Ciliary beat and intraflagellar transport depend on dynein and kinesin motors. The kinesin-9 family members Kif6 and Kif9 are implicated in motile cilia motilities across protists and mammals. How they function and whether they act redundantly, however, remain unclear. Here, we show that Kif6 and Kif9 play distinct roles in mammals. Kif6 forms puncta that move bidirectionally along axonemes, whereas Kif9 appears to oscillate regionally on the ciliary central apparatus. Consistently, only Kif6 displays microtubule-based motor activity in vitro, and its ciliary localization requires its ATPase activity. Kif6 deficiency in mice disrupts coordinated ciliary beat across ependymal tissues and impairs cerebrospinal fluid flow, resulting in severe hydrocephalus and high mortality. Kif9 deficiency causes mild hydrocephalus without obviously affecting the ciliary beat or the lifespan. Kif6−/− and Kif9−/− males are infertile but exhibit oligozoospermia with poor sperm motility and defective forward motion of sperms, respectively. These results suggest Kif6 as a motor for cargo transport and Kif9 as a central apparatus regulator.
Introduction
Cilia and flagella in eukaryotes are evolutionarily conserved organelles based on basal bodies (BBs) that protrude out of the cell surface. They are generally categorized into immotile cilia and motile cilia/flagella. Immotile monocilia, or primary cilia, usually consist of nine peripheral doublet microtubules (DMTs) (9 + 0 arrangement), whereas most motile cilia or flagella additionally possess a central pair (CP) of MT singlets (9 + 2 arrangement). The two CP MTs (C1 and C2) are decorated with distinct proteinaceous projections to form the CP apparatus. The peripheral DMTs are equipped with dynein arms protruding toward the B-tubule of neighboring DMTs and radial spokes extending into the central lumen to contact the CP apparatus. Mechanical signals from the CP apparatus transmit through radial spokes to coordinate axonemal dynein activities, generating rhythmic planar beat forms of cilia or flagella (Ishikawa, 2017; Klena and Pigino, 2022; Lin and Nicastro, 2018; Loreng and Smith, 2017; Oda et al., 2014). Mammalian motile cilia are mainly present as multicilia, with dozens to hundreds of cilia existing in a single cell. Multicilia beat in a back-and-forth manner, each beat consisting of a fast, forward “effective stroke” and a relatively slow, backward “recovery stroke.” The beat direction of each cilium roughly matches the BB polarity, indicated by the orientation of the basal foot (BF), a conical projection on the BB wall (Gibbons, 1961; Lindemann and Lesich, 2021; Nguyen et al., 2020; Schneiter et al., 2021). Multicilia are found in the epithelia of various organs, such as the brain ventricles, the airway, and the oviduct, driving cerebrospinal fluid (CSF) circulation, mucus clearance, and ovum transport (Boutin and Kodjabachian, 2019; Lyu et al., 2024; Wallmeier et al., 2020).
To generate directional fluid flows, proper polarities must be established at both cellular and tissue levels so that multicilia across a tissue surface beat coordinately in a major direction. In ependymal cells, BBs of multicilia cluster into a patch close to one side of the cell border to establish translational polarity (Mirzadeh et al., 2010; Ohata and Alvarez-Buylla, 2016). Initially, ciliary beat directions in single cells are randomized. As the development progresses, BB polarities become largely unidirectional in both single cells and different cells to achieve rotational polarity. As a result, the cilia in different cells across the tissue beat coordinately toward the same direction to drive directional CSF flows (Mirzadeh et al., 2010; Ohata and Alvarez-Buylla, 2016). These planar polarities require proper activation of the planar cell polarity (PCP) pathway and directional hydrodynamic forces to establish (Butler and Wallingford, 2017; Guirao et al., 2010; Ohata and Alvarez-Buylla, 2016; Ohata et al., 2014). The rotational polarization is a laborious process: although multicilia in mouse ependyma form in the first neonatal week, the rotational polarity is not fully established until after postnatal day 21 (P21) (Guirao et al., 2010; Ohata and Alvarez-Buylla, 2016; Spassky et al., 2005).
Kinesins are molecular motors that play essential roles in directional cargo transport through processive movements along MTs or in the regulation of MT dynamics in various cellular processes such as mitosis, ciliogenesis, and intracellular transport across eukaryotes (Cason and Holzbaur, 2022; Lu and Gelfand, 2017; Ou and Scholey, 2022). They are classified into N-kinesins, M-kinesins, and C-kinesins based on the position of the motor domain. Two kinesin families, kinesin-2 and kinesin-9, are found to function in cilia or flagella. The kinesin-2 family motors (Kif3a/b/c) are well-known for their involvement in intraflagellar transport (IFT), a specialized bidirectional transportation machinery in cilia (Klena and Pigino, 2022; Ou and Scholey, 2022; Scholey, 2013). IFT relies on two multisubunit complexes, IFT-A and IFT-B, which form IFT trains that are powered by kinesin-2 or cytoplasmic dynein-2, respectively, for anterograde or retrograde trafficking along axonemal DMTs (Klena and Pigino, 2022; Nakayama and Katoh, 2018). IFT enables the transport of ciliary proteins in and out of cilia. It is critical for cilia assembly, maintenance, and function (Nakayama and Katoh, 2018; Pigino, 2021).
The kinesin-9 family consists of two members, Kif6 and Kif9, that share a high sequence identity in their motor domains. They belong to the N-kinesin group, whose members are typically involved in the plus-end-directed transport of cargo (Lu and Gelfand, 2017; Ou and Scholey, 2022). Unlike the kinesin-2 family, which functions in both primary and motile cilia, the kinesin-9 family is predominantly associated with flagellated species (Demonchy et al., 2009; Konjikusic et al., 2018, 2023; Meng et al., 2023; Scholey, 2013; Yokoyama et al., 2004), suggesting distinct or redundant roles in regulating flagella motility. In Chlamydomonas, only KLP1, the homolog of Kif9, has been studied. It specifically localizes to the CP MTs and is proposed as an active motor to regulate flagellar motility (Bernstein et al., 1994; Han et al., 2022; Yokoyama et al., 2004). Interestingly, in Trypanosoma, the kinesin-9 family displays distinct functions in flagella motility. Knockdown of KIF9A, the Trypanosoma homolog of Kif9, reduces flagellum beating and cell movement, while knockdown of KIF9B, the homolog of Kif6, results in cell paralysis and defective assembly of the paraflagellar rod (Demonchy et al., 2009). In Xenopus, Kif9 is proposed to maintain the integrity of the axonemal distal ends and regulate ciliary beating in epidermal multiciliated cells (Konjikusic et al., 2023). In mammals, mutations in Kif9 have been linked to defective sperm motility and impaired fertility (Meng et al., 2023; Miyata et al., 2020). Its functions in mammalian motile cilia, however, remain unexplored. In addition, a homozygous frameshift mutation of KIF6 (p.Leu398Glnfs*2) is identified in a patient with intellectual disability and macrocephaly, and mice carrying a similar frameshift mutation (Kif6p.G555fs) develop severe hydrocephalus, possibly due to defective ciliogenesis in the ependyma (Konjikusic et al., 2018). A recent study reports that mice carrying the Kif6p.G555fs mutation still generate ependymal multicilia with normal axonemal ultrastructure but manifest impaired planar polarization of ependymal cells, decreased ependymal cilia motility, and attenuated CSF flow (Takagishi et al., 2024). Despite these achievements, how Kif6 and Kif9 function in mammalian cilia remains unclear.
In this study, we investigated the functions of Kif6 and Kif9 in mice. Our results suggest that Kif6 is a motile cilia-specific motor involved in ciliary trafficking and essential for the planar polarity of ependymal multicilia, whereas Kif9 functions in the central apparatus to fine-tune ciliary and flagellar beat.
Results
Mammalian Kif6 and Kif9 are motile cilia-specific kinesins with distinct localizations
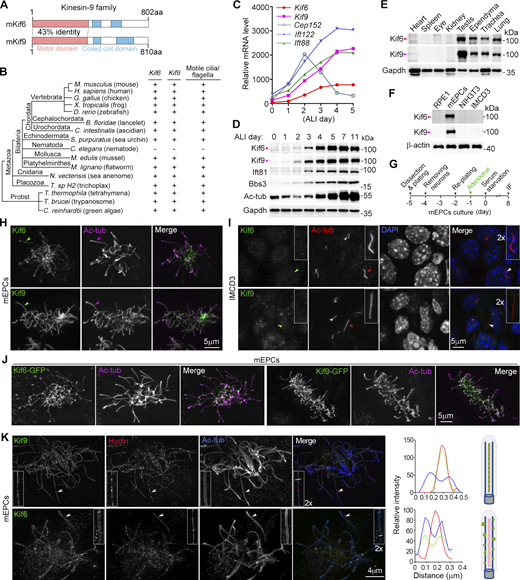
Murine full-length Kif6 (NP_796026.2) and Kif9 (NP_001157041.1) are highly conserved proteins composed of 802 and 810 amino acid residues, respectively. Structurally, both proteins have a motor domain located at the N-terminus, followed by two coiled-coil regions and a tail at the C-terminus (Fig. 1 A). Their motor domains share 43% sequence identity, while their C-termini are less conserved (Fig. 1 A; and Fig. S1, A and B). Phylogenetic analysis showed that Kif6 and Kif9 coexisted in eukaryotes with motile cilia/flagella, dating back to protists like Chlamydomonas and Tetrahymena (Fig. 1 B and Fig. S1 A) (Bernstein et al., 1994; Vincensini et al., 2011; Yokoyama et al., 2004). In contrast, they lacked homologs in Caenorhabditis elegans, which only contain immotile sensory cilia (Fig. 1 B) (Inglis et al., 2007; Vincensini et al., 2011). These data imply that Kif6 and Kif9 have evolved to be important for motile cilia.
Molecular characterizations and subcellular localizations of mammalian Kif6 and Kif9. (A) Schematics of full-length murine Kif6 and Kif9. The motor and coiled-coil domains were identified using the Conserved Domain Search Service of NCBI and SMART (Simple Modular Architecture Research Tool) (http://smart.embl.de). (B) Conservation of Kif6 and Kif9 across species with motile cilia and/or flagella in the evolution. The phylogenetic tree and taxonomic groups were based on literature (Cetkovic et al., 2018; Mukherjee and Brocchieri, 2013). (C) mRNA expression profiles of Kif6 and Kif9 during multiciliation. The gene expression profiles were obtained from our previous cDNA microarray analysis of mTECs cultured at the air–liquid interface (ALI) for the indicated days (Xu et al., 2015). Expression patterns of genes crucial for cilia formation (Ift88 and Ift122) and BB amplification (Cep152) are listed for comparison (Klena and Pigino, 2022; Zhao et al., 2013). (D) Expression patterns of Kif6 and Kif9 during multiciliation of mTECs. Multicilia formation is indicated by increased levels of Ift81, Bbs3, and acetylated tubulin (Ac-tub) (Klena and Pigino, 2022). Gapdh served as loading control. (E and F) Expression patterns of Kif6 and Kif9 in mouse tissues (E) and cultured cells (F). Tissues from a 2-month-old male mouse were dissected and lysed. mEPCs were harvested on day 8 post serum starvation. RPE1, NIH3T3, and IMCD3 cells were serum-starved for 48 h to induce primary (immotile) cilia formation. Gapdh or β-actin served as loading control. (G) Experimental scheme for H, J, and K. Precursors of mEPCs were isolated from P0 mouse brain and serum-starved on day 0 to induce differentiation into multiciliated cells. To express GFP-tagged proteins, the cells were infected with adenovirus on day 1. The cells were fixed on day 8 for immunofluorescence (IF) staining and imaged by confocal microscopy (H and J) or 3D-SIM (K). (H) Localizations of endogenous Kif6 and Kif9 in motile cilia. Ac-tub labeled ciliary axonemes. Arrowheads indicate typical cilia. (I) Kif6 and Kif9 were not detected in primary cilia. IMCD3 cells were serum-starved to induce primary ciliogenesis and fixed at day 2 after serum starvation for IF, followed by confocal microscopy. The nucleus was visualized by DAPI, a DNA-specific dye. Cilia pointed by arrowheads were zoomed in 2 × to show details. (J) Localizations of exogenous Kif6-GFP and Kif9-GFP in multicilia. (K) Typical 3D-SIM images of endogenous Kif9 and Kif6. Ac-tub and Hydin served as axoneme and CP markers, respectively. Cilia pointed by arrowheads were magnified by 200% to show details. Corresponding line scans at the indicated regions of the magnified insets showed colocalizations of Kif9 with Hydin and of Kif6 with Ac-tub. Illustrations are provided to aid comprehension. Source data are available for this figure: SourceData F1.
Molecular characterizations and subcellular localizations of mammalian Kif6 and Kif9. (A) Schematics of full-length murine Kif6 and Kif9. The motor and coiled-coil domains were identified using the Conserved Domain Search Service of NCBI and SMART (Simple Modular Architecture Research Tool) (http://smart.embl.de). (B) Conservation of Kif6 and Kif9 across species with motile cilia and/or flagella in the evolution. The phylogenetic tree and taxonomic groups were based on literature (Cetkovic et al., 2018; Mukherjee and Brocchieri, 2013). (C) mRNA expression profiles of Kif6 and Kif9 during multiciliation. The gene expression profiles were obtained from our previous cDNA microarray analysis of mTECs cultured at the air–liquid interface (ALI) for the indicated days (Xu et al., 2015). Expression patterns of genes crucial for cilia formation (Ift88 and Ift122) and BB amplification (Cep152) are listed for comparison (Klena and Pigino, 2022; Zhao et al., 2013). (D) Expression patterns of Kif6 and Kif9 during multiciliation of mTECs. Multicilia formation is indicated by increased levels of Ift81, Bbs3, and acetylated tubulin (Ac-tub) (Klena and Pigino, 2022). Gapdh served as loading control. (E and F) Expression patterns of Kif6 and Kif9 in mouse tissues (E) and cultured cells (F). Tissues from a 2-month-old male mouse were dissected and lysed. mEPCs were harvested on day 8 post serum starvation. RPE1, NIH3T3, and IMCD3 cells were serum-starved for 48 h to induce primary (immotile) cilia formation. Gapdh or β-actin served as loading control. (G) Experimental scheme for H, J, and K. Precursors of mEPCs were isolated from P0 mouse brain and serum-starved on day 0 to induce differentiation into multiciliated cells. To express GFP-tagged proteins, the cells were infected with adenovirus on day 1. The cells were fixed on day 8 for immunofluorescence (IF) staining and imaged by confocal microscopy (H and J) or 3D-SIM (K). (H) Localizations of endogenous Kif6 and Kif9 in motile cilia. Ac-tub labeled ciliary axonemes. Arrowheads indicate typical cilia. (I) Kif6 and Kif9 were not detected in primary cilia. IMCD3 cells were serum-starved to induce primary ciliogenesis and fixed at day 2 after serum starvation for IF, followed by confocal microscopy. The nucleus was visualized by DAPI, a DNA-specific dye. Cilia pointed by arrowheads were zoomed in 2 × to show details. (J) Localizations of exogenous Kif6-GFP and Kif9-GFP in multicilia. (K) Typical 3D-SIM images of endogenous Kif9 and Kif6. Ac-tub and Hydin served as axoneme and CP markers, respectively. Cilia pointed by arrowheads were magnified by 200% to show details. Corresponding line scans at the indicated regions of the magnified insets showed colocalizations of Kif9 with Hydin and of Kif6 with Ac-tub. Illustrations are provided to aid comprehension. Source data are available for this figure: SourceData F1.
Conservations and ciliary localizations of Kif6 and Kif9. Related to Fig. 1. (A) Similarities of Kif6 (left) and Kif9 (right) orthologues, obtained using constraint-based multiple alignment tool (COBALT) in NCBI. Highly conserved positions are shown in red and less conserved positions in blue. Protein sequences of Kif6 orthologues used are mouse (NP_796026.2), human (NP_659464.3), chicken (XP_003640993.5), frog (NP_001106482.1), zebrafish (NP_001070899.1), lancelet (XP_035685117.1), ascidian (XP_002124970.1), sea urchin (XP_030854411.1), mussel (CAG2221785.1), flatworm (PAA61917.1), sea anemone (XP_032228212.2), Trichoplax (RDD42533.1), Tetrahymena (XP_001024804.2), Trypanosoma (XP_846346.1), and green algae (XP_042928646.1). Protein sequences of Kif9 orthologues used were: mouse (NP_001157041.1), human (NP_001400904.1), chicken (XP_040522129.1), frog (XP_012821147.1), zebrafish (XP_001922460.1), lancelet (XP_035663761.1), ascidian (XP_018668035.1), sea urchin (XP_030830023.1), mussel (CAG2242108.1), flatworm (PAA61899.1), sea anemone (XP_001636554.1), Trichoplax (RDD45058.1), Tetrahymena (XP_001022313.1), Trypanosoma (RHW71316.1), and green algae (P46870.1). (B) Sequence alignment of Kif6 and Kif9 motor domains, generated by the COBALT sequence alignment program using default parameters. Their GenBank accession numbers are NP_796026.2 (Kif6) and NP_001157041.1 (Kif9). The motor domains were identified using SMART. (C) Localizations of exogenous Kif6 and Kif9 in motile cilia. Cultured mEPCs were treated as indicated in Fig. 1 G to express Kif6-GFP or Kif9-GFP and imaged by 3D-SIM. Spef1 and acetylated tubulin (Ac-tub) served as markers for the CP and the axoneme, respectively. Typical cilia (arrowheads) were magnified by 200% to highlight details. Line scans were generated at the indicated positions by white lines in merged images.
Conservations and ciliary localizations of Kif6 and Kif9. Related to Fig. 1. (A) Similarities of Kif6 (left) and Kif9 (right) orthologues, obtained using constraint-based multiple alignment tool (COBALT) in NCBI. Highly conserved positions are shown in red and less conserved positions in blue. Protein sequences of Kif6 orthologues used are mouse (NP_796026.2), human (NP_659464.3), chicken (XP_003640993.5), frog (NP_001106482.1), zebrafish (NP_001070899.1), lancelet (XP_035685117.1), ascidian (XP_002124970.1), sea urchin (XP_030854411.1), mussel (CAG2221785.1), flatworm (PAA61917.1), sea anemone (XP_032228212.2), Trichoplax (RDD42533.1), Tetrahymena (XP_001024804.2), Trypanosoma (XP_846346.1), and green algae (XP_042928646.1). Protein sequences of Kif9 orthologues used were: mouse (NP_001157041.1), human (NP_001400904.1), chicken (XP_040522129.1), frog (XP_012821147.1), zebrafish (XP_001922460.1), lancelet (XP_035663761.1), ascidian (XP_018668035.1), sea urchin (XP_030830023.1), mussel (CAG2242108.1), flatworm (PAA61899.1), sea anemone (XP_001636554.1), Trichoplax (RDD45058.1), Tetrahymena (XP_001022313.1), Trypanosoma (RHW71316.1), and green algae (P46870.1). (B) Sequence alignment of Kif6 and Kif9 motor domains, generated by the COBALT sequence alignment program using default parameters. Their GenBank accession numbers are NP_796026.2 (Kif6) and NP_001157041.1 (Kif9). The motor domains were identified using SMART. (C) Localizations of exogenous Kif6 and Kif9 in motile cilia. Cultured mEPCs were treated as indicated in Fig. 1 G to express Kif6-GFP or Kif9-GFP and imaged by 3D-SIM. Spef1 and acetylated tubulin (Ac-tub) served as markers for the CP and the axoneme, respectively. Typical cilia (arrowheads) were magnified by 200% to highlight details. Line scans were generated at the indicated positions by white lines in merged images.
We reanalyzed our previous microarray results (Xu et al., 2015) and found that Kif6 and Kif9 were highly upregulated during the multiciliation of mouse tracheal epithelial cells (mTECs) (Fig. 1 C). Immunoblots confirmed a substantial increase in the protein levels of Kif6 and Kif9 during multiciliation of mTECs (Fig. 1 D). Furthermore, Kif6 and Kif9 were specifically expressed in tissues and cells abundant in motile cilia or flagella, such as the ependyma, the trachea, the lung, the testis, and primary cultured mouse ependymal cells (mEPCs), but were hardly detected in tissues and cells with only immotile cilia (Fig. 1, E and F). Consistently, immunostaining indicated their localizations in motile cilia of mEPCs (Fig. 1, G and H) but not in primary cilia of IMCD3 cells (Fig. 1 I). To validate their ciliary localization, we infected mEPCs with adenovirus to express Kif6-GFP or Kif9-GFP (Fig. 1 G) and clearly observed localizations of the exogenous proteins in motile cilia (Fig. 1 J).
To visualize their detailed localizations, we performed three-dimensional structured illumination microscopy (3D-SIM). Ciliary Kif9 localized in the central lumen of axonemes marked with acetylated tubulin (Ac-tub) and colocalized well with the CP marker Hydin (Fig. 1 K) (Lechtreck et al., 2008), suggesting a conserved localization in the C2 projection as Chlamydomonas KLP1 (Bernstein et al., 1994; Han et al., 2022; Yokoyama et al., 2004). In sharp contrast, Kif6 mainly localized along the axonemes as puncta (Fig. 1 K). Similar ciliary localization patterns were observed for GFP-tagged Kif6 and Kif9, using Spef1 as a CP marker (Fig. S1 C) (Zheng et al., 2019). Therefore, Kif6 and Kif9 appear to play different roles, specifically in motile cilia.
Kif6, but not Kif9, displays IFT-like behaviors along ciliary axonemes
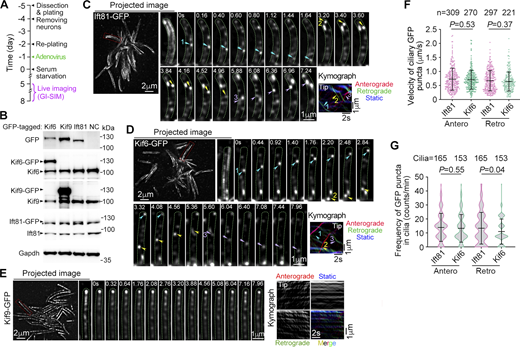
As kinesins with a motor domain at their N-terminus typically function as plus-end-directed motor proteins (Hirokawa et al., 2009), we examined whether GFP-tagged Kif6 and Kif9 expressed in mEPCs (Fig. 2 A) could move along DMTs and central MTs, respectively. Immunoblotting demonstrated comparable expression levels of GFP-tagged Kif6 and Ift81, an IFT-B component (Klena and Pigino, 2022), with their endogenous proteins, whereas Kif9-GFP showed relatively higher expression than endogenous Kif9 (Fig. 2 B). To achieve superior spatiotemporal resolution, we employed grazing incidence structured illumination microscopy (GI-SIM) (Guo et al., 2018; Qiao et al., 2023). As shown previously (Qiao et al., 2023), ciliary Ift81-GFP particles displayed clear bidirectional movements along axonemes (Fig. 2 C, Fig. S2 A, and Video 1). Strikingly, ciliary Kif6-GFP puncta also underwent robust and processive bidirectional movements resembling the IFT (Fig. 2 D, Fig. S2 A, and Video 2). Ciliary Kif9-GFP puncta did not exhibit apparent long-distance movements along axonemes but appeared to mainly oscillate on the CP apparatus (150 cilia from 10 cells) (Fig. 2 E, Fig. S2 A, and Video 3). Interestingly, some puncta appeared to exhibit short positional changes (Fig. 2 E, Fig. S2 A, and Video 3), suggesting that they may undergo short-distance movements.
Kif6, but not Kif9, displays IFT-like movements along DMTs. Quantification results are presented as mean ± SD and subjected to unpaired two-tailed Student’s t test. (A) Experimental scheme. Cultured mEPCs were infected with adenovirus at day −1 to express GFP-tagged Ift81, Kif6, or Kif9 and live imaged during days 5–8 by using GI-SIM to record the motilities of these proteins in cilia. (B) Expression levels of GFP-tagged proteins in mEPCs harvested at day 7. Immunoblotting was performed using antibodies against GFP, Kif6, Kif9, and Ift81, respectively. The asterisk indicates the non-specific band closed to Ift81-GFP. (C–E) Motilities of Ift81-GFP (C), Kif6-GFP (D), and Kif9-GFP (E) puncta in motile cilia. Images were recorded at 25 frames per sec (fps). The trafficking trajectories of GFP-positive puncta projected using the first 200 frames from Videos 1, 2, and 3 were shown as projected images. Typical trajectories framed by dashed red lines in the projected images were magnified by 300% so that the cilia could be roughly outlined (dashed green lines). Corresponding representative image sequences of these cilia are presented, in which typical, clearly traceable GFP-positive puncta are denoted to show their anterograde (arrowheads) or retrograde (arrows) movements. The corresponding kymographs were also presented. (F and G) Quantification results of velocities and frequencies from the indicated numbers of particles (F) and cilia (G). Antero: Anterograde transport; Retro: Retrograde transport; s: second. Source data are available for this figure: SourceData F2.
Kif6, but not Kif9, displays IFT-like movements along DMTs. Quantification results are presented as mean ± SD and subjected to unpaired two-tailed Student’s t test. (A) Experimental scheme. Cultured mEPCs were infected with adenovirus at day −1 to express GFP-tagged Ift81, Kif6, or Kif9 and live imaged during days 5–8 by using GI-SIM to record the motilities of these proteins in cilia. (B) Expression levels of GFP-tagged proteins in mEPCs harvested at day 7. Immunoblotting was performed using antibodies against GFP, Kif6, Kif9, and Ift81, respectively. The asterisk indicates the non-specific band closed to Ift81-GFP. (C–E) Motilities of Ift81-GFP (C), Kif6-GFP (D), and Kif9-GFP (E) puncta in motile cilia. Images were recorded at 25 frames per sec (fps). The trafficking trajectories of GFP-positive puncta projected using the first 200 frames from Videos 1, 2, and 3 were shown as projected images. Typical trajectories framed by dashed red lines in the projected images were magnified by 300% so that the cilia could be roughly outlined (dashed green lines). Corresponding representative image sequences of these cilia are presented, in which typical, clearly traceable GFP-positive puncta are denoted to show their anterograde (arrowheads) or retrograde (arrows) movements. The corresponding kymographs were also presented. (F and G) Quantification results of velocities and frequencies from the indicated numbers of particles (F) and cilia (G). Antero: Anterograde transport; Retro: Retrograde transport; s: second. Source data are available for this figure: SourceData F2.
Localizations and behaviors of exogenous Kif6 or Kif9 in ependymal cilia. Related to Figs. 2 and 3. (A) Additional examples for motility behaviors of ciliary Kif6-GFP and Kif9-GFP. mEPCs were infected with adenovirus as illustrated in Fig. 2 A to express the indicated GFP-tagged proteins. Ift81-GFP served as a positive control. GI-SIM images were recorded and processed as described in Fig. 2, C–E. Arrows indicate typical clearly traceable GFP-positive puncta to show their movements. (B) 3D-SIM images showing spatial relationships between Kif6-GFP and Ift56 or Ift88. Acetylated tubulin (Ac-tub) marked ciliary axonemes. The cilia magnified from the framed regions are presented in Fig. 3 A. (C) Typical images of microscope calibrations. Instrument calibration was conducted routinely with fluorescent beads prior to dual-color GI-SIM imaging experiments. A line scan was performed along the white line to determine the alignment. (D) Additional examples for bidirectional movements of ciliary Kif6-GFP-alone (Kif6+) and Ift27-2Halo-alone (Ift27+) puncta. mEPCs were treated and imaged as described in Fig. 3, B–F.
Localizations and behaviors of exogenous Kif6 or Kif9 in ependymal cilia. Related to Figs. 2 and 3. (A) Additional examples for motility behaviors of ciliary Kif6-GFP and Kif9-GFP. mEPCs were infected with adenovirus as illustrated in Fig. 2 A to express the indicated GFP-tagged proteins. Ift81-GFP served as a positive control. GI-SIM images were recorded and processed as described in Fig. 2, C–E. Arrows indicate typical clearly traceable GFP-positive puncta to show their movements. (B) 3D-SIM images showing spatial relationships between Kif6-GFP and Ift56 or Ift88. Acetylated tubulin (Ac-tub) marked ciliary axonemes. The cilia magnified from the framed regions are presented in Fig. 3 A. (C) Typical images of microscope calibrations. Instrument calibration was conducted routinely with fluorescent beads prior to dual-color GI-SIM imaging experiments. A line scan was performed along the white line to determine the alignment. (D) Additional examples for bidirectional movements of ciliary Kif6-GFP-alone (Kif6+) and Ift27-2Halo-alone (Ift27+) puncta. mEPCs were treated and imaged as described in Fig. 3, B–F.
Motilities of ciliary Ift81-GFP. Related to Fig. 2 C. mEPCs were treated to express Ift81-GFP and imaged using GI-SIM as illustrated in Fig. 2 A. Images were recorded at 25 frames per sec (fps) and the playback speed is 20 fps. The outlined region in the first frame was magnified by 300% to show details. Arrowheads or arrows mark typical and clearly traceable puncta to show their anterograde or retrograde movements, respectively.
Motilities of ciliary Ift81-GFP. Related to Fig. 2 C. mEPCs were treated to express Ift81-GFP and imaged using GI-SIM as illustrated in Fig. 2 A. Images were recorded at 25 frames per sec (fps) and the playback speed is 20 fps. The outlined region in the first frame was magnified by 300% to show details. Arrowheads or arrows mark typical and clearly traceable puncta to show their anterograde or retrograde movements, respectively.
Motilities of ciliary Kif6-GFP. Related to Fig. 2 D. mEPCs were treated as described in Video 1. Images were recorded at 25 fps and the playback speed is 20 fps.
Motilities of ciliary Kif9-GFP. Related to Fig. 2 E. mEPCs were treated as described in Video 1. Images were recorded at 25 fps and the playback speed is 20 fps.
Quantification results revealed that the Kif6-GFP puncta moved at 0.71 ± 0.34 μm/s anterogradely and 0.64 ± 0.34 μm/s retrogradely, similar to those of the Ift81-GFP particles (0.73 ± 0.40 μm/s anterogradely and 0.67 ± 0.36 μm/s retrogradely) (Fig. 2 F). Frequencies of movements were also similar between the two (13.3 ± 11.3 and 14.0 ± 10.0 counts/min, respectively, for anterograde Kif6-GFP puncta and Ift81-GFP particles; 11.0 ± 9.0 and 13.2 ± 9.9 counts/min for retrograde Kif6-GFP puncta and Ift81-GFP particles) (Fig. 2 G). These results suggest that Kif6 is involved in IFT trafficking.
Ciliary Kif6 puncta traffic with or without accompanying Ift27 puncta
To understand the relationship between Kif6 and IFT, we immunostained Kif6-GFP-expressing mEPCs to visualize the IFT-B component Ift88 or Ift56 (Klena and Pigino, 2022). 3D-SIM images showed that some Kif6 puncta were located adjacent to IFT-B particles while others appeared to be alone (Fig. 3 A and Fig. S2 B).
Kif6-GFP puncta move with or without accompanying Ift27-2Halo puncta along DMTs. (A) Typical 3D-SIM images of Kif6-GFP and IFT proteins (Ift56 and Ift88). Cilia cropped from Fig. S2 B were magnified by 400% to show details. Ac-tub marked ciliary axoneme. Line scans at the indicated regions (I–IV) help to identify Kif6-GFP puncta with (arrowheads) or without (arrows) an obviously colocalized IFT particle. (B–F) Ciliary trafficking of fluorescent particles. mEPCs were infected with adenovirus to express Kif6-GFP and Ift27-2Halo as illustrated in Fig. 2 A, followed by dual-color live imaging at 14 fps using GI-SIM. GFP- or Halo-positive puncta in the first 200 frames of Video 4 were projected to show trafficking trajectories (projected images). Typical trajectories (I–II) were magnified by 300% so that the cilia could be roughly outlined (dashed green lines). Corresponding representative image sequences are presented, in which typical, clearly traceable puncta are denoted to show anterograde (arrowheads) or retrograde (arrows) movements of Ift27+ Kif6+ duo-particles (B), Kif6+ particles (C), or Ift27+ particles (C). For each cilium, the corresponding kymograph is also presented. The ratios (D) and velocities (E) of these three types of trafficking particles were quantified from 45 cilia in four mEPCs. The velocities for each particle type with the indicated numbers (E) are presented as mean ± SD. These data were statistically analyzed using an unpaired two-tailed Student’s t test. Refer to Fig. S2 D for additional examples of bidirectional movements of Kif6+ or Ift27+ puncta. The illustration (F) is provided to aid comprehension. (G) Prediction of T104 and F105 as critical residues for nucleotide-binding in the motor domain of Kif6. Superposition of Kif6 (AlphaFold prediction, blue, highlighting the side chains of residues T104 and F105) and the structure of AMPPNP-bound Kif4 (PDB 3ZFD, gray) (Cao et al., 2017) indicate that both motor domains share the same overall conformation. AMPPNP is an analogue of ATP. (H) The AlphaFold predictions of 3D structures of Kif6 and Kif6-mut. pTM: the predicted template modeling (pTM). A pTM score above 0.5 indicates that the overall predicted fold of the protein is likely similar to the actual structure. (I) Kif6 bearing T104A/F105A mutations did not localize to ependymal cilia. mEPCs were infected with adenovirus on day −1 to express GFP-tagged Kif6 and Kif6-mut, a T104A/F105A mutant, and fixed on day 8 for fluorescent microscopy. Ac-tub served as a ciliary marker. Nuclei were stained with DAPI. Cilia-containing and cell body-containing zones of the same microscopic field are presented separately to show fluorescent signals in the two regions. Arrows point to GFP-positive cells.
Kif6-GFP puncta move with or without accompanying Ift27-2Halo puncta along DMTs. (A) Typical 3D-SIM images of Kif6-GFP and IFT proteins (Ift56 and Ift88). Cilia cropped from Fig. S2 B were magnified by 400% to show details. Ac-tub marked ciliary axoneme. Line scans at the indicated regions (I–IV) help to identify Kif6-GFP puncta with (arrowheads) or without (arrows) an obviously colocalized IFT particle. (B–F) Ciliary trafficking of fluorescent particles. mEPCs were infected with adenovirus to express Kif6-GFP and Ift27-2Halo as illustrated in Fig. 2 A, followed by dual-color live imaging at 14 fps using GI-SIM. GFP- or Halo-positive puncta in the first 200 frames of Video 4 were projected to show trafficking trajectories (projected images). Typical trajectories (I–II) were magnified by 300% so that the cilia could be roughly outlined (dashed green lines). Corresponding representative image sequences are presented, in which typical, clearly traceable puncta are denoted to show anterograde (arrowheads) or retrograde (arrows) movements of Ift27+ Kif6+ duo-particles (B), Kif6+ particles (C), or Ift27+ particles (C). For each cilium, the corresponding kymograph is also presented. The ratios (D) and velocities (E) of these three types of trafficking particles were quantified from 45 cilia in four mEPCs. The velocities for each particle type with the indicated numbers (E) are presented as mean ± SD. These data were statistically analyzed using an unpaired two-tailed Student’s t test. Refer to Fig. S2 D for additional examples of bidirectional movements of Kif6+ or Ift27+ puncta. The illustration (F) is provided to aid comprehension. (G) Prediction of T104 and F105 as critical residues for nucleotide-binding in the motor domain of Kif6. Superposition of Kif6 (AlphaFold prediction, blue, highlighting the side chains of residues T104 and F105) and the structure of AMPPNP-bound Kif4 (PDB 3ZFD, gray) (Cao et al., 2017) indicate that both motor domains share the same overall conformation. AMPPNP is an analogue of ATP. (H) The AlphaFold predictions of 3D structures of Kif6 and Kif6-mut. pTM: the predicted template modeling (pTM). A pTM score above 0.5 indicates that the overall predicted fold of the protein is likely similar to the actual structure. (I) Kif6 bearing T104A/F105A mutations did not localize to ependymal cilia. mEPCs were infected with adenovirus on day −1 to express GFP-tagged Kif6 and Kif6-mut, a T104A/F105A mutant, and fixed on day 8 for fluorescent microscopy. Ac-tub served as a ciliary marker. Nuclei were stained with DAPI. Cilia-containing and cell body-containing zones of the same microscopic field are presented separately to show fluorescent signals in the two regions. Arrows point to GFP-positive cells.
Next, we coexpressed Kif6-GFP and Ift27-2Halo, another IFT-B component (Klena and Pigino, 2022), in mEPCs through adenoviral infection and performed dual-color time-lapse GI-SIM imaging. To ensure proper alignment of different fluorescent channels, the microscopic system was calibrated routinely with fluorescent beads prior to imaging (Fig. S2 C). Interestingly, we observed three distinct types of bidirectional movements within cilia: co-moved Kif6-GFP puncta and Ift27-2Halo puncta (Ift27+ Kif6+ duo-particles), Kif6-GFP puncta alone (Kif6+ particles), and Ift27-2Halo puncta alone (Ift27+ particles) (Fig. 3, B–D, Fig. S2 D, and Video 4). The moving Kif6+ puncta usually left two separate tracks in cilia, similar to the moving Ift27+ puncta (Fig. 3, B and C; and Fig. S2 D), indicating that their movements are along peripheral DMTs rather than along central MTs.
Motilities of ciliary Kif6-GFP and Ift27-2Halo. Related to Fig. 3, B and C. mEPCs were infected with adenovirus to express Kif6-GFP and Ift27-2Halo as illustrated in Fig. 2 A. Dual-color live imaging was performed using GI-SIM. Images were recorded at 14 fps and the playback speed is 20 fps. Typical cilia denoted by arrowheads (I and II) in the first frame were magnified by 300% to show anterograde (arrowheads) and retrograde (arrows) movements of Ift27+ Kif6+ duo-particles, Kif6+ particles or Ift27+ particles.
Motilities of ciliary Kif6-GFP and Ift27-2Halo. Related to Fig. 3, B and C. mEPCs were infected with adenovirus to express Kif6-GFP and Ift27-2Halo as illustrated in Fig. 2 A. Dual-color live imaging was performed using GI-SIM. Images were recorded at 14 fps and the playback speed is 20 fps. Typical cilia denoted by arrowheads (I and II) in the first frame were magnified by 300% to show anterograde (arrowheads) and retrograde (arrows) movements of Ift27+ Kif6+ duo-particles, Kif6+ particles or Ift27+ particles.
Quantification analyses on 45 cilia in four mEPCs indicated that the ratios of these three trafficking types were ∼1:1.5:1 for both anterograde and retrograde movements (Fig. 3 D). These three types of particles exhibited comparable velocities: 0.75 ± 0.32 μm/s (Ift27+ Kif6+), 0.74 ± 0.32 μm/s (Kif6+), and 0.70 ± 0.31 μm/s (Ift27+) anterogradely, or 0.66 ± 0.28 μm/s, 0.65 ± 0.34 μm/s, and 0.62 ± 0.28 μm/s retrogradely (Fig. 3 E), similar to those measured from mEPCs expressing only one exogenous protein (Fig. 2 F).
Taken together, we conclude that Kif6 can traffic bidirectionally in motile cilia as a discrete punctum with an IFT-B particle (Fig. 3 F). Interestingly, a significant portion of Kif6 puncta also appears to move without an accompanying IFT27 punctum, suggesting a possibility of independent movement (Fig. 3 F).
Kif6 requires its ATPase activity to achieve ciliary localization
The trafficking behaviors of Kif6 (Fig. 3) suggested two possibilities: (1) Kif6 might function actively as a processive motor to transport cargos in motile cilia; (2) Kif6 might be transported as a passive cargo by IFT trains. As active motility must require the ATPase activity of a kinesin while passive transport does not, we reasoned that an ATPase-defective mutant of Kif6 would be able to discriminate between the two situations. We found that the AlphaFold-predicted motor domain of Kif6 shared the same overall conformation with the motor domain structure of AMPPNP-bound Kif4 (Fig. 3 G) (Cao et al., 2017). Accordingly, threonine-104 (T104) and phenylalanine-105 (F105) in the predicted ATP-binding pocket of Kif6 were predicted as critical residues for ATP binding (Fig. 3 G). As AlphaFold predicted that mutating these residues into alanine would not alter the overall folding of Kif6 (Fig. 3 H), we created a T104A/F105A mutant (Kif6-mut). Kif6-mut-GFP was indeed expressed as a soluble protein, rather than as aggregates of misfolded proteins, in the soma of mEPCs but failed to localize to cilia (Fig. 3 I). This requirement of the ATPase activity for the ciliary localization strongly suggests that Kif6 enters the cilia as an active motor, though it is also possible that the ATPase activity is required for the transport of Kif6 as a cargo of the IFT machinery.
Kif6 is a self-inhibited slow, processive MT-based motor in vitro
Next, we investigated the motor properties of Kif6 and Kif9. Typically, processive kinesins are autoinhibited by their C-termini and can be activated either physiologically through cargo binding or artificially by removing inhibitory domains (Cason and Holzbaur, 2022; Hirokawa et al., 2009). We transiently expressed GFP-Flag-tagged Kif6, Kif9, and different truncation constructs containing the motor domain (Fig. S3 A) to examine their MT-binding activities in HEK293T cells. We observed that, while the full-length Kif6 did not localize to MTs, all its truncation constructs (Kif6-CC1, Kif6-CC2, and Kif6-MD) exhibited strong MT localizations, especially on the spindle of mitotic cells (Fig. S3 B). In contrast, neither Kif9 nor its truncation constructs (Kif9-CC1, Kif9-CC2, and Kif9-MD) displayed obvious MT associations (Fig. S3, A and B). To clarify whether the robust MT association in vivo was a direct effect, we purified GFP-Flag-tagged Kif6 and its truncation mutants, Kif9-CC1, and rKin430, a rat kinesin construct serving as a positive control (Rogers et al., 2001), from HEK293T cells (Fig. 4, A–C) and performed MT binding assays in vitro (Fig. 4 D) (Diao et al., 2022). Consistently, Kif6-CC1, Kif6-CC2, Kif6-MD, and rKin430 bound to MTs immobilized on glass coverslips in the presence of ATP, whereas the full-length Kif6 and Kif9-CC1 did not show detectable MT binding (Fig. 4 E).
The C-terminal tail of Kif6 inhibits its spindle MT-association activity. Related to Fig. 4. (A) Diagrams of Kif6 and Kif9 constructs and a summary of the results in B. (B) Spindle MT association properties of Kif6 and Kif9. HEK293T cells were transiently transfected to express the indicated constructs (A) as illustrated in Fig. 4 B and fixed for fluorescent microscopy. α-tubulin (α-tub) was immunostained to show MTs. Chromosomes and nuclei were stained with DAPI, a DNA-specific dye. Arrowheads point to spindles in representative mitotic cells (B). Note that none of the Kif9 constructs showed obvious spindle MT associations.
The C-terminal tail of Kif6 inhibits its spindle MT-association activity. Related to Fig. 4. (A) Diagrams of Kif6 and Kif9 constructs and a summary of the results in B. (B) Spindle MT association properties of Kif6 and Kif9. HEK293T cells were transiently transfected to express the indicated constructs (A) as illustrated in Fig. 4 B and fixed for fluorescent microscopy. α-tubulin (α-tub) was immunostained to show MTs. Chromosomes and nuclei were stained with DAPI, a DNA-specific dye. Arrowheads point to spindles in representative mitotic cells (B). Note that none of the Kif9 constructs showed obvious spindle MT associations.
Kif6 is a slow progressive motor. (A) Diagrams of kinesin constructs and summaries of their properties based on the results in this figure. rKin430 served as a positive control. N/D, not done. (B) Experimental scheme for protein expressions in HEK293T cells. Harvested cells were lysed for protein purifications using anti-Flag beads. (C) Coomassie blue-stained polyacrylamide gel showing a typical batch of purified proteins. (D) Schematic of invitro MT binding and single-molecule motility assays. Rhodamine-labeled, taxol-stabilized MTs were immobilized on Pluronic F127-treated glass surface of flow chambers via neutravidin-biotin interaction, followed by the addition of GFP-tagged kinesins at concentrations of 1–2 μM and ATP at 1–2 mM. Imaging was performed using TIRF microscopy. (E) Representative images of MT-binding assays. (F) Representative time-lapse images of single-molecule motility assays, cropped from Video 5. GFP-tagged full-length Kif6, Kif6-CC2, and rKin430 were added to immobilized MTs at 50, 5, and 1 nM, respectively. Typical GFP-positive puncta on MTs are tracked with arrows to show their behaviors over time. The corresponding kymographs are presented. (G) Velocities of indicated motor proteins on MTs. 180 puncta from three biological replicates were measured for each protein. Data are presented as mean ± SD plus sample dots. (H) Schematic of in vitro MT gliding assays. Motor proteins at concentrations of 2–4 μM were coated on the glass surface of flow chambers. After blocking the remaining non-specific binding sites with Pluronic F127, Rhodamine-labeled, taxol-stabilized MTs, and 1–2 mM ATP were added, followed by live imaging. (I) Representative image sequences of MT gliding assays, cropped from Video 6. Arrowheads denote the leading ends of representative gliding MTs, whereas asterisks indicate representative stationary MTs. (J) MT gliding velocities. 50 MTs from three biological replicates were measured for each protein. Data are presented as mean ± SD plus sample dots.
Kif6 is a slow progressive motor. (A) Diagrams of kinesin constructs and summaries of their properties based on the results in this figure. rKin430 served as a positive control. N/D, not done. (B) Experimental scheme for protein expressions in HEK293T cells. Harvested cells were lysed for protein purifications using anti-Flag beads. (C) Coomassie blue-stained polyacrylamide gel showing a typical batch of purified proteins. (D) Schematic of invitro MT binding and single-molecule motility assays. Rhodamine-labeled, taxol-stabilized MTs were immobilized on Pluronic F127-treated glass surface of flow chambers via neutravidin-biotin interaction, followed by the addition of GFP-tagged kinesins at concentrations of 1–2 μM and ATP at 1–2 mM. Imaging was performed using TIRF microscopy. (E) Representative images of MT-binding assays. (F) Representative time-lapse images of single-molecule motility assays, cropped from Video 5. GFP-tagged full-length Kif6, Kif6-CC2, and rKin430 were added to immobilized MTs at 50, 5, and 1 nM, respectively. Typical GFP-positive puncta on MTs are tracked with arrows to show their behaviors over time. The corresponding kymographs are presented. (G) Velocities of indicated motor proteins on MTs. 180 puncta from three biological replicates were measured for each protein. Data are presented as mean ± SD plus sample dots. (H) Schematic of in vitro MT gliding assays. Motor proteins at concentrations of 2–4 μM were coated on the glass surface of flow chambers. After blocking the remaining non-specific binding sites with Pluronic F127, Rhodamine-labeled, taxol-stabilized MTs, and 1–2 mM ATP were added, followed by live imaging. (I) Representative image sequences of MT gliding assays, cropped from Video 6. Arrowheads denote the leading ends of representative gliding MTs, whereas asterisks indicate representative stationary MTs. (J) MT gliding velocities. 50 MTs from three biological replicates were measured for each protein. Data are presented as mean ± SD plus sample dots.
Next, we directly visualized single-molecule motilities of Kif6 on MTs immobilized on a glass surface (Fig. 4 D). Time-lapse imaging revealed that Kif6-CC2 puncta displayed processive movement along MTs, whereas full-length Kif6 puncta were basically immotile (Fig. 4, F and G; and Video 5). Quantifications indicated an average velocity of 23.1 ± 5.3 nm/s for Kif6-CC2, which was much slower than that of rKin430 (1,050 ± 293 nm/s) (Fig. 4 G).
Single-molecule motilities of Kif6, Kif6-CC2, and rKin430 on MTs in vitro. Related to Fig. 4 F. GFP-tagged full-length Kif6 (50 nM), Kif6-CC2 (5 nM), or rKin430 (1 nM; positive control) (green) was added to rhodamine-labeled, taxol-stabilized MTs (red) immobilized on a glass coverslip. Time-lapse TIRF images were captured at 1-s intervals for Kif6 and Kif6-CC2, and at 0.1-sec intervals for rKin430. The playback speed is 20 fps.
Single-molecule motilities of Kif6, Kif6-CC2, and rKin430 on MTs in vitro. Related to Fig. 4 F. GFP-tagged full-length Kif6 (50 nM), Kif6-CC2 (5 nM), or rKin430 (1 nM; positive control) (green) was added to rhodamine-labeled, taxol-stabilized MTs (red) immobilized on a glass coverslip. Time-lapse TIRF images were captured at 1-s intervals for Kif6 and Kif6-CC2, and at 0.1-sec intervals for rKin430. The playback speed is 20 fps.
Several autoinhibited kinesins are activated to glide MTs when the inhibitory C-terminal tail is immobilized on a glass surface to alleviate the inhibitory effect, analogous to the case of cargo binding (Coy et al., 1999; Du and Su, 2019; Imanishi et al., 2006). To verify that Kif6 is self-inhibited by its C-terminal region, we performed MT gliding assays (Fig. 4 H). Indeed, both full-length Kif6 and Kif6-CC2 were able to glide MTs (Fig. 4 I and Video 6). The velocities of MTs were 7.3 ± 1.1 nm/s and 10.0 ± 1.7 nm/s, respectively (Fig. 4 J), still in sharp contrast to the rapid MT gliding velocity (429.6 ± 49.2 nm/s) driven by rKin430 (Fig. 4, I and J; and Video 6) (Rogers et al., 2001). In contrast, Kif6-mut did not propel MT gliding (Fig. 4, A, C, I, and J; and Video 6), confirming the loss of ATPase activity.
Full-length Kif6 and Kif6-CC2, but not Kif6-mut, drive MT gliding. Related to Fig. 4 I. GFP-tagged full-length Kif6, Kif6-CC2, Kif6-mut, or rKin430 (positive control) was coated on a glass surface, followed by the addition of rhodamine-labeled, taxol-stabilized MTs. Time-lapse TIRF images were captured at 10-sec intervals for Kif6, Kif6-CC2, and Kif6-mut, or at 0.1-s intervals for rKin430. The playback speed is 20 fps.
Full-length Kif6 and Kif6-CC2, but not Kif6-mut, drive MT gliding. Related to Fig. 4 I. GFP-tagged full-length Kif6, Kif6-CC2, Kif6-mut, or rKin430 (positive control) was coated on a glass surface, followed by the addition of rhodamine-labeled, taxol-stabilized MTs. Time-lapse TIRF images were captured at 10-sec intervals for Kif6, Kif6-CC2, and Kif6-mut, or at 0.1-s intervals for rKin430. The playback speed is 20 fps.
Taken together, we conclude that Kif6 is a slow, processive MT-based motor that is autoinhibited by its C-terminal tail. In sharp contrast, Kif9 does not display an obvious MT-binding activity in both HEK293T cells and in vitro, even in the absence of its C-terminus. These results indicate that Kif6 differs strikingly from Kif9 in motor properties, consistent with their distinct ciliary localizations.
Mice lacking Kif6 or Kif9 develop hydrocephalus and exhibit male infertility
To uncover their physiological functions, we generated Kif6 and Kif9 knockout mice (Fig. 5, A and B) using the CRISPR/Cas9 system (Joung et al., 2017). Immunoblotting confirmed the complete depletion of Kif6 or Kif9 in motile cilia-enriched tissues (Fig. 5 C). Both Kif6−/− and Kif9−/− mice were born at the expected Mendelian ratio of genotypes. The majority of Kif6−/− mice experienced growth failure and often exhibited a dome-shaped skull compared with wild-type littermates after P21, suggestive of severe hydrocephalus (Fig. 5 D; arrows). Some of these mice also displayed a forward curvature of the spine and were smaller in size compared to their littermates (Fig. 5 D; arrowhead). Careful examination of brain dissections or coronal sections revealed that every analyzed Kif6−/− mouse (100%; total n = 60) displayed lateral ventricle expansion compared to the wild-type controls (Fig. 5, F and H). This ventricle enlargement was evident even in mice that displayed a normal skull appearance and a longer lifespan (Fig. 5 F; bottom). In sharp contrast to Kif6−/− mice, Kif9−/− mice appeared normal, similar to their wild-type littermates, based on macroscopic examination (Fig. 5 E). However, coronal brain sections revealed enlarged ventricles in over half of Kif9−/− mice (Fig. 5, G and H), indicative of hydrocephalus. Survival curve analysis revealed that ∼77% of Kif6−/− mice died before reaching P90, while Kif9−/− mice exhibited a lifespan similar to their wild-type littermates during a 12-month observation period (Fig. 5 I).
Knockout of Kif6 or Kif9 leads to hydrocephalus and male infertility. (A) Schematic for generating Kif6 (top) and Kif9 (bottom) knockout mice using the CRISPR/Cas9 technique. Targeting sites of sgRNAs (scissors) and genotyping PCR primers are indicated for each knockout model. (B) Representative genotyping PCR results. Genomic DNAs were extracted from P21 mouse tails and amplified by PCR using the indicated primer pairs in A. (C) Complete depletion of Kif6 and Kif9 in the indicated tissues. Tissue lysates were prepared from 2-month-old mice of the indicated genotypes, followed by immunoblotting. Gapdh served as a loading control. (D and E) Morphologies of Kif6- and Kif9-deficient mice. Kif6−/− mice exhibited a dome-shaped skull (arrow) and kyphosis (arrowhead) with skinny bodies (D). In contrast, Kif9−/− mice showed a normal appearance (E). (F and G) Representative images of coronal brain sections of Kif6−/− (F) or Kif9−/− (G) and their corresponding wide-type littermates. M: month. (H) Quantification of mice with enlarged brain ventricles (n = 60 for Kif6−/−; n = 11 for Kif9−/− mice). (I) Survival curves of mice with the indicated genotypes during a 12-month observation period. The number (n) of mice used for each genotype was indicated. (J) Sperm morphologies from wide-type and Kif9−/− littermates were similar. (K)Kif9−/− sperms exhibited forward motility defects. Sperm-head displacements in 1 s from the indicated numbers (n) of sperms collected from two P80 mice of each genotype (10 videos per mouse) were pooled together (mean ± SD plus sample dots) and subjected to unpaired two-tailed Student’s t test. (L) Sperm morphologies from wide-type and Kif6−/− littermates were similar. The images are representative of sperms from two pairs of P88 Kif6−/− and wild-type littermates. Source data are available for this figure: SourceData F5.
Knockout of Kif6 or Kif9 leads to hydrocephalus and male infertility. (A) Schematic for generating Kif6 (top) and Kif9 (bottom) knockout mice using the CRISPR/Cas9 technique. Targeting sites of sgRNAs (scissors) and genotyping PCR primers are indicated for each knockout model. (B) Representative genotyping PCR results. Genomic DNAs were extracted from P21 mouse tails and amplified by PCR using the indicated primer pairs in A. (C) Complete depletion of Kif6 and Kif9 in the indicated tissues. Tissue lysates were prepared from 2-month-old mice of the indicated genotypes, followed by immunoblotting. Gapdh served as a loading control. (D and E) Morphologies of Kif6- and Kif9-deficient mice. Kif6−/− mice exhibited a dome-shaped skull (arrow) and kyphosis (arrowhead) with skinny bodies (D). In contrast, Kif9−/− mice showed a normal appearance (E). (F and G) Representative images of coronal brain sections of Kif6−/− (F) or Kif9−/− (G) and their corresponding wide-type littermates. M: month. (H) Quantification of mice with enlarged brain ventricles (n = 60 for Kif6−/−; n = 11 for Kif9−/− mice). (I) Survival curves of mice with the indicated genotypes during a 12-month observation period. The number (n) of mice used for each genotype was indicated. (J) Sperm morphologies from wide-type and Kif9−/− littermates were similar. (K)Kif9−/− sperms exhibited forward motility defects. Sperm-head displacements in 1 s from the indicated numbers (n) of sperms collected from two P80 mice of each genotype (10 videos per mouse) were pooled together (mean ± SD plus sample dots) and subjected to unpaired two-tailed Student’s t test. (L) Sperm morphologies from wide-type and Kif6−/− littermates were similar. The images are representative of sperms from two pairs of P88 Kif6−/− and wild-type littermates. Source data are available for this figure: SourceData F5.
While female Kif9−/− and Kif6−/− mice were fertile, Kif9−/− and Kif6−/− males were sterile, even when those with normal appearance were used for mating (n = 3 for each genotype). Subsequently, we isolated sperm from the cauda epididymis of wild-type, Kif9−/−, and Kif6−/− mice at reproductive age (∼2 months) for motility analysis. Kif9−/− male mice showed a slight decrease in sperm count compared with wild-type controls (Fig. S4, A and B). Although Kif9−/− sperm had typical morphologies, they displayed impaired progressive motilities (Fig. 5, J and K; and Video 7), consistent with a previous report (Miyata et al., 2020). Kif6−/− mice, however, showed a significant reduction in sperm count (Fig. S4, C and D). Interestingly, while Kif6−/− sperm appeared to be normal in morphologies (Fig. 5 K), most of them completely lacked motility (67%, total n = 46) compared to wild-type sperm (3%, n = 131) (Video 8). Despite these findings, we did not observe noticeable defects, such as polydactyly or polycystic kidney, in organs where primary cilia are critical, suggesting intact primary cilia function. These observations emphasize the critical yet distinct roles of Kif6 and Kif9 in motile cilia and sperm flagella.
Kif6-deficient and Kif9-deficient males show decreased sperm numbers. Related to Fig. 5, J and L. Sperms were released from cauda epididymis and imaged with a spinning disk confocal microscope at a fixed z-plane. (A and C) Representative images were from the cauda epididymis of littermates. (B and D) Quantification results from two mice of each genotype (5 full-size micrographs per mouse) were pooled together (mean ± SD plus sample dots) and subjected to unpaired two-tailed Student’s t test.
Kif6-deficient and Kif9-deficient males show decreased sperm numbers. Related to Fig. 5, J and L. Sperms were released from cauda epididymis and imaged with a spinning disk confocal microscope at a fixed z-plane. (A and C) Representative images were from the cauda epididymis of littermates. (B and D) Quantification results from two mice of each genotype (5 full-size micrographs per mouse) were pooled together (mean ± SD plus sample dots) and subjected to unpaired two-tailed Student’s t test.
Morphologies and motilities of Kif9+/+and Kif9−/−sperms. Related to Fig. 5, J and K. Sperms were released from the cauda epididymis of a pair of P80 male littermates, followed by live imaging with a spinning disk confocal microscope at a fixed z-plane and at 25-ms intervals. The playback speed is 20 fps.
Morphologies and motilities of Kif9+/+and Kif9−/−sperms. Related to Fig. 5, J and K. Sperms were released from the cauda epididymis of a pair of P80 male littermates, followed by live imaging with a spinning disk confocal microscope at a fixed z-plane and at 25-ms intervals. The playback speed is 20 fps.
Morphologies and motilities of Kif6+/+and Kif6−/−sperms. Related to Fig. 5 L. Sperms were released from the cauda epididymis of a pair of P88 male littermates and imaged as described in Video 7. The playback speed is 20 fps.
Kif6 but not Kif9 deficiency disrupts the planar polarity of ependymal motile cilia
As abnormalities in mouse ependymal cilia cause hydrocephalus (Lechtreck et al., 2008; Liu et al., 2021; Ohata et al., 2014), we dissected the brain of mice older than P21 and stained the multicilia in living ependymal tissues with SiR-tubulin, a fluorescent probe for MTs (Lukinavicius et al., 2014), followed by live imaging using high-speed spinning disk confocal microscopy (Fig. 6 A). We did not observe gross differences in multiciliogenesis, the back-and-forth beat pattern of multicilia, and their beat frequencies between Kif6+/+ and Kif6−/− or Kif9+/+ and Kif9−/− littermates (Fig. 6, B–E). Nevertheless, while multicilia beat directionally across different cells in the wild-type or Kif9−/− ependymal tissues, their beat directions varied among individual cells in Kif6−/− ependymal tissues, regardless of the severity of hydrocephalus in the mice (Fig. 6, F and G; and Video 9). This indicates a disruption of the planar polarity of Kif6−/− multicilia.
Kif6 deficiency impairs the planar polarity of multicilia across the ependyma. Quantification results were pooled together (mean ± SD plus sample dots) and subjected to unpaired two-tailed Student’s t test. Mi, Mild hydrocephalus; Se, Severe hydrocephalus. (A) Experimental scheme. Whole-mounts of ventricle walls were freshly dissected and soaked in a culture medium. The ex-vivo tissues were stained with SiR-tubulin, a fluorescent dye for MTs, to label cilia. A fixed z-plane was imaged at 10-ms intervals to visualize ciliary motilities. (B–E) Motilities of multicilia in ependymal tissues of the indicated genotypes. Representative image sequences (B and D) show ciliary motilities in two consecutive beat cycles. Color-coded arrows indicate directions of effective and recovery strokes, respectively. Color images are overlaid pseudo-colored image sequences covering the first beat cycle. Multiciliary beat frequencies were quantified from five pairs of littermates (60 cells per mouse) (C) or three pairs of littermates (50 cells per mouse) (E). No gross differences were observed in the back-and-forth beat pattern of multicilia (B and D) and their beat frequencies (C and E) between Kif6+/+ and Kif6−/− or Kif9+/+ and Kif9−/− littermates. (F)Kif6 deficiency abolished the planar polarity of ependymal multicilia. The first three consecutive frames cropped from Video 9 were pseudocolored and overlaid to show ciliary motilities. Beat directions of multicilia are indicated by arrows. (G)Kif9 deficiency did not impair the planar polarity of ependymal multicilia. (H) Schematic for assays on multicilia-driven liquid flows. Fresh ependymal tissues were positioned on glass-bottomed culture dishes with the multicilia-containing side down. Fluorescent beads were added into culture medium. Beads in a fixed z-plane beneath ex-vivo ependymal tissues were imaged at 100-ms intervals. (I) Liquid flows and flow directions are shown by merging image sequences in the first 500 ms of Video 10. Colored arrows indicate trajectories of representative, rapidly moving fluorescent puncta during the period of time. (J) Quantification results for bead velocities. Data were from two pairs of P36 and one pair of P48 littermates (60 traceable beads for each mouse).
Kif6 deficiency impairs the planar polarity of multicilia across the ependyma. Quantification results were pooled together (mean ± SD plus sample dots) and subjected to unpaired two-tailed Student’s t test. Mi, Mild hydrocephalus; Se, Severe hydrocephalus. (A) Experimental scheme. Whole-mounts of ventricle walls were freshly dissected and soaked in a culture medium. The ex-vivo tissues were stained with SiR-tubulin, a fluorescent dye for MTs, to label cilia. A fixed z-plane was imaged at 10-ms intervals to visualize ciliary motilities. (B–E) Motilities of multicilia in ependymal tissues of the indicated genotypes. Representative image sequences (B and D) show ciliary motilities in two consecutive beat cycles. Color-coded arrows indicate directions of effective and recovery strokes, respectively. Color images are overlaid pseudo-colored image sequences covering the first beat cycle. Multiciliary beat frequencies were quantified from five pairs of littermates (60 cells per mouse) (C) or three pairs of littermates (50 cells per mouse) (E). No gross differences were observed in the back-and-forth beat pattern of multicilia (B and D) and their beat frequencies (C and E) between Kif6+/+ and Kif6−/− or Kif9+/+ and Kif9−/− littermates. (F)Kif6 deficiency abolished the planar polarity of ependymal multicilia. The first three consecutive frames cropped from Video 9 were pseudocolored and overlaid to show ciliary motilities. Beat directions of multicilia are indicated by arrows. (G)Kif9 deficiency did not impair the planar polarity of ependymal multicilia. (H) Schematic for assays on multicilia-driven liquid flows. Fresh ependymal tissues were positioned on glass-bottomed culture dishes with the multicilia-containing side down. Fluorescent beads were added into culture medium. Beads in a fixed z-plane beneath ex-vivo ependymal tissues were imaged at 100-ms intervals. (I) Liquid flows and flow directions are shown by merging image sequences in the first 500 ms of Video 10. Colored arrows indicate trajectories of representative, rapidly moving fluorescent puncta during the period of time. (J) Quantification results for bead velocities. Data were from two pairs of P36 and one pair of P48 littermates (60 traceable beads for each mouse).
Ciliary beat patterns in Kif6+/+and Kif6−/−ependymal tissues. Related to Fig. 6 F. Freshly dissected ependymal tissues from a pair of P31 littermates were labeled with SiR-tubulin to visualize ciliary axonemes, followed by live imaging with a spinning disk confocal microscope at a fixed z-plane and at 10-ms intervals. The playback speed is 5 fps. Images from the first three frames are presented in Fig. 6 F.
Ciliary beat patterns in Kif6+/+and Kif6−/−ependymal tissues. Related to Fig. 6 F. Freshly dissected ependymal tissues from a pair of P31 littermates were labeled with SiR-tubulin to visualize ciliary axonemes, followed by live imaging with a spinning disk confocal microscope at a fixed z-plane and at 10-ms intervals. The playback speed is 5 fps. Images from the first three frames are presented in Fig. 6 F.
Next, we verified whether the loss of ciliary planar polarity impaired the directional fluid flow across ex-vivoKif6−/− ependymal tissues by adding latex fluorescent microbeads to the culture medium and tracking their movements by live imaging (Fig. 6 H). We observed that the beads beneath ex-vivo wide-type ependymal tissues flowed in one direction with a velocity of 63.6 ± 27.1 μm/s (Fig. 6, I and J; and Video 10). In contrast, the beads beneath the Kif6−/− tissues mainly oscillated or whirled regionally, with a velocity of 46.3 ± 15.4 μm/s (Fig. 6, I and J; and Video 10). Therefore, we attribute the hydrocephalus of Kif6−/− mice to impaired CSF flows.
Liquid flows driven by ciliary beat in Kif6+/+and Kif6−/−ependymal tissues. Related to Fig. 6 I. Fresh ependymal tissues from a pair of P36 littermates were incubated with SiR-tubulin to label cilia and fluorescent latex beads as flow indicators as illustrated in Fig. 6 H. Motilities of the beads beneath the tissues were imaged with a spinning disk confocal microscope at 100-ms intervals and at a fixed z-plane. The playback speed is 5 fps. Colored lines in the first 500 ms indicate trajectories of representative, rapidly moving fluorescent puncta.
Liquid flows driven by ciliary beat in Kif6+/+and Kif6−/−ependymal tissues. Related to Fig. 6 I. Fresh ependymal tissues from a pair of P36 littermates were incubated with SiR-tubulin to label cilia and fluorescent latex beads as flow indicators as illustrated in Fig. 6 H. Motilities of the beads beneath the tissues were imaged with a spinning disk confocal microscope at 100-ms intervals and at a fixed z-plane. The playback speed is 5 fps. Colored lines in the first 500 ms indicate trajectories of representative, rapidly moving fluorescent puncta.
Kif6 is crucial for the rotational polarity of BBs in ependymal tissues
For insights into why Kif6−/− multicilia lost the planar polarity of beat directions (Fig. 6), we performed transmission electron microscopy on ependymal tissues to examine cilia-related ultrastructure (Fig. 7 A). Cross-sections indicated that Kif6−/− ependymal cilia still contained 9 + 2 axonemes, with outer and inner dynein arms on DMTs (Fig. 7 B). Nevertheless, the image resolutions were still insufficient for a definite conclusion as to whether the entire axonemal ultrastructure was identical to the wild-type one (Fig. 7 B). Interestingly, we observed that, while BFs were oriented in a similar direction in wild-type ependymal cells, BFs in Kif6−/− ependymal cells were misoriented regardless of the severity of hydrocephalus (Fig. 7 C), indicating a defect in the rotational polarity. To quantify the extent of the rotational polarity, we considered each arrow marking a BF orientation as a unit vector and measured the mean vector length for each electron micrograph (Bustamante-Marin et al., 2019). The proper rotational polarity of BBs in the wild-type ependymal tissues was confirmed by a mean vector length of 0.97 ± 0.07. In sharp contrast, the value was 0.35 ± 0.17 and 0.31 ± 0.18 for ependymal tissues from mice with mild hydrocephalus and severe hydrocephalus, respectively (Fig. 7 D).
Deficiency of Kif6, but not Kif9, impairs the rotational polarity of BBs in ependymal tissues. Quantification results were pooled together (mean ± SD plus sample dots) and subjected to unpaired two-tailed student’s t test. Se, Severe hydrocephalus. Mi, Mild hydrocephalus. (A) Schematic diagrams of a motile cilium and an array of rotationally polarized BBs. (B)Kif6 deficiency did not alter the overall axonemal ultrastructure. Shown are typical transverse views of ependymal cilia, representing transmission electron microscopic results from two wild-type and two Kif6−/− mice. (C and D) BB polarities assessed using transmission electron microscopy. Arrows indicate BB polarities in the representative electron micrographs (C), based on the BF orientation. At least ten full-size electron micrographs, each containing at least 6 BBs with clear BFs, were used for quantification. The extent of the rotational polarity in each micrograph was calculated as the mean vector length of all the BBs by considering each arrow marking their polarities as a unit vector (D). (E and F) BB polarities assessed using 3D-SIM. Ependymal tissues from the indicated mice were immunostained for Cep164 to visualize BBs and γ-tubulin to visualize BFs. Framed regions in E were magnified to show detailed BB polarities (arrows). The extent of the rotational polarity for a BB patch (BBs in a cell) was calculated as the mean vector length of all the BBs with clear BFs by considering each arrow marking their polarities as a unit vector. Quantification results (F) were from two P34 and one P43 Kif6−/− mice with severe (Se) hydrocephalus, one P62 Kif6−/− mouse with mild (Mi) hydrocephalus, and three of their wild-type littermates; and two 6-month-old (6 M) and one 7-month-old Kif9−/− mice and three wild-type littermates. 20 multiciliated ependymal cells were scored for each mouse. (G) Representative 3D-SIM images of ependymal tissues from the indicated mice. BB patches magnified from the framed regions are presented in E. Arrows indicate directions of the rotational polarity of BB patches, based on the major polarities of the BBs. Note that the arrows in each micrograph point to similar directions. No arrows are drawn for the Kif6−/− BB patches due to their lack of the rotational polarity (F). (H) A summarization model. Kif6 may act as an accessory motor to assist anterograde IFT or be transported by the IFT machinery as a cargo for speedy delivery. It may also function as an independent motor on DMTs to transport cargos into motile cilia to facilitate the establishment of rotational polarity. On the other hand, Kif9 localizes on the C2 MT to fine-tune ciliary or flagellar beat by inducing conformational changes in the central apparatus, similar to its Chlamydomonas counterpart KLP1 (Han et al., 2022). TZ, transition zone.
Deficiency of Kif6, but not Kif9, impairs the rotational polarity of BBs in ependymal tissues. Quantification results were pooled together (mean ± SD plus sample dots) and subjected to unpaired two-tailed student’s t test. Se, Severe hydrocephalus. Mi, Mild hydrocephalus. (A) Schematic diagrams of a motile cilium and an array of rotationally polarized BBs. (B)Kif6 deficiency did not alter the overall axonemal ultrastructure. Shown are typical transverse views of ependymal cilia, representing transmission electron microscopic results from two wild-type and two Kif6−/− mice. (C and D) BB polarities assessed using transmission electron microscopy. Arrows indicate BB polarities in the representative electron micrographs (C), based on the BF orientation. At least ten full-size electron micrographs, each containing at least 6 BBs with clear BFs, were used for quantification. The extent of the rotational polarity in each micrograph was calculated as the mean vector length of all the BBs by considering each arrow marking their polarities as a unit vector (D). (E and F) BB polarities assessed using 3D-SIM. Ependymal tissues from the indicated mice were immunostained for Cep164 to visualize BBs and γ-tubulin to visualize BFs. Framed regions in E were magnified to show detailed BB polarities (arrows). The extent of the rotational polarity for a BB patch (BBs in a cell) was calculated as the mean vector length of all the BBs with clear BFs by considering each arrow marking their polarities as a unit vector. Quantification results (F) were from two P34 and one P43 Kif6−/− mice with severe (Se) hydrocephalus, one P62 Kif6−/− mouse with mild (Mi) hydrocephalus, and three of their wild-type littermates; and two 6-month-old (6 M) and one 7-month-old Kif9−/− mice and three wild-type littermates. 20 multiciliated ependymal cells were scored for each mouse. (G) Representative 3D-SIM images of ependymal tissues from the indicated mice. BB patches magnified from the framed regions are presented in E. Arrows indicate directions of the rotational polarity of BB patches, based on the major polarities of the BBs. Note that the arrows in each micrograph point to similar directions. No arrows are drawn for the Kif6−/− BB patches due to their lack of the rotational polarity (F). (H) A summarization model. Kif6 may act as an accessory motor to assist anterograde IFT or be transported by the IFT machinery as a cargo for speedy delivery. It may also function as an independent motor on DMTs to transport cargos into motile cilia to facilitate the establishment of rotational polarity. On the other hand, Kif9 localizes on the C2 MT to fine-tune ciliary or flagellar beat by inducing conformational changes in the central apparatus, similar to its Chlamydomonas counterpart KLP1 (Han et al., 2022). TZ, transition zone.
As transmission electron microscopy is not ideal for assessing BB polarities in entire cells, letting alone planar polarities, we immunostained whole-mounts of ependymal tissues from wild-type, Kif6−/−, and Kif9−/− mice using Cep164, a transition fiber component (Siller et al., 2017), to mark BBs, and γ-tubulin or Centriolin to label BFs (Nguyen et al., 2020; Ohata et al., 2014). 3D-SIM revealed a uniform rotational polarity of BBs in individual cells and a planar polarity among neighboring cells in both wild-type and Kif9−/− whole-mounts (Fig. 7, E–G and Fig. S5, A–C). Both the rotational and the planar polarities of BBs, however, were markedly impaired in Kif6−/− ependymal tissues (Fig. 7, E–G and Fig. S5, A–C). We also scored BB numbers and observed that the deficiency of neither Kif6 nor Kif9 interfered with the BB biogenesis (Fig. S5 D), consistent with the normal ciliogenesis (Fig. 6) but different from the reported defective ciliogenesis in Kif6p.G555fs mice (Konjikusic et al., 2018).
Kif6 deficiency disrupts rotational polarity but not the translational polarity of BBs in the ependyma. Related to Fig. 7. Se, Severe hydrocephalus; Mi, Mild hydrocephalus. (A and B) BBs in Kif6−/− but not in Kif9−/− ependymal cells displayed impaired rotational polarity. Ependymal tissues from the indicated littermates were co-immunostained for Cep164 to visualize BBs and Centriolin to visualize BFs. Framed regions (A) were magnified to show BF orientations (arrows). The extent of the rotational polarity for a BB patch (BBs in a cell) was calculated as the mean vector length of all the BBs with clear BFs by considering each arrow marking their polarities as a unit vector. Quantification results from three mice per genotype are presented as mean ± SD plus sample dots and subjected to unpaired two-tailed student’s t test. Mice used were two 6-month-old (6 M) and one 7-month-old mice for Kif9−/− and three of their wild-type littermates; three P35 mice with severe hydrocephalus for Kif6−/− and three of their wild-type littermates. 20 ependymal cells (BB patches) were scored for each mouse. (C) Representative 3D-SIM images of ependymal tissues from the indicated mice. BB patches magnified from the framed regions are presented in A. Arrows indicate directions of the rotational polarity of BB patches, based on major polarities of the BBs. Note that the arrows in each micrograph point to similar directions. No arrows are drawn for the Kif6−/− BB patches due to their lack of rotational polarity (B). (D)Kif6 or Kif9-deficiency did not alter BB number in ependymal cells. 50 cells were measured per mouse. (E–H)Kif6 deficiency did not impair the translational polarity of BBs. Ependymal tissues from Kif6−/− mice with mild hydrocephalus (two P62 and one P68) and severe hydrocephalus (P34, P38, and P43) or wild-type mice (P62, P38, and P43) were immunostained for ZO1 and Cep164 to visualize cell borders (tight junctions) and BBs, respectively (E). BB patch displacement relative to the cellular geographic center (G) and relative BB patch area (H) were quantified as illustrated in (F). 50 ependymal cells were scored for each mouse.
Kif6 deficiency disrupts rotational polarity but not the translational polarity of BBs in the ependyma. Related to Fig. 7. Se, Severe hydrocephalus; Mi, Mild hydrocephalus. (A and B) BBs in Kif6−/− but not in Kif9−/− ependymal cells displayed impaired rotational polarity. Ependymal tissues from the indicated littermates were co-immunostained for Cep164 to visualize BBs and Centriolin to visualize BFs. Framed regions (A) were magnified to show BF orientations (arrows). The extent of the rotational polarity for a BB patch (BBs in a cell) was calculated as the mean vector length of all the BBs with clear BFs by considering each arrow marking their polarities as a unit vector. Quantification results from three mice per genotype are presented as mean ± SD plus sample dots and subjected to unpaired two-tailed student’s t test. Mice used were two 6-month-old (6 M) and one 7-month-old mice for Kif9−/− and three of their wild-type littermates; three P35 mice with severe hydrocephalus for Kif6−/− and three of their wild-type littermates. 20 ependymal cells (BB patches) were scored for each mouse. (C) Representative 3D-SIM images of ependymal tissues from the indicated mice. BB patches magnified from the framed regions are presented in A. Arrows indicate directions of the rotational polarity of BB patches, based on major polarities of the BBs. Note that the arrows in each micrograph point to similar directions. No arrows are drawn for the Kif6−/− BB patches due to their lack of rotational polarity (B). (D)Kif6 or Kif9-deficiency did not alter BB number in ependymal cells. 50 cells were measured per mouse. (E–H)Kif6 deficiency did not impair the translational polarity of BBs. Ependymal tissues from Kif6−/− mice with mild hydrocephalus (two P62 and one P68) and severe hydrocephalus (P34, P38, and P43) or wild-type mice (P62, P38, and P43) were immunostained for ZO1 and Cep164 to visualize cell borders (tight junctions) and BBs, respectively (E). BB patch displacement relative to the cellular geographic center (G) and relative BB patch area (H) were quantified as illustrated in (F). 50 ependymal cells were scored for each mouse.
We also examined the translational polarity by quantifying the extent of BB patch displacement relative to the cell center (Mirzadeh et al., 2010) but did not observe a significant difference between the wild-type mice and their Kif6−/− littermates (Fig. S5, E–G). The area of BB patches was also unaffected in Kif6−/− mice with mild hydrocephalus but slightly increased in mice with severe hydrocephalus (Fig. S5, E, F, and H), possibly due to the pathological effects of the hydrocephalus.
Taken together, we conclude that Kif6 is critical for the rotational polarity of ependymal multicilia but dispensable for the translational polarity. Such a defect abolishes the planar polarity of a ciliary beat in the Kif6−/− ependyma, leading to abnormal CSF flows and hydrocephalus.
Discussion
In this study, we investigated the roles of mouse Kif6 and Kif9, two members of the kinesin-9 family with expression patterns highly correlated with multicilia and flagella (Fig. 1, A and C–F). Phylogenetic analysis supports their specific function in motile cilia and flagella (Fig. 1 B). Consistently, they are detected in motile cilia but not in immotile cilia (Fig. 1, H–J). Mouse Kif9 localizes along the CP of cilia (Fig. 1 K and Fig. 2 E), similar to its Chlamydomonas homolog KLP1 (Han et al., 2022; Yokoyama et al., 2004), whereas mouse Kif6 is distributed as puncta along peripheral DMTs (Fig. 1 K, Fig. 2 D, and Fig. 3, A–C) (Takagishi et al., 2024), suggesting their distinct physiological functions.
We propose that Kif6 is actively involved in cargo transport within motile cilia (Fig. 7 H). First, Kif6 moves along MTs in vitro, and its motor activity is autoinhibited by its C-terminal tail (Fig. 4, Fig. S3; and Videos 5 and 6) (Takagishi et al., 2024), similar to many other active kinesins (Cason and Holzbaur, 2022; Lu and Gelfand, 2017; Ou and Scholey, 2022). Second, ciliary Kif6 puncta display bidirectional IFT-like movements along DMTs with or without an accompanying Ift27 puncta (Figs. 2 and 3; Fig. S2; and Videos 2 and 4). Third, its ATPase activity is required for its ciliary localization (Fig. 3, G–I), suggesting that Kif6 enters motile cilia as an active motor. The requirement for ATPase activity is also supported by ciliary localization of the motor-active construct, Kif6(1–493) (Takagishi et al., 2024). Given its relatively low in vitro speed, measured at 23.1 ± 5.3 nm/s for Kif6-CC2-GFP (Fig. 4, F and G) and 12.2 ± 2.0 nm/s for Kif6(1–493)-mNeonGreen (Takagishi et al., 2024), a construct one residue shorter than Kif6-CC2 (Fig. 4 A), Kif6 may act as an accessory IFT motor, similar to KLP-6 in C. elegans (Morsci and Barr, 2011), to modulate anterograde IFT (Fig. 7 H). Alternatively, Kif6 may be transported as a cargo of IFT trains for speedy delivery. As the motor-active Kif6(1–493) enters cilia independently of IFT (Takagishi et al., 2024) and we observed Ift27-free movements of Kif6 puncta (Fig. 3), Kif6 may also transport cargos independently of the anterograde IFT machinery (Fig. 7 H). Whether the motilities of IFT train-free Kif6 could be dramatically enhanced to what we observed in cilia (Fig. 3, C–E), however, still requires future clarifications. Currently, we were unable to exclude the possibility that accompanying IFT-B particles were present but not captured in the instances of ciliary Kif6-alone movements (Fig. 3, C–E) due to limitations of fluorescent microscopy, even though the cutting-edge GI-SIM with superior spatiotemporal resolutions (Guo et al., 2018; Qiao et al., 2023) was used. The observation of retrograde Kif6 puncta (Fig. 2 D, Fig. 3, B–F; and Videos 2 and 4) is likely due to Kif6 being transported out of cilia by the retrograde IFT machinery, as N-kinesins are usually MT plus end-directed (Cason and Holzbaur, 2022; Lu and Gelfand, 2017; Ou and Scholey, 2022).
Ciliary Kif6 may be activated upon binding to IFT trains or other regulatory factors, including cargos. C-terminal tails of kinesins are usually involved in interactions with cargos and/or regulatory proteins to simultaneously relieve their autoinhibitory effects (Cason and Holzbaur, 2022; Lu and Gelfand, 2017; Ou and Scholey, 2022). The activation of full-length Kif6 in MT-gliding assays, but not in single-molecule assays (Fig. 4), suggests this is also true for Kif6. Consistently, ciliary Kif6(1–493) loses the punctate pattern of full-length Kif6, displays no IFT-like movement, and is mainly enriched at the ciliary tip (Takagishi et al., 2024), indicating that the ciliary Kif6(1–493) molecules no longer bind to the IFT machinery and other proteins that could enhance its motility. As a result, they can only walk slowly along ciliary DMTs by themselves until they reach the tip. Nevertheless, as Takagishi and colleagues performed the live imaging with a confocal microscope at 5-s intervals (Takagishi et al., 2024), it is unlikely that they were able to capture the movement of individual Kif6(1–493) molecules along the nine DMTs in cilia. Detailed underlying mechanisms, however, still await future investigations.
The severe postnatal-onset hydrocephalus and sterility of our Kif6−/− mice (Fig. 5; and Fig. S4, C and D) further strengthen the physiological importance of Kif6 in mammals, as demonstrated previously with both the human patient and Kif6p.G555fs homozygous mice (Konjikusic et al., 2018). Furthermore, hydrocephalus phenotypes in both genetic mouse models are mainly attributed to defects in the planar polarity of ependymal multicilia (Figs. 6 and 7) (Takagishi et al., 2024), though the Kif6p.G555fs mice additionally manifest reduced ciliary numbers and beat frequencies (Konjikusic et al., 2018; Takagishi et al., 2024), probably due to their expression of a truncated, motor-active Kif6 mutant. As hydrodynamic forces on beating cilia are required to eventually achieve planar polarity (Guirao et al., 2010; Ohata and Alvarez-Buylla, 2016), Kif6 may help to transport proteins involved in sensing hydrodynamic forces and other environmental cues into cilia. Similarly, the severe motility defects in Kif6−/− sperms (Fig. S4, C and D; and Video 8) may also be attributed to the lack of certain sensory proteins during spermatogenesis. It will be intriguing to identify these cargo proteins for future investigations.
We propose that Kif9 acts to fine-tune cilia beat, possibly as a C2 MT projection to generate conformational changes in the central apparatus (Fig. 7 H), analogous to the C2 MT localization and proposed functions of its Chlamydomonas counterpart KLP1 (Han et al., 2022). Consistently, only roughly half of Kif9−/− mice displayed mild to moderate hydrocephalus (Fig. 5, E, G, and H). We were unable to identify apparent abnormalities in ciliary motility and planar polarity in Kif9−/− ependymal tissues (Fig. 6, D, E, and G; Fig. 7, E–G, and Fig. S5, A–D), possibly due to our technical limitations. Nevertheless, the impaired progressive motility of Kif9−/− sperms (Fig. 5, J and K; and Video 7) (Miyata et al., 2020) is consistent with the role of Kif9 in modulating axonemal beat patterns. Furthermore, in Kif9-knockdown Xenopus embryos, epidermal multicilia display abnormal beat patterns and reduced beat frequencies (Konjikusic et al., 2023). Cryo-electron tomography (Cryo-ET) studies on Chlamydomonas CP apparatus indicate that KLP1 forms an asymmetric dimer with a trailing head and a leading head on the C2 MT (Han et al., 2022). Furthermore, the trailing heads of KLP1 arrays can manifest a cooperative 16-nm movement forward (Han et al., 2022), though it is unclear whether the KLP1 arrays move persistently forward or just swing back and forth on the CP apparatus. Our GI-SIM results (Fig. 2 E, Fig. S2 A, and Video 3) suggest that Kif9-GFP puncta, which possibly represent clustered Kif9 arrays, appear to mainly swing regionally and may sometimes undergo short movements. Nevertheless, as the optical resolution of GI-SIM (97 nm) is far below the periodicity of Kif9 on the CP apparatus, which according to KLP1 is 16 nm (Han et al., 2022), future studies are definitely required to verify these observations. Interestingly, Xenopus Kif9(1–461)-mNeonGreen binds to MTs in vitro and walks along MTs at 7.2 nm/s (Konjikusic et al., 2023). Nevertheless, we did not observe obvious MT binding of similar mouse Kif9 constructs tagged with GFP both in vitro and in HEK293T cells, indicating that such activities, if any, must be much weaker than the comparable Kif6 constructs (Fig. 4 E and Fig. S3). Possibly the use of mNeonGreen, a fluorescent protein brighter than GFP (Shaner et al., 2013), enables the detection of weak MT-binding signals that we missed. The differences in constructs, species, and experimental conditions may also be causes that should be clarified in the future.
Materials and methods
Plasmids
The DNA fragments encoding full-length Kif6 (GenBank: NM_177052.3), Kif9 (GenBank: NM_001163569.1), Ift81 (GenBank: NM_001358917.1), and Ift27 (GenBank: NM_025931.3) were amplified by PCR using cDNAs of cultured mEPCs (Day 10) as the template. These DNA fragments were used to generate adenoviral expression constructs by cloning them into the entry vector pYr-1.1-GFP (YRBio) for GFP-tagged proteins or into pYr-1.1-Halo for Halo-tagged proteins. The LR recombination reactions between the entry constructs and the destination vector pAd/BLOCK-iT-DEST (YRBio) were carried out using LR Clonase II enzyme mix (11791020; Thermo Fisher Scientific), as described previously (Zhao et al., 2021).
To express GFP-fusion proteins, the DNA fragments of Kif6 (1–802 aa), Kif6-MD (1–353 aa), Kif6-CC1 (1–385 aa), Kif6-CC2 (1–494 aa), Kif9 (1–810 aa), Kif9-MD (1–348 aa), Kif9-CC1 (1–442 aa), and Kif9-CC2 (1–694 aa) were amplified by PCR and cloned into pEGFP-N1. To express GFP-Flag-fusion proteins for in vitro assays, the DNA fragments for Kif6-GFP, Kif6-MD-GFP, Kif6-CC1-GFP, Kif6-CC2-GFP, Kif9-GFP, Kif9-MD-GFP, Kif9-CC1-GFP, Kif9-CC2-GFP, and rKin430-GFP were PCR-amplified and constructed into pCDAN3.1-cFlag. To generate polyclonal antibodies against Kif9, the DNA fragment encoding amino acids 348–810 was amplified by PCR and subcloned into pET-28a or pGEX-4T-1 to express His-tagged or GST-tagged Kif9 (348–810 aa), respectively. The Kif6 construct containing T104A and F105A mutations (referred to as Kif6-mut) was produced by PCR.
All constructs were validated by sequencing. The primers used for PCR amplification are listed in Table S1.
Mice
All animal experiments were performed following the guidelines approved by the Institutional Animal Care and Use Committee of the Shanghai Institute of Biochemistry and Cell Biology. The mice used in this study had a C57BL/6J genetic background. Kif6+/− and Kif9+/− mice were obtained from GemPharmatech, China. The ages of the mice used in this study are provided in the figures or the corresponding legends. The guide RNA (gRNA) sequences for targeting Kif6 or Kif9 genes and the primers used for genotyping are listed in Table S1.
Cell culture and transfection
HEK293T and HEK293A cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (12430-054; Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) (VS500T; Ausbian), 0.3 mg/ml glutamine (G8540; Sigma-Aldrich), 100 U/ml penicillin (P8420; Solarbio), and 100 U/ml streptomycin (S8290; Solarbio). NIH3T3 cells were cultured in DMEM with 10% normal goat serum (NGS), 0.3 mg/ml glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin. hTERT-RPE1 and IMCD3 cells were cultured in DMEM/F12 (GE Healthcare) supplemented with 10% FBS, 0.3 mg/ml glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin. Additionally, the hTERT-RPE1 culture medium was supplemented with 10 μg/ml hygromycin B (10687010; Thermo Fisher Scientific). All cell lines were passaged at a 1:4 ratio upon reaching 80–90% confluence. HEK293A cells were used for adenovirus packaging following the guidelines (YRBio). Routine mycoplasma testing was performed on all cell lines.
The primary culture of mouse ependymal cells (mEPCs) was prepared and cultured as previously described (Delgehyr et al., 2015; Zhao et al., 2021). Newborn C57BL/6J mice were anesthetized on ice, and the cerebellum, olfactory bulbs, meninges, and hippocampus were carefully removed using sharp tweezers (1214Y84; Dumont) in a cold dissection solution (5 mM Hepes, 161 mM NaCl, 5 mM KCl, 1 mM MgSO4, 3.7 mM CaCl2, and 5.5 mM Glucose, pH 7.4) under a stereo microscope. The remaining telencephala were then digested in a dissection solution containing 10 U/ml papain (LS003126; Worthington-Biochem), 0.2 mg/ml L-cysteine, 1.5 mM NaOH, 1 mM CaCl2, 0.5 mM EDTA, and 0.15% DNase I (D5025; Sigma-Aldrich) for 30 min at 37°C. After gentle pipetting and centrifugation at 400 × g for 5 min at room temperature (r.t.), the cells were resuspended in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin and seeded into flasks (L2020; Sigma-Aldrich) precoated with 10 μg/ml Human Plasma Fibronectin (FC010; Sigma-Aldrich). Neurons were mechanically removed by patting the flask ∼2 days post-inoculation. When reaching ∼90% confluence in flasks, cells were digested with trypsin and replated into fibronectin-coated 29-mm glass-bottomed dishes (D29-14-1.5-N; Cellvis) for immunofluorescence staining. Once cells reached ∼90% confluence in dishes, FBS was removed from the medium to induce differentiation.
Mouse tracheal epithelial cells (mTECs) were cultured following the method described (Zhao et al., 2021). Tracheas isolated from 4-week-old C57BL/6J mice were cleaned of connective and muscle tissues, cut into small pieces, and digested overnight at 4°C in Ham’s F-12K medium containing 0.15% Pronase E (P6911; Sigma-Aldrich) and 0.1 mg/ml DNase I (D5025; Sigma-Aldrich). After digestion, the cells were harvested by centrifugation at 400 × g for 5 min at r.t., then resuspended in DMEM-Ham’s F-12 (11330-032; Thermo Fisher Scientific) supplemented with 3.6 mM sodium bicarbonate, 4 mM L-glutamine, 1% penicillin/streptomycin, and 0.25 μg/ml fungizone and 10% FBS (mTEC basic medium), and incubated at 37°C for 4 h. Non-adherent mTECs were collected by centrifugation at 400 × g for 5 min, resuspended in mTEC basic medium supplemented with 10 μg/ml insulin (I6634; Sigma-Aldrich), 5 μg/ml transferrin (T8158; Sigma-Aldrich), 0.1 μg/ml cholera toxin (C8052; Sigma-Aldrich), 25 ng/ml epidermal growth factor (E4127; Sigma-Aldrich), 30 μg/ml bovine pituitary extract (P1167; Sigma-Aldrich), 5% FBS, and 0.05 μM retinoic acid (freshly added; R2625; Sigma-Aldrich) (mTEC plus medium), and seeded onto collagen (C8897; Sigma-Aldrich)-coated 6.5-mm Transwell inserts with a 0.4-μm-pore polyester membrane (3470; Corning). Once the cells achieved full confluence, an air–liquid interface (ALI) was created by removing the medium from the Transwell insert and replacing the medium in the lower compartment with mTEC basic medium supplemented with 2% Nu-Serum (355100; BD) and 0.05 μM retinoic acid (freshly added) to induce differentiation. DAPT (D5942; Sigma-Aldrich) (mTEC differentiation medium) was added to a final concentration of 10 μM at day 1 after ALI to enhance the efficiency of multiciliated cell differentiation. mTECs were used to investigate the expression profiles of Kif6 and Kif9 during multiciliation due to their high multiciliation efficiency.
For transfection of plasmids, cells were transfected at ∼70% confluence with Lipofectamine 2000 (11668500; Thermo Fisher Scientific).
Preparation and culture of whole mounts of lateral ventricle walls
The whole mounts of lateral ventricles were dissected as previously described (Ohata et al., 2014) with minor modifications. After mice were euthanized with CO2, the lateral ventricles were carefully dissected at a thickness of 500–1,000 μm using Vannas Scissors (66VT, 54140B) in a prewarmed (37°C) dissection solution (25 mM Hepes,117.2 mM NaCl, 5.3 mM KCl, 0.81 mM MgSO4, 1.8 mM CaCl2, 26.1 mM NaHCO3, 1 mM NaH2PO4·2H2O, and 5.6 mM Glucose, pH 7.4). The dissected samples were cultured in DMEM supplemented with 0.3 mg/ml glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin.
Viral production and transfection
Adenovirus particles were produced and utilized to infect mEPCs as previously described (Zhao et al., 2021). In brief, the adenovirus expression constructs were linearized using PacI restriction enzyme (NEB) and transfected into HEK293A cells cultured in six-well plates using Lipofectamine 2000 to produce the initial adenovirus. When ∼80% of cells exhibited a cytopathic effect (CPE), the culture medium was collected and infected HEK293A cells in a 10-cm dish. This infection cycle was repeated until about 80% of cells in successive cultures showed a CPE. After the final cycle, both cells and culture medium were collected. To ensure complete release of the viral particles, the mixture underwent three freeze–thaw cycles between −80 and 37°C, followed by centrifugation at 400 × g for 5 min to remove cell debris. The harvested adenoviral particles were then used at dilutions of 1:1,000 or 1:200 for experiments.
Immunofluorescence staining and imaging
IMCD3 cells were fixed with 4% paraformaldehyde (PFA) in PBS, whereas mEPCs grown on 29-mm glass-bottom dishes and freshly dissected whole-mounts were prepermeabilized with 0.5% Triton X-100 in PBS for 30 s to remove soluble proteins before fixation. After fixation, cells were extracted with 0.5% Triton X-100 in PBS for 15 min and blocked using 4% BSA in TBST at r.t. for 1 h. Cells were then incubated with primary antibodies overnight at 4°C. After three 5-min washes with the blocking solution, the cells were incubated with secondary antibodies at r.t. for 1 h and mounted using Dako (S3023; Dako) or ProLong (2273640; Thermo Fisher Scientific). All the antibodies used are listed in Table S2.
Fluorescent images were captured at 18–20°C either using a Leica TCS SP8 WLL system with an HC PL APO CS2 63×/1.40 OIL objective or an Olympus Xplore SpinSR10 microscope with UPLAPO OHR 60×/1.50 Objective and an ORCA-Fusion camera (Hamamatsu). Images were processed with maximum intensity projections. 3D-SIM super-resolution images were taken at 18–20°C with a DeltaVision OMX SR system (GE Healthcare) equipped with a Plan Apo × 60/1.42 NA oil-immersion objective lens (Olympus). The z-axis scanning step was 0.125 μm. Original images were processed for OMX SI Reconstruction, OMX Image Registration, and maximum intensity projection with SoftWoRx software.
Live cell imaging
To perform grazing incidence structured illumination microscopy (GI-SIM) imaging for fluorescent particle movements in mEPCs, cells were seeded onto fibronectin-coated 12-mm coverslips and infected with adenovirus expressing fluorescent proteins a day before serum starvation. To label Ift27-2Halo, mEPCs were incubated with 200 nM Janelia Fluor 549 HaloTag ligand (GA1110; Promega) for 20 min before live imaging. Coverslips with confluent cells were carefully positioned, with the cell side down, onto a large microscope cover glass precoated with poly-L-lysine (P1399; Sigma-Aldrich). Approximately 400 μl of culture medium was then gently dispensed onto the surface. mEPCs were then captured using GI-SIM system under the physiological conditions of 37°C and 5% CO2 as described (Guo et al., 2018; Qiao et al., 2023). GI-SIM was built on an inverted fluorescence microscope (IX83; Olympus) with an Olympus 100×/1.49-NA objective and a sCMOS camera (Orca Flash 4.0 v3 sCMOS; Hamamatsu). The beam from a laser combiner equipped with 488 nm (500 mW, Genesis Max 488–500 STM; Coherent) and 560 nm (1 W, VFL-P-500-560; MPB Communications) lasers is passed through an acousto-optic tunable filter (AOTF; AA Quanta Tech, AOTFnc-400.650-CPch-TN). The beam is then expanded to a 1/e2 diameter of 12-mm and sent to a phase-only modulator consisting of a polarizing beam splitter, an achromatic half-wave plate (HWP; Bolder Vision Optik, BVO AHWP3), and a ferroelectric spatial light modulator (SLM; ForthDimension Displays, QXGA-3DM). The excitation intensity is 30 W/cm2 (Guo et al., 2018). Dual-color imaging calibration was routinely conducted using fluorescent beads. Images and videos were processed using Fiji and Huygens software. The velocities and frequencies of GFP or Halo-tagged puncta were analyzed using kymographs in Fiji software, considering only the first 200 frames to avoid signal bleaching.
To record ciliary beating, the whole-mounts of lateral ventricles were dissected at a thickness of 500–1,000 μm as previously described (Ohata et al., 2014; Zhao et al., 2021) and cilia were labeled with 100 nM SiR-tubulin (SC002; Spirochrome) before imaging. The high-speed live imaging was conducted with a spinning disk confocal microscope (Xplore SpinSR 10; Olympus) equipped with a UPLAPO OHR 60×/1.50 Objective, an ORCA-Fusion camera (Hamamatsu), OBIS solid-state Laser, 4,000 rpm CSU Disk Speed, and incubation chamber (37°C, 5% CO2, and 80% humidity). The ciliary beat was captured at 100 frames per second (fps) by a time-lapse collection of single optical sections with 80% laser power (640 nm) to achieve an exposure time of 5 ms. Ciliary beat frequencies were analyzed using kymographs generated for the first 500 ms in Fiji software.
The ependymal flow assay was performed as described (Liu et al., 2021; Mirzadeh et al., 2010) with minor modifications. Briefly, the freshly dissected ependymal tissues were sectioned using a vibratome (VT1000S; Leica) to obtain slices with a thickness of 250 μm. Before imaging, multicilia were labeled with 100 nM SiR-tubulin for 1 h, and small fluorescent latex beads with a diameter of 250 nm at 1:500 dilution (F8809; Invitrogen) were added to the culture medium. Time-lapse images of bead movements were captured with the same microscope used for recording ciliary beating. The laser setting for the beads channel (561 nm) was adjusted based on fluorescent intensity with an exposure time set to 100 ms. The SiR-tubulin channel (640 nm) used 80% laser power to achieve a 5-ms exposure time. During the recording, the motility of fluorescent beads suspended in the culture medium containing the living ependymal tissues was captured at a fixed z-plane. Multicilia were then visualized by adjusting the focus and live imaging of a single optical section was conducted to record ciliary motility. Frames capturing beads from Video 10 within the same area were combined and overlaid with the trajectories of rapidly moving, traceable bead aggregates during the first 500 ms to depict flow directions and paths. The velocities of beads were measured using Fiji software.
To record sperm motility, sperms were released from the cauda epididymis into a buffer (M1130; Njabsw), followed by live imaging with a spinning disk confocal microscope (Xplore SpinSR 10; Olympus), equipped with a UPLAPO OHR 60×/1.50 Objective, an ORCA-Fusion camera (Hamamatsu), Bright Field (BF), 4,000 rpm CSU Disk Speed, and incubation chamber (37°C, 5% CO2, and 80% humidity). Images were taken at a fixed z-plane with 25-ms intervals. Sperms from two pairs of P88 wild-type and Kif6−/− littermates and two pairs of P80 wild-type and Kif9−/− littermates were examined. To assess sperm count, we randomly selected the first frame (full micrograph with 2,304 × 2,304 pixels) of recordings and counted only the sperm with heads from each genotype. For sperm-head displacement analysis, frames displaying clear sperm movement were randomly selected to measure the head displacement during the first 1 sec using Fiji software.
Protein purification
Protein purification was performed as described (Liu et al., 2021). Cells were lysed with cold lysis buffer (20 mM Tris-HCl, pH 7.5, 100 mM KCl, 0.1% NP-40, 1 mM EDTA, 10 mM Na4O7P2, 10% Glycerol, and protease inhibitors [539134; Sigma-Aldrich]). The lysates were collected after centrifugation for 20 min at 14,000 × g at 4°C to remove debris. The lysates were incubated with 20 μl of anti-Flag beads (A2220; Sigma-Aldrich) for 2 h at 4°C under rotary agitation. The beads were washed three times with lysis buffer and three times with wash buffer (20 mM Tris-HCl, pH 7.5, 150 mM KCl, 0.5% NP-40, 1 mM EDTA, 10 mM Na4P2O7, 10% Glycerol). Proteins bound to the Flag beads were eluted with 30 μl of 1 mg/ml Flag peptide and were ready for in vitro MT binding, gliding, and single-molecule motility assays.
Immunoblotting
Immunoblotting experiments were performed as described (Zhao et al., 2021). To prepare tissue lysates, freshly dissected mouse tissues were lysed in ice-cold RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and 1 mM EDTA, pH7.5) supplemented with protein inhibitors cocktail (539134; Calbiochem). The tissues were homogenized using the homogenizer Precellys 24 system (Bertin). The lysates were cleared by centrifugation at 14,000 × g at 4°C for 15 min and mixed with an equal volume of 2 × SDS-PAGE loading buffer (100 mM Tris-HCl, 4% SDS, 20% glycerol, 2% 2-mercaptoethanol, 0.1% bromophenol blue, pH 6.8). For cultured cells, cells were washed with prewarmed PBS and lysed directly in 2 × SDS-PAGE loading buffer. The lysates were boiled at 100°C for 10 min and then subjected to immunoblotting.
Invitro MT binding and gliding assays
Imaging flow chambers were assembled using clean, silanized microscope slides and 18 × 18 mm glass coverslips, held together by two strips of double-sided adhesive tape as previously described (Gell et al., 2010; Stanhope and Ross, 2015).
Rhodamine-labeled, taxol-stabilized MTs were polymerized as described previously with some modifications (Gell et al., 2010). A mixture of 1 μl of 5% rhodamine-labeled tubulin (TL590M; Cytoskeleton), 1 μl of 5% biotin-labeled tubulin (T333P; Cytoskeleton), and 1 μl of 90% unlabeled tubulin (T240; Cytoskeleton) was incubated with BRB80 buffer (80 mM PIPES, 1 mM EGTA, 1 mM MgCl2, pH 6.8) containing 1 mM GTP at 37°C for 30 min. An additional 30-min incubation with 10 μM taxol (Sigma-Aldrich) at 37°C was performed to stabilize the MTs. After stabilization, the MTs were pelleted by centrifugation at 13,000 × g for 25 min at r.t. to remove free tubulin. The pellet was resuspended in 40 μl BRB80 buffer containing 10 μM taxol.
For MT binding assays, flow chambers were treated with neutravidin (31000; Thermo Fisher Scientific). A solution of 5–10 μl of 10 μg/ml of neutravidin in BRB80 buffer was pipetted into the imaging chamber and incubated for 10 min at r.t. Excess neutravidin was washed out using two times the chamber volumes of BRB80 buffer, which was between 5 and 10 μl. The flow chambers were blocked with 1% Pluronic F127 in BRB80 buffer for 20 min to prevent non-specific binding. Rhodamine-labeled MTs in BRB80 buffer were introduced into the imaging chamber and incubated for 5 min. Subsequently, purified kinesin proteins diluted in BRB80 buffer were flowed into the chamber and incubated for 5 min, followed by a wash with two times the chamber volumes of BRB80 buffer containing 1–2 mM ATP. The images were taken at r.t. using a total internal reflection fluorescent (TIRF) microscope equipped with a Zeiss Cell Observer spinning disk system consisting of an Evolve Electron-Multiplying CCD (EMCCD) camera and a 63×/1.46 NA oil lens.
For MT gliding assays, the purified kinesin proteins in BRB80 buffer were flowed into the chamber and incubated for 10 min. Free kinesin proteins were washed out with BRB80 buffer. Rhodamine-labeled MTs were diluted in a basic reaction mix (RM buffer: BRB80 buffer supplemented with 0.08 mg/ml glucose oxidase, 0.032 mg/ml catalase, 0.16 mg/ml casein, 1% β-mercaptoethanol, 0.001% tween, 10 μM taxol and 80 mM D-glucose) containing 1–2 mM Mg-ATP. The MTs were introduced into the chamber, and the MT gliding videos were captured at r.t. using the same TIRF microscope used in MT binding assays. For rKin430, time-lapse TIRF images were acquired at 0.1-sec intervals for 2 min. For full-length Kif6, Kif6-CC2, and Kif6-mut (a T104A/F105A mutant), time-lapse TIRF images were taken at 10-s intervals for 10 min. The position and movement of fluorescent MTs were manually tracked using Fiji software. The velocities of gliding MTs were calculated using kymograph analysis.
Single-molecule motility assay
MTs extracted from porcine brains (5% Alexa Fluor 647 labeled and 20% biotin-labeled) were polymerized as previously described (Gell et al., 2010). Briefly, taxol-stabilized MTs were polymerized using a mixture of 40 μM tubulin, 1 mM GTP (10106399001; Roche), 4 mM MgCl2, and 4% DMSO (276855; Sigma-Aldrich). The mixture was incubated for 30 min at 37°C. Polymerized MTs were collected using an Air-Driven Ultracentrifuge (340401; Beckman) and resuspended in BRB80 buffer with 20 μM taxol (9807; Cell Signaling Technology) and stored at 37°C. For single-molecule motility assays, MTs were immobilized on the surface of a cover glass using biotin-neutravidin protein links (31000; Thermo Fisher Scientific). GFP-tagged full-length Kif6 or Kif6-CC2 were added into the flow chamber in the imaging buffer (BRB80 supplemented with 1 mM ATP, 20 μM taxol, 80 mM D-glucose, 0.4 mg/ml glucose oxidase, 0.2 mg/ml catalase, 0.8 mg/ml casein, 1% β-mercaptoethanol, 0.001% Tween 20), with rKin430 serving as a control. All samples were kept at 35°C using a temperature controller (Tokai Hit). Images were recorded using a TIRF Microscope (Olympus) equipped with a 100×/1.49-NA Oil TIRF objective (Olympus) and an Andor 897 Ultra EMCCD camera (Andor). For rKin430, time-lapse TIRF images were acquired at 0.1-s intervals with a 50-ms exposure for 2 min. For full-length Kif6 and Kif6-CC2, images were captured at 1-s intervals with a 100-ms exposure for 2 min. The velocities of fluorescent motor puncta were calculated using kymograph analysis in Fiji software.
Transmission electron microscopy
The ependymal tissues were fixed in 2.5% glutaraldehyde in phosphate-buffered saline (PBS) at 4°C overnight, followed by a wash with PBS and treatment with 1% OsO4 for 1.5 h at r.t. The tissues were dehydrated with a graded series of ethanol and further dehydrated with acetone. Subsequently, the samples were embedded in Epon 812 resin at 60°C for 48 h. Thin sections of 70 nm thickness were obtained using an ultramicrotome (EM PACT2; Leica) and stained with 2% uranyl acetate for 15 min, followed by 1% lead citrate for 5 min. The images were captured using a FEI Tecnai G2 Spirit Twin transmission electron microscope and analyzed using Fiji software.
Coronal brain section
Coronal brain slices with a thickness of 250 μm were achieved using a vibratome sectioning technique, as previously described (Chen et al., 2020). Mice were deeply anesthetized with 360 mg/kg body weight of avertin administered via intraperitoneal injections. Transcardial perfusion was performed using PBS followed by 4% PFA in PBS, utilizing an injection pump (WZS-50F6, 250 ml/h; Smiths Medical). Whole brains were dissected from the mice and sectioned using a vibratome to obtain coronal slices with a thickness of 250 μm. These slices were placed into 35-mm glass-bottom dishes (D35-20-1.5-N; Cellvis) and were imaged with an Olympus SZX16 Stereo Microscope.
Reproductive performance test
The mating test was performed as previously described (Miyata et al., 2020). Single 2–3 month-old Kif6−/− or Kif9−/− male mice with normal appearance were individually caged with two age-appropriate heterozygous female mice for 4 months. Two Kif6−/− or Kif9−/− female mice were separately housed with a single heterozygous male mouse. All groups were routinely monitored every few days and the number of pups was counted. Three independent experiments were carried out.
Phylogenomic analysis and multiple alignment
The phylogenetic tree and taxonomic groups were constructed based on the literature and Taxonomy Browser of the National Center for Biotechnology Information (NCBI) (Cetkovic et al., 2018; Mukherjee and Brocchieri, 2013). The protein database of NCBI was searched to identify orthologous proteins to Kif6 and Kif9 across a wide range of species (such as mammals, birds, amphibians, fishes, jawless vertebrates, cephalochordates, urochordates, echinoderms, nematodes, mollusks, flatworms, cnidarians, placozoans, and protozoans). The search used the full-length murine protein sequences of Kif6 and Kif9 as queries.
The selected sequences were aligned using COBALT searches with default settings. Genebank accession numbers of Kif6 and Kif9 orthologues were provided in Fig. S1 A. In the alignment, columns that were gap-free were highlighted, with highly conserved amino acid positions marked in red and less conserved positions shown in blue.
Analysis of BB rotational polarity
BB rotational polarity was analyzed as previously described (Bustamante-Marin et al., 2019; Guirao et al., 2010; Mirzadeh et al., 2010; Ryu et al., 2021). In cross-sections of transmission electron microscopy, BB rotational polarity was established by a unit vector extending from the center of a BB to the apex of its corresponding BF. The mean vector length for each field in the full-size electron micrographs (2,048 pixels × 2,048 pixels), containing a minimum of 6 BBs with clearly discernible BFs, was calculated using Excel software (Microsoft) to quantify the rotational polarity. In 3D-SIM images, the BB rotational polarity was determined by drawing a unit vector from the geographic center of the Cep164 ring (BB distal end marker) to the center of either Centriolin or γ−tubulin signals (BF marker). The mean vector length for BBs within a cell was calculated using Excel software (Microsoft) to define the rotational polarity.
Quantification and statistical analysis
BB numbers were measured from 3D-SIM images according to the signal of Cep164. The BB center and cell border center were determined using the default setting of the “ROI Manager” function in Fiji.
All experiments, unless otherwise stated, were independently repeated at least twice for microscopic or biochemical results. Statistical results are presented as mean ± SD. Unpaired two-tailed student’s t test was performed using GraphPad Prism 9.0 software, and differences were considered significant at P < 0.05. Data analyses were blinded, with the researchers performing the quantification being unaware of the genotype.
Online supplemental material
The supplementary material consists of five figures, ten videos, and two tables. Fig. S1 presents the conservations and ciliary localizations of Kif6 and Kif9. Fig. S2 shows the localizations and behaviors of exogenous Kif6 or Kif9 in ependymal cilia. Fig. S3 shows that Kif6 but not Kif9 exhibits spindle MT associations in HEK293T cells. Fig. S4 shows a decreased sperm count in Kif9-deficient and Kif6-deficient males. Fig. S5 shows that Kif6 deficiency disrupts rotational polarity but not the translational polarity of BBs in ependyma. Video 1 shows the motilities of ciliary Ift81-GFP. Video 2 shows the motilities of ciliary Kif6-GFP puncta. Video 3 shows the motilities of ciliary Kif9-GFP. Video 4 shows the motilities of ciliary Kif6-GFP and Ift27-2Halo. Video 5 shows single-molecule motilities of full-length Kif6, Kif6-CC2, and rKin430 on MTs in vitro. Video 6 shows that full-length Kif6 and Kif6-CC2, but not Kif6-mut, drive MTs gliding. Video 7 shows morphologies and motilities of Kif9+/+ and Kif9−/− sperms. Video 8 shows morphologies and motilities of Kif6+/+ and Kif6−/− sperms. Video 9 shows ciliary beat patterns in Kif6+/+ and Kif6−/− ependymal tissues. Video 10 shows liquid flows driven by ciliary beat in Kif6+/+ and Kif6−/− ependyma. Table S1 lists the sequences of primers and gRNAs used in this study. Table S2 lists the antibodies used in this study.
Data availability
Data are available in the article itself and its supplementary materials.
Acknowledgments
We thank Prof. Lan Bao, Dr. Lei Diao (Shanghai Institute of Biochemistry and Cell Biology, CAS, Shanghai, China), and Dr. Dong Li (Tsinghua University, Beijing, China) for the reagents and technical assistance, Prof. Wei Feng (Institute of Biophysics, CAS, Beijing, China) for valuable discussions, and the staff members of the Integrated Laser Microscopy System at the National Facility for Protein Science in Shanghai (NFPS), Shanghai Advanced Research Institute, Chinese Academy of Sciences, China, for sample preparation, data collection and analysis, and core facilities of the Center for Excellence in Molecular Cell Science (CEMCS) for instrumental and technical supports.
This work was supported by the National Natural Science Foundation of China (31991192 to X. Zhu, 32230027 to X. Zhu, and 32270725 to X. Yan).
Author contributions: C. Fang: Formal analysis, Investigation, Validation, Visualization, Writing—original draft, Writing—review & editing, X. Pan: Formal analysis, Investigation, Validation, Visualization, Writing—original draft, Writing—review & editing, D. Li: Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing—original draft, W. Chen: Investigation, Y. Huang: Investigation, Y. Chen: Investigation, Methodology, L. Li: Investigation, Q. Gao: Investigation, X. Liang: Investigation, Methodology, D. Li: Investigation, Methodology, Validation, Visualization, Writing—original draft, X. Zhu: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing—review & editing, X. Yan: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing.
References
Author notes
C. Fang, X. Pan, and D. Li are co-first authors.
Disclosures: The authors declare no competing interests exist.