The transmembrane serine protease matriptase is a key regulator of both barrier-disruptive and protective epithelial cell–cell interactions. Elevated matriptase is a consistent feature of epithelial ovarian cancers (OvCa), where multicellular spheroids shed from the primary tumor into the peritoneal cavity are critical drivers of metastasis. Dynamic cell-to-cell adhesive contacts are required for spheroid formation and maintenance. Here, we show that overactive matriptase, reflected in an increased ratio of matriptase to its inhibitor hepatocyte growth factor activator inhibitor 1 (HAI-1), disrupts cell–cell contacts to produce loose prometastatic spheroids that display increased mesothelial cell adhesion and submesothelial invasion. We show that these activities are dependent on the matriptase activation of a protease-activated receptor-2 (PAR-2) signaling pathway involving PI3K/Akt and MMP9-induced disruption of cell–cell adhesion by the release of the soluble E-cadherin ectodomain. These data reveal a novel pathological connection between matriptase activation of PAR-2 and disruption of cell–cell adhesion, and support the clinical investigation of this signaling axis as a therapeutic strategy for aggressive metastatic OvCa.
Introduction
Ovarian cancer (OvCa) is the fifth leading cause of cancer deaths in women in the United States and the deadliest gynecological malignancy. Although the 5-yr survival rate for women diagnosed with localized disease is 93%, over ∼60% of OvCa patients present with peritoneal disseminated disease at the time of diagnosis, which dramatically reduces the relative 5-yr survival to <30% (ACS, 2022). While recent studies have revealed much about OvCa tumor initiation and origin (Kim et al., 2018), a major obstacle to effective treatment lies in the lack of knowledge of the biological underpinnings that govern tumor dissemination and metastasis (Fares et al., 2020).
In contrast to other cancers that spread by hematogenous or lymphatic routes, OvCa predominantly metastasizes intraperitoneally due to the anatomic location of the primary tumor. During disease progression, OvCa cells may be shed as floating single cells or multicellular spheroid clusters from the primary tumor mass (Allen et al., 1987; Goyeneche et al., 2020). The tumor cells disseminate within the peritoneum and interact with the mesothelium lining of the peritoneal cavity where they drive changes in vascular permeability and increase abnormal abdominal fluid accumulation (ascites). Ascites fluid provides a rich source of nutrients and a microenvironment for tumor cell survival and proliferation (Lengyel, 2010; van Baal et al., 2018). Intraperitoneal fluid movement facilitates tumor distribution, spread, and subsequent adhesion of malignant tumor cells and spheroids to the mesothelium lining of the peritoneal cavity. Finally, malignant tumor cells anchor within the submesothelial matrix and proliferate to form lesions at secondary sites, commonly the omentum, diaphragm, and bowel serosa (Lengyel, 2010).
Malignant multicellular spheroid aggregates or clusters are an understudied aspect of OvCa propagation, metastasis, and recurrence (Al Habyan et al., 2018). The molecular drivers of peritoneal dissemination and the invasive transitions of malignant spheroids remain poorly understood. OvCa spheroid clusters can be generated by three-dimensional (3D) culture from OvCa cell lines or patient tumors and retain and amplify many of the features and functionality of disseminated metastatic OvCa (Heredia-Soto et al., 2018). Spheroids derived from patient ascites are found to be inherently heterogeneous in maintaining phenotypic plasticity. They can transition dynamically between epithelial, mesenchymal, and intermediate phenotypes (Al Habyan et al., 2018; Capellero et al., 2022; Klymenko et al., 2017b), and these changes do not seem to be correlated with classical epithelial to mesenchymal transition (EMT) patterns and biomarkers (Heredia-Soto et al., 2018). E-cadherin is indispensable for spheroid formation due to its stabilization of epithelial cell–cell contacts (Klymenko et al., 2017a; Smyrek et al., 2019; Takeichi, 1991); however, the mechanisms that regulate E-cadherin function to facilitate the dynamic properties of spheroids and propagation of malignant disease remain unclear (Gunay et al., 2020).
The type II transmembrane serine protease matriptase, encoded by the ST14 gene, is widely expressed in human epithelium where it is localized to cell–cell contacts in adherens junctions. Studies of complete or partial ablation of matriptase in mice demonstrate that matriptase is essential for the maintenance of epithelial barrier integrity (Friis et al., 2017; List et al., 2002, 2009). Misregulated or overactive matriptase disrupts epidermal and intestinal epithelial barriers, causing loss of cell–cell adhesion and inflammation (Kawaguchi et al., 2011; List et al., 2005; Nagaike et al., 2008; Szabo et al., 2007). Matriptase is reported to be overexpressed with high frequency in multiple OvCa subtypes relative to normal ovarian tissues, and matriptase immunoreactivity positively correlates with the TNM and FIGO stages of ovarian serous adenocarcinomas (Jin et al., 2006; Tanimoto et al., 2005). Matriptase overexpression has been shown to enhance in vitro migration and invasion of OvCa cell lines (Sun et al., 2016) and has been reported to promote malignant progression in a number of animal models (List et al., 2006). Matriptase differs from most tumor-associated proteases that have been studied to date in that it is predominantly overexpressed by tumor cells and not by surrounding tumor stroma (List, 2009). Whether matriptase contributes to cell–cell interactions in spheroids and mechanisms of spheroid plasticity has not been explored.
Molecular and cellular data identify matriptase to be a direct proteolytic activator of the protease-activated receptor, PAR-2 (Pawar et al., 2019); however, direct activation of PAR-2 has not been connected to many physiological and pathological functions of overactive matriptase. PAR-2 is a G-protein-coupled receptor that is activated by proteolytic cleavage of an extracellular N-terminal activation site that reveals a “tethered ligand,” which binds to an intramolecular docking domain to trigger intracellular signaling pathways. PAR-2 is implicated in the inflammatory effects of matriptase overactivity in zebrafish and the potentiation of matriptase-driven Ras-mediated squamous cell carcinogenesis in mice (Ma et al., 2021; Sales et al., 2015; Schepis et al., 2018). Both matriptase and PAR-2 exhibit substantially elevated expression in OvCa compared with normal ovary tissues (Pawar et al., 2019), which suggests a potentially important function for activation of this substrate in OvCa progression.
The endogenous proteolytic activity of matriptase is controlled by Kunitz-type serine protease inhibitors, specifically HAI-1/SPINT1 and HAI-2/SPINT2 (Friis et al., 2014; Lin et al., 1999; List et al., 2005; Oberst et al., 2003, 2005; Szabo et al., 2008). Genetic studies in mice show that HAI-1 is the major regulator of matriptase activity, whereas HAI-2 is a regulator of matriptase activation by a second protease, prostasin (Friis et al., 2014). Matriptase activity, unopposed by HAI-1, is increasingly recognized as important for the oncogenesis of multiple epithelial-derived tumors (Kataoka et al., 2018). Low levels of HAI-1 have been associated with poor OvCa patient prognosis (Nakamura et al., 2009). There is evidence that advanced-stage ovarian cancers that express matriptase are more likely to do so in the absence of HAI-1 (Oberst et al., 2002), leading to the notion that an imbalance in the matriptase:HAI-1 ratio may be important in the development of advanced disease.
In the present study, we demonstrate that unopposed matriptase hyperactivity, caused by increasing the levels of matriptase relative to its cognate inhibitor HAI-1, activates a PAR-2/PI3K/Akt/MMP9 signaling pathway that releases the E-cadherin extracellular adhesion domain from the surface of OvCa spheroids, thus connecting matriptase’s activation of PAR-2 with its role in disrupting cell adhesion. We show that matriptase activity confers a loose spheroid morphology, increased metastatic behavior, and is a driving force for metastatic peritoneal dissemination, revealing a pathological signaling network that could be targeted therapeutically for treatment of advanced and metastatic OvCa.
Results
Increased matriptase relative to HAI-1 is associated with poor patient outcome and more aggressive disease
Analysis of publicly available gene chip data by Kaplan–Meier plotter reveals that among patients stratified with above median matriptase mRNA expression, those with low HAI-1 expression exhibit poor progression-free survival (PFS; Fig. 1 A, left panel). Similarly, among patients stratified with below median HAI-1 mRNA expression, patients with high matriptase expression have poorer PFS (Fig. 1 A, right panel). Higher HAI-1 levels amongst patients with above median matriptase levels improve PFS (Fig. 1 A, left panel), supporting the protective role of HAI-1 in opposing deregulated matriptase activity. Multiple OvCa cell lines, including OVCAR3, CAOV3, and COV362, express elevated levels of matriptase mRNA and protein compared with immortalized ovarian surface epithelial cells (IOSE397; Fig. S1 A). The high matriptase levels are associated with increased cell surface serine protease activity compared with non-malignant IOSE397 cells (Table 1), measured by hydrolysis of a fluorogenic substrate.
Increased matriptase:HAI-1 ratio correlates with poor patient survival and enhanced late stage peritoneal dissemination in an orthotopic xenograft model. (A) Left panel: Kaplan–Meier progression-free survival (PFS) analysis based on low or high HAI-1 expression in a cohort of OvCa patients stratified by matriptase mRNA expression above the median using publicly available data obtained from KMPlotter (N = 326; log rank P = 0.0024). Right panel: Kaplan–Meier PFS analysis based on low or high matriptase expression in a cohort of OvCa patients stratified by HAI-1 mRNA expression below the median (N = 326; log-rank P = 0.026). All patients also expressed CA125 mRNA below the lower quartile and biased arrays were excluded from analysis. (B) ES-2 Vec and Mat cells were analyzed for AEBSF-sensitive cell surface serine protease activity using the Boc-QAR-AMC fluorogenic substrate over 4 h; representative time course performed in triplicate is shown; error bars represent ±SEM of cell surface serine protease activity amongst triplicate wells (left panel). Right panel shows the average fold change of activity at the endpoint relative to Vec from three independent experiments (error bars represent ±SEM) performed in triplicate (*P < 0.05, two-tailed unpaired Student’s t test). (C) ES-2 Vec and Mat cells were injected i.p. into nude mice (n = 5 per group) and tumor burden was measured by IVIS over time. Mean photon intensity measured on the abdomen was quantified per mouse on the days indicated (error bars represent ± SEM; **P < 0.01, ***P < 0.005, two-tailed unpaired Student’s t test). (D) Volume of ascites fluid recovered from peritoneal cavity upon necropsy was measured per mouse (error bars represent ±SEM; *P < 0.05, two-tailed unpaired Student’s t test). Ascites fluid was undetectable in ES-2 Vec tumor-bearing mice. (E) Tumor samples recovered from metastatic sites in peritoneal cavities in mice upon necropsy were analyzed by qPCR for matriptase mRNA expression and normalized to luciferase mRNA expression to only account for tumor cells (error bars represent ±SEM; ****P < 0.001, two-tailed unpaired Student’s t test). (F) Photos of intraperitoneal tumor burden in mice bearing ES-2 Vec and Mat tumors on the diaphragms upon necropsy. Differences in tumor morphology are indicated by yellow arrows. (G) Tumorsphere formation. ES-2 and NCI/ADR-Res Vec or Mat cells were seeded at low density onto poly-HEMA coated 24-well plates in Mammocult media and allowed to form tumorspheres for 10 d. Tumorspheres were visualized by EVOS FL Auto Cell Imaging (representative images are enlarged images of 10× magnification). The total tumorsphere number was counted and the percentage of tumorspheres <200 or >200 μm2 (measured by gridlines on EVOS, scale bars represent 200 μm) was calculated per well. Data represent the average of three independent experiments (error bars are ±SEM) performed with four to six replicates each (*P < 0.05, **P < 0.01, two-tailed unpaired Student’s t test). (H) ES-2, NCI/ADR-Res, and SKOV3 Vec or Mat cells were seeded onto 0.75% agarose hydrogel in 96-well plates and allowed to form spheroids overnight. Spheroid morphology was visualized by EVOS FL Auto Cell Imaging and representative images are shown at 4× magnification (top images; scale bar = 500 μm for ES-2 and SKOV3, 650 μm for NCI/ADR-Res). Graphs below the images show quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represent the average of three independent experiments (error bars are ±SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, two-tailed unpaired Student’s t test). (I) Non-malignant IOSE397 cells, OvCa cell lines CAOV3, OVCAR3, and COV362 were seeded on agarose hydrogel in 96-well plates and allowed to form spheroids overnight. Representative images are shown at 4× magnification (top images). Graphs below the images show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represent the average of three independent experiments (error bars are ±SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, two-tailed unpaired Student’s t test). (J) Patient ascites-derived tumor cells from two patients (Patient 36 and Patient 37; see Materials and methods) were seeded on agarose hydrogel in 96-well plates and allowed to form spheroids overnight. Representative images are shown at 4× magnification. Scale bars for H–J represent 650 μm.
Increased matriptase:HAI-1 ratio correlates with poor patient survival and enhanced late stage peritoneal dissemination in an orthotopic xenograft model. (A) Left panel: Kaplan–Meier progression-free survival (PFS) analysis based on low or high HAI-1 expression in a cohort of OvCa patients stratified by matriptase mRNA expression above the median using publicly available data obtained from KMPlotter (N = 326; log rank P = 0.0024). Right panel: Kaplan–Meier PFS analysis based on low or high matriptase expression in a cohort of OvCa patients stratified by HAI-1 mRNA expression below the median (N = 326; log-rank P = 0.026). All patients also expressed CA125 mRNA below the lower quartile and biased arrays were excluded from analysis. (B) ES-2 Vec and Mat cells were analyzed for AEBSF-sensitive cell surface serine protease activity using the Boc-QAR-AMC fluorogenic substrate over 4 h; representative time course performed in triplicate is shown; error bars represent ±SEM of cell surface serine protease activity amongst triplicate wells (left panel). Right panel shows the average fold change of activity at the endpoint relative to Vec from three independent experiments (error bars represent ±SEM) performed in triplicate (*P < 0.05, two-tailed unpaired Student’s t test). (C) ES-2 Vec and Mat cells were injected i.p. into nude mice (n = 5 per group) and tumor burden was measured by IVIS over time. Mean photon intensity measured on the abdomen was quantified per mouse on the days indicated (error bars represent ± SEM; **P < 0.01, ***P < 0.005, two-tailed unpaired Student’s t test). (D) Volume of ascites fluid recovered from peritoneal cavity upon necropsy was measured per mouse (error bars represent ±SEM; *P < 0.05, two-tailed unpaired Student’s t test). Ascites fluid was undetectable in ES-2 Vec tumor-bearing mice. (E) Tumor samples recovered from metastatic sites in peritoneal cavities in mice upon necropsy were analyzed by qPCR for matriptase mRNA expression and normalized to luciferase mRNA expression to only account for tumor cells (error bars represent ±SEM; ****P < 0.001, two-tailed unpaired Student’s t test). (F) Photos of intraperitoneal tumor burden in mice bearing ES-2 Vec and Mat tumors on the diaphragms upon necropsy. Differences in tumor morphology are indicated by yellow arrows. (G) Tumorsphere formation. ES-2 and NCI/ADR-Res Vec or Mat cells were seeded at low density onto poly-HEMA coated 24-well plates in Mammocult media and allowed to form tumorspheres for 10 d. Tumorspheres were visualized by EVOS FL Auto Cell Imaging (representative images are enlarged images of 10× magnification). The total tumorsphere number was counted and the percentage of tumorspheres <200 or >200 μm2 (measured by gridlines on EVOS, scale bars represent 200 μm) was calculated per well. Data represent the average of three independent experiments (error bars are ±SEM) performed with four to six replicates each (*P < 0.05, **P < 0.01, two-tailed unpaired Student’s t test). (H) ES-2, NCI/ADR-Res, and SKOV3 Vec or Mat cells were seeded onto 0.75% agarose hydrogel in 96-well plates and allowed to form spheroids overnight. Spheroid morphology was visualized by EVOS FL Auto Cell Imaging and representative images are shown at 4× magnification (top images; scale bar = 500 μm for ES-2 and SKOV3, 650 μm for NCI/ADR-Res). Graphs below the images show quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represent the average of three independent experiments (error bars are ±SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, two-tailed unpaired Student’s t test). (I) Non-malignant IOSE397 cells, OvCa cell lines CAOV3, OVCAR3, and COV362 were seeded on agarose hydrogel in 96-well plates and allowed to form spheroids overnight. Representative images are shown at 4× magnification (top images). Graphs below the images show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represent the average of three independent experiments (error bars are ±SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, two-tailed unpaired Student’s t test). (J) Patient ascites-derived tumor cells from two patients (Patient 36 and Patient 37; see Materials and methods) were seeded on agarose hydrogel in 96-well plates and allowed to form spheroids overnight. Representative images are shown at 4× magnification. Scale bars for H–J represent 650 μm.
OvCa cell lines express varying levels of matriptase and HAI-1 mRNA and protein. (A) Whole cell RNA lysates were prepared from IOSE397 and five OvCa cell lines and analyzed by qPCR for matriptase (ST14) and HAI-1 (SPINT1) mRNA expression. Data represents average mRNA expression relative to GAPDH (error bars are ± SEM from three independent experiments performed in triplicate; *P < 0.05 relative to IOSE397 cells). (B) Whole cell lysates were collected from IOSE397 and five OvCa cell lines with RIPA lysis buffer, assessed by SDS-PAGE, and immunoblotted for rabbit anti-human matriptase, mouse anti-HAI-1, and rabbit anti-human β-tubulin (representative immunoblot shown in left panel). Upper matriptase band (∼90 kD) represents full-length matriptase, while 70 kD fragment represents matriptase zymogen. Densitometric analyses (right panels) were conducted using ImageJ; data represents average densitometric units from three independent experiments (error bars are ± SEM; *P < 0.05, **P < 0.01, ns = not significant relative to IOSE397). (C) Whole-cell RNA lysates were prepared from ES-2-, NCI/ADR-Res, and SKOV3- Vec/Mat cells and analyzed by qPCR for matriptase (ST14) and HAI-1 (SPINT1) mRNA expression. Data represents average mRNA expression relative to GAPDH from three independent experiments performed in triplicate (error bars are ± SEM; *P < 0.05, ****P < 0.001 relative to corresponding Vec cells). (D) Whole-cell lysates were collected from ES-2-, NCI/ADR-Res, and SKOV3- Vec/Mat cells with RIPA lysis buffer, assessed by SDS-PAGE and immunoblotted for rabbit anti-human matriptase, mouse anti-HAI-1, and rabbit anti-human β-tubulin (representative immunoblot shown in left panel). Densitometric analyses (right panels) were conducted using ImageJ; data represents average densitometric units from three independent experiments (errors bars are ± SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ns = not significant relative to respective Vec controls). All P values were calculated according to two-tailed, unpaired Student’s t tests. Source data are available for this figure: SourceData FS1.
OvCa cell lines express varying levels of matriptase and HAI-1 mRNA and protein. (A) Whole cell RNA lysates were prepared from IOSE397 and five OvCa cell lines and analyzed by qPCR for matriptase (ST14) and HAI-1 (SPINT1) mRNA expression. Data represents average mRNA expression relative to GAPDH (error bars are ± SEM from three independent experiments performed in triplicate; *P < 0.05 relative to IOSE397 cells). (B) Whole cell lysates were collected from IOSE397 and five OvCa cell lines with RIPA lysis buffer, assessed by SDS-PAGE, and immunoblotted for rabbit anti-human matriptase, mouse anti-HAI-1, and rabbit anti-human β-tubulin (representative immunoblot shown in left panel). Upper matriptase band (∼90 kD) represents full-length matriptase, while 70 kD fragment represents matriptase zymogen. Densitometric analyses (right panels) were conducted using ImageJ; data represents average densitometric units from three independent experiments (error bars are ± SEM; *P < 0.05, **P < 0.01, ns = not significant relative to IOSE397). (C) Whole-cell RNA lysates were prepared from ES-2-, NCI/ADR-Res, and SKOV3- Vec/Mat cells and analyzed by qPCR for matriptase (ST14) and HAI-1 (SPINT1) mRNA expression. Data represents average mRNA expression relative to GAPDH from three independent experiments performed in triplicate (error bars are ± SEM; *P < 0.05, ****P < 0.001 relative to corresponding Vec cells). (D) Whole-cell lysates were collected from ES-2-, NCI/ADR-Res, and SKOV3- Vec/Mat cells with RIPA lysis buffer, assessed by SDS-PAGE and immunoblotted for rabbit anti-human matriptase, mouse anti-HAI-1, and rabbit anti-human β-tubulin (representative immunoblot shown in left panel). Densitometric analyses (right panels) were conducted using ImageJ; data represents average densitometric units from three independent experiments (errors bars are ± SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ns = not significant relative to respective Vec controls). All P values were calculated according to two-tailed, unpaired Student’s t tests. Source data are available for this figure: SourceData FS1.
Matriptase:HAI-1 ratios and effects on cell surface serine protease activity and spheroid phenotype across OvCa cell lines
| Cell line . | Relative Matriptase:HAI-1 protein ratioa . | Cell surface serine protease activity (fold change ± SEM)b . | Spheroid morphology . | |
|---|---|---|---|---|
| Spheroid phenotypec . | % empty space (rel. to IOSE)d . | |||
| IOSE397 | 1.0 | 1.00 | Tight | 1.00 |
| ES-2 | 3.2 | 3.19 (±0.14) | Tight | 1.27 |
| NCI/ADR-Res | 6.6 | 6.70 (±0.88) | Tight | 1.86 |
| OVCAR3 | 704.9 | 20.64 (±0.10) | Loose | 4.56 |
| CAOV3 | 144.6 | 11.94 (±1.93) | Loose | 4.60 |
| COV362 | 98.5 | 38.98 (±3.78) | Loose | 6.43 |
| Cell line . | Relative Matriptase:HAI-1 protein ratioa . | Cell surface serine protease activity (fold change ± SEM)b . | Spheroid morphology . | |
|---|---|---|---|---|
| Spheroid phenotypec . | % empty space (rel. to IOSE)d . | |||
| IOSE397 | 1.0 | 1.00 | Tight | 1.00 |
| ES-2 | 3.2 | 3.19 (±0.14) | Tight | 1.27 |
| NCI/ADR-Res | 6.6 | 6.70 (±0.88) | Tight | 1.86 |
| OVCAR3 | 704.9 | 20.64 (±0.10) | Loose | 4.56 |
| CAOV3 | 144.6 | 11.94 (±1.93) | Loose | 4.60 |
| COV362 | 98.5 | 38.98 (±3.78) | Loose | 6.43 |
Matriptase and HAI-1 protein expression were determined by immunoblot analysis; levels were quantitated by densitometric analysis, normalized to β-tubulin, and the matriptase:HAI-1 ratio was calculated (see Fig. S1 B). The data is expressed relative to control immortalized IOSE397 cells, run on the same gels.
AEBSF-sensitive cell surface serine protease activity was measured using Boc-QAR-AMC fluorogenic peptide. Fold change of endpoint fluorescent values (normalized to cell number) was calculated relative to IOSE397 cells.
Spheroids were allowed to form on nonadhesive agarose hydrogels overnight, visualized using the EVOS microscope, and qualitatively scored as tight or loose as defined in the Materials and methods.
As a quantitative measure of spheroid morphology, the percentage empty space in EVOS images of spheroids was determined using ImageJ as described in the Materials and methods and expressed relative to IOSE397 cells.
Elevated matriptase activity confers enhanced late-stage peritoneal dissemination of ovarian tumors in a xenograft model of OvCa
To determine the specific effect of deregulated matriptase on OvCa peritoneal dissemination and metastasis, plasmids encoding matriptase or vector alone were cotransfected along with HAI-1 into ES-2-Luc cells to generate stable high matriptase–expressing (Mat) and vector control (Vec) cell lines (Fig. S1 B). The ES-2 OvCa cell line expresses low to negligible endogenous levels of both matriptase and HAI-1 mRNA (Fig. S1 A) and has a relatively low matriptase:HAI-1 protein ratio (Table 1). The cotransfection with HAI-1 ensures appropriate expression, intracellular trafficking, activation, and regulation of matriptase (Oberst et al., 2005). Mat cells displayed increased matriptase expression (Fig. S1, C and D) and increased cell surface serine protease activity compared with Vec cells (Fig. 1 B and Table 2).
Elevated matriptase:HAI-1 ratios increase cell surface serine protease activity and promote spheroid phenotype in three OvCa models
| Cell line . | Relative Matriptase:HAI-1 protein ratioa . | Cell surface serine protease activity (fold change ± SEM)b . | Spheroid morphology . | |
|---|---|---|---|---|
| Spheroid phenotypec . | % empty space (rel. to Vec)d . | |||
| ES-2 Vec | 0.0e | 1.00 | Tight | 1.00 |
| ES-2 Mat | 23.0 | 3.26 (±0.22) | Loose | 4.85 |
| NCI/ADR-Res Vec | 0.0e | 1.00 | Tight | 1.00 |
| NCI/ADR-Res Mat | 19.6 | 4.02 (±0.84) | Loose | 2.24 |
| SKOV3 Vec | 0.0e | 1.00 | Tight | 1.00 |
| SKOV3 Mat | 5.4 | 2.78 (±0.002) | Loose | 2.56 |
| Cell line . | Relative Matriptase:HAI-1 protein ratioa . | Cell surface serine protease activity (fold change ± SEM)b . | Spheroid morphology . | |
|---|---|---|---|---|
| Spheroid phenotypec . | % empty space (rel. to Vec)d . | |||
| ES-2 Vec | 0.0e | 1.00 | Tight | 1.00 |
| ES-2 Mat | 23.0 | 3.26 (±0.22) | Loose | 4.85 |
| NCI/ADR-Res Vec | 0.0e | 1.00 | Tight | 1.00 |
| NCI/ADR-Res Mat | 19.6 | 4.02 (±0.84) | Loose | 2.24 |
| SKOV3 Vec | 0.0e | 1.00 | Tight | 1.00 |
| SKOV3 Mat | 5.4 | 2.78 (±0.002) | Loose | 2.56 |
Matriptase and HAI-1 protein expression were determined by immunoblot analysis; levels were quantitated by densitometric analysis, normalized to β-tubulin, and the matriptase:HAI-1 ratio was calculated (see Fig. S1 B).
AEBSF-sensitive cell surface serine protease activity was measured using Boc-QAR-AMC fluorogenic peptide. Fold change of endpoint fluorescent values (normalized to cell number) was calculated relative to each respective Vec control.
Spheroids were allowed to form on nonadhesive agarose hydrogels overnight, visualized using the EVOS microscope and qualitatively scored as tight or loose as defined in the Materials and methods.
As a quantitative measure of spheroid morphology, the percentage of empty space in EVOS images of spheroids was determined using ImageJ as described in the Materials and methods and expressed relative to each respective Vec control.
Matriptase protein was undetectable in Vec cells by immunoblotting, thus resulting in a zero value for the relative matriptase:HAI-1 ratio (see Fig. S1 D).
We have developed an ES-2-Luc xenograft model that reproduces key events of late-stage ovarian cancer with the development of malignant ascites fluid containing floating tumor spheroids and disseminated tumor foci attached to the walls of the abdominal cavity and surrounding peritoneal organs (Conway et al., 2019). To investigate the role of overactive matriptase in vivo, female athymic nude mice were injected intraperitoneally (i.p.) with Mat or Vec cells, and tumor burden was monitored longitudinally by luminescence using the in vitro imaging system (IVIS) over the course of 8 d (Fig. S2 A). Mice bearing Mat tumors exhibited significantly increased tumor burden by day 4 (approximately twofold) and day 8 after tumor cell injection (2.2-fold) compared with mice bearing Vec tumors (Fig. 1 C and Fig. S2 B). Mice bearing Mat tumors developed significant ascites fluid accumulation, indicative of substantial i.p. disseminated tumor burden, whereas ascites fluid was not found in mice bearing Vec tumors (Fig. 1 D). Upon necropsy, Mat tumor–bearing mice showed increased tumor lesions throughout the peritoneal cavity and were loosely attached to the surface of peritoneal organs as well as invading some tissues, similar to the dissemination patterns found in advanced human OvCa patients (Ahmed and Stenvers, 2013; Lengyel, 2010; van Baal et al., 2018). Molecular analysis of Vec and Mat tumor cells recovered from mouse diaphragms verified that Mat tumors had retained increased matriptase expression (Fig. 1 E), confirming that the ascites accumulation and increased metastatic tumor burden were due to enhanced matriptase.
Elevated matriptase activity enhances peritoneal dissemination in an orthotopic xenograft model. (A) Experimental timeline: Stable ES-2 Vec and Mat cell lines expressing luciferase were injected i.p. into female nude mice (5 × 106 cells per mouse, n = 5 mice/group) and tumor burden was monitored by IVIS imaging (Xenogen) at days 1, 4, and 8 after sorting into cohorts of similar tumor burden. (B) Images of the peak luciferase activity levels of individual mice in the Vec and Mat cohorts. (C) Mouse diaphragms were fixed with paraformaldehyde, paraffin-embedded, and stained with H&E. Representative images of ES-2 Vec and Mat tumor-bearing diaphragms were taken at 4× (scale bar = 1,000 μm; left panels), 10× (scale bar = 500 μm; middle panels), and 20× (scale bar = 200 μm; right panels) magnification to show areas of tumor (T), necrotic tissue (N), and smooth muscle cells (SM). Images show expansive areas of invasive fronts (Vec) and infiltrated areas of tumor lesions that have seeded dispersed areas of tissue (Mat). (D) Morphological analysis of ascites fluid recovered from ES-2-Mat tumor-bearing mice was analyzed using cytospins stained with Kwik-Diff (no ascites was recovered from ES-2-Vec bearing mice). A representative whole scan at 4× magnification (scale bar = 1,000 μm and 10× image; scale bar = 500 μm) is shown.
Elevated matriptase activity enhances peritoneal dissemination in an orthotopic xenograft model. (A) Experimental timeline: Stable ES-2 Vec and Mat cell lines expressing luciferase were injected i.p. into female nude mice (5 × 106 cells per mouse, n = 5 mice/group) and tumor burden was monitored by IVIS imaging (Xenogen) at days 1, 4, and 8 after sorting into cohorts of similar tumor burden. (B) Images of the peak luciferase activity levels of individual mice in the Vec and Mat cohorts. (C) Mouse diaphragms were fixed with paraformaldehyde, paraffin-embedded, and stained with H&E. Representative images of ES-2 Vec and Mat tumor-bearing diaphragms were taken at 4× (scale bar = 1,000 μm; left panels), 10× (scale bar = 500 μm; middle panels), and 20× (scale bar = 200 μm; right panels) magnification to show areas of tumor (T), necrotic tissue (N), and smooth muscle cells (SM). Images show expansive areas of invasive fronts (Vec) and infiltrated areas of tumor lesions that have seeded dispersed areas of tissue (Mat). (D) Morphological analysis of ascites fluid recovered from ES-2-Mat tumor-bearing mice was analyzed using cytospins stained with Kwik-Diff (no ascites was recovered from ES-2-Vec bearing mice). A representative whole scan at 4× magnification (scale bar = 1,000 μm and 10× image; scale bar = 500 μm) is shown.
OvCa tumor xenografts expressing elevated matriptase activity form smaller, more punctate disseminated tumor nodules compared with control xenografts
Increased tumor burden in Mat tumor–bearing mice was associated with substantially increased numbers of distinctive, small grape-like tumor nodules on the diaphragms that spread throughout the peritoneal cavity, compared with the larger more compact tumors in mice bearing Vec tumors (Fig. 1 F). Examination of fixed mouse diaphragm tissue sections did not distinguish any apparent histological differences between embedded Mat and Vec tumors; both Mat and Vec tumor lesions contained invasive fronts as well as dispersed areas of tumor infiltration into the tissue (Fig. S2 C). Thus, the major effect of overactive matriptase on increased tumor burden appeared to be related to the distinctive morphological difference in tumor nodules between Mat and Vec tumors and the development of ascites by the Mat tumors only, suggesting that enhanced matriptase may influence the phenotypic properties of shed multicellular tumor clusters, propagating a looser, more easily disseminated, and malignant tumor population.
Matriptase activity promotes tumorsphere formation and propagation of smaller spheroids
To determine whether the role of overactive matriptase phenotype was generally observed, we stably expressed Vec control and Mat in two additional OvCa cell lines, NCI/ADR-Res and SKOV3 (Fig. S1, C and D). The characterized Mat cell lines demonstrated elevated matriptase levels and low HAI-1 levels compared with control Vec cells when normalized to the housekeeping gene GAPDH (Table 2) and also demonstrated elevated cell surface serine protease activity (Fig. S3 A). Overactive matriptase did not affect the doubling time of cultured OvCa cells (Fig. S3 B); however, disseminated OvCa spheroid populations can retain self-renewal and proliferative capabilities while being maintained in a favorable growth environment like ascites fluid, thus propagating minimal residual disease that can emerge later as an aggressive disease after cytoreductive surgery. We tested the proliferative capacities of ES-2 and NCI/ADR-Res Mat cells by tumorsphere formation assays, in which cells plated at low density were grown under non-adherent conditions in stem-cell enriching media. ES-2 and NCI/ADR-Res Mat cells both formed significantly more tumorspheres, growing as spherical clusters of tumor cells, compared with their respective Vec controls (Fig. 1 G), demonstrating enhanced tumor propagation capabilities, consistent with the enhanced disseminated tumor burden observed in vivo. In addition, a significantly higher percentage of Mat tumorspheres were <200 μm2 in area, whereas Vec spheroids had a higher percentage that was >200 μm2 (Fig. 1 G), highlighting the activity of matriptase in causing the propagation of smaller spheroids.
Characterization of stable NCI/ADR-Res and SKOV3 matriptase-expressing (Mat) or vector alone (Vec) cell lines. (A) Activity. NCI/ADR-Res and SKOV3 Vec and Mat cells were characterized for cell surface protease activity using the Boc-QAR-AMC fluorogenic substrate over 4 h; representative time course is shown, and the plot shows average fold change of activity at the endpoint relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM; *P < 0.05). (B) Proliferation. Stable ES-2, NCI/ADR-Res, and SKOV3 matriptase-expressing (Mat) or vector alone (Vec) cell lines were seeded onto six-well plates in triplicate and cells were counted after 48 and 72 h. Cell counts at 0, 48, and 72 h showed no significant difference (ns). (C) A11 antibody blocks Boc-QAR-AMC matriptase activity. ES-2 Mat cells were analyzed for AEBSF-sensitive cell surface serine protease activity in the presence of the matriptase-blocking antibody A11 to determine the optimal dose for matriptase inhibition. Representative time course over 4 h shows average cell surface serine protease activity; error bars show ± SEM from triplicate wells (left panel). Right panel graph shows the average fold change of cell surface protease activity at the endpoint from three independent experiments performed in triplicate (error bars are ± SEM; *P < 0.05; ns = not significant). All P values were calculated according to two-tailed, unpaired Student’s t tests.
Characterization of stable NCI/ADR-Res and SKOV3 matriptase-expressing (Mat) or vector alone (Vec) cell lines. (A) Activity. NCI/ADR-Res and SKOV3 Vec and Mat cells were characterized for cell surface protease activity using the Boc-QAR-AMC fluorogenic substrate over 4 h; representative time course is shown, and the plot shows average fold change of activity at the endpoint relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM; *P < 0.05). (B) Proliferation. Stable ES-2, NCI/ADR-Res, and SKOV3 matriptase-expressing (Mat) or vector alone (Vec) cell lines were seeded onto six-well plates in triplicate and cells were counted after 48 and 72 h. Cell counts at 0, 48, and 72 h showed no significant difference (ns). (C) A11 antibody blocks Boc-QAR-AMC matriptase activity. ES-2 Mat cells were analyzed for AEBSF-sensitive cell surface serine protease activity in the presence of the matriptase-blocking antibody A11 to determine the optimal dose for matriptase inhibition. Representative time course over 4 h shows average cell surface serine protease activity; error bars show ± SEM from triplicate wells (left panel). Right panel graph shows the average fold change of cell surface protease activity at the endpoint from three independent experiments performed in triplicate (error bars are ± SEM; *P < 0.05; ns = not significant). All P values were calculated according to two-tailed, unpaired Student’s t tests.
Elevated matriptase confers a loose, scattered multicellular spheroid phenotype on non-adhesive agarose hydrogels
Fixed cytospins prepared from the ascites fluid of Mat tumor bearing mice contained single cells as well as a range of tumor cell clusters that varied in size and shape (Fig. S2 D). To investigate the possible functional effects of overactive matriptase on the development of multicellular tumor clusters, we established an assay to propagate Mat and Vec multicellular spheroids in vitro using 3D cell culture on non-adhesive hydrogels. The method utilizes a liquid overlay technique with a non-adhesive, inert agarose hydrogel to induce spheroid aggregation in a low-attachment environment. Spheroid “tightness” was qualitatively assessed and quantitatively evaluated by calculating the percentage of empty space as described in Materials and methods. Analysis of the resulting self-assembled spheroids revealed that ES-2, NCI/ADR-Res, and SKOV3 Vec control cells all formed tight, compact spheroids, while Mat cells formed loose, scattered, and more grape-like 3D spheroids (Fig. 1 H and Table 2).
Elevated matriptase in OvCa cell lines and patient-derived tumors are associated with loose, scattered multicellular spheroids
The OvCa cell lines, CAOV3, OVCAR3, and COV362, have a relatively high endogenous ratio of matriptase:HAI-1 protein levels and display enhanced serine protease activity (Table 1). We found that these cell lines also formed loosely aggregated spheroids on hydrogels compared with the control IOSE397 cells (Fig. 1 I and Table 1) and OvCa cell lines with low to negligible matriptase, ES-2, NCI/ADR-Res, or SKOV3 (Vec) cells (Fig. 1 H), which all formed tight, compact spheroids. Tumor cells recovered from the ascites fluids of OvCa patients (Patients #36 and #37; see Materials and methods) were found to form loosely aggregated spheroids and expressed elevated levels of matriptase, demonstrating that this phenotype occurs in primary human OvCa (Fig. 1 J). Together, these data implicate elevated and dysregulated matriptase as a potential determinant of the loose spheroid morphology across a range of OvCa cells.
Elevated matriptase confers loose, grape-like multicellular spheroid morphologies in hanging drop suspensions that are dependent on matriptase activity
In addition to spheroid clustering on non-adhesive hydrogels, spheroids can be generated under free-floating conditions using the hanging drop method, thus avoiding any substratum contacts (Kelm et al., 2003; Klymenko et al., 2017a; Timmins and Nielsen, 2007). This method enables cells to accumulate at the free-liquid–air interface, mimicking aspects of the fluid microenvironment in ascites (Weiswald et al., 2015). When formed by the hanging drop method, ES-2 and NCI/ADR-Res Mat cells form loose, dispersed, and grape-like 3D clusters compared with the tighter spheroids formed with Vec cells (Fig. 2 A), similar to our observations on non-adhesive hydrogels. When incubated with the A11 blocking antibody that inhibits matriptase catalytic activity (Schneider et al., 2012; Fig. S3 C), ES-2 and NCI/ADR-Res Mat spheroids reverted to the tighter phenotype, similar to their respective Vec controls (Fig. 2 A), demonstrating the dependence of the loose spheroid phenotype on matriptase proteolytic activity.
Matriptase activity promotes the formation of smaller loose, disaggregated multicellular spheroids via disrupted homotypic cell–cell interactions, enhanced spheroid disaggregation, and increased tumorsphere formation. (A) Hanging-drop spheroids. ES-2 and NCI/ADR-Res Vec or Mat cells were seeded in 10 μl droplets (2 × 104 cells/drop), inverted on the lid of a 10-cm dish, and allowed to form hanging drop spheroids for 4 d. Some were formed in the presence of matriptase-blocking antibody A11 (100 nM; see Fig. S3 C for titration of A11). Hanging drop spheroids were visualized by EVOS (representative images shown at 4× magnification; scale bars represent 650 μm; left images) and spheroid morphology was determined under each condition. Graphs show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represent the average of three independent experiments (error bars are ± SEM; **P < 0.01, ***P < 0.005, ****P < 0.001, ns = not significant). (B) Cell–cell adhesion. ES-2 and NCI/ADR-Res Vec or Mat cells were seeded onto a black-walled 96-well plate and allowed to form a monolayer for 48 h. Cells were labeled with CellTrackerOrange as per the manufacturer’s instructions and seeded on top of corresponding homotypic monolayers and allowed to adhere for 2 h, treated with either vehicle (PBS) or with 100 nM A11. Fluorescent cells were visualized by EVOS and fluorescence intensity was measured by FlexStation3 plate reader; cells were washed twice with PBS and remaining fluorescence of adherent cells was measured and visualized. Representative images of adherent cells (red) on homotypic monolayers (grayscale) are shown at 4× magnification; scale bars represent 650 μm. Data represents average percentage of remaining fluorescence from three independent experiments performed with at least three replicates each (error bars are ± SEM; *P < 0.05, ***P < 0.005, ns = not significant). (C) Spheroid disaggregation. LP9 mesothelial cells were seeded onto collagen-coated 96-well plates and allowed to form a confluent monolayer for 48 h. ES-2 and NCI/ADR-Res Vec or Mat spheroids were formed on agarose hydrogel overnight and labeled with calcein-AM. Labeled spheroids were seeded onto formed LP9 monolayers in the presence of vehicle control (DMSO) or 100 nM A11 and visualized for 72 h by EVOS; representative images are shown at 4× magnification, scale bars represent 650 μm. The percentage increase in spheroid area from starting point (0 h) was quantified by ImageJ; data represents average of three independent experiments performed with six replicates each (error bars are ± SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ns = not significant). All P values were calculated according to two-tailed unpaired Student’s t tests.
Matriptase activity promotes the formation of smaller loose, disaggregated multicellular spheroids via disrupted homotypic cell–cell interactions, enhanced spheroid disaggregation, and increased tumorsphere formation. (A) Hanging-drop spheroids. ES-2 and NCI/ADR-Res Vec or Mat cells were seeded in 10 μl droplets (2 × 104 cells/drop), inverted on the lid of a 10-cm dish, and allowed to form hanging drop spheroids for 4 d. Some were formed in the presence of matriptase-blocking antibody A11 (100 nM; see Fig. S3 C for titration of A11). Hanging drop spheroids were visualized by EVOS (representative images shown at 4× magnification; scale bars represent 650 μm; left images) and spheroid morphology was determined under each condition. Graphs show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represent the average of three independent experiments (error bars are ± SEM; **P < 0.01, ***P < 0.005, ****P < 0.001, ns = not significant). (B) Cell–cell adhesion. ES-2 and NCI/ADR-Res Vec or Mat cells were seeded onto a black-walled 96-well plate and allowed to form a monolayer for 48 h. Cells were labeled with CellTrackerOrange as per the manufacturer’s instructions and seeded on top of corresponding homotypic monolayers and allowed to adhere for 2 h, treated with either vehicle (PBS) or with 100 nM A11. Fluorescent cells were visualized by EVOS and fluorescence intensity was measured by FlexStation3 plate reader; cells were washed twice with PBS and remaining fluorescence of adherent cells was measured and visualized. Representative images of adherent cells (red) on homotypic monolayers (grayscale) are shown at 4× magnification; scale bars represent 650 μm. Data represents average percentage of remaining fluorescence from three independent experiments performed with at least three replicates each (error bars are ± SEM; *P < 0.05, ***P < 0.005, ns = not significant). (C) Spheroid disaggregation. LP9 mesothelial cells were seeded onto collagen-coated 96-well plates and allowed to form a confluent monolayer for 48 h. ES-2 and NCI/ADR-Res Vec or Mat spheroids were formed on agarose hydrogel overnight and labeled with calcein-AM. Labeled spheroids were seeded onto formed LP9 monolayers in the presence of vehicle control (DMSO) or 100 nM A11 and visualized for 72 h by EVOS; representative images are shown at 4× magnification, scale bars represent 650 μm. The percentage increase in spheroid area from starting point (0 h) was quantified by ImageJ; data represents average of three independent experiments performed with six replicates each (error bars are ± SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ns = not significant). All P values were calculated according to two-tailed unpaired Student’s t tests.
Cell–cell adhesive properties of loose spheroids with elevated and overactive matriptase
Multicellular spheroid formation and “tightness” is dependent on homotypic cell adhesion (Klymenko et al., 2017a). The effect of enhanced matriptase activity on the cell–cell adhesive properties of ES-2 and NCI/ADR-Res Mat and Vec spheroids was investigated using several methods.
Matriptase activity disrupts homotypic cell–cell interactions
Fluorescently labeled ES-2 and NCI/ADR-Res Vec or Mat cells were seeded onto their respective homotypic cell monolayers, washed, and the remaining adherent cells measured. Mat cells were significantly less cell-adhesive compared with their Vec counterparts (Fig. 2 B). Inhibition of matriptase activity by A11 attenuated this decrease in cell–cell interactions (Fig. 2 B), indicating that matriptase proteolytic activity is directly involved in disrupting homotypic cell–cell adhesion to promote loose spheroids.
Matriptase activity enhances spheroid disaggregation
When spheroids come in contact with the mesothelium, they disaggregate, and previous published studies have shown that spheroids with extensive and tight cell–cell connections have a hindered ability to disaggregate (Burleson et al., 2006). To investigate the effect of enhanced matriptase on spheroid disaggregation and to mimic interactions with the mesothelium, spheroids were seeded on top of a monolayer of LP9 cells, a cell line propagated from human primary peritoneal mesothelial cells. ES-2 and NCI/ADR-Res Mat spheroids exhibited a significantly higher percentage increase in the spheroid area after 72 h compared with Vec spheroids (Fig. 2 C), demonstrating enhanced propensity for disaggregation and decreased spheroid tightness. Inhibition of matriptase activity with A11 attenuated the matriptase-mediated increase in spheroid disaggregation in both ES-2 and NCI/ADR-Res Mat spheroids relative to Vec controls (Fig. 2 C), further showing that matriptase proteolytic activity is involved in the disruption of spheroid cell–cell interactions.
Together, these data identify that elevated matriptase expression and unhindered proteolytic activity play functional roles in disrupting cell–cell adhesions and promoting loose spheroid morphology in two different OvCa cell models, and that matriptase-mediated disruption of cell–cell adhesions may be a novel mechanism to facilitate OvCa dissemination.
Elevated matriptase enhances metastasis-associated spheroid behaviors
Loss of spheroid forming capabilities has been associated with increased migration and invasion abilities (Stadler et al., 2018). Based on the observed matriptase-mediated loose spheroid morphology and disrupted cell–cell interactions, we investigated the role of matriptase in processes of OvCa metastasis that may be utilized in the transition from free-floating spheroids to the establishment of secondary peritoneal lesions.
Matriptase activity enhances adhesion to mesothelial monolayers
Once disseminated within the peritoneal cavity, OvCa spheroids typically metastasize by adhering to the mesothelium lining the peritoneal cavity as a first step to the seeding of secondary lesions. To mimic interactions with the peritoneal lining during OvCa dissemination, adhesion of ES-2 and NCI/ADR-Res Vec and Mat spheroids plated on a confluent monolayer of LP9 mesothelial cells was measured. Matriptase-expressing ES-2 (Fig. 3 A) and NCI/ADR-Res (Fig. 3 A and Fig. S4 A) spheroids were significantly more adhesive to the LP9 mesothelial monolayer compared with the respective Vec control spheroids.
Matriptase expression promotes metastasis-associated behaviors: adhesion and clearance of mesothelial monolayer and invasion through mesothelial monolayer into submesothelial matrix. (A) Spheroid adhesion to mesothelial monolayer. ES-2 and NCI/ADR-Res Vec or Mat spheroids formed on agarose hydrogel and labeled with calcein-AM were seeded onto LP9 monolayers (formed on thin collagen-coated 24-well plates). Spheroids were allowed to adhere for 1 h and washed three times with PBS; adherent spheroids were visualized and counted before and after wash by EVOS (representative whole-well scans at 4× magnification of ES-2 experiment are shown in left panels; scale bars represent 1.4 mm). Right panel: Data represents the average fold change of the percentage of adherent spheroids relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM, *P < 0.05, **P < 0.01). (B) Mesothelial clearance. ES-2 and NCI/ADR-Res Vec or Mat spheroids formed on agarose hydrogel (shown in “Spheroid [Brightfield]” panels) were seeded onto LP9 mesothelial monolayers labeled with calcein-AM (shown in “LP9 [Green]” panels). “Overlay” panels are Brightfield and Green channel views superimposed. Mesothelial clearance was visualized by EVOS, representative images of ES-2 experiment are shown at 4× magnification, scale bars represent 650 μm; top panel. The area of clearance was quantified by ImageJ and calculated as a percentage of the total spheroid area. Data in the bar graph (bottom panel) represent the average fold change of the percentage of clearance area relative to Vec from three independent experiments performed with at least three replicates each (error bars are ± SEM; *P < 0.05, **P < 0.01). (C) Migration and invasion. ES-2 Vec and Mat cells were seeded onto uncoated or Matrigel-coated Transwells, allowed to migrate or invade for 16–24 h, stained by Kwik-Diff, and quantified by ImageJ; data represents average migration/invasion relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM, *P < 0.05, **P < 0.01). (D) Invasion of mesothelial monolayers. ES-2 and NCI/ADR-Res Vec or Mat cells labeled with calcein-AM were seeded onto Transwell filters coated with thin collagen and an LP9 mesothelial monolayer; after 24 h, invaded cells were fixed with PFA, visualized by EVOS, and quantified by ImageJ. A representative image of one field of invaded cells is shown (4× magnification; scale bars represent 650 μm). Data represents the average ratio of invaded cells relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM, **P < 0.01, ***P < 0.005). (E) 3D collagen invasion. ES-2 and NCI/ADR-Res Vec or Mat spheroids formed on agarose hydrogel were embedded into a 3D type I collagen matrix in chamber slides and allowed to invade for 48 or 72 h respectively; spheroids were visualized by EVOS (representative images shown at 4× magnification; scale bars represent 650 μm). The average fold change of the percentage increase in spheroid size relative to Vec was quantified by ImageJ from three experiments performed with six to eight replicates (error bars are ± SEM, *P < 0.05 ***P < 0.005). All P values were calculated according to two-tailed unpaired Student’s t tests.
Matriptase expression promotes metastasis-associated behaviors: adhesion and clearance of mesothelial monolayer and invasion through mesothelial monolayer into submesothelial matrix. (A) Spheroid adhesion to mesothelial monolayer. ES-2 and NCI/ADR-Res Vec or Mat spheroids formed on agarose hydrogel and labeled with calcein-AM were seeded onto LP9 monolayers (formed on thin collagen-coated 24-well plates). Spheroids were allowed to adhere for 1 h and washed three times with PBS; adherent spheroids were visualized and counted before and after wash by EVOS (representative whole-well scans at 4× magnification of ES-2 experiment are shown in left panels; scale bars represent 1.4 mm). Right panel: Data represents the average fold change of the percentage of adherent spheroids relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM, *P < 0.05, **P < 0.01). (B) Mesothelial clearance. ES-2 and NCI/ADR-Res Vec or Mat spheroids formed on agarose hydrogel (shown in “Spheroid [Brightfield]” panels) were seeded onto LP9 mesothelial monolayers labeled with calcein-AM (shown in “LP9 [Green]” panels). “Overlay” panels are Brightfield and Green channel views superimposed. Mesothelial clearance was visualized by EVOS, representative images of ES-2 experiment are shown at 4× magnification, scale bars represent 650 μm; top panel. The area of clearance was quantified by ImageJ and calculated as a percentage of the total spheroid area. Data in the bar graph (bottom panel) represent the average fold change of the percentage of clearance area relative to Vec from three independent experiments performed with at least three replicates each (error bars are ± SEM; *P < 0.05, **P < 0.01). (C) Migration and invasion. ES-2 Vec and Mat cells were seeded onto uncoated or Matrigel-coated Transwells, allowed to migrate or invade for 16–24 h, stained by Kwik-Diff, and quantified by ImageJ; data represents average migration/invasion relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM, *P < 0.05, **P < 0.01). (D) Invasion of mesothelial monolayers. ES-2 and NCI/ADR-Res Vec or Mat cells labeled with calcein-AM were seeded onto Transwell filters coated with thin collagen and an LP9 mesothelial monolayer; after 24 h, invaded cells were fixed with PFA, visualized by EVOS, and quantified by ImageJ. A representative image of one field of invaded cells is shown (4× magnification; scale bars represent 650 μm). Data represents the average ratio of invaded cells relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM, **P < 0.01, ***P < 0.005). (E) 3D collagen invasion. ES-2 and NCI/ADR-Res Vec or Mat spheroids formed on agarose hydrogel were embedded into a 3D type I collagen matrix in chamber slides and allowed to invade for 48 or 72 h respectively; spheroids were visualized by EVOS (representative images shown at 4× magnification; scale bars represent 650 μm). The average fold change of the percentage increase in spheroid size relative to Vec was quantified by ImageJ from three experiments performed with six to eight replicates (error bars are ± SEM, *P < 0.05 ***P < 0.005). All P values were calculated according to two-tailed unpaired Student’s t tests.
Matriptase expression enhances spheroid adhesion, mesothelial clearance, and migration/invasion. (A) Representative image of spheroid adhesion to mesothelial monolayers. NCI/ADR-Res Vec and Mat spheroids were formed on agarose hydrogel and seeded onto confluent LP9 monolayers (formed on thin collagen), allowed to adhere for 1 h, washed, and adherent spheroids visualized by EVOS; representative stitched images taken at 4× magnification of whole-well scans are shown (scale bars represent 1.4 mm). A quantitative analysis of three independent experiments performed in triplicate is provided in Fig. 3 A. (B) Mesothelial clearance. NCI/ADR-Res Vec or Mat spheroids were formed on agarose hydrogel, seeded onto confluent, fluorescently labeled LP9 monolayers (calcein-AM), and mesothelial clearance was visualized up to 5 h by EVOS; representative images are shown at 4× magnification, scale bars represent 650 μm. (C) Migration and invasion. SKOV3 Vec and Mat cells were seeded onto uncoated or Matrigel-coated transwells, allowed to migrate or invade for 16–24 h, stained by Kwik-Diff, and quantified by ImageJ. Data represents average migration/invasion relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM; **P < 0.01, two-tailed unpaired Student’s t test).
Matriptase expression enhances spheroid adhesion, mesothelial clearance, and migration/invasion. (A) Representative image of spheroid adhesion to mesothelial monolayers. NCI/ADR-Res Vec and Mat spheroids were formed on agarose hydrogel and seeded onto confluent LP9 monolayers (formed on thin collagen), allowed to adhere for 1 h, washed, and adherent spheroids visualized by EVOS; representative stitched images taken at 4× magnification of whole-well scans are shown (scale bars represent 1.4 mm). A quantitative analysis of three independent experiments performed in triplicate is provided in Fig. 3 A. (B) Mesothelial clearance. NCI/ADR-Res Vec or Mat spheroids were formed on agarose hydrogel, seeded onto confluent, fluorescently labeled LP9 monolayers (calcein-AM), and mesothelial clearance was visualized up to 5 h by EVOS; representative images are shown at 4× magnification, scale bars represent 650 μm. (C) Migration and invasion. SKOV3 Vec and Mat cells were seeded onto uncoated or Matrigel-coated transwells, allowed to migrate or invade for 16–24 h, stained by Kwik-Diff, and quantified by ImageJ. Data represents average migration/invasion relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM; **P < 0.01, two-tailed unpaired Student’s t test).
Matriptase activity enhances spheroid-mediated mesothelial clearance
Upon successful initial adhesion, OvCa spheroids have been shown to retract and displace the mesothelial cell lining before entering the submesothelial environment (Iwanicki et al., 2011). 3 h after adhesion, ES-2 (Fig. 3 B) and NCI/ADR-Res (Fig. S4 B) Mat spheroids demonstrated significantly enhanced displacement of LP9 mesothelial monolayers compared with Vec control spheroids, ∼5- and 3.5-fold, respectively, indicating enhanced mesothelial clearance capabilities.
Matriptase activity enhances migration and invasion
In a further set of experiments, we asked whether overactive matriptase affected cell migration and invasion. Cell migration through Transwell filters was significantly increased in all Mat cell lines, displaying over twofold higher migratory ability than their corresponding Vec controls (Fig. 3 C and Fig. S4 C). Similarly, invasion through a Matrigel matrix was significantly elevated in all the cell lines as compared with their corresponding Vec controls (Fig. 3 C and Fig. S4 C).
Matriptase activity enhances invasion through mesothelial monolayers and into the submesothelial matrix
Upon successful penetration of the mesothelial cell layer and basal lamina, advanced ovarian tumors invade through the collagen-laden submesothelial matrix to establish secondary lesions. When ES-2 and NCI/ADR-Res Mat cells were seeded on LP9 mesothelial monolayers grown on a thin layer of collagen on Transwell filters, they were significantly more invasive than Vec control cells (Fig. 3 D). Further, when ES-2 and NCI/ADR-Res Vec and Mat spheroids were embedded into a 3D type I collagen gel, Mat spheroids displayed a significantly increased area of invasion relative to Vec spheroids (Fig. 3 E).
Altogether, these data demonstrate a functional role for increased matriptase activity to drive the transition from condensed, tight spheroids to a loose, invasive spheroid phenotype in an in vivo orthotopic xenograft model of advanced OvCa and in in vitro OvCa cell culture models.
The matriptase-mediated loose spheroid morphology is dependent on the activation of PAR-2
Downstream substrates and signaling pathways activated by matriptase that have been implicated in tumor malignancy are found to be cell-type- and context-specific (List et al., 2006). Previous studies have shown that matriptase is an important activator of the G-protein coupled receptor, protease-activated receptor-2 (PAR-2) that can induce a myriad of signaling responses that depend on the cell type and context, including malignant transformation, tumorigenic signaling, inflammation, and prometastatic signaling (Pawar et al., 2019). PAR-2 is elevated in OvCa compared with normal tissues (Jahan et al., 2007), and increased expression has been correlated with decreased OvCa patient survival (Aman et al., 2017); however, the functional significance of PAR-2 signaling in OvCa progression is as yet not known. Differential gene chip analysis of PAR-2 mRNA expression in nonpaired ovarian tumor and normal tissues using TNM plot shows significantly elevated PAR-2 in ovarian tumors (Fig. 4 A). In addition, high PAR-2 expression correlates with poor overall progression-free survival for advanced-stage OvCa patients by Kaplan–Meier survival analysis (Fig. 4 B), consistent with other reports (Aman et al., 2017; Jahan et al., 2007). Gene expression profiling of human OvCa patient tissues across all stages by TissueScan array (Origene) also demonstrated increased PAR-2 and matriptase mRNA compared with the normal ovary (Fig. S5 A). Similar to matriptase expression, PAR-2 is also expressed at varying levels in a panel of five OvCa cell lines (Fig. S5 B). Enhanced expression of matriptase and its substrate PAR-2 in OvCa tissues compared with the normal ovary suggests potential involvement or crosstalk of these two important signaling molecules (Pawar et al., 2019) in the promotion of advanced OvCa.
Matriptase-mediated loose spheroid morphology is attenuated by PAR-2 inhibition. (A) Analysis of PAR-2 mRNA expression amongst normal ovary tissue (n = 46) and ovarian tumors (n = 744) using publicly available gene chip data obtained from TNM Plotter (P = 1.54 e−06). (B) Kaplan–Meier progression-free survival analysis of a cohort of stage III and IV OvCa patients with high and low PAR-2 expression using publicly available data obtained from KMPlotter (outlier arrays excluded; n = 220; logrank P = 0.012). (C and D) ES-2 and NCI/ADR-Res Vec or Mat hanging-drop spheroids were formed in the presence of vehicle control (DMSO), GB-83 (5 and 25 μM respectively), or SAM11 (2 ng/ml and 1 μg/ml, respectively) for 4 d. Spheroids were visualized by EVOS (representative images shown at 4× magnification, scale bars represent 650 μm in left image panels) and morphology was assessed under each condition. Graphs show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represents the average of three independent experiments (error bars are ± SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, ns = not significant, two-tailed unpaired Student’s t tests). (E) Homotypic cell–cell interactions. ES-2 and NCI/ADR-Res Vec and Mat cells labeled with CellTrackerOrange were seeded onto respective homotypic monolayers in the presence of vehicle control (DMSO) or GB-83 (25 μM); fluorescence was measured after 1 h of adhesion, before and after wash with PBS. Fold change of remaining fluorescence relative to Vec is represented as an average of three independent experiments performed with at least three replicates each (error bars are ± SEM; *P < 0.05, ***P < 0.005, ns = not significant, two-tailed unpaired Student’s t tests). (F) Spheroid disaggregation. ES-2 and NCI/ADR-Res Vec and Mat spheroids generated on agarose hydrogel and labeled with calcein-AM were seeded onto LP9 mesothelial monolayers and disaggregation in the presence of vehicle control (DMSO) or GB-83 (25 μM) was monitored over 72 h by EVOS. The percentage increase in spheroid area was measured by ImageJ and represented as an average of three independent experiments performed in triplicate (error bars are ± SEM; **P < 0.01, ns = not significant, two-tailed unpaired Student’s t tests).
Matriptase-mediated loose spheroid morphology is attenuated by PAR-2 inhibition. (A) Analysis of PAR-2 mRNA expression amongst normal ovary tissue (n = 46) and ovarian tumors (n = 744) using publicly available gene chip data obtained from TNM Plotter (P = 1.54 e−06). (B) Kaplan–Meier progression-free survival analysis of a cohort of stage III and IV OvCa patients with high and low PAR-2 expression using publicly available data obtained from KMPlotter (outlier arrays excluded; n = 220; logrank P = 0.012). (C and D) ES-2 and NCI/ADR-Res Vec or Mat hanging-drop spheroids were formed in the presence of vehicle control (DMSO), GB-83 (5 and 25 μM respectively), or SAM11 (2 ng/ml and 1 μg/ml, respectively) for 4 d. Spheroids were visualized by EVOS (representative images shown at 4× magnification, scale bars represent 650 μm in left image panels) and morphology was assessed under each condition. Graphs show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represents the average of three independent experiments (error bars are ± SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, ns = not significant, two-tailed unpaired Student’s t tests). (E) Homotypic cell–cell interactions. ES-2 and NCI/ADR-Res Vec and Mat cells labeled with CellTrackerOrange were seeded onto respective homotypic monolayers in the presence of vehicle control (DMSO) or GB-83 (25 μM); fluorescence was measured after 1 h of adhesion, before and after wash with PBS. Fold change of remaining fluorescence relative to Vec is represented as an average of three independent experiments performed with at least three replicates each (error bars are ± SEM; *P < 0.05, ***P < 0.005, ns = not significant, two-tailed unpaired Student’s t tests). (F) Spheroid disaggregation. ES-2 and NCI/ADR-Res Vec and Mat spheroids generated on agarose hydrogel and labeled with calcein-AM were seeded onto LP9 mesothelial monolayers and disaggregation in the presence of vehicle control (DMSO) or GB-83 (25 μM) was monitored over 72 h by EVOS. The percentage increase in spheroid area was measured by ImageJ and represented as an average of three independent experiments performed in triplicate (error bars are ± SEM; **P < 0.01, ns = not significant, two-tailed unpaired Student’s t tests).
PAR-2 and matriptase are both overexpressed in human OvCa tumors compared to normal ovary tissue. (A) Human TissueScan Ovarian Cancer cDNA Array of 40 ovarian tumor specimens across stages I–IV and 8 normal ovary tissue specimens were assessed for PAR-2 (F2RL1) and matriptase (ST14) mRNA expression by qPCR. Data is normalized to a housekeeping control and represented relative to normal tissue. (B) PAR-2 is expressed in OvCa cell lines. Whole-cell lysates were collected from IOSE397 cells and 5 OvCa cell lines, analyzed by SDS-PAGE, and immunoblotted with mouse anti-human PAR-2 (SAM11) or rabbit anti-human β-tubulin antibodies. (C and D) Matriptase-mediated loose spheroid morphology is attenuated by PAR-2 blocking antibody SAM11. ES-2 and NCI/ADR-Res Vec and Mat hanging-drop spheroids were formed for 4 d in the presence of vehicle control (DMSO) or PAR-2 blocking antibody SAM11. Representative images of DMSO and SAM11 treated Vec and Mat spheroids are shown at 4× magnification using EVOS; scale bars represent 650 μm. Source data are available for this figure: SourceData FS5.
PAR-2 and matriptase are both overexpressed in human OvCa tumors compared to normal ovary tissue. (A) Human TissueScan Ovarian Cancer cDNA Array of 40 ovarian tumor specimens across stages I–IV and 8 normal ovary tissue specimens were assessed for PAR-2 (F2RL1) and matriptase (ST14) mRNA expression by qPCR. Data is normalized to a housekeeping control and represented relative to normal tissue. (B) PAR-2 is expressed in OvCa cell lines. Whole-cell lysates were collected from IOSE397 cells and 5 OvCa cell lines, analyzed by SDS-PAGE, and immunoblotted with mouse anti-human PAR-2 (SAM11) or rabbit anti-human β-tubulin antibodies. (C and D) Matriptase-mediated loose spheroid morphology is attenuated by PAR-2 blocking antibody SAM11. ES-2 and NCI/ADR-Res Vec and Mat hanging-drop spheroids were formed for 4 d in the presence of vehicle control (DMSO) or PAR-2 blocking antibody SAM11. Representative images of DMSO and SAM11 treated Vec and Mat spheroids are shown at 4× magnification using EVOS; scale bars represent 650 μm. Source data are available for this figure: SourceData FS5.
To determine the involvement of PAR-2 in the disruptive functions of matriptase activity in OvCa spheroids, we utilized two inhibitors of PAR-2 activation and signaling: GB-83, a specific reversible chemical antagonist of PAR-2 (Bang et al., 2021; Barry et al., 2010) and SAM11, a PAR-2 blocking antibody directed against the tethered ligand sequence that inhibits cleavage by PAR-2 activating proteases (Crilly et al., 2012). Treatment of ES-2 and NCI/ADR-Res Mat and Vec hanging-drop spheroids with either GB-83 or SAM11 significantly reverted the predominantly loose Mat spheroid phenotype to the more compact Vec phenotype (Fig. 4, C and D; and Fig. S5, C and D). Treatment with GB-83 also abrogated the disrupted homotypic cell–cell interactions (Fig. 4 E) and the increased spheroid disaggregation (Fig. 4 F) characterized in ES-2 and NCI/ADR-Res Mat spheroids compared with Vec controls. Together, these data show that matriptase-mediated loose spheroid morphology and disruption of cell–cell homotypic adhesion are dependent on PAR-2 activation.
Matriptase activation of PAR-2 in loose spheroids triggers PI3K/Akt signaling
To identify downstream signaling pathways triggered by matriptase/PAR-2 activation in loose spheroids, we performed a screen of signaling pathway inhibitors previously reported to selectively block pathways downstream of PAR-2 activation (Adams et al., 2011). Neither the MEK1/2 inhibitor (U0126) nor the p38 MAPK inhibitor (SB202190) affected the loose morphology of Mat spheroids (Fig. 5 A). However, the specific PI3K pathway inhibitor LY294002 abrogated the loose morphology of both ES-2 and NCI/ADR-Res Mat spheroids, reverting them to a tighter phenotype (Fig. 5, A and B). Mat spheroids also expressed increased levels of phosphorylated Akt (p-Akt [Ser473]), a downstream target of activated PI3K (Altomare et al., 2004; Gao et al., 2004; Wu et al., 2020), compared with the respective Vec controls (Fig. 5 C; lanes 1 and 2). This increase was abrogated by LY294002 (lanes 5 and 6), demonstrating PI3K activation by matriptase-mediated signaling. Treatment with the PAR-2 inhibitor GB-83 prevented the matriptase-induced increase in p-Akt (Ser473) protein levels (Fig. 5 C, lanes 3 and 4), indicating that Akt pathway activation by matriptase is dependent on PAR-2. This was particularly evident in NCI/ADR-Res spheroids whose endogenous p-Akt (Ser473) levels were not affected by GB-83. Of note, levels of p-Akt (Ser473) in Vec cells remain unaffected by LY294002, suggesting that endogenous baseline p-Akt (Ser473) in these cell lines is likely regulated by alternative signaling pathways that are not fully or specifically inhibited by LY294002.
Matriptase activation of PAR-2 triggers PI3K/Akt signaling to mediate loose spheroid morphology. (A) ES-2 Vec or Mat cells were treated with vehicle control (DMSO), 10 μM U0126 (MEK1/2 inhibitor), 10 μM SB202190 (p38 MAPK inhibitor), or 10 μM LY294002 (PI3K inhibitor) and allowed to form hanging drop spheroids for 4 d. Graphs show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represents the average of three independent experiments (error bars are ± SEM, *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, ns = not significant). Right: Representative images of ES-2 Vec and Mat hanging drop spheroids treated with vehicle control (DMSO) or LY294002 are shown at 4× magnification; scale bars represent 650 μm. (B) NCI/ADR-Res Vec or Mat spheroids were treated with vehicle control (DMSO) or LY294002 (10 μM) for 4 d. Graphs show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represents the average of three independent experiments (error bars are ± SEM, *P < 0.05, **P < 0.01, ns = not significant). Right: Representative images at 4× magnification are shown; scale bars represent 650 μm. (C) Whole-cell lysates from ES-2 (left panels) and NCI/ADR-Res Vec and Mat (right panels) hanging drop spheroids treated with vehicle control (DMSO), GB-83 (5 μM for ES-2 and 25 μM for NCI/ADR-Res) or LY294002 (10 μM) were collected with RIPA lysis buffer, analyzed by SDS-PAGE and immunoblotted with rabbit anti-human p-Akt (Ser473), total Akt, and β-tubulin. GB-83 slightly affected total Akt levels in both ES-2 Mat and Vec spheroids. Densitometric analysis was conducted with ImageJ and reported as relative densitometric units normalized to β-tubulin and compared to Vec; average of three independent experiments (error bars are ± SEM, *P < 0.05, **P < 0.01, ns = not significant). All P values were calculated according to two-tailed unpaired Student’s t tests. Source data are available for this figure: SourceData F5.
Matriptase activation of PAR-2 triggers PI3K/Akt signaling to mediate loose spheroid morphology. (A) ES-2 Vec or Mat cells were treated with vehicle control (DMSO), 10 μM U0126 (MEK1/2 inhibitor), 10 μM SB202190 (p38 MAPK inhibitor), or 10 μM LY294002 (PI3K inhibitor) and allowed to form hanging drop spheroids for 4 d. Graphs show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represents the average of three independent experiments (error bars are ± SEM, *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, ns = not significant). Right: Representative images of ES-2 Vec and Mat hanging drop spheroids treated with vehicle control (DMSO) or LY294002 are shown at 4× magnification; scale bars represent 650 μm. (B) NCI/ADR-Res Vec or Mat spheroids were treated with vehicle control (DMSO) or LY294002 (10 μM) for 4 d. Graphs show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represents the average of three independent experiments (error bars are ± SEM, *P < 0.05, **P < 0.01, ns = not significant). Right: Representative images at 4× magnification are shown; scale bars represent 650 μm. (C) Whole-cell lysates from ES-2 (left panels) and NCI/ADR-Res Vec and Mat (right panels) hanging drop spheroids treated with vehicle control (DMSO), GB-83 (5 μM for ES-2 and 25 μM for NCI/ADR-Res) or LY294002 (10 μM) were collected with RIPA lysis buffer, analyzed by SDS-PAGE and immunoblotted with rabbit anti-human p-Akt (Ser473), total Akt, and β-tubulin. GB-83 slightly affected total Akt levels in both ES-2 Mat and Vec spheroids. Densitometric analysis was conducted with ImageJ and reported as relative densitometric units normalized to β-tubulin and compared to Vec; average of three independent experiments (error bars are ± SEM, *P < 0.05, **P < 0.01, ns = not significant). All P values were calculated according to two-tailed unpaired Student’s t tests. Source data are available for this figure: SourceData F5.
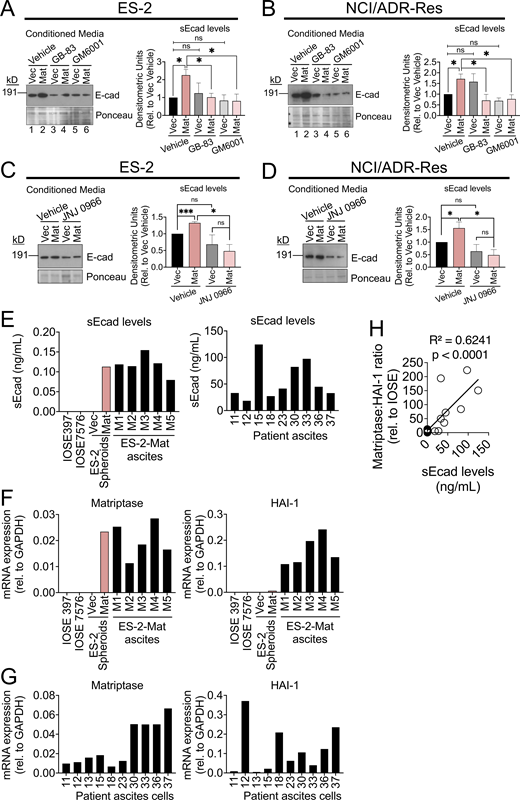
Matriptase activation of PAR-2 increases matrix-metalloprotease-9 (MMP9) expression, which cleaves E-cadherin to mediate a loose spheroid phenotype
Elevated levels of several matrix-metalloproteases (MMPs) have been associated with poor patient survival and have been proposed as reliable prognostic biomarkers in advanced-stage OvCa (Zeng et al., 2020). Specifically, the gelatinases MMP2 and MMP9 have been widely characterized as secreted and highly active in advanced OvCa, with elevated expression contributing to migration, invasion, ascites development, and ability to metastasize (Al-Alem and Curry, 2015). Treatment of ES-2 and NCI/ADR-Res Mat and Vec spheroids with the broad-spectrum MMP inhibitor GM6001 abrogated the loose spheroid morphology mediated by Mat expression (Fig. 6 A), demonstrating the involvement of MMP activity in spheroid disruption. We found that ES-2 and NCI/ADR-Res Mat cells express significant levels of MMP9 compared with Vec controls, whereas the levels of MMP2 were more modestly increased in ES-2 Mat cells compared with Vec controls and not elevated in NCI/ADR-Res Mat cells compared with controls (Fig. 6 B). The membrane-type MMP14, an activator of MMP2, was downregulated in both ES-2 and NCI/ADR-Res Mat cells compared with Vec cells. To further investigate the involvement of MMP9, ES-2 and NCI/ADR-Res Mat and Vec spheroids were treated with a specific inhibitor of MMP9, JNJ 0966, which inhibits MMP9 by blocking zymogen activation (Scannevin et al., 2017). JNJ 0966 attenuated loose spheroid formation (Fig. 6 C), demonstrating that MMP9 is specifically involved in spheroid disruption. By comparison, the MMP2 inhibitor, SB-3CT (Kleifeld et al., 2001), failed to affect loose Mat spheroid formation (Fig. S6 A). Treatment with the PAR-2 inhibitor GB-83 prevented the increase in MMP9 mRNA in both ES-2 and NCI/ADR-Res Mat cells (Fig. 6 D), showing that elevated MMP9 is PAR-2 dependent.
Matriptase activation of PAR-2 increases MMP9 expression to mediate a loose spheroid phenotype. (A) ES-2 and NCI/ADR-Res Vec and Mat hanging drop spheroids were grown in the presence of vehicle control (DMSO) or MMP inhibitor GM6001 (50 μM) for 4 d. Graphs show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represents the average of three independent experiments (error bars are ±SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, ns = not significant). (B) mRNA from ES-2 and NCI/ADR-Res Vec and Mat cells was collected and analyzed for MMP14, MMP2, and MMP9 expression by qPCR. mRNA expression was normalized to GAPDH and represented as average fold change relative to Vec (error bars are ± SEM from at least three independent experiments performed in triplicate; *P < 0.05, **P < 0.01). (C) ES-2 and NCI/ADR-Res Vec and Mat hanging drop spheroids were grown in the presence of vehicle control (DMSO) or MMP9 inhibitor JNJ 0966 (10 μM). Representative images at 4× magnification are shown in top panels; scale bars represent 650 μm. Graphs below the images show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represents the average of three independent experiments (error bars are ± SEM **P < 0.01, ***P < 0.005, ****P < 0.001, ns = not significant). (D) mRNA from ES-2 or NCI/ADR-Res Vec and Mat hanging drop spheroids treated with vehicle control (DMSO) or GB-83 (5 or 25 μM, respectively) was collected and analyzed by qPCR for MMP9 mRNA expression. mRNA expression was normalized to GAPDH and represented as average fold change relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM; *P < 0.05, **P < 0.01, ns = not significant). All P values were calculated according to two-tailed unpaired Student’s t tests.
Matriptase activation of PAR-2 increases MMP9 expression to mediate a loose spheroid phenotype. (A) ES-2 and NCI/ADR-Res Vec and Mat hanging drop spheroids were grown in the presence of vehicle control (DMSO) or MMP inhibitor GM6001 (50 μM) for 4 d. Graphs show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represents the average of three independent experiments (error bars are ±SEM; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, ns = not significant). (B) mRNA from ES-2 and NCI/ADR-Res Vec and Mat cells was collected and analyzed for MMP14, MMP2, and MMP9 expression by qPCR. mRNA expression was normalized to GAPDH and represented as average fold change relative to Vec (error bars are ± SEM from at least three independent experiments performed in triplicate; *P < 0.05, **P < 0.01). (C) ES-2 and NCI/ADR-Res Vec and Mat hanging drop spheroids were grown in the presence of vehicle control (DMSO) or MMP9 inhibitor JNJ 0966 (10 μM). Representative images at 4× magnification are shown in top panels; scale bars represent 650 μm. Graphs below the images show the quantitation of the spheroid morphology (% loose spheroids and % empty space) as described in the Materials and methods. Data represents the average of three independent experiments (error bars are ± SEM **P < 0.01, ***P < 0.005, ****P < 0.001, ns = not significant). (D) mRNA from ES-2 or NCI/ADR-Res Vec and Mat hanging drop spheroids treated with vehicle control (DMSO) or GB-83 (5 or 25 μM, respectively) was collected and analyzed by qPCR for MMP9 mRNA expression. mRNA expression was normalized to GAPDH and represented as average fold change relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM; *P < 0.05, **P < 0.01, ns = not significant). All P values were calculated according to two-tailed unpaired Student’s t tests.
Matriptase-mediated loose spheroids are dependent on MMP-9 activity. (A) ES-2 and NCI/ADR-Res Vec and Mat hanging drop spheroids were formed in the presence of MMP-2 inhibitor SB-3CT (2 μM) for 4 d. At least five representative images were taken from three independent experiments and the average percentage of loose morphology was quantified (error bars are ± SEM, **P < 0.01, ***P < 0.005, ns = not significant). (B) mRNA was collected from ES-2 and NCI/ADR-Res Vec and Mat cells and analyzed by qPCR for α5-integrin expression. Data represents average fold change relative to Vec controls normalized to GAPDH from three independent experiments performed in triplicate (error bars are ± SEM; ns = not significant). All P values were calculated according to two-tailed, unpaired Student’s t tests.
Matriptase-mediated loose spheroids are dependent on MMP-9 activity. (A) ES-2 and NCI/ADR-Res Vec and Mat hanging drop spheroids were formed in the presence of MMP-2 inhibitor SB-3CT (2 μM) for 4 d. At least five representative images were taken from three independent experiments and the average percentage of loose morphology was quantified (error bars are ± SEM, **P < 0.01, ***P < 0.005, ns = not significant). (B) mRNA was collected from ES-2 and NCI/ADR-Res Vec and Mat cells and analyzed by qPCR for α5-integrin expression. Data represents average fold change relative to Vec controls normalized to GAPDH from three independent experiments performed in triplicate (error bars are ± SEM; ns = not significant). All P values were calculated according to two-tailed, unpaired Student’s t tests.
Elevated MMP9 has been reported to lead to a loss of E-cadherin and disrupted cell–cell junctional integrity that can promote a migratory and invasive phenotype (Cowden Dahl et al., 2008). MMP9 directly cleaves E-cadherin (Symowicz et al., 2007), releasing the ectodomain and resulting in soluble-E-cadherin (sEcad) accumulation, which can be detected using an ectodomain-specific E-cadherin antibody (HECD-1). We detected elevated levels of sEcad in conditioned media from ES-2 and NCI/ADR-Res Mat hanging drop spheroids compared with Vec spheroids (Fig. 7, A and B, lanes 1–2), indicating enhanced E-cadherin cleavage in the presence of overactive matriptase. When spheroids were treated with GB-83 (Fig. 7, A and B, lanes 3–4) or GM6001 (Fig. 7, A and B, lanes 5–6), the release of sEcad into the conditioned media was significantly reduced. Moreover, treatment of spheroids with JNJ 0966 (Fig. 7, C and D) also significantly reduced the release of sEcad into the conditioned media of ES-2 and NCI/ADR-Res Mat spheroids, demonstrating that matriptase activation of a PAR-2/MMP9 signaling axis is involved in E-cadherin cleavage and release of the extracellular domain. While it has been reported that downregulation of E-cadherin increases the expression of α5-integrin to facilitate OvCa invasion and metastasis (Sawada et al., 2008), α5-integrin levels were not affected by Mat overexpression in ES-2 and NCI/ADR-Res cells compared with Vec controls (Fig. S6 B). Together, these data demonstrate that matriptase activation of PAR-2 signaling stimulates upregulation of MMP9 to cause cleavage and release of the E-cadherin extracellular domain, promoting loose spheroids by disrupting cell–cell adhesive contacts.
Matriptase activation of a PAR-2/MMP9 signaling axis cleaves E-cadherin to mediate loose spheroid formation. (A and B) ES-2 and NCI/ADR-Res Vec and Mat hanging-drop spheroids were formed in serum-free media for 4 d in the presence of vehicle control (DMSO), GB-83 (5 μM), and GM6001 (50 μM) or DMSO and JNJ 0966 (10 μM; C and D). Conditioned media was concentrated ∼10×, analyzed by SDS-PAGE, and immunoblotted for mouse-anti human E-cadherin recognizing the ectodomain region (HECD1-Invitrogen) or total protein Ponceau stain. Densitometric analysis was conducted using ImageJ and represents average soluble E-cadherin (sEcad) levels normalized to total protein (Ponceau staining) and relative to Vec DMSO (error bars are ± SEM of three independent experiments; *P < 0.05, ***P < 0.005, ns = not significant, two-tailed unpaired Student’s t tests). (E) Left panel: Conditioned media from IOSE397 and IOSE7576 cells, ES-2-Mat and ES-2-Vec spheroids, and clarified ascites fluid from mice bearing ES-2 Mat tumors were assessed for sEcad levels by quantitative ELISA (0.5–0.15 ng/ml). Right panel: Clarified ascites fluid from nine OvCa patients were assessed for sEcad levels by quantitative ELISA (10–125 ng/ml). Patient information is in the Materials and methods. (F) Matriptase (left panel) and HAI-1 (right panel) mRNA levels were determined by qPCR analysis of IOSE397 and IOSE7576 cells, ES-2 Vec and Mat spheroids, and tumor cells isolated from ascites of ES-2-Mat tumor-bearing mice. qPCR analysis was conducted in triplicate and mRNA expression was normalized to GAPDH. (G) Matriptase (left panel) and HAI-1 (right panel) mRNA levels were determined by qPCR analysis of tumor cells recovered from ascites fluid from nine independent patients. qPCR analysis was conducted in triplicate and mRNA expression was normalized to GAPDH. (H) Linear regression analysis of sEcad levels versus matriptase:HAI-1 ratios revealed a significant positive correlation (R2 = 0.6241, P <0.0001). Source data are available for this figure: SourceData F7.
Matriptase activation of a PAR-2/MMP9 signaling axis cleaves E-cadherin to mediate loose spheroid formation. (A and B) ES-2 and NCI/ADR-Res Vec and Mat hanging-drop spheroids were formed in serum-free media for 4 d in the presence of vehicle control (DMSO), GB-83 (5 μM), and GM6001 (50 μM) or DMSO and JNJ 0966 (10 μM; C and D). Conditioned media was concentrated ∼10×, analyzed by SDS-PAGE, and immunoblotted for mouse-anti human E-cadherin recognizing the ectodomain region (HECD1-Invitrogen) or total protein Ponceau stain. Densitometric analysis was conducted using ImageJ and represents average soluble E-cadherin (sEcad) levels normalized to total protein (Ponceau staining) and relative to Vec DMSO (error bars are ± SEM of three independent experiments; *P < 0.05, ***P < 0.005, ns = not significant, two-tailed unpaired Student’s t tests). (E) Left panel: Conditioned media from IOSE397 and IOSE7576 cells, ES-2-Mat and ES-2-Vec spheroids, and clarified ascites fluid from mice bearing ES-2 Mat tumors were assessed for sEcad levels by quantitative ELISA (0.5–0.15 ng/ml). Right panel: Clarified ascites fluid from nine OvCa patients were assessed for sEcad levels by quantitative ELISA (10–125 ng/ml). Patient information is in the Materials and methods. (F) Matriptase (left panel) and HAI-1 (right panel) mRNA levels were determined by qPCR analysis of IOSE397 and IOSE7576 cells, ES-2 Vec and Mat spheroids, and tumor cells isolated from ascites of ES-2-Mat tumor-bearing mice. qPCR analysis was conducted in triplicate and mRNA expression was normalized to GAPDH. (G) Matriptase (left panel) and HAI-1 (right panel) mRNA levels were determined by qPCR analysis of tumor cells recovered from ascites fluid from nine independent patients. qPCR analysis was conducted in triplicate and mRNA expression was normalized to GAPDH. (H) Linear regression analysis of sEcad levels versus matriptase:HAI-1 ratios revealed a significant positive correlation (R2 = 0.6241, P <0.0001). Source data are available for this figure: SourceData F7.
E-cadherin cleavage and release by matriptase/PAR-2 signaling was further investigated by quantitating sEcad levels by ELISA in (1) conditioned media of ES-2 Mat and Vec spheroids and (2) clarified ascites fluids from mice bearing ES-2-Mat tumors (see Fig. 1 E). sEcad (0.08–0.15 ng/ml) was detected in the conditioned media of Mat spheroids and clarified mouse ascites fluids, whereas sEcad was undetectable in conditioned media from ES-2-Vec and two control non-malignant IOSE cell lines (Fig. 7 E). In addition, clarified ascites fluids recovered from nine OvCa patients representing different stages, subtypes, and chemotherapy treatment status (see Materials and methods) displayed elevated sEcad levels (Fig. 7 E).
To further investigate the relationship between matriptase levels and sEcad release, the relative Matriptase and HAI-1 mRNA levels in (1) tumor cells recovered from the ascites fluids of the five ES-2-Mat tumor-bearing mice (from Fig. 1 D) and (2) human tumor cells isolated from the nine patients’ ascites fluids were determined (Fig. 7, F and G). Linear regression analysis of the qPCR data comparing the ratio of matriptase to HAI-1 mRNA (Fig. 7, F and G) with the sEcad levels (Fig. 7 E) revealed a positive correlation (Fig. 7 H). Together, these data implicate elevated matriptase expression and activity in increased shedding of sEcad, providing a plausible mechanism for the loss of cell–cell adhesion and the increased invasive properties of Mat overexpressing spheroids.
Together, these data identify a novel matriptase-activated PAR-2/PI3K/Akt/MMP9 signaling axis that results in E-cadherin cleavage and sEcad release, disrupting cell–cell interactions and resulting in a loose, spheroid-like morphology that is highly prone to peritoneal dissemination (Fig. 8 A).
Matriptase/PAR-2 signaling and promotion of metastatic OvCa dissemination. (A) Proposed model of a matriptase/PAR-2/PI3K/Akt/MMP9/E-cadherin signaling axis which promotes OvCa spheroid dissemination. (B) Kaplan–Meier progression-free survival analysis of a cohort of OvCa patients stratified by matriptase mRNA expression above the median (n = 281; logrank P = 0.0072) or matriptase mRNA expression below the median (n = 281; logrank P = 0.15; C) using publicly available data obtained from KMPlotter. All patients who expressed CA125 mRNA levels in lower quartile and outlier arrays were excluded.
Matriptase/PAR-2 signaling and promotion of metastatic OvCa dissemination. (A) Proposed model of a matriptase/PAR-2/PI3K/Akt/MMP9/E-cadherin signaling axis which promotes OvCa spheroid dissemination. (B) Kaplan–Meier progression-free survival analysis of a cohort of OvCa patients stratified by matriptase mRNA expression above the median (n = 281; logrank P = 0.0072) or matriptase mRNA expression below the median (n = 281; logrank P = 0.15; C) using publicly available data obtained from KMPlotter. All patients who expressed CA125 mRNA levels in lower quartile and outlier arrays were excluded.
Discussion
High fatality in OvCa is attributed to passive OvCa dissemination, propagated by multicellular spheroid clusters in the peritoneal cavity and subsequent seeding and metastatic invasion into peritoneal organs. Here, we identify a novel function for tumor-associated overactive matriptase as an orchestrator of disrupted cell–cell adhesion in OvCa spheroids that propagates enhanced dissemination and metastasis. Matriptase exerts these effects by constitutive proteolytic activation of PAR-2, triggering a PI3K/Akt/MMP9 signaling axis which ultimately cleaves and releases the soluble E-cadherin ectodomain, resulting in disruption of homotypic cell–cell interactions, promoting OvCa invasion and driving advanced disease.
HAI-1 is the major regulator of matriptase proteolytic activity (Friis et al., 2014). The importance of matriptase activity, when unopposed by HAI-1, is highlighted by survival data (Fig. 1 A), where patients with low HAI-1 expression and high matriptase expression exhibit poor progression-free survival, while patients with high matriptase and high HAI-1 expression exhibit more favorable progression-free survival. The importance of the ratio of matriptase to HAI-1 may explain why some studies find that increased matriptase expression correlates with advanced clinical stages of OvCa (Jin et al., 2006; Tanimoto et al., 2005), while others report no significant changes in matriptase expression across OvCa stages (Köbel et al., 2008).
Studies examining broad biomarker signatures indicate that different OvCa subtypes may represent distinct diseases (Köbel et al., 2008). We find that the prometastatic phenotype conferred by overactive matriptase occurs across multiple OvCa cell lines in collected ascites-derived patient tumor samples spanning multiple histological subtypes and is correlated with poor patient survival across multiple tumor subtypes in publicly available datasets. These data suggest that the adverse effects of overactive matriptase are broadly applicable. Importantly, they highlight that a focus on gene or protein abundance alone can be misleading for identifying key signaling molecules that execute metastatic functions. Deregulated overactivity of tumorigenic proteases appears in fact to be the detrimental factor for patient outcomes.
The present studies uniquely identify activation of prometastatic signaling pathways downstream of PAR-2 resulting from constitutively enhanced matriptase on the surface of OvCa cells, a phenomenon that is highly likely to occur in OvCa cells that aberrantly overexpress PAR-2 and overactive matriptase. OvCa patients with high PAR-2 expression and high matriptase expression experience significantly decreased progression-free survival (Fig. 8 B), whereas patients with high PAR-2 expression and low matriptase expression do not experience any significantly different progression-free survival outcomes (Fig. 8 C). It would appear that elevated PAR-2 levels are only detrimental to patient survival when matriptase overactivity enables the activation of prometastatic signaling.
Heterogenous multicellular spheroid populations are present in malignant patients’ ascites fluid (Klymenko et al., 2017a, 2017b), but molecular determinants and how they influence aggressive diseases have been unclear. Here, we have identified for the first time a molecular pathway that can drive the transition from a compact spheroid to a loose, more metastatic tumor phenotype and define a mechanism for the disruption of cell–cell adhesive contacts. The loose spheroid morphology and enhanced migratory and invasive properties were demonstrated to be dependent on a matriptase-activated PAR-2 dependent PI3K/Akt signaling axis. Deregulation of the PI3K/Akt signaling pathway has been identified as the most frequent alteration in OvCa, with over 70% of OvCa cases exhibiting excessive pathway activation (Li et al., 2014), leading to enhanced proliferative, migratory, and invasive tumors (Bai et al., 2015). PI3K/Akt pathway activation has been demonstrated to upregulate MMP9 to enhance migration and invasion in several tumor types (Ellerbroek et al., 2001; Thant et al., 2000) and PAR-2 activation can upregulate MMP9 (Vliagoftis et al., 2000; Wilson et al., 2004), consistent with our findings in OvCa. In other tumor contexts, PAR-2 and PI3K/Akt signal activation have been reported to regulate cytoskeletal tension by actin and actomyosin remodeling (Das et al., 2018) and are postulated to directly oppose cell–cell adhesion required for cell aggregation (Saias et al., 2015). In accordance with this, overactive matriptase in zebrafish hai-1a morphants is correlated with enhanced PAR-2 dependent cell extrusion in periderm keratinocytes (Schepis et al., 2018). E-cadherin plays a critical role in maintaining cohesive contacts during spheroid formation (Na et al., 2020; Symowicz et al., 2007). It is likely that matriptase overactivity activates constitutive PAR-2 signaling and downstream E-cadherin cleavage by MMP9 to orchestrate a shift in cytoskeletal tension, disrupting E-cadherin-mediated cell–cell adhesion and conferring mesenchymal and invasive properties to OvCa spheroids. While several studies have associated loss of E-cadherin expression to advanced-stage ovarian tumors (Hudson et al., 2008), increased levels of soluble E-cadherin in patients’ ascites fluid (Sundfeldt et al., 2001; Symowicz et al., 2007) suggests that mechanisms of cleavage and release of the ectodomain play a crucial role in OvCa progression. The matriptase-directed signaling mechanism uncovered here is significant for enhancing the broader understanding of dynamic cell–cell interactions that influence epithelial barrier integrity and function in tissues and in disease states where matriptase is overactive, and uniquely focuses on posttranscriptional alterations of E-cadherin to disrupt cell–cell adhesions in OvCa spheroid behaviors.
In summary, this study demonstrates a critical function and molecular mechanism by which hyperactive matriptase executes prometastatic activities to advance OvCa dissemination. The inherent heterogeneity and phenotypic plasticity of advanced disseminated OvCa remain a challenge for standard patient therapies, which are largely focused on inhibiting tumor cell proliferation. Recent studies show that ascites-derived tumor cells from patients with advanced, chemoresistant disease form irregular and loosely clustered spheroids ex vivo (Goyeneche et al., 2020), emphasizing the potential importance of this morphology for advanced disease. The identification of matriptase expression and activity as a tumorigenic biomarker (LeBeau et al., 2013) and the findings of this study demonstrating upregulated PAR-2 as an essential signaling node, along with the PI3K/Akt/MMP9/E-cadherin axis, will enable potential interventions for targeting of multiple levels of this complex signaling cascade to improve outcomes for women diagnosed with advanced OvCa.
Materials and methods
OvCa cell lines
OvCa cell lines were cultured and maintained at 37°C in a 5% CO2/95% air environment. The cell lines, ES-2 (originally identified as clear-cell carcinoma [Lengyel et al., 2014] but potentially high-grade serous carcinoma [HGSC; Devor et al., 2021; Kwok et al., 2014]), SKOV3 (ovarian serous cystadenocarcinoma derived from ascites), CAOV3 (HGSC derived from primary ovarian carcinoma), and COV362 (HGSC derived from pleural effusion) were obtained from American Type Culture Collection (ATCC) and cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (P/S/G). NCI/ADR-Res (multidrug resistant HGSC derived from OVCAR8 [Domcke et al., 2013; Liscovitch and Ravid, 2007]) cells were obtained from the National Cancer Institute Division of Cancer Treatment and Diagnosis (NCI-DCTD) repository and cultured in RPMI 1640 supplemented with 10% FBS and P/S/G. OVCAR3 (HGSC derived from ascites) cells were cultured in RPMI 1640 supplemented with 20% FBS and 0.01 mg/ml insulin. IOSE397 and IOSE7576 (immortalized ovarian surface epithelial cells) were obtained from the Canadian Ovarian Tissue Bank (University of British Columbia, Vancouver, Canada) and cultured in medium 199:MCDB 105 (1:1) supplemented with 15% FBS, 1% non-essential amino acids, and P/S/G. LP9 (normal human peritoneal mesothelial cells) were obtained from Coriell Institute and cultured in Medium 199 and MCDB 105 (1:1) supplemented with 2 mM L-glutamine, 15% FBS, human epidermal growth factor (10 ng/ml), and hydrocortisone (0.4 μg/ml). All cells were routinely tested and confirmed to be Mycoplasma negative using the MycoAlert Mycoplasma Detection Kit assay (Lonza). All cells were passaged routinely using the non-enzymatic cell dissociation reagent Versene (Gibco).
Patient-derived samples
Deidentified patient tumor cells and ascites fluids were used in this study. Tissues were recovered at the time of surgery in excess of pathology requirements with informed consent under protocol GCC1488. This study was approved by the Institutional Review Board of the University of Maryland, Baltimore and conducted in accordance with the Declaration of Helsinki. Collected ascites was centrifuged; the clarified ascites fluid and cellular content were frozen for subsequent analyses. Spheroids were generated using freshly isolated cells in Medium 199:MCDB 105 (1:1) supplemented with 15% FBS, 1% non-essential amino acids, and penicillin–streptomycin–glutamine (10378-016; Gibco) on agarose hydrogels. Patient details are as follows: Patient 11: HGSC, chemoresistant, stage IIIB; Patient 12: low-grade serous carcinoma (LGSC), chemo-naïve, stage IIIC; Patient 15: endometrioid, chemo-naïve, stage IIIA(ii); Patient 18: HGSC, chemo-naïve, stage IIIB; Patient 23: HGSC, chemoresistant, stage IVB; Patient 30: HGSC, chemo-naïve, stage IVB; Patient 33: HGSC/endometrioid, chemo-naïve, stage IIIB; Patient 36: HGSC, chemo-naïve, stage IV; and Patient 37: Mullerian, chemo-naïve, stage undetermined.
Cell lysis and immunoblot analysis
Cells and pooled spheroids (∼100 spheroids per condition) were lysed with RIPA lysis buffer (R0278-50 Ml; Sigma-Aldrich) with Complete Mini Protease Inhibitor Cocktail (11697498001; Roche) & PhosSTOP Phosphatase Inhibitor Cocktail (4906845001; Roche). Protein concentrations were determined using Protein Assay Dye (5000006; Bio-Rad), and samples containing equal protein were prepared with 6× LDS sample buffer containing 5% β-mercaptoethanol and heated at 70°C for 10 min. Samples were separated on SDS-PAGE gels (NuPAGE 4–12% Bis-Tris Protein Gels, NP0322BOX; Invitrogen) according to standard methods, transferred to 0.45-μm PVDF membrane (EMD Millipore), and blocked in 5% milk in PBST, prior to incubation with primary and secondary antibodies. HRP activity was detected by SuperSignal West Pico PLUS Chemiluminescent Substrate (34577; Thermo Fisher Scientific). Serum-free conditioned media from hanging-drop spheroid cultures was collected, centrifuged to remove cellular contents, and concentrated using Pierce protein concentrators PES (10 K MWCO; 88513; Thermo Fisher Scientific). Densitometry analysis of immunoblots was performed using ImageJ (National Institutes of Health) software and signals were normalized to β-tubulin loading control or total protein by Ponceau S staining. Antibodies used were rabbit anti-human matriptase polyclonal antibody (pAb; IM1014; Millipore), mouse anti-HAI-1 monoclonal antibody (mAb; 67031-1-Ig; ProteinTech), rabbit anti-human p-Akt (Ser473; #9271; Cell Signaling), mouse anti-human Total Akt mAb (#40D8; Cell Signaling), mouse anti-human E-cadherin (ectodomain) mAb (HECD-1), rabbit anti-human β-tubulin pAb (H-235; Santa Cruz), goat anti-mouse-HRP, and mouse anti-rabbit-HRP secondary antibodies (Jackson Immunoresearch Laboratories). A11 (rabbit recombinant monoclonal matriptase inhibiting antibody) was obtained from MilliporeSigma (MABC115) and SAM11 (mouse monoclonal PAR-2 inhibiting antibody) from SantaCruz Biotechnology (sc-13504).
Real-time quantitative PCR (qPCR)
RNA was prepared from cells and pooled spheroid cultures (∼100 spheroid replicates per condition) using RNeasy Mini Kit (74106; Qiagen). cDNA was generated using High-Capacity cDNA Reverse Transcription Kit (4368814; Life Technologies). RT-qPCR analysis was performed with Taqman Fast Advanced Master Mix (4444557) and Taqman primers using QuantStudio 3 Real Time-PCR System (Thermo Fisher Scientific). RT-qPCR was performed using Taqman primers (Thermo Fisher Scientific) for human matriptase (ST14 Hs00222707_m1), HAI-1 (SPINT1 Hs00173678_m1), PAR-2 (F2RL1 Hs_00608346), MMP14 (Hs01037003_g1), MMP2 Hs01548727_m1, MMP9 (Hs_00957562), Luciferase (Mr03987587_mr), and GAPDH (Hs02758991_g1). α5-integrin expression was analyzed using SYBR Green primers (ITGA5 Fwd 5′-TCCACTGGGGCTTGGGGGTC-3′, ITGA5 Rev 5′-GGGTACGGCGGGCACTGTTC-3′; Neiman et al., 2019) and normalized to RPLP0 expression (Fwd 5′-GGTGCCTCTGGAGATTTTAG-3′, Rev 5-CACTGGTCTCGGGCCGAGAA-3′). The TissueScan Ovarian Cancer cDNA Array II (HORT102; Origene), containing 40 samples covering four disease stages (8 stage I, 9 stage II, 17 stage III, and 6 stage IV) of varying OvCa histopathologies and eight normal ovary tissues was screened for matriptase mRNA expression using the primers listed above.
Measurement of cell surface serine protease activity
Cells were grown to ∼40% confluence in 96-well, black-walled, clear-bottom plates, washed in Opti-MEM I (31985062; Thermo Fisher Scientific), and incubated with 100 µM Boc-QAR-AMC fluorogenic peptide substrate (ES014; R&D Systems) in Opti-MEM I in the absence or presence of the serine protease inhibitor AEBSF (100 µM). Cleavage of the peptide and release of the AMC group was monitored at excitation 380 nm and emission 460 nm using a FlexStation 3 microplate reader at 15- or 30-min intervals. Background fluorescence from wells containing substrate alone was subtracted from the readings. AEBSF-insensitive values were subtracted from the relative fluorescence in the absence of AEBSF at each time point. Fluorescence was also normalized to the average cell number per well counted at the end of each time course. The specificity for matriptase was determined by titration with the blocking antibody A11 (MABC115; Sigma-Aldrich) that inhibits matriptase activity (LeBeau et al., 2013; Schneider et al., 2012).
Generation of stable matriptase expressing ES-2 and NCI/ADR-Res cell lines
Human matriptase cDNA (Buzza et al., 2013) was amplified by PCR and cloned into EcoRI/Sal I sites of the pEF1a-IRES-AcGFP1 plasmid (ClonTech Laboratories). The matriptase-expressing plasmid (Mat) and vector alone (Vec) were cotransfected along with human pcDNA3.1-HAI-1 (27). HAI-1 is a Kunitz-type protease inhibitor that regulates matriptase activity and serves as a chaperone for efficient matriptase protein expression and translocation to the cell surface (List et al., 2006; Oberst et al., 2003, 2005). Plasmids were transfected into ES-2, NCI/ADR-Res, and SKOV3 cell lines stably expressing a luciferase reporter as in Conway et al. (2019) using Lipofectamine (11668027; Thermo Fisher Scientific) as per manufacturer’s instructions. Transfected cells were selected with G418 (15040066; 400–800 μg/ml; Gibco) and sorted by flow cytometry for GFP-positive cells indicative of matriptase expression (University of Maryland Greenebaum Comprehensive Cancer Center Flow Cytometry Core). Cells were cultured with a low dose of G418 (150 μg/ml) to maintain stable expression.
Orthotopic xenograft model of OvCa metastasis
Animal experiments were conducted in compliance with Public Health Services (PHS) guidelines and approved by the UMB Institutional Animal Care and Use Committee (IACUC). Luciferase expressing ES-2-Luc cells (Conway et al., 2019) expressing matriptase (Mat) or vector control (Vec) were injected intraperitoneally (i.p.; 5 × 106 cells in 400 μl) into female athymic nude mice (Nu/Nu; 6- to 8-wk old, five mice/group), and tumor burden was monitored longitudinally over time by IVIS. On Day 11 after tumor cell injection, mice were euthanized and photographed, ascites was collected, and necropsies were performed (Conway et al., 2019). Tumor tissues, ascites fluids and isolated cells, and major organs were collected for histological analysis or snap-frozen for RNA analysis.
Histopathological analyses
Mouse diaphragm tissues were fixed in 4% paraformaldehyde, stored in 70% ethanol, and embedded in paraffin. 5-μm-thick sections were cut, deparaffinized, and stained with hematoxylin and eosin (H&E) using standard procedures. Ascites fluid samples from ES-2-Mat tumor-bearing mice were collected upon necropsy, and cells were isolated from supernatant by centrifugation. Cytospins were generated from harvested ascites fluid samples using a cytocentrifuge at 800 × g for 10 min. Cytospins were air-dried and stained using Kwik-Diff according to the manufacturer’s instructions. Images of stained tissues and cytospins were obtained by the EVOS FL Auto Cell Imaging System (Life Technologies). All tissues were completed in a single run for consistency.
Scaffold-free 3D spheroid formation assays
Non-adhesive agarose hydrogels
Spheroid clusters were generated by seeding cells at 1–3 × 104 cells per well on 0.75% agarose-coated 96-well plates. Spheroids self-assembled after overnight incubation and were visualized using an EVOS FL Auto Cell Imaging System (Life Technologies).
Hanging drop spheroids
Aggregation and growth of spheroids in suspension in the absence of underlying matrices were evaluated by the hanging drop method (Klymenko et al., 2017a; Timmins and Nielsen, 2007). Tumor cells were seeded at 2 × 104 cells per 10 μl drop on the inner surface of 100 × 20 mm tissue culture dish lids, with 10 ml PBS in the lower dish, and the lid was inverted and placed on top of the dish. Hanging-drop spheroids were allowed to form over the course of 4 d and were visualized and quantified by EVOS.
Spheroid morphology and quantitation
Under each condition, cells were plated on agarose with ∼48 spheroid replicates or as hanging drops with at least 100 spheroid replicates. Spheroid morphology was assessed by two methods.
% of loose spheroids
Each spheroid was visualized and qualitatively scored as 1-tight, 2-loose, or 3-undeterminable. “Tight” spheroids were defined as confined to one compact mass of cells, while “loose” spheroids were defined as dispersed, discontinuous, small, scattered cell aggregates (Allen et al., 1987; Goyeneche et al., 2020). Less than ∼5% of spheroids per condition were scored 3-undeterminable and were not included in the total. The % of 2-loose spheroids of the total spheroids counted for each condition was calculated, and the condition was considered to result in “loose” morphology if the percentage was over 60%.
Image analysis of % of empty space
For a quantitative assessment of spheroid morphologies, three to six representative “loose” or “tight” spheroids from each of the three independent experiments were imaged per condition. For each image, the maximum spheroid area was drawn using the oval selection tool in ImageJ. The freehand selection tool in ImageJ was used to draw the actual spheroid area. The empty space was calculated by subtracting the actual spheroid area from the maximum spheroid area and the % of the total maximum spheroid area was calculated. Tight spheroids had a range of ∼20–30% empty space, while loose spheroids had a range of 65–80% empty space.
Tumorsphere assay
Tumor cells were seeded at 5,000 cells per well onto poly-HEMA-coated 24-well plates (Corning) in Mammocult media (StemCell Technologies) and tumorspheres formed over 10 d. The total number of tumorspheres was counted per well. Tumorspheres that were contained within a 200 μm2 grid square were counted as small (<200 μm2) and those that spanned more than one 200 μm2 grid square were counted as large (>200 μm2). The percentage of large and small tumorspheres per total number of tumorspheres was calculated in each well.
Chemical inhibitors
For chemical inhibitor screening, reagents were added at the time of spheroid seeding. GB-83 (PAR-2 inhibitor) was obtained from Axon Medchem (1622). U0126 (MEK1/2 inhibitor; 662005), SB202190 (p38 MAPK inhibitor; 440204), LY294002 (PI3K inhibitor; 559397), and GM6001 (MMP inhibitor; 364205) were obtained from Calbiochem. JNJ 0966 (MMP-9 inhibitor; HY-103482) and SB-3CT (MMP-2/-9 inhibitor; HY-12354) were obtained from MedChemExpress.
Transwell migration and matrigel invasion assays
Tumor cells were harvested in serum-free media and seeded at 0.5–1 × 105 cells per well onto 6.5 mm × 8 μm pore size Transwell filters (3422; Corning), either uncoated for migration or Matrigel-coated (354234; Corning) for Matrigel invasion. For LP9 invasion, LP9 cells were seeded at 6 × 104 cells per well and grown to a confluent monolayer on thin type I collagen-coated (10 μg/cm2) Transwell filters for 24 h. OvCa cells were harvested, stained with calcein-AM (C1430; Invitrogen), and seeded at 1 × 105 cells per well onto LP9 and collagen-coated Transwell filters. Bottom chambers were filled with 400 μl of serum-containing media to allow cells to invade toward a serum gradient. After 24 h of tumor cell invasion, the top of the Transwell filters were wiped with a cotton swab and the bottom side was either stained with Kwik-Diff (9990705; Thermo Fisher Scientific) according to manufacturer’s instructions (for migration and Matrigel invasion assays) or fixed with PFA for 15 min, and fluorescently labeled invaded cells were visualized by EVOS FL Auto Cell Imaging (LP9 invasion assays). All migrated and invaded cells per Transwell were counted using the multipoint tool on ImageJ; fluorescence intensity of labeled invaded cells in LP9 invasion assays was measured by ImageJ.
Cell proliferation and doubling time
Cells were seeded at 1.5–2 × 105 cells/ml in triplicate wells onto six-well plates. Cells were harvested using non-enzymatic versene dissociation and counted using the Countess 3 Automated Cell Counter (Thermo Fisher Scientific) at 48 and 72 h after seeding. Cell counts are represented as the average of three independent experiments.
Homotypic cell–cell adhesion assay
Confluent tumor cell monolayers were grown on thin-collagen-coated, black-walled, 96-well plates for 48 h. Cells labeled with CellTracker Orange CMTMR Dye (C2927; Thermo Fisher Scientific), according to the manufacturer’s instructions, were seeded on top of homotypic cell monolayers (e.g., labeled ES-2-Vec cells seeded onto unlabeled ES-2-Vec formed monolayers) and allowed to adhere for 1 h at 37°C. Wells with unlabeled and labeled cells were imaged by EVOS and the fluorescence signal was measured at excitation/emission 541/565 nm using the FlexStation 3 plate reader (prewash). Wells were washed three times with PBS to remove non-adherent cells. The remaining labeled adherent cells were imaged by EVOS and fluorescence was measured (after wash). Cell–cell adhesion is presented as the remaining fluorescent values of adherent cells after wash relative to before wash and represents percentage cell–cell adhesion.
Spheroid disaggregation assays on mesothelial cell monolayers
LP9 cells were seeded on black-walled 96-well plates at 6 × 104 cells per well and cultured for 48 h to form confluent monolayers. Tumor cells were labeled with calcein-AM (50 nM final concentration for 30 min) and spheroids were formed using the agarose-hydrogel method. Spheroids were removed by pipette and seeded onto LP9 monolayers, allowed to adhere for 1 h, and images of spheroid disaggregation were taken by EVOS every 24 h for a total of 72 h. Spheroid circumference was measured using the freehand selection tool on ImageJ (National Institutes of Health), and the percentage increase at 72 h relative to the starting point (0 h) was calculated to determine disaggregation.
Spheroid adhesion to mesothelial monolayers
Tumor cells were labeled with calcein-AM (50 nM) and spheroids were formed using the agarose-hydrogel method. LP9 mesothelial cells were seeded at 6 × 104 cells per well onto 24-well plates and allowed to form confluent monolayers for 48 h. Fluorescently labeled tumor spheroids (∼30/well) were seeded onto LP9 monolayers, allowed to adhere for 2 h, and visualized by EVOS. Non-adherent spheroids were washed off with PBS and remaining adherent spheroids were visualized by EVOS. The numbers of spheroids present on the images of spheroids before and after wash were quantified using the multipoint tool on ImageJ.
Mesothelial cell clearance assay
LP9 mesothelial cells were seeded at 6 × 104 cells per well onto 0.2% gelatin-coated 96-well plates, allowed to form confluent monolayers for 48 h, and the confluent monolayers were labeled with calcein-AM (10 μM) according to manufacturer’s instructions. Spheroids formed using the agarose–hydrogel method were seeded onto LP9 monolayers and allowed to adhere for 1 h. Clearance of the fluorescently labeled LP9 monolayer was visualized by EVOS for 5 h, and the area of clearance was measured and quantified using the freehand selection tool on ImageJ. The area of mesothelial clearance at 5 h after seeding is calculated as the percentage of the total mesothelial area under the adherent spheroid at the time of seeding (0 h) relative to vector control.
3D collagen invasion assay
Spheroids formed using the agarose-hydrogel method were embedded into a 3D type I collagen matrix (consisting of DMEM, 7% NaHCO3, HEPES, 0.2% FBS) formed on eight-well chamber slides. Spheroids were monitored and visualized by EVOS for 48 h. The area of spheroid invasion was measured by the freehand selection tool on ImageJ and is represented as a percentage area increase compared with the starting point (0 h).
Soluble E-cadherin ELISA
The conditioned media from ∼70% confluent IOSE397 and IOSE7576 cells and clarified ascites fluid samples from the mouse xenograft model and human OvCa patients were analyzed using the Human Soluble E-cadherin Quantikine ELISA kit (DCADE0; R&D Systems) according to the manufacturer’s instructions. Absorbance signals were measured at 450/540 nm using a FlexStation 3 Microplate Reader and the concentration of samples was interpolated using a generated standard curve from standards included in the Quantikine ELISA kit.
Database analyses
The KMplot web-based survival analysis tool (https://KMPlot.com; Gyorffy et al., 2012) was utilized to analyze gene expression data and survival information. Sources for the databases include Gene Expression Omnibus (GEO; NCBI), European Genome-Phenome Archive (EGA; EMBL-EBI), and The Cancer Genome Atlas (TCGA; NCI). “ST14,” “SPINT1,” and “F2RL1” were entered as gene symbols and all probe sets were used per gene. For “high” and “low,” the data were stratified to include only patient expression data above or below, respectively, the median expression of the genes specified. Analysis was restricted to patients with average CA125 mRNA levels below the lower quartile, and outlier or biased arrays were excluded. The number of patients for each Kaplan–Meier plot is provided in the respective figure legends. Differential gene expression analysis of non-paired normal (n = 46) and tumor (n = 744) tissues was conducted using gene array data from GEO-NCBI using the TNMPlot webtool (https://TNMPlot.com [Bartha and Gyorffy, 2021]). All specimens of each category were grouped and differential PAR-2 expression was analyzed by the Mann–Whitney U test; gene expression is displayed by violin plot to account for the distribution amongst patient samples.
Microscope image acquisition
All microscope images were acquired using the EVOS M7000 microscope. All objective lenses are the Plan fluorite type: 4× EVOS 16.9 lens (0.13NA), 10× EVOS 9.2 lens (0.25NA), and 20× EVOS 2.2 lens (0.4NA). All images were taken at room temperature (20–22°C) in a complete growth medium, Opti-MEM, or dPBS. Cubes for fluorescent imaging were GFP (Ex470/Em525 nm) for calcein-AM stained cells/spheroids and RFP (Ex531/Em593 nm) for CellTrackerOrange stained cells. The EVOS M7000 Imaging System utilizes internal monochrome (for fluorescence imaging and color (for brightfield imaging i.e., H&E) cameras, both with the following specifications: high-sensitivity 3.2 MP (2,048 × 1,536) CMOS sensor with 3.45 μm pixel resolution. The EVOS FL Auto 2 Cell Imaging System Software was used for acquisition and image tiling. No other software was needed for imaging processing subsequent to data acquisition.
Statistical analysis
Student’s two-tailed unpaired t test was used to evaluate significant differences in test conditions as indicated. Data distribution was assumed to be normal, but this was not formally tested. Statistical analyses were conducted using GraphPad Prism 8.0 (GraphPad Software Inc.). The significance between Kaplan–Meier survival curves was tested by log-rank (Mantel-Cox) test. Simple linear regression analysis was used for correlation analysis of sEcad levels and matriptase:HAI-1 ratios. A threshold of P < 0.05 was considered statistically significant.
Online supplemental material
Fig. S1 demonstrates matriptase and HAI-1 mRNA and protein expression levels across OvCa cell lines and stably transfected ES-2, NCI/ADR-Res, and SKOV3 Vec and Mat cells. Fig. S2 displays the experimental timeline for the orthotopic xenograft model and shows IVIS imaging and histological analysis of ES-2 Vec and Mat disseminated tumor burden. Fig. S3 shows the characterization of cell surface protease activity and proliferation of matriptase-expressing stable cell lines. Fig. S4 shows that matriptase expression enhances spheroid adhesion, mesothelial clearance, and Matrigel invasion. Fig. S5 shows that PAR-2 and matriptase are both overexpressed in human OvCa tumors compared with normal ovary tissue and that the matriptase-mediated loose spheroid morphology is attenuated by the PAR-2 antibody, SAM11. Fig. S6 shows that the MMP2 inhibitor SB-3CT does not inhibit loose spheroid formation and there is no difference in α5-integrin mRNA levels in Mat-expressing cells.
Data availability
The data generated in this study are available within the article and its supplementary data files or from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank members of the University of Maryland Greenebaum Comprehensive Cancer Center Translational Laboratory Shared Service (TLSS) for assistance with the in vivo orthotopic xenograft model, and Dr. Dana Roque and Dr. Gautam Rao for providing patient tissues and their helpful clinical perspective. The authors also thank the patients for making this research possible.
This work was supported in part by National Institutes of Health (NIH) grants R01CA196988 (T.M. Antalis) and R01HL118390 (T.M. Antalis), the UMGCCC Cancer Center Support Grant (CCSG) P30CA134274 by funds through the Maryland Department of Health’s Cigarette Restitution Fund Program (CH-649-CRF) and the 2022 Rosser Family Pilot Study Award from the Rivkin Center for Ovarian Cancer (30034041/3003404). M.S. Buzza was supported by an Institutional Research Grant IRG-18-160-16 award from the American Cancer Society. N.R. Pawar was supported by an NIH T32 Training Grant in Cancer Biology fellowship (T32CA154274). A.A. Strong was supported by the Meyerhoff Fellowship (R25-GM055036). T.M. Antalis is also an employee of the VA Maryland Health Care System. The views reported in this paper do not reflect the views of the Department of Veterans Affairs or the United States Government.
Author contributions: N.R. Pawar: Conceptualization, investigation, data curation, visualization, methodology, writing-original draft, and writing-review & editing. M.S. Buzza: Investigation, data curation, methodology, and writing-review & editing. N. Duru: Investigation, data curation, methodology, and writing-review & editing. A.A. Strong: Investigation and data curation. T.M. Antalis: Conceptualization, data curation, funding acquisition, investigation, supervision, resources, writing-original draft, writing-review & editing, and project administration.
References
Author notes
Disclosures: The authors declare no competing interests exist.



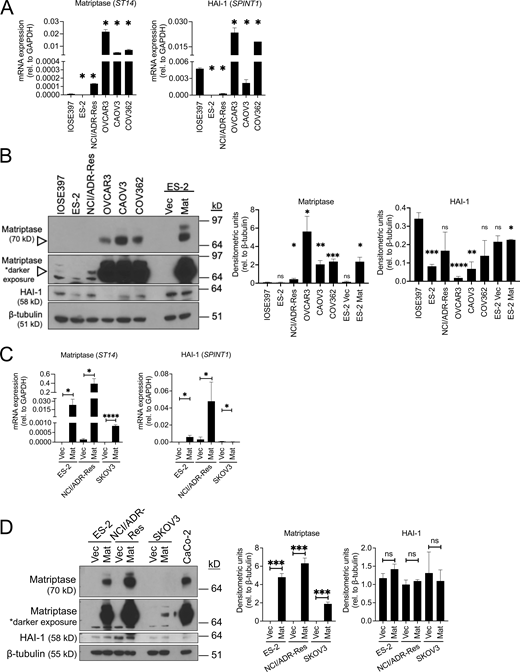



![Matriptase expression promotes metastasis-associated behaviors: adhesion and clearance of mesothelial monolayer and invasion through mesothelial monolayer into submesothelial matrix. (A) Spheroid adhesion to mesothelial monolayer. ES-2 and NCI/ADR-Res Vec or Mat spheroids formed on agarose hydrogel and labeled with calcein-AM were seeded onto LP9 monolayers (formed on thin collagen-coated 24-well plates). Spheroids were allowed to adhere for 1 h and washed three times with PBS; adherent spheroids were visualized and counted before and after wash by EVOS (representative whole-well scans at 4× magnification of ES-2 experiment are shown in left panels; scale bars represent 1.4 mm). Right panel: Data represents the average fold change of the percentage of adherent spheroids relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM, *P < 0.05, **P < 0.01). (B) Mesothelial clearance. ES-2 and NCI/ADR-Res Vec or Mat spheroids formed on agarose hydrogel (shown in “Spheroid [Brightfield]” panels) were seeded onto LP9 mesothelial monolayers labeled with calcein-AM (shown in “LP9 [Green]” panels). “Overlay” panels are Brightfield and Green channel views superimposed. Mesothelial clearance was visualized by EVOS, representative images of ES-2 experiment are shown at 4× magnification, scale bars represent 650 μm; top panel. The area of clearance was quantified by ImageJ and calculated as a percentage of the total spheroid area. Data in the bar graph (bottom panel) represent the average fold change of the percentage of clearance area relative to Vec from three independent experiments performed with at least three replicates each (error bars are ± SEM; *P < 0.05, **P < 0.01). (C) Migration and invasion. ES-2 Vec and Mat cells were seeded onto uncoated or Matrigel-coated Transwells, allowed to migrate or invade for 16–24 h, stained by Kwik-Diff, and quantified by ImageJ; data represents average migration/invasion relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM, *P < 0.05, **P < 0.01). (D) Invasion of mesothelial monolayers. ES-2 and NCI/ADR-Res Vec or Mat cells labeled with calcein-AM were seeded onto Transwell filters coated with thin collagen and an LP9 mesothelial monolayer; after 24 h, invaded cells were fixed with PFA, visualized by EVOS, and quantified by ImageJ. A representative image of one field of invaded cells is shown (4× magnification; scale bars represent 650 μm). Data represents the average ratio of invaded cells relative to Vec from three independent experiments performed in triplicate (error bars are ± SEM, **P < 0.01, ***P < 0.005). (E) 3D collagen invasion. ES-2 and NCI/ADR-Res Vec or Mat spheroids formed on agarose hydrogel were embedded into a 3D type I collagen matrix in chamber slides and allowed to invade for 48 or 72 h respectively; spheroids were visualized by EVOS (representative images shown at 4× magnification; scale bars represent 650 μm). The average fold change of the percentage increase in spheroid size relative to Vec was quantified by ImageJ from three experiments performed with six to eight replicates (error bars are ± SEM, *P < 0.05 ***P < 0.005). All P values were calculated according to two-tailed unpaired Student’s t tests.](https://cdn.rupress.org/rup/content_public/journal/jcb/222/11/10.1083_jcb.202209114/2/m_jcb_202209114_fig3.png?Expires=1773825984&Signature=HAoghVhGhkRyGcEzAeY6X4E1zP32fUb94GmQ50OqYiSYtX1ByNhww2evEfWpMLI1A73n29Lr9FULa17g0OCldkQSxES-aWI9~VP-ruAmofqxU4-VEqnBYb4xEPk-cIFgMXq8priwR0HlguTVQI57KwKbS4QlskvjrViQDn-4aKrilMA8Ay06KSTPXIEt7GfVMSIu30q935lOrQ-lpC5Ow-QyxpvjNht3QEJr-IXlII1k1lmNT5jshZXqqMiVH4O3gQOQ9Y2F7fr2fENVXr0RItjyKffs2IhqTJ00DiiedzxrcqZbjUb06sZEoM9~0M9tUGWHIruGPydJlZaciEWs9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)