The essential COPI coat mediates retrieval of transmembrane proteins at the Golgi and endosomes following recruitment by the small GTPase, Arf1. ArfGAP proteins regulate COPI coats, but molecular details for COPI recognition by ArfGAPs remain elusive. Biochemical and biophysical data reveal how β′-COP propeller domains directly engage the yeast ArfGAP, Glo3, with a low micromolar binding affinity. Calorimetry data demonstrate that both β′-COP propeller domains are required to bind Glo3. An acidic patch on β′-COP (D437/D450) interacts with Glo3 lysine residues located within the BoCCS (binding of coatomer, cargo, and SNAREs) region. Targeted point mutations in either Glo3 BoCCS or β′-COP abrogate the interaction in vitro, and loss of the β′-COP/Glo3 interaction drives Ste2 missorting to the vacuole and aberrant Golgi morphology in budding yeast. These data suggest that cells require the β′-COP/Glo3 interaction for cargo recycling via endosomes and the TGN, where β′-COP serves as a molecular platform to coordinate binding to multiple proteins, including Glo3, Arf1, and the COPI F-subcomplex.
Introduction
The highly conserved COPI coat (Duden, 2003; Jackson, 2014; Arakel and Schwappach, 2018) is essential for vesicular membrane trafficking in eukaryotes. COPI has many established roles, including retrieval of ER proteins from the Golgi back to the ER; cycling of proteins between the ER and Golgi; retrograde (Popoff et al., 2011) and anterograde (Yang et al., 2011) trafficking within the Golgi stack; and from endosomes to the TGN (Xu et al., 2017). COPI has been implicated in lipid homeostasis (Beller et al., 2008), viral replication (Cureton et al., 2012), and pathogen entry (Guo et al., 2008; Misselwitz et al., 2011; Park et al., 2019). Mutations or upregulation of COPI subunits have been specifically linked to both inherited and acquired diseases, including microcephaly (DiStasio et al., 2017) and cancer (Bhandari et al., 2019). Viral glycoproteins use established COPI motifs to circumvent host immunity (Pääbo et al., 1986; Goepfert et al., 1997).
The COPI heptamer (α−/β−/β′-/γ−/δ−/ε−/ζ−COP subunits) is recruited en bloc onto Golgi membranes (Hara-Kuge et al., 1994) following recruitment by the small GTPase, Arf1, and cargo. COPI historically has been conceptually divided into the B-subcomplex (α/β′/ε) and F-subcomplex (β/δ/γ/ζ). The F-subcomplex is both structurally and functionally related to the AP clathrin adaptor complexes (Schledzewski et al., 1999; Yu et al., 2012). The B-subcomplex is often compared to clathrin, but it shares no evolutionary history (Schledzewski et al., 1999). Structural data demonstrate that COPI is an interwoven coat with multiple contacts between subunits (Dodonova et al., 2015; Dodonova et al., 2017; Bykov et al., 2017), in contrast to the layered structure adopted by clathrin (Kovtun et al., 2020) and COPII (Noble et al., 2013; Hutchings et al., 2018) coats. The COPI B-subcomplex contains four WD-repeat (also known as β-propeller) domains, which are interaction platforms that can accommodate multiple protein binding partners. The α- and β′-COP N-terminal WD-repeat domains bind dilysine motifs in transmembrane cargoes (Jackson et al., 2012; Ma and Goldberg, 2013) and K63-linked ubiquitin chains (Xu et al., 2017). The COPI WD-repeat domains found in α- and β′-COP are strong candidates for recognizing other proteins, including regulators.
ArfGAP proteins are critical regulators of COPI function, but their precise molecular roles remain poorly understood. ArfGAPs have been implicated in coat assembly (Yang et al., 2002; Lewis et al., 2004), cargo/SNARE sorting (Lee et al., 2005; Robinson et al., 2006; Schindler et al., 2009), and coat disassembly (Reinhard et al., 2003). Yeast contain two essential ArfGAP proteins, Glo3 (Fig. 1 A) and Gcs1, which have overlapping functions (Poon et al., 1999). Both ArfGAPs have homologs/orthologs in mammalians cells: Gcs1 corresponds to ArfGAP1, while Glo3 corresponds to ArfGAP2/3. Budding yeast tolerate the deletion of either GLO3 or GCS1 individually, but deletion of both genes is lethal (Poon et al., 1999). Glo3 GAP activity is an essential function (Lewis et al., 2004; Arakel et al., 2019). Strains harboring the GAP-dead version of Glo3 (R59K) are not viable, even in the presence of Gcs1, which implies a dominant role for Glo3. In mammalian cultured cell lines, mutations in key basic residues of ArfGAP2/3 have been shown to affect localization and function (Kliouchnikov et al., 2009).
There are very limited molecular data indicating how ArfGAPs interact with the COPI coat and the precise roles they play in trafficking. Classically, ArfGAPs were thought to drive vesicle uncoating by hydrolyzing GTP and releasing Arf1(GTP) from membranes; in this model, the ArfGAP serves to recycle COPI. However, Glo3 has been shown to play important roles in coat assembly, including cargo selection and SNARE binding via the BoCCS region in Glo3 (Schindler et al., 2009). Biochemical data from cell lysates (Arakel et al., 2019) suggest Glo3, but not Gcs1, stably associates with COPI. Both genetic (Surma et al., 2013; Costanzo et al., 2016) and affinity-capture data (Gavin et al., 2002) in budding yeast suggest an interaction between COPI and Glo3, while the ArfGAP2/3 GAP domain has been visualized in a cryo-electron tomography (cryo-ET) reconstructions (Dodonova et al., 2017) near the B-subcomplex. In yeast cell lysates, Glo3 interacts with γ-COP appendage domain (Watson et al., 2004) and associates stably with COPI (Arakel et al., 2019). Recent data in yeast indicate that posttranslational modifications affect interactions between Glo3, SNAREs, and COPI, with Glo3 and COPI interacting more robustly when SNAREs are absent (Date et al., 2022). Despite multiple lines of evidence, the biochemical basis for a direct interaction between COPI subunits and Glo3 has not been established.
Previous work (Jackson et al., 2012) and data on other coat proteins (Miele et al., 2004; Lemmon and Traub, 2012; Muenzner et al., 2017) led to our hypothesis that β′-COP functions as a molecular platform to engage multiple protein partners within the COPI coat. In this work, we set out to identify additional binding partners for the β′-COP WD-repeat (also known as propeller) domains. We identified ArfGAP, Glo3, and Arf1 as direct β′-COP binding partners in vitro. We characterized the β′-COP/Glo3 interaction by mapping specific residues on both proteins, quantifying binding affinities, and testing the effects of structure-based mutants in vitro and in budding yeast. We tested whether disrupting the β′-COP/Glo3 interaction affects dilysine cargo binding in vitro, as well as whether the loss of interaction affects cargo sorting and Golgi morphology in budding yeast. Together, these new data identify an expanded role for the β′-COP subunit and suggest a compelling model for the β′-COP/Glo3/Arf1 complex on Golgi membranes.
Results
β′-COP directly binds Glo3 and Arf1 in vitro
WD-repeat (also known as β-propeller) domains are known protein binding platforms (Jain and Pandey, 2018). In preliminary work, we sought to identify other direct binding partners for the β′-COP propeller domains beyond the well-established dilysine motif cargo (Jackson et al., 2012; Ma and Goldberg, 2013). We used recombinant purified β′-COP protein (residues 1–604 with a C-terminal GST tag or β′604-GST; Fig. 1 A) as bait in pulldown experiments from budding yeast cell lysates and identified both Glo3 and Arf1 as potential binding partners (Fig. S1 A). We next undertook biochemical pulldown experiments to ascertain whether purified recombinant proteins directly bind to each other (Fig. 1 B). For pulldown experiments, 50 μg GST-tagged bait was incubated on glutathione Sepharose with a fivefold molar excess of prey protein. The data indicate β′604-GST binds directly to both Glo3 and Arf1 in either nucleotide-bound state (Fig. 1 B and Fig. S1 B). The β′-COP/Glo3 interaction could be visualized with Coomassie staining, which suggested the binding affinity (KD) between β′-COP and Glo3 might lie in the low micromolar range (see next section). A three-way pulldown suggests all three proteins interact simultaneously with each other (Fig. S1 B). When fivefold molar excess Arf1 is added, the β′604-GST/Glo3 complex does not appear to favor the nucleotide-bound state (GDP-locked T31N or GTP-locked Q71L). These data provide the first biochemical evidence for a direct interaction between β′-COP and Glo3, and between β′-COP and Arf1. These data are further supported by structural data from COPI coats reconstituted in vitro, which place β′-COP and Arf1 adjacent to each other in reconstructions (Dodonova et al., 2015).
β′-COP propeller (WD-repeat) domains directly bind Glo3 and Arf1 in vitro. (A) Schematics of yeast Glo3 and β′-COP proteins. Glo3 contains a GAP domain (residues 1–140), BoCCS region (defined as residues 214–375), and GRM region (residues 375–493). β′-COP contains two WD-repeat (also known as β-propeller) domains followed by an α-solenoid. The N-terminal propeller domain binds dilysine motifs in transmembrane cargo, while the α-solenoid interacts with α-COP. (B) Pulldown experiments using GST-tagged β′-COP propeller domains (residues 1–604 with C-terminal GST tag) as bait and either full-length Arf1-H6 or full-length Glo3-H8 as prey. We tested binding to both GDP-locked (T31N) Arf1 and GTP-locked (Q71L) Arf1. β′-COP interacts directly with both Arf1 constructs and with Glo3 in vitro. The top panel shows a Coomassie-stained SDS-PAGE gel, while the bottom two panels show Western blots against the Glo3 His8 tag (α-His; Abcam NB100-63173) or against yeast Arf1 (α-Arf1, Todd Graham lab, Vanderbilt University).
β′-COP propeller (WD-repeat) domains directly bind Glo3 and Arf1 in vitro. (A) Schematics of yeast Glo3 and β′-COP proteins. Glo3 contains a GAP domain (residues 1–140), BoCCS region (defined as residues 214–375), and GRM region (residues 375–493). β′-COP contains two WD-repeat (also known as β-propeller) domains followed by an α-solenoid. The N-terminal propeller domain binds dilysine motifs in transmembrane cargo, while the α-solenoid interacts with α-COP. (B) Pulldown experiments using GST-tagged β′-COP propeller domains (residues 1–604 with C-terminal GST tag) as bait and either full-length Arf1-H6 or full-length Glo3-H8 as prey. We tested binding to both GDP-locked (T31N) Arf1 and GTP-locked (Q71L) Arf1. β′-COP interacts directly with both Arf1 constructs and with Glo3 in vitro. The top panel shows a Coomassie-stained SDS-PAGE gel, while the bottom two panels show Western blots against the Glo3 His8 tag (α-His; Abcam NB100-63173) or against yeast Arf1 (α-Arf1, Todd Graham lab, Vanderbilt University).
β′-COP, Arf1, and Glo3 form a ternary complex in vitro. (A) Glo3 and Arf1 were identified as potential direct binding partners using GST-tagged β′-COP protein as bait in yeast cell lysates. The table summarizes mass spectrometry results for these two hits. (B) GST-pulldown experiments using GST-tagged β′-COP propeller domains (residues 1–604 with C-terminal GST tag) as bait and full-length Arf1-H6 with and without full-length Glo3-H8 as prey. We tested binding to both nucleotide-bound forms of Arf1: GDP-locked (T31N) or GTP-locked (Q71L). β′-COP can pull down both Arf1 and Glo3 simultaneously. β′-COP does not appear to show a preference for Arf1 nucleotide state; it pulls down Arf1 T31N or Q71L equally well when Arf1 is added at a 5:1 molar ratio.
β′-COP, Arf1, and Glo3 form a ternary complex in vitro. (A) Glo3 and Arf1 were identified as potential direct binding partners using GST-tagged β′-COP protein as bait in yeast cell lysates. The table summarizes mass spectrometry results for these two hits. (B) GST-pulldown experiments using GST-tagged β′-COP propeller domains (residues 1–604 with C-terminal GST tag) as bait and full-length Arf1-H6 with and without full-length Glo3-H8 as prey. We tested binding to both nucleotide-bound forms of Arf1: GDP-locked (T31N) or GTP-locked (Q71L). β′-COP can pull down both Arf1 and Glo3 simultaneously. β′-COP does not appear to show a preference for Arf1 nucleotide state; it pulls down Arf1 T31N or Q71L equally well when Arf1 is added at a 5:1 molar ratio.
We next set out to ascertain which part of Glo3 binds β′-COP. Glo3 (Fig. 1 A) contains a GAP domain; an unstructured middle region called the BoCCS (for binding of coatomer, cargo, and SNAREs; Schindler et al., 2009); and a C-terminal GRM domain (Golgi regulatory motif). We purified recombinant GST-fusion proteins encompassing each portion of Glo3 and undertook pulldown experiments (Fig. S2). These data indicate only the Glo3 BoCCS region can pull down β′604-H6. The BoCCS region has been previously implicated in binding TAP-purified COPI from yeast cell lysates (Arakel et al., 2019), but the COPI subunit mediating the interaction was unknown. Our data reveal for the first time how the β′-COP subunit directly binds Glo3 BoCCS.
β′-COP WD-repeat domains directly bind the Glo3 BoCCS region in vitro. GST-tagged Glo3 fragments (GAP-GST, residues 1–150; GST-BoCCS, residues 208–383; or GST-GRM, residues 350–493) were used as bait with β′-COP-H6 to determine which portion of Glo3 binds β′-COP. Only the GST-BoCCS fragment exhibited direct binding. Both the BoCCS and GRM fragments are unstable in solution, most likely because they contain long regions predicted to be unstructured. Mass spectrometry data (not shown) confirms the top two bands in the GST-BoCCS input lane correspond to Glo3 BoCCS peptides.
β′-COP WD-repeat domains directly bind the Glo3 BoCCS region in vitro. GST-tagged Glo3 fragments (GAP-GST, residues 1–150; GST-BoCCS, residues 208–383; or GST-GRM, residues 350–493) were used as bait with β′-COP-H6 to determine which portion of Glo3 binds β′-COP. Only the GST-BoCCS fragment exhibited direct binding. Both the BoCCS and GRM fragments are unstable in solution, most likely because they contain long regions predicted to be unstructured. Mass spectrometry data (not shown) confirms the top two bands in the GST-BoCCS input lane correspond to Glo3 BoCCS peptides.
Glo3 BoCCS binds both β′-COP propellers with low micromolar affinity
Our initial biochemical data indicated the interaction between β′-COP and Glo3 is strong enough to be quantified using calorimetry. We thus mapped the binding region within Glo3 using isothermal titration calorimetry (ITC) to quantify binding affinities between purified recombinant proteins (Fig. 2 A, Fig. S3, and Table S1). All affinity tags were removed from both Glo3 and β′1-604 prior to running calorimetry experiments, and the initial goal was to ascertain the minimal binding fragment for pursuing structural studies. Pulldown experiments suggested a low micromolar interaction between full-length Glo3 and the β′-COP propeller domains (residues 1–604), and only the BoCCS region appeared to interact directly with β′-COP (Fig. S2). Initial experiments using the full BoCCS region (residues 208–375) revealed a low micromolar KD (Fig. S3 A) and 1:1 stoichiometry. We generated a series of Glo3 constructs truncated at either the N- or C-terminal end, purified each protein, and quantified binding to β′604 by ITC (Fig. 2 A and Fig. S3 B). Although longer purified Glo3 constructs are unstable over time (Fig. S2), shorter Glo3 fragments used in ITC experiments exhibited high purity and stability after purification (representative example SDS-PAGE gel shown in Fig. S3 C).
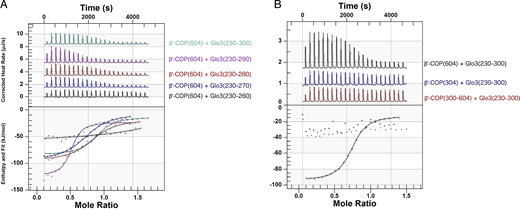
Residues within the Glo3 BoCCS region directly bind both β′-COP propellers with low micromolar affinity. (A) Purified recombinant proteins (untagged β′-COP residues 1–604 and Glo3 fragments as labeled) were used in isothermal titration calorimetry (ITC) experiments to quantify binding affinities; representative traces are shown. β′-COP binds a Glo3 fragment located within the BoCCS region. The highest affinity interaction occurs between β′-COP 1-604 and Glo3 residues 230–290, but all fragments exhibit low micromolar KD values (0.8–6 μM) and 1:1 stoichiometry (Table S1). (B) Representative ITC experiments between untagged Glo3 BoCCS fragment (residues 230–300) and N-terminal β′-COP propeller (residues 1–304), C-terminal β′-COP propeller (residues 300–604), or both β′-COP propellers (residues 1–604). Each propeller domain on its own is insufficient to produce measurable binding by calorimetry, which suggests both propellers are required to bind Glo3. Unless otherwise noted, 0.05 mM β′-COP protein was placed in the cell and 0.3 mM Glo3 protein in the syringe (see Materials and methods).
Residues within the Glo3 BoCCS region directly bind both β′-COP propellers with low micromolar affinity. (A) Purified recombinant proteins (untagged β′-COP residues 1–604 and Glo3 fragments as labeled) were used in isothermal titration calorimetry (ITC) experiments to quantify binding affinities; representative traces are shown. β′-COP binds a Glo3 fragment located within the BoCCS region. The highest affinity interaction occurs between β′-COP 1-604 and Glo3 residues 230–290, but all fragments exhibit low micromolar KD values (0.8–6 μM) and 1:1 stoichiometry (Table S1). (B) Representative ITC experiments between untagged Glo3 BoCCS fragment (residues 230–300) and N-terminal β′-COP propeller (residues 1–304), C-terminal β′-COP propeller (residues 300–604), or both β′-COP propellers (residues 1–604). Each propeller domain on its own is insufficient to produce measurable binding by calorimetry, which suggests both propellers are required to bind Glo3. Unless otherwise noted, 0.05 mM β′-COP protein was placed in the cell and 0.3 mM Glo3 protein in the syringe (see Materials and methods).
The Glo3 BoCCS region interacts directly with β′-COP. (A) An ITC experiment with full BoCCS Glo3 fragment (residues 208–383) shows a low micromolar KD. (B) ITC experiments to ascertain the minimum Glo3 fragment required for binding. Glo3 N-terminal residues 230–240 are required for measurable binding in the calorimeter, while removing residues 220–230 exhibits no measurable effect on binding affinity. n.b. denotes no measurable binding in the calorimeter (KD > 300 μM). (C) Representative SDS-PAGE gel of β′604/Glo3 (residues 230–300) complex following purification; the complex elutes together over gel filtration columns (data not shown). Although some Glo3 fragments are unstable over time, the recombinant fragments used for ITC runs exhibit high levels of purity and stability. (D–F) Representative models generated using AlphaFold2. The well-established interaction between β′-COP and dilysine motifs served as a positive control computational experiment, but AlphaFold failed to predict binding between dilysine motifs and the N-terminal β′-COP propeller (D) or to both propeller domains (E). The known dilysine binding site is marked by black asterisks. (F) One representative model from an AlphaFold experiment with β′-COP 1-604 and Glo3 residues 230–290. Results from modeling experiments are reported in Table S3.
The Glo3 BoCCS region interacts directly with β′-COP. (A) An ITC experiment with full BoCCS Glo3 fragment (residues 208–383) shows a low micromolar KD. (B) ITC experiments to ascertain the minimum Glo3 fragment required for binding. Glo3 N-terminal residues 230–240 are required for measurable binding in the calorimeter, while removing residues 220–230 exhibits no measurable effect on binding affinity. n.b. denotes no measurable binding in the calorimeter (KD > 300 μM). (C) Representative SDS-PAGE gel of β′604/Glo3 (residues 230–300) complex following purification; the complex elutes together over gel filtration columns (data not shown). Although some Glo3 fragments are unstable over time, the recombinant fragments used for ITC runs exhibit high levels of purity and stability. (D–F) Representative models generated using AlphaFold2. The well-established interaction between β′-COP and dilysine motifs served as a positive control computational experiment, but AlphaFold failed to predict binding between dilysine motifs and the N-terminal β′-COP propeller (D) or to both propeller domains (E). The known dilysine binding site is marked by black asterisks. (F) One representative model from an AlphaFold experiment with β′-COP 1-604 and Glo3 residues 230–290. Results from modeling experiments are reported in Table S3.
We observed the strongest interaction between the β′-COP propeller domains and Glo3 residues 230–290 (average KD = 0.6 ± 0.2 μM, n = 3 runs; representative trace in Fig. 2 A and Table S1). We saw no difference in binding affinity when comparing β′-COP binding to Glo3 residues 220–290 or Glo3 residues 230–290, but the loss of Glo3 residues 230–240 abrogated measurable ITC binding (Fig. S3 B). Truncations at the C-terminal end resulted in progressively weaker interactions (Fig. 2 A). These data define Glo3 residues 230–290 as the key region that interacts directly with β′-COP propellers.
We next tested which β′-COP propeller domain is required for the interaction with Glo3 (Fig. 2 B). Specifically, we tested Glo3 residues 230–300 with each propeller domain on its own (either β′-COP residues 1–304 or residues 305–604) and with both propeller domains (residues 1–604). Neither the N- nor C-terminal β′-COP propeller domains alone were sufficient to measure binding using calorimetry (KD < ∼300 μM). However, when both propeller domains were present, β′-COP bound Glo3 with a KD ∼1 μM and 1:1 stoichiometry. These data suggest the Glo3 interaction requires the surface of both propeller domains, in contrast to dilysine cargo motifs, which interact only with the N-terminal propeller domain (Jackson et al., 2012; Ma and Goldberg, 2013).
Point mutations in β′-COP or Glo3 abrogate binding in vitro
Mutagenesis mapping
We took a systematic mutagenesis approach to identify β′-COP and Glo3 residues that mediate the interaction. Sequence alignments revealed that the Glo3 BoCCS region contains two highly conserved clusters of lysine residues (Fig. S4 A). We first tested the salt dependence of the β′-COP/Glo3 interaction by calorimetry and found that high salt concentrations (500 mM NaCl) substantially weakened the interaction (Fig. S4 B). We concluded that the interaction was likely to be mediated by electrostatic contacts. The first cluster (K233/K234/K235; Fig. S4 A) was proposed to interact with COPI (Schindler et al., 2009) in cell lysates, although the COPI subunit has never been identified. The second cluster (K251/K252/K255) has not been previously implicated. We tested both clusters of lysine residues (Fig. 3 A). The Glo3 K233E single point mutant binds β′-COP ∼40-fold more weakly than does wild type, and the Glo3 BoCCS K233E/K234E/K235E triple mutant reduces binding below detectable levels (Fig. 3 A). Mutating the second cluster of residues (Glo3 BoCCS K251E/K252E/K255E) also reduces binding affinity by ∼40-fold; this second patch may correspond to mammalian ArfGAP3 residues shown to be important for COPI binding (Kliouchnikov et al., 2009). Together, these data suggest that both basic clusters within the Glo3 BoCCS region mediate the interaction with β′-COP, and the first set of lysine residues is particularly important.
Conserved Glo3 lysine residues mediate an electrostatic interaction with β′-COP C-terminal propeller domain. (A) Glo3 partial sequence alignment highlighting key conserved residues between Saccharomyces cerevisiae Glo3 and mammalian ArfGAP2/3 homologs in Mus musculus and Homo sapiens. Glo3 residues 230–300 are labeled. (B) ITC experiment between wild-type β′-COP residues 1–604 and Glo3 residues 230–270 in 100 mM NaCl and 500 mM NaCl. Near physiological salt concentrations, the two proteins interact with a low micromolar KD. The same experiment in high-salt buffer (500 mM NaCl) disrupts the interaction, suggesting electrostatic residues play an important role. In high salt, binding was too weak to determine a KD. (C) Side view from membrane showing the β′-COP D437/D450 acidic patch that binds Glo3.
Conserved Glo3 lysine residues mediate an electrostatic interaction with β′-COP C-terminal propeller domain. (A) Glo3 partial sequence alignment highlighting key conserved residues between Saccharomyces cerevisiae Glo3 and mammalian ArfGAP2/3 homologs in Mus musculus and Homo sapiens. Glo3 residues 230–300 are labeled. (B) ITC experiment between wild-type β′-COP residues 1–604 and Glo3 residues 230–270 in 100 mM NaCl and 500 mM NaCl. Near physiological salt concentrations, the two proteins interact with a low micromolar KD. The same experiment in high-salt buffer (500 mM NaCl) disrupts the interaction, suggesting electrostatic residues play an important role. In high salt, binding was too weak to determine a KD. (C) Side view from membrane showing the β′-COP D437/D450 acidic patch that binds Glo3.
Key Glo3 lysine residues mediate an electrostatic interaction with an acidic patch on β′-COP. (A) Representative ITC experiments between wild-type β′-COP 1-604 and mutant versions of Glo3 (residues 230–290). Two Glo3 mutants substantially reduce binding in the calorimeter: a single point mutation at K233E and the K251E/K252E/K255E triple mutant. Both mutants exhibit 40-fold weaker binding as compared with wild-type Glo3 (KD ∼ 12 μM; Table S2). The Glo3 K233E/K234E/K235E triple mutant exhibits no measurable binding by calorimetry (KD < 300 μM). (B) Representative ITC experiment with wild-type untagged Glo3 (residues 230–290) and mutant β′-COP (D437A/D450A) proteins; the calculated KD for this interaction is 18 μM. The D437/D450 mutant exhibits 60-fold weaker binding to Glo3, suggesting this acidic patch plays a critical role in the interaction. For these experiments, 0.04 mM wild-type or 0.05 mM mutant β′-COP was placed in the cell. (C) Glo3 associates with COPI primarily using its interaction with the β′-COP subunit. Wild-type GST-Glo3 BoCCS pulled down COPI (B- and F-subcomplexes) while disrupting all six lysine residues disrupted Glo3 association with COPI. (D) Disruption of the β′-COP D437/D450 patch reduces the interaction between COPI and Glo3 in yeast cells. Glo3-6xHis-TEV-3xFLAG (Glo3-HTF) efficiently immunoprecipitates COPI from wild-type SEC27 cell lysates, in contrast to the β′-COP D437A/D450A mutant strain.
Key Glo3 lysine residues mediate an electrostatic interaction with an acidic patch on β′-COP. (A) Representative ITC experiments between wild-type β′-COP 1-604 and mutant versions of Glo3 (residues 230–290). Two Glo3 mutants substantially reduce binding in the calorimeter: a single point mutation at K233E and the K251E/K252E/K255E triple mutant. Both mutants exhibit 40-fold weaker binding as compared with wild-type Glo3 (KD ∼ 12 μM; Table S2). The Glo3 K233E/K234E/K235E triple mutant exhibits no measurable binding by calorimetry (KD < 300 μM). (B) Representative ITC experiment with wild-type untagged Glo3 (residues 230–290) and mutant β′-COP (D437A/D450A) proteins; the calculated KD for this interaction is 18 μM. The D437/D450 mutant exhibits 60-fold weaker binding to Glo3, suggesting this acidic patch plays a critical role in the interaction. For these experiments, 0.04 mM wild-type or 0.05 mM mutant β′-COP was placed in the cell. (C) Glo3 associates with COPI primarily using its interaction with the β′-COP subunit. Wild-type GST-Glo3 BoCCS pulled down COPI (B- and F-subcomplexes) while disrupting all six lysine residues disrupted Glo3 association with COPI. (D) Disruption of the β′-COP D437/D450 patch reduces the interaction between COPI and Glo3 in yeast cells. Glo3-6xHis-TEV-3xFLAG (Glo3-HTF) efficiently immunoprecipitates COPI from wild-type SEC27 cell lysates, in contrast to the β′-COP D437A/D450A mutant strain.
We next tested whether these Glo3 lysine residues are important in binding to COPI in yeast (Fig. 3 C). Purified GST-tagged Glo3 fragments (wild-type and lysine mutants) were used as bait in pulldown experiments from budding yeast cell lysates in which both His and FLAG tags were integrated into the β-COP gene under its endogenous promoter. Full-length purified Glo3 or the Glo3 BoCCS region alone immunoprecipitated COPI from yeast cells (Fig. 3 C). The data indicated that mutating all six lysine residues (K233E/K234E/K235E/K251E/K252E/K255E) in the Glo3 BoCCS substantially reduced the ability of Glo3 to pull down COPI. These results suggest that Glo3 engages the COPI coat mainly using the β′-COP subunit. Glo3 has been proposed to interact with γ-COP appendage (Watson et al., 2004), but these data suggest that the β′-COP/Glo3 BOCCS interaction may be the primary binding site required for the stable association between Glo3 and COPI.
Having identified key Glo3 lysine residues, we set out to find their counterparts on β′-COP, which were most likely to be acidic residues. We systematically mutated acidic patches located throughout both the N- and C-terminal propeller domains to change aspartate and glutamate residues to alanines. The vast majority of the mutant β′-COP proteins bound the Glo3 fragment with similar affinity as wild-type β′-COP (Table S2). We identified one patch on the C-terminal propeller (D437/D450) that reduced binding to Glo3 by ∼60-fold when mutated to alanines (D437A/D450A; KD = 18 μΜ; Fig. 3 B, Fig. S4 C, and Table S2). Several N-terminal mutant propeller proteins exhibited slightly weaker affinities (approximately eightfold weaker; Table S2). The N-terminal mutant with the largest effect was the R13A/K15A/R59A mutant that disrupts binding to dilysine motifs; this mutant exhibits ∼16-fold weaker binding to Glo3 (Table S2; discussed further below). Finally, we tested whether disrupting the β′-COP D437/D450 acidic patch affects binding to Glo3 in budding yeast (Fig. 3 D). We generated both wild-type SEC27 and D437A/D450A mutant sec27 yeast strains containing Glo3-6xHis-TEV-3xFLAG (Glo3-HTF; Date et al., 2022) using plasmid shuffling. Immunoprecipitations were performed using the 3xFLAG tag on Glo3. The strain containing wild-type SEC27 efficiently immunoprecipitated COPI subunits (Fig. 3 D) in contrast to the D437A/D450A mutant strain. These data together suggest the β′-COP acidic patch is a key site for binding Glo3 in budding yeast.
Structural and modeling approaches
Unfortunately, attempts to determine a wide variety of β′-COP/Glo3 x-ray structures failed. ITC experiments revealed Glo3 binds robustly to β′-COP, so we initially used the Glo3 fragment (residues 230–290) exhibiting the greatest binding affinity for co-crystallization experiments with β′-COP. We repeatedly found that the β′-COP double propeller packing within the crystal lattice excluded Glo3 fragments, which are likely to be flexible. We then systematically set up crystallization trials with Glo3 fragments and each β′-COP propeller individually; with both propellers together; with multiple synthesized Glo3 peptides of varying lengths (8–12 residues centered around two basic clusters); and using different β′-COP/Glo3 fusion constructs. We grew crystals of several protein/peptide complexes, but in each case, structure determination revealed that the Glo3 peptide partially occupied the well-established dilysine cargo binding site (Jackson et al., 2012; Ma and Goldberg, 2013; discussed further below). Attempts to grow crystals in the presence of both dilysine and Glo3 peptides were unsuccessful.
We also attempted to generate structural models of the β′-COP/Glo3 interaction using AlphaFold2 (details in Materials and methods). Briefly, we undertook multiple runs using the β′-COP N-terminal, C-terminal, or both propellers with a variety of Glo3 fragments (summarized in Table S3; representative models in Fig. S3, D–F). AlphaFold did not converge on a solution for any attempted run. In some runs, AlphaFold placed specific Glo3 lysine residues (K234, K252, or K255) near the established β′-COP dilysine binding patch (D98/D117; Table S3). In parallel, we tested whether AlphaFold predicts the interaction between the β′-COP N-terminal propeller and dilysine motifs (Fig. S3 D). This served as a “positive control” for computational experiments because the interaction has been observed in multiple x-ray and cryo-ET structures from different labs (Jackson et al., 2012; Ma and Goldberg, 2013). AlphaFold also consistently fails to predict the β′-COP/dilysine motif interaction. We note that AlphaFold successfully predicts interactions between folded domains and short peptides in other trafficking proteins exhibiting similar affinities. One example is the SNX27 FERM interaction with DxF motifs (Chandra et al., 2022; Simonetti et al., 2022); AlphaFold models and the experimentally determined x-ray structure are very similar (Yong et al., 2021). Overall, the systematic mutagenesis approach combined with biophysical experiments provided the most compelling evidence for where Glo3 binds β′-COP.
β′-COP can bind Glo3 and dilysine motifs simultaneously in vitro
The biochemical and biophysical data presented here show a new direct interaction between the β′-COP subunit and the ArfGAP, Glo3. COPI sorts many important transmembrane proteins from the Golgi and endosomes, including cargoes with short amino acid motifs (Jackson et al., 1990; Letourneur et al., 1994; Townsley and Pelham, 1994) and SNARE proteins with more complex molecular signatures (Xu et al., 2017). The β′-COP subunit recognizes dilysine motifs (KKxx or KxKxx) found in transmembrane proteins that require recycling to the ER (Jackson et al., 2012; Ma and Goldberg, 2013). Both x-ray data (not shown) and AlphaFold modeling placed Glo3 lysine residues near the dilysine binding site. We thus tested in vitro whether β′-COP binds dilysine cargo and Glo3 simultaneously. Both crystal structures and cryo-ET reconstructions reveal that β′-COP can exist in multiple conformations, so we wondered whether Glo3 might “lock” β′-COP into a cargo-binding conformation. Alternatively, Glo3 and dilysine cargo could compete for binding to the same patch on β′-COP. However, calorimetry data (Fig. S5 A) showed no difference in the affinity of recombinant purified β′-COP for dilysine motifs whether Glo3 is absent or present (Fig. S5 A). The published β′-COP RKR dilysine binding mutant (Jackson et al., 2012) binds Glo3 with slightly weaker affinity, while the D98A/D117A mutant binds as well as the wild-type protein (Fig. S5 B). Together, these data suggest two things. First, both Glo3 and dilysine cargo can bind β′-COP simultaneously, at least in the context of this in vitro assay. Second, the Glo3 binding site on the N-terminal propeller may be located near the dilysine site. This N-terminal patch may contribute directly to binding Glo3, or alternatively, mutating it may have altered the overall electrostatic charge distribution across the propeller surface. We predict there must be a second patch on the N-terminal propeller since both β′-COP propellers are required for binding (Fig. 2 B; see Discussion). We could not conclusively identify the patch, but current data suggest that it may be located relatively close to the dilysine binding site.
Glo3 BoCCS and dilysine cargo motifs bind β′-COP simultaneously in vitro. (A) Representative ITC experiments with untagged β′-COP 1–604; Glo3 residues 230–290; and dilysine motif (KTKLL) peptide. The presence of Glo3 does not alter the binding affinity of β′-COP to dilysine motifs in vitro. (B) ITC experiments with β′-COP dilysine binding mutants. The R13A/K15A/R59A (RKR mutant) disrupts binding to the dilysine motif C-terminus, while D98A/D117A disrupts binding to lysine residues. The RKR mutant exhibits weaker binding to Glo3, while D98A/D117A binds Glo3 as well as wild-type protein. These data suggest Glo3 and dilysine motifs do not compete for binding β′-COP in vitro. However, disrupting the overall charge distribution on the N-terminal propeller in the RKR mutant suggests it may play some role in Glo3 binding.
Glo3 BoCCS and dilysine cargo motifs bind β′-COP simultaneously in vitro. (A) Representative ITC experiments with untagged β′-COP 1–604; Glo3 residues 230–290; and dilysine motif (KTKLL) peptide. The presence of Glo3 does not alter the binding affinity of β′-COP to dilysine motifs in vitro. (B) ITC experiments with β′-COP dilysine binding mutants. The R13A/K15A/R59A (RKR mutant) disrupts binding to the dilysine motif C-terminus, while D98A/D117A disrupts binding to lysine residues. The RKR mutant exhibits weaker binding to Glo3, while D98A/D117A binds Glo3 as well as wild-type protein. These data suggest Glo3 and dilysine motifs do not compete for binding β′-COP in vitro. However, disrupting the overall charge distribution on the N-terminal propeller in the RKR mutant suggests it may play some role in Glo3 binding.
COPI cargo sorting in yeast
We then turned to budding yeast to test whether abrogating the β′-COP/Glo3 interaction would affect Glo3 function or COPI-dependent cargo sorting in vivo. We tested multiple known COPI- and Glo3-dependent cargoes, including Ste2, Rer1, Emp47, and SNARE proteins.
Growth assays
Using the glo3 K233E/K234E/K235E and glo3 K251E/K252E/K255E mutations, we first tested if disrupting the β′-COP/Glo3 interaction would affect cell growth in a glo3Δgcs1Δ strain background (Fig. S6). We observed no difference in growth between the glo3 K233E/K234E/K235E and glo3 K251E/K252E/K255E mutants compared to the GLO3 strain. Thus, mutations disrupting the β′-COP/Glo3 interaction do not abrogate the essential growth function of Glo3 at 30°C.
Yeast growth assays. Yeast growth assays in glo3Δgcs1Δ background strains with GLO3 or glo3 mutants (K233E/K234E/K235E or K251E/K252E/K255E). The glo3∆ gcs1∆ double mutant is inviable but can be sustained with a wild-type copy of GLO3 on a URA3 marked plasmid (pRS416). Both the wild-type GLO3 and the mutant forms were introduced into this background on a LEU2 marked (pRS315) plasmid. On media that selects for both plasmids, all transformed strains grew like a wild-type strain because they contain the wild-type pRS416-GLO3 plasmid. However, upon switching to 5-FOA media, which selects against the pRS416-GLO3 plasmid, the glo3∆gcs1∆ strain harboring the empty pRS415 plasmid failed to grow, as expected.
Yeast growth assays. Yeast growth assays in glo3Δgcs1Δ background strains with GLO3 or glo3 mutants (K233E/K234E/K235E or K251E/K252E/K255E). The glo3∆ gcs1∆ double mutant is inviable but can be sustained with a wild-type copy of GLO3 on a URA3 marked plasmid (pRS416). Both the wild-type GLO3 and the mutant forms were introduced into this background on a LEU2 marked (pRS315) plasmid. On media that selects for both plasmids, all transformed strains grew like a wild-type strain because they contain the wild-type pRS416-GLO3 plasmid. However, upon switching to 5-FOA media, which selects against the pRS416-GLO3 plasmid, the glo3∆gcs1∆ strain harboring the empty pRS415 plasmid failed to grow, as expected.
Ste2
Yeast α-factor receptor (Ste2) is a G-protein coupled receptor that cycles between the cell surface and internal compartments. Previous work demonstrated how postendocytic Ste2-GFP trafficking back to the cell surface depends on the presence of Glo3 (Kawada et al., 2015). We examined Ste2-GFP localization in glo3Δgcs1Δ cells expressing wild-type GLO3, glo3 K233E/K234E/K235E, or glo3 K251E/K252E/K255E mutants (Fig. 4). We observed a significant difference in the amount of Ste2-GFP at the plasma membrane in GLO3 versus glo3 mutant cells. Less Ste2-GFP was observed at the plasma membrane in both glo3 mutant strains, and mislocalization to the vacuole was apparent (Fig. 4 A).
Ste2 is missorted to the vacuole when the β′-COP/Glo3 interaction is disrupted in S. cerevisiae. (A) Fluorescence imaging of Ste2-GFP in glo3Δgcs1Δ strains with GLO3 or glo3 mutants (K233E/K234E/K235E or K251E/K252E/K255E) introduced on a plasmid. Scale bar represents 2 μm. (B) Box plots showing the percentage of Ste2-GFP observed at the plasma membrane in each strain with median marked (black line). Mutating either lysine cluster in yeast cells causes a significant difference in Ste2-GFP sorting compared to the GLO3 strain. Statistical comparisons were pairwise between GLO3 and mutants; data were analyzed using a one-way ANOVA (Prism); and probability values of <0.001 are represented by ***.
Ste2 is missorted to the vacuole when the β′-COP/Glo3 interaction is disrupted in S. cerevisiae. (A) Fluorescence imaging of Ste2-GFP in glo3Δgcs1Δ strains with GLO3 or glo3 mutants (K233E/K234E/K235E or K251E/K252E/K255E) introduced on a plasmid. Scale bar represents 2 μm. (B) Box plots showing the percentage of Ste2-GFP observed at the plasma membrane in each strain with median marked (black line). Mutating either lysine cluster in yeast cells causes a significant difference in Ste2-GFP sorting compared to the GLO3 strain. Statistical comparisons were pairwise between GLO3 and mutants; data were analyzed using a one-way ANOVA (Prism); and probability values of <0.001 are represented by ***.
Emp47
α- and β′-COP recognize dilysine sorting motifs (KKxx or KxKxx) present at the C-terminus of cargo proteins. Emp47 is an established COPI cargo containing a KxKxx motif that can be recognized by β′-COP; it is therefore a suitable cargo to study COPI-mediated Golgi-to-ER retrieval involving the β′-COP subunit. Mutations that disrupt Emp47 sorting into COPI vesicles cause Emp47 mislocalization to the vacuole where it is degraded. We examined the stability of Emp47-myc over time in Δglo3Δgcs1 cells after inhibiting new protein synthesis with cycloheximide, but we did not observe an effect on Emp47 sorting. Emp47 appeared to remain stable over time in glo3 K233E/K234E/K235E and glo3 K251E/K252E/K255E cells (Fig. S7 A). We tested whether disrupting the β′-COP/Glo3 interaction would affect protein cargoes known to cycle between the Golgi and ER in COPI-coated vesicles.
Golgi/ER COPI cargo trafficking data. (A) The Emp47-myc reporter construct (visualized using α−myc) remains stable over time in both wild-type GLO3 and mutant glo3 strains. In contrast, the reporter is sent to the vacuole and degraded when the dilysine binding site on sec27 is mutated (sec27RKR; R13A/K15A/R59A) or the first sec27 propeller is deleted (sec27Δ2-304). (B) Representative fluorescence images of GFP-Rer1 in glo3Δgcs1Δ strains with GLO3 or glo3 mutants introduced on a plasmid. GFP-Rer1 trafficking does not appear to change when the β′-COP/Glo3 interaction is disrupted. Scale bar represents 2 μm.
Golgi/ER COPI cargo trafficking data. (A) The Emp47-myc reporter construct (visualized using α−myc) remains stable over time in both wild-type GLO3 and mutant glo3 strains. In contrast, the reporter is sent to the vacuole and degraded when the dilysine binding site on sec27 is mutated (sec27RKR; R13A/K15A/R59A) or the first sec27 propeller is deleted (sec27Δ2-304). (B) Representative fluorescence images of GFP-Rer1 in glo3Δgcs1Δ strains with GLO3 or glo3 mutants introduced on a plasmid. GFP-Rer1 trafficking does not appear to change when the β′-COP/Glo3 interaction is disrupted. Scale bar represents 2 μm.
Rer1
We examined the localization of GFP-Rer1 (Fig. S7 B), a protein that localizes to early Golgi cisternae at steady state but rapidly cycles between the ER and early Golgi in COPI and COPII vesicles. Disruption of COPI function causes mislocalization of Rer1-GFP to the vacuole (Sato et al., 2001; Xu et al., 2017). However, we found no difference in Rer1-GFP localization between the glo3 mutants and wild-type GLO3 in the strains tested. Together, data on Rer1 and Emp47 (previous section) suggest the glo3 basic patch mutants do not appear to disrupt COPI function in retrieving cargoes from the Golgi to ER.
SNAREs
The yeast R-SNARE, Snc1, is an exocytic SNARE that cycles between the Golgi and plasma membrane and has been shown to be mislocalized when Glo3 is deleted (Schindler and Spang, 2007; Kawada et al., 2015). We examined mNG-Snc1 localization in glo3 K233E/K234E/K235E and glo3 K251E/K252E/K255E mutants in the glo3Δgcs1Δ background (Fig. S8, A–C). mNG-Snc1 normally localizes to punctate structures inside the cell, as well as to the plasma membrane. We found no significant difference in the amount of mNG-Snc1 at the plasma membrane relative to internal structures. However, in the glo3 K233E/K234E/K235E cells, mNG-Snc1 localizes to ring or tubular structures that are less frequently observed in cells expressing wild-type GLO3 (Fig. S8 A). These structures may indicate abnormal Golgi and/or endosome morphology. This significant phenotype suggests that disrupting the β′-COP/Glo3 interaction affects the COPI function required to maintain Golgi morphology.
SNARE localization and Golgi morphology data. (A) Fluorescence imaging of mNG-Snc1 in glo3Δgcs1Δ strains with GLO3 or glo3 mutants (K233E/K234E/K235E or K251E/K252E/K255E) on a plasmid. Scale bar represents 2 μm. (B) Strip plot showing the percentage of abnormal structures (tubules and rings) with mean (black bar) observed in each strain. Mutating the first lysine cluster resulted in cells exhibiting a significant difference from wild type. Significance was determined using a Mann-Whitney test comparing wild-type and mutant strains. (C) Bar graph showing number of mNG-Snc1 ring structures with standard deviation (black lines) in each strain. (D) Fluorescence imaging of mNG-Bet1 in glo3Δgcs1Δ strains with GLO3 or glo3 mutants (K233E/K234E/K235E or K251E/K252E/K255E) on a plasmid. Scale bar represents 2 μm. (E) Strip plot showing percentage of abnormal structures (tubules and rings) with standard deviation observed in each strain. Although we sometimes observe abnormal structures, we do not find a significant difference, as determined by a Mann-Whitney test. (F) Bar graph showing number of mNG-Bet1 ring structures with standard deviation (black lines) in each strain.
SNARE localization and Golgi morphology data. (A) Fluorescence imaging of mNG-Snc1 in glo3Δgcs1Δ strains with GLO3 or glo3 mutants (K233E/K234E/K235E or K251E/K252E/K255E) on a plasmid. Scale bar represents 2 μm. (B) Strip plot showing the percentage of abnormal structures (tubules and rings) with mean (black bar) observed in each strain. Mutating the first lysine cluster resulted in cells exhibiting a significant difference from wild type. Significance was determined using a Mann-Whitney test comparing wild-type and mutant strains. (C) Bar graph showing number of mNG-Snc1 ring structures with standard deviation (black lines) in each strain. (D) Fluorescence imaging of mNG-Bet1 in glo3Δgcs1Δ strains with GLO3 or glo3 mutants (K233E/K234E/K235E or K251E/K252E/K255E) on a plasmid. Scale bar represents 2 μm. (E) Strip plot showing percentage of abnormal structures (tubules and rings) with standard deviation observed in each strain. Although we sometimes observe abnormal structures, we do not find a significant difference, as determined by a Mann-Whitney test. (F) Bar graph showing number of mNG-Bet1 ring structures with standard deviation (black lines) in each strain.
Bet1 is a Q-SNARE involved in ER–Golgi trafficking that localizes to early Golgi compartments at steady state and also interacts with the BoCCS region of Glo3 (Ossipov et al., 1999; Schindler et al., 2009). We examined mNG-Bet1 localization (Fig. S8, D–F) in glo3Δgcs1Δ cells expressing wild-type GLO3 or the basic cluster mutants. We identified a small number of abnormal ring structures in glo3 K233E/K234E/K235E cells. Data from the mutants were not significantly different from GLO3 cells (Fig. S7, E and F), and the number of abnormal structures was on the edge of significance, in contrast to data on mNG-Snc1.
Together, data from multiple COPI transmembrane receptor cargoes suggest that the β′-COP/Glo3 interaction is more important for cargoes recycling via the endosome/plasma membrane and less important for cargoes cycling between the cis-Golgi and ER (see Discussion).
Discussion
Molecular details of the β′-COP/Glo3 interaction
Published data (Arakel et al., 2019) previously demonstrated how Glo3, but not Gcs1, stably associates with COPI using affinity purification from yeast cell lysates. Here, we show for the first time that Glo3 and the β′-COP subunit exhibit a direct molecular interaction with a low micromolar KD. We mapped specific residues in both Glo3 and β′-COP propeller domains to identify an acidic patch (D437/D450) on the β′-COP C-terminal propeller that binds residues located in the Glo3 BoCCS region. The Glo3 BoCCS contains two basic lysine clusters that interact directly with β′-COP (Fig. 3 A). These two clusters are separated by ∼20 amino acids and could span up to 50 Å if this region is unconstrained by secondary structure. We have shown that both β′-COP propeller domains are required to bind Glo3 BoCCS (Fig. 2 B). Taken together, it is tempting to speculate that each Glo3 cluster binds an acidic patch on each β′-COP propeller domain. This implies there is a second acidic patch located on the N-terminal propeller. We were unable to identify this patch using a systematic mutagenesis approach, but circumstantial evidence implies a patch may be located near the R15/K17/R59 patch (Fig. S5 B). Attempts to determine structures experimentally or to use AlphaFold to generate structural models for the β′-COP/Glo3 interaction failed. Crystallography attempts were confounded by two primary factors. First, the minimal Glo3 fragment required to maintain the binding affinity is relatively long (50–60 amino acids), which makes it a difficult co-crystallization target. Second, short Glo3 peptides used in co-crystallization experiments are similar to dilysine binding motifs in cargo molecules that bind the N-terminal β′-COP propeller. These peptides partially occupied the established dilysine binding site (data not shown). In vitro calorimetry data (Fig. S5) suggest that Glo3 and dilysine cargo occupy distinct binding sites on the β′-COP propellers, so the structural data are very likely to be crystallization artifacts. It is more challenging to speculate why AlphaFold did not yield testable models. One possibility is the lack of sequence conservation among ArfGAPs. ArfGAP flexible linkers vary in both sequence and length among species, and there are multiple basic residues in the linkers. Algorithms may struggle to predict which clusters are conserved among species. Furthermore, it has not been shown that mammalian ArfGAP2/3 interacts with β′-COP in the same way, so it remains possible that mammalian COPI assembly differs from yeast. New structural approaches will be required to uncover precise molecular details of the β′-COP/Glo3 interaction, particularly with the N-terminal propeller.
β′-COP emerges as a molecular platform
Multiple lines of evidence now suggest that the β′-COP subunit acts as a molecular platform to coordinate binding to multiple protein partners within the COPI coat. Propeller domains in other trafficking proteins also act as platforms. For example, clathrin contains a single propeller called terminal domain (TD), which contains four binding sites for binding partners (ter Haar et al., 2000; Miele et al., 2004; Willox and Royle, 2012; Muenzner et al., 2017). The COPI coat contains four propeller domains: two in β′-COP and two in α-COP. Until now, only one specific binding site on both α- and β′-COP propeller domains has been identified: the N-terminal propeller domains bind short dilysine motifs (KKxx or KxKxx) found in transmembrane protein cargoes cycling between the Golgi and ER (Jackson et al., 2012; Ma and Goldberg, 2013; Bykov et al., 2017). β′-COP is located near γ-appendage in the F-subcomplex (Dodonova et al., 2017) in reconstituted coats, and β′-COP binds ubiquitinated Snc1 (Xu et al., 2017), although high-resolution structural data are not available. β′-COP thus directly engages multiple important proteins in assembling COPI coats.
In this work, we identify two new direct binding partners for β′-COP: the Glo3 BoCCS region and Arf1 (Fig. 1). We combine data on this new β′-COP/Glo3 BoCCS interaction with a published Glo3 GAP/Arf1 structural model (Xie et al., 2021) to propose how β′-COP may bind Glo3 and Arf1 on membranes in the presence of cargo (Fig. 5). Multiple lines of evidence support this model. In cryo-ET reconstructions, β′-COP is located adjacent to both Arf1 (Dodonova et al., 2015) and the ArfGAP2/3 GAP domain (Dodonova et al., 2017), although these structures did not report molecular details of a β′-COP/Arf1 interface. Pulldown data from this work demonstrate biochemically how β′-COP propeller domains can directly bind Arf1 and Glo3, and we ascribe the first function to the C-terminal β′-COP propeller domain. One caveat to this model is that the orientation of the BoCCS domain remains unknown. The model presented here is drawn based on reported interactions, including interaction with γ-COP appendage (Watson et al., 2004) in mammalian cells. Together, these data suggest that β′-COP may coordinate key events in COPI coat function, perhaps coupling cargo recognition with F-subcomplex, Arf1, and ArfGAP binding. It will be critical to test in future experiments whether cargo binding by β′-COP influences Arf1(GTP) hydrolysis by Glo3.
β′-COP is a molecular platform on Golgi membranes. Model for the interaction between β′-COP (blue ribbons; WD-repeat domains and solenoid shown), Arf1 (pink ribbons with N-terminal amphipathic helix shown as cylinder), γ-COP appendage (green ribbons), and Glo3 (GAP domain as red ribbons and BoCCS region as red line). The dashed line marks the start of Glo3 GRM region; its position and orientation remain unknown, but it is predicted to have a C-terminal amphipathic helix that may insert into the membrane. The dilysine motif in transmembrane cargoes is shown as grey cylinders. The position of γ-COP was generated based on cryo-ET reconstructions of COPI (PDB ID 5NZS). Our data suggest that the first Glo3 lysine cluster (K233/K234/K235) may interact with the D437/D450 patch on the C-terminal β′-COP propeller, and the second cluster (K251/K252/K255) may interact with the N-terminal β′-COP propeller. γ-COP appendage also binds Glo3; γ-COP F836 is implicated in binding, but the Glo3 residues remain unknown.
β′-COP is a molecular platform on Golgi membranes. Model for the interaction between β′-COP (blue ribbons; WD-repeat domains and solenoid shown), Arf1 (pink ribbons with N-terminal amphipathic helix shown as cylinder), γ-COP appendage (green ribbons), and Glo3 (GAP domain as red ribbons and BoCCS region as red line). The dashed line marks the start of Glo3 GRM region; its position and orientation remain unknown, but it is predicted to have a C-terminal amphipathic helix that may insert into the membrane. The dilysine motif in transmembrane cargoes is shown as grey cylinders. The position of γ-COP was generated based on cryo-ET reconstructions of COPI (PDB ID 5NZS). Our data suggest that the first Glo3 lysine cluster (K233/K234/K235) may interact with the D437/D450 patch on the C-terminal β′-COP propeller, and the second cluster (K251/K252/K255) may interact with the N-terminal β′-COP propeller. γ-COP appendage also binds Glo3; γ-COP F836 is implicated in binding, but the Glo3 residues remain unknown.
Cargo sorting and Golgi morphology
ArfGAP proteins have long been known to play important roles in membrane trafficking. Yeast can tolerate the loss of either Gcs1 or Glo3, but not both proteins. Despite their importance, the molecular roles of ArfGAP proteins have been difficult to define. This study defines a new molecular interaction between β′-COP and Glo3, which can now be used to generate molecular tools for separation of function studies to probe COPI function with Glo3 and Gcs1 more precisely.
When the β′-COP/Glo3 interaction was disrupted in budding yeast, we observed two distinct phenotypes. First, Ste2 (α-factor receptor) exhibited a trafficking defect at a steady state and tended to be sorted to the vacuole instead of cycling via the plasma membrane. Second, we observed defects in Golgi morphology. Published data have implicated Glo3 in Snc1 localization (Kawada et al., 2015) because the loss of the entire BoCCS region partially traps Snc1 in internal punctate structures. Here, we imaged tagged SNARE proteins (Snc1 or Bet1) and found the Golgi sometimes formed abnormal structures (Fig. S7) when we specifically disrupted the β′-COP/Glo3 interaction. In contrast, we did not identify mistrafficking of well-characterized COPI cargos that cycle between the cis-Golgi and ER, such as Rer1 and Emp47. β′-COP can bind both Glo3 BoCCS region and dilysine motifs simultaneously in vitro (Fig. S5), which supports the idea that β′-COP may directly engage Glo3 and some cargos simultaneously. However, binding Glo3 does not appear to be required for proper dilysine cargo sorting in the cis-Golgi (Fig. S6). Together, these data may implicate the β′-COP/Glo3 interaction in maintaining proper steady-state levels of receptor cargoes (e.g., Ste2) and SNAREs (e.g., Snc1) that cycle from endosomes via the TGN to the plasma membrane. These data may also suggest a separation of function between Glo3 and Gcs1, which will be important to test further.
Implications and future directions
Our data raise multiple new questions about how and why the COPI coat couples Glo3 binding to Arf1 hydrolysis and recognition of cargo and SNARE proteins. Classically, Arf1(GTP) has been reported to recruit the F-subcomplex (Hara-Kuge et al., 1994; Yu et al., 2012; Dodonova et al., 2015). Goldberg and colleagues showed how the β/δ-COP and γ/ζ-COP subcomplexes interact directly with Arf1(GTP) to promote an “open” conformation analogous to AP-1/AP-2 (Jackson et al., 2010; Ren et al., 2013) in clathrin-coated vesicles. Our data suggest that β′-COP shows no preference for either Arf1 nucleotide-bound state in vitro, so it is possible only the F-subcomplex “senses” Arf1 state to promote the open or hyper-open conformations observed in crystal structures (Yu et al., 2012) and reconstituted vesicles (Dodonova et al., 2015; Dodonova et al., 2017). We speculate that the relatively weak β′-COP/Arf1 interaction could further stabilize an assembling coat and that the interface must be located on the opposite face to the Arf1 switch I/II regions. The tripartite interaction between β′-COP, Glo3, and Arf1 bears further investigation to understand how Arf1 hydrolysis mediated by Glo3 may be coupled to cargo and/or SNARE binding.
Multiple lines of evidence suggest that the Glo3 BoCCS region plays an important regulatory role in COPI trafficking. The BoCCS region has been shown to be sufficient for rescuing growth defects in yeast strains lacking both GLO3 and GCS1 (Schindler and Spang, 2007). Arakel and colleagues (Arakel et al., 2019) demonstrated that reintroducing the BoCCS region alone rescues growth in the Glo3 GAP-dead mutant (R59K) strain. The BoCCS region coimmunoprecipitates Emp47, Bet1, and Bos1 (Schindler and Spang, 2007). This may represent a direct interaction, but our data suggest it may alternatively reflect the ability of Glo3 BoCCS to interact with Emp47 (Jackson et al., 2012; Ma and Goldberg, 2013) indirectly via β′-COP. In addition, Glo3 has been implicated in Sec22 binding (Rein et al., 2002) but the molecular details remain unknown. Based on both published and new data reported here, we speculate the β′-COP/Glo3 interaction may be necessary to ensure proper incorporation and recycling of specific receptor and SNARE proteins, even in the absence of a functional Glo3 GAP domain.
Materials and methods
Antibodies
The following antibodies were used in this study: mouse α-myc (9E10; Invitrogen), rabbit α-Arf1 (from Todd Graham, Vanderbilt University, Nashville, TN, USA), mouse α-His (NB100-63173; Abcam), and mouse α-CPY (10A5B5; Invitrogen). COPI anti-sera was provided by the Barlowe lab (Geisel School of Medicine at Dartmouth, Hanover, NH).
Cloning and mutagenesis
For structural and biochemical analyses, C-terminal His-tagged β′-COP and Glo3 were placed into the Nde I/BamHI or Nde I/HinD III sites of in-house vector pMW172 (Owen and Evans, 1998) under the control of the T7 promoter. N-terminal His-tagged yeast Arf1 was placed into the pET-21a+ vector in the Nde I/BamHI sites. The Glo3 BoCCS and GRM regions and β′-COP (residues 300–604) were ligated into BamHI/NotI sites of pGEX-6P-1 (GE Healthcare), resulting in N-terminal GST-tagged proteins with a 3C-protease cleavage site. Other GST-tagged Glo3 and β′-COP constructs were subcloned into Nde I/HinD III or Nde I/BamHI sites of pMWGST, a modified form of pMW172 incorporating a C-terminal, thrombin cleavable GST tag. A two-stage quick-change mutagenesis protocol was used to introduce mutations in Glo3 and β′-COP. In this protocol, mutagenic primers (Sigma-Aldrich) were created for the desired mutations. In the first step, two polymerase chain reactions (PCRs), with either the mutagenic 5′ or 3′ primer, were amplified around the plasmid. The two reactions were then combined in an additional PCR step, followed by Dpn I digest and transformation. All constructs were verified by sequencing (GENEWIZ) prior to use. Oligonucleotides used in this study may be found in Table S5.
Protein expression and purification
All constructs were expressed in BL21(DE3)pLysS cells (Invitrogen) for 16–20 h at 22°C following induction with 0.4 mM IPTG. His-tagged Arf1 constructs were purified in 20 mM Tris-HCl (pH 8.0), 200 mM NaCl, 0.5 mM TCEP, and 5 mM MgCl2. Full-length Glo3 was purified in 20 mM HEPES (pH 7.5), 500 mM NaCl, and 1 mM DTT. Other Arf1 and Glo3 constructs and all β′-COP constructs were purified in 20 mM HEPES (pH 7.5), 200 mM NaCl, and 1 mM DTT. AEBSF (Calbiochem) was incorporated in the early stages of all purifications. Cells were lysed by a disruptor (Constant Systems Limited) and proteins were affinity-purified using glutathione sepharose (GE Healthcare) or HisPur cobalt resin (Thermo Fisher Scientific) in the buffers listed above. GST-tagged proteins were cleaved overnight with thrombin (Recothrom, The Medicine Company) or 3C-protease (made in-house) at 4°C and eluted in batches. All proteins were further purified by gel filtration preparative Superdex HiLoad 26/600 or analytical (Superdex 200 10/300) columns (GE Healthcare).
GST pulldown and immunoprecipitation assays
50 μg of GST, GST-tagged β′-COP (residues 1–604), or GST-Glo3 proteins (GAP domain, BoCCS region, GRM region) were immobilized on glutathione sepharose resin (GE Healthcare) for 1 h on ice. The resin was incubated for 1 h on ice with a fivefold molar excess of prey proteins (Glo3-His8; T31N or Q71L Arf1His6; or β′-COP604His6) in 20 mM HEPES (pH 7.5), 200 mM NaCl, 2 mM DTT, and 0.5% NP40. Samples were washed three times with the same buffer. Proteins were eluted from the resin using buffer plus 30 mM reduced glutathione. Gel samples were prepared from the supernatant following elution and the assay was analyzed by Coomassie staining of SDS-PAGE gels. The gels were further analyzed by Western blotting using anti-His (NB100-63173; Abcam) and rabbit anti-Arf1 antibodies (from Todd Graham). For immunoprecipitation experiments, affinity isolation of FLAG-tagged Glo3 was performed using EZ view anti-FLAG M2 affinity gel. 800 OD600 of strains containing untagged wild-type SEC27 or mutant D437A/D450A sec27 with Glo3-HTF were grown in YPD and harvested by centrifugation when OD600 reached ∼0.8. After washing with cold water, pellets were resuspended in 3 ml lysis buffer (100 mM Tris, pH 7.4, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid [EDTA], 5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid [EGTA], 10% glycerol, 1% Triton X-100, and complete protease inhibitor tablet). Cells were broken using a Disruptor Genie (Scientific Industries) at 4°C with three cycles for 10 min at 3,000 setting with 0.5 mm diameter glass beads. Lysates were centrifuged at 13,000 rpm in Beckman Coulter Microfuge 18 for 15 min at 4°C and the supernatant was incubated with 50 μl anti-FLAG beads overnight at 4°C. The beads were washed three times with washing buffer (100 mM Tris, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% NP40, and 0.5% Triton X-100) and eluted in SDS running buffer.
Isothermal titration calorimetry
ITC experiments were conducted on a NanoITC instrument (TA Instruments) at 20°C. Briefly, 0.05 mM β′-COP protein was placed in the cell and 0.3 mM Glo3 proteins in the syringe; the molar concentration of protein in the syringe was at least five times that of protein in the cell. All experiments were carried out in 10 mM HEPES (pH 7.5), 100 mM NaCl, and 0.5 mM TCEP, and filtered and degassed. Incremental titrations were performed with a baseline of 100 s and injection intervals of 200 s. Titration data were analyzed in NANOANALYZE (TA instruments) to obtain a fit and value for stoichiometry (n) and equilibration association constant (Ka). KD values were then calculated from the association constant.
Sequence alignments
To map residue conservation of Glo3, sequences of full-length and BoCCS region alone of Glo3 from S. cerevisiae, M. musculus, H. sapiens, Drosophila melanogaster, Caenorhabditis elegans, Schizosaccharomyces pombe, and Arabidopsis thaliana were aligned using ClustalW (Madeira et al., 2019) and Praline (Simossis and Heringa, 2005). A partial alignment is shown in Fig. S4 to highlight key conserved lysine residues in yeast, murine, and human proteins required to interact with β′-COP.
Yeast strains and plasmids
Standard media and techniques for growing and transforming yeast were used. Strain CGY2 was derived from crossing BY4741 gcs1∆::kanMX with BY4742 glo3∆::kanMX pRS416-GLO3 to generate the double mutant covered by the WT GLO3 plasmid. Strains expressing glo3 mutants were constructed by shuffling in pRS315-glo3 plasmids into CGY2 using 5′-fluoro-orotic acid (5-FOA) plates to eliminate pRS416-GLO3. Yeast strains used in this study are listed in Table S4. Plasmid constructions were performed using standard molecular manipulation. Wild-type GLO3 gene was cloned into pRS315 yeast vectors with an endogenous promoter and terminator sequences. Mutations were introduced using the two-stage quick-change mutagenesis protocol.
Yeast growth assays
Strains containing pRS416-GLO3 (wild type) and pRS315-glo3 (mutant constructs) were grown at 30°C. Cells were then subcultured and an equal OD of each strain was loaded into a 96-well plate. Using a prong replicator, strains were stamped onto both appropriate synthetic media and 5-FOA plates to select against the pRS416-GLO3 plasmid. Cells were grown for 4 d and images of these plates were captured.
Fluoresence imaging
Three biological replicates of transformed strains were subcultured for imaging in appropriate synthetic media. Cells at mid-log phase were then mounted on glass slides and observed immediately at room temperature. Images were acquired using a DeltaVision Elite Imaging System (GE Healthcare Life Sciences): Olympus IX-71 inverted microscope with DAPI (EX: 390/18, EM: 435/48), GFP (EX: 475/28, EM: 525/48), and mCherry (EX: 575/25, EM: 632/60) filters; Olympus 100× oil objective 1.5 NA; oil n = 1.516 ± 0.0002 (Cargille Lab); DV Elite sCMOS camera. Z-stacks were collected for green channel and DIC, and Softworx software v7.000.01 (GE Healthcare) was used for image acquisition and deconvolution. Image processing was undertaken in FIJI (https://imagej.net/software/fiji).
Data analysis and statistics
To quantify levels of Ste2-GFP at the cell surface, traces were drawn just outside and inside the plasma membranes using the freehand drawing tool in ImageJ to measure the total cellular fluorescence (Cellfl) and Internal fluorescence (Intfl), respectively. The percent of Ste2-GFP at the plasma membrane was then calculated by (Cellfl − Intfl)/Cellfl. Plotted data exhibited a normal distribution, and statistical differences were determined using a one-way ANOVA test in GraphPad Prism version 8.0 (GraphPad Software, www.graphpad.com). Images of fluorescently tagged SNARE proteins were coded, and the number of tubules, puncta, and rings was counted in a blinded experiment. These data did not exhibit a normal Gaussian distribution, so non-parametric tests were used. Statistical differences against wild type were determined using a Mann–Whitney test, and data were visualized using Python. Probability values of <0.04, 0.01, or 0.001 were used to show statistically significant differences and are represented with *, **, or ***, respectively.
Dilysine reporter construct trafficking in yeast
Yeast Emp47 under the control of its endogenous promoter was used as the backbone for reporter constructs, and a myc-tag was incorporated following the transmembrane domain to facilitate Western blotting. The KxKxx reporter contained endogenous Emp47 transmembrane domain followed by the cytoplasmic sequence (RQEIIKTKLL). The reporter was introduced into appropriate GLO3 wild-type and glo3 mutant strains. Reporter levels were monitored by Western blotting against the myc-tag both prior to and following a 20 μg/ml cycloheximide chase at time points equal to 0, 30, and 60 min. Cells were lysed using glass beads with SDS buffer and then boiled at 95°C for 5 min. Western blots were probed with mouse monoclonal anti-myc (9E10; Invitrogen) for the reporter and with mouse anti-CPY (10A5B5; Invitrogen) as a loading control. The membrane was then incubated with HRP-goat anti-mouse IgG secondary antibody (Invitrogen) or IRDye 800CW goat anti-mouse IgG secondary antibody (LI-COR) to visualize.
Structural predictions using AlphaFold
To generate structural models of yeast Glo3 and β′-COP proteins, we used both AlphaFold2 Multimer neural-network (Jumper et al., 2021a; Jumper et al., 2021b) implemented within the freely accessible ColabFold pipeline (Mirdita et al., 2021) and standalone AlphaFold2 Multimer installed through SBGrid. Sequences of single or double propeller domains of β′-COP were used in complexes with different Glo3 lengths. For each structural prediction experiment, sequences from evolutionarily related proteins in the form of a multiple sequence alignment were created by Jackhammer (Eddy, 2009) in local AlphaFold or by MMseqs2 in ColabFold (Mirdita et al., 2021), and AlphaFold2 model building was executed using standard settings. Structural relaxation of final protein complexes was performed with Amber to generate five models per protein complex.
Online supplemental material
Fig. S1 shows that β′-COP, Arf1, and Glo3 form a ternary complex in vitro. Fig. S2 shows that β′-COP WD-repeat domains directly bind the Glo3 BoCCS region in vitro. Fig. S3 shows that the Glo3 BoCCS region interacts directly with β′-COP. Fig. S4 shows that conserved Glo3 lysine residues mediate an electrostatic interaction with β′-COP C-terminal propeller domain. Fig. S5 shows that Glo3 BoCCS and dilysine cargo motifs bind β′-COP simultaneously in vitro. Fig. S6 shows yeast growth assays. Fig. S7 shows Golgi/ER COPI cargo trafficking data. Fig. S8 gives SNARE localization and Golgi morphology data. Table S1 lists ITC data summary. Table S2 lists β′-COP mutant ITC data. Table S3 lists AlphaFold computational modeling summary. Table S4 lists yeast strains used in this study. Table S5 lists olignucleotides used in this study.
Acknowledgments
We sincerely thank David Owen and members of the Jackson and Graham labs for helpful feedback, discussion, and critical reading of the manuscript. We also acknowledge the Barlowe and Spang lab for providing the anti-COPI.
B. Xie, C. Jung, A.K. Kendall, and L.P. Jackson are supported by National Institutes of Health (NIH) grant R35GM119525. C.I. Cohen is supported by NIH grant T32GM008320-34. L.P. Jackson is a Pew Scholar in the Biomedical Sciences, supported by the Pew Charitable Trusts. C. Guillem, J.T. Best, S.S. Date, and T.R. Graham are supported by NIH R01GM118452.
Author contributions: B. Xie: Investigation; Formal Analysis; Validation; Writing- Original Draft; Writing-Review & Editing; Visualization. C. Guillem: Investigation; Formal Analysis; Visualization, S.S. Date: Investigation; Formal Analysis; Visualization, C.I. Cohen: Investigation; Formal Analysis; Visualization. C. Jung: Investigation. A.K. Kendall: Investigation. J.T. Best: Investigation. T.R. Graham: Resources; Supervision; Writing-Review & Editing; Funding acquisition. L.P. Jackson: Conceptualization; Writing- Original Draft; Writing-Review & Editing; Supervision; Project administration; Funding acquisition.
References
Author notes
Disclosures: The authors declare no competing interests exist.
Supplementary data
lists ITC data summary
summarizes β′-COP mutant ITC data