Plants often adapt to adverse or stress conditions via differential growth. The trans-Golgi network (TGN) has been implicated in stress responses, but it is not clear in what capacity it mediates adaptive growth decisions. In this study, we assess the role of the TGN in stress responses by exploring the previously identified interactome of the Transport Protein Particle II (TRAPPII) complex required for TGN structure and function. We identified physical and genetic interactions between AtTRAPPII and shaggy-like kinases (GSK3/AtSKs) and provided in vitro and in vivo evidence that the TRAPPII phosphostatus mediates adaptive responses to abiotic cues. AtSKs are multifunctional kinases that integrate a broad range of signals. Similarly, the AtTRAPPII interactome is vast and considerably enriched in signaling components. An AtSK–TRAPPII interaction would integrate all levels of cellular organization and instruct the TGN, a central and highly discriminate cellular hub, as to how to mobilize and allocate resources to optimize growth and survival under limiting or adverse conditions.
Introduction
Plant responses to environmental stimuli involve diverse forms of growth or movement. The slow, cyclic movement of shoots and stems helps climbing plants such as vines find supportive structures, and stem growth or tendrils are then used to wrap around and cling to such structures (Darwin, 1880). Leaf movements linked to the circadian rhythm enable plants to maximize their exposure to sunlight (McClung, 2006). Tropisms are additional examples of movement in plants: the shoot bends toward directional light, whereas the root bends away from the light. In addition to phototropism, plants have tropic responses to a range of stimuli including moisture, fluctuations in temperature, gravity, touch, and other mechanical cues (Garzón and Keijzer, 2011). Darwin argues that the movements of plants are driven by growth and the need to access resources, such as sunlight and water (Darwin, 1880). Gyrations, revolutions, and tropisms require some form of differential growth or bending at the organ level. Bending is achieved when one side of an organ grows more rapidly than the opposing side. This differential growth is a result, at least in part, of the differential sorting of PIN-FORMED (PIN) auxin transporters, resulting in the unequal distribution of auxin, a morphogen, at opposing sides of a cell (Friml et al., 2002; Ding et al., 2011). It follows that differential growth responses such as bending require differential sorting decisions. How environmental stimuli are translated into sorting decisions remains largely unclear.
The sorting of PIN transporters has been shown to require trans-Golgi network (TGN) function. Indeed, disruption of TGN function by mutation results in the ectopic distribution of PIN or AUX proteins (Naramoto et al., 2014; Qi et al., 2011; Rybak et al., 2014; Ravikumar et al., 2018). The TGN plays a key role not only in the sorting of macromolecules but also in exocytosis and endocytosis. In addition, the TGN performs specialized functions such as cytokinesis, cell differentiation, the establishment of cell polarity, and anisotropic growth (Gendre et al., 2015; Ravikumar et al., 2017). As an early endosome, the plant TGN is a central hub in the flow of information to and from the plant cell surface (Uemura, 2016). The plant TGN has been implicated in responses to abiotic stimuli such as drought, heat, salt stress and osmotic stress, and to biotic stimuli such as fungal attack (Rosquete and Drakakaki, 2018). Studies on the role of the TGN in stress responses have been carried out predominantly with core trafficking components required for membrane tethering, docking, and fusion (Rosquete and Drakakaki, 2018; Ravikumar et al., 2017). Trafficking mutants typically exhibit root growth defects and/or hypersensitivity to abiotic cues such as salt stress, osmotic stress, drought, or heat (Asaoka et al., 2013; Kim and Bassham, 2011; Lee et al., 2006; Rosquete et al., 2019; Uemura et al., 2012; Wang et al., 2011; Zhu et al., 2002). However, whether trafficking mutants have primary defects in growth with secondary consequences in stress responses or whether the primary defects lie in an impaired response to stress factors remains unclear. More broadly, in the context of stress responses, the question pertains as to whether the TGN is involved in decision-making processes per se or merely in the execution of adaptive growth decisions.
As regards decision-making processes, there is a growing body of evidence to suggest that plants have the ability to learn, process information, communicate, reach decisions, and in general exhibit behavior that could be considered cognitive (reviewed in Severino, 2021). Severino (2021) makes the case for experimental approaches to study the decisions plants make in complex environments. A recent experimental approach for the study of decision-making processes in germinating seedlings has incorporated two tools used in decision theory: the use of a limited budget and conflict-of-interest scenarios (Kalbfuß et al., 2022). A limited budget was achieved by germination in the dark in the absence of a carbon source, such that the only available energy source is that available in the seed (Kalbfuß et al., 2022). A conflict-of-interest scenario comprises the simultaneous withdrawal of light, which promotes hypocotyl elongation, and water, which promotes root elongation (Kalbfuß et al., 2022). As the severity of water stress increased, root length increased while hypocotyl length decreased; importantly, the total seedling length remained constant (Kalbfuß et al., 2022). Thus, trade-offs in hypocotyl versus root growth were observed and these comprise a binary readout for responses to these additive stress conditions. Decision mutants were defined as mutants that were either incapable of adjusting their hypocotyl/root ratios in response to additive stress, or that consistently reached the wrong growth decisions as compared to the wild type (Kalbfuß et al., 2022). By emphasizing growth trade-offs, the experimental approach developed by Kalbfuß et al. (2022) is aligned with the definition of decision-making as entailing an appraisal of the advantages and disadvantages of various courses of action (Karban and Orrock, 2018). While Kalbfuß et al. (2022) address decision-making at a cellular level, the literature on plant decision-making has, to our knowledge, not included considerations about the possible role of the TGN.
To understand the role of the TGN in adaptive or stress responses, it would be important to deploy a battery of gene products not only broadly associated with or localized to the TGN but also intrinsic to TGN structure and function. Two such proteins or complexes are ECHIDNA and the Transport Protein Particle II (TRAPPII) complex. ECHIDNA was identified as an upregulated transcript in elongating cells (Gendre et al., 2011). Yeast and metazoan TRAPPII is a hetero-oligomeric complex that acts as a guanine nucleotide exchange factor (GEF) for Rab GTPases, converting GDP-bound inactive Rab GTPases to active GTP-bound forms (Cai et al., 2005; Morozova et al., 2006; Pinar et al., 2015; Thomas and Fromme, 2016; Riedel et al., 2018). TRAPPII has been shown to play a key role in the regulation of the TGN in all eukaryotes, but our understanding of its potential physiological roles is incomplete (Pinar and Peñalva, 2020). The Arabidopsis TRAPPII (AtTRAPPII) complex was identified by mutation in screens for seedlings with aberrant morphogenesis or cytokinesis defects (Söllner et al., 2002; Thellmann et al., 2010; Jaber et al., 2010). AtTRAPPII consists of seven shared core subunits and three TRAPPII-specific subunits (AtTRS120/TRAPPC9, CLUB/AtTRS130/TRAPPC10, and the plant-specific TRIPP) and most resembles fungal and metazoan TRAPPII complexes (Garcia et al., 2020; Kalde et al., 2019; Pinar et al., 2019). We have previously shown that ECHIDNA and TRAPPII have overlapping yet distinct functions at the TGN in Arabidopsis (Ravikumar et al., 2018). ECHIDNA is primarily required for the genesis of secretory vesicles and, as a consequence, for cell expansion (Boutté et al., 2013; Gendre et al., 2013; McFarlane et al., 2013). AtTRAPPII plays a role not only in basal TGN functions—exocytosis, endocytosis, and protein sorting—but also in more specialized TGN functions such as cytokinesis and the establishment of cell polarity (Ravikumar et al., 2018). Whether or not AtTRAPPII plays a role in responses to abiotic cues such as osmotic or drought stress remains to be determined.
In this study, we focus on the TRAPPII complex as a starting point as it is required for all aspects of TGN function, including the sorting of proteins such as PINs to distinct membrane domains (Qi et al., 2011; Rybak et al., 2014; Ravikumar et al., 2018). We first explored the Arabidopsis TRAPPII interactome and also surveyed dynamic or conditional interactions. Together with yeast two-hybrid screens, this identified shaggy-like kinases such as AtSK21/BIN2 as TRAPPII interactors. We corroborated this finding with in vitro kinase assays and pharmacological inhibition in vivo. Shaggy-like kinases are multitaskers that integrate a vast number of biotic and abiotic cues (Lv and Li, 2020; Planas-Riverola et al., 2019; Youn and Kim, 2015; Li et al., 2021; Song et al., 2023). AtSK21/BIN2 has recently been implicated in decision-making in Arabidopsis seedlings (Kalbfuß et al., 2022). We explore the meaning of the AtSK–TRAPPII interaction using a variety of assays to monitor stress responses and differential growth decisions.
Results
The TRAPPII interactome contains a large number of signaling components
To gain insight into TGN function, we used the TRAPPII interactome as a starting point. We have previously identified TRAPP and EXOCYST subunits, cytoskeletal proteins, and Rab GTPases in the TRAPPII interactome (Rybak et al., 2014; Steiner et al., 2016; Kalde et al., 2019; Garcia et al., 2020). However, no global meta-analysis of the vast Arabidopsis TRAPPII interactome has been carried out to date. We, therefore, performed a gene ontology (GO) term enrichment analysis. We focused on the TRAPPII-specific CLUB:GFP interactome. This interactome was significantly enriched in proteins involved in cell division, trafficking or transport, root hair elongation, and microtubule organization, which is consistent with known trappii phenotypes (Fig. 1 A and Fig. S1 A; Jaber et al., 2010; Qi et al., 2011; Rybak et al., 2014; Ravikumar et al., 2018; Söllner et al., 2002; Thellmann et al., 2010; Steiner et al., 2016). Interestingly, a large number of enriched GO terms were implicated in signaling (Fig. 1 A and Fig. S1 A), suggesting that AtTRAPPII may act as a cellular hub.
The TRAPPII interactome. The data are derived from an analysis of IP-MS from inflorescences with the TRAPPII-specific subunit CLUB:GFP as bait (see Fig. 1 B; Kalde et al., 2019). Each protein was present in all three biological replicates. The soluble GFP empty vector was used as a negative control. (A) Gene ontology (GO) term enrichment analysis of the TRAPPII interactome. Depicted are highly enriched (fold enrichment ≥4) and significant (FDR-adjusted P value ≤0.003) GO term associations of biological processes of level 0 as bar plots. The length of each bar depicts the fold enrichment of GO terms associated with detected proteins, while the color intensity indicates the significance given as the P value adjusted for the false discovery rate (FDR). Interactors of intermediate intensity (>5 and <8) were used (see Fig. S1 A for an analysis of high-confidence interactors). In addition to the expected ontologies describing traffic, transport, cell division, and microtubule organization, significantly enriched GO categories describe responses to stimuli such as light and metal ions. (B and C) Volcano plots are presented. On the X axis: The ratio (or fold change) was calculated for each protein as the average intensity of the signal in the experiment divided by its average intensity in the control. On the Y axis the P values of the signal in the experiment versus the control, depicted along a negative log10 scale, are shown. Dotted gray lines represent cutoffs: P value ≤0.02 in B and C and ratio >8 for high fold change or >5 for intermediate fold change in B. (B) Note that TRAPPII subunits (light blue for core TRAPP; dark blue for TRAPPII-specific subunits; the CLUB/AtTRS130:GFP TRAPPII-specific bait is depicted as an open circle) are in the upper right field, indicating high abundance and good reproducibility. AtSKs (magenta), MAP65-1 (orange) and RAB-A2a GTPase (green) are all in the upper middle field; these may be transient interactors of TRAPPII. Note that AtSKs (AtSK11/12/32) are more significant than validated interactors such as MAP65 and RAB-A2a (Kalde et al., 2019; Steiner et al., 2016). (C) Highlighted in magenta are members of the brassinosteroid signaling pathway that were differentially enriched over light- versus dark-grown seedlings in a different IP-MS experiment. Note that BSK8, and ROC1 did not meet the significance cutoffs. The most significant interactors were GSK3/AtSK shaggy-like kinases and TOR. Related to Fig. S1 and Table S1.
The TRAPPII interactome. The data are derived from an analysis of IP-MS from inflorescences with the TRAPPII-specific subunit CLUB:GFP as bait (see Fig. 1 B; Kalde et al., 2019). Each protein was present in all three biological replicates. The soluble GFP empty vector was used as a negative control. (A) Gene ontology (GO) term enrichment analysis of the TRAPPII interactome. Depicted are highly enriched (fold enrichment ≥4) and significant (FDR-adjusted P value ≤0.003) GO term associations of biological processes of level 0 as bar plots. The length of each bar depicts the fold enrichment of GO terms associated with detected proteins, while the color intensity indicates the significance given as the P value adjusted for the false discovery rate (FDR). Interactors of intermediate intensity (>5 and <8) were used (see Fig. S1 A for an analysis of high-confidence interactors). In addition to the expected ontologies describing traffic, transport, cell division, and microtubule organization, significantly enriched GO categories describe responses to stimuli such as light and metal ions. (B and C) Volcano plots are presented. On the X axis: The ratio (or fold change) was calculated for each protein as the average intensity of the signal in the experiment divided by its average intensity in the control. On the Y axis the P values of the signal in the experiment versus the control, depicted along a negative log10 scale, are shown. Dotted gray lines represent cutoffs: P value ≤0.02 in B and C and ratio >8 for high fold change or >5 for intermediate fold change in B. (B) Note that TRAPPII subunits (light blue for core TRAPP; dark blue for TRAPPII-specific subunits; the CLUB/AtTRS130:GFP TRAPPII-specific bait is depicted as an open circle) are in the upper right field, indicating high abundance and good reproducibility. AtSKs (magenta), MAP65-1 (orange) and RAB-A2a GTPase (green) are all in the upper middle field; these may be transient interactors of TRAPPII. Note that AtSKs (AtSK11/12/32) are more significant than validated interactors such as MAP65 and RAB-A2a (Kalde et al., 2019; Steiner et al., 2016). (C) Highlighted in magenta are members of the brassinosteroid signaling pathway that were differentially enriched over light- versus dark-grown seedlings in a different IP-MS experiment. Note that BSK8, and ROC1 did not meet the significance cutoffs. The most significant interactors were GSK3/AtSK shaggy-like kinases and TOR. Related to Fig. S1 and Table S1.
GO term enrichment and selected brassinosteroid signaling components in IP-MS with the TRAPPII-specific CLUB:GFP subunit as bait. Light-grown inflorescences were used (see Fig. 1, B and C; Kalde et al., 2019). As control, the soluble GFP empty vector was used. Three replicates were carried out for the IP-MS experiments. (A) Gene ontology (GO) term enrichment analysis of the TRAPPII interactome. Depicted are highly abundant (fold enrichment ≥4) and significant (FDR-adjusted P value ≤0.003) GO term associations of biological processes of level 0. The length of each bar corresponds to the fold enrichment of GO terms associated with detected proteins, while the color intensity indicates the significance given as the P value adjusted for the false discovery rate (FDR). The GO term enrichment analysis was carried out with high-confidence interactors (intensity ratio >8 and P value ≤0.02; see Fig. 1 B). Note that the majority of GO terms are associated with trafficking and transport (12/30). GO terms associated with response to stimulus, light responses, and cell division were also enriched. (B) Individual intensity-based absolute quantification (iBAQ) values in log2 scale of brassinosteroid-related proteins (highlighted in Fig. 1 C) found in each CLUB:GFP IP-MS replicate. Selected brassinosteroid-related proteins were differentially enriched over light- versus dark-grown seedlings in a different IP-MS experiment. Dots clustered at the bottom of the graphs represent peptides that were not detected (or detected as very low intensity) in the sample. P values were obtained using the Welch’s t test. Related to Fig. 1, and Table S1.
GO term enrichment and selected brassinosteroid signaling components in IP-MS with the TRAPPII-specific CLUB:GFP subunit as bait. Light-grown inflorescences were used (see Fig. 1, B and C; Kalde et al., 2019). As control, the soluble GFP empty vector was used. Three replicates were carried out for the IP-MS experiments. (A) Gene ontology (GO) term enrichment analysis of the TRAPPII interactome. Depicted are highly abundant (fold enrichment ≥4) and significant (FDR-adjusted P value ≤0.003) GO term associations of biological processes of level 0. The length of each bar corresponds to the fold enrichment of GO terms associated with detected proteins, while the color intensity indicates the significance given as the P value adjusted for the false discovery rate (FDR). The GO term enrichment analysis was carried out with high-confidence interactors (intensity ratio >8 and P value ≤0.02; see Fig. 1 B). Note that the majority of GO terms are associated with trafficking and transport (12/30). GO terms associated with response to stimulus, light responses, and cell division were also enriched. (B) Individual intensity-based absolute quantification (iBAQ) values in log2 scale of brassinosteroid-related proteins (highlighted in Fig. 1 C) found in each CLUB:GFP IP-MS replicate. Selected brassinosteroid-related proteins were differentially enriched over light- versus dark-grown seedlings in a different IP-MS experiment. Dots clustered at the bottom of the graphs represent peptides that were not detected (or detected as very low intensity) in the sample. P values were obtained using the Welch’s t test. Related to Fig. 1, and Table S1.
To further explore a possible implication of the TRAPPII complex in signaling, we surveyed the dynamic interactome, by which we refer to interactors perceived under one environmental condition (for example in the light) but not in another (for example in the dark). By these criteria, brassinosteroid (BR) signaling components were significantly enriched (P = 0.016); this category encompasses 11 proteins, of which 9 were detected in CLUB:GFP immunoprecipitates from light-grown influorescences (Fig. 1, B and C; and Fig. S1 B and Table S1). Among these were the TOR kinase and a family of shaggy-like kinases (AtSKs; Fig. 1 C and Fig. S1 B; and Table S1). TOR signaling was also a significantly enriched GO term in the TRAPPII interactome (Fig. 1 A). TOR and AtSKs are highly significant interactors in the TRAPPII-specific subunit CLUB:GFP interactome (Fig. 1, B and C; and Fig. S1 B). They show a fold change that is lower than that seen for components of the TRAPPII complex, but similar to that seen for validated interactors such as RAB-A2a and MAP65, expected to form more transient associations (Fig. 1, B and C; Kalde et al., 2019; Steiner et al., 2016). We then used yeast two-hybrid (Y2H) to probe for binary interactions between TRAPPII and signaling components identified in the IP-MS. Y2H was carried out with TRAPPII subunits and truncations thereof (Fig. 2 A; Kalde et al., 2019; Steiner et al., 2016; Garcia et al., 2020). In a large-scale Y2H screen including 2,400 pair-wise tests, an interaction was detected between a TRS120499-1187 truncation (TRS120-T2) and the shaggy-like kinase BIN2 (AtSK21; Fig. 2 B). BIN2 interacted specifically with AtTRS120 and not with other tested TRAPPII subunits (Fig. 2 B). Furthermore, we did not detect any other TRAPPII-kinase interactions in our pairwise Y2H assays. In conclusion, mass spectrometry and Y2H identify physical interactions between TRAPPII and AtSKs, in planta and in a heterologous system.
GSK3/AtSK sites in TRAPPII and binary interactions. Yeast two-hybrid assays were carried out by pairwise one-on-one mating in four independent replicate experiments, of which three (B) or four (F) are shown. As a negative control, the respective AD constructs were tested with the empty DB vector. The panels are from different plates (in B, F). (A) TRAPPII-specific truncations for yeast two-hybrid were based on phylogenetic analysis (Steiner et al., 2016). Conserved sequences are depicted in red, intermediate degrees of conservation in orange, and plant-specific sequences are in green. All three GSK3 sites found to be phosphorylated in vivo reside in the plant-specific moiety of TRS120-T2 (green; a blue rectangle delineates the region of interest). (B) Yeast two-hybrid assays of interactions between BIN2 and TRAPPII subunits. BIN2 was fused to the GAL4 DNA-binding domain (DB) and TRAPPII subunits and truncations thereof (CLUB-C1, -C2, -C3 and TRS120-T1, -T2, -T3) to the GAL4 activation domain (AD). The results show interactions between BIN2 and AtTRS120-T2 wild type. BIN2 bound specifically to the TRAPPII subunit TRS120 and not with other TRAPPII subunits or truncations. In a total of >2,400 pairwise tests, BIN2 was the only kinase we found that interacted with a TRAPP subunit. The Y2H did not detect CLUB/AtTRS130-AtSK interaction shown in Fig. 1 B, which shows copurified proteins that can include indirect—as opposed to binary—interactors. (C) GSK3 sites in AtTRS120-T2. The α-phosphosite of AtTRS120 spans amino acid positions S922 and S923, the β-phosphosite amino acids S971-S975 and the γ-phosphosite amino acid residue S1165. The canonical GSK3 consensus sequence is: (pS/pT)XXX(S/T), and the β-phosphosite fits this definition (see underlined SXXXS). Even though they are annotated as GSK3 sites in the PPSP (https://www.phosphosite.org) database, the α-phosphosite deviates somewhat with SXXXXS, and the γ-phosphosite deviates completely. Amino acids in red were mutated to A or D via site-directed mutagenesis. Amino acids highlighted in yellow were found to be phosphorylated in vivo (see Fig. S2) and these were all serines. (D) The Arabidopsis TRAPPII complex consists of seven shared core subunits (TCA17, TRS33, BET3, BET5, TRS23, TRS31, TRS20; light blue) and three TRAPPII-specific subunits (CLUB/AtTRS130, AtTRS120, and the plant-specific subunit TRIPP), and forms a dimer with plant-specific domains (green) at the predicted dimer interface (Kalde et al., 2019; Garcia et al., 2020). This model is based on extensive pair-wise yeast two-hybrid analysis between TRAPPII subunits in Arabidopsis (Kalde et al., 2019; Garcia et al., 2020). (E) Mapping of GSK3/AtSK sites on an AlphaFold structural prediction (Jumper et al., 2021; Varadi et al., 2022) of AtTRS120. A lateral view and a frontal perspective are shown. Note that all three GSK3/AtSK sites (magenta sticks) reside in unstructured, flexible, and accessible regions of the protein. (F) Interactions between BIN2 and TRAPPII complex subunits (CLUB-C2, CLUB-C3, TRS120-T1, TRS120-T3 truncations), as positive controls, fused to the GAL4 DNA-binding domain (DB) and TRS120-T2 truncation and its phosphomutants TRS120-T2 SαD, SβD, SγD, SαβD, and SαβγD fused to the GAL4 activation domain (AD). Note the positive interactions between BIN2 and AtTRS120-T2 wild type and phosphovariants; there was, however, no reproducible interaction if all three target sites were phosphomimetic (TRS120-T2 SαβγD). Related to Figs. S2, S3, and S4.
GSK3/AtSK sites in TRAPPII and binary interactions. Yeast two-hybrid assays were carried out by pairwise one-on-one mating in four independent replicate experiments, of which three (B) or four (F) are shown. As a negative control, the respective AD constructs were tested with the empty DB vector. The panels are from different plates (in B, F). (A) TRAPPII-specific truncations for yeast two-hybrid were based on phylogenetic analysis (Steiner et al., 2016). Conserved sequences are depicted in red, intermediate degrees of conservation in orange, and plant-specific sequences are in green. All three GSK3 sites found to be phosphorylated in vivo reside in the plant-specific moiety of TRS120-T2 (green; a blue rectangle delineates the region of interest). (B) Yeast two-hybrid assays of interactions between BIN2 and TRAPPII subunits. BIN2 was fused to the GAL4 DNA-binding domain (DB) and TRAPPII subunits and truncations thereof (CLUB-C1, -C2, -C3 and TRS120-T1, -T2, -T3) to the GAL4 activation domain (AD). The results show interactions between BIN2 and AtTRS120-T2 wild type. BIN2 bound specifically to the TRAPPII subunit TRS120 and not with other TRAPPII subunits or truncations. In a total of >2,400 pairwise tests, BIN2 was the only kinase we found that interacted with a TRAPP subunit. The Y2H did not detect CLUB/AtTRS130-AtSK interaction shown in Fig. 1 B, which shows copurified proteins that can include indirect—as opposed to binary—interactors. (C) GSK3 sites in AtTRS120-T2. The α-phosphosite of AtTRS120 spans amino acid positions S922 and S923, the β-phosphosite amino acids S971-S975 and the γ-phosphosite amino acid residue S1165. The canonical GSK3 consensus sequence is: (pS/pT)XXX(S/T), and the β-phosphosite fits this definition (see underlined SXXXS). Even though they are annotated as GSK3 sites in the PPSP (https://www.phosphosite.org) database, the α-phosphosite deviates somewhat with SXXXXS, and the γ-phosphosite deviates completely. Amino acids in red were mutated to A or D via site-directed mutagenesis. Amino acids highlighted in yellow were found to be phosphorylated in vivo (see Fig. S2) and these were all serines. (D) The Arabidopsis TRAPPII complex consists of seven shared core subunits (TCA17, TRS33, BET3, BET5, TRS23, TRS31, TRS20; light blue) and three TRAPPII-specific subunits (CLUB/AtTRS130, AtTRS120, and the plant-specific subunit TRIPP), and forms a dimer with plant-specific domains (green) at the predicted dimer interface (Kalde et al., 2019; Garcia et al., 2020). This model is based on extensive pair-wise yeast two-hybrid analysis between TRAPPII subunits in Arabidopsis (Kalde et al., 2019; Garcia et al., 2020). (E) Mapping of GSK3/AtSK sites on an AlphaFold structural prediction (Jumper et al., 2021; Varadi et al., 2022) of AtTRS120. A lateral view and a frontal perspective are shown. Note that all three GSK3/AtSK sites (magenta sticks) reside in unstructured, flexible, and accessible regions of the protein. (F) Interactions between BIN2 and TRAPPII complex subunits (CLUB-C2, CLUB-C3, TRS120-T1, TRS120-T3 truncations), as positive controls, fused to the GAL4 DNA-binding domain (DB) and TRS120-T2 truncation and its phosphomutants TRS120-T2 SαD, SβD, SγD, SαβD, and SαβγD fused to the GAL4 activation domain (AD). Note the positive interactions between BIN2 and AtTRS120-T2 wild type and phosphovariants; there was, however, no reproducible interaction if all three target sites were phosphomimetic (TRS120-T2 SαβγD). Related to Figs. S2, S3, and S4.
The TRAPPII complex is a target of shaggy-like kinases
To assess whether the TRAPPII complex is a target of AtSK/GSK3 kinases, we first looked for the presence of phosphopeptides in AtTRS120 and CLUB/AtTRS130 coimmunoprecipitates via mass spectrometry. This provided ample in vivo evidence for TRAPPII AtTRS120 phosphorylation at AtSK/GSK3 sites (Fig. S2). Furthermore, shaggy-like kinases were detected in IP-MS not only with the TRAPPII-specific subunit CLUB/AtTRS130 (Fig. 1, B and C; and Fig. S1 B) but also with the TRAPPII-specific subunit AtTRS120 (Fig. S3). The Arabidopsis genome encodes ten shaggy-like kinases (AtSKs), which are classified into four clades (Fig. S3 A). Razor peptides covering all four clades were found in the AtTRS120:GFP interactome (Fig. S3, B–D).
Fragment ion mass spectra of AtTRS120 phosphopeptides found in AtTRS120:GFP IP-MS. Seedlings were grown in the light. (A) α-phosphosite of AtTRS120 at amino acid position S922. The spectrum with the phosphorylated S922 residue was identified with a 1% FDR, as can be seen in the deposited Skyline library. Note that the depicted y-ion series does not show the phosphorylation at S922. (B) β-phosphosite of AtTRS120 at amino acid position S971. (C) γ-phosphosite of AtTRS120 at amino acid position S1165. The y-ion series is highlighted in blue and non-annotated fragment ions in gray. Phosphorylated serines are written in bold letters. These sites are annotated as GSK3 sites in the PPSP (https://www.phosphosite.org) database. Note that not all phosphosites we detected in vivo (highlighted in yellow in Fig. 2 C) are depicted here. Spectra are taken from the deposited Skyline library, resulting from a MaxQuant search. At least three replicates were carried out for the IP-MS experiments. Related to Fig. 2.
Fragment ion mass spectra of AtTRS120 phosphopeptides found in AtTRS120:GFP IP-MS. Seedlings were grown in the light. (A) α-phosphosite of AtTRS120 at amino acid position S922. The spectrum with the phosphorylated S922 residue was identified with a 1% FDR, as can be seen in the deposited Skyline library. Note that the depicted y-ion series does not show the phosphorylation at S922. (B) β-phosphosite of AtTRS120 at amino acid position S971. (C) γ-phosphosite of AtTRS120 at amino acid position S1165. The y-ion series is highlighted in blue and non-annotated fragment ions in gray. Phosphorylated serines are written in bold letters. These sites are annotated as GSK3 sites in the PPSP (https://www.phosphosite.org) database. Note that not all phosphosites we detected in vivo (highlighted in yellow in Fig. 2 C) are depicted here. Spectra are taken from the deposited Skyline library, resulting from a MaxQuant search. At least three replicates were carried out for the IP-MS experiments. Related to Fig. 2.
Fragment ion mass spectra of AtSK/GSK3 kinases found in AtTRS120:GFP IP-MS. The AtSK/GSK3 kinase family in Arabidopsis consists of 10 isoforms. Therefore, mass-spectrometric evidence of different peptides can account for the presence of different kinases in a coimmunoprecipitation with light-grown TRS120:GFP seedlings as bait. The y-ion series is highlighted in blue and non-annotated fragment ions in gray. (A) The Arabidopsis genome encodes ten shaggy-like kinases, which are classified into four clades. Multiple sequence alignment in UniProt using full-length protein sequences. (B) VLGTPTREEIK is shared by AtSKs in clades I and IV. (C) LLQYSPNLR is shared by AtSKs in clades I and III. (D) QTISYMAER is shared by AtSKs in clades I and II. Spectra are taken from the deposited Skyline library, resulting from a MaxQuant search. At least three replicates were carried out for the IP-MS experiments. Related to Fig. 2.
Fragment ion mass spectra of AtSK/GSK3 kinases found in AtTRS120:GFP IP-MS. The AtSK/GSK3 kinase family in Arabidopsis consists of 10 isoforms. Therefore, mass-spectrometric evidence of different peptides can account for the presence of different kinases in a coimmunoprecipitation with light-grown TRS120:GFP seedlings as bait. The y-ion series is highlighted in blue and non-annotated fragment ions in gray. (A) The Arabidopsis genome encodes ten shaggy-like kinases, which are classified into four clades. Multiple sequence alignment in UniProt using full-length protein sequences. (B) VLGTPTREEIK is shared by AtSKs in clades I and IV. (C) LLQYSPNLR is shared by AtSKs in clades I and III. (D) QTISYMAER is shared by AtSKs in clades I and II. Spectra are taken from the deposited Skyline library, resulting from a MaxQuant search. At least three replicates were carried out for the IP-MS experiments. Related to Fig. 2.
The TRS120-T2 truncation contains the GSK3 sites we had found to be phosphorylated in vivo (Fig. S2; see yellow highlights in Fig. 2 C). Our nomenclature for these sites is α, β, and γ, which is short for TRS120-S922:S923 (α), TRS120-S971:S973:S974:S975 (β), and TRS120-S1165 (γ; Fig. 2 C). These reside in the plant-specific moiety of AtTRS120 and are embedded in plant-specific sequences (see green shading in Fig. 2 A and Fig. 2 D) at the dimer interface (Kalde et al., 2019). AlphaFold structural predictions (Varadi et al., 2022; Jumper et al., 2021) show that the three sites reside in unstructured, flexible, and accessible regions of the AtTRS120 protein, as is observed in the majority of modified amino acid residues (Fig. 2 E and S4 A; Jiménez et al., 2007). Furthermore, a cross-kingdom structural alignment of AtTRS120 and CLUB/AtTRS130 with cryo-EM-generated structures of yeast TRAPPII (Mi et al., 2022) showed that the β and γ phosphorylation sites face the active site chamber or Rab GTPase binding pocket predicted by Mi et al. (2022) and Bagde and Fromme (2022) (Fig. 3, Fig. S4, and Video 1). To study the three sites, we generated site-directed mutations in TRS120-T2, mutating the serine (S) and threonine (T) residues (depicted in red in Fig. 2 C) to non-phosphorylatable alanine (A) residues, or to aspartate (D) to mimic constitutive phosphorylation. In the case of the TRS120-Sβ site, for example, we designate these variants as TRS120-SβA or TRS120-SβD (Fig. 2 F and Fig. 4 A). The point mutations were introduced into cDNA sequences for expression in yeast and bacteria. In Y2H screens, the BIN2–TRS120 interaction, but not TRAPPII complex interactions, was almost abolished when all three sites were phosphomimetic (TRS120-T2 SαβγD; Fig. 2 F). As kinases typically have kiss-and-run interactions with their unphosphorylated substrates, and as BIN2 interacts more strongly with an unphosphorylated than a phosphorylated substrate (Pusch et al., 2012; Tang et al., 2011), this is consistent with AtTRS120/TRAPPC9 being targeted by the BIN2 kinase.
Cross-kingdom structural alignment of TRAPPII-specific subunits. (A and B) AlphaFold predictions (Jumper et al., 2021; Varadi et al., 2022) of Arabidopsis thaliana (At) (A) AtTRS120 and (B) CLUB/AtTRS130 aligned with the open formation of the TRAPPII monomer structure without substrate in Saccharomyces cerevisiae (Sc) resolved with cryo-electron microscopy (in vitro) from Mi et al. (2022). Arabidopsis subunits were mapped onto the ScTRAPPII structure with the align algorithm in PyMOL. Reported root-mean-square deviations (RMSD) were 16.966 Å (3,775 atoms) for AtTRS120/TRAPPC9 and 12.953 Å (1,660 atoms) for CLUB/AtTRS130/TRAPPC10. The ScTRAPPII monomer is depicted in different shades of gray and only the respective Sc subunit where the Arabidopsis subunits aligned with is highlighted in blue-white (ScTRS120 in A and ScTRS130 in B). AtTRS120 and CLUB/AtTRS130 are colored based on their sequence conservation (red: conserved sequences; orange: intermediate conservation; green: plant-specific sequences—as depicted in Fig. 2, A and D). Both Arabidopsis protein structures aligned with their respective yeast subunit. Despite their small protein length differences ScTRS120 (1,289 aa) and AtTRS120 (1,186 aa) share conserved sequences (Cox et al., 2007) and mapped onto each other in the cross-kingdom structural alignment. Note that the β and γ phosphorylation sites of AtTRS120 (magenta sticks) face inwards, toward the active site chamber (including the RAB11/Rab-A GTPase binding pocket) proposed by Mi et al. (2022) and Bagde and Fromme (2022). Related to Fig. S4 and Video 1.
Cross-kingdom structural alignment of TRAPPII-specific subunits. (A and B) AlphaFold predictions (Jumper et al., 2021; Varadi et al., 2022) of Arabidopsis thaliana (At) (A) AtTRS120 and (B) CLUB/AtTRS130 aligned with the open formation of the TRAPPII monomer structure without substrate in Saccharomyces cerevisiae (Sc) resolved with cryo-electron microscopy (in vitro) from Mi et al. (2022). Arabidopsis subunits were mapped onto the ScTRAPPII structure with the align algorithm in PyMOL. Reported root-mean-square deviations (RMSD) were 16.966 Å (3,775 atoms) for AtTRS120/TRAPPC9 and 12.953 Å (1,660 atoms) for CLUB/AtTRS130/TRAPPC10. The ScTRAPPII monomer is depicted in different shades of gray and only the respective Sc subunit where the Arabidopsis subunits aligned with is highlighted in blue-white (ScTRS120 in A and ScTRS130 in B). AtTRS120 and CLUB/AtTRS130 are colored based on their sequence conservation (red: conserved sequences; orange: intermediate conservation; green: plant-specific sequences—as depicted in Fig. 2, A and D). Both Arabidopsis protein structures aligned with their respective yeast subunit. Despite their small protein length differences ScTRS120 (1,289 aa) and AtTRS120 (1,186 aa) share conserved sequences (Cox et al., 2007) and mapped onto each other in the cross-kingdom structural alignment. Note that the β and γ phosphorylation sites of AtTRS120 (magenta sticks) face inwards, toward the active site chamber (including the RAB11/Rab-A GTPase binding pocket) proposed by Mi et al. (2022) and Bagde and Fromme (2022). Related to Fig. S4 and Video 1.
Predicted ali gned error graphs of the AlphaFold generated AtTRS120 and CLUB/AtTRS130 protein structures. (A and B) Predicted aligned error (PAE) graphs of the AlphaFold predictions (Jumper et al., 2021; Varadi et al., 2022) of A. thaliana (At) (A) AtTRS120 and (B) CLUB/AtTRS130 are shown for assessing the domain prediction confidence. The heatmaps are colored based on the expected positional error (given in Ångström) at residue x if the predicted and true structures are aligned on residue y. Related to Figs. 2 and 3; and Video 1.
Predicted ali gned error graphs of the AlphaFold generated AtTRS120 and CLUB/AtTRS130 protein structures. (A and B) Predicted aligned error (PAE) graphs of the AlphaFold predictions (Jumper et al., 2021; Varadi et al., 2022) of A. thaliana (At) (A) AtTRS120 and (B) CLUB/AtTRS130 are shown for assessing the domain prediction confidence. The heatmaps are colored based on the expected positional error (given in Ångström) at residue x if the predicted and true structures are aligned on residue y. Related to Figs. 2 and 3; and Video 1.
Cross-kingdom structural alignment of the TRAPPII-specific TRS120 subunit. AlphaFold prediction (Jumper et al., 2021; Varadi et al., 2022) of Arabidopsis thaliana (At) AtTRS120/TRAPPC9 aligned with the open formation of the TRAPPII monomer structure without substrate in Saccharomyces cerevisiae (Sc) resolved with cryo-electron microscopy (in vitro) from Mi et al. (2022). Alignment was performed with the align algorithm in PyMOL. Reported root-mean-square deviation (RMSD) was 16.966 Å (3,775 atoms). The ScTRAPPII monomer is depicted in different shades of gray, and only the ScTRS120 subunit on which AtTRS120 was mapped in the alignment is highlighted in blue-white. AtTRS120 is colored based on its sequence conservation (red: conserved sequences; orange: intermediate conservation; green: plant-specific sequences - as depicted in Fig. 2, A and D; and Fig. 3). Note that the β and γ phosphorylation sites of AtTRS120 (magenta sticks) face inward, toward the active site chamber (including the RAB11/Rab-A GTPase binding pocket) proposed by Mi et al. (2022) and Bagde and Fromme (2022). Image display rate: 30 frames/s. Related to Figs. 2, 3, and S4.
Cross-kingdom structural alignment of the TRAPPII-specific TRS120 subunit. AlphaFold prediction (Jumper et al., 2021; Varadi et al., 2022) of Arabidopsis thaliana (At) AtTRS120/TRAPPC9 aligned with the open formation of the TRAPPII monomer structure without substrate in Saccharomyces cerevisiae (Sc) resolved with cryo-electron microscopy (in vitro) from Mi et al. (2022). Alignment was performed with the align algorithm in PyMOL. Reported root-mean-square deviation (RMSD) was 16.966 Å (3,775 atoms). The ScTRAPPII monomer is depicted in different shades of gray, and only the ScTRS120 subunit on which AtTRS120 was mapped in the alignment is highlighted in blue-white. AtTRS120 is colored based on its sequence conservation (red: conserved sequences; orange: intermediate conservation; green: plant-specific sequences - as depicted in Fig. 2, A and D; and Fig. 3). Note that the β and γ phosphorylation sites of AtTRS120 (magenta sticks) face inward, toward the active site chamber (including the RAB11/Rab-A GTPase binding pocket) proposed by Mi et al. (2022) and Bagde and Fromme (2022). Image display rate: 30 frames/s. Related to Figs. 2, 3, and S4.
In vitro AtSK kinase assays with TRS120 as bait. (A) In vitro kinase assays using GST:AtBIN2 (69 kDa) and GST:TRS120-T2 (100 kDa). The change of phosphosignal is shown in a representative autoradiograph (upper panel) and the loaded protein amount in the corresponding CBB (Coomassie stain, lower panel). Non-phosphorylatable S to A TRS120-T2 variants were used as negative controls. The means ± SD of phosphosignals were normalized to the protein amount and related to non-mutated TRS120-T2 wild-type control. Note that BIN2 phosphorylated AtTRS120-T2 in vitro, with a preference for wild-type (WT) sequences over non-phosphorylatable AtTRS120-SγA, AtTRS120-SαγA, AtTRS120-SβγA and AtTRS120-SαβγA substrates. n = 3 independent experiments; *: P < 0.05 for significant differences to TRS120-T2 WT (set at 1.0 right panel) determined by using a one sample two-tailed t test. (B) Kinase assays were performed in vitro with mass-spectrometry readout. One member of each shaggy-like kinase clade (AtSKs; see Fig. S3 A) was used with a constant concentration of the TRS120-T2 truncation as substrate. The dilution series of the kinase are depicted in different shades of blue. AtTRS120-T2 has highly (red) and moderately (orange) conserved sequences, as well as plant-specific sequences (green). Three GSK3 sites (referred to as α, β, γ; see Fig. 2 C) can be found in the plant-specific T2 domain. AtSKs in clades I-III differentially phosphorylated the substrate at three GSK3 consensus sites (with a preference for the γ site) in a time-dependent and concentration-dependent manner. A clade IV AtSK did not phosphorylate at all. Samples incubated for 120 min in a kinase buffer without ATP, or samples in which the kinase was heat-inactivated (KD), served as negative controls. The numbers in grey in each plot denote the number of times the phosphorylation event was seen in the given number of independent replicates. Note the higher intensity of the TRS120-Sγ peptide, especially for Clade I/SK11 (1e8 for TRS120-γ versus 1e6 for TRS120-α and TRS120-β on the Y axis). Related to Figs. S2, S3, and S5. Source data are available for this figure: SourceData F4.
In vitro AtSK kinase assays with TRS120 as bait. (A) In vitro kinase assays using GST:AtBIN2 (69 kDa) and GST:TRS120-T2 (100 kDa). The change of phosphosignal is shown in a representative autoradiograph (upper panel) and the loaded protein amount in the corresponding CBB (Coomassie stain, lower panel). Non-phosphorylatable S to A TRS120-T2 variants were used as negative controls. The means ± SD of phosphosignals were normalized to the protein amount and related to non-mutated TRS120-T2 wild-type control. Note that BIN2 phosphorylated AtTRS120-T2 in vitro, with a preference for wild-type (WT) sequences over non-phosphorylatable AtTRS120-SγA, AtTRS120-SαγA, AtTRS120-SβγA and AtTRS120-SαβγA substrates. n = 3 independent experiments; *: P < 0.05 for significant differences to TRS120-T2 WT (set at 1.0 right panel) determined by using a one sample two-tailed t test. (B) Kinase assays were performed in vitro with mass-spectrometry readout. One member of each shaggy-like kinase clade (AtSKs; see Fig. S3 A) was used with a constant concentration of the TRS120-T2 truncation as substrate. The dilution series of the kinase are depicted in different shades of blue. AtTRS120-T2 has highly (red) and moderately (orange) conserved sequences, as well as plant-specific sequences (green). Three GSK3 sites (referred to as α, β, γ; see Fig. 2 C) can be found in the plant-specific T2 domain. AtSKs in clades I-III differentially phosphorylated the substrate at three GSK3 consensus sites (with a preference for the γ site) in a time-dependent and concentration-dependent manner. A clade IV AtSK did not phosphorylate at all. Samples incubated for 120 min in a kinase buffer without ATP, or samples in which the kinase was heat-inactivated (KD), served as negative controls. The numbers in grey in each plot denote the number of times the phosphorylation event was seen in the given number of independent replicates. Note the higher intensity of the TRS120-Sγ peptide, especially for Clade I/SK11 (1e8 for TRS120-γ versus 1e6 for TRS120-α and TRS120-β on the Y axis). Related to Figs. S2, S3, and S5. Source data are available for this figure: SourceData F4.
The IP-MS and Y2H interactions were validated with in vitro kinase assays, performed with a phosphorus radioisotope (Fig. 4 A and Fig. S5 A). This showed that BIN2 and AtSK11 phosphorylated AtTRS120-T2 in vitro, with a preference for wild-type sequences over non-phosphorylatable substrates such as AtTRS120-SαβγA (Fig. 4 A and Fig. S5 A). Further, the phosphorylation was confirmed with mass-spectrometry using non-radioactive assays. The mass-spectrometry results showed that the AtTRS120 α, β, and γ sites phosphorylated in vivo (Fig. S2; IP-MS on seedlings using TRS120:GFP as bait) were phosphorylated by AtSKs in vitro (Fig. 4 B and Fig. S5 B). Kinase assays showed that the phosphorylation events were time- and/or concentration-dependent (Fig. 4 B and Fig. S5 B). AtTRS120 was a substrate of AtSKs in clades I-III; we did not detect phosphorylation of AtTRS120 with a clade IV AtSK in vitro (Fig. 4 B). All AtSKs that targeted AtTRS120 had a marked and consistent preference for the TRS120-γ (S1165) site (Fig. 4 B and Fig. S5 B). In vivo, IP-MS performed on seedlings treated with the AtSK inhibitor bikinin showed a reduced extent of phosphorylation of the TRS120-Sγ peptide (Fig. S5, C and D). Conversely, seedlings treated with the BR biosynthesis inhibitor PPZ, which should relieve BR-mediated BIN2 inhibition, showed an increased extent of phosphorylation of the TRS120-Sγ peptide (Fig. S5, C and D). These in vivo observations are consistent with the in vitro kinase assays (Figs. 4 and S5). In summary, several lines of in vitro (Y2H, kinase assays) and in vivo (IP-MS, pharmacological inhibition) evidence support the conclusion that the TRAPPII subunit AtTRS120 is a substrate of shaggy-like kinases.
In vitro and in vivo evidence for TRAPPII phosphorylation by AtSKs. (A) In vitro kinase assays using GST:AtSK11 (72 kDa) and GST:TRS120-T2 (100 kDa). The change of phosphosignal is shown in a representative autoradiograph (upper panel) and the loaded protein amount in the corresponding CBB (Coomassie stain, lower panel). Non-phosphorylatable S to A TRS120-T2 variants were used as negative controls. The means ± SD of phosphosignals were normalized to the protein amount and related to non-mutated TRS120-T2 wild-type control. Note that AtSK11 phosphorylated AtTRS120-T2 in vitro, with a preference for wild-type (WT) sequences over non-phosphorylatable AtTRS120-SαβγA. n = 2–4 independent experiments; *: P < 0.05 for significant differences to TRS120-T2 WT (set at 1.0 right panel) determined by using a one sample two-tailed t test. (B) In vitro kinase assay with mass-spectrometry readout using BIN2 as kinase and TRS120-T2 truncation as substrate. Dilution series of the kinase are depicted in different shades of blue. BIN2 phosphorylated the β and γ site of TRS120 with a preference for the γ site (1e7 for TRS120-γ versus 1e6 for TRS120-β on the Y axis). Samples incubated for 120 min in a kinase buffer without ATP, or samples in which the kinase was heat-inactivated (KD), served as negative controls. The numbers in gray in each plot denote the number of times the phosphorylation event was seen in the given number of independent replicates. (C) Bikinin is an inhibitor of shaggy-like kinases (AtSKs), whereas PPZ is a BR biosynthesis inhibitor that relieves BR-mediated BIN2 inhibition. (D) Impact of the pharmacological inhibitors bikinin and PPZ on the TRS120 phosphorylation status in vivo. IP-MS was carried out on light-grown TRS120:GFP seedlings treated with bikinin, PPZ, or the respective mock-controls. The phosphorylated peptides were further analyzed via Skyline (MacLean et al., 2010). Normalized intensities were calculated as the ratio of the TRS120-Sγ phosphopeptide intensity over the sum of all TRS120 peptide intensities found in the respective experiment. The extent of phosphorylation at the TRS120-γ site was significantly decreased by bikinin. In contrast, PPZ treatment increased the phosphorylation of the TRS120-Sγ peptide in vivo. P values were computed with a two-tailed Student’s t test (*: P < 0.05; **: P < 0.01). Mean ± SD of four replicates are shown for the control and treatment. Related to Figs. 2, 3, and 4. Source data are available for this figure: SourceData FS5.
In vitro and in vivo evidence for TRAPPII phosphorylation by AtSKs. (A) In vitro kinase assays using GST:AtSK11 (72 kDa) and GST:TRS120-T2 (100 kDa). The change of phosphosignal is shown in a representative autoradiograph (upper panel) and the loaded protein amount in the corresponding CBB (Coomassie stain, lower panel). Non-phosphorylatable S to A TRS120-T2 variants were used as negative controls. The means ± SD of phosphosignals were normalized to the protein amount and related to non-mutated TRS120-T2 wild-type control. Note that AtSK11 phosphorylated AtTRS120-T2 in vitro, with a preference for wild-type (WT) sequences over non-phosphorylatable AtTRS120-SαβγA. n = 2–4 independent experiments; *: P < 0.05 for significant differences to TRS120-T2 WT (set at 1.0 right panel) determined by using a one sample two-tailed t test. (B) In vitro kinase assay with mass-spectrometry readout using BIN2 as kinase and TRS120-T2 truncation as substrate. Dilution series of the kinase are depicted in different shades of blue. BIN2 phosphorylated the β and γ site of TRS120 with a preference for the γ site (1e7 for TRS120-γ versus 1e6 for TRS120-β on the Y axis). Samples incubated for 120 min in a kinase buffer without ATP, or samples in which the kinase was heat-inactivated (KD), served as negative controls. The numbers in gray in each plot denote the number of times the phosphorylation event was seen in the given number of independent replicates. (C) Bikinin is an inhibitor of shaggy-like kinases (AtSKs), whereas PPZ is a BR biosynthesis inhibitor that relieves BR-mediated BIN2 inhibition. (D) Impact of the pharmacological inhibitors bikinin and PPZ on the TRS120 phosphorylation status in vivo. IP-MS was carried out on light-grown TRS120:GFP seedlings treated with bikinin, PPZ, or the respective mock-controls. The phosphorylated peptides were further analyzed via Skyline (MacLean et al., 2010). Normalized intensities were calculated as the ratio of the TRS120-Sγ phosphopeptide intensity over the sum of all TRS120 peptide intensities found in the respective experiment. The extent of phosphorylation at the TRS120-γ site was significantly decreased by bikinin. In contrast, PPZ treatment increased the phosphorylation of the TRS120-Sγ peptide in vivo. P values were computed with a two-tailed Student’s t test (*: P < 0.05; **: P < 0.01). Mean ± SD of four replicates are shown for the control and treatment. Related to Figs. 2, 3, and 4. Source data are available for this figure: SourceData FS5.
BIN2 and TRAPPII are required for differential growth decisions under additive stress
As TRAPPII is a BIN2 substrate, the question is whether bin2 and trappii mutants have related phenotypes. We were not able to detect cytokinesis or protein sorting defects, characteristic of trappii, in semi-dominant bin2-1 alleles (Fig. S6). We have recently shown that BIN2 is required for hypocotyl versus root trade-offs in the germinating seedling under additive stress conditions involving the simultaneous withdrawal of both light and water (Kalbfuß et al., 2022). Water stress in the dark is a “conflict-of-interest” scenario in which hypocotyl and root growth have competing interests (Kalbfuß et al., 2022). Kalbfuß et al. (2022) defined decision mutants as ones that had either insignificant hypocotyl/root-ratio responses (P > 0.05), or consistently wrong growth responses as compared with the wild type (P < 0.05 but for an opposite growth phenotype, as depicted by red asterisks in Fig. 5, B–D; Kalbfuß et al., 2022). Under additive stress, trappii null mutants failed to adjust their hypocotyl length along the same line as the wild type (red asterisks in Fig. 5, C and D; and Fig. 5 F; see Fig. S7, D and E for a direct comparison to the wild type and for the distribution of datapoints). In particular, trappii trs120-4 mutants had non-significant hypocotyl/root ratio responses to water withdrawal in the dark (Fig. 5 D and Fig. S7 E). In addition to comparing organ lengths, each mutant line was normalized to its corresponding wild-type ecotype on the same (PEG) plate, which helped us to take the variability between PEG plates and experiments into account and enabled us to pool biological replicates (see Materials and methods for further detail). To this end, the response to water stress in the dark was represented as a normalized response quotient (RQ). The RQ is an indication of how well each mutant responds to a given combination of stress cues and indicates how much each line deviates from the wild type. A value of 1.0 means that the mutant line behaves exactly like its corresponding wild type. This rendition shows that bin2 higher order and trappii null alleles considerably deviated from the wild type, with severely attenuated responses (Fig. 5 G and Fig. S8). We reason that decision mutants unable to integrate environmental cues might have highly variable hypocotyl versus root lengths. This high variance would, in turn, translate into an insignificant (i.e., high) P value, indicative of a low signal-to-noise ratio. We, therefore, plotted the median P values against the normalized response quotients (referred to as volcano plots; mean RQratio in Fig. 5 H). Wild-type ecotypes had significant P values <10−10 (gray shading on the red line in Fig. 5 H, green arrow). Mutants with insignificant P values and response quotients considerably smaller than 1.0 would be considered “confused” decision mutants, and these would map in the lower left quadrant of the RQratio volcano plot (see peach shading in Fig. 5 H). bin2 higher order null alleles and trs120-4 mutants clustered together in the lower left region of the volcano plot in contrast to the near-wild-type phenotype of higher order null mutants impaired in the perception of light or water stress (phyAphyBcry1cry2 and pyr1pyl1pyl2pyl4 in Fig. 5, G and H; and Fig. S7, A and B; Mazzella and Casal, 2001; Park et al., 2009). We conclude that bin2 higher order and trappii null alleles are decision mutants (cf. Kalbfuß et al., 2022).
Cytokinesis and protein sorting in bin2-1. Localization patterns of diverse markers in root tip cells of light-grown seedlings. Confocal Scanning Laser Microscopy. A protein that resides at the cell plate during cytokinesis in the wild type, PKEU::KEULE:GFP (Steiner et al., 2016b) was also seen at the cell plate in bin2-1 mutants (yellow arrowhead). Conversely, markers that are largely excluded from the cell plate (white star) in the wild type (PEXO84b::EXO84b:GFP [Fendrych et al., 2010], PVHAa1::VHAa1:GFP [Dettmer et al., 2006]) are also excluded in bin2-1 mutants. Cell plate formation and morphology in bin2-1 also did not visibly differ from the wild type. Sample numbers n = number of imaged cells. Scale bars represent 5 µm.
Cytokinesis and protein sorting in bin2-1. Localization patterns of diverse markers in root tip cells of light-grown seedlings. Confocal Scanning Laser Microscopy. A protein that resides at the cell plate during cytokinesis in the wild type, PKEU::KEULE:GFP (Steiner et al., 2016b) was also seen at the cell plate in bin2-1 mutants (yellow arrowhead). Conversely, markers that are largely excluded from the cell plate (white star) in the wild type (PEXO84b::EXO84b:GFP [Fendrych et al., 2010], PVHAa1::VHAa1:GFP [Dettmer et al., 2006]) are also excluded in bin2-1 mutants. Cell plate formation and morphology in bin2-1 also did not visibly differ from the wild type. Sample numbers n = number of imaged cells. Scale bars represent 5 µm.
Role of the TRAPPII complex versus ECHIDNA in hypocotyl/root trade-offs. Seedlings were germinated on ½ MS in the dark (dark) or in the dark with −0.4 MPa water stress (darkW). (A) Col-0 (wild type). (B) BR signaling mutant bin2-3bil1bil2 triple knockout (from Kalbfuß et al., 2022). (C)club-2, a null trappii mutant. (D)trs120-4, a null trappii allele. (E) Null echidna allele, impaired in TGN structure and function. At least three experiments were performed for each line, and a representative one is shown here based on RQ and P values. (F) Representative seedling images of Col-0 (wild type) and mutants shown in A–E. bin2-3bil1bil2 and trs120-4 mutants failed to correctly adjust their hypocotyl and root lengths from dark to darkW conditions. Dotted lines mark the hypocotyl-root junction, whereas arrows point to the end of the root. Scale bar is 1 cm. (G) Normalized response quotient RQratio. Each replicate is represented by a dot. A value of 1 (vertical red line) corresponds to an identical adaptation to darkW conditions as the wild type. Note that the triple bin2-3bil1bil2 knock out, trs120-4 and club-2 had attenuated responses (magenta arrows). (H) Volcano plot with the mean RQratio depicted on the X axis and the median P value of the response on the Y axis (negative log scale; a median of all replicates was used). The area shaded in gray on the red line (green arrow) is where wild-type ecotypes would theoretically map onto the plot. Mutants in the lower left quadrant (peach shading) were considered to have a “confused decision phenotype” (see text). trs120-4 mutants mapped to the lower left quadrant and qualified as decision mutants on two counts: (i) a consistently opposite hypocotyl response (red asterisk in D), (ii) failure to adjust the hypocotyl/root ratio to darkW (the ratio for darkW is the same as for dark in D), translating into a non-significant P value for the ratio response (H). The number (n) of seedlings measured per condition is in gray below the mean ± SD bar graphs. P values were computed with a two-tailed Student’s t test and are represented as follows: *: P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001; *****: P < 0.00001. Mutant alleles and the corresponding ecotypes are described in Table S2. All data points for A–E are shown in Fig. S7. Related to Figs. S7 and S8.
Role of the TRAPPII complex versus ECHIDNA in hypocotyl/root trade-offs. Seedlings were germinated on ½ MS in the dark (dark) or in the dark with −0.4 MPa water stress (darkW). (A) Col-0 (wild type). (B) BR signaling mutant bin2-3bil1bil2 triple knockout (from Kalbfuß et al., 2022). (C)club-2, a null trappii mutant. (D)trs120-4, a null trappii allele. (E) Null echidna allele, impaired in TGN structure and function. At least three experiments were performed for each line, and a representative one is shown here based on RQ and P values. (F) Representative seedling images of Col-0 (wild type) and mutants shown in A–E. bin2-3bil1bil2 and trs120-4 mutants failed to correctly adjust their hypocotyl and root lengths from dark to darkW conditions. Dotted lines mark the hypocotyl-root junction, whereas arrows point to the end of the root. Scale bar is 1 cm. (G) Normalized response quotient RQratio. Each replicate is represented by a dot. A value of 1 (vertical red line) corresponds to an identical adaptation to darkW conditions as the wild type. Note that the triple bin2-3bil1bil2 knock out, trs120-4 and club-2 had attenuated responses (magenta arrows). (H) Volcano plot with the mean RQratio depicted on the X axis and the median P value of the response on the Y axis (negative log scale; a median of all replicates was used). The area shaded in gray on the red line (green arrow) is where wild-type ecotypes would theoretically map onto the plot. Mutants in the lower left quadrant (peach shading) were considered to have a “confused decision phenotype” (see text). trs120-4 mutants mapped to the lower left quadrant and qualified as decision mutants on two counts: (i) a consistently opposite hypocotyl response (red asterisk in D), (ii) failure to adjust the hypocotyl/root ratio to darkW (the ratio for darkW is the same as for dark in D), translating into a non-significant P value for the ratio response (H). The number (n) of seedlings measured per condition is in gray below the mean ± SD bar graphs. P values were computed with a two-tailed Student’s t test and are represented as follows: *: P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001; *****: P < 0.00001. Mutant alleles and the corresponding ecotypes are described in Table S2. All data points for A–E are shown in Fig. S7. Related to Figs. S7 and S8.
Response to single versus additive stress: violin plots. Seedlings were germinated on ½ MS in the light (light), in the dark (dark), or in the dark with −0.4 MPa water stress (darkW). (A–F) Violin plots depict a direct comparison of organ lengths or the hypocotyl/root ratio between the wild type and (A) phyAphyBcry1cry2; (B) pyrpyl1pyl2pyl4; (C) bin2-3bil1bil2; (D) club-2; (E) trs120-4; (F) echidna in the same experiment and on the same PEG plates. The same datasets as shown in Fig. 5, B–E and Fig. S9, B–E were used. Decision phenotypes of bin2-3bil1bil2 and trs120-4 mutants are highlighted as follows: bin2-3bil1bil2 showed an opposite root adaptation (red asterisks) and no hypocotyl/root adaptation (non-significant [n.s.] in red) to the multiple stress conditions of darkW. trappii null mutants, club-2 and trs120-4, had an attenuated, but significant etiolation response but failed to correctly adjust their hypocotyl length under darkW (red asterisks). Additionally, trs120-4 mutants showed no root and no hypocotyl/root ratio adaptation (n.s. in red) to darkW conditions. At least three experiments were performed for each line, and a representative one is shown here on the basis of RQ and P values. P values for decision phenotypes were computed with a two-tailed Student’s t test (*: P < 0.05; ***: P < 0.001) Related to Figs. 5 and S9.
Response to single versus additive stress: violin plots. Seedlings were germinated on ½ MS in the light (light), in the dark (dark), or in the dark with −0.4 MPa water stress (darkW). (A–F) Violin plots depict a direct comparison of organ lengths or the hypocotyl/root ratio between the wild type and (A) phyAphyBcry1cry2; (B) pyrpyl1pyl2pyl4; (C) bin2-3bil1bil2; (D) club-2; (E) trs120-4; (F) echidna in the same experiment and on the same PEG plates. The same datasets as shown in Fig. 5, B–E and Fig. S9, B–E were used. Decision phenotypes of bin2-3bil1bil2 and trs120-4 mutants are highlighted as follows: bin2-3bil1bil2 showed an opposite root adaptation (red asterisks) and no hypocotyl/root adaptation (non-significant [n.s.] in red) to the multiple stress conditions of darkW. trappii null mutants, club-2 and trs120-4, had an attenuated, but significant etiolation response but failed to correctly adjust their hypocotyl length under darkW (red asterisks). Additionally, trs120-4 mutants showed no root and no hypocotyl/root ratio adaptation (n.s. in red) to darkW conditions. At least three experiments were performed for each line, and a representative one is shown here on the basis of RQ and P values. P values for decision phenotypes were computed with a two-tailed Student’s t test (*: P < 0.05; ***: P < 0.001) Related to Figs. 5 and S9.
Response to additive stress: response quotients and volcano plots for the hypocotyl and root. Response quotients (RQ, left) and volcano plots (right) of the hypocotyl and root adaptations to dark-to-dark with water stress (−0.4 MPa, darkW). RQ response quotients are normalized to the respective wild-type response; a value of 1 (vertical red line) indicates that the response to a shift from dark to darkW is identical to that of the respective wild-type ecotype. Each replicate is represented by a dot. Mean RQ values are given on the right. Volcano plots show the mean RQ depicted on the X axis and the median P value of the response on the Y axis (negative log scale). (A) Hypocotyl responses to dark versus darkW conditions. Note that club-2 and trs120-4 had attenuated responses (RQhypocotyl < 0.8, magenta arrows). (B) Root responses to dark versus darkW conditions. Note that the triple bin2-3bil1bil2 knock out (from Kalbfuß et al., 2022) had the strongest attenuated RQroot phenotype (mean RQroot = 2.42, magenta arrow) with an insignificant root response. phyAphyBcry1cry2, club-2, trs120-4, and echidna mutants show also an attenuated root response (RQroot > 1.2, magenta arrows). Quadruple pyrpyl mutants did not have a phenotype, possibly because only four genes are knocked out in a family with 14 members (Ma et al., 2009; Park et al., 2009). Due to the opposite adaptations of the hypocotyl and root under the additive stress conditions (decrease in hypocotyl and increase in root length from dark-to-darkW), the respective thresholds for attenuated responses are opposite (Fig. S8 A, compared to Fig. S8 B). Related to Fig. 5.
Response to additive stress: response quotients and volcano plots for the hypocotyl and root. Response quotients (RQ, left) and volcano plots (right) of the hypocotyl and root adaptations to dark-to-dark with water stress (−0.4 MPa, darkW). RQ response quotients are normalized to the respective wild-type response; a value of 1 (vertical red line) indicates that the response to a shift from dark to darkW is identical to that of the respective wild-type ecotype. Each replicate is represented by a dot. Mean RQ values are given on the right. Volcano plots show the mean RQ depicted on the X axis and the median P value of the response on the Y axis (negative log scale). (A) Hypocotyl responses to dark versus darkW conditions. Note that club-2 and trs120-4 had attenuated responses (RQhypocotyl < 0.8, magenta arrows). (B) Root responses to dark versus darkW conditions. Note that the triple bin2-3bil1bil2 knock out (from Kalbfuß et al., 2022) had the strongest attenuated RQroot phenotype (mean RQroot = 2.42, magenta arrow) with an insignificant root response. phyAphyBcry1cry2, club-2, trs120-4, and echidna mutants show also an attenuated root response (RQroot > 1.2, magenta arrows). Quadruple pyrpyl mutants did not have a phenotype, possibly because only four genes are knocked out in a family with 14 members (Ma et al., 2009; Park et al., 2009). Due to the opposite adaptations of the hypocotyl and root under the additive stress conditions (decrease in hypocotyl and increase in root length from dark-to-darkW), the respective thresholds for attenuated responses are opposite (Fig. S8 A, compared to Fig. S8 B). Related to Fig. 5.
A question that arises is whether trappii mutants are impaired in differential growth decisions as a secondary consequence of primary defects in morphogenesis or cytokinesis (Jaber et al., 2010; Rybak et al., 2014; Thellmann et al., 2010). The etiolation response was severely attenuated in trappii mutants, but nonetheless highly significant (P < 0.00001; Fig. S9, C, D, and F–I). Similarly, an attenuated but clear etiolation response has been shown for other cytokinesis-defective mutants including keule and knolle (Assaad et al., 2001). This shows that, despite a severe impairment in cell division and morphogenesis, cytokinesis-defective mutants are nonetheless capable of differential growth. In addition to their cytokinesis defect, trappii mutants are impaired in TGN function (Ravikumar et al., 2018). We, therefore, compared trappii mutants to echidna mutants, which are severely impaired in TGN structure and function (Boutté et al., 2013; Gendre et al., 2013; McFarlane et al., 2013). Both echidna and trappii mutants exhibited a severe impairment in root elongation in the light (Fig. S9, C–F and H). In contrast to trappii, however, echidna mutants had highly significant responses to additive stress that resembled the wild type in all respects (Fig. 5, E–H; and Fig. S7 F). Thus, echidna mutants do not qualify as decision mutants. In conclusion, a comparison to other cytokinesis-defective or TGN mutants suggests that neither cytokinesis defects nor TGN malfunction suffices to explain the trappii trs120-4 decision phenotype.
Light responses in bin2-3bil1bil2, trappii, and echidna mutants. Seedlings were germinated on ½ MS in the light or dark. (A) Col-0 (wild type). (B) BR signaling mutant bin2-3bil1bil2 triple knockout (from Kalbfuß et al., 2022). (C)club-2, a null trappii allele. (D)trs120-4, a null trappii allele. (E) Null echidna allele, impaired in TGN structure and function. At least three experiments were performed for each line, and a representative one is shown here on the basis of RQ and P values. (F) Representative images of light-grown seedlings of Col-0 (wild type) and mutants shown in A–E. Dotted lines mark the hypocotyl-root junction, whereas arrows point to the end of the root. For corresponding dark-grown seedlings see Fig. 5 F. Scale bar is 1 cm. (G–I) Response quotients (RQ, left) and volcano plots (right) of (G) the hypocotyl, (H) the root, and (I) the hypocotyl/root ratio responses to light versus dark. RQs are normalized to the wild-type quotient; a value of 1 (vertical red line) indicates that the response to a shift from light to dark is identical to that of the respective wild-type ecotype. Each replicate is represented by a dot. Volcano plots show the mean RQ depicted on the X axis and the P value of the response on the Y axis (negative log scale; a median of all replicates was used). trappii mutants trs120-4 and club-2, the TGN mutant echidna and the higher order light perception null mutant phyAphyBcry1cry2 show a severely attenuated etiolation response (mean RQhypocotyl and mean RQratio >> 1.2 as well as RQroot << 0.8). Note that due to the opposite adaptations of the hypocotyl and root from light-to-dark conditions (increase in hypocotyl, decrease in root length and therefore increased hypocotyl/root ratio), the respective thresholds for attenuated responses are opposite (Fig. S9, G and I, compared with Fig. S9 H). The number (n) of seedlings measured per condition is in gray below the mean ± SD bar graphs. P values were computed with a two-tailed Student’s t test (*: P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001; *****: P < 0.00001). Ecotypes are described in Table S2. All data points for A–E are shown in Fig. S7. Related to Fig. 5.
Light responses in bin2-3bil1bil2, trappii, and echidna mutants. Seedlings were germinated on ½ MS in the light or dark. (A) Col-0 (wild type). (B) BR signaling mutant bin2-3bil1bil2 triple knockout (from Kalbfuß et al., 2022). (C)club-2, a null trappii allele. (D)trs120-4, a null trappii allele. (E) Null echidna allele, impaired in TGN structure and function. At least three experiments were performed for each line, and a representative one is shown here on the basis of RQ and P values. (F) Representative images of light-grown seedlings of Col-0 (wild type) and mutants shown in A–E. Dotted lines mark the hypocotyl-root junction, whereas arrows point to the end of the root. For corresponding dark-grown seedlings see Fig. 5 F. Scale bar is 1 cm. (G–I) Response quotients (RQ, left) and volcano plots (right) of (G) the hypocotyl, (H) the root, and (I) the hypocotyl/root ratio responses to light versus dark. RQs are normalized to the wild-type quotient; a value of 1 (vertical red line) indicates that the response to a shift from light to dark is identical to that of the respective wild-type ecotype. Each replicate is represented by a dot. Volcano plots show the mean RQ depicted on the X axis and the P value of the response on the Y axis (negative log scale; a median of all replicates was used). trappii mutants trs120-4 and club-2, the TGN mutant echidna and the higher order light perception null mutant phyAphyBcry1cry2 show a severely attenuated etiolation response (mean RQhypocotyl and mean RQratio >> 1.2 as well as RQroot << 0.8). Note that due to the opposite adaptations of the hypocotyl and root from light-to-dark conditions (increase in hypocotyl, decrease in root length and therefore increased hypocotyl/root ratio), the respective thresholds for attenuated responses are opposite (Fig. S9, G and I, compared with Fig. S9 H). The number (n) of seedlings measured per condition is in gray below the mean ± SD bar graphs. P values were computed with a two-tailed Student’s t test (*: P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001; *****: P < 0.00001). Ecotypes are described in Table S2. All data points for A–E are shown in Fig. S7. Related to Fig. 5.
Cellular growth parameters in trappii were assessed under single versus additive stress in both the hypocotyl and root tip. The width, height, and surface area of trappii hypocotyl cells grown in the light did not show any deviation from the wild type (Fig. 6, A and B light; Fig. S10, A–D light). In wild-type hypocotyls, both organ and cell length decreased in response to water stress in the dark (Fig. 5 A, Fig. 6 A, and Fig. S10 C). In contrast, in trappii mutants, organ and cell length significantly increased (red asterisks or compact letter displays in Fig. 5, C and D, Fig. 6 B, and Fig. S10, A, C, and D highlight a phenotype consistently opposite to the wild type). In root tips, we monitored meristem properties and cell length along single cortical cell files as a function of distance from the quiescent center. In the wild type, meristem size was large in the light, intermediate in the dark, and shortest under water stress in the dark (Fig. 6 C, darkW). In contrast, meristem size in trs120-4 remained constant under the three environmental conditions tested (Fig. 6 D). We have recently shown that root growth in response to water stress in the dark is due to a combination of cell division and rapid exit from the meristem (Kalbfuß et al., 2022). An early exit from the meristem can be visualized as cell elongation in cells close to the quiescent center. This was observed under dark and darkW conditions in the wild type (green arrows in Fig. 6 C) but not in the trappii mutant trs120-4 (magenta arrows in Fig. 6 D). While the curves differed under the different environmental conditions in the wild type (Fig. 6 C), these were fairly similar regardless of the environmental cue in trs120-4 (magenta arrows in Fig. 6 D; note that the gray shading, which designates the 95% confidence interval, overlaps). We conclude that, at the cellular level, trappii trs120-4 mutants are unable to differentially regulate their growth parameters in response to additive stress (Figs. 6 and S10). This would suffice to explain the growth defects we observed at the organ level (Fig. 5). The trappii cellular phenotype in the decision screen is reminiscent of that reported for bin2 (Kalbfuß et al., 2022). In summary, bin2 and trappii alleles have related phenotypes with respect to an inability to differentially regulate cell growth in both the hypocotyl and root tip in response to additive stress (Figs. 5 and 6; cf. Kalbfuß et al., 2022).
Cellular growth parameters of trappii mutants under single versus additive stress conditions. Seedlings were grown in the light (orange), dark (black), or dark with −0.4 MPa water stress (darkW; blue). (A and B) Scanning electron micrographs (SEM) of hypocotyls of (A) Col-0 wild type and (B) trs120-4, null trappii allele. The cell surface area was calculated as the product of cell width and length. trs120-4 mutants showed the opposite hypocotyl cell height and cell surface area adaptations to dark-to-darkW conditions (red asterisks in B) than the wild-type control. Representative SEM images of seedlings grown under the specified conditions are shown (scale bar = 40 µm). Outlines of representative cells are highlighted by white dashed lines. Shown are means ± SD. P values were computed with a two-tailed Student’s t test and are represented as follows: ***: P < 0.001; *****: P < 0.00001. (C and D) Confocal micrographs of mPS-PI stained (C) Col-0 wild type and (D) trs120-4 null mutant root tips under single or additive stress conditions; black arrowheads mark the junction between the meristematic and elongation zones for representative root tips. 10 days after incubation, the cell lengths were measured in single cortex cell files, starting at the cortex/endodermis initials. Cell lengths of consecutive cells were mapped as a function of cell number from the quiescent center (QC). The fitted lines were generated with Local Polynomial Regression Fitting with the “loess” method in R; gray shading designates the 95% confidence interval. Col-0 seedlings grown in the dark with and without water stress show steep slopes (green arrows). In the trappii mutant, the curves exhibit minimal to no difference between the different screen conditions (magenta arrows). Scale bar is 50 µm. The sample size (n) is given in each panel as the number of cells/number of seedlings that were analyzed (A–D). Related to Fig. S10.
Cellular growth parameters of trappii mutants under single versus additive stress conditions. Seedlings were grown in the light (orange), dark (black), or dark with −0.4 MPa water stress (darkW; blue). (A and B) Scanning electron micrographs (SEM) of hypocotyls of (A) Col-0 wild type and (B) trs120-4, null trappii allele. The cell surface area was calculated as the product of cell width and length. trs120-4 mutants showed the opposite hypocotyl cell height and cell surface area adaptations to dark-to-darkW conditions (red asterisks in B) than the wild-type control. Representative SEM images of seedlings grown under the specified conditions are shown (scale bar = 40 µm). Outlines of representative cells are highlighted by white dashed lines. Shown are means ± SD. P values were computed with a two-tailed Student’s t test and are represented as follows: ***: P < 0.001; *****: P < 0.00001. (C and D) Confocal micrographs of mPS-PI stained (C) Col-0 wild type and (D) trs120-4 null mutant root tips under single or additive stress conditions; black arrowheads mark the junction between the meristematic and elongation zones for representative root tips. 10 days after incubation, the cell lengths were measured in single cortex cell files, starting at the cortex/endodermis initials. Cell lengths of consecutive cells were mapped as a function of cell number from the quiescent center (QC). The fitted lines were generated with Local Polynomial Regression Fitting with the “loess” method in R; gray shading designates the 95% confidence interval. Col-0 seedlings grown in the dark with and without water stress show steep slopes (green arrows). In the trappii mutant, the curves exhibit minimal to no difference between the different screen conditions (magenta arrows). Scale bar is 50 µm. The sample size (n) is given in each panel as the number of cells/number of seedlings that were analyzed (A–D). Related to Fig. S10.
Cellular hypocotyl parameters of trappii mutants under single and additive stress conditions. Seedlings were grown in the light (orange), dark (black), or dark with −0.4 MPa water stress (darkW; blue). (A) Scanning electron micrographs (SEM) of hypocotyls of club-2 mutants. The cell surface area was calculated as the product of cell width and length. club-2 mutants showed, identical to trs120-4 (Fig. 6 B), the opposite hypocotyl cell height and cell surface area adaptations to dark-to-darkW conditions (red asterisks) than the wild-type control (Fig. 6 A). Representative SEM images of seedlings grown under the specified conditions are shown (scale bar = 40 µm). The sample size (n) is given as the number of cells/number of seedlings that were analyzed in gray below the graph. Shown are means ± SD. P values were computed with a two-tailed Student’s t test and are represented as follows: ***: P < 0.001; *****: P < 0.00001. (B–D) Bar plots for a direct comparison of the hypocotyl (B) cell width, (C) cell height, and (D) cell surface area of trappii mutants and the wild-type control (Col-0) shown in Fig. 6, A and B; and Fig. S10 A. The cellular hypocotyl parameters in the light did not significantly differ between trappii mutants and the wild type. club-2 and trs120-4 showed a compromised, but significant adjustment of the cellular parameters under single stress conditions (light to dark). Only under multiple stress (dark to darkW), trappii mutants failed to correctly adjust their cellular hypocotyl parameters with the opposite adjustment of the cell height and surface area compared with the wild type (red letters). Shown are means ± SD. Letters indicate statistical significance in two-way ANOVA with Tukey’s post hoc test. Related to Fig. 6.
Cellular hypocotyl parameters of trappii mutants under single and additive stress conditions. Seedlings were grown in the light (orange), dark (black), or dark with −0.4 MPa water stress (darkW; blue). (A) Scanning electron micrographs (SEM) of hypocotyls of club-2 mutants. The cell surface area was calculated as the product of cell width and length. club-2 mutants showed, identical to trs120-4 (Fig. 6 B), the opposite hypocotyl cell height and cell surface area adaptations to dark-to-darkW conditions (red asterisks) than the wild-type control (Fig. 6 A). Representative SEM images of seedlings grown under the specified conditions are shown (scale bar = 40 µm). The sample size (n) is given as the number of cells/number of seedlings that were analyzed in gray below the graph. Shown are means ± SD. P values were computed with a two-tailed Student’s t test and are represented as follows: ***: P < 0.001; *****: P < 0.00001. (B–D) Bar plots for a direct comparison of the hypocotyl (B) cell width, (C) cell height, and (D) cell surface area of trappii mutants and the wild-type control (Col-0) shown in Fig. 6, A and B; and Fig. S10 A. The cellular hypocotyl parameters in the light did not significantly differ between trappii mutants and the wild type. club-2 and trs120-4 showed a compromised, but significant adjustment of the cellular parameters under single stress conditions (light to dark). Only under multiple stress (dark to darkW), trappii mutants failed to correctly adjust their cellular hypocotyl parameters with the opposite adjustment of the cell height and surface area compared with the wild type (red letters). Shown are means ± SD. Letters indicate statistical significance in two-way ANOVA with Tukey’s post hoc test. Related to Fig. 6.
AtTRS120 phosphovariants are functional
To assess the in vivo impact of the TRS120 phosphorylation status on intracellular localization, targeted point mutations (Fig. 2 C) were introduced into AtTRS120 genomic sequences fused to a C-terminal GFP tag. We refer to the ensuing site-directed mutants as TRS120 phosphovariants. The constructs were expressed and capable of rescuing the null trs120-4 allele in the T1 and T2 generations (Fig. S11, A and B); upon further propagation, however, signs of silencing were evident in seedlings (Fig. S11, C and D). The wild-type TRAPPII complex resides in the cytosol, at the TGN and at the cell plate (Fig. S11 E; Naramoto et al., 2014; Qi et al., 2011; Ravikumar et al., 2018; Rybak et al., 2014). Phosphovariants had a similar appearance, but the phosphomimetic TRS120-SαβγD variant tended to be mostly membrane associated, with almost no detectable cytosolic signal (Fig. S11 E).
Characterization and localization of TRS120:GFP phosphovariants. (A) Western blot depicting protein expression of the phosphovariants TRS120-SαβγA and TRS120-SαβγD in trs120-4/trs120-4. Presence of the GFP-fused phosphovariants was detected with an anti-GFP antibody (upper panel). Shown are the expression levels in different primary transformants for each variant. Loaded protein amounts are shown by the corresponding CBB staining (Coomassie stain, lower panel). Protein sizes are given in kDa on the left. (B) Complementation analysis. The TRS120-SαβγA and TRS120-SαβγD phosphovariants in trs120-4/trs120-4 could rescue the null trs120-4 seedling lethal phenotype (third seedling from the left; white arrow) and did not differ from the wild-type Col-0 control in the T1 and T2 generations. Kanamycin selection was used to select for the presence of the phosphovariant construct. Images taken 13 days after stratification. Scale bar is 1 cm. (C and D) Silencing of the phosphovariant constructs upon propagation beyond the T2 generation. 2-week-old seedlings were imaged using a camera-equipped binocular microscope. (C) TRS120-SαβγA and (D) TRS120-SαβγD seedlings grown on kanamycin had some white sectors due to loss of chlorophyll in the shoot apical meristem and first true leaves; this was presumably due to gene silencing. Scale bars represent 200 µm. (E) Localization patterns of TRS120:GFP phosphovariants in root tip cells of light-grown seedlings. Confocal Scanning Laser Microscopy. PTRS120::TRS120:GFP (Rybak et al., 2014) and phosphovariants thereof. Scale bars represent 5 µm. Wild-type TRS120 resides in the cytosol and at the TGN, it labels endomembrane compartments (white arrowheads) and the cell plate (yellow arrowhead); the phosphovariants have a similar appearance. Sample numbers n = interphase or cytokinetic cells imaged. 44 TRS120-WT, 13 -SαβγA, and 10 -SαβγD root tips were imaged. Source data are available for this figure: SourceData FS11.
Characterization and localization of TRS120:GFP phosphovariants. (A) Western blot depicting protein expression of the phosphovariants TRS120-SαβγA and TRS120-SαβγD in trs120-4/trs120-4. Presence of the GFP-fused phosphovariants was detected with an anti-GFP antibody (upper panel). Shown are the expression levels in different primary transformants for each variant. Loaded protein amounts are shown by the corresponding CBB staining (Coomassie stain, lower panel). Protein sizes are given in kDa on the left. (B) Complementation analysis. The TRS120-SαβγA and TRS120-SαβγD phosphovariants in trs120-4/trs120-4 could rescue the null trs120-4 seedling lethal phenotype (third seedling from the left; white arrow) and did not differ from the wild-type Col-0 control in the T1 and T2 generations. Kanamycin selection was used to select for the presence of the phosphovariant construct. Images taken 13 days after stratification. Scale bar is 1 cm. (C and D) Silencing of the phosphovariant constructs upon propagation beyond the T2 generation. 2-week-old seedlings were imaged using a camera-equipped binocular microscope. (C) TRS120-SαβγA and (D) TRS120-SαβγD seedlings grown on kanamycin had some white sectors due to loss of chlorophyll in the shoot apical meristem and first true leaves; this was presumably due to gene silencing. Scale bars represent 200 µm. (E) Localization patterns of TRS120:GFP phosphovariants in root tip cells of light-grown seedlings. Confocal Scanning Laser Microscopy. PTRS120::TRS120:GFP (Rybak et al., 2014) and phosphovariants thereof. Scale bars represent 5 µm. Wild-type TRS120 resides in the cytosol and at the TGN, it labels endomembrane compartments (white arrowheads) and the cell plate (yellow arrowhead); the phosphovariants have a similar appearance. Sample numbers n = interphase or cytokinetic cells imaged. 44 TRS120-WT, 13 -SαβγA, and 10 -SαβγD root tips were imaged. Source data are available for this figure: SourceData FS11.
TRS120-SαβγA and TRS120-SαβγD have opposite effects on seed germination under osmotic stress
We first analyzed the impact of osmotic stress on the germination frequencies of TRS120-SαβγA and TRS120-SαβγD phosphovariants (Fig. 7, A–C). As compared with the control (Col-0), the TRS120-SαβγA phosphovariant had higher seed germination rates on mannitol (Fig. 7 C). In this respect, TRS120-SαβγA was similar to the ABA-deficient mutant aba2-1, known to be osmotolerant with respect to seed germination (Fig. 7 C; González-Guzmán et al., 2002). In contrast, the TRS120-SαβγD phosphovariant exhibited delayed and reduced germination with lower maximal germination rates, even in the absence of mannitol (Fig. 7, A–C). With respect to its delay in germination, TRS120-SαβγD was similar to the ABA coreceptor higher order mutant hab1-1 abi1-2 pp2ca-1, known to be osmosensitive at germination (Fig. 7 C; Rubio et al., 2009). In summary, the non-phosphorylatable TRS120-SαβγA mutations enhanced whereas the phosphomimetic TRS120-SαβγD mutations reduced germination on mannitol.
Responses of TRS120 phosphovariants to osmotic or additive stress. Phosphovariants of PTRS120::TRS120:GFP (Rybak et al., 2014) were in trs120-4 homozygous backgrounds with the exception of the TRS120-SαβγD variant in A–C, which was in a hemizygous trs120-4/+ background. (A–C) Germination of TRS120 phosphovariants under osmotic stress. Effect of (A) 0 mM, (B) 200 mM, and (C) 400 mM mannitol on seed germination of the TRS120-SαβγA, the TRS120-SαβγD mutants, and the following controls: Col-0 wild type; the ABA deficient mutant aba2-1 and the ABA PP2C coreceptor triple knock-out line hab1-1 abi1-2 pp2ca-1. The non-phosphorylatable TRS120-SαβγA mutation increased seed germination on higher mannitol concentrations (on day 4, we observed 70% germination in TRS120-SαβγA versus 35% germination for the control on 400 mM mannitol, C), whereas the phosphomimetic TRS120-SαβγD decreased seed germination. Shown are the means ± SD of three technical replicates. Similar results were obtained in three independent biological replicates. Stars indicate statistical significance compared with the wild-type Col-0 calculated with a two-tailed Student’s t test (*: P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001). DAS: days after stratification. (D–F) Response quotients (RQ) of (D) the hypocotyl, (E) the root, and (F) the hypocotyl/root ratio of the non-phosphorylatable TRS120-SαβγA and the phosphomimetic TRS120-SαβγD mutants for dark-to-dark with water stress (−0.4 MPa, darkW) adaptation. The divergent hypocotyl and root responses of TRS120-SαβγA and TRS120-SαβγD mutants resulted in a strong hypocotyl/root ratio phenotype under darkW conditions compared with the TRS120-WT control. TRS120-SαβγA showed an enhanced ratio adaption, whereas TRS120-SαβγD mutants had an attenuated ratio response. RQ response quotients are normalized to the rescue mutant TRS120-WT (PTRS120::TRS120:GFP in trs120-4/trs120-4). A value of 1 (vertical red line) corresponds to an identical adaptation to darkW conditions as the rescue mutant. Note that due to the opposite adaptations of the hypocotyl and root under the additive stress conditions (decrease in hypocotyl and increase in root length from dark-to-darkW), the respective thresholds for attenuated and enhanced responses are opposite. Dots represent biological replicates, of which there were a total of four primary transformants per line. Mean RQ values are given on the right. Related to Figs. S12 and S13.
Responses of TRS120 phosphovariants to osmotic or additive stress. Phosphovariants of PTRS120::TRS120:GFP (Rybak et al., 2014) were in trs120-4 homozygous backgrounds with the exception of the TRS120-SαβγD variant in A–C, which was in a hemizygous trs120-4/+ background. (A–C) Germination of TRS120 phosphovariants under osmotic stress. Effect of (A) 0 mM, (B) 200 mM, and (C) 400 mM mannitol on seed germination of the TRS120-SαβγA, the TRS120-SαβγD mutants, and the following controls: Col-0 wild type; the ABA deficient mutant aba2-1 and the ABA PP2C coreceptor triple knock-out line hab1-1 abi1-2 pp2ca-1. The non-phosphorylatable TRS120-SαβγA mutation increased seed germination on higher mannitol concentrations (on day 4, we observed 70% germination in TRS120-SαβγA versus 35% germination for the control on 400 mM mannitol, C), whereas the phosphomimetic TRS120-SαβγD decreased seed germination. Shown are the means ± SD of three technical replicates. Similar results were obtained in three independent biological replicates. Stars indicate statistical significance compared with the wild-type Col-0 calculated with a two-tailed Student’s t test (*: P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001). DAS: days after stratification. (D–F) Response quotients (RQ) of (D) the hypocotyl, (E) the root, and (F) the hypocotyl/root ratio of the non-phosphorylatable TRS120-SαβγA and the phosphomimetic TRS120-SαβγD mutants for dark-to-dark with water stress (−0.4 MPa, darkW) adaptation. The divergent hypocotyl and root responses of TRS120-SαβγA and TRS120-SαβγD mutants resulted in a strong hypocotyl/root ratio phenotype under darkW conditions compared with the TRS120-WT control. TRS120-SαβγA showed an enhanced ratio adaption, whereas TRS120-SαβγD mutants had an attenuated ratio response. RQ response quotients are normalized to the rescue mutant TRS120-WT (PTRS120::TRS120:GFP in trs120-4/trs120-4). A value of 1 (vertical red line) corresponds to an identical adaptation to darkW conditions as the rescue mutant. Note that due to the opposite adaptations of the hypocotyl and root under the additive stress conditions (decrease in hypocotyl and increase in root length from dark-to-darkW), the respective thresholds for attenuated and enhanced responses are opposite. Dots represent biological replicates, of which there were a total of four primary transformants per line. Mean RQ values are given on the right. Related to Figs. S12 and S13.
TRS120 phosphorylation status affects hypocotyl versus root trade-offs under additive stress conditions
In light of the decision phenotypes of bin2 and trappii mutants and of the observation that AtSKs such as BIN2 target TRAPPII, the question arises as to whether shaggy-like kinases regulate differential growth decisions via TRS120 phosphorylation. To test this hypothesis, AtTRS120 phosphovariants homozygous for the null trs120-4 allele and hemizygous for the phosphovariant construct were studied under different environmental or stress conditions. The etiolation response was not impacted by the phosphorylation status of AtTRS120 (Fig. S12). In contrast, TRS120 phosphomutants had phenotypes under additive stress conditions (Fig. 7, D–F and Fig. S13, A–C). TRS120-SαβγA non-phosphorylatable variants exhibited an enhanced root response under darkW conditions (Fig. 7 E) and a strongly enhanced ratio response to water stress in the dark (mean RQratio = 1.49; Fig. 7 F). Conversely, the phosphomimetic TRS120-SαβγD mutants exhibited severely attenuated responses to water stress in the dark (Fig. 7, D–F; mean RQratio = 0.44). Thus, the non-phosphorylatable TRS120-SαβγA and the phosphomimetic TRS120-SαβγD mutations had opposite effects on hypocotyl versus root lengths under water stress in the dark, with TRS120-SαβγA exhibiting an enhanced and TRS120-SαβγD an attenuated ratio response (Fig. 7 F). Under all environmental conditions, the total seedling length of the phosphovariants did not significantly differ from that of the wild-type AtTRS120 construct (Fig. S13 D), which highlights the absence of a growth defect. We conclude that the TRS120 phosphorylation status impacts the seedling’s ability to differentially regulate its hypocotyl and root lengths in response to additive stress conditions. Taken together, the data show opposite impacts of non-phosphorylatable versus phosphomimetic mutations at AtTRS120 AtSK sites in terms of seed germination under osmotic stress and responses to additive stress. This suggests that TRAPPII phosphorylation by shaggy-like kinases mediates adaptive responses to osmotic stress and to light and water availability.
The etiolation response in TRS120 phosphovariants. (A) TRS120-WT (PTRS120::TRS120:GFP in trs120-4/trs120-4) (Rybak et al., 2014). (B) The non-phosphorylatable TRS120 mutant TRS120-SαβγA in trs120-4/trs120-4. (C) The phosphomimetic TRS120 mutant TRS120-SαβγD in trs120-4/trs120-4. Seedlings were grown on ½ MS media under light and dark conditions. All mutants showed a significant adaptation to light versus dark conditions, i.e., increased hypocotyl growth and reduced root growth leading to a higher hypocotyl/root ratio under dark conditions. For each mutant, four biological replicates were analyzed, and the representative line based on the RQ and P values is depicted here. Values are means ± SD of measured seedlings per condition, number (n) of seedlings are given in gray below the graphs. Stars indicate statistical significance with a two-tailed Student’s t test (*****: P < 0.00001). (D–F) Response quotients (RQ) of (D) the hypocotyl, (E) the root, and (F) the hypocotyl/root ratio of the non-phosphorylatable TRS120-SαβγA and the phosphomimetic TRS120-SαβγD mutants for light-to-dark adaptation. RQ response quotients are normalized to the TRS120-WT control, PTRS120::TRS120:GFP in trs120-4/trs120-4. A value of 1 (vertical red line) corresponds to an identical adaptation to dark conditions as the control. TRS120-SαβγA and TRS120-SαβγD phosphovariants did not measurably differ from the control (TRS120-WT) line, with RQ values close to 1 (TRS120-SαβγA: mean RQratio = 0.86; TRS120-SαβγD: mean RQratio = 0.83). Note that due to the opposite adaptations of the hypocotyl and root from light-to-dark conditions (increase in hypocotyl, decrease in root length and therefore increased hypocotyl/root ratio), the respective thresholds for attenuated and enhanced responses are opposite. Dots represent biological replicates, mean RQ values are given on the right. Related to Fig. 7.
The etiolation response in TRS120 phosphovariants. (A) TRS120-WT (PTRS120::TRS120:GFP in trs120-4/trs120-4) (Rybak et al., 2014). (B) The non-phosphorylatable TRS120 mutant TRS120-SαβγA in trs120-4/trs120-4. (C) The phosphomimetic TRS120 mutant TRS120-SαβγD in trs120-4/trs120-4. Seedlings were grown on ½ MS media under light and dark conditions. All mutants showed a significant adaptation to light versus dark conditions, i.e., increased hypocotyl growth and reduced root growth leading to a higher hypocotyl/root ratio under dark conditions. For each mutant, four biological replicates were analyzed, and the representative line based on the RQ and P values is depicted here. Values are means ± SD of measured seedlings per condition, number (n) of seedlings are given in gray below the graphs. Stars indicate statistical significance with a two-tailed Student’s t test (*****: P < 0.00001). (D–F) Response quotients (RQ) of (D) the hypocotyl, (E) the root, and (F) the hypocotyl/root ratio of the non-phosphorylatable TRS120-SαβγA and the phosphomimetic TRS120-SαβγD mutants for light-to-dark adaptation. RQ response quotients are normalized to the TRS120-WT control, PTRS120::TRS120:GFP in trs120-4/trs120-4. A value of 1 (vertical red line) corresponds to an identical adaptation to dark conditions as the control. TRS120-SαβγA and TRS120-SαβγD phosphovariants did not measurably differ from the control (TRS120-WT) line, with RQ values close to 1 (TRS120-SαβγA: mean RQratio = 0.86; TRS120-SαβγD: mean RQratio = 0.83). Note that due to the opposite adaptations of the hypocotyl and root from light-to-dark conditions (increase in hypocotyl, decrease in root length and therefore increased hypocotyl/root ratio), the respective thresholds for attenuated and enhanced responses are opposite. Dots represent biological replicates, mean RQ values are given on the right. Related to Fig. 7.
Role of TRS120 phosphorylation status on adaptive growth responses under additive stress conditions. (A) TRS120-WT (PTRS120::TRS120:GFP in trs120-4/trs120-4) (Rybak et al., 2014). (B) The non-phosphorylatable TRS120-SαβγA in trs120-4/trs120-4. (C) The phosphomimetic TRS120-SαβγD in trs120-4/trs120-4. TRS120-SαβγD mutants had attenuated root and ratio responses under darkW, whereas TRS120-SαβγA mutants showed a slightly enhanced root response. (D) Total seedling lengths of TRS120-WT, TRS120-SαβγA, and TRS120-SαβγD. The hypocotyl length is depicted in green and the root in beige-brown shades with an increasing opacity gradient for the different conditions. The total seedling length did not vary from the TRS120-WT control, highlighting the adaptation defect of TRS120-SαβγD to darkW. Seedlings were grown on ½ MS media under light, dark and dark with −0.4 MPa water stress (darkW) conditions. For each line, four biological replicates were analyzed. Values are means ± SD of measured seedlings per condition, number (n) of seedlings are given in gray below the graphs. A representative line, based on the RQ and P values, is depicted for A–C. For total seedling lengths, means ± SD of four biological replicates are shown. Stars indicate statistical significance with a two-tailed Student’s t test (*: P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001; *****: P < 0.00001). Related to Fig. 7.
Role of TRS120 phosphorylation status on adaptive growth responses under additive stress conditions. (A) TRS120-WT (PTRS120::TRS120:GFP in trs120-4/trs120-4) (Rybak et al., 2014). (B) The non-phosphorylatable TRS120-SαβγA in trs120-4/trs120-4. (C) The phosphomimetic TRS120-SαβγD in trs120-4/trs120-4. TRS120-SαβγD mutants had attenuated root and ratio responses under darkW, whereas TRS120-SαβγA mutants showed a slightly enhanced root response. (D) Total seedling lengths of TRS120-WT, TRS120-SαβγA, and TRS120-SαβγD. The hypocotyl length is depicted in green and the root in beige-brown shades with an increasing opacity gradient for the different conditions. The total seedling length did not vary from the TRS120-WT control, highlighting the adaptation defect of TRS120-SαβγD to darkW. Seedlings were grown on ½ MS media under light, dark and dark with −0.4 MPa water stress (darkW) conditions. For each line, four biological replicates were analyzed. Values are means ± SD of measured seedlings per condition, number (n) of seedlings are given in gray below the graphs. A representative line, based on the RQ and P values, is depicted for A–C. For total seedling lengths, means ± SD of four biological replicates are shown. Stars indicate statistical significance with a two-tailed Student’s t test (*: P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001; *****: P < 0.00001). Related to Fig. 7.
bin2 higher order and trappii conditional mutants exhibit a synergistic genetic interaction with respect to root gravitropism
To further address the physiological significance of the BIN2-TRAPPII interaction in vivo, we deployed higher-order mutant analysis. This approach, however, was challenged by (i) the functional redundancy between BIN2 and its homologues (Vert and Chory, 2006; Yan et al., 2009), (ii) the semi-dominant nature of bin2-1, (iii) the seedling lethality of trappii null alleles, as well as (iv) the pleiotropic phenotypes of both bin2-1 and trappii dwarfs (Li et al., 2001; Thellmann et al., 2010; Ravikumar et al., 2018; Garcia et al., 2020). To address these challenges, we engineered a conditional trs120 knock-down allele, named trs120i. This features an artificial microRNA that targets 5′ AtTRS120/TRAPPC9 sequences, expressed under an estradiol-inducible promoter (Fig. 8 A; Curtis and Grossniklaus, 2003). The trs120i construct was introduced into the wild type (Col-0) and into the bin2-3bil1bil2 triple knock-out mutant. Upon induction, trs120i exhibited a mild and bin2-3bil1bil2 a more pronounced root agravitropism (Fig. 8, B and C). The bin2-3bil1bil2 trs120i higher order mutant had a more than additively enhanced agravitropic response upon induction as compared with trs120i or bin2-3bil1bil2 alone (Fig. 8, B, C, and E). This was evidenced as primary roots growing in all directions, often against gravity at 180° in bin2-3bil1bil2 trs120i (Fig. 8, B and C). We observed synthetic enhancement specifically upon estradiol induction and not in the mock control (Fig. 8 D, compared with Fig. 8 E). This is indicative of a synergistic genetic interaction. Furthermore, it suggests that adaptive growth decisions such as gravitropism are mediated by the BIN2/AtSK–TRAPPII interaction.
Genetic interaction between bin2 higher order and trappii conditional mutants. Seedlings were transferred to estradiol (Est; induced) or EtOH (mock) containing media and grown vertically for 9 days. (A) A conditional trs120 knock-down allele, trs120i. This features an artificial microRNA targeting the 5′ end of the AtTRS120/TRAPPC9 coding sequence, expressed under an estradiol-inducible promoter. LB: left border; RB: right border; G10-90: synthetic constitutive promoter; XVE: encoding a chimeric transcription factor composed of the DNA-binding domain of the bacterial repressor LexA (X), the acidic transactivating domain of VP16 (V), and the regulatory region of the human estrogen receptor (E); T3A: poly(A) addition sequence; HygR: hygromycin resistance marker; OlexA-46: estradiol inducible promoter system with multiple copies of the lexA binding domain. (B) Representative seedling images of Col-0 (wild type), trs120i mutant, bin2-3bil1bil2 and bin2-3bil1bil2 trs120i higher order mutants grown on estradiol. Upon induction, trs120i exhibited a mild phenotype, with a wavy primary root, and bin2-3bil1bil2 a more pronounced root agravitropism. In contrast, bin2-3bil1bil2 trs120i mutants had an enhanced root agravitropism, growing against gravity or in loops (yellow arrow points to a 360° loop in the primary root). Scale bar is 1 cm. (C) Visualization of the primary root growth orientation. Primary root tip angles (excluding lateral roots) of Col-0, trs120i, bin2-3bil1bil2 and bin2-3bil1bil2 trs120i seedlings grown on Est were measured and a frequency count with a bin width of 20° was performed. Frequency plots were generated by using a polar coordinate system. A root tip angle of 0° corresponds to vertical root tip growth in line with the gravitropic vector. Primary roots exhibiting looping (see yellow arrow in B) were scored as −180° due to their growth against gravity. A representative plot for each genotype is shown. (D and E) Quantification of the frequency of agravitropic primary roots upon growth on (D) mock (EtOH) or (E) induced (Est) conditions. Frequency of primary root tips growing against gravity (angles < −90° and >90° in C) was counted per experiment. The bin2-3bil1bil2 trs120i higher order mutant had a more than additively enhanced agravitropic response upon induction as compared with trs120i or bin2-3bil1bil2 alone (E). Note the synthetic enhancement specifically upon estradiol induction and not in the mock control (Fig. 8 D, compared with Fig. 8 E). This is indicative of a synergistic genetic interaction. Mean ± SD bar graphs; **: P ≤ 0.01 two-tailed Student’s t test. n = 2 independent experiments for Col-0 and bin2-3bil1bil2, n = 4 for bin2-3bil1bil2 trs120i, and n = 6 for trs120i with at least 11 seedlings per experiment.
Genetic interaction between bin2 higher order and trappii conditional mutants. Seedlings were transferred to estradiol (Est; induced) or EtOH (mock) containing media and grown vertically for 9 days. (A) A conditional trs120 knock-down allele, trs120i. This features an artificial microRNA targeting the 5′ end of the AtTRS120/TRAPPC9 coding sequence, expressed under an estradiol-inducible promoter. LB: left border; RB: right border; G10-90: synthetic constitutive promoter; XVE: encoding a chimeric transcription factor composed of the DNA-binding domain of the bacterial repressor LexA (X), the acidic transactivating domain of VP16 (V), and the regulatory region of the human estrogen receptor (E); T3A: poly(A) addition sequence; HygR: hygromycin resistance marker; OlexA-46: estradiol inducible promoter system with multiple copies of the lexA binding domain. (B) Representative seedling images of Col-0 (wild type), trs120i mutant, bin2-3bil1bil2 and bin2-3bil1bil2 trs120i higher order mutants grown on estradiol. Upon induction, trs120i exhibited a mild phenotype, with a wavy primary root, and bin2-3bil1bil2 a more pronounced root agravitropism. In contrast, bin2-3bil1bil2 trs120i mutants had an enhanced root agravitropism, growing against gravity or in loops (yellow arrow points to a 360° loop in the primary root). Scale bar is 1 cm. (C) Visualization of the primary root growth orientation. Primary root tip angles (excluding lateral roots) of Col-0, trs120i, bin2-3bil1bil2 and bin2-3bil1bil2 trs120i seedlings grown on Est were measured and a frequency count with a bin width of 20° was performed. Frequency plots were generated by using a polar coordinate system. A root tip angle of 0° corresponds to vertical root tip growth in line with the gravitropic vector. Primary roots exhibiting looping (see yellow arrow in B) were scored as −180° due to their growth against gravity. A representative plot for each genotype is shown. (D and E) Quantification of the frequency of agravitropic primary roots upon growth on (D) mock (EtOH) or (E) induced (Est) conditions. Frequency of primary root tips growing against gravity (angles < −90° and >90° in C) was counted per experiment. The bin2-3bil1bil2 trs120i higher order mutant had a more than additively enhanced agravitropic response upon induction as compared with trs120i or bin2-3bil1bil2 alone (E). Note the synthetic enhancement specifically upon estradiol induction and not in the mock control (Fig. 8 D, compared with Fig. 8 E). This is indicative of a synergistic genetic interaction. Mean ± SD bar graphs; **: P ≤ 0.01 two-tailed Student’s t test. n = 2 independent experiments for Col-0 and bin2-3bil1bil2, n = 4 for bin2-3bil1bil2 trs120i, and n = 6 for trs120i with at least 11 seedlings per experiment.
Discussion
In this study, we explored the role of the TGN in stress responses in Arabidopsis. Our point of entry is the TRAPPII complex, which has been shown to be required for TGN structure and function (Qi et al., 2011; Ravikumar et al., 2018). We performed proteomic and yeast two-hybrid screens and presented several lines of in vitro (Y2H, kinase assays) and in vivo (IP-MS, pharmacological inhibition) evidence that the TRAPPII subunit AtTRS120/TRAPPC9 is the target of AtSK kinases, including BIN2. We document differential phosphorylation of three distinct AtSK/GSK3 sites by three of the four AtSK clades. The phosphorylation status of AtTRS120 impacted seed germination under osmotic stress as well as adaptive responses to additive stress in planta. We show that bin2 and trappii alleles have related phenotypes with respect to an impaired adaptation to additive stress conditions, in this instance, achieved by the simultaneous withdrawal of light and water. Furthermore, bin2 higher order and trappii conditional mutants exhibited a synergistic genetic interaction with respect to root gravitropism.
Like other tropisms, root gravitropism requires organ bending as a result, at least in part, of the differential sorting of PIN transporters (Friml et al., 2002; Ding et al., 2011; Konstantinova et al., 2021). PIN2 polarity has been shown to be abolished in trappii mutants (Qi et al., 2011; Rybak et al., 2014) and to require TGN function (Naramoto et al., 2014; Qi et al., 2011; Rybak et al., 2014; Ravikumar et al., 2018). A concern when assessing the role of the TGN in adaptive or stress responses is that TGN-related mutants typically have pleiotropic phenotypes as a consequence of their impairment in fundamental processes such as secretion, endocytosis, or sorting (Rosquete and Drakakaki, 2018; Ravikumar et al., 2018). To distinguish between primary defects in growth on the one hand and adaptive responses on the other, we monitored trade-offs between hypocotyl and root growth under additive stress conditions (Kalbfuß et al., 2022). We focus on echidna and trappii mutants as these have been shown to impact both the structure and the function of the TGN (Boutté et al., 2013; Gendre et al., 2011, 2013; McFarlane et al., 2013; Qi et al., 2011; Ravikumar et al., 2018). These TGN mutants had hypocotyl and root growth defects, which are characteristic of trafficking mutants (Fig. S9). In addition to their growth defects, trappii trs120-4 mutants had a significant hypocotyl response but in the wrong orientation as compared with the wild type (denoted by red asterisks in Figs. 5, 6, and S10) as well as an insignificant hypocotyl/root ratio response to light and water deprivation (Fig. 5). The trappii phenotypes described in this study are difficult to explain as mere growth defects. Rather, the inability to mediate growth trade-offs in response to additive stress provides a compelling argument for TRAPPII as having a role in adaptive growth decisions.
Conceptually, the optimization of hypocotyl-to-root ratios can be deconstructed into four distinct stages: (i) perception, (ii) signal integration, (iii) decision making, and (iv) the implementation of resulting actions (Kalbfuß et al., 2022). To address the first stage, perception, we looked at, for example, quadruple phyAphyBcry1cry2 photoreceptor mutants; these failed to adjust their organ lengths to light versus dark conditions but had a highly significant response to additive stress (Fig. 5, G and H, Fig. S7 A, Fig. S8, and Fig. S9, G–I; Kalbfuß et al., 2022). The opposite was true for bin2-3bil1bil2 and trappii mutants, which were able to respond to single stress factors but not to additive stress (Figs. 5, 6, and S10; Kalbfuß et al., 2022). Shaggy-like kinases integrate a vast number of signaling pathways (Lv and Li, 2020; Youn and Kim, 2015; Li et al., 2021; Song et al., 2023; Planas-Riverola et al., 2019). Accordingly, we have postulated that the bin2-3bil1bil2 “decision” phenotype under conflict-of-interest scenarios is primarily due to signal integration (Kalbfuß et al., 2022). Prime candidates for the fourth stage, execution or implementation of the action, are echidna mutants, which are impaired in the formation of secretory vesicles and cell elongation (Boutté et al., 2013; Gendre et al., 2013; McFarlane et al., 2013); these had highly significant adaptive responses —in the correct orientation—despite their growth defects (Fig. 5 E). The question arises as to how to categorize trappii mutants in the above conceptual framework. Despite having abnormally short hypocotyls or roots, trappii mutants still exhibited differential length increase depending on light availability (Fig. S9). This shows that they are able to perceive and respond to light.
If TRAPPII is primarily involved neither in perception nor in execution, this would place it at the signal integration and/or decision-making steps of adaptive growth decisions. The TRAPPII interactome is vast and complex, with a surprising number of signaling components implicated in responses to abiotic cues (Figs. 1 and S1). This suggests a role for TRAPPII in signal integration. Conversely, the BIN2 signaling network includes secretion, endocytosis, autophagy, TGN, endoplasmic reticulum (ER), cell wall, and cytoskeleton (Kim et al., 2023). Thus, there is a considerable compartmental overlap between the BIN2 network and the TRAPPII interactome. Nonetheless, TRAPPII was not reported among the 482 members of the BIN2 signaling network identified by proximity labeling (Kim et al., 2023). The discrepancy could be due to the different methods used: yeast two-hybrid and proteomics in this study versus proximity labeling and phosphoproteomics in Kim et al. (2023). However, a multiomics approach identified AtTRS120-S971 (encompassed by our identified β site AtTRS120-S971:S973:S974:S975; Fig. 2 C) as being differentially phosphorylated in response to brassinolide treatment (Clark et al., 2021). This provides an additional line of in vivo evidence for a BIN2-AtTRS120 interaction and, consistently with our PPZ experiment (Fig. S5 D), suggests that TRAPPII phosphorylation is, at least in part, brassinosteroid-regulated.
To explore the biological significance of the AtSK–TRAPPII interaction, we probed the phenotypes of TRAPPII phosphovariants. We found that these had an impact on hypocotyl versus root growth trade-offs in response to additive stress (Fig. 7, D–F). Furthermore, the non-phosphorylatable TRS120-SαβγA variant had an enhanced root adaptation whereas the phosphomimetic variant decreased root growth under additive stress (Fig. 7, D–F), which is consistent with the negative regulation of growth by BIN2 and other AtSKs. We have previously described plant-specific domains or subunits predicted to be at the dimer interface of the Arabidopsis TRAPPII complex (marked in green in Fig. 2 D; Garcia et al., 2020; Kalde et al., 2019). Interestingly, BIN2 interacts with the plant-specific C-terminal domain of AtTRS120, while MAP65-3 interacts with plant-specific C-terminal domains of the TRAPPII-specific subunits CLUB/AtTRS130 and AtTRS120 (Steiner et al., 2016). This presents intriguing implications regarding the potential role of the AtSK–TRAPPII module in meeting the unique demands of endomembrane traffic in plants. Plant and animal cells differ in numerous ways, many of which can be attributed to the presence of the plant cell wall. Novel or expanded families of vesicle-trafficking genes are implicated in cell wall assembly (Assaad, 2001). Cell wall deposition and remodeling underly any growth response in plants. Growth in the face of challenging, restrictive environments is a distinctive survival strategy unique to plants (Assaad, 2001). In this study, we look at the tight regulation of differential growth that enables plants to thrive under multiple stress conditions, in the absence of a carbon or energy source.
The observation that the trappii decision phenotype was not shared by echidna mutants raises the question as to what facet of TGN function is required for responses to additive stress. ECHIDNA and TRAPPII have been shown to have overlapping roles in basal TGN functions such as secretion (Ravikumar et al., 2018). In addition, ECHIDNA regulates ER stress and immunity whereas TRAPPII has roles in cytokinesis and in the establishment of cell polarity that appear to be independent of ECHIDNA (Liu et al., 2023; Ravikumar et al., 2018). In the literature, there are a few examples of null mutants that are associated with the TGN and that have a positive influence on growth. One such example is a null allele of a core TRAPPII subunit that enhances root growth also in the absence of stress in maize (Zhao et al., 2023). The TRAPPII complex has two distinct possible molecular functions. First, it has been postulated to act as a multisubunit tethering complex, even though direct evidence is lacking (Ravikumar et al., 2017; Brunet and Sacher, 2014; Kim et al., 2016; Pinar et al., 2019). Second, it has been shown to act as a Rab GTPase guanine nucleotide exchange factor (Rab-GEF; Cai et al., 2005; Morozova et al., 2006; Pinar et al., 2015; Thomas and Fromme, 2016; Riedel et al., 2018). Rab-GEFs act as central cellular switches that can activate Rab GTPase cascades, which are in turn crucial for the development of polarity and directional growth in plants (Elliott et al., 2020). Indirect in vivo evidence suggests that Arabidopsis TRAPPII acts as a GEF for Rab-A GTPases, orthologues of the RAB11/Ypt31 families known to be activated by fungal and metazoan TRAPPII (Qi and Zheng, 2011; Kalde et al., 2019). In Arabidopsis, Rab-A GTPases are TGN-associated and comprise a highly expanded class with 26 members (Kalde et al., 2019; Elliott et al., 2020). In contrast to ECHIDNA, which is required for the biogenesis of secretory vesicles and for growth generically, as a putative, Rab-A GEF TRAPPII has the ability to mediate all the highly diverse facets of TGN function. In a judgment-decision model for plant behavior, judgment is a composite of discrimination, assessment, recognition, and categorization (Karban and Orrock, 2018). In this context, it is noteworthy that the most expanded Rab GTPase family is TGN-associated, suggestive of a tremendous degree of discrimination, specialization, and subcompartmentalization at the TGN. Atomic structures of yeast and metazoan TRAPPII depict a conserved triangular structure around the central active site chamber in which TRS120/TRAPPC9 and TRS130/TRAPPC10 form tongs that hold the core complex in place (Galindo et al., 2021; Mi et al., 2022; Bagde and Fromme, 2022). In a cross-kingdom structural alignment, AtTRS120 and CLUB/AtTRS130 aligned along their yeast orthologues in the same overall structure (Fig. 3). In yeast, TRS120/TRAPPC9 has been proposed to comprise a lid that encloses the active site chamber of the TRAPPII GEF (Bagde and Fromme, 2022). Interestingly, the β and γ phosphorylation sites we have identified in AtTRS120/TRAPPC9 face the active site chamber, including the RAB11/Rab-A binding pocket, proposed by Mi et al. (2022) and Bagde and Fromme (2022) (Fig. 3 A). It is, therefore, tempting to speculate that the phosphorylation status of AtTRS120/TRAPPC9 modulates the specificity of the putative GEF activity of Arabidopsis TRAPPII (Kalde et al., 2019).
Our study highlights the possible relevance of the AtSKs-TRAPPII interaction for adaptive growth decisions (Fig. 9). Shaggy-like kinases such as BIN2 integrate a vast number of signaling pathways and, together with receptor complexes at the cell surface, comprise a surveillance system fine-tuned to both biotic and abiotic cues (Lv and Li, 2020; Planas-Riverola et al., 2019; Youn and Kim, 2015; Li et al., 2021; Song et al., 2023). Via the cytosol, the AtSKs would then transmit this information by mediating the phosphorylation status of the TRAPPII complex. TRAPPII would, in turn, mediate TGN function. As an early endosome, the TGN is a central hub in the flow of information to and from the cell surface. It is also intimately connected to the late endosome (or the pre-vacuolar compartment) and to the Golgi, which provides complex polysaccharides as building blocks for the deposition of new cell walls. Thus, the AtSK–TRAPPII interaction would integrate all levels of cellular organization. We posit that signal integration and decision-making occur at the AtSK–TRAPPII interface and that, downstream of TRAPPII, Rab GTPase cascades are implicated in implementing decisions reached at the AtSK–TRAPPII module (Fig. 9). Sorting and trafficking decisions at the TGN would enable plants to respond to developmental or environmental signals via differential growth or various forms of movement.
Flow diagram suggesting how BIN2 might mediate plant adaptive responses by phosphorylating the TRAPPII complex. Shaggy-like kinases such as BIN2 integrate a vast number of signaling pathways and, together with receptor complexes at the cell surface, comprise a surveillance system fine-tuned to both biotic and abiotic cues (see text for further detail). BIN2 resides in the plasma membrane (PM) and in the cytosol and TRAPPII resides at the cytosol and in the TGN (Vert and Chory, 2006; Qi et al., 2011; Naramoto et al., 2014; Rybak et al., 2014; Ravikumar et al., 2018). We propose that, in the cytosol, the BIN2-TRAPPII interaction serves in the relay of information from the PM to the TGN. We posit that signal integration and decision-making occur at the AtSK–TRAPPII interface. A surface view of the AtTRAPPII complex with AtTRS120 GSK3 phosphorylation sites facing the predicted active site chamber is depicted as AlphaFold structures of AtTRS120/TRAPPC9 and CLUB/AtTRS130/TRAPPC10 mapped onto the cryo-EM-generated structures of yeast TRAPPII (Mi et al., 2022; see Fig. 3 for a cross-kingdom structural alignment). Downstream of TRAPPII, Rab GTPase cascades are postulated to be implicated in implementing decisions reached by the AtSK–TRAPPII module. We speculate that the phosphorylation status of AtTRS120 modulates the specificity of the putative TRAPPPII GEF activity, thereby differentially activating distinct Rab GTPase cascades. In the lower panel, we show one example of an adaptive growth decision in 10-day-old seedlings (Col-0 wild type). Bar plots depict hypocotyl (green) versus root (beige-brown) lengths. Light: Light-grown seedling with a short hypocotyl and a long root. Dark: A seedling germinated in the dark with a long hypocotyl and a short root. DarkW: Water deprivation in the dark; the hypocotyl is shorter and the root longer than under dark conditions. The dotted green arrow (dark, darkW) represents the incentive for hypocotyl elongation in search of light and the brown arrow (darkW) the incentive for root growth in search of water. Thus, darkW is a “conflict-of-interest” scenario in which hypocotyl and root growth have competing interests (Kalbfuß et al., 2022). Figure generated with https://BioRender.com.
Flow diagram suggesting how BIN2 might mediate plant adaptive responses by phosphorylating the TRAPPII complex. Shaggy-like kinases such as BIN2 integrate a vast number of signaling pathways and, together with receptor complexes at the cell surface, comprise a surveillance system fine-tuned to both biotic and abiotic cues (see text for further detail). BIN2 resides in the plasma membrane (PM) and in the cytosol and TRAPPII resides at the cytosol and in the TGN (Vert and Chory, 2006; Qi et al., 2011; Naramoto et al., 2014; Rybak et al., 2014; Ravikumar et al., 2018). We propose that, in the cytosol, the BIN2-TRAPPII interaction serves in the relay of information from the PM to the TGN. We posit that signal integration and decision-making occur at the AtSK–TRAPPII interface. A surface view of the AtTRAPPII complex with AtTRS120 GSK3 phosphorylation sites facing the predicted active site chamber is depicted as AlphaFold structures of AtTRS120/TRAPPC9 and CLUB/AtTRS130/TRAPPC10 mapped onto the cryo-EM-generated structures of yeast TRAPPII (Mi et al., 2022; see Fig. 3 for a cross-kingdom structural alignment). Downstream of TRAPPII, Rab GTPase cascades are postulated to be implicated in implementing decisions reached by the AtSK–TRAPPII module. We speculate that the phosphorylation status of AtTRS120 modulates the specificity of the putative TRAPPPII GEF activity, thereby differentially activating distinct Rab GTPase cascades. In the lower panel, we show one example of an adaptive growth decision in 10-day-old seedlings (Col-0 wild type). Bar plots depict hypocotyl (green) versus root (beige-brown) lengths. Light: Light-grown seedling with a short hypocotyl and a long root. Dark: A seedling germinated in the dark with a long hypocotyl and a short root. DarkW: Water deprivation in the dark; the hypocotyl is shorter and the root longer than under dark conditions. The dotted green arrow (dark, darkW) represents the incentive for hypocotyl elongation in search of light and the brown arrow (darkW) the incentive for root growth in search of water. Thus, darkW is a “conflict-of-interest” scenario in which hypocotyl and root growth have competing interests (Kalbfuß et al., 2022). Figure generated with https://BioRender.com.
Plants exhibit plasticity in their growth, meaning they can adjust their growth patterns in response to environmental cues such as light, temperature, and nutrient availability. It has been argued that plant phenotypic plasticity “is the result of signal integration—a process that requires cell–cell communication, and that results in adaptive forms of movement not to be interpreted as automatic and programmed” (Garzón and Keijzer, 2011). Movement in plants involving organ bending requires the polar distribution of morphogens such as auxin. This, in turn, requires the polarized localization of auxin transporters such as PINs. The Arabidopsis TRAPPII complex has been shown to play a pivotal role in a variety of sorting decisions including the polar localization of PIN2 proteins (Qi et al., 2011; Rybak et al., 2014; Ravikumar et al., 2018). This study presents evidence that AtSK–TRAPPII interactions regulate plant adaptation. Whether or not different AtSKs have distinct roles in TRAPPII phosphorylation could not be resolved due to high sequence similarity between AtSK homologs and some experimental limitations. Validation will be required to address whether the Arabidopsis TRAPPII complex shares the conserved triangular structure with yeast and metazoan TRAPPII, as suggested by extensive pair-wise Y2H tests and by AlphaFold predicted structures of individual subunits (Kalde et al., 2019; this study). How environmental or developmental cues regulate the cargo composition and sorting of TGN vesicles via the AtSK–TRAPPII module remains unclear. Future experiments will explore the impact of the TRAPPII phosphorylation status on protein sorting, cell polarity, and the Rab specificity of TRAPPII as a putative GEF. Furthermore, downstream components that govern physiological changes in response to TRAPPII phosphorylation will need to be determined. The presence of the TOR kinase in the TRAPPII interactome (Fig. 1) is intriguing, as TOR integrates information about nutrient and energy availability, and has recently been shown to regulate actin dynamics as a function of ATP levels (Dai et al., 2022); it is tempting to speculate that TOR could provide information on nutrient and energy levels to the AtSK–TRAPPII decision module. In plants, the trans-Golgi network (TGN) plays a critical role in the relay of information between the cell surface and intracellular compartments. An AtSK–TRAPPII interaction would instruct the TGN, a central and highly discriminate cellular hub, as to how to mobilize and allocate resources to optimize survival under limiting or adverse conditions.
Materials and methods
Lines and growth conditions
All the mutant lines used in this study are listed in Table S2. Seedling-lethal mutants were propagated as hetero- or hemizygotes. Insertion lines were selected via the TAIR and NASC websites (Swarbreck et al., 2008). Plants were grown in the greenhouse under controlled temperature conditions and with supplemental light, or under controlled growth chamber conditions at the TUMmesa ecotron (16/8 h photoperiod at 180 μmol m−2s−1). Seeds were surface sterilized, stratified at 4°C for 2 days, and plated on ½ MS medium supplemented with B5 vitamins (G1019; Sigma-Aldrich). For confocal microscopy, the media was additionally supplemented with 1% sucrose. Plates were incubated at 22°C in constant light (80 μmol m−2s−1). The root tips of 5-day-old plate-grown seedlings were used for confocal microscopy. 7-day-old plate-grown seedlings were used for coimmunoprecipitation.
Coimmunoprecipitation (IP)
For CLUB/AtTRS130:GFP, we used 3 g of inflorescences per IP experiment. For TRS120:GFP, 3 g of light-grown seedlings were harvested on day 7. For bikinin treatment, seedlings were treated for 30 min with 25 µM bikinin at day 7. For PPZ treatment, 5-day-old seedlings were treated with 2 µM PPZ for 48 h; respective mock treatments were conducted in parallel.
Coimmunoprecipitation experiments were carried out as described previously (Rybak et al., 2014). Briefly, seedling or inflorescence lysates were incubated with GFP-trap beads (gta, Chromotek). After washing away all non-binding proteins, 70 μl 2× NuPAGE LDS + 25 mM dithiothreitol (DTT) buffer (Thermo Fisher Scientific) was added and boiled at 70°C for 10 min to denature the bait and all interaction partners.
In-gel trypsin digestion was performed according to standard procedures (Shevchenko et al., 2006). Briefly, the samples were run on a NuPAGE 4–12% Bis-Tris Protein Gel (Thermo Fisher Scientific) for 3 min. Subsequently, the still not size-separated single protein band per sample was cut out of the gel, reduced (50 mM DTT), alkylated (55 mM chloroacetamide), and digested overnight with trypsin (Trypsin Gold, Promega). The resulting peptides were analyzed by mass spectrometry. Detailed information on the mass-spectrometric data acquisition and analysis is provided in the respective LC-MS/MS sections below. See data availability for access to the raw data.
Molecular techniques and site-directed mutagenesis (SDM)
Standard molecular techniques were used for subcloning (Sambrook et al., 1989). For subcloning for expression in Escherichia coli (E. coli) or yeast, we used cDNA clones developed by the plant genome project of RIKEN Genomic Sciences Center (Seki et al., 1998, 2002).
Non-phosphorylatable and phosphomimetic TRS120 phosphosite variants (SαA, SβA, SγA, SαβA, SαγA, SβγA, SαβγA and SαD, SβD, SγD, SαβD, and SαβγD; Table S3) were generated using a DpnI-mediated Site-Directed Mutagenesis protocol and the GATEWAY cloning system. Briefly, site-directed mutations were introduced into the template construct via polymerase chain reaction using mutagenic primers with the desired mutations (see Table S3 for substituted amino acids and used primers) and the KOD Hot Start DNA Polymerase (Novagen) for strand extension. Subsequently, the methylated non-mutated DNA template was digested with the DpnI endonuclease (Thermo Fisher Scientific). Mutated vectors were transformed in E. coli DH5α for nick repair and amplification of the plasmids. After subsequent purification, the constructs were sequenced to ensure correct mutagenesis. For the mutation of two or three phosphorylation sites, sequential mutagenesis was carried out using already mutated vectors as templates.
For Y2H assays, mutations of phosphorylation sites were introduced into TRS120-T2 cDNA sequences (spanning amino acids 499–1187; Rybak et al., 2014) fused to GAL4-AD (pAD-GAL4). N-terminal GST-fused TRS120-T2 phosphovariants (pDEST15) were used for in vitro kinase assays. For in planta experiments (confocal imaging, stress assays, protein expression, etc.) the genomic construct PTRS120::TRS120:GFP in the pCAMBIA2300 plasmid (Rybak et al., 2014) was used as an SDM template. The final phosphovariant constructs were transformed into the Agrobacterium tumefaciens strain GV3101 (pMP90) and introduced into the Arabidopsis hemizygous null trs120-4 background using the floral dip method (Clough and Bent, 1998). Transgenic lines were selected on ½ MS medium supplemented with 50 µg/ml kanamycin.
Generation of inducible trs120 knock-down lines
To generate an inducible trs120 knock-down mutant, named trs120i, an artificial microRNA targeting the 5′ end of AtTRS120 was designed with the Web MicroRNA Designer 3 (Schwab et al., 2006). The designed amiRNA sequence (5′-TATAACTCTTACAAGCGGCAT-3′) was introduced in the miR319a precursor and synthesized as a gene strand (Eurofins) with attached attB sites for GATEWAY compatibility (see Table S4 for full gene strand sequence). The TRS120 amiRNA precursor was first cloned into the pDONR207 entry vector and subsequently into the estradiol inducible pMDC7 vector (Curtis and Grossniklaus, 2003) using Gateway cloning. The construct was introduced into the A. tumefaciens strain GV3101 (pMP90) and inserted into Col-0 wild-type and bin2-3bil1bil2 mutant plants using the floral dip method (Table S2; Clough and Bent, 1998). Transgenic lines were selected on ½ MS medium supplemented with 20 µg/ml hygromycin B.
Yeast two-hybrid (Y2H)
Y2H pairwise tests were performed as described in Altmann et al. (2018). Briefly, open reading frames (ORFs) encoding CLUB/AtTRS130 truncations (C2, C3), TRS120 truncations (T1, T3), and BIN2 were transferred by Gateway cloning into the GAL4 DNA-binding domain (DB) encoding Y2H vector pDEST-pPC97, and subsequently transformed into the yeast strain Y8930. These constructs were screened by yeast mating against TRAPPII subunits and truncations thereof (CLUB-C1, -C2, -C3 and TRS120-T1, -T2, -T3) or the TRS120-T2 truncation and its phosphomutants TRS120-T2 SαD, SβD, SγD, SαβD, and SαβγD fused to the GAL4 activation domain (AD) in the yeast strain Y8800. The interaction was assayed by growth on selective plates using the HIS3 reporter and using 1 mM 3-Amino-1,2,4-triazole (3-AT) to suppress background growth. All candidate interactions were verified by pairwise one-on-one mating in four independent experiments. Only pairs scoring positives in all four assays were considered as bona fide interaction partners. The use of low-copy plasmids, weak promoters, the counter-selectable marker cyh2S on the AD-Y plasmid as well as semiquantitative scoring of quadruplicate tests has been shown to reliably eliminate experimental artifacts and hence false-positives. With the exception of the CLUB-C1 truncation, all TRAPPII truncations and catalytic core subunits used in pair-wise tests yielded at least one positive interaction (see also Kalde et al., 2019; Garcia et al., 2020), and this was used as an internal positive control for the interpretation of negative interaction data.
In vitro kinase assays
Glutathione S-transferase (GST):BIN2 (Li and Nam, 2002) and GST:TRS120-T2 WT or phosphovariants were expressed in E. coli (Rosetta-gami strain; 71351-M; Novagen) under constant shaking for 20 h at 25°C or 20 h at 18°C, respectively. The expressed proteins were affinity-purified with GST-tags. After sonication of the samples in 1× PBS, 1 mM PMSF, 1 mg/ml lysozyme, and 1% Triton X-100, the bacterial cell rests were centrifuged for 30 min at 16,000 g. Supernatants were incubated for 2 h with glutathione Sepharose 4 Fast Flow Beads (GE Healthcare) while rotating. After washing the samples five times, the GST-tagged proteins were eluted with 10 mM glutathione and concentrated by ultrafiltration.
In vitro kinase assays with radiograph readout were performed as described by Kim et al. (2012), with some adaptations. In short, ∼0.1 µg GST:BIN2 and ∼0.5 µg of GST:TRS120-T2 WT or phosphovariant were incubated in 20 mM Tris pH 7.5, 1 mM MgCl2, 100 mM NaCl, 1 mM DTT, 100 µM ATP, and 10 µCi ATP[γ-32P] for 3 h at 22°C in motion. The kinase reaction was stopped by adding 2× SDS sample buffer and by boiling the samples for 10 min. Protein phosphorylation was analyzed by SDS-PAGE followed by autoradiography acquired on a Typhoon Trio imager (GE Healthcare) or a Personal Molecular Imager (PMI) system (Bio-Rad). For quantification, mean gray values of phosphosignal bands were measured using Fiji – ImageJ, and the background signal was subtracted. Phosphosignals were normalized to the protein amount, and relative intensities to the non-mutated TRS120-T2 wild-type control were calculated.
In vitro kinase assays with mass-spectrometry readout were performed to determine specific phosphorylation sites. For each reaction, 10 µg of the substrate (TRS120-T2) and different dilutions of the kinase (undiluted, 1:1, 1:5, 1:10, 1:20, or 1:100 as indicated in the figure panels) were incubated for 0, 15, 30, 120, 240, and 360 min in a kinase buffer (20 mM Tris HCl pH 7.8, 100 mM NaCl, 1 mM MgCl2, 1 mM DTT, 1 mM ATP). For the negative controls, one sample with the highest kinase concentration and the longest incubation time was incubated in a kinase buffer without ATP. For the kinase-dead control, the kinase was heat-inactivated prior to the incubation with its substrate. To stop the reaction, samples were heated at 95°C for 5 min. Samples were prepared for mass spectrometry by reduction with 10 mM dithiothreitol (DTT) (30 min at 30°C) and alkylation with 55 mM chloracetamide (CAA) in the dark (30 min at 25°C). Afterward, the samples were diluted four-fold in 50 mM NH4HCO3 and proteolytically digested with trypsin (1 h at 30°C, Trypsin Gold Mass Spectrometry Grade, Promega) in a ratio of 1:100, i.e., 10 µg protein sample were digested with 0.1 µg trypsin. After the 1 h trypsin incubation time, the same amount of trypsin was added a second time. After 16 h of incubation, the enzymatic reaction was stopped with 1% formic acid (FA). Afterward, stage tip purification was performed. To this end, the pH of the samples was measured (pH < 3) with pH strips (MColorpHast; Merck). The in-house built C18 tips (three disks, ø 1.5 mm, C18 material, 3 M Empore) were equilibrated consecutively with 250 μl 100% ACN, 250 μl elution solution (40% ACN, 0.1% FA) and 250 μl washing solution (2% ACN, 0.1% FA) at 1,500 g. The sample was loaded onto the column (5 min at 500 g) and desalted with three washing steps (washing buffer: 2% ACN, 0.1% FA; 2 min at 1,500 g, 250 μl). Finally, the peptides were eluted with two times 40 μl elution solution (40% ACN, 0.1% FA) for 2 min at 500 g. The solvent of all samples was completely subtracted in a centrifugal evaporator (Centrivap Cold Trap -50; Labconco), freshly suspended before MS measurement in washing solution (2% ACN, 0.1% FA), and ∼0.25 µg of the digest was injected into the mass spectrometer per measurement.
LC-MS/MS data acquisition for in vitro kinase assays and coimmunoprecipitations
Generated peptides were analyzed on a Dionex Ultimate 3000 RSLCnano system coupled to a Q-Exactive HF-X mass spectrometer (Thermo Fisher Scientific). Peptides were delivered to a trap column (ReproSil-pur C18-AQ, 5 μm, Dr. Maisch, 20 mm × 75 μm, self-packed) at a flow rate of 5 μl/min in HPLC grade water with 0.1% formic acid. After 10 min of loading, peptides were transferred to an analytical column (ReproSil Gold C18-AQ, 3 μm, Dr. Maisch, 450 mm × 75 μm, self-packed) and separated using a linear gradient (50 min for coimmunoprecipitation samples and 30 min for kinase assay samples) from 4% to 32% of solvent B (0.1% formic acid in acetonitrile and 5% (vol/vol) DMSO) at 300 nl/min flow rate. Both nanoLC solvents (solvent A: 0.1% formic acid in HPLC grade water and 5% (vol/vol) DMSO) contained 5% DMSO to boost MS intensity. The Q-Exactive HF-X mass spectrometer was operated in a data-dependent acquisition (DDA) and positive ionization mode. MS1 spectra (360–1,300 m/z) were recorded at a resolution of 60,000 using an automatic gain control (AGC) target value of 3e6 and a maximum injection time (maxIT) of 45 ms. Up to 18 peptide precursors were selected for fragmentation in the case of the full proteome analyses. Only precursors with a charge state 2–6 were selected and dynamic exclusion of 25 s was enabled. Peptide fragmentation was performed using higher energy collision-induced dissociation (HCD) and a normalized collision energy (NCE) of 26%. The precursor isolation window width was set to 1.3 m/z. MS2 resolution was 15,000 with automatic gain control (AGC) target value of 1e5 and maximum injection time (maxIT) of 25 ms.
LC-MS/MS data analysis for coimmunoprecipitations
For AtTRS120 coimmunoprecipitations, peptide identification and quantification were performed using the software MaxQuant (version 1.6.1.0) (Cox and Mann, 2008; Tyanova et al., 2016a) with its built-in search engine Andromeda (Cox et al., 2011). MS raw data were searched against an Arabidopsis thaliana reference database (Araport, updated 2016-06) supplemented with common contaminants (built-in option in MaxQuant). Carbamidomethylated cysteine was used as a fixed modification; variable modifications included oxidation of methionine and N-terminal protein acetylation. Trypsin/P was specified as a proteolytic enzyme with up to two missed cleavage sites. Label-free quantification (Cox et al., 2014), match-between-runs intensity-based absolute quantification (iBAQ), and label-free quantification (LFQ) options were enabled. Precursor tolerance was set to 4.5 ppm, and fragment ion tolerance to 20 ppm. Results were adjusted to 1% false discovery rate (FDR) on peptide spectrum match (PSM) level and protein level employing a target-decoy approach using reversed protein sequences.
Perseus (Tyanova et al., 2016b) and Python with its packages matplotlib (Hunter, 2007), pandas (McKinney, 2010), and seaborn (Waskom, 2021) were used for statistical data analysis. Normalization was performed by median centering. LFQ intensities were log2-transformed. Protein groups identified only in a single sample were removed from the analysis. Missing values were replaced from a normal distribution (width = 0.3, down shift = 1.8). Protein fold changes and their significance were computed via a two-sample t test. A fold change of >3 and a P value <0.05 was regarded as significant.
For CLUB/AtTRS130, IP-MS data were analyzed following the method used in Kalde et al. (2019). In short, raw MS files were loaded into the MaxQuant software (version 1.5.7.4; Cox and Mann, 2008) and searched against an A. thaliana RefSeq database (Araport11_genes.201606.pep.fasta). Trypsin was specified as a proteolytic enzyme and up to two missed cleavages were allowed. Protein identifications were filtered at a 1% protein false discovery rate on PSM and protein levels. In downstream analysis, we used the intensity-based absolute quantification (iBAQ) (Schwanhäusser et al., 2011), whose values were calculated using the implemented iBAQ algorithm in the MaxQuant software. Before statistical analysis, iBAQ intensities were normalized according to the iBAQ of the bait protein used in coimmunoprecipitation, and missing values were replaced by a constant (1,000). Then the iBAQ intensities were log2-transformed. The protein abundance between control and IP samples was compared using a t test to evaluate the statistical significance. Ratios of protein abundance between control and IP were considered high if larger than 8 in the log2 scale, and intermediate if in the 5–8 range (log2 scale).
LC-MS/MS phosphopeptide analysis
For a targeted analysis of the AtTRS120 phosphostatus in kinase assays (Fig. 4 B and Fig. S5 B) and in the bikinin and PPZ data sets (Fig. S5 D), MS1 chromatograms from selected phosphopeptides were extracted and analyzed using the Skyline software (MacLean et al., 2010). Peak integration, interferences, and integration boundaries were reviewed manually for all precursors. For the bikinin and PPZ treatments, a two-tailed Student’s t test was performed to determine statistical significance. For the kinase assay, raw intensities as exported from Skyline were plotted.
Light and electron microscopy
For scanning electron microscopy, a Zeiss (LEO) VP 438 microscope was operated at 15 kV. 10-day-old seedlings from the differential growth decision assay were placed onto stubs and examined immediately in low vacuum. Confocal microscopy imaging was performed at controlled room temperature (22°C) using an Olympus (https://www.olympus-ims.com) Fluoview 1000 confocal laser scanning microscope (CSLM) with a 40× 0.9 NA or a 60× 1.2 NA water immersion objective and a Leica (https://www.leica-microsystems.com) TCS SP8 X Hyvolution CSLM with a 40× 1.1 NA or 63× 1.2 NA water immersion objective. Imaging data were acquired using LAS X software (Leica) and FV10-ASW software (Olympus). Images taken with the Leica SP8 microscope were deconvolved using the built-in Huygens Scientific deconvolution software (https://www.leica-microsystems.com) operated in 2D. GFP fluorescent proteins were imaged with 488 nm excitation and 500–550 nm emission. For consistency, we selected cortical root tip cells at a height of 6–22 cells above the quiescent center in the root apical meristem for localization analysis. For cellular root parameters, 10-day-old seedlings from the differential growth decision assay were stained with modified pseudo-Schiff propidium iodide staining (Truernit et al., 2008). The excitation laser was set to 488 nm. The emission was detected at 520 nm. The junction between the meristematic and elongation zones was determined by marking the first cell in a single cortex cell file that was double the length of the previous cell (González-García et al., 2011). For seedling images, a Leica S APO stereo microscope with a Leica MC170 HD camera was used.
Differential growth decision assay
The differential growth decision experiment was performed as described by Kalbfuß et al. (2022). In brief, seeds were surface-sterilized using a brief 80% ethanol rinse followed by 15 min incubation in sterilization buffer (0.01% SDS, 3% NaOCl). After five washes in mQ water, seeds were resuspended in 0.15% agar and stratified in the dark at 4°C for 7 days to break seed dormancy. Afterward, seeds were plated on squared plates with exactly 45 ml ½ MS medium supplemented with B5 Vitamins (Sigma-Aldrich). Culture media for TRS120 phosphomutants was additionally supplemented with 50 μg/ml kanamycin to select for transgenic seedlings. For −0.4 MPa water deficit, media plates were infused with sterile-filtrated 45 ml 2× PEG-6000 (Merck group; dissolved in liquid ½ MS media) for exactly 24 h at RT, and afterward the PEG-solution was completely decanted. Seeds were plated at the interface between the culture media and sterilized plastic strips on the media such that, upon germination, only the root touched the agar. For water stress, seeds in each biological replicate were sown onto two plates for technical replicates, which were pooled for analysis. After plating, plates were sealed with breathable tape. Plates for dark conditions were wrapped with two layers of thick aluminum foil. All plates were incubated for 10 days at 22°C with permanent light (80 μmol m−2s−1). Note that the plates were negatively inclined by 4° to promote root growth on the media surface rather than in the agar.
After 10 days of incubation, seedlings were either used for organ length determination or for assessing cellular hypocotyl and root parameters. For organ length measurements, seedlings were transferred onto cold 1.2% agar plates, scanned at 1,200 ppi, and saved as tiff files. Hypocotyl and root lengths were analyzed with Fiji - ImageJ using the free-hand tool. The hypocotyl/root ratio was calculated as hypocotyl length divided by root length.
Because the hypocotyl and root have opposite responses to our additive stress conditions (hypocotyl length decreases but root length increases in response to water stress in the dark, as shown in Fig. 5) the RQs move in opposite directions.
RQhypocotyl and RQroot as well as light versus dark responses were computed in a similar fashion (Figs. S8, S9, and S12). These normalized organ and ratio adjustments were referred to as response quotients (RQ) to light-to-dark or dark-to-darkW conditions. The mean RQratio of at least three biological replicates (i.e., the seed stocks from different mother plants) is shown. Please see Table S5 for thresholds for attenuated, normal, versus enhanced organ or ratio responses. For volcano plots we plotted the mean RQratio on the X axis and the median Pratio value on the Y axis. Organ and ratio adjustments for light-to-dark comparisons were computed as organ length (or ratio) in the light divided by organ length (or ratio) in the dark. P values were computed with the two-tailed Student’s t test. Responses were considered to be insignificant for P values ≥0.05 and attenuated for P values ≥0.00001. The median P value for at least three replicates is shown in the volcano plots.
Germination assay under osmotic stress
For germination curve experiments, surface-sterilized seeds were stratified in the dark at 4°C for 2 days. Seeds were plated on ½ MS media supplemented with 0.05% B5 Vitamins (Sigma-Aldrich) and either 0, 200, or 400 mM mannitol (Millipore). Approximately 100 seeds were plated per condition. Plates were incubated at 22°C under constant light conditions (80 μmol m−2s−1) and rotated every day to avoid light effects. Seed germination rates were recorded every 24 h. Seeds were scored as germinated when the radicle emerged. Data were obtained for three biological replicates, each with three technical replicates.
Root gravitropism assay
trs120i lines were selected on ½ MS media supplemented with 20 µg/ml hygromycin B. Control lines were grown in parallel on media without antibiotics. After 4 days, seedlings were transferred to ½ MS media supplemented with 20 µM β-estradiol (Est; 20 mM stock dissolved in EtOH; Sigma-Aldrich) or 0.1% EtOH as mock control and grown vertically for an additional 9 days. The primary root tip angles (excluding lateral roots) were analyzed with Fiji - ImageJ. Primary roots exhibiting looping (see yellow arrow in Fig. 8 B) were scored as −180° due to their growth against gravity. Primary root growth orientation was visualized as frequency plots in a polar coordinate system in R. To quantify agravitropic roots, the frequency of primary root tips growing against gravity (angles < −90° and >90°) was counted per experiment.
Western blot analysis
Standard Western blot analysis was performed according to Sambrook et al. (1989). 7.5% SDS-PAGE gels (Mini-PROTEAN TGX gels, Bio-Rad) were blotted onto PVDF Immobilon-FL membranes (Millipore). Polyclonal rabbit anti-GFP antibody (1:2,000; A11122; Invitrogen) was used as primary antibody. As secondary antibody, we used a goat anti-rabbit Horseradish peroxidase-conjugated antibody (1:6,000; 1858415, Pierce; Thermo Fisher Scientific) together with the SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific). Chemiluminescence images were acquired using an ImageQuant LAS 4000 mini (GE Healthcare). To check for equal loading, blots were stained with Coomassie (10% acetic acid, 50% methanol, 0.25% Coomassie Blue R-250) and subsequently destained (10% acetic acid, 50% methanol).
Gene ontology (GO) enrichment analysis of CLUB:GFP interactome
The CLUB:GFP interactome (Kalde et al., 2019) was used for GO term enrichment analysis. To reduce the TRAPPII interactome complexity, the GO enrichment analysis of biological processes of level 0 was done separately for high-confidence interactors (intensity ratio >8) and for intermediate-intensity interactors (intensity ratio >5 and <8) (see cutoffs Fig. 1 B). The values were computed based on a comparison between observed versus random protein occurrences for each GO term. Each term is part of the hierarchical structure of GO and has defined relationships to one or more other terms in the GO enrichment. Metabolic, transcriptional, and translational processes were excluded for simplification. Terms were grouped by manually annotated super-categories. Cutoffs for enriched GO terms were set at ≥4 for fold enrichment and ≤0.003 for the FDR-adjusted P value.
Structural and multiple sequence alignments
AlphaFold predicted structures for A. thaliana AtTRS120 (UniProt: Q9FY61) and CLUB/AtTRS130 (UniProt: F4K0C4) were aligned with the Saccharomyces cerevisiae open formation of the TRAPPII monomer structure without substrate resolved with cryo-electron microscopy (in vitro) (PDB: 7E2C; Mi et al., 2022). The alignments were carried out with the align algorithm in PyMOL, which first performs a sequence alignment, followed by a structural superposition and then iteratively refines the alignment. The tree of the AtSK clades was created by a multiple sequence alignment with the Clustal Omega program in UniProt, using full-length protein sequences.
Statistical analysis and image processing
Statistical analyses were performed in Microsoft Excel and RStudio. All statistical information is described in the figure legends. Data distribution was assumed to be normal, but this was not formally tested. One sample two-tailed t test was used to determine statistical differences when data relative to the wild-type control were computed (Fig. 4 A and Fig. S5 A). To compare two independent groups, an unpaired two-tailed Student’s t test was used. Differences between groups across conditions were compared using two-way ANOVA (genotype × condition) with Tukey’s post hoc test in Fig. S10. Local Polynomial Regression Fitting for root cell lengths in Fig. 6, C and D was carried out with the loess method in R and reported with the 95% confidence interval. Statistical information for MS analyses is given in the LC-MS/MS analysis sections. False discovery rates, determined with the standard two-tailed t test, were set at a cutoff of 1%. Significance is represented as follows: *: P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001; *****: P < 0.00001. Images were processed with Adobe photoshop (https://www.adobe.com) and GIMP (https://www.gimp.org) and analyzed with ImageJ (https://imagej.nih.gov). Graphs were created with RStudio’s ggplot2 package and figures assembled with Inkscape (https://inkscape.org).
Online supplemental material
Fig. S1 shows the GO term enrichment of high-confidence interactors and brassinosteroid related proteins in the CLUB:GFP interactome. Fig. S2 contains ion mass spectra of AtTRS120 phosphopeptides. Fig. S3 highlights the Arabidopsis family of shaggy-like kinases and AtSK peptides found in AtTRS120:GFP IP-MS. Fig. S4 shows the predicted aligned error graphs of the used AlphaFold generated AtTRS120 and CLUB/AtTRS130 protein structures. Fig. S5 contains in vitro and in vivo evidence for TRAPPII phosphorylation by AtSKs. Fig. S6 emphasizes that bin2-1 mutants show no cytokinesis or protein sorting defects, characteristic of trappii mutants. Violin plots of the organ lengths or the hypocotyl/root ratio under single and additive stress in Fig. S7 enable a direct comparison between mutants and their respective wild-type control. Fig. S8 validates the attenuated hypocotyl and root phenotype of bin2-3bil1bil2 and trappii mutants under additive stress. Fig. S9 highlights the attenuated, but highly significant etiolation phenotype of trappii mutants. Fig. S10 shows the cellular hypocotyl parameters of club mutants and a direct comparison between the wild-type and trappii cellular hypocotyl parameters in an ANOVA analysis. Fig. S11 validates the expression and functionality of the TRS120 phosphovariants till the T2 generation and shows the TRS120:GFP localization in phosphomutants. Fig. S12 emphasizes the normal etiolation response in TRS120 phosphovariants. Fig. S13 contains the organ lengths and the hypocotyl/root ratio of TRS120 phosphovariants under additive stress and the total seedling lengths under the tested conditions. Table S1 shows details of the brassinosteroid related proteins found in the CLUB:GFP interactome, related to Fig. 1 C and Fig. S1 B. Table S2 lists the mutant lines used in this study. Table S3 lists the mutated sites in TRS120 phosphovariants and primer sequences used for mutagenesis. Table S4 contains the gene strand sequence carrying the TRS120 amiRNA precursor. Table S5 provides the thresholds for attenuated, normal, and enhanced response quotients. Video 1 shows a 3D projection of the cross-kingdom structural alignment of the TRAPPII-specific TRS120 subunit.
Data availability
The CLUB:GFP IP-MS data has previously been published (Kalde et al., 2019) and deposited to the ProteomeXchange Consortium via the PRoteomics IDEntification (PRIDE) partner repository (Perez-Riverol et al., 2019). The dataset can be accessed with the identifier PXD013016. All other IP-MS or MS datasets are being deposited here for the first time. New proteomics raw data (in vitro kinase assays and in vivo evidence with AtTRS120:GFP IP-MS of bikinin and PPZ treated seedlings) including MaxQuant search results and used protein sequence databases can be accessed via Panorama Public (Sharma et al., 2014, 2018) with the following link https://panoramaweb.org/phosphoTRAPP_Ara.url. All other data are available in the article, together with its supplemental materials.
Acknowledgments
We thank Prof. Wilfried Schwab, Prof. Erwin Grill, and members of their departments for support. Thanks to Prof. Arne Skerra, Martin Schlapschy, and Veder Garcia for their useful suggestions. We thank Yannik Schreckenberg, Andreas Czempiel, Theo Kalmbach, Hermine Kienberger, Nina Lomp, and Franziska Hackbarth for technical assistance. We thank the WZW/TUM Centre for Advanced Light Microscopy (CALM), headed by Ramon Torres-Ruiz and Klaus Michel, for access to confocal microscopes, and Prof. Kay Schneitz for access to their Leica S APO stereo microscope. Thanks to Roman Meier at the TUMmesa facility, directed by Leonardo Teixeira and Bálint Jákli, for supporting us with optimal growth conditions for our plants. Jorge José Casal, Sean Cutler, and Ueli Grossniklaus shared published resources.
This work was supported by Deutsche Forschungsgemeinschaft DFG grants AS110/5-2, AS110/8-1, and AS110/10-1 as well as BaCaTEC Nr. 14 [2018-2] to F.F. Assaad, by the European Research Council’s Horizon 2020 Research and Innovation Programme (Grant Agreement 648420) grant to P. Falter-Braun, and by National Institutes of Health (R01GM066258 to Z.-Y. Wang). M. Abele and C. Ludwig were supported by the EU Horizon 2020 grant Epic-XS. TUMmesa was funded with the support of the Deutsche Forschungsgemeinschaft (DFG, INST 95/1184-1 FUGG).
Author contributions: C. Wiese: methodology, investigation, visualization, writing—original draft, and writing—review & editing; M. Abele, B. Al, N. Kalbfuß, A. Strohmayr, C.H. Park, B. Brunschweiger, and C. Meng: methodology, investigation, visualization, and writing—review & editing; M. Altmann: investigation and visualization; A. Steiner: methodology, investigation, and visualization; R. Ravikumar: investigation, visualization, and writing—review & editing; E. Facher: methodology, investigation, and supervision; D.W. Ehrhardt: funding acquisition, and supervision; P. Falter-Braun: methodology, funding acquisition, supervision and writing—review & editing; Z.-Y. Wang: funding acquisition, supervision, and writing—review & editing; C. Ludwig: methodology, funding acquisition, supervision, writing—review & editing; F.F. Assaad: conceptualization, methodology, investigation, funding acquisition, project administration, supervision, writing—original draft, and writing—review & editing.
References
Author notes
Disclosures: The authors declare no competing interests exist.




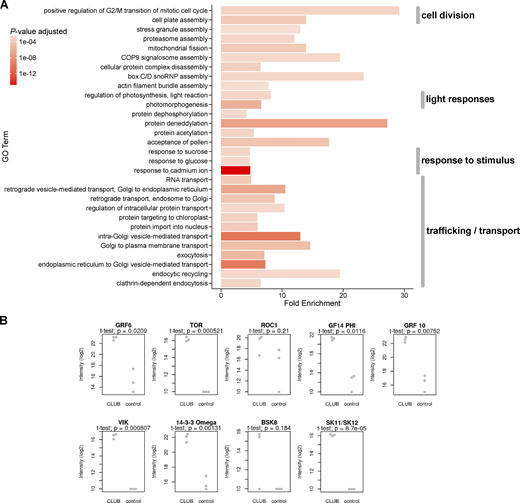







![Cytokinesis and protein sorting in bin2-1. Localization patterns of diverse markers in root tip cells of light-grown seedlings. Confocal Scanning Laser Microscopy. A protein that resides at the cell plate during cytokinesis in the wild type, PKEU::KEULE:GFP (Steiner et al., 2016b) was also seen at the cell plate in bin2-1 mutants (yellow arrowhead). Conversely, markers that are largely excluded from the cell plate (white star) in the wild type (PEXO84b::EXO84b:GFP [Fendrych et al., 2010], PVHAa1::VHAa1:GFP [Dettmer et al., 2006]) are also excluded in bin2-1 mutants. Cell plate formation and morphology in bin2-1 also did not visibly differ from the wild type. Sample numbers n = number of imaged cells. Scale bars represent 5 µm.](https://cdn.rupress.org/rup/content_public/journal/jcb/223/5/10.1083_jcb.202311125/2/m_jcb_202311125_figs6.png?Expires=1773817238&Signature=i7A3~FN9DZssbXv6HAfTBds2tab9bfXamwSIsGe1HCW1ao8ynG6Up1W70MF2UsP3FV5bS9WW0JdEBNh2CJGt8zXBReidz~BKOPqPWqnz5s6QP9s51Mzqmrd-nnS87nFFxkhcp~Egn4BicSWmvUFHuBZkhUDhNiIStpKieWnlDexFA6dMk4LsAbXOSnKOKuvI87PS-DJjUufdKeQKHIVS3MzecxV1mDrvtN2xpsI5QG2fBhE16o4CJuS8Z2EDdnK1rofkr4-ZnRIpgt7MQ121c4Z2hheWIqJ29OOgfnl7NFlvvmgyTCZeSWo4X1nREx1sT-PuINZW9daWt41TX7~qAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Response to single versus additive stress: violin plots. Seedlings were germinated on ½ MS in the light (light), in the dark (dark), or in the dark with −0.4 MPa water stress (darkW). (A–F) Violin plots depict a direct comparison of organ lengths or the hypocotyl/root ratio between the wild type and (A) phyAphyBcry1cry2; (B) pyrpyl1pyl2pyl4; (C) bin2-3bil1bil2; (D) club-2; (E) trs120-4; (F) echidna in the same experiment and on the same PEG plates. The same datasets as shown in Fig. 5, B–E and Fig. S9, B–E were used. Decision phenotypes of bin2-3bil1bil2 and trs120-4 mutants are highlighted as follows: bin2-3bil1bil2 showed an opposite root adaptation (red asterisks) and no hypocotyl/root adaptation (non-significant [n.s.] in red) to the multiple stress conditions of darkW. trappii null mutants, club-2 and trs120-4, had an attenuated, but significant etiolation response but failed to correctly adjust their hypocotyl length under darkW (red asterisks). Additionally, trs120-4 mutants showed no root and no hypocotyl/root ratio adaptation (n.s. in red) to darkW conditions. At least three experiments were performed for each line, and a representative one is shown here on the basis of RQ and P values. P values for decision phenotypes were computed with a two-tailed Student’s t test (*: P < 0.05; ***: P < 0.001) Related to Figs. 5 and S9.](https://cdn.rupress.org/rup/content_public/journal/jcb/223/5/10.1083_jcb.202311125/2/m_jcb_202311125_figs7.png?Expires=1773817238&Signature=fI762V7bfHHSjM7Iy0uT2DD72pAvV~pwuVasDC1qetDN~jusl478kVoDC51L-vo0wUVC0SARZmbevrzShWb8QAVxJvHDQjfzF9FnBtOuKJcnOqbrWuwjcI~LjKcwlHg3fK6fUUdzo~78hg2RGc8PyMG8Mc-SeO5aOj9~bagUipLlWAdjMW0MmBx7GSnfI4nHZi5Br6g1kyaEzDDfJH0DXoPTO7TKXsrv2KWJe5lVQnT-7Wlu5TAXzgnkakDTnoVwS3ddSw3~-1czQdSZaUt9k8utv3gmQDhhLoxPruvNbuILgf-EqqK87O9n4XbrF4ee8ZT0qMxcIgcUrev0KlMADg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)












