The chaperone-mediated sequestration of misfolded proteins into inclusions is a pivotal cellular strategy to maintain proteostasis in Saccharomyces cerevisiae, executed by small heat shock proteins (sHsps) Hsp42 and Btn2. Direct homologs of Hsp42 and Btn2 are absent in other organisms, questioning whether sequestration represents a conserved proteostasis strategy and, if so, which factors are involved. We examined sHsps from Escherchia coli, Caenorhabditis elegans, and humans for their ability to complement the defects of yeast sequestrase mutants. We show that sequestration of misfolded proteins is an original and widespread activity among sHsps executed by specific family members. Sequestrase positive C. elegans’ sHsps harbor specific sequence features, including a high content of aromatic and methionine residues in disordered N-terminal extensions. Those sHsps buffer limitations in Hsp70 capacity in C. elegans WT animals and are upregulated in long-lived daf-2 mutants, contributing to lifespan extension. Cellular protection by sequestration of misfolded proteins is, therefore, an evolutionarily conserved activity of the sHsp family.
Introduction
Cells employ a multifaceted network of quality control machinery to prevent the accumulation of misfolded proteins and maintain proteostasis. This network relies on three main strategies to cope with misfolded proteins: chaperone-mediated refolding, degradation by proteolytic systems, and sequestration into large inclusions (Chen et al., 2011; Tyedmers et al., 2010).
The organized sequestration of misfolded proteins, actively promoted by a set of chaperones termed sequestrases (Hill et al., 2017; Miller et al., 2015b; Saarikangas and Barral, 2016), is typically observed upon cell exposure to proteotoxic stress like heat shock or cellular aging. These conditions either cause the rapid generation of misfolded proteins or lead to their continuous accumulation due to the functional decline of the cellular proteostasis machinery. The deposition of misfolded proteins at inclusions confines their accessible sticky surface, thereby reducing cytotoxicity, trapping of other proteins, and binding of proteostasis components, which otherwise would exhaust the limited chaperone resources (Ho et al., 2019). Protein sequestration might also aid in asymmetric damage inheritance (Coelho et al., 2014; Hill et al., 2016) and facilitate downstream processing of deposited proteins by disaggregases or selective autophagy (Lu et al., 2014; Marshall et al., 2016).
The mechanism of misfolded protein sequestration has been best elucidated in Saccharomyces cerevisiae, where the two heat shock proteins Hsp42 and Btn2 mediate sequestration at cytosolic and nuclear deposition sites (CytoQ/Q-bodies and INQ, respectively; Escusa-Toret et al., 2013; Malinovska et al., 2012; Miller et al., 2015a; Saarikangas and Barral, 2015; Song et al., 2014; Specht et al., 2011).
Btn2 and Hsp42 share the ability for self-assembly and both harbor long disordered segments, crucial for sequestrase activity (Grousl et al., 2018; Ho et al., 2019). Notably, Btn2 and Hsp42 do not exhibit sequence homology and do not have direct homologs in bacteria, plants, or metazoa. This raises the question of whether active protein sequestration represents an evolutionarily conserved strategy of proteostasis networks and if so, which factors could act as executors in non-fungal cells.
Hsp42 is a small heat shock protein (sHsp), which suggests that the sequestrase activity is a feature that some other members of this family share. sHsps are considered the first line of cellular defense against proteotoxic stress as they capture early unfolding intermediates (Basha et al., 2012; Ungelenk et al., 2016). They protect substrates from further unfolding and uncontrolled aggregation and facilitate their reactivation by the ATP-dependent Hsp70 chaperone system (Haslbeck et al., 2019; Mogk et al., 2019). sHsps share the conserved α-crystallin domain and possess variable disordered N-terminal and C-terminal extensions (NTE, CTE), which drive sHsp differentiation during evolution (Kriehuber et al., 2010).
In comparison to canonical sHsps, Hsp42 has an unusually long N-terminal extension that includes a functionally essential prion-like domain (Grousl et al., 2018). This unique sequence feature of Hsp42 makes it difficult to predict whether other sHsp family members act as sequestrases as well. On the other hand, sHsps are frequently found associated with insoluble misfolded proteins in heat-stressed or aged cells and can even represent the most abundant single protein species of these aggregates (Coelho et al., 2014; Laskowska et al., 1996; Lee et al., 2005; Walther et al., 2015). This points to the critical role of sHsps in modulating protein aggregation, though direct evidence for a general sequestrase function is lacking.
The analysis of the function of sHsps in proteostasis networks is typically hampered by the absence of strong phenotypes of respective mutants. This can be explained by compensatory activities of other proteostasis components (Ho et al., 2019) and also by the large expansion of the sHsp family during evolution (e.g., C. elegans: 16 members, human: 10 members; Haslbeck and Vierling, 2015). In this study, we overcame this obstacle by using the strong growth defects of a combination of yeast sequestrase and refoldase mutants. This allowed us to screen for sHsps from diverse species that restore growth and misfolded protein sequestration in yeast. We showed that four out of twelve C. elegans sHsps tested exhibit robust sequestrase activity and define specific sequence features of their disordered N-terminal extensions that are crucial for activity. The sHsps E. coli IbpA and human HspB2/HspB3 are also able to partially rescue the defects of a yeast sequestrase mutant. These findings demonstrate that sHsp sequestrase function is evolutionarily conserved and represents an originally cytoprotective activity of this chaperone family.
Results
A subset of C. elegans sHsps rescues growth defects of yeast sequestrase mutants
We recently demonstrated that Btn2 and Hsp42 become essential for the growth of S. cerevisiae fes1Δ hsp104Δ (termed ΔΔ) mutant cells, lacking the major nucleotide exchange factor (NEF) of Hsp70 in the nucleus, Fes1, and the AAA+ disaggregase Hsp104 (Ho et al., 2019). Both Fes1 and Hsp104 displace substrates from Hsp70, and in consequence, the levels of free Hsp70 become low in ΔΔ cells. The sequestration of soluble misfolded proteins into large inclusions by Btn2 or Hsp42 restricts their accessibility for Hsp70, thereby preventing the overload of the Hsp70 system. The lack of sequestrase activity in ΔΔbtn2Δ or ΔΔhsp42Δ cells therefore further reduces Hsp70 capacity, causing a temperature-sensitive growth phenotype (Ho et al., 2019). ΔΔbtn2Δ mutant cells are most affected and hardly grow at 30°C, representing the strongest phenotype of a sequestrase mutant reported so far. This provides the unique possibility to screen proteins from other organisms for a potential sequestrase function. We focused on members of the sHsp family and chose C. elegans as the model organism for the following reasons: (i) C. elegans expresses 16 sHsps, allowing us to explore the functional consequences of diversification and to identify common sequence features that might mediate sequestrase activity; (ii) expression profiles of C. elegans sHsp genes are well characterized; (iii) various C. elegans sHsps are found in aggregated protein fractions of aged C. elegans wild-type (wt) and long-lived daf-2 mutant animals (Walther et al., 2015).
To identify potential sequestrase activities of C. elegans sHsps we tested for their ability to rescue the growth defects of yeast ΔΔbtn2Δ mutant cells. We integrated 12 out of the 16 C. elegans sHsp-encoding genes (Fig. S1 A) in the genome of ΔΔbtn2Δ cells and expressed them from the constitutive GPD promoter. Since Btn2 is a nuclear sequestrase, we targeted the C. elegans sHsps to the nucleus by C-terminal fusion with the SV40 nuclear localization sequence (NLS). This strategy was previously shown to rescue the ΔΔbtn2Δ phenotype by expressing Hsp42-NLS as an additional Hsp42 copy (Ho et al., 2019), demonstrating that sequestration activity is not compartment-specific and can be re-directed from the cytosol to the nucleus by an NLS fusion. To allow for evaluation of C. elegans sHsp expression levels, we additionally fused a FLAG-tag to the sHsp C-termini. Hsp42-FLAG is functional in misfolded protein sequestration, indicating that FLAG-tag fusion does not cause sHsp inactivation (Specht et al., 2011).
Expression of C. elegans sHsps in yeast sequestrase mutant cells. (A) Cartoon representation of screened C. elegans sHsps with indicated boundaries between N-terminal extension (NTE), α-crystallin domain (ACD), and C-terminal extension (CTE). A red asterisk in the CTE indicates presence of the conserved IXI motif. (B) Western blot analysis depicting the levels of all tested or (C) selected sequestrase-positive and -negative C. elegans sHsps in comparison to Hsp42 (FLAG-tagged allele) in ∆∆btn2∆ cells. α-FLAG and α-Zwf1 antibodies were used to probe for sHsp and Zwf1 (loading control) levels, respectively. A red asterisk (B) indicates the correct molecular weight of the respective sHsp. Source data are available for this figure: SourceData FS1.
Expression of C. elegans sHsps in yeast sequestrase mutant cells. (A) Cartoon representation of screened C. elegans sHsps with indicated boundaries between N-terminal extension (NTE), α-crystallin domain (ACD), and C-terminal extension (CTE). A red asterisk in the CTE indicates presence of the conserved IXI motif. (B) Western blot analysis depicting the levels of all tested or (C) selected sequestrase-positive and -negative C. elegans sHsps in comparison to Hsp42 (FLAG-tagged allele) in ∆∆btn2∆ cells. α-FLAG and α-Zwf1 antibodies were used to probe for sHsp and Zwf1 (loading control) levels, respectively. A red asterisk (B) indicates the correct molecular weight of the respective sHsp. Source data are available for this figure: SourceData FS1.
All C. elegans sHsps were expressed in ΔΔbtn2Δ cells, but at varying levels (Fig. S1 B). The highest production levels were determined for Hsp-12.1, Hsp-12.2, and Hsp-12.3, and their amounts were comparable to endogenous Hsp42 levels (Fig. S1 C).
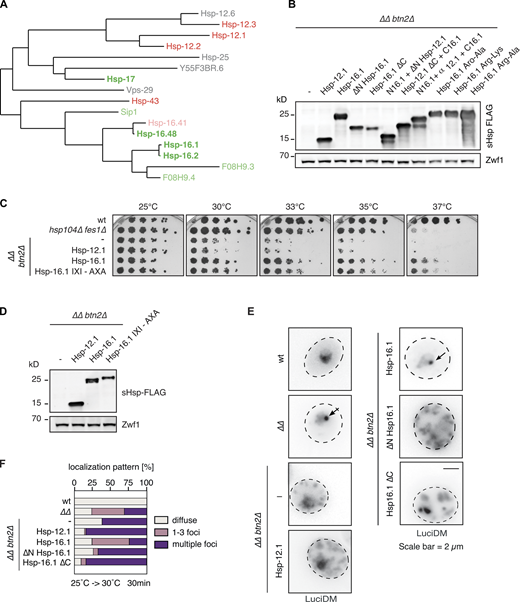
Seven out of 12 C. elegans sHsps restored the growth of yeast ΔΔbtn2Δ cells on YPD plates to varying degrees (Fig. 1 A). Hsp-16.1 was the most active and it robustly rescued growth at all growth temperatures tested. The growth profile of ΔΔbtn2Δ cells expressing Hsp-16.1 was comparable to ΔΔ cells that overproduce the nuclear sequestrase Btn2 due to activation of the heat shock response (Ho et al., 2019). This suggests similar protective activities of Hsp-16.1 and Btn2. Similarly, Hsp-16.1 complementation activity was comparable to Hsp42-NLS, suggesting an underlying robust sequestrase activity (Fig. S2 A). Hsp-16.2, F08H9.3, F08H9.4, Hsp-17, Sip1, and Hsp-16.48 showed moderate activity and allowed for partial growth at 33–37°C (Fig. 1 A). F08H9.3, F08H9.4, Hsp-17, Sip1, and Hsp-16.48 were produced at lower levels, thus potentially restricting their activities. Highly expressed Hsp-12.1, Hsp-12.2, and Hsp-12.3 did not improve growth, indicating that complementation activities are not necessarily correlated to sHsp production levels.
A subset of C. elegans sHsps complements the growth deficits of yeast sequestrase mutants. (A) Fivefold serial dilutions of S. cerevisiae WT, hsp104∆ fes1∆ (∆∆), and ∆∆ btn2∆ cells expressing the indicated C. elegans sHsps were spotted on YPD agar plates and incubated at indicated temperatures for 3 d. Dashed lines indicate that spot tests from different plates are cropped and shown together for comparative analysis. (B) Yeast strains as in A were grown in YPD liquid media at 25°C. Log phase cultures were normalized to OD600 = 0.2 and shifted to either 30 or 33°C. The OD600 value for each strain at the time point when the hsp104∆ fes1∆ reference cell culture reached saturation was noted and plotted as a bar graph normalized to the OD600 value corresponding to ∆∆btn2∆ cells without complementing C. elegans sHsp. A growth ratio of 1 denotes that the growth curves of ∆∆btn2∆ cells with and without C. elegans sHsp are equal. The dashed line corresponds to the set threshold (growth ratio 1.5). Error bars depict ±SD from n = 3 independent experiments. One-way ANOVA test (Dunnett) was used to assess the statistical significance of hits passing the set threshold (**, P < 0.01, ****, P < 0.0001). Data distribution was assumed to be normal, but this was not formally tested. (C) Venn diagram summarizing the results of C. elegans sHsp growth complementation tests of yeast sequestrase mutant cells (∆∆btn2 and ∆∆hsp42∆) both on solid agar and in liquid growth medium.
A subset of C. elegans sHsps complements the growth deficits of yeast sequestrase mutants. (A) Fivefold serial dilutions of S. cerevisiae WT, hsp104∆ fes1∆ (∆∆), and ∆∆ btn2∆ cells expressing the indicated C. elegans sHsps were spotted on YPD agar plates and incubated at indicated temperatures for 3 d. Dashed lines indicate that spot tests from different plates are cropped and shown together for comparative analysis. (B) Yeast strains as in A were grown in YPD liquid media at 25°C. Log phase cultures were normalized to OD600 = 0.2 and shifted to either 30 or 33°C. The OD600 value for each strain at the time point when the hsp104∆ fes1∆ reference cell culture reached saturation was noted and plotted as a bar graph normalized to the OD600 value corresponding to ∆∆btn2∆ cells without complementing C. elegans sHsp. A growth ratio of 1 denotes that the growth curves of ∆∆btn2∆ cells with and without C. elegans sHsp are equal. The dashed line corresponds to the set threshold (growth ratio 1.5). Error bars depict ±SD from n = 3 independent experiments. One-way ANOVA test (Dunnett) was used to assess the statistical significance of hits passing the set threshold (**, P < 0.01, ****, P < 0.0001). Data distribution was assumed to be normal, but this was not formally tested. (C) Venn diagram summarizing the results of C. elegans sHsp growth complementation tests of yeast sequestrase mutant cells (∆∆btn2 and ∆∆hsp42∆) both on solid agar and in liquid growth medium.
A subset of sHsps complements growth deficits of yeast sequestrase mutant cells. (A) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ (∆∆), and ∆∆btn2∆ cells expressing the indicated C. elegans sHsps or an additional HSP42 allele (SV40NLS-FLAG tagged) were spotted on YPD plates and incubated at indicated temperatures for 3 d. (B)S. cerevisiae WT and indicated mutant strains with or without indicated C. elegans sHsps were grown in YPD media at 25°C. Log phase cultures were normalized to OD600 = 0.2, shifted to 33°C and then growth (OD600 value) of each yeast strain was monitored for 25 h. (C and D) Western blot analysis depicting the levels of all (C) tested or (D) selected sequestrase positive and negative C. elegans sHsps in comparison to Hsp42 (FLAG-tagged allele) in the ∆∆hsp42∆ cells. α-FLAG and α-Zwf1 antibodies were used to probe for sHsp and Zwf1 (loading control) levels, respectively. A red asterisk (A) indicates the correct molecular weight of the respective sHsp. (E) Five-fold serial dilutions of S. cerevisiae WT, hsp104∆ fes1∆ (∆∆), and ∆∆hsp42∆ cells expressing the indicated C. elegans sHsps were spotted on YPD plates and incubated at indicated temperatures for 3 d. Dashed lines indicate that spot tests from different plates are cropped and shown together for comparative analysis. Source data are available for this figure: SourceData FS2.
A subset of sHsps complements growth deficits of yeast sequestrase mutant cells. (A) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ (∆∆), and ∆∆btn2∆ cells expressing the indicated C. elegans sHsps or an additional HSP42 allele (SV40NLS-FLAG tagged) were spotted on YPD plates and incubated at indicated temperatures for 3 d. (B)S. cerevisiae WT and indicated mutant strains with or without indicated C. elegans sHsps were grown in YPD media at 25°C. Log phase cultures were normalized to OD600 = 0.2, shifted to 33°C and then growth (OD600 value) of each yeast strain was monitored for 25 h. (C and D) Western blot analysis depicting the levels of all (C) tested or (D) selected sequestrase positive and negative C. elegans sHsps in comparison to Hsp42 (FLAG-tagged allele) in the ∆∆hsp42∆ cells. α-FLAG and α-Zwf1 antibodies were used to probe for sHsp and Zwf1 (loading control) levels, respectively. A red asterisk (A) indicates the correct molecular weight of the respective sHsp. (E) Five-fold serial dilutions of S. cerevisiae WT, hsp104∆ fes1∆ (∆∆), and ∆∆hsp42∆ cells expressing the indicated C. elegans sHsps were spotted on YPD plates and incubated at indicated temperatures for 3 d. Dashed lines indicate that spot tests from different plates are cropped and shown together for comparative analysis. Source data are available for this figure: SourceData FS2.
To substantiate the results obtained from spot tests, we compared the growth curves of ΔΔbtn2Δ cells expressing diverse C. elegans sHsps in liquid YPD media at 30 and 33°C (Fig. 1 B and Fig. S2 B). We determined the ratio of OD600 values of ΔΔbtn2Δ cells expressing C. elegans sHsps and ΔΔbtn2Δ reference cells at the time point when the growth curve of ΔΔ cells reached saturation. A value larger than 1 indicates improved growth upon the expression of C. elegans sHsps, and we set a threshold value of 1.5 for analysis. The same subset of C. elegans sHsps that was tested positive in spot tests partially improved the growth of ΔΔbtn2Δ cells at 30 or 33°C except for Sip1, which was just below the threshold, and F08H9.3.
To generalize our findings, we repeated the growth complementation experiments in ΔΔhsp42Δ cells that lack the cytosolic sequestrase Hsp42. C. elegans sHsp expression was achieved by the same strategy as described for ΔΔbtn2Δ cells, yet without fusing a C-terminal NLS allowing sHsp expression in the yeast cytosol. The sHsp expression levels were overall similar to the ones determined in ΔΔbtn2Δ cells and to levels of Hsp42-FLAG expressed from its authentic promoter (Fig. S1, C and D). Two (Hsp-16.1, Hsp-16.48) out of the 12 C. elegans sHsps robustly restored the growth of ΔΔhsp42Δ cells up to 37°C, while four (Hsp-16.2, Hsp-17, F08H9.3, F08H9.4) only moderately improved growth at 33°C (Fig. S1 E). This subset of sHsps is almost identical to the one partially rescuing growth of ΔΔbtn2Δ cells, except for Sip1, which showed moderate growth improvement only in ΔΔbtn2Δ cells. Three (Hsp-16.1, Hsp-16.2, and Hsp-17) out of the six sHsps significantly improved the growth of ΔΔhsp42Δ cells at 33°C in liquid medium (Fig. S3, A and B).
Rescue of yeast sequestrase mutant phenotypes by C. elegans and human sHsps. (A)S. cerevisiae WT and indicated mutant strains with or without indicated C. elegans sHsps were grown in YPD media at 25°C. Log phase cultures were normalized to OD600 = 0.2, shifted to 33°C, and the growth (OD600 value) of each yeast strain was monitored for 25 h. (B) The OD600 value for each strain at the time point when the hsp104∆ fes1∆ reference cell culture reached saturation was noted (see [I]) and plotted as a bar graph normalized to the OD600 value corresponding to ∆∆hsp42∆ cells without complementing C. elegans sHsp. A growth ratio of 1 denotes that the growth curves of ∆∆hsp42∆ cells with and without C. elegans sHsp are equal. Error bars represent ± SD calculated based on n = 3 independent experiments. One-way ANOVA test (Dunnett) was used to assess the statistical significance (*, P < 0.05, ****, P < 0.0001). Data distribution was assumed to be normal, but this was not formally tested. (C) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ and ∆∆hsp42∆ cells expressing Hsp-16.1 or Hsp-12.1 (tagged or non-tagged) were spotted on YPD plates and incubated at indicated temperatures for 3 d. The dashed line indicates that spot tests from different plates are cropped and shown together for comparative analysis. (D) Fivefold serial dilutions of S. cerevisiae WT and indicated mutant strains were done as described in (C and F). (E) Western blot depicting the levels of E. coli IbpA and C. elegans Hsp-12.1 and Hsp-16.1 that served as reference in ∆∆btn2∆ cells. α-FLAG and α-Zwf1 antibodies were used to probe for sHsp and Zwf1 (loading control) levels respectively. (F) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ and ∆∆btn2∆ cells expressing human sHsps were spotted on YPD plates and incubated at indicated temperatures for 3 d. The dashed line indicates that spot tests from different plates are cropped and shown together for comparative analysis. (G)S. cerevisiae ∆∆btn2∆ cells strains with or without indicated human sHsps were grown in YPD media at 25°C. Log phase cultures were normalized to OD600 = 0.2, shifted to 30 or 33°C and then growth (OD600 value) of each yeast strain was monitored for 25 h. The growth ratio was determined as described in (J). OD600 value for each strain at the time point when the hsp104∆ fes1∆ reference cell culture reached saturation was noted and plotted as a bar graph normalized to the OD600 value corresponding to ∆∆btn2∆ ells without complementing human sHsp. A growth ratio of >1 indicates improved growth upon expression of humans sHsps. Error bars represent ± SD calculated based on n = 3 independent experiments. A threshold ratio of 1.5 was defined and a one-way ANOVA test (Dunnett) was used to assess the statistical significance (****, P < 0.0001) only for those sHsps passing the threshold. (H) Western blot analysis depicting the levels of all human sHsps in ∆∆btn2∆ cells. α-FLAG and α-Zwf1 antibodies were used to probe for sHsp and Zwf1 (loading control) levels, respectively. (I)S. cerevisiae ∆∆ and ∆∆btn2∆ cells expressing selected human sHsps and GFP-VHL (green) were grown at 25°C and shifted to 30°C for 1 h. Temperature-upshifted samples were collected and analyzed by fluorescence microscopy. DNA was stained by DAPI. Maximum intensity Z-projection images of selected growth complementing and non-complementing human sHsps are shown. Scale bar: 2 μm. (J) Cellular localization of GFP-VHL in respective yeast cells was quantified (n > 100; 2 replicates with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 foci/cell), and multiple inclusions (>3 foci/cell). Source data are available for this figure: SourceData FS3.
Rescue of yeast sequestrase mutant phenotypes by C. elegans and human sHsps. (A)S. cerevisiae WT and indicated mutant strains with or without indicated C. elegans sHsps were grown in YPD media at 25°C. Log phase cultures were normalized to OD600 = 0.2, shifted to 33°C, and the growth (OD600 value) of each yeast strain was monitored for 25 h. (B) The OD600 value for each strain at the time point when the hsp104∆ fes1∆ reference cell culture reached saturation was noted (see [I]) and plotted as a bar graph normalized to the OD600 value corresponding to ∆∆hsp42∆ cells without complementing C. elegans sHsp. A growth ratio of 1 denotes that the growth curves of ∆∆hsp42∆ cells with and without C. elegans sHsp are equal. Error bars represent ± SD calculated based on n = 3 independent experiments. One-way ANOVA test (Dunnett) was used to assess the statistical significance (*, P < 0.05, ****, P < 0.0001). Data distribution was assumed to be normal, but this was not formally tested. (C) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ and ∆∆hsp42∆ cells expressing Hsp-16.1 or Hsp-12.1 (tagged or non-tagged) were spotted on YPD plates and incubated at indicated temperatures for 3 d. The dashed line indicates that spot tests from different plates are cropped and shown together for comparative analysis. (D) Fivefold serial dilutions of S. cerevisiae WT and indicated mutant strains were done as described in (C and F). (E) Western blot depicting the levels of E. coli IbpA and C. elegans Hsp-12.1 and Hsp-16.1 that served as reference in ∆∆btn2∆ cells. α-FLAG and α-Zwf1 antibodies were used to probe for sHsp and Zwf1 (loading control) levels respectively. (F) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ and ∆∆btn2∆ cells expressing human sHsps were spotted on YPD plates and incubated at indicated temperatures for 3 d. The dashed line indicates that spot tests from different plates are cropped and shown together for comparative analysis. (G)S. cerevisiae ∆∆btn2∆ cells strains with or without indicated human sHsps were grown in YPD media at 25°C. Log phase cultures were normalized to OD600 = 0.2, shifted to 30 or 33°C and then growth (OD600 value) of each yeast strain was monitored for 25 h. The growth ratio was determined as described in (J). OD600 value for each strain at the time point when the hsp104∆ fes1∆ reference cell culture reached saturation was noted and plotted as a bar graph normalized to the OD600 value corresponding to ∆∆btn2∆ ells without complementing human sHsp. A growth ratio of >1 indicates improved growth upon expression of humans sHsps. Error bars represent ± SD calculated based on n = 3 independent experiments. A threshold ratio of 1.5 was defined and a one-way ANOVA test (Dunnett) was used to assess the statistical significance (****, P < 0.0001) only for those sHsps passing the threshold. (H) Western blot analysis depicting the levels of all human sHsps in ∆∆btn2∆ cells. α-FLAG and α-Zwf1 antibodies were used to probe for sHsp and Zwf1 (loading control) levels, respectively. (I)S. cerevisiae ∆∆ and ∆∆btn2∆ cells expressing selected human sHsps and GFP-VHL (green) were grown at 25°C and shifted to 30°C for 1 h. Temperature-upshifted samples were collected and analyzed by fluorescence microscopy. DNA was stained by DAPI. Maximum intensity Z-projection images of selected growth complementing and non-complementing human sHsps are shown. Scale bar: 2 μm. (J) Cellular localization of GFP-VHL in respective yeast cells was quantified (n > 100; 2 replicates with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 foci/cell), and multiple inclusions (>3 foci/cell). Source data are available for this figure: SourceData FS3.
Hsp-16.1 showed the best complementation activity in all assays. To make sure that the C-terminal FLAG tagging did not artificially increase Hsp-16.1 activity, we expressed untagged Hsp-16.1 in ΔΔhsp42Δ cells (Fig. S3 C). The complementation activities of FLAG-tagged and untagged Hsp-16.1 were largely comparable, whereas the expression of untagged Hsp-12.1 did not allow for growth rescue. This excludes that the C-terminal tagging of Hsp-16.1 substantially alters its chaperone activity in vivo.
When comparing the results from the four complementation assays, Hsp-16.1, Hsp-16.2, and Hsp-17 always showed activity, while F08H9.4 and Hsp-16.48 were functional in three out of four tests (Fig. 1 C). This defines a core set of five C. elegans sHsps that can partially rescue the growth of yeast sequestrase mutants at elevated temperatures.
C. elegans sHsps sequester misfolded GFP-VHL into large nuclear inclusions
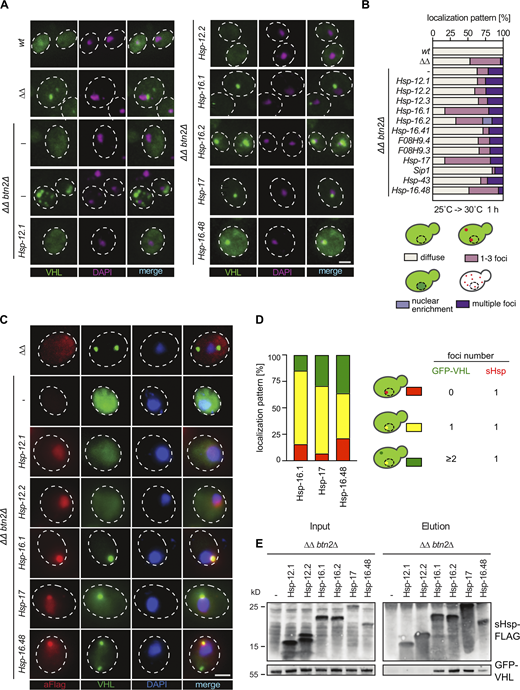
Btn2 and Hsp42 maintain the growth of fes1Δ hsp104Δ cells (ΔΔ) at elevated temperatures by depositing misfolded proteins into inclusions (Ho et al., 2019). Loss of Btn2 in ΔΔbtn2Δ cells largely abrogates the formation of those organized inclusions and instead results in uncontrolled protein aggregation of the misfolded reporter GFP-VHL. This becomes microscopically apparent in a subpopulation (24.4%, 1 h after a shift to 30°C) of ΔΔbtn2Δ cells, which harbors multiple GFP-VHL aggregates distributed throughout the cell (Ho et al., 2019; Fig. 2, A and B). We analyzed whether the growth complementation activities of C. elegans sHsps in ΔΔbtn2Δ cells correlate with their abilities to sequester GFP-VHL into large nuclear inclusions. The ΔΔbtn2Δ cells expressing GFP-VHL and the individual C. elegans sHsps were grown at 25°C and then shifted to 30°C for 1 h, and GFP-VHL localization was determined (Fig. 2, A and B). Overall, there is a good correlation between the ability of specific C. elegans sHsps to rescue the growth of ΔΔbtn2Δ cells and to restore misfolded protein sequestration. Four (Hsp-16.1, Hsp-16.2, Hsp-17, Hsp-16.48) out of the five C. elegans sHsps that showed the most robust growth complementation activity triggered the formation of a large GFP-VHL inclusion located adjacent to chromatin stained by DAPI, defining the inclusion as nuclear INQ (Miller et al., 2015a; Fig. 2, A and B). None of the C. elegans sHsps that were non-complementing in growth tests changed the GFP-VHL localization pattern in ΔΔbtn2Δ cells. F08H9.3 and F08H9.4, which showed moderate growth complementation activity, did not affect the GFP-VHL localization pattern. Sip1 reduced the number of cells showing multiple GFP-VHL foci, implying that Sip1 impairs uncontrolled GFP-VHL aggregation by forming submicroscopic substrate complexes (Fig. 2 B). From these data, we infer that a subset of C. elegans sHsps (Hsp-16.1, Hsp-16.2, Hsp-16.48, and Hsp-17) improves the growth of ΔΔbtn2Δ cells by sequestering misfolded proteins.
Most growth complementing C. elegans sHsps function as sequestrases. (A)S. cerevisiae wt, ∆∆, and ∆∆btn2∆ cells expressing C. elegans sHsps and GFP-VHL (green) were grown at 25°C and shifted to 30°C for 1 h. Temperature-upshifted samples were collected and analyzed by fluorescence microscopy. DNA was stained by DAPI. Maximum intensity Z-projection images of selected growth complementing and non-complementing C. elegans sHsps are shown. ∆∆btn2∆ cells showing diffuse staining or multiple GFP-VHL foci are depicted. Scale bar: 2 μm. (B) Cellular localization of GFP-VHL in respective yeast cells was categorized into diffuse staining, organized inclusions (1–3 foci/cell), nuclear enrichment, and multiple inclusions (>3 foci/cell) and quantified (n > 100; three replicates, all with a similar outcome). The cartoon representation illustrates the different phenotypes scored. (C) Yeast cells expressing selected growth complementing and non-complementing C. elegans sHsps were subjected to temperature upshift as in A. Samples were collected and sHsp localizations were determined by immunofluorescence microscopy using α-Flag antibodies (red). Maximum intensity Z-projection images are shown. DNA was stained by DAPI. Scale bar: 2 µm. (D) Co-localization of GFP-VHL and sequestrase positive sHsp foci were quantified (n > 50; 2 replicates with a similar outcome). Cells harboring (i) no, (ii) a single nuclear, and (iii) cytosolic plus nuclear GFP-VHL foci were analyzed for the presence of nuclear sHsp foci. The cartoon representation illustrates the different phenotypes scored. (E) Yeast cells expressing selected growth complementing and non-complementing C. elegans Flag-tagged sHsps were subjected to a temperature upshift (as in A). Samples were collected, lysed, and subjected to immunoprecipitation of sHsps using α-FLAG antibody-conjugated agarose beads. Input and elution samples were collected and analyzed by Western blotting for sHsp levels using α-FLAG antibodies and for co-immunoprecipitated GFP-VHL levels using α-GFP antibodies. Source data are available for this figure: SourceData F2.
Most growth complementing C. elegans sHsps function as sequestrases. (A)S. cerevisiae wt, ∆∆, and ∆∆btn2∆ cells expressing C. elegans sHsps and GFP-VHL (green) were grown at 25°C and shifted to 30°C for 1 h. Temperature-upshifted samples were collected and analyzed by fluorescence microscopy. DNA was stained by DAPI. Maximum intensity Z-projection images of selected growth complementing and non-complementing C. elegans sHsps are shown. ∆∆btn2∆ cells showing diffuse staining or multiple GFP-VHL foci are depicted. Scale bar: 2 μm. (B) Cellular localization of GFP-VHL in respective yeast cells was categorized into diffuse staining, organized inclusions (1–3 foci/cell), nuclear enrichment, and multiple inclusions (>3 foci/cell) and quantified (n > 100; three replicates, all with a similar outcome). The cartoon representation illustrates the different phenotypes scored. (C) Yeast cells expressing selected growth complementing and non-complementing C. elegans sHsps were subjected to temperature upshift as in A. Samples were collected and sHsp localizations were determined by immunofluorescence microscopy using α-Flag antibodies (red). Maximum intensity Z-projection images are shown. DNA was stained by DAPI. Scale bar: 2 µm. (D) Co-localization of GFP-VHL and sequestrase positive sHsp foci were quantified (n > 50; 2 replicates with a similar outcome). Cells harboring (i) no, (ii) a single nuclear, and (iii) cytosolic plus nuclear GFP-VHL foci were analyzed for the presence of nuclear sHsp foci. The cartoon representation illustrates the different phenotypes scored. (E) Yeast cells expressing selected growth complementing and non-complementing C. elegans Flag-tagged sHsps were subjected to a temperature upshift (as in A). Samples were collected, lysed, and subjected to immunoprecipitation of sHsps using α-FLAG antibody-conjugated agarose beads. Input and elution samples were collected and analyzed by Western blotting for sHsp levels using α-FLAG antibodies and for co-immunoprecipitated GFP-VHL levels using α-GFP antibodies. Source data are available for this figure: SourceData F2.
To demonstrate that the formation of the nuclear GFP-VHL inclusion relies on interactions between the misfolded protein and a C. elegans sHsp, we tested for co-localization by immunofluorescence (Fig. 2, C and D) and co-purification by pulldown experiments (Fig. 2 E) in stressed ΔΔbtn2Δ cells. Quantification of co-localization experiments revealed that all nuclear GFP-VHL foci were stained positive for the particular C. elegans sHsp (Hsp-16.1, Hsp-17 and Hsp-16.48), triggering its formation (Fig. 2, C and D). In contrast, C. elegans sHsps (Hsp-12.1 and Hsp-12.2) tested negative before they were enriched in the nucleus but did not form foci (Fig. 2 C). Foci formation by sequestrase positive sHsps was also observed in cells showing no or only diffuse GFP-VHL fluorescence (Fig. 2 D). This indicates that sHsp inclusions can form in the absence of reporter aggregation, likely reflecting sequestration of authentic substrates that misfold in the mutant cells. In some cells, we noticed the formation of cytosolic and nuclear GFP-VHL inclusions. Here, only the nuclear GFP-VHL foci co-localized with the sHsp sequestrases (Fig. 2 D). This specific colocalization is explained by the nuclear localization of the NLS-tagged sHsps.
Pulldown experiments of FLAG-tagged C. elegans sHsps showed interactions between sequestrase positive sHsps and GFP-VHL (Fig. 2 E), while non-complementing sHsps failed to interact. We infer that the ability of C. elegans sHsps to complement growth defects of ΔΔbtn2Δ cells is linked to their ability to bind and sequester misfolded proteins.
Selected C. elegans sHsps exhibit robust sequestrase activity
To substantiate and generalize our finding that a specific subset of C. elegans sHsps exhibits sequestrase activity, we pursued two strategies. First, we employed nuclear NLS-GFP-Luciferase-DM as an alternative misfolded reporter and monitored its localization in ΔΔbtn2Δ cells. Second, we determined the localization of GFP-VHL in btn2Δ hsp42Δ cells, which lack both yeast sequestrases, but do not have impaired Hsp70 capacity.
NLS-GFP-Luciferase-DM is a nuclear, hyper-thermolabile Luciferase variant that harbors destabilizing mutations (R188Q/R261Q; Gupta et al., 2011). It misfolds in ΔΔ cells at 30°C (Ho et al., 2019). Similar to GFP-VHL, this leads to its Btn2-dependent sequestration into nuclear foci (INQ) at 30°C in a large fraction (26%) of ΔΔ cells. Lack of Btn2 activity in ΔΔbtn2Δ cells causes proteostasis collapse at 30°C, resulting in an uncontrolled formation of multiple nuclear and cytosolic NLS-GFP-Luciferase-DM aggregates in 34% of cells as compared to 2% in ΔΔ reference cells (Fig. 3, A and B). All sequestrase positive C. elegans sHsps induced INQ formation of NLS–GFP–Luciferase–DM in ΔΔbtn2Δ cells, but to varying degrees. Hsp-16.1 and Hsp-17 showed the highest sequestration activity and restored a NLS–GFP–Luciferase–DM localization pattern similar to the one determined in Btn2-harboring ΔΔ cells (Fig. 3 B): 33–38% of these cells showed the formation of INQ, while the frequency of cells harboring multiple NLS–GFP–Luciferase–DM foci was reduced to 7–10%. INQ formation upon co-expression of Hsp-16.2 and Hsp-16.48 was less efficient (9 and 20% of cells, respectively), but both sHsps reduced the fraction of cells harboring multiple NLS–GFP–Luciferase–DM aggregates to 17%. No INQ formation was observed upon co-expression of sequestrase negative Hsp-12.1 or Hsp-12.2 (Fig. 3, A and B).
A specific core set of C. elegans sHsps exhibits robust sequestrase activity. (A) S. cerevisiae wt, ∆∆, and ∆∆btn2∆ cells expressing C. elegans sHsps and thermolabile NLS–GFP–Luci–DM were grown at 25°C and shifted to 30°C for 30 min, followed by analysis by fluorescence microscopy. Maximum intensity Z-projection images of selected cells expressing growth complementing and non-complementing C. elegans sHsps are shown. Arrows illustrate INQ formation. Scale bar: 2 µm. (B) Cellular localization of NLS–GFP–Luci–DM in respective yeast cells were quantified (n > 50; three replicates, all with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 foci/cell), and multiple inclusions (>3 foci/cell). The cartoon representation illustrates the different phenotypes scored. (C)S. cerevisiae btn2∆ hsp42∆ cells expressing selected C. elegans sHsps and GFP-VHL were grown at 30°C and shifted to 38°C for 1 h, followed by fluorescence microscopy analysis. Maximum intensity Z-projection images are shown. Nuclear localization of GFP-VHL was determined by expression of fluorescently tagged nuclear pore protein, Nic96-mCherry (red). Scale bar: 2 µm. (D) Cellular localizations of GFP-VHL in respective yeast cells were quantified (n > 100, 2 replicates with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 foci/cell) and nuclear enrichment. (E) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ (∆∆), and ∆∆btn2∆ cells expressing indicated C. elegans sHsps or E. coli IbpA were spotted on YPD agar plates and incubated at indicated temperatures for 3 d. (F) Indicated yeast strains expressing GFP-VHL (green) were grown at 25°C and shifted to 30°C for 1 h, followed by fluorescence microscopy analysis. Maximum intensity Z-projection images of selected sHsps are shown. DNA is stained by DAPI. Scale bar: 2 µm. (G) Cellular localizations of GFP-VHL in respective yeast cells were quantified (n > 100; three replicates, all with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 in number), and multiple inclusions (more than three in number).
A specific core set of C. elegans sHsps exhibits robust sequestrase activity. (A) S. cerevisiae wt, ∆∆, and ∆∆btn2∆ cells expressing C. elegans sHsps and thermolabile NLS–GFP–Luci–DM were grown at 25°C and shifted to 30°C for 30 min, followed by analysis by fluorescence microscopy. Maximum intensity Z-projection images of selected cells expressing growth complementing and non-complementing C. elegans sHsps are shown. Arrows illustrate INQ formation. Scale bar: 2 µm. (B) Cellular localization of NLS–GFP–Luci–DM in respective yeast cells were quantified (n > 50; three replicates, all with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 foci/cell), and multiple inclusions (>3 foci/cell). The cartoon representation illustrates the different phenotypes scored. (C)S. cerevisiae btn2∆ hsp42∆ cells expressing selected C. elegans sHsps and GFP-VHL were grown at 30°C and shifted to 38°C for 1 h, followed by fluorescence microscopy analysis. Maximum intensity Z-projection images are shown. Nuclear localization of GFP-VHL was determined by expression of fluorescently tagged nuclear pore protein, Nic96-mCherry (red). Scale bar: 2 µm. (D) Cellular localizations of GFP-VHL in respective yeast cells were quantified (n > 100, 2 replicates with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 foci/cell) and nuclear enrichment. (E) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ (∆∆), and ∆∆btn2∆ cells expressing indicated C. elegans sHsps or E. coli IbpA were spotted on YPD agar plates and incubated at indicated temperatures for 3 d. (F) Indicated yeast strains expressing GFP-VHL (green) were grown at 25°C and shifted to 30°C for 1 h, followed by fluorescence microscopy analysis. Maximum intensity Z-projection images of selected sHsps are shown. DNA is stained by DAPI. Scale bar: 2 µm. (G) Cellular localizations of GFP-VHL in respective yeast cells were quantified (n > 100; three replicates, all with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 in number), and multiple inclusions (more than three in number).
To exclude the possibility that the C. elegans sHsp sequestration activity documented so far can only be observed in yeast cells with limited Hsp70 capacity (ΔΔbtn2Δ cells), we tested selected C. elegans sHsps, harboring C-terminal NLS and FLAG sequences, in btn2Δ hsp42Δ cells. These cells lack both yeast sequestrases; however, they do not exhibit a stress-sensitive phenotype on plates as they have normal Hsp70 activity (Fig. S3 D). To visualize sHsp sequestrase activity, we co-expressed the GFP-VHL reporter and subjected the cells to heat shock at 38°C for 30 min (Fig. 3, C and D). Co-expressed Nic96-mCherry stained the nuclear envelope, enabling monitoring the formation of nuclear GFP-VHL inclusions. btn2Δ hsp42Δ cells exhibited a small proportion of GFP-VHL foci (6%), and GFP-VHL staining remained predominantly diffuse (56%) or was enriched in the nucleus (38%; Fig. 3, C and D) due to preferential import of misfolded proteins into the nucleus as also described before (Miller et al., 2015a; Park et al., 2013). Expression of sequestrase negative Hsp-12.2 did not change the GFP-VHL localization pattern and, similarly, Hsp-12.1 caused GFP-VHL foci formation only in a low number (14%) of cells. In contrast, all sequestrase positive sHsps (Hsp-16.1, Hsp-16.2, Hsp-16.48 and Hsp-17) strongly increased the fraction of cells showing nuclear GFP-VHL foci to 53% (Hsp-16.2) and up to 89% (Hsp-17; Fig. 3, C and D).
Together these findings document that C. elegans possesses a core set of sHsps, which exhibit robust sequestrase activity, irrespective of the reporter protein or the yeast strain background used to test functionality. Consistent with our findings, C. elegans Hsp-17 has been recently shown to exhibit sequestrase activity in vitro and in vivo (Iburg et al., 2020), validating our screening approach.
Bacterial and selected human sHsps exhibit sequestrase activity
To assess how widespread sHsp sequestrase activity is across different phylogenetic taxa, we analyzed the ability of E. coli and human sHsps (IbpA/B and HspB1-HspB10, respectively; Fig. S1 A) to complement the phenotypes of ΔΔbtn2Δ cells. We applied the same strategy as for C. elegans sHsps and integrated the E. coli ibpA and ibpB and human hspB1 - hspB10 genes encoding sHsps into the chromosome of yeast ΔΔbtn2Δ cells such that the encoded proteins harbor C-terminal NLS and FLAG sequences for nuclear targeting and determination of expression levels. Expression was detected for IbpA but not IbpB, and IbpA levels were similar to Hsp-16.1 (Fig. S3 E). We observed moderate growth rescue by E. coli IbpA that could be linked to IbpA triggered sequestration of GFP-VHL into nuclear inclusions (Fig. 3, E–G). Similarly, we noticed partial growth rescue upon the expression of selected human sHsps. Growth rescue was strongest and most robust for HspB2 and HspB3 (Fig. S3, F and G). Protein levels of HspB2 and HspB3 were not higher as compared to most other human sHsps, largely excluding that their complementation activities are based on increased protein production (Fig. S3 H). The moderate growth rescue by HspB2 and HspB3 again correlated with their abilities to sequester GFP-VHL into a nuclear inclusion, while the non-rescuing HspB1 and HspB4 did not enhance GFV-VHL foci formation (Fig. S3, I and J). Together, these findings indicate that substrate sequestration is an originally and evolutionarily conserved function of sHsps.
C. elegans sHsp domains governing sequestrase function
We next sought to define the specific sequence determinants that underlie the sequestrase activity of sHsps. We focused our analysis on C. elegans sHsps as they showed the most pronounced differences in growth complementation and substrate sequestration activities, and included family members providing the strongest growth rescue. Phylogenetic analysis of C. elegans sHsps revealed that except for Hsp-17, the sHsps that tested positive for growth complementation belong to one clade of a phylogenetic tree (Fig. S4 A). To reveal common sequence features among these sHsps we focused on NTEs and CTEs as they are the most variable among sHsps and define functional specificity (Kriehuber et al., 2010). Sequestrase positive sHsps typically harbor longer NTEs and CTEs as compared to negative ones (mean NTE length: 39 vs. 25 residues, mean CTE length: 21 vs. 6 residues; Fig. 4 A) except for Hsp-43, which has an atypically long NTE (108 residues). Furthermore, the negative Hsp-12.1, Hsp-12.2, and Hsp-12.3 did not harbor the IXI motif in their CTEs (Fig. S1 A), which mediates the formation of larger sHsp oligomers via interaction with adjacent α-crystallin domains (Haslbeck et al., 2019; Mogk et al., 2019).
Hsp-16.1 NTE and CTE are crucial for sequestrase activity. (A) Phylogenetic tree depicting that C. elegans sHsps populate two major clades and most sHsps with sequestrase activity (depicted in dark green) except Hsp-17 fall into one clade. The phylogenetic analysis was performed at http://www.phylogeny.fr using the complete sHsp sequence. sHsps that partially rescued the growth deficits of ∆∆btn2∆ mutant cells but showed no sequestrase activity are depicted in light green. sHsps were tested negative in all assays are depicted in red and sHsps that were not included in the screen are depicted in grey. (B) Expression levels of Hsp-12.1 and Hsp-16.1 wild type, mutant derivatives and fusion constructs were determined in S. cerevisiae ∆∆btn2∆ cells using α-FLAG antibodies. Zwf1 levels were determined using α-Zwf1 antibodies and served as loading control. (C) Fivefold serial dilutions of S. cerevisae WT, hsp104∆ fes1∆, and ∆∆btn2∆ cells expressing Hsp-12.1, Hsp-16.1, or a Hsp-16.1 IXI mutant were spotted on YPD plates and incubated at indicated temperatures for 3 d. (D) Expression levels of sHsps tested in C were determined as described in B. (E)∆∆btn2∆ cells expressing indicated Hsp-16.1 deletion mutants together with GFP-Luciferase-DM-NLS were grown at 25°C and shifted to 30°C for 30 min. GFP-Luciferase-DM-NLS localization was analyzed after heat shock using fluorescence microscopy. Maximum intensity Z-projection images are shown. Arrows indicate foci formation. Scale bar: 2 µm. (F) Cellular localizations of GFP-Luciferase-DM-NLS in respective yeast cells (see E) were quantified (n > 100; 3 replicates with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 foci) and multiple inclusions (>3 foci). Source data are available for this figure: SourceData FS4.
Hsp-16.1 NTE and CTE are crucial for sequestrase activity. (A) Phylogenetic tree depicting that C. elegans sHsps populate two major clades and most sHsps with sequestrase activity (depicted in dark green) except Hsp-17 fall into one clade. The phylogenetic analysis was performed at http://www.phylogeny.fr using the complete sHsp sequence. sHsps that partially rescued the growth deficits of ∆∆btn2∆ mutant cells but showed no sequestrase activity are depicted in light green. sHsps were tested negative in all assays are depicted in red and sHsps that were not included in the screen are depicted in grey. (B) Expression levels of Hsp-12.1 and Hsp-16.1 wild type, mutant derivatives and fusion constructs were determined in S. cerevisiae ∆∆btn2∆ cells using α-FLAG antibodies. Zwf1 levels were determined using α-Zwf1 antibodies and served as loading control. (C) Fivefold serial dilutions of S. cerevisae WT, hsp104∆ fes1∆, and ∆∆btn2∆ cells expressing Hsp-12.1, Hsp-16.1, or a Hsp-16.1 IXI mutant were spotted on YPD plates and incubated at indicated temperatures for 3 d. (D) Expression levels of sHsps tested in C were determined as described in B. (E)∆∆btn2∆ cells expressing indicated Hsp-16.1 deletion mutants together with GFP-Luciferase-DM-NLS were grown at 25°C and shifted to 30°C for 30 min. GFP-Luciferase-DM-NLS localization was analyzed after heat shock using fluorescence microscopy. Maximum intensity Z-projection images are shown. Arrows indicate foci formation. Scale bar: 2 µm. (F) Cellular localizations of GFP-Luciferase-DM-NLS in respective yeast cells (see E) were quantified (n > 100; 3 replicates with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 foci) and multiple inclusions (>3 foci). Source data are available for this figure: SourceData FS4.
Hsp-16.1 NTE and CTE are necessary but not sufficient for sequestrase activity. (A) Comparison of the lengths of the NTEs and CTEs of C. elegans sHsps that belong to one classification based on growth complementation and sequestrase activities (good: Hsp-16.1, Hsp-16.2. Hsp-17, Hsp-16.48; weak: Sip1, F08H9.3, F08H9.4; none: Hsp-12.1, Hsp-12.2, Hsp-12.3, Hsp-16.41; not included: Hsp-43) as mean ± SD. Each point represents the NTE or CTE length of one sHsp. An unpaired t test (two-tailed) was used to assess the statistical significance of NTE and CTE length differences between the classified sHsps (*, P < 0.05). (B) Cartoon representation of sequestrase-negative (Hsp-12.1) and -positive (Hsp-16.1) sHsp models. Domain boundaries between NTE, α-crystallin domain, and CTE are indicated. (C) Five-fold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ (∆∆), and ∆∆btn2∆ cells expressing indicated deletion mutants of Hsp-16.1 and Hsp-12.1/Hsp-16.1 chimera were spotted on YPD agar plates and incubated at indicated temperatures for 3 d. Dashed lines indicate that spot tests from different plates are cropped and shown together for comparative analysis. Domain organizations of Hsp-16.1 and Hsp-12.1 indicating variable NTE/CTE lengths are indicated (top). (D)S. cerevisiae ∆∆btn2∆ cells expressing indicated Hsp-16.1 deletion mutants and GFP-VHL were grown at 25°C and shifted to 30°C for 1 h, followed by fluorescence microscopy analysis. DNA was stained with DAPI. Maximum intensity Z-projection images are shown. Scale bar: 2 µm. (E) Proportion of cells (n > 100; 2 replicates with a similar outcome) from C showing diffuse fluorescence, organized inclusions (1–3/cell), and multiple foci. Quantification of GFP-VHL localization in S. cerevisiae WT and hsp104∆ fes1∆ (∆∆) cells is provided as the control. (F) Temperature upshifted samples from C were collected, lysed, and subjected to immunoprecipitation of Hsp-16.1 deletion mutants and Hsp-12.1/Hsp-16.1 chimera using α-FLAG antibody-conjugated agarose beads. Input and elution samples were collected and analyzed by Western blot for sHsp levels using α-FLAG antibodies and for co-immunoprecipitated GFP-VHL levels using α-GFP antibodies. Source data are available for this figure: SourceData F4.
Hsp-16.1 NTE and CTE are necessary but not sufficient for sequestrase activity. (A) Comparison of the lengths of the NTEs and CTEs of C. elegans sHsps that belong to one classification based on growth complementation and sequestrase activities (good: Hsp-16.1, Hsp-16.2. Hsp-17, Hsp-16.48; weak: Sip1, F08H9.3, F08H9.4; none: Hsp-12.1, Hsp-12.2, Hsp-12.3, Hsp-16.41; not included: Hsp-43) as mean ± SD. Each point represents the NTE or CTE length of one sHsp. An unpaired t test (two-tailed) was used to assess the statistical significance of NTE and CTE length differences between the classified sHsps (*, P < 0.05). (B) Cartoon representation of sequestrase-negative (Hsp-12.1) and -positive (Hsp-16.1) sHsp models. Domain boundaries between NTE, α-crystallin domain, and CTE are indicated. (C) Five-fold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ (∆∆), and ∆∆btn2∆ cells expressing indicated deletion mutants of Hsp-16.1 and Hsp-12.1/Hsp-16.1 chimera were spotted on YPD agar plates and incubated at indicated temperatures for 3 d. Dashed lines indicate that spot tests from different plates are cropped and shown together for comparative analysis. Domain organizations of Hsp-16.1 and Hsp-12.1 indicating variable NTE/CTE lengths are indicated (top). (D)S. cerevisiae ∆∆btn2∆ cells expressing indicated Hsp-16.1 deletion mutants and GFP-VHL were grown at 25°C and shifted to 30°C for 1 h, followed by fluorescence microscopy analysis. DNA was stained with DAPI. Maximum intensity Z-projection images are shown. Scale bar: 2 µm. (E) Proportion of cells (n > 100; 2 replicates with a similar outcome) from C showing diffuse fluorescence, organized inclusions (1–3/cell), and multiple foci. Quantification of GFP-VHL localization in S. cerevisiae WT and hsp104∆ fes1∆ (∆∆) cells is provided as the control. (F) Temperature upshifted samples from C were collected, lysed, and subjected to immunoprecipitation of Hsp-16.1 deletion mutants and Hsp-12.1/Hsp-16.1 chimera using α-FLAG antibody-conjugated agarose beads. Input and elution samples were collected and analyzed by Western blot for sHsp levels using α-FLAG antibodies and for co-immunoprecipitated GFP-VHL levels using α-GFP antibodies. Source data are available for this figure: SourceData F4.
We probed for the roles of NTEs and CTEs in two ways. First, we expressed NTE and CTE deletion and IXI motif mutant variants of Hsp-16.1 (Fig. 4, B and C; and Fig. S4 C), which we used as the model since it showed the highest activities in all previous assays. Second, we generated hybrid sHsps by swapping NTEs and CTEs between sequestrase positive Hsp-16.1 and negative Hsp-12.1 (Fig. 4, B and C). All constructs were expressed in ΔΔbtn2Δ cells to similar levels, except for Hsp-16.1ΔC lacking its CTE, which exhibited lower expression (Fig. S4, B and D). None of the Hsp-16.1 deletion mutants rescued the growth of ΔΔbtn2Δ cells, implying crucial functions for NTE and CTE (Fig. 4 C). The potential role of the CTE does not require the IXI motif as the Hsp-16.1-AXA variant complemented the growth defects of ΔΔbtn2Δ cells (Fig. S4C). All Hsp-16.1/Hsp-12.1 fusion constructs failed to rescue the growth of ΔΔbtn2Δ cells, and only a very minor complementation activity was observed for the N16.1-α12.1-C16.1 fusion harboring NTE and CTE of Hsp-16.1 (Fig. 4 C). According to the growth tests, none of the deletion and hybrid sHsps triggered efficient GFP-VHL inclusion formation in ΔΔbtn2Δ cells (Fig. 4, C and D). Similar results were obtained for Hsp-16.1 deletion constructs when monitoring the localization of NLS–GFP–Luciferase–DM in ΔΔbtn2Δ cells (Fig. S4, E and F).
To determine the molecular basis for the non-functionality of the deletion and fusion constructs we tested for their ability to bind GFP-VHL (Fig. 4 F). ΔN-Hsp-16.1 was largely deficient for substrate interaction, suggesting the NTE serves as a binding site for misfolded proteins and rationalizes why the mutant does not exhibit sequestrase activity. Transferring the NTE of Hsp-16.1 onto Hsp-12.1 (N16.1+ΔNHsp-12.1 and N16.1+α12.1+C16.1) enabled the fusion construct to bind GFP-VHL (Fig. 4 F); however, it was not sufficient to trigger substrate sequestration into large inclusions (Fig. 4, D and E). For N16.1+α12.1+C16.1 we noticed reduced levels upon expression of GFP-VHL, which might also affected GFP-VHL sequestration.
Together these findings allow the separation of substrate sequestration from a mere binding event mediated via the Hsp-16.1 NTE. While substrate binding is essential for substrate sequestration, it is clearly not sufficient. This suggests that substrate sequestration involves an intricate interplay between all three sHsp domains with crucial contributions from the Hsp-16.1 α-crystallin domain and CTE.
Aromatic and methionine residues of the NTE are crucial for sequestrase function in vivo
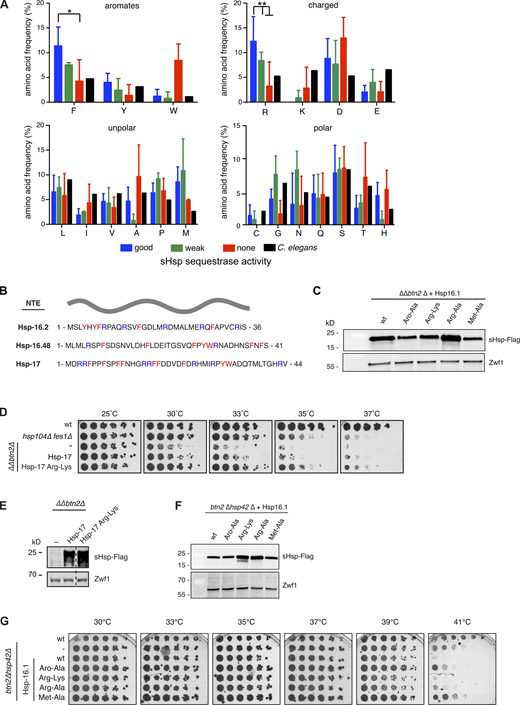
As the NTE of Hsp-16.1 mediates binding to misfolded proteins and is essential for sequestrase activity, we analyzed the amino acid composition of NTEs from sequestrase positive and negative C. elegans sHsps in greater detail. When comparing the chemical characters of amino acids (e.g., nonpolar, polar, and charged), no particular enrichment or disenrichment was observed for sequestrase positive sHsps. However, analysis of individual amino acids revealed that NTEs of sequestrase positive sHsps are significantly enriched for phenylalanine and arginine residues as compared to sequestrase negative sHsps (Fig. S5 A; Tsuji et al., 2010). We also noticed an enrichment for methionines that was however just barely above the significance threshold when taking the N-terminal methionine residue into account. The NTE of Hsp-16.48 represents an exception as it is only enriched for phenylalanines. Clustering of enriched residues within the NTEs is not pronounced, though the very N-termini of sequestrase-positive Hsp-16.1 and Hsp-16.2 show some local enrichment of aromatic residues (Fig. 5 A and Fig. S5 B). To test the role of aromatic residues in Hsp-16.1 sequestrase function, we replaced all five aromates of the NTE with alanines (Hsp-16.1-Aro/Ala). Additionally, we analyzed the role of enriched methionine and arginine residues by mutating them to either alanines (Met/Ala, Arg/Ala) or lysines (Arg/Lys). All Hsp-16.1 mutants were expressed in ΔΔbtn2Δ cells at reasonably similar levels (Fig. S5 C) and tested for growth complementation (Fig. 5 B). This activity was lost for Hsp-16.1-Aro/Ala and reduced for Hsp-16.1-Met/Ala, demonstrating the functional importance of the NTE aromatic and methionine residues. In contrast, the Hsp-16.1-Arg/Lys and Hsp-16.1-Arg/Ala variants showed higher complementation activity as compared to WTHsp-16.1 at all temperatures (Fig. 5 B). This suggests that the arginine residues of Hsp-16.1 NTE are not essential, but appear to modulate this activity. To generalize this unexpected finding, we repeated the experiment with an Hsp-17-Arg/Lys mutant expressed in ΔΔbtn2Δ cells and found that it was also more proficient in rescuing growth at elevated temperatures as compared to WTHsp-17 (Fig. S5, D and E).
Aromatic, methionine, and arginine residues enriched in the NTE of sequestrase positive sHsps regulate sequestrase activity. (A)C. elegans sHsps were classified according to their determined growth complementation and sequestrase activities (good: Hsp-16.1, Hsp-16.2. Hsp-17, Hsp-16.48; weak: Sip1, F08H9.3, F08H9.4; none: Hsp-12.1, Hsp-12.2, Hsp-12.3, Hsp-16.41, Hsp-43). NTE boundaries were identified by sequence alignment and frequency distributions of amino acids present in the NTE were determined. The frequencies (%) of individual amino acids represent the ratio of the number of a particular residue and the total length of the respective NTE. The average frequency of each amino acid in the total C. elegans proteome is given as reference (Tsuji et al., 2010). Error bars indicate ± SD of the respective amino acid frequencies of sHsps grouped into the same category. An unpaired t test (two-tailed) was used to assess the statistical significance of phenylalanine and arginine enrichment in the NTE of sequestrase positive sHsps (*, P < 0.05; **, P < 0.01). (B) Color-coded amino acid sequence of the NTE of Hsp-16.2, Hsp-17 and Hsp-16.48. Aromatic and arginine residues are highlighted in red and blue, respectively. (C) Expression levels of Δ1-7 Hsp-16.1 (right) and Hsp-16.1 NTE point mutants (left) were determined in S. cerevisiae ∆∆btn2∆ cells by Western blot analysis using α-FLAG antibodies. Zwf1 levels were determined using α-Zwf1 antibodies and served as loading control. (D) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ (∆∆), and ∆∆btn2∆ cells expressing Hsp-17 or Hsp-17-Arg/Lys were spotted on YPD agar plates and incubated at indicated temperatures for 3 d. (E) Expression levels of Hsp-17 and Hsp-17-Arg/Lys were determined in S. cerevisiae ∆∆btn2∆ cells by Western blot analysis using α-FLAG antibodies. Zwf1 levels were determined using α-Zwf1 antibodies and served as loading control. (F) Expression levels of Hsp-16.1 NTE point mutants (left) were determined in S. cerevisiae btn2∆ hsp42∆ cells by Western blot analysis using α-FLAG antibodies. Zwf1 levels were determined using α-Zwf1 antibodies and served as loading control. (G) Fivefold serial dilutions of indicated yeast strains expressing indicated C. elegans Hsp-16.1 WT and mutant derivatives in btn2∆ hsp42∆ cells were spotted on YPD plates and incubated at indicated temperatures for 3 d. Source data are available for this figure: SourceData FS5.
Aromatic, methionine, and arginine residues enriched in the NTE of sequestrase positive sHsps regulate sequestrase activity. (A)C. elegans sHsps were classified according to their determined growth complementation and sequestrase activities (good: Hsp-16.1, Hsp-16.2. Hsp-17, Hsp-16.48; weak: Sip1, F08H9.3, F08H9.4; none: Hsp-12.1, Hsp-12.2, Hsp-12.3, Hsp-16.41, Hsp-43). NTE boundaries were identified by sequence alignment and frequency distributions of amino acids present in the NTE were determined. The frequencies (%) of individual amino acids represent the ratio of the number of a particular residue and the total length of the respective NTE. The average frequency of each amino acid in the total C. elegans proteome is given as reference (Tsuji et al., 2010). Error bars indicate ± SD of the respective amino acid frequencies of sHsps grouped into the same category. An unpaired t test (two-tailed) was used to assess the statistical significance of phenylalanine and arginine enrichment in the NTE of sequestrase positive sHsps (*, P < 0.05; **, P < 0.01). (B) Color-coded amino acid sequence of the NTE of Hsp-16.2, Hsp-17 and Hsp-16.48. Aromatic and arginine residues are highlighted in red and blue, respectively. (C) Expression levels of Δ1-7 Hsp-16.1 (right) and Hsp-16.1 NTE point mutants (left) were determined in S. cerevisiae ∆∆btn2∆ cells by Western blot analysis using α-FLAG antibodies. Zwf1 levels were determined using α-Zwf1 antibodies and served as loading control. (D) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ (∆∆), and ∆∆btn2∆ cells expressing Hsp-17 or Hsp-17-Arg/Lys were spotted on YPD agar plates and incubated at indicated temperatures for 3 d. (E) Expression levels of Hsp-17 and Hsp-17-Arg/Lys were determined in S. cerevisiae ∆∆btn2∆ cells by Western blot analysis using α-FLAG antibodies. Zwf1 levels were determined using α-Zwf1 antibodies and served as loading control. (F) Expression levels of Hsp-16.1 NTE point mutants (left) were determined in S. cerevisiae btn2∆ hsp42∆ cells by Western blot analysis using α-FLAG antibodies. Zwf1 levels were determined using α-Zwf1 antibodies and served as loading control. (G) Fivefold serial dilutions of indicated yeast strains expressing indicated C. elegans Hsp-16.1 WT and mutant derivatives in btn2∆ hsp42∆ cells were spotted on YPD plates and incubated at indicated temperatures for 3 d. Source data are available for this figure: SourceData FS5.
Roles of Hsp-16.1 NTE residues for sequestrase activity. (A) Color-coded amino acid sequence of the Hsp-16.1 NTE. Aromatic and arginine residues are colored in red and blue, respectively. (B) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ (∆∆), and ∆∆btn2∆ cells expressing Hsp-16.1 NTE point mutants or truncations were spotted on YPD agar plates and incubated at indicated temperatures for 3 d. Dashed line indicates that spot tests from different plates are cropped and shown together for comparative analysis. (C)S. cerevisiae ∆∆btn2∆ expressing Hsp-16.1 NTE point mutants and NLS-GFP-Luciferase-DM were grown at 25°C and shifted to 30°C for 30 min, followed by fluorescence microscopy analysis. Maximum intensity Z-projection images are shown. (D) Cellular localization of NLS–GFP–Luciferase–DM in respective yeast cells was quantified (n > 100; 3 replicates, all with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 foci/cell), and unorganized aggregation (multiple foci). (E)btn2∆ hsp42∆ cells expressing Hsp-16.1 NTE point mutants and GFP-VHL (green) were grown at 30°C and shifted to 38°C for 30 min, followed by fluorescence microscopy analysis. Maximum intensity Z-projection images are shown. The nuclear envelope is marked by fluorescently tagged nuclear pore protein, Nic96-mCherry (red). Scale bar: 2 µm. (F) Cellular localization of GFP-VHL in respective yeast cells was quantified (n > 100; 3 replicates, all with similar outcome) and categorized into diffuse staining, organized inclusions (1–3/cell), and nuclear enrichment.
Roles of Hsp-16.1 NTE residues for sequestrase activity. (A) Color-coded amino acid sequence of the Hsp-16.1 NTE. Aromatic and arginine residues are colored in red and blue, respectively. (B) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ (∆∆), and ∆∆btn2∆ cells expressing Hsp-16.1 NTE point mutants or truncations were spotted on YPD agar plates and incubated at indicated temperatures for 3 d. Dashed line indicates that spot tests from different plates are cropped and shown together for comparative analysis. (C)S. cerevisiae ∆∆btn2∆ expressing Hsp-16.1 NTE point mutants and NLS-GFP-Luciferase-DM were grown at 25°C and shifted to 30°C for 30 min, followed by fluorescence microscopy analysis. Maximum intensity Z-projection images are shown. (D) Cellular localization of NLS–GFP–Luciferase–DM in respective yeast cells was quantified (n > 100; 3 replicates, all with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 foci/cell), and unorganized aggregation (multiple foci). (E)btn2∆ hsp42∆ cells expressing Hsp-16.1 NTE point mutants and GFP-VHL (green) were grown at 30°C and shifted to 38°C for 30 min, followed by fluorescence microscopy analysis. Maximum intensity Z-projection images are shown. The nuclear envelope is marked by fluorescently tagged nuclear pore protein, Nic96-mCherry (red). Scale bar: 2 µm. (F) Cellular localization of GFP-VHL in respective yeast cells was quantified (n > 100; 3 replicates, all with similar outcome) and categorized into diffuse staining, organized inclusions (1–3/cell), and nuclear enrichment.
To correlate growth complementation and sequestrase activities of Hsp-16.1 mutants, we monitored NLS–GFP–Luciferase–DM localization in ΔΔbtn2Δ cells (Fig. 5, C and D). Hsp-16.1-Arg/Lys and Hsp-16.1-Arg/Ala, but not Hsp-16.1-Aro/Ala and Hsp-16.1-Met/Ala, sequestered the reporter at nuclear INQ and strongly reduced its unorganized aggregation. We additionally probed for GFP-VHL localization in btn2Δ hsp42Δ cells (Fig. 5, E and F; and Fig. S5 F). Hsp-16.1-Arg/Lys and Hsp-16.1-Arg/Ala again restored sequestration of GFP-VHL into a large nuclear inclusion, while Hsp-16.1-Aro/Ala and Hsp-16.1-Met/Ala were unable to do so. Notably, the expression of sequestrase active Hsp-16.1 WTand NTE arginine mutants abrogated the growth of btn2Δ hsp42Δ cells at 41°C, while inactive NTE aromate and methionine mutants did not (Fig. S5 G). This suggests that an extensive protein sequestration can have detrimental effects on growth. This finding also underlines the critical role of NTE aromatic and methionine residues for sequestrase function.
Hsp-16.1, but not Hsp-12.1, displays sHsp chaperone activity in vitro
We next tested whether the differences in sequestrase activities between C. elegans sHsps can be reconstituted in vitro. We purified Hsp-16.1 and Hsp-12.1 and determined their assembly states and chaperone activities. Hsp-16.1 formed larger assemblies (∼34-mers) while Hsp-12.1 only formed tetramers, which can be explained by the absence of a C-terminal IxI motif (Fig. S6 A). Hsp-16.1, but not Hsp-12.1, exhibited classical sHsp chaperone function and prevented or reduced the formation of turbid aggregates of the thermolabile reporters, citrate synthase (CS) and firefly Luciferase, at increased temperatures (Fig. 6 A and Fig. S6 B). Light scattering experiments were confirmed by monitoring the formation of insoluble CS aggregates in the absence and presence of sHsps. Hsp-12.1 could not suppress CS aggregation and was also not found in the insoluble fraction (Fig. S6 C), indicating that it is deficient in binding to misfolded CS, which is consistent with our in vivo findings.
Biochemical analysis of C. elegans Hsp-16.1 and Hsp-12.1. (A) Determination of sHsp oligomeric states by size exclusion chromatography. Assembly size was estimated by fitting a calibration curve from standard proteins and referencing sHsp elution peaks. The number of subunits was calculated by dividing the estimated molecular weight of the assembly by the size of a monomer. (B) Prevention of firefly Luciferase aggregation at increasing sHsp/substrate ratios (left: Hsp-16.1, right: Hsp-12.1) was monitored by turbidity measurements at 42.5°C. The sHsp/substrate ratio is indicated. sHsp only controls (Hsp-16.1, Hsp-12.1) are provided. (C) Distribution of citrate synthase after incubation at 43°C in the presence and absence of Hsp-16.1 and Hsp-12.1 between supernatant and pellet fractions. (D) Light scattering measurements of 1 µM Hsp-16.1 variants (equivalent to highest concentration used in prevention of CS aggregation assays) were performed in absence of CS at 43°C. (E) Prevention of firefly Luciferase aggregation by Hsp-16.1 WT and NTE mutants at 4:1 sHsp/substrate ratio was monitored at 42.5°C. Representative curves from at least three independent experiments are provided (B, D, and E). Source data are available for this figure: SourceData FS6.
Biochemical analysis of C. elegans Hsp-16.1 and Hsp-12.1. (A) Determination of sHsp oligomeric states by size exclusion chromatography. Assembly size was estimated by fitting a calibration curve from standard proteins and referencing sHsp elution peaks. The number of subunits was calculated by dividing the estimated molecular weight of the assembly by the size of a monomer. (B) Prevention of firefly Luciferase aggregation at increasing sHsp/substrate ratios (left: Hsp-16.1, right: Hsp-12.1) was monitored by turbidity measurements at 42.5°C. The sHsp/substrate ratio is indicated. sHsp only controls (Hsp-16.1, Hsp-12.1) are provided. (C) Distribution of citrate synthase after incubation at 43°C in the presence and absence of Hsp-16.1 and Hsp-12.1 between supernatant and pellet fractions. (D) Light scattering measurements of 1 µM Hsp-16.1 variants (equivalent to highest concentration used in prevention of CS aggregation assays) were performed in absence of CS at 43°C. (E) Prevention of firefly Luciferase aggregation by Hsp-16.1 WT and NTE mutants at 4:1 sHsp/substrate ratio was monitored at 42.5°C. Representative curves from at least three independent experiments are provided (B, D, and E). Source data are available for this figure: SourceData FS6.
Hsp-16.1 but not Hsp-12.1 shows in vitro chaperone activity. (A) Prevention of citrate synthase (CS) aggregation at increasing sHsp/substrate ratios (left: Hsp-16.1, right: Hsp-12.1) was monitored by turbidity measurements at 43°C. The sHsp/substrate ratio is indicated. sHsp-only controls (Hsp-16.1, Hsp-12.1) are provided. (B) Increase in lysozyme (LZ) aggregation by increasing Hsp-16.1 and Hsp-12.1 concentrations was determined by light scattering measurements at 37°C. The sHsp/substrate ratio is indicated. sHsp only controls (Hsp16.1, Hsp-12.1) are provided. S. cerevisiae Hsp42 served as a positive control and was used at 1:1 (MDH:Hsp42) ratio. (C) Comparison of prevention of CS aggregation activity between Hsp-16.1 WT , and NTE mutants (Aro-Ala: AA, Arg-Lys: RK, Arg-Ala: RA, Met-Ala: MA) at 4:1 sHsp/substrate ratio. (D) Comparison of Lysozyme aggregase activity between Hsp-16.1 WT and NTE mutants at 1:1 sHsp/substrate ratio. Representative curves from at least three independent experiments are provided (A–D).
Hsp-16.1 but not Hsp-12.1 shows in vitro chaperone activity. (A) Prevention of citrate synthase (CS) aggregation at increasing sHsp/substrate ratios (left: Hsp-16.1, right: Hsp-12.1) was monitored by turbidity measurements at 43°C. The sHsp/substrate ratio is indicated. sHsp-only controls (Hsp-16.1, Hsp-12.1) are provided. (B) Increase in lysozyme (LZ) aggregation by increasing Hsp-16.1 and Hsp-12.1 concentrations was determined by light scattering measurements at 37°C. The sHsp/substrate ratio is indicated. sHsp only controls (Hsp16.1, Hsp-12.1) are provided. S. cerevisiae Hsp42 served as a positive control and was used at 1:1 (MDH:Hsp42) ratio. (C) Comparison of prevention of CS aggregation activity between Hsp-16.1 WT , and NTE mutants (Aro-Ala: AA, Arg-Lys: RK, Arg-Ala: RA, Met-Ala: MA) at 4:1 sHsp/substrate ratio. (D) Comparison of Lysozyme aggregase activity between Hsp-16.1 WT and NTE mutants at 1:1 sHsp/substrate ratio. Representative curves from at least three independent experiments are provided (A–D).
We next tested for potential sequestrase function by monitoring the aggregation of lysozyme upon its unfolding triggered by the reducing agent TCEP. Hsp-16.1, but not Hsp-12.1, triggered the formation of turbid lysozyme (LZ) assemblies in a concentration-dependent manner, documenting Hsp-16.1 sequestrase activity in vitro (Fig. 6 B). Why Hsp-16.1 forms smaller and non-scattering complexes with CS as opposed to large and turbid complexes with LZ is not understood. The diverse sizes of sHsp/substrate complexes likely depend on substrate identity and its structural state upon denaturation. Our findings on Hsp-16.1 also underline that “holdase” and “sequestrase” functions of sHsps cannot be simply separated, but likely involve at least partially overlapping activities.
We then investigated the impact of Hsp-16.1 NTE mutants (Aro/Ala, Met/Ala, Arg/Lys, and Arg/Ala) on oligomerization and chaperone activities. All mutants formed only smaller oligomers (4–8 mers), which can be explained by NTE contributions to sHsp oligomerization (Fig. S6 A; Klevit, 2020). Hsp-16.1-Aro/Ala and Met/Ala were deficient in preventing CS and Luciferase aggregation and displayed strongly reduced (Aro/Ala) or reduced (Met/Ala) activities in a sequestrase assay (Fig. 6, C and D; and Fig. S6, D and E). The chaperone activities of Hsp-16.1-Arg/Lys and Hsp-16.1-Arg/Ala were diverse and substrate dependent. Both were proficient in preventing CS aggregation but did not suppress Luciferase aggregation (Fig. 6 C and Fig. S6 E). In the lysozyme sequestrase assay, both showed an activity comparable to Hsp-16.1 WT (Fig. 6 D). Together these findings underline the crucial role of aromatic and methionine NTE residues for sHsp activity.
sHsps with sequestrase activity contribute to the longevity of daf-2 mutant animals
We next searched the literature for commonalities between the expression patterns of sequestrase positive sHsps in C. elegans. All positive C. elegans sHsps are heat shock inducible (Jovic et al., 2017; Liang et al., 2014; Shim et al., 2003; Fig. 7 A). For the hsp-16.1/hsp-16.48 and hsp-16.2/hsp-16.41 gene pairs, which are each controlled by bidirectional Hsf-1 dependent promoters, a very strong upregulation upon stress exposure has been reported (Brunquell et al., 2016; Stringham et al., 1992). Furthermore, the expression of various sequestrase-positive C. elegans sHsp genes is induced in WT animals during aging, some of which (hsp-16.2, hsp-16.48) are particularly upregulated in the long-lived daf-2 mutant (Halaschek-Wiener et al., 2005; Hsu et al., 2003; Walther et al., 2015; Fig. 7 A). daf-2 encodes for an insulin receptor, and daf-2 mutant animals are long-lived due to reduced insulin signaling (Kimura et al., 1997). Notably, upregulated sHsps are specifically enriched in the aggregated proteome of aged daf-2 mutant animals (Walther et al., 2015). We, therefore, postulated that sequestrase-positive sHsps contribute to the long-lived phenotype of daf-2 mutants. We tested the effect of RNAi-induced knockdown of Hsp-16.1, Hsp-16.2, Hsp-16.48, and Hsp-17 on the longevity of daf-2 animals (Fig. 7 B and Fig. S7 A). Reducing the expression of hsp-16.2 and hsp-16.48, but not hsp-16.1 or hsp-17, slightly but significantly reduced the median lifespan of daf-2 mutant worms by 10 ± 6% and 11 ± 5.8%, respectively (Fig. 7 B and Fig. S7 A). The specific impact of hsp-16.2 and hsp-16.48, but not of hsp-16.1 and hsp-17, can be explained by their particularly high upregulation in daf-2 animals in comparison to the latter sHsps (Halaschek-Wiener et al., 2005). The simultaneous knockdown of hsp-16.2 and hsp-16.48 had an additive effect and further shortened the lifespan compared to the respective individual RNAi knockdown controls (Fig. 7 C). This indicates functional redundancy between these sHsps and likely explains why the single knockdowns had only little effect. In the case of WT animals, we observed a significant reduction in median lifespan only upon hsp-17 knockdown (Fig. S7 B), in line with recent findings (Iburg et al., 2020). Knockdown of hsp-16.1, hsp-16.2, and hsp-16.48 had no effect (Fig. S7 B), supporting the idea that the specific upregulation of the sequestrases Hsp-16.2 and Hsp-16.48 in daf-2 animals contributes to their prolonged lifespan.
C. elegans sHsps with sequestrase activity contribute to increased life span of daf-2 animals. (A) Relation of the growth complementation and sequestrase activities of C. elegans sHsps in S. cerevisiae ∆∆btn2∆ cells and their in vivo characteristics. The relative activities of C. elegans sHsps in yeast assays (this work), their degree of upregulation during the indicated stress conditions, and their presence in the insoluble cell fraction of aged C. elegans daf-2 animals are illustrated by color code (dark blue, moderately active/strong induction; bright blue, weakly active/moderate induction; gray, not active/not induced; white, not tested/no information available). Data on C. elegans sHsps were derived from Walther et al. (2015); Liang et al. (2014); Hsu et al. (2003); Brunquell et al. (2016); Jovic et al. (2017); Lund et al. (2002); Shim et al. (2003). (B) Scatter plot of the median lifespans ± SEM from all experiments of daf-2(e1370) animals on empty vector (EV) control (n = 5) or 100% sHsp RNAi plates (n = 3). Median lifespans were extracted with GraphPad Prism 6.h and tested for statistically significant differences using one-way ANOVA with Dunnett’s adjustment for multiple comparisons. n ≥ 3; * P < 0.05. Data distribution was assumed to be normal, but this was not formally tested. (C) Representative lifespan curves of starting populations of 100-101 daf-2(e1370) animals on EV plates, 50% diluted RNAi plates against hsp-16.2, hsp-16.48/hsp-16.49, or a 1:1 combination of these from a single experiment. The experiment was repeated an additional time with the same outcome. (D) Composite pictures of brightfield and collapsed confocal z-stacks of either whole nematodes or the indicated tissues. Differential expression of (i) Hsp-16.2::mCh or (ii) Hsp-16.48::mCh/Hsp-16.49::mCh between Wt and daf-2 mutant background at the specified ages. (E) Representative lifespan curves of starting populations of 110 Wt animals on EV plates, 50% diluted RNAi plates against hsp-1 only, or in a 1:1 combination with RNAi against (i) hsp-16.1, (ii) hsp-16.2, or (iii) hsp-16.48/hsp-16.49 from a single experiment. The experiment was repeated two additional times with the same outcome. (C and D) P values of the lifespans were calculated using the log-rank method utilizing Prism 6.h. Survival statistics for all lifespan assays including repeats are summarized in Table S5.
C. elegans sHsps with sequestrase activity contribute to increased life span of daf-2 animals. (A) Relation of the growth complementation and sequestrase activities of C. elegans sHsps in S. cerevisiae ∆∆btn2∆ cells and their in vivo characteristics. The relative activities of C. elegans sHsps in yeast assays (this work), their degree of upregulation during the indicated stress conditions, and their presence in the insoluble cell fraction of aged C. elegans daf-2 animals are illustrated by color code (dark blue, moderately active/strong induction; bright blue, weakly active/moderate induction; gray, not active/not induced; white, not tested/no information available). Data on C. elegans sHsps were derived from Walther et al. (2015); Liang et al. (2014); Hsu et al. (2003); Brunquell et al. (2016); Jovic et al. (2017); Lund et al. (2002); Shim et al. (2003). (B) Scatter plot of the median lifespans ± SEM from all experiments of daf-2(e1370) animals on empty vector (EV) control (n = 5) or 100% sHsp RNAi plates (n = 3). Median lifespans were extracted with GraphPad Prism 6.h and tested for statistically significant differences using one-way ANOVA with Dunnett’s adjustment for multiple comparisons. n ≥ 3; * P < 0.05. Data distribution was assumed to be normal, but this was not formally tested. (C) Representative lifespan curves of starting populations of 100-101 daf-2(e1370) animals on EV plates, 50% diluted RNAi plates against hsp-16.2, hsp-16.48/hsp-16.49, or a 1:1 combination of these from a single experiment. The experiment was repeated an additional time with the same outcome. (D) Composite pictures of brightfield and collapsed confocal z-stacks of either whole nematodes or the indicated tissues. Differential expression of (i) Hsp-16.2::mCh or (ii) Hsp-16.48::mCh/Hsp-16.49::mCh between Wt and daf-2 mutant background at the specified ages. (E) Representative lifespan curves of starting populations of 110 Wt animals on EV plates, 50% diluted RNAi plates against hsp-1 only, or in a 1:1 combination with RNAi against (i) hsp-16.1, (ii) hsp-16.2, or (iii) hsp-16.48/hsp-16.49 from a single experiment. The experiment was repeated two additional times with the same outcome. (C and D) P values of the lifespans were calculated using the log-rank method utilizing Prism 6.h. Survival statistics for all lifespan assays including repeats are summarized in Table S5.
sHsp sequestrases affect aging of C. elegans WT and daf-2 animals. (A) Representative Kaplan-Meier lifespan curves of starting populations of 98-103 WT animals on EV control plates or RNAi plates against hsp-16.1, hsp-16.2, hsp-16.48, or hsp-17 from one assay. This experiment was repeated an additional time with similar outcome. (B) Representative Kaplan-Meier lifespan curves of starting populations of 94-101 daf-2(e1370) animals on EV control plates or RNAi plates against hsp-16.1, hsp-16.2, hsp-16.48, or hsp-17 from one assay. This experiment was repeated two additional times with similar outcome. (A and B) P values of the lifespans were calculated using the log-rank method utilizing Prism 6.h. Survival statistics for all lifespan assays including repeats are summarized in Table S5. (C and D) Composite pictures of brightfield and collapsed confocal z-stacks of either the indicated tissues or whole animals. Differential expression of (C) Hsp-16.2::mCh or (D) Hsp-16.48/Hsp-16.49::mCh between WT and daf-2 mutants at the specified ages. Scale bars: 20 µm.
sHsp sequestrases affect aging of C. elegans WT and daf-2 animals. (A) Representative Kaplan-Meier lifespan curves of starting populations of 98-103 WT animals on EV control plates or RNAi plates against hsp-16.1, hsp-16.2, hsp-16.48, or hsp-17 from one assay. This experiment was repeated an additional time with similar outcome. (B) Representative Kaplan-Meier lifespan curves of starting populations of 94-101 daf-2(e1370) animals on EV control plates or RNAi plates against hsp-16.1, hsp-16.2, hsp-16.48, or hsp-17 from one assay. This experiment was repeated two additional times with similar outcome. (A and B) P values of the lifespans were calculated using the log-rank method utilizing Prism 6.h. Survival statistics for all lifespan assays including repeats are summarized in Table S5. (C and D) Composite pictures of brightfield and collapsed confocal z-stacks of either the indicated tissues or whole animals. Differential expression of (C) Hsp-16.2::mCh or (D) Hsp-16.48/Hsp-16.49::mCh between WT and daf-2 mutants at the specified ages. Scale bars: 20 µm.
We next aimed to identify the tissues in which the Hsp-16.2 and Hsp-16.48 sequestrases are induced to extend the lifespan of daf-2 animals, and which are therefore most relevant for lifespan extension. We determined their expression in WT and daf-2 mutants during aging by engineering their endogenous gene loci to yield C-terminally tagged sHsp-mCherry (mCh) fusions. In young animals (day 1 of adulthood) hsp-16.2::mCh and hsp-16.48::mCh were not or hardly expressed, while upon aging their expression was increased in both WT and daf-2 animals (Fig. 7 D; and Fig. S7, C and D). However, remarkable differences existed for specific tissues. An increased expression of both sHsps was observed in age-matched animals in a few head neurons, the gonads, and the excretory system (Fig. S7, C and D). hsp-16.48::mCh was additionally expressed during aging in the pharyngeal muscle of WT and daf-2 animals (Fig. S7, C and D). In contrast, hsp-16.48::mCh was specifically upregulated in body wall muscle (BWM) cells and the hypodermis of daf-2 animals, whereas its expression remained low in WT animals (Fig. 7 D and Fig. S7 D). hsp-16.2::mCh was also only detectable in BWM cells of daf-2 mutants but not in WT animals (Fig. 7 D). Thus, only body wall muscles and the hypodermis exhibited a strong daf-2-specific upregulation of both sHsps, suggesting that sequestrase activity is required particularly in these tissues for the observed effect on the lifespan of daf-2 mutants.
Lastly, we asked if the absence of a lifespan phenotype in WT worms under knockdown of hsp-16.1, hsp-16.2, and hsp-16.48 could be due to a redundancy between the sequestrase and refolding systems of the proteostasis network. We previously reported that a deletion of the S. cerevisiae sequestrases, Btn2 or Hsp42, only leads to reduced viability when the capacity of the Hsp70 refolding system is limited simultaneously (Ho et al., 2019). We, therefore, tested for genetic interactions between sHsp sequestrases and Hsp-1, the major cytosolic Hsp70 chaperone of C. elegans (Fig. 7 E). In line with published data (Kirstein et al., 2017), the knockdown of hsp-1 resulted in a strong reduction in lifespan. However, combining the hsp-1 knockdown with the knockdown of either hsp-16.1, hsp-16.2, or hsp-16.48 significantly reduced maximum lifespan further (Fig. 7 E). This suggests that sHsp sequestrases serve an important role as a buffer for maintaining a critical cellular Hsp70 capacity in C. elegans similar to yeast.
Discussion
In this work, we probed for sequestrase activity of sHsps originating from diverse kingdoms of life by taking advantage of the growth defects of yeast mutants that have combined defects in organized sequestration of misfolded proteins and limited capacity of Hsp70 activity. Our findings demonstrate an evolutionarily conserved function of the sHsp family members as cellular sequestrases. The analysis of twelve C. elegans and ten human sHsps revealed that a subset partially rescues the growth of yeast sequestrase mutants and restores sequestration of misfolded reporter proteins. The fact that only a subset of sHsps restores sequestration activity suggests functional diversification among family members, consistent with earlier findings that human sHsps substantially differ in their chaperone activities in vitro (Mymrikov et al., 2017). Specialization of sHsps is also observed in bacteria and unicellular eukaryotes, all of which encode for more than one sHsp. In E. coli, IbpA interacts with substrates stably, while IbpB facilitates the transfer of IbpA-bound substrates to Hsp70/Hsp100 disaggregases for refolding (Obuchowski et al., 2019; Ratajczak et al., 2009). We show here that IbpA is able to actively promote the formation of foci of misfolded proteins and hence acts as a cellular sequestrase. Similarly, Deinococcus radiodurans Hsp20.2, but not Hsp17.7, stably binds the substrate (Bepperling et al., 2012). We showed before that S. cerevisiae Hsp42, but not Hsp26, functions as sequestrase (Specht et al., 2011; Ungelenk et al., 2016). We, therefore, propose that proteostasis networks are typically equipped with at least one sHsp with sequestrase activity, while additional sHsps fulfill alternative functions in proteostasis networks.
We cannot exclude the possibility that some C. elegans or human sHsps tested negative on our screen do exhibit sequestrase activity that remained undetected. The C-terminal tagging of sHsps, which was necessary for our screening approach, might alter their activities, though this was not observed for yeast Hsp42 (Ho et al., 2019; Specht et al., 2011) or C. elegans Hsp-16.1. Post-translational modifications or stress parameters, like high temperatures or low pH, can increase sHsp activity (Fleckenstein et al., 2015; Franzmann et al., 2008; Peschek et al., 2013), and some sHsps, e.g., Sip1, whose activity is pH-dependent (Fleckenstein et al., 2015), might therefore not display full activity in our yeast setup. Furthermore, we expressed human sHsps individually, but some are known to form mixed complexes, which differ in chaperone activities compared to single sHsps species (Morelli et al., 2017; Mymrikov et al., 2020). Given these limitations, the list of sHsps that tested positive for sequestrase activity might be incomplete. Regardless of these potential limitations, our screen reveals that multiple metazoan sHsps exhibit sequestrase activity, documenting how widespread this function is and defining it as a core activity of sHsps.
Sequence analysis revealed that sequestrase positive C. elegans sHsps harbor longer NTEs and CTEs in comparison to sHsps tested negative. Deletion analysis confirmed the crucial contribution of the NTE to Hsp-16.1 sequestrase activity. Deletion of the CTE also caused loss of activity; however, we cannot exclude that the reduced expression level of Hsp-16.1-ΔCTE also contributes to this phenotype. NTEs of sequestrase-positive sHsps were enriched for phenylalanine, methionine, and arginine residues. Using Hsp-16.1 as the model, we show that aromates are required for substrate binding and thus sequestrase function in vivo and in vitro. Competence for substrate binding can be transferred from a sequestrase-positive (Hsp-16.1) to a sequestrase-negative (Hsp-12.1) sHsp by swapping NTEs; however, it is not sufficient for achieving sequestrase function. Our analysis of sHsp fusion constructs suggests that a more complex interplay of NTE and α-crystallin domain, likely also involving the CTE, dictates sHsp sequestrase activity.
This interplay might control the size and dynamics of sHsp oligomers and the accessibility of substrate binding sites.
Surprisingly, mutating N-terminal arginine residues (NTE-Arg) enhanced growth complementation activities of Hsp-16.1 and Hsp-17 mutants. This suggests a regulatory function of these residues in controlling sHsp activity, though we did not observe enhanced chaperone activities of respective mutants in vitro. How arginine residues of the NTE modulate sHsp activity remain elusive.
What is the physiological importance of the sequestrase activity of sHsps? Several findings underscore a critical role to maintain basal Hsp70 activity in cells. Yeast mutant cells with low Hsp70 capacity rely on sequestrases for growth even at intermediate temperatures (Ho et al., 2019). Sequestrases reduce the accessibility of the misfolded proteins when sequestering them into inclusions, thus preventing the exhaustion of limited Hsp70 resources. Our finding that the expression of sHsp sequestrases from diverse species partially restores the growth of yeast mutants suffering from low Hsp70 capacity suggests that these sHsps fulfill similar functions in the respective organisms. Indeed, we demonstrate a negative genetic interaction between the major C. elegans Hsp70, Hsp-1 and the sHsp sequestrases Hsp-16.1, Hsp-16.2, and Hsp-16.48. In E. coli, cells with low DnaK levels rely on the presence of IbpA/IbpB for growth at elevated temperatures (Mogk et al., 2003), and recent findings indicate that the expression of IbpA alone is sufficient to rescue the growth of these mutant cells (Obuchowski et al., 2019). Here, we identify IbpA as a cellular sequestrase, supporting that sequestrases act to prevent exhaustion of Hsp70 capacity. Along the same line, differentiated human neural cells downregulate the central ATP-dependent TRiC chaperone while upregulating sHsps, including HspB5, which promotes protein sequestration in these cells (Vonk et al., 2020). We, therefore, suggest that sHsp sequestrases buffer against the limitations of major ATP-dependent chaperone systems.
C. elegans sHsps with sequestrase activity are upregulated upon heat stress but also during the aging process in WT and daf-2 mutant animals (Fig. 7 A; Halaschek-Wiener et al., 2005; Walker et al., 2001). Here, we show that RNAi knockdown of hsp-16.2 and hsp-16.48 reduces the longevity of daf-2 mutants. The specific impact of these sHsp sequestrases is consistent with their particularly strong upregulation in daf-2 mutants (Halaschek-Wiener et al., 2005). Both sHsps revealed functional overlap as a double RNAi knockdown further shortened the lifespan of daf-2 animals. The functional redundancy of sequestrase-positive sHsps explains why the reduction of individual sequestrases had little or no effect in daf-2 animals and suggests that the combined level of sHsp sequestrases is most important for the cytoprotective effects. Accordingly, extra copies of Hsp-16.1 modestly increase longevity of WT animals, in which sequestrase levels are considerably lower than in daf-2 mutants (Walker and Lithgow, 2003).
Our results extend previous findings documenting the roles of other sHsps (e.g., Sip-1) in daf-2 lifespan extension (Hsu et al., 2003). Of note, the long-lived C. elegans age-1 mutants also exhibit increased expression of sequestrase-positive sHsps (Hsp-16.1, Hsp-16.2, and Hsp-16.48), which contributes to lifespan extension (Morley and Morimoto, 2004; Seo et al., 2013). This suggests that diverse aging pathways enhance sHsp sequestrase activity as a strategy to improve cellular proteostasis. We infer that the sequestrase function of these sHsps sets the basis for their role as longevity proteins (Hsu et al., 2003). This role might not be restricted to C. elegans as overexpression of diverse sHsps also extends lifespan in flies (Aigaki et al., 2002; Morrow et al., 2004; Vos et al., 2016).
Why is protein sequestration cytoprotective and particularly in demand in aging animals? Cellular aging of C. elegans WT animals does not cause upregulation of major ATP-dependent chaperones including Hsp70, Hsp90, and TRiC/CCT (Walther et al., 2015). Similarly, the ability of cells to react to proteotoxic stress diminishes with age and the capacity of proteostasis networks ultimately declines (Ben-Zvi et al., 2009; Douglas and Dillin, 2010). We, therefore, envision two possibilities for how sHsp sequestrase activity exerts beneficial effects on protein homeostasis in aged cells. First, sHsps deposit toxic aberrant proteins that might accumulate in aged cells, making them more inert. Second, the sequestration of misfolded proteins relieves major chaperone systems (e.g., Hsp70), allowing them to fulfill housekeeping activities and boosting proteostasis capacity. Notably, aged C. elegans animals reduce protein synthesis, which has been linked to increased lifespan (Kirstein-Miles et al., 2013; Laplante and Sabatini, 2012). A reduction in the number of nascent polypeptides can also reduce the burden on major chaperone systems. Lowering the amount of soluble, non-native proteins by either reduction of protein synthesis or sHsp-mediated sequestration might therefore represent adaptive and ATP-independent measures of aged organisms to balance the decline of proteostasis networks.
Materials and methods
Yeast strains, plasmids, and growth conditions
All yeast strains and plasmids used in this study are listed in Tables S1 and S2, respectively. Yeast genome was edited using the homologous recombination technique as described (Janke et al., 2004). In brief, desired genomic modifications were PCR amplified using primer pairs listed in Table S3 with flanking sequences homologous to the target genomic loci and suitable selection marker. PCR products were transformed into yeast cells. For transformation, yeast cells were grown in a suitable media (YPD or synthetic complete) until log phase growth (OD600 = 0.8) and then harvested by centrifugation (4,000 g). The cell pellet from 10 ml of culture was used for one transformation experiment. The pellet was first washed with 5 ml dH2O, then with 2.5 ml LiSorb buffer (100 mM LiOAc, 10 mM Tris pH 8.1 mM EDTA, 1 M Sorbitol, filter sterilized), and was finally resuspended in 50 μl LiSorb buffer. The desired DNA for transformation (10 μl PCR product or 100 ng plasmid) and 10 μl Yeastmarker Carrier DNA (Clontech Laboratories) were added to the resuspended pellet and incubated at RT for 15 min. Then 30 μl of LiPEG buffer (100 mM LiOAc, 10 mM Tris pH 8.1 mM EDTA, 50% [w/v] PEG 3350, filter sterilized) was added and further incubated at RT for 15 min. A total of 30 μl of DMSO was then added and the suspension was heat-shocked (WT or wt-like growing cells: 42°C, 10 min; ΔΔbtn2Δ and ΔΔhsp42Δ cells: 35°C, 20 min). Yeast cells were selected for appropriate markers (auxotrophy or drug resistance) and the correct integration of the PCR product was verified by PCR using primer pairs (listed in Table S3) that anneal within the amplicon and upstream or downstream of the target locus.
Yeast cells were grown in liquid YPD medium or synthetic dropout (SD) media at indicated temperatures. SD media was supplemented with either 2% (w/v) glucose (Glu) or 2% (w/v) raffinose (Raf)/galactose (Gal). Growth plates contained bacto-agar made to a final concentration of 2% (w/v).
Protein purifications
Hsp-16.1 and Hsp-12.1 were purified after overproduction at 15°C in E. coli MH1 cells using cold shock inducible pCool6-derived expression vectors. All sHsps were produced as His6-SUMO fusions and were purified using Protino Ni-IDA (Macherey-Nagel). Proteins were washed on the Ni-IDA column with high salt buffer A (50 mM Na-phosphate pH 8.0, 300 mM NaCl, 1 mM ATP, 1 mM MgCl2) and low salt buffer B (50 mM Tris pH 8.0, 25 mM NaCl) and eluted with low salt elution buffer C (50 mM Tris pH 8.0, 25 mM NaCl, 250 mM imidazole). Protein-containing fractions were pooled and the His6-SUMO tag was removed by cleavage with Ulp1 protease for 1 h at room temperature, followed by anion exchange chromatography (ResourceQ; Cytiva) with buffer B and a gradient of 20 CV to a salt concentration of 500 mM. Protein-containing fractions were pooled and concentrated by dialysis against Aquacide II powder (Merck).
Light scattering assays
(i) Prevention of aggregation: 250 nM citrate synthase (CS) or 250 nM firefly luciferase were incubated in buffer D (25 mM Tris pH 7.5, 15 mM KCl, 1 mM CaCl2, 2 mM DTT) in the presence or absence of varying ratios of sHsps at 43°C (CS) and 42.5°C (luciferase). Turbidity was measured at an excitation and emission wavelength of 600 nm (CS) or 590 nm (luciferase) using a Perkin-Elmer spectrometer LS50B. (ii) Aggregase assay: Unfolding of 250 nM chicken lysozyme (LZ) was induced upon the addition of 0.5 mM TCEP in 25 mM Tris pH 7.5, 15 mM KCl, and 1 mM CaCl2 at 37°C. Turbidity was measured at 600 nm in the absence or presence of sHsps.
Supernatant/pellet assay
500 nM citrate synthase in buffer D was incubated for 90 min at 43°C in the presence or absence of sHsps followed by centrifugation (14,000 rpm, 30 min, 4 C). Supernatant and pellet fractions were separated and analyzed by SDS–PAGE followed by SYPRO Ruby (Invitrogen) staining.
Oligomeric state of sHsps
Assembly sizes of sHsps (1 µM) were determined by size exclusion chromatography (S200; GE Healthcare) in buffer E (50 mM Tris pH7.5, 20 mM NaCl, 5% glycerol [v/v], 2 mM DTT, pH 7.5). Molecular weights and oligomeric states of sHsps were calculated from a calibration curve generated from a size exclusion standard (Biorad).
C. elegans strains and maintenance
All strains used or generated in this study are listed in Table S4. Animals were cultured using standard methods (Brenner, 1974). If not otherwise indicated, worms were grown on nematode growth medium (NGM) plates seeded with E. coli strain OP50 at 20°C. Age-synchronization was achieved by bleaching or egg laying as previously described (Nussbaum-Krammer et al., 2015). For bleach-synchronization, gravid adults were dissolved in 20% sodium hypochlorite solution. The surviving C. elegans embryos were washed with M9 buffer two times and let to hatch with gentle rocking in M9 buffer at 20°C overnight. The next day, L1 larvae were distributed onto NGM or RNAi plates and grown at 20°C. Alternatively, animals were age-synchronized by egg laying (Nussbaum-Krammer et al., 2013); adult animals were allowed to lay eggs for 2–3 h and removed again from the plates. The remaining embryos were grown to the desired age.
Preparation of NGM and RNAi plates
Per liter standard NGM 15 g Bacto-Agar (BD Biosciences), 2.5 g Bacto-Peptone (BD Biosciences), and 3 g NaCl were made upto 1 liter using distilled water and autoclaved. The media was cooled to 60°C in a water bath and 1 ml of 1 M MgSO4, 25 ml of 1 M phosphate buffer (pH = 6), 1 ml of 1 M CaCl2, and 1 ml of 5 mg/ml cholesterol dissolved in EtOH were added while stirring. Plates were poured next to a flame and left to dry at RT for 2 to 3 d before seeding with overnight cultures of OP50 E. coli.
For RNAi plates, 1 ml of 100 mg/ml Ampicillin, 2.5 ml 5 mg/ml Tetracycline, and 240 mg IPTG freshly dissolved in 5 ml dH2O and sterile-filtered were added in addition to the NGM after autoclaving. RNAi plates were dried for 1 to 2 d at RT protected from light before being seeded.
Generation of transgenic animals
The genomic hsp-16.2 and hsp-16.48/hsp-16.49 loci were tagged with mCherry at the C-terminus following a published CRISPR/Cas9 protocol (Dokshin et al., 2018). In short, the mCherry ORF containing three artificial C. elegans introns was ordered as gBlocks dsDNA fragment from Thermo Fisher Scientific. Target-specific DNA donors were generated by PCR. sgRNAs were designed using the online tool CCTop (Stemmer et al., 2015), and corresponding crRNAs and tracrRNAs were ordered from Thermo Fisher Scientific and then assembled into sgRNAs. sgRNAs were preincubated with the TrueCut Cas9 Protein v2 (Thermo Fisher Scientific) and injected into young adult N2 hermaphrodites together with the dsDNA donor. After injection, the animals were singled onto individual plates and the progeny was screened 3 d later for successful endogenous tagging by induction of mCherry fluorescence after mild heat shock. Positive animals were singled and examined for potential mutations at the insertion side by PCR amplification of the gene locus and sequencing. Correctly tagged animals were outcrossed into WT N2s three times and subsequently crossed into the daf-2 strain. Since hsp-16.48 and hsp-16.49 are identical in their cDNA sequence, both genes were tagged. All primers, RNA sequences, and the nucleotide sequence of the mCherry tag are listed in Table S3.
Yeast growth assays
Spot test: Indicated yeast strains were grown in liquid YPD medium until log phase (OD600 = 0.6 – 1.0) at 25°C. All cultures were normalized to OD600 = 0.2. A dilution series of cultures was prepared in a sterile 96-well microtiter plate with five fivefold dilution steps. The resultant dilution series was spotted on YPD plates using a pinning tool. Spots were first allowed to be absorbed on plates, and then plates were incubated for 3 d at indicated temperatures.
Liquid growth assay: Indicated yeast strains were grown in YPD medium until log phase (OD600 = 0.6 – 1.0) at 25°C. All cultures were normalized to OD600 = 0.2 with a final volume of 1 ml in a 24-well sterile plate. Yeast growth curves were measured in a temperature-controlled microplate reader (SPECTROstar Nano; BMG Labtech) at either 30 or 33°C. Culture growths (OD600 value) were monitored every 6 min for the next 25 h, and cultures were mixed well by orbital shaking before each reading. Statistic tests were done using GraphPad Prism 5 software.
C. elegans lifespan assay
RNAi-mediated knockdown of target genes was achieved by feeding nematodes with E. coli HT115(DE3) bacteria that express the corresponding dsRNA. The respective bacteria were selected from the Ahringer RNAi library (Source BioScience; Kamath and Ahringer, 2003). Since hsp-16.48 and hsp-16.49 are identical in their cDNA sequence, both are targeted by the hsp-16.48 RNAi clone. RNAi plates were prepared as described previously (Sandhof et al., 2020). In the case of double knockdown experiments, the RNAi cultures were adjusted to the same OD and mixed in a 1:1 ratio before seeding. To keep single knockdown conditions comparable to the double knockdown, the respective single knockdown plates were seeded with RNAi cultures diluted with the empty vector control to the same OD as present on double RNAi plates. Adult-only RNAi lifespan was carried out as described in Sandhof et al. (2020). In short, animals were synchronized by egg-laying on OP50 plates. Two days later roughly 100 L4 larvae per genotype and knockdown were transferred to RNAi or empty vector control plates. Animals were passaged to fresh plates every second day until progeny production ceased and later only when necessary to avoid starvation or when plates started to dry out. Animals were checked for viability during passaging and subsequently every other day by gentle prodding of the head. Animals that exhibited internal hatching, an exploding vulva phenotype, that burrowed into the agar or crawled up the petri dish side were censored from the lifespan assay and included as such in the statistical analysis. All experiments were performed independently two to three times. Kaplan-Meier estimates and median lifespan of survival data were extracted with GraphPad Prims version 6.0 h and tested for significant differences using the log-rank test. Data of all lifespan assays conducted are summarized in Table S5.
Co-immunoprecipitation
Yeast cells expressing FLAG-tagged sHsps and GFP-VHL were grown at 25°C (30°C for btn2∆ hsp42∆ strains) for 24 h in SC-Leu (2% Raf) media. Cultures were diluted in SC-Leu (2% Raf + 2% Gal) as such that their growth at 25°C (30°C for btn2∆ hsp42∆ strains) after 18 h reached log phase (OD600 = 0.6 – 1.0). Cultures were then shifted to 30°C for 1 h (38°C for 30 min in case of btn2∆ hsp42∆ strains), harvested by centrifugation (1,300 g, 4°C, 5 min), resuspended in 1 ml lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, cOmplete EDTA free protease inhibitors), and snap-frozen as drops in liquid nitrogen. Cell lysis was achieved by mixer milling (frequency = 30/s and time = 2 min). Pulldown of sHsps was performed with ANTI-FLAGM2 affinity gel (Sigma-Aldrich). A total of 50 μl of bead slurry was used for individual pulldown reactions. Beads were first washed two times with 1 ml TBS buffer, including complete EDTA-free protease inhibitors, and their sedimentation was achieved by centrifugation (5,000 g, 30 s). A total of 1.5 mg of total protein extract (after removing cell debris through centrifugation) from individual cell culture was mixed with sedimented beads and the total volume was normalized to 1 ml using TBS-I buffer. The reaction tube was then incubated at 4°C for 1 h in a turning wheel for facilitating binding. Beads were then sedimented and washed three times with 1 ml TBS-I buffer at RT. During each wash step, beads were transferred to a fresh microcentrifuge tube. After the final sedimentation, beads were mixed with sample buffer and heated at 95°C for 5 min to elute bound proteins, which were analyzed by SDS-PAGE and Western blot.
Microscopy and image processing
Yeast cells were harvested by centrifugation (1,000 g, 4°C, 3 min) and fixed with 70% (v/v) ethanol for 5 min on ice. Cells were washed once with sterile deionized water and suspended in 50 μl PBS. DNA was stained by the addition of either DAPI or Hoechst 33442 in the cell suspension to a final concentration of 50 ng/ml or 20 μg/ml, respectively. Fixed cells were imaged as stacks of optical sections with 0.2 μm width using the Olympus widefield microscope (XCellence IX81) equipped with a MT20 illumination system. Images were acquired for each channel using an EM-CCD C9100-02 camera (Hamamatsu) and a UApo N 100×/1.49 oil immersion objective. All yeast cell image acquisition was done at RT. Images were later deconvolved with XCellence software (Olympus) using the Wiener filter and further processed using ImageJ software (NIH).
Synchronized C. elegans were mounted on 10% agarose pads and immobilized in a 2.5 mM levamisole solution containing 100 nm nanosphere size standard polystyrene beads as described before (Sandhof et al., 2020). Microscopy was performed using a Leica DMi8SD AF spinning disc microscope (Leica Microsystems) equipped with a 561 nm diode laser (50 mW) and the MetaMorph Advanced Acquisition software (Molecular Devices). Images were acquired for each channel using an Orca Flash 4.0 LT (C11440-42U) camera (Hamamatsu) and a HC PL APO 63×/1.40 (NA)-0.60 oil objective. Whole animal microscopy was done using an Olympus IXplore83 Spin SR system (Olympus) equipped with a Coherent OBIS LX 561 nm laser (100 mW) and the cellSens Dimension Version 1 software (Olympus), including the CS-S-MP-VF Multi Position module (Olympus). Images were acquired using a 60× UPLSAPO60XS2 NA 1.3 objective (Olympus) in combination with an Hamamatsu CAM-ORCA-FLASH4.0V3 camera (Hamamatsu). All the imaging of C. elegans was done at RT. Further processing of images was done using the Fiji distribution of the ImageJ software. Laser power and exposure times of images acquired at the same magnification were kept identical between genotypes and ages. Acquired z-stacks were flattened using maximum z-projections and are shown with the same display range to enable comparison of the fluorescently tagged sHsp signal intensities.
Immunofluorescence microscopy
Yeast cells were grown and treated as indicated. At different times of treatment, cell cultures were fixed with 4% PFA for 1 h before cell wall digestion. To achieve this, 37% formalin solution (Merck) was added to the culture to reach a final concentration of 4%. The cell suspension was gently mixed by inverting the microcentrifuge tube and then incubated at RT for 15 min. Cells were pelleted (1,000 g, 4°C, 3 min), resuspended in freshly prepared 4% PFA % ([w/v] paraformaldehyde in 100 mM KPi pH 6.5), and incubated further for 1 h at RT in a turning wheel. Cells were washed three times after fixation with 100 mM KPi pH 6.5 and once with wash buffer (100 mM KPi pH 6.5, 1.2 M Sorbitol). The cell wall was digested for 30 min at 30°C using 500 μg/ml Zymolase T-100 in wash buffer added with 20 mM ß-mercaptoethanol. The resultant spheroblasts were attached to poly-lysine-coated microscopy slides, washed three times with wash buffer (plus 1% Triton X-100), and blocked with 1% (w/v) BSA in 100 mM KPi pH6.5 for 1 h. Both primary and secondary antibody dilutions were made in the blocking buffer. Incubation with primary antibody was done either for 1 h at RT or overnight at 4°C. Cells were then washed two times with blocking buffer and subsequently incubated with secondary antibody for 1 h at RT. Following incubation with secondary antibody, cells were stained with 50 ng/ml DAPI and washed with PBS. Finally, cells were embedded in 55% (v/v) glycerol and coverslips were sealed with nail polish.
Protein extraction and Western blot
Log phase yeast cultures were harvested through centrifugation, and total protein extracts were prepared as described previously. In short, 200 μl 1.85 M NaOH was added to 900 μl culture sample and incubated for 10 min on ice. Then 200 μl of 55% TCA was added and mixed gently by inverting the sample tubes. The suspension was incubated further for 10 min on ice. Cells were pelleted using centrifuge (13,000 g) and later resuspended in 100 μl HU buffer (8 M Urea, 5% SDS, 200 mM Tris pH 6.8, 1 mM EDTA, 1.5% [w/v] DDT, 0.1% [w/v] bromophenol blue) per 1 OD600 equivalent of sample culture. Samples were incubated at 65°C for 10 min before analysis by SDS-PAGE.
Western blot analysis: Proteins resolved by SDS-PAGE were transferred to PVDF membranes by TransBlot Turbo semi-dry blotter from Bio-Rad. Transferred membranes were then blocked with 3% BSA (w/v) in TBS-T. Specific primary and secondary antibodies (conjugated with Alkaline Phosphatase) were used to detect proteins of interest (antibodies are listed in Table S6). Protein bands were visualized by probing the membrane with ECF reagent (GE Healthcare) and later imaging with ImageQuant LAS-5000 (Fujifilm) and the software Image-Reader LAS-4000 (Fujifilm).
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 6 software. Tests used to analyze differences between conditions for statistical significance are indicated in the corresponding figure legends. Data distribution was assumed to be normal but was not formally tested.
Online supplemental material
Fig. S1: Domain organization of all sHsps tested in this study and expression of C. elegans sHsps in yeast sequestrase mutant cells. Fig. S2: Identification of C. elegans sHsps that rescue growth defects of yeast sequestrase mutants. Fig. S3: C. elegans and human sHsps rescue defects of yeast sequestrase mutants. Fig. S4: Dissection of Hsp-16.1 sequestration activity in yeast sequestrase mutants. Fig. S5: Analysis of the function of the disordered N-terminal extension of Hsp-16.1 in protein sequestration. Fig. S6: Characterization of Hsp-16.1 and Hsp-12.1 chaperone activities in vitro. Fig. S7: Role of sequestrase-positive sHsps in C. elegans’ lifespan and their age-dependent expression in diverse tissues. Table S1 summarizes the diverse yeast strains used in this study. Table S2 summarizes the diverse plasmids used for yeast studies. Table S3 summarizes the primers used for genetic manipulation of C. elegans and yeast. Table S4 summarizes the diverse C. elegans animals used in this study. Table S5 summarizes the results of all C. elegans lifespan experiments. Table S6 summarizes the antibodies used in this study.
Acknowledgments
We thank Chi-Ting Ho for valuable help and support during the initial phase of the project, and Regina Zahn and Silke Druffel-Augustin for excellent technical support. We acknowledge the support of A. Shrivastava, C.A. Sandhof, and K. Reinle by the Heidelberg Biosciences International Graduate School of Molecular and Cellular Biology (HBIGS), and A. Jawed by the Helmholtz International Graduate School for Cancer Research of the DKFZ.
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - Project-ID 201348542 - SFB1036 (TP8 to A. Mogk and B. Bukau and TP20 to C. Nussbaum-Krammer) and BU617/20 to B. Bukau and MO970/6-1 to A. Mogk. This work was supported by the Helmholtz Zukunftsthema Aging and Metabolic Programming (AMPro) ZT-0026.
The authors declare no competing financial interests.
Author contributions: Conceptualization: A. Shrivastava, C. Nussbaum-Krammer, A. Mogk, B. Bukau: Methodology: A. Shrivastava, C.A. Sandhof, K. Reinle, A. Jawed: Formal analysis: A. Shrivastava, C.A. Sandhof, K. Reinle. A. Jased: Investigation: A. Shrivastava, C.A. Sandhof, K. Reinle. A. Jased, C. Ruger-Herreros, D. Schwarz, D. Creamer: Writing original draft: C. Nussbaum-Krammer, A. Mogk, B. Bukau: Writing—Review & Editing: A. Mogk, B. Bukau: Visualization: A. Shrivastava, C.A. Sandhof, K. Reinle. A. Jased, A. Mogk: Supervision: C. Nussbaum-Krammer, A. Mogk, B. Bukau: Funding acquisition: C. Nussbaum-Krammer, A. Mogk, B. Bukau.
References
Author notes
Dominic Schwarz’s present address is Institute of Pharmacy and Molecular Biotechnology, University of Heidelberg, Heidelberg, Germany.
Declan Creamer’s present address is Division of Molecular and Cellular Function, School of Biological Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK.
Carmen Nussbaum-Krammer’s present address is Department of Anatomy II, Ludwig-Maximilians-University (LMU) Munich, Munich, Germany.





![Rescue of yeast sequestrase mutant phenotypes by C. elegans and human sHsps. (A)S. cerevisiae WT and indicated mutant strains with or without indicated C. elegans sHsps were grown in YPD media at 25°C. Log phase cultures were normalized to OD600 = 0.2, shifted to 33°C, and the growth (OD600 value) of each yeast strain was monitored for 25 h. (B) The OD600 value for each strain at the time point when the hsp104∆ fes1∆ reference cell culture reached saturation was noted (see [I]) and plotted as a bar graph normalized to the OD600 value corresponding to ∆∆hsp42∆ cells without complementing C. elegans sHsp. A growth ratio of 1 denotes that the growth curves of ∆∆hsp42∆ cells with and without C. elegans sHsp are equal. Error bars represent ± SD calculated based on n = 3 independent experiments. One-way ANOVA test (Dunnett) was used to assess the statistical significance (*, P < 0.05, ****, P < 0.0001). Data distribution was assumed to be normal, but this was not formally tested. (C) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ and ∆∆hsp42∆ cells expressing Hsp-16.1 or Hsp-12.1 (tagged or non-tagged) were spotted on YPD plates and incubated at indicated temperatures for 3 d. The dashed line indicates that spot tests from different plates are cropped and shown together for comparative analysis. (D) Fivefold serial dilutions of S. cerevisiae WT and indicated mutant strains were done as described in (C and F). (E) Western blot depicting the levels of E. coli IbpA and C. elegans Hsp-12.1 and Hsp-16.1 that served as reference in ∆∆btn2∆ cells. α-FLAG and α-Zwf1 antibodies were used to probe for sHsp and Zwf1 (loading control) levels respectively. (F) Fivefold serial dilutions of S. cerevisiae wt, hsp104∆ fes1∆ and ∆∆btn2∆ cells expressing human sHsps were spotted on YPD plates and incubated at indicated temperatures for 3 d. The dashed line indicates that spot tests from different plates are cropped and shown together for comparative analysis. (G)S. cerevisiae ∆∆btn2∆ cells strains with or without indicated human sHsps were grown in YPD media at 25°C. Log phase cultures were normalized to OD600 = 0.2, shifted to 30 or 33°C and then growth (OD600 value) of each yeast strain was monitored for 25 h. The growth ratio was determined as described in (J). OD600 value for each strain at the time point when the hsp104∆ fes1∆ reference cell culture reached saturation was noted and plotted as a bar graph normalized to the OD600 value corresponding to ∆∆btn2∆ ells without complementing human sHsp. A growth ratio of >1 indicates improved growth upon expression of humans sHsps. Error bars represent ± SD calculated based on n = 3 independent experiments. A threshold ratio of 1.5 was defined and a one-way ANOVA test (Dunnett) was used to assess the statistical significance (****, P < 0.0001) only for those sHsps passing the threshold. (H) Western blot analysis depicting the levels of all human sHsps in ∆∆btn2∆ cells. α-FLAG and α-Zwf1 antibodies were used to probe for sHsp and Zwf1 (loading control) levels, respectively. (I)S. cerevisiae ∆∆ and ∆∆btn2∆ cells expressing selected human sHsps and GFP-VHL (green) were grown at 25°C and shifted to 30°C for 1 h. Temperature-upshifted samples were collected and analyzed by fluorescence microscopy. DNA was stained by DAPI. Maximum intensity Z-projection images of selected growth complementing and non-complementing human sHsps are shown. Scale bar: 2 μm. (J) Cellular localization of GFP-VHL in respective yeast cells was quantified (n > 100; 2 replicates with similar outcome) and categorized into diffuse staining, organized inclusions (1–3 foci/cell), and multiple inclusions (>3 foci/cell). Source data are available for this figure: SourceData FS3.](https://cdn.rupress.org/rup/content_public/journal/jcb/221/10/10.1083_jcb.202202149/1/m_jcb_202202149_figs3.png?Expires=1773754754&Signature=jCqacmOOWffOPvxTYRkCGPNfN5GD6VKEBU3BWcFQpmXj0r-Q7czLUHryRhVnPuq9N3efPAAjtsEil3t8-wD4cHJJoc28GUJL7FjFrHvQhL7T2sIXPK10uFT3JSM9djeHT9W256XWU51~Sd~1GCsvg~xiscfMW6LShVANlOcPaaveOFhfnzvogVyoxC65oQyVkZm8g5t1IhBYn9RSbtfNIMEwtb~i0CDCzfQNrc-px8D~zIz~gJkuvwqpwFV7TOjsO7R3IGqnpAiQVlofWkG2j5ZrcFr~MJ9FqAX8ashBTBdo0GU-yeaiobJoGEtBzAXbARqXGb8UpTBjr4sGCEN4Rg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)