Neuronal extracellular vesicles (EVs) play important roles in intercellular communication and pathogenic protein propagation in neurological disease. However, it remains unclear how cargoes are selectively packaged into neuronal EVs. Here, we show that loss of the endosomal retromer complex leads to accumulation of EV cargoes including amyloid precursor protein (APP), synaptotagmin-4 (Syt4), and neuroglian (Nrg) at Drosophila motor neuron presynaptic terminals, resulting in increased release of these cargoes in EVs. By systematically exploring known retromer-dependent trafficking mechanisms, we show that EV regulation is separable from several previously identified roles of neuronal retromer. Conversely, mutations in rab11 and rab4, regulators of endosome-plasma membrane recycling, cause reduced EV cargo levels, and rab11 suppresses cargo accumulation in retromer mutants. Thus, EV traffic reflects a balance between Rab4/Rab11 recycling and retromer-dependent removal from EV precursor compartments. Our data shed light on previous studies implicating Rab11 and retromer in competing pathways in Alzheimer’s disease, and suggest that misregulated EV traffic may be an underlying defect.
Introduction
Transmembrane proteins are routed from the plasma membrane through endolysosomal trafficking pathways that determine whether cargoes are degraded or recycled (Naslavsky and Caplan, 2018). Neuronal function and survival depend on sorting between these pathways, and aberrant endosomal trafficking is linked to many neurodegenerative diseases, including Alzheimer’s disease (AD; Winckler et al., 2018). One possible fate for endosomal cargo is incorporation into extracellular vesicles (EVs). EVs include exosomes (derived when multivesicular bodies [MVBs] fuse with the plasma membrane, releasing their intralumenal vesicles [ILVs] from the cell) and microvesicles (which bud directly from the plasma membrane; van Niel et al., 2018). EVs contain signaling proteins, lipids, and nucleic acids, and mediate communication between cell types in the nervous system (Holm et al., 2018). Neuronal EVs also function in both intercellular propagation and clearance of pathogenic proteins, including the Aβ fragment of amyloid precursor protein (APP), a central player in AD (Arbo et al., 2020; Song et al., 2020). However, the mechanisms for EV sorting of APP and other neuronal cargoes are poorly understood, and are challenging to study in vivo at presynaptic terminals, which may be important sites of APP fragment release (Lazarov et al., 2002; Lundgren et al., 2014; Yu et al., 2018).
Retromer complex, composed of Vps35, Vps26, and Vps29, mediates endosomal cargo sorting into membrane tubules and buds (Seaman, 2021). Retromer retrieves cargoes from endosomes to the Golgi and plasma membrane, controls endosome maturation, facilitates autophagy, and influences mitochondrial fusion and function (Chen et al., 2019). The role of retromer in endosome-derived EV traffic is less clear. While depletion of retromer leads to increased APP in EVs released from HEK293T cells (Sullivan et al., 2011), others have found decreased EV levels of Wnt3A upon loss of retromer from this same cell type (Gross et al., 2012), and little is known about the role of retromer in neuronal EV traffic.
Neuronal retromer localizes to cell bodies, dendrites, and axons, and is required for proper synaptic morphology, synaptic transmission, synaptic vesicle cycling, and neurotransmitter receptor and transporter traffic (Brodin and Shupliakov, 2018). In cellular and animal models of AD, loss of retromer causes mislocalization of APP and its amyloidogenic protease BACE1, leads to increased Aβ, and exacerbates synaptic and cognitive defects (Brodin and Shupliakov, 2018). Retromer levels are reduced in the AD patient entorhinal cortex, and it has therefore been proposed as a therapeutic target (Berman et al., 2015). However, despite the importance of retromer in AD and endosome biology, and of endosome-derived EVs in neurological function and disease, it is unknown if or how neuronal retromer influences the traffic of APP and other cargoes into EVs. This will be particularly important for retromer-directed therapies, which should target specific pathological functions of retromer rather than all of its functions.
Here, we test the hypothesis that retromer is involved in EV cargo sorting using the Drosophila larval neuromuscular junction (NMJ), a powerful system for studying mechanisms of synaptic EV traffic in vivo (Koles et al., 2012).
Results
Neuronally expressed APP trafficks into EVs
To examine trafficking of APP at synapses, we used the GAL4/UAS system to express human APP-EGFP in Drosophila motor neurons (Fig. 1 A). Surprisingly, in addition to its expected presynaptic localization at the NMJ, we found neuronally expressed APP-EGFP in stationary extra-neuronal puncta (Fig. 1, B and C). Since the EGFP tag is on the intracellular C terminus of APP, these puncta do not represent the shed extracellular domain of APP, but instead are likely EVs encapsulated by neuronally derived membrane. Indeed, postsynaptic APP-EGFP puncta colocalized with α-HRP (which recognizes neuronal membrane glycoproteins; Snow et al., 1987), and with the neuronal EV markers Tsp42Ej (the Drosophila homologue of tetraspanin CD63) and Evi/Wntless (Korkut et al., 2009; Fig. 1, C and D), but not with the cytosolic presynaptic protein Complexin (Cpx; Fig. 1 B). APP-EGFP puncta were also labeled with α-Aβ antibodies, indicating that they contain membrane-spanning APP sequences (Fig. 1 D). Finally, we confirmed that postsynaptic puncta of neuronally driven APP-EGFP did not come from its leaky expression in muscles, since APP-EGFP expressed in muscle localized diffusely in the subsynaptic reticulum (SSR; Fig. S1 A).
APP localizes to neuronally derived EVs. (A) Schematic of APP-EGFP construct. AICD, APP intracellular domain. (B–D) Spinning-disk confocal images from muscle 4 NMJs expressing neuronally driven UAS-APP-EGFP and labeled with the indicated antibodies. Arrows highlight examples of colocalization. (B) (MaxIP.) Motor neuron–derived APP-EGFP localizes presynaptically (marked by the cytoplasmic neuronal protein Cpx), as well as to extraneuronal puncta. (C) (MaxIP.) Motor neuron–derived APP-EGFP colocalizes pre- and postsynaptically with the neuronal membrane marker α-HRP. (D) (Single confocal slices.) Motor neuron–derived APP-EGFP colocalization with neuronally expressed EV cargoes Tsp42Ej and Evi, and a transmembrane epitope in APP (4G8). Arrows highlight examples of colocalization. (E and F) α-GFP immunoblots of sucrose gradient fractions from S2 cell lysates. (G) Negative-stain EM of an APP CTF-containing 1.14 mg/ml fraction. Scale bars are 5 µm (B–D) or 100 nm (G).
APP localizes to neuronally derived EVs. (A) Schematic of APP-EGFP construct. AICD, APP intracellular domain. (B–D) Spinning-disk confocal images from muscle 4 NMJs expressing neuronally driven UAS-APP-EGFP and labeled with the indicated antibodies. Arrows highlight examples of colocalization. (B) (MaxIP.) Motor neuron–derived APP-EGFP localizes presynaptically (marked by the cytoplasmic neuronal protein Cpx), as well as to extraneuronal puncta. (C) (MaxIP.) Motor neuron–derived APP-EGFP colocalizes pre- and postsynaptically with the neuronal membrane marker α-HRP. (D) (Single confocal slices.) Motor neuron–derived APP-EGFP colocalization with neuronally expressed EV cargoes Tsp42Ej and Evi, and a transmembrane epitope in APP (4G8). Arrows highlight examples of colocalization. (E and F) α-GFP immunoblots of sucrose gradient fractions from S2 cell lysates. (G) Negative-stain EM of an APP CTF-containing 1.14 mg/ml fraction. Scale bars are 5 µm (B–D) or 100 nm (G).
Presynaptic APP and Appl localize to NMJ EVs. (A) Confocal images of NMJs from animals expressing muscle-driven (GAL4C57) UAS-APP-EGFP, stained with α-HRP. Arrows indicate presynaptically derived HRP-positive puncta that do not colocalize with muscle-derived APP. (B) Schematic of domain organization and mutants in Drosophila Appl. AICD, APP intracellular domain; E, extracellular; CTF, C-terminal fragment. (C) MaxIPs showing EV localization of Drosophila Appl. Yellow arrows indicate puncta that are positive for both Appl and α-HRP. White arrows indicate puncta that are solely α-HRP–positive. Cpx (presynaptic marker) is absent from Appl and α-HRP puncta. (D) MaxIP showing EV localization of Drosophila ApplSD, and absence of α-Appl C-terminal (C-term) antibody immunostaining for ApplSDΔC. Yellow arrows indicate puncta that are positive for both Appl and α-HRP. Note that image display settings for α-Appl are different between C and D in order to visualize ApplSD, which expresses at high levels. All scale bars are 5 µm. Associated with Fig. 1.
Presynaptic APP and Appl localize to NMJ EVs. (A) Confocal images of NMJs from animals expressing muscle-driven (GAL4C57) UAS-APP-EGFP, stained with α-HRP. Arrows indicate presynaptically derived HRP-positive puncta that do not colocalize with muscle-derived APP. (B) Schematic of domain organization and mutants in Drosophila Appl. AICD, APP intracellular domain; E, extracellular; CTF, C-terminal fragment. (C) MaxIPs showing EV localization of Drosophila Appl. Yellow arrows indicate puncta that are positive for both Appl and α-HRP. White arrows indicate puncta that are solely α-HRP–positive. Cpx (presynaptic marker) is absent from Appl and α-HRP puncta. (D) MaxIP showing EV localization of Drosophila ApplSD, and absence of α-Appl C-terminal (C-term) antibody immunostaining for ApplSDΔC. Yellow arrows indicate puncta that are positive for both Appl and α-HRP. Note that image display settings for α-Appl are different between C and D in order to visualize ApplSD, which expresses at high levels. All scale bars are 5 µm. Associated with Fig. 1.
We next tested if APP could sort to bona fide EVs in other cell types. We generated a stable Drosophila S2 cell line expressing APP-EGFP, fractionated cell culture supernatants, and immunoblotted the EV fraction for APP-EGFP. EVs pellet from cell culture supernatant at 100,000 × g, fractionate on gradients at 1.1–1.2 mg/ml sucrose, and exhibit a cup-shaped morphology by negative-stain EM (Théry et al., 2018). We found the APP-EGFP–containing EV fraction from S2 cell supernatants met these criteria (Fig. 1, E and F). Notably, a C-terminal fragment (CTF) of APP-EGFP was enriched in this EV fraction relative to full-length APP-EGFP, while EGFP alone did not fractionate with EVs. Indeed, S2 cells express proteases capable of processing APP family members (Luo et al., 1990), and similar CTFs of endogenous APP are enriched in EVs from cortical neurons (Laulagnier et al., 2018). Since CTFs are generated by endosomally localized proteases (Tan and Gleeson, 2019), this suggests that APP EVs are endosomally derived exosomes.
Finally, we asked if synaptic EV localization is conserved for the Drosophila APP homologue Appl, which acts as a signaling factor in neuronal development and maintenance (Cassar and Kretzschmar, 2016). At wild-type and Appl-overexpressing (but not appld null) NMJs, Appl α-C-terminal antibodies labeled α-HRP–positive extraneuronal puncta (Fig. S1, A–C). Further, cleavage-resistant Appl (ApplSD) localized to α-HRP–positive extraneuronal puncta, while ApplSD lacking the C-terminal intracellular epitope did not (Fig. S1 D), indicating that these puncta do not represent shed extracellular domain. These results suggest that EVs are a normal trajectory for synaptic APP and Appl in vivo.
Neuronal retromer restricts accumulation of EV cargoes
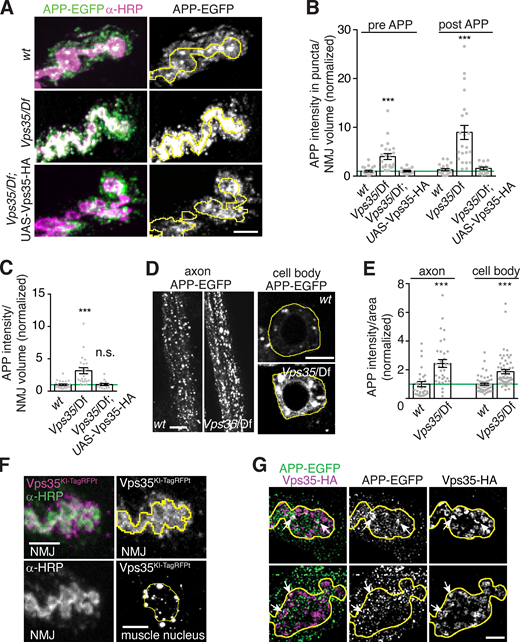
At the Drosophila NMJ, retromer regulates synaptic vesicle size and recycling, as well as synaptic growth (Inoshita et al., 2017; Korolchuk et al., 2007; Ye et al., 2020). Retromer could also be involved in EV-mediated release of APP from neurons, as had been suggested previously in HEK293T cells (Sullivan et al., 2011), but this hypothesis has not yet been tested at synapses in vivo. To address this question, we measured APP-EGFP intensity in control and Vps35 mutants, in the presynaptic neuron, and in EVs in the surrounding postsynaptic region. The mean intensity of APP-EGFP was strongly increased at Vps35 mutant NMJs, in presynaptic puncta, in the overall presynaptic region, and in EVs (Fig. 2, A–C), as well as in cell bodies and axons (Fig. 2, D and E). Since synapses are a site of EV release in these neurons (Korkut et al., 2009; Korkut et al., 2013), we focused our subsequent studies on the mechanisms by which Vps35 mutants lead to cargo accumulation and release from this site.
Neuronal retromer restricts accumulation of APP presynaptically and in EVs. (A) MaxIPs of NMJs expressing neuronally driven UAS-APP-EGFP in the indicated genotypes. Yellow line indicates presynaptic region; scale bar is 5 µm. (B) Quantification of presynaptic and postsynaptic (within 3 µm of presynaptic membrane) APP-EGFP in thresholded puncta. (C) Quantification of overall (unthresholded) APP-EGFP in the presynaptic volume. (D) Single confocal slices of APP-EGFP in cell bodies in the ventral ganglion (right, outlined in yellow) and axons (left). Scale bars are 20 µm. (E) Quantification of D. (F) Endogenously tagged Vps35-TagRFPT shows both pre- and postsynaptic localization at the NMJ (MaxIP, presynaptic terminal marked with yellow line) as well as in perinuclear puncta in the muscle (MaxIP, nucleus marked with yellow line). Scale bar is 5 µm for NMJ and 10 µm for muscle. (G) Partial colocalization (arrows) of APP-EGFP and Vps35-HA in MaxIPs of SIM images; presynaptic terminal marked with yellow line; scale bar is 2 µm. Bar graphs show mean ± SEM; n represents NMJs. See Table S3 for genotypes and statistical tests. ***, P < 0.001.
Neuronal retromer restricts accumulation of APP presynaptically and in EVs. (A) MaxIPs of NMJs expressing neuronally driven UAS-APP-EGFP in the indicated genotypes. Yellow line indicates presynaptic region; scale bar is 5 µm. (B) Quantification of presynaptic and postsynaptic (within 3 µm of presynaptic membrane) APP-EGFP in thresholded puncta. (C) Quantification of overall (unthresholded) APP-EGFP in the presynaptic volume. (D) Single confocal slices of APP-EGFP in cell bodies in the ventral ganglion (right, outlined in yellow) and axons (left). Scale bars are 20 µm. (E) Quantification of D. (F) Endogenously tagged Vps35-TagRFPT shows both pre- and postsynaptic localization at the NMJ (MaxIP, presynaptic terminal marked with yellow line) as well as in perinuclear puncta in the muscle (MaxIP, nucleus marked with yellow line). Scale bar is 5 µm for NMJ and 10 µm for muscle. (G) Partial colocalization (arrows) of APP-EGFP and Vps35-HA in MaxIPs of SIM images; presynaptic terminal marked with yellow line; scale bar is 2 µm. Bar graphs show mean ± SEM; n represents NMJs. See Table S3 for genotypes and statistical tests. ***, P < 0.001.
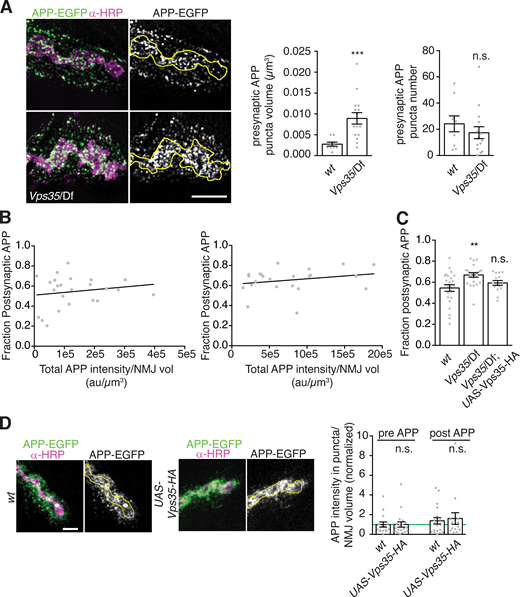
Using structured illumination microscopy (SIM), we found that increased presynaptic APP-EGFP levels manifested in larger and more intense puncta, rather than greater density of puncta (Fig. S2 A). Importantly, the overall increase in APP levels was insufficient to explain higher postsynaptic APP EV abundance: At both control and Vps35 mutant NMJs, the fraction of postsynaptic APP did not correlate strongly with total (i.e., presynaptic plus postsynaptic) APP levels (Fig. S2 B). Further, the ratio of postsynaptic to total APP was significantly increased in Vps35 mutants (Fig. S2 C). This suggests that the EV phenotype of Vps35 mutants is mediated by a specific EV-regulatory pathway rather than simply by increased APP levels.
Neuronal retromer and APP trafficking.(A) APP-containing compartments are larger in Vps35 mutants. MaxIPs and quantification of representative SIM images of NMJs expressing APP-EGFP in the indicated genotypes. Yellow line indicates presynaptic region; scale bar is 2 µm. (B) Dataset from Fig. 2, B and C; the fraction of postsynaptic APP-EGFP versus total APP-EGFP at Vps35 mutant NMJs is not strongly correlated. (C) Dataset from Fig. 2, B and C; the fraction of APP-EGFP that is postsynaptic slightly increases in Vps35 NMJs. (D) Controls for Vps35-HA rescue. Neuronal UAS-Vps35-HA expression does not affect levels of APP-EGFP in a wild-type background. Scale bar is 5 µm. Measurements were normalized to presynaptic mean of control (green line). Yellow lines indicate presynaptic region; bar graphs show mean ± SEM; dots show all data points representing individual NMJs. See Table S3 for detailed genotypes and statistical tests. Associated with Fig. 2. **, P < 0.01; ***, P < 0.001.
Neuronal retromer and APP trafficking.(A) APP-containing compartments are larger in Vps35 mutants. MaxIPs and quantification of representative SIM images of NMJs expressing APP-EGFP in the indicated genotypes. Yellow line indicates presynaptic region; scale bar is 2 µm. (B) Dataset from Fig. 2, B and C; the fraction of postsynaptic APP-EGFP versus total APP-EGFP at Vps35 mutant NMJs is not strongly correlated. (C) Dataset from Fig. 2, B and C; the fraction of APP-EGFP that is postsynaptic slightly increases in Vps35 NMJs. (D) Controls for Vps35-HA rescue. Neuronal UAS-Vps35-HA expression does not affect levels of APP-EGFP in a wild-type background. Scale bar is 5 µm. Measurements were normalized to presynaptic mean of control (green line). Yellow lines indicate presynaptic region; bar graphs show mean ± SEM; dots show all data points representing individual NMJs. See Table S3 for detailed genotypes and statistical tests. Associated with Fig. 2. **, P < 0.01; ***, P < 0.001.
Retromer is ubiquitously expressed, and both neuronal and muscle retromer contribute to Drosophila synaptic morphology and transmission (Inoshita et al., 2017; Korolchuk et al., 2007). Indeed, endogenously tagged Vps35-TagRFPt (Koles et al., 2016) localizes to both neurons and muscles (Fig. 2 F). Presynaptic Vps35-TagRFPt was faint and difficult to resolve from postsynaptic structures, so to further explore its localization by SIM, we expressed Drosophila Vps35-HA (Vps35-HA) in neurons using GAL4-UAS (Fig. 2 G). Vps35-HA accumulated at the periphery of endosome-like structures and colocalized with a subset of APP-EGFP puncta. We then tested the cell autonomy of retromer’s EV function. Neuronal restoration of Vps35-HA in the Vps35 mutant background was sufficient to rescue both presynaptic and postsynaptic APP-EGFP to wild-type levels (Fig. 2, A–C). By contrast, Vps35-HA expression in wild-type neurons had no effect on APP levels, indicating that rescue in the mutant was not confounded by Vps35 overexpression (Fig. S2 D). Therefore, presynaptic Vps35 is sufficient to restrict cargo trafficking into EVs and to prevent EV cargo accumulation.
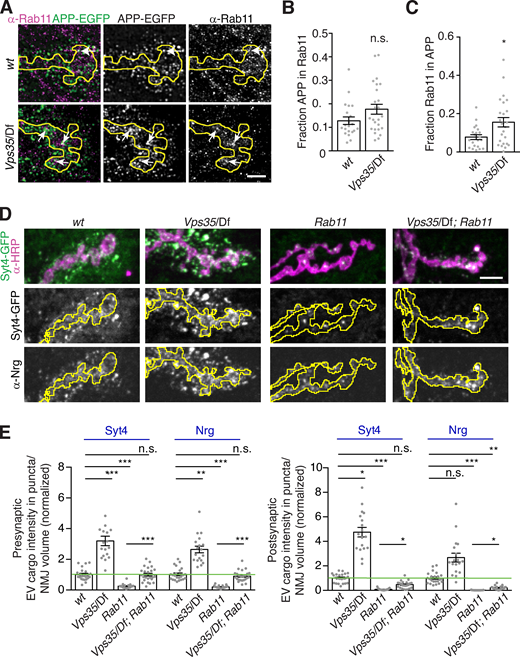
To determine the cargo specificity of retromer-dependent EV accumulation, we compared the effect of retromer loss on synaptotagmin-4 (Syt4, an established EV cargo; Korkut et al., 2013), Neuroglian (Nrg, which we confirmed is an EV cargo as previously observed; Enneking et al., 2013; Fig. S3, A–D), and the bone morphogenetic protein receptor Thickveins (Tkv, which localizes to the presynaptic plasma membrane and endosomes and is not normally found in EVs; Deshpande et al., 2016). Endogenously tagged Syt4-EGFP exhibited increased pre- and postsynaptic accumulation in Vps35 mutants, as well as in mutants of Vps26, another retromer component (Fig. 3 A). Endogenous Nrg levels were similarly increased in Vps26 mutants, indicating that this phenotype was not a function of the GFP tag (Fig. 3 B). Surprisingly, we observed both presynaptic and postsynaptic punctate accumulation of Tkv-mCherry in Vps35 mutants (Fig. 3 C). Thus, retromer loss leads to the general accumulation of multiple EV and endosomal cargoes and aberrant inclusion of non-EV cargoes into EVs. By contrast, another neuronal transmembrane cargo, Synaptotagmin-1 (Syt1, which does not undergo extensive endosomal trafficking), did not accumulate presynaptically and was not detected in EVs in Vps35 mutants (Fig. 3 D), indicating that retromer may specifically sequester endosomally enriched transmembrane cargoes from the EV pathway.
Characterization of Nrg as an EV cargo. Nrg is a cell adhesion molecule for which an excellent monoclonal antibody exists. Previous reports indicate an EV-like localization for Nrg (Enneking et al., 2013), which we further validate here to enable its use as a convenient EV marker. (A) Schematic of domain organization, RNAi target, and antibody binding site for Drosophila Nrg. FN III, fibronectin type III. (B) MaxIPs of Airyscan confocal stacks showing EV localization of Nrg. Yellow arrows indicate puncta that are positive for both α-Nrg and α-HRP signals, suggesting that postsynaptic Nrg is in EVs surrounded by neuronally derived membrane; white line outlines the α-HRP–labeled presynaptic terminal. Scale bar is 5 µm. (C) Presynaptic knockdown of Nrg depletes presynaptic and postsynaptic signal, indicating that all NMJ Nrg is derived from the neuron and released in EVs. (D) Postsynaptic knockdown of Nrg causes a mild presynaptic reduction, but no change to postsynaptic signal (as previously observed; Enneking et al., 2013). MaxIPs of representative SDCM images (left) and quantification (right), normalized to mean intensity of wild-type control. Scale bars in C and D are 10 µm. All bar graphs show mean ± SEM; dots show all data points representing individual NMJs. See Table S3 for detailed genotypes and statistical tests. Associated with Fig. 3. **, P < 0.01; ***, P < 0.001.
Characterization of Nrg as an EV cargo. Nrg is a cell adhesion molecule for which an excellent monoclonal antibody exists. Previous reports indicate an EV-like localization for Nrg (Enneking et al., 2013), which we further validate here to enable its use as a convenient EV marker. (A) Schematic of domain organization, RNAi target, and antibody binding site for Drosophila Nrg. FN III, fibronectin type III. (B) MaxIPs of Airyscan confocal stacks showing EV localization of Nrg. Yellow arrows indicate puncta that are positive for both α-Nrg and α-HRP signals, suggesting that postsynaptic Nrg is in EVs surrounded by neuronally derived membrane; white line outlines the α-HRP–labeled presynaptic terminal. Scale bar is 5 µm. (C) Presynaptic knockdown of Nrg depletes presynaptic and postsynaptic signal, indicating that all NMJ Nrg is derived from the neuron and released in EVs. (D) Postsynaptic knockdown of Nrg causes a mild presynaptic reduction, but no change to postsynaptic signal (as previously observed; Enneking et al., 2013). MaxIPs of representative SDCM images (left) and quantification (right), normalized to mean intensity of wild-type control. Scale bars in C and D are 10 µm. All bar graphs show mean ± SEM; dots show all data points representing individual NMJs. See Table S3 for detailed genotypes and statistical tests. Associated with Fig. 3. **, P < 0.01; ***, P < 0.001.
Retromer mutants exhibit synaptic accumulation of multiple EV and endosomally sorted cargoes.(A–D) Representative MaxIPs and quantification of EV cargoes Syt4-EGFP and Nrg (A and B) and non-EV cargoes Tkv-mCherry and Syt1 (C and D) in control and Vps35 mutants. Bar graphs show mean ± SEM; n represents NMJs. Scale bars are 5 µm. All measurements were normalized to presynaptic mean of their respective control (green line). See Table S3 for genotypes and statistical tests. ***, P < 0.001.
Retromer mutants exhibit synaptic accumulation of multiple EV and endosomally sorted cargoes.(A–D) Representative MaxIPs and quantification of EV cargoes Syt4-EGFP and Nrg (A and B) and non-EV cargoes Tkv-mCherry and Syt1 (C and D) in control and Vps35 mutants. Bar graphs show mean ± SEM; n represents NMJs. Scale bars are 5 µm. All measurements were normalized to presynaptic mean of their respective control (green line). See Table S3 for genotypes and statistical tests. ***, P < 0.001.
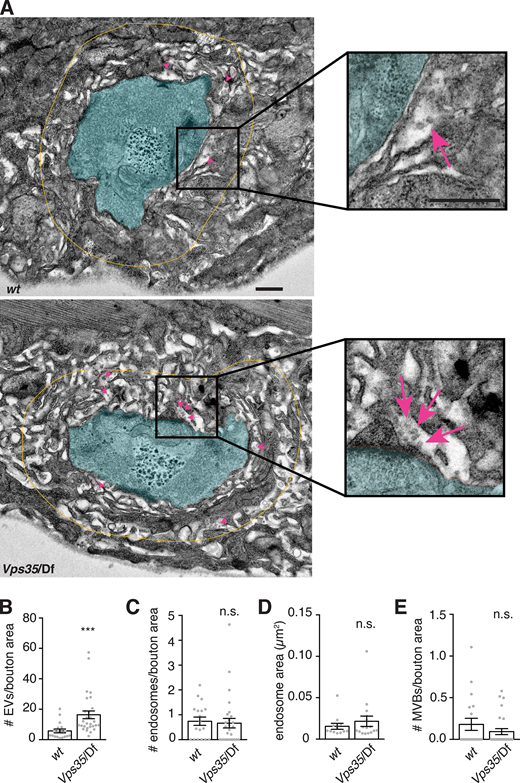
Vps35 mutants may either have more EVs or else may package more cargo into the same number of EVs. To distinguish between these possibilities, we performed EM on control and Vps35 mutant NMJs. We noted a significant increase in postsynaptic 50–100 nm vesicles (consistent with the expected size for endosomally derived exosomes) in the muscle SSR immediately surrounding the neuron (Fig. 4, A and B), suggesting that retromer mutant NMJs release more EVs. However, we did not observe a significant increase in presynaptic MVBs (defined as structures with ILVs) or in the number or size of endosomes (defined as structures >100 nm in diameter, to distinguish them from the enlarged ∼70 nm synaptic vesicles in Vps35 mutants; Inoshita et al., 2017; Fig. 4, C–E). This result suggests that altered kinetics of MVB maturation or fusion with the plasma membrane (which would lead to MVB accumulation) is not a primary phenotype in Vps35 mutants, or that this step may not be rate limiting for synaptic cargo and EV accumulation.
Retromer mutants exhibit more EV-sized vesicles in the postsynaptic cleft, but do not exhibit changes in neuronal endosome profiles. (A) Transmission EM of thin sections showing muscle 6/7. Boutons are pseudocolored in cyan; yellow line indicates the perimeter of the region <1 µm from bouton plasma membrane, in which 40–100-nm vesicular structures were counted in the extracellular space. Magenta arrows indicate several examples of such EV-sized structures. Scale bar is 500 nm. (B)Vps35 mutants exhibit a greater number of 50–100-nm EV-sized vesicles in the postsynaptic cleft. (C and D) However, neither the number (C) nor size (D) of presynaptic endosomes (>100-nm vesicles) increases in a Vps35 mutant. (E) Presynaptic MVBs are rare at both control and Vps35 mutant synapses. Bar graphs show mean ± SEM; each data point represents measurements derived from a single bouton. See Table S3 for genotypes and statistical tests. ***, P < 0.001.
Retromer mutants exhibit more EV-sized vesicles in the postsynaptic cleft, but do not exhibit changes in neuronal endosome profiles. (A) Transmission EM of thin sections showing muscle 6/7. Boutons are pseudocolored in cyan; yellow line indicates the perimeter of the region <1 µm from bouton plasma membrane, in which 40–100-nm vesicular structures were counted in the extracellular space. Magenta arrows indicate several examples of such EV-sized structures. Scale bar is 500 nm. (B)Vps35 mutants exhibit a greater number of 50–100-nm EV-sized vesicles in the postsynaptic cleft. (C and D) However, neither the number (C) nor size (D) of presynaptic endosomes (>100-nm vesicles) increases in a Vps35 mutant. (E) Presynaptic MVBs are rare at both control and Vps35 mutant synapses. Bar graphs show mean ± SEM; each data point represents measurements derived from a single bouton. See Table S3 for genotypes and statistical tests. ***, P < 0.001.
Retromer has separable functions in synaptic growth and EV traffic
One shared function of Appl and Vps35 is to regulate the growth of synaptic arbors during larval development (Cassar and Kretzschmar, 2016; Korolchuk et al., 2007; Torroja et al., 1999; Fig. 5, A and B). We considered the possibility that synaptic overgrowth in Vps35 mutants is caused by accumulation of synaptic growth-promoting EV cargoes. To challenge this hypothesis, we asked if we could separate the functions of retromer in EV cargo traffic and synaptic growth by genetically manipulating retromer accessory factors. Retromer acts directly or indirectly with distinct combinations of sorting nexins (SNXs) to direct specific trafficking events (Seaman, 2021). We measured synaptic growth in null mutants of Snx27, Snx3, and double mutants lacking endosomal SNX–BAR sorting complex for promoting exit 1 (ESCPE-1) complex components Snx1 and Snx6 (Strutt et al., 2019; Zhang et al., 2011). We found that Snx3 mutants showed significant synaptic overgrowth, while Snx27 single and Snx1, Snx6 double mutants did not (Fig. 5, B and C). Retromer also interacts with the actin-regulating Wiskott-Aldrich Syndrome Protein and SCAR Homolog complex (Follett et al., 2014; McGough et al., 2014; Zavodszky et al., 2014), and a Parkinson’s disease–associated mutant of human Vps35 (Vps35D620N) disrupts this association (Harbour et al., 2010; Jia et al., 2012; Seaman et al., 2013). Similar to previous findings (Inoshita et al., 2017; Malik et al., 2015), we found that knock-in of the analogous mutation (D628N) at the Drosophila Vps35 locus (Koles et al., 2016) phenocopied the null mutant synaptic overgrowth (Fig. 5, A and B). Thus, SNX3 and Wiskott-Aldrich Syndrome Protein and SCAR Homolog–associated functions of retromer are required to constrain synaptic growth, while SNX27 and SNX1/6-associated functions are not.
Control of synaptic growth depends on specific retromer-associated pathways.Vps35 null, Snx3, and Vps35D628N mutations cause an increase in bouton number at the NMJ, while mutations in retromer-associated Snx1, Snx6, and Snx27 have no effect. (A) MaxIPs of α-HRP–labeled muscle 4 NMJs in the indicated genotypes. Scale bar is 20 µm. (B) Quantification of bouton number for muscle 4, segments A2 and A3. (C) MaxIPs of α-HRP–labeled muscle 4 NMJs in the indicated genotypes. Scale is identical to A. See Table S3 for detailed genotypes and statistical tests. ***, P < 0.001.
Control of synaptic growth depends on specific retromer-associated pathways.Vps35 null, Snx3, and Vps35D628N mutations cause an increase in bouton number at the NMJ, while mutations in retromer-associated Snx1, Snx6, and Snx27 have no effect. (A) MaxIPs of α-HRP–labeled muscle 4 NMJs in the indicated genotypes. Scale bar is 20 µm. (B) Quantification of bouton number for muscle 4, segments A2 and A3. (C) MaxIPs of α-HRP–labeled muscle 4 NMJs in the indicated genotypes. Scale is identical to A. See Table S3 for detailed genotypes and statistical tests. ***, P < 0.001.
We asked if these separable functions of Vps35 in synaptic growth correlated with its EV cargo phenotypes. Remarkably, Snx27 and Vps35D628N mutants did not phenocopy the increased EV cargo levels of Vps35 null mutants, while Snx1/6 double mutants recapitulated the accumulation of presynaptic and postsynaptic APP-EGFP, though to a lesser extent than Vps35 (Fig. 6, A–C). These results demonstrate that synaptic growth and EV trafficking are distinct, uncorrelated functions of retromer that can be genetically uncoupled, and that rely on separate components of the Vps35-associated machinery.
Control of EV Cargo levels depends on the Snx1/Snx6 ESCPE-1 complex but not Vps35D628N or Snx27. (A–C) Representative MaxIPs and quantification of APP-EGFP levels at muscle 6/7 NMJs in the indicated genotypes. Each condition is normalized to the mean intensity of wild-type control (green line). Bar graphs show mean ± SEM; dots show all data points representing individual NMJs. Yellow line indicates presynaptic region; scale bars are 5 µm. See Table S3 for detailed genotypes and statistical tests. *, P < 0.05; **, P < 0.01.
Control of EV Cargo levels depends on the Snx1/Snx6 ESCPE-1 complex but not Vps35D628N or Snx27. (A–C) Representative MaxIPs and quantification of APP-EGFP levels at muscle 6/7 NMJs in the indicated genotypes. Each condition is normalized to the mean intensity of wild-type control (green line). Bar graphs show mean ± SEM; dots show all data points representing individual NMJs. Yellow line indicates presynaptic region; scale bars are 5 µm. See Table S3 for detailed genotypes and statistical tests. *, P < 0.05; **, P < 0.01.
Lysosome dysfunction does not recapitulate Vps35 EV phenotypes
We next investigated the mechanisms by which loss of retromer results in increased levels of EV cargo. Since retromer is required to maintain lysosomal hydrolase activity (Arighi et al., 2004; Cui et al., 2019; Seaman et al., 1998), general lysosomal dysfunction in retromer mutants might lead to the accumulation and release of material that would otherwise be targeted for degradation (Bécot et al., 2020). We tested this by examining the lysosomal marker Spinster (Spin; Dermaut et al., 2005; Sweeney and Davis, 2002). Spin-GFP intensity was significantly reduced at Vps35 mutant NMJs (Fig. S4, A and B), arguing against lysosomal expansion, and consistent with the absence of enlarged lysosomal profiles in our EM data. We next asked if lysosomes were functionally impaired in Vps35 mutants by measuring the levels of Atg8-GFP, a marker of autophagosomes, which accumulate during lysosomal dysfunction. Instead, we observed a significant decrease in Atg8-GFP intensity in Vps35 NMJs (Fig. S4, A and B). Finally, staining with LysoTracker Deep Red, which labels acidic compartments, was reduced in Vps35 mutant muscle (Fig. S4 C), and undetectable in control or Vps35 mutant presynaptic terminals, again suggesting that acidic compartments are not amplified at Vps35 mutant NMJs. These results contrast with previous findings of increased LysoTracker and Atg8 fluorescence in Drosophila Vps35 mutant fat body cells (Maruzs et al., 2015), and suggest that unlike in other tissues, Vps35 mutant synapses do not accumulate acidified endolysosomes.
Lysosome dysfunction does not cause retromer-dependent EV cargo sorting defects. (A–C) Retromer loss does not cause accumulation of lysosome-like compartments. (A) MaxIPs of NMJs from larvae expressing neuronally driven Spin-GFP or ATG8-GFP. Yellow line outlines α-HRP–labeled presynaptic terminal. (B) Quantification of A, normalized to mean intensity of wild-type control (green line). (C) MaxIPs of NMJs and the muscle perinuclear region labeled with LysoTracker Deep Red. Muscles exhibit a decrease in LysoTracker intensity indicating a reduction in acidified lysosomal compartments. Yellow line outlines muscle nucleus. (D and E) Mutants that disrupt lysosome function do not exhibit EV cargo accumulation or increased postsynaptic EV cargo. (D) MaxIPs of NMJs from larvae expressing endogenously tagged Syt4-EGFP. Yellow line outlines α-HRP–labeled presynaptic terminal. (E) Quantification of D. (F and G) Pharmacological inhibition of lysosome function does not affect EV cargo levels. (F) MaxIPs of NMJs from larvae expressing endogenously tagged Syt4-EGFP and treated with the indicated concentration of chloroquine (CQ). Yellow line outlines α-HRP–labeled presynaptic terminal. (G) Quantification of F. All conditions normalized to control. Red line indicates mean Syt4-EGFP levels in Vps35 mutants from Fig. 3. Yellow lines indicate presynaptic regions; scale bars are 5 µm. Bar graphs show mean ± SEM; dots show all data points representing individual NMJs. See Table S3 for detailed genotypes and statistical tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Lysosome dysfunction does not cause retromer-dependent EV cargo sorting defects. (A–C) Retromer loss does not cause accumulation of lysosome-like compartments. (A) MaxIPs of NMJs from larvae expressing neuronally driven Spin-GFP or ATG8-GFP. Yellow line outlines α-HRP–labeled presynaptic terminal. (B) Quantification of A, normalized to mean intensity of wild-type control (green line). (C) MaxIPs of NMJs and the muscle perinuclear region labeled with LysoTracker Deep Red. Muscles exhibit a decrease in LysoTracker intensity indicating a reduction in acidified lysosomal compartments. Yellow line outlines muscle nucleus. (D and E) Mutants that disrupt lysosome function do not exhibit EV cargo accumulation or increased postsynaptic EV cargo. (D) MaxIPs of NMJs from larvae expressing endogenously tagged Syt4-EGFP. Yellow line outlines α-HRP–labeled presynaptic terminal. (E) Quantification of D. (F and G) Pharmacological inhibition of lysosome function does not affect EV cargo levels. (F) MaxIPs of NMJs from larvae expressing endogenously tagged Syt4-EGFP and treated with the indicated concentration of chloroquine (CQ). Yellow line outlines α-HRP–labeled presynaptic terminal. (G) Quantification of F. All conditions normalized to control. Red line indicates mean Syt4-EGFP levels in Vps35 mutants from Fig. 3. Yellow lines indicate presynaptic regions; scale bars are 5 µm. Bar graphs show mean ± SEM; dots show all data points representing individual NMJs. See Table S3 for detailed genotypes and statistical tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We then asked if disruption of lysosomal function affected EV cargo traffic similarly to the Vps35 mutant. We measured pre- and postsynaptic Syt4-EGFP levels in mutants of spin and deep orange (dor/Vps18), which are both required for lysosome function at the NMJ (Dermaut et al., 2005; Fernandes et al., 2014; Narayanan et al., 2000; Sweeney and Davis, 2002). In contrast to elevated Syt4-EGFP levels in Vps35 mutant NMJs, pre- and postsynaptic Syt4-EGFP were significantly reduced in spin and dor mutant NMJs compared with controls (Fig. S4, D and E). Finally, we pharmacologically disrupted lysosome function with chloroquine, which blocks lysosome acidification (Gonzalez-Noriega et al., 1980). Chloroquine treatment caused a decrease in larval viability as well as enlarged presynaptic Syt4-EGFP puncta, indicating lysosome dysfunction. However, Syt4-EGFP levels did not change either pre- or postsynaptically (Fig. S4, F and G). Taken together, these results suggest that defects in the lysosomal pathway do not promote EV cargo accumulation in Vps35 mutants.
A Rab11-dependent recycling pathway maintains EV cargo levels
Since generalized endolysosomal dysfunction is unlikely to account for Vps35 EV phenotypes, we next asked whether and how specific subtypes of endosomes were altered in Vps35 synapses. We examined the localization and levels of Rab GTPases implicated in retromer function, using antibodies or tags knocked in to their endogenous loci (Fig. 7 A). GFP-Rab5, which is associated with early endosomes, showed a decrease in mean puncta intensity and number (but not size) in Vps35 mutant NMJs compared with controls (Fig. 7, B–D). By contrast, YFP-Rab7, which is associated with late endosomes, showed an increase in intensity, puncta number, and puncta size in both Vps35 (Fig. 7, B–D) and Vps26 (Fig. S5 A) mutants. This indicates an amplification of late endosomes, consistent with the role of retromer in attenuating Rab7 activity (Jimenez-Orgaz et al., 2018; Kvainickas et al., 2019; Seaman et al., 2018; Ye et al., 2020). Levels of α-Rab11 immunostaining, marking the recycling endosome and post-Golgi secretory vesicles, remained unchanged, but Rab11 structures increased in size (but not number) in Vps35 (Fig. 7, A, B, and E–G) and Vps26 (Fig. S5 B) mutants. Thus, one or several of these endosomal changes might underlie retromer-dependent EV trafficking phenotypes and define the site at which EV cargo fate is determined.
Endosome distribution in Vps35 mutants.(A) MaxIPs of confocal images of NMJs from larvae expressing endogenously tagged YFP-Rab7 or GFP-Rab5, or labeled with α-Rab11 antibodies, in control or Vps35/Df genotypes. (B) Quantification of fluorescence intensity from spinning-disk confocal microscopy (SDCM), normalized to mean intensity of wild-type control (green line). (C) Rab5 and Rab7-positive puncta density by SDCM. (D) Rab5 and Rab7-positive puncta size by SDCM. (E) SIM MaxIPs of NMJs from control or Vps35/Df genotypes, labeled with α-Rab11 antibodies. Rab11 puncta were too dense to distinguish by SDCM, so we used SIM to analyze individual endosomes. (F and G) Quantification of puncta density and size from E. Scale bars are 5 µm for SDCM, 2 µm for SIM. Presynaptic terminal marked with yellow line; bar graphs show mean ± SEM; dots show all data points representing individual NMJs. See Table S3 for genotypes and statistical tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Endosome distribution in Vps35 mutants.(A) MaxIPs of confocal images of NMJs from larvae expressing endogenously tagged YFP-Rab7 or GFP-Rab5, or labeled with α-Rab11 antibodies, in control or Vps35/Df genotypes. (B) Quantification of fluorescence intensity from spinning-disk confocal microscopy (SDCM), normalized to mean intensity of wild-type control (green line). (C) Rab5 and Rab7-positive puncta density by SDCM. (D) Rab5 and Rab7-positive puncta size by SDCM. (E) SIM MaxIPs of NMJs from control or Vps35/Df genotypes, labeled with α-Rab11 antibodies. Rab11 puncta were too dense to distinguish by SDCM, so we used SIM to analyze individual endosomes. (F and G) Quantification of puncta density and size from E. Scale bars are 5 µm for SDCM, 2 µm for SIM. Presynaptic terminal marked with yellow line; bar graphs show mean ± SEM; dots show all data points representing individual NMJs. See Table S3 for genotypes and statistical tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Endosome distribution in Vps26 and Snx1, Snx6 mutants.(A) Airyscan microscopy MaxIPs and quantification of presynaptic (endogenously tagged) YFP-Rab7 intensity, and endosome number and size in control or Vps26 mutant backgrounds. (B) Airyscan microscopy MaxIPs and quantification of presynaptic α-Rab11 intensity, and endosome number and size in control or Vps26 mutant backgrounds. (C) SDCM MaxIPs and quantification of presynaptic (endogenously tagged) YFP-Rab7 intensity, and endosome number and size in control, Vps35, or Snx1, Snx6 mutant backgrounds. Yellow lines indicate presynaptic regions; scale bars are 5 µm. Bar graphs show mean ± SEM; dots show all data points representing individual NMJs. See Table S3 for genotypes and statistical tests. Associated with Fig. 7. *, P < 0.05; ***, P < 0.001.
Endosome distribution in Vps26 and Snx1, Snx6 mutants.(A) Airyscan microscopy MaxIPs and quantification of presynaptic (endogenously tagged) YFP-Rab7 intensity, and endosome number and size in control or Vps26 mutant backgrounds. (B) Airyscan microscopy MaxIPs and quantification of presynaptic α-Rab11 intensity, and endosome number and size in control or Vps26 mutant backgrounds. (C) SDCM MaxIPs and quantification of presynaptic (endogenously tagged) YFP-Rab7 intensity, and endosome number and size in control, Vps35, or Snx1, Snx6 mutant backgrounds. Yellow lines indicate presynaptic regions; scale bars are 5 µm. Bar graphs show mean ± SEM; dots show all data points representing individual NMJs. See Table S3 for genotypes and statistical tests. Associated with Fig. 7. *, P < 0.05; ***, P < 0.001.
We then separately perturbed the function of each type of endosome using neuron-specific expression of constitutively active (CA) or dominant negative (DN) forms of their resident Rab GTPases and measured pre- and postsynaptic levels of Syt4-EGFP (Fig. 8, A–D). Overexpression of Rab5CA, which promotes homotypic fusion but blocks conversion to the Rab7-positive state (Rink et al., 2005; Stenmark et al., 1994), did not change presynaptic Syt4 levels, although Syt4 was redistributed into aberrant bright puncta. Notably, we observed decreased EVs in this condition. This suggests that trapping cargo in early endosomes inhibits EV release, and that retromer sorts cargo away from the EV pathway downstream of Rab5. Overexpression of Rab5DN blocks homotypic endosome fusion and impairs early endosome maturation (Stenmark et al., 1994), so we used this manipulation to test if the decreased Rab5 levels that we observed in Vps35 mutants could account for increased EV cargo levels. Indeed, we saw a twofold increase in presynaptic Syt4 levels, similar to Vps35 mutants. However, unlike Vps35 mutants, postsynaptic Syt4 was unaffected (Fig. 8, A and C). Thus, though inhibiting Rab5 function causes accumulation of EV cargoes in the presynaptic neuron, it does not affect release of these cargoes in EVs. This supports our previous conclusion that accumulation of cargo alone is not sufficient to drive EV sorting (Fig. S2 B), and further suggests that reduction in Rab5 levels in Vps35 mutants is unlikely to account for EV cargo sorting phenotypes.
EV cargo accumulation depends on Rab11 and Rab4 activity.(A and B) MaxIPs and quantification of NMJs from larvae expressing endogenously tagged Syt4-EGFP with neuronally driven (GAL4C380) Rab5, Rab7, and Rab11 transgenes. (C and D) Quantification of A and B. (E) MaxIPs and quantification of NMJs expressing APP-EGFP in control or Rab11 mutant backgrounds. Control dataset is identical to that in Fig. 2 B. Yellow line indicates presynaptic region. (F) MaxIPs and quantification of NMJs from larvae with neuronal GAL4Vglut-driven YFP-Rab11 and YFP-Rab4 transgenes. All bar graphs show mean ± SEM; independent experiments are shown separately, each normalized to the mean presynaptic intensity of their respective wild-type controls (green line). Dots show all data points representing individual NMJs. Scale bars are 5 µm. See Table S3 for detailed genotypes and statistical tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
EV cargo accumulation depends on Rab11 and Rab4 activity.(A and B) MaxIPs and quantification of NMJs from larvae expressing endogenously tagged Syt4-EGFP with neuronally driven (GAL4C380) Rab5, Rab7, and Rab11 transgenes. (C and D) Quantification of A and B. (E) MaxIPs and quantification of NMJs expressing APP-EGFP in control or Rab11 mutant backgrounds. Control dataset is identical to that in Fig. 2 B. Yellow line indicates presynaptic region. (F) MaxIPs and quantification of NMJs from larvae with neuronal GAL4Vglut-driven YFP-Rab11 and YFP-Rab4 transgenes. All bar graphs show mean ± SEM; independent experiments are shown separately, each normalized to the mean presynaptic intensity of their respective wild-type controls (green line). Dots show all data points representing individual NMJs. Scale bars are 5 µm. See Table S3 for detailed genotypes and statistical tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Manipulations of Rab7 had a milder influence on Syt4 trafficking compared with Rab5. Overexpression of Rab7CA caused a modest decrease in EV Syt4 (Fig. 8, A and C). Rab7CA increases the rate by which the late endosome merges with the lysosome (Méresse et al., 1995), so this phenotype may reflect accelerated degradation of EV cargoes. Rab7DN overexpression, which inhibits recruitment of Vps35 to late endosomes (Rojas et al., 2008; Seaman et al., 2009) and prevents the Rab5-to-Rab7 switch that is a hallmark of endosome maturation (Rink et al., 2005), had no effect on pre- or postsynaptic Syt4 levels (Fig. 8, A and C). Finally, we examined the Rab7 compartment in Snx1, Snx6 ESCPE-1 double mutants, which exhibit increased EV cargo levels. Rab7-positive endosome intensity, size, and number were not significantly different from wild-type in ESCPE-1 mutants (Fig. S5 C). Together, these data argue that the accumulation of Rab7 and Rab7-associated compartments in Vps35 mutant is unlikely to be necessary for the EV cargo trafficking defect, and that synaptic EV cargo sorting does not depend on recruitment of Vps35 to late endosomes by Rab7.
Next, we examined the effects of manipulating Rab11. DN and loss-of-function alleles of Rab11 block recycling of endosome- and Golgi-derived carriers to the plasma membrane (Ullrich et al., 1996) and reduce EV levels of the transferrin receptor (TfR), Evi, Syt4, and Arc (Ashley et al., 2018; Beckett et al., 2013; Koles et al., 2012; Korkut et al., 2013; Savina et al., 2002). This led to the hypothesis that Rab11 is involved in MVB fusion with the plasma membrane, and the prediction that EV cargoes should accumulate presynaptically in Rab11 mutants, though this has not yet been directly tested. Surprisingly, opposite to this prediction, we found decreased pre- and postsynaptic Syt4-EGFP upon Rab11DN expression (Fig. 8, B and D), as well as APP-EGFP in a hypomorphic rab11 loss-of-function allelic combination (Fig. 8 E). By contrast, we previously found that Rab11DN has no effect on presynaptic levels of the non-EV cargo Tkv, indicating that this depletion is specific to EV cargo (Deshpande et al., 2016). Constitutively active Rab11 (Rab11CA) is predicted to enlarge the recycling endosome and also disrupts recycling activity (Ullrich et al., 1996; Wilcke et al., 2000). We found that neuronal expression of Rab11CA dramatically altered neuronal morphology including reduced α-HRP staining, synaptic bouton size, and overall arbor size (as previously described; Akbergenova and Littleton, 2017). With the caveat that this extreme phenotype likely causes pleiotropic defects in membrane traffic, Syt4-EGFP levels were also reduced both presynaptically and postsynaptically in Rab11CA-expressing animals. Finally, we examined the “fast recycling” pathway mediated by Rab4 (D’Souza et al., 2014). Rab4CA and Rab4DN both caused significant reductions in Nrg levels both pre- and postsynaptically, similar to Rab11 (Fig. 8 F), indicating that Rab4 and Rab11 pathways may act together or in parallel. Since multiple recycling pathway manipulations caused EV phenotypes opposite from Vps35 mutants, we hypothesized that recycling may regulate EV cargo levels by counteracting the Vps35 pathway, and further investigated the role of Rab11 compartments in the Vps35 mutant phenotype.
APP accumulates in a Rab11-dependent compartment in Vps35 mutants
Depending on the specific cell type and cargo, loss of retromer can result in altered cargo levels in early endosomes, in late endosomes, or on the plasma membrane (Seaman, 2021). The specific site of cargo accumulation at Vps35 mutant synapses may thus reveal which compartments are EV permissive in neurons. Our genetic data indicated that this may be the Rab11 compartment, so we asked if retromer regulates APP traffic through presynaptic Rab11-positive structures. In control animals, APP-EGFP and Rab11 partially colocalized by SIM (Fig. 9 A), and in Vps35 mutants, we found an increased fraction of Rab11 signal in APP-positive compartments (Fig. 9, A–C). These data suggest that when EV cargo is retained in a recycling regimen, it is more likely to be incorporated into EVs.
EV cargo accumulates in a Rab11-positive and -dependent compartment in Vps35 mutants.(A) MaxIPs of SIM images showing control and Vps35 larvae expressing GAL4C155-driven UAS-APP-EGFP and labeled with α-Rab11. Arrows indicate colocalization. Scale bar is 2 µm. (B and C) Quantification of Mander’s coefficients from A. (D) Rab11 and Vps35 act in opposing pathways. MaxIPs of NMJs from larvae expressing endogenously tagged Syt4-EGFP in the indicated genotypes. (E) Quantification of D, normalized to presynaptic mean intensity (left) or postsynaptic mean intensity (right) of wild-type control (green line). All bar graphs show mean ± SEM; dots show all data points representing individual NMJs. Yellow lines indicate presynaptic regions. See Table S3 for detailed genotypes and statistical tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
EV cargo accumulates in a Rab11-positive and -dependent compartment in Vps35 mutants.(A) MaxIPs of SIM images showing control and Vps35 larvae expressing GAL4C155-driven UAS-APP-EGFP and labeled with α-Rab11. Arrows indicate colocalization. Scale bar is 2 µm. (B and C) Quantification of Mander’s coefficients from A. (D) Rab11 and Vps35 act in opposing pathways. MaxIPs of NMJs from larvae expressing endogenously tagged Syt4-EGFP in the indicated genotypes. (E) Quantification of D, normalized to presynaptic mean intensity (left) or postsynaptic mean intensity (right) of wild-type control (green line). All bar graphs show mean ± SEM; dots show all data points representing individual NMJs. Yellow lines indicate presynaptic regions. See Table S3 for detailed genotypes and statistical tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We then asked whether aberrant sorting of EV cargo through this Rab11-positive compartment in Vps35 mutants caused its accumulation, by testing if levels could be restored by rebalancing the opposing activities of Rab11 and Vps35 in Vps35; rab11 double mutants. rab11 null mutants are embryonic lethal (Dollar et al., 2002), precluding clean epistasis experiments, so we tested how these mechanisms interact using hypomorphic mutants. Strikingly, reduction of rab11 function in the Vps35 mutant significantly rescued both pre- and postsynaptic Syt4 and Nrg levels (Fig. 9, D and E). These results suggest that accumulation of Syt4 in a Rab11-dependent compartment (possibly in the Rab11-positive compartment itself) in Vps35 mutants is causative of EV cargo accumulation and release. Finally, to ask whether Rab11 and Vps35 might be acting on the same compartment, we performed SIM and found Rab11-Vps35–positive puncta in all NMJs we examined (n = 8; Fig. 10 A).
Synaptic Vps35 and Rab11 compartments and model. (A) Vps35 and Rab11 colocalize on a subset of endosomes. MaxIP SIM images from NMJs expressing neuronally driven Vps35-HA immunolabeled for α-HA and α-Rab11. Yellow line indicates presynaptic region; arrows indicate Vps35-HA and Rab11-positive structures. Scale bar is 2 µm. (B) Model for the balance of retromer-mediated removal and recycling traffic in populating the EV pathway. EV cargo is maintained in a recycling pool between the early endosome (EE), the recycling endosome (RE), and the plasma membrane. MVBs populated by this pathway preferentially fuse with the plasma membrane and release their contents as EVs. In Vps35 mutants, cargo is retained in the recycling pathway. Increased levels of cargo on the endosome membrane may drive ILV formation, resulting in EV secretion and pre- and postsynaptic cargo accumulation. In a rab11 mutant, the recycling pool of cargo is not maintained. Cargo proceeds to the lysosome for degradation and is depleted from the synapse.
Synaptic Vps35 and Rab11 compartments and model. (A) Vps35 and Rab11 colocalize on a subset of endosomes. MaxIP SIM images from NMJs expressing neuronally driven Vps35-HA immunolabeled for α-HA and α-Rab11. Yellow line indicates presynaptic region; arrows indicate Vps35-HA and Rab11-positive structures. Scale bar is 2 µm. (B) Model for the balance of retromer-mediated removal and recycling traffic in populating the EV pathway. EV cargo is maintained in a recycling pool between the early endosome (EE), the recycling endosome (RE), and the plasma membrane. MVBs populated by this pathway preferentially fuse with the plasma membrane and release their contents as EVs. In Vps35 mutants, cargo is retained in the recycling pathway. Increased levels of cargo on the endosome membrane may drive ILV formation, resulting in EV secretion and pre- and postsynaptic cargo accumulation. In a rab11 mutant, the recycling pool of cargo is not maintained. Cargo proceeds to the lysosome for degradation and is depleted from the synapse.
Discussion
Here we find that trafficking of multiple synaptic EV cargoes is restricted by the neuron-autonomous activity of retromer. Rather than playing a general role in maintaining overall endolysosomal homeostasis, we find that retromer has separable roles in neurons and specifically restricts traffic within the EV pathway by regulating accumulation of cargoes in Rab4/Rab11-dependent endosomes. Our results indicate that retromer and Rab4/Rab11 recycling balance traffic of AD-relevant cargoes such as APP into the EV pathway, and may therefore be critical for disease propagation in the brain.
Neuronal retromer has multiple, separable functions
Our EV trafficking findings add to the retromer’s previously described roles in synaptic morphogenesis and transmission, synaptic vesicle loading and recycling, and neurotransmitter receptor and transporter traffic (Brodin and Shupliakov, 2018). Interestingly, photoreceptor degeneration and synaptic transmission defects in retromer mutants are opposed by Rab11, similar to our findings for EV phenotypes, suggesting that balance between these pathways is of general importance in neurons (Inoshita et al., 2017; Satoh et al., 2005; Wang et al., 2014). However, our data also show that the role of retromer in EV traffic is separable from its other functions. Compared with synaptic transmission and synaptic growth defects, which require both neuronal and muscle retromer (Inoshita et al., 2017; Korolchuk et al., 2007), presynaptic retromer is sufficient for EV cargo regulation. Further, synaptic growth, Rab7 endosome maturation, and EV defects are genetically separable: Snx3 and Vps35D628N mutants phenocopy Vps35 null mutants for synaptic growth (likely via excess bone morphogenetic protein signaling; Korolchuk et al., 2007) but not for EV traffic, while ESCPE-1 mutants recapitulate EV but not synaptic growth or Rab7 turnover phenotypes of Vps35.
Given these diverse phenotypes, it will be critical to determine which of these many cellular functions of retromer underlie neurological disease. Indeed, acute depletion of Vps35 in hippocampal neurons leads only to endosomal trafficking defects, while chronic retromer depletion also affects synaptic vesicle cycling and neuronal morphogenesis (Vazquez-Sanchez et al., 2018). These chronic phenotypes remain interesting in light of retromer depletion in AD but raise the possibility that some retromer-associated neuronal defects are indirect or compensatory. Overall, the diverse cellular roles of neuronal retromer could differentially contribute to neurological disease, and individually manipulating these functions via distinct retromer-associated molecular pathways may improve therapeutic strategies (Berman et al., 2015).
Retromer and Rab4/Rab11–dependent mechanisms of EV traffic in neurons
Our data highlight the importance of studying the cell biological functions of retromer in neurons in vivo. The canonical role of retromer is to rescue endosomal cargoes from lysosomal degradation and to redirect them to the Golgi or plasma membrane; in many cell types, retromer loss causes these cargoes to be misrouted to the lysosome and degraded (Arighi et al., 2004; Cui et al., 2019; Seaman et al., 1998; Steinberg et al., 2013; Temkin et al., 2011). By contrast, we observe accumulation of cargoes in Vps35-deficient Drosophila neurons, similar to hippocampal neurons (Bhalla et al., 2012) and secretory cells of the Drosophila salivary gland (Neuman et al., 2021). A prevailing hypothesis that could account for this accumulation is retromer-dependent lysosome dysfunction (Maruzs et al., 2015; Miura et al., 2014; Wang et al., 2014). Indeed, lysosomal damage increases APP-CTFs in secreted EVs from neuroblastoma cells (Miranda et al., 2018; Vingtdeux et al., 2007). However, in our in vivo system, we did not observe functional or ultrastructural indications of lysosome defects. Further, manipulating late endosome or lysosome function did not phenocopy retromer loss, suggesting that, like salivary gland secretory cells (Neuman et al., 2021), lysosome dysfunction is not sufficient to account for the observed cargo increase at synapses (though we cannot exclude a contributing role). Finally, we found that retromer-mediated Rab7 turnover and late endosome maturation is unlikely to be involved in synaptic EV traffic, though Rab7 does contribute to EV release in other neuronal cell types (Song et al., 2016).
Our data instead suggest that in retromer mutant neurons, endosomal cargoes are diverted to the EV pathway, rather than to lysosomal degradation. This could be beneficial, since EV recipient cells (i.e., muscles and glia) may be better suited for cargo degradation than the donor neuron, which has limited lysosomal capability (Eitan et al., 2016; Ferguson, 2018; French et al., 2017; Fuentes-Medel et al., 2009). According to this model, in presynaptic nerve terminals, MVBs would preferentially fuse with the plasma membrane rather than with lysosomes. Why, then, do we not observe excess MVBs by EM in retromer mutants, and why do EV cargoes accumulate in Rab11-positive compartments?
We propose that EV traffic is not rate limited by MVB–plasma membrane fusion (Lauwers et al., 2018), but instead by loading of endosomes with EV cargo via recycling pathways (Fig. 10 B). This mechanism may be conserved, as EV-directed p75-neurotrophin receptor accumulates in Rab11-positive compartments in PC12 cells and sympathetic neurons (Escudero et al., 2014). Rab11 has previously been implicated in the MVB–plasma membrane fusion step of EV traffic (Escudero et al., 2014; Fan et al., 2020; Koles et al., 2012; Korkut et al., 2013; Savina et al., 2002). Our results instead show that the primary cause of EV trafficking defects in rab11 mutants is cargo depletion in the donor cell (as observed for the EV cargo Arc; Ashley et al., 2018). We hypothesize that recycling maintains a pool of EV cargo that fluxes between early endosomes, recycling endosomes, and the plasma membrane; this recycling pool of cargo is protected from lysosomal degradation and is incorporated into MVBs destined to become EVs. Alternatively, recycling cargo could be released from the plasma membrane by direct budding of microvesicles. An alternative hypothesis is that the recycling pathway directly loads cargo into Rab11-positive MVBs, similar to EV-releasing Drosophila accessory gland secondary cells (Corrigan et al., 2014; Fan et al., 2020; Marie et al., 2020 Preprint).
Retromer may oppose EV formation in several ways: It might retrieve or protect cargo from Rab11-dependent trafficking through the recycling endosome (perhaps via direct recycling to the plasma membrane; Naslavsky and Caplan, 2018; Steinberg et al., 2013), either by interacting directly with cargo (Simonetti et al., 2017) or via carriers such as SorLA (Fjorback et al., 2012; Nielsen et al., 2007). Our finding that ESCPE-1 acts in EV traffic may enable identification of EV cargo sorting determinants, given recent insights into SNX1-SNX6–dependent sorting motifs (Simonetti et al., 2019; Yong et al., 2020). In support of direct sorting, we found that Rab11 and Vps35 partly colocalize at the synapse. It will also be interesting to further unravel the role of Rab4 in this process, and ask if it acts in parallel or together with Rab11. Alternatively, retromer may nonspecifically restrict MVB formation by creating domains protected from ESCRT-mediated ILV formation (Derivery et al., 2012; Norris et al., 2017), or by limiting overall cargo accumulation on endosomes, thus suppressing ESCRT activity (Baietti et al., 2012). In support of this indirect role, we found an overall increase in EV-like postsynaptic structures in Vps35 mutants, as well as aberrant trafficking of the non-EV cargo Tkv into EVs.
Role of the retromer-Rab11 EV pathway in neuronal function and disease
Our results have implications for the role of Vps35 in human disease. We found that the Parkinson’s disease Vps35D628N mutant does not recapitulate Vps35 null EV trafficking defects. Instead, our results may relate more directly to AD, which is associated with reduced levels of retromer, based on genetic and pathological data from patients (Small et al., 2005; Vardarajan et al., 2012), and findings that Vps35 mutants enhance pathology in AD animal models (Li et al., 2020; Muhammad et al., 2008; Wen et al., 2011). The specific role of retromer in APP traffic is unclear, due to differing results for trafficking itineraries and intersections of APP and its processing proteases in various cell types (Arbo et al., 2020; Tan and Gleeson, 2019). In neurons, loss of retromer increases colocalization of APP and BACE1 in early and late endosomes, resulting in higher Aβ levels (Bhalla et al., 2012; Toh et al., 2017; Wang et al., 2012a; Wen et al., 2011). Our results now extend the role of neuronal retromer to the EV pathway, and show that retromer loss promotes traffic of endosomally derived APP CTFs into synaptic EVs, potentially driving intercellular propagation of Aβ, and other EV cargoes such as tau (Kanmert et al., 2015).
Conversely, Rab11 is also genetically linked to AD, but exhibits the opposite behavior from Vps35: Rab11 loss causes decreased Aβ, while Rab11 activation causes increased Aβ (Li et al., 2012; Udayar et al., 2013). These effects were previously ascribed to Rab11-mediated intracellular traffic (e.g., by regulating the endocytosis of Aβ and trafficking of BACE1; Buggia-Prévot et al., 2014; Li et al., 2012; Udayar et al., 2013). Our data suggest a new interpretation of these previous disease model results; loss of Rab11 may suppress AD phenotypes by counteracting pathological EV cargo traffic and shunting synaptic APP for degradation.
What, then, is the physiological function of the retromer-dependent EV pathway? Our results suggest that an important role of EV traffic is to deliver neuronal cargo to recipient cells for disposal, as an alternative to intraneuronal lysosomal degradation. However, increasing evidence suggests that neuronal EV cargoes also play functional roles in recipient cells, driving structural and functional plasticity as well as morphogen signaling (Holm et al., 2018). In the future, it will be exciting to test whether neurons control these functional processes by directly tuning EV traffic via the endosomal machinery, lending these trafficking mechanisms interesting new roles beyond general synaptic proteostasis.
Materials and methods
Drosophila strains and methods
To create human APP-EGFP, the APP695 isoform was cloned into pBI-UASc-gateway-EGFP (Wang et al., 2012b) and injected into flies using Φc381 recombinase at the Attp40 locus (Ni et al., 2008). The endogenous Syt4 locus was tagged with a tissue-specific convertible TagRFPt to EGFP tag (T-STEP; Koles et al., 2016). Briefly, the following genomic sequence was used to target DNA cleavage by Cas9: 5′-TGAACGAGTAGGGGAGGGGC-3′ (at location 3R:7269302-7269321), with the 5′ T converted to the obligatory G, thus yielding the 20-bp sequence 5′-GGAACGAGTAGGGGAGGGGC-3′. The T-STEP cassette was flanked with 5′ homology (3R: 7267814–7269309) and 3′ homology (3R:7269313-7270955) arms, and gene targeting was performed as described (Koles et al. [2016], based on Chen et al. [2015]). Syt4–T-STEP flies were crossed to bam-Gal4;;UAS-Rippase::PEST @attP2 flies to isolate germline ripouts of the TagRFPt cassette, leaving a C-terminal EGFP knock-in with the following linker (Syt4 sequence in lowercase, EGFP in italics, Prescission protease site underlined): prrqiaewhklneSYALMKEYVILSSSSGSSLEVLFQGPGSGSGSMVSKGEELFTGV. The EGFP knockin strain was created by germline conversion of the T-STEP tag. All other fly stocks and sources are described in Table S1.
Drosophila larvae were cultured using standard media at controlled density at 25° for all experiments. Wandering third instar larvae were dissected in calcium-free HL3.1 saline (Feng et al., 2004) and fixed in HL3.1 containing 4% formaldehyde before antibody staining. Male larvae were used for all experiments, except for chloroquine treatment (Fig. S4, F and G) and Vps35 rab11 genetic interaction experiments (Fig. 9, D and E), for which both male and female larvae were used. For chloroquine treatment, Formula 4–24 Instant Drosophila Medium (Carolina Biological Supply Company) was prepared at a 1:2 ratio with 0–8 mM chloroquine diluted in water and allowed to solidify at room temperature. Four male and four female larvae homozygous for Syt4-EGFP were added to each vial, and vials were incubated for up to 9 d at 25°C.
Immunohistochemistry and imaging
For primary antibody information and concentrations, see Table S2. FluoTag-X4 α-GFP nanobodies (Nanotag Biotechnologies) were used to amplify EGFP signal for SIM imaging only; all other EGFP imaging reflects endogenous EGFP fluorescence. Primary and secondary incubations and washes were conducted in PBS with 0.5% Triton X-100, 2% (wt/vol) BSA, and 2% (vol/vol) normal goat serum, except Nrg staining, which was conducted with PBS with 0.2% Triton X-100 (no blocking agent). α-HRP antibodies and secondary antibodies for imaging were conjugated to Dylight 488, Rhodamine Red-X, or Alexa 647 (Jackson Immunoresearch) and were used at a concentration of 1:250 (Alexa 647) or 1:100 (Dylight 488 and Rhodamine Red-X). For LysoTracker staining, fillets were incubated with 1:100 LysoTracker Deep Red (Thermo Fisher Scientific; cat no. L12492) in PBS with no detergent for 10 min before fixation and mounting. Subsequent processing for immunohistochemistry was conducted as indicated above with the exception that no detergent was used. Samples were mounted in Vectashield (Vector Labs) for spinning-disk confocal and Airyscan imaging, and in Prolong Diamond (Thermo Fisher Scientific; cat no. P36965) for SIM.
NMJs on muscle 6/7 from segments A2 and A3 were selected for EV and colocalization analyses, and muscle 4 from the same segments was used for bouton counting. Spinning-disk confocal stacks were collected at room temperature, using Nikon Elements AR software, on a Nikon Ni-E upright microscope equipped with 60× (NA, 1.4) and 100× (NA, 1.45) oil immersion objectives, a Yokogawa CSU-W1 spinning-disk head, and an Andor iXon 897U EMCCD camera. Images for each independent experiment were acquired and are shown with identical settings for all conditions (except where indicated otherwise, and for all α-HRP immunostaining, which was used to analyze morphology and not for intensity quantifications).
3D-SIM was performed at room temperature on a Nikon N-SIM E system (consisting of an inverted Eclipse Ti-E microscope, 100× [NA, 1.45] oil-immersion objective, and a Hamamatsu OrcaFLASH4 sCMOS camera). Channel alignment was calculated for each imaging session using TetraSpeck beads (Invitrogen; cat no. T-7284). Images were collected at room temperature with a regimen of three grid orientations and five phases and were reconstructed using Nikon Elements software, using a theoretical, ideal optical transfer function generated by the software.
Airyscan microscopy was conducted at room temperature on a Zeiss 880 laser scanning microscope equipped with a Fast Airyscan detector, and operated in super resolution acquisition mode, using a 63× NA 1.4 objective.
Image analysis and statistics
3D analyses of presynaptic and postsynaptic EV cargo at NMJs were conducted using Volocity 6.0 software (Perkin Elmer). Manual thresholding of the α-HRP signal was used to define presynaptic volume (HRP-positive objects >7 µm3, with holes filled) and postsynaptic volume (a dilation of 3 µm around the presynaptic volume). The signal to be measured (see Table S3) was manually thresholded to distinguish postsynaptic puncta from background, and sum intensity measurements were made from EV cargo-positive objects intersecting with either presynaptic or postsynaptic volumes as defined above. All intensity values were then normalized to the presynaptic volume. Puncta number and volume were measured in presynaptic volumes that were thresholded and defined as above.
Analysis of APP-EGFP in axons and motor neuron cell bodies was conducted using ImageJ. Axon intensity measurements were conducted from maximum intensity projections (MaxIPs); HRP was used as a threshold to select a region of axon. APP intensity in motor neuron cell bodies was measured from a middle slice through each cell body.
Co-localization between APP and Rab11 was performed in Volocity. As above, the α-HRP channel was used as a mask to highlight the presynaptic area. For quantitative analysis of SIM images, the following unbiased criteria were applied to select images with sufficiently high-quality staining and signal:noise ratio, and that were adequately free of reconstruction artifacts. Both channels required a presynaptic coefficient of variation (CoV) >0.1, and ratio of presynaptic APP CoV:postsynaptic APP CoV of >1.5. A threshold was set to identify all APP or Rab11-positive volumes in the HRP area, and the fraction of the sum intensity of APP signal that coincided with Rab11 signal was calculated and vice versa.
EM of larval NMJs
Wandering third instar larvae (three animals per condition) were dissected and fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 1% sodium cacodylate buffer overnight at 4°C. Samples were postfixed in 1% osmium tetroxide and 1.5% potassium ferrocyanide for 1 h, then 1% aqueous uranyl acetate for 1 h. Stepwise dehydration was conducted for 10 min each in 50%, 70%, and 90% ethanol, followed by 2× 10 min in 100% ethanol. Samples were transferred to 100% propylenoxide for 1 h and then left overnight in a 1:1 mixture of propylenoxide and 812 TAAB Epon Resin (TAAB Laboratories Equipment Ltd.). Samples were then transferred to fresh epon, flat-embedded, polymerized at 60°C for 72 h, and remounted for sectioning. 70-µm-thin sections were cut on a Leica UC6 Ultramicrotome (Leica Microsystems), collected onto copper grids coated with formvar and carbon, and then poststained with lead citrate (Venable and Coggeshall, 1965). Grids were imaged using a FEI Morgagni transmission electron microscope (FEI) operating at 80 kV and equipped with a 1k × 1k charge coupled device camera (Gatan), or on a CM12 transmission electron microscope operating at 120 keV and equipped with LaB6 electron source and a Gatan Ultrascan 2k × 2k charge coupled device camera.
EM image analysis was performed on blinded images using FIJI. For quantification of endosomes and MVBs, endosomes were defined as presynaptic unilamellar compartments >100 nm at their longest axis. MVBs were defined as endosomes that contained one or more 50–100-nm intraluminal vesicles. Extracellular vesicles were measured as objects that were between 50 and 100 nm in diameter, within 1 µm of the neuron, and apparently free-floating (no contiguity with the SSR membrane). Numbers of endosomes, MVBs, and EVs were normalized to the area of the bouton.
Statistical methods
All statistical measurements were performed in GraphPad Prism 6 (see Table S3). Comparisons were made separately for presynaptic and postsynaptic datasets, due to differences between these compartments for intensity, signal-to-noise ratio, and variance. Datasets were tested for normality, and statistical significance was tested using unpaired two-tailed Student’s t tests or Mann–Whitney tests (if number of conditions was two) or ANOVA followed by Tukey’s tests or Kruskal–Wallis followed by Dunn’s test (if number of conditions was greater than two). Statistical significance is indicated as *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Online supplemental material
Fig. S1 shows that presynaptic APP and Appl localize to NMJ EVs. Fig. S2 shows neuronal retromer and APP trafficking. Fig. S3 depicts the characterization of Nrg as an EV cargo. Fig. S4 shows that lysosome dysfunction does not cause retromer-dependent EV cargo sorting defects. Fig. S5 depicts endosome distribution in Vps26 and Snx1, Snx6 mutants. Table S1 lists fly strains. Table S2 lists antibodies and reagents. Table S3 provides genotypes and statistics by dataset.
Acknowledgments
We thank the Bloomington Drosophila Stock Center (National Institutes of Health P40OD018537), the Developmental Studies Hybridoma Bank created by the National Institute of Child Health and Human Development of the National Institutes of Health, Konrad Bassler (University of Zurich, Zurich, Switzerland), Vivian Budnik (University of Massachusetts Medical School, Worcester, MA), Pete Cullen (University of Bristol, Bristol, UK), Xinhua Lin (Cincinnati Children's Hospital Medical Center, Cincinnati, OH), Kalpana White (Brandeis University, Waltham, MA), and Philip Copenhaver (Oregon Health and Science University, Portland, OR) for strains and reagents; and Crystal Yu, Troy Zhao, Agnieska Collins, and the Brandeis EM Facility for technical assistance.
This work was supported by National Institute of Neurological Disorders and Stroke grants DP2 NS082127 and R01 NS103967 to A.A. Rodal, T32 GM007122 to R.B. Walsh, T32 NS007292 to E.C. Dresselhaus, T32 MH019929 to A.L. Scalera, and F32 NS110123 to C.R. Blanchette, and the Brandeis National Science Foundation Materials Research Science and Engineering Center, Bioinspired Soft Materials (NSF-DMR 2011846).
The authors declare no competing financial interests.
Author contributions: R.B. Walsh, A.N. Becalska, M.J. Zunitch, and A.A. Rodal designed the study and experiments. R.B. Walsh, E.C. Dresselhaus, A.N. Becalska, C.R. Blanchette, M.J. Zunitch, A.L. Scalera, J. Apiki, T. Lemos, S.M. Lee, S. Wang, B. Isaac, A. Yeh, and A.A. Rodal conducted the experiments and performed analyses. K. Koles generated critical reagents. R.B. Walsh and A.A. Rodal wrote the manuscript.