Vigorous proliferative CD4+ T cell responses are the hallmark of spontaneous clearance of acute hepatitis C virus (HCV) infection, whereas comparable responses are absent in chronically evolving infection. Here, we comprehensively characterized the breadth, specificity, and quality of the HCV-specific CD4+ T cell response in 31 patients with acute HCV infection and varying clinical outcomes. We analyzed in vitro T cell expansion in the presence of interleukin-2, and ex vivo staining with HCV peptide-loaded MHC class II tetramers. Surprisingly, broadly directed HCV-specific CD4+ T cell responses were universally detectable at early stages of infection, regardless of the clinical outcome. However, persistent viremia was associated with early proliferative defects of the HCV-specific CD4+ T cells, followed by rapid deletion of the HCV-specific response. Only early initiation of antiviral therapy was able to preserve CD4+ T cell responses in acute, chronically evolving infection. Our results challenge the paradigm that HCV persistence is the result of a failure to prime HCV-specific CD4+ T cells. Instead, broadly directed HCV-specific CD4+ T cell responses are usually generated, but rapid exhaustion and deletion of these cells occurs in the majority of patients. The data further suggest a short window of opportunity to prevent the loss of CD4+ T cell responses through antiviral therapy.
Infection with the hepatitis C virus is a major health burden worldwide (Alter and Seeff, 2000; Lauer and Walker, 2001). The virus persists in the majority of infected persons, although a significant minority of individuals is able to spontaneously control viral replication, as indicated by lack of detectable viremia over years of follow up (Takaki et al., 2000). The exact mechanisms that lead to these distinct outcomes have not been fully defined, but the presence or absence of a vigorous and multispecific proliferative CD4+ T cell response against hepatitis C virus (HCV) proteins is a strong immunological correlate of the outcome of acute HCV infection (Diepolder et al., 1995, 1996; Chang et al., 2001; Day et al., 2002; Grakoui et al., 2003; Rehermann, 2009). Proliferative HCV-specific CD4+ T cell responses are usually not detectable in acute persisting and chronic HCV infection (Chang et al., 2001; Day et al., 2002; Gerlach et al., 1999; Shoukry et al., 2004; Schulze zur Wiesch et al., 2005), with the exception of chronically infected patients who previously resolved an HCV infection with a heterologous HCV genotype (Schulze Zur Wiesch et al., 2007). The analysis of HCV-specific CD4+ T cells has historically relied on standard tritium uptake lymphoproliferative assays using whole HCV proteins, yielding very limited information about the breadth and fine specificities of the HCV-specific CD4+ T cell response (Diepolder et al., 1995; Missale et al., 1996; Gerlach et al., 1999; Kamal et al., 2004; Rahman et al., 2004; Lauer et al., 2005). The standard lymphoproliferative assay (LPA) is also dependent on in vitro proliferation of T cells. Hence, a negative result does not preclude the presence of antigen-specific cells nor does the test assess frequencies and phenotypic properties of the T cells of interest. A few studies using direct ex vivo techniques, such as cytokine ELISpot or class II tetramer staining, have also suggested a limited presence of HCV-specific CD4+ T cells in patients developing chronic infection, but lack either detail or comprehensiveness (Day et al., 2003; Ulsenheimer et al., 2006; Urbani et al., 2006; Semmo and Klenerman, 2007; Smyk-Pearson et al., 2008). Thus, it remains unclear whether the absence of CD4+ proliferative responses observed during acute persistent HCV infection is indicative of a complete lack of priming of HCV-specific CD4+ T cells, or whether HCV-specific CD4+ T cells are present, but are functionally deficient (Diepolder, 1995, 2009; Thimme et al., 2001; Shoukry et al., 2004; Klenerman and Hill, 2005; Day et al., 2006; Folgori et al., 2006; Chang, 2007; Urbani et al., 2008; Golden-Mason et al., 2009; Nakamoto et al., 2009).
This study combines novel approaches to comprehensively analyze the virus-specific CD4+ T cell response with high resolution in a large and well-defined cohort with acute HCV infection to test the hypothesis that HCV persistence is associated with an absence of virus-specific CD4+ T cell responses. HCV-specific CD4+ T cells were analyzed using the following techniques: (a) standard tritium uptake LPAs against recombinant HCV proteins; (b) single-peptide intracellular cytokine secretion assays of in vitro expanded HCV-specific CD4+ T cell lines; and (c) ex vivo class II tetramer analyses with tetramers restricted by several different DR molecules [eight different HCV class II tetramers for epitopes restricted by five different molecules (DRB1*0101, DRB1*0401, DRB0*404, DRB1*1101, and DRB1*1501)].
When using advanced CD4+ T cell assays, we find that broadly directed HCV-specific CD4+ T cells are universally detectable during early stages of infection, regardless of outcome. We also find a similar repertoire of targeted epitopes in all subjects. In chronically evolving HCV infection, we find that HCV-specific CD4+ T cells show early functional defects that are partially reversible in vitro by addition of exogenous recombinant IL-2 (rIL-2) at early stages of infection. In addition, the CD4+ T cell response rapidly collapses in vivo in the presence of persistent HCV viremia and becomes completely undetectable by the most sensitive detection methods within months of the acute infection. Of note, early initiation of antiviral treatment was able to protect HCV-specific CD4+ T cells from complete deletion. Our data demonstrate that HCV infection generally induces a broadly directed HCV-specific CD4+ T cell response that is short-lived in the majority of subjects. Future research needs to elucidate the mechanisms of secondary CD4+ T cell failure (Diepolder, 2009) and the fate of HCV-specific CD4+ T cells in persisting viremia.
RESULTS
Multispecific HCV CD4+ T cell responses are universally detectable during acute HCV infection despite absent LPA responses in acute chronically evolving (AC) HCV
The virus-specific CD4+ T cell response during acute HCV infection has not been well characterized beyond proliferative responses against whole HCV proteins. We therefore performed a comprehensive assessment of the early HCV-specific CD4+ T cell response using different technologies in a large cohort of 31 acutely infected patients: 18 patients who went on to spontaneously resolve infection, and 13 patients with acute, chronically evolving infection. Clinical characteristics of the patients are summarized in Table I.
Clinical and immunological characteristics of the study cohort
| Label | Course of infection | Peak ALT | Age, sex | Therapy | Therapy outcome | Genoytpe | Risk factor | First sample | No of CD4+ responses | First sample VL | Peak VL | Nadir VL |
| d | IU/ml | IU/ml | IU/ml | |||||||||
| AC1 | AC | 473 | 35, M | None | Chronic | 1 | IDU | 94 | 2 | 42,000 | 42,000 | 42,000 |
| AC2 | AC | 2915 | 20, F | PEG/RBV 24W | SVR | 1a | IDU | 125 | 1 | 358,000 | >700,000 | 235,000 |
| AC3 | AC | 206 | 19, F | PEG/RBV 24W | SVR | 1b | IDU | 49 | 13 | 2800 | 358,000 | 1080 |
| AC4 | AC | 201 | 30, M | PEG/RBV 48W | SVR | 1a | IDU | 107 | 1 | 7470 | 7470 | 7470 |
| AC5 | AC | 720 | 28, M | IFN 6 mIU tiw 48W | Relapse | 1 | Hospitalization | 137 | 3 | >700,000 | >700,000 | 194,000 |
| AC6 | AC | 420 | 26, M | PEG 24W | SVR | 1a | IDU | 100 | 1 | 234,000 | 234,000 | 120,647 |
| AC7 | AC | 1074 | 37, M | PEG/RBV 24W | SVR | 1b | IDU | 0 | 13 | >700,000 | >700,000 | +* |
| AC8 | AC | 2145 | 23, F | PEG/RBV 12W | SVR | 1 | IDU | 35 | 17 | 902 | 902 | +* |
| AC9 | AC | 678 | 38, M | None | Chronic | 1 | IDU | 62 | 6 | 380,000 | 380,000 | 380,000 |
| AC10 | AC | 308 | 20, F | PEG/RBV 24W | SVR | 1b | IDU | 53 | 12 | >700,000 | >700,000 | 342 |
| AC11 | AC | 427 | 40, F | IFN 3 mIU tiw/RBV | SVR | 1a | Needle Stick | 14 | 12 | 900 | >700,000 | 900 |
| AC12 | AC | 1074 | 37, M | None | Chronic | 1a | IDU | 54 | 6 | >700,000 | >700,000 | 456,999 |
| AC13 | AC | 1204 | 55, F | PEG/RBV 24W | SVR | 2** | Needle stick | 7 | 13 | >700,000 | >700,000 | +* |
| AR1 | AR | 2237 | 27, M | NA | NA | 3** | IDU | 16 | 7 | +* | +* | NA |
| AR2 | AR | 331 | 33, M | NA | NA | NA | Cocaine | 41 | 2 | +* | +* | NA |
| AR3 | AR | 1630 | 28, F | NA | NA | 1** | IDU | 30 | 22 | 7215 | 7215 | NA |
| AR4 | AR | 1673 | 21, F | NA | NA | 1a | IDU | 7 | 20 | >700,000 | >700,000 | NA |
| AR5 | AR | 443 | 41, F | NA | NA | 1** | Blood Transfusion | 56 | 26 | 260,830 | 260,830 | NA |
| AR6 | AR | 1845 | 38, F | NA | NA | 1** | Surgery | 156 | 12 | +* | +* | NA |
| AR7 | AR | 1235 | 31, M | NA | NA | 1** | Surgery | 27 | 17 | +* | +* | NA |
| AR8 | AR | 1073 | 42, M | NA | NA | 1** | IDU | 20 | 5 | 55,700 | 55,700 | NA |
| AR9 | AR | 918 | 35, F | NA | NA | 1** | Hospitalization | 26 | 14 | 4000 | 4000 | NA |
| AR10 | AR | 463 | 29, M | NA | NA | 3a | IDU | 15 | 8 | 211,195 | 211,195 | NA |
| AR11 | AR | 1327 | 47, F | NA | NA | 1** | Sexual Transmission | 112 | 16 | +* | +* | NA |
| AR12 | AR | 2235 | 31, F | NA | NA | 1** | Hospitalization | 44 | 5 | 9700 | 9700 | NA |
| AR13 | AR | 1694 | 59, F | NA | NA | 1** | Hospitalization | 18 | 11 | 342000 | 342000 | NA |
| AR14 | AR | 2659 | 39, F | NA | NA | 1a | IDU | 12 | 9 | 734,400 | 734,400 | NA |
| AR15 | AR | 201 | 23, F | NA | NA | 1a | IDU/Cocaine | 14 | 5 | 3200 | 3200 | NA |
| AR16 | AR | 1624 | 22, F | NA | NA | 1** | IDU | 108 | 17 | +* | +* | NA |
| AR17 | AR | 2435 | 63, F | NA | NA | 1** | Unknown | 177 | 9 | +* | +* | NA |
| AR18 | AR | 1060 | 52, F | NA | NA | NA | Sexual Transmission | 57 | 5 | +* | +* | NA |
| Label | Course of infection | Peak ALT | Age, sex | Therapy | Therapy outcome | Genoytpe | Risk factor | First sample | No of CD4+ responses | First sample VL | Peak VL | Nadir VL |
| d | IU/ml | IU/ml | IU/ml | |||||||||
| AC1 | AC | 473 | 35, M | None | Chronic | 1 | IDU | 94 | 2 | 42,000 | 42,000 | 42,000 |
| AC2 | AC | 2915 | 20, F | PEG/RBV 24W | SVR | 1a | IDU | 125 | 1 | 358,000 | >700,000 | 235,000 |
| AC3 | AC | 206 | 19, F | PEG/RBV 24W | SVR | 1b | IDU | 49 | 13 | 2800 | 358,000 | 1080 |
| AC4 | AC | 201 | 30, M | PEG/RBV 48W | SVR | 1a | IDU | 107 | 1 | 7470 | 7470 | 7470 |
| AC5 | AC | 720 | 28, M | IFN 6 mIU tiw 48W | Relapse | 1 | Hospitalization | 137 | 3 | >700,000 | >700,000 | 194,000 |
| AC6 | AC | 420 | 26, M | PEG 24W | SVR | 1a | IDU | 100 | 1 | 234,000 | 234,000 | 120,647 |
| AC7 | AC | 1074 | 37, M | PEG/RBV 24W | SVR | 1b | IDU | 0 | 13 | >700,000 | >700,000 | +* |
| AC8 | AC | 2145 | 23, F | PEG/RBV 12W | SVR | 1 | IDU | 35 | 17 | 902 | 902 | +* |
| AC9 | AC | 678 | 38, M | None | Chronic | 1 | IDU | 62 | 6 | 380,000 | 380,000 | 380,000 |
| AC10 | AC | 308 | 20, F | PEG/RBV 24W | SVR | 1b | IDU | 53 | 12 | >700,000 | >700,000 | 342 |
| AC11 | AC | 427 | 40, F | IFN 3 mIU tiw/RBV | SVR | 1a | Needle Stick | 14 | 12 | 900 | >700,000 | 900 |
| AC12 | AC | 1074 | 37, M | None | Chronic | 1a | IDU | 54 | 6 | >700,000 | >700,000 | 456,999 |
| AC13 | AC | 1204 | 55, F | PEG/RBV 24W | SVR | 2** | Needle stick | 7 | 13 | >700,000 | >700,000 | +* |
| AR1 | AR | 2237 | 27, M | NA | NA | 3** | IDU | 16 | 7 | +* | +* | NA |
| AR2 | AR | 331 | 33, M | NA | NA | NA | Cocaine | 41 | 2 | +* | +* | NA |
| AR3 | AR | 1630 | 28, F | NA | NA | 1** | IDU | 30 | 22 | 7215 | 7215 | NA |
| AR4 | AR | 1673 | 21, F | NA | NA | 1a | IDU | 7 | 20 | >700,000 | >700,000 | NA |
| AR5 | AR | 443 | 41, F | NA | NA | 1** | Blood Transfusion | 56 | 26 | 260,830 | 260,830 | NA |
| AR6 | AR | 1845 | 38, F | NA | NA | 1** | Surgery | 156 | 12 | +* | +* | NA |
| AR7 | AR | 1235 | 31, M | NA | NA | 1** | Surgery | 27 | 17 | +* | +* | NA |
| AR8 | AR | 1073 | 42, M | NA | NA | 1** | IDU | 20 | 5 | 55,700 | 55,700 | NA |
| AR9 | AR | 918 | 35, F | NA | NA | 1** | Hospitalization | 26 | 14 | 4000 | 4000 | NA |
| AR10 | AR | 463 | 29, M | NA | NA | 3a | IDU | 15 | 8 | 211,195 | 211,195 | NA |
| AR11 | AR | 1327 | 47, F | NA | NA | 1** | Sexual Transmission | 112 | 16 | +* | +* | NA |
| AR12 | AR | 2235 | 31, F | NA | NA | 1** | Hospitalization | 44 | 5 | 9700 | 9700 | NA |
| AR13 | AR | 1694 | 59, F | NA | NA | 1** | Hospitalization | 18 | 11 | 342000 | 342000 | NA |
| AR14 | AR | 2659 | 39, F | NA | NA | 1a | IDU | 12 | 9 | 734,400 | 734,400 | NA |
| AR15 | AR | 201 | 23, F | NA | NA | 1a | IDU/Cocaine | 14 | 5 | 3200 | 3200 | NA |
| AR16 | AR | 1624 | 22, F | NA | NA | 1** | IDU | 108 | 17 | +* | +* | NA |
| AR17 | AR | 2435 | 63, F | NA | NA | 1** | Unknown | 177 | 9 | +* | +* | NA |
| AR18 | AR | 1060 | 52, F | NA | NA | NA | Sexual Transmission | 57 | 5 | +* | +* | NA |
AC patient was recruited and studied within 6 mo after onset of symptoms and remained viremic after 6 mo; AR patient was recruited and studied within 6 mo after onset of symptoms, and tested HCV RNA negative on at least two occasions after 6 mo and later; ALT, alanine transaminase; M, male; F, female; PEG, pegylated interferon α; RBV, Ribavirin; tiw, three times weekly; IU, international units; IDU, intravenous drug use; VL, viral load, NA, not applicable. *, + HCV PCR, too low to quantify. **, serotype.
For direct comparison, we initially performed 6-d LPAs, the historic “standard” for the analysis of CD4+ T cell responses (Diepolder et al., 1995; Rosenberg et al., 1997; Lauer et al., 2002), using recombinant HCV proteins and fresh PBMCs from the earliest time point for which PBMCs were available for 29 of the 31 patients, all within the first 6 mo after onset of symptoms (Table I). In accordance with many immunological studies of acute infection (Gerlach et al., 1999; Urbani et al., 2006; Kaplan et al., 2007), we detected strong proliferative responses against all of the HCV nonstructural antigens in patients with AR HCV. These responses were mostly absent in patients with acute, chronically evolving HCV (mean NS3 stimulation index [SI] in acute resolvers 15 vs. 2.1 in AC infection, P < 0.0017; NS4 SI 13.2 vs. 3.2, P < 0.016; NS5 SI 7.15 vs. 1.93 P < 0.0026; Fig. 1 A).
Responsiveness to HCV antigens by CD4+ T cells from patients with acute HCV infection and spontaneously resolving versus persistent viremia. (A) PBMCs obtained from patients within 6 mo of symptomatic HCV infection were stimulated for 6 d in vitro using 3 different recombinant HCV proteins (NS3, NS4, and NS5) in the absence of rIL-2. Proliferation was measured using thymidine uptake and expressed as SI over negative control. Each dot represents a single patient. The dotted line delineates an SI value of 5 that is considered the cut-off for a positive proliferative response. (B and C) PBMCs obtained as in A were depleted of CD8+ T cells, and short-term CD4+ T cell lines were generated using recombinant HCV antigens and overlapping HCV peptides together with rIL-2. After 10–14 d, cell lines were tested for IFN-γ production, initially with pools of 20 overlapping peptides, and then, if positive, by single 20-mer peptides. (B) For each subject, the number of single peptides that tested positive is shown, plotted separately for the AR versus AC cohort. (C) The percentage of IFN-γ–secreting cells for each reactive cell line as determined by ICS and ro compare the relative size of the expanded HCV-specific populations between AR and AC subjects. (D) Detailed results for subject AC9 with acute infection followed by chronic viremia. The bar graph on the left shows results (in SI) from the standard proliferation assay without rIL-2 as in (A), showing no response against any of the HCV proteins, but robust proliferative responses against cytomegalovirus lysate, tetanus toxoid (TT) and phytohemagglutinin (PHA) controls. On the right, six dot blots showing positive ICS assays from the experiments outlined in B and C, confirming expansion of HCV-specific CD4+ T cells targeting six different epitopes in the same individual using short-term in vitro culture under the addition of rIL-2.
Responsiveness to HCV antigens by CD4+ T cells from patients with acute HCV infection and spontaneously resolving versus persistent viremia. (A) PBMCs obtained from patients within 6 mo of symptomatic HCV infection were stimulated for 6 d in vitro using 3 different recombinant HCV proteins (NS3, NS4, and NS5) in the absence of rIL-2. Proliferation was measured using thymidine uptake and expressed as SI over negative control. Each dot represents a single patient. The dotted line delineates an SI value of 5 that is considered the cut-off for a positive proliferative response. (B and C) PBMCs obtained as in A were depleted of CD8+ T cells, and short-term CD4+ T cell lines were generated using recombinant HCV antigens and overlapping HCV peptides together with rIL-2. After 10–14 d, cell lines were tested for IFN-γ production, initially with pools of 20 overlapping peptides, and then, if positive, by single 20-mer peptides. (B) For each subject, the number of single peptides that tested positive is shown, plotted separately for the AR versus AC cohort. (C) The percentage of IFN-γ–secreting cells for each reactive cell line as determined by ICS and ro compare the relative size of the expanded HCV-specific populations between AR and AC subjects. (D) Detailed results for subject AC9 with acute infection followed by chronic viremia. The bar graph on the left shows results (in SI) from the standard proliferation assay without rIL-2 as in (A), showing no response against any of the HCV proteins, but robust proliferative responses against cytomegalovirus lysate, tetanus toxoid (TT) and phytohemagglutinin (PHA) controls. On the right, six dot blots showing positive ICS assays from the experiments outlined in B and C, confirming expansion of HCV-specific CD4+ T cells targeting six different epitopes in the same individual using short-term in vitro culture under the addition of rIL-2.
We had previously demonstrated that we can define the HCV-specific CD4+ T cell response in subjects with resolved infection on a single epitope level using short-term in vitro culture with HCV proteins and a comprehensive, overlapping HCV peptide set covering the entire HCV genome under supplementation of rIL-2 (Day et al., 2002; Schulze zur Wiesch et al., 2005, 2007). Using this approach, we next tested all subjects with acute HCV infection using PBMCs from the first available time point (≤ 6 mo after onset of symptoms; median, 57 d; range, 3–179; Table I); all patients were treatment naive at the time of analysis. We detected broad responses in almost all individuals, with between 1 and 26 HCV CD4+ T cell epitopes targeted.
In contrast to the negative results observed in the standard LPA assay, all 13 patients with chronic evolution of acute HCV infection demonstrated CD4+ T cell responses, with most subjects displaying broad reactivity against numerous CD4+ T cell epitopes (range, 1–18 specificities; Fig. 1, A–D). Broad responses were detectable in the majority of patients over a wide range of viral loads at the time point of the assay, with some subjects with the highest viral loads targeting >10 HCV CD4+ T cell epitopes (Table I). None of the subjects with a chronic course of infection achieved complete temporary control of HCV (defined as a negative qualitative HCV PCR) during the observation period, an uncommon occurrence that has been demonstrated to be associated with temporary detection of CD4 responses in standard LPA (Gerlach et al., 1999). At the same time, partial control of HCV was displayed by many of the subjects at select time points of viremia, leading to a “yo-yo pattern,” which we and others have described as the most common pattern of viremia during acute HCV infection (Cox et al., 2009; McGovern et al., 2009). Overall, there was no correlation between the nadir viral load and the number of targeted epitopes (r = 0.34; P = 0.25). These results demonstrate that HCV-specific CD4+ T cells are not absent in early persisting infection, but are usually broadly directed, though with functional deficits as they do not proliferate in vitro upon restimulation with recombinant proteins in the standard LPA assay.
The breadth of the response detected in patients with early AC infection was not significantly different from what we observed in the 18 subjects with spontaneously resolving infection (median, 10 responses; range, 2–26; P > 0.05; Fig. 1 B). There was also no difference between subjects with resolving and persistent infection in the size of the expanded CD4+ T cell populations as measured by intracellular cytokine staining (ICS) for interferon-γ after stimulation with single HCV peptides (Fig. 1 C).
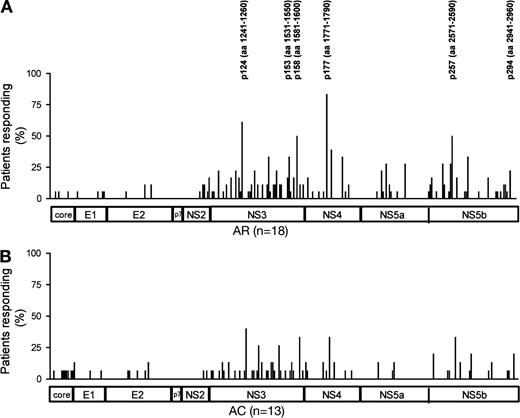
When we compared the repertoire of targeted epitopes in subjects with AR versus persisting infection, we observed similar patterns of recognition in both groups, with a majority of responses located in the NS3 region (Fig. 2, A and B; and Table S2). Responses against previously reported immunodominant epitopes frequently detected in resolved infection and thought to be crucial for resolution of infection (e.g., HCV aa 1241-1260, HCV aa 1771-1790; Fig. 2, A and B; Table S2; Diepolder et al., 1997; Wertheimer et al., 2003; Schulze zur Wiesch et al., 2005) were also detected in subjects who ultimately failed to control viremia.
Frequency of detection of single HCV CD4+ epitopes using short-term in vitro culture with the addition of rIL-2. (A and B) The relative location of each epitope detected in the assays from Fig. 1 (B and C) within the HCV polyprotein and the frequency with which the respective peptide was recognized in the cohort of patients with self-limited (AR) and chronic (AC) infection. The six most frequently recognized peptides are marked with their exact location in the HCV protein sequence. More detailed information on all epitopes detected in each individual patient of the cohort can be found in Table S2.
Frequency of detection of single HCV CD4+ epitopes using short-term in vitro culture with the addition of rIL-2. (A and B) The relative location of each epitope detected in the assays from Fig. 1 (B and C) within the HCV polyprotein and the frequency with which the respective peptide was recognized in the cohort of patients with self-limited (AR) and chronic (AC) infection. The six most frequently recognized peptides are marked with their exact location in the HCV protein sequence. More detailed information on all epitopes detected in each individual patient of the cohort can be found in Table S2.
These data suggest that neither breadth nor specificity of the HCV-specific CD4+ T cell response during the early stages of infection determine the outcome of infection.
Supplementation of rIL-2 reverses the proliferative defects of HCV-specific CD4+ T cell responses in persisting acute HCV infection
To dissect which difference in the experimental setup determined the improved proliferation of HCV-specific CD4+ T cell responses in our in vitro assays as compared with standard LPA, we performed a CFSE proliferation assay combined with class II tetramer staining. This experiment demonstrated HCV-specific T cell proliferation only after peptide stimulation together with IL-2 support. HCV-specific cells of acute patient AC7 (week 16 after symptoms of infection) were unable to proliferate after restimulation with peptides p124 (HCV aa1241-1260) and p177 (HCV aa1771-1790) alone, but proliferated vigorously after addition of recombinant rIL-2 to the cell culture (Fig. 3 A).
HCV-specific proliferation of virus-specific CD4+ T cells in the presence or absence of IL-2. (A) PBMCs of an early time point from patient AC7 with AC HCV infection were labeled with CFSE dye and stimulated with no peptide (−) or peptides p124 (aa 1241-1260) and p177 (aa 1771–1790) in the presence (left) or absence (right) of rIL-2. After 7 d, cells were stained with HCV-specific tetramers Tet124 and Tet177. (B) CFSE proliferation assay in a patient with acute HCV infection and self-limited disease. PBMCs were stained with CFSE dye and stimulated with HIV peptides (control peptides) or a pool of the six most frequently detected immunodominant HCV peptides (Fig. 2) for 7 d with or without addition of rIL-2. (C) Summary of the CFSE proliferation experiments performed as in A on six patients (filled circles) with AC infection and six patients (filled squares) with AR infection either in the absence or presence of IL-2. (SI, stimulation index with the frequency of proliferating cells without addition of peptide and rIL-2 as the denominator).
HCV-specific proliferation of virus-specific CD4+ T cells in the presence or absence of IL-2. (A) PBMCs of an early time point from patient AC7 with AC HCV infection were labeled with CFSE dye and stimulated with no peptide (−) or peptides p124 (aa 1241-1260) and p177 (aa 1771–1790) in the presence (left) or absence (right) of rIL-2. After 7 d, cells were stained with HCV-specific tetramers Tet124 and Tet177. (B) CFSE proliferation assay in a patient with acute HCV infection and self-limited disease. PBMCs were stained with CFSE dye and stimulated with HIV peptides (control peptides) or a pool of the six most frequently detected immunodominant HCV peptides (Fig. 2) for 7 d with or without addition of rIL-2. (C) Summary of the CFSE proliferation experiments performed as in A on six patients (filled circles) with AC infection and six patients (filled squares) with AR infection either in the absence or presence of IL-2. (SI, stimulation index with the frequency of proliferating cells without addition of peptide and rIL-2 as the denominator).
To confirm and extend these results, we performed CFSE proliferative assays after 7-d in vitro stimulation with a standardized HCV CD4+ T cell epitope peptide pool in the presence and absence of exogenous rIL-2. Although patients with AR infection had proliferative responses (in the standard LPA, but also in CFSE experiments without addition of rIL-2; Fig. 3, B and C), HCV-specific CD4+ T cells from subjects with early chronically evolving infection (<6 mo after onset of symptoms) only proliferated when stimulated with HCV peptides after addition of rIL-2 (Fig. 3 C). The importance of IL-2 for proliferation of HCV-specific CD4+ T cells was further confirmed through an experiment in which in vitro blockade with an IL-2–blocking antibody (Lichterfeld et al., 2004) abrogated the HCV-specific proliferation in a patient with self-limited infection (Fig. 4). Finally, we also examined the effect of exogenous rIL-7 and/or rIL-21 on CD4+ T cell proliferation induced by HCV antigens. In five AC and two acute spontaneously resolving (AR) subjects, we performed proliferative assays with rIL-2 or with rIL-2 in combination with rIL-7 and rIL-21. The additional cytokines did not increase the proliferative response in subjects that had detectable responses with rIL-2 alone. Nor did we detect responses in cases when the assay with rIL-2 was negative (unpublished data). Together, these data suggest that IL-2 is critical for the proliferative capacity of HCV-specific CD4+ T cells during early acute HCV infection.
Abortion of HCV-specific T cell proliferation by an anti–IL-2 blocking antibody. CFSE assay in a patient with resolution of the acute infection. PBMCs from a patient with resolved HCV infection were depleted of CD8+ T cells, stained with CFSE dye, and stimulated with recombinant c200 (NS3+NS4) protein in the presence of IL-2–blocking antibody or isotype control. At day 7, the number of CFSElow cells was assessed by flow cytometry. Superoxide dismutase served as negative control and phytohemagglutinin and c200 plus IL-2 served as positive controls.
Abortion of HCV-specific T cell proliferation by an anti–IL-2 blocking antibody. CFSE assay in a patient with resolution of the acute infection. PBMCs from a patient with resolved HCV infection were depleted of CD8+ T cells, stained with CFSE dye, and stimulated with recombinant c200 (NS3+NS4) protein in the presence of IL-2–blocking antibody or isotype control. At day 7, the number of CFSElow cells was assessed by flow cytometry. Superoxide dismutase served as negative control and phytohemagglutinin and c200 plus IL-2 served as positive controls.
Detection of HCV-specific CD4+ T cell responses is dependent on the duration of viremia in acute HCV infection with a chronic outcome
Because we had been mostly unable to detect any HCV-specific CD4+ T cell responses in our previous studies analyzing subjects with established chronic infection (Chang et al., 2001; Day et al., 2002, 2003; Schulze zur Wiesch et al., 2005), we aimed to establish how early the loss of CD4+ T cell responses occurs with persistence of viremia. Analyzing the cross-sectional data of in vitro expanded cell lines in acute infection, we found a significant inverse correlation between the number of detected responses and time elapsed between the onset of symptoms and performance of the experiment in subjects with chronically evolving infection; only very few responses could be expanded after a 10–12-wk duration of viremia (Fig. 5 A; P < 0.02) and none after 6 mo of viremia (not depicted). The observation of HCV-specific CD4+ T cell responses early in AC infection, followed by a loss of the response with persisting viremia held true for all HCV CD4+ T cell specificities, including responses against two HCV epitopes that we and others had previously described as immunodominant in resolving HCV infection (HCV aa 1241-1260 and HCV aa 1771-1790; Diepolder et al., 1997; Day et al., 2002; Wertheimer et al., 2003; Schulze zur Wiesch et al., 2005, 2007).
Time dependency of detection of HCV-specific CD4+ T cell responses in patients with acute HCV infection. The number of HCV-specific CD4+ responses detected in HCV-antigen stimulated short-term T cell lines with rIL-2 (as shown in Fig. 1 B) was plotted against the time elapsed between symptom onset and sampling of blood for the experiment in AC infection (A) and patients with AR course (B). Each square represents the number of HCV peptide-specific CD4+ T cell responses of a single patient.
Time dependency of detection of HCV-specific CD4+ T cell responses in patients with acute HCV infection. The number of HCV-specific CD4+ responses detected in HCV-antigen stimulated short-term T cell lines with rIL-2 (as shown in Fig. 1 B) was plotted against the time elapsed between symptom onset and sampling of blood for the experiment in AC infection (A) and patients with AR course (B). Each square represents the number of HCV peptide-specific CD4+ T cell responses of a single patient.
In contrast, for patients with spontaneous resolution of HCV we observed no such correlation, and the number of HCV-specific CD4+ T cell responses detected in the in vitro cell culture system remained stable over time (Fig. 5 B). Indeed, we and others were able to recover in vitro HCV-specific CD4+ T cell responses in patients with spontaneously controlled infection many months and even years after resolution of HCV viremia (Chang et al., 2001; Schulze zur Wiesch et al., 2005; Smyk-Pearson et al., 2006).
Direct ex vivo tetramer analysis confirms circulating HCV-specific CD4+ T cells during early acute infection irrespective of outcome
To confirm the results obtained with in vitro culture of HCV-specific CD4+ T cells, we analyzed these cells directly ex vivo, using class II tetrameric complexes. Based on previous studies (Gerlach et al., 2005; Schulze zur Wiesch et al., 2005; Kasprowicz et al., 2008) we were able to synthesize a total of 8 class II tetramers for some of the most frequently targeted HCV epitopes restricted by 5 different human leukocyte antigen (HLA) molecules (Table S3). In Fig. 6 A, representative flow cytometry dot plots of tetramer stainings for patients AC1 with chronically evolving course and AR3 with AR course are depicted. Because only a limited number of subjects in the original cohort of 31 patients expressed HLA molecules for which we have class II tetramers available, we performed tetramer analysis on PBMCs from an additional 18 patients with documented acute infection (Table S1). This allowed us to test for a total of 61 HCV CD4+ T cell specificities directly ex vivo in 40 patients using HLA-matched class II tetramers.
Analysis of the frequency of HCV-specific CD4+ T cells by direct ex vivo class II tetramer staining in patients with acute HCV infection. (A) Sample dot blots for ex vivo tetramer staining with tetramer p177 in a patient with self-limited acute HCV infection (AR3) and a patient with chronically evolving HCV infection (AC1). (B) The ex vivo frequencies of all HCV MHC class II tetramers stainings performed on all samples of a total of 38 patients are plotted against the time elapsed between onset of symptoms and sampling of blood for each assay, depicted separately for AR infection and AC infection.
Analysis of the frequency of HCV-specific CD4+ T cells by direct ex vivo class II tetramer staining in patients with acute HCV infection. (A) Sample dot blots for ex vivo tetramer staining with tetramer p177 in a patient with self-limited acute HCV infection (AR3) and a patient with chronically evolving HCV infection (AC1). (B) The ex vivo frequencies of all HCV MHC class II tetramers stainings performed on all samples of a total of 38 patients are plotted against the time elapsed between onset of symptoms and sampling of blood for each assay, depicted separately for AR infection and AC infection.
Testing the first available PBMC sample after infection for each subject, at least one ex vivo tetramer response was detectable in 37 of 40 subjects with acute infection (93%). Of those, 14/17 patients with AC infection and 23/23 of patients with AR infection showed at least one tetramer response. Overall, we detected 55 of 61 (90%) individual positive tetramer tests, with 20/26 positive tetramer responses in patients with AC infection. In patients with AR infection, we detected 35/35 individual tetramer responses, and these were typically of higher frequency than those in subjects with persisting viremia. In addition, the three subjects without any detectable tetramer response all developed persistent viremia. However, these patients could only be tested at later time points (>75 d after the onset of symptoms), a timeframe in which the in vitro expansion assays usually do not recover HCV CD4+ T cell responses in AC infection either.
Tetramer assays also allowed us to examine the expression of memory T cell markers on HCV-specific CD4+ T cell populations. Overall, we saw low expression of CD127 in all subjects during early infection, in contrast to responses after spontaneous resolution of infection that uniformly express the IL-7 receptor (unpublished data). The activation marker CD38 was highly expressed during early infection in all patients, but was subsequently lost with the emergence of CD127+ memory cells. Expression of the inhibitory T cell receptor programmed death 1 was uniformly high, independent of HCV outcome, as we have previously shown (Kasprowicz et al., 2008). Overall these results are similar to what we have observed for HCV-specific CD8+ T cell responses (Lucas et al., 2004; Kasprowicz et al., 2008, 2010).
HCV-specific CD4+ T cell responses are deleted from the blood during persistence of HCV viremia, but early initiation of therapy can preserve CD4+ T cell populations
We were unable to detect CD4+ T cell responses by in vitro expansion once HCV viremia had been established for >6 mo, despite addition of exogenous rIL-2 (unpublished data; Schulze zur Wiesch et al., 2005). To determine whether this failure of detection was based on increasing deficits in proliferative capacity of HCV-specific T cells or on physical deletion of this T cell population from the blood, we longitudinally analyzed the kinetics of the CD4+ T cell response during acute HCV ex vivo using class II tetramers. In AR infection (Fig. 7 A), a strong expansion of HCV-specific CD4+ T cells was followed by a quick contraction of these populations after resolution of HCV, but core populations of tetramer-positive cells remained detectable long after the acute phase of infection. Throughout the observation period, proliferative responses remained strongly positive and cells were readily expandable for years.
Longitudinal assessment of HCV-specific CD4+ T cell responses by MHC class II tetramer staining. Detailed longitudinal results for three subjects with different clinical courses using standard proliferation assay and short-term cell lines with rIL-2, as in Fig. 1 D, as well as tetramer assays as shown in Fig. 6 (A and B). Frequencies of two class II tetramers (Tet124 and Tet177; triangles) over time for patient AR8 with AR infection blotted together with HCV viral load (gray; A) and as individual dot blots for each time point (B). (bottom) Results for the in vitro standard proliferation assay for comparison with the direct ex vivo tetramer results above. (C–F) Patients AC7 and AC10 were both diagnosed with acute HCV infection and persisting viremia. AC7 was treated at an early time point after developing symptomatic disease, whereas AC10 was treated only almost a year after the onset of symptoms. Both achieved a sustained virological response (SVR). Frequencies for detected tetramer responses (triangles) are plotted together with HCV viral load (gray) and the treatment period (red) in the top panel of C for AC7 and E for AC10. Asterisks in E mark time points at which tetramer assays were negative even with more sensitive tetramer staining protocols based on magnetic bead capture enrichment (Day et al., 2003). (C and E, bottom) Results from the standard proliferation assay over the same time periods as the tetramer results above. (D and F) ICS results detecting IFN-γ secretion are shown for short-term cultures at different time points of infection using rIL-2 and single HCV peptide that had tested positive in the first available blood sample.
Longitudinal assessment of HCV-specific CD4+ T cell responses by MHC class II tetramer staining. Detailed longitudinal results for three subjects with different clinical courses using standard proliferation assay and short-term cell lines with rIL-2, as in Fig. 1 D, as well as tetramer assays as shown in Fig. 6 (A and B). Frequencies of two class II tetramers (Tet124 and Tet177; triangles) over time for patient AR8 with AR infection blotted together with HCV viral load (gray; A) and as individual dot blots for each time point (B). (bottom) Results for the in vitro standard proliferation assay for comparison with the direct ex vivo tetramer results above. (C–F) Patients AC7 and AC10 were both diagnosed with acute HCV infection and persisting viremia. AC7 was treated at an early time point after developing symptomatic disease, whereas AC10 was treated only almost a year after the onset of symptoms. Both achieved a sustained virological response (SVR). Frequencies for detected tetramer responses (triangles) are plotted together with HCV viral load (gray) and the treatment period (red) in the top panel of C for AC7 and E for AC10. Asterisks in E mark time points at which tetramer assays were negative even with more sensitive tetramer staining protocols based on magnetic bead capture enrichment (Day et al., 2003). (C and E, bottom) Results from the standard proliferation assay over the same time periods as the tetramer results above. (D and F) ICS results detecting IFN-γ secretion are shown for short-term cultures at different time points of infection using rIL-2 and single HCV peptide that had tested positive in the first available blood sample.
In persisting acute infection (Fig. 7, C and E) a different picture emerged. Tetramer+ cells were detectable only initially during acute infection and then disappeared completely from the blood with persisting viremia, even if viral loads were relatively low (Fig. 7 C). Similarly, at early time points we were able to expand HCV-specific CD4+ T cell populations and confirm by ICS in these subjects, but not at later time points, when ex vivo tetramer assays were also negative. The standard LPA assays that we performed in parallel remained negative throughout the observation period.
Fig. 6 B summarizes the results for subjects with AC versus AR infection separately, plotting the results from all tetramer assays we performed in relationship to the day after onset of symptoms the sample was obtained. These ex vivo results look strikingly similar to the results obtained using in vitro HCV CD4+ T cell lines (Fig. 5, A and B), in that early during infection we see universally positive responses, followed by a rapid decline and disappearance of HCV-specific populations with persisting viremia. Barely any responses are detectable later than 3 mo after onset of symptoms by either method in AC subjects. In contrast, although the magnitude of individual responses decreases over time in AR infection, HCV-specific MHC class II tetramer+ T cells remain detectable directly ex vivo up to 1 yr and longer. Overall, the frequencies of tetramer+ populations are significantly higher in subjects with AR infection compared with those in individuals with persisting viremia. However, during the first 30–60 d of observation, these differences are much less pronounced than at later time points, and one observes a wide overlap between the two groups. Together, these data show that HCV-specific CD4+ T cell responses have two distinct fates. Spontaneous resolution of infection is associated with lasting CD4+ T cell populations that retain or even increase their proliferative capacity. In contrast the expansion of HCV-specific CD4+ T cells in persistent infection is short-lived, and the cell populations are already limited in their proliferative capacity at early time points, and then are completely undetectable in the blood by ex vivo tetramer technology or by in vitro expansion.
We were also able to follow subjects with persisting acute infection through antiviral therapy, with some subjects being treated relatively early (<24 wk after onset of symptoms; Fig. 7, C and D) and others receiving delayed treatment for a variety of clinical reasons (>24 wk after onset of symptoms; Fig. 7, E and F). Not only did tetramer responses reemerge after successful early treatment within the first 3 mo after onset of symptoms (Fig. 7 C and Fig. 8 B), but, in parallel, we were also able to detect broad responses through the assays using in vitro stimulation followed by confirmation of HCV-specific CD4+ T cell responses with ICS (Fig. 7 D and Fig. 8 A). In contrast, successful therapy during the subacute phase (later than 6 mo after onset of symptoms) or untreated AC infection were not associated with a reconstitution of HCV-specific CD4+ T cell responses (Fig. 7, E and F; and Fig. 8, A and B), similar to what we observe in patients after successful treatment in the chronic phase (not depicted). Overall, our data suggest that the deletion of HCV-specific CD4+ T cell responses is directly dependent on HCV viremia and that removal of the virus by antiviral therapy is able to prevent the complete loss of HCV-specific CD4+ T cell responses during a short window of opportunity.
Analysis of HCV-specific CD4+ T cell responses in patients with AC infection and a sustained virological response (SVR) after early versus late induction of antiviral treatment. (A) PBMCs obtained after successful antiviral therapy of acute infection and either early (<6 mo after symptom onset; n = 5) or late (<6 mo after symptom onset; n = 3) initiation of treatment were depleted of CD8+ T cells, stimulated with recombinant antigens and overlapping peptides, and after 10–14 d the number of single peptides eliciting an IFN-γ response was measured (as in Fig. 1 B). Each dot represents the number of different responses in each patient. (B) Ex vivo HCV MHC class II tetramer frequencies before and after treatment. Frequencies of HCV-specific CD4+ T cells were assessed before and after treatment using HLA class II tetramer staining in subjects with chronically evolving infection who were treated early. Untreated patients with AC served as controls. Each dot or square represents one tetramer response in a single individual.
Analysis of HCV-specific CD4+ T cell responses in patients with AC infection and a sustained virological response (SVR) after early versus late induction of antiviral treatment. (A) PBMCs obtained after successful antiviral therapy of acute infection and either early (<6 mo after symptom onset; n = 5) or late (<6 mo after symptom onset; n = 3) initiation of treatment were depleted of CD8+ T cells, stimulated with recombinant antigens and overlapping peptides, and after 10–14 d the number of single peptides eliciting an IFN-γ response was measured (as in Fig. 1 B). Each dot represents the number of different responses in each patient. (B) Ex vivo HCV MHC class II tetramer frequencies before and after treatment. Frequencies of HCV-specific CD4+ T cells were assessed before and after treatment using HLA class II tetramer staining in subjects with chronically evolving infection who were treated early. Untreated patients with AC served as controls. Each dot or square represents one tetramer response in a single individual.
DISCUSSION
There is a general consensus that vigorous CD4+ T cell proliferative responses are correlated with spontaneous resolution of HCV infection. Since the initial study by Diepolder et al. (1995), this observation has been confirmed by a multitude of studies (Chang et al., 2001; Schulze zur Wiesch et al., 2005; Smyk-Pearson et al., 2006). Nevertheless, little is known about the quantity and the quality of the early CD4+ T cell response beyond the ability of HCV-specific CD4+ T cells to proliferate against HCV proteins in vitro in standard LPA assays. It also remains unknown whether HCV-specific CD4+ T cell responses are generally absent in chronically evolving infection (with the exception of subjects with complete temporary control or patients that previously spontaneously cleared a heterologous HCV genotype), or whether responses are present but functionally disabled in persisting viremia. Another important question is whether targeting of certain epitopes (Diepolder et al., 1997; Schulze zur Wiesch et al., 2005; Smyk-Pearson et al., 2006) is necessary or sufficient to achieve a favorable outcome. In the present study, we have combined distinct methods characterized by high sensitivity, specificity, and resolution to characterize the CD4+ T cell response in a large cohort with early acute HCV infection and diverging clinical outcomes.
Our first surprising finding is that the breadth of the CD4+ T cell response during early acute infection is comparable between patients who become chronically infected and those with spontaneously resolving viremia. In all 31 subjects studied, we detected HCV-specific CD4+ T cells in HCV-stimulated T cell lines, with up to 26 epitopes targeted in a single individual. Although there was a trend toward a greater number of epitopes targeted in self-limited infection, this is most likely explained by the important observation that with persisting infection the breadth of the response is tightly correlated to the time elapsed after infection, as responses become undetectable within weeks (Fig. 5 A, Fig. 6 B, and Fig. 7 E). In contrast, the HCV-specific CD4+ T cell response repertoire in AR infection remains stable for months, and as we and others have previously shown, even years after infection (Takaki et al., 2000; Chang et al., 2001; Day et al., 2002). Hence, patients with persisting infection who were recruited into our study during the second half of our recruitment window for acute infection (between 3 and 6 mo after onset of symptoms) fully account for the trend toward somewhat fewer responses in persons with persisting infection. It seems very likely that analysis of subjects that were all recruited within the initial 8–12 wk after infection would show even less distinguishable results of the CD4+ T cell response over different clinical outcomes. Our data indicate that the progression of acute HCV infection into chronic hepatitis C is not a result of a primary failure to mount a broad CD4+ T cell response, but is caused by secondary mechanisms leading to the collapse of the HCV-specific CD4+ T cell response. Furthermore, our data highlight the importance of studying well-defined cohorts of early acute HCV infection (Cox et al., 2009) and of carefully differentiating between results obtained in the true acute phase of infection versus those in the subacute (early chronic) phase, e.g., between 6 and 12 mo after infection, as the CD4+ T cell response is clearly already profoundly altered at these later time points. Only at the earliest time points will it be possible to identify key mechanisms contributing to the outcome of infection, rather than just studying the consequences of viral persistence or control.
Our experimental approach allowed us to define not just the HCV protein targets of the CD4+ T cell response but also to identify for each subject the individual peptides that were recognized. The results confirm our previous study of resolved infection in that NS3, NS4, and NS5 are most frequently targeted, with 6 peptides resembling immunological “hotspots” that were recognized by >25% of subjects with spontaneous resolution of viremia. Importantly, we were able to detect these dominant responses in patients with early acute HCV infection that were not successful in controlling viremia. The recognition pattern in these subjects was very similar to that found in AR infection, raising the possibility that control of viremia is more a consequence of the quality, timeliness, kinetics, and maintenance of the CD4+ T cell response and not targeting of certain immunodominant epitopes.
The in vitro cell culture assay used for this study differed from the standard proliferation assay (LPA) by three important parameters: a higher input number of cells per test, an in vitro incubation time that is doubled, and the addition of rIL-2 to the culture. We sought to further define the relevance of these changes by performing additional CFSE experiments in which we compared the assay when performed with and without the addition of rIL-2. The results suggest that rIL-2 is a critical factor for the enhanced sensitivity of this assay and promote the hypothesis that a lack of IL-2 support is an important step in the failure to maintain CD4+ T cell response against persisting viruses (Iyasere et al., 2003; Lichterfeld et al., 2004; Klenerman and Hill, 2005; Semmo et al., 2005).
Based on our previous comprehensive screening for HCV CD4+ T epitopes (Schulze zur Wiesch et al., 2005), we constructed a set of novel class II tetramers that enabled us to detect HCV-specific CD4+ T cells directly ex vivo, with no need for in vitro culture. In contrast to previous studies, we were able to forego enrichment of the tetramer+ cells via magnetic beads caused by the higher numbers of specific cells during acute infection and improved quality of the tetrameric complexes. These experiments confirmed the presence of significant numbers of HCV-specific CD4+ T cells during the early stages of acute infection, irrespective of infection outcome and usually targeting multiple epitopes. Our results for several epitopes restricted by different class II molecules confirm and extend the findings by Lucas et al. (Lucas et al., 2007) and Diepolder (Ulsenheimer et al., 2006) who previously described tetramer responses in acute persisting HCV infection using a single class II tetramer restricted by HLA-DR1.
Even during early acute infection, frequencies of the ex vivo HCV-specific CD4+ T cells were somewhat lower in subjects with acute persisting infection; however, these differences seem to be gradual and there was substantial overlap between both groups. This difference could be explained by the distinct dynamics of the tetramer+ cells that become so rapidly undetectable (usually within weeks) in persisting viremia (Fig. 6 B and Fig. 7 E); thus, it seems possible that at the earliest stages of infection, before individuals were recruited into our study, the frequency and vigor of HCV-specific CD4+ T cells might have been comparable between the different patient groups. Alternatively, a marked difference in the vigor of the CD4+ T cell response at the earliest days of infection might be critical for the outcome of infection. To fully define the importance of the magnitude of HCV-specific CD4+ T cell responses, it would be helpful to study patients from prospective cohorts of acute HCV infection (Cox et al., 2005a), potentially allowing analysis of even earlier events after infection.
In contrast to the scenario in acute infection with persisting viremia, HCV-specific CD4+ T cell populations contract after spontaneous resolution of infection, but a core population remains detectable, often for years. Although our current hypothesis is that HCV-specific CD4+ T cells are mostly deleted through persistent viremia, it is also possible that the lack of detectable responses in PBMCs is at least partially caused by compartmentalization to the liver. In contrast to the CD8+ T cell response, for which there is ample evidence for this phenomenon (Nelson et al., 1998; Wong et al., 1998; Spangenberg et al., 2005; Neumann-Haefelin et al., 2008), little is known about compartmentalization of HCV-specific CD4+ T cells in chronic infection (Minutello et al., 1993; Penna et al., 2002). However, in our own preliminary studies, we could barely detect any intrahepatic HCV-specific CD4+ T cell responses using in vitro expansion protocols (n = 10 patients; unpublished data), despite our observation in the present study that the breadth of the peripheral CD4+ T cell response against HCV is generally greater than that of the CD8+ T cell response in acute infection (Lauer et al., 2005).
Another explanation for the collapse of the HCV-specific CD4+ T cell response in patients with acute HCV infection and persisting viremia could be in the development of viral escape mutations leading to a lack of stimulation of the T cell receptor. We have started to generate longitudinal viral sequences corresponding to the CD4+ epitopes targeted by individuals with chronic evolution of infection. Our preliminary results for 13 epitopes in 8 individuals do not indicate any viral evolution that could be attributed to CD4+ T cell responses (unpublished data), in contrast to observations for CD8+ responses (Timm et al., 2004; Cox et al., 2005b; Tester et al., 2005). Together with data from the chimpanzee model of HCV infection (Fuller et al., 2010), this suggests a limited role for viral escape mutations in the loss of the CD4+ T cell response in acute HCV infection that persists chronically.
Our observations in subjects undergoing antiviral therapy support the theory that the loss of proliferative function followed by deletion of HCV-specific CD4+ T cells is directly associated with HCV viremia. After successful control of viremia in early therapy, we were able to continuously detect HCV-specific CD4+ T cell responses against HCV both by extended in vitro culture and ex vivo by class II tetramers, comparable to what we have observed after spontaneous resolution of infection (Day et al., 2003; Schulze zur Wiesch et al., 2005). In contrast, HCV-specific CD4+ T cell responses were detectable neither by tetramer technology nor by in vitro cell lines and ICS in patients that had been treated at later stages of chronically evolving infection (>24 wk after onset of disease), suggesting that damage to the HCV-specific CD4+ T cell response occurs very early with persistent viremia, progresses rapidly, and becomes difficult to revert once chronicity has been established.
It is also noteworthy that, even with early therapy, the recovery of the CD4+ T cell response is not necessarily functionally complete; in patient AC10 (Fig. 7, C and D), HCV-specific responses remained undetectable by standard proliferation assay (without IL-2), but were detectable after in vitro expansion under addition of IL-2 and also directly ex vivo using class II tetramers. In subjects who were successfully treated at later stages of acute infection (>6 mo after symptoms), we could not detect CD4+ T cell responses against HCV by any method (Fig. 7, E and F; and Fig. 8, A and B). Together this suggests that the loss of CD4+ T cell function occurs before the physical deletion of the T cells and that fully functional CD4+ T cell responses are not required for the success of antiviral therapy (Rahman et al., 2004; Lauer et al., 2005; Barnes et al., 2009). Whether the rescue of CD4+ T cell responses through early treatment would be beneficial during a subsequent reexposure to HCV remains an open question.
One of the remaining challenges in unraveling the mystery of the secondary failure of the CD4+ T cell response in AC HCV infection will be to define the reasons for the functional differences of the HCV-specific CD4+ T cells, and also to define the exact mechanisms leading to deletion of these cells. Despite our ability to visualize HCV-specific T cells directly ex vivo by tetramer technology, the extremely low frequencies of these cell populations, even in the acute phase of infection, has prevented a more detailed ex vivo functional analysis at this time. However, based on the results from this study, we have now successfully begun to live-sort HCV-specific CD4+ T cells using tetramers for use in large scale gene expression analyses that have now become feasible even on very low cell numbers.
In summary, we demonstrate here for the first time that the HCV-specific CD4+ T cell response in persisting early acute infection is comparable in breadth, vigor, and repertoire to that observed in spontaneously resolving infection. Our findings challenge the current paradigm that suggests an initial failure to prime CD4+ T cell responses as the primary cause of viral persistence in most patients. Rather, persistent viremia is associated with a secondary failure of the CD4+ T cell response, which is characterized by early functional deficits and, later, complete deletion of the CD4+ T cell response from the blood through mechanisms not yet fully understood. Interestingly, the failure of the CD4+ T cell response can at least be partially reversed through early antiviral therapy, suggesting a direct role of HCV in subverting the immune response (Folgori et al., 2006; Badr et al., 2008). To understand the requirements of a protective CD4+ T cell immune response to be elicited by a successful HCV vaccine or immunotherapy, we must define the critical mechanisms that lead to the early functional impairment followed by the physical deletion of HCV-specific CD4+ T cells in the context of persisting viremia.
MATERIALS AND METHODS
Patients.
Acute hepatitis C was diagnosed by documented seroconversion to anti-HCV antibodies or all of the following: acute onset of hepatitis in previously healthy individual, aminotransferases at least 10× the upper limit of normal, exclusion of other infectious, metabolic, or toxic causes of hepatitis and recent exposure or source of infection identified (Corey et al., 2006; McGovern et al., 2006). 18 patients with spontaneously controlled HCV infection and 13 patients with chronically evolving HCV infection were enrolled in this study (Table I). Because only a limited number of subjects in this cohort of 31 patients expressed HLA class II molecules, allowing HCV class II tetramer analysis, we recruited a second cohort of 18 subjects for tetramer studies (Table S1). These individuals fulfilled the same criteria for acute HCV infection, and all expressed class II molecules for which HCV tetramers were available. The study was approved by the Institutional Review Boards of the Massachusetts General Hospital, the Lemuel Shattuck Hospital, and the Oswaldo Cruz Institute. All patients gave written informed consent.
HCV peptides and recombinant HCV proteins.
Peptides were synthesized corresponding to the amino acid sequences of the HCV-1a strain HCV-1 (available from GenBank/EMBL/DDBJ under accession no. M62321). A total of 301 20-mers overlapping by 10 aa and spanning the entire polyprotein were used for screening responses (Day et al., 2002). Additional peptides were synthesized according to autologous viral sequences. Recombinant HCV proteins used in this study were expressed as C-terminal fusion proteins with human superoxide dismutase in Saccharomyces cerevisiae or Escherichia coli and were provided by M. Houghton (Novartis Vaccines and Diagnostics). These proteins were derived from the HCV-1 sequence and encoded core (C22-3 aa 2 to 120), NS3 (C33C aa 1192–1457), NS4 (C100.3 aa 1569–1931), NS3/NS4 (C200 aa 1192–1931), and NS5 (NS5 aa 2054–2995).
HLA typing.
DNA for typing was extracted using the PUREGENE DNA isolation kit for blood (Gentra Systems) according to the manufacturer’s instructions. HLA class II typing was performed at the MGH Tissue Typing Laboratory using sequence-specific primer PCR, as previously described (Bunce et al., 1995). High-resolution HLA class II typing was performed by Dynal Biotech HLA Diagnostics (Invitrogen) using sequence-specific primer PCR.
Sequencing of autologous virus.
Sequences were determined as previously described (Timm et al., 2004; Schulze Zur Wiesch et al., 2007). In brief, viral RNA was extracted from plasma using the vRNA extraction kit (QIAGEN). Genotype-specific primers were designed based on alignments of all available sequences from public HCV databases. Reverse transcription was performed with the QIAGEN One-Step RT-PCR kit, followed by nested PCR using Titanium Taq DNA polymerase (CLONTECH Laboratories). PCR fragments were gel- or PCR-purified (QIAGEN QIA-Quick Gel Extraction/PCR Purification kit), and the population was sequenced bidirectionally on an ABI 3100 PRISM automated sequencer (Applied Biosystems). Sequencher (Gene Codes) and MacVector 4.1 (Oxford Molecular) software programs were used to edit and align sequences.
Lymphocyte proliferation assays.
Lymphocyte proliferation assays were performed as described previously (Day et al., 2002; Lauer et al., 2002) using c22.3, c33c, c100.3, C200 and NS5 HCV proteins (Chiron) at concentrations of 10 µg/ml (Day et al., 2002). CFSE proliferation assays were performed as previously described (Lichterfeld et al., 2004; Kasprowicz et al., 2008; Schulze Zur Wiesch et al., 2009). Where indicated, IL-2–neutralizing antibodies (clone MQ1-17H12; BD) or isotype control antibodies (clone A4A; Neomarkers) were added at 10 µg/ml.
Bulk stimulation of PBMCs.
15-20 million fresh or thawed PBMCs were depleted from CD8+ cells according to manufacturer’s instructions (Invitrogen) and the CD8+-depleted cells were stimulated with 1 µg/ml recombinant HCV antigens (either C22-3, C33C, C100, C200, or NS5) in 2 ml of R10 medium supplemented with recombinant IL-2 (50 U/ml) as previously described (Day et al., 2002; Schulze zur Wiesch et al., 2005, 2007). Alternatively, for the regions of the HCV genome for which recombinant antigens were not available (E1, E2, p7, and NS2) PBMCs were stimulated with overlapping 20-mer peptides (1 µg/ml each) in pools of 10 peptides.
To test the effect of additional stimulation with different interleukins, further HCV CD4+ T cell cultures were generated and rIL-7 and rIL-21 were added at day 1 and 3 at a final concentration of 50 ng/ml. After 10–14 d, the cells were assayed for IFN-γ production by ICS in response to stimulation with pools of overlapping 20-mer HCV peptides. After 10–14 d, cells were assayed for IFN-γ production by ELISpot or ICS in response to stimulation with HCV peptides.
ELISpot assay.
ELISpot assays were performed as previously described (Lauer et al., 2002, 2005; Schulze zur Wiesch et al., 2005). Responses were considered positive if the number of spots was three times greater than the background; phytohemagglutinin served as a positive control. All positive responses were reconfirmed by intracellular cytokine assay after stimulation with the specific peptide.
Intracellular IFN-γ staining and flow cytometry.
In brief, 5 × 105 bulk expanded PBMCs were stimulated with 4 µM peptide and anti-CD28 and anti-CD49d MAbs (1 µg/ml each; BD) at 37°C and 5% CO2 for 1 h before the addition of Brefeldin (1 µl/ml; BD). The cells were incubated for an additional 5 h at 37°C and 5% CO2. PBMCs were then washed and stained with surface antibodies, allophycocyanin-conjugated anti-CD3, and peridin-chlorophyll protein-conjugated anti-CD4 (BD) at room temperature for 20 min. After washing, the PBMCs were fixed and permeabilized (Invitrogen), and the FITC-conjugated anti–IFN-γ mAb (BD) were added as previously described (Day et al., 2002; Rosen et al., 2002; Lauer et al., 2004; Schulze zur Wiesch et al., 2005). Cells were then washed and analyzed on a FACSCalibur flow cytometer using CELLQuest software (BD).
MHC class II tetramer staining of PBMCs.
MHC class II tetramer staining was performed as previously described (Day et al., 2003; Kasprowicz et al., 2008). Cells (either fresh PBMCs, cryopreserved PBMCs, or cells from a short-term stimulated line) were stained in 100 µl medium (RPMI, 10% fetal calf serum, 10 mM Hepes, 2 mM l-glutamine, and 50 U/ml penicillin-streptomycin) with 2 µg of PE-conjugated MHC class II tetramer (Beckman Coulter) for 1 h at room at 37°C, and cells were washed once. Cells were then stained with APC Cy5.5-conjugated anti-CD4, PECy5.5 anti-CD30, CD14 PERCP, CD19 PERCP, and the respective fluorochrome-conjugated (APC, FITC, PE Cy7) CD27, CD38, CD45RA, or CCR7 MAbs were added and for the last 20 min (Day et al., 2003; Kasprowicz et al., 2008). Cells were analyzed by flow cytometry on a LSR2 machine (BD) using FlowJo (Tree Star) software. Cells were gated on CD4+, CD3+, CD14−, CD19−, and Via-Probe− cells. Multiple control tetramers with irrelevant peptides were used in most experiments to demonstrate specificity of binding by the relevant tetramers. Specificity of ex vivo tetramer responses was confirmed on epitope-specific cell lines of the same patient. All tetramers used were tested on cell lines of the respective patients, and we did not detect tetramer+ populations above background (>0.005%) in HLA-matched PBMCs of healthy volunteers or patients with chronic HCV infection (Day et al., 2003).
Statistical analysis.
Nonparametric Mann-Whitney U tests and regression analyses were performed using GraphPad Prism 4.0 software (GraphPad Software). To avoid overestimation of the total breadth of antiviral responses, responses to adjacent overlapping peptides were counted as responses to 1 epitopic region, with only the stronger response shown.
Online supplemental material.
Table S1 shows the clinical characteristics of a cohort of 18 additional patients with acute HCV infection studied with HCV MHC Class II tetramers. Table S2 shows individual HCV CD4+ epitopes targeted during acute HCV infection. Table S3 is a list of the HCV-specific MHC class II tetramers used in this study.
Acknowledgments
We thank foremost all patients who generously participated in this study and the dedicated clinical research staff and nurses who helped to recruit them. We thank Steven E. Longworth, Andrea Jones, Andrew Berical, Jared E. Duncan, Kei Ouchi and Jenna Blum for technical help with the experiments.
This study was supported by the Howard Hughes Medical Institute (J. Schulze zur Wiesch), Deutsche Forschungsgemeinschaft Schu 2482/1-1, SFB 841 A6 (J. Schulze zur Wiesch), Swiss National Science Foundation (D. Ciuffreda), and National Institutes of Health grants U19-AI066345 (L. Lewis-Ximenez, T.M. Allen, A.Y. Kim, and G.M. Lauer), U19-AI082630 (R.T. Chung and G.M. Lauer), and R01-AI067926-01 (T.M. Allen).
None of the authors declared any competing financial interests.
References
Author notes
J. Schulze zur Wiesch and D. Ciuffreda contributed equally to this paper.