N-degrons are short sequences located at protein N-terminus that mediate the interaction of E3 ligases (E3s) with substrates to promote their proteolysis. It is well established that N-degrons can be exposed following protease cleavage to allow recognition by E3s. However, our knowledge regarding how proteases and E3s cooperate in protein quality control mechanisms remains minimal. Using a systematic approach to monitor the protein stability of an N-terminome library, we found that proline residue at the third N-terminal position (hereafter “P+3”) promotes instability. Genetic perturbations identified the dipeptidyl peptidases DPP8 and DPP9 and the primary E3s of N-degron pathways, UBR proteins, as regulators of P+3 bearing substrate turnover. Interestingly, P+3 UBR substrates are significantly enriched for secretory proteins. We found that secretory proteins relying on a signal peptide (SP) for their targeting contain a “built-in” N-degron within their SP. This degron becomes exposed by DPP8/9 upon translocation failure to the designated compartments, thus enabling clearance of mislocalized proteins by UBRs to maintain proteostasis.
Introduction
Protein degradation within the ubiquitin-proteasome system (UPS) is highly specific. In most cases, it is thought that E3 ligases (E3s) bind their substrates through the recognition of specific short peptide motifs termed degrons (Varshavsky, 1991; Timms and Koren, 2020; Ella et al., 2019; Mészáros et al., 2017). The first degrons discovered were N-terminal degrons (N-degrons), leading to the discovery of the N-degron pathway (Bachmair et al., 1986) (formerly known as N-end rule [Varshavsky, 2019]). The N-degron pathway relates protein stability to the identity of the N-terminal residues and a large body of work over the past three decades has characterized a collection of N-degron pathways that target proteins for degradation through N-degron motifs (Varshavsky, 2011, 2019; Timms and Koren, 2020). In eukaryotes, there are several branches of N-degron pathways recognizing distinct N-terminal residues. In the Arg/N-degron pathway, UBR-family E3s target N-terminal primary degrons (Arg, Lys, His, Leu, Trp, Phe, Ile, Tyr, or Met followed by a bulky hydrophobic residue), secondary degrons (Asp or Glu), and tertiary degrons (Cys, Asn, or Gln) (Bartel et al., 1990; Varshavsky, 2011) (Fig. S1 A). Additional pathways include the Ac/N-degron pathway, through which proteins bearing acetylated N-termini are targeted for degradation by the E3 MARCH6 (also known as TEB4) (Shemorry et al., 2013; Hwang et al., 2010) (Fig. S1 B); and the Pro/N-degron pathway, which uses the GID E3 complex to recognize proteins containing an N-terminal proline for degradation (Chen et al., 2017) (Fig. S1 C). Recently, we identified a new branch of the N-degron pathway, the Gly/N-degron pathway (Timms et al., 2019) (Fig. S1 D). An exposed N-terminal glycine residue is recognized and mediates the degradation of substrates by Cul2ZYG11B/ZER1 E3 complexes to regulate quality control of protein N-myristoylation and apoptosis (Timms et al., 2019).
N-degron pathways in eukaryotes. (A) Arg/N-degron pathway. Substrate recognition by UBR family E3 ubiquitin ligases. UBR proteins harbor two distinct substrate binding sites: one accommodates the positively charged primary type I destabilizing residues (R, K, and H), whilst the second recognizes the bulky hydrophobic primary type II destabilizing residues (W, Y, F, L, I). Specificity for the remaining N-terminal (Nt) residues comes as a result of further Nt processing pathways: the tertiary destabilizing residues (N and Q) can be deamidated to form the secondary destabilizing residues (D and E), which are subject to Nt arginylation by ATE1. Oxidized cysteine (C*) is also subject to Nt arginylation. (B) Ac/N-degron pathway. In certain contexts, acetylated N-termini can serve as degrons and are recognized by MARCH6 (NOT4). (C) Pro/N-degron pathway. The GID E3 complex targets Nt proline degrons. Proline at the penultimate position can be exposed at the Nt by MetAP1- and MetAP2-mediated cleavage of iMet. In S. cerevisiae, following MetAPs processing, Nt-containing proline at the second position can be further trimmed by aminopeptidases Fra1 and/or Icp55 to yield substrates bearing Nt-Pro residue that are targeted for degradation by the GID complex. (D) Gly/N-degron pathway. Two Cullin 2 (Cul2) complexes target Nt glycine degrons via the substrate receptors ZYG11B and ZER1. Glycine is exposed at the Nt following iMet cleavage by MetAPs.
N-degron pathways in eukaryotes. (A) Arg/N-degron pathway. Substrate recognition by UBR family E3 ubiquitin ligases. UBR proteins harbor two distinct substrate binding sites: one accommodates the positively charged primary type I destabilizing residues (R, K, and H), whilst the second recognizes the bulky hydrophobic primary type II destabilizing residues (W, Y, F, L, I). Specificity for the remaining N-terminal (Nt) residues comes as a result of further Nt processing pathways: the tertiary destabilizing residues (N and Q) can be deamidated to form the secondary destabilizing residues (D and E), which are subject to Nt arginylation by ATE1. Oxidized cysteine (C*) is also subject to Nt arginylation. (B) Ac/N-degron pathway. In certain contexts, acetylated N-termini can serve as degrons and are recognized by MARCH6 (NOT4). (C) Pro/N-degron pathway. The GID E3 complex targets Nt proline degrons. Proline at the penultimate position can be exposed at the Nt by MetAP1- and MetAP2-mediated cleavage of iMet. In S. cerevisiae, following MetAPs processing, Nt-containing proline at the second position can be further trimmed by aminopeptidases Fra1 and/or Icp55 to yield substrates bearing Nt-Pro residue that are targeted for degradation by the GID complex. (D) Gly/N-degron pathway. Two Cullin 2 (Cul2) complexes target Nt glycine degrons via the substrate receptors ZYG11B and ZER1. Glycine is exposed at the Nt following iMet cleavage by MetAPs.
Regulated degradation of proteins by the Arg/N-degron pathway is involved in a broad range of biological functions, including protein quality control (PQC) mechanisms (Varshavsky, 2011, 2019; Timms and Koren, 2020). PQC mechanisms deal with different types of aberrant proteins within cells, such as misfolded, orphaned, and mislocalized proteins to limit their proteotoxic effect (Balchin et al., 2016; Hipp et al., 2019; Adams et al., 2019; Kong et al., 2021; Hegde and Zavodszky, 2019).
Roughly, one-fourth of human proteins are secretory proteins, proteins that are translocated across or embedded into the endoplasmic reticulum (ER) membrane before selective targeting to subcellular destinations (Juszkiewicz and Hegde, 2018). The signal for ER targeting is composed of a stretch of hydrophobic residues located in either a signal peptide (SP) or a transmembrane domain (TMD) (von Heijne, 1985) that can be recognized by multiple targeting pathways (Aviram and Schuldiner, 2017). However, even under optimal conditions, the limited capacity of targeting machinery can result in the retention of secretory proteins in the cytoplasm (Rane et al., 2010). Exposed hydrophobic degrons within the mislocalized proteins are believed to be recognized by dedicated chaperones and E3s that together promote their ubiquitination and proteasomal degradation (Hegde and Zavodszky, 2019). In mammalian cells, the hydrophobic SP or TMD of mislocalized membrane proteins are recognized by the quality control factors BAG6 or Ubiquilin family members, which recruit E3s to facilitate substrate ubiquitination and degradation by the proteasome (Suzuki and Kawahara, 2016; Rodrigo-Brenni et al., 2014; Hessa et al., 2011; Itakura et al., 2016; Yamamoto et al., 2017). Since BAG6 and Ubiquilins are believed to recognize exposed hydrophobic residues in their substrates, they are unlikely able to recognize the whole arsenal of mislocalized proteins. Other classes of exposed degrons in mislocalized proteins may exist and be targeted by different PQC mechanisms, suggesting that more PQC factors for proteins mislocalized to the cytosol likely remain to be identified.
Previously, we modified the global protein stability (GPS) system (Yen et al., 2008) to develop the “GPS-peptidome” approach (Koren et al., 2018; Timms et al., 2019, 2023; Zhang et al., 2023; Makaros et al., 2023), a high-throughput method to characterize degron motifs in human proteins. The GPS systematic method monitors protein stability and is based on a lentiviral expression vector that encodes two fluorescent proteins: green fluorescent protein (GFP) fused to a short peptide of interest, and DsRed, which serves as an internal reference and is translated from an internal ribosome entry site (IRES). Because both DsRed and the GFP-peptide fusion protein are expressed from the same transcript, the GFP/DsRed ratio can be used to quantify the effect of the peptide sequence on the stability of GFP (Yen et al., 2008). To explore mammalian N-degron determinants, we recently screened a human N-terminome library and revealed that proline at the N-terminal +3 position (hereafter “P+3”) promoted substrate instability (Timms et al., 2019).
Here, we investigated the molecular mechanism that controls the stability of proteins bearing P+3 motifs and found them to be physiological Arg/N-degron substrates. Interestingly, P+3 substrates expose N-degrons following cleavage by dipeptidyl peptidases 8 and 9 (DPP8/9), the cytoplasmic members of the DPP family of proteases (Wilson et al., 2016; Ajami et al., 2004; Abbott et al., 2000). Beyond DPP8/9, studies have revealed that caspases, calpains, and deubiquitinases also unmask N-degrons, allowing N-degron pathways to regulate diverse cellular processes (Rao et al., 2001; Ditzel et al., 2003; Piatkov et al., 2012a, 2012b, 2014; Brower et al., 2013; Tasaki et al., 2012; Timms et al., 2019). However, the DPP8/9 connection to regulated protein degradation has only begun to emerge in the past several years (Finger et al., 2020; Justa-Schuch et al., 2016; Bolgi et al., 2022). Intriguingly, substrates bearing P+3 are enriched for secretory proteins. This suggests a specific role for DPP8/9 in concert with UBR E3s and possibly other redundant PQC pathways in the destruction of mislocalized secretory proteins that depend on SP for their localization. Moreover, a systematic screen to identify DPP8/9 substrates delineated DPP8/9 cleavage specificity and substrate scope and revealed that DPP8/9 could also function with additional N-degron E3s such as Cul2ZYG11B/ZER1 to control the turnover of Gly/N-degron bearing substrates. Altogether, our study uncovers a global role for DPP8/9 as regulators of N-degron pathways to control homeostatic and regulatory functions.
Results
Proline at the N-terminal +3 position promotes proteasomal degradation
To discover N-degrons, we recently exploited the ubiquitin-fusion technique (Bachmair et al., 1986) to adapt the GPS-peptidome approach by fusing the first 24 amino acids (hereafter “N24mer”) of all human proteins between ubiquitin and GFP (“Ub-GPS”) (Fig. 1 A) (Timms et al., 2019). Upon expression of the constructs in human embryonic kidney (HEK) 293T cells, proteolytic cleavage of the ubiquitin moiety by endogenous deubiquitinating enzymes led to the exposure of the peptides at the N-terminus of GFP. Interestingly, P+3 scored as a highly destabilizing residue (Timms et al., 2019); however, this N-terminal motif was not previously explored and the identity of the N-degron pathway responsible for its recognition is currently unknown.
A P+3 motif is critical for peptide instability and ubiquitin-dependent proteasomal degradation. (A) Schematic representation of the Ub-GPS lentiviral vector and Ub-GPS N24mer screen. Oligonucleotides encoding the first 24 residues of human proteins were synthesized on a high-density chip and cloned between ubiquitin (Ub) and GFP in the Ub-GPS vector. The lentiviral library was introduced into HEK293T cells at a multiplicity of infection (MOI) of ∼0.2, followed by selection. To isolate peptide-GFP fusions based on their stability, fluorescence-activated cell sorting (FACS) was utilized to sort cells into six populations ("Bins") based on their GFP/DsRed fluorescence ratio. Finally, next-generation sequencing was used to identify the peptides enriched in each bin. (B) Scanning mutagenesis experiment of N24mer peptides containing a P+3 motif. For each of the indicated genes, data is presented for mutagenesis of single residues (top) or three consecutive residues (bottom). In each case, darker colors represent a greater degree of stabilization conferred by the mutation. Gene’s name is indicated at the top and a universal scale of stabilization is shown on the right. (C) Saturation mutagenesis of N24mer peptides harboring a P+3 motif. In each case, darker colors represent a greater degree of stabilization conferred by the mutation or N-terminal single amino acid addition (A, D, L, or S only). The name of the gene is indicated at the top and a universal scale of stabilization is shown on the right. (D) Cells expressing Ub-GPS constructs in which GFP was fused C-terminally to the first 24 residues of the indicated genes were treated with 1 μM MLN7243 or bortezomib for 7 h and analyzed by flow cytometry. DMSO (vehicle) served as the untreated control. GFP/DsRed ratio represents the stability of the indicated GFP-fusion proteins. Stabilization of the target protein by the indicated inhibitors is indicated by a sharp peak to the right side of each panel. For each gene, its name and the first N-terminal four residues are indicated.
A P+3 motif is critical for peptide instability and ubiquitin-dependent proteasomal degradation. (A) Schematic representation of the Ub-GPS lentiviral vector and Ub-GPS N24mer screen. Oligonucleotides encoding the first 24 residues of human proteins were synthesized on a high-density chip and cloned between ubiquitin (Ub) and GFP in the Ub-GPS vector. The lentiviral library was introduced into HEK293T cells at a multiplicity of infection (MOI) of ∼0.2, followed by selection. To isolate peptide-GFP fusions based on their stability, fluorescence-activated cell sorting (FACS) was utilized to sort cells into six populations ("Bins") based on their GFP/DsRed fluorescence ratio. Finally, next-generation sequencing was used to identify the peptides enriched in each bin. (B) Scanning mutagenesis experiment of N24mer peptides containing a P+3 motif. For each of the indicated genes, data is presented for mutagenesis of single residues (top) or three consecutive residues (bottom). In each case, darker colors represent a greater degree of stabilization conferred by the mutation. Gene’s name is indicated at the top and a universal scale of stabilization is shown on the right. (C) Saturation mutagenesis of N24mer peptides harboring a P+3 motif. In each case, darker colors represent a greater degree of stabilization conferred by the mutation or N-terminal single amino acid addition (A, D, L, or S only). The name of the gene is indicated at the top and a universal scale of stabilization is shown on the right. (D) Cells expressing Ub-GPS constructs in which GFP was fused C-terminally to the first 24 residues of the indicated genes were treated with 1 μM MLN7243 or bortezomib for 7 h and analyzed by flow cytometry. DMSO (vehicle) served as the untreated control. GFP/DsRed ratio represents the stability of the indicated GFP-fusion proteins. Stabilization of the target protein by the indicated inhibitors is indicated by a sharp peak to the right side of each panel. For each gene, its name and the first N-terminal four residues are indicated.
To investigate the functional significance of P+3 in promoting protein instability and to systematically delineate the residues critical for substrate degradation, we performed a series of mutagenesis experiments on a panel of N24mers starting with the sequence MxP. In this set of experiments, we generated peptide libraries in which single or triple amino acids across all positions within the N24mer are mutated (see Materials and methods). The two mutagenesis libraries were cloned into the Ub-GPS vector followed by expression in HEK293T cells. To separate the peptides based on stability, the library was sorted into six bins based on the GFP/DsRed ratio, followed by next-generation sequencing to recover the peptides in each bin (Fig. 1 A). The mutagenesis revealed that, in each case, mutating the extreme N-terminal residues disrupted substrate degradation, indicating the importance of P+3 and adjacent residues in promoting substrate instability (Fig. 1 B; Fig. S2; and Table S1).
Peptide instability is controlled by the P+3 motif. Scanning mutagenesis of N24mer peptides containing P+3 motif. For each of the indicated genes, two independent mutagenesis experiments were performed: mutagenesis of single residues (top) and mutagenesis of three consecutive residues (bottom). In each case, darker colors represent a greater degree of stabilization conferred by the mutation. The name of the gene is indicated at the top and a universal scale of stabilization is displayed at the bottom.
Peptide instability is controlled by the P+3 motif. Scanning mutagenesis of N24mer peptides containing P+3 motif. For each of the indicated genes, two independent mutagenesis experiments were performed: mutagenesis of single residues (top) and mutagenesis of three consecutive residues (bottom). In each case, darker colors represent a greater degree of stabilization conferred by the mutation. The name of the gene is indicated at the top and a universal scale of stabilization is displayed at the bottom.
Next, saturation mutagenesis was conducted on selected substrates. In this experiment, each amino acid along the N24mer was substituted for each of the remaining 19 amino acids. We also included mutant versions in which a single amino acid was added upstream of peptides bearing MxP (thus generating MyxP) to examine the importance of proline being precisely located at +3 position. The saturation library validated the scanning mutagenesis results and showed that the first three positions mediate substrate instability, with the precise location of the proline residue at the +3 N-terminal position being critical for degradation (Fig. 1 C and Table S1).
All N24mers harboring P+3 were stabilized following treatment with both the proteasome inhibitor bortezomib and the E1 enzymes inhibitor MLN7243 (Fig. 1 D), indicating that the P+3 motif can act as a degron to promote ubiquitin-mediated proteasomal degradation.
UBR E3s mediate the degradation of P+3 bearing substrates
While the yeast GID E3 complex promotes degradation of substrates bearing proline at the +2 or +3 position (Fig. S1 C) (Chen et al., 2017, 2021), we found that the stability of P+3 peptides did not change in cells lacking the human GID complex subunits GID4 and RMND5A (Fig. S3, A and B). Thus, to identify the E3(s) responsible for the degradation of P+3 bearing peptides, a CRISPR screen was performed on three representative peptide-GFP fusions using a CRISPR library that targets all known E3s (Fig. 2 A). The screens identified two UBR family E3s—UBR2 and UBR4—as the primary E3s regulating the stability of peptide-GFP fusion bearing P+3 (Fig. 2 B and Table S2). These results were intriguing as UBR1, UBR2, and UBR4 are the main regulators of N-degron pathways (Tasaki et al., 2005), but none have yet been implicated in the recognition of N-terminal proline residues. However, a reanalysis of previous GPS N-terminome screen data in cells lacking UBR1/2/4 (Timms et al., 2019) uncovered 142 candidate UBR substrates that contain P+3 (Table S3). A few representative P+3 candidate UBR substrates’ performance in the screen are shown in Fig. S3 C.
UBRs, not the GID complex, regulate the stability of P+3 N24mers. (A) Genomic DNA sequencing of KO cell line populations was performed, and KO efficiency was analyzed by Inference of CRISPR Edits (ICE) CRISPR analysis tool. KO score of the human GID complex subunits RMND5A or GID4 is calculated by out-of-frame insertion and deletion (indel) % compared with wild-type sequences. (B) Ablation of RMND5A or GID4 does not affect the stability of the indicated P+3 N24mers-GFP fusions as indicated by flow cytometry. (C) GPS screen profiles for representative N24mers showing the distribution of Illumina sequencing reads across the 6 bins in control KO (gray) versus three independent UBR KO clonal cells (magenta). (D) Stability profiling of N24mers expressed in the context of Ub-GPS or GPS lacking Ub fusion in control or UBR KO cells.
UBRs, not the GID complex, regulate the stability of P+3 N24mers. (A) Genomic DNA sequencing of KO cell line populations was performed, and KO efficiency was analyzed by Inference of CRISPR Edits (ICE) CRISPR analysis tool. KO score of the human GID complex subunits RMND5A or GID4 is calculated by out-of-frame insertion and deletion (indel) % compared with wild-type sequences. (B) Ablation of RMND5A or GID4 does not affect the stability of the indicated P+3 N24mers-GFP fusions as indicated by flow cytometry. (C) GPS screen profiles for representative N24mers showing the distribution of Illumina sequencing reads across the 6 bins in control KO (gray) versus three independent UBR KO clonal cells (magenta). (D) Stability profiling of N24mers expressed in the context of Ub-GPS or GPS lacking Ub fusion in control or UBR KO cells.
UBR E3s regulate the stability of substrates containing a P+3 motif. (A) Schematic representation of the CRISPR screen. Three clones expressing P+3 containing peptide-GFP fusion were transduced with a CRISPR library targeting all known E3s. 7 days after transduction, the population of cells in which the peptide-GFP fusion was stabilized (top 1% GFP positive cells) was then isolated by FACS, and the enriched sgRNAs were identified by Illumina sequencing. (B) CRISPR screen results for three clones expressing P+3 containing peptide-GFP fusion. Significantly enriched genes across multiple screens as determined using the Model-based Analysis of genome-wide CRISPR-Cas9 Knockout (MAGeCK) tool are annotated. (C) Ablation of UBR1/2/4 genes in selected P+3–containing clones resulted in the stabilization of the peptide–GFP fusion as indicated by flow cytometry. Control KO- sgRNA targeting AAVS1. (D) Stability analysis in UBR or control KO cells expressing the indicated wild-type (WT) or mutated N24mer–GFP fusions.
UBR E3s regulate the stability of substrates containing a P+3 motif. (A) Schematic representation of the CRISPR screen. Three clones expressing P+3 containing peptide-GFP fusion were transduced with a CRISPR library targeting all known E3s. 7 days after transduction, the population of cells in which the peptide-GFP fusion was stabilized (top 1% GFP positive cells) was then isolated by FACS, and the enriched sgRNAs were identified by Illumina sequencing. (B) CRISPR screen results for three clones expressing P+3 containing peptide-GFP fusion. Significantly enriched genes across multiple screens as determined using the Model-based Analysis of genome-wide CRISPR-Cas9 Knockout (MAGeCK) tool are annotated. (C) Ablation of UBR1/2/4 genes in selected P+3–containing clones resulted in the stabilization of the peptide–GFP fusion as indicated by flow cytometry. Control KO- sgRNA targeting AAVS1. (D) Stability analysis in UBR or control KO cells expressing the indicated wild-type (WT) or mutated N24mer–GFP fusions.
To validate the screens’ results, we tested the stability of example P+3 peptides using a HEK293T clone that lacks UBR1/2/4 (“UBR KO”) (Timms et al., 2019). In each case, the loss of UBRs resulted in the stabilization of the Ub-GPS reporters (Fig. 2 C). Notably, UBR-mediated degradation of P+3 substrates also occurred independent of the ubiquitin-fusion technique (Fig. S3 D). Finally, we found that mutation of the P+3 residue stabilized the peptide-GFP fusion substrates to the same extent as UBR ablation, with no further stabilization observed in UBR KO cells (Fig. 2 D). Overall, these data demonstrate the importance of the P+3 residue for UBR-mediated degradation.
DPP enzymes cleave following P+3 to expose N-degron for UBR proteins
Our observation that UBR proteins regulate the stability of P+3 substrates was intriguing since UBRs have not been demonstrated to bind N-terminal proline (Tasaki et al., 2005). Interestingly, among the P+3 bearing UBR substrates identified in the N-terminome screen (Table S3), most contained a small amino acid (A, G, S, or P) in the second position, while the fourth position was significantly enriched for the positively charged residues, R/K, and the hydrophobic residues W and L (P < 0.05, t test) (Fig. 3 A). Thus, we reasoned that potential cleavage events that removed the first three residues would, in most cases, create an optimal N-degron for UBRs. Candidate proteases are the DPP cytosolic family members, DPP8 and DPP9 (Wilson et al., 2016; Ajami et al., 2004; Abbott et al., 2000), that recognize and cleave N-terminal xPy motifs after the proline bond (where x cannot be D/E, and y cannot be P) (Wilson et al., 2013; Zhang et al., 2015). Following initiator methionine (iMet) removal from “MxP-” bearing proteins by methionine aminopeptidases (MetAPs), if the second residue (“x”) is A, C, S, T, V, G, or P (Arfin et al., 1995; Varshavsky, 2011), N-terminal dipeptides “xP-” containing proline at the second position can be trimmed by DPP8/9 (Rosenblum and Kozarich, 2003; Waumans et al., 2015).
Degradation of P+3 substrates is dependent on sequential action by MetAPs, DPP8/9, and UBRs. (A) Logoplots showing the consensus N-terminal amino acid sequences among P+3 UBRs substrates. (B and C) Flow cytometry analysis revealed stabilization of the selected P+3 containing clones upon DPP inhibition for 6 h with Talabostat (B) or CRISPR-mediated ablation of DPP8 and/or DPP9 (C). (D and E) Stability analysis in DPP8/9, UBR, or control KO cells expressing the indicated Ub-GPS N24mer-GFP fusion. (D) WT substrate compared to iMet mutated to Thr variant. (E) Truncated substrate versions mimicking DPP8/9 processing. (F) Stability analysis in cells simultaneously ablated for UBR and DPP8/9 proteins compared with DPP8/9, UBR, or control KO cells.
Degradation of P+3 substrates is dependent on sequential action by MetAPs, DPP8/9, and UBRs. (A) Logoplots showing the consensus N-terminal amino acid sequences among P+3 UBRs substrates. (B and C) Flow cytometry analysis revealed stabilization of the selected P+3 containing clones upon DPP inhibition for 6 h with Talabostat (B) or CRISPR-mediated ablation of DPP8 and/or DPP9 (C). (D and E) Stability analysis in DPP8/9, UBR, or control KO cells expressing the indicated Ub-GPS N24mer-GFP fusion. (D) WT substrate compared to iMet mutated to Thr variant. (E) Truncated substrate versions mimicking DPP8/9 processing. (F) Stability analysis in cells simultaneously ablated for UBR and DPP8/9 proteins compared with DPP8/9, UBR, or control KO cells.
Several lines of evidence supported this hypothesis. First, we observed robust stabilization of each P+3 peptide-GFP fusion in cells treated with the DPP inhibitor Talabostat (Jones et al., 2003) and in CRISPR KO HEK293T cells lacking DPP8/9 (Fig. 3, B and C; and Fig. S4, A–C). DPP8 ablation alone did not affect the stability of any of the example substrates, whereas loss of DPP9 did result in significant stabilization. However, the largest effect was observed in DPP8/9 double knockout (DKO) cells (Fig. 3 C and Fig. S4 C), suggesting that, although DPP9 may be dominant, some redundancy exists (Geiss-Friedlander et al., 2009; Finger et al., 2020).
Knock out efficiency across various genetic backgrounds. (A) KO score calculated by ICE CRISPR analysis tool of DPP8 and DPP9 in single or double KO cells is indicated. (B and C) DPP8/9 regulates P+3–bearing substrates’ stability. Stability analysis of the indicated N24mer-GFP fusions in cells treated with Talabostat for 6 h (B) or expressed in the indicated genetic backgrounds (C). (D) KO score of DPP8 and DPP9 on the background of UBR KO clonal cells. (E) BAG6 protein levels analyzed by immunoblot with a BAG6 antibody in the various genetic backgrounds confirmed efficient BAG6 KO (top). KO score of DPP8/9 in the triple KO of BAG6 + DPP8/9 cells (bottom). Source data are available for this figure: SourceData FS4.
Knock out efficiency across various genetic backgrounds. (A) KO score calculated by ICE CRISPR analysis tool of DPP8 and DPP9 in single or double KO cells is indicated. (B and C) DPP8/9 regulates P+3–bearing substrates’ stability. Stability analysis of the indicated N24mer-GFP fusions in cells treated with Talabostat for 6 h (B) or expressed in the indicated genetic backgrounds (C). (D) KO score of DPP8 and DPP9 on the background of UBR KO clonal cells. (E) BAG6 protein levels analyzed by immunoblot with a BAG6 antibody in the various genetic backgrounds confirmed efficient BAG6 KO (top). KO score of DPP8/9 in the triple KO of BAG6 + DPP8/9 cells (bottom). Source data are available for this figure: SourceData FS4.
Second, interfering with MetAPs-mediated cleavage of the iMet by substituting Met with non-cleavable Thr residue resulted in marked stabilization of peptides expressed in the context of Ub-GPS, with no further stabilization observed in KO cells (Fig. 3 D).
Third, for two DPPs peptide substrates, TOMM34 (MAPK---GFP) and ANGEL1 (MAPR---GFP), we generated shorter versions mimicking the effect of DPP8/9 cleavage (K---GFP) and (R---GFP), respectively. Whilst the full-length peptide was stabilized in both DPP8/9 and UBR KO cells, the shorter variants were only stabilized in UBR KO but not in DPPs KO cells (Fig. 3 E).
Fourth, no further stabilization was observed in penta KO cells ablated for both DPP8/9 and UBR proteins compared with DPP ablation alone (Fig. 3 F and Fig. S4 D), suggesting DPP-UBR cooperation in the elimination of P+3 substrates.
Fifth, mass spectrometry (MS) analysis was performed to analyze the in vivo cleavage events of two P+3-containing peptide–GFP fusions (Fig. 4 A). The experiments clearly showed that, in wild-type (WT) cells, most peptides are cleaved following the P+3 residue to expose the fourth residue at the new N-terminus (“R-” for ZNF750 and “A-” for POMT2). In DPP8/9 DKO cells, on the other hand, MetAPs-mediated cleavage did occur but further processing was prevented, with the peptides mostly commencing with “SPR-” for ZNF750 and “PPA-” for POMT2 (Fig. 4, B and C and Table S4). Altogether, the MS results indicated that cleavage following P+3 is dependent on the activity of DPP8/9.
DPP8/9 cleave P+3 substrates post proline as revealed by mass spectrometry experiment. (A) Overview of the experiment. Peptide–GFP UBR and DPP substrates expressed in DPP8/9 DKO or control KO (“WT”) cells treated with bortezomib for 6 h were immunoprecipitated using an anti-GFP antibody and then analyzed by mass spectrometry (MS). (B) Proteomic analysis of indicated N24mer peptide-GFP immunoprecipitated from HEK293T cells revealed mostly the presence of the indicated three N-termini: “MSPR-,” “SPR-,” and “R-” (ZNF750); “MPPA-,” “PPA-,” and “A-” (POMT2). The processing from “MSPR-” to “SPR-” (ZNF750) and “MPPA-” to “PPA-” (POMT2) is caused by MetAPs. The second processing event is executed by DPP8/9 as CRISPR/Cas9 ablation of the genes prevented this processing as revealed by MS. (C) Peptide MS spectra of the indicated N24mers obtained from DPP8/9 or WT cells as revealed by LC-MS/MS analysis. Species without the first three residues were mostly abundant in WT cells, while N-terminal peptides missing the iMet can be detected in DPP8/9 DKO cells.
DPP8/9 cleave P+3 substrates post proline as revealed by mass spectrometry experiment. (A) Overview of the experiment. Peptide–GFP UBR and DPP substrates expressed in DPP8/9 DKO or control KO (“WT”) cells treated with bortezomib for 6 h were immunoprecipitated using an anti-GFP antibody and then analyzed by mass spectrometry (MS). (B) Proteomic analysis of indicated N24mer peptide-GFP immunoprecipitated from HEK293T cells revealed mostly the presence of the indicated three N-termini: “MSPR-,” “SPR-,” and “R-” (ZNF750); “MPPA-,” “PPA-,” and “A-” (POMT2). The processing from “MSPR-” to “SPR-” (ZNF750) and “MPPA-” to “PPA-” (POMT2) is caused by MetAPs. The second processing event is executed by DPP8/9 as CRISPR/Cas9 ablation of the genes prevented this processing as revealed by MS. (C) Peptide MS spectra of the indicated N24mers obtained from DPP8/9 or WT cells as revealed by LC-MS/MS analysis. Species without the first three residues were mostly abundant in WT cells, while N-terminal peptides missing the iMet can be detected in DPP8/9 DKO cells.
Finally, in cells ablated for UBRs or DPPs, we observed substantially reduced substrates’ ubiquitination (Fig. 5 A) and decreased rate of turnover (Fig. 5 B). Thus, a DPP–UBR axis controls the ubiquitin-mediated proteasomal degradation of substrates bearing P+3 motifs. The DPP–UBR-mediated degradation pathway is not restricted to N24mer GFP-fused peptide substrates but also operates on full-length proteins, independent of the GFP fusion. As effective commercial antibodies were not available, we cloned five HA-tagged full-length proteins corresponding to P+3 bearing N24mers that scored as UBR substrates and used single-copy lentiviral integrants as a proxy for the endogenous proteins. Protein abundance was substantially increased in DPPs or UBRs KO cells (Fig. 5 C), indicating DPP–UBR axis control over the degradation of full-length substrates containing a P+3 motif.
DPP8/9 and UBR E3s mediate the ubiquitination and degradation of P+3 substrates. (A) Immunoblot of HA-ubiquitin conjugates and peptide-GFP fusions in total cell extract (TCE) or GFP immunoprecipitates (IP:GFP) using anti-HA or -GFP antibodies, respectively. Cont.: control KO (sgRNA targeting AAVS1). (B) Western blot analysis of cycloheximide (CHX) chase assay to monitor the turnover of the indicated N24mer GFP-fusions in DPP8/9, UBR, or control KO cells. Immunoblot with anti-GFP antibody was used to detect the peptide-GFP fusions. Endogenous TSPYL1 is a short-lived protein used as a positive control for CHX assay. Vinculin serves as a loading control. h: hours. (C) Immunoblot of the indicated C-terminal HA-tagged full-length proteins using anti-HA antibody. Vinculin serves as a loading control. Source data are available for this figure: SourceData F5.
DPP8/9 and UBR E3s mediate the ubiquitination and degradation of P+3 substrates. (A) Immunoblot of HA-ubiquitin conjugates and peptide-GFP fusions in total cell extract (TCE) or GFP immunoprecipitates (IP:GFP) using anti-HA or -GFP antibodies, respectively. Cont.: control KO (sgRNA targeting AAVS1). (B) Western blot analysis of cycloheximide (CHX) chase assay to monitor the turnover of the indicated N24mer GFP-fusions in DPP8/9, UBR, or control KO cells. Immunoblot with anti-GFP antibody was used to detect the peptide-GFP fusions. Endogenous TSPYL1 is a short-lived protein used as a positive control for CHX assay. Vinculin serves as a loading control. h: hours. (C) Immunoblot of the indicated C-terminal HA-tagged full-length proteins using anti-HA antibody. Vinculin serves as a loading control. Source data are available for this figure: SourceData F5.
DPP and UBR proteins cooperate in the destruction of mislocalized secretory proteins
Gene-annotation enrichment analysis using DAVID (Huang et al., 2009) revealed a significant enrichment score of 6.45 (P < 1e−5) of “signal peptide” annotation among UBRs P+3 substrates (Table S5). Furthermore, bioinformatic analysis revealed that while 7.6% of human proteins contain proline at +3 position, ∼30% of SP-containing proteins encode P+3 (∼fourfold enrichment; P < 1e−5, Fisher’s exact test). We thus hypothesized that an important function of UBR proteins may be to eliminate mislocalized secretory proteins that fail to transport into the ER.
To explore our hypothesis, we employed a protein mislocalization strategy by deleting the targeting signal of two example reporters containing P+3: the secreted protein ARSJ and the single-pass membrane protein RELL1. Notably, both scored as UBR substrates in the N-terminome screen (Fig. 2, C and D; Fig. S3, C and D; and Table S3), and mutagenesis experiments confirmed the critical role of their N-termini in promoting instability (Fig. 1 B; Fig. S2; and Table S1). To monitor mislocalization, we cloned each full-length protein as an N-terminal fusion to GFP. We further engineered mislocalized variants: for ARSJ, the SP was truncated to retain the first ten N-degron residues while deleting residues 11–49 (“ΔSP”) (Fig. 6 A). Similarly, the SP of RELL1 was truncated and its TMD was further deleted to enforce mislocalization (“ΔSPΔTM”) (Fig. 7 A).
DPP-UBR axis mediates degradation of mislocalized secretory protein ARSJ. (A) Scheme of full-length ARSJ WT and mutant variant. The amino acid sequence is shown for signal peptide (SP). The mutant ARSJ contains the first 10 residues of the SP (underlined) to maintain the N-degron activity, while residues 11–49 were deleted to promote mislocalization (“ΔSP”). (B) WT or mutant (Mut) ARSJ-GFP were expressed in cells. Immunoblot was used to measure ARSJ-GFP level in media and total cell extract (TCE) using GFP antibody. COX-IV serves as a non-secreted control protein. Ponceau is used to show equal loading of media. (C) Representative confocal images of WT or mutated ARSJ-GFP in the context of the GPS construct in cells treated with Talabostat for 6 h. Scale bar = 25 μM. Green, GFP-fusion; red, DsRed uniformly expressed; magenta, ER localization (Calnexin); and blue, nucleus (DAPI). (D and E) Stability analysis of ARSJ-GFP, WT versus ΔSP mutant (D), or ΔSP versus ΔSP mutant with P+3 substitution (E) expressed in DPP8/9, UBR or control KO cells. Source data are available for this figure: SourceData F6.
DPP-UBR axis mediates degradation of mislocalized secretory protein ARSJ. (A) Scheme of full-length ARSJ WT and mutant variant. The amino acid sequence is shown for signal peptide (SP). The mutant ARSJ contains the first 10 residues of the SP (underlined) to maintain the N-degron activity, while residues 11–49 were deleted to promote mislocalization (“ΔSP”). (B) WT or mutant (Mut) ARSJ-GFP were expressed in cells. Immunoblot was used to measure ARSJ-GFP level in media and total cell extract (TCE) using GFP antibody. COX-IV serves as a non-secreted control protein. Ponceau is used to show equal loading of media. (C) Representative confocal images of WT or mutated ARSJ-GFP in the context of the GPS construct in cells treated with Talabostat for 6 h. Scale bar = 25 μM. Green, GFP-fusion; red, DsRed uniformly expressed; magenta, ER localization (Calnexin); and blue, nucleus (DAPI). (D and E) Stability analysis of ARSJ-GFP, WT versus ΔSP mutant (D), or ΔSP versus ΔSP mutant with P+3 substitution (E) expressed in DPP8/9, UBR or control KO cells. Source data are available for this figure: SourceData F6.
RELL1, a model mislocalized membrane protein is eliminated by DPP and UBR proteins. (A) Scheme of RELL1 WT and mutant variant that contains the first 10 resides of the SP (underlined) with TMD (resides 58–78) deletion (“ΔSPΔTM”). (B and C) Representative confocal images of WT or mutant RELL1-GFP in the context of the GPS construct, in cells treated with Talabostat for 6 h (B) or of mutant RELL1-GFP in DPP, UBR, or control KO cells (C). Scale bar = 25 μM. Green, RELL1-GFP; red, DsRed; and blue, nucleus (DAPI). (D and E) Stability analysis of RELL1-GFP WT, ΔSPΔTMD mutant (D), or ΔSPΔTMD mutant with P+3 substitution (E) expressed in DPP8/9, UBRs or control KO cells.
RELL1, a model mislocalized membrane protein is eliminated by DPP and UBR proteins. (A) Scheme of RELL1 WT and mutant variant that contains the first 10 resides of the SP (underlined) with TMD (resides 58–78) deletion (“ΔSPΔTM”). (B and C) Representative confocal images of WT or mutant RELL1-GFP in the context of the GPS construct, in cells treated with Talabostat for 6 h (B) or of mutant RELL1-GFP in DPP, UBR, or control KO cells (C). Scale bar = 25 μM. Green, RELL1-GFP; red, DsRed; and blue, nucleus (DAPI). (D and E) Stability analysis of RELL1-GFP WT, ΔSPΔTMD mutant (D), or ΔSPΔTMD mutant with P+3 substitution (E) expressed in DPP8/9, UBRs or control KO cells.
As expected, WT ARSJ was secreted, while the mutant protein was not (Fig. 6 B). Intracellular immunofluorescence staining demonstrated that the WT protein could be detected in the ER (Fig. 6 C) and that its abundance does not change in response to Talabostat (Fig. 6, B and C). Conversely, the abundance of the mutated protein increased dramatically in response to Talabostat (Fig. 6 B) and could only be detected by microscopy analysis in the cytoplasm upon DPP inhibition (Fig. 6 C) or when expressed in cells ablated for DPPs or UBRs (Fig. S5). Flow cytometry revealed that the stability of the mutant protein, but not of the WT protein, was substantially increased in both UBR and DPP8/9 KO cells (Fig. 6 D) and that its degradation was dependent on the P+3 residue (Fig. 6 E).
ARSJ, a model of mislocalized secreted proteins is degraded by DPP and UBR proteins. Representative confocal images of mislocalized ΔSP mutant ARSJ-GFP expressed in the context of GPS in DPP, UBR or control KO cells. Scale bar = 20 μM. Green, ARSJ-GFP; red, DsRed uniformly expressed; and blue, nucleus (DAPI).
ARSJ, a model of mislocalized secreted proteins is degraded by DPP and UBR proteins. Representative confocal images of mislocalized ΔSP mutant ARSJ-GFP expressed in the context of GPS in DPP, UBR or control KO cells. Scale bar = 20 μM. Green, ARSJ-GFP; red, DsRed uniformly expressed; and blue, nucleus (DAPI).
Similar results were obtained for RELL1. WT RELL1 localized to the plasma membrane (Fig. 7 B) and its stability was not affected by Talabostat or by ablating UBRs or DPP8/9 (Fig. 7, B–D). Mutant RELL1, however, was mislocalized and was only detected in the nucleocytoplasm of cells upon treatment with Talabostat (Fig. 7 B) or in cells ablated for DPPs or UBRs (Fig. 7 C). Stability analysis revealed that the mutated RELL1 variant exhibits substantially lower stability compared with its WT counterpart, and furthermore, its stability was dramatically increased in UBR or DPP8/9 KO cells (Fig. 7 D) in a P+3–dependent manner (Fig. 7 E).
Altogether, our data suggests that DPP8/9 and UBRs work in concert to enforce the correct localization of secretory proteins (Fig. 8 A).
ER import blockade triggers differential survival responses. (A) Model- DPP8/9 and UBRs work in concert to enforce the correct localization of secretory proteins. Secretory proteins contain a cleavable N-degron that is exposed upon their mislocalization. MetAP1/2 cleave first the initiating Met followed by cleavage of N-terminal di-peptide post proline by DPP8/9. Cleavage events expose an N-degron for UBR E3s. UBRs recognize now the mislocalized protein and promote its ubiquitination and its destruction in the proteasome. (B) Cells were treated with 25 nM Apratoxin A (Apra) for 48 h. To assess the effect on ER import blockade, Western blotting was performed on both the culture media (“Media”) and total cell extracts (“TCE”). Proteins were probed with antibodies against GFP to detect the presence of ARSJ-GFP, a secreted reporter protein or COX-IV, a non-secreted control protein. Ponceau staining was used to verify equal protein loading in the media samples. (C and D) Survival assay was performed on DPP8/9 and UBR KO (C) or BAG6, DPP8/9, and BAG6 + DPP8/9 TKO lines (D) following treatment with 25 nM Apra for 72 h. Data are presented as percent of surviving cells by dividing Apra treated to untreated cells (incubated with DMSO [vehicle]). (E) Colony formation assay on the same genetic backgrounds as in D following 10 days of Apra treatment. Colony size was measured and presented as ratio between Apra treated cells and untreated controls. For C–E, n = 3. ns = not significant; * P value< 0.0001, ** P value< 0.007, one-way ANOVA test. Source data are available for this figure: SourceData F8.
ER import blockade triggers differential survival responses. (A) Model- DPP8/9 and UBRs work in concert to enforce the correct localization of secretory proteins. Secretory proteins contain a cleavable N-degron that is exposed upon their mislocalization. MetAP1/2 cleave first the initiating Met followed by cleavage of N-terminal di-peptide post proline by DPP8/9. Cleavage events expose an N-degron for UBR E3s. UBRs recognize now the mislocalized protein and promote its ubiquitination and its destruction in the proteasome. (B) Cells were treated with 25 nM Apratoxin A (Apra) for 48 h. To assess the effect on ER import blockade, Western blotting was performed on both the culture media (“Media”) and total cell extracts (“TCE”). Proteins were probed with antibodies against GFP to detect the presence of ARSJ-GFP, a secreted reporter protein or COX-IV, a non-secreted control protein. Ponceau staining was used to verify equal protein loading in the media samples. (C and D) Survival assay was performed on DPP8/9 and UBR KO (C) or BAG6, DPP8/9, and BAG6 + DPP8/9 TKO lines (D) following treatment with 25 nM Apra for 72 h. Data are presented as percent of surviving cells by dividing Apra treated to untreated cells (incubated with DMSO [vehicle]). (E) Colony formation assay on the same genetic backgrounds as in D following 10 days of Apra treatment. Colony size was measured and presented as ratio between Apra treated cells and untreated controls. For C–E, n = 3. ns = not significant; * P value< 0.0001, ** P value< 0.007, one-way ANOVA test. Source data are available for this figure: SourceData F8.
To assess the adaptive advantage conferred by the DPP-UBR pathway, we analyzed cell survival in conditions that inhibit secretory proteins ER import. Consistent with established function, treatment with the Sec61 translocon inhibitor, Apratoxin A (Apra) (Paatero et al., 2016; Thornburg et al., 2013), effectively blocked the translocation of secretory proteins to the ER measured by the reduced secretion of the ARSJ-GFP reporter (Fig. 8 B). Then, under Apra-induced ER import stress conditions, we observed no significant difference in the viability of cells lacking DPP8/9 or UBR (Fig. 8 C). This finding suggests the presence of alternative PQC pathways, potentially those regulated by the cellular main PQC player BAG6 (Rodrigo-Brenni et al., 2014; Hessa et al., 2011; Yamamoto et al., 2017; Kesner et al., 2023; Müller et al., 2023), that function redundantly to eliminate mislocalized secretory proteins and maintain cellular health. To investigate this further, we generated cells ablated for BAG6 alone or in combination with DPP8/9 (“DPP8/9 + BAG6 TKO”) (Fig. S4 E). Subsequently, we assessed their survival upon Apra treatment as before. While individual knockout of DPP8/9 or BAG6 resulted in minimal or no impact on cell survival, the combined knockout of BAG6 and DPP8/9 resulted in the most profound and significant reduction in viability as evident by both cell survival (Fig. 8 D) and colony formation assays (Fig. 8 E). These findings collectively suggest that BAG6 and DPP8/9 likely function as parallel PQC pathways for the clearance of mislocalized secretory proteins. While DPP8/9 appears dispensable for cell survival when BAG6 is functional, it can compensate for BAG6 deficiency, highlighting their cooperative role in maintaining protein homeostasis.
DPP8/9 cooperate with additional E3s of N-degron pathways
To globally analyze the substrate scope of DPP8/9, we performed an N-terminome GPS screen in the background of DPP8/9 DKO cells. This screen identified 157 potential DPP8/9 substrates (Table S6), revealing some key insights:
- a
MxPy motif preference: it is believed that in addition to MxPy (which becomes xPy following iMet cleavage), DPP8/9 recognition motifs could also include substrates that commence MPy if the cleavage of the iMet was inefficient (Wilson et al., 2013; Zhang et al., 2015). We found that 72% of the DPP substrates harbored P+3 (MxPy) while only 13% harbored P+2 (MPy) (Table S6). Thus, at least in the context of stability regulation, DPPs act primarily on MxPy substrates following iMet cleavage.
- b
Collaboration with UBR E3s: Among the 113 P+3 DPP substrates, ∼35% overlapped with the UBR screen, indicating that a substantial fraction of DPP substrates is regulated by UBRs. Indeed, most DPP substrates bear canonical UBR N-degrons at the fourth position (Fig. 9 A).
- c
Beyond UBRs—cooperation with other E3s: DPPs appear to work with additional E3s in degrading substrates. DPP substrates bearing Gly at the fourth position represent candidate substrates for Cul2ZYG11B/ZER1 (Fig. S1 D) (Timms et al., 2019). Indeed, combined ablation of ZYG11B and ZER1 resulted in the stabilization of two example “MxPG-” substrates, and mutating the P+3 or G+4 residue abolished this effect (Fig. 9 B and Fig. S2). Thus, following exposure by DPP-mediated cleavage, Gly serves as N-degron for ZYG11B/ZER1.
DPP8/9 cooperation with ZYG11B/ZER1 in substrates degradation. (A) Logoplots showing the consensus N-terminal amino acid sequences among DPPs N24mer substrates. (B) Stability analysis in ZYG11B/ZER1, DPP8/9, or control KO cells expressing the indicated WT or mutated N24mer-GFP fusions.
DPP8/9 cooperation with ZYG11B/ZER1 in substrates degradation. (A) Logoplots showing the consensus N-terminal amino acid sequences among DPPs N24mer substrates. (B) Stability analysis in ZYG11B/ZER1, DPP8/9, or control KO cells expressing the indicated WT or mutated N24mer-GFP fusions.
In conclusion, our comprehensive analysis expands the known functional repertoire of DPP8/9 enzymes. We demonstrate their critical role in mediating the turnover of P+3 motif-containing substrates by diverse N-degron E3s, including UBRs and Cul2ZYG11B/ZER1, to regulate particular biological programs and safeguard the cellular proteome.
Discussion
The importance of proteostasis maintenance is underscored by the fact that nearly 5% of mammalian genes are dedicated to PQC pathways (Clague et al., 2015; Peng et al., 2003; Wolff et al., 2014). However, in many cases, the molecular mechanisms and repertoire of players involved in proteostasis-related pathways are not known. This study revealed cooperation between the primary E3s regulating N-degron pathways, the UBR E3s, and DPPs in a PQC mechanism that serves to eliminate mislocalized secretory proteins. Interestingly, DPPs may also collaborate with other E3s such as Cul2ZYG11B/ZER1 for broader control of proteome turnover under homeostatic or regulatory conditions.
Although DPP8 and DPP9 are widely expressed in mammals including humans (Cui et al., 2022), their substrates are not yet fully defined and thus their physiological and pathological roles remain to be fully explored. A functional link between DPP8/9 substrate cleavage and its subsequent proteasomal degradation has been demonstrated recently by two studies (Bolgi et al., 2022; Finger et al., 2020), however, the E3s involved were not revealed. Here, we found that both DPP8 and DPP9 could control the stability of a large coverage of substrates by cleaving the N-terminus of substrates bearing proline at the +3 N-terminal position, with DPP9 playing the more dominant role. Indeed, although DPP8 and DPP9 exhibit high sequence similarity (77% amino acid similarity, 57% amino acid identity, and higher similarity within the active site) (van Goethem et al., 2011), DPP9 is more abundant than DPP8 and its depletion leads to a large reduction in the capacity of cells to process proline-containing peptides (Geiss-Friedlander et al., 2009; Finger et al., 2020). Interestingly, DPP9 interaction with its substrates Syk and BRAC2 requires an actin-binding protein, FLNA, that acts as a scaffold linking DPP9 to its substrates (Justa-Schuch et al., 2016; Bolgi et al., 2022). Whether FLNA or other cellular factors are needed to promote the interaction between DPP8/9 and the substrate identified here awaits future studies.
Besides interacting partners regulating DPP8/9 substrate recruitment, N-terminal modifications such as N-terminal acetylation, a widespread protein modification, might also play a regulatory role in DPP8/9 N-terminal processing. Interestingly, following iMet cleavage by MetAPs, a proline residue in the first or second position, prevents N-terminal acetylation (referred to as the (X)PX rule) (Goetze et al., 2009). This will create a modification-free N-terminal that allows the interaction with DPPs. Thus, the N-terminal proline may play a dual role: preventing N-terminal acetylation and serving as a recognition site for DPPs. Indeed, we did not detect the N-terminal acetylation of our substrates by MS (Fig. 4 C and Table S4).
As the machinery of the UPS is absent from the secretory pathway lumen and mitochondria matrix, endowing proteins destined for these sites with terminal degrons might offer an attractive strategy to ensure their correct localization. Indeed, organellular proteins are significantly more likely to harbor N-degrons favored by yeast Ubr1 than their cytosolic counterparts (Tran, 2019), and as such the Arg/N-degron pathway may play a global role in the degradation of proteins aberrantly localized to the cytosol. Recently, DPP9-dependent N-terminal processing of mitochondrial precursors was demonstrated to prevent their cytosolic accumulation (Finger et al., 2020). Interestingly, around 60% of mitochondrial proteins containing a DPP9 motif also encode optimal N-degrons for UBR proteins after DPP9-mediated cleavage. This suggests a potential role for the DPP–UBR axis in the elimination of mislocalized mitochondria proteins. This pathway might function in parallel to the recently identified silencing factor of the integrated stress response (SIFI) complex, a large E3 complex that functions in mitochondrial protein import stress (Haakonsen et al., 2024). Similar to secretory proteins, mitochondrial proteins appear to encode dual-function motifs that determine both their localization and stability. These motifs enable recognition by PQC pathways when translocation fails.
Ablation of DPP8/9 alone did not increase cell death susceptibility upon treatment with ER translocation inhibitors. This finding highlights the intricate redundancy within the numerous cellular PQC pathways that act in concert to maintain proteostasis. Indeed, combined knockout of DPP8/9 and BAG6, a key PQC pathway for mislocalized membrane proteins (Rodrigo-Brenni et al., 2014; Hessa et al., 2011; Yamamoto et al., 2017), resulted in a dramatic decrease in cell viability, surpassing the effect observed with BAG6 knockout alone. While BAG6 appears to be the primary PQC mechanism for highly hydrophobic SP- or TMD -containing secretory proteins, the DPP–E3 axis might be responsible for the clearance of secretory proteins with less hydrophobic SPs. Beyond the redundant protein degradation mechanisms exemplified by BAG6 and DPP8/9, additional PQC pathways likely function in parallel, such as those involving Ubiquilin family members (Itakura et al., 2016; Suzuki and Kawahara, 2016). Furthermore, multiple, redundant targeting mechanisms provide additional layers of safeguards, ensuring accurate protein delivery to the secretory pathway (Aviram and Schuldiner, 2017).
Given the potential link between mislocalized protein clearance deficiencies and disease development (Popovic et al., 2014), elucidating the substrate selection mechanisms employed by PQC pathways could provide crucial insights into the molecular mechanism of such diseases with therapeutic implications.
Meterials and methods
Cell lines
HEK293T (ATCC CRL-3216) and HeLa cells (a gift from Kimchi A., Weizmann Institute of Science, Rehovot, Israel) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (Life Technologies) supplemented with 10% fetal bovine serum (FBS) (Gibco) and penicillin/streptomycin (Life Technologies). HEK293T cells were used in all experiments except for those in Fig. 7 B. In Fig. 7 B, HeLa cells were chosen to better visualize the membrane versus cytoplasmic localization of RELL1, the model mislocalized membrane protein.
Transfection and lentivirus production
Lentivirus was generated through the transfection of HEK293T cells using PolyJet In Vitro DNA Transfection Reagent (#SL100688; SignaGen Laboratories). Cells seeded at ∼80% confluency were transfected as recommended by the manufacturer with the lentiviral transfer vector plus four plasmids encoding Gag-Pol, Rev, Tat, and VSV-G. The media was changed 24 h after transfection and lentiviral supernatants were collected 24 h later. Cell debris was removed by centrifugation (800 × g, 5 min), and the virus was stored in single-use aliquots at −80°C. Transduction of target cells was achieved by adding the virus in the presence of 8 μg/ml hexadimethrine bromide (Polybrene) (# H9268; Sigma-Aldrich).
Inhibitors
The proteasome inhibitor Bortezomib was obtained from APExBio (#A2614) and the E1 inhibitor MLN7243 was obtained from Active Biochem (#A-1384); both were used at a final concentration of 1 µM. Talabostat was obtained from APExBio (#B3941) and was used at a final concentration of 20 µM. Cycloheximide was purchased from Sigma-Aldrich (CAS 66-81-9; Calbiochem) and was used at a final concentration of 100 μg/ml. Apratoxin A was a kind gift from V. Paavilainen (Helsinki, Finland) (Paatero et al, 2016) and was used at a final concentration of 10–25 nM.
Antibodies
Primary antibodies used in this study were the following: mouse anti-GFP (#ab290; Abcam), rabbit anti-TSPYL1 (#ab95943; Abcam), rabbit anti-COX-IV (#4850; Cell Signaling Technology), rabbit anti-HA-Tag (C29F4) (#3724; Cell Signaling Technology), rabbit anti-BAG6 (#8523; Cell Signaling Technology), and mouse anti-Vinculin (#V9131; Sigma-Aldrich). HRP-conjugated goat anti-rabbit and anti-mouse IgG secondary antibodies were obtained from Jackson ImmunoResearch (#111-035-003, #115-035-003, respectively).
Plasmids
Open reading frames (ORFs) encoding RELL1 and ARSJ were obtained from the Ultimate ORF Clone collection (Thermo Fisher Scientific). ORFs were amplified by PCR to include XhoI and BstBI sites. Mutant variants were encoded and synthesized by Integrated DNA Technologies (IDT). WT and mutant variants were cloned by ligation into pHAGE-GFP-IRES-DsRed vector N-terminal to GFP. ORFs encoding SRP54, PRODH2, ZNF750, ANGEL1, and SCRN3 were synthesized by Twist Bioscience and were cloned into pLenti vector under EF1α promoter. The pLenti vector also encodes for Crimson under the PGK promoter to enable the control of equal expression of the ORFs in various genetic backgrounds. Peptides used in this study were amplified by PCR from the N24mer peptidome library and cloned into the pHAGE-Ub-GPS or pHAGE-GPS vectors.
For individual CRISPR/Cas9-mediated gene disruption experiments, the lentiCRISPR v2 vector was used (#52961; Addgene). Oligonucleotides encoding the top and bottom strands of the sgRNAs were synthesized (IDT), annealed, and cloned into the lentiCRISPR v2 vector as previously described (Sanjana et al., 2014).
Nucleotide sequences of the sgRNAs used were:
sg-AAVS1: 5′-GGGGCCACTAGGGACAGGAT-3′.
sg1-UBR1: 5′-GTGAGAGGATGGAAATCAGCG-3′.
sg2-UBR1: 5′-GATTCTAACTTGTGGACCGAA-3′.
sg1-UBR2: 5′-GAGGAGGAGAGAAGATGGCGT-3′.
sg2-UBR2: 5′-GTACCCAAAATCTACTGCAG-3′.
sg1-UBR4: 5′-GCCTCTCGAAGATGAACACCG-3′.
sg2-UBR4: 5′-GCTGACCCCTGGACAGACAG-3′.
sg-DPP8: 5′-GATGATTTCATGTTTGTGAAG-3′.
sg-DPP9: 5′-GCGGACTCGTATCGGTACCCC-3′.
sg-RMND5A: 5′-GTGGAGCACTTCTTTCGACA-3′.
sg-GID4: 5′-GGACCCAGCTCAGGACTGGG-3′.
sg-ZYG11B: 5′-GCGCTCGTAAGGATCCTCGA-3′.
sg-ZER1: 5′-GTATGAGGAGGAGAACCCAGG-3′.
sg1-BAG6: 5′-GGAGGTGTTGGTGAAGACCT-3′.
sg2-BAG6: 5′-GAGGCTCCTCCACAGCGGTAC-3′.
Generation of CRISPR/Cas9 knockout cells
Lentivirus was generated through the transfection of HEK293T with lentiCRISPR v2 as explained before. 48 h following transduction, cells were selected with puromycin to eliminate non-transduced cells. UBR1/2/4 clone #2 is used in this study as it gave the strongest stabilization effect of known UBR substrates (Timms et al., 2019). UBR1/2/4 + DPP8/9 penta KO cells were generated by simultaneous transduction of UBR1/2/4 clone #2 with DPP8/9 sgRNAs. BAG6 KO cells were generated by simultaneous transduction with the two indicated BAG6 sgRNAs. BAG6 + DPP8/9 triple KO cells were generated by simultaneous transduction of BAG6 KO cells with DPP8/9 sgRNAs. ZYG11B/ZER1 double KO cells were generated via cotransduction with sgRNAs targeting both genes. 7 days after transduction, the genomic DNA of transduced cells was extracted, PCRs were performed to amplify an ∼500-base pair fragment flanking the edited site followed by Sanger sequencing. Inference of CRISPR Edits (ICE) CRISPR Analysis Tool was used to analyze the efficiency of editing (Synthego Performance Analysis, ICE Analysis. 2019. v3.0).
Microscopy
Cells grown on cover slips were fixed for 15 min with 4% formaldehyde, followed by permeabilizing and blocking with 3% BSA and 0.5% Triton X-100 in PBS. ER staining was done using anti-Calnexin antibody (#ab22595; Abcam) for 1 h at room temperature. Following three washes with PBS + 0.1% Tween-20 (PBS-T), DAPI staining (#D9542; Sigma-Aldrich) was added for 1 min. Finally, coverslips were mounted onto slides using mounting media (#F6182; Sigma-Aldrich) prior to imaging. Cells were imaged with Leica Stellaris 5 confocal microscope using LASX software and a 63× oil lens /1.4 N.A. UPlanSApo objective (Olympus).
Flow cytometry
Analysis of HEK293T cells by flow cytometry was performed on an LSRFortessa (Becton Dickinson) or CytoFlex (Beckman Coulter) instrument, and the resulting data were analyzed using FlowJo software. Cell sorting was performed on a MoFlo Astrios (Beckman Coulter) or BD FACSAria II (Becton Dickinson).
Protein secretion assay and immunoblotting
Five million HEK293T cells were plated in 10-cm plates. 24 h after seeding, the medium was changed to DMEM without FBS. Talabostat or DMSO were added to cells for 6 h followed by total media collection and concentration (to volume of 50 μl) using Amicon columns (Amicon Centrifugal 4 ml NMWL K10; Merck Millipore). Cells were harvested and lysed in ice-cold lysis buffer (10 mM NaPO4, 100 mM NaCl, 5 mM EDTA pH 8, 1% Triton X-100, 0.5% Deoxycholic acid sodium salt, 0.1% SDS) supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific) for 25 min at 4°C. Lysates were clarified by centrifugation (20,000 × g, 15 min, 4°C), and nuclear pellets were resuspended in lysis buffer, sonicated briefly, and reclarified. Protein concentration was determined by a standard Bradford assay (#500-0006; Bio-Rad), a linear bovine serum albumin (BSA) calibration curve, and an Epoch microplate spectrophotometer. 30 μg of total cell extract or 50 μl of concentrated media were subsequently resolved by SDS-PAGE (Mini-PROTEAN TGX Precast Protein Gels; Bio-Rad) and transferred to a nitrocellulose membrane (Trans-Blot Turbo System; Bio-Rad) which was then blocked in 10% nonfat dry milk in PBS-T. The membrane was incubated with primary antibody overnight at 4°C, and then, following three washes with PBS-T, HRP-conjugated secondary antibody was added for 1 h at room temperature. Following a further three washes in PBS-T, reactive bands were visualized using SuperSignal West Femto chemiluminescence substrate (#34095; Pierce) or an EZ-ECL (#20-500-171; Biological Industries) for 5 min. Reactive bands were visualized using the ImageQuant TL software v8.2 on Amersham Imager 680 (Cytiva).
Cycloheximide chase assays
Following treatment with 100 μg/ml cycloheximide, cells were harvested at the indicated time points and subjected to Western blot as explained before.
Analysis of ubiquitination
HEK293T cells stably expressing peptide-GFP fusions were grown in 10-cm plates and transfected with an HA-ubiquitin plasmid (Koren et al., 2018). 48 h after transfection, cells were treated with bortezomib (1 µM, 5 h) and then lysed in ice-cold lysis buffer (10 mM NaPO4, 100 mM NaCl, 5 mM EDTA pH 8, 1% Triton X-100, 0.5% Deoxycholic acid sodium salt, 0.1% SDS) supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific) and 50 µM of the deubiquitinating enzyme inhibitor PR-619 (#662141; EMD Millipore) for 30 min on ice. Nuclei were pelleted by centrifugation (14,000 × g, 10 min, 4°C). Protein concentration was determined by Bradford assay and equal amounts were taken for immunoprecipitation experiment by incubation for 1 h with 20 μl GFP-Trap magnetic agarose beads (#gtma; ChromoTek). The beads were then stringently washed three times using wash buffer (10 mM NaPO4, 300 mM NaCl, 5 mM EDTA pH 8, 1% Triton X-100, 0.5% Deoxycholic acid sodium salt, 0.1% SDS) supplemented with Halt Protease and Phosphatase Inhibitor Cocktail and 50 µM PR-619 before bound proteins were eluted upon incubation with SDS-PAGE sample buffer (95°C, 10 min). SDS-PAGE and immunoblot were done as explained before.
Immunoprecipitation of peptide-GFP for mass spectrometry analysis
DPP8/9 DKO or AAVS1 control KO cells stably expressing peptide-GFP fusions growing in 15-cm plates were treated with bortezomib for 6 h followed by lysis in ice-cold lysis buffer and immunoprecipitation using GFP-Trap magnetic agarose as explained before. The beads were then washed three times with lysis buffer before bound proteins were eluted by treatment with 2 M glycine for 1 min, followed by neutralization with 1 M Tris base, pH 10.4. Eluted proteins were further processed for mass spectrometry analysis.
Proteolysis and mass spectrometry analysis
Eluted peptide-GFP proteins were resuspended in 8 M Urea and 100 mM ammonium bicarbonate, reduced with 3 mM DTT (60°C for 30 min), modified with 10 mM iodoacetamide in 100 mM ammonium bicarbonate (room temperature 30 min in the dark) and digested in 2 M urea, 25 mM ammonium bicarbonate with Chymotrypsin or GluC (Promega) overnight at 37°C in a 1:50 (M/M) enzyme-to-substrate ratio. The resulting peptides were desalted using homemade C18 stage-tips, dried, and resuspended in 0.1% formic acid. The peptides were resolved by reverse-phase chromatography on 0.075 × 180-mm fused silica capillaries (J&W) packed with Reprosil reversed-phase material (Dr Maisch GmbH). The peptides were eluted with a linear 60-min gradient of 5–28% 15 min gradient of 28–95% and 15 min at 95% acetonitrile with 0.1% formic acid in water at flow rates of 0.15 μl/min. Mass spectrometry was performed by Q Exactive plus mass spectrometer (Thermo Fisher Scientific) in a positive mode (m/z 300–1,800 resolution 70,000 for MS1 and 17,500 for MS2) using repetitively full MS scan followed by high collision induces dissociation (HCD at 25 normalized collision energy) of the 10 most dominant ions selected from the first MS scan. The AGC settings were 3 × 106 for the full MS and 1 × 105 for the MS/MS scans. The mass spectrometry data were analyzed using Protein Discoverer 2.4 (Thermo Fisher Scientific) using Sequest search engine, searching against the sequence of the human section of the uniport database and the specific sequences of the proteins. Peptide- and protein-level false discovery rates (FDRs) were filtered to 1% using the target-decoy strategy.
Cell viability assay
Cells were plated at a density of 60,000 cells per well of a 12-well plate. The assay was performed in quadruplets. 24 h after seeding, cells were treated with 25 nM of Apratoxin A (Apra) or vehicle (DMSO) for 72 h. Viability was quantified using CellTiter-Glo assay (#G7571; Promega) according to manufacture protocol. Briefly, for each well containing 500 μl media, 500 μl of CellTiter-Glo reagent was added. The luminescence levels from 200 μl from each well were reordered using BioTek Synergy H1 microplate reader. Graphs were generated and statistical analysis was performed with GraphPad Software (GraphPad Prism v7.00-9.00).
Colony formation assay
To evaluate the ability of single cancer cells to form a colony, cells were plated at a concentration of 2,000 cells/well on six-well plates (day 0) in replicates. At day 2, the cells were treated with Apratoxin A (10 nM). At day 10, cells were fixed with methanol and acetic acid (3:1) for 5 min. Colonies were stained with 0.1% crystal violet working solution in methanol for 15 min. Colonies areas were measured for each well using ImageJ software (version 1.54 h; National Institutes of Health) and presented as a ratio between Apratoxin A–treated cells and untreated controls (incubated with DMSO).
Generation of Ub-GPS libraries
Oligonucleotides encoding single and triple substitution of selected genes were synthesized by Agilent Technologies and cloned into Ub-GPS vector as described previously (Timms et al., 2019). Briefly, synthesized oligonucleotides were amplified by PCR (Q5 Hot Start High-Fidelity DNA Polymerase, #M0493S; NEB). The PCR product was then cloned into the Ub-GPS vector between a unique SalI site engineered into the 3′ end of the ubiquitin gene and a unique NdeI site at the 5′ end of GFP using the Gibson assembly method (#E5520S; NEBuilder HiFi Cloning Kit), such that the resulting vector encoded the peptides immediately downstream of ubiquitin, followed by a short linker (ATSALGT) and GFP (commencing SKGEEL-). At least a 100-fold representation of the library was maintained at each step.
For the single and triple substitutions libraries the following substitution “code” was used:
“A”>“R,” “G”>“R,” “V”>“'R,” “L”>“R,” “I”>“'R,” #small nonpolar to big and charged (R)
“M”>“S,” “W”>“S,” “F”>“S,” “P”>“S,” #large nonpolar to small polar (S).
“S”>“R,” “T”>“R,” “C”>“R,” #small polar to big and charged (R).
“Y”>“A,” “N”>“A,” “Q”>“A,” #large polar to small nonpolar (A).
“D”>“R,” “E”>“R,” #acidic to basic (R).
“K”>“A,” “R”>“A,” “H”>“A” #basic to small non-polar (A).
This “code” was used to create maximum disruption to any potential degron, but only by replacing it with amino acids that we found previously to have a neutral effect on stability (hence using R, S, and A). In singles mutagenesis library, single mutations were used as above, whereas in triples mutagenesis library, each set of three consecutive amino acids was mutated as above. A recoded replicate of the wild-type peptide in each case was included as well. Fig. 1 B and Fig. S2 represent heatmaps comparing ΔPSI of mutant vs. wild-type peptides. In triples experiments, for each amino acid shown, the average ΔPSI of the three consecutive positions that contain the specific mutated amino acid is presented in the heatmaps.
The saturation mutagenesis library was cloned in an identical manner. For each peptide selected for analysis, each amino acid encoded at position 2 through to position 10 was mutated to all other possible 19 amino acids. For each peptide, nine reference sequences were also synthesized, in which the same wild-type amino acid sequence was encoded by different nucleotide sequences. In addition, N-terminal single amino acid addition (A, D, L, or S only) was also included. Fig. 1 C represents heatmaps comparing ΔPSI of mutant versus average of all wild-type peptides.
Ub-GPS screens
GPS plasmid libraries were packaged into lentiviral particles which were used to transduce HEK293T cells at a multiplicity of infection of ∼0.2 (achieving ∼20% DsRed positive cells) and at sufficient scale to achieve ∼500-fold coverage of the library. Hygromycin (100 µg/ml) was added two days after transduction to eliminate untransduced cells. Surviving cells were pooled, expanded, and then partitioned by FACS into bins 7 days after transduction based on the GFP/DsRed ratio. Genomic DNA was extracted from each of the pools separately (Gentra Puregene Cell Kit, #158767; Qiagen) and the fusion peptides were amplified by PCR (Q5 Hot Start Polymerase; NEB) using a forward primer annealing to the end of the ubiquitin gene and a reverse primer annealing to the front of GFP; sufficient reactions were performed to amplify a total mass of DNA equivalent to the mass of genomic DNA from cells representing 500-fold coverage of the library. All PCR products were pooled, and one-tenth of the mix was purified using a spin column (PCR purification kit, #28106; Qiagen). Finally, 200 ng of the purified PCR product was used as the template for a second PCR reaction using primers to add the Illumina P5 sequence and a 7 bp “stagger” region to the 5′ end, and Illumina indexes and P7 sequence at the 3′ end. Samples to be multiplexed were then pooled, purified on an agarose gel (QIAEXII Gel Extraction Kit, #20051; Qiagen), and sequenced on an Illumina NextSeq instrument.
CRISPR screens
A custom sgRNA library was designed targeting 850 human E3s at a depth of six sgRNAs per gene. The sgRNA sequences together with flanking BbsI restriction enzyme recognition sites were synthesized by Twist Bioscience. The oligonucleotide pool was amplified by PCR (Q5 Hot Start Polymerase; NEB), and the product was purified (Qiagen PCR purification kit) and digested with BbsI (NEB). The digested product was concentrated by ethanol precipitation and then visualized on a 10% TBE PAGE gel (Thermo Fisher Scientific) stained with SYBR Gold (Thermo Fisher Scientific). DNA was isolated from the 28 bp band using the “crush-and-soak” method, concentrated by ethanol precipitation, and then cloned into lentiCRISPR v2 (#52961; Addgene) digested with BsmBI (NEB).
The sgRNA library DNA was packaged into lentiviral particles. HEK293T cells stably expressing example peptide-GFP fusion proteins were transduced at a multiplicity of infection of ∼0.3 at sufficient scale to maintain at least a 1,000-fold representation of the library. Untransduced cells were eliminated through puromycin selection commencing 2 days after transduction. The top ∼1% of the surviving cells based on the GFP/DsRed ratio were isolated by FACS, which was performed 7 days after transduction. For each screen, genomic DNA was extracted from both the sorted cells and the unselected library as a reference. The sgRNAs in both pools were amplified by PCR and sequenced on an Illumina NextSeq instrument.
Bioinformatics
N-terminome Ub-GPS screens
Read counts and associated stability scores for each peptide–GFP fusion are detailed in Table S1 (mutagenesis screens) and Table S6 (DPPs screen).
For the mutagenesis libraries screen, a ΔPSI score was generated for each peptide–GFP fusion reflecting the difference in raw PSI scores between wild-type peptides and mutants.
For the classification of UBR substrates bearing P+3 originating from the Ub-GPS screen detailed by Timms et al. (2019), a ΔPSI score was generated for each peptide–GFP fusion reflecting the difference in raw PSI scores between the control KO sample and UBR KO clones #1–3. Peptide-GFP fusions were defined as UBR substrates if they were stabilized ≥0.3 PSI units in at least one UBR KO clone. In addition, GPS screen profiles for each of the representative Ub-GPS N24mers bearing P+3 substrates showing the distribution of Illumina sequencing reads across the six bins in control cells versus three independent UBR1/2/4 KO cells were plotted (examples are presented in Fig. S3 C). Those with a positive median geometric mean stability of the peptide in UBR KO cells compared with WT cells (indicating a stabilization shift in UBR KO cells) were filtered as substrates.
In the DPP8/9 N-terminome screen, candidate substrates were defined as whether they showed ΔPSI≥0.5 (PSIDPP8/9 DKO-PSIControl KO) for both replicates.
CRISPR screens
Illumina reads were first trimmed of constant regions derived from the backbone of lentiCRISPR v2 expression vector using Cutadapt (Martin, 2011). Count tables were generated from the remaining variable sgRNA sequences using Bowtie 2 (Langmead and Salzberg, 2012). The model-based analysis of genome-wide CRISPR/Cas9 Knockout (MAGeCK) method (Li et al., 2014) was used to rank the performance of individual genes targeted by multiple sgRNAs enriched in the selected cells versus the unsorted populations. The full MAGeCK results for each screen are presented in Table S2.
Online supplemental material
Five supplemental figures are provided. Fig. S1 shows the N-degron pathways in eukaryotes. Fig. S2 shows that the peptide instability is controlled by P+3 motif. Fig. S3 shows that UBRs, not the GID complex, regulate the stability of P+3 N24mers. Fig. S4 shows the knock-out efficiency across various genetic backgrounds. Fig. S5 shows that ARSJ, a model of mislocalized secreted proteins is degraded by DPP and UBR proteins. Six supplemental tables are provided. Table S1 shows the mutagenesis screens data. Table S2 shows the results from the CRISPR screens on the Ub-GPS-N24mer clones as analyzed by MAGeCK. Table S3 shows the P+3 candidate UBR substrates recovered from Ub-GPS N24mer library screen data. Table S4 shows high-confidence peptides recovered by LC-MS/MS analysis of N24mer-GFP purified from DPP8/9 DKO and WT cells. Table S5 shows the DAVID functional annotation analysis comparing UBR P+3 substrates versus the N24mer library. Table S6 shows the Ub-GPS N24mer library screen data in DPP8/9 DKO compared to AAVS1 control KO cells.
Data availability
All data originating from this study are available in the published article and its online supplemental material.
Acknowledgments
We thank T. Ziv at The Smoler Proteomics Center (Technion, Haifa, Israel) for MS. We thank V. Paavilainen (Helsinki, Finland) for Apratoxin A.
This study was supported by a United States-Israel Binational Science Foundation to I. Koren and S.J. Elledge (grant 2021029). I. Koren is supported by the European Research Council (ERC-2020-STG 947709). S.J. Elledge is an Investigator with the Howard Hughes Medical Institute. R.T. Timms is the recipient of a Pemberton-Trinity Fellowship.
Author contributions: A. Shimshon: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Visualization, K. Dahan: Data curation, Formal analysis, Investigation, Methodology, Resources, M. Israel Gueta: Investigation, Validation, D. Olmayev-Yaakobov: Formal analysis, Investigation, R.T. Timms: Formal analysis, Writing—review and editing, A. Bekturova: Investigation, Y. Makaros: Formal analysis, Investigation, Methodology, Project administration, S.J. Elledge: Conceptualization, Funding acquisition, Supervision, Writing—original draft, I. Koren: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing—original draft, Writing—review and editing.
References
Author notes
A. Shimshon and K. Dahan contributed equally to this paper.
M. Israel-Gueta, D. Olmayev-Yaakobov, and R.T. Timms contributed equally to this paper.
Disclosures: The authors declare no competing interests exist.








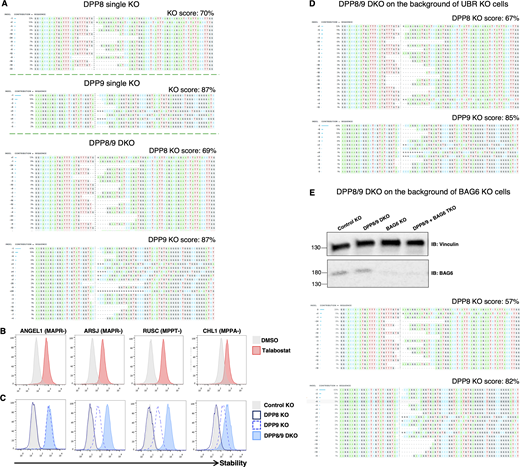





![ER import blockade triggers differential survival responses. (A) Model- DPP8/9 and UBRs work in concert to enforce the correct localization of secretory proteins. Secretory proteins contain a cleavable N-degron that is exposed upon their mislocalization. MetAP1/2 cleave first the initiating Met followed by cleavage of N-terminal di-peptide post proline by DPP8/9. Cleavage events expose an N-degron for UBR E3s. UBRs recognize now the mislocalized protein and promote its ubiquitination and its destruction in the proteasome. (B) Cells were treated with 25 nM Apratoxin A (Apra) for 48 h. To assess the effect on ER import blockade, Western blotting was performed on both the culture media (“Media”) and total cell extracts (“TCE”). Proteins were probed with antibodies against GFP to detect the presence of ARSJ-GFP, a secreted reporter protein or COX-IV, a non-secreted control protein. Ponceau staining was used to verify equal protein loading in the media samples. (C and D) Survival assay was performed on DPP8/9 and UBR KO (C) or BAG6, DPP8/9, and BAG6 + DPP8/9 TKO lines (D) following treatment with 25 nM Apra for 72 h. Data are presented as percent of surviving cells by dividing Apra treated to untreated cells (incubated with DMSO [vehicle]). (E) Colony formation assay on the same genetic backgrounds as in D following 10 days of Apra treatment. Colony size was measured and presented as ratio between Apra treated cells and untreated controls. For C–E, n = 3. ns = not significant; * P value< 0.0001, ** P value< 0.007, one-way ANOVA test. Source data are available for this figure: SourceData F8.](https://cdn.rupress.org/rup/content_public/journal/jcb/223/8/10.1083_jcb.202311035/1/m_jcb_202311035_fig8.png?Expires=1773903040&Signature=s4FA9otKjpg03RZhHeQpwASpYHmFKsuOLuMzZnzRrT3UNKunImKvltYrT3nC80uE4Qd62fkb0c10u0Pbdeva9hYleiO1swmvAGGQAdhENfTFMJmBHpCOSEDRyYRhb7mT64rev1J0x3xlKsVHdEYicXdJjj1PJjokxI4gj1JKepgc24cth3Ml2WaHmFTBaRX6ggf7ftpGbp6FXsJEWk8gEUvnz3HrkTl1Y1nJiOTTDCgluNeDwcgXcwAc5c~V~vhCaFfDOnsV~TrXrnuo6mFAfB58SkTSO2yZmxguJkjmYt-N06788m0vpCi8qkWbcViahhN-DKGZP8YLMr0GTdRD-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



