The signaling adaptor TNFR-associated factor 1 (TRAF1) is specifically lost from virus-specific CD8 T cells during the chronic phase of infection with HIV in humans or lymphocytic choriomeningitis virus (LCMV) clone 13 in mice. In contrast, TRAF1 is maintained at higher levels in virus-specific T cells of HIV controllers or after acute LCMV infection. TRAF1 expression negatively correlates with programmed death 1 expression and HIV load and knockdown of TRAF1 in CD8 T cells from viral controllers results in decreased HIV suppression ex vivo. Consistent with the desensitization of the TRAF1-binding co-stimulatory receptor 4-1BB, 4-1BBL–deficient mice have defects in viral control early, but not late, in chronic infection. TGFβ induces the posttranslational loss of TRAF1, whereas IL-7 restores TRAF1 levels. A combination treatment with IL-7 and agonist anti–4-1BB antibody at 3 wk after LCMV clone 13 infection expands T cells and reduces viral load in a TRAF1-dependent manner. Moreover, transfer of TRAF1+ but not TRAF1− memory T cells at the chronic stage of infection reduces viral load. These findings identify TRAF1 as a potential biomarker of HIV-specific CD8 T cell fitness during the chronic phase of disease and a target for therapy.
Immune dysregulation is a hallmark of chronic viral infection (Virgin et al., 2009). Chronic infection with human immunodeficiency virus (HIV) or hepatitis C virus in humans, or with lymphocytic choriomeningitis virus (LCMV) clone 13 in mice, results in up-regulation of inhibitory receptors such as programmed death 1 (PD-1) and TIM-3 on effector T cells, as well as the sustained production of immune regulatory cytokines such as TGFβ and IL-10 (Barber et al., 2006; Day et al., 2006; Freeman et al., 2006; Petrovas et al., 2006; Trautmann et al., 2006; Urbani et al., 2006; Brooks et al., 2008; Jones et al., 2008; Tinoco et al., 2009; Jin et al., 2010). It is thought that these regulatory mechanisms minimize immune pathology, but also contribute to the inability of the immune system to control viral load during progressive HIV infection.
T cell responses are controlled by a balance between stimulatory and inhibitory signaling pathways (Sharpe, 2009). This raises the question of why co-stimulation fails to overcome the effects of inhibitory signals on T cells during chronic infection. In this study, we show that during chronic infection a co-stimulatory pathway involving the TNFR family member 4-1BB is desensitized through loss of its signaling adaptor, TRAF1.
4-1BB signals by recruiting two TNFR-associated factors, TRAF1 and TRAF2 (Arch and Thompson, 1998; Jang et al., 1998; Saoulli et al., 1998). TRAF2 is a ubiquitously expressed protein that is required for NF-κB and mitogen-activated protein kinase activation downstream of several TNFR family members, including 4-1BB (Aggarwal, 2003). TRAF1 is an NF-κB–inducible protein with low expression in resting cells, and is primarily found in cells of the immune system (Lee and Choi, 2007). In T cells, overexpression of TRAF1 results in delayed contraction of LCMV-specific CD8 T cells (Speiser et al., 1997), and deficiency of TRAF1 impairs the survival of activated and memory CD8 T cells (Sabbagh et al., 2006, 2008; Wang et al., 2007).
In this study, we provide evidence that TRAF1 levels are significantly lower in HIV-specific CD8 T cells from chronically infected as compared with recently infected donors or viral controllers. Similarly, during chronic infection of mice with LCMV clone 13, TRAF1 is lost from virus-specific T cells between day 7 and 21 of infection. In contrast, TRAF1 protein is maintained at higher levels in memory T cells after acute infection with the Armstrong strain of LCMV. We show that the decreased TRAF1 expression can have functional consequences. Knockdown of TRAF1 in CD8 T cells from HIV controllers results in a decrease in T cell–dependent viral suppression and impairs HIV-specific, 4-1BB–dependent CD8 T cell responses. In addition, transfer of TRAF1-expressing, but not TRAF1-deficient, P14 memory CD8 T cells improves viral control at the chronic stage of clone 13 infection. Moreover, TRAF1-deficient mice show impaired responses to agonistic anti–4-1BB antibody treatment. Finally, 4-1BBL–deficient mice show early defects in T cell numbers and viral control, whereas these effects are lost at late time points consistent with the desensitization of the 4-1BB signaling pathway through loss of TRAF1. Together, these results identify a novel mechanism of immune dysfunction during chronic HIV infection through the posttranscriptional loss of a signaling adaptor from the virus-specific T cells, resulting in desensitization of a co-stimulatory pathway.
RESULTS
Defective TRAF1 expression during chronic HIV infection
As TRAF1 is critical for 4-1BB–induced survival signaling (Wang et al., 2007; Sabbagh et al., 2008), we examined TRAF1 expression in HIV-specific T cells from recently and chronically infected donors (Table S1) using flow cytometry (as described in Fig. S1 a). The proportion of HIV-tetramer+ T cells expressing TRAF1 was significantly lower in individuals at the chronic as compared with the early stage of the infection, whereas viral controllers showed an intermediate phenotype (Fig. 1 a). TRAF1 is expressed at low levels in resting T cells and inducible upon activation (Fig. S1 b); therefore, the level of activation of the T cells could be a confounding factor in comparing the different groups. To address this issue, we measured expression of the activation marker CD38 on the HIV-specific (tetramer+) T cells (Fig. S1 c). The early and chronic infection groups showed similar levels of CD38 expression and viral load, whereas the viral controllers showed lower CD38 expression, consistent with their lower viral load (Fig. S1 d). Overall, the frequency of TRAF1-expressing cells correlated with the frequency of activated T cells, as measured by CD38 staining (Fig. S1 e). Analysis of the frequency of TRAF1+ versus CD38+ HIV-specific CD8 T cells shows that HIV-specific T cells in viral controllers have higher levels of TRAF1 for a given level of activation than T cells from chronic progressors (P = 0.009; Fig. 1 b). We also followed TRAF1 levels longitudinally in three donors for whom consecutive samples were available (Fig. 1 c). We observed a 20–83% loss of TRAF1 over time for the 5 HIV epitopes examined (Fig. 1 c), despite a similar level of activation as measured by CD38 levels (unpublished data). These results demonstrate that TRAF1 levels decrease in HIV-specific CD8 T cells over time.
Loss of TRAF1 protein from HIV-specific T cells during chronic HIV infection. Freshly thawed PBMCs from HIV-infected individuals were stained for CD8, CD3, TRAF1, HIV-tetramer, and CD38 (a-d), and flow cytometry data were analyzed with the gating strategy described in Fig. S1. (a) Frequency of TRAF1+ HIV-specific CD8 T cells in recently or chronically infected donors or viral controllers (groups defined in Table S1). (top) Summary of the frequency of TRAF1+ HIV-tetramer+ cells for all donors analyze with each symbol representing a single epitope, with one to two epitopes per donor. Statistical analysis was performed by one-way ANOVA. (bottom) Representative histograms from each group, with shaded panels indicating FMO controls, open histograms indicating TRAF1 staining on CD3+CD8+tetramer+ T cells. Note: some donors showed bimodal staining for TRAF1 on their HIV-specific T cells. (b) Correlation between the frequency of TRAF1+ HIV-specific T cells and the level of activation as measured by the frequency of CD38+ HIV-specific T cells. Both viral controllers and chronic progressors are included in this analysis. Statistical analysis was performed using linear regression (P = 0.009). (c) TRAF1 expression in HIV-specific T cells using longitudinal samples from three donors with five HIV-specific epitopes. (top) Representative TRAF1 staining during early and later time point within the same donor (shown for two donors); (bottom) Summary plot with each donor represented by a different symbol and filled and open symbols distinguishing two epitopes within the same donor. FMO controls are indicated in the shaded histograms. (d) Frequency of TRAF1+ T cells of total CD8 T cells for the three groups of donors. (e) TRAF1 expression in total CD8+CD45RA− T cells before and after activation. Each symbol represents an individual donor. PBMCs were labeled with CFSE and stimulated with 1 µg/ml of anti-CD3 and 10 µg/ml of anti-CD28 for 6 d, and then stained for CD3, CD8, and TRAF1. The frequency of TRAF1+ T cells on the CFSE low (divided) CD8+CD45RA− population is reported for the stimulated cells (right axis). For the unstimulated cells, the frequency of TRAF1+ in the CD8+CD45RA− T cell population is reported (left axis). VC, viral controller.
Loss of TRAF1 protein from HIV-specific T cells during chronic HIV infection. Freshly thawed PBMCs from HIV-infected individuals were stained for CD8, CD3, TRAF1, HIV-tetramer, and CD38 (a-d), and flow cytometry data were analyzed with the gating strategy described in Fig. S1. (a) Frequency of TRAF1+ HIV-specific CD8 T cells in recently or chronically infected donors or viral controllers (groups defined in Table S1). (top) Summary of the frequency of TRAF1+ HIV-tetramer+ cells for all donors analyze with each symbol representing a single epitope, with one to two epitopes per donor. Statistical analysis was performed by one-way ANOVA. (bottom) Representative histograms from each group, with shaded panels indicating FMO controls, open histograms indicating TRAF1 staining on CD3+CD8+tetramer+ T cells. Note: some donors showed bimodal staining for TRAF1 on their HIV-specific T cells. (b) Correlation between the frequency of TRAF1+ HIV-specific T cells and the level of activation as measured by the frequency of CD38+ HIV-specific T cells. Both viral controllers and chronic progressors are included in this analysis. Statistical analysis was performed using linear regression (P = 0.009). (c) TRAF1 expression in HIV-specific T cells using longitudinal samples from three donors with five HIV-specific epitopes. (top) Representative TRAF1 staining during early and later time point within the same donor (shown for two donors); (bottom) Summary plot with each donor represented by a different symbol and filled and open symbols distinguishing two epitopes within the same donor. FMO controls are indicated in the shaded histograms. (d) Frequency of TRAF1+ T cells of total CD8 T cells for the three groups of donors. (e) TRAF1 expression in total CD8+CD45RA− T cells before and after activation. Each symbol represents an individual donor. PBMCs were labeled with CFSE and stimulated with 1 µg/ml of anti-CD3 and 10 µg/ml of anti-CD28 for 6 d, and then stained for CD3, CD8, and TRAF1. The frequency of TRAF1+ T cells on the CFSE low (divided) CD8+CD45RA− population is reported for the stimulated cells (right axis). For the unstimulated cells, the frequency of TRAF1+ in the CD8+CD45RA− T cell population is reported (left axis). VC, viral controller.
To determine whether the differences in TRAF1 expression were unique to the HIV-specific T cells, we measured the TRAF1 expression in total CD8 T cells directly ex vivo. We observed no differences in the overall frequency of TRAF1+CD8+ T cells between the groups (Fig. 1 d), in contrast to the differences observed when the HIV-specific (tetramer+) CD8 T cells were examined (Fig. 1 a). Moreover, for the longitudinal samples, there was slightly higher TRAF1 expression in total CD8 T cells at the chronic stage of the response than at the acute stage, perhaps reflecting bystander activation (Fig. S1 f). In several donors who were co-infected with CMV or EBV, we analyzed the TRAF1 expression in their CMV or EBV-specific (tetramer+) CD8 T cells. Although the sample size is too small for the result to be conclusive, we observed a trend toward a higher percentage of CMV or EBV-specific CD8 T cells expressing TRAF1 in chronic as compared with HIV viral controllers, perhaps reflecting reactivation of CMV or EBV during the chronic stage of HIV infection (Fig. S1 g).
We next examined the potential of T cells in donors from each group to up-regulate TRAF1 in response to T cell receptor signaling. All donors examined showed similar up-regulation of TRAF1 in their total CD45RA− memory population in response to anti-CD3/anti-CD28 signaling, regardless of their stage or control of HIV infection (Fig. 1 e). We conclude from these studies that TRAF1 is lost specifically from the HIV-specific CD8 T cells of chronically infected donors over time of infection, but is maintained in a higher frequency of cells and at higher levels in viral controllers.
TRAF1 shows a negative correlation with PD-1 expression and viral load
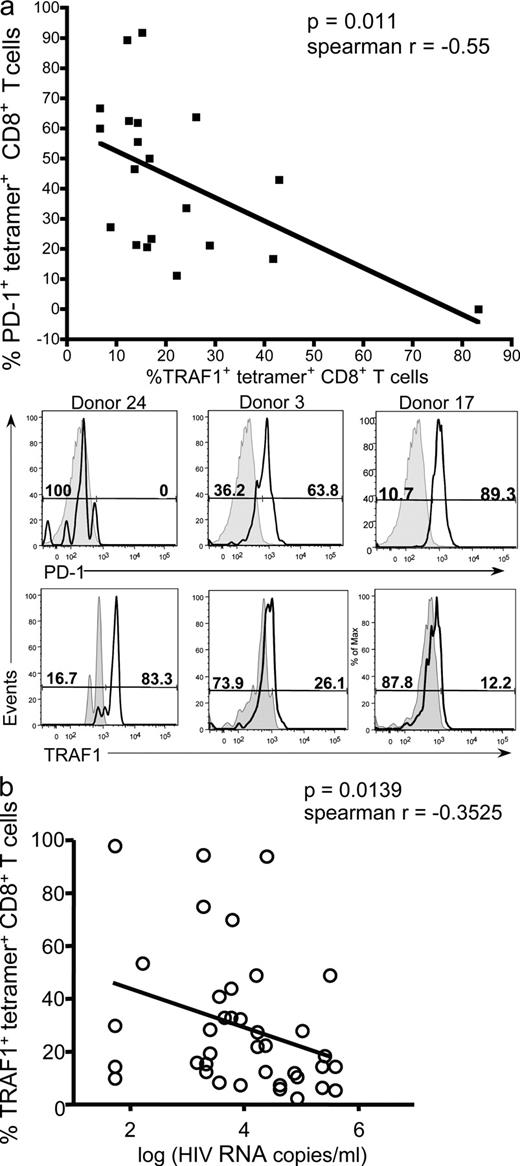
PD-1 up-regulation on virus-specific CD8 T cells is one of the hallmarks of T cell dysregulation during chronic infection (Barber et al., 2006; Day et al., 2006; Freeman et al., 2006; Trautmann et al., 2006; Urbani et al., 2006; Velu et al., 2007). We therefore asked whether low TRAF1 expression is indicative of high PD-1 levels on HIV-specific CD8 T cells from the chronic progressors. Indeed, we found that there is a significant negative correlation between the percentage of TRAF1+ tetramer+ and PD-1+ tetramer+ CD8 T cells from HIV-infected donors (Fig. 2 a; r = −0.55, P = 0.011).
TRAF1 expression negatively correlates with PD-1 levels and viral load. PBMCs from HIV-infected individuals were stained for CD3, CD8, HIV-tetramer, PD-1, and TRAF1. (a, top) Percentage of PD-1+ HIV-specific CD8 T cells is plotted against percentage of TRAF1+ HIV-specific CD8 T cells from the same chronically HIV-infected donors, with each symbol representing a single epitope from an individual donor. (bottom) Representative FACS plots of PD-1 and TRAF1 expression gated on HIV-tetramer+ CD8 T cells from three representative donors. Note: if we remove the extreme value point for the one donor with low PD-1 and high TRAF1, the correlation becomes r = −0.48, with a P value of 0.038. (b) Percentage of TRAF1 expression in HIV-specific CD8 T cells from the chronic stage of HIV infection, including both chronically infected donors and viral controllers, is plotted against log viral load recorded for each donor in Table S1. Each data point represents a single donor.
TRAF1 expression negatively correlates with PD-1 levels and viral load. PBMCs from HIV-infected individuals were stained for CD3, CD8, HIV-tetramer, PD-1, and TRAF1. (a, top) Percentage of PD-1+ HIV-specific CD8 T cells is plotted against percentage of TRAF1+ HIV-specific CD8 T cells from the same chronically HIV-infected donors, with each symbol representing a single epitope from an individual donor. (bottom) Representative FACS plots of PD-1 and TRAF1 expression gated on HIV-tetramer+ CD8 T cells from three representative donors. Note: if we remove the extreme value point for the one donor with low PD-1 and high TRAF1, the correlation becomes r = −0.48, with a P value of 0.038. (b) Percentage of TRAF1 expression in HIV-specific CD8 T cells from the chronic stage of HIV infection, including both chronically infected donors and viral controllers, is plotted against log viral load recorded for each donor in Table S1. Each data point represents a single donor.
We next asked whether the presence of TRAF1+ HIV-specific T cells is predictive of control of HIV viral load. Although there is no correlation during the early stage of HIV infection (Fig. S1 h), TRAF1 expression shows a weak, but statistically significant, negative correlation with viral load during the later stage of infection (Fig. 2 b; P = 0.014).
Requirement for TRAF1 in HIV-specific T cell responses
To more directly investigate whether decreased TRAF1 expression could play a role in loss of HIV control, we knocked down TRAF1 in CD8 T cells from viral controllers and assessed T cell function in a viral suppression assay. Purified CD8 T cells from viral controllers or an uninfected donor were transfected with small interfering RNA (siRNA) for TRAF1 or control RNA and then rested overnight before incubating with autologous HIV-infected CD4 T cells and autologous irradiated PBMCs, as a source of APC. The ability of CD8 T cells to control HIV-infected CD4 T cells was determined by measuring the frequency of Gag+ CD4 T cells via flow cytometry (Fig. S2) after a 5–7-d co-culture. For the HIV viral controllers, TRAF1 knockdown resulted in an increase in the frequency of HIV-infected (Gag+) CD4 T cells compared with cultures given control-treated CD8 T cells (Fig. 3 a). In contrast, as expected, there was no significant control of Gag+CD4 T cells in cultures of T cells from an HIV uninfected donor, with or without TRAF1 knockdown.
TRAF1 is required for HIV-specific CD8 T cell responses. (a) CD8 and CD4 T cells were separately purified from HIV+ or HIV− donors. CD4 T cells were infected with a primary isolate of HIV and at 48 h, co-cultured with their autologous CD8 T cells that had been transfected with either TRAF1-specific siRNA or a control scrambled RNA (ctrl). Irradiated autologous PBMCs were added as a source of APC. The frequency of Gag+ T cells was measured 5–7 d later by flow cytometry using CD3-, CD8-, and GAG-specific antibodies to assess the proportion of infected CD4 T cells. As CD4 is down-regulated on the infected cells, the absence of CD8 is used to determine the CD4 T cell population. Representative flow cytometry plots are shown in Fig. S2. (left and middle) representative suppression curves (based on three to five replicates at each effector-to-target ratio for each donor and representative of three viral controllers and a healthy uninfected control). Statistical significance was determined by linear regression of percentage of Gag+ T cells against log (effector: target ratio) using GraphPad (Prism) software. (right) Representative Western blot analysis of TRAF1 levels after knockdown, determined at 48 h after activation. (b). (left and middle) Viral suppression assay performed as in a. Bim-specific siRNA, TRAF1-specific siRNA, or both, or control scrambled RNA were used to transfect CD8 T cells from two viral controllers. CD8 T cells were plated at a ratio of 1:1 with infected CD4 T cells as in a. Open symbols on the right of each panel indicate the percentage of Gag expression in the CD4 T cells if no CD8 T cells were added at all. Cells were harvested for analysis of percentage of Gag+ CD4 T cells (CD8− T cells) after 7 d of co-culture. Statistical analysis was performed using one-way ANOVA. (right) Representative Western blot analysis of Bim levels after knockdown, determined at 48 h after activation. (c) Purified CD8 T cells from viral controllers were nucleofected with either control RNA or TRAF1 siRNA and incubated with autologous monocytes that had been pulsed with control or HIV peptide and pretreated with replication defective adenovirus expressing 4-1BBL or CD80 at a multiplicity of infection of 200, as previously described (Bukczynski et al., 2004). 8 d later, the cells were harvested for FACS analysis. (top left) Representative Western blot analysis of TRAF1 levels at the end of the 8-d culture. (bottom) Representative FACS plots. (top right) Summary plot for three experiments with cells from two different donors, shown as the number of HIV-tetramer+ CD8 T cells recovered in the TRAF1 siRNA-transfected population over those transfected with control RNA after stimulation with HIV peptide-pulsed 4-1BBL or CD80-expressing monocytes.
TRAF1 is required for HIV-specific CD8 T cell responses. (a) CD8 and CD4 T cells were separately purified from HIV+ or HIV− donors. CD4 T cells were infected with a primary isolate of HIV and at 48 h, co-cultured with their autologous CD8 T cells that had been transfected with either TRAF1-specific siRNA or a control scrambled RNA (ctrl). Irradiated autologous PBMCs were added as a source of APC. The frequency of Gag+ T cells was measured 5–7 d later by flow cytometry using CD3-, CD8-, and GAG-specific antibodies to assess the proportion of infected CD4 T cells. As CD4 is down-regulated on the infected cells, the absence of CD8 is used to determine the CD4 T cell population. Representative flow cytometry plots are shown in Fig. S2. (left and middle) representative suppression curves (based on three to five replicates at each effector-to-target ratio for each donor and representative of three viral controllers and a healthy uninfected control). Statistical significance was determined by linear regression of percentage of Gag+ T cells against log (effector: target ratio) using GraphPad (Prism) software. (right) Representative Western blot analysis of TRAF1 levels after knockdown, determined at 48 h after activation. (b). (left and middle) Viral suppression assay performed as in a. Bim-specific siRNA, TRAF1-specific siRNA, or both, or control scrambled RNA were used to transfect CD8 T cells from two viral controllers. CD8 T cells were plated at a ratio of 1:1 with infected CD4 T cells as in a. Open symbols on the right of each panel indicate the percentage of Gag expression in the CD4 T cells if no CD8 T cells were added at all. Cells were harvested for analysis of percentage of Gag+ CD4 T cells (CD8− T cells) after 7 d of co-culture. Statistical analysis was performed using one-way ANOVA. (right) Representative Western blot analysis of Bim levels after knockdown, determined at 48 h after activation. (c) Purified CD8 T cells from viral controllers were nucleofected with either control RNA or TRAF1 siRNA and incubated with autologous monocytes that had been pulsed with control or HIV peptide and pretreated with replication defective adenovirus expressing 4-1BBL or CD80 at a multiplicity of infection of 200, as previously described (Bukczynski et al., 2004). 8 d later, the cells were harvested for FACS analysis. (top left) Representative Western blot analysis of TRAF1 levels at the end of the 8-d culture. (bottom) Representative FACS plots. (top right) Summary plot for three experiments with cells from two different donors, shown as the number of HIV-tetramer+ CD8 T cells recovered in the TRAF1 siRNA-transfected population over those transfected with control RNA after stimulation with HIV peptide-pulsed 4-1BBL or CD80-expressing monocytes.
BIM (O’Connor et al., 1998) is a proapoptotic member of the Bcl2 family that has been implicated in contraction of CD8 T cell responses (Green, 2008). Previous studies have linked survival effects of TRAF1 in T cells to TRAF1-dependent BIM down-modulation (Sabbagh et al., 2006, 2008; Wang et al., 2007). Moreover, BIM has been shown to be important for CD8 T cell viability during chronic LCMV infection in mice (Grayson et al., 2006). Therefore, we analyzed whether knockdown of BIM in the CD8 T cells could overcome the lack of viral control observed when TRAF1 alone was knocked down. The simultaneous knockdown of BIM and TRAF1 in the CD8 T cells restored CD8 T cell–mediated elimination of Gag+ CD4 T cells (Fig. 3 b). These data suggest that TRAF1, via decreasing the proapoptotic molecule BIM, contributes to CD8 T cell–mediated control of HIV infection.
We next went on to assess whether the loss of TRAF1 could affect a 4-1BBL–dependent CD8 T cell response to HIV. We knocked down TRAF1 in CD8 T cells from viral controllers with siRNA (Fig. 3 c), and then stimulated the CD8 T cells with autologous monocytes that had been pulsed with HIV peptide antigens and modified with replication defective recombinant adenoviruses to express CD80 or 4-1BBL. When TRAF1 was knocked down at the onset of culture, we observed substantially impaired expansion of HIV-specific CD8 T cells in response to overexpressed 4-1BBL, with lesser effects on the response to overexpressed CD80 (Fig. 3 c), which does not use TRAF1 in its signaling pathway. This result shows that loss of TRAF1 can desensitize the 4-1BB co-stimulatory pathway in HIV-specific T cells.
Selective down-regulation of TRAF1 during chronic LCMV infection in mice
To assess the mechanism of TRAF1 loss and to try to correct the defect during a chronic infection in vivo, we moved to a mouse model. Infection of mice with clone 13 of LCMV results in viral persistence and CD8 T cell exhaustion, whereas the Armstrong strain of LCMV causes an acute infection that is readily cleared (Ahmed et al., 1984; Salvato et al., 1991; Zajac et al., 1998; Barber et al., 2006). The LCMV model shares several features of chronic infection with HIV, including up-regulation of PD-1 and TIM3 and the up-regulation of inhibitory cytokines (Virgin et al., 2009). Of note, 4-1BB is expressed on a substantial fraction of LCMV-specific CD8 T cells from both spleen and liver during the chronic phase of clone 13 infection (Fig. S3 a). We infected 5-wk-old mice with either the Armstrong or clone 13 virus and sorted LCMV-tetramer+ CD8 T cells from spleens on day 7 and 21 after infection. Purified LCMV-specific T cells were analyzed for TRAF1 expression by Western blot. Similar to our findings with HIV-specific T cells, LCMV-specific CD8 T cells from mice chronically infected with clone 13 displayed reduced TRAF1 protein expression on day 21 compared with the LCMV-specific T cells from the acute Armstrong infection at the same time point, whereas at day 7 after infection no such decrease was observed (Fig. 4 a). In contrast, TRAF2 did not show differential expression with acute or chronic infection over time (Fig. 4 a). Moreover, the loss of TRAF1 was specific to the LCMV-specific cells, as TRAF1 levels in PD-1− cells (see also Fig. 7 d), or in B cells and MHC-II+B220− APC were not changed over time of infection (Fig. S3 b). It should be noted that TRAF1 levels are low in resting cells, up-regulated with TCR signaling, and present at an intermediate level in early memory T cells (Sabbagh et al., 2006). Thus, the finding that the level of TRAF1 in activated T cells at the chronic stage of infection was even lower than the level of TRAF1 in the resting memory T cells at day 21 of Armstrong infection argues that TRAF1 levels are abnormally low at the chronic stage of LCMV infection.
TRAF1 protein is selectively lost from antigen-specific CD8 T cells during chronic, but not acute, LCMV infection. Mice were infected with either acutely infecting LCMV Armstrong (A) or chronically infecting clone 13 (C). Tetramer+ (gp33+ gp276+ NP396+) CD8 T cells were sorted on day 7 and 21 after infection, and the pooled purified tetramer+ T cells were subjected to either Western blot analysis or semiquantitative RT-PCR analysis. (a) Representative blots of TRAF1, TRAF2, and actin protein levels (left) and summary of TRAF1 and TRAF2 levels normalized to actin for tetramer+ T cells isolated from individual mice at the times indicated for the two different viral infections (right). Total CD8 T cells were sorted from uninfected mice (U) as a control. (b) mRNA levels of TRAF1 relative to Actin on the sorted T cells for the indicated viruses and times of infection. Data are representative of three (for protein) or two (for mRNA) independent mouse experiments. Right side of the gel in (b) indicates a titration of the template, indicating nonsaturation of the signal.
TRAF1 protein is selectively lost from antigen-specific CD8 T cells during chronic, but not acute, LCMV infection. Mice were infected with either acutely infecting LCMV Armstrong (A) or chronically infecting clone 13 (C). Tetramer+ (gp33+ gp276+ NP396+) CD8 T cells were sorted on day 7 and 21 after infection, and the pooled purified tetramer+ T cells were subjected to either Western blot analysis or semiquantitative RT-PCR analysis. (a) Representative blots of TRAF1, TRAF2, and actin protein levels (left) and summary of TRAF1 and TRAF2 levels normalized to actin for tetramer+ T cells isolated from individual mice at the times indicated for the two different viral infections (right). Total CD8 T cells were sorted from uninfected mice (U) as a control. (b) mRNA levels of TRAF1 relative to Actin on the sorted T cells for the indicated viruses and times of infection. Data are representative of three (for protein) or two (for mRNA) independent mouse experiments. Right side of the gel in (b) indicates a titration of the template, indicating nonsaturation of the signal.
We next asked whether the loss of TRAF1 is a transcriptional or posttranscriptional event. Mice were infected and tetramer+ cells sorted as in Fig. 4 a. Using semiquantitative RT-PCR, we observed similar TRAF1 mRNA levels in LCMV-specific T cells from Armstrong and clone 13 infections at day 21 (Fig. 4 b). These results suggest that TRAF1 protein loss during chronic infection occurs posttranscriptionally.
4-1BBL plays a role in control of LCMV early, but has limited impact later in the infection
We next assessed whether a co-stimulatory pathway involving TRAF1 shows defects in the LCMV clone 13 model. As TRAF1 is important in 4-1BB–dependent signaling in CD8 T cells (Wang et al., 2007; Sabbagh et al., 2008), we compared viral load and T cell responses in 4-1BBL−/− and WT mice both early and late in infection. We observed that at day 8 after infection, 4-1BBL−/− mice had a significantly lower frequency of NP396-specific CD8 T cells in the blood (Fig. 5 a); NP396 is an epitope that is highly sensitive to deletion during LCMV clone 13 infection (Zajac et al., 1998). However, this difference was rapidly lost in the infected mice, and by day 35 after infection there were minimal NP396-specific T cells left in either WT or 4-1BBL−/− mice (Fig. 5 b). Consistent with the T cell data, 4-1BBL−/− mice had a higher viral load in both kidney and lung as compared with WT mice at day 8 (Fig. 5 a), but by day 60 the viral loads in WT and 4-1BBL−/− mice were similar (Fig. 5 c). Thus, 4-1BB/L plays a role in viral control early during LCMV clone 13 infection, but not later, a finding that could be explained by the loss of TRAF1 at the chronic phase of infection, thereby desensitizing 4-1BB’s signaling pathway.
4-1BBL is required for viral control early but has limited impact late in LCMV clone 13 infection. (a) Analysis of WT versus 4-1BBL−/− mice 8 d after LCMV clone 13 infection. (top) Percentage of LCMV-tetramer+ CD8 T cells in blood. Each data point represents one mouse. Data are pooled from three independent experiments. (bottom) Viral titer as measured in lung and kidney. Data are representative of two independent experiments. (b) Kinetic analysis of the frequencies of NP396-specific T cells in the blood of WT and 4-1BBL−/− mice after LCMV clone 13 infection. Note that the day 8 data from this experiment were included in the compiled day 8 data in panel a. (c) Analysis of WT versus 4-1BBL−/− mice 60 d after LCMV clone 13 infection. (left) Percentage of GP33-tetramer+ CD8 T cells in spleen. Data are pooled from two independent experiments. (right) Viral titers in kidney. Data are representative of two independent experiments.
4-1BBL is required for viral control early but has limited impact late in LCMV clone 13 infection. (a) Analysis of WT versus 4-1BBL−/− mice 8 d after LCMV clone 13 infection. (top) Percentage of LCMV-tetramer+ CD8 T cells in blood. Each data point represents one mouse. Data are pooled from three independent experiments. (bottom) Viral titer as measured in lung and kidney. Data are representative of two independent experiments. (b) Kinetic analysis of the frequencies of NP396-specific T cells in the blood of WT and 4-1BBL−/− mice after LCMV clone 13 infection. Note that the day 8 data from this experiment were included in the compiled day 8 data in panel a. (c) Analysis of WT versus 4-1BBL−/− mice 60 d after LCMV clone 13 infection. (left) Percentage of GP33-tetramer+ CD8 T cells in spleen. Data are pooled from two independent experiments. (right) Viral titers in kidney. Data are representative of two independent experiments.
TGFβ causes loss of TRAF1 by a chloroquine-sensitive mechanism
Recent evidence has shown that production of the regulatory cytokine TGFβ is sustained during chronic as compared with acute LCMV infection, resulting in increased expression of BIM by the T cells and their loss by apoptosis (Tinoco et al., 2009). As TRAF1 can maintain lower levels of BIM in antigen-activated T cells (Sabbagh et al., 2006; Wang et al., 2007), we hypothesized that TGFβ might influence TRAF1 levels. To test this hypothesis, we infected mice with LCMV clone 13, and 21 d later mice were given 200 µg of anti-TGFβ1 by the i.p. route. On day 24, we sorted LCMV-tetramer+ PD1+ CD8 T cells and analyzed TRAF1 levels by Western blot (Fig. 6 a). The results showed that a single treatment with anti-TGFβ1 could increase TRAF1 levels as measured 3 d later.
TGFβ regulation of TRAF1 levels in T cells. (a) Mice were infected with LCMV clone 13 for 21 d. 200 µg/mouse of anti-TGFβ1 or control antibody was injected i.p. on day 21. On day 24, CD8 T cells were purified from total splenocytes, stained, and sorted for tetramer+PD-1+ CD8 T cells (GP33 + GP276 + NP396) and subjected to Western blot analysis. Left panel shows a representative blot, and the right panel shows the summary of results with each symbol representing a single mouse from the same experiment. A similar increase in TRAF1 upon TGFβ blockade was obtained in two additional smaller experiments. (b) Splenocytes from OT-1 mice were stimulated with SIINFEKL peptide for 36 h with or without TGFβ. Live CD8 T cells were purified and lysed for Western blotting for TRAF1, TRAF2, and Actin. Data are representative of 3 experiments. (c) Splenocytes from OT-1 mice were stimulated with SIINFEKL peptide for 20 h with or without TGFβ, followed by addition of 1 µg/ml of CHX in the continued presence of TGFβ. Note that if TGFβ was added at the same time as CHX, without the pretreatment, it did not cause loss of TRAF1 (not depicted). Cells were harvested at 0, 1, 2, 3, and 4 h after drug treatment. Live CD8 T cells were purified, and then subjected to Western blot. Data are representative of two experiments. (d) Same as in c, except that 25 nM of chloroquine (Clq) or 25 nM of lactacystin (Lac) was added to the cells at the time of CHX addition, where indicated. Cells were harvested and purified at 2 h after drug treatment. Data are representative of two experiments.
TGFβ regulation of TRAF1 levels in T cells. (a) Mice were infected with LCMV clone 13 for 21 d. 200 µg/mouse of anti-TGFβ1 or control antibody was injected i.p. on day 21. On day 24, CD8 T cells were purified from total splenocytes, stained, and sorted for tetramer+PD-1+ CD8 T cells (GP33 + GP276 + NP396) and subjected to Western blot analysis. Left panel shows a representative blot, and the right panel shows the summary of results with each symbol representing a single mouse from the same experiment. A similar increase in TRAF1 upon TGFβ blockade was obtained in two additional smaller experiments. (b) Splenocytes from OT-1 mice were stimulated with SIINFEKL peptide for 36 h with or without TGFβ. Live CD8 T cells were purified and lysed for Western blotting for TRAF1, TRAF2, and Actin. Data are representative of 3 experiments. (c) Splenocytes from OT-1 mice were stimulated with SIINFEKL peptide for 20 h with or without TGFβ, followed by addition of 1 µg/ml of CHX in the continued presence of TGFβ. Note that if TGFβ was added at the same time as CHX, without the pretreatment, it did not cause loss of TRAF1 (not depicted). Cells were harvested at 0, 1, 2, 3, and 4 h after drug treatment. Live CD8 T cells were purified, and then subjected to Western blot. Data are representative of two experiments. (d) Same as in c, except that 25 nM of chloroquine (Clq) or 25 nM of lactacystin (Lac) was added to the cells at the time of CHX addition, where indicated. Cells were harvested and purified at 2 h after drug treatment. Data are representative of two experiments.
To further explore the mechanism of TGFβ-induced loss of TRAF1, we incubated TCR transgenic CD8 T cells with peptide antigen with or without recombinant TGFβ for 36 h and observed that TRAF1, but not TRAF2, protein was significantly reduced upon TGFβ treatment (Fig. 6 b). We next tested whether TGFβ can regulate TRAF1 at the posttranslational level. Cells were treated with TGFβ for 20 h, and then cycloheximide (CHX) was added to block protein synthesis. TRAF1 stability was followed for 4 h after addition of CHX. The results show that TRAF1 protein has a shorter half-life if the cells had been pretreated with TGFβ (Fig. 6 c). Notably, if TGFβ was only added at the time of CHX treatment, TRAF1 protein was stable (unpublished data), indicating that protein translation downstream of TGFβ signaling is required for TRAF1 loss.
To determine the mechanism of TGFβ-induced TRAF1 protein loss, we added the proteasome inhibitor lactacystin or the lysosome inhibitor chloroquine at the same time as CHX to the untreated or TGFβ-treated cultures (Fig. 6 d). Whereas lactacystin increased the level of TRAF1 in the absence of TGFβ, likely reflecting a block in normal TRAF1 protein turnover, it did not prevent TGFβ-induced loss of TRAF1. In contrast, the addition of chloroquine resulted in inhibition of TGFβ-induced TRAF1 protein loss, suggesting the involvement of an endosome-dependent process. Thus, TGFβ can cause TRAF1 protein loss by a chloroquine-sensitive mechanism. It should be noted that TGFβ can also regulate TRAF1 mRNA levels when a higher dose is used (Fig. S4), suggesting that a more complicated mechanism could be at play in TRAF1 dysregulation during chronic viral infection in vivo.
Restoration of TRAF1 levels by IL-7
Although TGFβ blockade shows promise in increasing TRAF1 expression, the pleiotropic effects of TGFβ makes its use in therapy complex. IL-7 was previously reported to antagonize the effects of TGFβ on T cells (Pellegrini et al., 2009). IL-7 is also in clinical trials for treating HIV patients (Levy et al., 2009). We therefore tested whether IL-7 can up-regulate TRAF1 expression. Indeed, CD45RA− memory CD8 T cells from both healthy and HIV-infected individuals exhibit an antigen-independent increase in TRAF1 expression in response to IL-7 and other common γ chain family cytokines, such as IL-2 and 15, but not IL-21 (Fig. 7, a and b). Similarly, HIV-specific CD8 T cells showed enhanced TRAF1 expression when treated with IL-7 (Fig. 7 c).
Cytokine regulation of TRAF1 levels in T cells. (a) Purified CD8 T cells from healthy donors were stimulated with either anti-CD3 (1 µg/ml)/CD28 (10 µg/ml) as a positive control or 20 ng/ml of IL-2, IL-7, IL-15, or IL-21, or media alone for 6 d. At day 6, cells were analyzed by flow cytometry. The right graph shows summary data, gated on CD45RA− CD8+ T cells, reported as the difference in median fluorescence intensity (dMFI) of TRAF1 compared with FMO controls, with each symbol representing a different donor. Statistical analysis was performed using one-way ANOVA. (b) PBMCs from HIV-infected donors were CFSE-labeled and incubated with cytokines as described in a. Data are shown gated on CD45RA−CFSElow (divided) CD8 T cells except for unstimulated samples, which were gated on CD45RA− CD8 T cells, as they did not undergo division. Data are reported as dMFI relative to FMO controls averaged for four different donors per group. Statistical analysis was performed using one-way ANOVA within each group of HIV donors (early, viral controller, and chronic). (c) TRAF1 levels in HIV-specific CD8 T cells in response to IL-7. To detect HIV-specific CD8 T cells over time, purified CD8 T cells were expanded in response to autologous monocytes pulsed with respective HIV peptides and 4-1BBL-AdV for 8 d with or without IL-7, as described in Materials and methods. The dMFI of TRAF1 against FMO in HIV-specific CD8 T cells for four donors, two early and two chronic, are reported. (d) Mice were infected with LCMV clone 13. At day 21-after infection, mice were treated with 10 µg/mouse of IL-7 or PBS. Tetramer+ and PD-1− CD8 T cells were sorted on day 23 as described in Fig. 4 and lysed for Western blot. CD8 T cells from uninfected TRAF1−/− and WT mice were used as controls. The left plot shows representative data and the bottom right plot shows the summary of TRAF1/actin ratio on the sorted cells which each data point representing an individual mouse. Data in d are the summary of two independent mouse experiments.
Cytokine regulation of TRAF1 levels in T cells. (a) Purified CD8 T cells from healthy donors were stimulated with either anti-CD3 (1 µg/ml)/CD28 (10 µg/ml) as a positive control or 20 ng/ml of IL-2, IL-7, IL-15, or IL-21, or media alone for 6 d. At day 6, cells were analyzed by flow cytometry. The right graph shows summary data, gated on CD45RA− CD8+ T cells, reported as the difference in median fluorescence intensity (dMFI) of TRAF1 compared with FMO controls, with each symbol representing a different donor. Statistical analysis was performed using one-way ANOVA. (b) PBMCs from HIV-infected donors were CFSE-labeled and incubated with cytokines as described in a. Data are shown gated on CD45RA−CFSElow (divided) CD8 T cells except for unstimulated samples, which were gated on CD45RA− CD8 T cells, as they did not undergo division. Data are reported as dMFI relative to FMO controls averaged for four different donors per group. Statistical analysis was performed using one-way ANOVA within each group of HIV donors (early, viral controller, and chronic). (c) TRAF1 levels in HIV-specific CD8 T cells in response to IL-7. To detect HIV-specific CD8 T cells over time, purified CD8 T cells were expanded in response to autologous monocytes pulsed with respective HIV peptides and 4-1BBL-AdV for 8 d with or without IL-7, as described in Materials and methods. The dMFI of TRAF1 against FMO in HIV-specific CD8 T cells for four donors, two early and two chronic, are reported. (d) Mice were infected with LCMV clone 13. At day 21-after infection, mice were treated with 10 µg/mouse of IL-7 or PBS. Tetramer+ and PD-1− CD8 T cells were sorted on day 23 as described in Fig. 4 and lysed for Western blot. CD8 T cells from uninfected TRAF1−/− and WT mice were used as controls. The left plot shows representative data and the bottom right plot shows the summary of TRAF1/actin ratio on the sorted cells which each data point representing an individual mouse. Data in d are the summary of two independent mouse experiments.
We next asked whether IL-7 could restore TRAF1 expression in T cells in vivo during the chronic phase of LCMV infection. We treated mice with IL-7 on day 21 after clone 13 infection, harvested them, and then sorted LCMV-specific CD8 T cells from the splenocytes on day 23. Although this brief IL-7 treatment did not reduce viral load (not depicted), we observed a significant increase in TRAF1 expression in tetramer+ CD8 T cells (Fig. 7 d), which is consistent with the in vitro data.
IL-7 combined with agonistic anti–4-1BB antibody expands functional virus-specific CD8 T cells and decreases viral load in a TRAF1-dependent manner
As IL-7 restores TRAF1 levels in T cells of chronically infected mice in vivo, we reasoned that a combined therapy involving 4-1BB and IL-7 might be of benefit. Notably, anti–4-1BB alone did not increase TRAF1 levels when delivered at the chronic phase of infection (unpublished data). We treated clone 13–infected mice at 25 d after infection with an agonistic anti–4-1BB antibody with or without recombinant IL-7 (delivered at day 21, 23, and 25) and sacrificed mice 5 d later for analysis of effects on T cells. IL-7 alone had little or no effect on virus-specific T cell recovery, whereas stimulatory anti–4-1BB antibody alone induced a limited increase in LCMV-specific lymphocyte numbers (one out of four epitopes; Fig. 8 a), consistent with the hypothesis that the 4-1BB signaling pathway is desensitized. In contrast, the combination of IL-7 and anti–4-1BB increased the total number of epitope-specific T cells recovered (Fig. 8 a). However, the frequency of virus-specific T cells was unchanged, as IL-7 increased the overall cellularity of the spleen and 4-1BB increased the frequency of all CD8 T cells (unpublished data). Nonetheless, the therapy did result in an increase in the frequency of multifunctional LCMV-specific T cells that can produce IFN-γ and express the degranulation marker CD107a in response to LCMV antigen (Fig. 8 b).
Combined treatment with IL-7 and agonistic anti–4-1BB increases the number of functional CD8 T cells and decreases viral load in a TRAF1-dependent manner. Mice were infected with LCMV clone 13 for 21 d and treated with either IL-7 alone, agonistic anti–4-1BB (3H3) alone, or in combination as indicated, using the following treatment regimen: 10 µg/mouse of IL-7 on day 21, 23, and 25, and 100 µg/mouse of 3H3 on day 25. Mice were sacrificed on d30 (a and b) or day 37 (c). (a) The number of tetramer+ CD8 T cells specific for LCMV epitopes was examined for each treatment group, with each data point representing a single mouse. (b) Splenocytes from mice in each group were subjected to LCMV peptide restimulation with brefeldin A, monensin, and anti-CD107a or isotype control for 6 h. Cells were then harvested for surface and intracellular staining and FACS analysis. (c) Organs were harvested at day 37, and viral titers were measured. Data in a–c are representative of three similar experiments for tetramer analysis and viral clearance, the latter presented as the median of seven or eight individual mice. The dotted line indicates the limit of detection of the assay. (d) WT and TRAF1−/− mice were treated with IL-7/anti–4-1BB or left untreated as described in a. At day 37 (12 d after treatment), the number of gp33-specific CD8 T cells were enumerated in the spleen (left). Viral titers were evaluated in spleen and liver (right). Data in d are pooled from two independent experiments with 7 TRAF1−/− and 10 WT mice per group.
Combined treatment with IL-7 and agonistic anti–4-1BB increases the number of functional CD8 T cells and decreases viral load in a TRAF1-dependent manner. Mice were infected with LCMV clone 13 for 21 d and treated with either IL-7 alone, agonistic anti–4-1BB (3H3) alone, or in combination as indicated, using the following treatment regimen: 10 µg/mouse of IL-7 on day 21, 23, and 25, and 100 µg/mouse of 3H3 on day 25. Mice were sacrificed on d30 (a and b) or day 37 (c). (a) The number of tetramer+ CD8 T cells specific for LCMV epitopes was examined for each treatment group, with each data point representing a single mouse. (b) Splenocytes from mice in each group were subjected to LCMV peptide restimulation with brefeldin A, monensin, and anti-CD107a or isotype control for 6 h. Cells were then harvested for surface and intracellular staining and FACS analysis. (c) Organs were harvested at day 37, and viral titers were measured. Data in a–c are representative of three similar experiments for tetramer analysis and viral clearance, the latter presented as the median of seven or eight individual mice. The dotted line indicates the limit of detection of the assay. (d) WT and TRAF1−/− mice were treated with IL-7/anti–4-1BB or left untreated as described in a. At day 37 (12 d after treatment), the number of gp33-specific CD8 T cells were enumerated in the spleen (left). Viral titers were evaluated in spleen and liver (right). Data in d are pooled from two independent experiments with 7 TRAF1−/− and 10 WT mice per group.
We next analyzed the impact of the therapy on viral load. Mice were infected and treated as in Fig. 8 (a and b), and at day 37 organs were harvested for analysis of viral load. The combination of anti–4-1BB and IL-7, but neither treatment alone, resulted in viral clearance in the liver and a significant decrease in viral load in the lung (Fig. 8 c). The effect on viral load in the kidney was more modest, but significant. It should be noted that when we looked earlier, at 5 d after treatment, there was a larger reduction of viral load in the kidney (Fig. S5), suggesting that there had been a transient effect in that organ. These data show that 4-1BB and IL-7 can cooperate to improve T cell function and viral clearance in chronic LCMV infection of mice.
To test whether TRAF1 was important for the effects of anti–4-1BB/IL-7 therapy, we infected WT or TRAF1−/− mice with LCMV clone 13, treated as in Fig. 8 a, and then analyzed the response on day 37 (Fig. 8 d). The IL-7 and anti–4-1BB treated TRAF1−/− mice did not respond to therapy, exhibiting T cell numbers equivalent to the untreated controls (Fig. 8 d). The lack of increase in the number of gp33-specific T cells in the spleen after therapy of TRAF1−/− mice correlated with increased viral load in the spleen and liver (Fig. 8 d, right), but not in the kidney or lung (unpublished data). The increased viral load in TRAF1−/− treated mice was not caused by higher viral load in TRAF1−/− mice before treatment, as in the absence of treatment WT or TRAF1−/− mice infected with clone 13 showed a similar viral load at day 30 after infection (unpublished data). These results provide evidence that TRAF1 is required for the IL-7 plus 4-1BB–mediated effects on viral control.
Transfer of TRAF1-expressing memory T cells enhances viral control
We next set out to determine whether T cell–intrinsic TRAF1 was indeed important in viral control and whether TRAF1-expressing T cells delivered at the chronic stage of infection could provide any benefit. To this end, we took advantage of the P14 transgenic model and generated P14.TRAF1−/− mice. To obtain a population of TRAF1-expressing and control memory T cells, we stimulated the P14.WT and P14.TRAF1−/− splenocytes with antigen, followed by 6 d of IL-15 treatment (Pulle et al., 2006). We confirmed that P14.WT memory-like CD8 T cells express TRAF1 at higher levels than LCMV-specific CD8 T cells sorted from mice at day 21 after clone 13 infection (Fig. 9 a). P14.WT or P14.TRAF1−/− memory T cells were then injected into mice that had been infected with LCMV clone 13 21 d before. At 2 wk after transfer, we observed a higher frequency of functional LCMV-specific CD8 T cells in mice that had received the TRAF1-expressing as compared with the TRAF1−/− P14 cells (Fig. 9 b). Consistent with the T cell functional data, delivery of the TRAF1-expressing T cells also resulted in a significant reduction in viral load in the kidneys (Fig. 9 c). These results demonstrate that T cell–intrinsic TRAF1 is important for control of LCMV at the chronic stage of infection.
Transfer of TRAF1-expressing memory T cells enhances viral control. WT P14 or TRAF1−/− P14 splenocytes were stimulated with GP33 peptide, and then washed and incubated with IL-15 for 6 d to generate memory like T cells as described in the Materials and methods. (a) Western blot analysis of TRAF1 levels in P14.TRAF1−/− and P14.WT T cells before transfer, compared with TRAF1 levels in LCMV tetramer+ (GP33, GP276, and NP396) CD8 T cells sorted from LCMV clone 13–infected mice at day 21 after infection (labeled as d21.tet+ in blot). (b and c) Mice were infected with 2 × 106 PFU/mouse LCMV clone 13. On day 21 post-infection, one million in-vitro-generated memory P14.WT or P14.TRAF1−/− T cells were transferred via the intravenous route. Mice were sacrificed 14 d later for analysis of T cells and viral titers. (b) Representative FACS plots are shown for GP33-specific CD8 T cell function as measured by production of IFN-γ and TNF in response to GP33 peptide restimulation as outlined in Materials and methods. Similar results were observed in three mice. In a separate and independent experiment, a higher percentage of GP33-tetramer+ CD8 T cells were found in P14.WT-transferred mice as compared with the P14.TRAF1−/−-transferred mice or mice without transfer. (c) Viral titer in kidney at 2 wk after P14 T cell transfer. Data are pooled from two independent experiments with a total of six mice without transfer, five mice with P14.WT transfer, and five mice with P14.TRAF1−/− transfer.
Transfer of TRAF1-expressing memory T cells enhances viral control. WT P14 or TRAF1−/− P14 splenocytes were stimulated with GP33 peptide, and then washed and incubated with IL-15 for 6 d to generate memory like T cells as described in the Materials and methods. (a) Western blot analysis of TRAF1 levels in P14.TRAF1−/− and P14.WT T cells before transfer, compared with TRAF1 levels in LCMV tetramer+ (GP33, GP276, and NP396) CD8 T cells sorted from LCMV clone 13–infected mice at day 21 after infection (labeled as d21.tet+ in blot). (b and c) Mice were infected with 2 × 106 PFU/mouse LCMV clone 13. On day 21 post-infection, one million in-vitro-generated memory P14.WT or P14.TRAF1−/− T cells were transferred via the intravenous route. Mice were sacrificed 14 d later for analysis of T cells and viral titers. (b) Representative FACS plots are shown for GP33-specific CD8 T cell function as measured by production of IFN-γ and TNF in response to GP33 peptide restimulation as outlined in Materials and methods. Similar results were observed in three mice. In a separate and independent experiment, a higher percentage of GP33-tetramer+ CD8 T cells were found in P14.WT-transferred mice as compared with the P14.TRAF1−/−-transferred mice or mice without transfer. (c) Viral titer in kidney at 2 wk after P14 T cell transfer. Data are pooled from two independent experiments with a total of six mice without transfer, five mice with P14.WT transfer, and five mice with P14.TRAF1−/− transfer.
DISCUSSION
This study reveals a novel mechanism by which T cells can become dysfunctional during chronic viral infection, through the down-regulation of a signaling adaptor, TRAF1. In contrast to other members of the TRAF family, TRAF1 lacks the RING domain required for NF-κB activation (Rothe et al., 1994). However, structural analysis of the TRAF1/2 coiled coil domains suggests that the TRAF1 (TRAF2)2 heterotrimer is a better recruiter of cellular inhibitor of apoptosis proteins than a TRAF2 homotrimer (Zheng et al., 2010), providing a structural explanation for enhanced NF-κB activation in the presence of TRAF1 (Arron et al., 2002). We showed that TRAF1 is selectively lost from antigen-specific T cells with progression of chronic infection for both HIV infection in humans and LCMV clone 13 infection in mice and that there is a negative correlation of the frequency of TRAF1+ cells with PD-1 expression and viral load during the later stage of HIV infection. The knockdown of TRAF1 in CD8 T cells from viral controllers results in a decrease in the ability of these T cells to control viral infection ex vivo, an effect that was compensated for by simultaneous knockdown of BIM. Moreover, TRAF1 deficiency impairs the ability of a combined treatment with IL-7 and anti–4-1BB to lower viral load in mice. Finally we showed that transfer of TRAF1 expressing, but not TRAF1-deficient, memory T cells at the chronic stage of infection can lower viral load, indicating that T cell intrinsic TRAF1 has a role in viral control. Collectively, these findings demonstrate that the lower level of TRAF1 in antigen-specific CD8 T cells during chronic infection has functional consequences for viral control.
The decreased frequency of TRAF1+ T cells with disease progression does not reveal whether loss of TRAF1 is a cause or an effect of chronic infection. However, the correlation of viral load with TRAF1 late, but not early, in infection is consistent with the loss of TRAF1 being a secondary rather than an initiating event. Our results show that the TRAF1 expression defect is at the protein level, but not the mRNA level (Fig. 4 b), a finding that has been made for other molecules in exhausted CD8 T cells, including IFN-γ (Wherry et al., 2007). Although the cause of TRAF1 protein loss could be multifaceted, we showed that blocking TGFβ at the chronic stage of infection can increase TRAF1 levels in vivo, which is consistent with the production of TGFβ during chronic LCMV and SIV infection (Cumont et al., 2007; Tinoco et al., 2009). Moreover, we show that TGFβ can cause the disappearance of TRAF1 protein from antigen-activated T cells by a chloroquine-sensitive mechanism in vitro. Interestingly, it appears that a TGFβ-induced protein (or proteins) controls TRAF1 protein stability, as pretreatment with TGFβ before the addition of CHX is required to reduce TRAF1 protein half-life. The loss of TRAF1 in the presence of cycloheximide is blocked by chloroquine but not by lactacystin, suggesting an endosome-dependent mechanism. As TGFβ has been shown to induce autophagy of specific substrates (Ding et al., 2010), it is possible that TGFβ causes loss of TRAF1 in the endosome, although this remains to be further investigated. Thus, a likely scenario is that early during infection, excessive viral replication leads to sustained expression of IL-10, PD-1, and TGFβ, which in turn results in loss of TRAF1 from the antigen-specific T cells, which further exacerbates their loss and/or dysfunction. Thus, the level of TRAF1 in the HIV-specific T cells may be a useful indicator of the state of these cells during the chronic phase of HIV infection.
TRAF1 knockdown specifically impairs the ability of HIV-specific CD8 T cells from viral controllers to expand in response to a co-stimulatory ligand, 4-1BBL. Moreover, TRAF1 was critical for IL-7 plus anti–4-1BB–induced treatment of chronic infection in vivo. Thus, the loss of TRAF1 from the CD8 T cells impairs their 4-1BB–dependent responses to both HIV and LCMV clone 13. Consistent with these findings, 4-1BBL deficiency resulted in reduced viral control early but not late in LCMV clone 13 infection. Although we have specifically identified the loss of TRAF1 as desensitizing the 4-1BB signaling pathway, it is also possible that the loss of TRAF1 affects signaling through other TNFRs on T cells (Croft, 2009; Watts, 2005).
Given their importance in T cell homeostasis, we tested IL-7 and other members of the common γ chain family of cytokines for restoration of TRAF1 levels. We found that IL-2, IL-7, and IL-15, but not IL-21 up-regulated TRAF1 protein in CD8 T cells in an antigen-independent manner. IL-7 in particular has been shown to antagonize the TGFβ signaling pathway (Pellegrini et al., 2009) and was selected for further study. One caveat of using these cytokines as a therapy for chronic viral infection is the suboptimal expression of receptors for both IL-7 and IL-15 on chronically stimulated T cells (Fuller et al., 2005; Lang et al., 2005). However, both IL-7Rlow and IL-15Rlow LCMV-specific CD8 T cells can still divide in response to IL-7 in vitro and IL-15 in vivo, albeit to a lesser extent than their receptor high counterparts (Wherry et al., 2004). Importantly, we showed that purified T cells from HIV-infected donors respond to these cytokines by up-regulating TRAF1 in vitro, despite their reduced proliferation compared with T cells from donors at early stage of infection (unpublished data). Moreover, IL-7 increased the level of TRAF1 protein in LCMV-specific CD8 T cells during clone 13 infection in vivo, although indirect effects of IL-7 cannot be ruled out. Given the pleiotropic effects of IL-7 on the immune system (Pellegrini et al., 2009), it is likely that IL-7 has additional effects besides restoring TRAF1 levels in this model. Indeed, although our data clearly show that TRAF1 contributes to T cell control of LCMV at the chronic stage of infection, we have not demonstrated that it is sufficient and in fact we think it unlikely that induction of TRAF1 alone would restore function to chronically dysfunctional T cells.
IL-7 monotherapy was recently shown to expand functional T cells and cure chronic infection with LCMV clone 13 when given daily for 3 wk starting at day 8 after infection (Pellegrini et al., 2011). In that study, IL-7 therapy was started at a time when PD-1 levels are already high on the T cells, but before the time when we observe the loss of TRAF1 protein (between day 7 and 21 of the LCMV clone 13 infection). The present study shows IL-7 alone had a limited effect at later stage of infection and that a single dose of 100 µg/mouse of anti–4-1BB, given at day 25 after infection in conjunction with IL-7, can result in a decreased viral load at day 37. IL-7 had a major effect on the overall cellularity of the spleen, and 4-1BB also expands CD8 T cells in general. However, the combined therapy clearly enhanced the frequency of functional LCMV-specific T cells and contributed to viral control. These data provide proof of principal that a 4-1BB–specific therapy could be of some efficacy in combination with IL-7 treatment at the later stage of infection.
A recent study showed that HAART is unable to restore CD8 T cell effector function to the level of those from HIV controllers (Migueles et al., 2009). Therefore, treatment that directly improves CD8 T cell function may further benefit viral control. In addition to its effects on T cell survival via NF-κB (Lee et al., 2002), as well as by Erk-dependent BIM down-modulation (Sabbagh et al., 2008), 4-1BB signaling on Ag-experienced T cells increases the level of effector function per CD8 T cell (Bukczynski et al., 2004, 2005; Wang et al., 2007), likely contributing to its therapeutic effect. There is concern, however, that agonistic anti–4-1BB antibodies show dose-dependent toxicities and must be used with caution (Niu et al., 2007; Ascierto et al., 2010). On the other hand, recent studies have shown that other forms of 4-1BB stimulation may be less toxic (Schabowsky et al., 2009).
There are several hints that TRAF1 is important in human disease. TRAF1 is overexpressed in >50% of human cancers of B cell origin (Zapata et al., 2000) and single nucleotide polymorphisms in TRAF1 or in the TRAF1/C5 region have been linked to non-Hodgkin’s lymphoma (Cerhan et al., 2007) and rheumatic disease (Kurreeman et al., 2007; Plenge et al., 2007), respectively. Our results suggest that TRAF1 may be useful as a prognostic marker for CD8 T cell fitness during the chronic phase of HIV infection. The finding that IL-7 and other common γ chain cytokines up-regulate TRAF1 also suggests that TRAF1 levels in human T cells could be a useful biomarker for cytokine therapy.
MATERIALS AND METHODS
Reagents.
Biotinylated MHC/HIV peptide monomers: A2-SLYNTVATL, A2-ILKEPVHGV, A2-FLGKIWPSYK, B7-TPGPGVRYPL, B7-IPRRIRQGL, B8-FLKEKGGL, B27-KRWIILGLNK, and B57-KAFSPEVIPMF were generated at the National Immune Monitoring Laboratory, St. Laurent, Quebec (http://www.niml.org/) or at the National Institute for Allergy and Infectious Disease tetramer facility (Emory University, Atlanta, GA) and mixed with PE or APC-streptavidin. LCMV-tetramers H-2Kb-GP34: AVYNFATM, H-2Db-GP33: KAVYNFATM, H-2Db-GP276: SGVENPGGYCL, and H-2Db-NP396: FQPQNGQFI were obtained from the MHC tetramer core laboratory, Baylor College of Medicine. Human TRAF1-specific antibody 1F3 (Siegler et al., 2003; Serviceeinheit Monoklonale Antikörper; Institut für Molekulare Immunologie, Munich, Germany) was labeled with Alexa Fluor 430 (Invitrogen) for flow cytometry analysis of human TRAF1. Anti–4-1BB (3H3; Shuford et al., 1997) was purified from the hybridoma, provided by R. Mittler (Emory University, Atlanta, GA) and was determined to contain <5 (0.9–4.2) U/ml endotoxin by the limulus amebocyte assay (Sigma-Aldrich). Anti–PD-1 antibody was purchased from BioLegend. Anti-TGFβ1 was purchased from Sigma-Aldrich (clone 9016.2). All other antibodies for flow cytometry were purchased from eBioscience. The following Western blot antibodies were used: anti-mTRAF1 (Santa Cruz Biotechnology, Inc.), anti-mTRAF2 (Cell Signaling Technology), anti-hBIM (Cell Signaling Technology), anti-hTRAF1 (Cell Signaling Technology), and anti-Actin (Sigma-Aldrich).
HIV-infected donors.
PBMCs from HIV+ donors were obtained by leukapheresis and cryopreserved until use. Healthy donor samples were obtained by venipuncture. All donors gave informed consent as approved by the research ethics boards of all participating institutions. Donors were HLA typed and ELISPOT analysis was used to determine their CD8 T cell specificities as previously described (Bukczynski et al., 2005), and the dominant epitopes were selected for further study. Human donor information is summarized in Table S1.
Mice and LCMV.
5-wk-old C57BL/6 mice (Charles River) were infected by intravenous infusion of LCMV Armstrong strain (5,000 PFU per mouse) or LCMV clone 13 strain (2 × 106 PFU/mouse). For quantification of TRAF1/2 levels, splenocytes were harvested from day 7 and 21 after Armstrong or clone 13 infections. Pooled GP33-, GP276-, and NP396-tetramer+ CD8 T cells, PD-1low CD8 T cells, B220+MHCII+ B cells, or B220−MHCII+ cells were sorted and subject to Western blots or semiquantitative RT-PCR. To test restoration of TRAF1 by blocking TGFβ, 200 µg of anti-TGFβ antibody was injected intraperitoneally on day 21 after clone 13 infection. To test induction of TRAF1 by IL-7 in vivo, 10 µg of recombinant human IL-7 (Cytheris) or PBS were injected i.p. on day 21 and 22 after clone 13 infection. For IL-7 therapy, 10 µg of IL-7 or PBS were given i.p. on day 21, 23, and 25 with 100 µg of agonistic anti–4-1BB or rat Ig (Sigma-Aldrich) on day 25. Mice were sacrificed on day 30 or day 37 for flow cytometry analysis and viral titer assays. TRAF1−/− mice, which had been backcrossed 8 times onto the C57BL/6 background (Tsitsikov et al., 2001; now available from The Jackson Laboratory), were obtained from E. Tsitsikov (Harvard Medical School, Boston, MA) and were bred in our facility. 4-1BBL−/− mice were originally obtained under materials transfer agreement from Immunex, now Amgen and have been backcrossed at least 8 times onto the C57BL/6 background. WT controls were obtained directly from Charles River or were bred in-house from Charles River C57BL/6 mice. All animal experiments were approved by the University of Toronto and/or University Health Network animal care committee according to the guidelines of the Canadian Council on animal care.
Intracellular cytokine and degranulation assay.
Splenocytes were incubated for 6 h with 100 nM LCMV-specific peptide, brefeldin, and monensin and anti-CD107a antibody. Cells were first stained with anti-CD3 and anti-CD8 antibody (eBioscience), fixed, permeabilized (BD), and stained with anti–IFN-γ antibody, followed by flow cytometry analysis.
Co-stimulation assay.
Autologous monocytes were infected with replication-deficient adenoviruses expressing 4-1BBL, CD80, or control, as previously described (Bukczynski et al., 2004, 2005). CD8 T cells were purified and transfected (Amaxa) with 600 nM TRAF1 siRNA or control scrambled RNA (Integrated DNA technology), rested overnight, and incubated with activated monocytes for 8 d. T cells were then harvested for FACS analysis.
HIV suppression assay.
CD4 T cells were enriched from PBMCs using negative selection (StemCell), activated with 1 µg/ml each of anti-CD3 (OKT3), anti-CD28 (28.2) antibodies (eBioscience), and PHA (Sigma-Aldrich) for 24 h, and then washed extensively. The primary isolate of HIV 91_US4 was obtained from the National Institutes of Health AIDS reagent program and expanded on activated primary CD4 T cells. Virus was purified through a 20% sucrose cushion and used to magnetofect CD4 T cells using Viromag beads following manufacturer’s instructions (Oz Biosciences). CD8 T cells were enriched from PBMCs using negative selection (Miltenyi Biotec); transfected (Lonza) with 600 nM of TRAF1 siRNA, BIM siRNA, or control scrambled RNA alone or in combination (Integrated DNA technology); and rested overnight. TRAF1 and BIM levels were confirmed by Western blot and the modified CD8 T cells were incubated with 25,000 CD4 T cells/well at a serial dilution with effector CD8 to target CD4 ratio of 8:1 (or 2:1), 1:1, 1:4, 1:16, and no effector in 3 to 5 replicates. PBMCs were enriched for APC (STEMCELL Technologies), irradiated, and plated at 5,000/well with the CD8 and infected CD4 T cells in the presence of 20 U/ml IL-2 (Hoffmann-La Roche). At day 5–7 of co-culture, cells were stained for surface markers and intracellular Gag, and subjected to flow cytometry for analysis of the frequency of Gag+ T cells.
Measurement of effect of TGFβ on TRAF1 levels.
Splenocytes from OT-1 mice were incubated with 10−7 M SIINFEKL peptide at 2.5 million/ml with or without TGFβ (5 ng/ml). CD8 T cells were purified at 36 h after co-incubation, and subjected to Western blots or semiquantitative RT-PCR. For blocking protein translation, 1 µg/ml CHX was added to the culture at 20 h after co-incubation, and CD8 T cells harvested and purified at 0, 1, 2, 3, and 4 h after addition of CHX. For analysis of the mechanism of protein turnover, 25 μM lactacystin or 25 nM chloroquine was added in the presence of CHX at 20 h after co-incubation, and CD8 T cells were harvested and purified at 2 h after drug addition.
Semiquantitative RT-PCR.
The following mouse TRAF1 primer pair was used: 5′-CATGCAGGAGCATGAGGCTACC–3′ and 5′-CCACCACCCTCTGCTCCAAGC-3′.
Viral immunoplaque assay.
To determine LCMV titers, organs were homogenized and supernatant dilutions were used to infect a monolayer of MC57 cells under an overlay of methylcellulose in DME. 48 h later, cells were fixed and stained with VL-4 rat anti-LCMV mAb, and a color reaction of ortho-phenylenediamine was used to determine the focus-forming unit. Focus-forming unit was used to calculate the viral titer in the original supernatant and was divided by the weight of the respective organs from individual mice. As the various organs showed different levels of background in the immunoplaque assay, this leads to a different limit of detection for each organ. This empirically determined limit is indicated in Fig. 8.
P14 in vitro memory generation and transfer experiment.
P14.TRAF1−/− mice were generated by crossing TRAF1−/− with P14.WT mice (Pircher et al., 1989) that had been backcrossed at least 20 times onto the C57BL/6 background. Splenocytes from both P14.WT and P14.TRAF1−/− mice were plated at 5 × 106 cells/ml and stimulated with 0.1 µg/ml GP33 peptide and 1 µg/ml LPS for 2 d and replated at 1 × 106 for 1 d. Viable cells were isolated using density gradient and plated at 1 × 106 with 20 ng/ml of hIL-15 (Sigma-Aldrich) for 6 d (replacing IL-15 every 2 d). Cells were harvested and injected at 1 × 106 i.v. into mice that had been infected 21 d earlier with LCMV clone 13.
Statistics.
All statistical analysis was done using GraphPad software (Prism) using one-way analysis of variance (ANOVA) for comparison of multiple groups, or nonpaired Student’s t test for two groups, with p-values as indicated on the figures. Correlation was performed using the Spearman coefficient.
Online supplemental material.
Fig. S1 shows the gating strategy and additional data for Fig. 1. Fig. S2 shows representative flow cytometry data for the experiments shown in Fig. 3 (a and b). Fig. S3 shows 4-1BB and TRAF1 levels during LCMV infection in mice. Fig. S4 analyzes the effect of TGFβ regulation of TRAF1 protein and message and shows that at low dose TGFβ mainly affects TRAF1 expression. Fig. S5 provides the day 30 viral load data for the experiment in Fig. 8.
Acknowledgments
We thank Cytheris S.A. for providing recombinasnt human IL-7 and advice in its use; Robert A. Mittler for provision of the 3H3, anti–4-1BB producing, hybridoma; Gabor Gyenes for HIV donor information mining and Dionne White for help with flow cytometry.
This work was funded by grant number MOP-74492 and -84419 from the Canadian Institutes of Health Research (CIHR) to T.H. Watts; CIHR grant MOP-106529 to P.S. Ohashi, CIHR MOP MOP-93787, and the Ontario HIV treatment network (MO), as well as by the Fonds de la Recherche en Santé du Québec AIDS and Infectious Diseases Network. KRWI monomer and the HIV 91_US4 primary isolate were obtained through the AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health). C. Wang was funded by an OHTN studentship; G.H.Y. Lin holds a CIHR doctoral award; and T.H. Watts holds the Sanofi Pasteur chair in Human Immunology at the University of Toronto.
References
Author notes
M. Pellegrini’s present address is Walter and Eliza Hall Institute of Medical Research, Parkville, Australia.
T. Calzascia’s present address is Novartis Institute for Biomedical Research, Basel, Switzerland.