Most mitochondrial proteins originate from the cytosol and require transport into the organelle. Such precursor proteins must be unfolded to pass through translocation channels in mitochondrial membranes. Misfolding of transported proteins can result in their arrest and translocation failure. Arrested proteins block further import, disturbing mitochondrial functions and cellular proteostasis. Cellular responses to translocation failure have been defined in yeast. We developed the cell line-based translocase clogging model to discover molecular mechanisms that resolve failed import events in humans. The mechanism we uncover differs significantly from these described in fungi, where ATPase-driven extraction of blocked protein is directly coupled with proteasomal processing. We found human cells to rely primarily on mitochondrial factors to clear translocation channel blockage. The mitochondrial membrane depolarization triggered proteolytic cleavage of the stalled protein, which involved mitochondrial protease OMA1. The cleavage allowed releasing the protein fragment that blocked the translocase. The released fragment was further cleared in the cytosol by VCP/p97 and the proteasome.
Introduction
Biogenesis and maintenance of cellular proteomes are challenging tasks. Proteins must be produced with a correct amino acid sequence, transported to their specific destination, and folded into mature, functional structures. Failure of any of these steps not only results in the formation of erroneous proteins but also has the capacity to disturb protein homeostasis. This necessitates quality control (QC) mechanisms that repair or remove aberrant proteins.
Protein transport is critical for the biogenesis of cellular organelles with sharply defined boundaries. This is apparent for mitochondria with two membrane systems, the outer mitochondrial membrane (OM) and the inner mitochondrial membrane (IM), sculpting the aqueous subcompartments called the mitochondrial matrix and the intermembrane space (IMS). The architecture of mitochondria allows these organelles to embody vital metabolic processes. The proteome of human mitochondria comprises over a thousand diverse proteins (Rath et al., 2021; Morgenstern et al., 2021). Nearly all of these are produced in the cytosol as protein precursors, which require selective and accurate transport to their destination sites within the organelle. The diversity of mitochondrial proteins and their submitochondrial locations is reflected by the diversity of their mitochondrial targeting signals (MTS) and sorting mechanisms (Becker et al., 2019; Bykov et al., 2020). Still, the primary step of the import is common to most precursors. They cross the OM via the translocase of the outer membrane (TOM) complex that serves as a passage, precursor receptor, and import regulator (Wasilewski et al., 2017; Becker et al., 2019). Protein-conducting channels across the OM are formed by the β-barrel protein Tom40 (TOMM40). After crossing the OM via TOM, proteins are routed to their final destinations by several protein-sorting and -assembly pathways. The largest group of precursors possess N-terminal cleavable MTS sequences and rely on the IM proton gradient to initiate and assist their translocation operated by the IM translocase TIM23 and presequence translocase-associated motor (Schendzielorz et al., 2017). TIM23 substrates are fully translocated into the matrix or laterally released for IM integration and undergo proteolytic removal of the MTS (Mossmann et al., 2012; Vögtle et al., 2009). Other protein translocation pathways include TIM22 for IM integration, the sorting and assembly machinery (SAM) for integration into the OM, and the mitochondrial import and assembly (MIA) pathway of the IMS (Wasilewski et al., 2017; Becker et al., 2019).
Protein import routes within mitochondria differ by their driving force and throughput (Schäfer et al., 2022; Morgenstern et al., 2017). Common is that precursor folding occurs after they reach their destination. Proteins must be unfolded while passing the translocases due to limitations of the lumen of the protein-conducting channel of Tom40 protein (Wiedemann and Pfanner, 2017; Kater et al., 2020; Tucker and Park, 2019; Ahting et al., 2001). Thus, a folded domain present in a precursor can stall during import, preventing the completion of the translocation (Schleyer and Neupert, 1985; Rassow et al., 1989; Wienhues et al., 1991; Schülke et al., 1997; Gaume et al., 1998; Voisine et al., 1999; Gold et al., 2014). The TOM channel allows the backward movement of proteins so arrested precursors can be released back (Bragoszewski et al., 2015). However, precursors that stall during import can span OM and IM in a stable complex with TOM and TIM translocases (Chacinska et al., 2003; Gomkale et al., 2021). Such stabilization prevents further mobility of the arrested protein. Substrates of both TIM23 and TIM22 could be affected by such arrests (Shiota et al., 2015). In vitro studies provided evidence that arrested import intermediates sequester available translocases, while studies in yeast revealed the associated growth defect (Wienhues et al., 1991; Schülke et al., 1997). The impact of protein import blockage goes beyond disturbing mitochondria’s bioenergetic and metabolic functions. Unimported mitochondrial proteins accumulate in the cytosol, increasing the risk of misfolding and aggregation (Nowicka et al., 2021). Importantly, native precursor proteins were found to stall during import (Weidberg and Amon, 2018; Boos et al., 2019; Glick et al., 1993).
The severe consequences of clogging necessitate efficient response mechanisms. As a first tier of defense against precursor protein mislocalization, yeast cells increase proteasome assembly and activity (Wrobel et al., 2015) and upregulate the expression of proteasome components (Boos et al., 2019). Simultaneously, transcripts encoding multiple mitochondrial proteins are downregulated, decreasing the load on mitochondrial translocases. In parallel with increased degradation, general protein synthesis becomes attenuated to limit the build-up of mislocalized proteins (Wang and Chen, 2015).
Resolving a translocase blockage requires the removal of the stalled cargo. In yeast, it is mediated by at least two mechanisms: constitutively active mitochondrial protein translocation-associated degradation (mitoTAD) (Mårtensson et al., 2019) and inducible mechanism of mitochondrial compromised protein import response (mitoCPR), which reacts to translocase overload by the IM-targeted proteins (Weidberg and Amon, 2018). MitoTAD employs a ubiquitin-dependent chaperone complex built around Cdc48 AAA ATPase (VCP, or p97 in humans), while in mitoCPR another AAA ATPase, Msp1, is recruited to extract the arrested precursor, allowing its further proteasomal degradation.
Several reported molecular mechanisms responding to mitochondrial protein translocase clogging show that cells are equipped with redundant defense paths, highlighting the biological significance of the problem. However, our understanding of these QC mechanisms originates vastly from studying yeast. To understand how the mechanisms discovered in fungi translate to higher eukaryotes, we investigated translocase clogging in human cells. In this work, we developed and validated a cellular model of translocase clogging based on stably folding GFP protein (i.e., superfolder GFP; sfGFP). The sfGFP tag within the mitochondria-targeted fusion caused its arrest in the TOM channel, interfering with the import of other proteins and disturbing mitochondrial functions. Using this model, we reported a significant role of mitochondrial proteases in clearing arrested precursors. Surprisingly, despite its negative impact, the arrested protein proved stable in a steady state and was rapidly cleared only upon dissipation of the inner membrane potential. The depolarization triggered proteolytic cleavage of the arrested precursor, allowing the release of the unimported fragment from the translocase and restoration of mitochondrial architecture. The released protein became a substrate of cytosolic degradation, mediated by VCP and proteasome. We conclude that in human mitochondria, precursors in transit may become stably bound and require intramitochondrial cleavage for effective release. The observed mechanism differs from those described in fungi, where AAA ATPases are recruited to extract arrested cargo. Also, the role of the proteasome is less direct as the initial cleavage of arrested protein is executed by mitochondrial proteases, including OMA1.
Results
Model in human cells
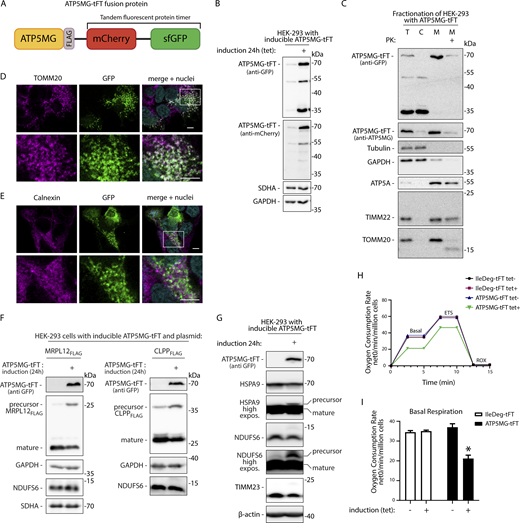
To study the QC of mitochondrial protein translocation, we established an inducible import failure model in a human cell line. We based the model on our earlier finding that tagging mitochondrial proteins with the tandem fluorescent protein timer (tFT; a fusion of mCherry and sfGFP) interferes with their import (Kowalski et al., 2018). Import disturbance likely could be attributed to sfGFP’s resistance to unfolding (Khmelinskii et al., 2016). This agrees with a recent study showing that sfGFP stalls in the mitochondrial translocation machinery of yeast if fused to the N-terminal targeting sequence (Gomkale et al., 2021). Here, we designed a precursor protein consisting of the ATP5MG fused to a C-terminal “clogging” tFT tag (Fig. 1 A) for expression in human cells. We used the Flp-In T-REx HEK-293 cell line, which allowed for integration and inducible expression of the transgene upon tetracycline treatment (Fig. 1 B). Cells expressing ATP5MG-tFT fusion accumulated a full-size fusion protein but also its smaller fragments that were detected by an anti-GFP antibody (Fig. 1, B and C).
ATP5MG-tFT fusion causes mitochondrial protein translocase clogging and impairs mitochondrial protein import. (A) Schematic representation of ATP5MG-tFT fusion protein used to model translocase blocking. ATP5MG was expressed in fusion with the tFT consisting of mCherry and sfGFP proteins. (B) Tetracycline-inducible expression of ATP5MG-tFT in HEK-293 cells. The cells were grown with or without the addition of tetracycline (1 µg/ml) for 24 h. Total protein extracts were analyzed with indicated antibodies. Expression of the fusion protein was tested with anti-GFP and anti-mCherry antibodies. (C) Subcellular fractionation of HEK-293 cells expressing ATP5MG-tFT protein. After differential centrifugation, samples were analyzed by SDS-PAGE and western blotting. T, total; C, cytoplasm; M, mitochondria; PK, proteinase K treatment (25 µg/ml). (D and E) Intracellular distribution of ATP5MG-tFT in HEK-293 cells visualized by confocal imaging. ATP5MG-tFT was detected by GFP fluorescence. Cells were immunolabeled using antibodies against TOMM20 (D) or calnexin (E) to visualize mitochondria and ER, respectively. Cell nuclei were stained with DAPI. Scale bars: 5 μm. The bottom panels show enlarged fragments marked in the top panel. (F) Co-expression of ATP5MG-tFT and plasmid-encoded CLPPFLAG or MRPL12FLAG proteins. Total cell extracts were analyzed by SDS-PAGE and western blotting. Precursor and mature forms processed in mitochondria were detected by anti-FLAG antibodies. (G) Cells with or without induction of ATP5MG-tFT were analyzed as in B. High-exposure images for HSPA9 and NDUFS6 western blots are shown. (H) OCR in cells expressing different tFT fusions. ETS, maximal respiration. (I) Basal respiration quantified from H. OCR was normalized to the number of cells. Data are presented as the mean ± SEM (number of independent experiments n = 3); *P < 0.05 (two-way ANOVA followed by multicomparision test). Source data are available for this figure: SourceData F1.
ATP5MG-tFT fusion causes mitochondrial protein translocase clogging and impairs mitochondrial protein import. (A) Schematic representation of ATP5MG-tFT fusion protein used to model translocase blocking. ATP5MG was expressed in fusion with the tFT consisting of mCherry and sfGFP proteins. (B) Tetracycline-inducible expression of ATP5MG-tFT in HEK-293 cells. The cells were grown with or without the addition of tetracycline (1 µg/ml) for 24 h. Total protein extracts were analyzed with indicated antibodies. Expression of the fusion protein was tested with anti-GFP and anti-mCherry antibodies. (C) Subcellular fractionation of HEK-293 cells expressing ATP5MG-tFT protein. After differential centrifugation, samples were analyzed by SDS-PAGE and western blotting. T, total; C, cytoplasm; M, mitochondria; PK, proteinase K treatment (25 µg/ml). (D and E) Intracellular distribution of ATP5MG-tFT in HEK-293 cells visualized by confocal imaging. ATP5MG-tFT was detected by GFP fluorescence. Cells were immunolabeled using antibodies against TOMM20 (D) or calnexin (E) to visualize mitochondria and ER, respectively. Cell nuclei were stained with DAPI. Scale bars: 5 μm. The bottom panels show enlarged fragments marked in the top panel. (F) Co-expression of ATP5MG-tFT and plasmid-encoded CLPPFLAG or MRPL12FLAG proteins. Total cell extracts were analyzed by SDS-PAGE and western blotting. Precursor and mature forms processed in mitochondria were detected by anti-FLAG antibodies. (G) Cells with or without induction of ATP5MG-tFT were analyzed as in B. High-exposure images for HSPA9 and NDUFS6 western blots are shown. (H) OCR in cells expressing different tFT fusions. ETS, maximal respiration. (I) Basal respiration quantified from H. OCR was normalized to the number of cells. Data are presented as the mean ± SEM (number of independent experiments n = 3); *P < 0.05 (two-way ANOVA followed by multicomparision test). Source data are available for this figure: SourceData F1.
Efficient targeting into mitochondria is a prerequisite for a fusion protein to stall in the translocation machinery. Accordingly, ATP5MG-tFT protein was recovered in the mitochondrial fraction, while faster-migrating fragments were located in the cytosol (Fig. 1 C). The clogging fusion was susceptible to externally added protease, as was the OM protein TOMM20 but not the internal mitochondrial proteins (ATP5A, TIMM22) (Fig. 1 C). This indicates that at least part of the fusion was exposed on the outer side of the OM. We confirmed the subcellular localization of the ATP5MG-tFT by confocal microscopy. The sfGFP fluorescent signal colocalized with the OM marker TOMM20 but not with the endoplasmic reticulum (ER) marker calnexin (Fig. 1, D and E). The sfGFP signal also colocalized with the mitochondrial stain Mitotracker (Fig. S1 A).
Related toFig. 1,. (A) Confocal imaging of HEK-293 cells with ATP5MG-tFT fusion. Cells were live-stained with Mitotracker Deep Red FM. ATP5MG-tFT was detected by GFP fluorescence. The bottom panels show enlarged fragments marked in the top panel. Scale bars: 5 μm. (B) A schematic representation of IleDeg-tFT fusion protein (Khmelinskii et al., 2012) used in this work. N-degron tFT fusion proteins are encoded as pro-N-degron with an N-terminal ubiquitin. Ubiquitin is removed from the translated protein, exposing the new N-termini—isoleucine (Ile) amino acid residue. Isoleucine was shown to confer a moderate turnover rate of the fusion (Khmelinskii et al., 2012). (C) Related to Fig. 1 F; co-expression of IleDeg-tFT (non-mitochondrial protein) or ATP5MG-tFT and plasmid-encoded CLPPFLAG or MRPL12FLAG proteins. Total cell extracts were analyzed by SDS-PAGE and western blotting. Precursor and mature forms processed in mitochondria were detected by anti-FLAG antibodies. (D) ETS respiration based on Fig. 1 H. OCR was normalized to the number of cells. Data are presented as the mean ± SEM (n = 3 independent experiments); two-way ANOVA, no significant changes for P threshold of 0.05. (E) HEK-293 Flp-In T-REx cells with ATP5MG-tFT, IleDeg-tFT, or empty were cultured with or without the addition of 1 µg/ml tetracycline for 48 h. Cell proliferation was measured by direct cell counting. The graph presents cell doubling time. Mean ± SEM (n = 4 independent experiments). *P < 0.05 (two-sided, paired Student’s t test). Source data are available for this figure: SourceData FS1.
Related toFig. 1,. (A) Confocal imaging of HEK-293 cells with ATP5MG-tFT fusion. Cells were live-stained with Mitotracker Deep Red FM. ATP5MG-tFT was detected by GFP fluorescence. The bottom panels show enlarged fragments marked in the top panel. Scale bars: 5 μm. (B) A schematic representation of IleDeg-tFT fusion protein (Khmelinskii et al., 2012) used in this work. N-degron tFT fusion proteins are encoded as pro-N-degron with an N-terminal ubiquitin. Ubiquitin is removed from the translated protein, exposing the new N-termini—isoleucine (Ile) amino acid residue. Isoleucine was shown to confer a moderate turnover rate of the fusion (Khmelinskii et al., 2012). (C) Related to Fig. 1 F; co-expression of IleDeg-tFT (non-mitochondrial protein) or ATP5MG-tFT and plasmid-encoded CLPPFLAG or MRPL12FLAG proteins. Total cell extracts were analyzed by SDS-PAGE and western blotting. Precursor and mature forms processed in mitochondria were detected by anti-FLAG antibodies. (D) ETS respiration based on Fig. 1 H. OCR was normalized to the number of cells. Data are presented as the mean ± SEM (n = 3 independent experiments); two-way ANOVA, no significant changes for P threshold of 0.05. (E) HEK-293 Flp-In T-REx cells with ATP5MG-tFT, IleDeg-tFT, or empty were cultured with or without the addition of 1 µg/ml tetracycline for 48 h. Cell proliferation was measured by direct cell counting. The graph presents cell doubling time. Mean ± SEM (n = 4 independent experiments). *P < 0.05 (two-sided, paired Student’s t test). Source data are available for this figure: SourceData FS1.
To verify that the fusion protein blocks mitochondrial translocases, we tested its effect on the import of other mitochondrial proteins. We co-expressed ATP5MG-tFT together with mitochondria-targeted proteins CLPPFLAG or MRPLFLAG. Both of these proteins contain relatively large N-terminal MTS signals, which make up ∼20% of their molecular mass and are cleaved off upon maturation in the matrix (Calvo et al., 2017). Thus, mature and precursor forms can be easily separated by electrophoresis. Indeed, upon the expression of CLPPFLAG and MRPLFLAG in HEK293 cells, well-separated bands could be detected with anti-FLAG antibodies, corresponding to precursor and mature forms (Fig. 1 F and Fig. S1 C). In standard conditions, CLPPFLAG or MRPLFLAG were effectively imported and processed in mitochondria, as manifested by the accumulation of smaller mature forms of these proteins. However, when expressed along ATP5MG-tFT fusion, we observed a decreased accumulation of mature forms and a noticeable increase in unprocessed precursors (Fig. 1 F). A control tFT fusion not directed to mitochondria (IleDeg-tFT) had no effect on the processing of mitochondria-directed proteins (Fig. S1, B and C).
We also looked for the accumulation of unprocessed precursor forms of native mitochondrial proteins. In physiological conditions, cellular levels of most mitochondrial protein precursors are low and often undetectable by western blot. However, translocase blocking can lead to the accumulation of unimported proteins (Weidberg and Amon, 2018; Boos et al., 2019; Mårtensson et al., 2019). Indeed, longer exposure of western blot images revealed the accumulation of higher molecular mass forms of mitochondrial heat shock protein HSPA9 and NDUFS6 (Fig. 1 G). Such forms, likely unprocessed precursors, were detected only in the cells expressing ATP5MG-tFT but not in control cells. Together, our observations provided evidence for a mitochondrial protein import defect related to the expression of the model protein.
Translocase blocking in yeast was demonstrated to cause a general decline in mitochondrial respiratory function. To check whether our fusion protein induces a similar defect, we compared oxygen utilization in untreated cells or 24 h after inducing ATP5MG-tFT production (Fig. 1 H). Cells expressing the mitochondria-directed fusion displayed significantly lower basal respiration (Fig. 1 I) and a tendency toward decreased maximum respiration (Fig. S1 D), while the expression of control IleDeg-tFT fusion did not affect respiratory parameters.
The expression of ATP5MG-tFT clogging fusion decreased cell proliferation (Fig. S1 E). The number of cells in the population after 48 h of expression induction was 79% of the population without induction. The decrease was specific to the clogging fusion. Expression of IleDeg-tFT fusion did not affect cell numbers. Similarly, HEK-293 Flp-In cells with no inducible transgene remained unaffected by inducing agent treatment. Still, the proliferation reduction appears mild compared with severe growth defects reported in yeast (Boos et al., 2019). This possibly reflects modest fusion protein expression levels characteristic of Flp-In T-REx cells.
Together, we found that the model fusion was directed to mitochondria, which interfered with the import of other proteins, disturbed mitochondrial functions, and impacted cell proliferation. These properties substantiate the probability of translocase clogging by ATP5MG-tFT protein.
Molecular interactions of the clogging protein validate translocase blocking
To define binding partners of the model fusion, we developed a co-immunoprecipitation (co-IP) protocol. We used GFP-Trap beads to purify ATP5MG-tFT from total cell extracts (Fig. 2 A). Using antibodies against TOMM40 and TOMM20 proteins, we confirmed that the model protein interacted with the TOM translocase. This membrane complex was copurified only with ATP5MG-tFT but not with the control IleDeg-tFT fusion or from the cells with no GFP. Interestingly, we did not observe similar co-purification of TIMM23 protein of the IM translocase. Instead, we found the ATP5A protein of the ATP synthase complex among proteins copurified with the clogging fusion. This indicates that the N-terminal part of the fusion likely reached its destination. We also observed the enrichment of components of mitochondrial contact site and cristae organizing system (MICOS) and SAM complexes. These are known partners of TOM and of each other, and might represent indirect interactions (Colina-Tenorio et al., 2020; Schorr and van der Laan, 2018). ATP5MG-tFT also might span cristae junction and thus interact with MICOS. Having established an effective co-IP protocol, we used mass spectrometry (MS) to define the interactome of the clogging protein. The experiments compared cells expressing the clogging protein and control cells not expressing any fusion protein (Fig. 2 B) or non-mitochondrial tFT fusion (Fig. S2). We analyzed co-IP eluates and corresponding total protein extracts. Out of 3,063 identified proteins, 86 were significantly enriched in clogger IP samples. Among the most enriched were components of the mitochondrial import machinery, namely TOM translocase subunits (Fig. 2 B and Fig. S2, see eluate). Although ATP5MG directs the clogging construct for IM integration, we did not detect enrichment of the components of the IM translocases TIM23 or TIM22. Instead, subunits of the ATP synthase were among the significantly enriched genes. This indicates that the N-terminal part of ATP5MG-tFT could complete its import, becoming integrated into the ATP synthase complex. Other identified interactors of the fusion protein could not be grouped by molecular function (Table S1). The analysis of protein changes in cell lysates that were inputs for the co-IP experiment (Fig. 2 B and Fig. S2, see load) indicated only a few proteins that showed a differential expression in lysates originating from ATP5MG-tFT–expressing vs. control cells, none of which was identified as an ATP5MG-tFT binder. Thus, the short-time expression of the clogging fusion (24 h) did not trigger substantial proteome remodeling. Importantly, none of the ATP synthase or TOM components were upregulated in ATP5MG-tFT cells, supporting the specificity of the IP enrichment.
Mitochondria-directed tFT fusion protein interacts with mitochondrial protein translocation channel. (A) Lysates of HEK-293 cells expressing ATP5MG-tFT or IleDeg-tFT (i.e., tFT fusion not directed to mitochondria), and control cells with no transgene were immunoprecipitated with anti-GFP beads. Proteins from the initial sample (load, 10%), the eluate (100%), and the sample remaining after IP (unbound, 10%) were analyzed using SDS-PAGE and immunoblotting with the indicated antibodies. (B) Proteins from cellular lysates (load) and purified with anti-GFP beads (eluate) were analyzed by LC-MS/MS (number of independent experiments n = 4). In volcano plots, the x-axis represents the log2 fold change of protein levels in ATP5MG-tFT samples compared with control cells samples. Total lysates (top) and eluates (bottom) were analyzed. Orange, magenta, and blue indicate proteins belonging to ATP synthase, translocases of the inner mitochondrial membrane (TIM), and TOM complexes, respectively; of these, significantly changed proteins (two-sided, paired Student’s t test P < 0.05, |log2 fold change| >1) were labeled. (C) Lysates of mitochondria isolated from HEK-293 cells expressing ATP5MG-tFT and/or TOMM22-HA, were immunoprecipitated with anti-HA beads. Samples were analyzed using SDS-PAGE and immunoblotting with the indicated antibodies (load, 5%; eluate, 100%). (D) Proteins of mitochondria isolated from HEK-293 cells with or without 24 h induction of ATP5MGtFT were analyzed by BN-PAGE and immunoblotting with the indicated antibodies. (E) Schematic depiction of ATP5MG-tFT plausible topology, interacting with ATP synthase and TOM translocase. *, ** indicate co-migration of ATP5MG-tFT, TOM, and ATP synthase. Source data are available for this figure: SourceData F2.
Mitochondria-directed tFT fusion protein interacts with mitochondrial protein translocation channel. (A) Lysates of HEK-293 cells expressing ATP5MG-tFT or IleDeg-tFT (i.e., tFT fusion not directed to mitochondria), and control cells with no transgene were immunoprecipitated with anti-GFP beads. Proteins from the initial sample (load, 10%), the eluate (100%), and the sample remaining after IP (unbound, 10%) were analyzed using SDS-PAGE and immunoblotting with the indicated antibodies. (B) Proteins from cellular lysates (load) and purified with anti-GFP beads (eluate) were analyzed by LC-MS/MS (number of independent experiments n = 4). In volcano plots, the x-axis represents the log2 fold change of protein levels in ATP5MG-tFT samples compared with control cells samples. Total lysates (top) and eluates (bottom) were analyzed. Orange, magenta, and blue indicate proteins belonging to ATP synthase, translocases of the inner mitochondrial membrane (TIM), and TOM complexes, respectively; of these, significantly changed proteins (two-sided, paired Student’s t test P < 0.05, |log2 fold change| >1) were labeled. (C) Lysates of mitochondria isolated from HEK-293 cells expressing ATP5MG-tFT and/or TOMM22-HA, were immunoprecipitated with anti-HA beads. Samples were analyzed using SDS-PAGE and immunoblotting with the indicated antibodies (load, 5%; eluate, 100%). (D) Proteins of mitochondria isolated from HEK-293 cells with or without 24 h induction of ATP5MGtFT were analyzed by BN-PAGE and immunoblotting with the indicated antibodies. (E) Schematic depiction of ATP5MG-tFT plausible topology, interacting with ATP synthase and TOM translocase. *, ** indicate co-migration of ATP5MG-tFT, TOM, and ATP synthase. Source data are available for this figure: SourceData F2.
Related to Fig. 2 . Proteins from cellular lysates (load) and purified with anti-GFP beads (eluate) were analyzed by LC-MS/MS (number of independent experiments n = 4). Volcano plot x-axis represents log2 fold change of protein levels in lysates and eluates of HEK-293 cells with ATP5MG-tFT compared to cells with IleDeg-tFT fusion proteins. Orange, magenta, and blue indicate proteins belonging to ATP synthase, TIM, and TOM complexes, respectively; significantly changed (two-sided, paired Student’s t test P < 0.05, |log2 fold change| >1) were labeled.
Related to Fig. 2 . Proteins from cellular lysates (load) and purified with anti-GFP beads (eluate) were analyzed by LC-MS/MS (number of independent experiments n = 4). Volcano plot x-axis represents log2 fold change of protein levels in lysates and eluates of HEK-293 cells with ATP5MG-tFT compared to cells with IleDeg-tFT fusion proteins. Orange, magenta, and blue indicate proteins belonging to ATP synthase, TIM, and TOM complexes, respectively; significantly changed (two-sided, paired Student’s t test P < 0.05, |log2 fold change| >1) were labeled.
Based on the cryo-EM structure of the mammalian ATP synthase, we expect ATP5MG to be located mainly within the IM, with its C-terminus facing IMS (Spikes et al., 2020). The robust interaction of ATP5MG-tFT with TOM and ATP synthase components raised the question of whether fusion protein can tether these complexes. To test this, we purified TOM complex using transiently expressed TOMM22-HA (Fig. 2 C). ATP5MG-tFT was effectively copurified with TOM. Also, ATP synthase component ATP5B was enriched with TOMM22-HA in the presence of ATP5MG-tFT but not when TOM22-HA was expressed alone. This shows that the clogging fusion can bind TOM and ATP synthase simultaneously. Furthermore, we could detect ATP5MG-tFT migrating as a broad range of high molecular weight complexes in Blue Native (BN)-PAGE (Fig. 2 D), indicating its involvement in protein–protein interactions. Using anti-TOMM40 and anti-ATP5B antibodies as TOM and ATP synthase markers, we observed an increase in higher molecular weight forms of these complexes. Moreover, in the migration of ATP5MG-tFT, we could observe three local maxima of the western blot signal—three overlapped with TOM complex and two with ATP synthase signals (Fig. 2 D). Combining this data, we outline the topology of the arrested protein in Fig. 2 E.
The stability of the clogging fusion depends on the mitochondrial membrane potential
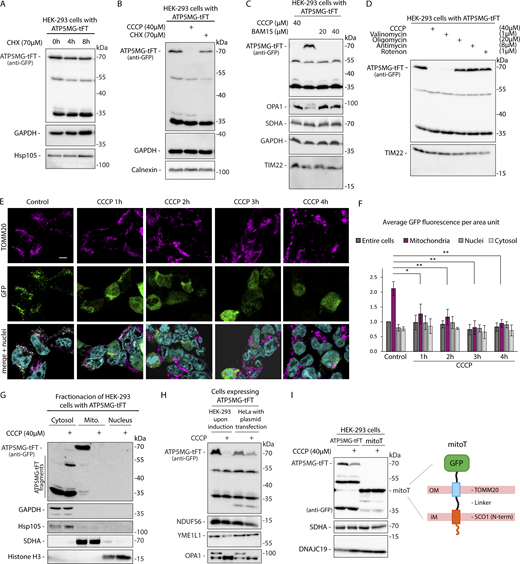
Studies in yeast found proteins that stall in mitochondrial translocases to be destabilized by the action of proteolytic QC machinery. We tested the stability of the clogging fusion using cycloheximide (CHX) to block cellular protein synthesis (Fig. 3 A). Surprisingly, despite being blocked in the TOM channel, ATP5MG-tFT remained relatively stable in basal conditions, with its half-life exceeding 6 h (Fig. S3 A). However, once we dissipated mitochondrial membrane potential using carbonyl cyanide m-chlorophenyl hydrazone (CCCP), the full-size fusion protein was destabilized with a 10-fold decrease in its half-life (Fig. 3 B and Fig. S3 B). The rapid removal of the clogging protein was specific to the membrane’s potential dissipation. Accordingly, treatment with BAM15 protonophore, which unlike CCCP is more selective to the mitochondrial membrane (Kenwood et al., 2013), resulted in degradation of the arrested fusion protein (Fig. 3 C). Another ionophore, valinomycin, which also depolarizes mitochondria, impacted the accumulation of the ATP5MG-tFT similarly to CCCP and BAM15 (Fig. 3 D). At the same time, mitochondrial poisons that have weaker or no effect on mitochondrial membrane potential: oligomycin A, antimycin A, and rotenone, did not cause clogging fusion destabilization. Washing out the uncoupling agent restored the accumulation of ATP5MG-tFT (Fig. S3 C).
Protein blocked in the translocase becomes destabilized upon dissipation of mitochondrial membrane potential. (A–D) Cellular levels of ATP5MG-tFT protein in HEK-293 cells following indicated treatments: (A) cells were treated with CHX (70 µM) to inhibit protein synthesis for the specified time; (B) cells were treated for 4 h with CCCP (40 µM) to dissipate mitochondrial membrane potential; (C) cells were treated with indicated concentrations of protonophores CCCP or BAM15 for 2 h; (D) cells were treated for 2 h with CCCP, valinomycin, oligomycin, antimycin A, or rotenone at the indicated concentrations. (E) Confocal imaging of HEK-293 cells expressing ATP5MG-tFT (GFP signal) treated with 40 µM CCCP for the indicated time. Mitochondria were visualized by TOMM20 immunostaining; cell nuclei were stained with DAPI. Scale bar: 5 μm. (F) Quantification of GFP fluorescence intensity in different cellular compartments. Graphs present means ± SD of n = 3 independent experiments. *P < 0.05, **P < 0.01 in two-sided Student’s t test. (G) Impact of 2 h of CCCP treatment on the presence of ATP5MG-tFT in cytosolic, mitochondrial (Mito.), and nuclear fractions of HEK-293 cells. Fractions were analyzed by western blotting using anti-GFP antibody to detect ATP5MG-tFT. For validation, cytosolic proteins GAPDH and Hsp105, mitochondrial marker SDHA, and nuclear marker Histone H3 were detected. (H) HEK-293 (after tetracycline induction) and HeLa (plasmid transfected) cells expressing ATP5MG-tFT were treated or not treated with 40 µM CCCP for the final 2 h of the culture. Total protein extracts were analyzed by SDS-PAGE and western blotting. Expression of the fusion proteins was tested with an anti-GFP antibody. (I) HEK-293 cells were transfected with mitoT (Viana et al., 2021) or ATP5MG-tFT expression vectors. CCCP treatment was applied for 2 h. Total protein extracts were analyzed by SDS-PAGE and western blotting. Expression of the fusion proteins was tested with an anti-GFP antibody; schematic depiction of the mitoT membrane tether construct (Viana et al., 2021). All panels: the expression of fusion proteins was induced for 24 h by adding tetracycline or by plasmid transfection 24 h before indicated treatments. Source data are available for this figure: SourceData F3.
Protein blocked in the translocase becomes destabilized upon dissipation of mitochondrial membrane potential. (A–D) Cellular levels of ATP5MG-tFT protein in HEK-293 cells following indicated treatments: (A) cells were treated with CHX (70 µM) to inhibit protein synthesis for the specified time; (B) cells were treated for 4 h with CCCP (40 µM) to dissipate mitochondrial membrane potential; (C) cells were treated with indicated concentrations of protonophores CCCP or BAM15 for 2 h; (D) cells were treated for 2 h with CCCP, valinomycin, oligomycin, antimycin A, or rotenone at the indicated concentrations. (E) Confocal imaging of HEK-293 cells expressing ATP5MG-tFT (GFP signal) treated with 40 µM CCCP for the indicated time. Mitochondria were visualized by TOMM20 immunostaining; cell nuclei were stained with DAPI. Scale bar: 5 μm. (F) Quantification of GFP fluorescence intensity in different cellular compartments. Graphs present means ± SD of n = 3 independent experiments. *P < 0.05, **P < 0.01 in two-sided Student’s t test. (G) Impact of 2 h of CCCP treatment on the presence of ATP5MG-tFT in cytosolic, mitochondrial (Mito.), and nuclear fractions of HEK-293 cells. Fractions were analyzed by western blotting using anti-GFP antibody to detect ATP5MG-tFT. For validation, cytosolic proteins GAPDH and Hsp105, mitochondrial marker SDHA, and nuclear marker Histone H3 were detected. (H) HEK-293 (after tetracycline induction) and HeLa (plasmid transfected) cells expressing ATP5MG-tFT were treated or not treated with 40 µM CCCP for the final 2 h of the culture. Total protein extracts were analyzed by SDS-PAGE and western blotting. Expression of the fusion proteins was tested with an anti-GFP antibody. (I) HEK-293 cells were transfected with mitoT (Viana et al., 2021) or ATP5MG-tFT expression vectors. CCCP treatment was applied for 2 h. Total protein extracts were analyzed by SDS-PAGE and western blotting. Expression of the fusion proteins was tested with an anti-GFP antibody; schematic depiction of the mitoT membrane tether construct (Viana et al., 2021). All panels: the expression of fusion proteins was induced for 24 h by adding tetracycline or by plasmid transfection 24 h before indicated treatments. Source data are available for this figure: SourceData F3.
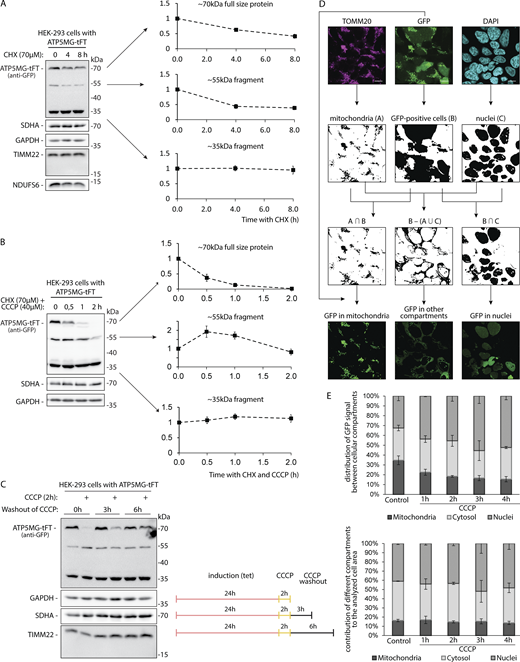
Related toFig. 3 . (A and B) Levels of ATP5MG-tFT protein and its fragments upon inhibition of protein synthesis using CHX. Charts show densitometry measurements of the indicated band signal intensities after the indicated time of CHX treatment. Data are presented as the mean ± SEM. (A) HEK-293 cells expressing ATP5MG-tFT were treated with CHX for the specified time (number of independent experiments n = 4). (B) HEK-293 cells expressing ATP5MG-tFT treated with CHX and CCCP for the specified time (number of independent experiments n = 5). (C) Levels of ATP5MG-tFT protein in HEK-293 cells treated with CCCP, followed by CCCP washout with fresh culture media for the indicated time. Tet, tetracycline. (D) The scheme of microscopic image analysis for GFP fluorescence quantification in specified cell compartments. First, the masks corresponding to mitochondria, GFP-expressing cells, and cell nuclei were created based on confocal images of TOMM20 immunostaining, GFP fluorescence visualization, and DAPI staining, respectively. Then, the final masks used in GFP quantification were calculated as follows: the mask for mitochondria in GFP-expressing cells (intersection of “mitochondria” and “GFP-positive cells” masks), for cell nuclei in GFP-expressing cells (intersection of “nuclei” and “GFP-positive cells” masks) and for cytosol and other compartments (“GFP-positive cells” mask upon subtraction of “mitochondria” and “nuclei” areas). Such obtained masks were superimposed on the original GFP fluorescence image to extract GFP signal derived from particular cellular compartments. Signal quantification included the average GFP fluorescence intensity and the integrated GFP fluorescence signal in the masked areas. Scale bars: 10 μm. (E) Quantification of GFP signal in immunocytochemistry of HEK-293 cells expressing ATP5MG-tFT, treated for indicated times with 40 µM CCCP. The distribution of the GFP signal between the analyzed compartments is presented as means ± SD from n = 3 independent experiments (upper panel). In the lower panel, the relative areas of the masks used for cell images segmentation into nuclei, mitochondria and other compartments are presented. Source data are available for this figure: SourceData FS3.
Related toFig. 3 . (A and B) Levels of ATP5MG-tFT protein and its fragments upon inhibition of protein synthesis using CHX. Charts show densitometry measurements of the indicated band signal intensities after the indicated time of CHX treatment. Data are presented as the mean ± SEM. (A) HEK-293 cells expressing ATP5MG-tFT were treated with CHX for the specified time (number of independent experiments n = 4). (B) HEK-293 cells expressing ATP5MG-tFT treated with CHX and CCCP for the specified time (number of independent experiments n = 5). (C) Levels of ATP5MG-tFT protein in HEK-293 cells treated with CCCP, followed by CCCP washout with fresh culture media for the indicated time. Tet, tetracycline. (D) The scheme of microscopic image analysis for GFP fluorescence quantification in specified cell compartments. First, the masks corresponding to mitochondria, GFP-expressing cells, and cell nuclei were created based on confocal images of TOMM20 immunostaining, GFP fluorescence visualization, and DAPI staining, respectively. Then, the final masks used in GFP quantification were calculated as follows: the mask for mitochondria in GFP-expressing cells (intersection of “mitochondria” and “GFP-positive cells” masks), for cell nuclei in GFP-expressing cells (intersection of “nuclei” and “GFP-positive cells” masks) and for cytosol and other compartments (“GFP-positive cells” mask upon subtraction of “mitochondria” and “nuclei” areas). Such obtained masks were superimposed on the original GFP fluorescence image to extract GFP signal derived from particular cellular compartments. Signal quantification included the average GFP fluorescence intensity and the integrated GFP fluorescence signal in the masked areas. Scale bars: 10 μm. (E) Quantification of GFP signal in immunocytochemistry of HEK-293 cells expressing ATP5MG-tFT, treated for indicated times with 40 µM CCCP. The distribution of the GFP signal between the analyzed compartments is presented as means ± SD from n = 3 independent experiments (upper panel). In the lower panel, the relative areas of the masks used for cell images segmentation into nuclei, mitochondria and other compartments are presented. Source data are available for this figure: SourceData FS3.
We have monitored the uncoupling-induced changes of the GFP signal (i.e., part of the tFT) by confocal imaging at different times after CCCP addition (Fig. 3, E and F; and Fig. S3, D and E). Before treatment, GFP fluorescence was most intense in the defined foci that colocalized with the mitochondrial marker TOMM20. After 1 h of CCCP treatment, the fluorescent signal became diffused, while after 2 h, GFP appeared evenly distributed in the cytosol, showing no apparent enhancement with TOMM20. During more prolonged CCCP treatment, the GFP signal gradually diminished.
To gain an additional perspective on how depolarization-induced degradation affects the localization of fusion protein and its fragments, we analyzed their presence in cytosolic, mitochondrial, and nuclear fractions (Fig. 3 G). We detected full-size clogging fusion only in the mitochondria-enriched fraction. Upon 2 h of treatment with CCCP, this band was depleted from mitochondria and was not recovered in any other fraction. However, CCCP treatment increased the level of lower molecular mass fusion fragments in the cytosol, which we consider degradation products. No GFP-related signal was detected in the nuclear fraction.
To verify that depolarization-activated degradation is not specific to HEK-293 cells, we transiently expressed ATP5MG-tFT clogging fusion in HeLa cells. Both cell lines responded to uncoupler treatment as indicated by OPA1 processing (Baker et al., 2014; Zhang et al., 2014). Similarly, we observed a full-size protein ATP5MG-tFT level reduction in both cell types when adding CCCP (Fig. 3 H).
Being threaded through the TOM complex and bound with ATP synthase, ATP5MG-tFT connects two mitochondrial membranes. We asked if protein connecting two membranes but not stuck in the translocase would also be processed upon depolarization. To test this, we expressed mitoT tether fusion, which was designed to span two membranes (Viana et al., 2021). MitoT also includes GFP moiety, which is preceded by a transmembrane fragment of TOMM20 protein. Thus, the GFP of mitoT remains outside the organelle, not colliding with the fusion’s import (Fig. 3 I, see scheme). In contrast to ATP5MG-tFT, mitoT remained stable during CCCP treatment (Fig. 3 I), evidencing that depolarization-induced degradation is not universal to membrane tethering proteins. Thus, the depolarization-activated degradation might be specific for proteins arrested in the TOM translocase.
Proteasome and VCP/p97 are not involved in the initial proteolytic clearance of the clogging fusion
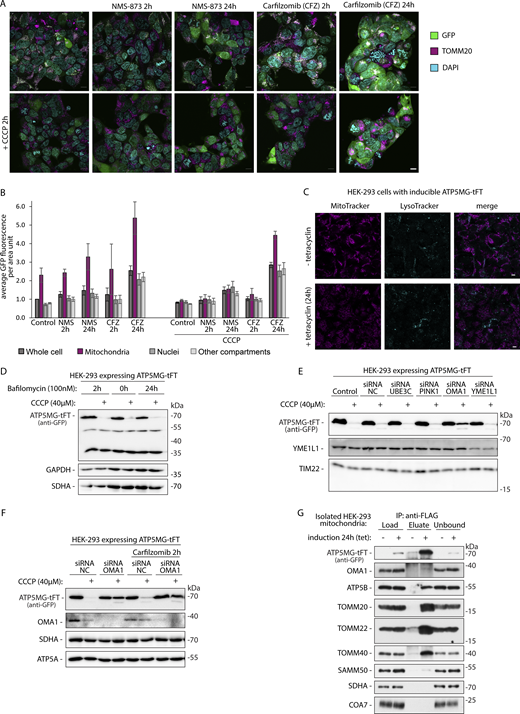
In yeast, the ubiquitin-dependent chaperone Cdc48 is recruited to clogged TOM translocases to extract the stalled cargo and facilitate its processing by the proteasome (Mårtensson et al., 2019). To test if an analogous mechanism clears clogging fusion in our human model, we targeted VCP/p97, a human ortholog of Cdc48, using its inhibitor NMS-873 (Fig. 4 A). VCP inhibition showed no effect on the levels of full-size ATP5MG-tFT, but it increased the accumulation of processing forms of the fusion. Increased accumulation of the fusion fragments indicates that these are VCP substrates. Notably, the NMS-873 inhibitor did not affect depolarization-induced degradation. The full-size protein was also effectively cleared when VCP was inhibited.
Mitochondrial depolarization-triggered processing of the stalled protein is not proteasome or VCP/P97 dependent. (A–C) HEK-293 cells expressing ATP5MG-tFT (24 h tetracycline induction) were treated with indicated inhibitors: (A) P97 inhibitor NMS-873 was applied as indicated; (B) E1 ubiquitin-activating enzyme inhibitor TAK-243 was applied for 2 h with or without CCCP; (C) proteasome inhibitors Carfilzomib and Ixazomib were applied for 24 h, parallel to induction; CCCP was added for 2 h. Cell extracts were analyzed by SDS-PAGE, western blotting, and tested with anti-GFP and control antibodies as indicated. (D) Jitter plot of protein log2 fold change (measured by LC-MS) in lysates (Load) or IP eluates from HEK-293 cells with ATP5MG-tFT vs. control cells not expressing the fusion (data available in Table S1). Proteasome components and other identified proteins are represented in red and gray, respectively. Filled circles indicate significant changes (two-sided, paired Student’s t test P < 0.05, |log2 fold change| >1). Source data are available for this figure: SourceData F4.
Mitochondrial depolarization-triggered processing of the stalled protein is not proteasome or VCP/P97 dependent. (A–C) HEK-293 cells expressing ATP5MG-tFT (24 h tetracycline induction) were treated with indicated inhibitors: (A) P97 inhibitor NMS-873 was applied as indicated; (B) E1 ubiquitin-activating enzyme inhibitor TAK-243 was applied for 2 h with or without CCCP; (C) proteasome inhibitors Carfilzomib and Ixazomib were applied for 24 h, parallel to induction; CCCP was added for 2 h. Cell extracts were analyzed by SDS-PAGE, western blotting, and tested with anti-GFP and control antibodies as indicated. (D) Jitter plot of protein log2 fold change (measured by LC-MS) in lysates (Load) or IP eluates from HEK-293 cells with ATP5MG-tFT vs. control cells not expressing the fusion (data available in Table S1). Proteasome components and other identified proteins are represented in red and gray, respectively. Filled circles indicate significant changes (two-sided, paired Student’s t test P < 0.05, |log2 fold change| >1). Source data are available for this figure: SourceData F4.
To test whether ubiquitination is involved in ATP5MG-tFT processing, we treated the cells with E1 ubiquitin-activating enzyme inhibitor TAK-243 (Szulc et al., 2023). The TAK-243 treatment effectively reduced cellular ubiquitinated species, but depolarization-induced processing of the arrested protein was unaffected (Fig. 4 B). Next, we investigated the impact of the proteasome, treating the cells with Carfilzomib and Ixazomib inhibitors (Fig. 4 C). Again, we observed no significant influence on the accumulation of full-size clogging protein. Mitochondria uncoupling still effectively cleared the translocase-blocking protein despite the proteasome being inhibited. Fragments of the fusion protein appeared to be substrates of proteasomal degradation as they were stabilized similarly to VCP inhibition.
Also, with confocal imaging of ATP5MG-tFT, we observed no apparent effects of VCP or proteasome inhibitors when applied in parallel to CCCP (Fig. S4, A and B). During 2 h treatments, the GFP signal fades from mitochondria. Upon 24 h pretreatment with the inhibitors, we observed an increase in GFP fluorescence levels in the cells. Still, 2 h of CCCP treatment resulted in a reduction of the signal in mitochondria, confirming that VCP and proteasome are not required for depolarization-induced processing. In addition, in proteomic data (Fig. 2 B), the abundance of proteasome subunits remained unchanged in the cells expressing ATP5MG-tFT as compared with control cells (Fig. 4 D, load). Thus, the expression of clogging fusion did not result in stress-induced proteasome upregulation, contrary to what was observed in yeast (Boos et al., 2019). In the co-IP with GFP-Trap, proteasome subunits appear to be slightly enriched with ATP5MG-tFT. However, only PSMA2 20S core subunit passed the significance criteria (Fig. 4 D). The enrichment could result from the proteasome involvement in degrading fusion fragments that are present in the cytosol and copurified with GFP-trap beads in parallel to the complete clogging protein (see Fig. 2 A).
Related toFigs. 4 and 6,. (A) Confocal imaging of HEK-293 cells expressing ATP5MG-tFT treated with NMS-873 or Carfilzomib with and without CCCP as indicated. ATP5MG-tFT expression was induced for 24 h and detected by GFP fluorescence. Cells were immunolabeled using antibodies against TOMM20, and cell nuclei were stained with DAPI. Scale bars: 10 μm. (B) Quantification of GFP fluorescence intensity from panel A, in different cellular compartments. Quantification was conducted as shown in Fig. S3 D. Graphs present means ± SD, n = 3 independent experiments. (C) Confocal imaging of HEK-293 cells with or without 24 h tetracycline induction of ATP5MG-tFT expression. Cells were live-stained with Lysotracker and Mitotracker Deep Red FM. Scale bar: 5 μm. (D) ATP5MG-tFT levels in HEK-293 cells are not affected by autophagy inhibition using bafilomycin (100 nM). (E) Representative western blot results used to calculate the impact of siRNAs targeting selected mitochondrial proteases presented in Fig. 6 C. HEK-293 cells with ATP5MG-tFT were treated with indicated siRNA for 48 h, fusion protein expression was induced for 24 h using tetracycline, and finally, cells were treated with or without CCCP for 2 h. Western blot signal representing full-size fusion was used for quantification. (F) HEK-293 cells with ATP5MG-tFT were treated with siRNA targeting OMA1 as in E. In addition to CCCP treatment, proteasome inhibitor Carfilzomib was applied as indicated. (G) Lysates of mitochondria isolated from HEK-293 cells with or without ATP5MG-tFT expression induction were immunoprecipitated with anti-FLAG beads. Samples were analyzed using SDS-PAGE and immunoblotting with the indicated antibodies (load, 2.5%; eluate, 100%; unbound, 2.5%). Source data are available for this figure: SourceData FS4.
Related toFigs. 4 and 6,. (A) Confocal imaging of HEK-293 cells expressing ATP5MG-tFT treated with NMS-873 or Carfilzomib with and without CCCP as indicated. ATP5MG-tFT expression was induced for 24 h and detected by GFP fluorescence. Cells were immunolabeled using antibodies against TOMM20, and cell nuclei were stained with DAPI. Scale bars: 10 μm. (B) Quantification of GFP fluorescence intensity from panel A, in different cellular compartments. Quantification was conducted as shown in Fig. S3 D. Graphs present means ± SD, n = 3 independent experiments. (C) Confocal imaging of HEK-293 cells with or without 24 h tetracycline induction of ATP5MG-tFT expression. Cells were live-stained with Lysotracker and Mitotracker Deep Red FM. Scale bar: 5 μm. (D) ATP5MG-tFT levels in HEK-293 cells are not affected by autophagy inhibition using bafilomycin (100 nM). (E) Representative western blot results used to calculate the impact of siRNAs targeting selected mitochondrial proteases presented in Fig. 6 C. HEK-293 cells with ATP5MG-tFT were treated with indicated siRNA for 48 h, fusion protein expression was induced for 24 h using tetracycline, and finally, cells were treated with or without CCCP for 2 h. Western blot signal representing full-size fusion was used for quantification. (F) HEK-293 cells with ATP5MG-tFT were treated with siRNA targeting OMA1 as in E. In addition to CCCP treatment, proteasome inhibitor Carfilzomib was applied as indicated. (G) Lysates of mitochondria isolated from HEK-293 cells with or without ATP5MG-tFT expression induction were immunoprecipitated with anti-FLAG beads. Samples were analyzed using SDS-PAGE and immunoblotting with the indicated antibodies (load, 2.5%; eluate, 100%; unbound, 2.5%). Source data are available for this figure: SourceData FS4.
In tandem with the ubiquitin-proteasome system, bulk degradation by autophagy provides clearance of cellular proteins. However, we did not observe any changes to the lysosome compartment as assayed with Lysotracker staining when comparing the cells with or without fusion induction (Fig. S4 C). Moreover, treatment with Bafilomycin A, a lysosomal proteolysis inhibitor, had no apparent impact on the accumulation of ATP5MG-tFT protein (Fig. S4 D). Bafilomycin A did not alter the fusion protein clearance upon depolarization. We thus concluded that autophagy did not significantly contribute to the translocase clogging response in our model.
Mitochondria-dependent processing of the clogging fusion
Our observations indicated that the initial proteolytic processing of the clogging fusion does not depend on the cytosolic QC machinery. This process is regulated by mitochondrial membrane polarization and, thus, possibly mediated by internal proteases. Only following this internal processing, would fragments of the clogging protein be further degraded outside the organelle.
To verify this internal processing hypothesis, we developed an assay in which we monitored the state of the clogging protein in isolated mitochondria incubated in conditions differentially affecting the membrane potential (Fig. 5 A). We used: (i) a trehalose-based isolation buffer, which assures correct osmotic conditions but does not contain metabolic substrates necessary to maintain mitochondrial membrane potential during incubation (Gnaiger et al., 2000; Pesta and Gnaiger, 2012; Hattori et al., 2005), and (ii) high-resolution respirometry buffer MiR05, supplemented with ADP+Mg2+, pyruvate, malate, and glutamate. The presence of respiratory substrates in the MiR05 maintained mitochondria polarized during incubation. After 1 h incubation of mitochondria at 37°C in the isolation trehalose buffer, the amount of clogging fusion in mitochondria was strongly decreased (Fig. 5 B). In the case of mitochondria incubated in MiR05 buffer, the ATP5MG-tFT fusion remained largely unaffected.
Isolated mitochondria process and release stalled protein when deprived of metabolic substrates. (A) Experimental scheme for B–D. (B–D) Mitochondria isolated from HEK-293 cells expressing ATP5MG-tFT were incubated in the isolation buffer or Mitochondrial Respiration Medium (MiR05) buffer supplemented with respiration substrates for the indicated time at 37°C. Next, mitochondria were separated from the buffers by centrifugation. Both fractions were analyzed by SDS-PAGE and western blot, and tested with anti-GFP and antibodies for mitochondrial proteins, as indicated. Anti-ATP5MG antibody was used in C to visualize the N-terminal part of the fusion. In D, isolation buffer was supplemented with NMS-873 (10 μM) as indicated. (E) Schematic representation of the intramitochondrial cleavage of the protein stalled in the TOM translocase. Source data are available for this figure: SourceData F5.
Isolated mitochondria process and release stalled protein when deprived of metabolic substrates. (A) Experimental scheme for B–D. (B–D) Mitochondria isolated from HEK-293 cells expressing ATP5MG-tFT were incubated in the isolation buffer or Mitochondrial Respiration Medium (MiR05) buffer supplemented with respiration substrates for the indicated time at 37°C. Next, mitochondria were separated from the buffers by centrifugation. Both fractions were analyzed by SDS-PAGE and western blot, and tested with anti-GFP and antibodies for mitochondrial proteins, as indicated. Anti-ATP5MG antibody was used in C to visualize the N-terminal part of the fusion. In D, isolation buffer was supplemented with NMS-873 (10 μM) as indicated. (E) Schematic representation of the intramitochondrial cleavage of the protein stalled in the TOM translocase. Source data are available for this figure: SourceData F5.
The loss of ATP5MG-tFT protein from mitochondria upon incubation in trehalose buffer was accompanied by the processing of proteases OMA1 and YME1L1 and their substrate OPA1. These are hallmarks of mitochondrial response to stress, including depolarization (Baker et al., 2014; Zhang et al., 2014). Mitochondria incubated in MiR05 buffer, where ATP5MG-tFT was preserved, did not display pronounced processing of stress markers.
Importantly, the depletion of full-size clogging protein from mitochondria under stress was accompanied by the recovery of its smaller fragments in the release fraction (i.e., precipitated from the incubation buffer; Fig. 5 B, see release). This indicates that stress-activated proteolytic cleavage allows the release of the cargo stalled in the OM translocase.
The anti-GFP antibody decorates the C-terminal part of the fusion protein (Fig. 1 A). To test the fate of its N-terminal part, we used ATP5MG-specific antibodies (Fig. 5 C). Both antibodies detected the full-size clogging fusion, which becomes depleted upon mitochondrial stress. However, contrary to the C-terminal fragment, which was released from the organelle, the N-terminal part was retained in mitochondria with ATP5MG likely built into the IM.
Effective clearance of the clogging protein from isolated mitochondria indicates that the process does not require the recruitment of external factors. The release of the C-terminal fragment was VCP/p97-independent (Fig. 5 D). The necessary factors are present within the mitochondrial fraction and become activated by IM depolarization. Notably, the model clogging protein was not cleared as a whole following proteolytic cleavage. The part arrested in the TOM translocase was released from the organelle, while the part integral to the IM remained inside mitochondria (Fig. 5 E).
Mitochondrial metallopeptidase OMA1 is involved in the depolarization-dependent degradation of the clogging protein
Many mitochondrial proteases contain metal ions in their catalytic centers. We treated the cells with metal ion chelator o-phenanthroline to inhibit the functions of metalloproteases. This treatment largely prevented CCCP-induced degradation of the clogger (Fig. 6 A). The chelating agent was also effective in organello, limiting ATP5MG-tFT processing upon mitochondrial stress resulting from the incubation in the trehalose-based isolation buffer (Fig. 6 B). These results suggest that the uncoupling-induced processing of clogged proteins, at least partly, depends on metalloprotease activity.
Mitochondrial metallopeptidase OMA1 contributes to depolarization-dependent degradation of the mitochondrial protein translocase stalled cargo. (A) HEK-293 cells with ATP5MG-tFT were treated with CCCP in the presence or absence of o-phenanthroline for 2 h. (B) Isolated mitochondria with ATP5MG-tFT were incubated in isolation buffer with or without o-phenanthroline for the indicated time at 37°C. Mitochondria and release fractions were analyzed by western blot. (C) HEK-293 cells with ATP5MG-tFT were treated with siRNA targeting selected mitochondrial proteases. Levels of the fusion protein were tested by western blotting and quantified by densitometry. Graph indicates fold change in the levels of full-size fusion protein during 2 h of CCCP treatment. Mean values ± SEM from n = 3 independent experiments are presented. (D) HEK-293 cells expressing ATP5MG-tFT were treated with OMA1 targeting or control siRNAs for 72 h, induced with tetracycline for 24 h; CCCP treatment was applied for 2 h. (E) Mitochondria isolated from HEK-293 cells expressing ATP5MG-tFT and treated with OMA1 targeting or control siRNAs for 48 h were incubated in isolation buffer for the indicated time at 37°C. Re-isolated mitochondria were analyzed by western blotting with indicated antibodies. NC, negative control. (F) Mitochondria isolated from WT or OMA1 KO HEK-293 cells transfected with ATP5MG-tFT expressing plasmid were incubated in isolation buffer for indicated time at 37°C. Re-isolated mitochondria and corresponding release fractions were analyzed by western blotting with indicated antibodies. (G) HEK-293 OMA1 KO and HEK-293 cells transfected with plasmid encoding ATP5MG-tFT were treated with or without CCCP for 2 h. Levels of the fusion protein were tested by western blotting. Graph indicates the levels of full-size fusion protein following CCCP treatment; densitometry values for untreated cells were set to 1. Mean values ± SEM (number of independent experiments n = 4, P value of two-sided, paired Student’s t test). Source data are available for this figure: SourceData F6.
Mitochondrial metallopeptidase OMA1 contributes to depolarization-dependent degradation of the mitochondrial protein translocase stalled cargo. (A) HEK-293 cells with ATP5MG-tFT were treated with CCCP in the presence or absence of o-phenanthroline for 2 h. (B) Isolated mitochondria with ATP5MG-tFT were incubated in isolation buffer with or without o-phenanthroline for the indicated time at 37°C. Mitochondria and release fractions were analyzed by western blot. (C) HEK-293 cells with ATP5MG-tFT were treated with siRNA targeting selected mitochondrial proteases. Levels of the fusion protein were tested by western blotting and quantified by densitometry. Graph indicates fold change in the levels of full-size fusion protein during 2 h of CCCP treatment. Mean values ± SEM from n = 3 independent experiments are presented. (D) HEK-293 cells expressing ATP5MG-tFT were treated with OMA1 targeting or control siRNAs for 72 h, induced with tetracycline for 24 h; CCCP treatment was applied for 2 h. (E) Mitochondria isolated from HEK-293 cells expressing ATP5MG-tFT and treated with OMA1 targeting or control siRNAs for 48 h were incubated in isolation buffer for the indicated time at 37°C. Re-isolated mitochondria were analyzed by western blotting with indicated antibodies. NC, negative control. (F) Mitochondria isolated from WT or OMA1 KO HEK-293 cells transfected with ATP5MG-tFT expressing plasmid were incubated in isolation buffer for indicated time at 37°C. Re-isolated mitochondria and corresponding release fractions were analyzed by western blotting with indicated antibodies. (G) HEK-293 OMA1 KO and HEK-293 cells transfected with plasmid encoding ATP5MG-tFT were treated with or without CCCP for 2 h. Levels of the fusion protein were tested by western blotting. Graph indicates the levels of full-size fusion protein following CCCP treatment; densitometry values for untreated cells were set to 1. Mean values ± SEM (number of independent experiments n = 4, P value of two-sided, paired Student’s t test). Source data are available for this figure: SourceData F6.
We targeted an array of mitochondrial proteases with siRNA to test their potential role in clogging fusion degradation. Following cell treatment with specific siRNA, we have compared the levels of full-size ATP5MG-tFT fusion in standard conditions and after CCCP treatment (Fig. 6 C). Out of the tested siRNA, only the one targeting OMA1 metalloprotease partially prevented the CCCP-triggered processing (Fig. 6 C and Fig. S4 E). Silencing OMA1 decreased the degradation of arrested protein in CCCP-treated cells (Fig. 6 D and Fig. S4 F) and isolated mitochondria (Fig. 6 E). The silencing effect was only partial. This could result from the incomplete depletion of OMA1 (see Fig. 6 E OMA1) or of another protease contributing to the process. We observed no or residual binding of ATP5MG-tFT and OMA1 in co-IP (Fig. S4 G). However, the substrate–protease interaction is likely transient. To investigate the role of OMA1 further, we turned to a knockout cell line (OMA1 KO) (Baker et al., 2014). We compared mitochondria isolated from wild-type and OMA1 KO HEK-293 cells following transfection with ATP5MG-tFT expressing plasmid (Fig. 6 F). Upon incubation in stress-inducing conditions, ATP5MG-tFT fusion was largely protected from cleavage and release in organelles lacking OMA1 as compared with those isolated from unmodified cells. Accordingly, in a cell-based assay, we observed increased stability of the full-size clogging fusion in the absence of OMA1 protease (Fig. 6 G). However, even in OMA1 KO cells, ATP5MG-tFT fusion was destabilized upon treatment with the uncoupling agent. The western blot signal decreased to 62% of the signal before adding any uncoupling agent (CCCP, Fig. 6 G, see graph). This finding indicates that while OMA1 is a major processor, the depolarization-triggered processing of protein arrested in transit is likely not restricted to OMA1 activity and could be executed by another protease.
Stalled import intermediates disturb cristae morphology
Our observation that ATP5MG-tFT can physically bind OM-located TOM complex and IM-located ATP synthase (Fig. 2) drove us to investigate its impact on mitochondrial ultrastructure. A protein stalled in transit and spanning two membranes is likely to impact mitochondrial morphology. This is especially relevant since ATP synthase complexes are distal to the OM as they locate in crista membranes often assembled into rows of dimers along the curved crista lamellae edges (Strauss et al., 2008; Rampelt et al., 2022).
Using transmission EM (TEM), we compared the architecture of mitochondrial membranes in the cells with or without expression of ATP5MG-tFT (Fig. 7, A and B). We found that the clogging protein strongly altered mitochondrial morphology. In cells expressing the clogging construct, most of the mitochondria had a significantly reduced number of cristae (69.9 % of mitochondria with abnormal cristae; Fig. 7 B). The remaining crista membranes were often positioned along organelles’ perimeters, resulting in a void appearance of the matrix. In some cases, crista lamellae formed elongated round structures (abnormal type B). At the same time, the control cells not expressing ATP5MG-tFT predominantly contained normal mitochondria (89.2 %). The observed changes were unrelated to the tetracycline used for expression induction (Fig. S5 A).
Disturbance of cristae morphology caused by stalled import intermediates is reversed by mitochondria uncoupling. (A) TEM imaging of mitochondrial ultrastructure in HEK-293 cells with or without ATP5MG-tFT expression and with or without CCCP treatment. Representative images are shown. Scale bar: 500 nm. (B) The percentage of mitochondria with normal or aberrant cristae was quantified. Mean + error bars (SEM), n = 4 biological replicates with 8–11 profiles per replicate; Treatments were compared by Mann–Whitney U test; *P < 0.05. The experimental scheme is shown at the bottom right.
Disturbance of cristae morphology caused by stalled import intermediates is reversed by mitochondria uncoupling. (A) TEM imaging of mitochondrial ultrastructure in HEK-293 cells with or without ATP5MG-tFT expression and with or without CCCP treatment. Representative images are shown. Scale bar: 500 nm. (B) The percentage of mitochondria with normal or aberrant cristae was quantified. Mean + error bars (SEM), n = 4 biological replicates with 8–11 profiles per replicate; Treatments were compared by Mann–Whitney U test; *P < 0.05. The experimental scheme is shown at the bottom right.
Related to Fig. 7 . (A) The percentage of mitochondria with normal or aberrant cristae was quantified from TEM imaging of mitochondrial ultrastructure in HEK-293 cells with or without tetracycline treatment (24 h, 1 µg/ml). Mean + error bars (SEM), n = 3 biological replicates, with 8–11 profiles per replicate. Treatments were compared using the Mann–Whitney U test; no effect of tetracycline on mitochondrial morphology was observed. (B and C) Live confocal imaging of HEK-293 cells expressing ATP5MG-tFT stained with ΔΨ indicator TMRE (B) before and after CCCP treatment; C presents an enlarged section of B. ATP5MG-tFT expression was driven by plasmid transfection to produce cells with diversified expression levels. (D) CLEM of HEK-293 cells expressing ATP5MG-tFT (green, after 24 h of tetracycline induction) and stained with TMRE to visualize membrane potential (red) and DAPI (nuclei, white). Live cell imaging by confocal microscopy was followed by fixation protocol and TEM imaging of respective cells to visualize mitochondrial ultrastructure.
Related to Fig. 7 . (A) The percentage of mitochondria with normal or aberrant cristae was quantified from TEM imaging of mitochondrial ultrastructure in HEK-293 cells with or without tetracycline treatment (24 h, 1 µg/ml). Mean + error bars (SEM), n = 3 biological replicates, with 8–11 profiles per replicate. Treatments were compared using the Mann–Whitney U test; no effect of tetracycline on mitochondrial morphology was observed. (B and C) Live confocal imaging of HEK-293 cells expressing ATP5MG-tFT stained with ΔΨ indicator TMRE (B) before and after CCCP treatment; C presents an enlarged section of B. ATP5MG-tFT expression was driven by plasmid transfection to produce cells with diversified expression levels. (D) CLEM of HEK-293 cells expressing ATP5MG-tFT (green, after 24 h of tetracycline induction) and stained with TMRE to visualize membrane potential (red) and DAPI (nuclei, white). Live cell imaging by confocal microscopy was followed by fixation protocol and TEM imaging of respective cells to visualize mitochondrial ultrastructure.
As uncoupling-induced cleavage releases the fusion protein, it should effectively disengage the two mitochondrial membranes. To test this possibility, we examined the effect of CCCP treatment on mitochondrial ultrastructure in cells expressing clogging protein (Fig. 7 B). The uncoupler treatment is known to disturb mitochondrial morphology and reduce the number of normal cristae (Viana et al., 2021; Miyazono et al., 2018). However, we observed that uncoupling-induced clogger release was accompanied by a significant restoration of the cristae structure, seen in TEM (during 1 h of CCCP treatment, the fraction of mitochondria with normal cristae increased from 30.1 % to 55.2 %). This observation supports the assumption that arrested precursors might disrupt mitochondrial structure by clasping OM and cristae membranes. Using ΔΨ indicator TMRE, we confirmed that mitochondria distorted by ATP5MG-tFT maintain membrane potential and respond to CCCP uncoupler (Fig. S5, B–D). Together, this proves that clogging-induced damage is not permanent and can be reversed by the mitochondrial QC machinery.
Discussion
Import of proteins into mitochondria is a critical component of the cellular proteostasis network. The undisturbed flow of incoming proteins is essential to maintain organellar functions and prevent protein mislocalization. We and others provide clear evidence of the detrimental consequences of import disturbance. Thus, protein translocation failure requires adequate QC responses. Such responses have primarily been defined in the yeast model (Lenkiewicz et al., 2021). Fusion proteins designed to stall during the import proved to be effective tools to characterize mechanisms that resolve translocation failure in yeast (Boos et al., 2019; Mårtensson et al., 2019; Weidberg and Amon, 2018). Using a similar strategy, we created an import arrest model in a cell line to study import QC in human cells. We fused ATP5MG protein, a FO ATP synthase component, with a tFT tag. The stably folding sfGFP, a part of tFT, is not translocated effectively (Gomkale et al., 2021; Kowalski et al., 2018). Our results confirmed the translocation arrest of ATP5MG-tFT. The fusion localized to mitochondria, but its C-terminus remained exposed on the organelle’s outside. Proteomic analysis revealed a pronounced interaction of the fusion not only with TOM translocase but also with the ATP synthase complexes, supporting IM integration of its N-terminal part. As expected in translocase blockage, model fusion protein interfered with the import of native mitochondrial proteins, impaired mitochondrial function, and reduced cellular proliferation.
Yeast cells respond to import disturbance by adjusting protein synthesis and degradation rates. Disturbed protein import inhibits protein synthesis and simultaneously boosts the activity of the proteasome in the processes called unfolded protein response activated by mistargeting of proteins (Topf et al., 2016) or mitochondrial precursor overaccumulation stress (Wang and Chen, 2015). Proteasome upregulation is also mediated transcriptionally and is not limited to yeast (Boos et al., 2019; Sladowska et al., 2021; Kim et al., 2023). Individual mitochondrial stresses can differentially alter the transcriptome but frequently trigger the integrated stress response (ISR) (Quirós et al., 2017; Topf et al., 2019; Kaspar et al., 2021; Fessler et al., 2020; Guo et al., 2020). ISR-induced changes alleviate import stress by decreasing the synthesis of mitochondria-targeted proteins and stimulating general proteostasis restoration. However, more direct mechanisms are required to unblock translocases. In yeast, such mechanisms depend on active extraction of translocase-clogging proteins coupled with their degradation by the ubiquitin–proteasome system (Mårtensson et al., 2019; Schulte et al., 2023; Weidberg and Amon, 2018). The QC mechanism we report here fundamentally differs from those described in yeast cells. It is based on proteolytic cleavage of the arrested protein allowing back movement and release of its blocked portion from the TOM translocase. We observed that the arrested protein remains relatively stable and is cleared effectively only after depolarizing the IM. We provide evidence that the cleavage of arrested protein is mediated by mitochondrial proteases, likely within the IMS. Mitochondria have multiple proteolytic enzymes that regulate the organelle’s proteome and respond to protein damage (Quirós et al., 2015; Szczepanowska and Trifunovic, 2021). We confirmed the involvement of OMA1 metalloprotease in arrested precursor processing. OMA1 is characterized by a low basal activity until it is activated by stress, such as mitochondrial membrane depolarization (Baker et al., 2014; Zhang et al., 2014). The adaptable activity of OMA1 corresponds to the observed low basal degradation of the clogging fusion protein, amplified by ΔΨ dissipation. The proximity interactome of OMA1 included TOM components, further supporting this protease’s role in translocation QC (Botham et al., 2019). Similar to the cleavage of our model clogging protein, OMA1 was reported to cleave proteins that mediate mitochondrial stress signaling, PINK1, and DELE1 upon their disturbed import, potentially indicating a broad substrate range of the QC mechanism (Akabane et al., 2023; Sekine et al., 2019; Fessler et al., 2020; Guo et al., 2020). Still, the stabilization of the translocase-arrested protein was only partial in the absence of OMA1, suggesting that another enzyme also contributes to the cleavage. Mitochondrial proteases share part of their substrates (Botham et al., 2019). A well-established example is the OMA1 and YME1L proteases pair, mediating the processing of OPA1 (Rainbolt et al., 2016). A yeast homolog of YME1L, Yme1, was reported to clear mutant forms of Aac1 protein, which can stall during import (Coyne et al., 2023). Moreover, the activity of other proteases, like PARL, AFG3L2, LONP1, or ClpXP, is also regulated by the membrane potential (Sekine et al., 2012; Pryde et al., 2016; Patron et al., 2022). Our siRNA-based experiments did not support the involvement of any of these proteases, but silencing might not sufficiently reduce targeted enzyme levels, which is a limitation of the method. Although, the redundancy of proteases appears likely as it ensures the robustness of the QC machinery, it is also conceivable that OMA1 acts indirectly by activating another protease.
Remarkably, the role of proteasomal degradation appears indirect and more limited in comparison to QC mechanisms discovered in yeast. We did not observe increased proteasome subunit levels in cells expressing ATP5MG-tFT. We also did not find the proteasome among significantly enriched partners of the fusion protein. Still, ATP5MG-tFT underwent degradation even without uncoupler treatment, as manifested by the accumulation of smaller protein fragments. Such fragments are characteristic of tFT fusions and represent degradation intermediates (Khmelinskii et al., 2016). In contrast to the whole fusion protein, these fragments were significantly stabilized in response to the inhibition of the ubiquitin-proteasome system or VCP/p97, a human homolog of yeast Cdc48. At the same time, the depolarization-triggered processing of the full-size protein was unaffected by the inhibitors. Processing intermediates were found outside mitochondria but originated from the mitochondrially localized full-size ATP5MG-tFT fusion. Notably, their release did not require the recruitment of external factors, a fundamental difference in comparison to QC mechanisms identified in yeast.
The QC response mediated by internal components of mitochondria appears largely independent of cytosolic translation modulation. In the time frame of our experiments, we did not observe substantial proteome remodeling that would indicate ISR activation. We also did not detect the activation of alternative stress-response programs, like the mitochondrial unfolded protein response (Fessler et al., 2020). Still, we cannot entirely exclude ISR activation as only a subset of known targets was detected in our proteomic dataset (Table S1) (Neill and Masson, 2023). The induction of ATP5MG-tFT expression only mildly affected cell proliferation, which may reflect its moderate expression. Due to adaptive mechanisms, cells can tolerate a certain degree of mitochondrial defects (Wallace and Chalkia, 2013). Assuming that a fraction of translocation events continually fail also under physiological conditions, no impact at the cellular level may be detectable due to their number staying below a tolerated threshold.
Stalled precursor proteins can form tethers connecting mitochondrial membranes and distort the membrane’s architecture. This appears to be the case for the model protein we used. The expression of ATP5MG-tFT resulted in a significantly reduced number of cristae and their location alongside the inner boundary membrane. Cristae morphology is pivotal for respiratory chain function. Consequently, arrested proteins can impair mitochondrial respiration and, without effective QC, further impair the import. Remarkably, the uncoupler-induced clearance of the arrested ATP5MG-tFT restored the cristae architecture. Mitochondrial proteases, including OMA1, are among the regulators of organellar ultrastructure. OMA1 KO mitochondria display strongly disturbed morphology (Viana et al., 2021), resembling the phenotype we observed upon ATP5MG-tFT expression. OMA1 is a well-established regulator of OPA1, a part of the machinery shaping IM (Quintana-Cabrera and Scorrano, 2023). Given the role of OMA1 in releasing arrested proteins, the tethering of mitochondrial membranes by stalled import intermediates should be considered among mitochondria shaping factors.
Our approach employing an engineered protein proved effective but might be regarded as synthetic. However, multiple data substantiate that the phenomenon we modeled also affects native mitochondrial proteins that can become arrested upon import, particularly when expressed in excess or mutated (Weidberg and Amon, 2018; Boos et al., 2019; Coyne et al., 2023). Moreover, mitochondrial translocases were shown to engage with misfolded or aggregated proteins from the cytosol even if such proteins were not destined for mitochondria (Ruan et al., 2017). Such events were observed in neurodegenerative disorders associated with protein misfolding, including Alzheimer’s disease (AD) and Parkinson’s disease (PD). In AD, amyloid precursor protein was found to interact with TOMM40 and TIMM23 (Anandatheerthavarada et al., 2003), disturb mitochondrial protein import (Devi et al., 2006), and suppress mitochondrial precursor processing (Mossmann et al., 2014). Likewise, the PD-associated variant of α-synuclein was observed to associate with mitochondria, interact with TOM translocase, and inhibit mitochondrial protein import (Gallardo et al., 2008; Di Maio et al., 2016). Similarly, huntingtin, another disease-linked protein, was proposed to block mitochondrial translocases (Yano et al., 2014). The above examples of clinically relevant proteins substantiate the risk of clogging for mitochondrial protein translocases. A possible link between these observations and our discoveries is that the uncoupling agent DNP was found to improve functioning in animal models of neurodegenerative diseases related to protein misfolding (Wu et al., 2017). Partial depolarization of mitochondria was proposed to counteract aging-related decline (Vyssokikh et al., 2020). It is an intriguing possibility that drugs altering mitochondrial membrane potential, generally regarded as protein import inhibitors, could help in import restoration. Transient depolarization of mitochondria occurs in physiology and can be induced by stress, such as ischemia or reperfusion, potentially activating proteolysis (Lee and Yoon, 2014; Kurz et al., 2010; Ashok and O’Rourke, 2021). We thus propose that applying models of clogging is necessary to streamline the discovery and understanding of protein import QC in mammalian cells.
Our research leaves open points that require addressing such as the fate of parts of arrested proteins remaining inside mitochondria after the cleavage. The released fragments appear to fall under the general control of the ubiquitin-proteasome system. However, their impact on proteostasis should not be overlooked as unimported mitochondrial proteins can aggregate disturbing proteostasis (Nowicka et al., 2021). Hence, the cytosolic handling of mitochondria-released fragments is a significant part of their QC, requiring further attention. It also remains to be elucidated whether the gradual processing of arrested precursor under basal conditions represents a parallel QC pathway, perhaps involving active extraction, or involves mitochondrial proteases.
Materials and methods
Cell lines and growth conditions
HEK-293 (CRL-1573; ATCC), HEK-293 Flp-In T-REx (Invitrogen), and HeLa (CCL-2; ATCC) cells were cultured at 37°C with 5% CO2 in standard Dulbecco’s modified Eagle’s medium (DMEM) containing 4,500 mg/liter glucose, supplemented with 10% (vol/vol) fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate. Flp-In T-REx HEK-293 with inducible expression of ATP5MG-tFT or IleDeg-tFT were generated by co-transfection with pcDNA5/FRT/TO (Invitrogen) plasmid encoding respective fusion protein and a helper plasmid pOG44 (Invitrogen). Stable transgenic cells were selected with hygromycin treatment. The resulting cell lines were tested for inducible expression of tFT fusion by adding tetracycline (1 µg/ml) for 24 h as described previously (Chojnacka et al., 2022). OMA1 KO T-Rex HEK-293 cells were kindly provided by Thomas Langer (Baker et al., 2014). Cell proliferation was measured by direct cell counting using a cell counter (Countess II; Life Technologies) or manually with a hemocytometer. P values were calculated with a two-sided, paired Student’s t test. Data distribution was assumed to be normal but this was not formally tested. ATP5MG-tFT fusion consists of a coding sequence (CDS) of ATP5MG (NM_006476.5, NCBI) without a stop codon, followed by the FLAG tag coding sequence (5′-GATTACAAGGATGACGACGATAAG-3′), followed by the tFT as in pMaM17, originally published by Khmelinskii et al. (i.e., mCherry sequence without a stop codon, followed by a 5× GlyAla linker (5′-GGAGCAGGTGCTGGTGCTGGTGCTGGAGCA-3′), followed by the sfGFP (Khmelinskii et al., 2012). IleDeg-tFT fusion was re-cloned from pMaM98 plasmid (Khmelinskii et al., 2012) into pcDNA5/FRT/TO plasmid and transformed into Flp-In T-REx HEK-293. TOMM22-HA is NM_020243.5 CDS (NCBI) without a stop codon followed by SerGly linker (TCAGGT) and a sequence encoding human influenza HA tag (5′-TACCCATACGATGTTCCAGATTACGCT-3′) with stop codon cloned in pcDNA3.1 plasmid (this work).
Total cellular protein lysates and western blotting
Cells were harvested with trypsin and lysed in radioimmunoprecipitation assay buffer (65 mM Tris–HCl [pH 7.4], 150 mM NaCl, 1% vol/vol NP 40, 0.25% sodium deoxycholate, 1 mM ethylenediaminetetraacetic acid [EDTA], and protease inhibitor cocktail [P8340; Merck]) for 30 min at 4°C. After 10 min centrifugation at 5,000 × g at 4°C, supernatants were collected and the protein concentration was measured by Bradford assay (ROTI Quant, ROTH). Proteins of the lysate were precipitated by the addition of trichloroacetic acid (TCA) to the concentration of 12.5% and pelleted by centrifugation at 20,000 × g at 4°C. The pellets were washed with acetone, followed by solubilization and denaturation with urea sample buffer (6 M urea, 6% SDS [288.38 g/mol], 125 mM Tris-HCl, pH 6.8, 0.01% Bromophenol Blue, and 50 mM DTT) at 65°C. Samples were analyzed by SDS-PAGE and western blotting with polyvinylidene difluoride membrane (Immobilon-FL Transfer Membrane; IPFL00010; Millipore). Primary antibodies used were anti-Actin (66009-1-Ig; Proteintech), anti-ATP5MG (HPA044629; Sigma-Aldrich), anti-Calnexin (ab22595; Abcam), anti-CLPP (WH0008192M1; Sigma-Aldrich), anti-DNAJC19 (12096-1-AP; Proteintech), anti-FLAG (F1804-200UG; Sigma-Aldrich), anti-GAPDH (sc-47724; Santa Cruz), anti-GFP (11814460001; Sigma-Aldrich), anti-Histone H3 (H0164-25UL; Sigma-Aldrich), anti-HSPA9 (sc-133137; Santa Cruz), anti-mCherry (ab167453; Abcam), anti-MRPL-12 (sc-100839; Santa Cruz), anti-NDUFS6 (ab195808; Abcam), anti-OMA1 (sc-515788; Santa Cruz), anti-OPA1 (612606; BD Biosciences), anti-PARL (26679-1-AP; Proteintech), anti-SDHA (sc-166947; Santa Cruz), anti-TIM22 (14927-1-AP; Proteintech), anti-TOM40 (sc-365467; Santa Cruz), anti-Tubulin (sc-134239; Santa Cruz), anti-Ubiquitin (sc-8017; Santa Cruz), anti-YME1L1 (11510-1-AP; Proteintech). Secondary antibodies with peroxidase used were goat anti-mouse (A4416-1ML; Sigma-Aldrich) and goat anti-rabbit (111-035-144; Jackson Immunoresearch). Immunoreactivity was detected by ECL. The chemiluminescent signals were detected using ChemiDoc MP (Bio-Rad), Imager 680 (Amersham), or x-ray films (Fujifilm, Super RX-N) digitized with Perfection V850 Pro scanner (Epson). Immunoblot quantification was performed using ImageLab software (Bio-Rad). Where indicated, P values were calculated using the Student’s t test. Data distribution was assumed to be normal, but this was not formally tested.
Cell transfection
Cells were transfected with plasmid DNA using Gene Juice Transfection Reagent (Merck Millipore) 24 h after plating. Transfection was performed according to the manufacturer’s protocol. Cells were harvested 24–48 h after transfection as indicated.
Cell fractionation
Cells were harvested with trypsin and resuspended in isolation buffer (20 mM Hepes, pH 7.6, 220 mM mannitol, 70 mM sucrose, 1 mM EDTA, 2 mM PMSF). Cells were homogenized in a Dounce glass homogenizer and the homogenate was subjected to centrifugation at 1,000 × g for 10 min at 4°C to pellet the cellular debris. The supernatant was carefully removed. Part of it was stored as a total fraction and the rest was subjected to subsequent centrifugation at 10,000 × g for 10 min at 4°C. After centrifugation, supernatant containing cytosolic proteins and mitochondria-enriched pellets were separated. To test for protease accessibility, mitochondrial samples were treated with proteinase K (25 μg/ml) for 15 min on ice. Proteinase K was inhibited by 2 mM PMSF followed by pelleting mitochondria at 20,000 × g for 10 min at 4°C. Aqueous fractions (i.e., total and cytosol) were precipitated with TCA as described for total cell extracts. Pelleted proteins or mitochondria were solubilized and denatured with urea sample buffer and analyzed by western blotting. For nuclear fraction, Abcam Cell Fractionation Kit (ab109719-1) was used in accordance with the manufacturer’s recommendations.
Mitochondrial isolation and model protein processing assay
Mitochondria isolation was performed as described previously (Mohanraj et al., 2019). Briefly, cells were harvested, resuspended in ice-cold isolation trehalose buffer (300 mM trehalose, 10 mM HEPES-KOH, pH 7.7, 10 mM KCl, and 2 mg/ml of bovine serum albumin [BSA]), and homogenized in a Dounce homogenizer. Homogenates were clarified by centrifugation at 650 × g at 4°C. The resulting supernatant was centrifuged at 14,000 × g for 15 min at 4°C to obtain a mitochondrial pellet. The mitochondria-enriched pellet was resuspended in isolation trehalose buffer without BSA or MiR05 Mitochondrial Respiration Medium (Gnaiger et al., 2000), as indicated. Mitochondria were used directly without storage. Samples were incubated at 37°C for 1 h or analyzed without incubation to observe the processing and release of the model protein. Mitochondria were re-isolated by centrifugation, and the supernatant was collected as a release fraction. Release fractions were precipitated by the addition of TCA as described for total cell extracts. Both mitochondria and release samples were denatured with the urea sample buffer and analyzed by western blotting.
siRNA-mediated knockdown
Cells were reverse transfected with siRNA targeting selected genes (MISSION esiRNA; Merck: targeting human OMA1—EHU072451; AFG3L2—EHU014171; ATP23—EHU004231; CLPP—EHU011941; HTRA2—EHU151841; IMMP1L—EHU046151; LACTB—EHU094481; LONP1—EHU072201; NLN—EHU007831; PARL—EHU115721; YME1L1—EHU115921) or non-targeting siRNA control (MISSION siRNA universal negative control UNC2; Merck) using Lipofectamine RNAiMAX Reagent (Thermo Fisher Scientific). For each well of a 6-well plate, 3 μl of Lipofectamine RNAiMAX Reagent (Thermo Fisher Scientific) was diluted in 250 μl of Opti-MEM reduced serum medium (Gibco), and siRNA was diluted in 250 μl Opti-MEM medium. Subsequently, both were mixed inside the well to prepare complexes. After 5 min of incubation, 2 ml of cell suspension in growth media was added. Cells were harvested after 48 h or 72 h, as indicated. The final concentration of siRNA was 20 nM.
BN-PAGE
Isolated mitochondria were solubilized 1 μg protein/μl in lysis buffer containing 1% digitonin, Tris-HCl, pH 7.4, 20 mM, NaCl 50 mM, EDTA 0.1 mM, glycerol 10%, and PMSF 1 mM. The samples were vortexed and incubated at 4°C for 30 min, then they were cleared by centrifugation at 20,000 × g for 5 min. The supernatant was mixed with 10× loading dye (5% Coomassie brilliant blue G-250, 500 mM 6-aminohexanoic acid, and 100 mM Bis-Tris, pH 7.0) and loaded on the gel. The samples were resolved at 4°C using a gradient gel (3–13%). The high molecular weight calibration kit for native electrophoresis (Amersham) was used as a molecular weight standard.
Immunopurification of proteins
For HA- and FLAG-tag affinity purifications, isolated mitochondria were solubilized 1 µg protein/µl in lysis buffer containing 1% digitonin, Tris-HCl, pH 7.4, 20 mM, NaCl 50 mM, EDTA 0.1 mM, glycerol 10%, and PMSF 1 mM. The samples were incubated on a rotator at 4°C for 20 min and centrifuged at 20,000 × g for 15 min. For GFP-tag affinity purification, cells were lysed in NP40 buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5 mM EDTA, 0.5% NP-40, protease inhibitor cocktail 1:500 dilution) for 30 min on ice. For MS analysis, before lysis, pellets of five million cells were crosslinked by incubation in 0.5 ml of 0.5% formaldehyde in PBS for 30 min at RT. Next, cell pellets were incubated with 1.25 M glycine solution in PBS for 10 min at RT to quench any remaining formaldehyde. After glycine treatment, cells were washed three times with PBS. Three million cells were lysed in 200 μl of lysis buffer, and lysates were clarified by centrifugation at 20,000 × g for 10 min 4°C. A sample of supernatant was kept as LOAD fraction while lysate was incubated 2 h (for anti-HA and anti-FLAG) or 1 h (GFP-Trap) on a rotator at 4°C with the affinity beads. Beads were collected by centrifugation for 3 min at 500 × g and a sample of supernatant was kept as UNBOUND fraction. Beads were washed five times (for anti-HA and anti-FLAG; washing buffer 150 mM NaCl, 10% glycerol, 20 mM MgCl2, 1 mM PMSF, 50 mM Tris-HCl, pH 7.4, with 0.1% digitonin) or three times (GFP-trap), and proteins were eluted by denaturation in 2× sample buffer with 50 mM DTT at 65°C. For MS analysis, beads were incubated with trypsin (sequencing grade from Promega, 1 µg) in 50 mM Tris-HCL buffer, pH 8.2, containing 5 mM tris(2-carboxyethyl)phosphine (TCEP) and 10 mM iodoacetamide overnight at 37°C. Proteins in the INPUT fractions were subjected to chloroform/methanol precipitation. Protein pellets were resuspended in 50 mM Tris-HCL buffer, pH 8.2, containing 5 mM TCEP and 10 mM iodoacetamide. Trypsin (sequencing grade from Promega, 0.5 µg) was added and the samples were incubated overnight at 37°C. Proteolysis reactions were quenched by the addition of trifluoroacetic acid (TFA, to the final concentration 1%), and the samples were centrifuged at 12,000 × g for 3 min at RT to pellet undigested proteins. Tryptic peptides were desalted with the use of AttractSPE Disks Bio C18 (Affinisep). Before liquid chromatography-mass spectrometry (LC-MS) measurement, the peptide fractions were resuspended in 0.1% TFA and 2% acetonitrile in water.
LC-MS/MS analysis and data processing
Chromatographic separation was performed on an Easy-Spray Acclaim PepMap column 50 cm × 75 µm inner diameter (Thermo Fisher Scientific Scientific) at 45°C by applying 135 min acetonitrile gradients in 0.1% aqueous formic acid at a flow rate of 300 nl/min. An UltiMate 3000 nano-LC system was coupled to a Q Exactive HF-X mass spectrometer via an easy-spray source (all Thermo Fisher Scientific Scientific). The Q Exactive HF-X was operated in data-dependent mode with survey scans acquired at a resolution of 60,000 at m/z 200. Up to 12 of the most abundant isotope patterns with charges z = 2–6 from the survey scan were selected with an isolation window of 1.3 m/z and fragmented by higher-energy collision dissociation with a normalized collision energy of 27, while the dynamic exclusion was set to 30 s. The maximum ion injection times for the survey scan and the MS/MS scans (acquired with a resolution of 15,000 at m/z 200) were 45 and 22 ms, respectively. The ion target value for MS was set to 3e6 and for MS/MS to 1e5, and the intensity threshold for MS/MS was set to 8e2. The obtained data were processed with MaxQuant v. 1.6.7. 0 (Cox and Mann, 2008), and the peptides were identified from the MS/MS spectra searched against Human Reference Proteome UP000005640 using the built-in Andromeda search engine. Raw files corresponding to 12 affinity-purified samples (three conditions, four replicates) and 12 input samples (three conditions, four replicates) were loaded into MaxQuant and processed. Cysteine carbamidomethylation was set as a fixed modification and methionine oxidation as well as protein N-terminal acetylation were set as variable modifications. For in silico digests of the reference proteome, cleavages of arginine or lysine followed by any amino acid were allowed (trypsin/P), and up to two missed cleavages were allowed. The false discovery rate was set to 0.01 for peptides, proteins, and sites. Match between runs and label-free quantification (LFQ) were enabled. Other parameters were used as preset in the software. Unique and razor peptides were used for quantification enabling protein grouping (razor peptides are the peptides uniquely assigned to protein groups and not to individual proteins).
LFQ intensities for protein groups were loaded into Perseus v. 1.6.6.0 (Tyanova et al., 2016) and log2-transformed. Proteins that were only identified by site, reverse, or potential contaminant were removed. Protein groups with LFQ intensity values in <2 out of 4 ATP5MG-tFT eluate samples were removed. Further data analysis and visualizations were performed in R v. 4.2.1 using the libraries tidyverse v. 1.3.2, fuzzyjoin v. 0.1.6, ggrepel v. 0.9.1. Missing values were attributed to low abundances and imputed by drawing from a normal distribution with a mean shifted down by 1.8 standard deviations and a standard deviation narrowed by a factor of 0.3. Imputation was performed separately for each group (i.e., total cell lysate or IP) to account for different global compositions. Fold changes were calculated as the difference between each group’s log2 average value. P values were calculated with a two-sided, paired Student’s t test. Data distribution was assumed to be normal, but this was not formally tested. No multiple comparison correction was applied. Statistical significance was assigned above an absolute fold change of 1 and a P value < 0.05 unless otherwise stated.
The raw mass spectrometry data have been deposited to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al., 2022) partner repository with the dataset identifier PXD038583.
TEM
HEK-293 cells (60–70% of confluency) were fixed by 2.5% glutaraldehyde and 2% paraformaldehyde (PFA; both from Electron Microscopy Science, Inc.) solution for 1 h at 4°C. After fixation, cells were rinsed three times for 10 min with 0.1 M cacodylate buffer (BDH Chemicals Ltd.). Next, the cells were postfixed in 1% osmium tetroxide (Polsciences, Inc.) for 1 h at RT, rinsed three times for 10 min with water, and stained with 2% uranyl acetate (Serva Electrophoresis GmbH) for 30 min. Dehydration was performed by incubating the sample in increasing acetone concentrations (25%, 50%, 70%, 90%, 96%, and 2 × 100%; each 10 min). After that, the cells were embedded in the mixture of pure acetone and Epon (Serva Electrophoresis GmbH) resin (1:1 and 1:2, each mixture for 30 min), then in pure Epon resin exchanged three times (incubation: 1 h, overnight, and 1 h). After resin polymerization at 60°C, 60-nm-thick sections were collected on TEM grids. Electron micrographs were obtained with Morada camera using a JEM 1400 transmission electron microscope at 80 kV (JEOL Co.) in the Laboratory of EM, Nencki Institute of Experimental Biology of Polish Academy of Sciences, Warsaw, Poland.
TEM image analysis
TEM cellular studies were repeated four times for each experimental group (n = 4). Quantification was obtained from 8 to 11 cell profiles for each repeated group. The number of mitochondria was established using Cell Counter plugin of ImageJ Fiji software (Schindelin et al., 2012), then the percentage of normal and abnormal mitochondria was determined. Results are presented as arithmetic means with standard errors. The significance of differences between the groups was tested with the Mann–Whitney U test using GraphPad Prism 8. The standard value of P < 0.05 was adopted as the critical level of significance. The significance level was marked in the graphs as: * for P < 0.05, ** for P < 0.01, and *** for P < 0.001.
Correlative light and EM (CLEM)
Cells were seeded onto 35-mm gridded polymer coverslip bottom dishes (Ibidi) to 60% confluency. ATP5MG-tFT expression was induced with tetracycline. 24 h after the induction, cells were loaded in DMEM with 50 nM tetramethylrhodamine ethyl ester perchlorate (TMRE) for 30 min at 37°C. Then TMRE was washed out and PBS containing 0.9 mM CaCl2, 0.49 mM MgCl2, 4.5 g/liter glucose, 2 mM L-glutamine, and 10 µM DRAQ5 nuclear stain was added. High-resolution live cell confocal stacks with a 0.25-µm step size were obtained at 37°C with a Leica SP8 confocal microscope (Leica Microsystems) with HC PL APO CS2 63×/1.20 water objective. Following confocal acquisition, cells were fixed for 1 h in 2.5% glutaraldehyde and 4% PFA in 0.1 M sodium cacodylate buffer at RT. Samples were then postfixed in 2% osmium tetroxide in 0.1 M sodium cacodylate buffer, followed by en-block staining with 2% uranyl acetate. This was followed by rinsing the sample with Milli-Q water and dehydration with a graded series of ethanol (25%, 50%, 75%, 2 × 100%). For TEM, samples were infiltrated with Glycid ether 100 epoxy resin (Serve Electrophoresis) and polymerized using an oven at 60°C for 48 h. Cells of interest were re-registered with the coordinate grid, and unwanted areas of the block face were trimmed away using a razor blade. Approximately, 180 µm of the polymer base of the Ibidi dish was sectioned away to reach the interface between the plastic bottom and resin. Following this, 70-nm ultrathin sections were collected onto formvar-coated copper slot TEM grids and post-stained using aqueous 2% uranyl acetate and Reynold’s lead citrate solutions. Cells were examined using a Morada camera on a JEM 1400 transmission electron microscope operating at 80 kV (JEOL Co.).
Life cell staining
Cells were incubated for 2 h at 37°C in a culture medium containing 50 nM Mitotracker Deep Red (Thermo Fisher Scientific) and/or 75 nM Lysotracker Blue DND-22. Then, the stained cells were visualized at 37°C using a confocal fluorescent microscope Leica SP8 with HC PL APO CS2 63×/1.2 water objective.
Immunofluorescent staining
Cells seeded on glass coverslips were fixed for 10 min at RT with 4% PFA in PBS. Then, they were washed with PBS three times for 5 min. Cell membranes were permeabilized with 0.1% Triton-X100 in PBS for 10 min, followed by three washes with PBS and blocking with 5% BSA in PBS for 1 h at RT. Incubation with primary antibodies was performed overnight at 4°C in a wet chamber. The antibodies were appropriately diluted in PBS with 2% BSA 1:50 for TOMM20 (Santa Cruz) and 1:1,000 for calnexin (Abcam). After the incubation, three washes with PBS were applied and coverslips were incubated for 1 h at RT with secondary antibodies diluted 1:1,000 in 2% BSA in PBS. Then, three washes with PBS were applied once again and the coverslips were mounted on microscopic slides using ProLong Diamond Antifade Mountant with DAPI (Thermo Fisher Scientific). The stained cells were imaged at RT using a confocal fluorescent microscope Leica SP8 with HC PL APO CS2 63×/1.4 oil objective.
Quantification of GFP signal in different subcellular compartments using ImageJ
In the obtained images, GFP signal originating from different subcellular compartments was quantified using ImageJ software. Based on the images of TOMM20 immunostaining, DAPI staining, and GFP fluorescence visualization, the masks were created to define the regions corresponding to mitochondria, cell nuclei, and all other cellular compartments. To segment mitochondria, the image of TOMM20 staining underwent background subtraction using a rolling ball radius of 20 pixels, and then automatic thresholding was performed using the Otsu method. In the case of cell nuclei segmentation from DAPI staining, the preprocessing included median filtering (5 × 5 filter size) and background subtraction (rolling ball radius of 200 pixels). After that, automatic thresholding with the Otsu method was performed. For GFP image, mean filtering with 5 × 5 filter was applied, after which the GFP-positive cells were segmented using manual thresholding. The threshold value was selected arbitrarily based on background fluorescence measurements in several randomly chosen areas devoid of cells and was the same for all of the images acquired in the particular experiment. The resolution of microscopic images used for segmentation was 0.26 µm/pixel. Such initial masks were used to calculate the final masks: the mask for mitochondria in GFP-expressing cells, for cell nuclei in GFP-expressing cells, and for cytosol and other compartments (see Fig. S3 D). The masks were then superimposed on the GFP fluorescence image to extract the GFP signal originating from individual compartments. For each of the masked areas, the average GFP fluorescence intensity, as well as the integrated GFP fluorescence signal, were quantified. Where indicated, P values were calculated using the Student’s t test. Data distribution was assumed to be normal, but this was not formally tested.
High-resolution respirometry
High-resolution respirometry was performed as previously described (Chojnacka et al., 2022). Oxygen consumption was measured in intact cells using Oxygraph-2k. Data were digitally recorded using DatLab v. 5.1.0.20 (Oroboros Instruments) and expressed as pmol of O2/min per 106 cells following the manufacturer’s instruction for air calibration and background correction. Trypsinized cells were suspended at 1–2 × 106 cells/ml in 2 ml of MiR05 medium (0.5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 3 mM MgCl2, 60 mM lactobionic acid, 20 mM taurine, 10 mM KH2PO4, 20 mM Hepes, 110 mM d-sucrose, 1 mg/ml BSA–fatty acid-free added freshly, pH 7.1), and immediately placed into the Oxygraph chamber to access basal respiration. HEK-293 Flp-In T-REx derivative proved susceptible to blocking ATP-synthase (irrespective of ATP5MG-tFT expression). Concentrations of oligomycin A as low as 0.25 μM abrogated O2 consumption in these cells (data not shown). Therefore, we assessed the overall oxygen consumption rate (OCR) cell profile without oligomycin A. The maximum respiration (electron transport system [ETS] capacity) was determined upon titration of the uncoupler CCCP (0.20 μM/step). Next, complexes I, III, and IV were inhibited by rotenone (0.5 μM), antimycin A (5 μM), and sodium azide (50 μM), respectively, to detect residual oxygen consumption (ROX). Stable O2 flux plateaus were used for the calculation of oxygen consumption, and the obtained values were corrected for ROX. Results were analyzed by two-way ANOVA followed by a multicomparision test. Data distribution was assumed to be normal, but this was not formally tested.
Online supplemental material
Fig. S1 covers the colocalization of ATP5MG-tFT fluorescence with Mitotracker stain and compares the impact of ATP5MG-tFT and a control fusion protein IleDeg-tFT expression on mitochondrial protein import, cellular oxygen consumption, and proliferation. Fig. S2 includes volcano plots representing log2 fold change of protein levels in lysates and eluates of HEK-293 cells with ATP5MG-tFT compared to cells with IleDeg-tFT fusion. Fig. S3 presents levels of ATP5MG-tFT protein and its fragments during CCCP and CHX treatments, CCCP washout and recovery, a scheme, and results of microscopic image analysis of GFP fluorescence intensity and distribution in specified cell compartments. Fig. S4 shows confocal imaging of HEK-293 cells expressing ATP5MG-tFT treated with NMS-873 or Carfilzomib inhibitors, a Lysotracker staining of ATP5MG-tFT expressing cells, and western blots related to Figs. 4 and 6. Fig. S5 tests the impact of tetracycline on mitochondrial ultrastructure and shows staining with membrane potential indicator TMRE combined with CLEM analysis and TEM control related to Fig. 7. Table S1 is a spreadsheet file with the complete set of MS results presented in Fig. 2 B, Fig. 4 D, and Fig. S2.
Data availability
The mass spectrometry data is available at the PRIDE repository with the dataset identifier PXD038583. Code used for data analysis and visualization is available at https://github.com/vanilink/ArrestedProteins/. Cell lines and plasmid encoding ATP5MG-tFT fusion protein will be made available upon reasonable request.
Acknowledgments
The authors thank Prof. Thomas Langer from the Max-Planck-Institute for Biology of Ageing, Cologne, Germany, for providing the OMA1 KO cell line.
This work was supported by the Foundation for Polish Science First TEAM Programme (POIR.04.04.00-00-3F36/17); the International Research Agendas Programme “Regenerative Mechanisms for Health—ReMedy” project (MAB/2017/2) co-financed by the European Union under the European Regional Development Fund; and the National Science Centre Poland grants SONATA BIS (2019/34/E/NZ1/00321), and SONATINA (2021/40/C/NZ3/00283). Proteomic measurements were performed at the Proteomics Core Facility, IMol Polish Academy of Sciences. TEM analyses were performed at the Laboratory of EM of the Nencki Institute using infrastructure supported by EuBI Polish Node “Advanced Light Microscopy Node Poland.” Confocal imaging was supported by the project financed by the Minister of Education and Science based on contract No 2022/WK/05. V. Linke was supported by EMBO Postdoctoral Fellowship ALTF 474-2021 and FNP START 064.2022.
Author contributions: P. Bragoszewski designed the study and performed experiments. M. Krakowczyk performed live-cell experiments and western blot analysis. A.M. Lenkiewicz performed experiments including microscopy. T. Sitarz generated cell lines and contributed to the MS experiment. D. Malinska acquired and processed microscopy images. B.H.M. Mussulini analyzed oxygen consumption rates. M. Borrero carried out part of co-IP and BN-PAGE. A.A. Szczepankiewicz, J.M. Biazik, and H.Nieznanska prepared TEM and CLEM images and statistics. R.A. Serwa and V. Linke acquired and analyzed MS-based proteomics data. A. Wydrych contributed to fractionation experiments. P. Bragoszewski, A. Chacinska, R.A. Serwa, and H. Nieznanska interpreted data. P. Bragoszewski and A. Chacinska provided funding. M. Krakowczyk assembled figures with the input of V. Linke, D. Malinska, B.H.M. Mussulini, and P. Bragoszewski. P. Bragoszewski and A. Krakowczyk wrote the manuscript with the input of all authors.
References
Author notes
Disclosures: The authors declare no competing interests exist.