, we asked whether VPS13B is involved in ER–Golgi trafficking of procollagen, the most abundant protein in the human body. We tracked the ER-to-Golgi trafficking of procollagen using primary mouse embryonic fibroblasts (MEF) as a cell model. In IF assays, we used an antibody (SP1.D8) that specifically recognized intracellular procollagen IA. The release of procollagen from the ER was synchronized by the addition of ascorbic acid (Fig. 6 A), which was a key factor for hydroxyproline formation in procollagen (Pinnell et al., 1987). As a control, the ER exit of procollagen IA was blocked by siRNA-mediated depletion of Sar1A, a master regulator of ER–Golgi trafficking (Fig. 6 B). Importantly, siRNA-mediated depletion of VPS13B (Fig. 6 C) or Sec23IP (Fig. 6 D) did not block the trafficking but caused accumulation of procollagen in the ER prior to release (Fig. 6 F) and resulted in a significant delay in ER export of procollagen compared with the control, as shown by the changes in intracellular procollagen IA fluorescence over time (Fig. 6 G). Of note, the effect was more evident in Sec23IP depletion compared with VPS13B, suggesting that Sec23IP may affect ER export of procollagen via other pathways independently of VPS13B, for instance, by modulation of the organization of ERES (Ong et al., 2010; Shimoi et al., 2005).
Depletion of VPS13B or Sec23IP delays ER exit of procollagen. (A–E) Representative images of fixed control (A), Sar1A-depleted (B), VPS13B-depleted (C), Sec23IP-depleted (D), Tango1-depleted (E) primary MEF cells labeled with anti-procollagen (magenta) and anti-GM130 (green) upon ascorbic acid (50 μM) stimulation with five timepoints (0, 30, 60, 90, 120 min). (F) The fluorescence intensity of procollagen prior to ascorbic acid stimulation. 23 control cells, 10 Sec23IP depleted cells, 17 VPS13B depleted cells, 10 Sar1A depleted cells, and 9 Tango1 depleted cells in three independent experiments were quantified. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (G) The changes of fluorescence intensity of procollagen upon ascorbic acid stimulation as in A–E. 23 control cells, 10 Sec23IP depleted cells, 17 VPS13B depleted cells, 10 Sar1A depleted cells, and 9 Tango1 depleted cells in three independent experiments were quantified. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (H) Volcano plot of proteins in the medium of control compared to VPS13B-depleted primary MEF cells. Candidates that were considered significant (−log [P value] >1.3; P < 0.05) were labeled in orange (Log2 [fold change] >1.2; increased in abundance) or blue (Log2 [fold change] less than −1.2; decreased in abundance). All collagens identified in the quantitative MS were labeled. (I) qPCR assays indicated the efficiency of siRNA-mediated suppression of VPS13B, Sec23IP, Sar1A, and Tango1 in primary MEF cells from three independent experiments. Mean ± SD. Two tailed unpaired Student’s t test. (J and K) Representative confocal images of fixed primary MEF cells from WT (J) or VPS13B KO (K) mice labeled with anti-procollagen (magenta) and anti-GM130 (green) upon ascorbic acid (50 μM) stimulation with five timepoints (0, 30, 60, 90, 120 min). (L) The fluorescence intensity of anti-procollagen prior to ascorbic acid stimulation. 24 control cells, 29 VPS13B KO cells in 3 independent experiments were quantified. Mean ± SD. Two-tailed unpaired Student’s t test. (M) The changes in fluorescence intensity of procollagen upon ascorbic acid stimulation as in J and K. 24 control cells, 29 VPS13B KO cells in three independent experiments were analyzed. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Scale bar, 10 μm in A–E, J, and K.
Depletion of VPS13B or Sec23IP delays ER exit of procollagen. (A–E) Representative images of fixed control (A), Sar1A-depleted (B), VPS13B-depleted (C), Sec23IP-depleted (D), Tango1-depleted (E) primary MEF cells labeled with anti-procollagen (magenta) and anti-GM130 (green) upon ascorbic acid (50 μM) stimulation with five timepoints (0, 30, 60, 90, 120 min). (F) The fluorescence intensity of procollagen prior to ascorbic acid stimulation. 23 control cells, 10 Sec23IP depleted cells, 17 VPS13B depleted cells, 10 Sar1A depleted cells, and 9 Tango1 depleted cells in three independent experiments were quantified. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (G) The changes of fluorescence intensity of procollagen upon ascorbic acid stimulation as in A–E. 23 control cells, 10 Sec23IP depleted cells, 17 VPS13B depleted cells, 10 Sar1A depleted cells, and 9 Tango1 depleted cells in three independent experiments were quantified. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (H) Volcano plot of proteins in the medium of control compared to VPS13B-depleted primary MEF cells. Candidates that were considered significant (−log [P value] >1.3; P < 0.05) were labeled in orange (Log2 [fold change] >1.2; increased in abundance) or blue (Log2 [fold change] less than −1.2; decreased in abundance). All collagens identified in the quantitative MS were labeled. (I) qPCR assays indicated the efficiency of siRNA-mediated suppression of VPS13B, Sec23IP, Sar1A, and Tango1 in primary MEF cells from three independent experiments. Mean ± SD. Two tailed unpaired Student’s t test. (J and K) Representative confocal images of fixed primary MEF cells from WT (J) or VPS13B KO (K) mice labeled with anti-procollagen (magenta) and anti-GM130 (green) upon ascorbic acid (50 μM) stimulation with five timepoints (0, 30, 60, 90, 120 min). (L) The fluorescence intensity of anti-procollagen prior to ascorbic acid stimulation. 24 control cells, 29 VPS13B KO cells in 3 independent experiments were quantified. Mean ± SD. Two-tailed unpaired Student’s t test. (M) The changes in fluorescence intensity of procollagen upon ascorbic acid stimulation as in J and K. 24 control cells, 29 VPS13B KO cells in three independent experiments were analyzed. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Scale bar, 10 μm in A–E, J, and K.
To further confirm the effects of VPS13B depletion in collagen secretion, we examined the secreted collagens in the medium of primary MEFs synchronized by ascorbic acid using quantitative mass spectrometry. Several collagen species, including collagen IA, were successfully identified in our MS assays (Fig. 6 H), and the levels of all these collagens were significantly reduced upon VPS13B depletion compared with the control (Fig. 6 H). Of note, VPS13B depletion led to aberrant increases in the secretion of some proteins, which may be due to indirect consequences of disorganized tERGIC or the Golgi resulting from VPS13B depletion.
We next investigated the ER export of procollagen in primary MEFs from VPS13B−/− mice (Fig. S5, M and N). Accordingly, procollagen trafficking was substantially delayed in the primary VPS13B−/− MEF cells compared with MEF cells from WT littermates (Fig. 6, J–M). Therefore, these results suggest that VPS13B is required for efficient ER-to-Golgi trafficking of procollagen.
Next, we compared the role of VPS13B and Sec23IP with Tango1, an ERES regulator that was shown to have a major effect on collagen secretion (Saito et al., 2011; Santos et al., 2015; Liu et al., 2017; Raote et al., 2018). siRNA-mediated Tango1 depletion inhibited procollagen secretion (Fig. 6 E) to a much greater extent compared with VPS13B or Sec23IP (Fig. 6 G), supporting the notion that VPS13B and Sec23IP are possibly more general regulators of ER-to-Golgi trafficking than being specific to procollagen.
Discussion
Our study describes the associations of VPS13B with cellular membranes, including the Golgi, the ER, and ERES (Fig. 7 A), and reveals a direct interaction between VPS13B and Sec23IP that promotes ERES–Golgi interfaces (Fig. 7 B). Cohen syndrome–associated missense mutations in the VAB domain of VPS13B impair the interaction with Sec23IP. VPS13B KO abolishes the formation of tERGIC, the unconventional ER-to-Golgi cargo carrier. Using primary MEF cells as a system to study collagen secretion, we found that depletion of VPS13B significantly delays ER export of procollagens, establishing a potential link between procollagen secretion and joint laxity in patients with the Cohen disease. While the questions of whether and how VPS13B transports lipids are currently unclear, we speculate that VPS13B mediates a transient interaction with the ER, thereby enabling the extraction of lipids from the ER to deliver lipids to either the Golgi or growing tubular ERGIC, or both. The loss of VPS13B lipid transfer activity or its interaction with Sec23IP impairs tERGIC formation and reduces the efficiency of membrane and protein trafficking in the early secretory pathway required for the nervous system, sperm and joint development at certain developmental stages, ultimately contributing to the pathogenesis of Cohen syndrome (Fig. 7 C; working model).
Working model of VPS13B at ERES-Golgi interface. (A) VPS13B associates with three types of cellular membranes, including the ER, ERES and the Golgi in mammal cells. (B) The putative Sec23IP-interacting surface in the AlphaFold-predicted structure of VPS13B-CT contains the VAB domain and the PH domain. (C) The working model of VPS13B functions at ERES–Golgi interface. Currently, the question of whether and how VPS13B transfers lipids at this site remains unclear. We speculate that VPS13B may mediate a transient and dynamic interaction with the ER, thereby enabling the extraction of lipids from the ER to deliver lipids to either the Golgi or growing tubular ERGIC, or both. The loss of VPS13B lipid transfer activity or its interaction with Sec23IP impairs tERGIC formation, and reduces the efficiency of membrane and protein trafficking required for the nervous system, sperm and joint development at certain developmental stages, ultimately contributing to the pathogenesis of Cohen syndrome. The AlphaFold-predicted VPS13B structure is shown in the model but it is not to scale.
Working model of VPS13B at ERES-Golgi interface. (A) VPS13B associates with three types of cellular membranes, including the ER, ERES and the Golgi in mammal cells. (B) The putative Sec23IP-interacting surface in the AlphaFold-predicted structure of VPS13B-CT contains the VAB domain and the PH domain. (C) The working model of VPS13B functions at ERES–Golgi interface. Currently, the question of whether and how VPS13B transfers lipids at this site remains unclear. We speculate that VPS13B may mediate a transient and dynamic interaction with the ER, thereby enabling the extraction of lipids from the ER to deliver lipids to either the Golgi or growing tubular ERGIC, or both. The loss of VPS13B lipid transfer activity or its interaction with Sec23IP impairs tERGIC formation, and reduces the efficiency of membrane and protein trafficking required for the nervous system, sperm and joint development at certain developmental stages, ultimately contributing to the pathogenesis of Cohen syndrome. The AlphaFold-predicted VPS13B structure is shown in the model but it is not to scale.
VPS13B has been reported to play an essential role in the formation of acrosomes during sperm development in mice (Da Costa et al., 2020), but the molecular mechanisms were unclear. Interestingly, Sec23IP, the VPS13B adaptor identified in this study, was also required for acrosome biogenesis (Arimitsu et al., 2011). Therefore, we speculate that the VPS13B–Sec23IP interaction at ERES-Golgi interface may also play an important role in acrosome formation in spermiogenesis. Overall, our findings may reveal a conserved mechanism in the early secretory pathway for Cohen disease pathogenesis and acrosome biogenesis, mechanistically linking the two previously unrelated cellular processes. Given that collagen is not required for acrosome biogenesis, VPS13B and its interactor Sec23IP, in response to developmental signals, may expedite the ER-to-Golgi trafficking of a wide range of proteins and lipids, but not a certain type of cargo.
While the cellular functions of the early secretory pathway are generally conserved among all eukaryotes, the organization of the ER–Golgi interface varies widely among species (Barlowe and Helenius, 2016). For example, plants and some yeast species have a compact organization of the ER and Golgi, in which Golgi mini stacks are dispersed throughout the cytosol and moved along actin cables adjacent to the ER. In contrast to the scattered ministacks, the Golgi of animal cells is present in the form of a ribbon that assembles in the perinuclear. Interestingly, the deletion of VPS13B or Sec23IP in mammalian cells resulted in dispersed Golgi mini stacks over the cytosol (Fig. S4 C), which is similar to the organization of ERES and Golgi in plants and yeasts. Since both VPS13B and Sec23IP have no ortholog in plants and yeasts, VPS13B and Sec23IP may contribute to the unique ER–Golgi organization in mammals.
It is controversial how procollagen is exported from the ER. Newly synthesized procollagen is exported from the ERES under the control of a small GTPase Sar1 in coordination with other factors, including TANGO1 (Liu et al., 2017; Raote et al., 2018), cTAGE5 (Saito et al., 2011), Seldin1 (Venditti et al., 2012), and KLHL12 (Jin et al., 2012). Due to the size of procollagen triple helices, it is assumed that they are transported from the ER to the Golgi in specialized large COPII-dependent carriers (>300 nm). However, in animal cells, the traditional vesicle carrier model is challenged by the recent nano-resolved structure of ERES identified as a continuous network of interwoven membrane tubules connecting the ER and extruding pearl-shaped extensions towards the Golgi (Weigel et al., 2021). Furthermore, in a recent study, McCaughey et al. (2019) suggested that transport of procollagen to the Golgi may not involve long-range transport of large vesicular structures, and thus propose a short-loop model of COPII-dependent transport that facilitated local transfer of procollagen from the juxtanuclear ERES to the Golgi through the local formation of budding structures at ERES in close proximity to Golgi membranes. In this study, our results show that the VPS13B–Sec23IP interaction promotes ERES–Golgi interfaces and facilitates ER export of procollagens, supporting the notion that ER export of procollagen may not require large vesicles. Furthermore, our findings are also in line with models of ERGIC-dependent expansion of COPII carriers (Kurokawa et al., 2014; Santos et al., 2015), in which procollagen does not utilize vesicles during transport between Golgi stacks but remained within cisternae (Bonfanti et al., 1998).
We reason that the delayed but not blocked procollagen secretion upon Sec23IP or VPS13B depletion may be explained by the following possibilities. First, these two proteins may be a general regulator of ER export other than specific regulators of procollagen. In accord with this notion, collagen secretion appears to be intact in C. elegans, where both VPS13B and Sec23IP are absent (unpublished data). Clinically, patients with Cohen syndrome appear not to specifically display some key features (e.g., blue sclera and osteoporosis) of osteogenesis imperfecta, which is caused by mutations in the COL1A1 and COL1A2 genes, encoding type I procollagen (Marini et al., 2017). However, the delayed procollagen trafficking in VPS13B KO cells is in line with the clinical manifestations of Cohen syndrome. Specifically, Cohen syndrome patients indeed shared some features with patients who suffered from osteogenesis imperfecta type I. These features include joint hypermobility, short stature, and hypotonia (Carey and Hall, 1978; Cohen et al., 1973), and all of these features are linked to collagen-related function. Together, consistent with the general role of VPS13B in ER export, Cohen syndrome is a developmental disorder implicating multiple organs and tissues including the brain, bone, connective tissue, and testes in male, while osteogenesis imperfecta is a genetic disorder specifically affecting bone. Second, VPS13B may be not strictly required for basal ER export of procollagen, but it can accelerate its secretion under certain functional contexts, for instance, at certain development stages or in certain tissues that require high intensity of procollagen secretion. Supporting the notion, VPS13B loss of function mutants are not lethal in mice or in cell cultures, but its loss indeed profoundly impairs neurite outgrowth and acrosome biogenesis, both of which require a high intensity of protein and lipid trafficking between the ER and the Golgi. In addition, tERGIC was not always present and its biogenesis could be induced either by Rab1 activity or overload of certain types of cargoes (Yan et al., 2022), both stimuli could be considered as cellular stresses impacted on the early secretory pathway. This therefore suggests that VPS13B may also function in a stress-induced manner. Last, cells may compensate for the loss of VPS13B by upregulating other proteins. For instance, VPS13D, the paralog of VPS13B in mammals, may play a moonlighting role in the early secretory pathway, as a previous study showed that part of VPS13D was localized in the Golgi (Guillen-Samander et al., 2021). Further studies are needed to investigate the functional relationship between VPS13 proteins in procollagen trafficking.
Another question not answered in this study is whether tERGIC carries procollagen triple helices. tERGIC is a highly elongated tubular membrane, with a length of 2–20 μm and a diameter of <30 nm (Yan et al., 2022). Meanwhile, a procollagen triple helix was ∼300 nm long and ∼1.5 nm in diameter. Physically, tERGIC was an optimal carrier for procollagen triple helices because of its high surface-to-volume ratio, high intracellular movement speed, and ER–Golgi recycling abilities (Yan et al., 2022). However, we cannot observe the evident appearance of tERGIC during the tracking of procollagen secretion in primary MEFs in IF because the fixation process may disrupt the elongated thin membranes of tERGIC (unpublished results), which may make the observation of tERGIC technically difficult. Noteworthy, given that VPS13B depletion delayed but did not block the procollagen trafficking, we proposed that tERGIC may indirectly regulate procollagen trafficking because the high ER–Golgi recycling capabilities of tERGIC may regulate the protein or lipid compositions of the early secretory pathway specialized for procollagen secretion.
Materials and methods
Reagent and siRNA used in this study
| Reagent or resource . | Source . | Identifier . |
|---|---|---|
| Antibodies | ||
| GFP antibody | CST | 2555 |
| Anti-Halo tag pAB | Promega | G928A |
| VAPA rabbit pAb | ABclonal | A12939 |
| VAPB rabbit pAb | ABclonal | A5363 |
| MOSPD3 antibody | Santa Cruz | sc-514923 |
| His Tag antibody, mAb, mouse | Genscript | A00186 |
| Purified mouse anti-GM130 | BD Biosciences | 610822 |
| Anti-VPS13B antibody produced in rabbit | Sigma-Aldrich | HPA043865 |
| GM130 (D6B1) XP rabbit mAb | CST | 12480 |
| TGN46/TGOLN2 rabbit mAb | ABclona | A19618 |
| Sec31A antibody | Santa Cruz | sc-376587 |
| Sec31A polyclonal antibody | Proteintech | 17913-1-AP |
| Sec23IP polyclonal antibody | Proteintech | 20892-1-AP |
| Anti-GFP | abcam | ab6556 |
| COL1A1 | DSHB | SP1.D8 |
| GST-Tag | Sinobiological | 11213-MM01 |
| ERGIC-53 antibody | Santa Cruz | sc-271517 |
| COPA antibody (H-3) | Santa Cruz | sc-398099 |
| anti-alpha tubulin | abcam | Ab52866 |
| Purified anti-PtdIns(4)P IgM | Echelon | Z-P004 |
| RAB2A monoclonal antibody | Proteintech | 67501-1-Ig |
| RAB6A polyclonal antibody | Proteintech | 10187-2-AP |
| Beta actin monoclonal antibody | Proteintech | 66009-1-Ig |
| HRP-conjugated Goat anti-mouse IgG | Sangon Biotech | D110087 |
| HRP-conjugated Goat anti-Rabbit IgG | Sangon Biotech | D110058 |
| Reagents | ||
| PAO | Sigma-Aldrich | P3075 |
| Y294002 | Sigma-Aldrich | 440202 |
| Vps34-IN-1 | MCE | HY-12795 |
| Wortmannin | MCE | HY-10197 |
| BFA | MCE | HY-16592 |
| L-ascorbic acid | MCE | HY-B0166 |
| Hoechst 33342 | Invitrogen | H1399 |
| Briain PI(4)P | Avanti | 840045P |
| DMSO | Sigma-Aldrich | D2650 |
| siRNA | ||
| siRNA scrambled 5′-CGTTAATCGCGTATAATACGCGTAT-3′ | Ribobio | N/A |
| siRNA human VPS13B#1: 5′-GACCTTACTTGTCATAATA-3′ | ||
| siRNA human VPS13B#2: 5′-GCCTATGTTTATTCGTATA-3′ | Ribobio | N/A |
| siRNA human VPS13B#3: 5′-GAAGGATCCTATTGTTTCT-3′ | Ribobio | N/A |
| siRNA human RAB6A#1: 5′-GACATCTTTGATCACCAGA-3′ | Ribobio | N/A |
| siRNA human RAB6A#2: 5′-TTACATCCGTGATTCTGCT-3′ | Ribobio | N/A |
| siRNA human RAB6A#3: 5′-CAGCTGTAGTAGTTTACGA-3′ | Ribobio | N/A |
| siRNA human RAB2A#1: 5′-GAAGGAGTCTTTGACATTA-3′ | Ribobio | N/A |
| siRNA human RAB2A#2: 5′-GCAGGAGCTTTACTAGTTT-3′ | Ribobio | N/A |
| siRNA human RAB2A#3: 5′-GTTCGGTGCTCGAATGATA-3′ | Ribobio | N/A |
| siRNA house mouse Vps13b#1: 5′-CCAGCTTAGTGAGAAGCAT-3′ | Ribobio | N/A |
| siRNA house mouse Vps13b#2: 5′-GCACCATCGGAACTGCTAA-3′ | Ribobio | N/A |
| siRNA house mouse Vps13b#3: 5′-CCATGAACTGAGAATTCAT-3′ | Ribobio | N/A |
| siRNA human SEC23IP#1: 5′-GGCTAATCCTTACATTGCA-3′ | Ribobio | N/A |
| siRNA human SEC23IP#2: 5′-TGGGAATATTGGACAGTCA-3′ | Ribobio | N/A |
| siRNA human SEC23IP#3: 5′-CAGGGCTAATCCTTACATT-3′ | Ribobio | N/A |
| siRNA human PI4KA: 5′-CCGCCATGTTCTCAGATAA-3′ | Ribobio | N/A |
| siRNA human PI4KB: 5′-TGTTGGGGCTTCCCTGCCC-3′ | Ribobio | N/A |
| siRNA house mouse Sec23IP: 5′-AGAAGATTGATATGGAGTCTC-3′ | Ribobio | N/A |
| siRNA house mouse Sar1a#1: 5′-AGAAGAACTGACAATTGCTGG-3′ | Ribobio | N/A |
| siRNA house mouse Sar1a#2: 5′-GCTGAAAGATGACAGACTAGG-3′ | Ribobio | N/A |
| siRNA house mouse Tango1#1: 5′-GAGAAGCTGCTAACTTAAGGC-3′ | Ribobio | N/A |
| siRNA house mouse Tango1#2: 5′-ACATACAAGACCTGATCTATT-3′ | Ribobio | N/A |
| siRNA human ERGIC53: 5′-GGACAGAATCGTATTCATC-3′ | Ribobio | N/A |
| Reagent or resource . | Source . | Identifier . |
|---|---|---|
| Antibodies | ||
| GFP antibody | CST | 2555 |
| Anti-Halo tag pAB | Promega | G928A |
| VAPA rabbit pAb | ABclonal | A12939 |
| VAPB rabbit pAb | ABclonal | A5363 |
| MOSPD3 antibody | Santa Cruz | sc-514923 |
| His Tag antibody, mAb, mouse | Genscript | A00186 |
| Purified mouse anti-GM130 | BD Biosciences | 610822 |
| Anti-VPS13B antibody produced in rabbit | Sigma-Aldrich | HPA043865 |
| GM130 (D6B1) XP rabbit mAb | CST | 12480 |
| TGN46/TGOLN2 rabbit mAb | ABclona | A19618 |
| Sec31A antibody | Santa Cruz | sc-376587 |
| Sec31A polyclonal antibody | Proteintech | 17913-1-AP |
| Sec23IP polyclonal antibody | Proteintech | 20892-1-AP |
| Anti-GFP | abcam | ab6556 |
| COL1A1 | DSHB | SP1.D8 |
| GST-Tag | Sinobiological | 11213-MM01 |
| ERGIC-53 antibody | Santa Cruz | sc-271517 |
| COPA antibody (H-3) | Santa Cruz | sc-398099 |
| anti-alpha tubulin | abcam | Ab52866 |
| Purified anti-PtdIns(4)P IgM | Echelon | Z-P004 |
| RAB2A monoclonal antibody | Proteintech | 67501-1-Ig |
| RAB6A polyclonal antibody | Proteintech | 10187-2-AP |
| Beta actin monoclonal antibody | Proteintech | 66009-1-Ig |
| HRP-conjugated Goat anti-mouse IgG | Sangon Biotech | D110087 |
| HRP-conjugated Goat anti-Rabbit IgG | Sangon Biotech | D110058 |
| Reagents | ||
| PAO | Sigma-Aldrich | P3075 |
| Y294002 | Sigma-Aldrich | 440202 |
| Vps34-IN-1 | MCE | HY-12795 |
| Wortmannin | MCE | HY-10197 |
| BFA | MCE | HY-16592 |
| L-ascorbic acid | MCE | HY-B0166 |
| Hoechst 33342 | Invitrogen | H1399 |
| Briain PI(4)P | Avanti | 840045P |
| DMSO | Sigma-Aldrich | D2650 |
| siRNA | ||
| siRNA scrambled 5′-CGTTAATCGCGTATAATACGCGTAT-3′ | Ribobio | N/A |
| siRNA human VPS13B#1: 5′-GACCTTACTTGTCATAATA-3′ | ||
| siRNA human VPS13B#2: 5′-GCCTATGTTTATTCGTATA-3′ | Ribobio | N/A |
| siRNA human VPS13B#3: 5′-GAAGGATCCTATTGTTTCT-3′ | Ribobio | N/A |
| siRNA human RAB6A#1: 5′-GACATCTTTGATCACCAGA-3′ | Ribobio | N/A |
| siRNA human RAB6A#2: 5′-TTACATCCGTGATTCTGCT-3′ | Ribobio | N/A |
| siRNA human RAB6A#3: 5′-CAGCTGTAGTAGTTTACGA-3′ | Ribobio | N/A |
| siRNA human RAB2A#1: 5′-GAAGGAGTCTTTGACATTA-3′ | Ribobio | N/A |
| siRNA human RAB2A#2: 5′-GCAGGAGCTTTACTAGTTT-3′ | Ribobio | N/A |
| siRNA human RAB2A#3: 5′-GTTCGGTGCTCGAATGATA-3′ | Ribobio | N/A |
| siRNA house mouse Vps13b#1: 5′-CCAGCTTAGTGAGAAGCAT-3′ | Ribobio | N/A |
| siRNA house mouse Vps13b#2: 5′-GCACCATCGGAACTGCTAA-3′ | Ribobio | N/A |
| siRNA house mouse Vps13b#3: 5′-CCATGAACTGAGAATTCAT-3′ | Ribobio | N/A |
| siRNA human SEC23IP#1: 5′-GGCTAATCCTTACATTGCA-3′ | Ribobio | N/A |
| siRNA human SEC23IP#2: 5′-TGGGAATATTGGACAGTCA-3′ | Ribobio | N/A |
| siRNA human SEC23IP#3: 5′-CAGGGCTAATCCTTACATT-3′ | Ribobio | N/A |
| siRNA human PI4KA: 5′-CCGCCATGTTCTCAGATAA-3′ | Ribobio | N/A |
| siRNA human PI4KB: 5′-TGTTGGGGCTTCCCTGCCC-3′ | Ribobio | N/A |
| siRNA house mouse Sec23IP: 5′-AGAAGATTGATATGGAGTCTC-3′ | Ribobio | N/A |
| siRNA house mouse Sar1a#1: 5′-AGAAGAACTGACAATTGCTGG-3′ | Ribobio | N/A |
| siRNA house mouse Sar1a#2: 5′-GCTGAAAGATGACAGACTAGG-3′ | Ribobio | N/A |
| siRNA house mouse Tango1#1: 5′-GAGAAGCTGCTAACTTAAGGC-3′ | Ribobio | N/A |
| siRNA house mouse Tango1#2: 5′-ACATACAAGACCTGATCTATT-3′ | Ribobio | N/A |
| siRNA human ERGIC53: 5′-GGACAGAATCGTATTCATC-3′ | Ribobio | N/A |
Primers used for plasmid construction
| Primers . | Sequences (5′-3′) . |
|---|---|
| Vector-VPS13B-F | 5′-TCGAGCTCAAGCTTCGAATTCGCCACCATGCTGGAGTCATATG-3′ |
| VPS13B(1265AA)-R | 5′-CCTTTGCTCATAACTGGAGAAGTAGGAACAGGCC-3′ |
| VPS13B(1265AA)^sfGFP-F | 5′-TCTCCAGTTATGAGCAAAGGAGAAGAACTTTTCA-3′ |
| SfGFP^VPS13B(1266AA)-R | 5′-TGCCTATACTGCTTCTTTTGTAGAGCTCATCCATG-3′ |
| VPS13B(1266AA)-F | 5′-AGAAGCAGTATAGGCACAGCTCC-3′ |
| Vector-VPS13B-R | 5′-CAGAATTCGAAGCTTGAGCTCTCAAGGAAACCCTTTCCTCAGG-3′ |
| GFP-VPS13B-NT(1–1,500)-F | 5′-TACAAGTCCGGACTCAGATCTATGCTGGAGTCATATGTAACTCCAA-3′ |
| GFP-VPS13B-NT(1–1,500)-R | 5′-CAGAATTCGAAGCTTGAGCTCTCAATCAAAAGCTTGTGCTGAAA-3′ |
| GFP-VPS13B-N,S560D-F | 5′-GACTTTTCTGATGGGAAAAGTGAAGATTTGGGAA-3′ |
| GFP-VPS13B-N,S560D-R | 5′-TTCCCATCAGAAAAGTCTTGTTGATTTGTGGAAC-3′ |
| GFP-VPS13B-N,S503D,P504A-F | 5′-GATTACCGAGATGCCGAAAATAATGGTACTCGCGCAGA-3′ |
| GFP-VPS13B-N,S503D,P504A-R | 5′-TTCGGCATCTCGGTAATCAAACAATGAATTTGTAA-3′ |
| GFP-VPS13B-N,S1403D-F | 5′-CTTCCTTGACTGTACTGACAAGCTGAACAGACGC-3′ |
| GFP-VPS13B-N,S1403D-R | 5′-CAGTACAGTCAAGGAAGACACCTCCACATTGC-3′ |
| GFP-VPS13B-N,S14033D-F | 5′-GCTTCTTTCCTGACACAACTACAAAACTTCTAGATGGCACTC-3′ |
| GFP-VPS13B-N,S14033D-R | 5′-TGTGTCAGGAAAGAAGCCAGAGCAATTACTGA-3′ |
| Halo-VAPB-F | 5′-GAGATTTCCCTCGAGCTCATGGCGAAGGTGGAGCAGG-3′ |
| Halo-VAPB-R | 5′-TTATCTAGATCCGGTGGATCCCTACAAGGCAATCTTCCCAATAATT-3′ |
| Halo-MOSPD2-F | 5′-CTGGAGATTTCCCTCGAGCTCATGGCAGAGAATCACGCCC-3′ |
| Halo-MOSPD2-R | 5′-TTATCTAGATCCGGTGGATCCTTAACTGTACAATAAATAGAAGAAAGAGG-3′ |
| MGAT2-Halo-F | 5′-TGAACCGTCAGATCCGCTAGCGCCACCATGAGGTTCCGCATCTACAAACG-3′ |
| MGAT2-Halo-R | 5′-CGACTGCAGAATTCGAAGCTTCTGCAGTCTTCTATAACTTTTACAGAGTTC-3′ |
| GFP-Sec31A-F | 5′-TACAAGTCCGGACTCAGATCTATGAAGTTAAAGGAAGTAGATCGTACAGC-3′ |
| GFP-Sec31A-R | 5′-GCCTGAGTAATTAAACCATCAATGTCCCCACTGA-3′ |
| mCherry-Sec31A-F | 5′-CTGCAGTCGACGGTACCGCGGATGAAGTTAAAGGAAGTAGATCGTACAGC-3′ |
| mCherry-Sec31A-R | 5′-TTATCTAGATCCGGTGGATCCTTAGACACCCAGCTTATTGGCC-3′ |
| GFP-Sec23IP-F | 5′-TACAAGTCCGGACTCAGATCTATGGCCGAGAGAAAACCTAACG-3′ |
| GFP-Sec23IP-R | 5′-CAGAATTCGAAGCTTGAGCTCTCAATGCTGGGGCTGTTCTG-3′ |
| Halo-Sec23IP-F | 5′-CTGGAGATTTCCCTCGAGCTCATGGCCGAGAGAAAACCTAACG-3′ |
| Halo-Sec23IP-R | 5′-TTATCTAGATCCGGTGGATCCTCAATGCTGGGGCTGTTCTG-3′ |
| GST-Sec23IP-F | 5′-GATCTGGTTCCGCGTGGATCCATGGCCGAGAGAAAACCTAACG-3′ |
| GST-Sec23IP-R | 5′-TCAGTCAGTCACGATGAATTCTCAATGCTGGGGCTGTTCTG-3′ |
| GST-Sec23IP NT-R | 5′-CGATGAATTCTCACTCTAGTTTTTCACTGAACTCCTCAGTAT-3′ |
| GST-Sec23IP ΔNT-F | 5′-GATCTGGTTCCGCGTGGATCCGCTGAATATAAAAAAGCTGTAACCACT-3′ |
| Halo-Sec23IP NT-R | 5′-TTATCTAGATCCGGTGGATCCCTACTCTAGTTTTTCACTGAACTCCTCAG-3′ |
| Halo-Sec23IP ΔNT-F | 5′-CTGGAGATTTCCCTCGAGCTCGCTGAATATAAAAAAGCTGTAACCACT-3′ |
| Halo-Sec23IP(Δ 368–642)-F | 5′-AGATCTCGAGCTCAAGCTTCGATGACAGTCGTAGGGAACCCTCG-3′ |
| Halo-Sec23IP Δ 368–642)-R | 5′-AAAACTAGAGAATAAAGAAGTCCTAACTTT-3′ |
| Halo-Sec23IP(Δ SAM)-F | 5′-GTTGAAAAGAAGGCAGCGTCAGAAAAGAAGGCA-3′ |
| Halo-Sec23IP Δ SAM)-R | 5′-CGCTGCCTTCTTTTCAACAACAAGGTCATACGACTCA-3′ |
| Halo-Sec23IP(Δ 708–779AA)-F | 5′-AGCCAAACTGGATTTTGAACCAGAGATATT-3′ |
| Halo-Sec23IP(Δ 708–779AA)-R | 5′-GTTCAAAATCCAGTTTGGCTGCTTTATGTT-3′ |
| Halo-Sec23IP Δ DDHD)-F | 5′-GCTTACAACTCATTAACAATGAACATTAGTCCAGAACAGCC-3′ |
| Halo-Sec23IP(Δ DDHD)-R | 5′-GTTAATGAGTTGTAAGCAACAGAAACCTGTCC-3′ |
| Halo-Sec23IP Δ 989–1000AA)-F | 5′-TATCGATGAGGATCCACCGGATCTAGATAACT-3′ |
| Halo-Sec23IP Δ 989–1000AA)-FR | 5′-GTGGATCCTCATCGATAAATTTCTTTAAGTAGTAACAGAGC-3′ |
| GFP-VPS13B-VAB-F | 5′-TACAAGTCCGGACTCAGATCTGTAGAGGAGTTGGTCTTCAGCCA-3′ |
| GFP-VPS13B-VAB-R | 5′-CAGAATTCGAAGCTTGAGCTCTCATAACCGAGCAGGTTTTAATTCAAA-3′ |
| GFP-VPS13B-PH-F | 5′-TACAAGTCCGGACTCAGATCTTGCTTGCTGCTGACATCAGAA-3′ |
| GFP-VPS13B-PH-R | 5′-CAGAATTCGAAGCTTGAGCTCTCACTTTGTGATGTAGTCCACCAGT-3′ |
| VPS13B(Δ PH)-GFP-F | 5′-CATGAAGGGACATCTTGTCACCTGGCCCCCAG-3′ |
| VPS13B(Δ PH)-GFP-R | 5′-CAAGATGTCCCTTCATGCTCCTGGCCTGAGCC-3′ |
| VPS13B G2729R-F | 5′-CATCTGTAGAAGACAGATCATCTGTAGTTACTTGTCTCAA-3′ |
| VPS13B G2729R-R | 5′-TCTGTCTTCTACAGATGATAATCTGTTTTTGTACTCCA-3′ |
| VPS13B I2820T-F | 5′-CCACTATTTATGTCTGGTGCACAGTTTTGACT-3′ |
| VPS13B I2820T-R | 5′-CCAGACATAAATAGTGGAACTGTTTGAAGATGGCAC-3′ |
| VPS13B Y2822C-F | 5′-ATTTGTGTCTGGTGCACAGTTTTGACTTTAGA-3′ |
| VPS13B Y2822C-R | 5′-GTGCACCAGACACAAATAATGGAACTGTTTGAAGATGGC-3′ |
| VPS13B N2993S-F | 5′-GCTTATCTCCGAATCCAAATGGGACCTCTGGC-3′ |
| VPS13B N2993S-R | 5′-TGGATTCGGAGATAAGCAGGGCCCAGGGCAGA-3′ |
| VPS13B A3033E-F | 5′-ACTGGGAAAATACAAACACTGTGCACAAGTCAG-3′ |
| VPS13B A3033E-R | 5′-GTTTGTATTTTCCCAGTATATTCCAATTTGAAAAGC-3′ |
| VPS13B N3113Y-F | 5′-CTACAATACACCATATCAAATATTTTATAAACCAC-3′ |
| VPS13B N3113Y-R | 5′-GATATGGTGTATTGTAGATTATTGTAGTCTTGTCTTCAATCTGAAT-3′ |
| VPS13B S3142R-F | 5′-CCAGACAGGGCTACTTTTAGCATTTGCCCAGG-3′ |
| VPS13B S3142R-R | 5′-AAAGTAGCCCTGTCTGGAACACGAAAATACTCC-3′ |
| VPS13B R3223W-F | 5′-CGGGAATTTCTGGGAAAATGGATTCTGTACCAGGGC-3′ |
| VPS13B R3223W-R | 5′-TTTCCCAGAAATTCCCGAGGGGTACTGCCACA-3′ |
| VPS13B S3328R-F | 5′-AGTGGAGGGATGCCATTGACATCAACAGTCAG-3′ |
| VPS13B S3328R-R | 5′-AATGGCATCCCTCCACTCAGTTGTTACTTCATCTAGAGG-3′ |
| VPS13B G3432R-F | 5′-TCCCTAGGGAAGAGCCTGTGGCTGCGTTGTTT-3′ |
| VPS13B G3432R-R | 5′-AGGCTCTTCCCTAGGGAGGGGACCAGCTCCCG-3′ |
| VPS13B V3470M-F | 5′-TGTCTTAATGTGCCAGGGAGAAAAAGCAGAAC-3′ |
| VPS13B V3470M-R | 5′-CCTGGCACATTAAGACAGCAAAGTGGAAATTGGA-3′ |
| GFP-VPS13B(VAB R1)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGGTCTTCAGCCATTTTGTGA-3′ |
| GFP-VPS13B(VAB R1)-R | 5′-TTATCTAGATCCGGTGGATCCTTATCCACAGATGATAATCTGTTTTTGT-3′ |
| GFP-VPS13B(VAB R2)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGCAGATCATCTGTAGTTACTTGTCTC-3′ |
| GFP-VPS13B(VAB R2)-R | 5′-TTATCTAGATCCGGTGGATCCTTAGCTGAACACAATCATTCGTTG-3′ |
| GFP-VPS13B(VAB R3)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGCTTTTTATCATGAGGAGTCATC-3′ |
| GFP-VPS13B(VAB R3)-R | 5′-TTATCTAGATCCGGTGGATCCTTACAGAAGTTCTATCAACAAAGTTCTAACA-3′ |
| GFP-VPS13B(VAB R4)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGGCCCTGCTTATCAATGAA-3′ |
| GFP-VPS13B(VAB R4)-R | 5′-TTATCTAGATCCGGTGGATCCTTAGTCTTCAATCTGAATAATTTGTATACCTT-3′ |
| GFP-VPS13B(VAB R5)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGACTACAATAATCAATAATACACCATATCA-3′ |
| GFP-VPS13B(VAB R5)-R | 5′-TTATCTAGATCCGGTGGATCCTTATTCTGAGAGGGTTAAATAAGTCACTC-3′ |
| GFP-VPS13B(VAB R6)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGGTAATTATCCACAATAGATGTCCA-3′ |
| GFP-VPS13B(VAB R6)-R | 5′-TTATCTAGATCCGGTGGATCCTTAAGTGATGAAGACTGTGCCACA-3′ |
| GFP-VPS13B(VAB Δ R1)-F | 5′-AGGAGTTGAGACAGATCATCTGTAGTTACTTGTCTCAA-3′ |
| GFP-VPS13B(VAB Δ R1)-R | 5′-GATCTGTCTCAACTCCTCTACAGATCTGAGTCCG-3′ |
| GFP-VPS13B(VAB Δ R6)-F | 5′-ACCCTAGTCCTCGAGTGGCCCCAGAAGGAAAAGC-3′ |
| GFP-VPS13B(VAB Δ R6)-R | 5′-CACTCGAGGACTAGGGTCTTCTGAGAGGGTTA-3′ |
| GFP-VPS13B(VAB Δ R2-5)-F | 5′-TCATCTGTGACCCTAGTCCTCGAGTAATTATCCA-3′ |
| GFP-VPS13B(VAB Δ R2-5)-R | 5′-ACTAGGGTCACAGATGATAATCTGTTTTTGTACTCCA-3′ |
| Sec23IP Δ 114–116AA-F | 5′-CAACCTCAGTTCTCCCTTTTACAACTGGATCCCA-3′ |
| Sec23IP Δ l 114–116AA-R | 5′-AAGGGAGAACTGAGGTTGCTGCAGTTGTTAATG-3′ |
| Sec23IP Δ 176–178AA-F | 5′-CCCCAAGGAATTGGATACAATCCATATCGCCATACC-3′ |
| Sec23IP Δ 176–178AA-R | 5′-GTATCCAATTCCTTGGGGTTGGTTCCCAAAATA-3′ |
| Sec23IP Δ 207–209AA-F | 5′-TGCCAAACACCTGCTCATCCTCCACCTTCTGGA-3′ |
| Sec23IP Δ 207–209AA-R | 5′-ATGAGCAGGTGTTTGGCACTGCTGCAGCTGGGG-3′ |
| Sec23IP Δ 213–215AA-F | 5′-TCCTGCTCATTCTGGACCCCCTGTTCAGATGTA-3′ |
| Sec23IP Δ 213–215AA-R | 5′-GGTCCAGAATGAGCAGGAGGGCCTGGTGTTTGG-3′ |
| Sec23IP Δ 232–234AA-F | 5′-CTTTGCCATCTTCAGTGCAGTCACCGGCACAGC-3′ |
| Sec23IP Δ 232–234AA-R | 5′-GCACTGAAGATGGCAAAGATCCTGGAGGCATCT-3′ |
| Sec23IP Δ 257–259AA-F | 5′-CTCCCTCTGTTCAAGTGTTTCTACTTCAAAACCAATATGAGCC-3′ |
| Sec23IP Δ 257–259AA-R | 5′-ACACTTGAACAGAGGGAGCCCCAGGTCTGGCAG-3′ |
| Sec23IP Δ 305–307AA-F | 5′-TTCAGTTCAGGAGAGCGTGGTTCTTGGCACGGA-3′ |
| Sec23IP Δ 305–307AA-R | 5′-ACGCTCTCCTGAACTGAATTATAGATTTCTTCAAGATT-3′ |
| Halo-Sec23A-F | 5′-CTGGAGATTTCCCTCGAGCTCATGACAACCTATTTGGAATTCATTCA-3′ |
| Halo-Sec23A-R | 5′-TTATCTAGATCCGGTGGATCCTCAAGCAGCACTGGACACAGC-3′ |
| Halo-Rab6A-F | 5′-GAGATTTCCCTCGAGCTCATGTCCACGGGCGGAGACTTC-3′ |
| Halo-Rab6A-R | 5′-GTTATCTAGATCCGGTGGATCCTTAGCAGGAACAGCCTC-3′ |
| Halo-Rab6A T27N-F | 5′-GCGTTGGAAAGAACTCTTTGATCACCAGATTCATGTATGA-3′ |
| Halo-Rab6A T27N-R | 5′-AGAGTTCTTTCCAACGCTTTGCTCCCCCAGGA-3′ |
| GFP-Rab1B-F | 5′-TACAAGTCCGGACTCAGATCTATGAACCCCGAATATGACTACCTG-3′ |
| GFP-Rab1B-R | 5′-CAGAATTCGAAGCTTGAGCTCCTAGCAACAGCCACCGCCA-3′ |
| Halo-Rab1B-F | 5′-CTGGAGATTTCCCTCGAGCTCATGAACCCCGAATATGACTACCTG-3′ |
| Halo-Rab1B-R | 5′-TTATCTAGATCCGGTGGATCCCTAGCAACAGCCACCGCCA-3′ |
| His-VPS13B-PH-F | 5′-CTGTACTTCCAATCCGCTAGCACCCTCACATCCATCACCAACC-3′ |
| His-VPS13B-PH-R | 5′-TCCTTTCGGGCTTTGGTCGACTCAAGGAAACCCTTTCCTCAGG-3′ |
| Primers . | Sequences (5′-3′) . |
|---|---|
| Vector-VPS13B-F | 5′-TCGAGCTCAAGCTTCGAATTCGCCACCATGCTGGAGTCATATG-3′ |
| VPS13B(1265AA)-R | 5′-CCTTTGCTCATAACTGGAGAAGTAGGAACAGGCC-3′ |
| VPS13B(1265AA)^sfGFP-F | 5′-TCTCCAGTTATGAGCAAAGGAGAAGAACTTTTCA-3′ |
| SfGFP^VPS13B(1266AA)-R | 5′-TGCCTATACTGCTTCTTTTGTAGAGCTCATCCATG-3′ |
| VPS13B(1266AA)-F | 5′-AGAAGCAGTATAGGCACAGCTCC-3′ |
| Vector-VPS13B-R | 5′-CAGAATTCGAAGCTTGAGCTCTCAAGGAAACCCTTTCCTCAGG-3′ |
| GFP-VPS13B-NT(1–1,500)-F | 5′-TACAAGTCCGGACTCAGATCTATGCTGGAGTCATATGTAACTCCAA-3′ |
| GFP-VPS13B-NT(1–1,500)-R | 5′-CAGAATTCGAAGCTTGAGCTCTCAATCAAAAGCTTGTGCTGAAA-3′ |
| GFP-VPS13B-N,S560D-F | 5′-GACTTTTCTGATGGGAAAAGTGAAGATTTGGGAA-3′ |
| GFP-VPS13B-N,S560D-R | 5′-TTCCCATCAGAAAAGTCTTGTTGATTTGTGGAAC-3′ |
| GFP-VPS13B-N,S503D,P504A-F | 5′-GATTACCGAGATGCCGAAAATAATGGTACTCGCGCAGA-3′ |
| GFP-VPS13B-N,S503D,P504A-R | 5′-TTCGGCATCTCGGTAATCAAACAATGAATTTGTAA-3′ |
| GFP-VPS13B-N,S1403D-F | 5′-CTTCCTTGACTGTACTGACAAGCTGAACAGACGC-3′ |
| GFP-VPS13B-N,S1403D-R | 5′-CAGTACAGTCAAGGAAGACACCTCCACATTGC-3′ |
| GFP-VPS13B-N,S14033D-F | 5′-GCTTCTTTCCTGACACAACTACAAAACTTCTAGATGGCACTC-3′ |
| GFP-VPS13B-N,S14033D-R | 5′-TGTGTCAGGAAAGAAGCCAGAGCAATTACTGA-3′ |
| Halo-VAPB-F | 5′-GAGATTTCCCTCGAGCTCATGGCGAAGGTGGAGCAGG-3′ |
| Halo-VAPB-R | 5′-TTATCTAGATCCGGTGGATCCCTACAAGGCAATCTTCCCAATAATT-3′ |
| Halo-MOSPD2-F | 5′-CTGGAGATTTCCCTCGAGCTCATGGCAGAGAATCACGCCC-3′ |
| Halo-MOSPD2-R | 5′-TTATCTAGATCCGGTGGATCCTTAACTGTACAATAAATAGAAGAAAGAGG-3′ |
| MGAT2-Halo-F | 5′-TGAACCGTCAGATCCGCTAGCGCCACCATGAGGTTCCGCATCTACAAACG-3′ |
| MGAT2-Halo-R | 5′-CGACTGCAGAATTCGAAGCTTCTGCAGTCTTCTATAACTTTTACAGAGTTC-3′ |
| GFP-Sec31A-F | 5′-TACAAGTCCGGACTCAGATCTATGAAGTTAAAGGAAGTAGATCGTACAGC-3′ |
| GFP-Sec31A-R | 5′-GCCTGAGTAATTAAACCATCAATGTCCCCACTGA-3′ |
| mCherry-Sec31A-F | 5′-CTGCAGTCGACGGTACCGCGGATGAAGTTAAAGGAAGTAGATCGTACAGC-3′ |
| mCherry-Sec31A-R | 5′-TTATCTAGATCCGGTGGATCCTTAGACACCCAGCTTATTGGCC-3′ |
| GFP-Sec23IP-F | 5′-TACAAGTCCGGACTCAGATCTATGGCCGAGAGAAAACCTAACG-3′ |
| GFP-Sec23IP-R | 5′-CAGAATTCGAAGCTTGAGCTCTCAATGCTGGGGCTGTTCTG-3′ |
| Halo-Sec23IP-F | 5′-CTGGAGATTTCCCTCGAGCTCATGGCCGAGAGAAAACCTAACG-3′ |
| Halo-Sec23IP-R | 5′-TTATCTAGATCCGGTGGATCCTCAATGCTGGGGCTGTTCTG-3′ |
| GST-Sec23IP-F | 5′-GATCTGGTTCCGCGTGGATCCATGGCCGAGAGAAAACCTAACG-3′ |
| GST-Sec23IP-R | 5′-TCAGTCAGTCACGATGAATTCTCAATGCTGGGGCTGTTCTG-3′ |
| GST-Sec23IP NT-R | 5′-CGATGAATTCTCACTCTAGTTTTTCACTGAACTCCTCAGTAT-3′ |
| GST-Sec23IP ΔNT-F | 5′-GATCTGGTTCCGCGTGGATCCGCTGAATATAAAAAAGCTGTAACCACT-3′ |
| Halo-Sec23IP NT-R | 5′-TTATCTAGATCCGGTGGATCCCTACTCTAGTTTTTCACTGAACTCCTCAG-3′ |
| Halo-Sec23IP ΔNT-F | 5′-CTGGAGATTTCCCTCGAGCTCGCTGAATATAAAAAAGCTGTAACCACT-3′ |
| Halo-Sec23IP(Δ 368–642)-F | 5′-AGATCTCGAGCTCAAGCTTCGATGACAGTCGTAGGGAACCCTCG-3′ |
| Halo-Sec23IP Δ 368–642)-R | 5′-AAAACTAGAGAATAAAGAAGTCCTAACTTT-3′ |
| Halo-Sec23IP(Δ SAM)-F | 5′-GTTGAAAAGAAGGCAGCGTCAGAAAAGAAGGCA-3′ |
| Halo-Sec23IP Δ SAM)-R | 5′-CGCTGCCTTCTTTTCAACAACAAGGTCATACGACTCA-3′ |
| Halo-Sec23IP(Δ 708–779AA)-F | 5′-AGCCAAACTGGATTTTGAACCAGAGATATT-3′ |
| Halo-Sec23IP(Δ 708–779AA)-R | 5′-GTTCAAAATCCAGTTTGGCTGCTTTATGTT-3′ |
| Halo-Sec23IP Δ DDHD)-F | 5′-GCTTACAACTCATTAACAATGAACATTAGTCCAGAACAGCC-3′ |
| Halo-Sec23IP(Δ DDHD)-R | 5′-GTTAATGAGTTGTAAGCAACAGAAACCTGTCC-3′ |
| Halo-Sec23IP Δ 989–1000AA)-F | 5′-TATCGATGAGGATCCACCGGATCTAGATAACT-3′ |
| Halo-Sec23IP Δ 989–1000AA)-FR | 5′-GTGGATCCTCATCGATAAATTTCTTTAAGTAGTAACAGAGC-3′ |
| GFP-VPS13B-VAB-F | 5′-TACAAGTCCGGACTCAGATCTGTAGAGGAGTTGGTCTTCAGCCA-3′ |
| GFP-VPS13B-VAB-R | 5′-CAGAATTCGAAGCTTGAGCTCTCATAACCGAGCAGGTTTTAATTCAAA-3′ |
| GFP-VPS13B-PH-F | 5′-TACAAGTCCGGACTCAGATCTTGCTTGCTGCTGACATCAGAA-3′ |
| GFP-VPS13B-PH-R | 5′-CAGAATTCGAAGCTTGAGCTCTCACTTTGTGATGTAGTCCACCAGT-3′ |
| VPS13B(Δ PH)-GFP-F | 5′-CATGAAGGGACATCTTGTCACCTGGCCCCCAG-3′ |
| VPS13B(Δ PH)-GFP-R | 5′-CAAGATGTCCCTTCATGCTCCTGGCCTGAGCC-3′ |
| VPS13B G2729R-F | 5′-CATCTGTAGAAGACAGATCATCTGTAGTTACTTGTCTCAA-3′ |
| VPS13B G2729R-R | 5′-TCTGTCTTCTACAGATGATAATCTGTTTTTGTACTCCA-3′ |
| VPS13B I2820T-F | 5′-CCACTATTTATGTCTGGTGCACAGTTTTGACT-3′ |
| VPS13B I2820T-R | 5′-CCAGACATAAATAGTGGAACTGTTTGAAGATGGCAC-3′ |
| VPS13B Y2822C-F | 5′-ATTTGTGTCTGGTGCACAGTTTTGACTTTAGA-3′ |
| VPS13B Y2822C-R | 5′-GTGCACCAGACACAAATAATGGAACTGTTTGAAGATGGC-3′ |
| VPS13B N2993S-F | 5′-GCTTATCTCCGAATCCAAATGGGACCTCTGGC-3′ |
| VPS13B N2993S-R | 5′-TGGATTCGGAGATAAGCAGGGCCCAGGGCAGA-3′ |
| VPS13B A3033E-F | 5′-ACTGGGAAAATACAAACACTGTGCACAAGTCAG-3′ |
| VPS13B A3033E-R | 5′-GTTTGTATTTTCCCAGTATATTCCAATTTGAAAAGC-3′ |
| VPS13B N3113Y-F | 5′-CTACAATACACCATATCAAATATTTTATAAACCAC-3′ |
| VPS13B N3113Y-R | 5′-GATATGGTGTATTGTAGATTATTGTAGTCTTGTCTTCAATCTGAAT-3′ |
| VPS13B S3142R-F | 5′-CCAGACAGGGCTACTTTTAGCATTTGCCCAGG-3′ |
| VPS13B S3142R-R | 5′-AAAGTAGCCCTGTCTGGAACACGAAAATACTCC-3′ |
| VPS13B R3223W-F | 5′-CGGGAATTTCTGGGAAAATGGATTCTGTACCAGGGC-3′ |
| VPS13B R3223W-R | 5′-TTTCCCAGAAATTCCCGAGGGGTACTGCCACA-3′ |
| VPS13B S3328R-F | 5′-AGTGGAGGGATGCCATTGACATCAACAGTCAG-3′ |
| VPS13B S3328R-R | 5′-AATGGCATCCCTCCACTCAGTTGTTACTTCATCTAGAGG-3′ |
| VPS13B G3432R-F | 5′-TCCCTAGGGAAGAGCCTGTGGCTGCGTTGTTT-3′ |
| VPS13B G3432R-R | 5′-AGGCTCTTCCCTAGGGAGGGGACCAGCTCCCG-3′ |
| VPS13B V3470M-F | 5′-TGTCTTAATGTGCCAGGGAGAAAAAGCAGAAC-3′ |
| VPS13B V3470M-R | 5′-CCTGGCACATTAAGACAGCAAAGTGGAAATTGGA-3′ |
| GFP-VPS13B(VAB R1)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGGTCTTCAGCCATTTTGTGA-3′ |
| GFP-VPS13B(VAB R1)-R | 5′-TTATCTAGATCCGGTGGATCCTTATCCACAGATGATAATCTGTTTTTGT-3′ |
| GFP-VPS13B(VAB R2)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGCAGATCATCTGTAGTTACTTGTCTC-3′ |
| GFP-VPS13B(VAB R2)-R | 5′-TTATCTAGATCCGGTGGATCCTTAGCTGAACACAATCATTCGTTG-3′ |
| GFP-VPS13B(VAB R3)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGCTTTTTATCATGAGGAGTCATC-3′ |
| GFP-VPS13B(VAB R3)-R | 5′-TTATCTAGATCCGGTGGATCCTTACAGAAGTTCTATCAACAAAGTTCTAACA-3′ |
| GFP-VPS13B(VAB R4)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGGCCCTGCTTATCAATGAA-3′ |
| GFP-VPS13B(VAB R4)-R | 5′-TTATCTAGATCCGGTGGATCCTTAGTCTTCAATCTGAATAATTTGTATACCTT-3′ |
| GFP-VPS13B(VAB R5)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGACTACAATAATCAATAATACACCATATCA-3′ |
| GFP-VPS13B(VAB R5)-R | 5′-TTATCTAGATCCGGTGGATCCTTATTCTGAGAGGGTTAAATAAGTCACTC-3′ |
| GFP-VPS13B(VAB R6)-F | 5′-TCAGATCTCGAGCTCAAGCTTCGGTAATTATCCACAATAGATGTCCA-3′ |
| GFP-VPS13B(VAB R6)-R | 5′-TTATCTAGATCCGGTGGATCCTTAAGTGATGAAGACTGTGCCACA-3′ |
| GFP-VPS13B(VAB Δ R1)-F | 5′-AGGAGTTGAGACAGATCATCTGTAGTTACTTGTCTCAA-3′ |
| GFP-VPS13B(VAB Δ R1)-R | 5′-GATCTGTCTCAACTCCTCTACAGATCTGAGTCCG-3′ |
| GFP-VPS13B(VAB Δ R6)-F | 5′-ACCCTAGTCCTCGAGTGGCCCCAGAAGGAAAAGC-3′ |
| GFP-VPS13B(VAB Δ R6)-R | 5′-CACTCGAGGACTAGGGTCTTCTGAGAGGGTTA-3′ |
| GFP-VPS13B(VAB Δ R2-5)-F | 5′-TCATCTGTGACCCTAGTCCTCGAGTAATTATCCA-3′ |
| GFP-VPS13B(VAB Δ R2-5)-R | 5′-ACTAGGGTCACAGATGATAATCTGTTTTTGTACTCCA-3′ |
| Sec23IP Δ 114–116AA-F | 5′-CAACCTCAGTTCTCCCTTTTACAACTGGATCCCA-3′ |
| Sec23IP Δ l 114–116AA-R | 5′-AAGGGAGAACTGAGGTTGCTGCAGTTGTTAATG-3′ |
| Sec23IP Δ 176–178AA-F | 5′-CCCCAAGGAATTGGATACAATCCATATCGCCATACC-3′ |
| Sec23IP Δ 176–178AA-R | 5′-GTATCCAATTCCTTGGGGTTGGTTCCCAAAATA-3′ |
| Sec23IP Δ 207–209AA-F | 5′-TGCCAAACACCTGCTCATCCTCCACCTTCTGGA-3′ |
| Sec23IP Δ 207–209AA-R | 5′-ATGAGCAGGTGTTTGGCACTGCTGCAGCTGGGG-3′ |
| Sec23IP Δ 213–215AA-F | 5′-TCCTGCTCATTCTGGACCCCCTGTTCAGATGTA-3′ |
| Sec23IP Δ 213–215AA-R | 5′-GGTCCAGAATGAGCAGGAGGGCCTGGTGTTTGG-3′ |
| Sec23IP Δ 232–234AA-F | 5′-CTTTGCCATCTTCAGTGCAGTCACCGGCACAGC-3′ |
| Sec23IP Δ 232–234AA-R | 5′-GCACTGAAGATGGCAAAGATCCTGGAGGCATCT-3′ |
| Sec23IP Δ 257–259AA-F | 5′-CTCCCTCTGTTCAAGTGTTTCTACTTCAAAACCAATATGAGCC-3′ |
| Sec23IP Δ 257–259AA-R | 5′-ACACTTGAACAGAGGGAGCCCCAGGTCTGGCAG-3′ |
| Sec23IP Δ 305–307AA-F | 5′-TTCAGTTCAGGAGAGCGTGGTTCTTGGCACGGA-3′ |
| Sec23IP Δ 305–307AA-R | 5′-ACGCTCTCCTGAACTGAATTATAGATTTCTTCAAGATT-3′ |
| Halo-Sec23A-F | 5′-CTGGAGATTTCCCTCGAGCTCATGACAACCTATTTGGAATTCATTCA-3′ |
| Halo-Sec23A-R | 5′-TTATCTAGATCCGGTGGATCCTCAAGCAGCACTGGACACAGC-3′ |
| Halo-Rab6A-F | 5′-GAGATTTCCCTCGAGCTCATGTCCACGGGCGGAGACTTC-3′ |
| Halo-Rab6A-R | 5′-GTTATCTAGATCCGGTGGATCCTTAGCAGGAACAGCCTC-3′ |
| Halo-Rab6A T27N-F | 5′-GCGTTGGAAAGAACTCTTTGATCACCAGATTCATGTATGA-3′ |
| Halo-Rab6A T27N-R | 5′-AGAGTTCTTTCCAACGCTTTGCTCCCCCAGGA-3′ |
| GFP-Rab1B-F | 5′-TACAAGTCCGGACTCAGATCTATGAACCCCGAATATGACTACCTG-3′ |
| GFP-Rab1B-R | 5′-CAGAATTCGAAGCTTGAGCTCCTAGCAACAGCCACCGCCA-3′ |
| Halo-Rab1B-F | 5′-CTGGAGATTTCCCTCGAGCTCATGAACCCCGAATATGACTACCTG-3′ |
| Halo-Rab1B-R | 5′-TTATCTAGATCCGGTGGATCCCTAGCAACAGCCACCGCCA-3′ |
| His-VPS13B-PH-F | 5′-CTGTACTTCCAATCCGCTAGCACCCTCACATCCATCACCAACC-3′ |
| His-VPS13B-PH-R | 5′-TCCTTTCGGGCTTTGGTCGACTCAAGGAAACCCTTTCCTCAGG-3′ |
Cell culture, transfection, and RNAi
The African green monkey kidney fibroblast-like COS7 cell line (ATCC), human cervical cancer HeLa cells (ATCC), and human embryonic kidney 293T (ATCC) were grown in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Gibco). All of the cell lines used in this study were confirmed free of mycoplasma contamination.
Mouse embryos were removed from pregnant C57BL/6 mice at day E13.5. The head, limb buds, and visible internal organs were removed from the embryo, followed by the treatment of 500 μl trypsin (0.05%) for 30 min at 37°C. The trypsin was inactivated using an equal volume of DMEM supplemented with 10% FBS, and the mixture was pipetted up and down several times to dissociate the embryo into single cells. The cells were suspended in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin and then were seeded into 10-cm culture dishes. The dishes were incubated under standard cell culture conditions at 37°C, 5% CO2.
Transfection of plasmids and RNAi oligos was carried out with Lipofectamine 2000 and RNAi MAX, respectively. For transfection, cells were seeded at 4 × 105 cells per well in a 6-well dish ∼16 h before transfection. Plasmid transfections were performed in OPTI- MEM (Invitrogen) with 2 μl Lipofectamine 2000 per well for 6 h, followed by trypsinization and replating onto glass-bottom confocal dishes at ∼3.5 × 105 cells per well. Cells were imaged in live-cell medium (DMEM with 10% FBS and 20 mM Hepes with no penicillin or streptomycin) ∼16–24 h after transfection. For siRNA transfections, cells were plated on 3.5-cm dishes at 30–40% density, and 2 μl Lipofectamine RNAimax (Invitrogen) and 50 ng siRNA were used per well. At 48 h after transfection, a second round of transfection was performed with 50 ng siRNAs. Cells were analyzed 24 h after the second transfection for suppression.
CRISPR-Cas9-mediated gene editing
To make VPS13B KO HeLa or HEK293 cell lines, two gRNAs (5′-AGACGTGACAGCTAGAGTGG-3′ and 5′-CTAGTGACTCTAGGTCAACA-3′) were used to delete ∼76 bp from exon23 of VPS13B gene. To make Sec23IP KO HeLa, two gRNAs (5′-TATGGATTGTATCCTGGTTG-3′ and 5′-ATTAGCCCTGCTGCTGCCAG-3′) were used to delete ∼31 bp from exon 2 of Sec23IP gene. Complementary gRNAs were annealed and subcloned into the pSpCas9(BB)-2A-GFP (pX-458) vector (#48138; Addgene) between BbsI endonuclease restriction sites. Upon transfection, cells were grown in an antibiotic-free medium for 48 h, followed by single-cell sorting by fluorescence-based flow cytometry.
VPS13B−/− mice
VPS13B conventional KO mice were generated in the C57BL/6J strain by the Shanghai Model Organisms Center (Shanghai, China) by deleting 51,822 bp between exon 1 and exon 7 of the VPS13B gene by CRISPR-Cas9 (Fig. S5 M).
Genotyping of VPS13B−/− mice
Genomic DNA was extracted from the harvested tail sample. The primers for genotyping were shown as following: P1 5′-and GCTGCGCCAGAACTTACAAAC-3′ (Forward), P2 5′-TCGCCATCCCACAGTCAAAA-3′ (Reverse), P3 5′-GGTTCGGAGCCCAATTTTGTC-3′ (Reverse). The length of PCR products using P1 and P2 was 386 bp in the KO but disappeared in the WT while the PCR products using P1 and P3 were 304 bp in length in the WT but did not exist in the KO (Fig. S5 N).
GFP-trap assay
GFP trap (GTA-100; ChromoTek) was used for the detection of protein–protein interactions, and the GFP-Trap assays were performed according to the manufacturer’s protocol. Briefly, after 24 h transfection with the indicated plasmids, cells were lysed in ice-cold lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 and protease inhibitor cocktail). Lysates were centrifuged at 13,000 rpm for 10 min at 4°C and pellets were removed. Supernatants were incubated with GFP-Trap agarose beads for 1 h at 4°C with gentle shaking. After washing four times with lysis buffer, beads were boiled with SDS sample buffer. Proteins of interest were analyzed by immunoblotting. 5% input was used in GFP traps unless otherwise indicated.
Protein purification
GST and His constructs were transformed into Escherichia coli BL21 (DE3) cells, and cells were incubated at 37°C until the optical density (OD) at 600 nm reached 0.6–0.8. Subsequently, cells were incubated at 16°C for another hour, followed by induction with 1 mM IPTG overnight at 16°C. Cells were lysed via sonication. GST fusion proteins were purified via the GST-tag Protein Purification kit (C600031-0025; Sangon), and His fusion proteins were purified via the Ni-NTA Sefinose (TM) Resin Purification kit (G600033-0100; Sangon).
In vitro pull-down assays of GFP-VPS13B(VAB) and GST-Sec23IP
HEK293 cells transiently transfected with GFP-VPS13B(VAB)were lysed in high-salt lysis buffer (RIPA buffer containing 500 mM NaCl, proteasome inhibitors, and PMSF). GFP-Trap beads were used to pellet GFP-VPS13B(VAB) from cell lysates, followed by washing with high-salt lysis buffer 10 times. The GFP-VPS13B(VAB) beads were incubated with Purified GST–SEC23IP or GST-only overnight at 4°C, respectively, followed by washing beads with freshly prepared HNM buffer (20 mM HEPES, pH 7.4, 0.1 M NaCl, 5 mM MgCl2, 1 mM DTT, and 0.2% NP-40). GFP-VPS13B(VAB) beads were resuspended in 100 μl 2 × SDS- sampling buffer. Re-suspended beads were boiled for 10 min at 95°C to dissociate protein complexes from beads. Western blotting was performed using anti-GFP, GST, or Sec23IP antibodies. The Coomassie staining was performed for purified GST-Sec23IP.
PIP strip assays
The PIP Strips (P-6001) were blocked by TBS-T + 3% fatty acid–free BSA, and then were gently agitated for 1 h at room temperature, followed by an incubation with purified His- VPS13B-PH (0.5 µg/ml) in TBS-T + 3% fatty acid–free BSA overnight at 4°C. After washing the PIP Strips with TBS-T + 3%fatty acid–free BSA three times under gentle agitation for 10 min each time, PIP strips were incubated with the anti-His antibodies overnight at 4°C, followed by repeated washing steps.
Dot blot assay
After the protein samples (2 µl containing 10 µg proteins) were spotted onto a nitrocellulose membrane, the membrane was placed in a plastic container and incubated in a blocking buffer (5% BSA in TBS-T) for 1 h at room temperature, followed by the incubation with a primary antibody in TBS-T overnight at 4°C, and then secondary antibody conjugated to HRP for 30 min at room temperature. The membrane was washed three times with TBS-T (1 × 15 and 2 × 5 min) and then once with TBS (5 min).
Cryosectioning, immunolabeling, and electron microscopy
We followed the Tokuyasu method as described previously (Slot and Geuze, 2007). HeLa cells were fixed in 2% formaldehyde and 0.01% glutaraldehyde in PB buffer at 4°C overnight and then washed with pre-cold PB/Glycine. The cells were scraped from the bottom of the plastic dishes in 1% gelatin of PB buffer, centrifuged at 1,000 rpm/min for 2 min, and suspended in 12% gelatin at 37°C for 10 min. The gelatin-cell mixture was solidified on ice for 15 min. Small blocks of about 0.5 mm3 were made and immersed in 2.3 M sucrose overnight at 4°C. Cryosections of 70 nm were made at 120°C with an ultratome (EM FC7; Leica). After sections were thawed at room temperature, immunolabeling was performed with anti-VPS13B antibodies followed by immune-Gold secondary antibody. The sections were treated with methyl cellulose/uranyl acetate and subsequently imaged under the H-7650 80kv transmission electron microscope.
Live imaging by high-resolution confocal microscopy
Cells were grown on 35-mm glass-bottom confocal MatTek dishes, and the dishes were loaded to a laser scanning confocal microscope (LSM980; Zeiss) equipped with multiple excitation lasers (405, 458, 488, 514, 561, and 633 nm) and a spectral fluorescence GaAsP array detector. Cells were imaged with the 63×1.4 NA iPlan-Apochromat 63× oil objective using the 405 nm laser for BFP, 488 nm for GFP, 561 nm for OFP, tagRFP or mCherry, and 633 nm for Janilia Fluo 646 HaloTag Ligand.
Mass spectrometry for identification of VPS13B-interacting proteins
The identification of VPS13B^sfGFP interacting proteins by MS was described in our previous study (Gao et al., 2022). Briefly, the bound proteins were extracted from GFP-Trap agarose beads using SDT lysis buffer (4% SDS, 100 mM DTT, 100 mM Tris-HCl pH 8.0), followed by sample boiling for 3 min and further ultrasonicated. Undissolved beads were removed by centrifugation at 16,000 g for 15 min. The supernatant, containing proteins, were collected. Protein digestion was performed with the FASP method. Briefly, the detergent, DTT, and IAA in the UA buffer were added to block-reduced cysteine. Finally, the protein suspension was digested with 2 µg trypsin (Promega) overnight at 37°C. The peptide was collected by centrifugation at 16,000 g for 15 min. The peptide was desalted with C18 StageTip for further LC-MS analysis. LC-MS/MS experiments were performed on a Q Exactive Plus mass spectrometer that was coupled to an Easy nLC (Thermo Fisher Scientific). Peptide was first loaded to a trap column (100 µm × 20 mm, 5 µm, C18; Dr Maisch GmbH) in buffer A (0.1% formic acid in water). Reverse-phase high-performance liquid chromatography (RP-HPLC) separation was performed using a self-packed column (75 µm × 150 mm; 3 µm ReproSil-Pur C18 beads, 120 Å; Dr Maisch GmbH) at a flow rate of 300 nl/min. The RP-HPLC mobile phase A was 0.1% formic acid in water, and B was 0.1% formic acid in 95% acetonitrile. The gradient was set as follows: 2–4% buffer B from 0 to 2 min, 4–30% buffer B from 2 to 47 min, 30–45% buffer B from 47 to 52 min, 45–90% buffer B from 52 min and to 54 min, and 90% buffer B kept until to 60 min. MS data were acquired using a data-dependent top20 method dynamically choosing the most abundant precursor ions from the survey scan (350–1,800 m/z) for HCD fragmentation. A lock mass of 445.120025 Da was used as the internal standard for mass calibration. The full MS scans were acquired at a resolution of 70,000 at m/z 200, and 17,500 at m/z 200 for MS/MS scan. The maximum injection time was set to 50 ms for MS and 50 ms for MS/MS. Normalized collision energy was 27 and the isolation window was set to 1.6 Th. Dynamic exclusion duration was 60 s. The MS data were analyzed using MaxQuant software version 1.6.1.0. MS data were searched against the UniProtKB Rattus norvegicus database (36,080 total entries, downloaded 08/14/2018). Trypsin was selected as the digestion enzyme. A maximum of two missed cleavage sites and the mass tolerance of 4.5 ppm for precursor ions and 20 ppm for fragment ions were defined for database search. Carbamidomethylating of cysteines was defined as a fixed modification, while acetylation of protein N-terminal, oxidation of Methionine was set as variable modifications for database searching. The database search results were filtered and exported with a <1% false discovery rate (FDR) at peptide-spectrum-matched level, and protein level, respectively.
Halo staining in live cell
Cells were incubated with a complete medium with 5 nM Janilia Fluo 646 HaloTag Ligand for 30 min. Cells were washed three times with the complete medium to remove extra ligands, followed by incubation for another 30 min. Medium was replaced with imaging medium to remove unconjugated Halo ligands that have diffused out of the cells prior to imaging.
Immunofluorescence staining
Cells were fixed with 4% PFA (paraformaldehyde; Sigma-Aldrich) in PBS for 10 min at room temperature. After washing with PBS three times, cells were permeabilized with 0.1% Triton X-100 in PBS for 15 min on ice. Cells were then washed three times with PBS, blocked with 0.5% BSA in PBS for 1 h, incubated with primary antibodies in diluted blocking buffer overnight, and washed with PBS three times. Secondary antibodies were applied for 1 h at room temperature. After washing with PBS three times, samples were mounted on Vectashield (H-1000; Vector Laboratories).
Image and statistical analysis
All image analysis and processing were performed using ImageJ (National Institutes of Health, Bethesda, MD). All statistical analyses and P value determinations were performed in GraphPad Prism6. All the error bars represent mean ± SD. To determine P values, ordinary one-way ANOVA with Tukey’s multiple comparisons test was performed among multiple groups and a two-tailed unpaired student t test was performed between two groups.
Online supplemental material
Fig. S1 shows the association of VPS13B with the Golgi and provides information on VPS13B KO HeLa cells by CRISPR-Cas9. Fig. S2 provides additional data to support that PI4P, but not Rab2 or Rab6, is required for the Golgi association of VPS13B. Fig. S3 shows the association of VPS13B with the ER possibly via a phospho-FFAT motif in its NT. Fig. S4 shows the information about Sec23IP KO HeLa cells and the effect of Sec23IP KO on Golgi integrity. This figure also provides additional data to support that the VPS13B–Sec23IP interaction promotes ERES-Golgi association. Fig. S5 shows the effects of VPS13B KO on ERES, coatomer, and ERGIC53, and shows the colocalization between VPS13B and tERGIC. It also provides information about the CRISPR-mediated VPS13B KO in HEK293 cells and the C57BL/6J strain, in addition to the information about a putative VPS13B lipid-transfer mutant.
Data availability
All the data and relevant materials, including reagents and primers, that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Anbing Shi and Yanling Yan (Huazhong University of Science and Technology) for discussions. We thank Ying Li (Cryo-EM Facility of Tsinghua University, Branch of National Protein Science Facility) for cryosection and immunolabeling and Jiansheng Guo (Center of CryoElectron Microscopy, Zhejiang University School of Medicine, China) for assistance in CLEM. We thank the Biomedical Research Core Facilities (BRCF)/Laboratory Animal Center, the Mass Spectrometry Core Facility (Mr. Cookson K.C. Chiu), and the Bio-imaging Core Facility (Dr. Zhenglong Sun and Ms. Mei Yu) of Shenzhen Bay Laboratory for providing technical supports.
L. Deng is supported by the National Key R&D Program of China (2022YFA1302800) and the National Natural Science Foundation of China (NNSFC) (32270779). W.-K. Ji was supported by NNSFC (92354304; 32371343; 32122025) and Shenzhen Bay Scholars Program. J. Xiong was supported by NNSFC (81901166).
Author contributions: Y. Du: Conceptualization, Investigation, Methodology, Resources, X. Fan: Data curation, Formal analysis, Investigation, Validation, Visualization, C. Song: Formal analysis, W. Chang: Funding acquisition, Resources, J. Xiong: Data curation, L. Deng: Formal analysis, Funding acquisition, Investigation, Project administration, Resources, W.-K. Ji: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing.
References
Author notes
Y. Du and X. Fan contributed equally to this paper.
Disclosures: The authors declare no competing interests exist.


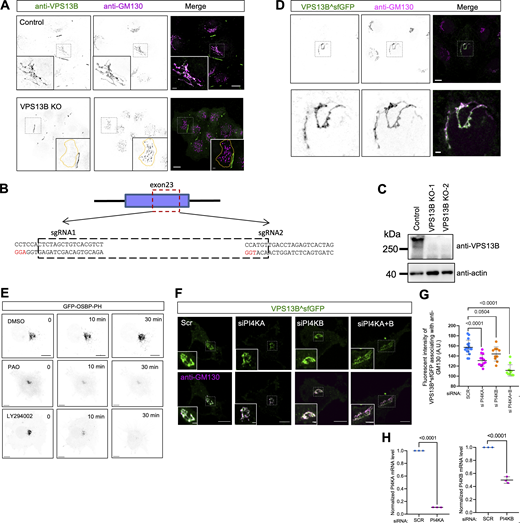
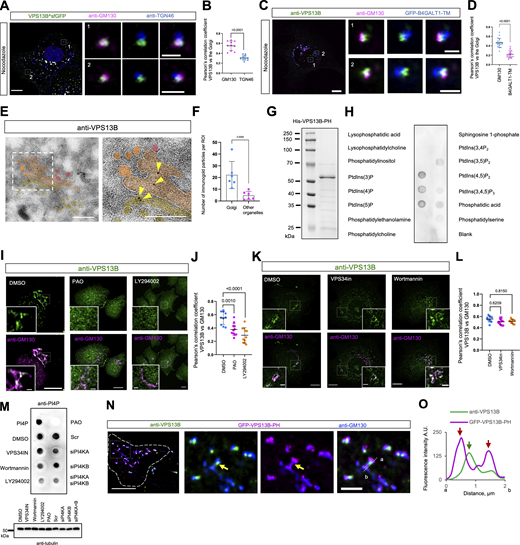

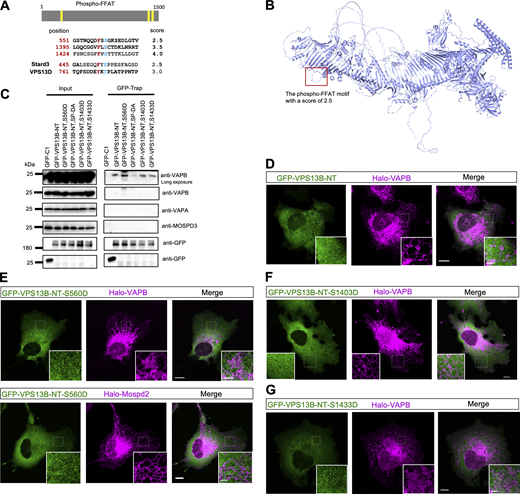
![VPS13B interacts with Sec23IP at ERES-Golgi interfaces. (A) Volcano plot of protein candidates coIPed with VPS13B^sfGFP in HEK293 cells compared with protein candidates coIPed with GFP tag only. After the removal of proteins that coIPed with the GFP tag, candidates that were considered significant (−log [P value] >1.3; P < 0.05) were labeled in orange (Log2 [fold change] >1.2; increased in abundance) or blue (Log2 [fold change] less than −1.2; decreased in abundance). (B) coIP assay showed an interaction between endogenous VPS13B and endogenous Sec23IP in HEK293 cells. Blue arrows denote VPS13B while red arrows indicate Sec23IP in the blots. (C) Representative images of a fixed HeLa cell expressing MGAT2-Halo (blue; a cis-/medial Golgi marker) stained with Sec23IP antibody (magenta) and Sec31A antibody (green) with two insets. Inset 1: Golgi area; inset 2: periphery area. (D) Representative images of a live HeLa cell co-expressing Halo-Sec23IP (magenta) and VPS13B^sfGFP (green) with two insets. (E) Representative images of a fixed HeLa cell co-expressing Halo-Sec23IP (magenta) and VPS13B^sfGFP (green) stained with Sec31A antibody (blue) with two insets on the bottom. (F) Percentage of Sec23IP puncta associated with the Golgi in either control (20 cells) or cells co-expressing Halo-Sec23IP and VPS13B^sfGFP (20 cells) as in E. Mean ± SD. Two-tailed unpaired Student’s t test. (G) Representative images of fixed HeLa cells expressing VPS13B^sfGFP (green) stained with GM130 antibody (blue) and Sec23IP antibody (magenta) with two insets on the bottom. Red arrows indicated a cell with the expression of VPS13B^sfGFP while blue arrows denoted a cell without VPS13B^sfGFP expression. (H) Percentage of Sec31A puncta associated with the Golgi in either control (n = 20), WT cells (n = 21) expressing VPS13B^sfGFP as in G or Sec23IP KO cells expressing VPS13B^sfGFP (n = 11; Fig. S4 G). Mean ± SD. Two-tailed unpaired Student’s t test. (I) Representative images of a fixed HeLa cell stained with VPS13B antibody (green) and Sec23IP antibody (magenta). One inset from the Golgi region was shown on the bottom while the other inset was shown to the right. (J) Pearson’s correlation coefficient of endogenous VPS13B versus endogenous Sec23IP at the Golgi or cell periphery (20 cells) in 3 independent experiments. Mean ± SD. Two tailed unpaired Student’s t test. (K) Representative images of fixed control, VPS13B KO, or siRNA-mediated Sec23IP knockdown in VPS13B KO HeLa cells stained with GM130 antibody (magenta) and Sec31A antibody (green) with insets. (L) Percentage of Sec31A puncta associated with the Golgi in either control (13 cells), VPS13B KO (20 cells), or VPS13B/Sec23IP double depleted cells (25 cells) as in K. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (M) Representative images of fixed HeLa cells co-expressing VPS13B^sfGFP (green) and ERES protein Sec16A-flag (magenta; top panel) or halo-Sec23A (magenta; bottom panel) stained with Sec31A antibody (blue) with two insets. (N) Percentage of Sec31A puncta associated with the Golgi in either control (n = 20), cells (n = 20) co-expressing Halo-Sec23IP and VPS13B^sfGFP as in E, cells (n = 13) co-expressing Halo-Sec23A and VPS13B^sfGFP or cells (n = 15) co-expressing sec16A-flag and VPS13B^sfGFP (20 cells) as in M. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (O) coIP assay showing Sec23IP, but not Sec16 or Sec23A, interacted with VPS13B^sfGFP. The blue arrow denoted VPS13B^sfGFP while the red arrows indicated Halo-Sec23IP in the blots. Scale bar, 10 μm in the whole cell images and 2 μm in the insets in C–E, G, I, K, and M. Source data are available for this figure: SourceData F2.](https://cdn.rupress.org/rup/content_public/journal/jcb/223/12/10.1083_jcb.202402083/1/m_jcb_202402083_fig2.png?Expires=1773832015&Signature=oAyX3qxZ7xRzMxK8HswKJlTXMECEXQcQZ2tpZ23hVWu7eT2~t3-Xacmt71FXGMkMXLOBHaydmZ4cpDI-p6WmFtlkAcKC7ZrSwpbLdnRjOLLnMUDKVg3m~EPgAluaJJEL~9GkLNVGmdI4NY41aiQWr9iPPK5qYW6XP-0AsXRdtr1Q87~Dk5Fl1rIfp0m53xci2zxOCUMbFdg50m0p5M8CS1OEx-gb-DiTD2p4Hj9LmsxY7FDjibZ1rQMTPuMX2SWNxADDf5HvmPCvYouKBRES9qoHmyxqp0y5EBlyYtJMH8IOoPaXecq-4oq2RqHqH6v4JFJKbNJqMfTnWrI1AAKt2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


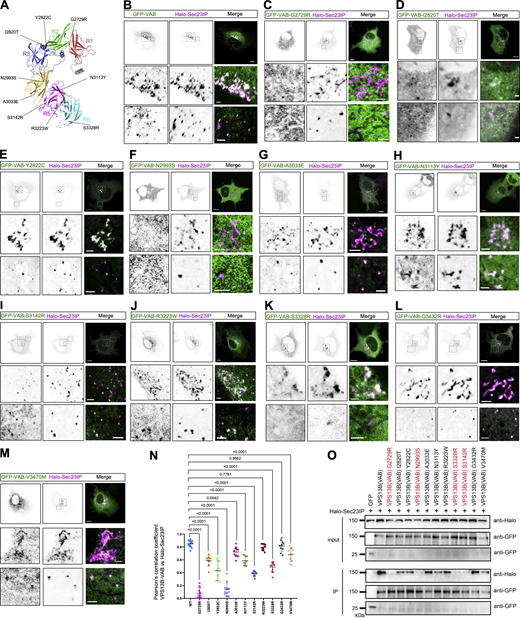


![Depletion of VPS13B or Sec23IP delays ER exit of procollagen. (A–E) Representative images of fixed control (A), Sar1A-depleted (B), VPS13B-depleted (C), Sec23IP-depleted (D), Tango1-depleted (E) primary MEF cells labeled with anti-procollagen (magenta) and anti-GM130 (green) upon ascorbic acid (50 μM) stimulation with five timepoints (0, 30, 60, 90, 120 min). (F) The fluorescence intensity of procollagen prior to ascorbic acid stimulation. 23 control cells, 10 Sec23IP depleted cells, 17 VPS13B depleted cells, 10 Sar1A depleted cells, and 9 Tango1 depleted cells in three independent experiments were quantified. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (G) The changes of fluorescence intensity of procollagen upon ascorbic acid stimulation as in A–E. 23 control cells, 10 Sec23IP depleted cells, 17 VPS13B depleted cells, 10 Sar1A depleted cells, and 9 Tango1 depleted cells in three independent experiments were quantified. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. (H) Volcano plot of proteins in the medium of control compared to VPS13B-depleted primary MEF cells. Candidates that were considered significant (−log [P value] >1.3; P < 0.05) were labeled in orange (Log2 [fold change] >1.2; increased in abundance) or blue (Log2 [fold change] less than −1.2; decreased in abundance). All collagens identified in the quantitative MS were labeled. (I) qPCR assays indicated the efficiency of siRNA-mediated suppression of VPS13B, Sec23IP, Sar1A, and Tango1 in primary MEF cells from three independent experiments. Mean ± SD. Two tailed unpaired Student’s t test. (J and K) Representative confocal images of fixed primary MEF cells from WT (J) or VPS13B KO (K) mice labeled with anti-procollagen (magenta) and anti-GM130 (green) upon ascorbic acid (50 μM) stimulation with five timepoints (0, 30, 60, 90, 120 min). (L) The fluorescence intensity of anti-procollagen prior to ascorbic acid stimulation. 24 control cells, 29 VPS13B KO cells in 3 independent experiments were quantified. Mean ± SD. Two-tailed unpaired Student’s t test. (M) The changes in fluorescence intensity of procollagen upon ascorbic acid stimulation as in J and K. 24 control cells, 29 VPS13B KO cells in three independent experiments were analyzed. Mean ± SD. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Scale bar, 10 μm in A–E, J, and K.](https://cdn.rupress.org/rup/content_public/journal/jcb/223/12/10.1083_jcb.202402083/1/m_jcb_202402083_fig6.png?Expires=1773832015&Signature=B2euf7LmGa75jhQ0W3wRqhkh8VW3-79hCm44TJL-NKKILy035zTVINV17fpxj4PpNhgQIdK0l09O3Dg8kzrLwblTjhRtKY-xxn2yBrJrVjGgLPuEhBG20YeL02uKFuZj5P90lQ9LFdN009SKGY6R9z1rFOvrXPgHKdu6hv-4nYp6ujbPtd~H0SPpAHBB--Q5ZnOcox0ogWFiD1QGrannlCXwhT7t54nOhMhKeQpNmpyvDt73yIQOji05aDzT5WvEMNelZUkxfM~GYmFPGNlCMrUNdwzNaGubwipAhY8EtXMQ-nEK87SvMU742LVDYX-TBWLD8VtXQdNQb4B8b51nvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



