The heart’s pumping capacity is determined by myofilament power generation. Power is work done per unit time and measured as the product of force and velocity. At a sarcomere level, these contractile properties are linked to the number of attached cross-bridges and their cycling rate, and many signaling pathways modulate one or both factors. We previously showed that power is increased in rodent permeabilized cardiac myocytes following PKA-mediated phosphorylation of myofibrillar proteins. The current study found that that PKA increased power by ∼30% in permeabilized cardiac myocyte preparations (n = 8) from human failing hearts. To address myofilament molecular specificity of PKA effects, mechanical properties were measured in rat permeabilized slow-twitch skeletal muscle fibers before and after exchange of endogenous slow skeletal troponin with recombinant human Tn complex that contains cardiac (c)TnT, cTnC and either wildtype (WT) cTnI or pseudo-phosphorylated cTnI at sites Ser23/24Asp, Tyr26Glu, or the combinatorial Ser23/24Asp and Tyr26Glu. We found that cTnI Ser23/24Asp, Tyr26Glu, and combinatorial Ser23/24Asp and Tyr26Glu were sufficient to increase power by ∼20%. Next, we determined whether pseudo-phosphorylated cTnI at Ser23/24 was sufficient to increase power in cardiac myocytes from human failing hearts. Following cTn exchange that included cTnI Ser23/24Asp, power output increased ∼20% in permeabilized cardiac myocyte preparations (n = 6) from the left ventricle of human failing hearts. These results implicate cTnI N-terminal phosphorylation as a molecular regulator of myocyte power and could serve as a regional target for small molecule therapy to unmask myocyte power reserve capacity in human failing hearts.
Introduction
The heart works as a functional syncytium, whereby cardiac muscle cells are connected end-to-end via intercalated discs, which provides strong mechanical and electrical coupling. Thus, during a normal heartbeat, all individual ventricular myocytes contract and relax in near synchrony. Since pumping capacity of a synchronized heart is highly variable, its regulation must be controlled within individual myocytes. Beat-to-beat alterations in cardiac myocyte contractility occur via modulation of the intracellular calcium transients, myocyte length, myocyte stress, and an array of posttranslational modifications of sarcomeric proteins. The interplay between intracellular Ca2+ levels and the Ca2+-induced activation of myofilament contraction allows for precise tuning of ventricular stroke output to hemodynamic demands. During ventricular ejection, individual myocytes generate force and shorten against an afterload, thus, stroke volume is limited by the power-generating capacity of the individual myocytes. Illumination of the molecular underpinnings that regulate the power generating capacity of individual myocytes is essential for understanding the intrinsic regulation of the heart in health and disease.
It is widely recognized that myocardial performance is enhanced by β-adrenergic stimulation. β-adrenergic positive inotropic and lusitropic responses are mediated by cAMP-dependent protein kinase (PKA). Intracellular PKA phosphorylates both calcium handling proteins such as sarcolemmal Ca2+ channel, ryanodine receptor, and phospholamban as well as myofilament proteins including cardiac troponin I (cTnI), cardiac myosin binding protein-C (cMyBP-C), and titin (Solaro, 2002). We have previously shown that PKA increases myofilament power but molecule-specific contributions among the aforementioned targets remains uncertain (Herron et al., 2001b). MyBP-C phosphorylation appears sufficient to increase power, at least in soleus fibers (Hanft et al., 2016; Robinett et al., 2019). It is plausible that cTnI phosphorylation also may be sufficient since thin filament proteins have been reported to increase the velocity of unloaded shortening (Sweeney et al., 1998; Homsher et al., 2000; Morris et al., 2003). However, the direct effects of cTnI phosphorylation per se on loaded shortening and power have not been tested. We performed experiments to address site-specific PKA phosphorylation effects at Ser23/24 as well as a more regional cTnI N-terminus effect by also probing a separate non-PKA phosphorylation site (i.e., Tyr26; Salhi et al., 2014; Biesiadecki and Westfall, 2019). While the healthy heart demonstrates beat-to-beat variability in stroke output in response to increased demand (e.g., exercise), there are also a number of myofilament alterations that occur in response to chronic adaptations to hypertension and other cardiomyopathies (Bodor et al., 1997; Kotlo et al., 2012; Walker et al., 2013; Wijnker et al., 2014; Hanft et al., 2017), which leads to heart failure. Heart failure is thought to arise from a spiraling constellation of unfavorable compensatory changes including altered Ca2+ handling, disrupted architecture and energetics, altered neurohormonal signaling, modified myofilament protein isoform expression patterns, and altered posttranslational modification of myofilament proteins (Eichhorn and Bristow, 1996; Messer et al., 2007; Hamdani et al., 2008; Copeland et al., 2010; Haynes et al., 2014). Myofilament preparations from human failing hearts have been shown to display (1) increased Ca2+ sensitivity of force, (2) depressed length dependence of force, and (3) decreased PKA phosphorylation compared to myofilament preparations from donor hearts (Schwinger et al., 1994; Wolff et al., 1996; Messer et al., 2007; Sequeira et al., 2013; Wijnker et al., 2013; Wijnker et al., 2014). Thus, decreased PKA phosphorylation may directly limit individual cardiac myocyte power.
For this study, we first tested the potential for PKA to increase power in the myofilament background of human heart failure. We next sought to define the molecule-specific role of PKA in augmenting power output. We tested the hypothesis that cTnI phosphorylation per se increases cardiac myofilament power. We tested whether the cTnI phosphorylation effect was specific to PKA phosphorylation sites (i.e., Ser23/24) or was a more generalized effect of phosphorylation of the cTnI N-terminal region (by testing Tyr26, a non-PKA substrate). Last, we tested the potential for PKA site-specific phosphorylation of cTnI to unmask power reserve capacity in the context of decompensatory myofibrillar changes associated with human heart failure.
Materials and methods
Recombinant troponin
Human cardiac troponin C (cTnC), troponin I (cTnI), and troponin T (cTnT) cDNA was isolated as previously described (Kobayashi and Solaro, 2006; Salhi et al., 2014). Resultant constructs were verified by DNA sequencing. The individual recombinant human cTn subunits were expressed in Escherichia coli and purified to homogeneity as previously described (Nixon et al., 2012a; Nixon et al., 2012b). The cTn complex was reconstituted by sequential dialysis and column purified as previously described (Biesiadecki et al., 2007; Nixon et al., 2012b). Column fractions containing pure cTn were extensively dialyzed against exchange buffer (200 mM KCl, 5 mM MgCl2, 5 mM EGTA, 1 mM DTT, and 20 mM MOPS, pH 6.5), and aliquots stored at −80°C until use.
Solutions
Compositions of relaxing and activating solutions used in mechanical measurements were as follows: 7 mM EGTA, 1 mM free Mg2+, 20 mM imidazole, 4 mM MgATP, 14.5 mM creatine phosphate, pH 7.0, various Ca2+ concentrations between 10−9 M (relaxing solution) and 10−4.5 M (maximal Ca2+ activating solution), and sufficient KCl to adjust ionic strength to 180 mM. The final concentrations of each metal, ligand, and metal–ligand complex were determined with the computer program of Fabiato (1988). Preceding each Ca2+ activation, myocyte preparations were immersed for 30 s in a solution of reduced Ca2+-EGTA buffering capacity, which was identical to normal relaxing solution except that EGTA was reduced to 0.5 mM. This protocol resulted in more rapid development of steady-state force during subsequent activation and helped preserve the striation pattern during activation. Relaxing solution contained 2 mM EGTA, 5 mM MgCl2, 4 mM ATP, 10 mM imidazole, and 100 mM KCl at pH 7.0 with the addition of a protease inhibitor cocktail (Set I Calbiochem). Troponin exchange was carried out in relaxing solution containing ∼0.5 mg/ml recombinant troponin.
Cardiac myocyte preparation
All procedures were approved by the University of Kentucky Institutional Review Board, and written informed consent was obtained from each patient who had heart failure. Human heart samples were obtained from patients undergoing heart transplants at the University of Kentucky. Hearts were handed to a researcher upon excision from the body. Mid-wall myocardial samples of distal anterior left ventricular free wall were dissected, snap frozen in liquid nitrogen, and stored at −150°C before shipping to the University of Missouri. The workflow for these experiments is illustrated in Fig. 1 A. A total of 14 permeabilized single cardiac myocyte preparations from hearts obtained from patients who had heart failure were analyzed for this work (see Tables 1 and 3). Single permeabilized myocyte preparations were prepared by cutting the frozen biopsies into 2–3 mm pieces and then further disrupted for 5–10 s in relaxing solution using a Waring blender. The resulting suspension of cells and cell fragments was centrifuged for 105 s at 165 × g. The myocytes were subsequently permeabilized by suspending the pellet for 4 min in 0.3% ultrapure Triton X-100 (Pierce Chemical Co.) in relaxing solution. The pellet was washed twice with cold relaxing solution, and the skinned cells were then resuspended in 10–20 ml of relaxing solution and kept on ice during the day of the experiment.
Experimental animals
All procedures involving animal use were performed according to the Animal Care and Use Committee of the University of Missouri. Male Sprague-Dawley rats (6 wk of age) were obtained from Envigo, housed in groups of two, and provided access to food and water ad libitum.
Skeletal muscle fiber preparation
We utilized slow-twitch skeletal muscle fibers as a tool to address the specific effect of cTnI phosphorylation on myofilament power output. This Tn exchange approach is the gold standard to study molecular mechanisms of regulation of contraction (Moss, 1992). The slow-twitch fiber was ideal for this approach since endogenous slow-skeletal (ss)TnI lacks the PKA N-terminal phosphorylation sites present in cTnI and the extent of Tn exchange could be quantified in the same fiber that mechanics were performed (Hanft et al., 2013; Hanft et al., 2016). Skeletal muscle fibers were obtained from Sprague-Dawley rats anesthetized by inhalation of isoflurane (20% [vol/vol] in olive oil; McDonald, 2000). Slow-twitch skeletal muscle fibers were obtained from the soleus muscle. The muscles were isolated, placed in relaxing solution at 4°C and bundles of ∼50 fibers were separated, tied to capillary tubes, and stored in relaxing solution containing 50% (vol/vol) glycerol for up to 4 wk. Single fibers for mechanical measurements were dissected by gently pulling individual fibers from the bundle.
Experimental apparatus
The experimental apparatus for mechanical measurements of skeletal muscle fiber and cardiac myocyte preparations was the same as previously described (McDonald et al., 1998b; McDonald, 2000). Fiber/myocyte preparations were attached between a force transducer and torque motor by placing the ends of the myocyte preparation into stainless steel troughs (25 gauge). The ends of the fiber/myocyte preparations were secured by overlaying a 0.5 mm length of 3-0 monofilament nylon suture (Ethicon, Inc.) onto each end of the fiber/myocyte, and then tying the suture into the troughs with two loops of 10-0 monofilament (Ethicon, Inc.). The attachment procedure was performed under a stereomicroscope (∼100× magnification) using finely shaped forceps.
Prior to mechanical measurements, the experimental apparatus was mounted on the stage of an inverted microscope (model IX-70, Olympus Instrument Co.), which was placed upon a pneumatic vibration isolation table having a cut-off frequency of ∼1 Hz. Mechanical measurements were performed using a capacitance-gauge transducer (Model 403-sensitivity of 20 mV/mg [plus a 10× amplifier for cardiac myocytes]; Aurora Scientific, Inc.). Length changes were introduced using a DC torque motor (Model 308, Aurora Scientific, Inc.) driven by voltage commands from a personal computer via a 16-bit D/A converter (AT-MIO-16E-1; National Instruments Corp.). Force and length signals were digitized at 1 kHz and stored on a personal computer using LabView for Windows (National Instruments Corp.). Sarcomere length was monitored using IonOptix SarcLen system (IonOptix), which used a fast Fourier transform algorithm of the video image of the myocyte. Microscopy was carried out using a 40× objective (Olympus UWD 40) and a 2.5× intermediate lens.
Permeabilized skeletal fiber/cardiac myocyte mechanical measurements
All mechanical measurements on skeletal muscle fibers and cardiac myocytes were performed at 15 ± 1°C. Following attachment, the relaxed preparation was adjusted to a sarcomere length of ∼2.50 µm for skeletal fibers and ∼2.30 µm for cardiac myocytes. The preparation was first transferred into pCa 4.5 solution for maximal activation and subsequently placed into a submaximal pCa solution that resulted in ∼50% of the maximal force at pCa 4.5. Force in submaximal activating solutions was expressed as a fraction of force obtained during maximal calcium activation. Force and kinetics of force development were obtained using a procedure previously described for skinned cardiac myocyte preparations (Korte et al., 2003; Hinken and McDonald, 2004; Hanft and McDonald, 2009). While in Ca2+ activating solution, the preparation was rapidly shortened by 10–20% of initial length (Lo) to yield zero force. The preparation was then allowed to shorten for ∼20 ms, after which the preparation was rapidly restretched to ∼105% of its Lo for 2 ms and then returned to Lo.
Force–velocity and power–load measurements were performed as previously described (McDonald, 2000). The preparation was transferred to a submaximal Ca2+ activating solution (which yielded ∼50% maximal force) and a series of sub-isometric force clamps were applied to determine isotonic shortening velocities. The isotonic force was maintained using a servo system for 150–250 ms while muscle length changes during this time were monitored. Following the force clamp, the myocyte was slackened to near zero force to estimate the relative load sustained during the isotonic shortening, after which the fiber/myocyte was reextended to its starting length. The preparations were kept in submaximal Ca2+ activating solution for 3–5 min, during which 10–20 force clamps were performed without significant loss of force. After obtaining a force–velocity relationship, the myocyte preparation was activated again in maximal Ca2+ activation solution and if force fell below 70% of initial force, data from that myocyte were discarded. Force–velocity measurements were obtained in this manner for all experimental conditions.
To assess the effects of PKA on force, rate of force development and loaded shortening in human failing myocytes, measurements were performed before and after 45 min incubation with PKA (0.5 U/μl; Sigma-Aldrich; Herron et al, 2001b; Hanft and McDonald, 2009; Hanft et al, 2013, 2016). To assess the effects of incorporation of pseudo-phosphorylated cTnI on force, rate of force, and loaded shortening, contractile properties were measured in each slow-twitch skeletal muscle fiber before and after 12 h of cTn exchange. For permeabilized cardiac myocytes from human failing hearts, contractile measurements were performed before and after 3 h of cTn exchange.
SDS-PAGE and Western blots
Permeabilized skeletal muscle fibers
cTn exchange was confirmed by Western blots of single skeletal muscle fibers after mechanical measurement using SDS-PAGE followed by Western blotting. The samples underwent SDS-PAGE using 12% polyacrylamide slab gels. After SDS-PAGE, the gels were placed on prewetted nitrocellulose membranes sandwiched between several sheets of 3 MM chromatography paper, and the size-separated proteins were transferred to nitrocellulose using a semidry blot apparatus at constant current (120 mA) for 1.5 h. Immediately following transfer, the blots were stained with Ponceau S (Pierce) to verify equivalent protein loads and then placed in blocking buffer consisting of 5% dry milk, pH 7.4, and rocked for 1 h. Blots were incubated with primary TnT antibodies (DSHB) diluted 1:1,000 in blocking buffer overnight. Blots were then washed in blocking buffer and incubated for 2 h with secondary peroxidase-conjugated goat anti-mouse antibodies (Thermo Fisher Scientific) diluted 1:1,000 in blocking buffer. Blots were then washed with PBS and subsequently coated with Supersignal West Pico-chemiluminescent substrate (Pierce) and imaged using a Bio-Rad ChemDoc imaging system, and signal intensity was quantified using ImageJ software (National Institutes of Health). The difference in TnT isoform size (between endogenous slow skeletal troponin T [ssTnT] and the human exogenous cardiac TnT [cTnT]) was used to assess relative level of Tn exchange (Fig. 2 A).
Human permeabilized cardiac myocytes
To assess troponin exchange in cardiac myocyte preparations, myofibrillar proteins were assessed using SDS-PAGE followed by Western blots. The methods for SDS-PAGE were similar to that previously described (Giulian et al., 1983). Since total myofilament protein content in a single cardiac myocyte-sized preparation is below detection levels, we could not assess troponin exchange in the same preparation that mechanics were performed. Rather, myofibrillar suspensions underwent batch cTn exchange and a sample was taken prior to cTn exchange, 1 h after cTn exchange, and 3 h after cTn exchange. Samples (∼1.0 μg) were separated by SDS-PAGE and subjected to Western blotting as describe above for permeabilized skeletal muscle fibers. The nitrocellulose blots were blocked as described above and then incubated overnight with primary antibody (Phospho cTnI 23/24; Abcam diluted 1:1,000 in 5% dry milk). Non-specific interactions were minimized by three washes in 5% dry milk. Secondary rabbit antibodies were diluted 1:1,000 in 5% dry milk and reacted with the blots for 2 h followed by three washes using PBS. Blots were then coated for 5 min with Supersignal West Pico-chemiluminescent substrate (Pierce), which reacts with the secondary antibody. The extent of cTn exchange was estimated by comparing relative amounts of phosphorylated cTnI before and after exchange with recombinant cTn, which is not detected by the phospho-specific cTnI antibody (Hanft et al., 2013). After 3 h of batch cTn exchange, there was ∼55% reduction in signal, i.e., cTn exchange (Fig. 4 A). This exchange level likely underestimates the cTn exchange in the single permeabilized cardiac myocyte preparations (for mechanics) since the same exogenous cTn concentration was used.
Data analysis and statistical methods
Curve fitting was performed using a customized program written in Qbasic, as well as commercial software (Sigmaplot).
Paired t tests were used to compare the effects PKA on contractile properties of permeabilized cardiac myocytes from human failing hearts. N equals the number of human hearts (biological replicates), with one cardiac myocyte preparation per heart. Two-way repeated measures ANOVA was used to compare group and interaction effects between Tn exchange and recombinant cTnI groups on contractile properties of permeabilized slow-twitch soleus fibers. n equals the number of slow-twitch skeletal muscle fibers (biological replicates). Paired t tests were used to compare the effects cTn exchange on contractile properties of permeabilized cardiac myocytes from human failing hearts. Values are expressed as means ± SEM. P < 0.05 is accepted as statistically significant.
Results
PKA effects on power in permeabilized cardiac myocytes from human failing hearts
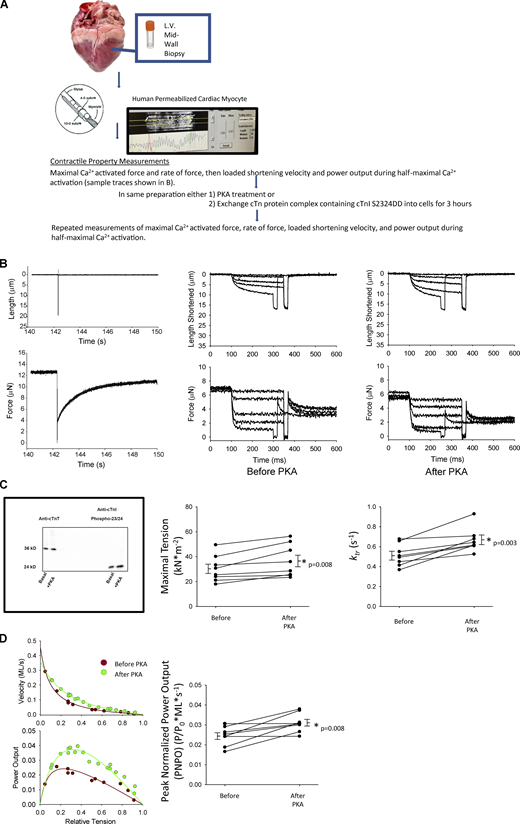
We addressed contractile reserve capacity of cardiac myofilaments from failing human hearts. We first tested the effects of PKA treatment since previous work from our lab observed increased force and power in rat permeabilized cardiac myocytes in response to PKA phosphorylation of myofilament proteins (Herron et al., 2001b; Hanft and McDonald, 2009). Representative cardiac myocyte preparation slack–restretch maneuver as well as length and force traces are shown during isotonic contractions before and after PKA in Fig. 1 B. PKA increased maximal Ca2+ activated tension by ∼20% in permeabilized cardiac myocyte preparations from failing human hearts (Table 1 and Fig. 1 C). PKA also increased the rate constant (ktr) of force development by ∼30% during maximal Ca2+ activation (Table 1 and Fig. 1 C). Furthermore, during half-maximal Ca2+ activations, PKA caused an upward shift in normalized power–load curves (Fig. 1 D). Overall, PKA increased PNPO ∼35% in cardiac myocytes from human failing hearts, indicative of a role for PKA-mediated phosphorylation of cardiac myofilament proteins in the contractile reserve capacity.
Assessment of reserve capacity of single permeabilized cardiac myocyte preparations from human failing hearts. (A) Illustration of the methods to measure the effects of PKA or phosphorylated cTnI on mechanical properties of single permeabilized cardiac myocytes from failing human hearts. (B) Left: Representative length and force traces during the slack–restretch maneuver. Right: Load clamps to measure force, rate of force, and loaded shortening velocities, respectively, before and after PKA. (C) Top: Western blot staining with either a cTnT ab or phospho 23/24-specific cTnI ab of human failing permeabilized cardiac myocytes before and after treatment with PKA. Bottom: Maximal Ca2+-activated tension and rates of force redevelopment before and after treatment with PKA. (D) Left: Representative force–velocity and power–load curves at submaximal force before and after PKA of a permeabilized myocyte preparation from a human failing heart. Right: Summary data measuring PNPO before and after PKA treatment. Myocyte preparations compared by paired t test (n = 8, biological replicates). Source data are available for this figure: SourceData F1.
Assessment of reserve capacity of single permeabilized cardiac myocyte preparations from human failing hearts. (A) Illustration of the methods to measure the effects of PKA or phosphorylated cTnI on mechanical properties of single permeabilized cardiac myocytes from failing human hearts. (B) Left: Representative length and force traces during the slack–restretch maneuver. Right: Load clamps to measure force, rate of force, and loaded shortening velocities, respectively, before and after PKA. (C) Top: Western blot staining with either a cTnT ab or phospho 23/24-specific cTnI ab of human failing permeabilized cardiac myocytes before and after treatment with PKA. Bottom: Maximal Ca2+-activated tension and rates of force redevelopment before and after treatment with PKA. (D) Left: Representative force–velocity and power–load curves at submaximal force before and after PKA of a permeabilized myocyte preparation from a human failing heart. Right: Summary data measuring PNPO before and after PKA treatment. Myocyte preparations compared by paired t test (n = 8, biological replicates). Source data are available for this figure: SourceData F1.
Characteristics of human failing permeabilized myocytes before and after PKA
| Human samples . | Myofibrillar preparations . | |
|---|---|---|
| Before . | After PKA . | |
| n | 8 | |
| Fiber length (µm) | 153 ± 8 | |
| Fiber width (µm) | 27 ± 2 | |
| Resting sarcomere length (µm) | 2.20 ± 0.02 | |
| Passive force (µN) | 0.53 ± 0.08 | 0.58 ± 0.08 |
| Passive tension (kN/m2) | 1.32 ± 0.18 | 1.39 ± 0.14 |
| Max force (µN) | 14.0 ± 2.3 | 14.7 ± 2.2 |
| Max tension (kN/m2) | 30.9 ± 3.5 | 36.6 ± 4.6a |
| ktr @ pCa 4.5 (s−1) | 0.515 ± 0.04 | 0.666 ± 0.04a |
| pCa for half-maximal force | 6.06 ± 0.03 | 5.96 ± 0.03 |
| Relative force (PO/P4.5) | 0.50 ± 0.04 | 0.52 ± 0.04 |
| a/PO | 0.16 ± 0.04 | 0.20 ± 0.07 |
| Fopt (P/PO) | 0.25 ± 0.02 | 0.27 ± 0.02 |
| Vopt (ML/s) | 0.100 ± 0.012 | 0.117 ± 0.009 |
| Vmax | 0.45 ± 0.09 | 0.47 ± 0.07 |
| PNPO (P/PO*ML/s) | 0.024 ± 0.002 | 0.031 ± 0.002a |
| Submax ktr | 0.456 ± 0.05 | 0.468 ± 0.05 |
| Human samples . | Myofibrillar preparations . | |
|---|---|---|
| Before . | After PKA . | |
| n | 8 | |
| Fiber length (µm) | 153 ± 8 | |
| Fiber width (µm) | 27 ± 2 | |
| Resting sarcomere length (µm) | 2.20 ± 0.02 | |
| Passive force (µN) | 0.53 ± 0.08 | 0.58 ± 0.08 |
| Passive tension (kN/m2) | 1.32 ± 0.18 | 1.39 ± 0.14 |
| Max force (µN) | 14.0 ± 2.3 | 14.7 ± 2.2 |
| Max tension (kN/m2) | 30.9 ± 3.5 | 36.6 ± 4.6a |
| ktr @ pCa 4.5 (s−1) | 0.515 ± 0.04 | 0.666 ± 0.04a |
| pCa for half-maximal force | 6.06 ± 0.03 | 5.96 ± 0.03 |
| Relative force (PO/P4.5) | 0.50 ± 0.04 | 0.52 ± 0.04 |
| a/PO | 0.16 ± 0.04 | 0.20 ± 0.07 |
| Fopt (P/PO) | 0.25 ± 0.02 | 0.27 ± 0.02 |
| Vopt (ML/s) | 0.100 ± 0.012 | 0.117 ± 0.009 |
| Vmax | 0.45 ± 0.09 | 0.47 ± 0.07 |
| PNPO (P/PO*ML/s) | 0.024 ± 0.002 | 0.031 ± 0.002a |
| Submax ktr | 0.456 ± 0.05 | 0.468 ± 0.05 |
Mechanical properties were tested before and after PKA treatment and compared via paired t test analysis. Values are mean ± SEM.
P ≤ 0.05. n equals the number of human hearts (biological replicates), with one cardiac myocyte preparation per heart.
Regulation of power by cardiac thin filament proteins in slow-twitch skeletal muscle fibers
To ascertain the mechanistic role of N-terminal cardiac TnI phosphorylation on myofilament mechanics, we employed a Tn complex exchange protocol using permeabilized rat slow-twitch skeletal muscle fibers. Mammalian slow-twitch skeletal muscle fibers naturally express a TnI isoform that lacks the N-terminal phosphorylation sites that are uniquely present in the cardiac isoform of TnI (cTnI). Exchange of endogenous slow skeletal Tn complex for recombinantly expressed cardiac Tn complex ensured that differences in mechanical measurements before and after exchange were due to molecule-specific alterations on the thin filament rather than, for instance, potential compensatory changes that might occur with a transgenic approach.
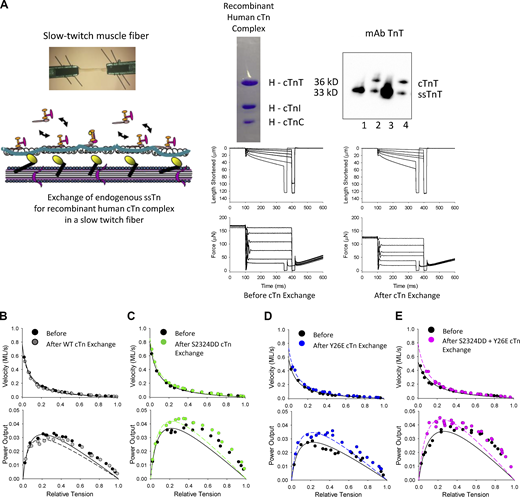
Representative fiber preparation length and force traces during isotonic contraction are shown in Fig. 2 A. Fig. 2, B–E shows representative force–velocity and power–load relationships for a fiber preparation from each cTn exchange group. Table 2 shows slow-twitch skeletal muscle fiber characteristics before and after 12 h of exchange of wildtype (WT) and pseudo-phosphorylated cTnI. cTn exchange with pseudo-phosphorylated cTnI significantly augmented power–load curves (Fig. 2, B–E). PNPO increased by ∼20% after cTn exchange with either pseudo-phosphorylated cTnI at sites Ser23/24Asp (S2323DD), Tyr26Glu (Y26E), or the combinatorial Ser23/24Asp and Tyr26Glu in slow-twitch skeletal muscle fibers (Fig. 3). These results indicate that pseudo-phosphorylation of cTnI is sufficient to increase mammalian striated muscle fiber power output. In addition, it appears that Ser23/24 and Tyr26Glu have a redundant biophysical role in regulating power output. Importantly, the control experiment (Fig. 2 B), which consisted of exchange with WT cTn (i.e., unphosphorylated cTnI), did not significantly alter power–load curves, i.e., PNPO was similar before and after WT cTn exchange in slow-twitch skeletal muscle fibers (Fig. 3).
PNPO of rat single permeabilized slow-twitch skeletal muscle fibers before and after exchange of human cardiac Tn complex. (A) Top left: Slow-twitch fiber attachment photomicrograph and illustration of Tn exchange protocol. Top right: Coomassie-stained gel of recombinant human cardiac Tn complex containing cTnT, cTnI, and cTnC and a Western blot to assess the relative amount of endogenous ssTn compared to exogenous cTn complex after Tn exchange. Lanes 1 and 3: Rat permeabilized slow-twitch fibers that did not undergo Tn exchange. Lanes 2 and 4: Rat permeabilized slow-twitch fibers that underwent Tn exchange. Bottom: Representative length and force traces during the load clamps to measure loaded shortening velocities. (B–E) Representative force–velocity and power–load curves before and after exchange of endogenous ssTn (which lacks the N-terminal TnI phosphorylation site) with recombinant human cardiac Tn complex which contained N-terminal cTnI phosphorylation sites. The WT cTnI has serines at sites 23 and 24, but those sites are not phosphorylated. The Ser23/24Asp (S2323DD) cTnI is PKA pseudo-phosphorylated, while the Tyr26Glu (Y26E) cTnI is non-PKA pseudo-phosphorylated. Source data are available for this figure: SourceData F2.
PNPO of rat single permeabilized slow-twitch skeletal muscle fibers before and after exchange of human cardiac Tn complex. (A) Top left: Slow-twitch fiber attachment photomicrograph and illustration of Tn exchange protocol. Top right: Coomassie-stained gel of recombinant human cardiac Tn complex containing cTnT, cTnI, and cTnC and a Western blot to assess the relative amount of endogenous ssTn compared to exogenous cTn complex after Tn exchange. Lanes 1 and 3: Rat permeabilized slow-twitch fibers that did not undergo Tn exchange. Lanes 2 and 4: Rat permeabilized slow-twitch fibers that underwent Tn exchange. Bottom: Representative length and force traces during the load clamps to measure loaded shortening velocities. (B–E) Representative force–velocity and power–load curves before and after exchange of endogenous ssTn (which lacks the N-terminal TnI phosphorylation site) with recombinant human cardiac Tn complex which contained N-terminal cTnI phosphorylation sites. The WT cTnI has serines at sites 23 and 24, but those sites are not phosphorylated. The Ser23/24Asp (S2323DD) cTnI is PKA pseudo-phosphorylated, while the Tyr26Glu (Y26E) cTnI is non-PKA pseudo-phosphorylated. Source data are available for this figure: SourceData F2.
Characteristics of slow-twitch skeletal muscle fibers before and after 12 h of exchange with WT or pseudo-phosphorylated cTnI
| Exchange . | WT . | S22/23DD . | Y26E . | S22/23DD + Y26E . | ||||
|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | Before . | After . | |
| n | 5 | 5 | 7 | 5 | ||||
| Fiber length (µm) | 1,103 ± 58 | 809 ± 60 | 1,030 ± 72 | 1,156 ± 132 | ||||
| Fiber width (µm) | 77 ± 9 | 68 ± 8 | 67 ± 4 | 68 ± 6 | ||||
| Resting sarcomere length (µm) | 2.52 ± 0.02 | 2.49 ± 0.05 | 2.51 ± 0.02 | 2.44 ± 0.03 | ||||
| Active sarcomere length (µm) | 2.34 ± 0.05 | 2.35 ± 0.04 | 2.39 ± 0.03 | 2.35 ± 0.03 | ||||
| Passive force (µN) | 2.75 ± 0.89 | 1.67 ± 0.33 | 1.86 ± 0.39 | 0.98 ± 0.27 | 2.03 ± 0.48 | 1.33 ± 0.30 | 2.45 ± 0.82 | 2.45 ± 1.02 |
| Passive tension (kN/m2) | 0.65 ± 0.17 | 0.41 ± 0.07 | 0.60 ± 0.15 | 0.28 ± 0.03 | 0.62 ± 0.10 | 0.44 ± 0.10 | 0.83 ± 0.12 | 0.66 ± 0.16 |
| Max force (µN) | 569 ± 66 | 450 ± 74a | 380 ± 57 | 308 ± 49a | 439 ± 52 | 320 ± 50a | 404 ± 86 | 354 ± 81a |
| Max tension (kN/m2) | 127 ± 19 | 103 ± 20a | 119 ± 14 | 95 ± 10a | 137 ± 10 | 95 ± 7a | 120 ± 17 | 105 ± 18a |
| ktr @ pCa 4.5 (s−1) | 4.30 ± 0.34 | 3.21 ± 0.19a | 5.10 ± 0.28 | 3.83 ± 0.26 | 4.75 ± 0.31 | 3.11 ± 0.27 | 4.83 ± 0.28 | 3.63 ± 0.26a |
| pCa for half-maximal force | 6.28 ± 0.04 | 6.28 ± 0.04 | 6.26 ± 0.05 | 6.10 ± 0.03 | 6.31 ± 0.01 | 6.09 ± 0.03a | 6.26 ± 0.04 | 6.08 ± 0.04 |
| Relative force (PO/P4.5) | 0.47 ± 0.01 | 0.45 ± 0.02 | 0.47 ± 0.03 | 0.42 ± 0.02 | 0.48 ± 0.03 | 0.51 ± 0.02 | 0.45 ± 0.02 | 0.47 ± 0.04 |
| Power output (µW/mg) | 2.60 ± 0.45 | 1.76 ± 0.31a | 2.24 ± 0.34 | 1.83 ± 0.19 | 2.36 ± 0.26 | 2.02 ± 0.16 | 2.15 ± 0.29 | 2.27 ± 0.31 |
| a/PO | 0.10 ± 0.02 | 0.06 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.02a | 0.11 ± 0.03 | 0.07 ± 0.01 | 0.11 ± 0.03 | 0.09 ± 0.01 |
| Fopt (P/PO) | 0.23 ± 0.01 | 0.19 ± 0.01a | 0.23 ± 0.01 | 0.21 ± 0.02 | 0.22 ± 0.02 | 0.19 ± 0.02 | 0.23 ± 0.02 | 0.22 ± 0.01 |
| Vopt (ML/s) | 0.14 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.19 ± 0.03 | 0.14 ± 0.01 | 0.20 ± 0.03a | 0.16 ± 0.01 | 0.19 ± 0.01a |
| Vmax | 0.62 ± 0.05 | 0.82 ± 0.04a | 0.64 ± 0.08 | 1.01 ± 0.26 | 0.66 ± 0.10 | 1.21 ± 0.28 | 0.70 ± 0.11 | 0.85 ± 0.09 |
| PNPO (P/PO*ML/s) | 0.032 ± 0.004 | 0.033 ± 0.003 | 0.033 ± 0.002 | 0.039 ± 0.002a | 0.030 ± 0.002 | 0.036 ± 0.002a | 0.036 ± 0.002 | 0.042 ± 0.003a,b |
| Submax ktr | 1.22 ± 0.14 | 1.40 ± 0.22 | 1.05 ± 0.26 | 1.10 ± 0.18 | 0.98 ± 0.12 | 1.31 ± 0.10a | 1.36 ± 0.24 | 1.37 ± 0.14 |
| Exchange . | WT . | S22/23DD . | Y26E . | S22/23DD + Y26E . | ||||
|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | Before . | After . | |
| n | 5 | 5 | 7 | 5 | ||||
| Fiber length (µm) | 1,103 ± 58 | 809 ± 60 | 1,030 ± 72 | 1,156 ± 132 | ||||
| Fiber width (µm) | 77 ± 9 | 68 ± 8 | 67 ± 4 | 68 ± 6 | ||||
| Resting sarcomere length (µm) | 2.52 ± 0.02 | 2.49 ± 0.05 | 2.51 ± 0.02 | 2.44 ± 0.03 | ||||
| Active sarcomere length (µm) | 2.34 ± 0.05 | 2.35 ± 0.04 | 2.39 ± 0.03 | 2.35 ± 0.03 | ||||
| Passive force (µN) | 2.75 ± 0.89 | 1.67 ± 0.33 | 1.86 ± 0.39 | 0.98 ± 0.27 | 2.03 ± 0.48 | 1.33 ± 0.30 | 2.45 ± 0.82 | 2.45 ± 1.02 |
| Passive tension (kN/m2) | 0.65 ± 0.17 | 0.41 ± 0.07 | 0.60 ± 0.15 | 0.28 ± 0.03 | 0.62 ± 0.10 | 0.44 ± 0.10 | 0.83 ± 0.12 | 0.66 ± 0.16 |
| Max force (µN) | 569 ± 66 | 450 ± 74a | 380 ± 57 | 308 ± 49a | 439 ± 52 | 320 ± 50a | 404 ± 86 | 354 ± 81a |
| Max tension (kN/m2) | 127 ± 19 | 103 ± 20a | 119 ± 14 | 95 ± 10a | 137 ± 10 | 95 ± 7a | 120 ± 17 | 105 ± 18a |
| ktr @ pCa 4.5 (s−1) | 4.30 ± 0.34 | 3.21 ± 0.19a | 5.10 ± 0.28 | 3.83 ± 0.26 | 4.75 ± 0.31 | 3.11 ± 0.27 | 4.83 ± 0.28 | 3.63 ± 0.26a |
| pCa for half-maximal force | 6.28 ± 0.04 | 6.28 ± 0.04 | 6.26 ± 0.05 | 6.10 ± 0.03 | 6.31 ± 0.01 | 6.09 ± 0.03a | 6.26 ± 0.04 | 6.08 ± 0.04 |
| Relative force (PO/P4.5) | 0.47 ± 0.01 | 0.45 ± 0.02 | 0.47 ± 0.03 | 0.42 ± 0.02 | 0.48 ± 0.03 | 0.51 ± 0.02 | 0.45 ± 0.02 | 0.47 ± 0.04 |
| Power output (µW/mg) | 2.60 ± 0.45 | 1.76 ± 0.31a | 2.24 ± 0.34 | 1.83 ± 0.19 | 2.36 ± 0.26 | 2.02 ± 0.16 | 2.15 ± 0.29 | 2.27 ± 0.31 |
| a/PO | 0.10 ± 0.02 | 0.06 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.02a | 0.11 ± 0.03 | 0.07 ± 0.01 | 0.11 ± 0.03 | 0.09 ± 0.01 |
| Fopt (P/PO) | 0.23 ± 0.01 | 0.19 ± 0.01a | 0.23 ± 0.01 | 0.21 ± 0.02 | 0.22 ± 0.02 | 0.19 ± 0.02 | 0.23 ± 0.02 | 0.22 ± 0.01 |
| Vopt (ML/s) | 0.14 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.19 ± 0.03 | 0.14 ± 0.01 | 0.20 ± 0.03a | 0.16 ± 0.01 | 0.19 ± 0.01a |
| Vmax | 0.62 ± 0.05 | 0.82 ± 0.04a | 0.64 ± 0.08 | 1.01 ± 0.26 | 0.66 ± 0.10 | 1.21 ± 0.28 | 0.70 ± 0.11 | 0.85 ± 0.09 |
| PNPO (P/PO*ML/s) | 0.032 ± 0.004 | 0.033 ± 0.003 | 0.033 ± 0.002 | 0.039 ± 0.002a | 0.030 ± 0.002 | 0.036 ± 0.002a | 0.036 ± 0.002 | 0.042 ± 0.003a,b |
| Submax ktr | 1.22 ± 0.14 | 1.40 ± 0.22 | 1.05 ± 0.26 | 1.10 ± 0.18 | 0.98 ± 0.12 | 1.31 ± 0.10a | 1.36 ± 0.24 | 1.37 ± 0.14 |
Mechanical properties were tested before and after recombinant cTn exchange and compared via two-way repeated measures ANOVA. Values are mean ± SEM.
P ≤ 0.05 vs. before same group.
P ≤ 0.05 vs. WT group. n equals the number of slow-twitch skeletal muscle fibers (biological replicates).
PNPO of rat single permeabilized slow-twitch skeletal muscle fibers before and after exchange of human cardiac Tn complex. (A–D) Summary data of PNPO before and after exchange of endogenous slow skeletal Tn with recombinant human cardiac Tn complex where A shows effects of exchange with WT cTnI, B shows effects of exchange with cTnI S23/24DD, C shows the effects of exchange with cTnI Y26E, and D shows the effects of the combination of cTnI S23/24 DD and Y26E. Fiber preparations were compared by two-way repeated measures ANOVA (n = 5–7 per group, biological replicates).
PNPO of rat single permeabilized slow-twitch skeletal muscle fibers before and after exchange of human cardiac Tn complex. (A–D) Summary data of PNPO before and after exchange of endogenous slow skeletal Tn with recombinant human cardiac Tn complex where A shows effects of exchange with WT cTnI, B shows effects of exchange with cTnI S23/24DD, C shows the effects of exchange with cTnI Y26E, and D shows the effects of the combination of cTnI S23/24 DD and Y26E. Fiber preparations were compared by two-way repeated measures ANOVA (n = 5–7 per group, biological replicates).
Regulation of power by cardiac thin filament proteins in cardiac myocytes from human failing hearts
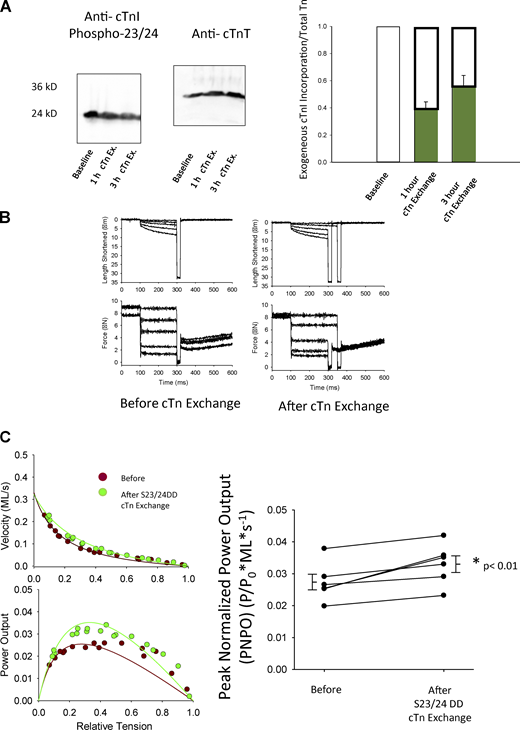
To address molecule specificity underlying PKA-mediated contractile reserve capacity in failing myocytes, we undertook a series of experiments whereby endogenous cTn was replaced with exogenous cTn (having pseudo-phosphorylated cTnI) in cardiac myocytes from failing human hearts (Fig. 4 A). Representative cardiac myocyte preparation length and force traces are shown during isotonic contractions before and after 3 h of cTn exchange, Fig. 4 B. Exchange with cTn containing cTnI Ser23/24Asp augmented power output in permeabilized cardiac myocyte preparations from human failing hearts (Fig. 4 C). PNPO increased ∼20% in cardiac myocytes from human failing hearts after Tn exchange containing pseudo-phosphorylated cTnI (Table 3 and Fig. 4 C). These results indicate N-terminal cTnI phosphorylation regulates cardiac myocyte power generation even in the context of a sarcomeric protein profile found within human failing myocardium.
PNPO of human failing permeabilized cardiac myocytes before and after exchange of human cardiac Tn complex containing S2324DD cTnI. (A) Relative assessment of recombinant pseudo-phosphorylated Ser23/24 (DD) cTnI exchanged (for 1 and 3 h) into myocyte preparations from human failing hearts. (B) Representative length and force traces during the load clamps to measure loaded shortening velocities before and after cTn exchange containing cTnI Ser23/24. (C) Representative force–velocity and power–load curves at submaximal force before and after 3 h of cTn exchange with pseudo-phosphorylated cTnI in a permeabilized myocyte preparation from a human failing heart. Right: Summary data measuring PNPO before and after 3 h cTn complex exchange. Myocyte preparations compared by paired t test (n = 6, biological replicates). Source data are available for this figure: SourceData F4.
PNPO of human failing permeabilized cardiac myocytes before and after exchange of human cardiac Tn complex containing S2324DD cTnI. (A) Relative assessment of recombinant pseudo-phosphorylated Ser23/24 (DD) cTnI exchanged (for 1 and 3 h) into myocyte preparations from human failing hearts. (B) Representative length and force traces during the load clamps to measure loaded shortening velocities before and after cTn exchange containing cTnI Ser23/24. (C) Representative force–velocity and power–load curves at submaximal force before and after 3 h of cTn exchange with pseudo-phosphorylated cTnI in a permeabilized myocyte preparation from a human failing heart. Right: Summary data measuring PNPO before and after 3 h cTn complex exchange. Myocyte preparations compared by paired t test (n = 6, biological replicates). Source data are available for this figure: SourceData F4.
Characteristics of human failing permeabilized myocytes before and after 3 h cTn exchange containing cTnI S23/24DD
| Human samples . | Myofibrillar preparations . | |
|---|---|---|
| Before . | After S2223DD . | |
| n | 6 | |
| Fiber length (µm) | 148 ± 10 | |
| Fiber width (µm) | 25 ± 3 | |
| Resting sarcomere length (µm) | 2.25 ± 0.02 | |
| Passive force (µN) | 0.52 ± 0.13 | 0.44 ± 0.16 |
| Passive tension (kN/m2) | 1.48 ± 0.21 | 0.96 ± 0.23 |
| Max force (µN) | 15.1 ± 4.6 | 12.6 ± 3.5a |
| Max tension (kN/m2) | 39.9 ± 3.4 | 34.9 ± 3.2a |
| ktr @ pCa 4.5 (s−1) | 0.712 ± 0.08 | 0.595 ± 0.06a |
| pCa for half-maximal force | 6.08 ± 0.03 | 5.88 ± 0.03a |
| Relative force (PO/P4.5) | 0.49 ± 0.04 | 0.55 ± 0.03 |
| a/PO | 0.16 ± 0.02 | 0.23 ± 0.05 |
| Fopt (P/PO) | 0.266 ± 0.01 | 0.291 ± 0.02 |
| Vopt (ML/s) | 0.104 ± 0.010 | 0.117 ± 0.013 |
| Vmax | 0.39 ± 0.04 | 0.42 ± 0.08 |
| PNPO (P/PO*ML/s) | 0.027 ± 0.002 | 0.033 ± 0.003a |
| Submax ktr | 0.552 ± 0.10 | 0.772 ± 0.11a |
| Human samples . | Myofibrillar preparations . | |
|---|---|---|
| Before . | After S2223DD . | |
| n | 6 | |
| Fiber length (µm) | 148 ± 10 | |
| Fiber width (µm) | 25 ± 3 | |
| Resting sarcomere length (µm) | 2.25 ± 0.02 | |
| Passive force (µN) | 0.52 ± 0.13 | 0.44 ± 0.16 |
| Passive tension (kN/m2) | 1.48 ± 0.21 | 0.96 ± 0.23 |
| Max force (µN) | 15.1 ± 4.6 | 12.6 ± 3.5a |
| Max tension (kN/m2) | 39.9 ± 3.4 | 34.9 ± 3.2a |
| ktr @ pCa 4.5 (s−1) | 0.712 ± 0.08 | 0.595 ± 0.06a |
| pCa for half-maximal force | 6.08 ± 0.03 | 5.88 ± 0.03a |
| Relative force (PO/P4.5) | 0.49 ± 0.04 | 0.55 ± 0.03 |
| a/PO | 0.16 ± 0.02 | 0.23 ± 0.05 |
| Fopt (P/PO) | 0.266 ± 0.01 | 0.291 ± 0.02 |
| Vopt (ML/s) | 0.104 ± 0.010 | 0.117 ± 0.013 |
| Vmax | 0.39 ± 0.04 | 0.42 ± 0.08 |
| PNPO (P/PO*ML/s) | 0.027 ± 0.002 | 0.033 ± 0.003a |
| Submax ktr | 0.552 ± 0.10 | 0.772 ± 0.11a |
Mechanical properties were tested before and after cTn exchange and compared via paired t test analysis. Values are mean ± SEM.
P ≤ 0.05. n equals the number of human hearts (biological replicates), with one cardiac myocyte preparation per heart.
Discussion
This study addressed thin filament regulation of loaded shortening and power output in mammalian striated muscle. We observed that pseudo-phosphorylation of the N-terminal region of cTnI was sufficient to speed loaded shortening and increase power output in rat slow-twitch skeletal muscle fibers. Furthermore, we observed that a similar cTnI residue-specific mechanism amplified power output in cardiac myofilament from failing human hearts.
Myofibrillar power generating capacity is regulated by several factors, including activator Ca2+ (McDonald, 2000), myosin heavy chain (Herron et al., 2001a; Herron and McDonald, 2002; Korte et al., 2005), protein kinase-mediated phosphorylation of myofilament proteins (Herron et al, 2001b; Hanft et al, 2016; Robinett et al, 2019), and sarcomere length (Korte and McDonald, 2007; Hanft and McDonald, 2009; McDonald et al., 2012; Hanft et al., 2021). Physiologically, there is a complex interplay between the intracellular calcium transient and the subsequent sarcomere level activation. The modulation of loaded shortening and power output on a beat-to-beat basis reveals the complex integration of post-translational modifications of both calcium handling proteins as well as myofilament contractile proteins. For instance, β-adrenergic stimulation is well known to initiate PKA-induced phosphorylation of the sarcolemmal L-type Ca2+ channel, the sarcoplasmic reticulum Ca2+ release channel (i.e., ryanodine receptor), and phospholamban, which culminates in elevated intracellular calcium transients with faster overall kinetics of both SR Ca2+ release and reuptake (Endoh and Blinks, 1988; Bers, 2002). PKA also phosphorylates the thin-filament protein cardiac troponin I (Solaro et al., 1976; Tardiff, 2011; Solaro et al., 2013), the thick-filament protein cardiac myosin binding protein C (Garvey et al., 1988; Gautel et al., 1995; Colson et al., 2012; Sadayappan and de Tombe, 2012), and titin (Yamasaki et al., 2002), and all together are thought to accelerate myofibrillar force production, loaded shortening, and increase power output. The mechanisms underlying PKA-induced increases in loaded shortening and power output likely involve increases in cooperative activation of the thin filament regulatory units, which, in turn, facilitate increases in both cross-bridge numbers and cross-bridge cycling kinetics to work against an afterload (McDonald, 2011).
The myofilaments serve as a convergent target for PKA-mediated phosphorylation, which results in altered contraction. PKA-mediated changes in contractile properties have been directly investigated using permeabilized muscle cell preparations, which allow PKA access to transfer phosphoryl groups to myofilament proteins including cTnI, cMyBP-C, and titin. PKA has been documented to decrease Ca2+ sensitivity of force (Solaro et al., 1976; Strang et al., 1994; Wattanapermpool et al., 1995), increase cooperative activation of force (as indexed by Hill coefficient; Konhilas et al., 2003; Hanft et al., 2013), speed maximum velocity of shortening (Strang et al., 1994), increase loaded shortening and power output (Herron et al., 2001b; Hanft and McDonald, 2009; Robinett et al., 2019), increase length dependence of Ca2+ sensitivity of force (Konhilas et al., 2003; Kooij et al., 2010; Wijnker et al., 2013; Wijnker et al., 2014) and steepen sarcomere length–tension relationships (Hanft and McDonald, 2010). Some studies have further investigated the molecule specificity underlying these changes in contractile properties. For instance, length-dependence of Ca2+ sensitivity was addressed at the molecular level via transgenic expression of pseudo-phosphorylated cTnI or cMyBP-C (Kumar et al., 2015). Both PKA phosphorylated molecules were found to independently alter length dependence of force with cTnI and cMyBP-C having 33 and 67% relative contribution, respectively. Maximum velocity of shortening, at least during submaximal Ca2+ activations, was elevated by pseudo-phosphorylation of cMyBP-C but not cTnI (Giles et al., 2021). Loaded shortening and power output are increased by either PKA-mediated or transgene-induced pseudo-phosphorylation of MyBP-C in permeabilized slow-twitch skeletal muscle fibers (Robinett et al., 2019) or cardiac myocytes (Hanft et al., 2021). Studies are lacking on the direct role of PKA phosphorylation of cTnI on loaded shortening and power output. We address this knowledge gap in this study and observed that pseudo-phosphorylation of human cTnI at PKA sites, as well as a nearby tyrosine site, was sufficient to speed loaded shortening and increase power output during submaximal Ca2+ activation of mammalian slow-twitch skeletal muscle fibers. In addition, pseudo-phosphorylation of human cTnI increased power generating capacity of cardiac myofilaments from human failing hearts. There is precedent for these findings as several studies have observed thin filament mediated alterations in myofilament shortening and/or myosin cross-bridge interaction kinetics (Sweeney et al., 1998; Homsher et al., 2000; Morris et al., 2003; Hinken et al., 2012). Interestingly, PKA increased power by 35% yet power increased by 20% following exchange with pseudo-phosphorylated cTnI, implicating a similar additive and/or synergistic role of cTnI and cMyBP-C phosphorylation on power. Another possibility to explain the differences between PKA and cTn exchange is the decline in maximal Ca2+-activated force that occurred after cTn exchange in all groups. The exact reason(s) for the fall in force is unknown but similar force changes with Tn exchange have been previously reported (Strauss et al., 1992; McDonald et al., 1998a; Chandra et al., 1999). Importantly, our experimental design controlled for the consistent fall in force by using WT cTnI exchange group. In WT cTn exchange fibers force declined but power did not increase. Thus, the increase in power output following exchange with pseudo-phosphorylated cTnI likely underestimates the full impact of phosphorylation of the N-terminus of cTnI on power output since power increased even in the presence of decreased force.
Regarding intermolecular mechanistic underpinnings for the increase in power by phosphorylation of cTnI, we observed similar changes in power following Tn exchange with Tn containing either TnI S23/24D or TnI Y26E. Consistent with this finding, the phosphorylation of TnI at Ser23/24 has been demonstrated to alter the TnI N-terminal structure disrupting TnI N-terminal interactions with TnC that decrease TnC Ca2+ binding (Ward et al., 2004; Howarth et al., 2007). Supporting the role of the TnI N-terminal TnC interactions, experiments that mutated TnI amino acids surrounding TnI Ser23/24 or removed the TnI N-terminal region (Ward et al., 2004; Barbato et al., 2005; Biesiadecki et al., 2010) both altered TnC Ca2+ binding similarly to that of TnI Ser23/24 phosphorylation. Together these data support that the TnI PKA-mediated Ser23/24 phosphorylation increase in power is not specific to TnI Ser23/24 phosphorylation per se, but rather results from the phosphorylation-induced structural alteration of the TnI N-terminal region that influences TnI interactions with TnC. This may explain our finding that TnI Ser23/24 phosphorylation and TnI Tyr26 phosphorylation similarly increase power and that their combination does not further augment power.
Human heart failure is characterized by a lack of cardiac reserve, which impacts workload capacity and worsens morbidity. Most heart failure medications alleviate symptoms at rest by decreasing hemodynamic loads but show minimal improvement on cardiac reserve. In theory, inotropic factors such as β-adrenergic pathway stimulation should augment cardiac reserve but this broad-spectrum approach is contraindicated because of arrhythmias, and catecholamine-driven myocyte death and adverse remodeling (Eichhorn and Bristow, 1996). Precision-based approaches are needed that circumvent adverse cardiac responses by targeting the myofilaments to reestablish cardiac reserve. At a molecular level, cTnI phosphorylation is depressed in myofilaments from human failing hearts (Bristow et al., 1982; Wolff et al., 1996; Messer et al., 2007; Kooij et al., 2010; Wijnker et al., 2013; Haynes et al., 2014; Blair et al., 2020). cTnI phosphorylation state inversely correlates with Ca2+ sensitivity of steady-state force. However, the direct effects of cTnI phosphorylation on power had not been addressed, especially in human failing myofilaments. We found cTnI N-domain phosphorylation is sufficient to augment myofilament power, which establishes rationale for a molecular target to improve cardiac reserve in human heart failure patients. Ongoing and future studies are aimed to mimic the biophysical and physiological changes caused by phosphorylation of the cTnI N-domain region, which hold promise as a means to augment contractile reserve capacity.
Data availability
The data are available from the corresponding author upon reasonable request.
Acknowledgments
Henk L. Granzier served as editor.
This work was supported by National Heart, Lung, and Blood Institute grants (R01-HL148785 to K.S. McDonald, R01 HL149164, K.S. Campbell, and R01 HL114940 to B.J. Biesiadecki). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions: All mechanical experiments were performed in the laboratory of K.S. McDonald, L.M. Hanft, and K.S. McDonald contributed equally to concept and design of experiments, collection, analysis, and interpretation of data, and writing the manuscript. J.C. Robinett and T.J. Kalogeris contribute to experiments and data analysis. L.M. Hanft performed Western blot analysis and assisted in concept and design of experiments, data analysis, and drafting and revising the manuscript. K.S. Campbell provided the human failing heart biopsies and B.J. Biesiadecki provided the human recombinant cardiac troponin complexes, all of which were necessary for the study. All authors contributed to editing and revising the manuscript.
References
This work is part of a special issue on Myofilament Function 2022.
Data availability
The data are available from the corresponding author upon reasonable request.
Competing Interests
Disclosures: The authors declare no competing interests exist.