Calcium (Ca2+) extrusion is an essential function of the enamel-forming ameloblasts, providing Ca2+ for extracellular mineralization. The plasma membrane Ca2+ ATPases (PMCAs) remove cytosolic Ca2+ (cCa2+) and were recently shown to be efficient when ameloblasts experienced low cCa2+ elevation. Sodium–calcium (Na+/Ca2+) exchange has higher capacity to extrude cCa2+, but there is limited evidence on the function of the two main families of Na+/Ca2+ exchangers in enamel formation. The purpose of this study was to analyze the function of the NCX (coded by SLC8) and the K+-dependent NCKX (coded by SLC24) exchangers in rat ameloblasts and to compare their efficacy in the two main stages of enamel formation: the enamel forming secretory stage and the mineralizing or maturation stage. mRNA expression profiling confirmed the expression of Slc8 and Slc24 genes in enamel cells, Slc24a4 being the most highly upregulated transcript during the maturation stage, when Ca2+ transport increases. Na+/Ca2+ exchange was analyzed in the Ca2+ influx mode in Fura-2 AM–loaded ameloblasts. We show that maturation-stage ameloblasts have a higher Na+/Ca2+ exchange capacity than secretory-stage cells. We also show that Na+/Ca2+ exchange in both stages is dominated by NCKX over NCX. The importance of NCKX function in ameloblasts may partly explain why mutations in the SLC24A4 gene, but not in SLC8 genes, result in enamel disease. Our results demonstrate that Na+/Ca2+ exchangers are fully operational in ameloblasts and that their contribution to Ca2+ homeostasis increases in the maturation stage, when Ca2+ transport need is higher.
Introduction
Calcium (Ca2+) is involved in many fundamental cellular processes and its regulation requires precise molecular control. Cytosolic calcium (cCa2+) is maintained low, ∼100 nM, compared with the significantly higher concentration outside the cell (∼2 mM) and, therefore, small changes in cCa2+ have important effects on cell function (Strehler, 1990). However, a rise in cCa2+ must be transient to prevent deleterious effects including mitochondrial overloading, activation of proteases, activation of DNA-fragmenting enzymes, and cell death (Guerini, 1998). Cells not only use Ca2+ as a signaling messenger in mineralizing cells, such as the enamel forming ameloblasts, Ca2+ is also needed to build the enamel crystals composed of carbonated hydroxyapatite.
Enamel forms in two stages, termed secretory and maturation (Smith, 1998). The enamel space where the enamel crystals grow de novo is adjacent to the apical/distal pole of the cells. This space is surrounded by the ameloblasts and hence these cells exert an important modulatory control on crystal growth (Lacruz, 2017; Lacruz et al., 2017; Nurbaeva et al., 2017). Ameloblasts are tall columnar cells forming a relatively tight sheet with cells ranging in height from ∼60 to 70 μm in the secretory stage to ∼35 μm in the maturation stage and maintaining a 5-μm diameter (Smith, 1998). Ameloblasts undergo cyclic changes from a distal ruffled-ended morphology characterized by membrane infoldings to a smooth-ended appearance devoid of these infoldings. The vast majority of maturation ameloblasts are in the ruffled-ended type (Smith, 1998), and this morphology has been linked with the ion transport mode of the ameloblasts, specifically Ca2+ transport (Ashrafi et al., 1989; Reith and Boyde, 1979, 1981). Secretory and ruffled-ended ameloblasts are bound distally by tight junctions sealing the paracellular space. The junctions near the basal/proximal pole are leaky (Sasaki et al., 1982), and because of this arrangement, it is widely considered that ion transport is primarily transcellular (Hubbard, 2000; Lacruz, 2017; Smith, 1998). The majority of Ca2+ transport occurs in the maturation stage as enamel crystal growth increases considerably and as protein fragments and water are removed (Smith, 1998). A previous evaluation of the volume of Ca2+ acquired during maturation reported that ∼86% is incorporated during that stage (Smith, 1998), which suggests that the molecular components of the Ca2+ extrusion system of maturation stage ameloblasts should also be enhanced to efficiently contribute to crystal growth (Lacruz, 2017; Lacruz et al., 2017). Ca2+ influx in ameloblasts is largely regulated by the store-operated Ca2+ entry (SOCE) pathway (Eckstein et al., 2017, 2019; Lacruz, 2017; Nurbaeva et al., 2015a, 2017). In line with the increased capacity for ion transport during maturation, the molecular components of SOCE and the magnitude of Ca2+ influx mediated by SOCE increase considerably during that stage (Aulestia et al., 2020; Nurbaeva et al., 2015a, 2018; Souza Bomfim et al., 2020). However, addressing Ca2+ homeostasis in enamel cells following the activation of SOCE remains under investigation. In most mammalian cells, Ca2+ extrusion is dominated by the activity of Ca2+ pumps and Na+/Ca2+ exchangers (Carafoli et al., 2001). In enamel cells, the functional role of the plasma membrane Ca2+-ATPase (PMCA) pumps was reported recently (Bomfim et al., 2023). PMCAs were found to be functional in both stages, but their role was more substantial when ameloblasts experienced low to moderate elevations in cCa2+, i.e., the secretory stage (Bomfim et al., 2023). However, the functional contribution of the Ca2+ exchangers in enamel formation is poorly defined and could be important given the difference in kinetics between the pumps and the exchangers. PMCAs have a high affinity for Ca2+ (Kd ∼0.2 μM) but low transport capacity, whereas the Na+/Ca2+ exchangers have lower Ca2+ affinity (Kd 1–20 μM) but have higher transport capacity, estimated to be ∼10 times faster than the PMCAs in tissues such as the heart (Carafoli et al., 2001).
The Na+/Ca2+ exchangers can be broadly separated into two main families sharing ∼20% homology (Lytton et al., 2002): the Na+/Ca2+ exchanger (NCX) and the K+-dependent Na+/Ca2+ exchangers (NCKX). The NCX family (coded by the SLC8 gene) came to light in the late 1960s, known primarily from studies in the contractility of the heart. The first member of the family of NCX (NCX1) was cloned in 1990 and since then the expression of NCXs has been described in most cells of higher animals (Blaustein and Lederer, 1999; Philipson and Nicoll, 2000). In the late 1980s, Na+/Ca2+ exchange in retinal rod photoreceptors was shown to require and transport K+ (Cervetto et al., 1989; Schnetkamp et al., 1989) and was later shown to represent the first member of the distinct SLC24 gene family (Reiländer et al., 1992). Both NCX and NCKX are predominantly involved in Ca2+ extrusion that requires the energy of the Na+ gradient produced by Na+ pumps; however, both are also capable of bidirectional exchange, e.g., influx and efflux (Blaustein and Lederer, 1999). In fact, the Ca2+ influx mode of operation in which Na+ moves out in exchange for the simultaneous influx of Ca2+ and K+ is widely used to study the function of NCKXs (Altimimi et al., 2013; Jalloul et al., 2016a; Li and Lytton, 2014; Lytton et al., 2002; Paillart et al., 2007; Schnetkamp et al., 1991). Two important features distinguish their function, the coupling ratio of NCX is 3 Na+:1 Ca2+, whereas NCKX exchanges 4 Na+:1 Ca2+ + 1 K+ (Schnetkamp et al., 1989, 1991). Further, the transport rates for NCXs are faster than those reported for NCKXs (Baazov et al., 1999; Hilgemann, 1996; Jalloul et al., 2016b). In enamel cells, beyond expression data for several of the SLC8 and SLC24 genes or proteins, only one study has previously addressed, by electrophysiology, the functional expression of NCX in ameloblasts (Okumura, et al., 2010). However, that study used ameloblasts derived from young mice which likely prevented the characterization of maturation-stage ameloblasts. Mutations in the SCL8 genes have not been associated with enamel defects to date. In 2012, we first identified the expression of Slc24a4 (coding for NCKX4) in the enamel organ by genome-wide transcript profiling (Lacruz et al., 2012a) and determined its localization in ameloblasts (Hu et al., 2012). Subsequent studies recognized that human mutations in SLC24A4 caused severe enamel defects (Parry et al., 2013; Wang et al., 2014) and that this isoform is considered essential in enamel mineralization (Hu et al., 2012; Lacruz et al., 2012a). More recently, it was reported that the introduction of three of the SLC24A4 mutations causing enamel defects (known as amelogenesis imperfecta) results in single-residue substitutions in the mutant NCKX4 proteins, and, when these mutant NCKX4 proteins were expressed into HEK293 cells, it resulted in the complete abolition of NCKX4-mediated exchanger activity, as measured in the Ca2+ influx mode (Jalloul et al., 2016a). These studies suggest that ameloblasts express both NCX and NCKX families of Ca2+ exchangers, raising the question about the necessity to have a dual system that is independent of the PMCA pumps.
To address the functional role of NCXs and NCKXs in ameloblasts and to determine whether the kinetics of Ca2+ exchange differ between the secretory and maturation stages that define enamel formation, we analyzed rat secretory and maturation stage ameloblasts to quantitate Ca2+ exchanger function in the Ca2+ influx mode. We first analyzed the expression profiles of the SCL8 and SLC24A genes at each stage and confirmed that SLC24A4 is the most highly upregulated transcript in maturation, as also further validated by Western blot. Ameloblasts of both stages expressed functional Ca2+ exchange that was more prominent in maturation. By comparing NCX and NCKX function using K+-free solutions, a pharmacological inhibitor of NCX, and ameloblasts of NCKX4-deficient mice, we determined that the contribution of NCKX is greater than that of NCX in ameloblasts. Our results are the first functional evidence that ameloblasts primarily rely on K+-dependent Na+/Ca2+ exchange.
Materials and methods
Animal use
All procedures employed in this study were conducted in accordance with guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of New York University College of Dentistry (protocol # IA16-00625). Experiments were carried out in male and/or female (100 ± 10 g) Sprague–Dawley rats ∼4.5- to 5.0-week-old obtained from Charles River Laboratories. The genetically engineered C57BL/6 mice harboring a homozygous Slc24a4−/− deletion were kindly provided by Dr. Haiqing Zhao (Stephan et al., 2011).
Primary culture of enamel ameloblast cells and immunohistochemistry
We used primary enamel organs to dissect ameloblasts because of the limited value of ameloblastcell lines (Klein et al., 2017). Secretory and maturation enamel organ cells were isolated from the rat lower incisors, as has been described in detail following anatomical landmarks in the lower jaw (Lacruz et al., 2012a; Nurbaeva et al., 2018; Smith and Nanci, 1989; Souza Bomfim et al., 2020). Moreover, the rat lower incisors are a well-established system for studying enamel development (Josephsen, 1974; Lacruz et al., 2017; Smith and Nanci, 1989). To obtain single ameloblasts populations, isolated cell clumps from secretory and maturation stages were transferred to Eppendorf tubes containing 450 μl of Hanks’ balanced salt solution (cat #14065-056; Thermo Fisher Scientific) with 1% antibiotic-antimycotic (cat #15240-062; Thermo Fisher Scientific). Cells were digested (0.25 mg/ml cat #414,654; Liberase, Roche) for 35 min at 37°C in a 5%-CO2 incubator and manually pipetted before stopping the enzymatic reaction by adding 2 ml of X-Vivo 15 (cat #m04418Q; Lonza Bioscience) cell media containing 10% FBS and 1% penicillin–streptomycin. Cells were centrifuged (1,500 × g for 5 min), washed with X-Vivo media, and plated on 25-mm borosilicate cover glass coated with Corning Cell-Tak (cat #CB40240; Thermo Fisher Scientific). Cells were used within 24 h after dissection. Single-cell imaging enabled us to increase the purity of the ameloblast population sampled by eliminating potential contamination of fibroblast/connective tissue cells using a fibroblastic marker, a PE-conjugated monoclonal anti-rat CD90 antibody (cat #202523; Biolegend), and by analyzing gene markers for secretory and maturation stage as we have reported (Bomfim et al., 2023; Lacruz et al., 2012b). In Slc24a4−/− mice, we did not separate between stages because of the smaller size of the incisors, which resulted in a significantly reduced number of ameloblasts compared with rats, and because the sampling method in mice has not been widely tested (Yin et al., 2014). NCKX4 protein expression in ameloblasts was detected by immunohistochemistry (IHC). Briefly, the lower jaw of 10-d-old mice was isolated and fixed in 4% PFA overnight, washed, embedded in paraffin, and sectioned. Tissue sections were stained with primary rabbit polyclonal anti-SLC24A4 (NCKX4) antibody 1:500 (cat #ab136968; Abcam) and detected by biotinylated anti-goat IgG secondary antibody 1:200 (cat #BA-1000-1.5; Vector Laboratories) and Pierce High Sensitivity NeutrAvidin-HRP 1:500 (cat #31030; Thermo Fisher Scientific). To increase the chromogenic signals and detection sensitivity, a rapid colorimetric detection system using diaminobenzidine (DAB) was used and a semiautomatic quantitative analysis of IHC staining was performed using IHC-ImageJ analysis toolbox.
Real-time PCR and Western blot
Total RNA from secretory and maturation enamel organ tissues were isolated using RLT lysis buffer from the RNeasy Mini Kit (cat #74106; Qiagen) with β-mercaptoethanol (1:100), as indicated by the manufacturer, followed by reverse transcription using the iScript cDNA Synthesis Kit (cat #1708891; Bio-Rad). For qRT-PCR, we used the SsoAdvanced Universal SYBR Green Supermix (cat #1725271; Bio-Rad), and experiments were performed in a CFX Connect Optics Module thermocycler (Bio-Rad). β-Actin was used as the housekeeping gene. Primer sequences for relative quantification are shown in Table S1. Expression levels were calculated relative to the secretory stage using the delta CT methods for a general comparison. For Western blot analysis, total protein was extracted from secretory and maturation enamel organ tissue and NCKX4−/− heart tissue using Pierce RIPA Lysis and Extraction Buffer (cat #89900; Thermo Fisher Scientific) with added 1:100 Halt Protease Inhibitor Cocktail (100×; cat #1861277; Thermo Fisher Scientific) and 1:1,000 phenylmethylsulfonyl fluoride (PMSF). Samples were homogenized manually with a pestle 8–10 times and then sonicated with a VIRTIS VirSonic 300 Ultrasonic Cell Disruptor (10–15% intensity, 10 s on and 10 s off; VIRTIS Co.). Samples were then cleared by centrifugation (15,000 g, 15 min, 4°C). Proteins were quantified using Pierce BCA Protein Assay Kit (cat #23227; Thermo Fisher Scientific). Protein samples were prepared by gentle heating at 37°C for 20 min and 20 μg of protein was equally loaded onto 10% SDS–PAGE resolving gels. Proteins were then transferred to a PVDF membrane and blocked with 5% milk in TBST for 1 h before loading with primary antibody overnight. The following day, membranes were washed three times for 15 min with TBST and loaded with goat anti-rabbit HRP conjugated secondary antibody for 75 min (cat #1705046; Bio-Rad). Membranes were washed three times for 15 min with TBST and imaged with SuperSignal West Pico PLUS Chemiluminescent Substrate (cat #34577; Thermo Fisher Scientific). For loading, control membranes were stripped (cat #21059; Thermo Fisher Scientific), reblocked with 5% milk in TBST for 1 h, and reloaded with a primary antibody overnight. Antibodies against GAPDH 1:1,000 (14C10 rabbit mAb, cat#2118S; Cell Signaling Technology), SLC24A4 (NCKX4) 1:500 (polyclonal, cat #136968; Abcam), and SLC8A3 (NCX3) 1:200 (polyclonal, cat #ANX-013; Alomone Labs) were used.
Measurements of intracellular Ca2+ and Na+ and membrane potential
Cytosolic Ca2+ measurements were performed as previously described (Nurbaeva et al., 2015a, 2018; Souza Bomfim et al., 2020). Cells were incubated for 1 h at room temperature with 1 µM of the ratiometric Ca2+ probe Fura-2 AM (cat #F1221; Thermo Fisher Scientific). To record the optical membrane potential imaging, preparations of secretory and maturation cells were loaded for 30 min at room temperature with FluoVolt Membrane Potential Kit (cat #F10488; Thermo Fisher Scientific). As indicated by the manufacturer, the fresh FluoVolt loading solution was prepared by adding 10 μl of FluoVolt dye 1,000× and 100 μl of 100× PowerLoad concentrate to 10 ml of regular Ringer’s solution (in mM): 155.0 NaCl; 4.5 KCl; 2.5 CaCl2; 1.0 MgCl2; 10 D-glucose; and 10 HEPES, pH 7.4. Cytosolic Na+ concentration was determined using the ratiometric Na+ probe SBFI-AM cell permeant (cat #S1264; Thermo Fisher Scientific). Secretory and maturation ameloblasts were loaded with 10 µM SBFI-AM plus Pluronic F-127 0.05% wt/vol (cat #P6866; Thermo Fisher Scientific) for 1.5 h at room temperature. SBFI ratios were calibrated to indicate c[Na+] as reported (Chabbert et al., 1995). Briefly, SBFI-loaded ameloblasts were perfused with an ionophore cocktail solution containing nigericin 6 µM (cat #SML1779; Sigma-Aldrich), monensin 6 µM (cat #M5273; Sigma-Aldrich), and gramicidin 2 mg/ml (cat #G5002; Sigma-Aldrich) to produce transmembrane pores and equilibrate internal monovalent cations with external concentrations. Sodium-free, 5, 10, 20, and 50 mM of NaCl (cat #S7653; Sigma-Aldrich) solutions were prepared replacing NaCl with N-methyl-D-glutamine (NMDG; cat #66930; Sigma-Aldrich) and maintaining the osmolarity and equimolar amounts of NMDG and NaCl. Steps to calibrate the SBFI probe fluorescence ratios followed the equations and procedures detailed in Chabbert et al. (1995). Changes in membrane potential or cytosolic Ca2+ levels were elicited by different isotonic high KCl concentrations (10, 40, or 75 mM, cat #P9541; Sigma-Aldrich). In the nominally Ca2+-free Ringer’s solution, CaCl2 was omitted and the osmolarity was maintained by adding 3.5 MgCl2. All reagents were obtained from Sigma-Aldrich. Ca2+, Na+, or optical membrane potential transients of secretory and maturation cells were measured at room temperature (24 ± 2°C) using a polycarbonate 260 μl chamber (cat #RC21BR; Harvard Bioscience Inc.) mounted on an inverted microscope coupled to a perfusion system electrically controlled. Fluorescence recordings were obtained using the Nikon Ti2-E Eclipse inverted light microscope, equipped with an objective (Nikon S Fluor × 20; numerical aperture: 0.75) and a digital SLR camera (DS-Qi2; Nikon) controlled by computer software (NIS Elements version 5.20.01). Cells were continuously perfused by a six-way perfusion system (VC-8 valve controller) at 5–6 ml per minute with a common outlet 0.28-mm tube driven by controlled valves (Harvard Bioscience Inc.). The Ca2+ probe Fura-2 AM and Na+ probe SBFI-AM were excited alternatively at 340 and 380 nm using a Lambda LS xenon-arc lamp (Sutter Instrument) and/or pE-340 Fura (Cool Led). Emitted fluorescence was collected through a 510-nm emission filter. Excitation/emission of FluoVolt dye was collected using standard GFP settings. All fluorescence images were generated at 5-s intervals, normalized, and the ratio values were calculated using Image J (1.53J).
Solutions used to alter chemical gradients and pharmacological inhibition of NCX
To address the possible effect of replacing KCl solutions on Ca2+ transients, we monitored the latter in Fura-2 AM–loaded cells during the addition or removal of solutions containing low (10 mM KCl) or high (40 mM KCl) K+. Osmolarity was maintained by adjusting the NaCl levels. Exchanger activity was monitored in the Ca2+ influx mode elicited by replacing external Na+ (122 mM NaCl) with N-methyl-D-glucamine (119.5 mM NMDG) to maintain osmolarity in the presence of external Ca2+ (2.5 mM CaCl2) and 40 mM KCl. To differentiate between NCX and NCKX function, the activity of the exchangers in Ca2+ influx mode was elicited by replacing Na+ (NaCl) with Li+ (LiCl) in the presence of Ca2+ (2.5 mM CaCl2), with or without 10 K+ (10 mM KCl), to selectively activate NCKX function. To inhibit the Ca2+ influx mode of NCX, we used the blocker KBR7943 (30 min, 5 μM, cat #K4144; Sigma-Aldrich) in a K+-free solution.
Data analyses and statistics
All data, mathematical analyses, and graphs were analyzed and/or generated using the GraphPad Prism software version 9.5.0 and/or 10.0 (GraphPad Inc.), as previously described (Bomfim et al., 2020; Souza Bomfim et al., 2020; Souza Bomfim et al., 2021). Basal Ca2+ and Na+ levels were calculated averaging the values from 0 to 60 s of each independent experiment. The Ca2+ transients were analyzed by measuring the Ca2+ influx mode calculated in each individual trace and fitted by the nonlinear regression curve. The plateau followed by one phase association equation has the following function: Y = IF(X < X0, Y0, Y0 + [Plateau-Y0]*{1 − exp[−K*(X − X0)]}). The delta (∆) Ca2+ peak parameter was calculated by the subtraction of maximum fluorescence from the basal/minimum fluorescence intensity, expressed in the same unit of Y-axis values. Data represent the mean ± SEM of the minimum of three independent experiments. Differences between the means of the group data that fit a normal distribution were analyzed using one-way ANOVA followed by Tukey’s multiple comparisons post-hoc test or two-tailed unpaired Student’s t test. The limit of significance was established *P < 0.05; **P < 0.01; ***P < 0.001; and n.s., non-significant, as indicated in each figure legend.
Online supplemental material
Fig. S1 shows gene expression levels of SLC8 and SLC24A genes in secretory and maturation stage ameloblasts. Fig. S2 shows a Western blot analysis of NCX3 and NCKX4 in secretory and maturation. Fig. S3 shows immunolocalization of NCKX4 in ameloblasts. Fig. S4 shows the calibration of SBFI. Fig. S5 shows changes in cytosolic [Ca2+] in response to the removal of extracellular K+. Table S1 shows the primer uses to detect gene expression by qRT-PCR.
Results
Expression profiles of SLC8 (NCX) and SLC24 (NCKX) in enamel cells
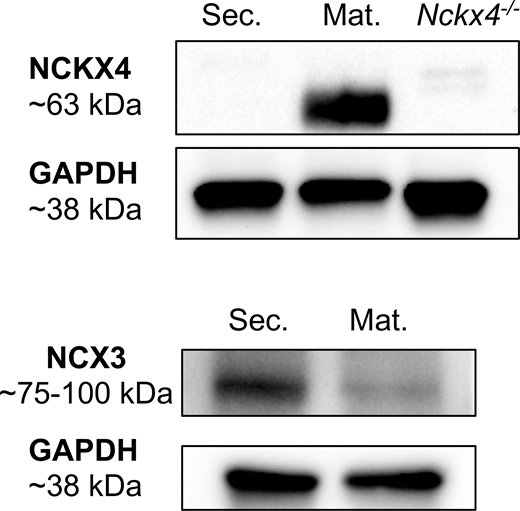
The relative abundance of the Ca2+ exchangers determines their functional relevance in various cell types (Philipson and Nicoll, 2000). The SLC8 gene family includes three members coding for NCX1–3 (Khananshvili, 2013) and five members are recognized in the SLC24 gene family coding for NCKX1–5 (Schnetkamp, 2004). Their expression patterns in enamel cells at the mRNA and protein level had been previously reported by us and others (Hu et al., 2012; Lacruz et al., 2012a, 2012b; Robertson et al., 2017), although results have been contradictory (Lacruz et al., 2012b; Robertson et al., 2017). Therefore, we reanalyzed mRNA expression levels and showed that NCX1 and NCX3 are the most abundant products in ameloblasts with NCX3 being more highly expressed in secretory ameloblasts (Fig. S1 A). NCKX1 and NCKX4 were upregulated in the maturation stage with the latter being the most abundant product showing a ∼50-fold increase (Fig. S1 B). Given that NCX3 and NCKX4 are the most abundant transcripts in each family, we further determined protein expression levels by Western blot. We show that NCXK4 is highly expressed in the maturation stage while NCX3 expression decreased at that stage (Fig. S2). We also confirmed the cellular localization of NCKX4 in the distal pole of maturation stage ameloblasts (Fig. S3) as previously reported, being absent in secretory stage ameloblasts and in cells surrounding the ameloblasts in the enamel organ (Fig. S3; Hu et al., 2012; Jalali et al., 2017; Wang et al., 2014). NCKX4 signals were also notably stronger in ruffled-ended ameloblasts than in smooth-ended cells (Fig. S3). This subcellular localization supports previous models suggesting a critical role of NCKX4 in the extrusion of Ca2+ out of the distal region of maturation stage ameloblasts and into the enamel space (Hu et al., 2012; Jalloul et al., 2016a).
Gene expression profiles of NCX (SLC8) and NCKX (SLC24) coding genes in ameloblasts. (A and B) Gene expression analyses of rat Scl8a1 (NCX1), Slc8a2 (NCX2), Slc8a3 (NCX3), Slc24a1 (NCKX1), Slc24a3 (NCKX3), and Slc24a4 (NCKX4) isoforms in secretory (SEC) and maturation (MAT) stage ameloblasts analyzed by qRT-PCR using the 2−ΔCT method using β-actin as the housekeeping control. Data represent the mean ± SEM of a minimum of four independent experiments analyzed by two-tailed unpaired Student’s t test at *P < 0.05; **P < 0.01; n.s, non-significant; n.d, not detected.
Gene expression profiles of NCX (SLC8) and NCKX (SLC24) coding genes in ameloblasts. (A and B) Gene expression analyses of rat Scl8a1 (NCX1), Slc8a2 (NCX2), Slc8a3 (NCX3), Slc24a1 (NCKX1), Slc24a3 (NCKX3), and Slc24a4 (NCKX4) isoforms in secretory (SEC) and maturation (MAT) stage ameloblasts analyzed by qRT-PCR using the 2−ΔCT method using β-actin as the housekeeping control. Data represent the mean ± SEM of a minimum of four independent experiments analyzed by two-tailed unpaired Student’s t test at *P < 0.05; **P < 0.01; n.s, non-significant; n.d, not detected.
Protein expression levels of NCX3 and NCKX4 in secretory and maturationstage ameloblasts. Western blot analysis of NCKX4 and NCX3 expression in rat secretory and maturation enamel cells. Heart tissue of Nckx4−/− mice was used as a control. The band sizes seen for NCKX4 and NCX3 were of predicted size, being ∼60–70 kD for NCKX4 and ∼75–100 kD for NCX3. Levels of GAPDH in each sample served as a control. Source data are available for this figure: SourceData FS2.
Protein expression levels of NCX3 and NCKX4 in secretory and maturationstage ameloblasts. Western blot analysis of NCKX4 and NCX3 expression in rat secretory and maturation enamel cells. Heart tissue of Nckx4−/− mice was used as a control. The band sizes seen for NCKX4 and NCX3 were of predicted size, being ∼60–70 kD for NCKX4 and ∼75–100 kD for NCX3. Levels of GAPDH in each sample served as a control. Source data are available for this figure: SourceData FS2.
Immunolocalization of NCKX4 in ameloblasts. (A–C) Immunofluorescence localization of NCKX4 (green) in tissue sections of a mouse incisor showing strong apical signals in ruffled-ended ameloblasts (A). NCKX4 becomes more diffuse in smooth-ended ameloblasts (B and C). Nuclear staining with DAPI (blue) Scale bar = A and B (30 μm), C (20 μm). (D–F) Immunostaining of NCKX4 (brown/reddish staining) in mouse molars in the secretory and maturation stage ameloblasts by peroxidase staining. Cells surrounding the ameloblasts within the enamel organ (EO) did not positively stain for NCKX4. The boundary of the EO is shown by a black dotted line. Secretory ameloblasts in E were imaged in D in the area bound by a dotted square. Maturation ameloblasts in F were imaged from the area in D bound by a solid square. The basal pole in E and F is close to the nucleus (top of the ameloblasts). Scale bars; E = 15 μm; F = 7 μm.
Immunolocalization of NCKX4 in ameloblasts. (A–C) Immunofluorescence localization of NCKX4 (green) in tissue sections of a mouse incisor showing strong apical signals in ruffled-ended ameloblasts (A). NCKX4 becomes more diffuse in smooth-ended ameloblasts (B and C). Nuclear staining with DAPI (blue) Scale bar = A and B (30 μm), C (20 μm). (D–F) Immunostaining of NCKX4 (brown/reddish staining) in mouse molars in the secretory and maturation stage ameloblasts by peroxidase staining. Cells surrounding the ameloblasts within the enamel organ (EO) did not positively stain for NCKX4. The boundary of the EO is shown by a black dotted line. Secretory ameloblasts in E were imaged in D in the area bound by a dotted square. Maturation ameloblasts in F were imaged from the area in D bound by a solid square. The basal pole in E and F is close to the nucleus (top of the ameloblasts). Scale bars; E = 15 μm; F = 7 μm.
Intracellular [Na+] is similar in both stages and K+ does induce depolarization
The NCX and NCKX families are capable of bidirectional exchange and can function in the Ca2+ influx mode if the Na+/Ca2+ gradients are reversed (Blaustein and Lederer, 1999). Because Na+ levels can affect the kinetics of this exchange, we first analyzed intracellular c[Na+] in secretory and maturation stage ameloblasts. Using the ratiometric Na+ probe, SBFI-AM calibrated as reported (Chabbert et al., 1995), we showed that secretory and maturation have similar c[Na+]I, averaging ∼9 mM (Fig. 1 A and Fig. S4), similar to levels found in other cells (Amorino and Fox, 1995; Fleysher et al., 2013). Because the study of NKCX function in the Ca2+ influx mode involves the addition and removal of extracellular [K+] and because it is well-established that extracellular [K+] can alter the resting membrane potential, we next analyzed if the addition of various concentrations of K+ in the solutions depolarized the enamel cells and if this could lead to an increase in cCa2+. To investigate membrane depolarization, we loaded the cells with the voltage-sensitive probe FluoVolt, and to address changes in cCa2+, cells were loaded in a separate experiment with Fura-2 AM. In the presence of external Ca2+ (2 mM), secretory and maturation stage ameloblasts were exposed to 10, 40, and 75 mM K+ (KCl). We show that perfusing the cells with 10 and 40 mM of KCl had no effect on membrane depolarization in either cell type (Fig. 1, C and E). These concentrations of KCl failed to evoke changes in cCa2+ (Fig. 1, D and F). However, the perfusion of 75 mM of KCl depolarized both cell types but without increasing cCa2+ (Fig. 1, G and H). These data indicate that membrane depolarization does not increase cCa2+. Moreover, because the experimental approach used involved the removal of solutions containing KCl, we addressed whether this affected intracellular Ca2+. We show that removing K+ (10 mM, 40 mM) had no effect on cCa2+ (Fig. S5). A potential caveat in our studies is that the concentration of solutes that ameloblast face at the basal and distal poles in vivo appear to be different (Aoba and Moreno, 1987), which may have a bearing in our in vitro studies where the cells are surrounded by the same solutions.
Basal c [Na + ], membrane potential, and Ca 2+ transients in response to changes in extracellular K + . (A) Quantification of basal cytosolic Na+ concentrations (c[Na+]) in secretory (SEC) and maturation (MAT) ameloblast stages loaded with the ratiometric cell-permeant Na+ probe SBFI. (B)c[Na+] calibrated to represent mM concentration based on data shown in A. Data represent the mean ± SEM of three independent experiments analyzed by two-tailed unpaired Student’s t test. n.s, non-significant. (C, E, and G) SEC and MAT ameloblasts loaded with the voltage probe FluoVolt to determine changes in membrane potential after perfusion with KCl (10, 40, 75 mM). (D, F, and H) Ca2+ transients in SEC and MAT ameloblasts loaded with the cytosolic Ca2+ probe Fura-2 AM in response to the KCl concentrations in C, E, and G. Data represent the mean ± SEM of ≥52 cells from three to six independent experiments analyzed by two-tailed unpaired Student’s t test; n.s, nonsignificant.
Basal c [Na + ], membrane potential, and Ca 2+ transients in response to changes in extracellular K + . (A) Quantification of basal cytosolic Na+ concentrations (c[Na+]) in secretory (SEC) and maturation (MAT) ameloblast stages loaded with the ratiometric cell-permeant Na+ probe SBFI. (B)c[Na+] calibrated to represent mM concentration based on data shown in A. Data represent the mean ± SEM of three independent experiments analyzed by two-tailed unpaired Student’s t test. n.s, non-significant. (C, E, and G) SEC and MAT ameloblasts loaded with the voltage probe FluoVolt to determine changes in membrane potential after perfusion with KCl (10, 40, 75 mM). (D, F, and H) Ca2+ transients in SEC and MAT ameloblasts loaded with the cytosolic Ca2+ probe Fura-2 AM in response to the KCl concentrations in C, E, and G. Data represent the mean ± SEM of ≥52 cells from three to six independent experiments analyzed by two-tailed unpaired Student’s t test; n.s, nonsignificant.
Calibration of SBFI. (A and B) An ionophore cocktail solution (nigericin 6 µM, monensin 6 µM, and gramicidin 2 mg/ml) was used to calibrate the probe SBFI. The traces represent averages of the fluorescence intensities of the ratiometric Na+ probe SBFI during stepwise changes in response to additions on [Na+] of 5, 10, 20, and 50 mM.
Calibration of SBFI. (A and B) An ionophore cocktail solution (nigericin 6 µM, monensin 6 µM, and gramicidin 2 mg/ml) was used to calibrate the probe SBFI. The traces represent averages of the fluorescence intensities of the ratiometric Na+ probe SBFI during stepwise changes in response to additions on [Na+] of 5, 10, 20, and 50 mM.
Removal of solutions containing K + (10 and 40 mM) had no effect on c Ca 2+ . (A and B) Ca2+ transients of Fura-2 AM loaded secretory and maturation stage ameloblasts in response to the removal of high K+ solution (10 or 40 mM KCl). Data represent the mean ± SEM of ≥52 cells from three to six independent experiments analyzed by two-tailed unpaired Student’s t test; n.s, non-significant.
Removal of solutions containing K + (10 and 40 mM) had no effect on c Ca 2+ . (A and B) Ca2+ transients of Fura-2 AM loaded secretory and maturation stage ameloblasts in response to the removal of high K+ solution (10 or 40 mM KCl). Data represent the mean ± SEM of ≥52 cells from three to six independent experiments analyzed by two-tailed unpaired Student’s t test; n.s, non-significant.
Overall Na+/Ca2+ exchange is more dominant in maturation
Next, to address the combined function of the two Na+/Ca2+ exchanger families in the Ca2+ influx mode, we loaded secretory and maturation stage ameloblasts with Fura-2 AM in a Ca2+ free Ringer solution containing 140 mM NaCl and 10 mM KCl to allow for a raise of intracellular Na+ levels (Kraev et al., 2001). This solution was replaced by a Ca2+-containing Ringer solution in the presence of K+, but Na+ was replaced by NMDG to maintain osmolarity. This experiment shows significantly higher Fura-2 AM fluorescence in maturation stage ameloblasts compared with secretory cells (Fig. 2 A), with the velocity of Ca2+ influx and the peak being significantly higher in maturation (Fig. 2, B and C). These results demonstrate that the combined function of NCX and NCKX is more dominant in the maturation stage.
Na + /Ca 2+ exchanger function is higher in maturation stage ameloblasts. (A) Original traces of Fura-2 AM–loaded secretory (SEC) and maturation (MAT) stage ameloblasts showing Ca2+ uptake by the Na+/Ca2+ exchangers in the Ca2+ influx mode. This function was elicited by maintaining cells in Na+- and K+-containing solution and replacing this with a solution containing NMDG (a Na+ replacement) in the presence of Ca2+ (2.5 mM CaCl) and K+ (40 mM KCl). (B) Quantification of the Ca2+ peak and Ca2+ influx rate. (C) Data represent the mean ± SEM of ≥84 cells from four independent experiments analyzed by two-tailed unpaired Student’s t test; *P < 0.05.
Na + /Ca 2+ exchanger function is higher in maturation stage ameloblasts. (A) Original traces of Fura-2 AM–loaded secretory (SEC) and maturation (MAT) stage ameloblasts showing Ca2+ uptake by the Na+/Ca2+ exchangers in the Ca2+ influx mode. This function was elicited by maintaining cells in Na+- and K+-containing solution and replacing this with a solution containing NMDG (a Na+ replacement) in the presence of Ca2+ (2.5 mM CaCl) and K+ (40 mM KCl). (B) Quantification of the Ca2+ peak and Ca2+ influx rate. (C) Data represent the mean ± SEM of ≥84 cells from four independent experiments analyzed by two-tailed unpaired Student’s t test; *P < 0.05.
NCKX is the main Na+/Ca2+ exchanger in ameloblasts
To further dissect the relative contribution of NCX and NCKX function in ameloblasts, we next stimulated secretory and maturation stage ameloblasts in the presence or absence of external K+ that is required for NCKX exchange (Cervetto et al., 1989; Schnetkamp et al., 1989). We showed above that perfusing ameloblasts with K+ containing bath solutions or removing K+ did not cause changes in cCa2+ (Fig. 1 D and Fig. S5) and, therefore, this approach could be used to differentiate between NCKX and NCX function. We perfused the cells with a Ca2+-free Ringer solution containing external Na+ before replacing the perfusate with Li+ to reverse the Na+ gradient, which is now high inside the cells (Blaustein and Lederer, 1999; Schnetkamp, 2004). We show that Fura-2 AM fluorescence is significantly higher in secretory and maturation stage ameloblasts in the presence of external K+ (Fig. 3, A and C, dark blue and red traces, respectively), required for NCKX activity, compared with the signals generated in the absence of K+, which rules out the involvement of NCKX (Fig. 3, A and C, light blue and red, respectively), as quantitated in Fig. 3, B and D. These data highlight an important distinction between NCX and NCKX function.
Differentiating NCKX and NCX function in ameloblasts. (A and C) Original traces of Fura-2 AM–loaded secretory (A; dark blue trace) and maturation (C; dark red trace) ameloblasts showing NCKX/NCX activity in Ca2+ influx mode. This mode was elicited by the replacement of Na+ with lithium (Li+) and the addition of Ca2+ in the presence of K+ solution (10 mM KCl). To distinguish NCX from NCKX function in Ca2+ influx mode, we halted NCKX activity by replacing the bath solutions with a K+-free solution evoking a decrease in Ca2+ transients in both secretory (light blue trace) and maturation (light red trace). Ca2+ transients were measured in the presence of the NCX inhibitor KBR7943 (30 min, 5 μM; gray traces in A and C). The black trace in A and C indicate Ca2+ fluxes in the absence of K+ but in the presence of KBR7943. (B and D) Quantification of the Ca2+ influx peak based on data from A and C. (E) Histogram comparing NCKX and NCX as a percentage of the decrease in the ∆Ca2+ peaks of Fura-2 AM fluorescence in B and D. Data represent the mean ± SEM of ≥41 cells from three to four independent experiments analyzed by one-way ANOVA followed by Tukey’s multiple comparison post-hoc test. *P < 0.05; **P < 0.01.
Differentiating NCKX and NCX function in ameloblasts. (A and C) Original traces of Fura-2 AM–loaded secretory (A; dark blue trace) and maturation (C; dark red trace) ameloblasts showing NCKX/NCX activity in Ca2+ influx mode. This mode was elicited by the replacement of Na+ with lithium (Li+) and the addition of Ca2+ in the presence of K+ solution (10 mM KCl). To distinguish NCX from NCKX function in Ca2+ influx mode, we halted NCKX activity by replacing the bath solutions with a K+-free solution evoking a decrease in Ca2+ transients in both secretory (light blue trace) and maturation (light red trace). Ca2+ transients were measured in the presence of the NCX inhibitor KBR7943 (30 min, 5 μM; gray traces in A and C). The black trace in A and C indicate Ca2+ fluxes in the absence of K+ but in the presence of KBR7943. (B and D) Quantification of the Ca2+ influx peak based on data from A and C. (E) Histogram comparing NCKX and NCX as a percentage of the decrease in the ∆Ca2+ peaks of Fura-2 AM fluorescence in B and D. Data represent the mean ± SEM of ≥41 cells from three to four independent experiments analyzed by one-way ANOVA followed by Tukey’s multiple comparison post-hoc test. *P < 0.05; **P < 0.01.
To further support that the Fura-2 AM signals generated in the K+-free bath solution were ascribed only to NCX, we reasoned that perfusing the cells with a specific NCX blocker would further decrease or abolish cCa2+ transients. Therefore, we maintained ameloblasts in a K+-free bath solution and treated them with an NCX-specific blocker, KB-R7943 (Iwamoto et al., 2001). This experiment showed that the Fura-2 AM signals were nearly abolished in secretory and maturation stage ameloblasts (Fig. 3, B and D, black traces in both). Additional validation resulted from exposing the cells to a K+-containing solution in the presence of KB-R7943, which elicited a decrease in the Fura-2 AM signal (Fig. 3 A and C, gray traces), reflecting the inhibition of NCX but not NCKX. These data confirm the functional participation of NCX in ameloblasts despite the lower mRNA expression levels of the SLC8A genes compared with the SLC24A genes. Based on the data shown in Fig. 3, B and D, we calculated the percentage of Fura-2 AM ratios generated in the presence or absence of K+, i.e., with/without NCKX involvement. We show that in both ameloblast stages, NCKX-mediated function was more pronounced compared with that of NCX-mediated function (Fig. 3 E). For example, in the maturation stage, NCKX accounted for 65% of Na+/Ca2+ exchanger function (Fig. 3 E).
Ameloblasts of Slc24a4−/− mice show significantly reduced cCa2+
We showed that NCKX-mediated function was more prominent than NCX-mediated function (Fig. 3 E). In addition, expression data indicate that NCKX4 is by far the dominant isoform of all the Na+/Ca2+ exchangers expressed in ameloblasts (Fig. S1). Therefore, it is expected that genetic deletion of SLC24A4 in ameloblasts would significantly reduce Na+/Ca2+ exchange. To address this and to determine the contribution of the NCKX4 isoform alone, we obtained the Slc24a4−/− mice described in Stephan et al. (2011), kindly shared by Dr. H. Zhao. The isolation of secretory and maturation stage ameloblasts in mice is more difficult than in rats because of the smaller size of the incisors in mice and because the delineation of secretory and maturation regions is not as well defined. Therefore, instead of separating cells by stage, we pooled secretory and maturation stage ameloblasts, as we have previously done in other KO mouse models (Eckstein et al., 2017, 2019), to gain an overall picture of the changes in Na+/Ca2+ exchange in the absence of NCKX4 alone. We show that loss of Slc24a4 in ameloblasts reduced Na+/Ca2+ exchange by ∼62% (Fig. 4, A and B). This percentage is similar to the reduction in Na+/Ca2+ exchange shown in Fig. 3 E (65%), confirming that the bulk of NCKX-mediated function in ameloblasts is driven by NCKX4.
Na + /Ca 2+ exchanger function is deficient in ameloblasts of Nckx4 −/− mice. (A) Representative original traces in Ca2+ influx mode of the Na+/Ca2+ exchangers in ameloblasts of Nckx4−/− mice and WT littermates. (B) Quantification of the Ca2+ influx peak based on data from A. Data represent the mean ± SEM of ≥38 cells from at least three independent experiments analyzed by two-tailed unpaired Student’s t test at *P < 0.05 versus control WT group.
Na + /Ca 2+ exchanger function is deficient in ameloblasts of Nckx4 −/− mice. (A) Representative original traces in Ca2+ influx mode of the Na+/Ca2+ exchangers in ameloblasts of Nckx4−/− mice and WT littermates. (B) Quantification of the Ca2+ influx peak based on data from A. Data represent the mean ± SEM of ≥38 cells from at least three independent experiments analyzed by two-tailed unpaired Student’s t test at *P < 0.05 versus control WT group.
Discussion
We have analyzed the expression and function of members of the Na+/Ca2+ exchanger families NCX and NCKX in rat ameloblasts. By far the most highly expressed gene in ameloblasts representing both exchanger families is Slc24a4 (coding for NCKX4; Figs. S1 and S2). Immunolocalization of NCKX4 in mouse molars and incisors showed that in the enamel organ, this protein is only found in ameloblasts, predominantly in the distal pole of maturation-stage ameloblasts near the enamel space where enamel crystals form (Fig. S3), a similar localization to what we and others have previously reported (Bronckers et al., 2015; Eckstein et al., 2017; Hu et al., 2012; Wang et al., 2014). A previous study had shown that NCX3, the most highly expressed gene of the NCX family in enamel cells (Fig. S1 A), was localized at the distal pole of the ameloblasts (Okumura et al., 2010). This subcellular localization of NCKX4 and NCX3 supports the notion that these exchangers participate in the extrusion of Ca2+ distally, providing the necessary Ca2+ required for crystal growth in the extracellular space. Because of the similar localization of both NCX3 and NCKX4, we next investigated protein expression levels by Western blot and showed that while NCX3 is relatively abundant in the secretory stage, its expression declines in the maturation stage (Fig. S2). Conversely, the expression of NCKX4 is difficult to detect in the secretory stage and vastly increases in maturation, the stage when the Ca2+ transport machinery is upregulated as crystals experience significant growth in thickness (Costiniti et al., 2021; Hubbard, 2000; Lacruz, 2017; Lacruz et al., 2017; Smith, 1998).
Next, to address whether the NCX and NCKX exchangers are in fact functional proteins in enamel cells, we isolated rat ameloblasts from the secretory and maturation stages. The isolation of single cells has been a very successful approach to investigating Ca2+ dynamics in ameloblasts, including the analysis of SOCE (Aulestia et al., 2020; Eckstein et al., 2017, 2019; Nurbaeva et al., 2018; Souza Bomfim et al., 2020), the modulation of cytosolic Ca2+ by mitochondria (Costiniti et al., 2022) as well as PMCA function (Bomfim et al., 2023). Although the isolated ameloblast preparations do not appear to maintain the morphology of the tall columnar, polarized cells in situ, they do retain the properties of the membrane channels they express, such as their ability to sustain Ca2+ influx via the ORAI1 channel, which is expressed at the basolateral membrane (Eckstein and Lacruz, 2018), as we have shown (Eckstein et al., 2017, 2019; Nurbaeva et al., 2015a). Thus, we aimed to determine if ameloblasts would also retain properties associated with Na+/Ca2+ exchange.
NCX and NCKX primarily mediate Ca2+ removal out of cells based on the Na+ gradient, the Ca2+ gradient, and the membrane potential (Philipson and Nicoll, 2000). However, this can be reversed in physiological conditions during membrane depolarization, although the physiological role of the Ca2+ influx mode is unclear (Philipson and Nicoll, 2000). In our study, to determine Na+/Ca2+ exchange, we analyzed Fura-2 AM fluorescence generated byboth exchangers in the Ca2+ influx mode by modifying ionic concentrations in the bath solutions as this is a commonly used method validated by several groups (Cervetto et al., 1989; Jalloul et al., 2016a, 2016b; Kraev et al., 2001; Lytton et al., 2002; Schnetkamp, 1986; Schnetkamp et al., 1991). First, we found that perfusing ameloblasts with a K+-containing bath solution or removing this perfusate had no effect on Fura-2 AM fluorescence in secretory or in maturation stage ameloblasts (Fig. 1 and Fig. S5). This is indicative of a lack of an apparent voltage-gated Ca2+ channel function in these epithelial cells. Our analysis showed that both secretory and maturation stage ameloblasts are capable of Na+/Ca2+ exchange, but maturation stage ameloblasts showed higher capacity (Fig. 2).
To further dissect possible differences in the contributions made by NCX and NCKX, we analyzed the Ca2+ influx mode in the absence of K+, to eliminate NCKX function, and in the presence of the NCX blocker KBR7943, to eliminate NCX function. In the absence of K+, there was a reduction of ~65% in Na+/Ca2+ exchange function in both stages, suggesting that the contribution of NCKX function is greater than NCX (Fig. 3). In conditions when the cells were exposed to no K+ in the presence of KBR7943, the Fura-2 AM signals were almost completely abolished (Fig. 3). Further, we also observed that ameloblasts of Scl24a4−/− mice showed a reduction in cCa2+ of ∼66% confirming the primacy of NKCX4 isoform in ameloblasts. This likely explains why patients with mutations in SLC24A4 suffer from severe enamel hypomineralization, clinically referred to as amelogenesis imperfecta (Parry et al., 2013; Wang et al., 2014), while to date, no mutations in the NCX coding genes have been linked to enamel defects. It is interesting to note that, based on the severe effects caused by NCKX4 mutations, it would appear that neither PMCA nor NCX3 can replace NCKX4 in proper ameloblast function.
Na+/Ca2+ exchange operates independently of the PMCA Ca2+ pumps. However, many cells express all three systems including the ameloblasts (Bomfim et al., 2023), posing the question of why this replication in Ca2+ clearance mechanisms might be required. A distinction in the Ca2+ clearance dynamics has been suggested as an obvious cause (Blaustein and Lederer, 1999). PMCA pumps have a high Ca2+ affinity but a Ca2+ extrusion capacity of ∼150 Ca2+/s, significantly lower than the exchangers (Brini et al., 2012; Cali et al., 2017; Baazov et al., 1999; Hilgemann, 1996; Jalloul et al., 2016b). We recently demonstrated that PMCA function in ameloblasts was dependent on cCa2+ availability whereby the pumps are more dominant at low levels of cCa2+ elevation and were more effective during the secretory stage (Bomfim et al., 2023). While data on ameloblasts and other cells reinforce the notion that PMCA is efficient at clearing moderate levels of cCa2+, the requirements for utilizing two families of Na+/Ca2+ exchangers remains an interesting research area.
Both NCKX4 and members of the NCX family are expressed at the distal pole of the ameloblasts (Hu et al., 2012; Okumura et al., 2010; Wang et al., 2014), the site that would be most efficient to extrude Ca2+ out of the ameloblasts into the enamel space. However, whether these exchangers cooperate synergistically to extrude Ca2+ is unknown. Although NCKX dominates Ca2+ extrusion function in ameloblasts, our data show that NCX also contributes to this task. This is evidenced by the presence of Fura-2 AM signals in ameloblasts of Slc24a4−/− mice or in rat ameloblasts after removal of external K+, indicative of a K+-independent exchanger, i.e., NCX. Perhaps the lack of reports on enamel defects associated with loss of NCX function might indicate redundancy of the NCX isoforms, whereas the expression of the NCKX4 isoform in maturation stage ameloblasts is so dominant that it prevents functional compensation by other members of the NCKX family.
A question that remains is why the contribution of NCKX appears critical when both NCKX4 and NCX3 are expressed in the ameloblasts and contribute to the Ca2+ fluxes described here. There are differences in Ca2+ kinetics and transport stoichiometry between these two exchanger families. Recently, the rate of transport of members of the NCKX family was described by one of us showing that NCKX4 had transport rates ∼560 Ca2+/s when expressed in HEK-293 cells (Jalloul et al., 2016b), significantly higher than PMCAs but lower than NCXs, which has been reported to be ∼5,000 Ca2+/s (Juhaszova et al., 2000). But why do ameloblasts predominantly use NCKX4 for cCa2+ clearance rather than the NCX proteins? We suggest that this is linked to the cellular demands of the ameloblasts. For instance, NCXs are well known for their role in cardiac excitation–contraction (Eisner et al., 2017; Ottolia et al., 2013), responding to the action of voltage-gated Ca2+ channels capable of generating very short inward fluxes of several tens of thousands of Ca2+/s (Catterall, 2011). By contrast, Ca2+ influx in ameloblasts relies on the SOCE mediated by the ORAI channels (Eckstein et al., 2019; Nurbaeva et al., 2015a, 2015b, 2018), whose unitary conductance is several orders of magnitude lower than the voltage-gated channels but results in more sustained Ca2+ fluxes (Prakriya and Lewis, 2015). This may be more like the well-understood role of NCKX1 in retinal rod photoreceptors where a sustained influx of Ca2+ entering the rod outer segment via the cGMP-gated channels in darkness under conditions of a depolarized membrane potential is extruded via NCKX1 as discussed (Cervetto et al., 1989; Schnetkamp et al., 1989, 1991) or via an NCKX2:NCKX4-mediated response as reported more recently for cone photoreceptors (Vinberg et al., 2017). Because enamel crystallites precipitate from a supersaturated solution in which the degree of saturation of the enamel fluid is regulated, with Ca2+ playing a central role in the thermodynamic properties of the process (Simmer and Fincham, 1995), we suggest that the sustained Ca2+ fluxes of relatively low magnitude by SOCE may have been coopted for with the slower extrusion rates by NCKX4 to meet the cellular demands of ameloblasts facilitating the precipitation of ions in a manner that supports the thermodynamic regulation of enamel mineralization. However, we cannot discount the possibility that the equilibrium potential of [Na+] and [K+] in the distal membrane of the maturation stage ameloblast may influence the energy required to extrude Ca2+ into the extracellular fluid such that the stoichiometry of NCKX4 provides the required energy to drive this exchange. However, the ionic concentrations in the extracellular space in the maturation stage are unknown, although we suspect that [Ca2+] should be higher than in secretory stage enamel fluid given the overall increase in Ca2+ influx and extrusion in the former.
Although the coupling ratio of the exchangers is the same in the influx and efflux modes (Blaustein and Lederer, 1999), a potential caveat in our interpretation is our assumption that the Ca2+ influx rates of NCX and NCKX are similar to the outward rates. While this could be true in ameloblasts, it is reported that Ca2+ binding in NCX is asymmetrical in the internal and external surfaces of the exchanger (Almagor et al., 2014; Blaustein and Lederer, 1999; Giladi et al., 2016). Studies in pig cardiomyocytes, squid and rat neurons, and all excitable cells, there are documented differences between the apparent inward- and outward-facing Ca2+ binding sites (DiPolo and Beaugé, 1988; Fontana et al., 1995; Miura and Kimura, 1989). Despite this, the dominant expression of NCKX4 in ameloblasts and the fact that this exchanger is essential for enamel mineralization (Wang et al., 2014) lends support to our observation that Ca2+ extrusion in ameloblasts is largely regulated by this exchanger. Therefore, it is plausible that the differences in the inward Ca2+ kinetics we observed between NCX and NCKX in these cells may reflect apparent differences in their physiological roles.
Data shown here contributes to our understanding of the Ca2+ signaling toolkit of ameloblasts and how this system contributes to enamel mineralization. Previous research highlighted that the bulk mineralization of the enamel crystals takes place during the maturation stage as mineral uptake rates and Ca2+ acquisition rates increase during that stage (Smith, 1998; Smith et al., 2005). Recent evidence indicates that the expression and function of the molecular components of SOCE are upregulated during the maturation stage (Aulestia et al., 2020; Nurbaeva et al., 2015a, 2018; Souza Bomfim et al., 2020). The Ca2+ extrusion toolkit of most cells includes the PMCA and Na+/Ca2+ exchange (Brini and Carafoli, 2011; Cali et al., 2017). In ameloblasts, PMCA function operates in both secretory and maturation stages but dominates Ca2+ extrusion during the secretory stage when Ca2+ requirements are low (Bomfim et al., 2023). By contrast, maturation stage ameloblasts have higher Na+/Ca2+ exchanger activity, with NCKX4 as the predominant exchanger function. Lack of data on mutations involving PMCA or NCX coding genes resulting in enamel disease contrasts with the severe hypomineralization defects in patients and mice with defective NCKX4 (Parry et al., 2013; Wang et al., 2014). Therefore, functional studies support the dominance of the K+-dependent NCKX proteins as important contributors to ameloblast Ca2+ extrusion and hence to enamel mineralization.
Data availability
The data that support the findings of this study are available in the methods and/or supplementary material of this article.
Acknowledgments
David A. Eisner served as editor.
This work was funded by the National Institute for Dental and Craniofacial Research grants DE025639 and DE027679 to R.S. Lacruz.
Author contributions: G.H. Souza Bomfim, P.P.M. Schnetkamp, and R.S. Lacruz designed the research studies. G.H. Souza Bomfim and E. Mitaishvili performed the experiments. G.H. Souza Bomfim, E. Mitaishvili, P.P.M. Schnetkamp, and R.S. Lacruz analyzed the data. G.H. Souza Bomfim, P.P.M. Schnetkamp, and R.S. Lacruz wrote the paper. All authors contributed to the final preparation of the manuscript.
References
Author notes
Disclosures: The authors declare no competing interests exist.




![Basalc[Na+], membrane potential, and Ca2+transients in response to changes in extracellular K+. (A) Quantification of basal cytosolic Na+ concentrations (c[Na+]) in secretory (SEC) and maturation (MAT) ameloblast stages loaded with the ratiometric cell-permeant Na+ probe SBFI. (B)c[Na+] calibrated to represent mM concentration based on data shown in A. Data represent the mean ± SEM of three independent experiments analyzed by two-tailed unpaired Student’s t test. n.s, non-significant. (C, E, and G) SEC and MAT ameloblasts loaded with the voltage probe FluoVolt to determine changes in membrane potential after perfusion with KCl (10, 40, 75 mM). (D, F, and H) Ca2+ transients in SEC and MAT ameloblasts loaded with the cytosolic Ca2+ probe Fura-2 AM in response to the KCl concentrations in C, E, and G. Data represent the mean ± SEM of ≥52 cells from three to six independent experiments analyzed by two-tailed unpaired Student’s t test; n.s, nonsignificant.](https://cdn.rupress.org/rup/content_public/journal/jgp/156/1/10.1085_jgp.202313372/1/m_jgp_202313372_fig1.png?Expires=1773766483&Signature=tC9FSrjMxW33~ckQ~HWX0c2BoMGwgtA8D65MpMz9ApmJuomFyNW0mQeBZsBhBx30tldIWplm0pGT8oUxhsi~J~PFG3Jfx8tgczXvlqhBzCJoxe-Q-zCnmB~9OcTo1T2XA4Rhn5kQx9HMxFtds7Kypl2QLy1NdS8APEoDSlb4PhFdoFs8mCUnf4Pn9EtDbZEBEkDX4wlpDU8oYAs4VKPkVrql2TK7FpHR41rtO7WroaGtB0v1FUIlkBXf8EU6yuoKhP4bWHkJk9g6UAKvYpQPNEd5fNWHAeFsBBjiNngtw3ZRKnu1XwaQuCbV1ZTIOf7HcLTDo316kY~Nq6OJ8I11ZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Calibration of SBFI. (A and B) An ionophore cocktail solution (nigericin 6 µM, monensin 6 µM, and gramicidin 2 mg/ml) was used to calibrate the probe SBFI. The traces represent averages of the fluorescence intensities of the ratiometric Na+ probe SBFI during stepwise changes in response to additions on [Na+] of 5, 10, 20, and 50 mM.](https://cdn.rupress.org/rup/content_public/journal/jgp/156/1/10.1085_jgp.202313372/1/m_jgp_202313372_figs4.png?Expires=1773766483&Signature=OpjLHnuViXDZVQDIA-vo7fY7DUVQLO2PPDll5A8e954wtwziRYaOUrw5yDPdhQkfdwiaggWgqcHB8G0ZIfMOl2vwA-ItR228Orf9jwjYMwyjIgPtls0jh7TTC9heHgIStzSy9zNT6Oso-~jgU18mHkFhm02q2k0-yU7PDmPLhcDWZ7EwlVKUjeb1KiQ05Ct~k4SZQGAfbYjz3jSoBsf6C7UmcW8-v2eQfLtoqCJ--Dp33xshywZARhFI3r9EfhiDcOv8uVTJ4zN35zSs8Bnp5Ndt2ak~aZZF2RSBj0bcNnCAq9uAvqVQNPgTPiXAgAvHXd4h800vULOnono84oDDww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)






