Peroxisomes are membrane-bound organelles harboring metabolic enzymes. In humans, peroxisomes are required for normal development, yet the genes regulating peroxisome function remain unclear. We performed a genome-wide CRISPRi screen to identify novel factors involved in peroxisomal homeostasis. We found that inhibition of RNF146, an E3 ligase activated by poly(ADP-ribose), reduced the import of proteins into peroxisomes. RNF146-mediated loss of peroxisome import depended on the stabilization and activity of the poly(ADP-ribose) polymerases TNKS and TNKS2, which bind the peroxisomal membrane protein PEX14. We propose that RNF146 and TNKS/2 regulate peroxisome import efficiency by PARsylation of proteins at the peroxisome membrane. Interestingly, we found that the loss of peroxisomes increased TNKS/2 and RNF146-dependent degradation of non-peroxisomal substrates, including the β-catenin destruction complex component AXIN1, which was sufficient to alter the amplitude of β-catenin transcription. Together, these observations not only suggest previously undescribed roles for RNF146 in peroxisomal regulation but also a novel role in bridging peroxisome function with Wnt/β-catenin signaling during development.
Introduction
The peroxisome is a membrane-bound organelle that harbors enzymes for specialized metabolic reactions. The most conserved peroxisomal functions include the β-oxidation of fatty acids and regulation of reactive oxygen species (Wanders and Waterham 2006); however, cells tune peroxisome function according to need. For example, peroxisomes in the large intestine of mice contain enzymes for optimal plasmalogen synthesis, while peroxisomes in the small intestines contain enzymes for optimal β-oxidation of fatty acids (Morvay et al., 2017). Peroxisome function differentiates alongside cell type: for example, in inner ear cells, sound-induced autophagy of peroxisomes protects against noise overexposure (Defourny et al., 2019), while in macrophages, peroxisomal metabolism improves phagocytosis (Di Cara et al., 2017). Accordingly, mutations in peroxisomal genes in humans cause a spectrum of peroxisome biogenesis disorders (PBDs) with phenotypes ranging in severity from early infant mortality, developmental abnormalities, and liver dysfunction to more specific metabolic syndromes, sensorineural hearing loss, and retinal degeneration (Braverman et al., 2016). It is therefore important to know both the genes dedicated to peroxisome function in human cells, as well as the mechanisms by which peroxisome abundance and function are coordinated to meet the needs of the cell.
Peroxisomes are made and maintained by ∼35 PEX proteins that coordinate the biogenesis of peroxisome membranes and the import of peroxisomal matrix localized enzymes. Protein import into peroxisomes depends on the presence of peroxisome structures, as well as on many of the best conserved PEX proteins that ensure the efficiency of import. Proteins tagged with a C-terminal peroxisomal targeting signal (PTS1) are recognized by the receptor PEX5, which shuttles the PTS1 cargo to the PEX13/PEX14 docking complex for import across the peroxisomal membrane (Dammai and Subramani, 2001; Skowyra and Rapoport, 2022). After import, PEX5 is recycled via extraction by PEX1/PEX6/PEX26 from the peroxisomal membrane following ubiquitination by the PEX2/PEX10/PEX12 E3 ligase complex (Platta et al., 2009; Platta et al., 2005). Cells fine-tune peroxisomal protein import, and therefore peroxisome function, according to need. The repertoire of imported enzymes is regulated through transcription, as well as ribosomal readthrough that can create protein isoforms with an appended PTS1 tag (Stiebler et al., 2014). The efficiency of import is also regulated cell-wide, for example, phosphorylation of PEX5 by ATM, a DNA repair kinase, can induce peroxisome-specific autophagy in response to oxidative stress (Zhang et al., 2015). Thus, peroxisome homeostasis is tightly regulated in cells and disruption of this regulation can have severe consequences on organismal development. However, the full regulatory network that governs the steady state equilibrium of peroxisome abundance, function, and homeostasis in human cells remains elusive.
Here, we performed a genome-wide CRISPR interference (CRISPRi) screen (Gilbert et al., 2013) in human cells to identify genes that influence the import of proteins targeted to peroxisomes. In addition to known PEX genes, we found that knockdown of the E3 ligase RNF146 reduces import of PTS1-tagged proteins into the peroxisome. RNF146 (Ring Finger Protein 146), also known as Iduna, is a RING-domain E3 ubiquitin ligase that recognizes and ubiquitinates proteins modified by poly(ADP-ribosyl)ation (PARsylation) (Zhang et al., 2011; DaRosa et al., 2015). RNF146 interacts directly with poly(ADP-ribose) polymerases, such as tankyrase-1 and tankyrase-2 (TNKS and TNKS2, referred to here as TNKS/2 together) (DaRosa et al., 2015) and PARP1 and PARP2 (Gerö et al., 2014; Kang et al., 2011). Together, the poly(ADP-ribose) polymerases and RNF146 specifically regulate the stability of numerous substrates, which are first PARsylated and subsequently polyubiquitinated by RNF146, triggering proteasomal degradation. We found that RNF146-mediated loss of peroxisomes was dependent on the accumulation of the poly(ADP-ribose) polymerases TNKS/2, specifically by impairing import into peroxisomes through a mechanism dependent on TNKS/2’s activity as poly(ADP-ribose) polymerases. We thus propose a model in which TNKS/2 binds and PARsylates PEX14 and neighboring proteins, inhibiting the import of PTS1-tagged proteins.
RNF146 and TNKS/2 are better known as coregulators of protein stability: TNKS/2 binds and PARsylates substrates with a tankyrase-binding motif (TBM), which then triggers polyubiquitination by RNF146 (DaRosa et al., 2015). Known RNF146/TNKS/2 substrates include AXIN1, BLZF1, 3BP2, and CASC3 (Nie et al., 2020; Levaot et al., 2011). Surprisingly, we found that in a variety of cell lines, a loss of PEX genes altered the stability of RNF146/TNKS/2 substrates and could therefore alter the output of downstream signaling pathways, including the Wnt/β-catenin pathway. These observations suggest that not only is peroxisome abundance and function integrally intertwined with cell signaling pathways but also that peroxisomes themselves regulate cellular responses to external stimuli.
Results
Sequestration of Zeocin resistance protein (ZeoR) in peroxisomes links peroxisome import to viability
Past screens for peroxisomal genes in mammalian cells have relied on peroxisome-localized enzymatic activity (Zoeller and Raetz 1986, Tsukamoto et al., 1990; Morand et al., 1990) and fluorescence microscopy of PTS1-tagged fluorescent proteins (Ito et al., 2000) since mammalian cells in tissue culture conditions do not require peroxisomes for growth. To facilitate a CRISPRi screening approach for regulators of peroxisome function, we engineered a cell line, which we term Pex-ZeoR, in which the efficiency of peroxisome import is linked to cell viability by fusing the fluorescent marker mVenus and a PTS1 to the gene encoding resistance to Zeocin, a 1,400 Dalton molecule in the bleomycin family that induces DNA double-strand breaks and causes cell death (Murray et al., 2014; Drocourt et al., 1990). With this fusion construct, mVenus-ZeoR-PTS1, cells with functional peroxisomes should sequester ZeoR, thereby preventing them from neutralizing Zeocin, which is too large to passively diffuse through peroxisome membranes (Antonenkov and Hiltunen 2006). By contrast, cells with reduced peroxisome import should accumulate mVenus-ZeoR-PTS1 in the cytoplasm where it can neutralize Zeocin, conferring a selective advantage in the presence of Zeocin (Fig. 1 A). To affirm our strategy, we transduced HCT116 CRISPRi (dCas9-KRAB) cells (Liang et al., 2018; Gilbert et al., 2014) to recombinantly express mVenus-ZeoR-PTS1. As predicted, cells expressing a non-targeting control (NTC) sgRNA had fluorescent mVenus foci, while cells expressing a PEX1 targeting sgRNA exhibited diffuse cytosolic mVenus signal (Fig. 1 B), consistent with mVenus-ZeoR-PTS1 targeting to the peroxisome. We then assessed cell growth of the HCT116 CRISPRi Pex-ZeoR cell line over a range of Zeocin concentrations, finding a clear growth advantage for cells with sgRNAs targeting PEX1 or PEX6 versus NTC at high concentrations of Zeocin (Fig. S1 A). To identify optimal selection conditions for the genome-wide screen, we performed a competition assay by coculturing either PEX1 or PEX6 CRISPRi Pex-ZeoR cells with NTC CRISPRi Pex-ZeoR cells at varying dosages of Zeocin and monitoring the abundance of each cell population by flow cytometry. PEX1 and PEX6 knockdown cells started at 5–10% of the cell population and were outcompeted by NTC cells in conditions without Zeocin. However, they displayed a marked competitive advantage in the presence of Zeocin (Fig. 1 C and Fig. S1 B). Together, these validation experiments suggest that peroxisomal sequestration of ZeoR allows for the selection of cells harboring sgRNAs that target peroxisomal genes.
A genome-wide screen uncovers genes that regulate peroxisome biology. (A) Design of the Pex-ZeoR cell line, which sequesters the Zeocin resistance protein in the peroxisome matrix. Loss of PEX genes causes cytosolic Zeocin resistance. (B) Representative fluorescence microscopy images of live HCT116 mVenus-ZeoR-PTS1 cells expressing either NTC or PEX1 sgRNAs. Fusion construct forms puncta in WT but not aperoxisomal (PEX1 knockdown) cells. Fluorescent microscopy data are representative of n = 49 images from m = 2 biological replicates. Scale bar: 10 μm. (C) Quantification of flow cytometry data of BFP− (NTC) and BFP+ (PEX1) cells grown in co-culture competition assay over t = 11 days in the presence of 0, 25, or 50 ng/μl of Zeocin. Timepoints are taken every t = 2 days. Data shown as the mean ± SD of n = 3 biological replicates. (D) Volcano plot of NGS data from genome-wide screen with significance (−log base 10 of P value, y-axis) and phenotype score (normalized fold change of cDNA guide count, x-axis) of guides targeting specific genes for cell cultures either untreated (DMSO mock treated) or treated (50 ng/μl Zeocin treated) for 14 days. Red data points represent known PEX genes and HIF1A. The green data point represents RNF146. Data displayed was calculated from m = 3 guides per gene and n = 2 biological replicates.
A genome-wide screen uncovers genes that regulate peroxisome biology. (A) Design of the Pex-ZeoR cell line, which sequesters the Zeocin resistance protein in the peroxisome matrix. Loss of PEX genes causes cytosolic Zeocin resistance. (B) Representative fluorescence microscopy images of live HCT116 mVenus-ZeoR-PTS1 cells expressing either NTC or PEX1 sgRNAs. Fusion construct forms puncta in WT but not aperoxisomal (PEX1 knockdown) cells. Fluorescent microscopy data are representative of n = 49 images from m = 2 biological replicates. Scale bar: 10 μm. (C) Quantification of flow cytometry data of BFP− (NTC) and BFP+ (PEX1) cells grown in co-culture competition assay over t = 11 days in the presence of 0, 25, or 50 ng/μl of Zeocin. Timepoints are taken every t = 2 days. Data shown as the mean ± SD of n = 3 biological replicates. (D) Volcano plot of NGS data from genome-wide screen with significance (−log base 10 of P value, y-axis) and phenotype score (normalized fold change of cDNA guide count, x-axis) of guides targeting specific genes for cell cultures either untreated (DMSO mock treated) or treated (50 ng/μl Zeocin treated) for 14 days. Red data points represent known PEX genes and HIF1A. The green data point represents RNF146. Data displayed was calculated from m = 3 guides per gene and n = 2 biological replicates.
Pex-ZeoR enables differential enrichment of genes regulating peroxisome homeostasis in a genome-wide screen. (A) Quantification of cell count by flow cytometry in different concentrations of Zeocin of HCT116 cells with sgRNAs targeting NTC, PEX1, or PEX6 over 72 h. Data is representative of n = 2 biological replicates. Cell count is normalized to untreated. (B) Quantification of flow cytometry data of BFP− (NTC) and BFP+ (PEX6) cells grown in coculture competition assay over t = 11 days in the presence of 0, 25, or 50 ng/μl of Zeocin. Time points are taken every t = 2 days. Data shown as the mean ± SD of n = 3 biological replicates. (C) Schematic of the CRISPRi screen. Pex-ZeoR cells were transformed with a genome-wide gRNA library, selected for expression of guides, and split into untreated and +Zeocin growth conditions. Genomic DNA takedowns for NGS sequencing at t = 0 and t = 7× for all conditions. (D) Heatmap showing Pearson’s correlation coefficient of guide abundance for all library elements between biological replicates of sequenced time points between treated and untreated conditions. T and Z represent untreated and Zeocin-treated conditions, respectively, while numbers represent time points (days). Highlighting indicates comparisons between day 14 samples. (E) Fold change of various PEX sgRNA abundances derived from genome-wide CRISPRi screen comparing Zeocin treated to untreated samples. Highlighting indicates comparisons between day 14 samples. Y-axis is phenotype score, a measure of the fold change of three of five significant guides per gene. X-axis is time (t) in days. Data is representative of n = 2 biological samples. (F) Volcano plot of NGS data from genome-wide screen with significance (−log base 10 of P value, y-axis) and phenotype score (normalized fold change of cDNA guide count, x-axis) of guides targeting specific genes for cell cultures either untreated (DMSO mock treated) or treated (50 ng/μl Zeocin treated) for 14 days. Pink data points are filtered to display genes with a Z-score value between [−0.5,5] in the screen by Olivieri et al. (2020), and a P-value <0.05 and a phenotype score ≥ 1 in our screening dataset.Gray data points are genes that did not pass filters. Red data points represent known PEX genes and RNF146. Data displayed was calculated from m = 3 guides per gene and n = 2 biological replicates.
Pex-ZeoR enables differential enrichment of genes regulating peroxisome homeostasis in a genome-wide screen. (A) Quantification of cell count by flow cytometry in different concentrations of Zeocin of HCT116 cells with sgRNAs targeting NTC, PEX1, or PEX6 over 72 h. Data is representative of n = 2 biological replicates. Cell count is normalized to untreated. (B) Quantification of flow cytometry data of BFP− (NTC) and BFP+ (PEX6) cells grown in coculture competition assay over t = 11 days in the presence of 0, 25, or 50 ng/μl of Zeocin. Time points are taken every t = 2 days. Data shown as the mean ± SD of n = 3 biological replicates. (C) Schematic of the CRISPRi screen. Pex-ZeoR cells were transformed with a genome-wide gRNA library, selected for expression of guides, and split into untreated and +Zeocin growth conditions. Genomic DNA takedowns for NGS sequencing at t = 0 and t = 7× for all conditions. (D) Heatmap showing Pearson’s correlation coefficient of guide abundance for all library elements between biological replicates of sequenced time points between treated and untreated conditions. T and Z represent untreated and Zeocin-treated conditions, respectively, while numbers represent time points (days). Highlighting indicates comparisons between day 14 samples. (E) Fold change of various PEX sgRNA abundances derived from genome-wide CRISPRi screen comparing Zeocin treated to untreated samples. Highlighting indicates comparisons between day 14 samples. Y-axis is phenotype score, a measure of the fold change of three of five significant guides per gene. X-axis is time (t) in days. Data is representative of n = 2 biological samples. (F) Volcano plot of NGS data from genome-wide screen with significance (−log base 10 of P value, y-axis) and phenotype score (normalized fold change of cDNA guide count, x-axis) of guides targeting specific genes for cell cultures either untreated (DMSO mock treated) or treated (50 ng/μl Zeocin treated) for 14 days. Pink data points are filtered to display genes with a Z-score value between [−0.5,5] in the screen by Olivieri et al. (2020), and a P-value <0.05 and a phenotype score ≥ 1 in our screening dataset.Gray data points are genes that did not pass filters. Red data points represent known PEX genes and RNF146. Data displayed was calculated from m = 3 guides per gene and n = 2 biological replicates.
A genome-wide CRISPRi screen in Pex-ZeoR cells enriches known PEX genes
Emboldened, we executed a genome-wide screen with the Pex-ZeoR cell line to identify novel genes that affect peroxisomal homeostasis. Infection with a genome-wide CRISPRi library was followed by chronic treatment with or without Zeocin, combined with regular passaging of cells over 35 days, with samples collected every 7 days for terminal Illumina sequencing preparation (Fig. S1 C). We found 1,717 genes that were significantly different (P < 0.05) between the treated and untreated conditions at the day 14 time point (Fig. 1 D). Day 14 serves as the optimal comparison time point because of the clear enrichment of the majority of known PEX genes while maintaining sufficient library diversity and replicate quality (Fig. S1, D and E).
We observed enrichment of guides targeting known PEX genes that facilitate PTS1 import (PEX5, PEX13, PEX14, PEX2/PEX12, PEX1/PEX6, PEX26) and peroxisome membrane protein targeting (PEX19) affirming the efficacy of our strategy (Fig. 1 D). Guides targeting PEX7 and α and β variants of PEX11 were not strongly enriched, consistent with roles in recognition of the alternative PTS2 targeting signal (PEX7) (Braverman et al., 1997) and peroxisomal membrane elongation (PEX11) (Koch et al., 2010). Guides targeting one component of the peroxisome RING finger complex, PEX10, were not enriched compared with the other constituents, PEX2 and PEX12, and guides targeting other peroxisome membrane biogenesis factors PEX3 and PEX16 were depleted in the screen (Fig. 1 D and Fig. S1 E). While initially unexpected, these results align with recent data that PEX10 and PEX16 CRISPR/Cas knockouts display only partial peroxisomal import defects (Yagita et al., 2022; Ott et al., 2022). Of the known factors regulating peroxisome-specific autophagy, such as NBR1, MARCH5, SQSTM1, HIF1A, and NIX (Kim et al., 2008; Deosaran et al., 2012; Zheng et al., 2022; Wilhelm et al., 2022), we found that only guides targeting HIF1A, the loss of which stabilizes peroxisomes (Wilhelm et al., 2022), were strongly depleted in our screen. Although most peroxisome-homeostasis related genes behaved according to our predictions, a handful did not align with our a priori prognosis. Our results suggest the possibility that not all of the aforementioned genes are simple or monotonic in their effect on peroxisome import or autophagy, representing potential new mechanisms for further investigation.
Guides targeting RNF146, INTS8, and KCNN4 reduce peroxisomal foci intensity
We anticipated that sgRNAs that improve resistance to Zeocin, independent of the peroxisomal localization of ZeoR, should also be significantly enriched in our dataset. Thus, to narrow the candidate list to genes relevant to peroxisomal localization of ZeoR, we filtered our screen results to exclude factors that modulated resistance to a related DNA damaging agent, bleomycin (Olivieri et al., 2020) (Fig. S1 F and Table S1, Z-score range [−0.5,0.5]). Gene Ontology (GO) analysis of the remaining genes with a fold change >2 and a Mann–Whitney P < 0.05 revealed a 100-fold enrichment of GO terms related to protein import into the peroxisome and a >20-fold enrichment related to RNA cleavage involved in mRNA processing (Table S2). We note that several PEX genes (PEX1, PEX6, and PEX12) modulate bleomycin resistance, possibly because there is a direct link between DNA repair and peroxisome biology through localization of the DNA repair kinase ATM to peroxisome membranes (Zhang et al., 2015).
We then used fluorescence microscopy of mVenus-PTS1 in the Pex-ZeoR cell line to assess how knockdown of candidate genes altered peroxisome abundance. For each candidate gene, we produced two unique constitutive knockdown cell lines per gene and quantified mVenus-PTS1 foci number, foci and cell area, and foci and cytoplasm fluorescence intensity using CellProfiler (Stirling et al., 2021). To estimate the efficiency of peroxisome import while accounting for different mVenus-PTS1 expression levels, we calculated the ratio of the intensity of mVenus-PTS1 in peroxisome foci to the intensity of mVenus-PTS1 in the cytoplasm (Fig. 2 A and Fig. S2, A and B). We found that several of the guides enriched by Zeocin selection decreased the ratio of peroxisomal to cytosolic mVenus-PTS1 intensity, including those targeting the E3 ligase RNF146, Integrator complex subunit INTS8, and calcium-activated potassium channel KCNN4 (Fig. 2 A).
Peroxisome abundance is regulated locally by RNF146. (A) CellProfiler quantification of the ratio of mVenus-PTS1 intensity in foci and in the cytoplasm in fluorescence microscopy images acquired of live HCT116 Pex-ZeoR cells expressing sgRNAs targeting various genes. Data per gene constitutes m = 2 unique sgRNAs with n = 49 images per gene. NTC sgRNA shown in yellow, PEX1 sgRNA shown in pink, sgRNAs significantly different from NTC (P < 0.0001, independent t test) are in blue, sgRNA with P > 0.05 are in white. (B) Representative fluorescence microscopy images of mVenus expression in HCT116 Pex-ZeoR cells harboring sgRNAs for NTC, PEX1, or RNF146 and quantification of the ratio of mVenus-PTS1 foci intensity to mVenus-PTS1 cytosolic intensity. Data are representative of m = 49 images. n = 2 biological replicates. Scale bars: 10 μm. (C) Representative fluorescence microscopy images of mVenus expression in HCT116 Pex-ZeoR cells treated with either scrambled (scr) siRNA or RNF146 siRNA, and quantification of the ratio of total mVenus-PTS1 foci intensity to mVenus-PTS1 cytosolic intensity. Data are representative of m = 49 images n = 2 biological replicates. Scale bar: 10 μm. (D) Representative fluorescence microscopy images of mVenus expression in H4 Pex-ZeoR cells harboring sgRNAs for NTC, PEX1, or RNF146 and quantification of the ratio of mVenus-PTS1 foci intensity to mVenus-PTS1 cytosolic intensity. Data are representative of m = 49 images. n = 2 biological replicates. Scale bars: 10 μm. Asterisks denote ****P < 0.0001. (E) Heatmap of RNA-seq data displaying significant (P < 0.05) fold change of PEX gene transcription in RNF146 knockdown cells versus NTC controls. Data are representative of n = 3 biological replicates.
Peroxisome abundance is regulated locally by RNF146. (A) CellProfiler quantification of the ratio of mVenus-PTS1 intensity in foci and in the cytoplasm in fluorescence microscopy images acquired of live HCT116 Pex-ZeoR cells expressing sgRNAs targeting various genes. Data per gene constitutes m = 2 unique sgRNAs with n = 49 images per gene. NTC sgRNA shown in yellow, PEX1 sgRNA shown in pink, sgRNAs significantly different from NTC (P < 0.0001, independent t test) are in blue, sgRNA with P > 0.05 are in white. (B) Representative fluorescence microscopy images of mVenus expression in HCT116 Pex-ZeoR cells harboring sgRNAs for NTC, PEX1, or RNF146 and quantification of the ratio of mVenus-PTS1 foci intensity to mVenus-PTS1 cytosolic intensity. Data are representative of m = 49 images. n = 2 biological replicates. Scale bars: 10 μm. (C) Representative fluorescence microscopy images of mVenus expression in HCT116 Pex-ZeoR cells treated with either scrambled (scr) siRNA or RNF146 siRNA, and quantification of the ratio of total mVenus-PTS1 foci intensity to mVenus-PTS1 cytosolic intensity. Data are representative of m = 49 images n = 2 biological replicates. Scale bar: 10 μm. (D) Representative fluorescence microscopy images of mVenus expression in H4 Pex-ZeoR cells harboring sgRNAs for NTC, PEX1, or RNF146 and quantification of the ratio of mVenus-PTS1 foci intensity to mVenus-PTS1 cytosolic intensity. Data are representative of m = 49 images. n = 2 biological replicates. Scale bars: 10 μm. Asterisks denote ****P < 0.0001. (E) Heatmap of RNA-seq data displaying significant (P < 0.05) fold change of PEX gene transcription in RNF146 knockdown cells versus NTC controls. Data are representative of n = 3 biological replicates.
A microscopy assay reveals positive and negative regulators of peroxisome import. (A and B) Additional data as in Fig. 2 A. CellProfiler quantification of the ratio of mVenus-PTS1 intensity in foci and in the cytoplasm in fluorescence microscopy images acquired of live HCT116 Pex-ZeoR cells expressing sgRNAs targeting various genes. (A) Positive phenotype score genes from the primary genetic screen. (B) Negative phenotype score genes from the primary genetic screen. Data per gene constitutes m = 2 unique sgRNAs with n = 49 images per gene. NTC sgRNA shown in yellow, PEX1 sgRNA shown in pink, sgRNAs significantly different from NTC are in blue (P < 0.0001, independent t test) or purple (P < 0.05, independent t test), and sgRNAs with P > 0.05 are in white.
A microscopy assay reveals positive and negative regulators of peroxisome import. (A and B) Additional data as in Fig. 2 A. CellProfiler quantification of the ratio of mVenus-PTS1 intensity in foci and in the cytoplasm in fluorescence microscopy images acquired of live HCT116 Pex-ZeoR cells expressing sgRNAs targeting various genes. (A) Positive phenotype score genes from the primary genetic screen. (B) Negative phenotype score genes from the primary genetic screen. Data per gene constitutes m = 2 unique sgRNAs with n = 49 images per gene. NTC sgRNA shown in yellow, PEX1 sgRNA shown in pink, sgRNAs significantly different from NTC are in blue (P < 0.0001, independent t test) or purple (P < 0.05, independent t test), and sgRNAs with P > 0.05 are in white.
RNF146 regulates peroxisome foci intensity in multiple cell lines
Given the magnitude of the impact of the RNF146 knockdown on mVenus-PTS1 foci (Fig. 2, A and B), we chose to focus our efforts on characterizing the effects of RNF146 on peroxisome homeostasis. We first ruled out possible off-target effects of the RNF146 sgRNA by treating our reporter cell line with RNF146 siRNA, which recapitulated the loss of mVenus foci signal within 24 h of siRNA treatment (Fig. 2 C). To determine if the peroxisomal effect of RNF146 knockdown was specific to the HCT116 cell line, we created a secondary cell line, the H4 astrocytoma cancer cell line, harboring the same CRISPRi machinery and our Pex-ZeoR reporter. We observed significant depletion of mVenus-PTS1 foci intensity in both the HCT116 and H4 RNF146 and PEX knockdown cell lines (Fig. 2, B and D). The significant depletion of PTS1 foci in two independent cell lines suggests that RNF146 has a bona fide role in regulating peroxisome homeostasis in human cells.
To determine if RNF146 knockdown impacted peroxisome biogenesis through an effect on PEX gene expression, we gathered RNA sequencing (RNA-seq) data of RNF146 knockdown HCT116 cell mRNA transcripts versus NTC cells. We found that knockdown of RNF146, which was confirmed in the data set, mildly repressed transcription of PEX3 and PEX10. Given that neither PEX3 nor PEX10 had positive phenotype scores in the CRISPRi screen, we found it unlikely that the RNF146 phenotype can be completely explained by these transcriptional changes, thereby indicating a posttranscriptional role for RNF146 with regard to peroxisomal homeostasis (Fig. 2 E).
RNF146-mediated loss of mVenus-PTS1 foci depends on TNKS/2, but not autophagy
RNF146 is known to collaborate with poly(ADP-ribose) polymerases to ubiquitinate PARsylated proteins and target them for degradation. Loss of RNF146 is therefore expected to stabilize PARsylated substrates, which could act to either inhibit peroxisome biogenesis or increase peroxisome-specific autophagy. We, therefore, tested if the observed loss of mVenus-PTS1 foci in response to RNF146 knockdown depended on changes in the RNF146 partners TNKS/2. We first assessed TNKS/2 levels in an RNF146 knockdown and found that knockdown of RNF146 expression in the HCT116 Pex-ZeoR cell line caused a marked increase in TNKS/2 protein levels (Fig. 3 A). To test if RNF146’s effect on peroxisomes depended on increased TNKS/2 levels, we performed a dual knockdown assay of RNF146 and TNKS/2 in our reporter cell line. We found that siRNA knockdown of TNKS and TNKS2 in RNF146 CRISPRi cells rescued the import of mVenus-PTS1 (Fig. 3, A and B), indicating that RNF146’s effect on peroxisomes depended on TNKS/2. In an extended assay, we attempted to swap the dual knockdown strategies of RNF146 and TNKS, such that only TNKS (and not TNKS2) was suppressed by CRISPRi, and RNF146 expression was suppressed by siRNA treatment. We observed that there was clear rescue in the TNKS CRISPRi and RNF146 siRNA treatment, but that this rescue was not as complete as the RNF146 CRISPRi and TNKS/2 siRNA treatment, suggesting that TNKS2 may also play a role in the RNF146 knockdown phenotype (Fig. S3 A). These results are consistent with previous reports that TNKS is significantly stabilized in cells lacking RNF146 (Nie et al., 2020). Although it was previously shown that TNKS mediates peroxisome-specific autophagy (Li et al., 2017), we found that siRNA inhibition of ATG7 did not prevent the accumulation of TNKS/2 nor the loss of mVenus-PTS1 foci intensity in RNF146 knockdown cells (Fig. 3, C and D). This lack of dependence on autophagy was further corroborated in multiple cell lines by the treatment of RNF146 knockdown cells with autophagy inhibitors bafilomycin or hydroxychloroquine, which, despite preventing LC3BII turnover, did not substantially rescue peroxisome foci number or intensity relative to control cells (Fig. S3, B–G). These observations suggest that while the effect of RNF146 knockdown on peroxisomes depends on TNKS/2, it does not depend on peroxisome-specific autophagy.
RNF146’s effect on peroxisomes is mediated by TNKS/2, but not autophagy. (A) Immunoblots for TNKS/2 and β-actin (ACTB, loading control) in lysate from scrambled (scr) or TNKS/2 siRNA-treated HCT116 Pex-ZeoR cells with sgRNAs for either NTC or RNF146. (B) Left panel: Representative mVenus-PTS1 fluorescence microscopy images of either NTC or RNF146 sgRNA cells treated with either scrambled siRNA or TNKS siRNA. Right panel: Quantification of mVenus-PTS1 microscopy images in the left panel for mVenus-PTS1 foci intensity versus total cytosol intensity in HCT116 Pex-ZeoR cells. Data are representative of 49 images per condition and two biological replicates. Scale bars: 20 μm. (C) Immunoblot for TNKS/2, ATG7, and LC3B in lysate from scrambled or ATG7 siRNA-treated HCT116 Pex-ZeoR cells with sgRNAs for either NTC or RNF146. (D) Left panel: Fluorescence microscopy data of scrambled or ATG7 siRNA-treated HCT116 Pex-ZeoR cells with sgRNAs for either NTC or RNF146.m = 32 images. n = 2 biological replicates. Right panel: Quantification of mVenus-PTS1 microscopy images for mVenus foci intensity versus cytosol intensity in HCT116 Pex-ZeoR cells. Scale bars: 10 μm. All blots are representative of n = 3 biological replicates. Asterisks denote P values *P < 0.05, ****P < 0.0001, whereas ns denotes not significant, calculated by independent t test. Source data are available for this figure: SourceData F3.
RNF146’s effect on peroxisomes is mediated by TNKS/2, but not autophagy. (A) Immunoblots for TNKS/2 and β-actin (ACTB, loading control) in lysate from scrambled (scr) or TNKS/2 siRNA-treated HCT116 Pex-ZeoR cells with sgRNAs for either NTC or RNF146. (B) Left panel: Representative mVenus-PTS1 fluorescence microscopy images of either NTC or RNF146 sgRNA cells treated with either scrambled siRNA or TNKS siRNA. Right panel: Quantification of mVenus-PTS1 microscopy images in the left panel for mVenus-PTS1 foci intensity versus total cytosol intensity in HCT116 Pex-ZeoR cells. Data are representative of 49 images per condition and two biological replicates. Scale bars: 20 μm. (C) Immunoblot for TNKS/2, ATG7, and LC3B in lysate from scrambled or ATG7 siRNA-treated HCT116 Pex-ZeoR cells with sgRNAs for either NTC or RNF146. (D) Left panel: Fluorescence microscopy data of scrambled or ATG7 siRNA-treated HCT116 Pex-ZeoR cells with sgRNAs for either NTC or RNF146.m = 32 images. n = 2 biological replicates. Right panel: Quantification of mVenus-PTS1 microscopy images for mVenus foci intensity versus cytosol intensity in HCT116 Pex-ZeoR cells. Scale bars: 10 μm. All blots are representative of n = 3 biological replicates. Asterisks denote P values *P < 0.05, ****P < 0.0001, whereas ns denotes not significant, calculated by independent t test. Source data are available for this figure: SourceData F3.
Regulation of peroxisome abundance by stabilized TNKS/2 is not altered by inhibition of autophagy. (A) Left panel: Representative fluorescence microscopy images of NTC, RNF146, and TNKS sgRNA expressing HCT116 Pex-ZeoR cells treated with either scrambled (scr) siRNA, RNF146 siRNA, or TNKS/2 siRNA (10 μM) for 24 h. X represents no sample/image. Scale bar: 10 μm. Right panel: Quantification of mVenus-PTS1 microscopy images in the left panel for mVenus foci intensity (peroxisomes) versus total cytosol intensity. Data are representative of m = 32 images per condition and n = 2 biological replicates. (B) Left panel: Representative immunofluorescence microscopy images of NTC and RNF146 sgRNA expressing HCT116 Pex-ZeoR cells treated with DMSO (mock) or 50 nM Bafilomycin A1 (Baf) for 15 h mVenus-PTS1 in green, DAPI in blue, and PMP70 in cyan. Scale bar: 10 μm. Right panel: Quantification of percentage foci area of mVenus-PTS1 and PMP70 versus cytosolic area for m = 21 images and n = 2 biological replicates. (C) Immunoblot of TNKS and LC3B of cell lysate from conditions in B. (D) Left panel: Representative immunofluorescence microscopy images of NTC and RNF146 sgRNA expressing HCT116 Pex-ZeoR cells treated with DMSO (mock), 5 µM hydroxychloroquine (HCQ), or 10 µM hydroxychloroquine for 24 h (5 µM HCQ not shown). mVenus-PTS1 in green, PMP70 in magenta, and DAPI in blue. Scale bar: 10 μm. Right panels: Quantification of immunofluorescence microscopy images for percentage foci area of mVenus and PMP70, respectively, versus cytosolic area for m = 32 images and n = 2 biological replicates. (E) Immunoblots of cellular lysate from D against TNKS/2 and LC3B. (F) Left panel: Representative fluorescence microscopy images of NTC and RNF146 sgRNA expressing H4 Pex-ZeoR cells treated with DMSO (mock) or 50 nM Bafilomycin A1 for 15 h. Scale bar: 10 μm. Right panel: Quantification of mVenus-PTS1 microscopy images in the left panel for mVenus foci intensity (peroxisomes) versus total cytosol intensity. Data is representative of m = 32 images per condition and n = 2 biological replicates. Immunoblots of cellular lysate from the left panel against TNKS and LC3B. All immunoblots are representative of n = 3 independent blots. Asterisks denote P values **P < 0.01, ****P < 0.0001, whereas ns denotes not significant, calculated by independent t test.
Regulation of peroxisome abundance by stabilized TNKS/2 is not altered by inhibition of autophagy. (A) Left panel: Representative fluorescence microscopy images of NTC, RNF146, and TNKS sgRNA expressing HCT116 Pex-ZeoR cells treated with either scrambled (scr) siRNA, RNF146 siRNA, or TNKS/2 siRNA (10 μM) for 24 h. X represents no sample/image. Scale bar: 10 μm. Right panel: Quantification of mVenus-PTS1 microscopy images in the left panel for mVenus foci intensity (peroxisomes) versus total cytosol intensity. Data are representative of m = 32 images per condition and n = 2 biological replicates. (B) Left panel: Representative immunofluorescence microscopy images of NTC and RNF146 sgRNA expressing HCT116 Pex-ZeoR cells treated with DMSO (mock) or 50 nM Bafilomycin A1 (Baf) for 15 h mVenus-PTS1 in green, DAPI in blue, and PMP70 in cyan. Scale bar: 10 μm. Right panel: Quantification of percentage foci area of mVenus-PTS1 and PMP70 versus cytosolic area for m = 21 images and n = 2 biological replicates. (C) Immunoblot of TNKS and LC3B of cell lysate from conditions in B. (D) Left panel: Representative immunofluorescence microscopy images of NTC and RNF146 sgRNA expressing HCT116 Pex-ZeoR cells treated with DMSO (mock), 5 µM hydroxychloroquine (HCQ), or 10 µM hydroxychloroquine for 24 h (5 µM HCQ not shown). mVenus-PTS1 in green, PMP70 in magenta, and DAPI in blue. Scale bar: 10 μm. Right panels: Quantification of immunofluorescence microscopy images for percentage foci area of mVenus and PMP70, respectively, versus cytosolic area for m = 32 images and n = 2 biological replicates. (E) Immunoblots of cellular lysate from D against TNKS/2 and LC3B. (F) Left panel: Representative fluorescence microscopy images of NTC and RNF146 sgRNA expressing H4 Pex-ZeoR cells treated with DMSO (mock) or 50 nM Bafilomycin A1 for 15 h. Scale bar: 10 μm. Right panel: Quantification of mVenus-PTS1 microscopy images in the left panel for mVenus foci intensity (peroxisomes) versus total cytosol intensity. Data is representative of m = 32 images per condition and n = 2 biological replicates. Immunoblots of cellular lysate from the left panel against TNKS and LC3B. All immunoblots are representative of n = 3 independent blots. Asterisks denote P values **P < 0.01, ****P < 0.0001, whereas ns denotes not significant, calculated by independent t test.
Loss of RNF146 specifically inhibits import into peroxisomes
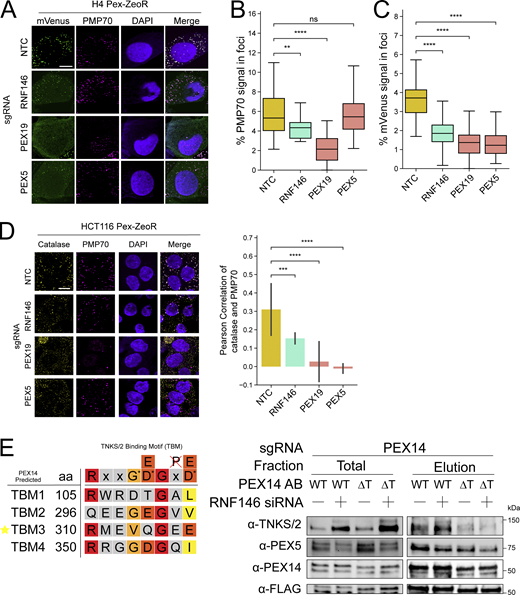
Since the loss of RNF146 did not appear to induce peroxisome-specific autophagy, we evaluated whether the loss of RNF146 could specifically impair peroxisome biogenesis at the stage of protein import into peroxisomes. We performed immunofluorescence microscopy on the HCT116 and H4 CRISPRi Pex-ZeoR cell lines harboring sgRNAs for NTC, RNF146, PEX5, and PEX19, where PEX5 and PEX19 are the receptors for PTS1-tagged matrix protein import and peroxisomal membrane protein insertion, respectively (Fig. 4 A and Fig. S4 A). We found that knockdown of RNF146 in both HCT116 and H4 cells resembled a PEX5 knockdown, in which a peroxisome membrane protein PMP70 remains present and punctate (Fig. 4, A and B; and Fig. S4, A and B), but matrix proteins, both mVenus-PTS1 and catalase, no longer form foci (Fig. 4, A and C; and Fig. S4, A and C) or co-localize with PMP70 (Fig. S4 D). These observations suggest that loss of RNF146 inhibits import of PEX5 client proteins into the peroxisome.
Loss of RNF146 impairs peroxisome protein import. (A) Representative immunofluorescence microscopy images of NTC, RNF146, PEX19, and PEX5 sgRNA expressing HCT116 Pex-ZeoR cells. mVenus-PTS1 in green, DAPI in blue, PMP70 in magenta. Scale bar: 10 μm. (B and C) Quantification of immunofluorescence microscopy images for percentage foci area of PMP70 (B) and mVenus-PTS1 (C) versus cytosolic area. m = 25 images. n = 2 biological replicates. Asterisks denote P values **P < 0.01, ***P < 0.001, ****P < 0.0001, whereas ns denotes not significant, calculated by independent t test. (D) Immunoblot of HCT116 Pex-ZeoR with sgRNAs targeting NTC, RNF146, and PEX5. Fractions represent total lysate (T), 20,000 × g supernatant (S), and 20,000 × g pellet (P). Densitometry quantification of blots represents the normalized (to NTC) fold change of the densitometric ratio of soluble versus pellet fractions (R = S/P) of selected proteins. Triangle for anti-PEX14 blot denotes the band that disappears with PEX14 sgRNA treatment. All blots are representative of n = 3 biological replicates. Source data are available for this figure: SourceData F4.
Loss of RNF146 impairs peroxisome protein import. (A) Representative immunofluorescence microscopy images of NTC, RNF146, PEX19, and PEX5 sgRNA expressing HCT116 Pex-ZeoR cells. mVenus-PTS1 in green, DAPI in blue, PMP70 in magenta. Scale bar: 10 μm. (B and C) Quantification of immunofluorescence microscopy images for percentage foci area of PMP70 (B) and mVenus-PTS1 (C) versus cytosolic area. m = 25 images. n = 2 biological replicates. Asterisks denote P values **P < 0.01, ***P < 0.001, ****P < 0.0001, whereas ns denotes not significant, calculated by independent t test. (D) Immunoblot of HCT116 Pex-ZeoR with sgRNAs targeting NTC, RNF146, and PEX5. Fractions represent total lysate (T), 20,000 × g supernatant (S), and 20,000 × g pellet (P). Densitometry quantification of blots represents the normalized (to NTC) fold change of the densitometric ratio of soluble versus pellet fractions (R = S/P) of selected proteins. Triangle for anti-PEX14 blot denotes the band that disappears with PEX14 sgRNA treatment. All blots are representative of n = 3 biological replicates. Source data are available for this figure: SourceData F4.
TNKS/2 stabilization decreases peroxisome import efficiency and increases TNKS/2 interaction with PEX14. (A) Left panel: Representative immunofluorescence microscopy images of NTC, RNF146, PEX19, and PEX5 sgRNA expressing H4 Pex-ZeoR cells. mVenus-PTS1 in green, DAPI in blue, PMP70 in magenta. Scale bar: 10 μm. (B and C) Quantification of immunofluorescence microscopy images for percentage foci area of PMP70 (B) and mVenus (C), respectively, versus cytosolic area. n = 25 images. (D) Additional analysis of the experiment in Fig. 4 A to show colocalization of catalase and PMP70. Left panel: Representative immunofluorescence microscopy images NTC, RNF146, PEX19, and PEX5 sgRNA expressing cells. Catalase in yellow, DAPI in blue, PMP70 in magenta. m = 25 images. n = 2 biological replicates. Right panel: Quantification of Pearson’s correlation coefficient of catalase and PMP70 colocalization of microscopy images. (E) Left panel: Schematic showing the predicted TBMs of PEX14 with amino acid positions, compared with the predicted consensus TBMs of Guettler et al., 2011; Pollock et al., 2017. Red = essential, dark orange = common/variable, light orange = variable, yellow = uncommon/accepted, gray = no pattern, G* = glycine or small non hydrophobic, D* = D/E with some variability, defaced P = no proline. Star = chosen ΔTBM. Right panel: Immunoblots of anti-FLAG immunoprecipitation total and elution fractions from HCT116 Pex-ZeoR cells expressing PEX14 sgRNAs with constitutive re-expression of either FLAG-PEX14 (WT) or FLAG-PEX14-ΔTBM3 (ΔT), treated with either NTC or RNF146 siRNA (10 nM) for 24 h, detecting TNKS/2, PEX5, and PEX14. Blots are representative of n = 3 biological replicates. Asterisks denote P values, **P < 0.01, ***P < 0.001, ****P < 0.0001, whereas ns denotes not significant, calculated by independent t test. Source data are available for this figure: SourceData FS4.
TNKS/2 stabilization decreases peroxisome import efficiency and increases TNKS/2 interaction with PEX14. (A) Left panel: Representative immunofluorescence microscopy images of NTC, RNF146, PEX19, and PEX5 sgRNA expressing H4 Pex-ZeoR cells. mVenus-PTS1 in green, DAPI in blue, PMP70 in magenta. Scale bar: 10 μm. (B and C) Quantification of immunofluorescence microscopy images for percentage foci area of PMP70 (B) and mVenus (C), respectively, versus cytosolic area. n = 25 images. (D) Additional analysis of the experiment in Fig. 4 A to show colocalization of catalase and PMP70. Left panel: Representative immunofluorescence microscopy images NTC, RNF146, PEX19, and PEX5 sgRNA expressing cells. Catalase in yellow, DAPI in blue, PMP70 in magenta. m = 25 images. n = 2 biological replicates. Right panel: Quantification of Pearson’s correlation coefficient of catalase and PMP70 colocalization of microscopy images. (E) Left panel: Schematic showing the predicted TBMs of PEX14 with amino acid positions, compared with the predicted consensus TBMs of Guettler et al., 2011; Pollock et al., 2017. Red = essential, dark orange = common/variable, light orange = variable, yellow = uncommon/accepted, gray = no pattern, G* = glycine or small non hydrophobic, D* = D/E with some variability, defaced P = no proline. Star = chosen ΔTBM. Right panel: Immunoblots of anti-FLAG immunoprecipitation total and elution fractions from HCT116 Pex-ZeoR cells expressing PEX14 sgRNAs with constitutive re-expression of either FLAG-PEX14 (WT) or FLAG-PEX14-ΔTBM3 (ΔT), treated with either NTC or RNF146 siRNA (10 nM) for 24 h, detecting TNKS/2, PEX5, and PEX14. Blots are representative of n = 3 biological replicates. Asterisks denote P values, **P < 0.01, ***P < 0.001, ****P < 0.0001, whereas ns denotes not significant, calculated by independent t test. Source data are available for this figure: SourceData FS4.
Efficient peroxisomal matrix protein import relies on PEX5 binding to the PTS1-tagged protein, PEX5 docking to PEX13/PEX14 at the peroxisome, and extraction of ubiquitinated PEX5 from the peroxisome membrane by the PEX1/PEX6/PEX26 motor complex for continued rounds of import. PEX5 is therefore typically distributed between both cytoplasmic and membrane fractions, with an increased proportion at the peroxisome membrane in mutants of the ubiquitination and extraction machinery (Platta et al., 2005). To determine if RNF146 knockdown alters the localization of PEX5, we probed for PEX5, mVenus-SKL, and catalase in soluble and membrane fractions after fractionation. As expected, we observed that PEX5 distributes between both membrane and soluble fractions in wild type cells. Interestingly, a larger proportion of PEX5 was soluble in RNF146 knockdown cells compared with control cells (Fig. 4 D). This suggests that the impairment of import of peroxisomes may be due to reduced recruitment of PEX5 and PTS1-cargo to the peroxisome membrane. Additionally, we observed that the soluble proportion of mVenus-SKL and catalase, both PEX5 client proteins with and without, respectively, a canonical PTS1 tag, increased in RNF146 and PEX5 knockdown cells, confirming that RNF146 knockdown also impedes import of endogenous matrix proteins (Fig. 4 D).
PARP activity of TNKS/2 impedes import into peroxisomes
TNKS/2 contains N-terminal ankyrin repeats that bind substrates with a TBM, a SAM domain that mediates oligomerization, and a C-terminal poly(ADP-ribose) polymerase domain (Guettler et al., 2011). There are predicted, conserved TBMs in PEX14, PEX5, PEX19, and PEX11G (Guettler et al., 2011). Specifically, PEX14 was predicted to have at least four purported TBMs (Fig. S4 E). We found that TNKS/2 coimmunoprecipitated both FLAG-PEX14 and PEX5 upon RNF146 knockdown (Fig. 5 A). Additionally, when the reciprocal experiment was performed, full-length FLAG-PEX14 coimmunoprecipitated TNKS/2 and PEX5 in NTC and RNF146 knockdown cells. Notably, when the TBM3 of PEX14 was mutated, FLAG-PEX14-ΔTBM3 cells had reduced affinity for TNKS/2 interaction (Fig. S4 E). These results suggest TNKS/2 associates with the peroxisome membrane and peroxisome import machinery, such as PEX14, upon RNF146 knockdown.
TNKS/2 PARP activity impairs peroxisome protein import. (A) Immunoblots of anti-TNKS/2 immunoprecipitation (IP) fractions from HCT116 Pex-ZeoR cells expressing PEX14 sgRNAs with constitutive re-expression for FLAG-PEX14 treated with either NTC or RNF146 siRNA (10 nM) for 24 h, detecting TNKS/2, PEX5, and PEX14. (B) Representative live-cell fluorescence microscopy images of NTC and RNF146 sgRNA expressing HCT116 Pex-ZeoR cells treated with DMSO (mock), 500 nM G007LK, or 10 μM XAV939 for 24 h mVenus-PTS1 in green. Scale bar: 10 μm. (C) Quantification of fluorescence microscopy images for the ratio of mVenus-PTS1 foci intensity to mVenus-PTS1 cytosolic intensity (left) and the number of foci per cell (right). m = 32 images and n = 2 biological replicates. Asterisks denote P values *P < 0.05, **P < 0.01, ****P < 0.0001, whereas ns denotes not significant, calculated by independent t test. (D) Immunoblots of anti-FLAG immunoprecipitation total lysate (input) and elution fractions from HCT116 Pex-ZeoR cells expressing NTC sgRNA (lane 4/8) or PEX14 sgRNAs with constitutive re-expression for PEX14-FLAG (lane 1/2/3/5/6/7) treated with either NTC or RNF146 siRNA (10 nM) for 24 h, with or without XAV939 (1 μM) for 24 h, and with carfilzomib (10 μM) for 4 h, detecting FLAG, Poly-(ADP)-ribose (PAR), TNKS/2, PEX5, PEX13, and PEX14. Representative of n = 2 biological replicates. (E) Immunoblots of lysates from HCT116 CRISPRi cells harboring NTC guides treated with either NTC or RNF146 siRNA (10 nM) for 48 h, and with or without XAV939 (1 μM) for 24 h. Representative of n = 4 biological replicates. (F) Proposed model: loss of RNF146 increases active TNKS/2, which binds PEX14 and PARsylates proteins at the peroxisome membrane impairing peroxisome import. Source data are available for this figure: SourceData F5.
TNKS/2 PARP activity impairs peroxisome protein import. (A) Immunoblots of anti-TNKS/2 immunoprecipitation (IP) fractions from HCT116 Pex-ZeoR cells expressing PEX14 sgRNAs with constitutive re-expression for FLAG-PEX14 treated with either NTC or RNF146 siRNA (10 nM) for 24 h, detecting TNKS/2, PEX5, and PEX14. (B) Representative live-cell fluorescence microscopy images of NTC and RNF146 sgRNA expressing HCT116 Pex-ZeoR cells treated with DMSO (mock), 500 nM G007LK, or 10 μM XAV939 for 24 h mVenus-PTS1 in green. Scale bar: 10 μm. (C) Quantification of fluorescence microscopy images for the ratio of mVenus-PTS1 foci intensity to mVenus-PTS1 cytosolic intensity (left) and the number of foci per cell (right). m = 32 images and n = 2 biological replicates. Asterisks denote P values *P < 0.05, **P < 0.01, ****P < 0.0001, whereas ns denotes not significant, calculated by independent t test. (D) Immunoblots of anti-FLAG immunoprecipitation total lysate (input) and elution fractions from HCT116 Pex-ZeoR cells expressing NTC sgRNA (lane 4/8) or PEX14 sgRNAs with constitutive re-expression for PEX14-FLAG (lane 1/2/3/5/6/7) treated with either NTC or RNF146 siRNA (10 nM) for 24 h, with or without XAV939 (1 μM) for 24 h, and with carfilzomib (10 μM) for 4 h, detecting FLAG, Poly-(ADP)-ribose (PAR), TNKS/2, PEX5, PEX13, and PEX14. Representative of n = 2 biological replicates. (E) Immunoblots of lysates from HCT116 CRISPRi cells harboring NTC guides treated with either NTC or RNF146 siRNA (10 nM) for 48 h, and with or without XAV939 (1 μM) for 24 h. Representative of n = 4 biological replicates. (F) Proposed model: loss of RNF146 increases active TNKS/2, which binds PEX14 and PARsylates proteins at the peroxisome membrane impairing peroxisome import. Source data are available for this figure: SourceData F5.
To test if RNF146’s effect on peroxisome import depended on the poly(ADP-ribose) polymerase activity of TNKS/2, we tested if the TNKS/2 inhibitors G007LK and XAV939 restored peroxisome foci in RNF146 knockdown cells (Fig. 5, B and C). We found that TNKS/2 inhibitors partially restored import of mVenus-PTS1 into foci in RNF146 knockdown cells as judged by the ratio of foci to cytosolic intensity of mVenus-PTS1, but did not fully recover peroxisome number (Fig. 5 C). To determine if TNKS/2 PARsylated proteins at the peroxisome membrane, we immunoprecipitated PEX14-FLAG. We found that proteins in the PEX14-FLAG elution, which included PEX14-FLAG, PEX13, PEX5, and TNKS, were PARsylated (Fig. 5 D). While it is unclear exactly which proteins are PARsylated, PARsylation was sensitive to TNKS/2 inhibitor XAV939 and amplified by RNF146 knockdown (Fig. 5 D). In addition, we found that suppression of RNF146 and the concomitant increase of TNKS/2 resulted in lowered steady-state levels of PEX14 and PEX13, but not peroxisome membrane protein PMP70, and that this effect was abrogated when TNKS/2 was inhibited by XAV939 (Fig. 5 E). Altogether, these observations suggest that TNKS/2’s PARsylation activity is important for RNF146’s effect on peroxisomes. We therefore propose a model in which high levels of active TNKS/2, induced by loss of RNF146, bind PEX14 and PARsylates proteins at the peroxisome membrane, which inhibits PEX5-mediated protein import into peroxisomes (Fig. 5 F).
PEX proteins alter RNF146/TNKS/2 activity toward other substrates
This model suggests that TNKS/2 binds peroxisome membrane protein PEX14 and can localize to the peroxisome. Other better-known substrates of TNKS/2, such as BLZF1, which localizes to the Golgi (Yue et al., 2021), and AXIN1, which partially localizes to centrosomes (Lach et al., 2022), have defined locations elsewhere in the cell. We thus wondered if peroxisomal recruitment of TNKS/2 could regulate access to other substrates. To test if the presence of peroxisome membranes and membrane proteins alters TNKS/2 substrate selection, we evaluated the stability of the TNKS/2/RNF146 substrates AXIN1, CASC3, and BLZF1 in cells with knockdown of the peroxisomal membrane protein PEX14, the peroxisomal membrane protein chaperone PEX19, or an NTC. We found that AXIN1 and CASC3 levels were significantly depleted in PEX19 knockdown HCT116 cells, and BLZF1 levels were depleted in both PEX19 and PEX14 knockdown HCT116 cells (Fig. 6 A). Furthermore, PEX14 and PEX19 knockdowns also depleted AXIN1 levels in HEK293T, induced pluripotent stem cells (iPSCs) AICS-0090-391, and H4 CRISPRi cells (Fig. 6, B and C; and Fig. S5 A), illustrating that this phenomenon is not specific to HCT116 cells. To confirm that the effect of PEX19 knockdown arises from loss of PEX19, we re-expressed PEX19 using a lentiviral vector to complement the knockdown of endogenous PEX19 and observed a rescue of AXIN1 stability (Fig. 6 D). Additionally, suppression of either RNF146 or TNKS/2 mRNA transcripts via siRNA, as well as XAV939-mediated catalytic inhibition of TNKS/2, restored AXIN1 stability in PEX19 knockdown cells, demonstrating that loss of PEX19 activates RNF146/TNKS/2-mediated destabilization of AXIN1 (Fig. 6 D). These observations suggest that functional peroxisomes repress TNKS/2 activity toward some substrates, including AXIN1, BLZF1, and CASC3.
Peroxisome abundance influences RNF146/TNKS substrate selection. (A–C) Immunoblots measuring the abundance of AXIN1, CASC3, and BLZF in (A) HCT116 (n = 3), (B) HEK293 (n = 3), and (C) iPSC AICS-0090-391 (n = 3) CRISPRi cells with indicated sgRNAs. (D) Western blot measuring abundance of AXIN1 and ACTB (loading control) in HCT116 cells with indicated sgRNAs, PEX19 knockdown cells are paired with treatments for PEX19 re-expression, TNKS siRNA (10 nM), RNF146 siRNA 10 nM), or XAV939 (10 μM). The blots shown are representative of n = 1 blot. (E and F) TOPFlash Dual Luciferase assays measuring the induction of Wnt signaling to downstream β-catenin transcription in PEX knockdown HCT116 cells (E) and HEK293T (F) harboring the indicated sgRNAs and treated with or without 315 ng/ml Wnt3a for 24 h (data shown is 48 h after transfection with TOPFlash constructs). Luciferase activity is measured versus a Renilla transfection control and data is normalized to untreated NTC samples. FOPFlash negative control performed in NTC sgRNA cells. Data is representative of n = 3 biological samples. Asterisks denote P values *P < 0.05, **P < 0.01, calculated by paired t test. Source data are available for this figure: SourceData F6.
Peroxisome abundance influences RNF146/TNKS substrate selection. (A–C) Immunoblots measuring the abundance of AXIN1, CASC3, and BLZF in (A) HCT116 (n = 3), (B) HEK293 (n = 3), and (C) iPSC AICS-0090-391 (n = 3) CRISPRi cells with indicated sgRNAs. (D) Western blot measuring abundance of AXIN1 and ACTB (loading control) in HCT116 cells with indicated sgRNAs, PEX19 knockdown cells are paired with treatments for PEX19 re-expression, TNKS siRNA (10 nM), RNF146 siRNA 10 nM), or XAV939 (10 μM). The blots shown are representative of n = 1 blot. (E and F) TOPFlash Dual Luciferase assays measuring the induction of Wnt signaling to downstream β-catenin transcription in PEX knockdown HCT116 cells (E) and HEK293T (F) harboring the indicated sgRNAs and treated with or without 315 ng/ml Wnt3a for 24 h (data shown is 48 h after transfection with TOPFlash constructs). Luciferase activity is measured versus a Renilla transfection control and data is normalized to untreated NTC samples. FOPFlash negative control performed in NTC sgRNA cells. Data is representative of n = 3 biological samples. Asterisks denote P values *P < 0.05, **P < 0.01, calculated by paired t test. Source data are available for this figure: SourceData F6.
PEX14 and PEX19 knockdown destabilizes AXIN1 levels. (A) Immunoblot measuring the abundance of AXIN1 in H4 CRISPRi cells expressing sgRNA for NTC, PEX5, PEX14, PEX19, and RNF146. Source data are available for this figure: SourceData FS5.
PEX14 and PEX19 knockdown destabilizes AXIN1 levels. (A) Immunoblot measuring the abundance of AXIN1 in H4 CRISPRi cells expressing sgRNA for NTC, PEX5, PEX14, PEX19, and RNF146. Source data are available for this figure: SourceData FS5.
Increased Wnt/β-catenin signaling in PEX knockdown cells
AXIN1 is the limiting component for the formation of the β-catenin destruction complex which induces the phosphorylation and subsequent degradation of the β-catenin transcription factor. In canonical Wnt signaling, Wnt ligand binding to the Frizzled receptor dissociates the β-catenin destruction complex, allowing β-catenin to accumulate, enter the nucleus, and induce transcription of Wnt-responsive genes. The stabilization of AXIN1, such as by TNKS/2 inhibitors, inhibits Wnt signaling by increasing levels of the destruction complex (Huang et al., 2009). Since AXIN1 was severely destabilized in PEX19 knockdown HCT116 cells and partially destabilized in PEX14 and PEX19 knockdown HEK293T, H4, and iPSC AICS-0090-391 cells, we tested if the knockdown of PEX genes can therefore influence the Wnt signaling pathway using the TOPFlash reporter for β-catenin transcriptional activity. We found that HCT116 cells had a greater transcriptional response to Wnt ligand in PEX14 and PEX19 knockdown cells (Fig. 6 E), as well as increased basal activity. Since HCT116 cells are derived from a colorectal carcinoma heterozygous for a dominant mutation in β-catenin that causes constitutively active β-catenin–TCF regulated transcription (Morin et al., 1997), we also tested the effect of the PEX knockdowns on the TOPFlash reporter in HEK293T cells. Both PEX14 and PEX19 knockdown HEK293Ts exhibited a partial loss of AXIN1 levels (Fig. 6 B), and consistently, also exhibited a greater response to Wnt ligand, though basal levels were not perturbed (Fig. 6 F). Our observations show that knockdown of PEX14 and PEX19 increases Wnt signaling consistent with the decreased levels of the core subunit of the β-catenin destruction complex, AXIN1.
Discussion
Here, we describe an approach to link cell viability to peroxisome import efficiency by sequestering the ZeoR in the peroxisome. We used this approach to screen for novel genes regulating peroxisome import efficiency. In addition to known PEX genes, we found that the E3 ligase RNF146 regulates peroxisome import through its control of the levels of the poly(ADP-ribose) polymerases TNKS/2. High levels of TNKS/2, which can bind PEX14 and possibly other PEX proteins, specifically inhibit import into peroxisomes. In our cell lines, inhibition of import depends on TNKS/2’s poly(ADP-ribose) polymerase activity. We note that Li and colleagues (Li et al., 2017) showed that increased levels of TNKS/2 due to treatment with TNKS/2 inhibitors such as XAV939 could induce peroxisome-specific autophagy in HEK-293T cells; however, this autophagy does not mediate the loss of mVenus-PTS1 foci in response to RNF146 knockdown in H4 or HCT116 cells. Instead, we find that TNKS/2’s polymerase activity is required for the observed inhibition of peroxisome import. We, therefore, propose a model in which loss of RNF146 stabilizes active TNKS/2, which PARsylates proteins at the peroxisome membrane and impairs their function in matrix protein import into the peroxisome.
This model suggests that any mechanism that inactivates RNF146 will inhibit import into peroxisomes. In mice, RNF146 transcription is repressed during RANKL-mediated osteoclastogenesis through an NF-κB binding site (Matsumoto et al., 2017), suggesting that peroxisome import may be coordinated with cell type specification through RNF146 and TNKS/2. RNF146 activity is also regulated by sumoylation (Li et al., 2023), localization to the nucleus (Gerö et al., 2014; Sheng et al., 2018), and direct interaction with other poly(ADP-ribose) polymerases such as PARP-1 (Gerö et al., 2014). It is therefore possible that temporary localization of RNF146 to the nucleus in response to DNA damage could impede peroxisome import, perhaps to increase concentrations of cytosolic catalase to reduce oxidative stress. The effect of this regulation on TNKS/2 activity and peroxisome import, and the consequences for RNF146’s protective role during DNA damage (Kang et al., 2011), oxidative stress (Xu et al., 2013), and PARsylation-induced cell death (Andrabi et al., 2011) warrants further investigation.
A second implication of our results is that RNF146/TNKS/2 together may regulate the stability of substrates at the peroxisome membrane, such as PEX14 itself, or neighboring proteins. Proteomic studies show that the loss of TNKS significantly stabilizes PEX14 and SLC27A2, a peroxisomal transporter for long-chain fatty acids (Bhardwaj et al., 2017), and indeed, we observed that high levels of TNKS induced by RNF146 knockdown destabilized PEX14 and PEX13 (Fig. 5 D). We did not observe a change in peroxisome protein import or peroxisome number in response to TNKS knockdown (Fig. 3 B). However, it is possible that this may be due to the relatively low levels of expression of endogenous TNKS in the HCT116 cell line, and in cells with high levels of TNKS, such as the brain, adipose tissue, and endocrine pancreas (Yeh et al., 2009), it is possible that a knockdown of TNKS could improve peroxisome import and abundance. Indeed, studies of TNKS-deficient mice show that they have increased fatty acid oxidation, which is consistent with improved peroxisomal function (Yeh et al., 2009). It is also possible that RNF146/TNKS activity at the peroxisome membrane regulates signaling from the peroxisome membrane. For example, RNF146/TNKS coordinate the degradation of the antiviral protein MAVS (Xu et al., 2022), which has been shown to localize to both the peroxisome and mitochondria and initiate disparate signaling pathways upon viral infection (Dixit et al., 2010).
An intriguing corollary of RNF146/TNKS localization to the peroxisome membrane is the impact of this localization on its access to other substrates. We found that the knockdown of different PEX proteins, particularly PEX14, which binds TNKS, and PEX19, which is generally required for peroxisome membrane protein stability, decreases the stability of RNF146/TNKS substrates that are not thought to be at the peroxisome. We propose that localization to the peroxisome membrane acts as a sink for RNF146/TNKS, keeping RNF146/TNKS away from other substrates such as AXIN1 and Golgi-localized BLZF1, and thereby stabilizing them. In this model, the absence of peroxisomes allows RNF146/TNKS to relocalize to induce the degradation of AXIN1 and BLZF1. Indeed, reports in the literature suggest that both RNF146 and TNKS can relocalize in response to perturbations; RNF146 moves between the cytoplasm and nucleus in response to oxidative stress and DNA damage (Gerö et al., 2014; Kang et al., 2011), and TNKS’s diffuse cytosolic localization becomes punctate with treatment with TNKS inhibitors (Martino-Echarri et al., 2016; Thorvaldsen et al., 2015) and infection with Sendai virus (Xu et al., 2022).
Finally, we demonstrated that the effect of PEX knockdowns on the RNF146/TNKS substrate AXIN1 was sufficient to alter the transcriptional response to Wnt ligand in two different cell lines. Our results suggest that peroxisomes may act as signaling platforms that can alter cell fate decisions by impacting Wnt signaling. The most severe forms of Zellweger syndrome have stereotyped neuronal migration disorders, chondrodysplasia punctata, renal cysts, and craniofacial dysmorphisms indicating disruptions to normal development (Braverman et al., 2016). Our findings raise the possibility that the perturbation of developmental signaling pathways contributes to the pathology of Zellweger Spectrum Disorders.
Materials and methods
Cell lines, culture conditions, lentiviral production, and transduction
H4 dCas9-KRAB (a gift from the laboratory of Diego Acosta-Alvear, University of California, Santa Barbara, Santa Barbara, CA, USA), HEK293T, and HCT116 dCas9-KRAB (a gift from the laboratory of J. Corn, ETH Zürich, Zürich, Switzerland) cells were cultured in Dulbecco’s Modified Eagle Media (10565018, DMEM; Gibco) supplemented with 10% fetal bovine serum (S11150H; R&D Systems), 1% penicillin/streptomycin (15140122; Gibco), and 2 mM L-Glutamine, and kept at 37°C and 5% CO2 in a humidified incubator. Generation of lentivirus was performed by transfecting HEK293T cells with standard delta viral protein R and vesicular stomatitis virus G protein packaging vectors paired with TransIT-LTI Transfection Reagent (MIR2305; Mirus). Lentivirus was harvested 72 h following transfection and frozen at −80°C.
Zeocin resistance harboring HCT116 CRISPRi cell lines were constructed by transducing cells with lentivirus expressing mVenus-ZeoR-PTS1 constructs with either a phosphoglycerate kinase (pCR2054) or hEF-1α (pCR2055) promoter and “spinfecting” cells in a centrifuge at 1,000 rpm for 2 h. ZeoR-expressing cells were single-cell sorted by flow cytometry (SH800S; Sony) for mVenus expression at 488-nm excitation, where modestly fluorescent monoclonal cells were selected for both promoter types.
Re-expression constructs were made by Gibson cloning desired coding sequences into the pLentiX-CD90 Thy1.1 vector backbone, with subsequent cell sorting of Thy1.1 positive cells by immunolabeling with CD90.1 Thy-1.1 antibody (17-0900-82; Thermo Fisher Scientific).
For drug treatment conditions, cells were treated with 50 nM bafilomycin (B1793; Sigma-Aldrich) for 15 h, 5–10 µM hydroxychloroquine (H0915; Sigma-Aldrich) for 24 h, 500 nM G007-LK (S7239; Selleck) for 24 h, 1 µM XAV939 (575545; EMD Millipore) for 24 h, and Carfilzomib (PR-171; Selleck) 10 µM for 4 h.
For siRNA treatment conditions, cells were transfected with 10 nM of desired siRNA using Lipofectamine RNAiMAX (13778150; Thermo Fisher Scientific) according to the manufacturer’s protocol for 24–48 h.
A list of siRNA and sgRNA sequences used in the manuscript is available in Table S3.
iPSCs
AICS-0090-391 (WTC-CLYBL-dCas9-TagBFP-KRAB-cl391) cells were cultured in 10 ml sterile-filtered mTeSR-Plus (100-0276; STEMCELL) on a Matrigel-coated plate (354277; Corning) and grown to 80% confluency, 5 days after thaw at 37°C and 5% CO2 in a humidified incubator. For routine passaging, at 80% confluency, media was aspirated and cells were washed with 4 ml room temperature Dulbecco's Phosphate Buffered Saline (DPBS) prior to dissociation. iPSCs were then treated with 2 ml prewarmed Accutase (AT104; Stem Cell Technologies) and the vessel was incubated at 37°C for 10 min. Once cells began to detach, 4 ml DMEM/F12 were added to the Accutase-treated cells and dissociated cells were triturated. Cells were rinsed with an additional 7 ml of DPBS for a final wash, and the dissociated cell suspension was transferred to a 15-ml conical tube and centrifuged at 500 g for 5 min at room temp. DMEM/F12/Accutase supernatant was carefully aspirated and cells were resuspended in 10 ml fresh mTeSR-Plus containing 10 µM Y-27632 2HCl (ROCK Inhibitor, S1049; Selleck) (ROCKi) and counted via flow cytometry. Cells were then seeded into a Matrigel-coated 6-well dish at a density of 1.5e + 05 per well in 3 ml mTeSR-Plus containing ROCKi. Old media containing ROCKi was aspirated from each well the next day and replaced with fresh mTeSR1 without ROCKi. mTeSR-Plus was changed daily, and ROCKi was used for each passaging event and always removed 24 h thereafter.
Genome-wide pooled CRISPRi screen and analysis
HCT116 CRISPRi pCR2054 cells were transduced with lentivirus harboring constructs expressing sgRNAs from the genome-wide pooled CRISPRi v2 library with 8 µg/ml of polybrene (TR-1003-G; EMD Millipore) at a multiplicity of infection of <1. hCRISPRi-v2 library was a gift from Jonathan Weissman (ID #83969; Addgene). Cells were then selected with 1.5 µg/ml puromycin (A1113803; Gibco) for 1 week in 15-cm dishes and expanded to 3.60 × 108 cells to allow for T0 condition takedowns as well as base seed for Zeocin (R25001; Invitrogen) treated and untreated samples. Treated cells were subjected to Zeocin 25 ng/μl final concentration and untreated cells were substituted with DMSO. Cells were maintained at >500× coverage per library element per replicate per condition throughout the screen. Cells were then cultured for 35 days in 5-Chamber CellStack vessels, splitting cells every 48–72 h and harvesting 2.40 × 108 cells every 7 days per condition, where the treated and untreated conditions reached ∼8 and 16 doublings at day 14, respectively. Genomic DNA was purified using Macherey-Nagel NucleoSpin Blood XL Maxi Kit (740950.50; Macherey-Nagel) and prepared as previously described (Kampmann et al., 2014) with modifications: Sbf1 (R3642S; NEB) was used instead of PvuII for the restriction digest. Next-generation sequencing (NGS) was performed using an Illumina NovaSeq SP with 2 × 50 paired-end reads using custom read primers:
Read 1:
5′-GTGTGTTTTGAGACTATAAGTATCCCTTGGAGAACCACCTTGTTGG-3′.
Read 2:
5′-CTAGCCTTATTTAAACTTGCTATGCTGTTTCCAGCTTAGCTCTTAAAC-3′.
NGS data was then quantified and phenotype scores were generated using python scripts from the Horlbeck Lab’s ScreenProcessing pipeline as previously described (Horlbeck et al., 2016).
Immunofluorescence staining
Cells were plated on glass bottom 96-well plates and fixed using 4% paraformaldehyde (15710; Electron Microscopy Sciences) in DPBS (14190250; Gibco) for 10 min and washed twice with DPBS. Cells were then permeabilized using 0.25% Triton X-100 (A16046.AP; Thermo Fisher Scientific) in DPBS for 10 min, blocked with 3% BSA (BP9703100; Thermo Fisher Scientific) in Phosphate Buffered Serum Tween (PBST) (DPBS, Gibco; 0.1% Tween 20, AAJ20605AP; Thermo Fisher Scientific) for 30 min, and then probed with desired antibody in 3% BSA PBST for 1 h at room temperature. Cells were then washed three times with PBST and incubated with secondary antibody and DAPI (D1306; Invitrogen) in 3% BSA PBST for 1 h at room temperature in darkness. Cells were then washed three times in PBST and stored in DPBS prior to image acquisition.
A list of antibodies used in the manuscript is available in Table S4.
Confocal microscopy and analysis
Fluorescent image acquisition was performed using a Nikon Eclipse Ti2 configured with a spinning disk confocal scanner (CSU-W1; Yokogawa), CFI Plan Apochromat Lambda D 40X air objective lens, CFI Apochromat TIRF 100×/1.49 oil-immersion objective lens, and NIS-Elements AR software (version 5.31.01; Nikon). Green (mVenus), blue (BFP, DAPI), red, and far red were excited with 488, 405, 561, and 640 nm lasers, respectively. Microscopy images were post-processed using ImageJ/FIJI software (version 2.0.0). Quantification and analysis of microscopy images were performed using CellProfiler (Stirling et al., 2021) (version 4.2.4). For live cell images, acquired images were thresholded by global minimum cross entropy to select for and differentiate between cell cytoplasm area and mVenus foci area in an unbiased manner; downstream mVenus foci number, area, and intensity was measured within a range of size and region of interest (ROI). For immunofluorescence microscopy, images were processed by, first, defining nuclei stained by DAPI by adaptive Otsu 3-class thresholding to differentiate between background and nuclei; second, by expanding from nuclei objects to define cytoplasm based on distance and Otsu 2-class thresholding and then subtracting nuclei from this area; third, by selecting, within the cytoplasm area, foci objects for mVenus, Catalase, or PMP70 of a defined size and ROI determined by adaptive Otsu 3-class thresholding. All of the previously mentioned objects are then measured for number, area, intensity, and colocalization by Pearson’s correlation.
Fluorescence-activated cell sorting
Flow cytometry was performed using an Attune NxT Flow Cytometer (Invitrogen) or SH800S (Sony). Excitation wavelengths of 488 nm (530/30 filter) and 405 nm (450/40 filter) were used to analyze mVenus and BFP expression, respectively. For selection of cells re-expressing PEX14 or PEX19, cells were sorted for Thy1.1-positive cells after immunolabeling with APC-conjugated CD90.1 Thy-1.1 antibody (17-0900-82; Thermo Fisher Scientific) at excitation wavelength of 638 nm (720/60 filter). Flow Cytometry Standard data were analyzed and visualized using FlowJo (version 10.6.2).
Immunoblotting
Cells were trypsinized (0.05% Trypsin, 25300062; Gibco), quenched, spun down at 300 × g for 5 min, decanted, and washed using DPBS (14190250; Gibco). Cells were lysed using radio-immunoprecipitation assay (RIPA) lysis buffer (0.1% SDS, BP8200100; Thermo Fisher Scientific, 1% octylphenoxypolyethoxyethanol (IPEGAL CA630), 8896; EMD-Millipore; 0.5% sodium deoxycholate, D6750; Sigma-Aldrich, 50 mM Tris, BP152-5; Thermo Fisher Scientific; 150 mM NaCl, S271-10; Thermo Fisher Scientific) with benzonase (101697; EMD Millipore) and protease inhibitor (78430; Thermo Fisher Scientific) for 30 min on ice and spun down at 14,000 rpm for 5 min and supernatant was collected. Total protein concentrations were quantified using Bio-Rad Protein Assay (5000006; Bio-Rad). Protein samples were normalized to 10–20 µg, mixed with 4X Laemmli sample buffer (62.5 mM Tris, 10% glycerol, 1% SDS, 0.005% bromophenol blue) containing β-mercaptoethanol (M6250; Sigma-Aldrich), and incubated for 5 min at 95°C. Samples were loaded and resolved on 4–20% SDS-PAGE gels (#4561095; Bio-Rad), semi-dry transferred to 0.45 µm low fluoresence polyvinylidene fluoride membranes (1620264; Bio-Rad), blocked in 5% milk (Nestle) in TBST (50 mM Tris, 150 mM NaCl, pH 7.4), and probed with desired antibody in 3% BSA TBST (BSA, BP9703100; Thermo Fisher Scientific) overnight at 4°C. Membranes were then washed and probed with secondary HRP-conjugated antibodies, with visualization of chemiluminescence using Pierce ECL2 Western Blotting Substrate (PI80196; Thermo Fisher Scientific) on a ChemiDoc MP Imaging System (Bio-Rad). Densitometry was performed using Fiji (Schindelin et al., 2012).
TOPFlash
HEK293T ZIM3-dCas9 and HCT116 dCas9-KRAB cells harboring NTC, PEX14, and PEX19 sgRNAs were transfected in 96-well plates by lipofectamine (TransIT LT-1; Mirus). A normalized 55 ng of total plasmid DNA was used at a ratio of 50:5 TOPFlash/FOPFlash:Renilla. Cells were treated with either BSA or human recombinant WNT3a (5036-WN-010; R&D Systems) 24 h later. Cells were then lysed 24 h after treatment and luciferase activity was measured using the Dual Luciferase Assay (E1910; Promega) according to the manufacturer’s protocol, and luminosity was read out using a microplate reader (SpectraMax M5; Molecular Devices). Plasmids used were TopFLASH (#12456; Addgene), FopFLASH (#12457; Addgene), and Renilla (#27163; Addgene). M50 Super 8x TOPFlash and M51 Super 8x FOPFlash (TOPFlash mutant) were a gift from Randall Moon (#12456, #12457; Addgene plasmid) (Veeman et al., 2003). pRL-SV40P was a gift from Ron Prywes (#27163; Addgene plasmid) (Chen and Prywes 1999).
Subcellular fractionation
Designated cell lines were harvested at 25–30 million cells (equalized among experimental replicates), spun down, washed, and resuspended in Homogenization Buffer (HB) (250 mM sucrose, 5 mM MOPS, 1 mM EDTA, 2 mM PMSF, 1 mM DTT, 1 mM ε-aminocaproic acid, pH 7.4 adjusted with KOH) based on Manner and Islinger (2017). Cells were quickly freeze-thawed and mechanically homogenized via dounce, with a minimum of 10 passes, to lyse the extracellular membrane while retaining intracellular organelles. Total lysate was collected. The remainder of the product was centrifuged at 600 × g max, 10 min, 4°C, the supernatant collected, pellet was then collected and resuspended with HB, homogenized via dounce, with a minimum of 10 passes again, centrifuged at 600 × g max, 10 min, 4°C, supernatant collected and combined with previously collected supernatant, whereas the remaining pellet is considered the nuclear pellet. The supernatant was then fractionated at 20,000 × g for 30 min at 4°C. The fractionated supernatant was harvested (cytoplasmic fraction), leaving behind the heavy mitochondrial/light mitochondrial/peroxisomal organellar pellet. The pellet is washed by resuspension in 1 ml of HB, spun down at 20,000 × g for 15 min at 4°C, the wash supernatant was discarded, and then washed again in the same manner. The resulting organellar pellet is lysed by RIPA lysis buffer; this is considered the cell pellet.
Co-immunoprecipitation
Designated cell lines were harvested at 10–15 million cells (equalized among experimental replicates), spun down, and resuspended in LB1 (60 mM HEPES pH 7.6, 150 mM NaCl, 150 mM KCl, 10 mM MgCl2, 0.2% IPEGAL CA630 [Sigma-Aldrich], 0.1% sodium deoxycholate [Sigma-Aldrich], and 1X Protease Inhibitor, with or without 1X benzonase). For FLAG-immunoprecipitation assaying for PARsylation: no benzonase, 1 µM PARGi, and 10 µM carfilzomib was included in the LB1. The suspension quickly undergoes freeze-thaw, is then dounce homogenized (with a minimum of 10 passes), and then incubated for 30 min at 4°C with inversion. Total lysate samples are acquired and the remaining lysate is centrifuged for 5 min, 20,000 × g at 4°C to separate the sample into soluble and insoluble fractions. The supernatant (soluble fraction) is collected and spun at 20,000 × g at 4°C for 30 min to clear out any remaining insoluble proteins or cell debris, and this is the lysate supernatant. The insoluble fraction is washed with LB1, spun down, decanted, and resuspended in LB1; this is the pellet. In an optional step, the lysate supernatant is precleared with protein G agarose beads for 30 min at 4°C and washed. For FLAG-immunoprecipitations, supernatant is incubated with M2 FLAG conjugated agarose beads (M8823; Millipore) for 2–3 h at 4°C with inversion. For TNKS immunoprecipitations, supernatant is incubated with 10 µg of TNKS antibody (sc-365897; Santa Cruz Biotech) for 3 h at 4°C with inversion and then conjugated to Protein G Dynabeads (10004D; Invitrogen) for 3 h at 4°C with inversion. Beads are then washed 5× in LB2 buffer (LB1 buffer without protease inhibitors or benzonase) with either centrifugation or a magnetic stand (where applicable). Beads are then eluted using 50 μl of freshly prepared (day of) Elution Buffer (100 mM NaHCO3, 1% SDS) at 65°C for 15 min on a heated shaker (1,200 rpm) twice, or for FLAG-IP assaying for PARsylation, 90 μl of 300 μg/ml FLAG peptide was dissolved in LB2, and samples were eluted at 4°C shaking at 1,100 rpm for 30 min, then spun down, and supernatant was collected.
RNA-seq
HCT116 Pex-ZeoR cell lines harboring either NTC or RNF146 sgRNAs were harvested, spun down, and RNA was extracted using RNeasy Mini Kit (#74104; Qiagen), according to manufacturer instructions, in triplicate. Purified RNA samples were poly-(A) enriched, reverse transcribed, and sequenced on an Illumina NovaSeq to produce paired-end 150 bp reads (Novogene). Raw fastq reads were trimmed using fastp v0.23.2 (Chen et al., 2018), alignment was done via STAR v 2.7.11a (Dobin et al., 2013), count tables were generated using htseq2 v. 2.0.2 (Putri et al., 2022), and differential expression analysis was performed using the R-package DESeq2 v. 1.40.1 (Love et al., 2014). Differential expression comparisons were made between experimental and nontargeting CRISPRi strains in biological triplicate.
Online supplemental material
Data availability
Acknowledgments
We thank Katelynn Kazane for assistance with engineering CRISPRi cell lines. We thank Amos Liang (University of Dundee, Dundee, Scotland, UK) and Jacob Corn (ETH Zurich, Zurich, Switzerland) for Hct116 CRISPRi cells. We thank Diego Acosta-Alvear (University of California, Santa Barbara, Santa Barbara, CA, USA) for H4 CRISPRi cells. We thank Mary West and Pingping He of the High-Throughput Screening Facility at University of California, Berkeley for preparation of the pooled CRISPRi library and Jonathan Weissman (Whitehead Institute for Biomedical Research, Boston, MA, USA) for the gift of the pooled library. We thank Griffin Kramer for assistance in data processing. We thank members of the Gardner and Richardson labs, Joel Rothman, and Chris Hayes for fruitful discussions. We thank Ron Prywes and Randall Moon for plasmid reagents.
B.M. Gardner acknowledges support from National Institutes of Health K99/R00GM121880, R35GM146784, and the Searle Scholars Program. J.T. Vu acknowledges support from the Connie Frank Fellowship. L. Mandjikian acknowledges support from the California Institute for Regenerative Medicine Educ4. C.D. Richardson acknowledges support from National Institutes of Health R35 GM142975. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions: J.T. Vu: Conceptualization, Formal analysis, Investigation, Visualization, Writing—original draft, Writing—review & editing, K.U. Tavasoli: Investigation, Validation, C.J. Sheedy: Investigation, Validation, S.P. Chowdhury: Investigation, Validation, L. Mandjikian: Methodology, J. Bacal: Resources, Visualization, M.A. Morrissey: Methodology, Resources, Writing—review & editing, C.D. Richardson: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Software, Supervision, Writing—original draft, Writing—review & editing, B.M. Gardner: Conceptualization, Funding acquisition, Project administration, Supervision, Visualization, Writing—original draft, Writing—review & editing.
References
Author notes
Disclosures: The authors declare no competing interests exist.



![Pex-ZeoR enables differential enrichment of genes regulating peroxisome homeostasis in a genome-wide screen. (A) Quantification of cell count by flow cytometry in different concentrations of Zeocin of HCT116 cells with sgRNAs targeting NTC, PEX1, or PEX6 over 72 h. Data is representative of n = 2 biological replicates. Cell count is normalized to untreated. (B) Quantification of flow cytometry data of BFP− (NTC) and BFP+ (PEX6) cells grown in coculture competition assay over t = 11 days in the presence of 0, 25, or 50 ng/μl of Zeocin. Time points are taken every t = 2 days. Data shown as the mean ± SD of n = 3 biological replicates. (C) Schematic of the CRISPRi screen. Pex-ZeoR cells were transformed with a genome-wide gRNA library, selected for expression of guides, and split into untreated and +Zeocin growth conditions. Genomic DNA takedowns for NGS sequencing at t = 0 and t = 7× for all conditions. (D) Heatmap showing Pearson’s correlation coefficient of guide abundance for all library elements between biological replicates of sequenced time points between treated and untreated conditions. T and Z represent untreated and Zeocin-treated conditions, respectively, while numbers represent time points (days). Highlighting indicates comparisons between day 14 samples. (E) Fold change of various PEX sgRNA abundances derived from genome-wide CRISPRi screen comparing Zeocin treated to untreated samples. Highlighting indicates comparisons between day 14 samples. Y-axis is phenotype score, a measure of the fold change of three of five significant guides per gene. X-axis is time (t) in days. Data is representative of n = 2 biological samples. (F) Volcano plot of NGS data from genome-wide screen with significance (−log base 10 of P value, y-axis) and phenotype score (normalized fold change of cDNA guide count, x-axis) of guides targeting specific genes for cell cultures either untreated (DMSO mock treated) or treated (50 ng/μl Zeocin treated) for 14 days. Pink data points are filtered to display genes with a Z-score value between [−0.5,5] in the screen by Olivieri et al. (2020), and a P-value <0.05 and a phenotype score ≥ 1 in our screening dataset.Gray data points are genes that did not pass filters. Red data points represent known PEX genes and RNF146. Data displayed was calculated from m = 3 guides per gene and n = 2 biological replicates.](https://cdn.rupress.org/rup/content_public/journal/jcb/223/10/10.1083_jcb.202312069/1/m_jcb_202312069_figs1.png?Expires=1773825037&Signature=j54gocBTgFI9kFUls02bxx~EZcr5YYBBpV4MkqmdvYwb5wUxvMM0NcsZEtdtzNLFZ0yGFIkUewsA8c2GWQvxN6sKfUm6IE5Ahuu~LiZaAIgdLgoEKqQwzvNetIu0btdc5Ume6AJej8VR1HG1mAIwEP6NRI96j~CGcDG961L4Pevm1rdeVYGcswp6UhH-qsKj9angl72nSg1XhbvmlD4YT9f09vSBb65aiHHVzobUuuK-TOLP190rwdPwhUymwwCXaxXc0oOhVZMaZ3f~ZUu5zkxHZO29Myj25wdIARFUp8QDMROwxqJaO1zw3zdRk6Ic0UuelT2FhDOzTFPz5JAkkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)