Aggressive solid malignancies, including pancreatic ductal adenocarcinoma (PDAC), can exploit lysosomal exocytosis to modify the tumor microenvironment, enhance motility, and promote invasiveness. However, the molecular pathways through which lysosomal functions are co-opted in malignant cells remain poorly understood. In this study, we demonstrate that inositol polyphosphate 4-phosphatase, Type II (INPP4B) overexpression in PDAC is associated with PDAC progression. We show that INPP4B overexpression promotes peripheral dispersion and exocytosis of lysosomes resulting in increased migratory and invasive potential of PDAC cells. Mechanistically, INPP4B overexpression drives the generation of PtdIns(3,5)P2 on lysosomes in a PIKfyve-dependent manner, which directs TRPML-1 to trigger the release of calcium ions (Ca2+). Our findings offer a molecular understanding of the prognostic significance of INPP4B overexpression in PDAC through the discovery of a novel oncogenic signaling axis that orchestrates migratory and invasive properties of PDAC via the regulation of lysosomal phosphoinositide homeostasis.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive and lethal malignancy with overall 5-year survival of <10% that is projected to become a leading cause of cancer-related death (Siegel et al., 2023; Connor and Gallinger, 2022; Rahib et al., 2014). Although surgery, chemotherapy, radiation, and targeted therapies are commonly employed for PDAC, success rates remain dismally low (Kamisawa et al., 2016; Sohal et al., 2020). The challenging prognosis of PDAC is attributed to its rapid asymptomatic progression, early invasion and metastatic development, tumor heterogeneity, immunosuppressive tumor microenvironment, and limited responses to chemotherapy (Peng et al., 2023). These clinical complexities underscore a pressing need to gain a deeper understanding of PDAC biology and to uncover innovative treatment strategies.
Lysosomes are membrane-bound organelles found in eukaryotic cells that are best recognized as cellular degradation centers that can recycle essential resources for cell growth (Xu and Ren, 2015; Davidson and Vander Heiden, 2017). However, lysosomes and their membrane-associated proteins also exert significant influence on diverse cellular signaling pathways including nutrient sensing, immune cell signaling, metabolism, and membrane repair (Davidson and Vander Heiden, 2017; Perera and Zoncu, 2016). In cancer cells, lysosomes also contribute to disease progression by participating in diverse processes such as calcium homeostasis, exocytosis, cell adhesion, cellular motility, invasion, angiogenesis, and facilitation of metastasis formation (Machado et al., 2021; Nomura and Katunuma, 2005). Nevertheless, our understanding of the specific molecular mechanisms by which lysosomal functions are hijacked in cancer cells remains limited.
Our lab previously discovered a significant association between elevated levels of Inositol polyphosphate 4-phosphatase type II (INPP4B) and unfavorable outcomes in PDAC (Dzneladze et al., 2018). Although subsequent studies have corroborated this observation (Zhai et al., 2019; Gao et al., 2021; Zhou et al., 2019; Luo et al., 2021; Wei et al., 2021; Chen et al., 2022), how altered INPP4B signaling promotes PDAC progression still remains unclear. INPP4B was first classified as a tumor suppressor that was lost in basal-like breast cancers (Gewinner et al., 2009; Fedele et al., 2010; Westbrook et al., 2005). Conversely, emerging studies show that high levels of INPP4B expression (INPP4Bhigh) are also associated with tumor progression in diverse cancers (Dzneladze et al., 2015; Rijal et al., 2015; Gasser et al., 2014; Rodgers et al., 2021b; Guo et al., 2016).
INPP4B is a phosphatidylinositol (PtdIns) phosphatase that catalyzes the dephosphorylation of PtdIns(3,4)P2 to form PtdIns(3)P on the plasma membrane and endosomal membranes (Li Chew et al., 2015; Liu et al., 2020; Rodgers et al., 2021a). Recent studies underscore the influence of INPP4B expression on the PtdIns composition of lysosomal membranes and the implications for various aspects of lysosomal function, including trafficking, permeability, reformation, and autophagy (Rodgers et al., 2021b, 2022; Woolley et al., 2021, Preprint; Saffi et al., 2022). In this study, we present a novel mechanism whereby elevated INPP4B levels in PDAC cells shift the balance of lysosomal PtdIns to favor peripheral lysosome localization and lysosomal exocytosis, which then promotes cellular motility and invasion, and thus PDAC progression.
Results
INPP4B overexpression is a common feature of PDAC
To provide evidence supporting a tumor-promoting role for INPP4B in PDAC, we examined INPP4B expression in various public PDAC databases. We observed consistently higher INPP4B transcript levels in PDAC specimens in comparison to non-tumoral pancreatic tissues across several PDAC datasets (Fig. 1 a). INPP4B protein levels were also increased in PDAC (Fig. S1 a). Moreover, PDAC ranks among cancers with the highest expression levels of INPP4B (Fig. S1 b), and similarly PDAC cell lines stand out as having some of the highest INPP4B expression levels among all cancer cell lines (Fig. S1 c). These data indicate that high levels of INPP4B (INPP4Bhigh) are a common feature of PDAC. Consistent with previous reports, survival analysis of PDAC patient data from The Cancer Genome Atlas-Pancreatic Ductal Adenocarcinoma study (TCGA-PAAD), the International Cancer Genome Consortium (ICGC) Canada study, and two GEO PDAC datasets (GSE21501 and GSE79670) confirm that INPP4Bhigh is indeed associated with decreased overall survival compared with INPP4Blow expression in PDAC (Fig. 1 b).
High levels of INPP4B expression are a common feature in primary PDAC patient samples and affects growth of PDAC cell lines. (a)INPP4B transcript expression from normal pancreas (N) versus PDAC patient specimens (P) in GEO datasets. (b) Kaplan–Meier survival analysis performed on four patient datasets where INPP4Bhigh versus INPP4Blow are compared using a median cutoff. (c and d) Depicted are cells with stable overexpression of INPP4B in INPP4Blow (c) PANC-1 or INPP4B KO in (d) INPP4Bhigh HPAC cell lines. Cells were tested for INPP4B protein expression by immunoblot (top left panels). Representative images of crystal violet–stained colonies and quantitation of average colony number and average colony size in clonogenic assay of INPP4B overexpressing (c) PANC-1 or INPP4B KO in (d) HPAC cells, with respective empty controls for PANC-1 cells or indicated control CRISPR-Cas9 KO for HPAC cells 19 days after seeding. Proliferation assays (bottom left images) and representative crystal violet–stained wells (bottom right graph) at day 0 and on the indicated day of the proliferation assay along with crystal violet quantification for the indicated days. Data are representative of three individual experiments, with unpaired two-tailed parametric t test performed for a and colony formation assays for c, log-rank (Mantel-Cox) test for b, one-way ANOVA Tukey’s post hoc test for growth curve assays from c and colony formation assays from d, two-way ANOVA and multiple unpaired t tests for growth curve assays from d. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Source data are available for this figure: SourceData F1.
High levels of INPP4B expression are a common feature in primary PDAC patient samples and affects growth of PDAC cell lines. (a)INPP4B transcript expression from normal pancreas (N) versus PDAC patient specimens (P) in GEO datasets. (b) Kaplan–Meier survival analysis performed on four patient datasets where INPP4Bhigh versus INPP4Blow are compared using a median cutoff. (c and d) Depicted are cells with stable overexpression of INPP4B in INPP4Blow (c) PANC-1 or INPP4B KO in (d) INPP4Bhigh HPAC cell lines. Cells were tested for INPP4B protein expression by immunoblot (top left panels). Representative images of crystal violet–stained colonies and quantitation of average colony number and average colony size in clonogenic assay of INPP4B overexpressing (c) PANC-1 or INPP4B KO in (d) HPAC cells, with respective empty controls for PANC-1 cells or indicated control CRISPR-Cas9 KO for HPAC cells 19 days after seeding. Proliferation assays (bottom left images) and representative crystal violet–stained wells (bottom right graph) at day 0 and on the indicated day of the proliferation assay along with crystal violet quantification for the indicated days. Data are representative of three individual experiments, with unpaired two-tailed parametric t test performed for a and colony formation assays for c, log-rank (Mantel-Cox) test for b, one-way ANOVA Tukey’s post hoc test for growth curve assays from c and colony formation assays from d, two-way ANOVA and multiple unpaired t tests for growth curve assays from d. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Source data are available for this figure: SourceData F1.
Effect of INPP4B expression on PDAC cell growth. (a) Differential analysis of INPP4B protein expression in normal and primary PDAC were assessed using Clinical Proteome Tumor Analysis Consortium (CPTAC) data accessed from the UALCAN data portal. (b) Dot blot of INPP4B transcript expression of INPP4B in normal (N) and tumor specimens (T) across 33 TCGA tumor samples and paired normal tissues. (c) Ranked means and total mean (inset) of INPP4B expression in PDAC cell lines compared to all other cancer cell lines in the Cancer Cell Line Encyclopedia. (d and e) Images representing cells with stable overexpression of INPP4B in INPP4Blow (d) PK-1 or INPP4B KO in (e) INPP4Bhigh PK8 cell lines. Cells were tested for INPP4B protein expression by IB (top left panels). Representative images of crystal violet–stained colonies and quantitation of average colony number and average colony size in clonogenic assay, with respective empty controls for PK-1 cells (d) or control CRISPR-Cas9 KO in (e) PK8 cells 19 days after seeding. Proliferation assays (bottom left images) and representative crystal violet–stained wells (bottom right graph) at day 0 and on the final day of the proliferation assay along with crystal violet quantification for the indicated days for PK-1 (d) and PK8 (e) cells. (f–h) Anchorage-independent growth capacity in soft agar (representative images, left images) was quantitated (right graphs) for empty control or INPP4B overexpressing PANC-1 (f) and PK-1 (g) cells, control or INPP4B CRISPR-Cas9 KO for PK8 (h) cells. (i)pCW-INPP4B PANC-1 and PK-1 cells were treated with Dox at the indicated concentrations, and HPAC cells stably expressing indicated sgRNAs were immunoblotted for INPP4B, LAMP1, and actin. (j) Quantitation of total LAMP1 intensity per cell for indicated cell lines and treatments. Data are representative of ± SD from three individual experiments for d–h, with 100–120 cells counted per treatment per condition for j, with unpaired two-tailed parametric t test performed for c, and colony formation assays for d, e, f–h, and j, one-way ANOVA Tukey’s post hoc test for j, and two-way ANOVA and multiple unpaired t tests for growth curve assays for d and e. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Source data are available for this figure: SourceData FS1.
Effect of INPP4B expression on PDAC cell growth. (a) Differential analysis of INPP4B protein expression in normal and primary PDAC were assessed using Clinical Proteome Tumor Analysis Consortium (CPTAC) data accessed from the UALCAN data portal. (b) Dot blot of INPP4B transcript expression of INPP4B in normal (N) and tumor specimens (T) across 33 TCGA tumor samples and paired normal tissues. (c) Ranked means and total mean (inset) of INPP4B expression in PDAC cell lines compared to all other cancer cell lines in the Cancer Cell Line Encyclopedia. (d and e) Images representing cells with stable overexpression of INPP4B in INPP4Blow (d) PK-1 or INPP4B KO in (e) INPP4Bhigh PK8 cell lines. Cells were tested for INPP4B protein expression by IB (top left panels). Representative images of crystal violet–stained colonies and quantitation of average colony number and average colony size in clonogenic assay, with respective empty controls for PK-1 cells (d) or control CRISPR-Cas9 KO in (e) PK8 cells 19 days after seeding. Proliferation assays (bottom left images) and representative crystal violet–stained wells (bottom right graph) at day 0 and on the final day of the proliferation assay along with crystal violet quantification for the indicated days for PK-1 (d) and PK8 (e) cells. (f–h) Anchorage-independent growth capacity in soft agar (representative images, left images) was quantitated (right graphs) for empty control or INPP4B overexpressing PANC-1 (f) and PK-1 (g) cells, control or INPP4B CRISPR-Cas9 KO for PK8 (h) cells. (i)pCW-INPP4B PANC-1 and PK-1 cells were treated with Dox at the indicated concentrations, and HPAC cells stably expressing indicated sgRNAs were immunoblotted for INPP4B, LAMP1, and actin. (j) Quantitation of total LAMP1 intensity per cell for indicated cell lines and treatments. Data are representative of ± SD from three individual experiments for d–h, with 100–120 cells counted per treatment per condition for j, with unpaired two-tailed parametric t test performed for c, and colony formation assays for d, e, f–h, and j, one-way ANOVA Tukey’s post hoc test for j, and two-way ANOVA and multiple unpaired t tests for growth curve assays for d and e. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Source data are available for this figure: SourceData FS1.
To provide experimental evidence for the association of INPP4Bhigh with PDAC progression, we performed proliferation, colony formation, and anchorage-independent growth assays in syngeneic gain- and loss-of-function PDAC cell line models. We observed that INPP4B overexpression in the INPP4Blow PANC-1 and PK-1 led to increased colony-forming potential, enhanced foci formation in soft-agar, and a greater proliferative capacity (Fig. 1 c; and Fig. S1, d, f, and g). On the other hand, CRISPR-Cas9–mediated knockout (KO) of INPP4B in INPP4Bhigh cell lines HPAC and PK8 diminished colony-forming potential, foci formation in soft-agar, and proliferative capacity (Fig. 1 d; and Fig. S1, e and h). Together, these data suggest that INPP4B is sufficient to promote growth, and necessary to maintain normal growth potential of PDAC cells.
INPP4B regulates lysosome localization in PDAC cell lines
To uncover cellular alterations by which INPP4Bhigh may promote PDAC progression, we examined the TCGA-PAAD patient dataset using Gene Set Enrichment Analysis (GSEA; https://www.gsea-msigdb.org) and INPP4B expression. The Kyoto Encyclopedia of Genes and Genomes (KEGG) lysosomal gene set demonstrated the highest ranking (Fig. 2 a), and related gene sets associated with autophagy and endocytosis were also significantly enriched in INPP4Bhigh patient samples (Fig. 2 b). These associations implicate INPP4B in lysosomal functions; however, specific mechanisms in PDAC remain unknown.
INPP4B expression regulates lysosomal localization in PDAC cells. (a) Top 20 KEGG gene sets enriched in INPP4Bhigh TCGA-PAAD patients as determined using GSEA. (b) KEGG lysosome, autophagy, and endocytosis enrichment plots from analysis of INPP4Bhigh GSEA analysis. NES, normalized enrichment score. (c) Micrographs of indicated PDAC cell lines immunostained for LAMP1 (green) and DAPI nuclear stain (blue). Scale bar: 10 µm. (d) Whole-cell lysates from indicated PDAC cell lines were subjected to IB for INPP4B and GAPDH. Quantification of INPP4B expression levels normalized to GAPDH is shown in red. (e) Illustration of image analysis technique used to segment cell areas for nucleus, inner shell (perinuclear), and outer shell (peripheral). (f) Quantitation of LAMP1 intensity distribution for images from c as measured by peripheral (outer shell)/perinuclear (inner shell) LAMP1 intensity ratio. (g) Scatterplot correlation of INPP4B protein expression in various PDAC cell lines versus outer/inner shell LAMP1 intensity. (h and j) LAMP1 immunostaining and DAPI nuclear staining of (h) PANC-1 and (j) PK-1 stably expressing pCW-INPP4B with and without 500 nM Dox. Scale bar: 20 µm for (h) PANC-1 cells and 15 µm for (j) PK-1 cells. (i and k) Quantitation of LAMP1 intensity distribution as measured by peripheral (outer shell)/perinuclear (inner shell) LAMP1 intensity ratio for (i) PANC-1 and (k) PK-1 cells. (l) HPAC cells stably expressing CRISPR-Cas9 and indicated sgRNAs for sgCDY1B, sgINPP4B-1, or sgINPP4B-2 immunostained for LAMP1 and nuclear stained with DAPI. Scale bar: 10 µm. (m) Quantitation of LAMP1 intensity distribution for l HPAC cells. Data represent ± SD from 100 to 120 cells from three independent experiments assessed per treatment per condition, with one-way ANOVA Tukey’s post hoc test performed for f and m, and unpaired two-tailed parametric t test for i and k. ***P < 0.001, ****P < 0.0001. Source data are available for this figure: SourceData F2.
INPP4B expression regulates lysosomal localization in PDAC cells. (a) Top 20 KEGG gene sets enriched in INPP4Bhigh TCGA-PAAD patients as determined using GSEA. (b) KEGG lysosome, autophagy, and endocytosis enrichment plots from analysis of INPP4Bhigh GSEA analysis. NES, normalized enrichment score. (c) Micrographs of indicated PDAC cell lines immunostained for LAMP1 (green) and DAPI nuclear stain (blue). Scale bar: 10 µm. (d) Whole-cell lysates from indicated PDAC cell lines were subjected to IB for INPP4B and GAPDH. Quantification of INPP4B expression levels normalized to GAPDH is shown in red. (e) Illustration of image analysis technique used to segment cell areas for nucleus, inner shell (perinuclear), and outer shell (peripheral). (f) Quantitation of LAMP1 intensity distribution for images from c as measured by peripheral (outer shell)/perinuclear (inner shell) LAMP1 intensity ratio. (g) Scatterplot correlation of INPP4B protein expression in various PDAC cell lines versus outer/inner shell LAMP1 intensity. (h and j) LAMP1 immunostaining and DAPI nuclear staining of (h) PANC-1 and (j) PK-1 stably expressing pCW-INPP4B with and without 500 nM Dox. Scale bar: 20 µm for (h) PANC-1 cells and 15 µm for (j) PK-1 cells. (i and k) Quantitation of LAMP1 intensity distribution as measured by peripheral (outer shell)/perinuclear (inner shell) LAMP1 intensity ratio for (i) PANC-1 and (k) PK-1 cells. (l) HPAC cells stably expressing CRISPR-Cas9 and indicated sgRNAs for sgCDY1B, sgINPP4B-1, or sgINPP4B-2 immunostained for LAMP1 and nuclear stained with DAPI. Scale bar: 10 µm. (m) Quantitation of LAMP1 intensity distribution for l HPAC cells. Data represent ± SD from 100 to 120 cells from three independent experiments assessed per treatment per condition, with one-way ANOVA Tukey’s post hoc test performed for f and m, and unpaired two-tailed parametric t test for i and k. ***P < 0.001, ****P < 0.0001. Source data are available for this figure: SourceData F2.
To experimentally validate these associations, we examined lysosomal-associated membrane protein 1 (LAMP1) by immunofluorescence (IF) in a panel of PDAC cell lines. We observed that INPP4Blow cell lines displayed a predominant perinuclear LAMP1 staining pattern compared with INPP4Bhigh cell lines that exhibited more peripheral LAMP1 staining (Fig. 2, c and d). To quantitate these observations, we adapted an image analysis techniques reported previously (Saric et al., 2016; Li et al., 2016; Johnson et al., 2016) where individual cell cytoplasms were divided into inner (perinuclear region) and outer (juxtamembrane region) shells allowing the calculation of a standardized outer/inner LAMP1 intensity ratio (Fig. 2 e). Quantitation of lysosomal localization in the panel of PDAC cell lines demonstrated that INPP4Bhigh cell lines have a greater outer/inner LAMP1 intensity ratio compared with INPP4Blow cell lines (Fig. 2 f). Remarkably, we observed a significant correlation between INPP4B expression levels and outer/inner shell LAMP1 intensity ratio (r2 = 0.83; P = 0.0044; Fig. 2 g). These results provide evidence that INPP4B expression levels are associated with lysosomal localization in PDAC cells.
Next, to examine if INPP4B has a direct role in regulating lysosomal residency, we measured lysosomal localization in INPP4B gain- and loss-of-function PDAC cells. Indeed, INPP4B overexpression in the INPP4Blow PANC-1 and PK-1 cells promoted the relocalization of lysosomes to the cell periphery (Fig. 2, h–k) and KO of INPP4B in INPP4Bhigh HPAC cells promoted the localization of lysosomes to perinuclear regions (Fig. 2, l and m). Despite changes in localization, lysosomal levels remained largely unchanged as measured by LAMP1 western blot and IF upon INPP4B overexpression or KO, with the exception of a small increase in PANC-1 cells (Fig. S1, i and j).
Peripheral dispersion of lysosomes has been associated with increased lysosomal pH and increased activity of lysosomal proteases (Brix et al., 2014; Rafn et al., 2012; Hämälistö and Jäättelä, 2016). To understand if INPP4B is involved in these processes, we measured lysosomal pH and protease activity in INPP4B-overexpressing PDAC cells using Lysotracker and dye quenched-bovine serun albumin (DQ-BSA), respectively. With these tools, we measured significant increases in the number of acidic organelles and lysosomal degradation capacity, suggesting that INPP4B overexpression can upregulate both lysosomal pH and lysosomal degradation functions (Fig. 2, a–l).
INPP4B activates lysosomal gene expression independently of mTORC1 or transcription factor EB (TFEB) localization
To build upon the GSEA results in PDAC (Fig. 2, a and b), we examined the consequences of INPP4B overexpression and KO on lysosomal transcript levels in PDAC cells. Qiantitative RT-PCR (qRT-PCR) identified upregulation of a panel of lysosomal transcripts (LAMP1, MCOLN-1, CTSD, ATPV61D, ATPV61H) upon INPP4B overexpression in PANC-1 and PK-1 cells, and general downregulation of those same transcripts upon INPP4B KO in HPAC cells (Fig. S3 a). Under the same conditions leading to lysosomal dispersion and transcriptional activation, we also examined AKT (Protein Kinase B)-mTORC1 signaling, a pathway that regulates transcription during lysosome biogenesis (Roczniak-Ferguson et al., 2012; Napolitano et al., 2018; Martina et al., 2012). No changes in the activation of AKT, mTORC1, and downstream effectors S6 kinase and 4EBP1 were observed by western blot upon INPP4B overexpression (Fig. S3 b). Additionally, we assessed the activation status of TFEB, a pivotal transcriptional regulator of most lysosomal transcripts and lysosomal biogenesis (Choy et al., 2018; Sardiello et al., 2009). Nuclear localization and subsequent transcriptional functions of TFEB are governed by a phosphorylation event at Ser211, mediated by mTORC1 (Roczniak-Ferguson et al., 2012). In our experiments, no changes in TFEB phosphorylation were observed upon INPP4B overexpression by western blot (Fig. S3 b). We also employed IF to examine nuclear translocation of TFEB in PANC-1 and PK-1 cells. Under amino acid starvation conditions, we observed the typical relocalization of TFEB to the nuclear compartment; however, INPP4B overexpression did not alter nuclear localization patterns in basal or starved states (Fig. S3, c and d). Similarly, nuclear-cytoplasmic fractionation did not reveal any role for INPP4B in TFEB localization (Fig. S3 e). Further analysis of transcription factor binding to IGHM enhancer 3 (TFE3) and microphthalmia-associated transcription factor (MITF), TFEB-related proteins belonging to the MiT/TFE subclass of basic helix-loop-helix family of transcription factors also involved in lysosome biogenesis (Martina et al., 2014; Perera et al., 2019), revealed no changes in subcellular localization upon INPP4B overexpression (Fig. S3 e).
Effect of INPP4B expression on lysosome function. (a–l) PANC-1 (a–f) or PK-1 (g–l) cells transiently expressing pmCherry or pmCherry-INPP4B were stained with Lysotracker Green (a and g) or DQ Green BSA (b and h) or Lucifer Yellow (c and i). Scale bar: 25 µm. Flow cytometry used to quantitate Lysotracker Green (lysosome acidity probe) (d and j) or DQ-BSA (probe for lysosomal proteolytic function) (e and k) or Lucifer Yellow (to assess endocytosis toward lysosomes) (f and l) intensity in non-transfected, mCherry, or INPP4B-mCherry expressing PANC-1 (a–f) or PK-1 (g–l) cells. Data represent ± SD from three independent experiments, with unpaired two-tailed parametric t test performed for statistical measures. *P < 0.05.
Effect of INPP4B expression on lysosome function. (a–l) PANC-1 (a–f) or PK-1 (g–l) cells transiently expressing pmCherry or pmCherry-INPP4B were stained with Lysotracker Green (a and g) or DQ Green BSA (b and h) or Lucifer Yellow (c and i). Scale bar: 25 µm. Flow cytometry used to quantitate Lysotracker Green (lysosome acidity probe) (d and j) or DQ-BSA (probe for lysosomal proteolytic function) (e and k) or Lucifer Yellow (to assess endocytosis toward lysosomes) (f and l) intensity in non-transfected, mCherry, or INPP4B-mCherry expressing PANC-1 (a–f) or PK-1 (g–l) cells. Data represent ± SD from three independent experiments, with unpaired two-tailed parametric t test performed for statistical measures. *P < 0.05.
Effect of INPP4B expression on lysosome gene transcription and TFEB function. (a) Stably expressing pCW-INPP4B PANC-1 and PK-1 cells treated with or without 500 nM Dox, or HPAC cells stably expressing the indicated gRNAs sgCDY1B (C), sgINPP4B-1 (1), or sgINPP4B-2 (2), and assessed for gene expression through qRT-PCR for the select lysosome genes as indicated and normalized to GAPDH. (b)pCW-INPP4B PANC-1 or PK-1 cells without or with 500 nM Dox immunoblotted for total and phosphorylated states for the proteins TFEB, mTOR, AKT, S6, 4EBP1, INPP4B, and β-actin. (c and d)pCW-INPP4B (c) PANC-1 cells or (d) PK-1 cells without or with 500 nM Dox or EBSS serum starvation and immunostained for endogenous TFEB (green) and DAPI nucleus (blue). Scale bar: 20 µm. Quantification of nuclear TFEB over cytosol TFEB intensity for PANC-1 (c) and PK-1 (d) cells. (e) Immunoblot of the isolated nucleus and cytosol fractions of no Dox or 500 nM Dox treated pCW-INPP4B PANC-1 cells or PK-1 cells for TFEB, MITF, and TFE3. (f and g)pCW-INPP4B PANC-1 cells treated with siCTRL or siTFEB and no Dox or 500 nM DOX and (f) immunoblot for INPP4B TFEB and actin, (g) examined for transcript expression of select lysosome genes through qRT-PCR. (h)pCW-INPP4B PANC-1 cells treated with siCTRL or siTFEB and without or with 500 nM Dox immunostained for LAMP1 (green) and DAPI nucleus (blue) and assessed for LAMP1 outer/inner shell intensity distribution. Scale bar: 20 µm. Data represent ± SD from three independent experiments for each treatment condition with 80–100 cells examined for c, d, and h, with unpaired two tailed parametric t test performed for PANC-1 and PK-1 cells for a, one-way ANOVA Tukey’s post hoc test for HPAC cells for a, c, d, g, and h. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Source data are available for this figure: SourceData FS3.
Effect of INPP4B expression on lysosome gene transcription and TFEB function. (a) Stably expressing pCW-INPP4B PANC-1 and PK-1 cells treated with or without 500 nM Dox, or HPAC cells stably expressing the indicated gRNAs sgCDY1B (C), sgINPP4B-1 (1), or sgINPP4B-2 (2), and assessed for gene expression through qRT-PCR for the select lysosome genes as indicated and normalized to GAPDH. (b)pCW-INPP4B PANC-1 or PK-1 cells without or with 500 nM Dox immunoblotted for total and phosphorylated states for the proteins TFEB, mTOR, AKT, S6, 4EBP1, INPP4B, and β-actin. (c and d)pCW-INPP4B (c) PANC-1 cells or (d) PK-1 cells without or with 500 nM Dox or EBSS serum starvation and immunostained for endogenous TFEB (green) and DAPI nucleus (blue). Scale bar: 20 µm. Quantification of nuclear TFEB over cytosol TFEB intensity for PANC-1 (c) and PK-1 (d) cells. (e) Immunoblot of the isolated nucleus and cytosol fractions of no Dox or 500 nM Dox treated pCW-INPP4B PANC-1 cells or PK-1 cells for TFEB, MITF, and TFE3. (f and g)pCW-INPP4B PANC-1 cells treated with siCTRL or siTFEB and no Dox or 500 nM DOX and (f) immunoblot for INPP4B TFEB and actin, (g) examined for transcript expression of select lysosome genes through qRT-PCR. (h)pCW-INPP4B PANC-1 cells treated with siCTRL or siTFEB and without or with 500 nM Dox immunostained for LAMP1 (green) and DAPI nucleus (blue) and assessed for LAMP1 outer/inner shell intensity distribution. Scale bar: 20 µm. Data represent ± SD from three independent experiments for each treatment condition with 80–100 cells examined for c, d, and h, with unpaired two tailed parametric t test performed for PANC-1 and PK-1 cells for a, one-way ANOVA Tukey’s post hoc test for HPAC cells for a, c, d, g, and h. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Source data are available for this figure: SourceData FS3.
Importantly, although TFEB localization patterns were unaltered by INPP4B overexpression, we observed that siRNA knockdown of TFEB inhibited the ability of INPP4B to induce lysosomal gene transcription (Fig. S3, f and g). Furthermore, siTFEB inhibited the lysosomal dispersion triggered by INPP4B overexpression (Fig. S3 h). Together, these results suggest that INPP4B-mediated induction of lysosomal gene transcription and lysosomal dispersion require TFEB function to proceed.
INPP4B expression affects cellular calcium ion (Ca2+) levels in a transient receptor potential cation channel, mucolipin subfamily, member 1 (TRPML-1/MCOLN1)–dependent manner
Cellular alterations in Ca2+ concentrations have been reported to influence the motility of intracellular organelles, including lysosomes (Li et al., 2016; Oyarzún et al., 2019). To investigate if INPP4B controls lysosome positioning through regulation of Ca2+, we assessed Ca2+ levels upon overexpression or KO of INPP4B in PDAC cells using the green fluorescent calcium indicator Fluo-4 AM (Saffi et al., 2021; Lloyd-Evans and Waller-Evans, 2020). Overexpression of INPP4B elevated total cellular Fluo-4 AM intensity and Lysotracker Red-colocalized (peri-lysosomal) Fluo-4 AM intensity in both PANC-1 and PK-1 cells (Fig. 3, a–c; and Fig. S4, a–e), and KO of INPP4B in HPAC cells significantly reduced both cellular and peri-lysosomal Fluo-4 AM intensity (Fig. 3, d–f; and Fig. S4 f). These results suggest that INPP4B may influence both cellular and peri-lysosomal Ca2+ levels. The potent calcium ionophore ionomycin served as a positive control in these experiments demonstrating rapid increases in Ca2+ levels with a pattern that is diffuse with some intense areas of Fluo-4 activation (Fig. 3, a–c) (Dayam et al., 2015). To further confirm that Ca2+ levels were induced in the immediate vicinity of lysosomes, we utilized the LAMP2-GCaMP6s biosensor, composed of the calcium indicator GCaMP6s fused to the cytosol-facing C terminus of LAMP2. With this tool, we can observe calcium release within proximity of LAMP2-positive lysosomal membranes. We observed that INPP4B overexpression increased peri-lysosomal and cytosol Ca2+ levels in PANC-1 and PK-1 cells (Fig. 3, g–i; and Fig. S4, g–i), consistent with previous data collected using Fluo-4 AM intensity. As a further corroboration, Glycyl-L-phenylalanine 2-naphthylamide (GPN), a lysosomotropic agent that selectively mobilizes lysosomal Ca2+ was used as a positive control in these experiments (Fig. 3, g–i; and Fig. S4, g–i). In sum, these data suggest that INPP4B promotes the release of lysosomal Ca2+ stores.
INPP4B expression regulates cellular Ca 2+ levels in PDAC cells through TRPML-1 function. (a) pCW-INPP4B PANC-1 cells without or with 500 nM Dox or ionomycin were co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green). Scale bar: 15 µm. (b and c) Quantitation of (b) Fluo-4 AM intensity on Lysotracker Red–positive structures or (c) cytosolic Fluo-4 AM intensity. (d–f) HPAC cells stably expressing indicated sgRNAs were (d) co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green) and quantitated (e and f) as above. Scale bar: 10 µm. (g–i) (g) pCW-INPP4B PANC-1 without or with 500 nM Dox or Gly-Phe β-naphthylamide (GPN) treatment, transfected with pBoBi-hLAMP2-C-GC6s (LAMP2-GCaMP6 [green]), labeled for lysosomes with Lysotracker Red (red), and quantitated (h and i) as above. Scale bar: 5 µm. (j–l) (j) pCW-INPP4B PANC-1 without or with 500 nM Dox were treated with vehicle or ML-SI3, co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green), and quantitated (k and l) as above. Scale bar: 15 µm. Data represent ± SD from 100 to 120 cells (a–f and j–l) or 50–60 cells (g–i) from three independent experiments assessed per treatment per condition, with one-way ANOVA Tukey’s post hoc test performed for statistical measures. **P < 0.01, ***P < 0.001, ****P < 0.0001.
INPP4B expression regulates cellular Ca 2+ levels in PDAC cells through TRPML-1 function. (a) pCW-INPP4B PANC-1 cells without or with 500 nM Dox or ionomycin were co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green). Scale bar: 15 µm. (b and c) Quantitation of (b) Fluo-4 AM intensity on Lysotracker Red–positive structures or (c) cytosolic Fluo-4 AM intensity. (d–f) HPAC cells stably expressing indicated sgRNAs were (d) co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green) and quantitated (e and f) as above. Scale bar: 10 µm. (g–i) (g) pCW-INPP4B PANC-1 without or with 500 nM Dox or Gly-Phe β-naphthylamide (GPN) treatment, transfected with pBoBi-hLAMP2-C-GC6s (LAMP2-GCaMP6 [green]), labeled for lysosomes with Lysotracker Red (red), and quantitated (h and i) as above. Scale bar: 5 µm. (j–l) (j) pCW-INPP4B PANC-1 without or with 500 nM Dox were treated with vehicle or ML-SI3, co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green), and quantitated (k and l) as above. Scale bar: 15 µm. Data represent ± SD from 100 to 120 cells (a–f and j–l) or 50–60 cells (g–i) from three independent experiments assessed per treatment per condition, with one-way ANOVA Tukey’s post hoc test performed for statistical measures. **P < 0.01, ***P < 0.001, ****P < 0.0001.
INPP4B expression regulates cellular Ca 2+ levels in PDAC cells. (a) pCW-INPP4B PK-1 cells treated without or with 500 nM Dox or ionomycin were co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green). Scale bar: 15 µm. (b and c) Quantitation of Fluo-4 AM intensity on Lysotracker Red–positive structures (b) or cytosolic Fluo-4 AM intensity (c). (d–f) Representative intensity distribution false-color images and intensity surface plots (inset) of pCW-INPP4B PANC-1 (d) or PK-1 (e) cells treated without or with 500 nM Dox or ionomycin, or HPAC cells stably expressing indicated gRNAs (f), and labeled with Fluo-4 AM for cellular calcium. (g–i) (g) pCW-INPP4B PK-1 cells treated without or with 500 nM Dox or GPN, transfected with pBoBi-hLAMP2-C-GC6s (LAMP2-GCaMP6 [green]), labeled for lysosomes with Lysotracker Red (red), and quantitated (h and i) as above. Scale bar: 5 µm. (j–l)pCW-INPP4B PK-1 cells treated without or with 500 nM Dox and vehicle or ML-SI3 (j), labeled for cellular Ca2+ with Fluo-4 AM and lysosomes with Lysotracker Red, and quantitated as above (k and l). Scale bar: 10 µm. (m–o) (m) pCW-INPP4B PK-1 cells treated with vehicle or ML-SA1 or MK6-83, labeled for cellular Ca2+ with Fluo-4 AM and lysosomes with Lysotracker Red, and quantitated as above (n and o). Scale bar: 10 µm. Data represent ± SD from 80 to 90 cells (a–c and j–o) or 50–60 cells (g–i) from three independent experiments assessed per treatment per condition, with one-way ANOVA Tukey’s post hoc test performed for statistical measures. ****P < 0.0001.
INPP4B expression regulates cellular Ca 2+ levels in PDAC cells. (a) pCW-INPP4B PK-1 cells treated without or with 500 nM Dox or ionomycin were co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green). Scale bar: 15 µm. (b and c) Quantitation of Fluo-4 AM intensity on Lysotracker Red–positive structures (b) or cytosolic Fluo-4 AM intensity (c). (d–f) Representative intensity distribution false-color images and intensity surface plots (inset) of pCW-INPP4B PANC-1 (d) or PK-1 (e) cells treated without or with 500 nM Dox or ionomycin, or HPAC cells stably expressing indicated gRNAs (f), and labeled with Fluo-4 AM for cellular calcium. (g–i) (g) pCW-INPP4B PK-1 cells treated without or with 500 nM Dox or GPN, transfected with pBoBi-hLAMP2-C-GC6s (LAMP2-GCaMP6 [green]), labeled for lysosomes with Lysotracker Red (red), and quantitated (h and i) as above. Scale bar: 5 µm. (j–l)pCW-INPP4B PK-1 cells treated without or with 500 nM Dox and vehicle or ML-SI3 (j), labeled for cellular Ca2+ with Fluo-4 AM and lysosomes with Lysotracker Red, and quantitated as above (k and l). Scale bar: 10 µm. (m–o) (m) pCW-INPP4B PK-1 cells treated with vehicle or ML-SA1 or MK6-83, labeled for cellular Ca2+ with Fluo-4 AM and lysosomes with Lysotracker Red, and quantitated as above (n and o). Scale bar: 10 µm. Data represent ± SD from 80 to 90 cells (a–c and j–o) or 50–60 cells (g–i) from three independent experiments assessed per treatment per condition, with one-way ANOVA Tukey’s post hoc test performed for statistical measures. ****P < 0.0001.
The transient receptor potential cation channel, mucolipin subfamily, member 1 (TRPML-1/MCOLN1) Ca2+ channel is highly expressed on lysosomal membranes and is implicated in maintaining Ca2+ homeostasis and lysosomal function (Cheng et al., 2010; Yang et al., 2020; Wang et al., 2015). Since MCOLN-1 transcript is induced upon INPP4B overexpression (Fig. S3 a), we hypothesized that lysosomal TRPML-1 may be an effector of the observed INPP4B-mediated Ca2+ upregulation. Indeed, the specific TRPML-1 inhibitor ML-SI3 (Schmiege et al., 2021) significantly reduced INPP4B overexpression-mediated cellular and peri-lysosomal Ca2+ induction (Fig. 3, j–l; and Fig. S4, j–l). TRPML-1 agonists ML-SA1 and MK6-83 (Wang et al., 2015) were used as positive controls and led to increased peri-lysosomal and cytosolic Fluo-4 AM intensity in all conditions (Fig. S4, m–o). Together, these experiments demonstrate that increased Ca2+ concentrations induced in response to INPP4B overexpression are dependent upon TRPML-1 function.
TRPML-1–associated Ca2+ mediates INPP4B-regulated lysosome positioning
We next posited that altered Ca2+ levels could be controlling INPP4B-mediated lysosome positioning in PDAC cells. As a proof-of-principle, we first demonstrated that robust activation of Ca2+ release with ionomycin caused significant relocalization of lysosomes to the cell periphery (Fig. 4, a and b). Next, we performed experiments using the cell-permeable selective Ca2+ chelator 1,2-bis(o-aminophenoxy)ethane-N',N',N',N'-tetraacetic acid (BAPTA) while simultaneously inducing the expression of INPP4B. In both PANC-1 and PK-1 cells, BAPTA treatment reversed the peripheral localization of lysosomes driven by INPP4B overexpression (Fig. 4, c and d). Similarly, treatment with the TRPML-1 antagonist ML-SI3 also reduced lysosomal dispersion driven by INPP4B overexpression (Fig. 4, e and f). To further support these findings, we showed that perinuclear lysosome repositioning induced by INPP4B KO in HPAC cells could be fully rescued with TRPML-1 agonists ML-SA1 and MK6-83 (Fig. 4, g and h). These data are consistent with the hypothesis that INPP4B-mediated peripheral lysosomal repositioning is regulated by TRPML-1–associated Ca2+ release.
INPP4B-regulated lysosome positioning is mediated by TRPML-1–associated Ca 2+ . (a and b) PANC-1 (a) or PK-1 (b) was treated with vehicle or ionomycin and immunostained for LAMP1 (green) and DAPI nuclear stain (blue). Scale bar: 15 µm. LAMP1 intensity distribution as measured by peripheral (outer shell)/perinuclear (inner shell) LAMP1 intensity ratio. (c and d)pCW-INPP4B (c) PANC-1 or (d) PK-1 cells without or with 500 nM Dox were treated with vehicle or BAPTA followed by immunostaining with LAMP1 (green) and DAPI nuclear stain (blue) and LAMP1 intensity distribution as indicated above. Scale bar: 20 µm. (e and f)pCW-INPP4B (e) PANC-1 or (f) PK-1 cells without or with 500 nM Dox were treated with vehicle or ML-SI3. Cells were immunostained as above and LAMP1 intensity distribution was measured. Scale bar: 20 µm. (g) HPAC cells stably expressing indicated sgRNAs were treated with vehicle, MK6-83, or ML-SA1. (h) Cells were immunostained as above and LAMP1 intensity distribution was measured. Data represent ± SD from 100 to 120 cells from three independent experiments assessed per treatment per condition, with unpaired two-tailed parametric t test for a and b, and one-way ANOVA Tukey’s post hoc test performed for c–h. *P < 0.05, ***P < 0.001, ****P < 0.0001.
INPP4B-regulated lysosome positioning is mediated by TRPML-1–associated Ca 2+ . (a and b) PANC-1 (a) or PK-1 (b) was treated with vehicle or ionomycin and immunostained for LAMP1 (green) and DAPI nuclear stain (blue). Scale bar: 15 µm. LAMP1 intensity distribution as measured by peripheral (outer shell)/perinuclear (inner shell) LAMP1 intensity ratio. (c and d)pCW-INPP4B (c) PANC-1 or (d) PK-1 cells without or with 500 nM Dox were treated with vehicle or BAPTA followed by immunostaining with LAMP1 (green) and DAPI nuclear stain (blue) and LAMP1 intensity distribution as indicated above. Scale bar: 20 µm. (e and f)pCW-INPP4B (e) PANC-1 or (f) PK-1 cells without or with 500 nM Dox were treated with vehicle or ML-SI3. Cells were immunostained as above and LAMP1 intensity distribution was measured. Scale bar: 20 µm. (g) HPAC cells stably expressing indicated sgRNAs were treated with vehicle, MK6-83, or ML-SA1. (h) Cells were immunostained as above and LAMP1 intensity distribution was measured. Data represent ± SD from 100 to 120 cells from three independent experiments assessed per treatment per condition, with unpaired two-tailed parametric t test for a and b, and one-way ANOVA Tukey’s post hoc test performed for c–h. *P < 0.05, ***P < 0.001, ****P < 0.0001.
INPP4B promotes lysosomal exocytosis in PDAC cells
Among its pleiotropic roles in the cell, Ca2+ is necessary to initiate fusion between lysosomal membranes and the plasma membrane during lysosomal exocytosis, a process whereby lysosomal contents are ejected from the cell (Vevea et al., 2021; Rao et al., 2004; Medina et al., 2011). During lysosomal exocytosis, Ca2+ interacts with the synaptotagmin (Syt) family member Syt-VII, which enhances Ptdns binding to SNARE complexes to promote lipid bilayer fusion (Tancini et al., 2020). Since we observed that INPP4B elevates Ca2+ in the vicinity of lysosomes, we tested if INPP4B overexpression also promotes lysosomal exocytosis.
The translocation of lysosomal membrane proteins to the plasma membrane is a hallmark of lysosomal exocytosis. This event is readily measurable by quantifying the levels of the LAMP1 protein on the surface of live, unfixed, unpermeabilized cells using IF or flow cytometry (Andrews, 2017). Ionomycin, used as a positive control for exocytosis, clearly demonstrates elevated levels of cell surface LAMP1 (Fig. 5, a, b, e, and f) (Rodríguez et al., 1997). Upon INPP4B overexpression, we consistently observed increased cell surface LAMP1 levels in both PANC-1 and PK-1 cells (Fig. 5, a–h) and KO of INPP4B in HPAC cells reduced cell surface LAMP1 levels (Fig. 5, i and j), consistent with a role for INPP4B in promoting lysosomal exocytosis. Moreover, our experiments demonstrate that the INPP4B-mediated exocytosis was Ca2+ dependent and TRPML-1 dependent, as it was blunted by BAPTA or ML-SI3 treatment, respectively (Fig. 5, c, d, g, and h). In support, TRPML-1 activation with ML-SA1 or MK6-83 elevated cell surface LAMP1 levels and completely reversed the effects of INPP4B KO (Fig. 5, i and j).
INPP4B regulates lysosomal exocytosis in a TRPML-1–dependent manner. (a–d and e–h) pCW-INPP4B PANC-1 (a–d) or PK-1 (e–h) cells without or with 500 nM Dox were treated with vehicle, ionomycin, BAPTA, or ML-SI3 followed by flow cytometry analysis of cell surface LAMP1. Representative immunostained images of cell surface LAMP1 and nuclear DAPI stain for PANC-1 (a) or PK-1 (e) cells. Scale bar: 15 µm. (i and j) HPAC cells stably expressing indicated sgRNAs were treated with vehicle, ML-SA1, or MK6-83 TRPML-1 agonists followed by flow cytometry analysis of cell surface LAMP1 (j). Representative immunostained images of cell surface LAMP1 and nuclear DAPI stain for HPAC cells (i). Scale bar: 15 µm. (k–m) Indicated cell models were cultured for cells for 6 days followed by measurement of extracellular lysosomal hexosaminidase activity. (n)pCW-INPP4B PK-1 cells were grown for 5 days, then exposed to vehicle or ML-SI3 for 24 h followed by measurement of extracellular lysosomal hexosaminidase activity. Data represent ± SD from three independent experiments, with one-way ANOVA Tukey’s post hoc test performed for b–j and m and n, and unpaired two-tailed parametric t test for k and l. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
INPP4B regulates lysosomal exocytosis in a TRPML-1–dependent manner. (a–d and e–h) pCW-INPP4B PANC-1 (a–d) or PK-1 (e–h) cells without or with 500 nM Dox were treated with vehicle, ionomycin, BAPTA, or ML-SI3 followed by flow cytometry analysis of cell surface LAMP1. Representative immunostained images of cell surface LAMP1 and nuclear DAPI stain for PANC-1 (a) or PK-1 (e) cells. Scale bar: 15 µm. (i and j) HPAC cells stably expressing indicated sgRNAs were treated with vehicle, ML-SA1, or MK6-83 TRPML-1 agonists followed by flow cytometry analysis of cell surface LAMP1 (j). Representative immunostained images of cell surface LAMP1 and nuclear DAPI stain for HPAC cells (i). Scale bar: 15 µm. (k–m) Indicated cell models were cultured for cells for 6 days followed by measurement of extracellular lysosomal hexosaminidase activity. (n)pCW-INPP4B PK-1 cells were grown for 5 days, then exposed to vehicle or ML-SI3 for 24 h followed by measurement of extracellular lysosomal hexosaminidase activity. Data represent ± SD from three independent experiments, with one-way ANOVA Tukey’s post hoc test performed for b–j and m and n, and unpaired two-tailed parametric t test for k and l. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
A second hallmark of exocytosis is the release of active lysosomal proteases into the extracellular space. Thus, as an independent assessment of exocytosis, we measured lysosomal hexosaminidase activity in the extracellular medium (Samie and Xu, 2014; Rodríguez et al., 1997). Upon INPP4B overexpression, we observed greater accumulation of hexosaminidase activity in the medium of cultured PANC-1 and PK-1 compared with controls (Fig. 5, k and l), and INPP4B KO in HPAC cells resulted in decreased hexosaminidase activity (Fig. 5 m). Furthermore, ML-SI3 reduced the INPP4B-dependent increase in extracellular hexosaminidase activity (Fig. 5 n), once again confirming the TRPML-1 dependency of this process. Together, these findings underscore the importance of TRPML-1–activated Ca2+ release in mediating INPP4B-mediated peripheral lysosomal translocation and exocytosis. Notably, these data are consistent with reports showing that TRPML-1 can contribute to tumor invasion, and may explain why LAMP1 is enriched on the cell surface of metastatic cells, together implicating INPP4B in tumor progression (Furuta et al., 2001; Grimm et al., 2018).
INPP4B promotes migration and invasion of PDAC cells in a TRPML-1–dependent manner
Thus far, our data support a model whereby high levels of INPP4B in PDAC cells promote peripheral lysosomal positioning and exocytosis through TRPML-1–mediated Ca2+ regulation. These data are reminiscent of features observed in advanced metastatic cancers, where lysosomes have been observed to relocate to the cell periphery and undergo exocytosis to release their enzymatic contents and help cancer cells modify the surrounding extracellular matrix (ECM) (Machado et al., 2015). These events have been demonstrated to promote migration and invasion, both of which are crucial for metastatic dissemination of cancer cells (Endres et al., 2016; Mohsen et al., 2017). However, whether INPP4B drives migration and invasion in PDAC cells through this axis is currently unknown.
Notably, Gene Ontology (GO) analysis of the TCGA-PAAD patient dataset revealed a strong association of high INPP4B levels with genes involved in cadherin binding, cell–cell adhesion, and focal adhesion, and several GO profiles related to actin cytoskeleton organization and cell motility, suggesting that INPP4B may have a role in regulating migratory phenotypes (Fig. 6, a–c). Cells utilize actin polymerization to engage in targeted movement or chemotaxis towards a soluble attractant in processes including wound healing, inflammatory responses, and various disease conditions, such as cancer metastasis (Franz et al., 2002; Wear et al., 2000). During these processes, the formation of filamentous actin (F-actin) promotes the outward extension of the cell membranes, giving rise to protrusive formations like lamellipodia and filopodia (Franz et al., 2002; Wear et al., 2000). To test if INPP4B is involved in such cell motility mechanisms, we examined F-actin organization at cell edges in PDAC cell models upon gain or loss of INPP4B using phalloidin, a well-known marker of F-actin and cell migration (Fig. 6 d) (Devi et al., 2021; Nguyen et al., 2016; DesMarais et al., 2019). Notably, overexpression of INPP4B in PDAC cells led to increased F-actin intensity levels around the cell membrane edge (Fig. 6, e–h), whereas INPP4B KO in HPAC cells demonstrated reduced F-actin intensity levels at the cell membrane edge (Fig. 6, i and j). Using ML-SI3, we determined that the ability of INPP4B to induce the formation of F-actin was dependent on TRPML-1 activity (Fig. 6, e–h). Conversely, TRPML-1 agonists ML-SA1 and MK6-83 rescued the decreased F-actin intensity observed in INPP4B KO cells (Fig. 6, i and j).
INPP4B affects cellular processes and surface F-actin related to migration. The most enriched GO terms among the top 200 correlated genes with INPP4B expression in TCGA-PAAD. (a–c) The top 20 GO terms for (a) Molecular Function, (b) Biological Process, and (c) Cellular Component, ranked by significance, with the top five terms colored. FDR, false discovery rate. (d) Image analysis technique to quantitate cell edge F-actin intensity. (e and g)pCW-INPP4B (e) PANC-1 and (g) PK-1 cells without or with 500 nM Dox-induced and vehicle or ML-SI3 treatment were assessed for cell edge F-actin expression through phalloidin staining. Scale bar: 20 µm. (f and h) Edge F-actin intensity analysis for (f) PANC-1 or (h) PK-1 cells. (i) HPAC cells stably expressing the indicated gRNAs were treated with vehicle or ML-SA1 or MK6-83 to assess the effect of TRPML-1 activation on cell edge F-actin. (j) Edge F-actin intensity analysis for i HPAC cells. Scale bar: 15 µm. Data represent ± SD from 90 to 100 cells from three independent experiments assessed per treatment per condition, with one-way ANOVA Tukey’s post hoc test performed for statistical measures. *P < 0.05, **P < 0.01, ****P < 0.0001.
INPP4B affects cellular processes and surface F-actin related to migration. The most enriched GO terms among the top 200 correlated genes with INPP4B expression in TCGA-PAAD. (a–c) The top 20 GO terms for (a) Molecular Function, (b) Biological Process, and (c) Cellular Component, ranked by significance, with the top five terms colored. FDR, false discovery rate. (d) Image analysis technique to quantitate cell edge F-actin intensity. (e and g)pCW-INPP4B (e) PANC-1 and (g) PK-1 cells without or with 500 nM Dox-induced and vehicle or ML-SI3 treatment were assessed for cell edge F-actin expression through phalloidin staining. Scale bar: 20 µm. (f and h) Edge F-actin intensity analysis for (f) PANC-1 or (h) PK-1 cells. (i) HPAC cells stably expressing the indicated gRNAs were treated with vehicle or ML-SA1 or MK6-83 to assess the effect of TRPML-1 activation on cell edge F-actin. (j) Edge F-actin intensity analysis for i HPAC cells. Scale bar: 15 µm. Data represent ± SD from 90 to 100 cells from three independent experiments assessed per treatment per condition, with one-way ANOVA Tukey’s post hoc test performed for statistical measures. *P < 0.05, **P < 0.01, ****P < 0.0001.
Next, to directly test whether INPP4B is sufficient and/or required to alter PDAC cell migration and invasion, wound healing/scratch assays, transwell migration assays, and transwell invasion assays were performed. We determined that INPP4B overexpression increased the rate of wound healing in scratch assays (Fig. 7, a and b), whereas INPP4B KO reduced wound healing rates (Fig. 7 c). Similarly, transwell migration (Fig. 7, d and f) and transwell invasion assays (Fig. 7, e and g) implicated INPP4B in promoting migratory and invasive phenotypes of PDAC cell lines, respectively. INPP4B KO in the INPP4Bhigh cell lines HPAC cells provide further data supporting that INPP4B is required for migration and invasion (Fig. 7, h and i).
INPP4B affects PDAC cell migration and invasion. (a–i) Depicted are cells with stable overexpression of empty control (pSMAL) or INPP4B (pSMAL-INPP4B) in INPP4Blow (a, d, and e) PK-1 or (b, f, and g) PANC-1 cells, or INPP4B KO with the indicated gRNAs in c, h, and i INPP4Bhigh HPAC cell lines. Respective cell models were tested for wound healing (a–c), transwell migration (d, f, and h), and transwell invasion (e, g, and i) assays. (j–m)pCW-INPP4B PANC-1 treated with siCTRL or siMCOLN-1 and no Dox or 500 nM Dox and assessed for transwell migration (j), transwell invasion (k), protein levels of MCOLN-1 (l), and transcripts of MCOLN-1 gene expression (m). Data represent ± SD from three independent experiments, with unpaired two-tailed parametric t test performed for a, b, and d–g, and one-way ANOVA Tukey’s post hoc test for c and h–m. **P < 0.01, ***P < 0.001, ****P < 0.0001. Source data are available for this figure: SourceData F7.
INPP4B affects PDAC cell migration and invasion. (a–i) Depicted are cells with stable overexpression of empty control (pSMAL) or INPP4B (pSMAL-INPP4B) in INPP4Blow (a, d, and e) PK-1 or (b, f, and g) PANC-1 cells, or INPP4B KO with the indicated gRNAs in c, h, and i INPP4Bhigh HPAC cell lines. Respective cell models were tested for wound healing (a–c), transwell migration (d, f, and h), and transwell invasion (e, g, and i) assays. (j–m)pCW-INPP4B PANC-1 treated with siCTRL or siMCOLN-1 and no Dox or 500 nM Dox and assessed for transwell migration (j), transwell invasion (k), protein levels of MCOLN-1 (l), and transcripts of MCOLN-1 gene expression (m). Data represent ± SD from three independent experiments, with unpaired two-tailed parametric t test performed for a, b, and d–g, and one-way ANOVA Tukey’s post hoc test for c and h–m. **P < 0.01, ***P < 0.001, ****P < 0.0001. Source data are available for this figure: SourceData F7.
Next, to examine if the migratory and invasive phenotypes driven by INPP4B overexpression were dependent on TRPML-1 function, we performed transwell migration and invasion assays in cells where the TRPML-1 transcript MCOLN-1 was knocked down (Fig. 7, j–m). These experiments demonstrated that INPP4B-mediated migration and invasion are inhibited upon MCOLN-1 knockdown. To further support these data, we performed transwell assays in the presence of the TRPML-1 inhibitor ML-SI3. We observed that the elevated levels of migration and invasion induced by INPP4B overexpression in PANC- and PK-1 cells were inhibited upon ML-SI3 treatment (Fig. 8, a–d). Interestingly, TRPML-1 activation with ML-SA1 or MK6-83 significantly accelerated migration and invasion phenotypes in HPAC cells and rescued the inhibition of migration or invasion mediated by INPP4B KO to control levels (Fig. 8, e and f). Together, these results suggest that INPP4B expression regulates PDAC progression by promoting migratory and invasive cell phenotypes through TRPML-1 activation.
INPP4B promotes PDAC cell migration and invasion through TRPML-1 function. (a–d) pCW-INPP4B PANC-1 (a and b) or PK-1 (c and d) cells without or with 500 nM Dox were treated with vehicle or ML-SI3 and assessed for cell migration (a and c) or invasion through a Matrigel basement membrane (b and d), followed by crystal violet staining of cells attached to ThinCert membrane. (e and f) HPAC cells stably expressing indicated sgRNAs were treated with vehicle ML-SA1 or MK6-83 and assessed for cell migration (e) or invasion (f) as above. Quantitation of crystal violet staining is displayed for each condition. Data represent ± SD from four independent experiments, with one-way ANOVA Tukey’s post hoc test performed for statistical measures. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
INPP4B promotes PDAC cell migration and invasion through TRPML-1 function. (a–d) pCW-INPP4B PANC-1 (a and b) or PK-1 (c and d) cells without or with 500 nM Dox were treated with vehicle or ML-SI3 and assessed for cell migration (a and c) or invasion through a Matrigel basement membrane (b and d), followed by crystal violet staining of cells attached to ThinCert membrane. (e and f) HPAC cells stably expressing indicated sgRNAs were treated with vehicle ML-SA1 or MK6-83 and assessed for cell migration (e) or invasion (f) as above. Quantitation of crystal violet staining is displayed for each condition. Data represent ± SD from four independent experiments, with one-way ANOVA Tukey’s post hoc test performed for statistical measures. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
INPP4B promotes the generation of lysosomal PtdIns(3,5)P2
Given its canonical function as a phosphatase that converts PtdIns(3,4)P2 to PtdIns(3)P, we examined the consequences of INPP4B overexpression on phosphoinositide levels in PDAC cells. First, INPP4B overexpression significantly reduced both whole cell and peri-lysosomal detection of PtdIns(3,4)P2, supporting its canonical role as a PtdIns(3,4)P2 phosphatase (Fig. S5, a and b). As expected, we also observed concomitantly increased levels of lysosomal PtdIns(3)P as measured using the GFP-2xFYVE biosensor (Fig. 9, a and b).
INPP4B regulates phosphoinositide levels in PDAC cells for migration. (a–d) pCW-INPP4B PANC-1 (a and c) or PK-1 (b and d) cells without or with 500 nM Dox immunostained for PtdIns(3,4)P2 (a,b) or PtdIns(3,5)P2 (c and d) and transiently expressing LAMP1-mCherry, with intensity quantification of respective phosphoinositide immunostain on LAMP1-mCherry–positive structures or total cell phosphoinositide intensity. (e) PANC-1 cells treated with vehicle or 20 nM apilimod, transfected with GFP-SnxA and stained for lysosomes with Lysotracker Red, and quantified as above. Scale bar for a–e: 5 µm. (f–h)pCW-INPP4B PK-1 treated with or without 500 nM Dox and vehicle or 10 nM apilimod assessed for (f) cell surface LAMP1 through microscopy imaging (left image panel, scale bar: 20 µm) and flow cytometry (right graph), and transwell migration (g) and invasion (h). Data represent ± SD from three independent experiments assessed per treatment per condition, with 80–90 cells (a–d) or 50–60 cells (e) counted, with unpaired two-tailed parametric t test performed for a–e, one-way ANOVA Tukey’s post hoc test for f–h. *P < 0.05, ***P < 0.001, ****P < 0.0001.
INPP4B regulates phosphoinositide levels in PDAC cells for migration. (a–d) pCW-INPP4B PANC-1 (a and c) or PK-1 (b and d) cells without or with 500 nM Dox immunostained for PtdIns(3,4)P2 (a,b) or PtdIns(3,5)P2 (c and d) and transiently expressing LAMP1-mCherry, with intensity quantification of respective phosphoinositide immunostain on LAMP1-mCherry–positive structures or total cell phosphoinositide intensity. (e) PANC-1 cells treated with vehicle or 20 nM apilimod, transfected with GFP-SnxA and stained for lysosomes with Lysotracker Red, and quantified as above. Scale bar for a–e: 5 µm. (f–h)pCW-INPP4B PK-1 treated with or without 500 nM Dox and vehicle or 10 nM apilimod assessed for (f) cell surface LAMP1 through microscopy imaging (left image panel, scale bar: 20 µm) and flow cytometry (right graph), and transwell migration (g) and invasion (h). Data represent ± SD from three independent experiments assessed per treatment per condition, with 80–90 cells (a–d) or 50–60 cells (e) counted, with unpaired two-tailed parametric t test performed for a–e, one-way ANOVA Tukey’s post hoc test for f–h. *P < 0.05, ***P < 0.001, ****P < 0.0001.
INPP4B promotes the generation of lysosomal PtdIns(3,5)P2 in PDAC cells. (a–d) pCW-INPP4B PANC-1 or PK1 cells without or with 500 nM Dox were transfected with GFP-2xFYVE (green) (a and b) to assess PtdIns(3)P or GFP-SnxA (green) (c and d) to assess PtdIns(3,5)P2 and labeled for lysosomes with Lysotracker Red (red), with quantification for the respective probes overlayed on Lysotracker Red–positive structures. Scale bar: 5 µm. Data represent ± SD from three independent experiments assessed per treatment per condition with 50–60 cells counted for a–d, with unpaired two-tailed parametric t test performed for statistical measures. ****P < 0.0001.
INPP4B promotes the generation of lysosomal PtdIns(3,5)P2 in PDAC cells. (a–d) pCW-INPP4B PANC-1 or PK1 cells without or with 500 nM Dox were transfected with GFP-2xFYVE (green) (a and b) to assess PtdIns(3)P or GFP-SnxA (green) (c and d) to assess PtdIns(3,5)P2 and labeled for lysosomes with Lysotracker Red (red), with quantification for the respective probes overlayed on Lysotracker Red–positive structures. Scale bar: 5 µm. Data represent ± SD from three independent experiments assessed per treatment per condition with 50–60 cells counted for a–d, with unpaired two-tailed parametric t test performed for statistical measures. ****P < 0.0001.
Crucially, significant increases in lysosomal PtdIns(3,5)P2 levels were also measured upon INPP4B overexpression in PANC-1 and PK-1 cell lines using the GFP-SnxA biosensor, and confirmed using IF with a PI(3,5)P2 antibody (Fig. 9, c and d; and Fig. S5, c and d). This data supports the notion that newly generated PtdIns(3)P by INPP4B on lysosomes is actively converted to PtdIns(3,5)P2.
INPP4B promotes lysosomal dispersion, exocytosis, migration, and invasion via the generation of lysosomal PtdIns(3,5)P2
To test that PtdIns(3,5)P2 generation is necessary for the observed TRPML-dependent INPP4B-induced phenotypes in PDAC cells, we used apilimod, a specific kinase inhibitor of PIKfyve to block the formation of PtdIns(3,5)P2. Upon confirming that apilimod significantly reduced levels of PtdIns(3,5)P2 (Fig. S5 e), we measured how this would affect INPP4B-induced phenotypes, including lysosomal dispersion, exocytosis, migration, and invasion. First, we observed that apilimod blocked the ability of INPP4B overexpression to induce peripheral lysosome dispersion (Fig. 10 a). Next, we observed that PIKfyve inhibition blocked INPP4B-induced lysosomal exocytosis as measured by cell surface LAMP1 flow cytometry (Fig. 10 b and Fig. S5 f). Finally, apilimod effectively blunted INPP4B-induced migration and invasion to baseline levels (Fig. 10, c and d; and Fig. S5, g and h). Altogether, these data demonstrate that INPP4B-induced phenotypes observed in PDAC cells including lysosomal dispersion, lysosomal exocytosis, migration, and invasion are dependent on the formation of lysosomal PtdIns(3,5)P2, an endogenous activator of TRPML-1 activity.
INPP4B promotes lysosomal phenotypes, migration, and invasion migration through PtdIns(3,5)P 2 generation. (a–d) pCW-INPP4B PANC-1 cells treated without or with 500 nM Dox and with vehicle or 10 nM apilimod and assessed for (a) LAMP1 intensity distribution through LAMP1 immunostain (green) and DAPI nuclear stain (blue) (scale bar: 20 µm), (b) cell surface LAMP1 through microscopy imaging (left panel images, scale bar: 20 µm) and flow cytometry (right graph), (c) transwell migration, and (d) transwell invasion. Data represent ± SD from three independent experiments assessed per treatment per condition with 100–120 cells counted for a with one-way ANOVA Tukey’s post hoc test performed for statistical measures. **P < 0.01, ****P < 0.0001.
INPP4B promotes lysosomal phenotypes, migration, and invasion migration through PtdIns(3,5)P 2 generation. (a–d) pCW-INPP4B PANC-1 cells treated without or with 500 nM Dox and with vehicle or 10 nM apilimod and assessed for (a) LAMP1 intensity distribution through LAMP1 immunostain (green) and DAPI nuclear stain (blue) (scale bar: 20 µm), (b) cell surface LAMP1 through microscopy imaging (left panel images, scale bar: 20 µm) and flow cytometry (right graph), (c) transwell migration, and (d) transwell invasion. Data represent ± SD from three independent experiments assessed per treatment per condition with 100–120 cells counted for a with one-way ANOVA Tukey’s post hoc test performed for statistical measures. **P < 0.01, ****P < 0.0001.
Discussion
Our study presents several lines of evidence pointing to a novel signal transduction pathway that links high INPP4B expression levels to enhanced migratory and invasive capacity of PDAC cells. We show that INPP4B promotes lysosomal Ca2+ release into the cytoplasm in a TRPML-1–dependent manner, an event that is necessary for peripheral lysosomal dispersion and lysosomal exocytosis. Enhanced migration and invasion of PDAC cells upon INPP4B expression also proceeded in a TRPML-1–dependent manner. Mechanistically, we show that INPP4B leads to the generation of lysosomal PtdIns(3,5)P2, which is essential to activate TRPML-1. Our discoveries offer an explanation for the observed clinical correlations associated with INPP4B overexpression in PDAC. Furthermore, these results open up new possibilities for therapeutic interventions in PDAC.
We previously discovered that PDAC patients with high levels INPP4B are more likely to have reduced overall survival (Dzneladze et al., 2018). This finding has been confirmed in other studies, and consequently, INPP4B has also been included in at least four predictive gene signatures for PDAC, underscoring its prognostic importance in this disease (Zhai et al., 2019; Zhou et al., 2019; Luo et al., 2021; Wei et al., 2021; Chen et al., 2022). Nevertheless, it remained unclear how this phosphoinositide phosphatase promotes the generation of aggressive PDAC. This study aimed to provide evidence supporting a direct role for INPP4B in driving PDAC progression.
Cancer cell invasion, a prerequisite for metastasis, is a complex, multistep process involving the abnormal migration of cancer cells from their primary tumor location into adjacent tissues through intricate matrix environments (Pijuan et al., 2019; Mejia et al., 2020). Invasion necessitates the coordination of various cellular processes that enhance processes that remove physical barriers limiting cell movements and spreading, including the loss of cell–cell adhesion, enabling cell dissociation from the primary tumor; the activation of cell motility and migration programs; and the secretion of substances that both promote migration and degrade basement membranes and the extracellular matrix (Martin et al., 2013). Notably, lysosomes offer key properties that can enhance all these processes. Peripherally localized lysosomes are believed to be poised for plasma membrane docking and exocytosis, which can release a cocktail of lysosomal contents including H+ ions that can acidify the tumor microenvironment, and hydrolases that stimulate the degradation of ECM (Machado et al., 2021).
This is reminiscent of previous reports that link plasma membrane proximal lysosomes to enhanced invasive and metastatic capabilities in malignant cells (Morgan et al., 2018; Machado et al., 2015, 2021). Detection of peripheral lysosomes has been shown to be associated with increased invasiveness in breast cancer, prostate cancer, or hepatocellular carcinomas (Wu et al., 2020; Dykes et al., 2016; Lyu et al., 2020). However, until now, INPP4B overexpression has not been linked to enhanced migration and invasion.
Lysosomal exocytosis directly promotes migratory and invasive properties through the regulation of cellular adhesion to the ECM, a crucial process for efficient cell motility dynamics (Machado et al., 2021). Furthermore, activated cathepsins are released outside the cell to trigger the degradation of extracellular matrix and basement membrane constituents like type IV collagen, fibronectin, and laminin (Hämälistö and Jäättelä, 2016; Buck et al., 1992). Our experiments utilizing gain-of-function and loss-of-function INPP4B models demonstrate a role for INPP4B in peripheral lysosome dispersion, exocytosis, cell migration, and invasion, findings that directly implicate INPP4B with metastasis and tumor progression in PDAC for the first time. Importantly, these results may provide a molecular explanation for the prognostic role played by INPP4B in PDAC.
Mechanistically, INPP4B overexpression in PDAC cells generated elevated levels of lysosomal PtdIns(3)P and PtdIns(3,5)P2. This finding is consistent with previous studies showing that PtdIns(3)P generated by INPP4B through the hydrolysis of PtdIns(3,4)P2 on endosomal membranes can persist on endolysosomes where it is then rapidly converted to PtdIns(3,5)P2 by PIKfyve (Rodgers et al., 2022). Together with the observation that alterations in INPP4B expression regulated peri-lysosomal Ca2+ concentrations led us to investigate the role of the predominant Ca2+ release channel on the lysosome, TRPML-1, a key driver of Ca2+-dependent processes such as calcineurin activation, autophagy, vesicle trafficking, lysosome reformation, and lysosomal exocytosis (Rodríguez et al., 1997; Tsunemi et al., 2019; Yang et al., 2020). Furthermore, the increased levels of PtdIns(3,5)P2 promoted by INPP4B provide a key mechanistic link between INPP4B and TRPML-1 activation since it is the only known endogenous activator of TRPML activity (Dong et al., 2010).
Crucially, the availability of ML-SI3 and apilimod, selective inhibitors of PIKyve and TRPML-1, respectively, enabled our studies tremendously. We were able to discover that lysosome positioning and lysosomal exocytosis mediated by INPP4B were reliant on the generation PtdIns(3,5)P2 and the subsequent activation of this specific Ca2+ channel. Similarly, ML-SI3 and apilimod were pivotal in demonstrating that migratory and invasive phenotypes driven by INPP4B were also mediated through PtdIns(3,5)P2 and TRPML-1. These findings align with established roles of TRPML-1 and PtdIns(3,5)P2 in governing cell migration and invasion (Xu et al., 2019; Edwards-Jorquera et al., 2020; Rühl et al., 2021; Giridharan et al., 2022; Gu et al., 2022). Similarly, in an assessment of TRPML-1 expression levels conducted in tumor tissues from 82 PDAC patients, Hu and colleagues revealed a correlation between high TRPML-1 expression levels and adverse clinical characteristics in PDAC (Hu et al., 2019).
In addition to TRPML-1, other members of the TRP superfamily TRPML-2 and TRPML-3 are also activated by PtdIns(3,5)P2 (Dong et al., 2010; Chen et al., 2020). Thus, although ML-SI3 effectively inhibits INPP4B effects in PDAC, we cannot exclude that TRPML-2 and TRPML-3 may also be involved. Nonetheless, our findings shed light on INPP4B as a previously unappreciated upstream regulator of TRPML-1 function with key relevance for PDAC migration and invasion in PDAC.
Notably, the initial indication that INPP4B played a role in lysosome regulation came from transcriptional analyses demonstrating that INPP4B was associated with lysosomal gene signatures and many Coordinated Lysosomal Expression and Regulation (CLEAR) network genes (Woolley et al., 2021, Preprint). Subsequent validation confirmed that ectopic INPP4B overexpression could indeed regulate lysosomal gene transcription. This finding begged the question: Does INPP4B activate TFEB activity? Lines of evidence presented herein demonstrate that INPP4B is unable to influence signaling upstream of TFEB, nor the translocation of TFEB and related family members TFE3 and MITF to the nucleus, which suggests that INPP4B functioned independently of TFEB. However, TFEB knockdown completely abrogated the transcriptional activation of lysosomal genes upon INPP4B overexpression, indicating that TFEB is indispensable for this function of INPP4B. An explanation for this could be that INPP4B influences posttranslational modifications on TFEB such as acetylation, which has been shown to promote TFEB function (Li et al., 2022). Remarkably, TFEB knockdown also inhibited INPP4B-mediated lysosomal dispersion. These findings indicate that the INPP4B-driven induction of lysosomal gene transcription and lysosomal dispersion depends on TFEB function. However, this process occurs through a non-canonical TFEB activation pathway that does not impact AKT-mTORC1 activation or TFEB localization, revealing a complex interaction between INPP4B, TFEB, and lysosomal signaling in PDAC cells. Further studies are needed to investigate a potential model where INPP4B could propel a feed-forward loop by simultaneously activating lysosomal biogenesis through TFEB and phosphoinositide signaling on the lysosomal membrane, leading to TRPML-1–mediated activation of lysosomal phenotypes, migration, and invasion.
Furthermore, our findings expand on the known roles of INPP4B in lysosomal biology, as demonstrated by our group and others (Rodgers et al., 2021b, 2022; Saffi et al., 2022; Woolley et al., 2021, Preprint). Recent work by Rodgers and colleagues in breast cancer cell lines has elegantly shown that INPP4B-mediated lysosomal signaling is crucial for lysosomal repopulation processes necessary for autophagy (Rodgers et al., 2021b, 2022). Our research has highlighted a correlation between INPP4B transcript levels and lysosomal gene sets in acute myeloid leukemia, as well as a role for INPP4B in regulating lysosomal membrane dynamics (Saffi et al., 2022; Woolley et al., 2021, Preprint). Collectively, these findings reinforce the role of INPP4B as a lysosomal regulator protein, while also indicating that much work remains to be done.
Our experimental results confirm that INPP4B plays a significant role in promoting aggressive PDAC cell phenotypes, such as increased growth, migration, and invasion. We propose that INPP4B functions, at least in part, via a novel and druggable pathway involving INPP4B→PIKfyve→TRPML-1. Importantly, selective inhibitors targeting this pathway have yet to be explored in the context of PDAC progression in vivo. Thus, further studies are needed to deepen our understanding of the biology of this signaling axis, and preclinical assessments should focus on identifying a therapeutic window for inhibiting this pathway in INPP4Bhigh PDAC patients.
Materials and methods
Cell lines
The pancreatic cancer cell lines BxPC-3, CAPAN-1, HPAC, PANC-1, PK-1, and PK-8 and HEK293T were used for this study. All cells were grown in Dulbecco’s Modified Eagle Medium (DMEM; Wisent) supplemented with 10% fetal bovine serum (FBS; Wisent) and 1% penicillin-streptomycin (Wisent) as complete DMEM media. Cells were grown at 37°C and 5% CO2.
Plasmids
pSMAL-INPP4B-puro was generated by replacing GFP with a puromycin sequence and cloning a codon optimized INPP4B sequence into the PacI-Sal sites downstream of the spleen focus forming virus promoter in pSMAL, a generous gift from John Dick and Peter van Galen (https://www.addgene.org/161785/). lentiCRISPR-V2 was a generous gift from Feng Zhang (https://www.addgene.org/52961/). pCW-INPP4B-Blast was generated by replacing Cas9 with codon optimized INPP4B using the NheI-BamHI sites of pCW-Cas9-Blast, a generous gift from Mohan Babu (https://www.addgene.org/83481/). The following sgRNA (single guide RNA) sequences were cloned into lentiCRISPR-V2 according to Zhang Lab protocols: sgINPP4B-1 (5′-ATACTCCAGCACCGAAATTG-3′) sgINPP4B-2 (5′-GATGTACAGGGACAAAAGGT-3′), and control sgRNAs were the non-targetting sgLacZ (5′-CCCGAATCTCTATCGTGCGG-3′) or targeting the non-essential gene sgCDY1B (5′-TCTGCACCAGGACGTGACAA-3′). pmCherry-INPP4B was generated by removing the FKBP sequence in mCherry-FKBP-INPP4B, a generous gift from Gerry Hammond (https://www.addgene.org/116864/). mCherry-Lysosomes-20 (mCherry-LAMP1) was a generous gift from Michael Davidson (https://www.addgene.org/55073/). pEGFP-2xFYVE was a generous gift from Herald Stenmark (https://www.addgene.org/140047/). pJSK659 (GFP-SnxA) was a generous gift from Jason King (https://www.addgene.org/205128/). pBoBi-hLAMP2-C-GC6s (LAMP2-GCaMP6) was a generous gift from Sheng-Cai Lin (https://www.addgene.org/154151/).
Lentiviral generation
psPAX2 (a generous gift from Didier Trono [https://www.addgene.org/12260/]) and VSVG (a generous gift from Tannishtha Reya [https://www.addgene.org/14888/]) lentiviral packaging plasmids were used to generate all lentiviral particles in HEK293T cells using Ca2+ chloride transfection (Life Technologies). Media containing lentivirus was collected at 48 and 72 h after transfection. PDAC cell lines were seeded at 2.5 × 105 cells per well in 6-well dishes and infected the next day with two 24-h cycles with lentiviral media supplemented with 8 µg/ml protamine sulfate. Following infection, media was replaced with fresh media for 24 h followed by appropriate antibiotic selection: 10 days with 25 µg/ml blasticidin or 2 days with 2 µg/ml puromycin.
Transient gene transfection and siRNA gene silencing
All transient transfections were performed using Fugene HD (Promega) at 3:1 of DNA:Fugene ratio for 48 h, followed by washing with 1X PBS and supplementing with complete DMEM media. pmCherry and pmCherry-INPP4B were introduced into parental PANC-1 and PK-1 cells through transient transfection. mCherry-LAMP1, pEGFP-2xFYVE, pJSK659 (GFP-SnxA), and pBoBi-hLAMP2-C-GC6s were transfected into pCW-INPP4B expressing PANC-1 and PK-1 cells. siRNA-mediated gene silencing for pCW-INPP4B expressing PANC-1 cells was performed by mixing 0.1 nmol of nontargeting or MCOLN1 or TFEB SMARTPool siRNA (GE Dharmacon) with 2 μl DharmaFECT1 Transfection reagent (GE Dharmacon) in FBS-free DMEM media for 24 h. The transfection mixture was washed off and treated for 48 h for respective transwell migration and invasion assays, qRT-PCR, and western blot.
Drug treatment of cell lines
To induce INPP4B expression in pCW-INPP4B PANC-1 and PK-1, cells were treated with Doxycycline (Dox) at indicated concentrations for at least 48 h. Ionomycin (Cell Signaling) was used at 10 µM for 10 min. BAPTA-AM (Thermo Fisher Scientific) was used at 40 µM for 50 min. TRPML-1 agonist MK6-83 (Selleckchem) was used at 5 µM or ML-SA1 (Selleckchem) at 20 µM for 24 h. The TRPML-1 antagonist ML-SI3 (Selleckchem) was used at 5 µM for 24 h. Apilimod (Selleckchem) was used to inhibit PIKfyve for 24 h at 10 nM or 20 nM as indicated. Gly-Phe β-naphthylamide (GPN) (Selleckchem) was used to release lysosomal calcium through 15 min treatment at 20 µM.
qRT-PCR
RNA was extracted using the Qiagen RNeasy mini kit (Qiagen). Reverse transcription to cDNA was performed using the Superscript IV VILO cDNA synthesis kit (Thermo Fisher Scientific). Amplification of cDNA was performed through quantitative PCR according to manufacturer instructions using Taqman Fast Advanced Master Mix (Applied Biosystems) in the presence of Taqman Assays with QuantStudio 3 Real-Time PCR system (Thermo Fisher Scientific) controlled by QuantStudio Design and Analysis Software version 1.2 (Thermo Fisher Scientific). The following Taqman assays were used: MCOLN1 (Hs01100661_g1), ATP6V1D (Hs00211133_m1), ATP6V1H (Hs00977530_m1), CTSD (Hs00924259_m1), LAMP1 (Hs05049889_s1), and GAPDH (Hs02786624_g1). Gene expression was determined using the relative quantification (ΔΔCt method) and normalized to GAPDH.
Immunoblotting
Total cell lysates were produced using 1X radioimmunoprecipitation assay buffer supplemented with protease inhibitors. Proteins were boiled for 5 min in 5X Protein Loading Buffer (Applied Biological Materials), then separated on a Bolt 4–12% Bis-Tris Plus gels (Thermo Fisher Scientific) and transferred onto nitrocellulose membrane (Bio-Rad). Non-specific binding was blocked using a blocking buffer containing Tris-buffered saline, 5% skim milk, and 0.1% Tween-20. The membranes were washed in Tris-buffered saline containing 0.1% Tween-20 and incubated overnight at 4°C with the primary antibodies in a 5% skim milk blocking buffer. Target proteins were identified with rabbit antibodies against human INPP4B, LAMP1, pTFEBSer211, TFEB, pmTORSer2448, mTOR, pAKTSer473, AKT, pS6Ser235/236, S6, p4-E-BP1Thr37/46, p4-E-BP1Ser65, 4-E-BP1, and β actin from Cell Signaling. The membranes were washed again and incubated with a horseradish peroxidase–conjugated anti-rabbit secondary antibody in a 5% skim milk blocking buffer for 60 min. Blots were developed and imaged using KwikQuant Digital Western Blot Detection System (Kindle Biosciences). Subcellular fractionation was performed according to manufacturer instructions from NE-PER Thermo Fisher Scientific nuclear and cytoplasmic fractionation kit. Separated nuclear and cytosol fractions were probed by antibodies for INPP4B, TFEB, MITF, TFE3, Vinculin, and HDAC2.
IF staining
For endogenous LAMP1 immunostaining, cells were fixed with 4% (vol/vol) paraformaldehyde for 15 min, permeabilized for 5 min with ice-cold methanol, and blocked in 3% bovine serum albumin (vol/vol) in PBS. Cells were then incubated with a rabbit monoclonal antibody against human LAMP1 (Cell Signaling) and Donkey anti-rabbit DyLight 488 (Bethyl) secondary antibody. For endogenous TFEB immunostain, cells were fixed with 4% (vol/vol) paraformaldehyde for 15 min, permeabilized for 10 min with 0.1% Triton X-100 at room temperature, and blocked in 3% bovine serum albumin (vol/vol) in PBS. Cells were incubated with mouse monoclonal antibody against human TFEB (R&D systems) and Goat anti-mouse DyLight 488 (Bethyl) secondary antibody and also nuclear stained with DAPI. For immunostaining of cell surface LAMP1, cells were treated and washed with PBS followed by incubating non-permeabilized and non-fixed cells with 1:100 dilution of APC anti-human CD107a (LAMP1) antibody clone H4A3 (BioLegend) for 30 min in the dark. Cells were washed with PBS and then fixed with 4% (vol/vol) paraformaldehyde for 15 min followed by DAPI nuclear staining. Cell surface LAMP1 was imaged through far-red confocal spinning disc microscopy. For immunostaining of cellular PdIns(3)P or PtdIns(3,5)P2 or PtdIns(3,4)P2, cells were fixed with 4% (vol/vol) paraformaldehyde for 15 min, permeabilized with 20 µM digitonin (Promega) in buffer A (20 mM PIPES, pH 6.8, 137 mM NaCl, 2.7 mM KCl) for 30 min, followed by blocking with buffer A containing 50 mM NH4Cl and 5% normal goat serum. Immunostaining was performed with anti-PtdIns(3)P IgG or PtdIns(3,4)P2 or anti-PtdIns(3,5)P2 IgG (Echelon) followed by Goat anti-mouse DyLight 488 (Bethyl) secondary antibody. For F-actin staining, cells were fixed with 4% (vol/vol) paraformaldehyde for 15 min, followed by permeabilization with 20 µM digitonin (Promega) for 10 min, and blocked in 3% bovine serum albumin (vol/vol) in PBS. Cells were incubated with Alexa Fluor 488 conjugated phalloidin (Thermo Fisher Scientific) for 10 min and DAPI for nuclear stain. To immunostain INPP4B and assess INPP4B at LAMP1-mCherry–positive lysosomes, pretreatment with saponin was performed before fixing cells to retain proteins interacting with intracellular membranes and to remove cytoplasmic proteins as described previously (Marat et al., 2017). Coverslip samples were mounted onto glass slides with DAKO fluorescent mounting media and imaged.
Lysosome labeling
Lysosomal Ca2+ or lysosomal proteolytic function was detected by pulsing cells with 25 nM Fluo-4 AM (Thermo Fisher Scientific) or DQ-BSA (Themo Fisher Scientific), respectively, for 2 h followed by washing with 1X PBS and replenishment with complete DMEM media for 1 h to chase the markers towards lysosomes. Endocytosis toward lysosomes was assessed by pulsing cells with 1 mg/ml Lucifer Yellow (Thermo Fisher Scientific) for 3 h followed by washing with 1X PBS and replenishment with complete DMEM media for 1 h to chase the marker toward lysosomes.
Live and fixed cell microscopy imaging
To observe LAMP1 or F-actin immunostained fixed cells, images were acquired with a ZEISS AxioImager M2 Epifluorescence microscope connected to AxioCam MRm CCD camera and controlled by AxioVision Software Version 4.8 at 40× 1.4 N.A. or 63× 1.4 N.A. objective (Carl Zeiss). To perform live cell imaging or assessing immunostained cells, spinning disc confocal microscopy was used with Olympus IX81 inverted microscope connected to Hamamatsu C900-13 EMCCD camera with 60× 1.35 N.A. objective and controlled by Volocity 6.3.0 software. Live imaging was performed in complete DMEM media at 37°C and 5% CO2 chamber. All microscopes used in this study were equipped with the appropriate standard filters for the fluorophores tested.
Image analysis
Lysosome distribution was quantified with ImageJ for LAMP1 immunostained cells using a technique adapted from previous reports (Saric et al., 2016; Li et al., 2016). To measure lysosomal localization, first, the nucleus and plasma membrane of individual cells were manually outlined. Next, we manually delineated inner and outer shells by drawing a line equidistant from the nucleus and membrane. Total fluorescence intensity was measured in the outer and the inner shell, and an inner/outer shell intensity ratio was computed as a measure of lysosome distribution. To quantify LAMP1 intensity, cells were individually outlined to identify regions of interest followed by measurement of mean LAMP1 intensity per cell. To measure nuclear TFEB intensity, ImageJ was used to apply intensity thresholding for DAPI nuclear structure and produce a mask. This mask was applied to the green (TFEB) channel to identify TFEB intensity over DAPI-positive regions. Whole-cell or cytosol TFEB intensity was identified by generating regions of interest around individual cells and quantifying total cell TFEB intensity. Ratio was obtained for nuclear-to-cytosol TFEB intensity. To quantify Fluo-4 AM, GFP-SnxA, GFP-2xFYVE, and LAMP2-GCaMP6 intensities over Lysotracker Red–positive structures, ImageJ was used to apply intensity thresholding for Lysotracker-positive structures and produce a mask. This mask was applied to the green (Fluo-4 AM, GFP-SnxA, GFP-2xFYVE, LAMP2-GCaMP6) channel to identify green channel intensity over Lysotracker-positive regions. Similar image analysis technique was applied to cells expressing LAMP1-mCherry to assess PtdIns(3)P, PtdIns(3,5)P2, or PtdIns(3,4)P2 fluorescence intensity on LAMP1-mCherry–positive regions in a cell. Whole-cell PtdIns(3)P, PtdIns(3,4)P2, or PtdIns(3,5)P2 fluorescence intensity was measured through intensity thresholding to identify PtdIns(3)P, PtdIns(3,4)P2, or PtdIns(3,5)P2 fluorescence regions within a cell, followed by measurement of mean fluorescence intensity. For image analysis of F-actin around the cell edge, a region of interest was drawn to specify the cell edge F-actin. Total raw integrated pixel intensity was measured within the region of interest and divided by the total number of pixels within the region of interest to measure mean F-actin intensity within the region of interest.
Flow cytometry
To monitor cell surface LAMP1 levels, cells were trypsinized and washed in 1X PBS, followed by incubating for 15 min in ice in the dark with 1:100 dilution of APC anti-human CD107a (LAMP1) antibody clone H4A3 (BioLegend). Cells were washed with 1X PBS followed by flow cytometry measurement using the APC channel. To measure lysosomal functions, DQ-BSA or Lysotracker Green–stained cells were trypsinized, followed by washing the cells with 1X PBS. The fluorescence measurements for pmCherry or pmCherry-INPP4B expression were detected using a phycoerythrin channel and the fluorescence measurements for DQ-BSA or Lysotracker Green or Lucifer Yellow intensity were detected with fluorescein isothiocyanate channel. Appropriate gating criteria was applied to isolate DQ-BSA or Lysotracker Green intensity in non-transfected or pmCherry or mCherry-INPP4B expressing PANC-1 or PK-1 cells. All flow cytometry was performed using the Beckman Coulter Cytoflex (Beckman), and a total of 10,000 events were counted per condition per sample and the background signal was identified using non-labeled cells.
Extracellular β hexosaminidase assay
To monitor extracellular β hexosaminidase activity, phenol red–free complete DMEM media (Thermo Fisher Scientific) was collected from treated cells from days 0, 2, 4, and 6. β Hexosaminidase activity from collected media was measured according to manufacturer instructions (Cell Biolabs).
Proliferation, clonogenic, and soft agar assays
Cells were seeded in each well of a 12-well plate at a density of 1.0 × 104 cells. At each time point, cells were fixed with 10% formalin for 10 min and stored in PBS at 4°C. Once all time points were collected, cells were stained with 0.1% crystal violet, 20% methanol solution, and then washed with water and dried for at least 4 h. Crystal violet–stained cells were first imaged, then crystal violet was solubilized by 10% acetic acid for 20 min, and the absorbance was measured at 590 nm with Spectramax M3.
For clonogenic assay, cells were seeded in each well of a 6-well plate at a density of 2.0 × 102 cells. Colonies were then grown over a period of 19 days. Cells were washed with PBS, fixed with 10% formalin, and stained with 0.1% crystal violet in 20% methanol. Crystal violet was washed off with distilled water followed by overnight drying. Stained cells were photographed. Images were imported into ImageJ and used to count colony size and number.
For soft agar assays, each well of a 6-well plate was coated with 0.6% noble agarose in complete DMEM media at 4°C for 30 min to generate a base. For each well, 5.0 × 103 cells were suspended in complete DMEM media containing 0.3% noble agarose and added as a top layer to the base. The top layer was solidified at 4°C for 30 min. Cells were grown over 19 days at 37°C and 5% CO2. Each well was segregated into four equal area sections and colonies from each section were imaged through differential interference contrast (DIC) light microscopy at 4× 0.13 N.A. objective using EVOS-FL inverted fluorescent microscope that was controlled by EVOS XL core imaging system. Images were imported into ImageJ, and colony size and number were quantified for each section.
Scratch-wound healing assay
Cells seeded onto each well of a 6-well plate were grown to complete confluency. Sterile plastic tips were used to scratch and achieve two vertical wounds or gaps. Cells were washed and imaged in PBS every 24 h using DIC light microscopy at 4× objective 0.13 N.A. using an EVOS-FL inverted fluorescent microscope. Images were imported into ImageJ and analysis was performed to measure the area of the wound in pixel units for each time point.
Transwell migration assay and transwell invasion assays
To assess PDAC cell migration, 1.0 × 104 cells were seeded in DMEM media supplemented with 1% FBS on upper chambers of 24-well ThinCerts with 8-µm pores (Greiner Bio-One). Complete DMEM media with 10% FBS was added beneath the ThinCerts to the well. Cells were allowed to migrate through pores over 48 h at 37°C. Moist cotton swabs were used to remove non-migrating cells attached to the upper side of the pores of the ThinCert membrane. Cells attached to the bottom of the membrane were fixed with 10% formalin and stained with 0.1% crystal violet in 20% methanol. Membranes were washed three times with 10 min 1X PBS per wash followed by overnight drying of membranes. Membranes were first photographed, then crystal violet from the membrane was dissolved in 10% acetic acid and quantified through SpectraMax M3 spectrometer plate reader at absorbance 595 nm.
To assess PDAC cell invasion, 24-well ThinCerts with 8-µm pores (Greiner Bio-One) were coated with 1:6 dilution of Matrigel Growth Factor Reduced Basement Membrane Matrix (Corning Life Science; Corning), followed by Matrigel polymerization at 37°C for 3 h. Following Matrigel polymerization, 5.0 × 104 PANC-1 or PK-1 cells, or 1.0 × 105 HPAC cells were seeded in DMEM media supplemented with 1% FBS on upper chambers of 24-well ThinCerts. Complete DMEM media with 10% FBS was added beneath the ThinCerts to the well. Cells were allowed to migrate through pores over 48 h in 37°C. Processing and imaging of membranes were performed as described above for transwell migration.
Data analysis
INPP4B protein levels in PDAC were assessed and downloaded from the UALCAN data portal (https://ualcan.path.uab.edu/index.html) (Chandrashekar et al., 2017). GSEA was performed on the TCGA-PAAD dataset using GSEA v.4.0.3 provided by the Broad Institute (https://www.gsea-msigdb.org/gsea/index.jsp). Samples were rank-ordered and split by median INPP4B expression. Enriched gene sets were identified by 1,000 phenotype permutations. Gene sets with a nominal P value < 0.05 were considered significantly enriched. The curated KEGG (CP:KEGG) gene set was obtained from MSigDB Collections (https://www.gsea-msigdb.org/gsea/msigdb/collections.jsp). GO was performed as follows. The interactive web service, GEPIA (Tang et al., 2017), was used to identify the top 200 genes whose expression was correlated with INPP4B in TCGA-PAAD (Cancer Genome Atlas Research Network, 2017). The DAVID analysis tool was then used to identify the top enriched GO terms among this list of genes (Huang et al., 2009b, 2009a). The top five GO terms for Biological Process, Cellular Component, and Molecular Function, ranked by false discovery rate, and the overlapping genes were displayed in chord diagrams generated with the circlize R package (Gu et al., 2014).
Statistical analysis
All experiments were performed at least three times. Graphpad Prism was used to plot and measure statistical significance between multiple conditions, and ANOVA analysis and Tukey’s post hoc analysis were performed. To compare statistical significance between two conditions, unpaired student’s t test was performed. Statistical significance was considered for P values < 0.05. In figures, ns = P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. The results of all statistical analyses are reported in Table S1.
Online supplemental material
Fig. S1 shows the effect of INPP4B expression on PDAC cell growth. Fig. S2 shows the effect of INPP4B expression on lysosome function. Fig. S3 shows the effect of INPP4B expression on lysosome gene transcription and TFEB function. Fig. S4 shows INPP4B expression regulates cellular Ca2+ levels in PDAC cells. Fig. S5 shows that INPP4B regulates phosphoinositide levels in PDAC cells for migration. Table S1 lists statistical tests and P values used for figure generation. Table S2 lists primary antibodies used for IB and IF experiments. Table S3 lists secondary antibodies used for IB and IF experiments. Table S4 is a list of other reagents used in this study.
Data availability
All data used in this study are included in the manuscript figures and available upon request.
Acknowledgments
We would like to thank Drs. Irakli Dzneladze, John F. Woolley, Mark Minden, and Julie Wilson for key contributions to the development of the project. We thank Drs. Rod Bremner, Arkaitz Carracedo, and John Clohessy for careful reading and editing of the manuscript. We thank all past and present members of the Salmena Lab for contributions.
L. Salmena is a recipient of Tier II Canada Research Chair and supported through Human Frontier Career Development Program award. Funding for this research was provided in part by Temerty Faculty of Medicine and Department of Pharmacology and Toxicology, University of Toronto, and awards received from Canada Foundation for Innovation (CFI-33505), Cancer Research Society Grant (PIN24261), and bridge funding from the Canadian Institute of Health Research (CIHR487599). Dr. R.J. Botelho is funded by Canada Research Chairs Program (950-23233) and Natural Sciences and Engineering Council of Canada (Discovery Grant RGPIN-2020-04343), and contributions from Toronto Metropolitan University.
Author contributions: G.T. Saffi: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing—original draft, Writing—review & editing, L. To: Conceptualization, Investigation, Methodology, N. Kleine: Investigation, Resources, C.M.P. Melo: Investigation, Methodology, K. Chen: Conceptualization, Investigation, G.E. Genc: Formal analysis, Validation, K.C.D. Lee: Formal analysis, Investigation, J. Tak-Sum Chow: Data curation, Formal analysis, G.H. Jang: Formal analysis, Software, Visualization, S. Gallinger: Data curation, Funding acquisition, Writing—review & editing, R.J. Botelho: Conceptualization, Funding acquisition, Resources, Supervision, Writing—review & editing, L. Salmena: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision.
References
Author notes
Disclosures: The authors declare no competing interests exist.



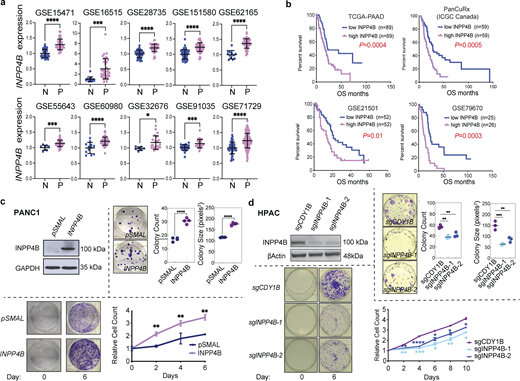



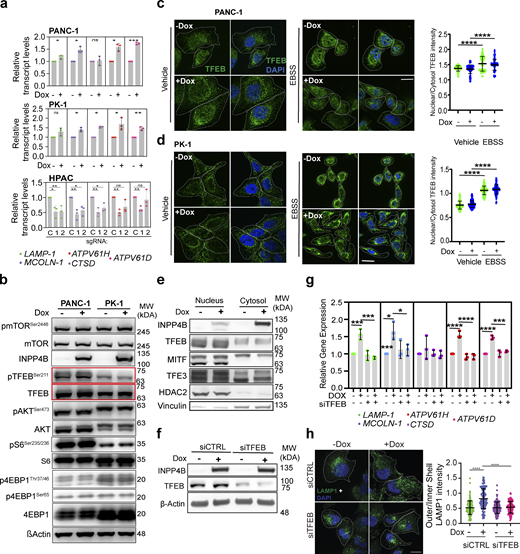
![INPP4B expression regulates cellular Ca2+levels in PDAC cells through TRPML-1 function. (a)pCW-INPP4B PANC-1 cells without or with 500 nM Dox or ionomycin were co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green). Scale bar: 15 µm. (b and c) Quantitation of (b) Fluo-4 AM intensity on Lysotracker Red–positive structures or (c) cytosolic Fluo-4 AM intensity. (d–f) HPAC cells stably expressing indicated sgRNAs were (d) co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green) and quantitated (e and f) as above. Scale bar: 10 µm. (g–i) (g) pCW-INPP4B PANC-1 without or with 500 nM Dox or Gly-Phe β-naphthylamide (GPN) treatment, transfected with pBoBi-hLAMP2-C-GC6s (LAMP2-GCaMP6 [green]), labeled for lysosomes with Lysotracker Red (red), and quantitated (h and i) as above. Scale bar: 5 µm. (j–l) (j) pCW-INPP4B PANC-1 without or with 500 nM Dox were treated with vehicle or ML-SI3, co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green), and quantitated (k and l) as above. Scale bar: 15 µm. Data represent ± SD from 100 to 120 cells (a–f and j–l) or 50–60 cells (g–i) from three independent experiments assessed per treatment per condition, with one-way ANOVA Tukey’s post hoc test performed for statistical measures. **P < 0.01, ***P < 0.001, ****P < 0.0001.](https://cdn.rupress.org/rup/content_public/journal/jcb/223/11/10.1083_jcb.202401012/2/m_jcb_202401012_fig3.png?Expires=1773754542&Signature=X1B6SvDCy7qnHjAwgvwN34efU0P0xqICAhL1ib75pVrClxEe1i~Qfg9achgbJr9yacIiYDDczlHv4xwOgoDwmW~i~nnpHAmFbGHVxVHsKFHyuq-Kgc9qaka0DmAZzlbRsC7WR4mJUWwssefCzpH4FjXLBMVlQH7Fc-S0q4UP9QlRtXGlP2PJbhXxQk1oDeEu-0jYinstAejMWdU6SsDc6QlF43xIxVEPCmMBM1SWIrKPBMHJ1UQKrj4~q5tfDETNqesjTgZ4PG28tWFFvrYtC5vzI-jHHfjvYBX6d9DnD3KDWMVoO3t3LWVd0jvvvNx4IkuV10HVlV8u2Yd4ZQD1Dw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![INPP4B expression regulates cellular Ca2+levels in PDAC cells. (a)pCW-INPP4B PK-1 cells treated without or with 500 nM Dox or ionomycin were co-stained for lysosomes with Lysotracker Red (red) and cellular Ca2+ with Fluo-4 AM (green). Scale bar: 15 µm. (b and c) Quantitation of Fluo-4 AM intensity on Lysotracker Red–positive structures (b) or cytosolic Fluo-4 AM intensity (c). (d–f) Representative intensity distribution false-color images and intensity surface plots (inset) of pCW-INPP4B PANC-1 (d) or PK-1 (e) cells treated without or with 500 nM Dox or ionomycin, or HPAC cells stably expressing indicated gRNAs (f), and labeled with Fluo-4 AM for cellular calcium. (g–i) (g) pCW-INPP4B PK-1 cells treated without or with 500 nM Dox or GPN, transfected with pBoBi-hLAMP2-C-GC6s (LAMP2-GCaMP6 [green]), labeled for lysosomes with Lysotracker Red (red), and quantitated (h and i) as above. Scale bar: 5 µm. (j–l)pCW-INPP4B PK-1 cells treated without or with 500 nM Dox and vehicle or ML-SI3 (j), labeled for cellular Ca2+ with Fluo-4 AM and lysosomes with Lysotracker Red, and quantitated as above (k and l). Scale bar: 10 µm. (m–o) (m) pCW-INPP4B PK-1 cells treated with vehicle or ML-SA1 or MK6-83, labeled for cellular Ca2+ with Fluo-4 AM and lysosomes with Lysotracker Red, and quantitated as above (n and o). Scale bar: 10 µm. Data represent ± SD from 80 to 90 cells (a–c and j–o) or 50–60 cells (g–i) from three independent experiments assessed per treatment per condition, with one-way ANOVA Tukey’s post hoc test performed for statistical measures. ****P < 0.0001.](https://cdn.rupress.org/rup/content_public/journal/jcb/223/11/10.1083_jcb.202401012/2/m_jcb_202401012_figs4.png?Expires=1773754542&Signature=37KEi7qTnTXfECyCwQNV2V~Bn6o2X8LAF5dPmRE0brU2vPNCmfBPKkCtAESVB7Sc~FGPjvs2lv1IomQXuT3OcBJWrTjve9WaoocCoVMPg8NR7eN6G8CJHECvlczycE30p8nRqvP80rpv7MQ3pDXtYpHs6gfxlpIV9Ee9uxqf5Rvlt8ROHjOv8-J5K6TAGVkSrO~tCFyXUSrV1QxViPKISpxx97dvsyBwgc4yVyn2dBv60i28dqgodq3hOsACHTnzWUZUkGXHKtXAiWEkdO3cgR8MqW1vCfTwLz0iH13j1i1KtufAmJtmqCMHBbgdnBSoHvA3yFAwx7i92SatNtA4kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)










