The centriole is the microtubule-based backbone that ensures integrity, function, and cell cycle–dependent duplication of centrosomes. Mostly unclear mechanisms control structural integrity of centrioles. Here, we show that the centrosome protein CEP350 functions as scaffold that coordinates distal-end properties of centrioles such as length, stability, and formation of distal and subdistal appendages. CEP350 fulfills these diverse functions by ensuring centriolar localization of WDR90, recruiting the proteins CEP78 and OFD1 to the distal end of centrioles and promoting the assembly of subdistal appendages that have a role in removing the daughter-specific protein Centrobin. The CEP350–FOP complex in association with CEP78 or OFD1 controls centriole microtubule length. Centrobin safeguards centriole distal end stability, especially in the compromised CEP350−/− cells, while the CEP350–FOP–WDR90 axis secures centriole integrity. This study identifies CEP350 as a guardian of the distal-end region of centrioles without having an impact on the proximal PCM part.
Introduction
The centrosome is the main microtubule (MT) organization center of animal cells. It consists of MT-based centrioles and the pericentriolar material (PCM) that surrounds centrioles at their proximal end. The PCM organizes MTs and is important for centriole duplication, while centrioles ensure centrosome stability (Bornens, 2002). Formation of the daughter centriole starts in G1/S phase on the proximal end of the two mother centrioles with the binding of the kinase PLK4. PLK4 eventually recruits the protein SAS6, which then assembles into a cartwheel-like structure. The cartwheel is removed from daughter centrioles in the middle of mitosis by proteolytic degradation of SAS6 (Arquint and Nigg, 2014). After formation of daughter centriole MTs and their elongation in S and G2, mother and daughter centrioles disengage at the end of mitosis. At this point in the cell cycle, mother centrioles can be distinguished from daughter centrioles by the distal and subdistal appendages, which assemble at daughter centrioles only after the second cell cycle of centriole formation (Tanos et al., 2013). Distal appendages have a role in cilia formation, whereas subdistal appendages organize stable MTs (Chong et al., 2020; Bowler et al., 2019).
According to the localization of proteins and their function, distinct structural and organizational centriolar regions were defined. PCM proteins such as pericentrin (PCNT), CDK5RAP2, and CEP192 localize to the outer proximal region of centrioles to create a matrix for MT nucleation and centriole duplication (Woodruff et al., 2014). The region inside centrioles toward the central and distal part contains the inner scaffold consisting of POC5, Centrin, and FAM161A (Hamel et al., 2017; Le Guennec et al., 2020). WDR90 functions as an MT cohesion protein bridging the MT triplets with the inner scaffold (Hamel et al., 2017).
The distal region of centrioles carries the proteins CP110, CEP78, OFD1, ODF2, and C2CD3 that function in centriole length control and/or cilia formation (Schmidt et al., 2009; Hossain et al., 2017; Singla et al., 2010; Thauvin-Robinet et al., 2014). Depletion of CP110 causes centrioles to overelongate, and its removal from centrioles is a prerequisite for cilia formation in serum-starved cells (Spektor et al., 2007; Schmidt et al., 2009). CEP78 also controls centriole length by regulating CP110 (Hossain et al., 2017; Gonçalves et al., 2021). It has been shown that loss of OFD1 promotes overelongation of centrioles; however, the molecular mechanism of this regulation is presently unclear (Singla et al., 2010). C2CD3, an antagonist and interaction partner of OFD1, functions as positive regulator of centriole length (Thauvin-Robinet et al., 2014; Wang et al., 2018). In addition, the MT-binding proteins, Centrosomal P4.1-associated protein (CPAP) and Centrobin, were shown to positively regulate centriole length (Schmidt et al., 2009; Gudi et al., 2011). Centrobin is required for centriole duplication and stability (Gudi et al., 2011) and it was suggested to associate with daughter centrioles from their assembly until they become mothers in the next S phase (Zou et al., 2005; Le Roux-Bourdieu et al., 2022). CPAP works downstream of Centrobin to support centriole elongation (Gudi et al., 2015).
CEP350, a centriolar MT binding protein that is mostly studied for its role in ciliogenesis (Kanie et al., 2017; Mojarad et al., 2017; Wang et al., 2018; Gonçalves et al., 2021), localizes between distal and subdistal appendages and forms a module with the centriole proteins CEP19 and FOP. CEP350 and FOP localize to mother and daughter centrioles, whereas CEP19 only associates with mother centrioles and initiates ciliation by recruiting the RABL2B GTPase to the ciliary base (Kanie et al., 2017; Mojarad et al., 2017). FOP and CEP350 require each other for correct localization, while CEP19 is dispensable for FOP and CEP350 localization, indicating that the CEP350–FOP complex has functions independent of CEP19 (Mojarad et al., 2017; Kanie et al., 2017; Yan et al., 2005). Interestingly, depletion of CEP350 is causing sensitivity of centriole MTs toward the MT-destabilizing drug nocodazole when centrioles were artificially amplified by the overexpression of PLK4 kinase (Le Clech, 2008). These observations suggest that CEP350 modulates centriole MT properties. How CEP350 regulates centriole MTs and how it cooperates with other distal end proteins on centrioles is mostly unclear.
In this study, we show that CEP350 is a central scaffold protein that functions in centriole wall stability via WDR90 and centriole length control via CEP78 and OFD1. In addition, CEP350 has an essential role in the assembly of distal and subdistal appendages. Subdistal appendage assembly is needed for the removal of Centrobin from matured centrioles. The persistent centriole binding of Centrobin stabilizes centrioles in CEP350−/− cells. These functions of CEP350 stabilize and control the length of the distal region of centrioles without influencing the proximal PCM part, establishing CEP350 as a specific coordinator of centriole distal-end properties.
Results
CEP350 is required for proper recruitment of distal-end proteins
To address cilia-unrelated functions of CEP350, we analyzed when CEP350 is recruited to assembling daughter centrioles and the centriole phenotype of CEP350 knockout cell lines. To follow assembling centrioles in cycling human telomerase-immortalized retinal pigment epithelial (RPE1) cells, we used the protein CEP97 as a marker, which associates with the distal end of the daughter centrioles in early S phase (Spektor et al., 2007; Fig. 1 a). In G1 cells, the mother and the daughter centrioles were decorated with CEP97 and CEP350 (Fig. 1 a). In early S phase, with the start of daughter centriole formation (four CEP97 signals), CEP97-positive daughter centrioles did not contain CEP350 while the two mothers both carried CEP350. In late G2 phase, when the linker between the two mother–daughter centriole pairs is resolved (Fry et al., 1998), mother and daughter centrioles were associated with CEP97 and CEP350. Thus, CEP350 is recruited to elongating daughter centrioles in S/G2 after CEP97.
CEP350 is stepwise recruited to assembling centrioles. (a) IF images of cycling CEP350 WT control cells that were fixed and stained for CEP97 (green), CEP350 (red), and DNA with DAPI (blue) to understand the relative recruitment time of CEP350. (b) u-ExM images showing the step-wise centriolar recruitment of CEP350. Centrioles of CEP350 WT control cells were stained with α-tubulin (gray) and CEP350 (red) antibodies. Green arrowheads indicate CEP350 signal at daughter centrioles. The green asterisk marks the mother centriole with CEP350 at its distal end. I, II, III, and IV represent recruitment phases of CEP350 that were ordered according to the length of the daughter centriole. (c) Centriole regions based on protein composition and structural compartmentalization are represented as a cartoon. (d) OFD1 (green), Centrin (red), and DAPI (blue) in control and CEP350−/− cells. (e) Quantification of the OFD1 signals at centrioles represented in d. (f) Image of ODF2 (green), Centrin (red), and DNA by DAPI (blue) in control and CEP350−/− cells. (g) Quantification of the ODF2 signals at centrioles in f. (h) Centrin (green), CEP164 (red), and DAPI (blue) in control and CEP350−/− cells. (i) Quantification of the CEP164 signals at centrioles in h. (j) C2CD3 (green), Centrin (red), and DAPI (blue) in control and CEP350−/− cells. (k) Quantification of the C2CD3 signals at centrioles in j. (a, b, d, f, h, and j) Cells were extracted by CSK (see Materials and methods), and then either methanol- (a, d, f, and h) or formalin-fixed (j). The enlargements on the bottom right of panels a, d, f, h, and j are magnifications of the centriole signals shown in the center. Scale bars: (a) 2 μm, (b) 100 nm, (d, f, h, and j) 10 μm in main panels, and (a, d, f, h, and j) 2 μm in inset magnifications. Data are presented as mean ± SD where error bars represent the SD. N = 3, n > 240 cells in total. Statistics were derived from two-tailed unpaired t test analysis of at least three biologically independent experiments. Each independent experiment repetition is represented with same color on different groups of graphs. Source data are provided in Data S1.
CEP350 is stepwise recruited to assembling centrioles. (a) IF images of cycling CEP350 WT control cells that were fixed and stained for CEP97 (green), CEP350 (red), and DNA with DAPI (blue) to understand the relative recruitment time of CEP350. (b) u-ExM images showing the step-wise centriolar recruitment of CEP350. Centrioles of CEP350 WT control cells were stained with α-tubulin (gray) and CEP350 (red) antibodies. Green arrowheads indicate CEP350 signal at daughter centrioles. The green asterisk marks the mother centriole with CEP350 at its distal end. I, II, III, and IV represent recruitment phases of CEP350 that were ordered according to the length of the daughter centriole. (c) Centriole regions based on protein composition and structural compartmentalization are represented as a cartoon. (d) OFD1 (green), Centrin (red), and DAPI (blue) in control and CEP350−/− cells. (e) Quantification of the OFD1 signals at centrioles represented in d. (f) Image of ODF2 (green), Centrin (red), and DNA by DAPI (blue) in control and CEP350−/− cells. (g) Quantification of the ODF2 signals at centrioles in f. (h) Centrin (green), CEP164 (red), and DAPI (blue) in control and CEP350−/− cells. (i) Quantification of the CEP164 signals at centrioles in h. (j) C2CD3 (green), Centrin (red), and DAPI (blue) in control and CEP350−/− cells. (k) Quantification of the C2CD3 signals at centrioles in j. (a, b, d, f, h, and j) Cells were extracted by CSK (see Materials and methods), and then either methanol- (a, d, f, and h) or formalin-fixed (j). The enlargements on the bottom right of panels a, d, f, h, and j are magnifications of the centriole signals shown in the center. Scale bars: (a) 2 μm, (b) 100 nm, (d, f, h, and j) 10 μm in main panels, and (a, d, f, h, and j) 2 μm in inset magnifications. Data are presented as mean ± SD where error bars represent the SD. N = 3, n > 240 cells in total. Statistics were derived from two-tailed unpaired t test analysis of at least three biologically independent experiments. Each independent experiment repetition is represented with same color on different groups of graphs. Source data are provided in Data S1.
Next, we used ultra-Expansion Microscopy (u-ExM), which considerably increases the resolution of conventional microscopy (Gambarotto et al., 2021), to determine the localization of CEP350 during centriole assembly. Staining the walls of centrioles with α-tubulin antibodies and using the daughter centriole length as an indicator for the time of centriole assembly, we observed that short daughter centrioles did not contain CEP350 (Fig. 1, b–I), consistent with Fig. 1 a. The first punctum of CEP350 appeared either on one or on both sides of medium-length daughter centrioles (Fig. 1, b-II and -III, green arrowheads). Later, on the elongated daughter centrioles, the CEP350 puncta increased to four (Fig. 1, b-IV). This dot-like CEP350 pattern was still seen on mother centrioles where CEP350 spans the distal halve of centrioles (Fig. 1 b, green asterisk). Thus, timing and localization suggest that CEP350 functions at the middle to distal end of centrioles.
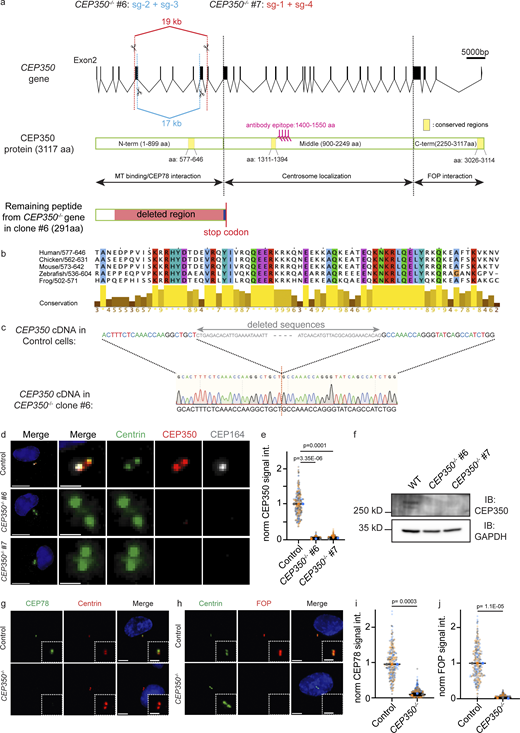
To study the function of CEP350 at centrioles beyond ciliogenesis, we constructed CEP350 knockout cell lines by CRISPR/Cas9 technology using a double-cut strategy (Fig. S1 a). CEP350 is listed as an essential gene in haploid human cells (Blomen et al., 2015). The tumor suppressor p53 (encoded by TP53) was reported as part of a control mechanism that monitors centriole integrity (Fong et al., 2016). Therefore, viable knockouts in genes that affect centrosome duplication or integrity can only be carried out in the TP53−/− background (Atorino et al., 2020). Thus, we used RPE1 TP53−/− cells (named “control”) together with RPE1 TP53+/+ for the knockout of CEP350. For construction of genetically different clones, two pairs of single guide RNAs (sgRNAs) were used, leading to 17 (CEP350−/−#6) and 19 kb (CEP350−/−#7) genomic deletions toward the 5′ end of the CEP350 gene (Fig. S1 a). Monoallelic deletions of CEP350 were obtained with a similar frequency of ∼1.5% in RPE1 TP53+/+ and TP53−/− cells. mRNA extraction and analysis of the cDNA by PCR and sequencing showed that biallelic CEP350 knockout cells were only obtained in TP53−/− background, but not in the TP53+/+ RPE1 cells. Sequencing of the cDNA confirmed deletion of exon 6-14 of CEP350 in CEP350−/−#6 (termed CEP350−/− in this manuscript) and termination of the ORF shortly after the introduced cut (Fig. S1, a and c). The strongly truncated N-terminal CEP350 fragment of CEP350−/− cells lacked the N-terminal MT binding site between amino acids 403 and 725 (Hoppeler-Lebel et al., 2007) and other conserved regions in CEP350 (Fig. S1, a and b). In addition, we confirmed the loss of CEP350 protein by immunofluorescence (IF) microscopy and immunoblotting (IB; Fig. S1, d–f). Furthermore, centriolar levels of CEP78 and FOP, whose recruitment to centrioles requires CEP350 (Gonçalves et al., 2021; Kanie et al., 2017), decreased to 15 and 2%, respectively, in CEP350−/− cells compared with the control (Fig. S1, g–j). These data together show that CEP350−/− cell lines have most likely lost the complete functions of CEP350.
Genetically different CEP350−/−cell lines were created by CRISPR/Cas9 and CEP350 loss was verified by IF and WB. (a) Different combinations of sgRNA guides were cloned in pX458 and electroporated in RPE-1 TP53−/− and RPE-1 TP53+/+ cells. 48 h after electroporation, single cells were FACS-sorted in 96-well plates and grown clones were analyzed by mRNA extraction and following cDNA production to use in PCR screen. WT CEP350 gene (ENST00000367607.8) is represented with coding exons and all introns to illustrate the sgRNA guided double CRISPR cuts, and the coding CEP350 protein (CCDS1336) is shown. CEP350 protein is divided into N-terminus (1–899 aa), middle (900–2249 aa), and C-terminus (2250–3117 aa), which are used to analyze domain interactions of CEP350 in rescue and IP experiments (see Fig. 6). Yellow marked domains are shown as the most conserved CEP350 regions in different species analyzed by Clustal-Omega tool of European Molecular Biology Laboratory. Remaining peptide from mutated CEP350 gene is shown for CEP350−/− clone #6 in comparison to the WT CEP350 protein to illustrate cut-out region and consecutive frame shift (represented in dark blue), which later leads to a premature stop codon at the 291 aa. (b) The result of CEP350 protein conservation among its orthologs was represented by Jalview tool to demonstrate conserved aa sequences at the N-terminus of CEP350 protein (577–646 aa). (c) After mRNA extraction and cDNA conversion, the PCR product of CEP350−/− clone #6 was sequenced to represent the missing sequences in CEP350 gene in comparison to WT CEP350. In absence of CEP350 WT band, cells were analyzed by IF. (d) IF analysis of control and CEP350−/− (clone #6 and #7) cells with Centrin (green), CEP350 (red), and CEP164 (gray) antibodies. (e) Loss of CEP350 signal was quantified in control (CEP350 WT) cells and CEP350−/− clones (2 and 6% of control in clone #6 and #7, respectively). (f) Absence of CEP350 protein was verified by WB with indicated antibodies. GAPDH is used as the loading control. (g and h) IF images of cycling control cells and CEP350−/− cells fixed with methanol and stained for (g) CEP78 (green), Centrin (red), and DNA with DAPI (blue) or (h) Centrin (green), FOP (red), and DNA with DAPI (blue). (i and j) Quantifications of the CEP78 and FOP signals at centrioles from cells in g and h. The enlargements on the bottom right are magnifications of the centriole signals shown in the center, and each independent experiment repetition is represented with the same color in CEP350−/− and control cells in respective graphs. Scale bars: (a) 5,000 bp, (d) 2 μm in panel and 1 μm in magnifications, (g and h) 5 μm in main panels and 2 μm in inset magnifications. (e, i, and j)N = 3, n > 240 cells. Data are presented as mean ± SD where error bars represent the SD. Statistics were from two-tailed unpaired t test analysis of indicated numbers of biologically independent experiments. Source data are provided in Data S1. Source data are available for this figure: SourceData FS1.
Genetically different CEP350−/−cell lines were created by CRISPR/Cas9 and CEP350 loss was verified by IF and WB. (a) Different combinations of sgRNA guides were cloned in pX458 and electroporated in RPE-1 TP53−/− and RPE-1 TP53+/+ cells. 48 h after electroporation, single cells were FACS-sorted in 96-well plates and grown clones were analyzed by mRNA extraction and following cDNA production to use in PCR screen. WT CEP350 gene (ENST00000367607.8) is represented with coding exons and all introns to illustrate the sgRNA guided double CRISPR cuts, and the coding CEP350 protein (CCDS1336) is shown. CEP350 protein is divided into N-terminus (1–899 aa), middle (900–2249 aa), and C-terminus (2250–3117 aa), which are used to analyze domain interactions of CEP350 in rescue and IP experiments (see Fig. 6). Yellow marked domains are shown as the most conserved CEP350 regions in different species analyzed by Clustal-Omega tool of European Molecular Biology Laboratory. Remaining peptide from mutated CEP350 gene is shown for CEP350−/− clone #6 in comparison to the WT CEP350 protein to illustrate cut-out region and consecutive frame shift (represented in dark blue), which later leads to a premature stop codon at the 291 aa. (b) The result of CEP350 protein conservation among its orthologs was represented by Jalview tool to demonstrate conserved aa sequences at the N-terminus of CEP350 protein (577–646 aa). (c) After mRNA extraction and cDNA conversion, the PCR product of CEP350−/− clone #6 was sequenced to represent the missing sequences in CEP350 gene in comparison to WT CEP350. In absence of CEP350 WT band, cells were analyzed by IF. (d) IF analysis of control and CEP350−/− (clone #6 and #7) cells with Centrin (green), CEP350 (red), and CEP164 (gray) antibodies. (e) Loss of CEP350 signal was quantified in control (CEP350 WT) cells and CEP350−/− clones (2 and 6% of control in clone #6 and #7, respectively). (f) Absence of CEP350 protein was verified by WB with indicated antibodies. GAPDH is used as the loading control. (g and h) IF images of cycling control cells and CEP350−/− cells fixed with methanol and stained for (g) CEP78 (green), Centrin (red), and DNA with DAPI (blue) or (h) Centrin (green), FOP (red), and DNA with DAPI (blue). (i and j) Quantifications of the CEP78 and FOP signals at centrioles from cells in g and h. The enlargements on the bottom right are magnifications of the centriole signals shown in the center, and each independent experiment repetition is represented with the same color in CEP350−/− and control cells in respective graphs. Scale bars: (a) 5,000 bp, (d) 2 μm in panel and 1 μm in magnifications, (g and h) 5 μm in main panels and 2 μm in inset magnifications. (e, i, and j)N = 3, n > 240 cells. Data are presented as mean ± SD where error bars represent the SD. Statistics were from two-tailed unpaired t test analysis of indicated numbers of biologically independent experiments. Source data are provided in Data S1. Source data are available for this figure: SourceData FS1.
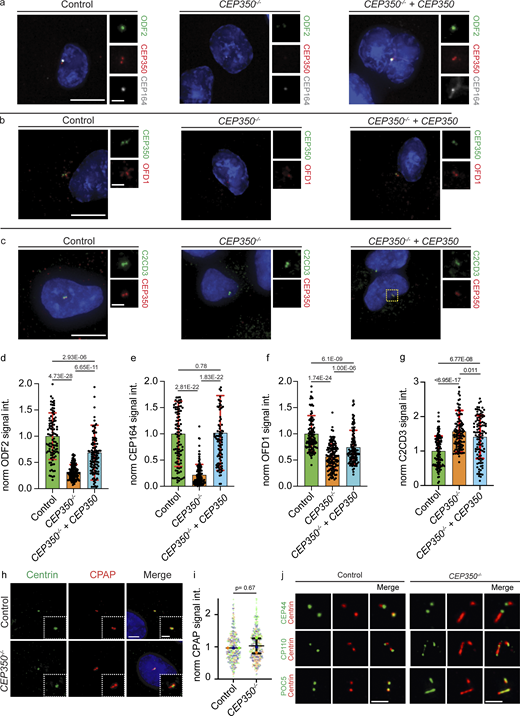
Since CEP350 is located on the distal half of centrioles (Fig. 1 c), we first analyzed the localization of the distal end proteins OFD1, ODF2, CEP164, and C2CD3. CEP350−/− cells had 40% less OFD1 and 74% less ODF2 compared with the control cells (Fig. 1, d–g). CEP164 at CEP350−/− centrioles dropped to 18% (Fig. 1, h and i), while C2CD3 levels were increased nearly twofold (178%; Fig. 1, j and k). These effects of CEP350 loss on the localization of centrosomal proteins were reversed when CEP350−/− cells were complemented by a WT copy of CEP350 (Fig. S2, a–g) indicating that they were caused by the loss of CEP350 and not by another mutation.
siRNA depletion of CEP350 verifies the distal protein recruitment defects observed in CEP350−/−cells. (a–c) Control, CEP350−/−, and CEP350−/− cells complemented with CEP350 (CEP350−/− + CEP350) were CSK extracted, fixed, and stained for IF by (a) ODF2 (green), CEP350 (red), CEP164 (gray), and DAPI (blue); (b) CEP350 (green) OFD1 (red), and DAPI (blue); and (c) C2CD3 (green), CEP350 (red), and DAPI (blue). (d–g) Quantifications of ODF2 (d), CEP164 (e), OFD1 (f), and C2CD3 (g). Data are presented as mean ± SD where error bars represent the SD of N = 2, n > 100 cells in total. (h) Centrin (green), CPAP (red), and DAPI (blue) in control and CEP350−/− cells are shown. The enlargements on the left of or bottom right of main panels are magnifications of the centriole signals shown in the center. (i) Quantification of CPAP levels from panel h presented as mean ± SD where error bars represent the SD of N = 3, n > 240 cells in total. Each independent experimental repetition is represented with the same color in CEP350−/− and control cells. (j) Cycling control cells and CEP350−/− cells were CSK extracted, fixed with methanol, and stained for IF with indicated antibodies to show abnormal localization of centriolar distal end proteins (CP110, POC5, and Centrin) in CEP350−/− cells. Scale bars: (a, b, c, and h) 5 μm in main panels and 2 μm in inset magnifications, (j) 1 μm. (d, e, f, g, and i) Statistics were derived from two-tailed unpaired t test analysis of indicated numbers of biologically independent experiments. Source data are provided in Data S1.
siRNA depletion of CEP350 verifies the distal protein recruitment defects observed in CEP350−/−cells. (a–c) Control, CEP350−/−, and CEP350−/− cells complemented with CEP350 (CEP350−/− + CEP350) were CSK extracted, fixed, and stained for IF by (a) ODF2 (green), CEP350 (red), CEP164 (gray), and DAPI (blue); (b) CEP350 (green) OFD1 (red), and DAPI (blue); and (c) C2CD3 (green), CEP350 (red), and DAPI (blue). (d–g) Quantifications of ODF2 (d), CEP164 (e), OFD1 (f), and C2CD3 (g). Data are presented as mean ± SD where error bars represent the SD of N = 2, n > 100 cells in total. (h) Centrin (green), CPAP (red), and DAPI (blue) in control and CEP350−/− cells are shown. The enlargements on the left of or bottom right of main panels are magnifications of the centriole signals shown in the center. (i) Quantification of CPAP levels from panel h presented as mean ± SD where error bars represent the SD of N = 3, n > 240 cells in total. Each independent experimental repetition is represented with the same color in CEP350−/− and control cells. (j) Cycling control cells and CEP350−/− cells were CSK extracted, fixed with methanol, and stained for IF with indicated antibodies to show abnormal localization of centriolar distal end proteins (CP110, POC5, and Centrin) in CEP350−/− cells. Scale bars: (a, b, c, and h) 5 μm in main panels and 2 μm in inset magnifications, (j) 1 μm. (d, e, f, g, and i) Statistics were derived from two-tailed unpaired t test analysis of indicated numbers of biologically independent experiments. Source data are provided in Data S1.
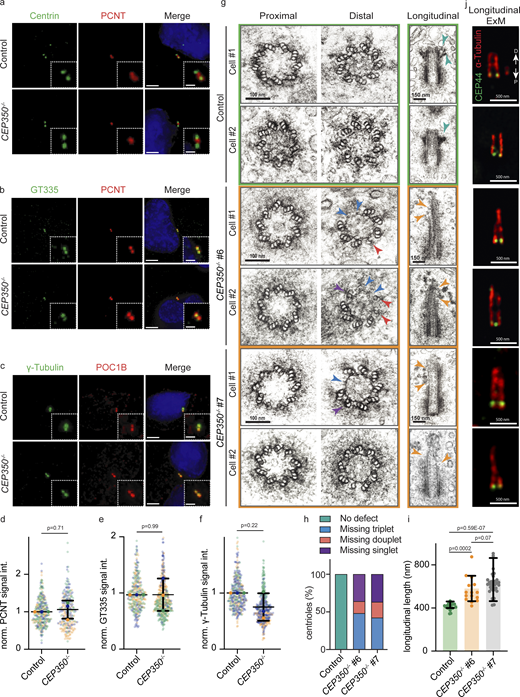
To understand whether the absence of CEP350 also affects the proximal-end proteins (Fig. 1 c), we checked PCNT, polyglutamylation of proximal tubulins (GT335), CPAP, and γ-tubulin in control and CEP350−/− cells. PCNT, the polyglutamylation of αβ-tubulin and CPAP did not show a difference between control and CEP350−/− centrioles (Fig. 2, a, b, d, and e; and Fig. S2, h and i). The reduction in γ-tubulin levels at CEP350−/− centrioles can be explained by a pool of γ-tubulin that localizes inside centrioles (Fig. 2, c and f; Schweizer et al., 2021). In addition, localization of the centriole proximal end protein CEP44 (Atorino et al., 2020) was not affected in CEP350−/− cells (Fig. S2 j). Depletion of CEP350 by siRNA affected the localization of distal and proximal centriole end proteins in a similar way as in CEP350−/− cells (Fig. S3, a–k), further confirming that the observed defects were indeed caused by the loss of CEP350 functions and not by an additional mutation in the CEP350−/− background. These data indicate a role for CEP350 in the organization and recruitment of centriole distal-end proteins.
CEP350 loss has no effect on the recruitment of proximal PCM proteins but distal-end centriole microtubule structures are defective. (a–c) IF images of cycling control cells and CEP350−/−cells. All cells were methanol fixed and stained with (a) Centrin (green), PCNT (red), and DAPI (blue); (b) GT335 for polyglutamylation (green), PCNT (red), and DAPI (blue); and (c) γ-tubulin (green), POC1B (red), and DAPI (blue). The enlargements on the bottom right are magnifications of the centriole signals shown in the center. (d–f) Quantifications of centriolar signals in panels a–c, respectively. (g) Electron micrographs of centrioles of control cells and CEP350−/− cells (clones #6 and #7) in cross and longitudinal sections. Two different cells (#1 and #2) are shown for each condition. The A-C linker was used to identify the end of centrioles. Microtubule damages are indicated by arrowheads in cross-sections (blue for missing triplets, red for missing doublets, and purple for missing singlets). Green arrowheads in the longitudinal sections of the control cells highlight the position of the distal and subdistal appendages. Ochre arrowheads indicate centriole microtubule defects in longitudinal sections of centrioles in CEP350−/− cells. (h) MT defects observed in centrioles in g were quantified (n = 5 for control, n = 21 for CEP350−/− clone #6, and n = 13 for CEP350−/− clone #7). Color of bars corresponds to arrowheads in g. (i) Longitudinal length analysis from centrioles in g of control and CEP350−/− clone #6 and clone #7 cells (n = 20 for control cells, n = 15 for clone #6, and n = 33 for clone #7). Data are presented as mean ± SD, where error bars represent the SD. (j) Fixed cells were expanded with an expansion factor of 4 for u-ExM and stained with CEP44 (green) and α-tubulin (red) antibodies. Proximal (P) and distal (D) directions are indicated by white arrows. All cells were CSK extracted. Scale bars: (a–c) 5 μm in main panels and 2 μm in inset magnifications, (g) 100 nm in cross-sections and 150 nm in longitudinal sections, (j) 500 nm. (d–f) Data are presented as mean ± SD of N = 3, n > 240 cells in total where error bars represent the SD. Statistics were derived from two-tail unpaired t test analysis of at least three biologically independent experiments. Each independent experiment repetition is represented with the same color on different groups of graphs. Source data are provided in Data S1.
CEP350 loss has no effect on the recruitment of proximal PCM proteins but distal-end centriole microtubule structures are defective. (a–c) IF images of cycling control cells and CEP350−/−cells. All cells were methanol fixed and stained with (a) Centrin (green), PCNT (red), and DAPI (blue); (b) GT335 for polyglutamylation (green), PCNT (red), and DAPI (blue); and (c) γ-tubulin (green), POC1B (red), and DAPI (blue). The enlargements on the bottom right are magnifications of the centriole signals shown in the center. (d–f) Quantifications of centriolar signals in panels a–c, respectively. (g) Electron micrographs of centrioles of control cells and CEP350−/− cells (clones #6 and #7) in cross and longitudinal sections. Two different cells (#1 and #2) are shown for each condition. The A-C linker was used to identify the end of centrioles. Microtubule damages are indicated by arrowheads in cross-sections (blue for missing triplets, red for missing doublets, and purple for missing singlets). Green arrowheads in the longitudinal sections of the control cells highlight the position of the distal and subdistal appendages. Ochre arrowheads indicate centriole microtubule defects in longitudinal sections of centrioles in CEP350−/− cells. (h) MT defects observed in centrioles in g were quantified (n = 5 for control, n = 21 for CEP350−/− clone #6, and n = 13 for CEP350−/− clone #7). Color of bars corresponds to arrowheads in g. (i) Longitudinal length analysis from centrioles in g of control and CEP350−/− clone #6 and clone #7 cells (n = 20 for control cells, n = 15 for clone #6, and n = 33 for clone #7). Data are presented as mean ± SD, where error bars represent the SD. (j) Fixed cells were expanded with an expansion factor of 4 for u-ExM and stained with CEP44 (green) and α-tubulin (red) antibodies. Proximal (P) and distal (D) directions are indicated by white arrows. All cells were CSK extracted. Scale bars: (a–c) 5 μm in main panels and 2 μm in inset magnifications, (g) 100 nm in cross-sections and 150 nm in longitudinal sections, (j) 500 nm. (d–f) Data are presented as mean ± SD of N = 3, n > 240 cells in total where error bars represent the SD. Statistics were derived from two-tail unpaired t test analysis of at least three biologically independent experiments. Each independent experiment repetition is represented with the same color on different groups of graphs. Source data are provided in Data S1.
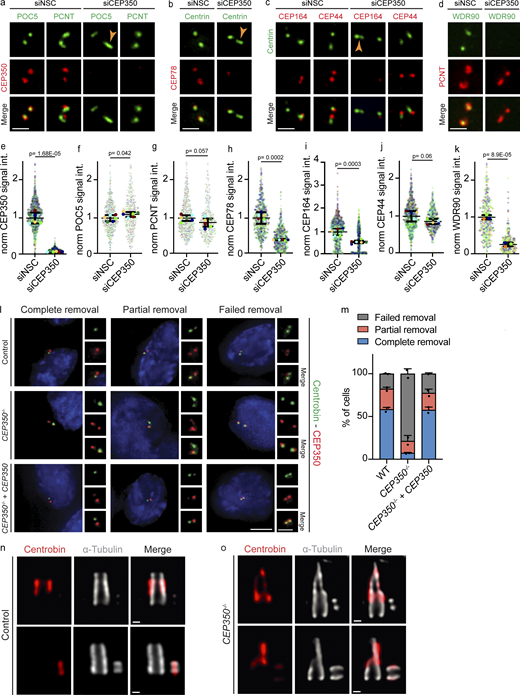
Centrobin mainly localizes to middle-subdistal regions of centrioles, and its depletion prevents Centrin elongation in CEP350−/−cells. (a–d) CEP350 was siRNA depleted from RPE-1 TP53−/− cells for 72 h, and the cells were CSK extracted, methanol fixed, and stained for IF with (a) CEP350 (red), POC5 (green), and PCNT (green); (b) CEP78 (red) and Centrin (green); (c) CEP164 (red), Centrin (green), and CEP44 (red); and (d) WDR90 (green) and PCNT (red). Ochre arrowheads point towards the visibly elongated centrioles. (e–k) Quantifications of CEP350 (e), POC5 (f), PCNT (g), CEP78 (h), CEP164 (i), CEP44 (j), WDR90 (k) from panels a–d. Each independent experiment repetition is represented with the same color in siCEP350 and siNSC cells in respective graphs. (l) Control, CEP350−/−, and CEP350−/− complemented with CEP350 (CEP350−/− + CEP350) cells were CSK extracted, fixed, and stained for IF by Centrobin (green), CEP350 (red), and DAPI (blue). G1 cells with two centrosomes were classified as complete removal (two CEP350, one Centrobin signal), partial removal (two CEP350, one potent, and one faint Centrobin signal), and failed removal (two CEP350 and two potent Centrobin signals). (m) Quantification of cells from l showing complete removal (blue bars), partial removal (red bars), and failed removal (gray bars). N = 2 and n > 48 cells. (n and o) (n) Control and (o) CEP350−/− cells were CSK extracted, fixed, and expanded with an expansion factor of 4 for u-ExM. Samples were stained for Centrobin (red) and α-tubulin (gray). Scale bars: (a–d) 1 μm, (l) 5 μm in main panels and 2 μm in inset magnifications, (n and o) 100 nm. (e–k and m) Data are presented as mean ± SD, where error bars represent the SD. (e–k)N = 3, n > 240 cells. (m)N = 2, n > 48. Statistics was derived from two-tailed unpaired t test analysis of at indicated numbers of biologically independent experiments. Source data are provided in Data S1.
Centrobin mainly localizes to middle-subdistal regions of centrioles, and its depletion prevents Centrin elongation in CEP350−/−cells. (a–d) CEP350 was siRNA depleted from RPE-1 TP53−/− cells for 72 h, and the cells were CSK extracted, methanol fixed, and stained for IF with (a) CEP350 (red), POC5 (green), and PCNT (green); (b) CEP78 (red) and Centrin (green); (c) CEP164 (red), Centrin (green), and CEP44 (red); and (d) WDR90 (green) and PCNT (red). Ochre arrowheads point towards the visibly elongated centrioles. (e–k) Quantifications of CEP350 (e), POC5 (f), PCNT (g), CEP78 (h), CEP164 (i), CEP44 (j), WDR90 (k) from panels a–d. Each independent experiment repetition is represented with the same color in siCEP350 and siNSC cells in respective graphs. (l) Control, CEP350−/−, and CEP350−/− complemented with CEP350 (CEP350−/− + CEP350) cells were CSK extracted, fixed, and stained for IF by Centrobin (green), CEP350 (red), and DAPI (blue). G1 cells with two centrosomes were classified as complete removal (two CEP350, one Centrobin signal), partial removal (two CEP350, one potent, and one faint Centrobin signal), and failed removal (two CEP350 and two potent Centrobin signals). (m) Quantification of cells from l showing complete removal (blue bars), partial removal (red bars), and failed removal (gray bars). N = 2 and n > 48 cells. (n and o) (n) Control and (o) CEP350−/− cells were CSK extracted, fixed, and expanded with an expansion factor of 4 for u-ExM. Samples were stained for Centrobin (red) and α-tubulin (gray). Scale bars: (a–d) 1 μm, (l) 5 μm in main panels and 2 μm in inset magnifications, (n and o) 100 nm. (e–k and m) Data are presented as mean ± SD, where error bars represent the SD. (e–k)N = 3, n > 240 cells. (m)N = 2, n > 48. Statistics was derived from two-tailed unpaired t test analysis of at indicated numbers of biologically independent experiments. Source data are provided in Data S1.
Loss of CEP350 disrupts length control, stability, and appendage formation of centrioles
To understand the functions of CEP350 protein in cyclin cells better, we analyzed the morphology of centrioles in CEP350−/− cells by EM. For this, we compared cross and longitudinal sections of the two independently constructed CEP350−/− cell lines (CEP350−/−#6 and CEP350−/−#7) with control cells. The centrioles of control cells showed the typical ninefold MT triplet conformation in cross-sections (Kitagawa et al., 2011). In contrast, both CEP350−/− clones showed MT damage starting from the middle-core centrioles till the distal end. Centriolar cross-sections of CEP350−/− cells indicated defective MT triplet structures with missing singlets (Fig. 2 g, purple arrowhead), doublets (Fig. 2 g, red arrowheads), and triplets (Fig. 2 g, blue arrowheads) at the distal end of centrioles. We also observed MT defects in longitudinal sections where the density of centriolar MTs decreased toward the distal end in both CEP350−/− clones (Fig. 2 g; ochre arrowheads), whereas proximal end MTs were still intact (Fig. 2 g). Our quantification showed similar defects for clone #6 and clone #7 of CEP350−/− cells (Fig. 2 h). These data indicate that CEP350 is required for middle-distal-end organization of centrioles, confirming our hypothesis that loss of CEP350 does not affect the centriole proximal end. We also observed that despite the MT damages, CEP350−/− centrioles were noticeably longer than control centrioles (Fig. 2 g, longitudinal panel). When we measured the longitudinal length of centrioles in EM images, we found that the average length of control centrioles was 429 nm, whereas CEP350−/−#6 centrioles were 580 nm and CEP350−/−#-7 centrioles were 664 nm (Fig. 2 i). u-ExM using CEP44 as proximal end marker confirmed the MT defect on the distal half of centrioles in CEP350−/− cells (Fig. 2 j). Additionally, the distal and subdistal appendage structures that were clearly visible in control cells were completely lost in CEP350−/− cells (Fig. 2 g, green arrowsheads in control), consistent with the strongly diminished ODF2 and CEP164 levels (Fig. 1, f–i).
We realized that the centriole elongation phenotype of CEP350−/− centrioles was also detectable by IF (Fig. S2 j). In IF pictures, the inner scaffold proteins, Centrin and POC5, showed elongated signals, whereas the signal of the proximal end protein CEP44 was similar to control cells. These phenotypes were also visible on CEP350 depleted RPE1 cells (Fig. S3, a–c, ochre arrowheads). This suggests that the inner scaffold is extending in CEP350−/− centrioles. Indeed, EM analysis of CEP350−/− cells showed that the inner scaffold was stable even in elongated regions that lacked MT triplets (Fig. 2 g, blue arrowheads).
Taken together, our results show that loss of CEP350 causes defects in recruitment of distal-end proteins, absence of distal and subdistal appendage structures, damaged centriolar MTs, and overall elongated centrioles.
CEP350−/− centrioles show defects in Centrobin removal due to lack of subdistal appendages
Recruitment of distal appendage proteins requires the previous removal of Centrobin from mother centrioles (Wang et al., 2018). This link between Centrobin removal and the assembly of distal appendages prompted us to check if the Centrobin removal mechanism was functional in CEP350−/− centrioles. To address this, we analyzed the cell cycle distribution of Centrobin on centrioles in RPE1 WT and CEP350−/− cells. Cells were categorized based on EdU staining and centriole number (CEP97 staining) into G1 (two CEP97 signals), S phase (four CEP97 and EdU signals), and G2/M (four CEP97, no EdU). Control RPE1 cells in G2/M carried two mother and two daughter centrioles, all stained by CEP97. Most G2/M centrioles contained only two Centrobin signals, likely corresponding to the two daughter centrioles (Fig. 3 a). In CEP350−/− cells, Centrobin stayed associated with the mother centrioles even when they assembled a daughter centriole as indicated by the four CEP97 signals that were all co-stained with Centrobin (Fig. 3 a). This Centrobin removal defect of CEP350−/− cells was rescued by a WT copy of CEP350 (Fig. S3, l and m).
Subdistal appendage proteins play an important role in Centrobin removal. (a) IF images of cycling control cells and CEP350−/− cells fixed with methanol and stained with CEP97 (green), EdU (magenta), and Centrobin (red). The enlargements on the bottom right are magnifications of the centriole signals shown in the center. (b) G2/M phase control cells and CEP350−/−, ODF2−/−, CEP128−/−, NIN−/− cells were stained for Centrin (green), Centrobin (red), DAPI (blue) to analyze Centrobin removal defects. The 4:2 ratio of Centrin:Centrobin indicates Centrobin removal from both mother centrioles, 4:3 a partial removal failure, and 4:4 a complete removal defect. (c) Cells in panel b were quantified for 4:2, 4:3, and 4:4 Centrin:Centrobin ratios to indicate Centrobin removal defects in different cell lines (N = 3, n = 50 for each condition). (d and e) Control cells (d) and CEP350−/− cells (e) were treated for 72 h with the siNSC and siCentrobin siRNA. Cells were analyzed by thin section EM. Shown are two longitudinal sections (sec1 and sec2) through centrioles of three cells each for CEP350−/ and control. (f) Centrioles in panels d and e were quantified for longitudinal centriole lengths. n = 16 for siNSC in WT, n = 25 for siCentrobinin WT, n = 38 for siNSC in CEP350−/−, and n = 11 for siCentrobin in CEP350−/−. Scale bars: (a and b) 5 μm in main panels, 2 μm in inset magnifications, and (d and e) 150 nm. (c and f) Data are presented as mean ± SD where error bars represent the SD. Statistics were from multiple comparison of two-way ANOVA multiple comparison analysis of three independent experiments (c) or two-tailed unpaired t test analysis of single measurements (f). Source data are provided in Data S1.
Subdistal appendage proteins play an important role in Centrobin removal. (a) IF images of cycling control cells and CEP350−/− cells fixed with methanol and stained with CEP97 (green), EdU (magenta), and Centrobin (red). The enlargements on the bottom right are magnifications of the centriole signals shown in the center. (b) G2/M phase control cells and CEP350−/−, ODF2−/−, CEP128−/−, NIN−/− cells were stained for Centrin (green), Centrobin (red), DAPI (blue) to analyze Centrobin removal defects. The 4:2 ratio of Centrin:Centrobin indicates Centrobin removal from both mother centrioles, 4:3 a partial removal failure, and 4:4 a complete removal defect. (c) Cells in panel b were quantified for 4:2, 4:3, and 4:4 Centrin:Centrobin ratios to indicate Centrobin removal defects in different cell lines (N = 3, n = 50 for each condition). (d and e) Control cells (d) and CEP350−/− cells (e) were treated for 72 h with the siNSC and siCentrobin siRNA. Cells were analyzed by thin section EM. Shown are two longitudinal sections (sec1 and sec2) through centrioles of three cells each for CEP350−/ and control. (f) Centrioles in panels d and e were quantified for longitudinal centriole lengths. n = 16 for siNSC in WT, n = 25 for siCentrobinin WT, n = 38 for siNSC in CEP350−/−, and n = 11 for siCentrobin in CEP350−/−. Scale bars: (a and b) 5 μm in main panels, 2 μm in inset magnifications, and (d and e) 150 nm. (c and f) Data are presented as mean ± SD where error bars represent the SD. Statistics were from multiple comparison of two-way ANOVA multiple comparison analysis of three independent experiments (c) or two-tailed unpaired t test analysis of single measurements (f). Source data are provided in Data S1.
To be able to uncover the possible reasons for the defect in the Centrobin removal mechanism, we analyzed the centriolar localization of Centrobin in control and CEP350−/− cells by u-ExM. The Centrobin signal concentrated at the middle centriole till the subdistal region of CEP350−/− mother centrioles (Fig. S3, n and o). Considering this overlap with the region that normally carries subdistal appendages and the lack of appendages in CEP350−/− centrioles, we asked whether subdistal appendages have a role in Centrobin removal. To test this, we analyzed cells with deletions in genes coding for subdistal appendage proteins ODF2−/−, CEP128−/−, and NIN−/− (coding for Ninein), where ODF2 is closest to the centriole MT wall and Ninein more toward the appendage tip (Fig. S4 a; Chong et al., 2020), together with CEP350−/− and control cells. EM analysis showed that ODF2−/− and CEP128−/− cells indeed failed to assemble subdistal appendages at mother centrioles and some centrioles even started to dock ciliary vesicles to the distal appendages confirming their function (Fig. S4 b). In contrast, the subdistal appendages of NIN−/− centrioles had a similar morphological appearance as those of WT centrioles, probably, because Ninein functions late in the subdistal appendage assembly hierarchy.
Centriole core proteins extend together with elongating CEP350−/−. (a) Cartoon representation of subdistal appendage formation in control, ODF2−/−, CEP128−/−, NIN−/−, and CEP350−/− cells according to Chong et al. (2020). (b) EM micrographs form control, CEP350−/−, ODF2−/−, CEP128−/−, and NIN−/− are shown to visualize subdistal appendage structures (magenta arrowheads) and distal appendage structures (cyan arrowheads). (c) Cells where Centrobin was removed from one of the centrioles (4:3 Centrin:Centrobin) were analyzed with ODF2. (d) Quantification of ODF2 centriole signal in 4:3 Centrin:Centrobin cells. In 83% of cells, ODF2 was localized only on the Centrobin lacking centriole, and in 17% of cells, second mother centriole was also showing an ODF2 signal despite the presence of Centrobin. (e) Cells where Centrobin is removed from two of the centrioles (4:2 Centrin:Centrobin) were analyzed with ODF2 to show presence of ODF2 on Centrobin lacking centrioles. (f) Quantification of ODF2 centriole signal in 4:2 Centrin:Centrobin cells. All the Centrobin lacking centrioles showed ODF2 presence. (d and f)N = 2, n > 50. (g) Control and CEP350−/− cells were depleted of Centrobin with siRNA, and after 72 h cells were CSK extracted, methanol-fixed, and stained with Centrin (green), Centrobin (red), and DNA with DAPI (blue). (h and i) Centrin signals were quantified from panel g with N = 3, n > 240 cells. Each independent experimental repetition is represented with the same color in CEP350−/− and control cells in respective graphs (h and i). Scale bars: (c, e, and g) 5 μm in main panels and 2 μm in inset magnifications, (b) 150 nm. Data are presented as mean ± SD where error bars represent the SD. Statistics was derived from two-tailed unpaired t test analysis of indicated numbers of biologically independent experiments. Source data are provided in Data S1.
Centriole core proteins extend together with elongating CEP350−/−. (a) Cartoon representation of subdistal appendage formation in control, ODF2−/−, CEP128−/−, NIN−/−, and CEP350−/− cells according to Chong et al. (2020). (b) EM micrographs form control, CEP350−/−, ODF2−/−, CEP128−/−, and NIN−/− are shown to visualize subdistal appendage structures (magenta arrowheads) and distal appendage structures (cyan arrowheads). (c) Cells where Centrobin was removed from one of the centrioles (4:3 Centrin:Centrobin) were analyzed with ODF2. (d) Quantification of ODF2 centriole signal in 4:3 Centrin:Centrobin cells. In 83% of cells, ODF2 was localized only on the Centrobin lacking centriole, and in 17% of cells, second mother centriole was also showing an ODF2 signal despite the presence of Centrobin. (e) Cells where Centrobin is removed from two of the centrioles (4:2 Centrin:Centrobin) were analyzed with ODF2 to show presence of ODF2 on Centrobin lacking centrioles. (f) Quantification of ODF2 centriole signal in 4:2 Centrin:Centrobin cells. All the Centrobin lacking centrioles showed ODF2 presence. (d and f)N = 2, n > 50. (g) Control and CEP350−/− cells were depleted of Centrobin with siRNA, and after 72 h cells were CSK extracted, methanol-fixed, and stained with Centrin (green), Centrobin (red), and DNA with DAPI (blue). (h and i) Centrin signals were quantified from panel g with N = 3, n > 240 cells. Each independent experimental repetition is represented with the same color in CEP350−/− and control cells in respective graphs (h and i). Scale bars: (c, e, and g) 5 μm in main panels and 2 μm in inset magnifications, (b) 150 nm. Data are presented as mean ± SD where error bars represent the SD. Statistics was derived from two-tailed unpaired t test analysis of indicated numbers of biologically independent experiments. Source data are provided in Data S1.
To judge Centrobin removal from centrioles better, we imaged RPE1 G2/M WT cells with Centrin and Centrobin staining to determine the Centrin:Centrobin ratio as an indication of Centrobin removal from mother centrioles. When Centrobin was removed from both mother centrioles, the Centrin:Centrobin ratio was 4:2 (two mother centrioles and two daughter centrioles), but when Centrobin was not removed, this ratio was 4:4 (Fig. 3, b and c). According to our results, complete failure of Centrobin (4:4) removal was rarely observed in control cells (11%). 30% of control cells showed partial Centrobin removal (4:3; Fig. 3 c), and analysis of these cells with ODF2 staining indicated that it was the mature ODF2 positive mother centriole that was devoid of Centrobin (Fig. S4 c, blue frame, and d). Only a small number of G2/M centrioles carried an ODF2 signal on a Centrobin-containing centriole (Fig. S4 c, red frame, and d). The majority of G2/M control cells contained a 4:2 Centrin to Centrobin ratio (Fig. 3, b and c), suggesting that Centrobin was removed from both mother centrioles. We confirmed the presence of an ODF2 signal at the two centrioles that lacked Centrobin in 4:2 cells (Fig. S4, e and f).
The G2/M pattern was quite different in the knockout cell lines. Centrobin removal from both mother centrioles failed in 78% of CEP350−/−, 46% of ODF2−/−, and 34% of CEP128−/− cells (4:4; Fig. 3, b and c). This complete defect of Centrobin removal was not observed for NIN−/− centrioles that showed a similar 4:4 Centrin:Centrobin frequency as control cells. However, 42% of NIN−/− centrioles had a partial Centrobin removal defect as indicated by the 4:3 Centrin:Centrobin ratio (Fig. 3 c). This suggests that subdistal appendage proteins, according to their localization hierarchy, are important for the removal of Centrobin from centrioles.
Next, we asked how the presence of unremoved Centrobin affects the morphology of CEP350−/− centrioles. Considering its function in centriole elongation and stability (Gudi et al., 2015; Gudi et al., 2011; Lee et al., 2010), we hypothesized that persistence of Centrobin might contribute to the overelongation phenotype of CEP350−/− centrioles. To test this hypothesis, we depleted Centrobin in CEP350−/− and control cells. The depletion of Centrobin reduced the length and intensity of the Centrin signal on CEP350−/− centrioles (Fig. S4, g–i). To be able to verify this role, we also analyzed Centrobin depleted centrioles by EM (Fig. 3, d–f). Depletion of Centrobin in control cells only mildly reduced the length of mother centrioles by 18% (410 nm in nonspecific control siRNA [siNSC], 335 nm in siCentrobin). In contrast, the length of CEP350−/− mother centrioles was reduced by 52% by Centrobin depletion (583 nm in siNSC and 280 nm in siCentrobin). In summary, Centrobin is especially important for centriole MT stability and thus also for MT length in CEP350−/− cells.
CEP350 loss prevents recruitment of the MT cohesion protein WDR90, whereas inner scaffold proteins accumulate on the elongated centrioles
We observed that CEP350−/− centrioles were not only longer but also had defective MTs (Fig. 2, g–j). To be able to understand the reasons for the centriole MT wall deformation, we checked the levels of centriolar MT cohesion protein WDR90 in CEP350−/− cells that before was described as a centriole MT cohesion factor (Steib et al., 2020). In CEP350−/− centrioles, WDR90 was diminished by 87% compared to the control (Fig. 4, a and b). Similarly, CEP350 siRNA depletion also reduced WDR90 at centrioles (Fig. 3, d and k). This indicates that in CEP350−/− cells, centriole MT instability might be caused by the absence of WDR90. To further support this notion, we overexpressed WDR90 in CEP350−/− cells to rescue the centriole MT integrity defect. Overexpressed WDR90 was able to localize to centrioles (Fig. S5, a–d). The centriolar MT defect as judged by the relative length difference of centriole MTs (Fig. S5 e) was significantly decreased in CEP350−/− cells overexpressing WDR90 (Fig. S5 f). Interestingly, WDR90 overexpression even slightly elongated centriole MT length compared with CEP350−/− centrioles (Fig. S5 g), probably because of reduced centriole MT breakage. This indicates that the reduced binding of WDR90 to centrioles is the cause of the MT integrity defect of CEP350−/− cells.
WDR90 recruitment to centriole wall is defected in CEP350−/−cells. (a, c, and f) Control cells and CEP350−/− cells are methanol fixed and stained for IF with (a) γ-tubulin (green) and WDR90 (red); (c) Centrin (green) and FAM161A (red); and (f) Centrin (green) and POC5 (red). DNA was stained with DAPI. The enlargements on the bottom right are magnifications of the centriole signals shown in the center. (b, d, e, and g) Quantifications of centriolar signals of panels a, c, and f. N = 3, n > 240 cells. Data are presented as mean ± SD where error bars represent the SD. Each independent experiment repetition is represented with the same color on different groups of graphs. Statistics were derived from two-tailed unpaired t test analysis of at least three biologically independent experiments. (h–j) Control cells and CEP350−/− cells were fixed, expanded with an expansion factor of 4 for u-ExM, and stained with (h) α-tubulin (gray) and WDR90 (red); (i) α-tubulin (gray) and FAM161A (red); and (j) α-tubulin (gray) and POC5 (red). All cells were CSK extracted. Scale bars: (a, c, and f) 5 μm in main panels and 2 μm in inset magnifications, (h–j) 100 nm. Source data are provided in Data S1.
WDR90 recruitment to centriole wall is defected in CEP350−/−cells. (a, c, and f) Control cells and CEP350−/− cells are methanol fixed and stained for IF with (a) γ-tubulin (green) and WDR90 (red); (c) Centrin (green) and FAM161A (red); and (f) Centrin (green) and POC5 (red). DNA was stained with DAPI. The enlargements on the bottom right are magnifications of the centriole signals shown in the center. (b, d, e, and g) Quantifications of centriolar signals of panels a, c, and f. N = 3, n > 240 cells. Data are presented as mean ± SD where error bars represent the SD. Each independent experiment repetition is represented with the same color on different groups of graphs. Statistics were derived from two-tailed unpaired t test analysis of at least three biologically independent experiments. (h–j) Control cells and CEP350−/− cells were fixed, expanded with an expansion factor of 4 for u-ExM, and stained with (h) α-tubulin (gray) and WDR90 (red); (i) α-tubulin (gray) and FAM161A (red); and (j) α-tubulin (gray) and POC5 (red). All cells were CSK extracted. Scale bars: (a, c, and f) 5 μm in main panels and 2 μm in inset magnifications, (h–j) 100 nm. Source data are provided in Data S1.
CEP350−/−centrioles do not overelongate in their first cell cycle. (a–d)CEP350−/− and WDR90 complemented CEP350−/− (CEP350−/−+ GFP-HA-WDR90) cells were CSK extracted, fixed and stained for IF by GFP (green), HA (red), and PCNT (gray; a and c), or expanded by a factor of 4 and stained for u-ExM with α-tubulin (gray) and GFP (green) to observe centriolar microtubules (b and d). (e) Centriole MT loss within each centriole was calculated by the length of the longest MT, minus the length of the shortest MT and then divided by the length of the longest MT. (f) Centriole MT loss within centrioles was quantified (n = 45 for CEP350−/− cells, n = 28 for CEP350−/− + GFP-HA-WDR90). Average MT loss was found as 40% for CEP350−/− cells and 14% for CEP350−/− + GFP-HA-WDR90 cells. (g) Average MT length of CEP350−/− and WDR90 complemented sCEP350−/− (CEP350−/− + GFP-HA-WDR90) cells was quantified as 631 nm in CEP350−/− cells and 789 nm in CEP350−/− + GFP-HA-WDR90 cells. (h) Control cells and CEP350−/− cells were CSK extracted, fixed, and expanded with an expansion factor of 4 and stained for u-ExM with antibodies of Centrin (red), POC5 (red), and FAM161A (red). The centriole wall was stained with α-tubulin (gray). The ochre arrowhead shows the peeling off of centriolar microtubule filaments from centrioles. (i)CEP350−/− and OFD1 complemented CEP350−/− (CEP350−/− + GFP-HA-OFD1) cells were CSK extracted, fixed, and stained for IF by GFP (green), HA (red), and PCNT (gray). (j)CEP350−/− and OFD1 complemented CEP350−/− (CEP350−/− + GFP-HA-OFD1) cells were also CSK extracted, fixed, and expanded by a factor of 4 and stained for u-ExM with α-tubulin (gray) and GFP (green) to observe centriolar MTs. (k) Centriole MT loss within centrioles was quantified (n = 45 for CEP350−/− cells, n = 38 for CEP350−/− + GFP-HA-OFD1). (l) Average MT length of CEP350−/− and OFD1 complemented CEP350−/− (CEP350−/− + GFP-HA-OFD1) cells was quantified as 631 nm in CEP350−/− cells and 600 nm for CEP350−/− + GFP-HA-OFD1 cells. Scale bars: (a, c, and i) 10 μm in main panels and 2 μm in inset magnifications, (b, d, and j) 400 nm, (h) 500 nm. Data are presented as mean ± SD where error bars represent the SD. Statistics was derived from two-tailed unpaired t test analysis of indicated numbers of single quantifications. Source data are provided in Data S1.
CEP350−/−centrioles do not overelongate in their first cell cycle. (a–d)CEP350−/− and WDR90 complemented CEP350−/− (CEP350−/−+ GFP-HA-WDR90) cells were CSK extracted, fixed and stained for IF by GFP (green), HA (red), and PCNT (gray; a and c), or expanded by a factor of 4 and stained for u-ExM with α-tubulin (gray) and GFP (green) to observe centriolar microtubules (b and d). (e) Centriole MT loss within each centriole was calculated by the length of the longest MT, minus the length of the shortest MT and then divided by the length of the longest MT. (f) Centriole MT loss within centrioles was quantified (n = 45 for CEP350−/− cells, n = 28 for CEP350−/− + GFP-HA-WDR90). Average MT loss was found as 40% for CEP350−/− cells and 14% for CEP350−/− + GFP-HA-WDR90 cells. (g) Average MT length of CEP350−/− and WDR90 complemented sCEP350−/− (CEP350−/− + GFP-HA-WDR90) cells was quantified as 631 nm in CEP350−/− cells and 789 nm in CEP350−/− + GFP-HA-WDR90 cells. (h) Control cells and CEP350−/− cells were CSK extracted, fixed, and expanded with an expansion factor of 4 and stained for u-ExM with antibodies of Centrin (red), POC5 (red), and FAM161A (red). The centriole wall was stained with α-tubulin (gray). The ochre arrowhead shows the peeling off of centriolar microtubule filaments from centrioles. (i)CEP350−/− and OFD1 complemented CEP350−/− (CEP350−/− + GFP-HA-OFD1) cells were CSK extracted, fixed, and stained for IF by GFP (green), HA (red), and PCNT (gray). (j)CEP350−/− and OFD1 complemented CEP350−/− (CEP350−/− + GFP-HA-OFD1) cells were also CSK extracted, fixed, and expanded by a factor of 4 and stained for u-ExM with α-tubulin (gray) and GFP (green) to observe centriolar MTs. (k) Centriole MT loss within centrioles was quantified (n = 45 for CEP350−/− cells, n = 38 for CEP350−/− + GFP-HA-OFD1). (l) Average MT length of CEP350−/− and OFD1 complemented CEP350−/− (CEP350−/− + GFP-HA-OFD1) cells was quantified as 631 nm in CEP350−/− cells and 600 nm for CEP350−/− + GFP-HA-OFD1 cells. Scale bars: (a, c, and i) 10 μm in main panels and 2 μm in inset magnifications, (b, d, and j) 400 nm, (h) 500 nm. Data are presented as mean ± SD where error bars represent the SD. Statistics was derived from two-tailed unpaired t test analysis of indicated numbers of single quantifications. Source data are provided in Data S1.
Apart from the damaged MT walls, the centriole inner scaffold was still intact even when the triplets were completely missing in the extended centrioles (Fig. 2 g). Thus, we analyzed CEP350−/− and control cells for the accumulation of inner scaffold proteins. Despite the diminished levels of WDR90, loss of CEP350 promoted accumulation of Centrin (187% of control; Fig. 4, c and d) and POC5 (204% of control; Fig. 4, f and g), while FAM161A was only slightly increased (107% of control; Fig. 4, c and e).
To be able to verify these results on WDR90, FAM161A, POC5, and Centrin and understand the reason for elevated levels of inner scaffold proteins, we employed u-ExM to visualize centrioles of CEP350−/− and control cells. We observed that absence of CEP350 reduced WDR90 binding to core and distal end regions of centrioles (Fig. 4 h). On the other hand, FAM161A, POC5, and Centrin signals were elongated in CEP350−/− cells starting from the end of the proximal region till the distal end (Fig. 4, i and j; and Fig. S5 h). Additionally, we were able to capture snapshots of MT breakage from centrioles. Centriolar MTs peeled off from centrioles in CEP350−/− cells, probably explaining the lack of MT triplets on CEP350−/− centrioles (Fig. S5 h, ochre arrowhead). Together these results confirm that loss of CEP350 elongates the centriole on the distal region despite defective MTs caused by the absence of WDR90. The inner scaffold is resisting the destabilization triggered by CEP350 loss. However, due to the loss of stability factors, first MTs and then the inner scaffold structure is breaking apart. The mechanisms of how the centriole MT wall and the inner scaffold are joined together and how these two structures support each other are still unclear.
The CEP350–FOP–WDR90 and CEP350–FOP–OFD1 modules ensure centriole stability and length control
We next asked how CEP350 regulates centriole stability and how CEP350 loss diminishes WDR90 at centrioles. We first employed u-ExM to characterize the localization of CEP350 and FOP in greater detail. The anti-CEP350 antibodies recognized an epitope in the middle region of CEP350 (Fig. S1 a). FOP and the middle CEP350 region covered similar regions on longitudinal centrioles (Fig. 5, a and b) consistent with the fact that CEP350 is working together with FOP (Mojarad et al., 2017; Yan et al., 2005). In top views, the FOP ring had a larger diameter than the ring corresponding to the middle region of CEP350. When measured, the centriolar tubulin wall diameter on distal centrioles was 177 nm, whereas CEP350 (middle region) and FOP diameters were 201 and 303 nm, respectively (Fig. 5 c). Taken together, CEP350 localizes between FOP and the tubulin wall of centrioles.
CEP350/FOP complex interacts with WDR90 and OFD1 to ensure centriole stability and length control. (a and b) Cycling control cells were fixed, expanded with an expansion factor of 4 and stained for u-ExM with (a) α-tubulin (gray) and CEP350 (red) and (b) α-tubulin (gray) and FOP (red). (c) Quantification of tubulin (centriole wall), CEP350, and FOP signal diameters (n = 57 for tubulin, n = 38 for CEP350, and n = 19 for FOP signal diameter quantification) presented as mean ± SD where error bars represent the SD. (d)2xHA-WDR90 was co-expressed with 2xFlag-CEP350-GFP, 2xFlag-FOP-GFP, and 2xFlag-GFP in HEK293T cells with transient transfection. Agarose GFP-Trap beads were used as baits and the presence of WDR90 was checked together with indicated proteins by IB using the indicated antibodies. Represented results are from three independent experiments. Asterisks indicate the endogenous CEP350 protein. (e) Protein interactions of CEP350/FOP complex based on co-IP experiments in d. All imaged cells were CSK extracted. Scale bars: (a and b) 100 nm. (c) Statistics were from two-tailed unpaired t test of single measurements. Source data are provided in Data S1. Source data are available for this figure: SourceData F5.
CEP350/FOP complex interacts with WDR90 and OFD1 to ensure centriole stability and length control. (a and b) Cycling control cells were fixed, expanded with an expansion factor of 4 and stained for u-ExM with (a) α-tubulin (gray) and CEP350 (red) and (b) α-tubulin (gray) and FOP (red). (c) Quantification of tubulin (centriole wall), CEP350, and FOP signal diameters (n = 57 for tubulin, n = 38 for CEP350, and n = 19 for FOP signal diameter quantification) presented as mean ± SD where error bars represent the SD. (d)2xHA-WDR90 was co-expressed with 2xFlag-CEP350-GFP, 2xFlag-FOP-GFP, and 2xFlag-GFP in HEK293T cells with transient transfection. Agarose GFP-Trap beads were used as baits and the presence of WDR90 was checked together with indicated proteins by IB using the indicated antibodies. Represented results are from three independent experiments. Asterisks indicate the endogenous CEP350 protein. (e) Protein interactions of CEP350/FOP complex based on co-IP experiments in d. All imaged cells were CSK extracted. Scale bars: (a and b) 100 nm. (c) Statistics were from two-tailed unpaired t test of single measurements. Source data are provided in Data S1. Source data are available for this figure: SourceData F5.
To uncover the protein network modulated by CEP350, we performed pull-down experiments using tagged CEP350, FOP, and WDR90 constructs (Mojarad et al., 2017; Yan et al., 2005). WDR90 was pulled down by FOP but not by CEP350, indicating that FOP interlinks CEP350 and WDR90 (Fig. 5, d and e) and further supporting the role of CEP350–FOP–WDR90 module in centriole stability. Additionally, we realized that OFD1, but not ODF2, was pulled down by FOP (Fig. 5 d). This interaction data together with the known role of OFD1 in centriole length control and CEP350-dependent centriole localization of OFD1 suggests that CEP350–FOP–OFD1 is important for centriole MT length control. However, overexpressed OFD1 only tended to decrease the centriole MT defect of CEP350−/− centrioles (Fig. S5, i–l; see Discussion).
CEP78 interacts with FOP, and N-CEP350 is sufficient for centriole length control
To uncover functional domains in CEP350, we divided CEP350 into N-terminus (1–899 aa), middle (900–2249 aa), and C-terminus (2250–3115 aa) and overexpressed them in CEP350-depleted cells for complementation. Then, we checked the POC5 levels as an indication of inner scaffold accumulation and centriole overelongation (Fig. 6 a). Only the N-terminal CEP350 region (1–899 aa) was able to suppress the centriole elongation and POC5 accumulation phenotype in CEP350-depleted cells, while middle and C-terminal fragments of CEP350 were unable to do so (Fig. 6, a and b). This indicates that the N-terminus of CEP350 has a role in the centriole elongation processes.
CEP350 N-terminus is sufficient to rescue centriole length control by recruiting CEP78 and FOP to CEP350−/−centrioles. (a) siCEP350-treated cells were complemented with siRNA-resistant CEP350 N-terminus (NT350-Flag), middle region (Mid350-Flag), and C-terminus (CT350-Flag) and stained with POC5 (red) and Flag (green). All imaged cells were CSK extracted. (b) Quantification of POC5 signal intensity at centrioles from a. N = 6, n > 50 cells for each condition. (c and d)FOP-GFP and GFP plasmids were coexpressed with (c) 2xFlag-CEP350-C-term and (d) 2xFlag-CEP350-N-term in HEK293T cells with transient transfection. Agarose GFP-Trap beads were used as baits and the presence of CEP350-C-terminus and CEP350-N-terminus was analyzed with anti-Flag antibodies. (e and f) (e) 2xFlag-CEP350-N-term, 2xFlag-CEP350-C-term, and (f) FOP-GFP and GFP were coexpressed with 2xHA-CEP78 in HEK293T cells with transient transfection. M2 flag beads and GFP trap beads were used as baits and the presence of CEP78 was checked with anti-HA antibodies. GAPDH was used as a loading control for input lanes in c–f. Asterisks indicate the unspecific bands of the stated antibodies. (g and h) (g) CEP350−/− cells and (h) CEP350−/− cells with overexpressed NT-CEP350 were CSK extracted, methanol-fixed, and stained for IF with the indicated antibodies. DNA was stained with DAPI. The enlargements on the bottom right are magnifications of the centriole signals shown in the center. (i and j) Cells represented in panels g and h were quantified for signal intensities. Data are presented as mean ± SD of N = 2, n > 100 cells in total where error bars represent the SD. Statistics were derived from two-tailed unpaired t test analysis of at least two biologically independent experiments. Each independent experiment repetition is represented with the same color on different groups of graphs. (a, g, and h) Scale bars: 5 μm in main panels and 2 μm in inset magnifications. (k) A model of protein interactions identified in c–f are represented. Source data are provided in Data S1. Source data are available for this figure: SourceData F6.
CEP350 N-terminus is sufficient to rescue centriole length control by recruiting CEP78 and FOP to CEP350−/−centrioles. (a) siCEP350-treated cells were complemented with siRNA-resistant CEP350 N-terminus (NT350-Flag), middle region (Mid350-Flag), and C-terminus (CT350-Flag) and stained with POC5 (red) and Flag (green). All imaged cells were CSK extracted. (b) Quantification of POC5 signal intensity at centrioles from a. N = 6, n > 50 cells for each condition. (c and d)FOP-GFP and GFP plasmids were coexpressed with (c) 2xFlag-CEP350-C-term and (d) 2xFlag-CEP350-N-term in HEK293T cells with transient transfection. Agarose GFP-Trap beads were used as baits and the presence of CEP350-C-terminus and CEP350-N-terminus was analyzed with anti-Flag antibodies. (e and f) (e) 2xFlag-CEP350-N-term, 2xFlag-CEP350-C-term, and (f) FOP-GFP and GFP were coexpressed with 2xHA-CEP78 in HEK293T cells with transient transfection. M2 flag beads and GFP trap beads were used as baits and the presence of CEP78 was checked with anti-HA antibodies. GAPDH was used as a loading control for input lanes in c–f. Asterisks indicate the unspecific bands of the stated antibodies. (g and h) (g) CEP350−/− cells and (h) CEP350−/− cells with overexpressed NT-CEP350 were CSK extracted, methanol-fixed, and stained for IF with the indicated antibodies. DNA was stained with DAPI. The enlargements on the bottom right are magnifications of the centriole signals shown in the center. (i and j) Cells represented in panels g and h were quantified for signal intensities. Data are presented as mean ± SD of N = 2, n > 100 cells in total where error bars represent the SD. Statistics were derived from two-tailed unpaired t test analysis of at least two biologically independent experiments. Each independent experiment repetition is represented with the same color on different groups of graphs. (a, g, and h) Scale bars: 5 μm in main panels and 2 μm in inset magnifications. (k) A model of protein interactions identified in c–f are represented. Source data are provided in Data S1. Source data are available for this figure: SourceData F6.
To be able to understand how the N-terminal domain rescues defects in cells with CEP350 depletion, we tested the ability of CEP350 domains to interact with FOP and CEP78 in IP experiments. We confirmed that the interaction between FOP and CEP350 was mediated by the C-terminal region of CEP350 (Fig. 6 c; Yan et al., 2005). Our results further showed that even though the CEP350 N-terminus was unable to pull down FOP (Fig. 6 d), it was able to pull down CEP78 as reported (Gonçalves et al., 2021; Fig. 6 e). Furthermore, we realized that in repeated experiments FOP was also able to pull down CEP78 (Fig. 6 f). These findings suggest formation of an interaction network between N-CEP350, FOP, and CEP78. To test this notion further, we checked the presence of FOP and CEP78 in CEP350−/− cells complemented with CEP350 N-terminus. When overexpressed, the N-terminus of CEP350 was able to increase the centriolar levels of FOP and CEP78 in CEP350−/− cells (Fig. 6, g–j). In conclusion, the N-terminus of CEP350, probably, in complex with CEP78 and FOP is sufficient to control centriole elongation (Fig. 6 k).
Centriole overelongation does not automatically trigger centriole instability
Since CEP350 takes part in centriole stability and centriole elongation, we asked whether these two functions are dependent on each other. To test this, we induced centriole elongation by CEP78 or OFD1 depletion and analyzed centriole length and centriole MT integrity. CEP78 and OFD1 depletion by siRNA visibly increased the centriole length compared with control (siNSC) when analyzed by IF and u-ExM (Fig. 7, a–e). u-ExM images indicated that the averaged centriole length was 414 nm in the siNSC centrioles, whereas it was 500 nm in CEP78-depleted cells and 646 nm in OFD1-depleted cells (Fig. 7 e). However, broken MTs were not observed in those cells (Fig. 7, c, d and f). Thus, CEP78 and OFD1 depletion can induce centriole elongation without disrupting the centriole MT wall structure.
Centriole length and stability are independent factors. (a–d) Cycling control cells were treated with indicated siRNAs to observe centriole elongation with IF and u-ExM. (a) siCEP78-treated cells were stained with Centrin (green) and CEP78 (red) and then analyzed by IF. (b) siOFD1-treated cells were stained with Centrin (green) and OFD1 (red) and then analyzed by IF. (c and d) Cells from panels a and b were fixed, expanded with an expansion factor of 4 for u-ExM, and stained with α-tubulin (gray). (e) Centriole length of siCEP78 and siOFD1 from u-ExM images represented in c and d was measured. (f) Relative length differences of centriole microtubules in c and d. Relative length difference was calculated as indicated in Fig. S5 e. (e and f) n = 14 for siNSC, n = 10 for siCEP78, and n = 12 for siOFD1. (g and j) The cells from a and b were stained for IF WDR90 (green) and Centrin (red). (h, i, k, and l) Levels of WDR90 (h and k), CEP78 (i), and OFD1 (l) were quantified. N = 3, n > 240 cells. All cells were extracted by CSK. (a, b, g, and j) The enlargements on the bottom right are magnifications of the centriole signals shown in the center. Scale bars: (a, b, g, and j) 5 μm in main panels and 2 μm in inset magnifications, (c and d) 100 nm. (e, f, h, i, k, and l) Data are presented as mean ± SD where error bars represent the SD. (e and f) From one experiment. (h, i, k, and l)N = 3, n > 240 cells. Statistics were from two-tailed unpaired t test analysis of single measurements or three independent experiments. Each independent experiment repetition is represented with the same color on different groups of graphs. Source data are provided in Data S1.
Centriole length and stability are independent factors. (a–d) Cycling control cells were treated with indicated siRNAs to observe centriole elongation with IF and u-ExM. (a) siCEP78-treated cells were stained with Centrin (green) and CEP78 (red) and then analyzed by IF. (b) siOFD1-treated cells were stained with Centrin (green) and OFD1 (red) and then analyzed by IF. (c and d) Cells from panels a and b were fixed, expanded with an expansion factor of 4 for u-ExM, and stained with α-tubulin (gray). (e) Centriole length of siCEP78 and siOFD1 from u-ExM images represented in c and d was measured. (f) Relative length differences of centriole microtubules in c and d. Relative length difference was calculated as indicated in Fig. S5 e. (e and f) n = 14 for siNSC, n = 10 for siCEP78, and n = 12 for siOFD1. (g and j) The cells from a and b were stained for IF WDR90 (green) and Centrin (red). (h, i, k, and l) Levels of WDR90 (h and k), CEP78 (i), and OFD1 (l) were quantified. N = 3, n > 240 cells. All cells were extracted by CSK. (a, b, g, and j) The enlargements on the bottom right are magnifications of the centriole signals shown in the center. Scale bars: (a, b, g, and j) 5 μm in main panels and 2 μm in inset magnifications, (c and d) 100 nm. (e, f, h, i, k, and l) Data are presented as mean ± SD where error bars represent the SD. (e and f) From one experiment. (h, i, k, and l)N = 3, n > 240 cells. Statistics were from two-tailed unpaired t test analysis of single measurements or three independent experiments. Each independent experiment repetition is represented with the same color on different groups of graphs. Source data are provided in Data S1.
For that reason, we speculated that depletion of CEP78 and OFD1 does not affect the levels of the centriole stabilizing factor WDR90. To test this, we stained CEP78 and OFD1-depleted cells for WDR90 (Fig. 7, g–l). Whereas CEP78 and OFD1 were efficiently depleted (Fig. 7, i and l), WDR90 levels were not significantly affected by CEP78 (105% of siNSC) and OFD1 (85% of siNSC) depletion compared to siNSC (Fig. 7, g, h, j, and k). We propose that centriole instability and elongation are triggered by the absence or mislocalization of distinct proteins in CEP350−/− cells.
CEP350−/− daughter centrioles do not overelongate in the first cell cycle
Next, we address when the centriole overelongation phenotype in CEP350−/− cells is arising. To be able to answer this, we analyzed the daughter centrioles of CEP350−/− and control cells with u-ExM for centriole overelongation and MT defects. As before, we determine the relative length difference of MTs within a centriole to observe a possible centriolar MT defect (Fig. S5 e). Daughter centrioles did not show a relative length difference of their MTs in CEP350−/− cells (Fig. 8 a, daughter centrioles marked by green asterisks, and Fig. 8 b) in clear contrast to the MTs of the mother centrioles of CEP350−/− cells that show length heterogenicity (Fig. S5 f).
CEP350−/−centrioles do not overelongate in their first cell cycle. (a) Control and CEP350−/− cells were fixed and expended by a factor of 4 and stained for u-ExM with α-tubulin (gray) to follow daughter centriole elongation. Green asterisks indicate the daughter centrioles. (b) Relative length differences within centrioles represented in panel a were measured (n = 11 for control and n = 15 for CEP350−/− cells) as outlined in Fig. S5 e. Data are presented as mean ± SD where error bars represent the SD. The statistic is from two-tailed unpaired t test analysis of indicated numbers of single measurements. (c) Electron micrographs of control and CEP350−/− centrioles were shown. Daughter centrioles were indicated with green asterisks, and defects on mother centrioles are indicated by ochre arrowheads. (d) Control and CEP350−/− cells were treated with Centrinone for 4 d. Centrinone was washed out after. 14 h after the washout, cells were methanol fixed for IF by Centrin (green) and Sas6 (red) in order to identify de novo centrioles (1:1 Centrin:Sas6 centrioles). (e) De novo centrioles were counted for elongated Centrin phenotype in control and CEP350−/− cells. n = 125 for control and n = 152 for CEP350−/− cells. Scale bars: (a) 100 nm, (c) 150 nm, and (d) 2 μm in main panels and 2 μm in inset magnifications. Source data are provided in Data S1.
CEP350−/−centrioles do not overelongate in their first cell cycle. (a) Control and CEP350−/− cells were fixed and expended by a factor of 4 and stained for u-ExM with α-tubulin (gray) to follow daughter centriole elongation. Green asterisks indicate the daughter centrioles. (b) Relative length differences within centrioles represented in panel a were measured (n = 11 for control and n = 15 for CEP350−/− cells) as outlined in Fig. S5 e. Data are presented as mean ± SD where error bars represent the SD. The statistic is from two-tailed unpaired t test analysis of indicated numbers of single measurements. (c) Electron micrographs of control and CEP350−/− centrioles were shown. Daughter centrioles were indicated with green asterisks, and defects on mother centrioles are indicated by ochre arrowheads. (d) Control and CEP350−/− cells were treated with Centrinone for 4 d. Centrinone was washed out after. 14 h after the washout, cells were methanol fixed for IF by Centrin (green) and Sas6 (red) in order to identify de novo centrioles (1:1 Centrin:Sas6 centrioles). (e) De novo centrioles were counted for elongated Centrin phenotype in control and CEP350−/− cells. n = 125 for control and n = 152 for CEP350−/− cells. Scale bars: (a) 100 nm, (c) 150 nm, and (d) 2 μm in main panels and 2 μm in inset magnifications. Source data are provided in Data S1.
To be able to visualize the structures of daughter centrioles in more detail, we employed EM in CEP350−/− and control cells. Verifying the results of the u-ExM, EM images confirmed that daughter centrioles had no MT defects, which is in contrast to the CEP350−/− mother centrioles (Fig. 8 c). To verify these results further, we enforced CEP350−/− cells to form de novo centrioles by incubating the cells with the PLK4 inhibitor Centrinone followed by Centrinone washout to allow de novo formation of centrioles (Wong et al., 2015). We analyzed centrioles that still carried Sas6, a marker for newly formed centrioles that did not pass through mitosis yet (Fong et al., 2014). None of the de novo formed centrioles with a Sas6 signal showed Centrin elongation as we observed in CEP350−/− mother centrioles (Fig. 8, d and e). Thus, we hypothesized that, rather than an overelongated daughter centriole that is formed in one single cell cycle, CEP350−/− centrioles are continuously extending even after they are disengaged from their mothers.
Continuous centriole elongation combined with centriole MT destabilization causes broken centrioles
After understanding that CEP350−/− daughter centrioles are not overelongated, we wanted to analyze when the MT defect arises in mother centrioles. For this purpose, we analyzed G2 phase cells, since they contain one older mother, one new mother, and two daughter centrioles, allowing us to compare centrioles of different ages in the same cell. In control cells, all four centrioles showed dot-like POC5 and Centrin signals (Fig. 9 a). In CEP350−/− cells we observed that based on the POC5 and Centrin signal dimensions, two centrioles were elongated, and between the elongated centrioles one was longer than the other (Fig. 9 a, right panel). It has been shown that a weak Centrin signal corresponds to the daughters on duplicating centrioles in S and G2 (Zou et al., 2005). Using Centrin signal intensity as a marker for mother centrioles, we observed that the two elongated centrioles were the two mother centrioles, which suggests that the daughter centrioles had normal length in CEP350−/− cells (Fig. 9 a).
Centriole elongation block after mitosis is defective in CEP350−/−cells. (a) Cycling control and CEP350−/− cells were fixed and stained for POC5 (red) and Centrin (green) antibodies. G2 cells were selected according to POC5 recruitment to daughter centrioles (weaker Centrin and POC5 signals). The images on the right-hand side are enlargements of the boxed areas in the main image numbered 1 and 2. (b) Control cells and CEP350−/− cells were treated with DMSO (solvent control) and 100 nM Palbociclib for 16 h. After 16 h, cells are fixed, expanded with an expansion factor of 4, and stained for u-ExM with α-tubulin. (c) Longitudinal length of centrioles from panel b was measured. n = 40 for control DMSO, n = 49 for control Palbociclib, n = 40 for CEP350−/− DMSO, and n = 46 for CEP350−/− Palbociclib. (d) G1 arrested control cells and CEP350−/− cells were methanol-fixed and stained for IF with POC5 (green), Centrin (red), and PCNT (gray). The enlargements on the bottom right are magnifications of the centriole signals shown in the center. (e) Quantification of d. Centrin and POC5 signal lengths that were observed in panel d were measured for each cell. n = 72 cells in DMSO control and n = 75 cells in Palbociclib treatment. MC, mother centriole; DC, daughter centriole. (f) Average length of mother centriole and daughter centriole from panel e is shown above the centriole cartoons. (g) A model for CEP350 driven centriole length and stability mechanisms is shown. Scale bars: (a) 5 and 1 μm in inset magnifications, (b) 100 nm, (d) 2 and 2 μm in inset magnifications. (c and e) Data are presented as mean ± SD where error bars represent the SD. Statistics were from two-tailed unpaired t test analysis of indicated numbers of biologically single measurements. Source data are provided in Data S1.
Centriole elongation block after mitosis is defective in CEP350−/−cells. (a) Cycling control and CEP350−/− cells were fixed and stained for POC5 (red) and Centrin (green) antibodies. G2 cells were selected according to POC5 recruitment to daughter centrioles (weaker Centrin and POC5 signals). The images on the right-hand side are enlargements of the boxed areas in the main image numbered 1 and 2. (b) Control cells and CEP350−/− cells were treated with DMSO (solvent control) and 100 nM Palbociclib for 16 h. After 16 h, cells are fixed, expanded with an expansion factor of 4, and stained for u-ExM with α-tubulin. (c) Longitudinal length of centrioles from panel b was measured. n = 40 for control DMSO, n = 49 for control Palbociclib, n = 40 for CEP350−/− DMSO, and n = 46 for CEP350−/− Palbociclib. (d) G1 arrested control cells and CEP350−/− cells were methanol-fixed and stained for IF with POC5 (green), Centrin (red), and PCNT (gray). The enlargements on the bottom right are magnifications of the centriole signals shown in the center. (e) Quantification of d. Centrin and POC5 signal lengths that were observed in panel d were measured for each cell. n = 72 cells in DMSO control and n = 75 cells in Palbociclib treatment. MC, mother centriole; DC, daughter centriole. (f) Average length of mother centriole and daughter centriole from panel e is shown above the centriole cartoons. (g) A model for CEP350 driven centriole length and stability mechanisms is shown. Scale bars: (a) 5 and 1 μm in inset magnifications, (b) 100 nm, (d) 2 and 2 μm in inset magnifications. (c and e) Data are presented as mean ± SD where error bars represent the SD. Statistics were from two-tailed unpaired t test analysis of indicated numbers of biologically single measurements. Source data are provided in Data S1.
Since CEP350−/− daughter centrioles are not overelongated, we hypothesized that CEP350−/− centrioles do not stop extending as this is the case in control cells (Vorobjev and YuS, 1982; Azimzadeh et al., 2009). To test this notion, we blocked the CEP350−/− and control cells with the CDK4/6 inhibitor Palbociclib (Trotter and Hagan, 2020) for 16 h in G1 and performed u-ExM to measure centriole length. G1 cells carried an older mother centriole and the previous daughter centriole. The length of CEP350−/− centrioles was 667 nm in DMSO and 863 nm in Palbociclib-treated cells. In contrast, the length of control centrioles was very similar under both conditions (401 nm in DMSO and 410 nm in Palbociclib; Fig. 9, b and c). This suggests that, unlike control cells, centrioles continue to elongate in G1-arrested CEP350−/− cells because centriole length control is no longer functional in CEP350−/− cells.
We repeated the Palbociclib experiment and analyzed by IF the behavior of the daughter centriole and mother centriole in CEP350−/− cells. Higher PCNT signal intensity was used as a marker for mother centrioles since CEP350−/− centrioles do not carry appendages (Fig. 2 g; Zou et al., 2005; Azimzadeh et al., 2009; Yamashita et al., 2007; Conduit et al., 2010). Centrin and POC5 signal extension were measured as an indication for centriole length. The overelongation phenotype in the G1 arrest was more pronounced on CEP350−/− mother centriole (20.3%; 476 nm in DMSO control and 598 nm in Palbociclib) that carried more PCNT than for daughter centriole (10.7%; 393 nm in DMSO control and 440 nm in Palbociclib; Fig. 9, d–f). These results confirm that in CEP350−/− cells, centriole length regulation is lost by the impairment of different control mechanisms (Fig. 9 g).
Discussion
Although centrioles mainly consist of MTs, knowingly dynamic assemblies of tubulin, centrioles are stable entities that resist mechanical forces and treatment with MT destabilizing agents, such as nocodazole (Le Clech, 2008). An additional feature of centriole MTs is their limitation to grow beyond a certain length after assembly in S phase and length extension in G2 phase. Although centriole length variation is modest in a certain cell type (<10% [Schmidt et al., 2009; Hossain et al., 2017; Singla et al., 2010; Thauvin-Robinet et al., 2014]), it varies considerably between different cell lines (Marteil et al., 2018) and is affected by cell transformation. This suggests that control mechanisms modulate and ensure centriole length and stability in a cell type–specific manner.
Here, we analyzed coordination between centriole length and stability and identified the centriolar protein CEP350, which before was mainly studied in the context of ciliogenesis (Mojarad et al., 2017; Gonçalves et al., 2021; Kanie et al., 2017) as a central scaffold that controls both events. It is most likely that the CEP350–FOP module executes these functions because the centriolar localization of both proteins is interdependent. CEP350 probably provides the centriolar binding site for FOP, and in return, FOP stabilizes the centriolar CEP350 (Kanie et al., 2017). This model is consistent with the closer centriole MT localization of CEP350 compared with FOP, which localizes more outwards.
Interestingly, CEP350, FOP, and CEP78 are embedded by a complex interaction network at the distal end of centrioles that coordinates centriole length, stability, distal and subdistal appendage formation, and ciliation (Fig. 6 k). First, the N-terminus of CEP350 interacts with CEP78 (Fig. 6 e; Gonçalves et al., 2021). Second, FOP and CEP78 show complex formation (Fig. 6 f). Third, FOP interacts with the C-terminus of CEP350 (Fig. 6 c; Yan et al., 2005). Consistent with an interaction between CEP78 and FOP is the observation that N-CEP350 overexpression, which lacks the FOP binding site but carries the CEP78 interaction motif (Gonçalves et al., 2021), recruited CEP78 and FOP to centrioles in CEP350−/− cells (Fig. 6, g–j). These findings together suggest a triple complex at centrioles consisting of CEP350, FOP, and CEP78 with interactions between N–CEP350–CEP78, C–CEP350–FOP, and CEP78–FOP. Within this complex, CEP78 functions downstream of CEP350, as indicated by the finding that CEP350 still localizes to centrioles in CEP78−/− cells, whereas CEP78 localization is strongly diminished at CEP350−/− centrioles (Fig. S1 i and Gonçalves et al., 2021). The CEP350–FOP module has additional roles in centriole recruitment of WDR90, OFD1 (Fig. 9 g), and CEP19. The latter is targeted specifically to mother centrioles where it functions in ciliogenesis (Kanie et al., 2017; Mojarad et al., 2017). CEP78 in turn recruits the EDD–DYRK2–DDB1 E3 ubiquitin ligase complex to centrioles (Hossain et al., 2017).
Previously, experiments came to different conclusions about the role of CEP78 in ciliogenesis. Hossain et al. (2017) reported that CEP78 negative regulates the EDD–DYRK2–DDB1 E3 ubiquitin ligase complex and thereby prevents CP110 degradation. Gonçalves et al. (2021) suggested that CEP78 activates EDD–DYRK2–DDB1 and thereby initiates ciliogenesis. These deviations leave the mechanism of how CEP78 controls ciliogenesis open. Here, we have analyzed centriole length in cycling RPE1 cells and observed that depletion of CEP78 elongates centrioles. In addition, expression of N-CEP350 that rescued the length defect of CEP350 depleted cells recruits CEP78 and FOP to CEP350−/− centrioles, indicating that under our experimental conditions CEP78 negatively regulates the length of centrioles. It is well possible that the function of CEP78 between cycling and serum-starved ciliated cells is different. Future experiments will show whether and how the activity of the E3 ligase EDD–DYRK2–DDB1 is involved in centriole length control by the CEP350–FOP–CEP78 complex.
OFD1 associated with the distal end of mother and daughter centrioles (Singla et al., 2010). We defined the connecting link between CEP350 and OFD1 as FOP (Fig. 5, d and e). Since the lack of OFD1 considerably elongates centrioles by a factor of 1.6 (Singla et al., 2010; Fig. 7 e), the partial loss of OFD1 from centrioles most likely is a contributing factor to the centriole length increase in CEP350−/− cells (Fig. 1, d and e). We have shown that persistence of Centrobin binding to centrioles also plays a role in centriole MT overelongation in CEP350−/− cells, and this stabilization might be the reason why OFD1 overexpression did not strongly reduce the length of CEP350−/− centrioles (Fig. S5 l). Another possible explanation is that CEP350 activates OFD1, and simple ectopic overexpression of OFD1 is therefore insufficient to restore full OFD1 function in the CEP350−/− background. How OFD1 functions in centriole length control is presently unclear; however, the mechanism is likely distinct from CPAP and CP110 (Singla et al., 2010).
Centrobin is believed to be a daughter centriole marker that becomes undetectable on mother centrioles once daughters start to form upon entering into S phase (Gudi et al., 2015). In this study, we report that Centrobin removal does not directly happen when centriole duplication starts in S phase, as indicated by a portion of G2/M mother centrioles that carried Centrobin (Fig. 3 c; and Fig. S4, c and d). Interestingly, of these G2/M centrioles it was always the old mother centriole (marked by the subdistal appendage marker ODF2) that was devoid of Centrobin. The young mothers that did not start subdistal appendage assembly (no ODF2 signal) still carried Centrobin. This hypothesis is supported by the observation that in all G2/M mother centrioles with a 4:2 Centrin:Centrobin ratio, the two Centrobin negative centrioles carried ODF2 (Fig. S4, c–f). In addition, lack of core subdistal appendage proteins such as ODF2 and CEP128 had a strong impact on Centrobin removal (Fig. 3, a–c). ODF2 is recruited to new mother centrioles as early as G1/S transition (Nakagawa et al., 2001), and this could then kick-start removal of Centrobin from these centrioles. However, it is not known how this mechanism functions together with previously reported regulators of Centrobin removal, such as polo-like kinase PLK1 and NEK2 kinases (Park and Rhee, 2013; Lee et al., 2010; Le Roux-Bourdieu et al., 2022). This together suggests that Centrobin is more of an immature centriole marker with respect to appendage assembly rather than a daughter centriole–specific marker, and subdistal appendages are specifically important for its removal.
A previous study showed that Centrobin removal from maturing centrioles is a requisite for the formation of distal appendages (Wang et al., 2018). However, we suggest that formation of intact subdistal appendages is a necessity for successful Centrobin removal. The same study also suggests that C2CD3 is the key to subdistal appendage structure formation (Wang et al., 2018). Our data indicate that the key player of subdistal appendage formation is CEP350 rather than C2CD3 since CEP350−/− cells do not form subdistal appendages despite the presence of elevated C2CD3 at centrioles (Fig. 1, j and k; and Fig. 2 g). The increase of centriolar C2CD3 could be part of a compensatory mechanism for the loss of CEP350.
It is known that Centrobin stabilizes MTs in vitro (Lee et al., 2010). Therefore, it is reasonable to assume that Centrobin fulfills a similar function on centriole MTs, and in this way it promotes centriole elongation in CEP350−/− centrioles enabling them to overelongate despite their damage. This function of Centrobin most likely overlaps with the MT stabilizing function of CEP350 (Le Clech, 2008; Yan et al., 2005) since Centrobin depletion had a stronger impact on centriole length in CEP350−/− cells compared with the control cells (Fig. 3, d–f).
Since lack of CEP350 triggers centriole elongation, it is formally possible that longer centriole MTs are automatically less stable than shorter ones, leading to the collapse of the centriole MT array on the distal end. However, our data argue against this simple extension/instability model. Depletion of CEP78 and OFD1 that both triggered centriole elongation in RPE1 cells did not generate the MT defects that were seen in CEP350−/− cells (Figs. 2 g and 7, c–f), indicating that overelongation of centrioles was not sufficient to cause centriole instability probably as long as centriole stability proteins associated with centrioles (Fig. 7, g–l). This raises the question: What is causing the centriole MT defect in CEP350−/− cells? Interestingly, in CEP350−/− cells MTs “peeling off” from centrioles (Fig. 2, g–j; and Fig. S5 h) lead to lack of MT triplets in CEP350−/− centrioles. A similar phenotype was also reported for WDR90 knockdown cells (Steib et al., 2020). Since WDR90 no longer associates with centrioles in CEP350−/− cells, probably because it requires the CEP350–FOP anchor for its efficient localization (Fig. 4, a, b, and h), we suggest that lack of WDR90 is provoking centriole distal instability in the absence of CEP350. Consistent with this is the observation that WDR90 overexpression was able to suppress the centriole MT defect but not the overelongation of centrioles in CEP350−/− cells (Fig. S5, c, f, and g). In fact, centrioles of CEP350−/− + WDR90 cells were slightly longer than CEP350−/− centrioles (Fig. S5 g), probably because of the abolished MT breakage. In the case of WDR90, its MT binding ability (Steib et al., 2020) is most likely sufficient to target enough overexpressed protein to the centrioles to fulfill its function even in the absence of CEP350. WDR90 stabilizes centriole MTs probably by connecting MT triplets or anchoring MT triplets to the stable inner scaffold of POC5, Centrin, and FAM161 (Hamel et al., 2017; Steib et al., 2020), which is more resistant to the destabilization effects of CEP350 loss than the centriole MTs (Fig. 2 g).
Elongation and stabilization of daughter centriole MTs in S/G2 are probably promoted by Centrobin and CEP350. As soon as cells have passed mitosis, disengaged centrioles stop elongating under the control of CEP78, and perhaps OFD1 (Sharma et al., 2021; Singla et al., 2010) CEP350−/− centrioles that are lacking these factors, and in addition, carrying the stabilizing Centrobin on the mother centrioles continuously grow at their distal end (Fig. 9, a–e). Eventually, centriole MTs of CEP350−/− cells peel off from the inner scaffold because WDR90 is absent. The proximal centriolar domain of centrioles is probably protected from destabilization by the A-C linker (Greenan et al., 2018), connecting adjacent centriole MT triplets at proximal end and the surrounding PCM.
In summary, we propose a model of how CEP350 orchestrates centriole length, stability, and architecture (Fig. 9 g). These functions of CEP350 make it a key factor for centriole sustainability, and it will be interesting to study whether the length variation of centrioles that is seen in different cell types and transformed cells is triggered by CEP350 tuning.
Materials and methods
Cell culture
Human telomerase-immortalized RPE1 cells (hTERT-RPE1 TP53−/−; Fong et al., 2014; Fong et al., 2016), ODF2−/− (Viol et al., 2020), CEP128−/− (Mönnich et al., 2018), NIN−/− (Li, 2020), and CEP350−/− cells were cultured in DMEM/F-12 (Gibco) medium supplemented with 10% FBS, 1% L-glutamine, and 1% penicillin–streptomycin. Human embryonic kidney 293 (T; HEK T293) cells and HEK GP2-293 (Clonetech) cells were cultured in the same medium as RPE1 cells.
Drug treatments
Cells were arrested in G1 by 100 nM Cdk4/6 inhibitor Palbociclib (Cat. #4786; Tocris) for 16 h (Atorino et al., 2020). For centriole depletion, 125 nM Centrinone was used as previously described (Wong et al., 2015). To detect the S phase cells, 10 nM EdU from Click-iT Plus EdU Alexa Fluor Imaging Kit (Cat. # C10638 and #C10640; Thermo Fisher Scientific) was used according to the manufacturer’s protocol 20 min before cell fixation.
Plasmid transfection and RNAi
Plasmid delivery into HEK GP2-293 and HEK T293 was achieved with polyethylenimine reagent (25 kD, #9002-98-6; Sigma-Aldrich). Transfection of synthetic siRNA oligos into RPE1 TP53−/− cells and RPE1 TP53−/−CEP350−/− cells were performed using Lipofectamine RNAiMAX Transfection Reagent from Life Technologies. Transfection reactions were prepared in Opti-MEM medium following the manufacturer’s protocol. All of the used siRNAs are obtained from Dharmacon as smartpools to efficiently deplete the proteins.
Plasmids and constructs
pRetroX-Tet3G and pVSVG plasmids (Retro-X Tet-On 3 G Inducible Expression System; Clontech) were used to generate RPE1 TP53−/− cells with Tet-On 3 G System. siRNA-resistant CEP350 fragments were Flag-tagged via PCR and cloned into pRetroX-TRE3G. To be able to create the CEP350−/− cell lines, a number of sgRNA pairs were used to create genetically different CEP350−/− clones. The sgRNA pairs used to create double cut in the CEP350 gene were designed by the sgRNA Design tool of Benchling (Biology Software). The oligos sg1: 5′-GGGAGCTTCTATGAGAACTG-3′; sg2: 5′-AGGAACGGATTAGAAAACAG-3′; sg3: 5′-CTGTTATTTCCAAAAGGCGC-3′; and sg4: 5′-CCAAGTGCATCTTCCAGTAG-3′ were cloned into the pX458 plasmid as described before (Ran et al., 2013). We used sg2 and sg3 for CEP350−/− clone #6 and sg1 and sg4 for clone #7 (Fig. S1 a).
2xFlag-CEP350-GFP, 2xFlag-FOP-GFP, 2xFlag-GFP, 2xHA-WDR90, and 2xHA-CEP78 (CEP78 ORF from Addgene #136827 [Kowarz et al., 2015]) were constructed by cloning the ORFs into pRetroX-TRE3G vector with NEBuilder HiFi DNA Assembly Master Mix. Additionally, we created siRNA-resistant fragments of the CEP350 gene: siRNA targeting regions were changed corresponding to the same amino acids but with different codon sequences. We mutated the siRNA targeting regions from 5′-GATGATAGGCAGTCGAGAA-3′ to 5′-GATGATTGGTTCCCGAGAG-3′, from 5′-AGTAGAGAACTGTATCGAGAT-3′ to 5′-TCCCGCGAGTTATACCGTGA-3′, and from 5′-AGATCTAAGTCGTCAGTAA-3′ to 5′-CGTAGCAAAAGCAGTGTGA-3′. CEP350 protein sequence was divided into N-terminus (1–899 aa), middle (900–2249 aa), and C-terminus (2250–3115 aa) regions and cloned into pRetroX-TRE3G to create stable cell lines.
For Sleeping Beauty constructs, the pSBtet-Pur (#60507; Addgene) plasmid has been modified to change antibiotic resistance to Zeocin. Then, CEP350 N-terminus, CEP350, WDR90, and OFD1 (OFD1 ORF from Addgene #24558 [Lukinavičius et al., 2013]) were cloned into SB vector to create SB-NtCep350-HA-GFP, SB-2xFL-GFP-CEP350, SB-WDR90-HA-GFP, and SB-OFD1-HA-GFP constructs.
Characterization of CEP350−/− cells
RPE1 TP53−/− and TP53+/+ cells are generated by electroporation of 106 cells with 10 µg gRNA inserted pX458 with Neon electroporation system (#MPK10025; Thermo Fisher Scientific) according to the manufacturer’s protocol. 48 h after electroporation, GFP-positive cells are single-sorted in 96-well plates containing full DMEMF12 medium. When cells are grown enough, 96-well plates are duplicated, and one plate was used for genomic DNA isolation by QuickExtraction DNA isolation solution (#QE09050; Epicentre). The cells with a negative WT band (obtained only in RPE1 TP53−/− background) were cultured further for mRNA extraction (RNeasy Plus Mini Kit, #74134; Qiagen). The resulting mRNA was converted to cDNA by RevertAid cDNA synthesis kit (#K1691; Thermo Fisher Scientific) according to the manufacturer’s protocol. 2 µl of produced cDNA (out of 20 µl) was used in PCR reactions to amplify with forward (5′-ATCCAAGGAACTCTCAAAGCAAGGATACTGT-3′) and reverse (5′-ATGAACGAGACGATGCAGCAGACTG-3′). PCR products were cloned into pJET2.1 (CloneJET Kit; #K1231; Thermo Fisher Scientific) for sequencing with pJET1.2 forward and reverse primers. Conserved sequences were identified by Clustal omega tool of European Molecular Biology Laboratory (Sievers et al., 2011) and aligned by Jalview tool (Waterhouse et al., 2009).
Stable cell line production with retrovirus and Sleeping Beauty system
For rescuing the CEP350−/− phenotype, cell lines expressing a siRNA-resistant N-terminus, C-terminus, and middle fragment of the CEP350 gene were made from RPE1 TP53−/− cells. Firstly, the Retro-X Tet-On 3G Inducible Expression System (Clontech) was introduced into RPE1 TP53−/− cells. CEP350 siRNA-resistant fragment constructs were integrated into RPE1 TP53−/− Tet3G cells under the control of the TRE3G promoter via retrovirus infection (Clontech).
CEP350−/− cell lines were generated via electroporation of RPE1 TP53−/− (Atorino et al., 2020; Fong et al., 2014) with pX458 cloned with the sequence for the sgRNA. 2 d after electroporation, GFP-positive cells were FACS-sorted as single cells in a 96-well plate. When colonies are grown, single clones were analyzed by PCR. The cells lacking WT CEP350 bands were used for mRNA extraction, cDNA conversion, followed by PCR for sequencing. The clones with successful knockout sequences are tested with antibodies for CEP350 IF and IB. Two CEP350−/− genetically different clones were used for the experiments.
Sleeping Beauty system was used to integrate and create stable cell lines to be used in complementation and rescue experiments as previously described (Kowarz et al., 2015; Mátés et al., 2009). Flag-GFP-CEP350, GFP-HA-WDR90, and GFP-HA-OFD1 constructs were cloned into the donor vector of Sleeping Beauty system. Afterward, donor vectors were electroporated into CEP350−/− cells together with the SB100X-transposase vector (1:1 donor:transposase). Cells were checked for presence of the gene of interest 2 d after electroporation. To enrich the successfully inserted cells, 100 μg/ml Zeocin (# R25001; Invitrogen) selection was done for 2 d and FACS sorting was employed.
Expansion microscopy
As described before (Gambarotto et al., 2021), cycling cells (regularly trypsinized) were extracted with CSK-extraction buffer (0.5% TritonX-100, 10 mM Hepes, 300 mM sucrose, 100 mM NaCl, and 3 mM MgCl2 in water) fixed on coverslips with fixation solution (formaldehyde [37%], acrylamide [40%], PBS [1×]) at 37°C for 5 h. After that, 35 μl of gelation solution (90 μl monomer solution, 5 μl ammonium persulfate and 5 μl tetramethylethylenediamine) was added on coverslips on ice (monomer solution: Na-Acrylate [38%], acrylamide [40%], N,N′-methylenebisacrylamide [2%], 10× PBS). Coverslips were incubated on ice for 20 min and then taken to 37°C for 1 h. After that the gel is removed from coverslips by incubation in denaturation solution (SDS [10%], NaCl [5 M], Tris-BASE, and water) for 15 min on a shaker. Later, gels are put into an Eppendorf and boiled at 98°C for 1 h. After denaturation, gels were washed with 1xPBS three times for 30 min and left overnight or for 3 h incubation at 37°C with primary antibodies (same dilution as IF). Then, gels were washed three times with 1xPBS again and incubated with secondary antibodies for 3 h at 37°C. Lastly, gels are washed with 1xPBS three times and consecutively three times with distilled water for expansion. When gels are properly expanded, pieces of gels were taken to microscope for imaging.
IF and expansion microscopy and image processing
Cycling cells (regularly trypsinized) were fixed on coverslips with methanol at −20°C for 5 min (C2CD3 stained cells were fixed with Formalin for 10 min) and the coverslips were blocked in 10% FBS, 0.1% Triton-X100 for 30 min. After incubation for 1 h with primary antibody (diluted in 3% BSA [wt/vol]), cells were incubated with secondary antibody (1:500 dilution in 3% BSA) and DAPI and mounted on glass slides with Moviol or ProLong Gold antifade mounting medium for super-resolution microscopy. Cells were extracted with CSK-extraction buffer for 3 s (0.5% TritonX-100, 10 mM Hepes, 300 mM sucrose, 100 mM NaCl, and 3 mM MgCl2 in water) prior to fixation as described before (Hass et al., 2012). The EdU stain detection reaction was accomplished after coverslip blocking and before primary antibody incubation, following the reaction instruction of Click-iT Plus EdU Alexa Fluor Imaging Kit (555 and 647 Kits; Thermo Fisher Scientific). All IF images were acquired by DeltaVision RT system (Applied Precision) with an Olympus IX71 microscope equipped with 60×/1.42 and 100×/1.40 oil objective lenses at room temperature.
Expansion microscopy samples were prepared according to the previously described protocol (Gambarotto et al., 2021) with the indicated antibodies. In Fig. 2 h, u-ExM samples and all IF samples were imaged on a DeltaVision RT system (Applied Precision) with an Olympus IX71 microscope equipped with 60×/1.42 and 100×/1.40 oil objective lenses, and the rest of the u-ExM samples were imaged on Leica TCS SP8 STED 3× microscope with FALCON FLIM with HC PL APO 100×/1.40 STED White Oil objectives in room temperature (Figs. 1 b; 4, h–j; 5, a and b; 7, c and d; 8 a; 9 b; S3 n, and o; and S5, b, d, h, and j). The IF images were deconvoluted and z-projected by the software of DV microscope itself (Applied precision), and raw u-ExM images were deconvoluted by Huygens’ Deconvolution software (SVI Inc.) using the standard 3D-deconvolution protocol. The z-stack spanning the centrioles was z-projected by ImageJ/Fiji software.
Antibodies
Primary antibodies directed against the indicated proteins were: γ-tubulin (mouse, 1:1,000, Ab27074; , Abcam), PCNT (rabbit, 1:2000, Ab4448; Abcam), CEP97 (rabbit, 1:300, A301-945A; Bethyl), Centrin (mouse, 1:1,000, MABC544; Millipore), Centrin (rabbit, 1:500, ab101332; Abcam), α-tubulin (mouse, 1:500, DM1A; Sigma-Aldrich), GT335 (mouse, 1:500, AG-2013-0020; AdipoGen), CEP164 (guinea pig, 1:2,000, gift from G. Pereira, Centre for Organismal Studies, Heidelberg University; [Schmidt et al., 2012]), SAS6 (mouse, 1:50, sc-81431; SCBT), Flag tag (mouse, IF 1:1,600; Western blot [WB] 1:1,000, 9A3; Cell Signaling), GAPDH (rabbit, WB 1:1,000, 14C10; Cell Signaling Technology), HA tag (rat, 1:1,000, No.1867423; Böhringer Mannheim), HA tag (rabbit, 1:1,000, 51064-2-AP; Proteintech), CEP350 (mouse, 1:500, CL3423; Abcam), α-tubulin (rabbit, 1:500, 11224-1-AP; Proteintech), α-tubulin (mouse, 1:500, 660311-1-Ig; Proteintech), Flag tag (rabbit, 1:500, 20543-1-AP; Proteintech), CEP78 (rabbit, 1:500, A301-800A-M; Bethyl), C2CD3 (rabbit, 1:500, A104062; Sigma-Aldrich), POC1B (guinea pig, 1:500, homemade [Atorino et al., 2020]), CEP44 (rabbit, 1:500, homemade [Atorino et al., 2020]), POC5 (rabbit, 1:500, A303-341A-T; Bethyl), WDR90 (rabbit, 1:500, NB2-31888; Novus), CP110 (rabbit, 1:500, A301-343A; Bethyl), Centrobin (mouse, 1:500, ab70448; Abcam), Centrobin (rabbit, 1:800; 26880-1-AP; Proteintech), OFD1 (rabbit, 1:500, NBP1-89355; Novus), ODF2 (rabbit, 1:500, A303-546A-T; Bethyl), ODF2 (guinea pig, 1:800, gift from G. Pereira [Viol et al., 2020]), FAM161A, (rabbit, 1:500, HPA-032119; Sigma-Aldrich), GFP (mouse, 1:1,000, 11814460001; Roche), and FOP (FGFR1OP; mouse, 1:500, H00011116-M01; Novus). Secondary antibodies used for immunofluorescence are Alexa Flour Plus 488 (rabbit, 1:500, A-11008; Thermo Fisher Scientific), Alexa Flour Plus 488 (mouse, 1:500, A-11001; Thermo Fisher Scientific), Alexa Flour Plus 555 (rabbit, 1:500, A32732; Thermo Fisher Scientific), Alexa Flour Plus 555 (mouse, 1:500, A32727; Thermo Fisher Scientific), Alexa Flour Plus 647 (rabbit, 1:500, A32733; Thermo Fisher Scientific), Alexa Flour Plus 647 (mouse, 1:500, A32728; Thermo Fisher Scientific), Alexa Flour Plus 647 (guinea pig, 1:500, A-21450; Thermo Fisher Scientific), and Alexa Flour Plus 680 (rabbit, 1:500, A32734; Thermo Fisher Scientific). For expansion microscopy with Leica SP8, we also used as secondary antibodies Abberior STAR 520SXP (mouse, 1:500, ST520L-1001-500UG; Abberior), Abberior STAR 580 (guinea pig, 1:500, ST580-1006-500UG; Abberior), and Abberior STAR 635P (mouse, 1:500, ST635P-1001-500UG; Abberior), which were used according to the primary antibody. Secondary HRP conjugated antibodies used in Western blotting are HRP anti-rabbit and anti-mouse were purchased from Proteintech (1:1,000, SA00001-2, SA00001-1) and anti–guinea pig was obtained from Jackson Immunoresearch (1:1,000, 706-035-148).
Protein pull-down and Western blotting
To test protein interactions, we overexpressed 2xFlag-CEP350-GFP, 2xFlag-FOP-GFP, and 2xFlag-GFP together with 2xHA-WDR90 in HEK T293 cells. 48 h after polyethylenimine transfection, cells covering the 10-cm dish confluently were harvested, lysed, and incubated with GFP-Trap agarose beads (Chromotech). In order to lyse the cells, we used 250 mM NaCl, 10 mM Tris-Cl, 0.5 mM EDTA, 0.5% NP-40, 1 mM PMSF, 10 U/μl Benzonase, and 1 tablet per 10 ml Roche protease inhibitor cocktail complete (EDTA free), pH 7.5, with pipetting. The lysate and the beads were incubated together for 3 h by rotating at 4°C. After washing the beads with 150 mM NaCl, 10 mM Tris-Cl, pH 7.5, buffer (300 mM NaCl for WDR90 immunoblot) three times, 40 μl of 4× Laemmli buffer was added on top of the beads, and samples were incubated for 5 min 98°C while shaking. Then, the samples were run on SDS-PAGE and transferred to polyvinylidene difluoride membrane for 90 min at 4°C. Then membranes were blocked with 3% milk solution overnight incubated with indicated primary antibodies, washed with 1xTBS, and incubated with secondary antibodies for 1 h for future imaging with GE Amersham imager 600.
Statistics
Each graph corresponds to either the pooled values of independent experiments in triplicates or duplicates, or single measurements of single experiments if not specified otherwise. Each sample of the triplicates, if not specified, was considered n ≥ 80 cells (total n ≥ 240). For statistical significance tests, unpaired two-tailed student t tests or two-way ANOVA (GraphPad Prism version 9.3.1, 2021) was used to test the statistical significance between the sample conditions. Student t-tests and ANOVA significance tests are parametric by default. The data distribution of all represented data was assumed to be normal, but this was not formally tested. All error bars show SD.
Online supplemental material
Fig. S1 shows the CRISPR/Cas9 strategy to create CEP350−/− clones and verification of CEP350 loss with mRNA sequencing, immunofluorescence, and immunoblotting. Fig. S2 shows the complementation experiments to rescue the observed phenotypes of CEP350 absence and mislocalization of distal proteins. Fig. S3 verifies the phenotypes of CEP350 loss by siRNA depletion and complementation of CEP350−/− cells for rescue of Centrobin removal. It also shows how persistent Centrobin localizes on CEP350−/− centrioles. Fig. S4 provides EM micrographs for control, CEP350−/−, ODF2−/−, CEP128−/−, and NIN−/− cells to visualize subdistal appendages. It also verifies that Centrobin is removed from ODF2-carrying centrioles and how Centrobin depletion affects CEP350−/− centrioles. Fig. S5 contains the effects of ectopically expressed WDR90 and OFD1 on centriole length and stability and u-ExM images of centriole inner scaffold proteins. Table S1 is a list of used genetic material and oligos. Data S1 is an OFD1 rescue analysis by ExM.
Acknowledgments
We thank Prof. Dr. B. Tsou (Sloan Kettering, USA) for TP53−/− RPE1 and cell line and Dr. X. Li (Riken BDR, Kobe, Japan) for RPE1 NIN−/− cell line, Prof. Dr. G. Pereira (Heidelberg University, Germany) for ODF2−/− cell line, and Prof. Dr. L.B. Pedersen (Copenhagen University, Denmark) for CEP128−/− cells used in this study. We thank the Flow Cytometry & FACS Core Facility, the Imaging Facility of Zentrum für Molekulare Biologie der Universität Heidelberg, and Electron Microscopy Core Facility of Heidelberg University for their technical support.
This work was supported by a grant from the German Research Council (Deutsche Forschungsgemeinschaft; Schi295/8-1). The work of G. Pereira was financed by the collaborative research grant of the Deutsche Forschungsgemeinschaft (SFB873, Project A14).
The authors declare no competing financial interests.
Author contributions: O.R. Karasu performed most of experiments. A. Neuner performed all of the EM images and analyzed the centriole microtubule defects and centriole elongation phenotypes with O.R. Karasu. E.S. Atorino developed the retroviral integration of CEP350 domains, modified SB donor plasmid, and performed the CEP350 siRNA experiments. G. Pereira contributed to input for experiments and the manuscript. O.R. Karasu designed all the experiments with input from E. Schiebel, A. Neuner, E.S. Atorino, and G. Pereira. O.R. Karasu and E. Schiebel wrote the manuscript.