Gain-of-function mutations in the LRRK2 gene cause Parkinson’s disease (PD), characterized by debilitating motor and non-motor symptoms. Increased phosphorylation of a subset of RAB GTPases by LRRK2 is implicated in PD pathogenesis. We find that increased phosphorylation of RAB3A, a cardinal synaptic vesicle precursor (SVP) protein, disrupts anterograde axonal transport of SVPs in iPSC-derived human neurons (iNeurons) expressing hyperactive LRRK2-p.R1441H. Knockout of the opposing protein phosphatase 1H (PPM1H) in iNeurons phenocopies this effect. In these models, the compartmental distribution of synaptic proteins is altered; synaptophysin and synaptobrevin-2 become sequestered in the neuronal soma with decreased delivery to presynaptic sites along the axon. We find that RAB3A phosphorylation disrupts binding to the motor adaptor MADD, potentially preventing the formation of the RAB3A–MADD-KIF1A/1Bβ complex driving anterograde SVP transport. RAB3A hyperphosphorylation also disrupts interactions with RAB3GAP and RAB-GDI1. Our results reveal a mechanism by which pathogenic hyperactive LRRK2 may contribute to the altered synaptic homeostasis associated with characteristic non-motor and cognitive manifestations of PD.
Introduction
Parkinson’s disease (PD) is a devastating neurodegenerative disease that causes cardinal motor symptoms: rest tremor, rigidity, bradykinesia, and postural instability (Kalia and Lang, 2015). These are manifestations of the loss of select neuronal populations, most prominently dopaminergic neurons in the substantia nigra pars compacta (SNc). In addition to these motor symptoms, PD is clinically characterized by debilitating non-motor symptoms such as cognitive decline, dementia, sleep disturbance, and depression (Kalia and Lang, 2015), suggesting that pathogenic mechanisms may also alter synaptic transmission in a broader set of neuronal populations.
Autosomal dominant missense mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are the most common genetic cause of PD, accounting for ∼5% of familial cases (Healy et al., 2008). Furthermore, genome-wide association studies have implicated LRRK2 noncoding variants in sporadic PD. Seven gain-of-function pathogenic mutations in LRRK2 increase LRRK2 kinase activity, resulting in elevated phosphorylation of a subset of RAB GTPases (RABs) (Steger et al., 2016). RABs coordinate vesicle trafficking by selectively associating with membrane compartments and recruiting effector proteins (Pfeffer, 2017). Mounting evidence shows that LRRK2-mediated phosphorylation of RABs alters their binding properties, either by introducing a new set of binding partners (Waschbüsch et al., 2020; Bonet-Ponce et al., 2020; Kluss et al., 2022; Boecker et al., 2021; Pal et al., 2023) or by impairing interaction with previous partners (Steger et al., 2016, 2017; Mamais et al., 2021). Therefore, the relative activity of LRRK2 and its opposing protein phosphatase 1H (PPM1H) regulates RAB binding to effectors (Dou et al., 2023; Berndsen et al., 2019).
In recent work, we demonstrated that LRRK2-mediated RAB hyperphosphorylation has consequences for retrograde axonal transport of autophagic vesicles (AVs), disrupting an interplay of motor regulators in a manner scaling with magnitude of LRRK2 kinase activity (Dou et al., 2023). Neurons require the directed transport of a wide range of distinct axonal cargoes to maintain homeostasis and synaptic function, in addition to AVs. Given the cognitive impairments and other non-motor manifestations of PD, an axonal cargo of particular interest is the synaptic vesicle precursor (SVP). SVPs arise in the neuronal soma and are transported anterogradely by kinesin-3 family members KIF1A and KIF1Bβ, carrying proteins that are fated for mature synaptic vesicles (SVs) at presynaptic sites (Nakata et al., 1998; Mundigl et al., 1993; Niwa et al., 2008, 2017; Hummel and Hoogenraad, 2021; De Pace et al., 2020; Goldstein et al., 2008), including numerous en passant synapses populating the complex axonal arbor. These SV proteins are only recycled for a limited time before being targeted for degradation, necessitating robust delivery of new SVPs to replenish SV machinery (Truckenbrodt et al., 2018; Guedes-Dias et al., 2019).
Anterograde SVP transport is initiated by the formation of a complex between RAB3, KIF1A/1Bβ, and a protein called “differentially expressed in normal and neoplastic cells/MAP kinase activating death domain” (DENN/MADD, henceforth referred to as MADD) (Niwa et al., 2008; Hummel and Hoogenraad, 2021). All three components of this complex are essential for the rapid, long-range transport of SVPs. MADD is a large ∼183 kDa protein that is also known as RAB3-GEP (guanine nucleotide exchange protein) due to its role as a GDP-GTP exchange factor (GEF) for RAB3 (Brown and Howe, 1998). Indeed, previous work has shown that the anterograde transport of SVPs depends on the GTP-bound state of RAB3 (Niwa et al., 2008; Südhof, 1997).
RAB3 exists in four isoforms (RAB3A/B/C/D), with RAB3A being the most abundant in the brain cortex, although all four isoforms act redundantly in neurons (Schlüter et al., 2002, 2004). Importantly, all four isoforms are endogenous substrates of LRRK2 (Steger et al., 2016, 2017) and are dephosphorylated by PPM1H (Berndsen et al., 2019). However, the downstream consequences of LRRK2-mediated RAB3 phosphorylation on axonal transport have not been explored.
Here, we demonstrate that the hyperactive LRRK2-p.R1441H mutation reduces the anterograde axonal flux of SVPs in gene-edited iPSC-derived human neurons (iNeurons). As an orthogonal model of RAB3 hyperphosphorylation, we show that knock-out (KO) of PPM1H phenocopies the effect of p.R1441H KI, indicating an important balance between LRRK2 and its opposing phosphatase for the regulation of SVP transport. This transport deficit alters the distribution of SVP-associated proteins within the neuron, causing somal sequestration and decreased delivery to presynaptic sites in a heterologous synaptic model. Increasing levels of active RAB3 induced by overexpression of a GTP-locked mutant rescued the SVP transport deficit in p.R1441H KI iNeurons. Using a coimmunoprecipitation approach, we show that phosphorylation of RAB3A at the threonine 86 (T86) residue disrupts its binding to the motor adaptor protein MADD and the regulatory proteins RAB-GDI1 and RAB3GAP but does not indiscriminately disrupt interactions with other known RAB3 binding partners. Together, our results uncover a mechanism by which pathogenic hyperactive LRRK2 mutations may contribute to synaptic dysfunction manifesting as debilitating motor and non-motor PD symptoms.
Results
Endogenous LRRK2-p.R1441H impairs anterograde axonal transport of synaptic vesicle precursors
Point mutations at the p.R1441 hotspot (p.R1441C/G/H) in LRRK2 are pathogenic for Parkinson’s disease (PD) and have high penetrance (Haugarvoll et al., 2008; Klein and Westenberger, 2012; Domingo and Klein, 2018; Ruiz-Martínez et al., 2010). Previous reports have shown that these gain-of-function mutations induce hyperactivity of the LRRK2 kinase domain in multiple systems (Steger et al., 2016; Dou et al., 2023; Kalogeropulou et al., 2022) including neurogenin-2 (NGN2)-induced human neurons that express endogenous LRRK2 (Boecker et al., 2021; Bieri et al., 2019; Fonseca-Ornelas et al., 2022). One RAB GTPase that is phosphorylated by LRRK2 is RAB3A, which is associated with SVPs (Schlüter et al., 2004) and is essential for the anterograde axonal transport of SVPs (Niwa et al., 2008; Hummel and Hoogenraad, 2021). Given reports of altered binding properties of pRABs (Steger et al., 2016; Waschbüsch et al., 2020; Bonet-Ponce et al., 2020; Kluss et al., 2022; Boecker et al., 2021; Pal et al., 2023; Steger et al., 2017), we hypothesized that LRRK2-p.R1441H impairs anterograde axonal SVP transport via aberrant phosphorylation of RAB3A.
To test this hypothesis, we employed human-induced pluripotent stem cells (iPSCs) with heterozygous KI of LRRK2-p.R1441H. These iPSCs had been gene-edited by the iPSC Neurodegenerative Disease Initiative (iNDI) at the NIH (Ramos et al., 2021) to introduce the p.R1441H mutation at the endogenous LRRK2 locus of the KOLF2.1J parental line. Using tetracycline-inducible expression of NGN2, we differentiated these iPSCs into excitatory glutamatergic neurons (iNeurons) (Pantazis et al., 2022). The resulting p.R1441H KI iNeurons exhibit elevated RAB phosphorylation, as previously shown using an antibody pan-specific to multiple phosphorylated RABs including RAB3A (Dou et al., 2023). To assess SVP trafficking in these mutant iNeurons and corresponding WT control iNeurons, we live-imaged SVPs labeled by the fluorescent reporter mScarlet-synaptophysin (SYP) (Fig. 1 A). We imaged each neuron at the proximal axon to limit variability caused by axonal branchpoints at a distance of ∼100 μm from the soma to avoid the axonal initial segment. To more clearly visualize SYP+ vesicles entering the imaged axonal region, we photobleached the field of view prior to imaging to deplete pre-existing mScarlet-SYP signal in the axon (Fig. 1 A).
LRRK2-p.R1441H knockin causes kinase-dependent decrease in anterograde axonal SVP flux. (A) Inset, below: example time-lapse images of mScarlet-SYP+ vesicles in the proximal axon of a DIV21 WT iPSC-derived neuron (iNeuron). Cytoplasmic mScarlet-SYP signal was photobleached at t = 0s. (B) Kymographs of axonal mScarlet-SYP+ vesicles in DIV21 WT and p.R1441H KI iNeurons. Inset, left: Example traces of anterograde and retrograde SYP+ vesicles. (C–E) Anterograde flux (C), anterograde velocity (D), and retrograde flux (E) of SYP+ vesicles in WT and p.R1441H KI iNeurons (n = 24–33 neurons from three independent experiments; ns, not significant, P = 0.8628 for antero velocity, P = 0.2978 for retro vesicles; ***P < 0.001; linear mixed effects model). (F) Cartoon depicting p.R1441H KI iNeuron treated overnight with DMSO or 100 nM MLi-2. (G) Kymographs of axonal mScarlet-SYP+ vesicles in p.R1441H KI iNeurons treated with DMSO or MLi-2. (H–J) Anterograde flux (H), anterograde velocity (I), and retrograde flux (J) of SYP+ vesicles in p.R1441H KI iNeurons treated with DMSO or MLi-2 (n = 29–30 neurons from three independent experiments; ns, not significant, P = 0.2344 for antero velocity, P = 0.6735 for retro vesicles; ***P < 0.001; linear mixed effects model). Scatter plot points indicate the means of three independent experiments and error bars show the mean ± SD of these points.
LRRK2-p.R1441H knockin causes kinase-dependent decrease in anterograde axonal SVP flux. (A) Inset, below: example time-lapse images of mScarlet-SYP+ vesicles in the proximal axon of a DIV21 WT iPSC-derived neuron (iNeuron). Cytoplasmic mScarlet-SYP signal was photobleached at t = 0s. (B) Kymographs of axonal mScarlet-SYP+ vesicles in DIV21 WT and p.R1441H KI iNeurons. Inset, left: Example traces of anterograde and retrograde SYP+ vesicles. (C–E) Anterograde flux (C), anterograde velocity (D), and retrograde flux (E) of SYP+ vesicles in WT and p.R1441H KI iNeurons (n = 24–33 neurons from three independent experiments; ns, not significant, P = 0.8628 for antero velocity, P = 0.2978 for retro vesicles; ***P < 0.001; linear mixed effects model). (F) Cartoon depicting p.R1441H KI iNeuron treated overnight with DMSO or 100 nM MLi-2. (G) Kymographs of axonal mScarlet-SYP+ vesicles in p.R1441H KI iNeurons treated with DMSO or MLi-2. (H–J) Anterograde flux (H), anterograde velocity (I), and retrograde flux (J) of SYP+ vesicles in p.R1441H KI iNeurons treated with DMSO or MLi-2 (n = 29–30 neurons from three independent experiments; ns, not significant, P = 0.2344 for antero velocity, P = 0.6735 for retro vesicles; ***P < 0.001; linear mixed effects model). Scatter plot points indicate the means of three independent experiments and error bars show the mean ± SD of these points.
In WT iNeurons, we observed rapid, highly-processive SVPs traveling in the anterograde direction (Fig. 1 B). To accommodate the high speed of these vesicles, we imaged each axon at five frames per second for 5 min. We observed the anterograde population of SYP+ vesicles to be more numerous, rapid, and processive than the retrograde population (Fig. 1 B, inset), consistent with our previous observations in WT primary mouse hippocampal neurons (Guedes-Dias et al., 2019) and in WT iNeurons from a different parental line (Aiken and Holzbaur, 2024). In LRRK2-p.R1441H KI iNeurons, we observed a significant decrease in anterograde SVP flux (Fig. 1, B and C). However, there was no effect on the velocity of anterograde vesicles (Fig. 1 D), indicating that SVPs that entered the axon were transported normally. We did not observe a change in the flux of retrograde SYP+ vesicles (Fig. 1 E), suggesting that the expression of mutant LRRK2 specifically affected the anterograde population.
To confirm that this effect is dependent on LRRK2 kinase activity, we applied the selective LRRK2 kinase inhibitor MLi-2 (Fell et al., 2015) to p.R1441H KI iNeurons (Fig. 1 F). Overnight treatment with 100 nM MLi-2 rescued anterograde SVP flux (Fig. 1, G and H) without affecting anterograde velocity (Fig. 1 I). MLi-2 treatment had no effect on the flux of retrograde SYP+ vesicles (Fig. 1 J). In parallel experiments, we also examined SVP flux in i3Neurons (Boecker et al., 2021; Fernandopulle et al., 2018; Boecker et al., 2020) gene-edited from the WTC11 parental line to express the common pathological p.G2019S variant of LRRK2. This mutation also hyperactivates kinase activity but to a lesser extent than the p.R1441H mutation (Steger et al., 2016; Dou et al., 2023; Kalogeropulou et al., 2022). In p.G2019S KI i3Neurons, we found that overnight treatment with 100 nM MLi-2 increased anterograde SVP flux compared with DMSO treatment (Fig. S1, A–C), albeit to a lesser extent than what was observed in iNeurons expressing p.R1441H.
Related to Fig. 1 . (A) Cartoon depicting p.G2019S KI i3Neuron treated overnight with DMSO or 100 nM MLi-2. (B) Kymographs of axonal mScarlet-SYP+ vesicles in p.G2019S KI i3Neurons treated with DMSO or MLi-2. (C) Anterograde flux of SYP+ vesicles in p.G2019S KI i3Neurons treated with DMSO or MLi-2 (n = 39–41 neurons from four independent experiments; ***P < 0.001; linear mixed effects model). Scatter plot points indicate the means of four independent experiments, and error bars show mean ± SD of these points.
Related to Fig. 1 . (A) Cartoon depicting p.G2019S KI i3Neuron treated overnight with DMSO or 100 nM MLi-2. (B) Kymographs of axonal mScarlet-SYP+ vesicles in p.G2019S KI i3Neurons treated with DMSO or MLi-2. (C) Anterograde flux of SYP+ vesicles in p.G2019S KI i3Neurons treated with DMSO or MLi-2 (n = 39–41 neurons from four independent experiments; ***P < 0.001; linear mixed effects model). Scatter plot points indicate the means of four independent experiments, and error bars show mean ± SD of these points.
Together, these results show a kinase activity-dependent decrease in anterograde axonal SVP flux caused by hyperactive LRRK2. Importantly, we did not observe altered anterograde SVP velocity, nor did we observe altered retrograde flux, suggesting that p.R1441H’s effect is specific to decreasing the number of SVPs that enter the axon from the soma.
PPM1H KO phenocopies the effect of hyperactive LRRK2 on anterograde SVP flux in the axon
PPM1H is a phosphatase that opposes the activity of LRRK2 through dephosphorylation of RAB GTPases (Fig. 2 A) (Berndsen et al., 2019; Jumper et al., 2021; Varadi et al., 2022). Therefore, the balance of LRRK2 and PPM1H activity has the potential to regulate neuronal pathways, including the transport of axonal cargoes, by modulating RAB phosphorylation levels (Dou et al., 2023). We previously generated PPM1H KO iPSCs from the same KOLF2.1J parental line as the p.R1441H KI iPSCs used here (Dou et al., 2023). To determine whether loss of PPM1H would phenocopy the effect of hyperactive LRRK2-p.R1441H on anterograde SVP flux, we compared mScarlet-SYP motility in WT and PPM1H KO iNeurons (Fig. 2 B). Indeed, we observed decreased anterograde axonal flux of the SYP+ population upon loss of PPM1H (Fig. 2, C and D). Similar to observations in p.R1441H KI neurons, there was no change in anterograde velocity in PPM1H KO cells (Fig. 2 E). While we did observe a significant decrease in retrograde SYP+ vesicles in PPM1H KO iNeurons (Fig. 2 F), the size of the effect was much smaller than the effect on the anterograde population (Fig. 2 D).
Loss of LRRK2-opposing PPM1H decreases anterograde axonal SVP flux. (A) Schematic depicting opposing regulation of RAB3A phosphorylation state at threonine 86 by LRRK2 kinase and PPM1H phosphatase. Protein structures: LRRK2 (PDB: 7LHT), PPM1H (PDB: 7L4J), RAB3A (AlphaFold prediction [Jumper et al., 2021; Varadi et al., 2022]). (B) Cartoon depicting WT or PPM1H KO iNeuron expressing mScarlet-SYP. (C) Kymographs of axonal mScarlet-SYP+ vesicles in DIV21 WT and PPM1H KO iNeurons. (D–F) Anterograde flux (D), anterograde velocity (E), and retrograde flux (F) of SYP+ vesicles in WT and PPM1H KO iNeurons (n = 28 neurons from three independent experiments; ns, not significant, P = 0.6872; *P = 0.0119; ***P < 0.001; linear mixed effects model). Scatter plot points indicate the means of three independent experiments, and error bars show mean ± SD of these points.
Loss of LRRK2-opposing PPM1H decreases anterograde axonal SVP flux. (A) Schematic depicting opposing regulation of RAB3A phosphorylation state at threonine 86 by LRRK2 kinase and PPM1H phosphatase. Protein structures: LRRK2 (PDB: 7LHT), PPM1H (PDB: 7L4J), RAB3A (AlphaFold prediction [Jumper et al., 2021; Varadi et al., 2022]). (B) Cartoon depicting WT or PPM1H KO iNeuron expressing mScarlet-SYP. (C) Kymographs of axonal mScarlet-SYP+ vesicles in DIV21 WT and PPM1H KO iNeurons. (D–F) Anterograde flux (D), anterograde velocity (E), and retrograde flux (F) of SYP+ vesicles in WT and PPM1H KO iNeurons (n = 28 neurons from three independent experiments; ns, not significant, P = 0.6872; *P = 0.0119; ***P < 0.001; linear mixed effects model). Scatter plot points indicate the means of three independent experiments, and error bars show mean ± SD of these points.
RAB hyperphosphorylation alters the compartmental distribution of SVP-associated proteins
SVP transport has the important role of replenishing presynaptic sites with SV proteins (Truckenbrodt et al., 2018; Guedes-Dias et al., 2019). We next asked whether decreased anterograde transport of SVPs caused by RAB hyperphosphorylation has consequences on the distribution of synaptic proteins within the neuron. Specifically, we interrogated the localization of two SV proteins known to be trafficked with SVPs and delivered to presynaptic sites: SYP and synaptobrevin-2 (SYB2).
To examine somal content, we stained endogenous SYP and SYB2 in p.R1441H KI and PPM1H KO iNeurons, as well as control WT KOLF2.1J iNeurons. An antibody to endogenous microtubule-associated protein-2 (MAP2) signal was used to visualize the somatodendritic compartment (Fig. 3 A). In both RAB-hyperphosphorylated conditions, we detected significant increases in the somal intensity of SYP (Fig. 3, A and B) and SYB2 (Fig. 3, A and C), with no associated changes in somal size, as quantified by the total area of somal MAP2 signal (Fig. S2 A). Western blots of neuronal lysates showed no evidence of changed levels of SYP, SYB2, or RAB3A (Fig. S2, B–D).
Delivery of synaptic vesicle proteins from soma to presynaptic sites is disrupted by RAB hyperphosphorylation. (A) Representative images of DIV14 WT, p.R1441H KI, and PPM1H KO iNeuron somas, stained for endogenous MAP2, SYP, and SYB2. (B and C) Normalized somal intensity (mean gray value) of SYP (B) and SYB2 (C) in WT, p.R1441H KI, and PPM1H KO iNeurons (n = 24 neurons from three independent experiments; **P = 0.0027; ***P < 0.001; linear mixed effects model). (D) Cartoon depicting heterologous synapse model set-up, where HEK293T cells expressing pBI-BFP-NL1 were introduced in coculture to WT, p.R1441H KI, or PPM1H KO iNeurons. Dishes were imaged at iNeuron DIV14, at which point βIII-tubulin+ iNeuron axons had selectively formed presynapses with NL1+ transfected HEK cells (inset, bottom), but not untransfected HEK cells (inset, top). (E) Representative images of heterologously modeled synapses including HEK293T cells expressing pBI-BFP-NL1 and WT, p.R1441H KI, or PPM1H KO iNeurons, stained for endogenous SYP, SYB2, and βIII-tubulin. (F) Normalized intensity of SYP at heterologous presynaptic sites in WT, p.R1441H KI, and PPM1H KO iNeurons (n = 492–840 presynaptic puncta in 17–20 fields of view from 4 independent experiments; **P = 0.0086; ***P < 0.001; linear mixed effects model). (G) Normalized intensity of SYB2 at heterologous presynaptic sites in WT, p.R1441H KI, and PPM1H KO iNeurons (n = 1,018–1,512 presynaptic puncta in 16–20 fields of view from four independent experiments; ***P < 0.001; linear mixed effects model). Scatter plot points indicate the means of three to four independent experiments, and error bars show mean ± SD of these points.
Delivery of synaptic vesicle proteins from soma to presynaptic sites is disrupted by RAB hyperphosphorylation. (A) Representative images of DIV14 WT, p.R1441H KI, and PPM1H KO iNeuron somas, stained for endogenous MAP2, SYP, and SYB2. (B and C) Normalized somal intensity (mean gray value) of SYP (B) and SYB2 (C) in WT, p.R1441H KI, and PPM1H KO iNeurons (n = 24 neurons from three independent experiments; **P = 0.0027; ***P < 0.001; linear mixed effects model). (D) Cartoon depicting heterologous synapse model set-up, where HEK293T cells expressing pBI-BFP-NL1 were introduced in coculture to WT, p.R1441H KI, or PPM1H KO iNeurons. Dishes were imaged at iNeuron DIV14, at which point βIII-tubulin+ iNeuron axons had selectively formed presynapses with NL1+ transfected HEK cells (inset, bottom), but not untransfected HEK cells (inset, top). (E) Representative images of heterologously modeled synapses including HEK293T cells expressing pBI-BFP-NL1 and WT, p.R1441H KI, or PPM1H KO iNeurons, stained for endogenous SYP, SYB2, and βIII-tubulin. (F) Normalized intensity of SYP at heterologous presynaptic sites in WT, p.R1441H KI, and PPM1H KO iNeurons (n = 492–840 presynaptic puncta in 17–20 fields of view from 4 independent experiments; **P = 0.0086; ***P < 0.001; linear mixed effects model). (G) Normalized intensity of SYB2 at heterologous presynaptic sites in WT, p.R1441H KI, and PPM1H KO iNeurons (n = 1,018–1,512 presynaptic puncta in 16–20 fields of view from four independent experiments; ***P < 0.001; linear mixed effects model). Scatter plot points indicate the means of three to four independent experiments, and error bars show mean ± SD of these points.
Related toFig. 3,. (A) Normalized somal MAP2 area of WT, p.R1441H KI, and PPM1H KO iNeurons for dataset shown in Fig. 3, A–C (n = 24 neurons from three independent experiments; ns > 0.0540; linear mixed effects model). (B–D) Example total protein stain and immunoblot of SYP (B), SYB2 (C), and RAB3A (D) in DIV21 WT, p.R1441H KI, and PPM1H KO iNeurons. (E) Representative images of DIV14 WT, p.R1441H KI, and PPM1H KO iNeuron somas, stained for endogenous MAP2, SYP, and golgin-97. (F) Ratio of SYP intensity (mean gray value) colocalized with golgin-97 signal/SYP intensity (mean gray value) of whole soma, in WT, p.R1441H KI, and PPM1H KO iNeurons (n = 24 neurons from three independent experiments; ns = 0.4638; ***P < 0.001; linear mixed effects model). Scatter plot points indicate the means of three independent experiments, and error bars show the mean ± SD of these points. Source data are available for this figure: SourceData FS2.
Related toFig. 3,. (A) Normalized somal MAP2 area of WT, p.R1441H KI, and PPM1H KO iNeurons for dataset shown in Fig. 3, A–C (n = 24 neurons from three independent experiments; ns > 0.0540; linear mixed effects model). (B–D) Example total protein stain and immunoblot of SYP (B), SYB2 (C), and RAB3A (D) in DIV21 WT, p.R1441H KI, and PPM1H KO iNeurons. (E) Representative images of DIV14 WT, p.R1441H KI, and PPM1H KO iNeuron somas, stained for endogenous MAP2, SYP, and golgin-97. (F) Ratio of SYP intensity (mean gray value) colocalized with golgin-97 signal/SYP intensity (mean gray value) of whole soma, in WT, p.R1441H KI, and PPM1H KO iNeurons (n = 24 neurons from three independent experiments; ns = 0.4638; ***P < 0.001; linear mixed effects model). Scatter plot points indicate the means of three independent experiments, and error bars show the mean ± SD of these points. Source data are available for this figure: SourceData FS2.
Proteins fated for SVPs are believed to be sorted at the trans-Golgi network (TGN) (Bonanomi et al., 2006; Hannah et al., 1999; Prado and Prado, 2002). We next explored whether a portion of somal SYP may be sequestered at the TGN in the context of elevated RAB3A phosphorylation. WT, p.R1441H KI, and PPM1H KO iNeurons were stained for endogenous SYP and golgin-97, a TGN marker (Fig. S2 E). Again, the MAP2 signal was used to visualize the somatodendritric compartment. In all three conditions, we observed that SYP intensity is enriched at the TGN relative to the whole soma (Fig. S2 F). Compared with WT neurons, PPM1H KO neurons displayed increased SYP intensity that colocalized with golgin-97 (Fig. S2 F). However, we did not detect this effect in p.R1441H KI iNeurons (Fig. S2 F).
Next, we sought to determine whether hyperphosphorylation of RABs disrupts the delivery of synaptic proteins to presynaptic sites. To accomplish this, we employed a recently developed heterologous synapse model for human neurons that allows unambiguous analysis of trafficking to the presynaptic compartment (Aiken and Holzbaur, 2024), introducing non-neuronal human embryonic kidney (HEK) 293T cells expressing the postsynaptic ligand neuroligin-1 (NL1) into coculture with iNeurons (Fig. 3 D). Within 24 h of introducing HEK cells, iNeuron axons specifically recognize NL1-expressing HEK cells and form connections where presynaptic proteins accumulate (Fig. 3 D, inset). This system provides both spatial and temporal control for quantification of SVP-associated protein accumulation. These heterologous presynapses contain SYP, SYB2, synapsin I/II (SYN), VGLUT1, and SVs that cycle upon neuronal depolarization (Aiken and Holzbaur, 2024). In heterologous synaptic cultures stained for endogenous SYP and SYB2 (Fig. 3 E), quantification revealed significantly decreased accumulation of both SYP (Fig. 3 F) and SYB2 (Fig. 3 G) at presynapses in PPM1H KO iNeurons relative to WT. In p.R1441H KI iNeurons, SYP presynaptic accumulation was also decreased, with SYB2 presynaptic accumulation trending downward but not achieving statistical significance (Fig. 3, F and G).
Together, these experiments show that the compartmental distribution of two different SVP-associated proteins is disrupted in p.R1441H KI and PPM1H KO iNeurons, with increased somal abundance and decreased presynaptic content. Together with our live-imaging findings, these data are consistent with somal sequestration and impaired presynaptic delivery of SVP-associated proteins as a consequence of disrupted anterograde SVP transport.
Phosphorylation of RAB3A impairs interaction with motor adaptor protein MADD
MADD has been previously shown to be essential for the transport of RAB3-containing SVPs by KIF1A and KIF1Bβ (Niwa et al., 2008). The same study showed that MADD directly interacts with KIF1A/1Bβ and RAB3. A more recent study showed that depletion of MADD levels decreased RAB3 abundance in the axon with a corresponding increase in somal sequestration (Hummel and Hoogenraad, 2021). This study further demonstrated that MADD interacts selectively with SVPs, not other axonal cargoes including dense core vesicles (DCVs) and lysosomes (Hummel and Hoogenraad, 2021). MADD is also known as RAB3-GEP (guanine nucleotide exchange protein) due to its role as a GDP-GTP exchange factor (GEF) for RAB3 (Brown and Howe, 1998). Notably, anterograde SVP transport has been shown to depend on the GTP-bound state of RAB3 (Niwa et al., 2008; Südhof, 1997). To probe the mechanism underlying LRRK2-p.R1441H’s effect on anterograde axonal SVP flux, we first tested whether overexpressing the GTP-locked mutant of RAB3 would rescue the deficit. Of the four RAB3 isoforms, we chose to focus on the best-characterized isoform, RAB3A, which is also the most abundant in the cortex (Schlüter et al., 2002, 2004). We found that transient expression of the glutamine-to-leucine (Q81L) mutant RAB3A, predicted to lock RAB3A into a GTP-bound state (Fig. 4 A) (Jumper et al., 2021; Varadi et al., 2022), abolished the inhibitory effect of the hyperactive LRRK2 mutant p.R1441H KI on anterograde SVP flux (Fig. 1, B and C; and Fig. 4, B–D).
Phosphorylation of RAB3A disrupts binding to motor adaptor MADD. (A) AlphaFold prediction (Jumper et al., 2021; Varadi et al., 2022) of RAB3A. Inset illustrates locations of site-directed mutagenesis for RAB3A mutants used in this study. (B) Cartoon depicting experimental approach. EGFP-RAB3AQL was transiently expressed in DIV21 WT or p.R1441H KI iNeurons expressing mScarlet-SYP. (C) Kymographs of axonal mScarlet-SYP+ vesicles in DIV21 WT and p.R1441H KI iNeurons, transiently expressing EGFP-RAB3AQL. (D) Anterograde flux of SYP+ vesicles in WT and p.R1441H KI iNeurons, transiently expressing EGFP-RAB3AQL (n = 23–24 neurons from 3 independent experiments; ns, not significant, P = 0.8993; linear mixed effects model). Scatter plot points indicate the means of three independent experiments, and error bars show mean ± SD of these points. (E and F) Example immunoblot and quantification of MADD coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, coexpressed in HEK293T cells (mean ± SEM; n = 5 independent experiments; **P = 0.0016 for WT versus T86E, P = 0.0021 for WT versus T86A; ***P < 0.0001; one-way ANOVA with Tukey’s multiple comparisons test). (G and H) Example immunoblot and quantification of pT RAB relative to total immunoprecipitated EGFP-RAB3AWT, expressed in HEK293T cells, with or without overnight treatment with 200 nM MLi-2 prior to lysis (mean ± SEM; n = 3 independent experiments; ***P = 0.0008; unpaired t test). (I and J) Example immunoblot and quantification of MADD coimmunoprecipitation by RAB3AWT, coexpressed in HEK293T cells, with or without 30 min treatment of lysate with lambda protein phosphatase (λPP; 200 units λPP per 50 μl reaction volume) (mean ± SEM; n = 3 independent experiments; *P = 0.0303; unpaired t test). For all co-IP experiments shown, samples were processed and immunoblotted in parallel. Source data are available for this figure: SourceData F4.
Phosphorylation of RAB3A disrupts binding to motor adaptor MADD. (A) AlphaFold prediction (Jumper et al., 2021; Varadi et al., 2022) of RAB3A. Inset illustrates locations of site-directed mutagenesis for RAB3A mutants used in this study. (B) Cartoon depicting experimental approach. EGFP-RAB3AQL was transiently expressed in DIV21 WT or p.R1441H KI iNeurons expressing mScarlet-SYP. (C) Kymographs of axonal mScarlet-SYP+ vesicles in DIV21 WT and p.R1441H KI iNeurons, transiently expressing EGFP-RAB3AQL. (D) Anterograde flux of SYP+ vesicles in WT and p.R1441H KI iNeurons, transiently expressing EGFP-RAB3AQL (n = 23–24 neurons from 3 independent experiments; ns, not significant, P = 0.8993; linear mixed effects model). Scatter plot points indicate the means of three independent experiments, and error bars show mean ± SD of these points. (E and F) Example immunoblot and quantification of MADD coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, coexpressed in HEK293T cells (mean ± SEM; n = 5 independent experiments; **P = 0.0016 for WT versus T86E, P = 0.0021 for WT versus T86A; ***P < 0.0001; one-way ANOVA with Tukey’s multiple comparisons test). (G and H) Example immunoblot and quantification of pT RAB relative to total immunoprecipitated EGFP-RAB3AWT, expressed in HEK293T cells, with or without overnight treatment with 200 nM MLi-2 prior to lysis (mean ± SEM; n = 3 independent experiments; ***P = 0.0008; unpaired t test). (I and J) Example immunoblot and quantification of MADD coimmunoprecipitation by RAB3AWT, coexpressed in HEK293T cells, with or without 30 min treatment of lysate with lambda protein phosphatase (λPP; 200 units λPP per 50 μl reaction volume) (mean ± SEM; n = 3 independent experiments; *P = 0.0303; unpaired t test). For all co-IP experiments shown, samples were processed and immunoblotted in parallel. Source data are available for this figure: SourceData F4.
It has previously been shown that LRRK2-mediated phosphorylation of RAB8A disrupts its ability to bind to RABIN8, its cognate GEF (Steger et al., 2016; Mamais et al., 2021). We therefore investigated whether the known interaction between RAB3A and MADD is altered by RAB3A phosphorylation. To test this, we coexpressed HA-MADD in HEK293T cells with EGFP-labeled RAB3A, with or without point mutations at the threonine residue that is phosphorylated by LRRK2 (Fig. 4 A) (Jumper et al., 2021; Varadi et al., 2022). Consistent with previous work (Niwa et al., 2008), RAB3AWT coimmunoprecipitated with MADD (Fig. 4 E). Threonine-to-alanine (T86A) mutant RAB3A, predicted to be non-phosphorylatable, exhibited the highest binding affinity to MADD (Fig. 4, E and F). Threonine-to-glutamic acid (T86E) mutant RAB3A, predicted to be a phosphomimetic, bound more weakly to MADD than RAB3AWT (Fig. 4, E and F).
Compared with the T86A and T86E mutants, RAB3AWT exhibited an intermediate binding affinity with MADD (Fig. 4, E and F). This raised the intriguing possibility that a fraction of the transiently expressed EGFP-RAB3AWT was phosphorylated by HEK cell endogenous LRRK2WT and thus exhibited an impaired ability to bind MADD. To confirm that endogenous LRRK2WT phosphorylates RAB3AWT in HEK293T cells, we applied MLi-2 prior to lysis and immunoprecipitation of EGFP-RAB3AWT. Application of MLi-2 reduced the phosphorylated fraction of RAB3A (Fig. 4, G and H), demonstrating endogenous LRRK2 activity given the high specificity of MLi-2 (Fell et al., 2015).
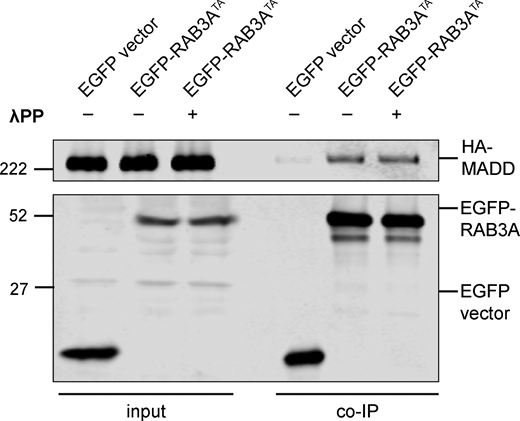
To confirm that phosphorylation of RAB3AWT affects MADD binding, we applied lambda protein phosphatase (λPP) to lysates prior to coimmunoprecipitation of EGFP-RAB3AWT and HA-MADD (Fig. 4 I). λPP treatment effectively decreased levels of phosphorylated EGFP-RAB3AWT in the bound fraction and increased binding to HA-MADD (Fig. 4, I and J). In contrast, we did not observe a change in MADD binding to non-phosphorylatable RAB3ATA with λPP treatment (Fig. S3).
Related toFig. 4 . Immunoblot of MADD coimmunoprecipitation by RAB3ATA, coexpressed in HEK293T cells, with or without 30 min treatment of lysate with lambda protein phosphatase (λPP; 200 units λPP per 50 μl reaction volume). For co-IP experiment shown, samples were processed and immunoblotted in parallel. Source data are available for this figure: SourceData FS3.
Related toFig. 4 . Immunoblot of MADD coimmunoprecipitation by RAB3ATA, coexpressed in HEK293T cells, with or without 30 min treatment of lysate with lambda protein phosphatase (λPP; 200 units λPP per 50 μl reaction volume). For co-IP experiment shown, samples were processed and immunoblotted in parallel. Source data are available for this figure: SourceData FS3.
In sum, our results show that the interaction between RAB3A and MADD is impaired by RAB3A phosphorylation at the T86 residue. Given MADD’s dual role as a GEF for RAB3A and a motor adaptor for RAB3A-positive SVPs, these data suggest that impaired RAB3A–MADD interaction contributes to the deficit of anterograde SVP flux in p.R1441H KI iNeurons (Fig. 1, A–C), which can be rescued by overexpression of GTP-locked RAB3A (Fig. 4 D).
Phosphorylation of RAB3A impairs interactions with RAB-GDI1 and RAB3GAP but not RIM2 or synapsin
Multiple regulatory proteins determine RAB GTPase localization and binding state (Pfeffer, 2017). GEFs such as MADD recruit and drive the conversion of RABs to the active GTP-bound state at membranes (Brown and Howe, 1998). Each RAB functions at specific membranes, contributing to membrane identity by selective effector recruitment (Pfeffer, 2013). However, cytosolic GDP-bound RABs have been shown to be “promiscuous” in terms of their ability to enter membranes belonging to a wide range of intracellular organelles, where they may fail to encounter their cognate GEF (Gomez et al., 2019). Two regulatory proteins called RAB3 GTPase-activating protein (RAB3GAP) and RAB GDP dissociation inhibitor-1 (RAB-GDI1) act in concert to retrieve RABs from inappropriate membranes. RAB3GAP converts GTP-bound RABs to the GDP-bound state, and RAB-GDI1 serves as a chaperone to return GDP-bound RABs from membranes to the cytosol (Pfeffer, 2017). Dysregulation of the RAB GTP binding state may therefore alter subcellular RAB localization and contribute to reduced effective availability of RABs (Pfeffer, 2017, 2023).
Previous work showed that phosphomimetic mutant RAB GTPases fail to bind RAB-GDI1 and that this is also true for directly phosphorylated RAB8AWT and RAB12WT (Steger et al., 2016, 2017; Liu et al., 2018). Consistent with these findings, in coimmunoprecipitation experiments, we observed that the phosphomimetic T86E mutation abolished the interaction between EGFP-RAB3A and endogenous RAB-GDI1 compared with the non-phosphorylatable T86A mutant (Fig. 5, A and B). Similar to the RAB3A–MADD interaction (Fig. 4, E and F), RAB3AWT exhibited intermediate binding affinity with GDI (Fig. 5, A and B). Notably, dephosphorylation of RAB3AWT by λPP treatment (Fig. 5 A) significantly increased binding to RAB-GDI1 (Fig. 5, A and B), confirming that direct RAB3A phosphorylation disrupts the interaction between RAB3A and RAB-GDI1.
Phosphorylation of RAB3A disrupts binding to RAB cycle regulators RAB-GDI1 and RAB3GAP. (A) Example immunoblot of endogenous RAB3GAP2 and endogenous RAB-GDI1 coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, expressed in HEK293T cells, with or without 30 min treatment of lysate with lambda protein phosphatase (λPP; 200 units λPP per 50 μl reaction volume). Lower panels (phosphothreonine RAB and EGFP) are separated for alignment purposes; no lanes that included sample were excluded. (B) Quantification of endogenous RAB-GDI1 coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, expressed in HEK293T cells, with or without 30 min treatment of lysate with λPP (mean ± SEM; n = 3 independent experiments; **P < 0.0048; ***P < 0.001; one-way ANOVA with Tukey’s multiple comparisons test). (C) Quantification of endogenous RAB3GAP2 coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, expressed in HEK293T cells, with or without 30 min treatment of lysate with λPP (mean ± SEM; n = 3 independent experiments; **P < 0.0094; one-way ANOVA with Tukey’s multiple comparisons test). For all co-IP experiments shown, samples were processed and immunoblotted in parallel. (D) Model: Dysregulated pRAB3A binding disrupts axonal transport of SVPs and distribution of synaptic proteins. In the neuronal soma, increased phosphorylation of RAB3A by hyperactive LRRK2 results in impaired formation of the RAB3A–MADD–KIF1A/1Bβ motor complex (inset) that is necessary for anterograde transport of SVPs out of the soma. As a result, there is increased somal sequestration of SYP and SYB2, and decreased anterograde SVP flux in the axon. Consequently, decreased SYP and SYB2 is delivered to presynaptic sites. Source data are available for this figure: SourceData F5.
Phosphorylation of RAB3A disrupts binding to RAB cycle regulators RAB-GDI1 and RAB3GAP. (A) Example immunoblot of endogenous RAB3GAP2 and endogenous RAB-GDI1 coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, expressed in HEK293T cells, with or without 30 min treatment of lysate with lambda protein phosphatase (λPP; 200 units λPP per 50 μl reaction volume). Lower panels (phosphothreonine RAB and EGFP) are separated for alignment purposes; no lanes that included sample were excluded. (B) Quantification of endogenous RAB-GDI1 coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, expressed in HEK293T cells, with or without 30 min treatment of lysate with λPP (mean ± SEM; n = 3 independent experiments; **P < 0.0048; ***P < 0.001; one-way ANOVA with Tukey’s multiple comparisons test). (C) Quantification of endogenous RAB3GAP2 coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, expressed in HEK293T cells, with or without 30 min treatment of lysate with λPP (mean ± SEM; n = 3 independent experiments; **P < 0.0094; one-way ANOVA with Tukey’s multiple comparisons test). For all co-IP experiments shown, samples were processed and immunoblotted in parallel. (D) Model: Dysregulated pRAB3A binding disrupts axonal transport of SVPs and distribution of synaptic proteins. In the neuronal soma, increased phosphorylation of RAB3A by hyperactive LRRK2 results in impaired formation of the RAB3A–MADD–KIF1A/1Bβ motor complex (inset) that is necessary for anterograde transport of SVPs out of the soma. As a result, there is increased somal sequestration of SYP and SYB2, and decreased anterograde SVP flux in the axon. Consequently, decreased SYP and SYB2 is delivered to presynaptic sites. Source data are available for this figure: SourceData F5.
In the same set of coimmunoprecipitation experiments, we also explored whether phosphorylation of RAB3A disrupts binding to RAB3GAP, quantified with an antibody for the non-catalytic subunit RAB3GAP2. We noted greater non-specific binding of RAB3GAP than GDI1 to the EGFP vector (Fig. 5 A), which was subtracted prior to quantification. We observed that the binding of EGFP–RAB3A to RAB3GAP was decreased by the phosphomimetic T86E mutation compared with both RAB3AWT and RAB3ATA (Fig. 5, A and C). Interestingly, while the T86E mutation strongly disrupted the RAB3A-GDI1 interaction (Fig. 5, A and B), this mutation had a more moderate effect on the RAB3A-RAB3GAP interaction (Fig. 5, A and C).
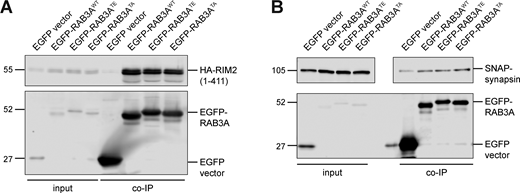
RAB3A has been implicated in mechanisms of SV release, acting in concert with effector proteins (van Weering et al., 2007; Persoon et al., 2019; Huang et al., 2011). Given the effect of RAB3A phosphorylation on binding to MADD, RAB–GDI1, and RAB3GAP, we wondered if RAB3A phosphorylation indiscriminately impaired interaction with all of its effectors. We therefore tested phosphomimetic mutant RAB3A binding to RAB3A-interacting molecule 2 (RIM2) and synapsin, two presynaptic proteins that have been shown to act as RAB3A effectors (Persoon et al., 2019; Giovedì et al., 2004). In agreement with these previous reports, we observed a pulldown of both RIM2 (Fig. S4 A) and synapsin (Fig. S4 B) by RAB3AWT. However, neither the phosphomimetic T86E mutation nor the non-phosphorylatable T86A mutation affected RAB3A binding to either RIM2 or synapsin, in contrast to MADD, GDI, and RAB3GAP.
Related toFig. 5 . (A) Example immunoblot of RIM2 (first 411 residues) coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, coexpressed in HEK293T cells. (B) Example immunoblot of synapsin coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, coexpressed in HEK293T cells. Upper panel is separated for alignment purposes; no lanes that included sample were excluded. For all co-IP experiments shown, samples were processed and immunoblotted in parallel. Source data are available for this figure: SourceData FS4.
Related toFig. 5 . (A) Example immunoblot of RIM2 (first 411 residues) coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, coexpressed in HEK293T cells. (B) Example immunoblot of synapsin coimmunoprecipitation by RAB3AWT, RAB3ATE, or RAB3ATA, coexpressed in HEK293T cells. Upper panel is separated for alignment purposes; no lanes that included sample were excluded. For all co-IP experiments shown, samples were processed and immunoblotted in parallel. Source data are available for this figure: SourceData FS4.
In summary, our results show that RAB3A phosphorylation at T86 selectively regulates binding to a subset of partners. Binding to the regulatory chaperone protein RAB–GDI1 was strongly impaired, while moderate disruption was observed for binding to the motor adaptor MADD and the regulatory protein RAB3GAP, and no effects were observed on binding to either RIM2 or synapsin.
Discussion
PD-linked pathogenic mutations in LRRK2 hyperphosphorylate a subset of RAB GTPases with a growing body of evidence linking this posttranslational modification to altered interactions with effectors. Though the LRRK2 substrate RAB3A has long been known to be essential for axonal SVP transport, the consequences of RAB3A phosphorylation on this trafficking pathway have not previously been explored. Here, we showed that in two iNeuron models of RAB hyperphosphorylation (LRRK2-p.R1441H KI and PPM1H KO), we observed impaired anterograde flux of SVPs (Figs. 1 and 2). We find that RAB3A phosphorylation at T86 disrupts binding to the motor adaptor protein MADD (Fig. 4). Phosphorylation of RAB3A also alters binding affinity to the regulatory proteins RAB3GAP and RAB-GDI1 (Fig. 5, A–C). Our findings support a model where pathogenic hyperactive LRRK2 causes dysregulated binding of RAB3A in the neuronal soma, including impairment of the formation of the RAB3A–MADD–KIF1A/Bβ complex (Fig. 5 D). We hypothesize that this contributes to the reduced availability of RAB3 to stimulate anterograde SVP transport. Consistent with this hypothesis, we find that the compartmental distribution of SVP-associated proteins is disrupted within neurons with hyperphosphorylated RABs, manifesting as increased somal content and decreased delivery to presynaptic sites along the axon (Fig. 3).
Our previous work linked hyperactive LRRK2 mutations to the disruption of the retrograde axonal transport of AVs (Boecker et al., 2021; Dou et al., 2023), most likely mediated by RAB10 and/or RAB35 (Waschbüsch et al., 2020; Bonet-Ponce et al., 2020; Berndsen et al., 2019; Goldsmith et al., 2022). In contrast, hyperactive LRRK2 does not alter the axonal transport of mitochondria (Dou et al., 2023), consistent with our current understanding that there is no known role for RABs in regulating the axonal transport of these organelles. Given the altered transport of RAB3A+ SVPs, our findings indicate a high degree of RAB-dependent selectivity for which axonal cargoes are perturbed by pathogenic hyperactive LRRK2. Furthermore, because PPM1H KO phenocopies these transport defects, this implies that the balance between LRRK2WT and PPM1H may regulate the transport of both SVPs and autophagosomes under physiologic conditions. As at least 10 different RABs are endogenously phosphorylated by LRRK2 (Steger et al., 2017), it remains to be explored whether other axonal cargoes rely on RAB-mediated transport mechanisms that are regulated by LRRK2.
Recent work indicates that the subcellular colocalization of PPM1H with specific RABs strongly influences levels of RAB phosphorylation (Yeshaw et al., 2023). Regulation of RAB-mediated pathways in neurons is therefore determined by the balance of LRRK2 and PPM1H activities at each specific membrane compartment, in ways that are difficult to predict from whole-cell pRAB levels alone. While our results show that both p.R1441H KI and PPM1H KO affect SVP transport and synaptic protein distribution, the effect size was generally more pronounced in PPM1H KO neurons (Fig. 3, F and G; and Fig. S2 F). Notably, PPM1H has been shown to strongly localize to the Golgi (Berndsen et al., 2019; Yeshaw et al., 2023), suggesting that its loss may cause more striking effects on protein sorting and cargo loading at the TGN. Further work could reveal how relative LRRK2-PPM1H abundance at specific subcellular membrane compartments differentially regulates other RAB-mediated pathways.
While we observed that RAB3A phosphorylation causes strong impairment of the RAB3A-GDI1 interaction (Fig. 5, A and B), we observed only moderate impairment of RAB3A–MADD (Fig. 4, E, F, I, and J) and RAB3A-RAB3GAP (Fig. 5, A and C) binding, and no impairment of RAB3A-RIM2 (Fig. S4 A) and RAB3A-synapsin (Fig. S4 B) interaction. Together, these results predict that RAB3A phosphorylation has a spectrum of effect sizes on effector binding properties and may alter the probability of successful binding in a manner depending on the specific interfaces of protein–protein interaction. Attempts to model the RAB3A–MADD and RAB3A–GDI1 complexes with AlphaFold-Multimer (Jumper et al., 2021; Varadi et al., 2022; Evans et al., 2022, Preprint; Cianfrocco et al., 2017a, 2017b) proved challenging, yielding only low confidence models. However, AlphaFold-Multimer generated a relatively high-confidence model of the complex between RAB3A and RAB3GAP1 (catalytic subunit of RAB3GAP) (Fig. S5 C; interface pTM + pTM score 0.794) (Jumper et al., 2021; Varadi et al., 2022; Evans et al., 2022, Preprint, 2022b; Cianfrocco et al., 2017a, 2017b). Modeling the RAB3A–MADD complex may be complicated by the presence of intrinsically disordered regions in the large MADD protein (UniProt Q8WXG6). However, previous work showed that the N-terminal 161 residues of MADD are necessary and sufficient for binding to GTP-bound RAB3 isoforms (Fig. S5 A) (Niwa et al., 2008). Moreover, MADD’s motor-binding death domain region is found toward its C-terminus (Niwa et al., 2008; Hummel and Hoogenraad, 2021). RABs are phosphorylated by LRRK2 at their characteristic switch II domain, which changes conformation in response to nucleotide binding to allow for interaction with effectors or regulatory proteins (Steger et al., 2016; Pfeffer, 2005). Thus, phosphorylation of RAB3A likely disrupts interaction between the switch II region of RAB3A and the N-terminus of MADD, without interrupting MADD’s ability to bind kinesin-3 (Fig. S5 A). Further work is required to elucidate the order of events and kinetics by which pRAB3A interrupts the loading of SVP cargo onto the MADD-kinesin motor complex. However, we observed that overexpression of GTP-locked RAB3A rescued anterograde SVP flux in p.R1441H KI iNeurons (Fig. 4, B–D), suggesting that increasing levels of active RAB3A in this system is sufficient to restore appropriate levels of the RAB3A–MADD–KIF1A/Bβ complex. This indicates that the increased fraction of phosphorylated RAB3A induced by p.R1441H KI reduces the abundance of eligible RAB3A required for the initiation of SVP transport.
Related to Figs. 4 and 5 . (A) AlphaFold predictions (Jumper et al., 2021; Varadi et al., 2022) of RAB3A and MADD. Left, annotated in purple: Putative switch II region of RAB3A (Singh et al., 2023). Right, annotated in blue: The N-terminal 161 residues that were previously shown (Niwa et al., 2008) to be necessary and sufficient for binding to RAB3. Right, annotated in magenta: The death domain toward the C-terminus of MADD that has been shown to be the motor-binding region (Niwa et al., 2008; Hummel and Hoogenraad, 2021). (B) AlphaFold predictions (Jumper et al., 2021; Varadi et al., 2022) of RAB3A and RAB-GDI1. (C) AlphaFold-Multimer (Jumper et al., 2021; Varadi et al., 2022; Evans et al., 2022, Preprint; Cianfrocco et al., 2017a, 2017b) prediction of complex formed by RAB3A and RAB3GAP1, the catalytic subunit of RAB3GAP. ipTM + pTM score for this prediction: 0.79404.
Related to Figs. 4 and 5 . (A) AlphaFold predictions (Jumper et al., 2021; Varadi et al., 2022) of RAB3A and MADD. Left, annotated in purple: Putative switch II region of RAB3A (Singh et al., 2023). Right, annotated in blue: The N-terminal 161 residues that were previously shown (Niwa et al., 2008) to be necessary and sufficient for binding to RAB3. Right, annotated in magenta: The death domain toward the C-terminus of MADD that has been shown to be the motor-binding region (Niwa et al., 2008; Hummel and Hoogenraad, 2021). (B) AlphaFold predictions (Jumper et al., 2021; Varadi et al., 2022) of RAB3A and RAB-GDI1. (C) AlphaFold-Multimer (Jumper et al., 2021; Varadi et al., 2022; Evans et al., 2022, Preprint; Cianfrocco et al., 2017a, 2017b) prediction of complex formed by RAB3A and RAB3GAP1, the catalytic subunit of RAB3GAP. ipTM + pTM score for this prediction: 0.79404.
Here, we primarily focused on the transport dynamics of the anterograde population of SYP+ vesicles, for which the mechanism of rapid, highly processive transport is known (Guedes-Dias et al., 2019). These results demonstrate that the major effect of RAB3A phosphorylation is on flux of the anterograde SYP+ population. The transport dynamics of the retrograde SYP+ population are not as well-characterized. Across multiple studies in mammalian neurons, we have observed it to be more heterogeneous, and overall less numerous, rapid, and processive than the anterograde population (Guedes-Dias et al., 2019; Aiken and Holzbaur, 2024). Recent work from our group has identified that SV proteins (including SYP and SYB2) make up a substantial proportion of autophagic cargoes (Goldsmith et al., 2022). Thus, a fraction of the retrograde SYP+ population we observe are likely to be synaptic proteins engulfed in AVs and thus moving processively in the retrograde direction along the axon.
Consistent with decreased anterograde SVP flux, our results using p.R1441H KI and PPM1H KO iNeurons also show decreased accumulation of SVP-associated proteins in a temporally controlled model of presynaptic site formation (Fig. 3, D–G). Interestingly, our results are consistent with recently published work showing that inhibition of LRRK2 increases the anterograde transport of α-synuclein to the presynapse (Brzozowski et al., 2021). As α-synuclein is believed to play a role in the presynapse in modulating neurotransmitter release (Bendor et al., 2013), hyperactive LRRK2 may decrease the presynaptic delivery of α-synuclein and other proteins important for synaptic function. Given the limited effective lifespan of SVs and the need for continuous replenishment of SVPs at presynapses (Truckenbrodt et al., 2018), it is predicted that RAB hyperphosphorylation and the resulting decrease in anterograde SVP flux would be detrimental to the size and health of the SV pool, especially the readily releasable and recycling pools. However, the specific ramifications of our findings on synaptic transmission are challenging to disentangle from other roles of LRRK2 activity at the presynaptic site. Despite numerous studies using multiple model systems of LRRK2 hyperactivity or loss (reviewed extensively by Pischedda and Piccoli [Pischedda and Piccoli, 2021]), no consensus has been reached on the effect of LRRK2 on SV exocytosis. LRRK2 has been reported to interact with or regulate actin, synapsin I, SNAP25, syntaxin, NSF, endophilin A, dynamin, auxillin, and synaptojanin, all of which contribute to the SV cycle (Pischedda and Piccoli, 2021; Meixner et al., 2011; Marte et al., 2019; Yun et al., 2013; Piccoli et al., 2011, 2014; Matta et al., 2012; Stafa et al., 2014; Nguyen and Krainc, 2018; Islam et al., 2016). Furthermore, LRRK2 substrates RAB3A (van Weering et al., 2007; Persoon et al., 2019; Huang et al., 2011; Fischer von Mollard et al., 1990; Schlüter et al., 2006), RAB5 (Shimizu et al., 2003; Wucherpfennig et al., 2003; Hoopmann et al., 2010), RAB10 (Dong et al., 2024, Preprint; Singh et al., 2023), and RAB35 (Sheehan et al., 2016; Uytterhoeven et al., 2011) have been implicated in membrane trafficking within the presynapse. The relative balance of LRRK2 and PPM1H activity at the presynapse will likely regulate some of these interactions, but others may involve scaffolding domains of LRRK2 that are not believed to be directly kinase-dependent (Piccoli et al., 2011; Cirnaru et al., 2014). RAB-mediated pathways at the presynapse may therefore be good candidates to be differentially affected by hyperactive LRRK2. Ultimately, the effect of pathogenic LRRK2 mutations on synaptic transmission is likely an integrated function of these different interactions, with more work needed to uncover how these intersecting pathways may contribute to the development of non-motor symptoms in PD.
Materials and methods
Plasmids and reagents
Plasmids and reagents used are detailed in Table 1, along with Addgene identification numbers. Antibodies used are detailed in Table 2, along with application and dilution. CMV HA-RIM2 was derived from GST-RIM2-RBD which was a gift from Ruben Bierings (Erasmus University Medical Center, Rotterdam, Netherlands). This construct contains the first 411 amino acids of RIM2, which contains the RAB binding domain (Kat et al., 2021).
Plasmids and reagents
| Reagent or resource . | Source . | Identifier . |
|---|---|---|
| Recombinant DNA | ||
| Plasmid: PB-TO-hNGN2 | Gift from iPSC Neurodegenerative Disease Initiative (iNDI) & Michael Ward | RRID:Addgene_172115 |
| Plasmid: PiggyBac transposase vector | Transposagen | N/A |
| Plasmid: PGK mScarlet-Synaptophysin | This paper | RRID:Addgene_206145 |
| Plasmid: PGK EGFP | This paper | RRID:Addgene_216110 |
| Plasmid: PGK EGFP-RAB3A-WT | This paper | RRID:Addgene_206146 |
| Plasmid: PGK EGFP-RAB3A-Q81L | This paper | RRID:Addgene_206147 |
| Plasmid: PGK EGFP-RAB3A-T86E | This paper | RRID:Addgene_206148 |
| Plasmid: PGK EGFP-RAB3A-T86A | This paper | RRID:Addgene_206149 |
| Plasmid: CMV HA-MADD | This paper | RRID:Addgene_206150 |
| Plasmid: CMV HA-RIM2 | This paper; GST-RIM2-RBD was a gift from Ruben Bierings (Erasmus University Medical Center) (Kat et al., 2021) | RRID:Addgene_206151 |
| Plasmid: CMV SNAP-synapsin | This paper | RRID:Addgene_206152 |
| Plasmid: pBI-BFP-NL1 | (Aiken and Holzbaur, 2024) | RRID:Addgene_206153 |
| Chemicals, peptides, and recombinant proteins | ||
| MLi-2 | Tocris | Cat# 5756 |
| DMSO | Sigma-Aldrich | Cat# D2650 |
| Matrigel growth factor reduced | Corning | Cat# 354230 |
| Essential 8 medium | Thermo Fisher Scientific | Cat# A1517001 |
| ReLeSR | Stemcell Technologies | Cat# 05872 |
| Accutase | Sigma-Aldrich | Cat# A6964 |
| ROCK inhibitor Y-27632 | Selleckchem | Cat# S1049 |
| Knockout serum replacement | Thermo Fisher Scientific | Cat# 10828010 |
| DMEM/F-12, HEPES | Thermo Fisher Scientific | Cat# 11330032 |
| N2 supplement | Thermo Fisher Scientific | Cat# 17502048 |
| Non-essential amino acids (NEAA) | Thermo Fisher Scientific | Cat# 11140050 |
| GlutaMAX | Thermo Fisher Scientific | Cat# 35050061 |
| Doxycycline | Sigma-Aldrich | Cat# D9891 |
| Poly-L-Ornithine | Sigma-Aldrich | Cat# P3655 |
| BrainPhys neuronal medium | Stemcell Technologies | Cat# 05790 |
| Laminin | Corning | Cat# 354232 |
| BDNF | PeproTech | Cat# 450-02 |
| NT-3 | PeproTech | Cat# 450-03 |
| B27 supplement | Thermo Fisher Scientific | Cat# 17504-044 |
| Lipofectamine stem transfection reagent | Thermo Fisher Scientific | Cat# STEM00003 |
| Microcystin-LR | Sigma-Aldrich | Cat# 475815 |
| Halt protease and phosphatase inhibitor cocktail | Thermo Fisher Scientific | Cat# 78442 |
| 5-Fluoro-2′-deoxyuridine | Sigma-Aldrich | Cat# F0503 |
| Uridine | Sigma-Aldrich | Cat# U3003 |
| DMEM (Dulbecco’s modified eagle’s medium) | Corning | Cat# MT10-013-CV |
| FuGENE 6 | Promega Corp | Cat# E2692 |
| GFP-trap magnetic particles M-270 | ChromoTek | Cat# gtd |
| GFP-trap magnetic agarose | ChromoTek | Cat# gtma |
| Lambda (λ) protein phosphatase | New England BioLabs | Cat# P0753S |
| Critical commercial assays | ||
| BCA protein assay kit | Thermo Fisher Scientific | Cat# 23225 |
| Plasmid maxi kit | QIAGEN | Cat# 12163 |
| Experimental models: Cell lines | ||
| Human: KOLF2.1J WT iPSCs | B. Skarnes (Jackson Laboratories, Connecticut) | RRID: CVCL_B5P3 |
| Human: KOLF2.1J LRRK2-R1441H iPSCs | B. Skarnes (Jackson Laboratories, Connecticut) | N/A |
| Human: KOLF2.1J PPM1H KO iPSCs | This paper | RRID: CVCL_C7TY |
| Human: HEK293T | ATCC | RRID: CVCL_0063 |
| Software and algorithms | ||
| FIJI (release 2.9.0) | NIH, USA | http://fiji.sc, RRID:SCR_002285 |
| Prism 9 | GraphPad | https://www.graphpad.com/scientific-software/prism/, RRID:SCR_002798 |
| RStudio: Integrated Development for R (2021.09.2 Build 382) | RStudio Team | http://www.rstudio.com/, RRID:SCR_000432 |
| R package: nlme | Pinheiro J, Bates D, R Core Team | http://CRAN.R-project.org/package=nlme, RRID:SCR_015655 |
| Matlab R2022a | MathWorks | https://www.mathworks.com/products/matlab.html, RRID:SCR_001622 |
| Volocity | PerkinElmer | https://www.perkinelmer.com, RRID:SCR_002668 |
| VisiView 5.0.0.24 | Visitron | https://www.visitron.de/products/visiviewr-software.html, RRID:SCR_022546 |
| LI-COR image studio | LI-COR | https://www.licor.com/bio/image-studio/, RRID:SCR_015795 |
| Adobe Illustrator 2022 | Adobe | https://www.adobe.com/products/illustrator.html, RRID:SCR_010279 |
| BioRender | BioRender | https://biorender.com/, RRID:SCR_018361 |
| Other | ||
| 35 mm #1.5 glass bottom imaging dishes | MatTek | Cat# P35G-1.5-20-C |
| ProLong gold antifade mountant | Thermo Fisher Scientific | Cat# P36930 |
| Reagent or resource . | Source . | Identifier . |
|---|---|---|
| Recombinant DNA | ||
| Plasmid: PB-TO-hNGN2 | Gift from iPSC Neurodegenerative Disease Initiative (iNDI) & Michael Ward | RRID:Addgene_172115 |
| Plasmid: PiggyBac transposase vector | Transposagen | N/A |
| Plasmid: PGK mScarlet-Synaptophysin | This paper | RRID:Addgene_206145 |
| Plasmid: PGK EGFP | This paper | RRID:Addgene_216110 |
| Plasmid: PGK EGFP-RAB3A-WT | This paper | RRID:Addgene_206146 |
| Plasmid: PGK EGFP-RAB3A-Q81L | This paper | RRID:Addgene_206147 |
| Plasmid: PGK EGFP-RAB3A-T86E | This paper | RRID:Addgene_206148 |
| Plasmid: PGK EGFP-RAB3A-T86A | This paper | RRID:Addgene_206149 |
| Plasmid: CMV HA-MADD | This paper | RRID:Addgene_206150 |
| Plasmid: CMV HA-RIM2 | This paper; GST-RIM2-RBD was a gift from Ruben Bierings (Erasmus University Medical Center) (Kat et al., 2021) | RRID:Addgene_206151 |
| Plasmid: CMV SNAP-synapsin | This paper | RRID:Addgene_206152 |
| Plasmid: pBI-BFP-NL1 | (Aiken and Holzbaur, 2024) | RRID:Addgene_206153 |
| Chemicals, peptides, and recombinant proteins | ||
| MLi-2 | Tocris | Cat# 5756 |
| DMSO | Sigma-Aldrich | Cat# D2650 |
| Matrigel growth factor reduced | Corning | Cat# 354230 |
| Essential 8 medium | Thermo Fisher Scientific | Cat# A1517001 |
| ReLeSR | Stemcell Technologies | Cat# 05872 |
| Accutase | Sigma-Aldrich | Cat# A6964 |
| ROCK inhibitor Y-27632 | Selleckchem | Cat# S1049 |
| Knockout serum replacement | Thermo Fisher Scientific | Cat# 10828010 |
| DMEM/F-12, HEPES | Thermo Fisher Scientific | Cat# 11330032 |
| N2 supplement | Thermo Fisher Scientific | Cat# 17502048 |
| Non-essential amino acids (NEAA) | Thermo Fisher Scientific | Cat# 11140050 |
| GlutaMAX | Thermo Fisher Scientific | Cat# 35050061 |
| Doxycycline | Sigma-Aldrich | Cat# D9891 |
| Poly-L-Ornithine | Sigma-Aldrich | Cat# P3655 |
| BrainPhys neuronal medium | Stemcell Technologies | Cat# 05790 |
| Laminin | Corning | Cat# 354232 |
| BDNF | PeproTech | Cat# 450-02 |
| NT-3 | PeproTech | Cat# 450-03 |
| B27 supplement | Thermo Fisher Scientific | Cat# 17504-044 |
| Lipofectamine stem transfection reagent | Thermo Fisher Scientific | Cat# STEM00003 |
| Microcystin-LR | Sigma-Aldrich | Cat# 475815 |
| Halt protease and phosphatase inhibitor cocktail | Thermo Fisher Scientific | Cat# 78442 |
| 5-Fluoro-2′-deoxyuridine | Sigma-Aldrich | Cat# F0503 |
| Uridine | Sigma-Aldrich | Cat# U3003 |
| DMEM (Dulbecco’s modified eagle’s medium) | Corning | Cat# MT10-013-CV |
| FuGENE 6 | Promega Corp | Cat# E2692 |
| GFP-trap magnetic particles M-270 | ChromoTek | Cat# gtd |
| GFP-trap magnetic agarose | ChromoTek | Cat# gtma |
| Lambda (λ) protein phosphatase | New England BioLabs | Cat# P0753S |
| Critical commercial assays | ||
| BCA protein assay kit | Thermo Fisher Scientific | Cat# 23225 |
| Plasmid maxi kit | QIAGEN | Cat# 12163 |
| Experimental models: Cell lines | ||
| Human: KOLF2.1J WT iPSCs | B. Skarnes (Jackson Laboratories, Connecticut) | RRID: CVCL_B5P3 |
| Human: KOLF2.1J LRRK2-R1441H iPSCs | B. Skarnes (Jackson Laboratories, Connecticut) | N/A |
| Human: KOLF2.1J PPM1H KO iPSCs | This paper | RRID: CVCL_C7TY |
| Human: HEK293T | ATCC | RRID: CVCL_0063 |
| Software and algorithms | ||
| FIJI (release 2.9.0) | NIH, USA | http://fiji.sc, RRID:SCR_002285 |
| Prism 9 | GraphPad | https://www.graphpad.com/scientific-software/prism/, RRID:SCR_002798 |
| RStudio: Integrated Development for R (2021.09.2 Build 382) | RStudio Team | http://www.rstudio.com/, RRID:SCR_000432 |
| R package: nlme | Pinheiro J, Bates D, R Core Team | http://CRAN.R-project.org/package=nlme, RRID:SCR_015655 |
| Matlab R2022a | MathWorks | https://www.mathworks.com/products/matlab.html, RRID:SCR_001622 |
| Volocity | PerkinElmer | https://www.perkinelmer.com, RRID:SCR_002668 |
| VisiView 5.0.0.24 | Visitron | https://www.visitron.de/products/visiviewr-software.html, RRID:SCR_022546 |
| LI-COR image studio | LI-COR | https://www.licor.com/bio/image-studio/, RRID:SCR_015795 |
| Adobe Illustrator 2022 | Adobe | https://www.adobe.com/products/illustrator.html, RRID:SCR_010279 |
| BioRender | BioRender | https://biorender.com/, RRID:SCR_018361 |
| Other | ||
| 35 mm #1.5 glass bottom imaging dishes | MatTek | Cat# P35G-1.5-20-C |
| ProLong gold antifade mountant | Thermo Fisher Scientific | Cat# P36930 |
Antibodies
| Antibody . | Source . | Application/Dilution . |
|---|---|---|
| Primary antibodies | ||
| Anti-RAB8A (phospho T72), rabbit monoclonal | Abcam (Cat# ab230260, RRID:AB_2814988) | WB @ 1 μg/ml |
| Anti-MAP2, mouse monoclonal | Sigma-Aldrich (Cat# MAB3418, RRID:AB_94856) | ICC @ 1:200 |
| Anti-synaptophysin, Guinea pig polyclonal | Synaptic Systems (Cat# 101-004, RRID:AB_1210382) | ICC @ 1:500 |
| Anti-synaptophysin, mouse monoclonal | Santa Cruz (Cat# sc-17750, RRID:AB_628311) | WB @ 1:2,000 |
| Anti-Synaptobrevin2 (VAMP2), rabbit monoclonal | Cell Signaling Technology (Cat# 13508, RRID:AB_2798240) | ICC @, WB @ 1:250 |
| Anti-RAB3A, rabbit polyclonal | Proteintech (Cat# 15029-1-AP, RRID:AB_2177378) | WB @ 1:1,000 |
| Anti-golgin-97, rabbit monoclonal | Cell Signaling Technology (Cat# 13192, RRID:AB_2798144) | ICC @ 1:200 |
| Anti-βIII-tubulin | Abcam (Cat# ab7751, RRID:AB_306045) | ICC @ 1:500 |
| Anti-HA, mouse monoclonal | BioLegend (Cat# 901501, RRID:AB_2565006) | WB @ 1:2000 |
| Anti-DENN, rabbit monoclonal | Abcam (Cat# ab134117, RRID:AB_2650580) | WB @ 1:1,000 |
| Anti-GFP, Chicken polyclonal | AvesLabs (Cat# GFP-1020, RRID:AB_10000240) | WB @ 1:5,000 |
| Anti-SNAP-tag, rabbit polyclonal | New England BioLabs (Cat# P9310S, RRID:AB_10631145) | WB @ 1:1,000 |
| Secondary antibodies | ||
| Anti-rabbit IgG-IRDye 800CW, donkey polyclonal | Li-COR Biosciences (Cat# 926-32213, RRID:AB_621848) | WB @ 1:20,000 |
| Anti-rabbit IgG-IRDye 680RD, donkey polyclonal | Li-COR Biosciences (Cat# 926-68073, RRID:AB_10954442) | WB @ 1:20,000 |
| Anti-mouse IgG-IRDye 800CW, donkey polyclonal | Li-COR Biosciences (Cat# 926-32212, RRID:AB_621847) | WB @ 1:20,000 |
| Anti-Chicken IRDye 680RD, donkey polyclonal | Li-COR Biosciences (Cat# 926-68075, RRID: AB_10974977) | WB @ 1:20,000 |
| Anti-rabbit IgG (H+L) alexa fluor 555, goat polyclonal | Thermo Fisher Scientific (Cat# A-32732, RRID: AB_2633281) | ICC @ 1:1,000 |
| Anti-Guinea pig IgG (H+L) alexa fluor 488, goat polyclonal | Thermo Fisher Scientific (Cat# A-11073, RRID: AB_2534117) | ICC @ 1:1,000 |
| Anti-mouse IgG (H+L) alexa fluor 647, goat polyclonal | Thermo Fisher Scientific (Cat# A-32728, RRID:AB_2633277) | ICC @ 1:1,000 |
| Antibody . | Source . | Application/Dilution . |
|---|---|---|
| Primary antibodies | ||
| Anti-RAB8A (phospho T72), rabbit monoclonal | Abcam (Cat# ab230260, RRID:AB_2814988) | WB @ 1 μg/ml |
| Anti-MAP2, mouse monoclonal | Sigma-Aldrich (Cat# MAB3418, RRID:AB_94856) | ICC @ 1:200 |
| Anti-synaptophysin, Guinea pig polyclonal | Synaptic Systems (Cat# 101-004, RRID:AB_1210382) | ICC @ 1:500 |
| Anti-synaptophysin, mouse monoclonal | Santa Cruz (Cat# sc-17750, RRID:AB_628311) | WB @ 1:2,000 |
| Anti-Synaptobrevin2 (VAMP2), rabbit monoclonal | Cell Signaling Technology (Cat# 13508, RRID:AB_2798240) | ICC @, WB @ 1:250 |
| Anti-RAB3A, rabbit polyclonal | Proteintech (Cat# 15029-1-AP, RRID:AB_2177378) | WB @ 1:1,000 |
| Anti-golgin-97, rabbit monoclonal | Cell Signaling Technology (Cat# 13192, RRID:AB_2798144) | ICC @ 1:200 |
| Anti-βIII-tubulin | Abcam (Cat# ab7751, RRID:AB_306045) | ICC @ 1:500 |
| Anti-HA, mouse monoclonal | BioLegend (Cat# 901501, RRID:AB_2565006) | WB @ 1:2000 |
| Anti-DENN, rabbit monoclonal | Abcam (Cat# ab134117, RRID:AB_2650580) | WB @ 1:1,000 |
| Anti-GFP, Chicken polyclonal | AvesLabs (Cat# GFP-1020, RRID:AB_10000240) | WB @ 1:5,000 |
| Anti-SNAP-tag, rabbit polyclonal | New England BioLabs (Cat# P9310S, RRID:AB_10631145) | WB @ 1:1,000 |
| Secondary antibodies | ||
| Anti-rabbit IgG-IRDye 800CW, donkey polyclonal | Li-COR Biosciences (Cat# 926-32213, RRID:AB_621848) | WB @ 1:20,000 |
| Anti-rabbit IgG-IRDye 680RD, donkey polyclonal | Li-COR Biosciences (Cat# 926-68073, RRID:AB_10954442) | WB @ 1:20,000 |
| Anti-mouse IgG-IRDye 800CW, donkey polyclonal | Li-COR Biosciences (Cat# 926-32212, RRID:AB_621847) | WB @ 1:20,000 |
| Anti-Chicken IRDye 680RD, donkey polyclonal | Li-COR Biosciences (Cat# 926-68075, RRID: AB_10974977) | WB @ 1:20,000 |
| Anti-rabbit IgG (H+L) alexa fluor 555, goat polyclonal | Thermo Fisher Scientific (Cat# A-32732, RRID: AB_2633281) | ICC @ 1:1,000 |
| Anti-Guinea pig IgG (H+L) alexa fluor 488, goat polyclonal | Thermo Fisher Scientific (Cat# A-11073, RRID: AB_2534117) | ICC @ 1:1,000 |
| Anti-mouse IgG (H+L) alexa fluor 647, goat polyclonal | Thermo Fisher Scientific (Cat# A-32728, RRID:AB_2633277) | ICC @ 1:1,000 |
Piggybac-mediated iPSC-derived neuron differentiation
KOLF2.1J-background WT and LRRK2-p.R1441H KI iPSCs were a gift from B. Skarnes (The Jackson Laboratory for Genomic Medicine, Farmington, CT, USA) as part of the iPSC Neurodegenerative Disease Initiative (iNDI) and have been described previously (Pantazis et al., 2022). KOLF2.1J-background PPM1H KO iPSCs were generated as described previously (Dou et al., 2023). Cytogenetic analysis of G-banded metaphase cells showed a normal male karyotype (Cell Line Genetics). Mycoplasma testing was negative. iPSCs were cultured on plates coated with Growth Factor Reduced Matrigel (Corning) and fed daily with Essential 8 media (Thermo Fisher Scientific). To stably express doxycycline-inducible hNGN2 using a PiggyBac delivery system, iPSCs were transfected with PB-TO-hNGN2 vector (gift from M. Ward, NIH, MD, USA) in a 1:2 ratio (transposase:vector) using Lipofectamine Stem (Thermo Fisher Scientific). After 72 h, transfected iPSCs were selected for 48 h with 0.5 μg/ml puromycin (Takara). Differentiation of iPSCs into iNeurons was performed using an established protocol (Pantazis et al., 2022; Fernandopulle et al., 2018). In brief, iPSCs were passaged using Accutase (Sigma-Aldrich) and plated on Matrigel-coated dishes in Induction Media (DMEM/F12 supplemented with 1% N2-supplement [Gibco], 1% NEAA [Gibco], and 1% GlutaMAX [Gibco], and containing 2 μg/ml doxycycline). After 72 h of doxycycline exposure, iNeurons were dissociated with Accutase and cryo-preserved in liquid N2. The published protocol can be found on Protocols.io (https://doi.org/10.17504/protocols.io.e6nvwj54dlmk/v1). Recent work has found that KOLF2.1J iPSCs carry small copy number variants in ASTN2 (Gracia-Diaz et al., 2023, Preprint; Ryan et al., 2024, Preprint). While changes in ASTN2 levels have the potential to alter synaptic strength, this is predicted to occur through postsynaptic rather than presynaptic mechanisms (Behesti et al., 2018) and is not expected to affect the phenotypes examined here.
i3Neuron differentiation
Pre-i3Neuron iPSCs (human iPSCs with an integrated doxycycline-inducible mNGN2 transgene in the AAVS1 safe-harbor locus) were a gift from M. Ward (NIH, MD, USA) and have been described previously (Boecker et al., 2021; Dou et al., 2023; Fernandopulle et al., 2018; Boecker et al., 2020). Cytogenetic analysis of G-banded metaphase cells showed a normal male karyotype (Cell Line Genetics). Mycoplasma testing was negative. Pre-i3N iPSCs were cultured on plates coated with Growth Factor Reduced Matrigel (Corning) and fed daily with Essential 8 media (Thermo Fisher Scientific). Induction into neuronal fate with doxycycline and cryopreservation of predifferentiated neurons was performed as described above (“Piggybac-mediated iPSC-derived neuron differentiation”). The published protocol can be found on Protocols.io (https://doi.org/10.17504/protocols.io.261ge348yl47/v1).
Culture and transfection of iPSC-derived neurons
Cryo-preserved, predifferentiated iNeurons (i3Neurons or Piggybac-delivered NGN2 neurons) were thawed and plated on live-imaging dishes (MatTek) coated with poly-L-ornithine at a density of 300,000 neurons per dish. For each experimental condition, cells from at least two different batches of induction were used over three or more independent experimental cultures. iPSC-derived neurons were cultured in BrainPhys Neuronal Media (StemCell) supplemented with 2% B-27 (Gibco), 10 ng/ml BDNF (PeproTech), 10 ng/ml NT-3 (PeproTech), and 1 μg/ml laminin (Corning). 40% of the media was replaced with fresh media twice per week. For Piggybac-delivered NGN2 neurons, 10 μM 5-Fluoro-2′-deoxyuridine and 10 μM uridine were included at the time of plating to prevent survival of mitotic cells. These drugs were removed 24 h after plating. Live imaging experiments were performed 21 days after thawing predifferentiated iPSC-derived neurons (DIV21). On DIV18, iPSC-derived neurons were transfected with Lipofectamine Stem (Thermo Fisher Scientific) and 1–2.5 μg total plasmid DNA. Immunostaining experiments were performed at DIV14. The published protocol can be found on Protocols.io (https://doi.org/10.17504/protocols.io.x54v9dj4zg3e/v1).
Live-cell imaging and motility quantification
iNeurons were imaged on DIV21 in low fluorescence Hibernate A medium (Brain Bits) supplemented with 2% B27, 10 ng/ml BDNF, and 10 ng/ml NT-3. Neurons were imaged in an environmental chamber at 37°C. Recordings of mScarlet-SYP+ vesicles for Figs. 1 and 4 were acquired on a PerkinElmer UltraView Vox Spinning Disk Confocal system with a Nikon Eclipse Ti inverted microscope, using a Plan Apochromat 60× 1.40 NA oil immersion objective and a Hamamatsu EMCCD C9100-50 camera controlled by Volocity software. Following a scheduled microscope upgrade, live imaging for Figs. 2 and 4 was instead performed using a Hamamatsu ORCA-Fusion C14440-20UP camera controlled by VisiView software. Axons were identified based on morphological parameters (Boecker et al., 2020; Kaech and Banker, 2006), and measurements were made to image ∼100–150 µm from the neuronal soma. After identifying this region, one photobleaching cycle was performed with the 405 nm laser for 3 ms/pixel using a ViRTEx Realtime Experiment Control Device. All time-lapse recordings were acquired at a frame rate of 5 frames/sec for 5 min. The published protocol can be found on Protocols.io (https://doi.org/10.17504/protocols.io.5jyl8p9mdg2w/v1).
Kymographs of axonal SVPs were generated using the Multiple Kymograph plugin (FIJI). The line width was set to five pixels. For flux quantification, anterograde and retrograde vesicle tracks were manually annotated and counted by a blinded investigator. For anterograde velocity quantification, 10–15 representative anterograde vesicle tracks from each kymograph were traced, and velocity was calculated from the average of their slopes.
Figure legends contain the statistical test used and specific P values for each quantification. RStudio version 2021.9.2.382 was used to perform a linear mixed effects model (LME; R package “nlme”). The genotype (or, in MLi-2 experiments, the treatment condition) was treated as the fixed effect. The independent experiment/culture being recorded was treated as the random effect. For all quantifications, at least three independent experiments were analyzed.
Immunostaining and quantification
At DIV14, human iNeurons were fixed in 4% paraformaldehyde supplemented with 4% sucrose for 15 min, washed four times with PBS, and permeabilized with 0.2% Triton-X in PBS for 15 min. Cells were then blocked for 1 h with 5% goat serum and 1% BSA in PBS. Neurons were then incubated in primary antibody (see Table 2) diluted in blocking solution at room temperature for 1 h, washed three times with PBS, and incubated in secondary antibody (see Table 2) diluted in blocking solution for 1 h at room temperature. After three washes with PBS, coverslips were mounted in ProLong Gold Antifade Mountant (Thermo Fisher Scientific). Images were acquired as z stacks at 200 nm step-size using a spinning disk confocal setup as described above. The published protocol can be found in Protocols.io (https://doi.org/10.17504/protocols.io.8epv5x91ng1b/v1).
For experiments shown in Fig. 3, A–C, the MAP2 channel was used to select an ROI around the somatic compartment by a blinded investigator. This ROI was used to measure the mean grey value of the SYP and SYB2 signals using sum projections. SYP/SYB2 intensity for each neuron was normalized to the average intensity of the WT neurons from that experimental replicate. Figure legends contain the statistical test used and specific P values for each quantification. RStudio version 2021.9.2.382 was used to perform a linear mixed effects model (LME; R package “nlme”). The genotype was treated as the fixed effect. The independent experiment/culture was treated as the random effect. For all quantifications, at least three independent experiments were analyzed.
For experiments shown in Fig. 3, D–G, HEK cells transfected 24 h prior with 6 µl:1 µg mix of FUGENE and pBI-BFP-NL1 (bicistronic vector expressing untagged NL1 and cytosolic BFP) were added to DIV13 iNeurons (100 K transfected HEK cells added to DIV13 iNeurons cultured in 35-mm imaging dishes). 24 h after the addition of transfected HEK cells, at iNeuron DIV14, cells were fixed for immunocytochemistry in 4% paraformaldehyde supplemented with 4% sucrose and stained as described above (see Tables 1 and 2 for antibody information). For analysis, the BFP-NL1 channel was used to select an ROI around an NL1+ HEK cell. To determine SYP and SYB2 intensity within presynaptic regions spanning the NL1+ HEK ROI, max-projection images were created and FIJI’s thresholding tool was used to segment an 8-bit object mask based on the top 1% intensity of the SYP or SYB2 channel. FIJI’s Analyze Particles tool was then used on the object mask redirected to the original SYP or SYB2 image channel to determine intensity values for individual presynaptic puncta. Figure legends contain the statistical test used and specific P values for each quantification. RStudio version 2021.9.2.382 was used to perform a linear mixed effects model (LME; R package “nlme”). The genotype was treated as the fixed effect. The independent experiment/culture and the field of view were treated as random effects, with the field of view nested within the experiment. For all quantifications, at least three independent experiments were analyzed.
For experiments shown in Fig. S2, E and F, the MAP2 channel was used to select an ROI around the somatic compartment by a blinded investigator. This ROI was used to measure the mean grey value of the somatic SYP signal using a sum projection. The “Adjust Threshold” function in ImageJ was used to create a mask on the region of high-intensity golgin-97 signal, and this ROI was used to measure the mean grey value of the SYP signal colocalized with the TGN. Figure legends contain the statistical test used and specific P values for each quantification. RStudio version 2021.9.2.382 was used to perform a linear mixed effects model (LME; R package “nlme”). The genotype was treated as the fixed effect. The independent experiment/culture was treated as the random effects. For all quantifications, at least three independent experiments were analyzed.
Neuronal lysis and immunoblotting
iNeurons were washed twice with ice-cold PBS and lysed with RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 2× Halt Protease and Phosphatase inhibitor, 2mg/ml microcystin-LR). Samples were centrifuged for 10 min at 17,000 g and protein concentration of the supernatant was determined by BCA assay. Neuronal proteins (SYP, SYB2, and RAB3A) were resolved on 15% acrylamide gels.
Proteins were transferred to Immobilon-FL PVDF membranes (Millipore) using a wet blot transfer system. Membranes were then stained for total protein using LI-COR Revert 700 Total Protein Stain. Following imaging of total protein stain, membranes were de-stained and blocked for 5 min with Bio-Rad EveryBlot blocking buffer. Membranes were incubated with primary antibody diluted in EveryBlot at 4°C overnight. After three washes with TBS (50 mM Tris-HCl [pH 7.4], 274 mM NaCl, 9 mM KCl) with 0.1% Tween-20, membranes were incubated with secondary antibodies diluted in EveryBlot with 0.01% SDS for 1 h at RT. Following three more washes with TBS with 0.1% Tween-20, membranes were imaged using an Odyssey CLx Infrared Imaging System (LI-COR). Western blots were analyzed with Image Studio Software (LI-COR). When necessary, stripping and reprobing were performed using LICOR NewBlot IR stripping buffer according to manufacturer instructions. The published protocol can be found in Protocols.io (https://doi.org/10.17504/protocols.io.5jyl8j5zrg2w/v1).
Cell line culture
HEK293T cells were maintained in DMEM (Corning) with 10% fetal bovine serum (HyClone). Cells were maintained at 37°C in a 5% CO2 incubator. Cells were tested for mycoplasma contamination routinely using a MycoAlert detection kit (LT07; Lonza). For coimmunoprecipitation experiments, cells were plated on three 10-cm tissue culture dishes per condition and transfected 24 h before lysis using FuGENE 6 (6–12 μg total plasmid DNA; Promega). The published protocol can be found in Protocols.io (https://doi.org/10.17504/protocols.io.kxygx3zeog8j/v1).
Coimmunoprecipitation experiments and quantification
HEK293T cells were lysed 24 h after transfection. For experiments not involving lambda protein phosphatase (λPP), cells were lysed in buffer containing 10 mM Tris-HCl pH 7.5, 150 mM NaCl, and 0.5 mM EDTA, with 0.5% NP-40 and protease inhibitors (1 mM PMSF, 0.01 mg/ml TAME, 0.01 mg/ml leupeptin, 0.001 mg/ml pepstatin A). Lysates were clarified at 10 × g at 4°C for 10 min. GFP-Trap Magnetic Particles M-270 were used for experiments involving MADD due to the protein’s large size; otherwise, GFP-Trap Magnetic Agarose beads were used. 25 μl of bead slurry per experimental condition were washed twice in wash buffer (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.5 mM EDTA; with 0.4% Triton X-100 for MADD pulldowns) for 5 min at 4°C and then equilibrated in lysis buffer for 5 min at 4°C. Beads were incubated with clarified lysate for 1 h at 4°C under rotating agitation. Following incubation, beads were washed three times for 10 min in wash buffer at 4°C and then resuspended in 60 μl denaturing buffer. The beads were then boiled to release the bound proteins.
For experiments using λPP, cells were lysed in 1× NEBuffer for Protein MetalloPhosphatases (New England BioLabs; supplied with λPP) with 0.5% NP-40 and 0.01 mg/ml leupeptin. Lysates were clarified at 10 × g at 4°C for 10 min. For conditions with λPP, 60 μl of 10 mM MnCl2 and 24 μl λPP (2,400 units) were added to clarified lysate for a final reaction volume of 600 μl and incubated at 30°C for 30 min. The same was performed for λPP-negative conditions, with 24 μl ddH2O instead. After incubation with λPP, incubation with beads was performed as described above. Published protocol can be found in Protocols.io (https://doi.org/10.17504/protocols.io.6qpvr36o2vmk/v1).
Coimmunoprecipitation was analyzed by Western blot (see “Immunoblotting”). Proteins from the same experiment were processed in parallel and resolved on different acrylamide gels based on protein size: 6% for HA-MADD; 10% for RAB3GAP2, RAB-GDI1, and SNAP-Synapsin; and 12% for EGFP-RAB3A, pT RAB, EGFP vector, and HA-RIM2. Figure legends contain the statistical test used and specific P values for each quantification. For quantifications, the residual signal caused by non-specific binding was subtracted, relative to the amount of GFP+ bait in each lane. Specific calculations for each lane can be found in the dataset accessible on Zenodo.
Online supplemental material
Data availability
Primary data that are presented in this study have been deposited in a Zenodo repository and are publicly available as of the date of publication. These can be accessed using the Digital Object Identifier https://doi.org/10.5281/zenodo.8156733. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Erika L.F. Holzbaur ([email protected]).
Acknowledgments
We thank Mariko Tokito for assistance in the cloning of plasmid constructs. We thank Michael Ward (National Institutes of Health), Erika Lara Flores (National Institutes of Health), and Bill Skarnes (The Jackson Laboratory) for their expertise in utilizing resources from the iPSC Neurodegeneration Initiative (iNDI). Cartoon schematics were created in part with http://BioRender.com. Protein structures in Fig. 2 were supplied from http://UniProt.org under the Creative Commons Attribution 4.0 International (CC BY 4.0) License (PDB: 7LHT for LRRK2, 7L4J for PPM1H). AlphaFold and AlphaFold-Multimer (accessed through COSMIC2 Gateway) were used for structural models and are cited in the main text. PPM1H KO iPSCs used in this study were previously generated by C. Alexander Boecker (Dou et al., 2023). Source plasmid DNA for HA-RIM2 was a gift from Ruben Bierings (Erasmus University Medical Center). We thank C. Alexander Boecker, Juliet Goldsmith, and Elizabeth Gallagher for helpful insights and discussions.
This work was supported by the National Institutes of Health (1 F31 NS124249-01 and T32-AG-000255 to D. Dou, F32 NS117672 to J. Aiken, and R35 GM126950 to E.L.F. Holzbaur) and the Michael J. Fox Foundation (MJFF) (MJFF-021130, MJFF-15100, and MJFF-019411 to E.L.F. Holzbaur). The E.L.F. Holzbaur laboratory is funded by the joint efforts of The MJFF and the Aligning Science Across Parkinson’s initiative. MJFF administers the grant ASAP-000350 on behalf of ASAP and itself.
Author contributions: Conceptualization, D. Dou, J. Aiken, and E.L.F. Holzbaur; methodology, D. Dou and J. Aiken; investigation, D. Dou and J. Aiken; writing—original draft, D. Dou, J. Aiken, and E.L.F. Holzbaur; writing—review and editing, D. Dou, J. Aiken, and E.L.F. Holzbaur; funding acquisition, D. Dou, J. Aiken, and E.L.F. Holzbaur; supervision, E.L.F. Holzbaur.
References
Author notes
Disclosures: The authors declare no competing interests exist.




![Loss of LRRK2-opposing PPM1H decreases anterograde axonal SVP flux. (A) Schematic depicting opposing regulation of RAB3A phosphorylation state at threonine 86 by LRRK2 kinase and PPM1H phosphatase. Protein structures: LRRK2 (PDB: 7LHT), PPM1H (PDB: 7L4J), RAB3A (AlphaFold prediction [Jumper et al., 2021; Varadi et al., 2022]). (B) Cartoon depicting WT or PPM1H KO iNeuron expressing mScarlet-SYP. (C) Kymographs of axonal mScarlet-SYP+ vesicles in DIV21 WT and PPM1H KO iNeurons. (D–F) Anterograde flux (D), anterograde velocity (E), and retrograde flux (F) of SYP+ vesicles in WT and PPM1H KO iNeurons (n = 28 neurons from three independent experiments; ns, not significant, P = 0.6872; *P = 0.0119; ***P < 0.001; linear mixed effects model). Scatter plot points indicate the means of three independent experiments, and error bars show mean ± SD of these points.](https://cdn.rupress.org/rup/content_public/journal/jcb/223/6/10.1083_jcb.202307092/1/m_jcb_202307092_fig2.png?Expires=1772497901&Signature=br3I4bkdb8hZ0espnZ2ptkcH6xaa-W6Tqo~MEwC6n-Gt5YJWXWBtWNmpq--l57H504VPpDd1-uKeeivQTqaRPBeA5zAr3MyvvDZ7MKCchsXpfCoXN-OortFreun9btRRpa02I3TWawZzpG-jsHH9vbBn-u8HdJSE~9ntW85HySrBwNVBv5qsjOBE5jahtiG9R2v5MAsO6AtBHmwrCCBJvRLW0LRtIfxXdiX9yqJLWPC5n3u8jbIb4NiWNYNwAx9eANfzLkVt6isRnaWXt00wFsti9A1j4Ep5-s-Pkx5fYqFwa8VXQzTY7yzZlhxkNbYZooI5mzCnseTCNeo-9McKQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)