In response to chromatin bridges, the abscission checkpoint delays completion of cytokinesis to prevent chromosome breakage or tetraploidization. Here, we show that spontaneous or replication stress-induced chromatin bridges exhibit “knots” of catenated and overtwisted DNA next to the midbody. Topoisomerase IIα (Top2α) forms abortive Top2–DNA cleavage complexes (Top2ccs) on DNA knots; furthermore, impaired Top2α–DNA cleavage activity correlates with chromatin bridge breakage in cytokinesis. Proteasomal degradation of Top2ccs is required for Rad17 localization to Top2-generated double-strand DNA ends on DNA knots; in turn, Rad17 promotes local recruitment of the MRN complex and downstream ATM-Chk2-INCENP signaling to delay abscission and prevent chromatin breakage. In contrast, dicentric chromosomes that do not exhibit knotted DNA fail to activate the abscission checkpoint in human cells. These findings are the first to describe a mechanism by which the abscission checkpoint detects chromatin bridges, through generation of abortive Top2ccs on DNA knots, to preserve genome integrity.
Introduction
Chromatin bridges are strings of chromatin connecting the anaphase poles or daughter nuclei, and have been linked to tumourigenesis (Ganem and Pellman, 2012; Hoffelder et al., 2004). DNA bridges can arise from the segregation of interlinked chromosomes, caused by incomplete DNA replication or imperfect resolution of double-strand DNA catenates, or from dicentric chromosomes generated by end-to-end chromosome fusions (Finardi et al., 2020; Liu et al., 2014; Maciejowski et al., 2015). DNA bridges increase in the presence of DNA replication, decatenation, or condensation inhibitors (Amaral et al., 2016; Chan et al., 2009; Wilhelm et al., 2014); however, they are also observed in the absence of exogenous stress, especially at centromeres in mammalian cells (Baumann et al., 2007; Chan et al., 2007).
In response to chromatin bridges in cytokinesis, eukaryotic cells delay abscission, the severing of the narrow cytoplasmic canal that connects the two daughter cells, to prevent chromatin breakage or tetraploidization by regression of the cleavage furrow (Amaral et al., 2016; Carlton et al., 2012; Steigemann et al., 2009; Thoresen et al., 2014), which is associated with genomic instability and cancer predisposition (Lens and Medema, 2019; Maciejowski et al., 2020; Petsalaki and Zachos, 2021a; Umbreit et al., 2020). In mammalian cells, this abscission delay is called the “abscission checkpoint” and is dependent on optimal localization and catalytic activity of Aurora B kinase at the midbody (Carlton et al., 2012; Petsalaki and Zachos, 2016; Petsalaki and Zachos, 2019; Petsalaki and Zachos, 2021a; Steigemann et al., 2009).
Aurora B is the catalytic subunit of the chromosomal passenger complex (CPC), also comprising the scaffolding protein INCENP and the non-enzymatic subunits Survivin and Borealin (Carmena et al., 2012). At the midbody, Aurora B phosphorylates the endosomal sorting complex required for transport-III (ESCRT-III) subunit Chmp4c (Capalbo et al., 2012; Carlton et al., 2012; Petsalaki and Zachos, 2016), which cooperates with several proteins to inhibit the activity of the ATPase Vps4 on ESCRT-III filaments at the abscission site to delay the final cut (Caballe et al., 2015; Morita et al., 2007; Thoresen et al., 2014). Recently, a signaling pathway that targets Aurora B to the midbody center (Flemming body; FB) to implement the abscission checkpoint was identified in human cells: in cytokinesis with chromatin bridges, the MRN (Mre11–Rad50–Nbs1) double-strand DNA break sensor complex activates the DNA damage response kinase ATM at the midbody (Petsalaki and Zachos, 2021b). Active ATM phosphorylates Chk2 kinase at threonine 68 (T68); in turn, phosphorylated (active) Chk2 phosphorylates INCENP-serine 91 (S91) to promote INCENP-Aurora B localization to the midbody and to delay abscission and prevent chromatin bridge breakage (Petsalaki and Zachos, 2021b). However, how chromatin bridges are detected by the abscission checkpoint and whether their molecular origin is important for checkpoint activation in human cells remain unresolved questions.
The DNA topoisomerase II (Top2) enzyme can relax DNA supercoils or untangle catenated DNA molecules during several processes, such as chromosome condensation and segregation, DNA replication, or transcription, by catalyzing a “strand passage” mechanism in which one double-stranded DNA molecule is passed through a double-stranded break in another DNA molecule (Berger et al., 1996; Deweese and Osheroff, 2009; Nitiss, 2009; Pommier et al., 2022; Wigley et al., 1991). For this purpose, Top2 forms an intermediate enzyme-linked DNA gate termed the Top2 cleavage complex (Top2cc) in which each monomer of the dimeric Top2 molecule is covalently bound to one end of the double-strand break through a 5′-phosphotyrosyl bond (Deweese and Osheroff, 2009; Liu et al., 1983; Nitiss, 2009; Sander and Hsieh, 1983; Zechiedrich et al., 1989). After strand passage, the break is religated and Top2 dissociates from the DNA. Catalytic intermediates of Top2 are normally transient because Top2ccs are self-reversible; however, pre-existing DNA alterations or anticancer drugs can generate abortive (irreversible) Top2ccs that can lead to topological defects (Deweese and Osheroff, 2009; Pommier et al., 2022). Top2ccs can be removed by the 5′-phosphodiesterase TDP2 (tyrosyl-DNA phosphodiesterase 2) after proteasomal degradation of the bulk of Top2 protein or, in the absence of Top2 degradation, by TDP2 in cooperation with the ZNF451 SUMO ligase (Gao et al., 2014; Cortes Ledesma et al., 2009; Schellenberg et al., 2017; Zhang et al., 2006). In contrast to yeast which has only one form of DNA Top2, mammals have two isoforms (α and β) that share similar N-terminal ATPase and core domains but differ in their C-terminal domains (Linka et al., 2007). Top2α strongly localizes to mitotic chromosomes and is absolutely required for chromosome condensation and segregation, whereas Top2β is required for transcription in differentiated cells (Grue et al., 1998; Linka et al., 2007; Pommier et al., 2016).
In response to damaged DNA, the replication factor C (RFC)-like DNA damage sensor protein Rad17 in association with the RFC2–5 subunits recruit Rad9 protein complexes onto chromatin to trigger checkpoint signaling cascades (Zou et al., 2002). Rad17 also interacts with Nbs1 to promote early recruitment of the MRN complex to double-strand DNA break sites to induce an efficient MRN–ATM signaling (Wang et al., 2014); furthermore, the Rad17-mediated localization of the MRN to double-strand DNA breaks is independent of the MDC1 adaptor protein that links Nbs1 with phosphorylated histone H2AX (γ-H2AX; Melander et al., 2008; Spycher et al., 2008; Stucki et al., 2005; Wang et al., 2014). However, a role for Rad17 or Top2 in the abscission checkpoint has not been previously reported.
In the present study, we show that spontaneous or replication stress-induced chromosome bridges derived from the segregation of catenated chromosomes exhibit DNA “knots” containing tangled and positively supercoiled DNA next to the midbody by high-resolution confocal microscopy in human cells. Top2α and Rad17 localize to the DNA knots; furthermore, Top2α forms covalent Top2–DNA complexes exhibiting Top2-associated double-strand DNA breaks (Top2ccs) on DNA knots. Inhibition of Top2α or expression of a mutant Top2α protein that binds to DNA but does not exhibit DNA cleavage activity impairs localization of Rad17, MRN, ATM, Chk2, and CPC proteins to the bridge DNA next to the midbody or the midbody ring and promotes chromatin bridge breakage in cytokinesis. Impaired ubiquitination or proteasomal degradation of Top2–DNA complexes also correlates with diminished localization of Rad17 to DNA knots. Furthermore, depletion of Rad17 impairs the recruitment of Mre11 and downstream abscission checkpoint proteins to DNA knots or to the midbody ring and promotes DNA bridge breakage. In contrast, expression of a Rad17 protein fragment that interacts with Nbs1 and is targeted to DNA knots rescues Mre11 localization and prevents chromatin breakage in Top2-depleted cells in cytokinesis. On the other hand, dicentric chromosomes generated by telomere end-fusion do not exhibit DNA knots, fail to recruit Top2α, Rad17, and downstream abscission checkpoint proteins to the bridge DNA next to the midbody or to the midbody ring, and exhibit increased breakage in cytokinesis compared with replication stress-induced chromatin bridges in the same cell line. Remarkably, generation of DNA knots on dicentric chromosome bridges by DNA replication stress rescues the localization of Top2α and Aurora B on chromatin bridges and prevents DNA bridge breakage. On the basis of these findings, we propose that, in cytokinesis with chromatin bridges exhibiting catenated DNA, but not in cytokinesis with dicentric chromosomes, the generation and proteolytic processing of Top2ccs on DNA knots next to the midbody promotes Rad17 localization to Top2α-generated double-strand DNA ends. In turn, Rad17 promotes the recruitment of the MRN complex on DNA knots and downstream abscission checkpoint signaling to delay abscission and prevent chromatin bridge breakage.
Results
Spontaneous or HU-induced chromatin bridges arise from catenated DNA in human cells
Three types of chromatin bridges were analyzed in this study: spontaneous (i.e., bridges that occur in the absence of exogenous stress), replication stress-induced bridges (after recovery of cells from treatment with the DNA replication inhibitor hydroxyurea, HU; Fig. 1, A and B), or dicentric chromosome bridges caused by dicentric fusions of sister chromatids or chromatids from different chromosomes, after induction of dominant-negative TRF2 by tetracycline (TC) in human retinal pigment epithelial-1 (RPE-1) cells (Fig. 9, A and B; and Fig. S5 J; Umbreit et al., 2020). Analysis of spontaneous or HU-induced chromatin bridges by fluorescence in situ hybridization (FISH) in human colon carcinoma BE cells using centromeric (cen) or telomere (tel) PNA probes showed that only 3–9% spontaneous or HU-induced chromatin bridges were positive for telomeric sequences on the bridge DNA (tel positive), indicating dicentric chromosome bridges derived from telomere fusions are relatively infrequent in BE cells under the above experimental conditions (Fig. 1, D and F; and Fig. S1, A and B; for an example of a tel positive bridge, see Fig. 9 C). Furthermore, ∼91% of spontaneous chromatin bridges exhibited centromeric DNA staining across the length of the bridge (cen positive), suggesting chromatin bridging occurs between sister centromeres (Fig. 1, B–D). On the other hand, only 6% HU-induced chromatin bridges exhibited cen-probe staining across the length of the bridge, although centromeric DNA foci were detectable at the bases of the chromatin bridge when the bridge DNA was detached from the main chromatin bulk, indicating that the majority of HU-induced DNA bridges arise from interlinked non-centromeric DNA (Fig. 1, B, E, and F; and Fig. S1 C). Furthermore, all spontaneous (n = 24) and HU-induced (n = 26) chromatin bridges tested lacked FANCD2 foci, which associate with late replication intermediates, at the base of the DNA bridges (Chan et al., 2009), suggesting these bridges are not caused by incomplete DNA replication but likely represent catenated DNA (Fig. S1, D and E). As a positive control, FANCD2 foci were detectable in interphase nuclei in cells treated with HU (Fig. S1, F; Hussain et al., 2004). These results suggest that the majority of spontaneous or HU-induced chromatin bridges are derived from catenated DNA, but not dicentrics, in human cells.
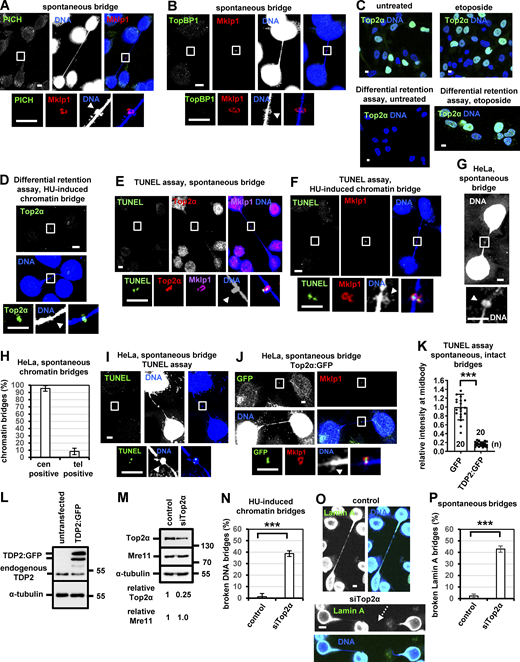
Localization of Top2α to DNA knots on spontaneous chromatin bridges. (A) Experimental procedure to study replication-stress-induced chromatin bridges in BE cells. (B) Schematic of potential mechanisms of spontaneous or replication-stress-induced chromatin bridges. Interlinked cen DNA is shown in red. (C and E) FISH analysis of chromatin bridges in BE cells using a cen probe. DNA bridges (boxed areas) are shown at higher magnification. Small arrows indicate a cen-positive chromatin bridge. (D and F) Frequency of chromatin bridges that are positive for cen or tel probes by FISH. Mean ± SD from three independent experiments (n > 60). (G) Chromatin bridges exhibiting DNA knots next to the midbody. Midbody rings are stained with Cep55 or Mklp1. (H) Localization of GapR:GFP and Top2α to DNA knots on chromatin bridges. (I) Localization of Top2α in cells extracted with the differential retention assay protocol. (J–L) Localization of double-strand DNA ends and mean intensity of TUNEL staining at the bridge DNA next to the midbody in untreated (control) cells or cells treated with Top2α siRNA. (M and N) Localization and mean intensity of Top2α in cells expressing GFP or TDP2:GFP. Mean ± SD, from n cells. Values in control or GFP were set to 1. (O) Frequency of broken chromatin bridges in cytokinesis. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (Student’s t test). Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Localization of Top2α to DNA knots on spontaneous chromatin bridges. (A) Experimental procedure to study replication-stress-induced chromatin bridges in BE cells. (B) Schematic of potential mechanisms of spontaneous or replication-stress-induced chromatin bridges. Interlinked cen DNA is shown in red. (C and E) FISH analysis of chromatin bridges in BE cells using a cen probe. DNA bridges (boxed areas) are shown at higher magnification. Small arrows indicate a cen-positive chromatin bridge. (D and F) Frequency of chromatin bridges that are positive for cen or tel probes by FISH. Mean ± SD from three independent experiments (n > 60). (G) Chromatin bridges exhibiting DNA knots next to the midbody. Midbody rings are stained with Cep55 or Mklp1. (H) Localization of GapR:GFP and Top2α to DNA knots on chromatin bridges. (I) Localization of Top2α in cells extracted with the differential retention assay protocol. (J–L) Localization of double-strand DNA ends and mean intensity of TUNEL staining at the bridge DNA next to the midbody in untreated (control) cells or cells treated with Top2α siRNA. (M and N) Localization and mean intensity of Top2α in cells expressing GFP or TDP2:GFP. Mean ± SD, from n cells. Values in control or GFP were set to 1. (O) Frequency of broken chromatin bridges in cytokinesis. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (Student’s t test). Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Top2α localizes to DNA knots on HU-induced chromatin bridges. (A–C) FISH analysis of spontaneous or HU-induced chromatin bridges in BE cells using a tel or cen probe. DNA bridges (boxed areas) are shown at higher magnification. (D–F) Localization of FANCD2. In F, cells were treated with 1 mM HU for 2 h. (G) A DNA knot from a spontaneous bridge. Small arrows indicate thin DNA strands at the DNA knot. Scale bar, 1 μm. (H and I) Frequency of chromatin bridges exhibiting DNA knots next to the midbody. Mean ± SD from three independent experiments (n > 150). (J–L) Localization of GapR:GFP, Top2α, and Top2β. (M) Mean intensity of Top2α at the bridge DNA next to the midbody in the absence (control) or presence of Top2α siRNA. Values in control were set to 1. (N) Localization of Top2α by using a different polyclonal antibody against Top2α. (O) Mean intensity of Top2α at DNA knots on spontaneous chromatin bridges exhibiting relatively strong or weak DNA staining at the midbody. In cells with chromatin bridges, the mean fluorescence intensity of DNA at the midbody was set to 1. Midbody DNA-fluorescence intensity >1 was taken as strong whereas midbody DNA-fluorescence intensity <1 was classified as relatively weak. The mean intensity of Top2α at the midbody in cells with strong or weak midbody DNA signal was plotted. Values in strong DNA signal were set to 1. (P) Mean intensity of Top2α at the bridge DNA next to the midbody on chromatin bridges of various lengths. Mean ± SD from n cells. Values in <20 μM were set to 1. ***, P < 0.001 (Student’s t test). Numbers below/next to each bar indicate n. (Q) Localization of KIF4A. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Scale bars, 5 μm.
Top2α localizes to DNA knots on HU-induced chromatin bridges. (A–C) FISH analysis of spontaneous or HU-induced chromatin bridges in BE cells using a tel or cen probe. DNA bridges (boxed areas) are shown at higher magnification. (D–F) Localization of FANCD2. In F, cells were treated with 1 mM HU for 2 h. (G) A DNA knot from a spontaneous bridge. Small arrows indicate thin DNA strands at the DNA knot. Scale bar, 1 μm. (H and I) Frequency of chromatin bridges exhibiting DNA knots next to the midbody. Mean ± SD from three independent experiments (n > 150). (J–L) Localization of GapR:GFP, Top2α, and Top2β. (M) Mean intensity of Top2α at the bridge DNA next to the midbody in the absence (control) or presence of Top2α siRNA. Values in control were set to 1. (N) Localization of Top2α by using a different polyclonal antibody against Top2α. (O) Mean intensity of Top2α at DNA knots on spontaneous chromatin bridges exhibiting relatively strong or weak DNA staining at the midbody. In cells with chromatin bridges, the mean fluorescence intensity of DNA at the midbody was set to 1. Midbody DNA-fluorescence intensity >1 was taken as strong whereas midbody DNA-fluorescence intensity <1 was classified as relatively weak. The mean intensity of Top2α at the midbody in cells with strong or weak midbody DNA signal was plotted. Values in strong DNA signal were set to 1. (P) Mean intensity of Top2α at the bridge DNA next to the midbody on chromatin bridges of various lengths. Mean ± SD from n cells. Values in <20 μM were set to 1. ***, P < 0.001 (Student’s t test). Numbers below/next to each bar indicate n. (Q) Localization of KIF4A. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Scale bars, 5 μm.
Top2α forms abortive cleavage complexes on DNA knots next to the midbody on spontaneous or HU-induced chromatin bridges
High-resolution confocal microscopy analysis of chromatin bridges in BE cells in cytokinesis showed that ∼82% of spontaneous or HU-induced chromatin bridges exhibited a DNA bulge containing tangled DNA strands next to the midbody ring. Although a complete understanding of the DNA topology requires further investigation, we call these structures “DNA knots” based on the apparent DNA entanglement (Fig. 1, B, and G; and Fig. S1, G and H). The frequency of the DNA knots did not depend on the length of chromatin bridges, suggesting that longer bridges do not tend to resolve the knot (Fig. S1 I). Furthermore, in cells expressing GapR protein fused to GFP (GapR:GFP), which binds to overtwisted DNA in bacterial and eukaryotic chromatin, 18 of 20 (90%) spontaneous and 22 of 23 (96%) HU-induced chromatin bridges exhibited GapR:GFP staining on DNA knots, suggesting that DNA knots contain positively supercoiled DNA (Fig. 1 H and Fig. S1 J; Guo et al., 2018, 2021). Positive DNA supercoils are substrates for Top2 (Baxter et al., 2011; McClendon et al., 2005). We found that Top2α, but not the Top2β isoform (0 of 30), localized to DNA knots in 51 of 56 (91%) spontaneous and in 45 of 47 (96%) HU-induced chromatin bridges; furthermore, Top2α colocalized with GapR:GFP at DNA knots in all spontaneous (n = 18) or HU-induced (n = 22) chromatin bridges tested (Fig. 1 H and Fig. S1, J–L). Depletion of Top2α by siRNA diminished Top2α-staining at the bridge DNA next to the midbody by ∼90% by immunofluorescence, indicating this signal was specific (Fig. S1 M). Furthermore, Top2α localized to DNA knots in all chromatin bridges (n = 20) by using a different anti-Top2α polyclonal antibody (Fig. S1 N). Top2α-associated fluorescence at DNA knots was independent of the intensity of the DNA signal at the midbody (relatively strong versus weak DNA signal) or the chromatin bridge length, suggesting that Top2α localization to DNA knots does not associate with the amount of DNA at the midbody or the cell-to-cell distance (Fig. S1, O and P). KIF4A, a chromokinesin that associates with mitotic chromosomes and with the midbody (Mazumdar et al., 2004), also localized to DNA knots next to the midbody in 18 of 20 (90%) chromatin bridges tested; however, understanding the significance of this localization requires further investigation (Fig. S1 Q). In contrast, PICH helicase and the Topoisomerase II binding protein TopBP1, which bind to non-chromatinized (ultrafine) DNA bridges in anaphase, were not detected at DNA knots on chromatin bridges in late cytokinesis (0 of 20 bridges tested; Fig. S2, A and B; Baumann et al., 2007; Germann et al., 2014).
Top2α forms Top2–DNA adducts on DNA knots on HU-induced chromatin bridges. (A and B) Localization of PICH and TopBP1 in BE cells. (C) Localization of Top2α in cells extracted with the differential retention assay protocol or the standard fixation protocol for immunofluorescence, in the absence of treatment (untreated) or after treatment of cells with 10 μM etoposide for 2 h. (D) Localization of Top2α on HU-induced chromatin bridges in cells extracted with the differential retention assay protocol. (E and F) TUNEL staining on spontaneous or HU-induced chromatin bridges. (G) A spontaneous bridge with a DNA knot in HeLa cells. (H) Frequency of chromatin bridges that are positive for cen or tel probes by FISH in HeLa cells. Mean ± SD from three independent experiments (n > 60). (I and J) Localization of double-strand DNA ends (TUNEL) and Top2α:GFP at the bridge DNA next to the midbody in HeLa cells. Midbodies (boxed areas) were reexported and are shown at higher magnification. (K) Mean intensity of TUNEL staining at the bridge DNA next to the midbody in BE cells expressing GFP or TDP2:GFP. Mean ± SD from n cells. Values in GFP were set to 1. Numbers below/next to each bar indicate n. (L) Western blot analysis of total GFP, TDP2, and tubulin in BE cells expressing TDP2:GFP. (M) Western blot analysis of total Top2α, Mre11, and tubulin in untreated (control) cells or cells transfected with Top2α siRNA. (N) Frequency of broken DNA bridges in HU-induced cells. (O) BE cells exhibiting Lamin A bridges in the absence (control) or presence of Top2α siRNA. (P) Frequency of broken Lamin A bridges. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Scale bars, 5 μm. Source data are available for this figure: SourceData FS2.
Top2α forms Top2–DNA adducts on DNA knots on HU-induced chromatin bridges. (A and B) Localization of PICH and TopBP1 in BE cells. (C) Localization of Top2α in cells extracted with the differential retention assay protocol or the standard fixation protocol for immunofluorescence, in the absence of treatment (untreated) or after treatment of cells with 10 μM etoposide for 2 h. (D) Localization of Top2α on HU-induced chromatin bridges in cells extracted with the differential retention assay protocol. (E and F) TUNEL staining on spontaneous or HU-induced chromatin bridges. (G) A spontaneous bridge with a DNA knot in HeLa cells. (H) Frequency of chromatin bridges that are positive for cen or tel probes by FISH in HeLa cells. Mean ± SD from three independent experiments (n > 60). (I and J) Localization of double-strand DNA ends (TUNEL) and Top2α:GFP at the bridge DNA next to the midbody in HeLa cells. Midbodies (boxed areas) were reexported and are shown at higher magnification. (K) Mean intensity of TUNEL staining at the bridge DNA next to the midbody in BE cells expressing GFP or TDP2:GFP. Mean ± SD from n cells. Values in GFP were set to 1. Numbers below/next to each bar indicate n. (L) Western blot analysis of total GFP, TDP2, and tubulin in BE cells expressing TDP2:GFP. (M) Western blot analysis of total Top2α, Mre11, and tubulin in untreated (control) cells or cells transfected with Top2α siRNA. (N) Frequency of broken DNA bridges in HU-induced cells. (O) BE cells exhibiting Lamin A bridges in the absence (control) or presence of Top2α siRNA. (P) Frequency of broken Lamin A bridges. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Scale bars, 5 μm. Source data are available for this figure: SourceData FS2.
In 16 of 16 spontaneous and in 17 of 18 (94%) HU-induced chromatin bridges examined, Top2α formed Top2–DNA adducts on DNA knots by Top2α-differential retention assay in which free topoisomerase and other cellular proteins are removed using salt-detergent extraction of unfixed cells and, after fixation, the specific trapping of covalent Top2α-DNA complexes onto DNA is detected and quantified by confocal microscopy (Fig. 1 I; and Fig. S2, C and D; Agostinho et al., 2004). Also, in control bridges that were intact by DAPI staining, DNA knots next to the midbody exhibited double-strand DNA ends with 3′-OH termini that could be labeled by TUNEL (Fig. 1 J). These DNA ends colocalized with Top2α on DNA knots and were Top2-dependent, suggesting they represent Top2-cleaved DNA intermediates (Fig. 1, J–L; and Fig. S2, E and F). Also in HeLa cells, 28 of 30 (93%) spontaneous chromatin bridges exhibited DNA knots (Fig. S2 G), and 95% of chromatin bridges were positive for the cen probe whereas only 8% were positive for the tel probe by FISH, showing that the majority of spontaneous chromatin bridges in HeLa cells are derived from interlinked centromeric DNA but not from dicentric chromosomes (Fig. S2 H). Furthermore, 20 of 20 chromatin bridges exhibited localization of Top2α:GFP to DNA knots and 18 of 20 (90%) chromatin bridges that were intact by DAPI staining exhibited double-strand DNA ends on DNA knots by TUNEL, in agreement with our findings in BE cells (Fig. S2, I and J). Overexpression of TDP2, an enzyme that removes abortive Top2ccs, fused to GFP (TDP2:GFP) in BE cells diminished the localization of Top2α to the bridge DNA next to the midbody, reduced TUNEL staining on DNA knots on intact chromatin bridges, and increased the frequency of broken chromatin bridges in cytokinesis compared with controls expressing GFP-only (Fig. 1, M–O; and Fig. S2, K and L). These results suggest that Top2α forms abortive Top2ccs on DNA knots next to the midbody on spontaneous or HU-induced chromatin bridges and that these Top2ccs are required for stable chromatin bridges in cytokinesis.
Top2α-inhibition promotes the breakage of spontaneous or HU-induced chromatin bridges
Top2α-depleted BE cells exhibited increased frequency of broken spontaneous or HU-induced chromatin bridges compared with controls as judged by DNA staining (Fig. 2, A and B; and Fig. S2, M and N). Also, Lamin A, which interacts with inner nuclear membrane proteins, efficiently visualizes chromatin bridges (Lamin A bridges always correlated with chromatin bridges in high-resolution still images in control cells; n = 60; Fig. S2 O). We found that Top2α-depleted cells exhibited an increased frequency of broken Lamin A bridges compared with controls (Fig. S2, O and P). Furthermore, HeLa cells expressing the inner nuclear envelope marker LAP2b fused to RFP (LAP2b:RFP) that correlates with chromatin bridges (Steigemann et al., 2009) and displaying spontaneous LAP2b:RFP bridges in cytokinesis were monitored for up to 180 min by time-lapse microscopy (Fig. 2 C). We found that 30 of 30 control cells exhibiting intercellular canals with LAP2b:RFP bridges sustained those canals and the LAP2b:RFP bridges for the duration of the experiment. In contrast, 25 of 49 (51%) cells treated with the catalytic Top2 inhibitor ICRF-193, which inhibits Top2 without stabilizing cleavable complexes (Classen et al., 2003; Roca et al., 1994), in cytokinesis exhibited breakage of LAP2b:RFP-positive intercellular canals and LAP2b:RFP bridges after ∼60 min (Fig. 2, C and D; and Videos 1, 2, 3, and 4). Consistently, Top2-depleted HeLa LAP2b:RFP cells exhibited increased frequency of spontaneous broken LAP2b:RFP bridges compared with controls by confocal microscopy analysis of fixed samples (Fig. 2, E and F). Expression of the dominant-negative mutant GFP:Vps4-K173Q, which inhibits abscission (Morita et al., 2007), diminished broken chromatin bridges after Top2α-depletion in BE cells compared with GFP-only controls (Fig. 2 G). Also, Top2 was not required for the formation of actin-rich structures (actin patches) at the bases of the intercellular canal, which stabilize chromatin bridges (Fig. S3, A–C; Bai et al., 2020; Dandoulaki et al., 2018; Steigemann et al., 2009). These results suggest that Top2α is required for the abscission checkpoint in cytokinesis with spontaneous or HU-induced chromatin bridges.
Top2-inhibition correlates with chromatin bridge breakage in cytokinesis. (A) Telophase BE cells with chromatin bridges. (B) Frequency of broken DNA bridges in untreated (control) cells or cells transfected with Top2α siRNA. (C) Fluorescence and phase-contrast live-cell imaging of HeLa cells expressing LAP2b:RFP. Cells were untreated (control) or treated with Top2-inhibitor ICRF-193 immediately before filming. Time is from the detection of the intercellular canals that contain LAP2b:RFP bridges. (D) Times to intercellular canal breakage in cells from C. (E) HeLa LAP2b:RFP cells exhibiting LAP2b:RFP bridges were analyzed by fluorescence microscopy of fixed samples. (F) Frequency of broken LAP2b:RFP bridges from E. (G) Percentage of broken DNA bridges in BE cells transfected with GFP or GFP:Vps4-K173Q in the absence or presence of Top2α siRNA. Mean ± SD from three independent experiments (n > 120). (H) Localization of Mre11 and Top2α on DNA knots. (I–Q) Localization and mean intensity of Mre11, Rad50, or Nbs1 at the bridge DNA next to the midbody in BE cells with chromatin bridges. Mean ± SD from n cells. Values in control were set to 1. ***, P < 0.001 (Student’s t test). Intact DNA bridges or intercellular canals are indicated by solid arrows; broken DNA bridges or canals by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Top2-inhibition correlates with chromatin bridge breakage in cytokinesis. (A) Telophase BE cells with chromatin bridges. (B) Frequency of broken DNA bridges in untreated (control) cells or cells transfected with Top2α siRNA. (C) Fluorescence and phase-contrast live-cell imaging of HeLa cells expressing LAP2b:RFP. Cells were untreated (control) or treated with Top2-inhibitor ICRF-193 immediately before filming. Time is from the detection of the intercellular canals that contain LAP2b:RFP bridges. (D) Times to intercellular canal breakage in cells from C. (E) HeLa LAP2b:RFP cells exhibiting LAP2b:RFP bridges were analyzed by fluorescence microscopy of fixed samples. (F) Frequency of broken LAP2b:RFP bridges from E. (G) Percentage of broken DNA bridges in BE cells transfected with GFP or GFP:Vps4-K173Q in the absence or presence of Top2α siRNA. Mean ± SD from three independent experiments (n > 120). (H) Localization of Mre11 and Top2α on DNA knots. (I–Q) Localization and mean intensity of Mre11, Rad50, or Nbs1 at the bridge DNA next to the midbody in BE cells with chromatin bridges. Mean ± SD from n cells. Values in control were set to 1. ***, P < 0.001 (Student’s t test). Intact DNA bridges or intercellular canals are indicated by solid arrows; broken DNA bridges or canals by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Control cells exhibit stable LAP2b:RFP bridges in cytokinesis. HeLa cells stably expressing LAP2b:RFP (white) were analyzed by time-lapse fluorescence microscopy. Frames were taken every 10 min for 190 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. 2 C.
Control cells exhibit stable LAP2b:RFP bridges in cytokinesis. HeLa cells stably expressing LAP2b:RFP (white) were analyzed by time-lapse fluorescence microscopy. Frames were taken every 10 min for 190 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. 2 C.
Control cells exhibit stable intercellular canals in cytokinesis. HeLa cells stably expressing LAP2b:RFP were analyzed by phase-contrast time-lapse microscopy. Frames were taken every 10 min for 190 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. 2 C.
Control cells exhibit stable intercellular canals in cytokinesis. HeLa cells stably expressing LAP2b:RFP were analyzed by phase-contrast time-lapse microscopy. Frames were taken every 10 min for 190 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. 2 C.
Breakage of LAP2b:RFP bridges after inhibition of Top2 enzyme activity. HeLa cells stably expressing LAP2b:RFP (white) were treated with 10 μM ICRF-193 and analyzed by time-lapse fluorescence microscopy. Frames were taken every 10 min for 120 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. 2 C.
Breakage of LAP2b:RFP bridges after inhibition of Top2 enzyme activity. HeLa cells stably expressing LAP2b:RFP (white) were treated with 10 μM ICRF-193 and analyzed by time-lapse fluorescence microscopy. Frames were taken every 10 min for 120 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. 2 C.
Breakage of intercellular canals after inhibition of Top2 enzyme activity. HeLa cells stably expressing LAP2b:RFP were treated with 10 μM ICRF-193 and analyzed by phase-contrast time-lapse microscopy. Frames were taken every 10 min for 120 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. 2 C.
Breakage of intercellular canals after inhibition of Top2 enzyme activity. HeLa cells stably expressing LAP2b:RFP were treated with 10 μM ICRF-193 and analyzed by phase-contrast time-lapse microscopy. Frames were taken every 10 min for 120 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. 2 C.
Depletion of Top2α does not impair actin-patch formation in cytokinesis with chromatin bridges. (A and B) Actin patches (empty arrowheads) in cytokinesis with chromatin bridges in BE cells, in the absence (control) or presence of Top2α siRNA. The bases of the canals are shown at higher magnification. Broken chromatin bridges are indicated by dotted arrows. (C) Relative actin-patch intensity values. (D) Mean intensity of INCENP at the midbody ring in cells with chromatin bridges. Values in control were set to 1. Mean ± SD from n cells. (E–I) Western blot analysis of total Rad50, ATM, Nbs1, Chk2, INCENP, Aurora B, actin, and tubulin. (J) Experimental procedure for Top2-inhibition by ICRF-193 or etoposide in cells enriched in cytokinesis after release from a nocodazole block. (K and L) Mean intensity of Mre11 or ATM at the bridge DNA next to the midbody in cells with chromatin bridges after nocodazole release, in the absence (control) or presence of 10 μM ICRF-193 or 10 μM etoposide. (M) Mean intensity of INCENP to the midbody ring in cells with chromatin bridges after nocodazole release. Mean ± SD from n cells. Values in control were set to 1. (N) Frequency of broken chromatin bridges in cytokinesis after nocodazole release. Mean ± SD from three independent experiments (n > 120). (O–Q) Mean intensity of Mre11 or ATM at the bridge DNA next to the midbody and of Aurora B at the midbody ring in HU-induced BE cells with chromatin bridges. Mean ± SD from n cells. Values in control were set to 1. ***, P < 0.001 (ANOVA and Student’s t test). Numbers below/next to each bar indicate n. Values in control were set to 1. (R–T) Localization of Top2α and Rad17 at the midbody in normally segregating cells. Tubulin values indicate midbody thickness. Midbodies (boxed areas) were reexported and are shown at higher magnification. Scale bars, 5 μm. Source data are available for this figure: SourceData FS3.
Depletion of Top2α does not impair actin-patch formation in cytokinesis with chromatin bridges. (A and B) Actin patches (empty arrowheads) in cytokinesis with chromatin bridges in BE cells, in the absence (control) or presence of Top2α siRNA. The bases of the canals are shown at higher magnification. Broken chromatin bridges are indicated by dotted arrows. (C) Relative actin-patch intensity values. (D) Mean intensity of INCENP at the midbody ring in cells with chromatin bridges. Values in control were set to 1. Mean ± SD from n cells. (E–I) Western blot analysis of total Rad50, ATM, Nbs1, Chk2, INCENP, Aurora B, actin, and tubulin. (J) Experimental procedure for Top2-inhibition by ICRF-193 or etoposide in cells enriched in cytokinesis after release from a nocodazole block. (K and L) Mean intensity of Mre11 or ATM at the bridge DNA next to the midbody in cells with chromatin bridges after nocodazole release, in the absence (control) or presence of 10 μM ICRF-193 or 10 μM etoposide. (M) Mean intensity of INCENP to the midbody ring in cells with chromatin bridges after nocodazole release. Mean ± SD from n cells. Values in control were set to 1. (N) Frequency of broken chromatin bridges in cytokinesis after nocodazole release. Mean ± SD from three independent experiments (n > 120). (O–Q) Mean intensity of Mre11 or ATM at the bridge DNA next to the midbody and of Aurora B at the midbody ring in HU-induced BE cells with chromatin bridges. Mean ± SD from n cells. Values in control were set to 1. ***, P < 0.001 (ANOVA and Student’s t test). Numbers below/next to each bar indicate n. Values in control were set to 1. (R–T) Localization of Top2α and Rad17 at the midbody in normally segregating cells. Tubulin values indicate midbody thickness. Midbodies (boxed areas) were reexported and are shown at higher magnification. Scale bars, 5 μm. Source data are available for this figure: SourceData FS3.
Top2α is required for abscission checkpoint signaling in cytokinesis with chromatin bridges
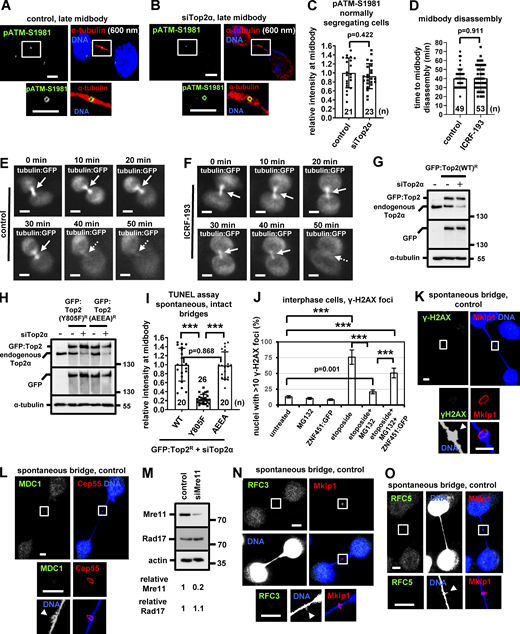
In response to chromatin bridges, the MRN-ATM-Chk2 signaling pathway implements the abscission checkpoint (Petsalaki and Zachos, 2021b). Top2α colocalized with Mre11 on DNA knots in all control cells examined (n = 18; Fig. 2 H). Depletion of Top2α diminished localization of Mre11, Rad50, Nbs1, ATM, or phosphorylated (active) Chk2-threonine 68 (pChk2-T68) to the bridge DNA next to the midbody, reduced localization of phosphorylated INCENP-serine 91 (pINCENP-S91), total INCENP, and Aurora B to the midbody ring compared with control cells in cytokinesis with spontaneous chromatin bridges, and this was not due to reduced levels of total Mre11, Rad50, Nbs1, ATM, Chk2, INCENP, or Aurora B proteins (Fig. 2, I–Q; Fig. 3, A–L; and Fig. S3, D–I). Acute inhibition of Top2 by ICRF-193 in cytokinesis after the release of cells from a nocodazole block also diminished localization of Mre11 or ATM to the bridge DNA next to the midbody, impaired localization of INCENP to the midbody ring, and induced chromatin bridge breakage compared with controls (Fig. S3, J–N). In contrast, treatment of cells with etoposide, which stabilizes Top2 cleavable complexes, after nocodazole release did not impair localization of Mre11 to the bridge DNA next to the midbody and did not induce chromatin bridge breakage compared with controls, suggesting that Top2:etoposide cleavable complexes can delay abscission in cytokinesis with chromatin bridges (Fig. S3, K and N). Depletion of Top2α impaired localization of total Mre11 or ATM to the bridge DNA next to the midbody and diminished Aurora B-localization to the midbody ring in cytokinesis with HU-induced chromatin bridges (Fig. S3, O–Q). Top2α or Rad17 did not localize to the midbody in relatively “early” midbodies exhibiting midbody thickness of 800–1,400 nm (n = 30) or in “late” cytokinesis exhibiting midbody thickness of 400–700 nm in normally segregating BE cells, i.e., in the absence of chromatin bridges (n = 30; Fig. S3, R–T; Petsalaki and Zachos, 2016, 2021b). Furthermore, Top2α-depleted cells exhibited similar levels of phosphorylated (active) ATM-serine 1981 (pATM-S1981) at the midbody ring compared with controls (Fig. S4, A–C). In addition, Top2-inhibition did not accelerate midbody disassembly by live-cell imaging in HeLa cells expressing tubulin:GFP (Fig. S4, D–F; and Videos 5 and 6; Petsalaki and Zachos, 2021b). These results show that Top2α is required for abscission checkpoint signaling in cytokinesis with spontaneous or HU-induced chromatin bridges but is dispensable for abscission timing in normally segregating cells.
Top2α inhibition correlates with impaired localization of phosphorylated INCENP-S91 and Aurora B to the midbody ring in cytokinesis with spontaneous chromatin bridges. (A–F) Localization and mean intensity of ATM or phosphorylated Chk2-T68 (pChk2) at the bridge DNA next to the midbody in BE cells with chromatin bridges, in the absence (control) or presence of Top2α siRNA. (G–L) Localization and mean intensity of phosphorylated INCENP-S91 (pINCENP) or Aurora B (AurB) at the midbody ring. Mean ± SD from n cells. Values in control were set to 1. ***, P < 0.001 (Student’s t test). (M–P) Localization and mean intensity of Mre11 at the bridge DNA next to the midbody in BE cells expressing siRNA-resistant (R) WT, Y805F, or AEEA GFP:Top2R proteins, or GFP-only, in the absence or presence of Top2α siRNA. Mean ± SD from n cells. Values in GFP were set to 1. ***, P < 0.001 (ANOVA and Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Top2α inhibition correlates with impaired localization of phosphorylated INCENP-S91 and Aurora B to the midbody ring in cytokinesis with spontaneous chromatin bridges. (A–F) Localization and mean intensity of ATM or phosphorylated Chk2-T68 (pChk2) at the bridge DNA next to the midbody in BE cells with chromatin bridges, in the absence (control) or presence of Top2α siRNA. (G–L) Localization and mean intensity of phosphorylated INCENP-S91 (pINCENP) or Aurora B (AurB) at the midbody ring. Mean ± SD from n cells. Values in control were set to 1. ***, P < 0.001 (Student’s t test). (M–P) Localization and mean intensity of Mre11 at the bridge DNA next to the midbody in BE cells expressing siRNA-resistant (R) WT, Y805F, or AEEA GFP:Top2R proteins, or GFP-only, in the absence or presence of Top2α siRNA. Mean ± SD from n cells. Values in GFP were set to 1. ***, P < 0.001 (ANOVA and Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Top2 inhibition does not accelerate midbody disassembly in normally segregating cells. (A–C) Localization and mean intensity of phosphorylated ATM-S1981 (pATM) at the midbody in normally segregating BE cells, in the absence (control) or presence of Top2α siRNA. Tubulin values indicate midbody thickness. Mean ± SD from n cells. Values in control were set to 1. (D–F) Time-lapse microscopy analysis of HeLa cells expressing tubulin:GFP. Cells were untreated (control) or treated with 10 μM ICRF-193 immediately before filming. Midbodies are shown by solid arrows. Time is from midbody formation to midbody disassembly (dotted arrows). (G and H) Western blot analysis of total Top2α, GFP, and tubulin in BE cells expressing siRNA-resistant (R) WT, Y805F, or AEEA GFP:Top2R, in the absence or presence of Top2α siRNA. (I) Mean intensity of TUNEL staining at the bridge DNA next to the midbody in cells expressing WT, Y805F, or AEEA GFP:Top2, in the presence of Top2α siRNA. Mean ± SD from n cells. Values in WT were set to 1. Numbers below/next to each bar indicate n. (J) Frequency of interphase cells exhibiting >10 γ-H2AX foci per nucleus after treatment with 10 μM MG132 and/or 10 μM etoposide for 4 h, in the absence or presence of ZNF451:GFP. Mean ± SD from three independent experiments (n > 300). ***, P < 0.001 (ANOVA and Student’s t test). (K and L) Localization of γ-H2AX or MDC1 in cytokinesis with chromatin bridges. (M) Western blot analysis of total Mre11, Rad17, and actin. (N and O) Localization of RFC3 or RFC5 in BE cells with chromatin bridges. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Scale bars, 5 μm. Source data are available for this figure: SourceData FS4.
Top2 inhibition does not accelerate midbody disassembly in normally segregating cells. (A–C) Localization and mean intensity of phosphorylated ATM-S1981 (pATM) at the midbody in normally segregating BE cells, in the absence (control) or presence of Top2α siRNA. Tubulin values indicate midbody thickness. Mean ± SD from n cells. Values in control were set to 1. (D–F) Time-lapse microscopy analysis of HeLa cells expressing tubulin:GFP. Cells were untreated (control) or treated with 10 μM ICRF-193 immediately before filming. Midbodies are shown by solid arrows. Time is from midbody formation to midbody disassembly (dotted arrows). (G and H) Western blot analysis of total Top2α, GFP, and tubulin in BE cells expressing siRNA-resistant (R) WT, Y805F, or AEEA GFP:Top2R, in the absence or presence of Top2α siRNA. (I) Mean intensity of TUNEL staining at the bridge DNA next to the midbody in cells expressing WT, Y805F, or AEEA GFP:Top2, in the presence of Top2α siRNA. Mean ± SD from n cells. Values in WT were set to 1. Numbers below/next to each bar indicate n. (J) Frequency of interphase cells exhibiting >10 γ-H2AX foci per nucleus after treatment with 10 μM MG132 and/or 10 μM etoposide for 4 h, in the absence or presence of ZNF451:GFP. Mean ± SD from three independent experiments (n > 300). ***, P < 0.001 (ANOVA and Student’s t test). (K and L) Localization of γ-H2AX or MDC1 in cytokinesis with chromatin bridges. (M) Western blot analysis of total Mre11, Rad17, and actin. (N and O) Localization of RFC3 or RFC5 in BE cells with chromatin bridges. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Scale bars, 5 μm. Source data are available for this figure: SourceData FS4.
Midbody disassembly in control cells. HeLa cells stably expressing tubulin:GFP (white) were analyzed by time-lapse fluorescence microscopy in cytokinesis. Frames were taken every 5 min for 60 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. S4 E.
Midbody disassembly in control cells. HeLa cells stably expressing tubulin:GFP (white) were analyzed by time-lapse fluorescence microscopy in cytokinesis. Frames were taken every 5 min for 60 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. S4 E.
Midbody disassembly after inhibition of Top2 enzyme activity. HeLa cells stably expressing tubulin:GFP (white) were treated with 10 μM ICRF-193 and analyzed by time-lapse fluorescence microscopy in cytokinesis. Frames were taken every 5 min for 60 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. S4 F.
Midbody disassembly after inhibition of Top2 enzyme activity. HeLa cells stably expressing tubulin:GFP (white) were treated with 10 μM ICRF-193 and analyzed by time-lapse fluorescence microscopy in cytokinesis. Frames were taken every 5 min for 60 min. Time counters show minutes:seconds. Display rate: one frame per second. Related image stills are shown in Fig. S4 F.
The DNA cleavage activity of Top2α is required for the abscission checkpoint
To further investigate the mechanism by which Top2α activates the abscission checkpoint in cytokinesis with chromatin bridges, Top2:GFP expression plasmids encoding the K-loop 342-KKKK-345 to 342-AEEA-345 mutant Top2α that interferes with strand passage and exhibits DNA cleavage, but not decatenation, activity (Schmidt et al., 2012), or coding the active site tyrosine-805 to phenylalanine (Y805F) mutant Top2α protein exhibiting DNA binding, but no DNA cleavage activity (Bromberg et al., 2002), were constructed and made resistant (R) to degradation by Top2α siRNA by introduction of appropriate silent point mutations. After the depletion of the endogenous Top2α, the expression of the wild type (WT) or AEEA, but not Y805F, Top2:GFP rescued localization of Mre11, ATM, or INCENP proteins to the bridge DNA next to the midbody or to the midbody ring and prevented chromatin bridge breakage in cytokinesis compared with control cells expressing GFP-only, showing that the DNA cleavage activity of Top2 is required for the abscission checkpoint (Fig. 3, M–P; Fig. 4, A–J; and Fig. S4, G and H). This correlated with impaired TUNEL staining on DNA knots on intact chromatin bridges (judged by DAPI staining) in cells expressing Y805F Top2:GFP compared with those expressing WT or AEEA Top2:GFP (Fig. S4 I). We propose that the generation of double-strand DNA ends on DNA knots by Top2α activates the abscission checkpoint in cytokinesis with chromatin bridges.
Expression of mutant Y805F, but not AEEA, GFP:Top2 correlates with chromatin bridge breakage in cytokinesis. (A–C) Localization of GFP or ATM at the bridge DNA next to the midbody in BE cells expressing siRNA-resistant (R) WT, Y805F, or AEEA GFP:Top2R proteins, or GFP-only, in the absence or presence of Top2α siRNA. (D–F) Localization of INCENP at the midbody ring. (G–I) Mean intensity of GFP, ATM, or INCENP at the bridge DNA next to the midbody or at the midbody ring. Mean ± SD from n cells. Values in WT (G) or GFP (H and I) were set to 1. (J) Percentage of broken DNA bridges in BE cells transfected as in H and I. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (ANOVA and Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Expression of mutant Y805F, but not AEEA, GFP:Top2 correlates with chromatin bridge breakage in cytokinesis. (A–C) Localization of GFP or ATM at the bridge DNA next to the midbody in BE cells expressing siRNA-resistant (R) WT, Y805F, or AEEA GFP:Top2R proteins, or GFP-only, in the absence or presence of Top2α siRNA. (D–F) Localization of INCENP at the midbody ring. (G–I) Mean intensity of GFP, ATM, or INCENP at the bridge DNA next to the midbody or at the midbody ring. Mean ± SD from n cells. Values in WT (G) or GFP (H and I) were set to 1. (J) Percentage of broken DNA bridges in BE cells transfected as in H and I. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (ANOVA and Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Proteasomal degradation of Top2α–DNA complexes is required for the abscission checkpoint
Top2-linked DNA ends are processed by the proteasome to activate downstream responses (Fan et al., 2008; Mao et al., 2001; Zhang et al., 2006). Control cells with spontaneous chromatin bridges exhibited ubiquitin–DNA adducts on DNA knots by ubiquitin-differential retention assay (Fig. 5 A). Treatment of cells with the ubiquitin-activating enzyme E1 inhibitor MLN7243 or depletion of Top2α by siRNA impaired formation of ubiquitin–DNA adducts on DNA knots on intact chromatin bridges compared with controls, suggesting these adducts represent ubiquitinated Top2α–DNA complexes (Fig. 5, A and C–E). Inhibition of the proteasome by MG132 also diminished ubiquitin–DNA adducts on DNA knots (Fig. 5, A, B, and E), which is consistent with proteasome activity being required for polyubiquitination of target proteins by promoting ubiquitin recycling (Ciechanover and Schwartz, 1998). Treatment of cells with MG132 or MLN7243 impaired localization of Mre11 or ATM to the bridge DNA next to the midbody and increased the frequency of broken chromatin bridges compared with controls, showing that ubiquitination and degradation of proteins by the proteasome is required for optimal Mre11 and ATM localization and stable chromatin bridges in cytokinesis (Fig. 5, F–O; and Fig. 6 A). This correlated with increased Top2α localization to DNA knots in cells treated with MG132 compared with untreated controls, showing that Top2α protein on DNA knots was stabilized by MG132 (Fig. 6, B–D). Simultaneous treatment of cells with MG132 and Top2α siRNA did not further increase chromatin bridge breakage in cytokinesis, which is consistent with Top2α and the proteasome regulating DNA breakage by acting in the same pathway (Fig. 6 A). Expression of ZNF451 fused to GFP (ZNF451:GFP) that promotes resolution of Top2 DNA–protein crosslinks independently of Top2α ubiquitination and proteasomal degradation (Schellenberg et al., 2017) did not impair localization of Mre11 or ATM to the bridge DNA next to the midbody and did not increase chromatin bridge breakage in cytokinesis compared with control cells expressing GFP-only, suggesting that degradation of Top2α by the proteasome is essential for the abscission checkpoint (Fig. 6, E–M). For comparison, in cells treated with etoposide and MG132, expression of ZNF451:GFP partially rescued the formation of phosphorylated H2AX (γ-H2AX) foci (a marker of DNA damage response signaling) compared with etoposide+MG132 only (Swan et al., 2020; Zhang et al., 2006), showing that the ZNF451:GFP plasmid is functional in our hands and that expression of ZNF451:GFP can promote processing of Top2α–DNA complexes under specific conditions (Fig. S4 J). Also, for comparison, overexpression of TDP2:GFP that can process Top2-linked DNA ends in both proteasome-modulated and proteasome-independent pathways (Gao et al., 2014; Cortes Ledesma et al., 2009; Schellenberg et al., 2017) impaired Mre11- and ATM-localization to the bridge DNA next to the midbody and increased chromatin bridge breakage in cytokinesis compared with GFP-only controls (Fig. 6, G, H, K, and L; and Fig. 1 O). We propose that ubiquitination and proteasomal degradation of Top2α–DNA complexes on the DNA knots is required for abscission checkpoint signaling and stable chromatin bridges in cytokinesis.
Proteasome inhibition correlates with impaired localization of Mre11 and ATM to the bridge DNA next to the midbody. (A–E) Localization and mean intensity of ubiquitin at DNA knots in intact chromatin bridges. BE cells were extracted with the differential retention assay protocol without treatment (control), after transfection with Top2α siRNA, or after treatment with 10 μM MG132 or 10 μM MLN7243 for 4 h. (F–O) Localization and mean intensity of Mre11 or ATM at the bridge DNA next to the midbody. Mean ± SD from n cells. Values in control were set to 1. ***, P < 0.001 (ANOVA and Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Proteasome inhibition correlates with impaired localization of Mre11 and ATM to the bridge DNA next to the midbody. (A–E) Localization and mean intensity of ubiquitin at DNA knots in intact chromatin bridges. BE cells were extracted with the differential retention assay protocol without treatment (control), after transfection with Top2α siRNA, or after treatment with 10 μM MG132 or 10 μM MLN7243 for 4 h. (F–O) Localization and mean intensity of Mre11 or ATM at the bridge DNA next to the midbody. Mean ± SD from n cells. Values in control were set to 1. ***, P < 0.001 (ANOVA and Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Expression of TDP2, but not ZNF451, correlates with impaired localization of Mre11 and ATM to the bridge DNA next to the midbody. (A) Percentage of broken DNA bridges in BE cells treated with 10 μM MG132 or 10 μM MLN7243 for 4 h, in the absence (control) or presence of Top2α siRNA. (B–D) Localization and mean intensity of Top2α at the bridge DNA next to the midbody. Mean ± SD from n cells. Values in MG132 were set to 1. (E–L) Localization and mean intensity of Mre11 or ATM at the bridge DNA next to the midbody in cells expressing GFP, ZNF451:GFP, or TDP2:GFP. Mean ± SD from n cells. Values in GFP were set to 1. ***, P < 0.001 (ANOVA and Student’s t test). (M) Percentage of broken DNA bridges in cells expressing GFP or ZNF451:GFP. Mean ± SD from three independent experiments (n > 120). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Expression of TDP2, but not ZNF451, correlates with impaired localization of Mre11 and ATM to the bridge DNA next to the midbody. (A) Percentage of broken DNA bridges in BE cells treated with 10 μM MG132 or 10 μM MLN7243 for 4 h, in the absence (control) or presence of Top2α siRNA. (B–D) Localization and mean intensity of Top2α at the bridge DNA next to the midbody. Mean ± SD from n cells. Values in MG132 were set to 1. (E–L) Localization and mean intensity of Mre11 or ATM at the bridge DNA next to the midbody in cells expressing GFP, ZNF451:GFP, or TDP2:GFP. Mean ± SD from n cells. Values in GFP were set to 1. ***, P < 0.001 (ANOVA and Student’s t test). (M) Percentage of broken DNA bridges in cells expressing GFP or ZNF451:GFP. Mean ± SD from three independent experiments (n > 120). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Top2α promotes the localization of Rad17 to DNA knots
Next, we investigated the mechanism of MRN localization to DNA knots. γ-H2AX or MDC1 foci were undetectable at DNA knots in all (n = 30) control cells with spontaneous intact chromatin bridges tested (Fig. S4, K and L). In contrast, Rad17 localized to DNA knots next to the midbody in spontaneous (25 of 25 cells) or HU-induced (20 of 20 cells) chromatin bridges, where it colocalized with double-strand DNA ends (shown by TUNEL, 20 of 20 cells) and with Mre11 (18 of 18 cells tested) in control cells (Fig. 7, A–D). Localization of Rad17 to the bridge DNA next to the midbody was diminished in cells depleted of Top2α by siRNA or treated with the proteasome inhibitor MG132 compared with controls (Fig. 7, D–G, and I), suggesting that generation and processing of Top2α-linked DNA ends by the proteasome is required for localization of Rad17 to DNA knots on chromatin bridges. Depletion of Mre11 also impaired localization of Rad17, but not Top2α, to the bridge DNA next to the midbody (Fig. 7, H–L; and Fig. S4 M), showing that Rad17 localization to DNA knots also requires Mre11. Rad17 associates with RFC proteins on the chromatin in the DNA damage response; however, RFC3, RFC4, or RFC5 were not detected on DNA knots next to the midbody on spontaneous chromatin bridges (n = 20; Fig. S4, N and O; and Fig. S5 A). Also, Rad17-associated fluorescence at DNA knots was independent of the chromatin bridge length, suggesting that Rad17 localization to DNA knots does not associate with cell-to-cell distance (Fig. S5 B). In addition, in cells labeled with BrdU, BrdU was not incorporated on DNA knots by immunofluorescence, thus showing that DNA knots are not regions of active DNA synthesis (n = 20; Fig. S5 C). These results show Top2α promotes the localization of Rad17 to DNA knots on chromatin bridges.
Top2α or proteasome inhibition correlates with impaired localization of Rad17 to the bridge DNA next to the midbody. (A–C) Localization of Rad17, Rad17:GFP, double-strand DNA ends (TUNEL), or Mre11 to DNA knots in BE cells, on spontaneous or HU-induced chromatin bridges. (D–I) Localization and mean intensity of Rad17 at the bridge DNA next to the midbody in the absence (control) or presence of Top2α or Mre11 siRNA, or after treatment of cells with 10 μM MG132 for 4 h. (J–L) Localization and mean intensity of Top2α at the bridge DNA next to the midbody in the absence (control) or presence of Mre11 siRNA. (M–O) Localization and mean intensity of Mre11 at the bridge DNA next to the midbody in the absence (control) or presence of Rad17 siRNA. Mean ± SD from n cells. Values in control were set to 1. ***, P < 0.001 (ANOVA and Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Top2α or proteasome inhibition correlates with impaired localization of Rad17 to the bridge DNA next to the midbody. (A–C) Localization of Rad17, Rad17:GFP, double-strand DNA ends (TUNEL), or Mre11 to DNA knots in BE cells, on spontaneous or HU-induced chromatin bridges. (D–I) Localization and mean intensity of Rad17 at the bridge DNA next to the midbody in the absence (control) or presence of Top2α or Mre11 siRNA, or after treatment of cells with 10 μM MG132 for 4 h. (J–L) Localization and mean intensity of Top2α at the bridge DNA next to the midbody in the absence (control) or presence of Mre11 siRNA. (M–O) Localization and mean intensity of Mre11 at the bridge DNA next to the midbody in the absence (control) or presence of Rad17 siRNA. Mean ± SD from n cells. Values in control were set to 1. ***, P < 0.001 (ANOVA and Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Dicentric chromatin bridges exhibit impaired formation of double-strand DNA ends at the bridge DNA next to the midbody. (A) Localization of RFC4 in BE cells with chromatin bridges. (B) Mean intensity of Rad17 at the bridge DNA next to the midbody on chromatin bridges of various lengths. Values in <20 μM were set to 1. (C) BrdU-associated fluorescence in cytokinesis with chromatin bridges. Midbodies (boxed areas) were reexported and are shown at higher magnification. (D) Mean intensity of Mre11 at the bridge DNA next to the midbody in cells induced by HU, in the absence (control) or presence of Rad17 siRNA. (E) Mean intensity of Aurora B at the midbody ring. Mean ± SD from n cells. Values in control were set to 1. (F) Frequency of broken chromatin bridges in cytokinesis. Mean ± SD from three independent experiments (n > 120). (G and H) Western blot analysis of total Rad17, ATM, INCENP, Mre11, Aurora B, Top2α, GFP, and tubulin in cells transfected with Rad17 or Top2α siRNA, and/or expressing the siRNA-resistant GFP:Top2(Y805F)R:Rad17Δ. (I) Μean intensity of GFP at the bridge DNA next to the midbody in BE cells expressing siRNA-resistant (R) WT, Y805F, or GFP:Top2(Y805F)R:Rad1Δ in the presence of Top2α siRNA. Mean ± SD from n cells. Values in WT were set to 1. (J) Schematic of a potential mechanism to generate dicentric chromosome bridges by fusion of chromatids from different chromosomes in RPE-1 cells after induction with TC. Deprotected tel sequences are shown in green. (K) FISH analysis of HU-induced chromatin bridges in RPE-1 cells using a tel probe. The DNA bridge (boxed area) is shown at higher magnification. (L) Frequency of HU-induced chromatin bridges that are positive for cen or tel probes by FISH. Mean ± SD from three independent experiments (n > 60). (M) Mean intensity of TUNEL staining at the bridge DNA next to the midbody in HU- or TC-induced cells. Mean ± SD from n cells. Values in HU-induced were set to 1. ***, P < 0.001 (Student’s t test). Numbers below/next to each bar indicate n. (N) Experimental procedure to generate dicentric bridges with DNA knots in RPE-1 cells. (O) Schematic of a potential mechanism that generates dicentric bridges with DNA knots. (P) FISH analysis of TC+HU-induced chromatin bridges using a tel probe. A tel-positive signal on the DNA bridge is indicated by an arrow and shown at higher magnification. Arrowheads indicate DNA knots. (Q) Frequency of TC+HU-induced chromatin bridges that are positive for tel probe by FISH. Mean ± SD from three independent experiments (n > 60). Scale bars, 5 μm. Source data are available for this figure: SourceData FS5.
Dicentric chromatin bridges exhibit impaired formation of double-strand DNA ends at the bridge DNA next to the midbody. (A) Localization of RFC4 in BE cells with chromatin bridges. (B) Mean intensity of Rad17 at the bridge DNA next to the midbody on chromatin bridges of various lengths. Values in <20 μM were set to 1. (C) BrdU-associated fluorescence in cytokinesis with chromatin bridges. Midbodies (boxed areas) were reexported and are shown at higher magnification. (D) Mean intensity of Mre11 at the bridge DNA next to the midbody in cells induced by HU, in the absence (control) or presence of Rad17 siRNA. (E) Mean intensity of Aurora B at the midbody ring. Mean ± SD from n cells. Values in control were set to 1. (F) Frequency of broken chromatin bridges in cytokinesis. Mean ± SD from three independent experiments (n > 120). (G and H) Western blot analysis of total Rad17, ATM, INCENP, Mre11, Aurora B, Top2α, GFP, and tubulin in cells transfected with Rad17 or Top2α siRNA, and/or expressing the siRNA-resistant GFP:Top2(Y805F)R:Rad17Δ. (I) Μean intensity of GFP at the bridge DNA next to the midbody in BE cells expressing siRNA-resistant (R) WT, Y805F, or GFP:Top2(Y805F)R:Rad1Δ in the presence of Top2α siRNA. Mean ± SD from n cells. Values in WT were set to 1. (J) Schematic of a potential mechanism to generate dicentric chromosome bridges by fusion of chromatids from different chromosomes in RPE-1 cells after induction with TC. Deprotected tel sequences are shown in green. (K) FISH analysis of HU-induced chromatin bridges in RPE-1 cells using a tel probe. The DNA bridge (boxed area) is shown at higher magnification. (L) Frequency of HU-induced chromatin bridges that are positive for cen or tel probes by FISH. Mean ± SD from three independent experiments (n > 60). (M) Mean intensity of TUNEL staining at the bridge DNA next to the midbody in HU- or TC-induced cells. Mean ± SD from n cells. Values in HU-induced were set to 1. ***, P < 0.001 (Student’s t test). Numbers below/next to each bar indicate n. (N) Experimental procedure to generate dicentric bridges with DNA knots in RPE-1 cells. (O) Schematic of a potential mechanism that generates dicentric bridges with DNA knots. (P) FISH analysis of TC+HU-induced chromatin bridges using a tel probe. A tel-positive signal on the DNA bridge is indicated by an arrow and shown at higher magnification. Arrowheads indicate DNA knots. (Q) Frequency of TC+HU-induced chromatin bridges that are positive for tel probe by FISH. Mean ± SD from three independent experiments (n > 60). Scale bars, 5 μm. Source data are available for this figure: SourceData FS5.
Rad17 promotes the binding of the MRN complex to DNA knots in cytokinesis with chromatin bridges
In cytokinesis with spontaneous or HU-induced chromatin bridges, the depletion of Rad17 diminished localization of Mre11 or ATM to the bridge DNA next to the midbody, impaired localization of Aurora B to the midbody ring, and increased the frequency of broken chromatin bridges compared with controls (Fig. 7, M–O; Fig. 8, A–G; and Fig. S5, D–G), showing that Rad17 is required for abscission checkpoint signaling. To further investigate the significance of Rad17 for the abscission checkpoint, a chimeric GFP:Top2(Y805F)R:Rad17Δ protein in which the siRNA-resistant, active site-mutant Y805F Top2:GFP was fused to Rad17(560–670) protein fragment (Rad17Δ) that interacts with Nbs1 and promotes MRN localization to sites of double-strand DNA breaks was constructed (Wang et al., 2014). After depletion of the endogenous Top2 by siRNA, expression of GFP:Top2(Y805F)R:Rad17Δ, but not GFP:Top2(Y805F)R, rescued localization of Mre11 or ATM to the bridge DNA next to the midbody and prevented chromatin bridge breakage in cytokinesis compared with control cells expressing WT GFP:Top2R (Fig. 8, H–N; and Fig. S5, H and I). These results suggest that Rad17 promotes binding of the MRN complex to the DNA knots next to the midbody to implement the Top2α-dependent abscission checkpoint in cytokinesis with chromatin bridges.
Expression of Y805F:Rad17Δ GFP:Top2 rescues localization of Mre11 and ATM to the bridge DNA next to the midbody in Top2α-depleted cells. (A–C) Localization and mean intensity of ATM at the bridge DNA next to the midbody in BE cells, in the absence (control) or presence of Rad17 siRNA. (D–F) Localization and mean intensity of Aurora B at the midbody ring. Mean ± SD from n cells. Values in control were set to 1. (G) Percentage of broken DNA bridges. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (Student’s t test). (H–M) Localization and mean intensity of Mre11 or ATM at the bridge DNA next to the midbody in BE cells expressing siRNA-resistant (R) WT, Y805F, or Y805F:Rad17Δ GFP:Top2R in the presence of Top2α siRNA. Mean ± SD from n cells. Values in WT were set to 1. (N) Percentage of broken DNA bridges in cells transfected as in J and M. Mean ± SD. SD from three independent experiments (n > 120). ***, P < 0.001 (ANOVA and Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were re-exported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Expression of Y805F:Rad17Δ GFP:Top2 rescues localization of Mre11 and ATM to the bridge DNA next to the midbody in Top2α-depleted cells. (A–C) Localization and mean intensity of ATM at the bridge DNA next to the midbody in BE cells, in the absence (control) or presence of Rad17 siRNA. (D–F) Localization and mean intensity of Aurora B at the midbody ring. Mean ± SD from n cells. Values in control were set to 1. (G) Percentage of broken DNA bridges. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (Student’s t test). (H–M) Localization and mean intensity of Mre11 or ATM at the bridge DNA next to the midbody in BE cells expressing siRNA-resistant (R) WT, Y805F, or Y805F:Rad17Δ GFP:Top2R in the presence of Top2α siRNA. Mean ± SD from n cells. Values in WT were set to 1. (N) Percentage of broken DNA bridges in cells transfected as in J and M. Mean ± SD. SD from three independent experiments (n > 120). ***, P < 0.001 (ANOVA and Student’s t test). Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were re-exported and are shown at higher magnification. Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Generation of dicentric chromosome bridges
To investigate whether chromatin bridges caused by dicentric chromosomes activate the abscission checkpoint in human cells, human RPE-1 cells conditionally expressing dominant-negative TRF2 after induction withTC were employed (Umbreit et al., 2020). Expression of dominant-negative TRF2 produces uncapped chromosome ends which can be fused by the DNA repair machinery to generate dicentric fusions (Fig. 9, A and B; and Fig. S5 J; Umbreit et al., 2020). After induction of RPE-1 cells with TC, ∼92% chromatin bridges exhibited tel probe-staining on the bridge DNA by FISH (Fig. 9, C and D), showing that these bridges represent mis-segregated dicentric chromosomes generated by tel fusion. In contrast, only 7% HU-induced chromatin bridges in RPE-1 cells were tel positive and 8% exhibited centromeric DNA staining across the length of the bridge, suggesting that the majority of chromatin bridges in HU-induced RPE-1 cells are derived from catenated non-centromeric DNA and not from dicentric chromosomes (Fig. S5, K, and L).
Dicentric chromosome bridges exhibit impaired localization of Top2α and Rad17 to the bridge DNA next to the midbody. (A) Experimental procedure to study replication stress-induced DNA bridges (by HU), or dicentric chromosome bridges (induced by TC) in RPE-1 cells. (B) Schematic of a potential mechanism to generate dicentric chromosome bridges by sister chromatid fusion in RPE-1 cells after induction with TC. Deprotected tel sequences are shown in green. (C) FISH analysis of a chromatin bridge in TC-induced RPE-1 cells using a tel probe. A tel-positive signal on the DNA bridge is indicated by an arrow and shown at higher magnification. (D) Frequency of TC-induced chromatin bridges that are positive for cen or tel probes by FISH. Mean ± SD from three independent experiments (n > 60). (E and F) Chromatin bridges and frequency of chromatin bridges exhibiting DNA knots next to the midbody in HU-, TC-, or TC+HU-induced RPE-1 cells. Mean ± SD from three independent experiments (n > 100). (G–M and O–Q) Localization and mean intensity of GapR:GFP, Top2α, or Rad17 at the bridge DNA next to the midbody in HU-, TC-, or TC+HU-induced cells. Mean ± SD from n cells. Values in HU-induced cells were set to 1. Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. (N) Percentage of broken DNA bridges HU-, TC-, or TC+HU-induced cells in the absence or presence of 300 nM VX-680 for 2 h. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (ANOVA and Student’s t test). Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Dicentric chromosome bridges exhibit impaired localization of Top2α and Rad17 to the bridge DNA next to the midbody. (A) Experimental procedure to study replication stress-induced DNA bridges (by HU), or dicentric chromosome bridges (induced by TC) in RPE-1 cells. (B) Schematic of a potential mechanism to generate dicentric chromosome bridges by sister chromatid fusion in RPE-1 cells after induction with TC. Deprotected tel sequences are shown in green. (C) FISH analysis of a chromatin bridge in TC-induced RPE-1 cells using a tel probe. A tel-positive signal on the DNA bridge is indicated by an arrow and shown at higher magnification. (D) Frequency of TC-induced chromatin bridges that are positive for cen or tel probes by FISH. Mean ± SD from three independent experiments (n > 60). (E and F) Chromatin bridges and frequency of chromatin bridges exhibiting DNA knots next to the midbody in HU-, TC-, or TC+HU-induced RPE-1 cells. Mean ± SD from three independent experiments (n > 100). (G–M and O–Q) Localization and mean intensity of GapR:GFP, Top2α, or Rad17 at the bridge DNA next to the midbody in HU-, TC-, or TC+HU-induced cells. Mean ± SD from n cells. Values in HU-induced cells were set to 1. Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. (N) Percentage of broken DNA bridges HU-, TC-, or TC+HU-induced cells in the absence or presence of 300 nM VX-680 for 2 h. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (ANOVA and Student’s t test). Numbers below/next to each bar indicate n. Scale bars, 5 μm.
Dicentric chromosome bridges fail to activate the abscission checkpoint in human cells
Approximately 85% of chromatin bridges in HU-induced RPE-1 cells exhibited DNA knots next to the midbody compared with 15% chromatin bridges in TC-induced cells by high-resolution confocal microscopy (Fig. 9, E and F). Intact TC-induced (dicentric chromosome) bridges exhibited relatively low levels of positively supercoiled DNA (by GapR:GFP staining) and reduced double-strand DNA ends (by TUNEL) at the bridge DNA next to the midbody compared with HU-induced bridges (Fig. 9, G–I; and Fig. S5 M). Also, TC-induced chromatin bridges exhibited diminished localization of Top2α, Rad17, and Mre11 to the bridge DNA next to the midbody, impaired localization of Aurora B to the midbody ring, and high frequency of breakage in cytokinesis compared with HU-induced chromatin bridges (Fig. 9, J–Q; and Fig. 10, A–E, and G). These results show that dicentric chromosome bridges exhibit impaired abscission checkpoint signaling. Inhibition of Aurora B by the catalytic inhibitor VX-680 did not exacerbate chromatin bridge breakage in cytokinesis in TC-induced cells, further supporting that dicentric chromosome bridges fail to activate the abscission checkpoint in human cells (Fig. 9 N). Importantly, the generation of tel-positive (dicentric) chromatin bridges that possess DNA knots after simultaneous treatment of RPE-1 cells with TC and HU (Fig. 9 F and Fig. S5, N–Q) rescued localization of Top2α and Aurora B, respectively, to the bridge DNA next to the midbody or to the midbody ring, and prevented chromatin bridge breakage in cytokinesis compared with RPE-1 cells induced with TC-only (Fig. 9, J–N; and Fig. 10, D–G). Also, after induction of cells with TC, expression of GFP:INCENP(FB) coding for a truncated INCENP protein that is constitutively targeted to the midbody (Petsalaki and Zachos, 2021b) rescued Aurora B-localization to the midbody ring in cells with chromatin bridges and prevented chromatin bridge breakage in cytokinesis compared with cells expressing GFP-only (Fig. 10, H–K). These results suggest that dicentric chromosomes fail to induce an Aurora B-mediated abscission delay because they lack DNA knots and escape detection by the checkpoint.
Expression of GFP:INCENP(FB) prevents breakage of dicentric chromosome bridges in cytokinesis. (A–C) Localization and mean intensity of Mre11 at the bridge DNA next to the midbody in HU- or TC-induced RPE-1 cells. (D–G) Localization and mean intensity of Aurora B (AurB) at the midbody ring in HU-, TC-, or TC+HU-induced cells. Mean ± SD from n cells. Values in HU-induced cells were set to 1. P < 0.001 (ANOVA and Student’s t test). (H–J) Localization and mean intensity of Aurora B at the midbody ring in TC-induced RPE-1 cells expressing GFP or GFP:INCENP(FB). Mean ± SD from n cells. Values in GFP:INCENP(FB) cells were set to 1. Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Scale bars, 5 μm. (K) Percentage of broken DNA bridges from cells treated as in H and I. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (Student’s t test). Numbers below/next to each bar indicate n. (L) Proposed mechanism by which the abscission checkpoint senses chromatin bridges derived from catenated DNA in human cells (left). Dicentric chromosome bridges on the other hand, fail to activate the abscission checkpoint (right). Y, Top2α-DNA bond.
Expression of GFP:INCENP(FB) prevents breakage of dicentric chromosome bridges in cytokinesis. (A–C) Localization and mean intensity of Mre11 at the bridge DNA next to the midbody in HU- or TC-induced RPE-1 cells. (D–G) Localization and mean intensity of Aurora B (AurB) at the midbody ring in HU-, TC-, or TC+HU-induced cells. Mean ± SD from n cells. Values in HU-induced cells were set to 1. P < 0.001 (ANOVA and Student’s t test). (H–J) Localization and mean intensity of Aurora B at the midbody ring in TC-induced RPE-1 cells expressing GFP or GFP:INCENP(FB). Mean ± SD from n cells. Values in GFP:INCENP(FB) cells were set to 1. Broken chromatin bridges are indicated by dotted arrows. Arrowheads indicate DNA knots. Midbodies (boxed areas) were reexported and are shown at higher magnification. Scale bars, 5 μm. (K) Percentage of broken DNA bridges from cells treated as in H and I. Mean ± SD from three independent experiments (n > 120). ***, P < 0.001 (Student’s t test). Numbers below/next to each bar indicate n. (L) Proposed mechanism by which the abscission checkpoint senses chromatin bridges derived from catenated DNA in human cells (left). Dicentric chromosome bridges on the other hand, fail to activate the abscission checkpoint (right). Y, Top2α-DNA bond.
Discussion
On the basis of the above findings, we propose the following model: spontaneous or replication stress-induced chromosome bridges that are derived from incomplete segregation of catenated chromosomes exhibit DNA knots containing tangled and supercoiled DNA next to the midbody (Fig. 10 L, left pathway). Top2α localizes to DNA knots where it forms irreversible Top2ccs exhibiting Top2-linked double-strand DNA ends. Ubiquitination and proteasomal degradation of Top2ccs exposes the Top2-generated DNA ends to recruit Rad17 on the DNA knots. In turn, Rad17 promotes localization of the MRN complex to DNA knots and downstream ATM-Chk2-INCENP signaling to delay abscission and prevent chromatin bridge breakage in cytokinesis. In contrast, dicentric chromosome bridges that are generated from telomere-end fusion do not exhibit DNA knots and do not recruit Top2α on the bridge DNA next to the midbody (Fig. 10 L, right pathway). As a result, dicentrics fail to activate the abscission checkpoint, leading to premature abscission and chromatin breakage in cytokinesis.
These findings are the first to describe a mechanism by which human cells detect chromatin bridges to activate the abscission checkpoint through the activation of a Top2α-Rad17 axis on DNA knots. Top2α localization to DNA knots is not related to the DNA mass at the midbody or to chromatin bridge length; instead, our results are consistent with Top2α recognizing catenated and overtwisted DNA inside these structures (Baxter et al., 2011; McClendon et al., 2005). By employing Top2α as a sensor for the abscission checkpoint in response to chromatin bridges, human cells can coordinate sister chromatid disjunction with the onset of abscission. During progression through anaphase, DNA bridges that exhibit double-strand DNA catenates can normally be resolved by Top2α, leading to Top2α removal from the bridge DNA thus allowing abscission to occur (Broderick et al., 2015; Spence et al., 2007; Wang et al., 2008). However, if bridge resolution fails, for example, due to specific topological challenges imposed by high bridge-tension and chromatin bridges persist in late cytokinesis, abortive Top2ccs on DNA knots can trigger the abscission checkpoint to delay abscission.
Our findings are consistent with the majority of spontaneous chromatin bridges representing catenated centromeric DNA, which is in agreement with previous observations from ultrafine DNA bridges in unstressed mitotic cells (Baumann et al., 2007; Chan et al., 2007, 2009; Wang et al., 2010). Our results also suggest that the majority of replication stress-induced chromatin bridges are not caused by unreplicated DNA in our experimental conditions but contain catenated DNA that is recognized by Top2α (Chan et al., 2009). Whether other types of DNA bridges that are not processed by Top2α (not dicentrics), such as ultrafine bridges generated by unresolved recombination or replication intermediates at fragile site loci, can activate the abscission checkpoint in human cells requires further investigation (Bhowmick et al., 2019; Chan et al., 2009, 2018). In this case, other bridge-associated proteins, such as Topoisomerase IIIα BLM, or PICH, may induce the abscission checkpoint in response to specific types of DNA bridges that do not involve Top2α (Chan et al., 2009, 2018). Because the abscission machinery may be wired differently in some normal cells compared with cancer cells (Tedeschi et al., 2020), it will be interesting to examine whether the Top2α–Rad17 axis activates the abscission checkpoint similarly also in non-transformed cells.
DNA knots were typically found next to the midbody in control cells with spontaneous or HU-induced chromatin bridges. One possibility is that, as chromatin bridges are stretched, DNA knots slide along the DNA molecule until they become “jammed” at the high protein density midbody region (Klotz et al., 2018). Alternatively, proteins at the DNA knot may somehow direct the position of midbody formation within the intercellular canal. Either way, close proximity of the DNA knot with the midbody may facilitate transmission of the abscission delay signal to the midbody where the CPC and other abscission control proteins localize.
Our results are also the first to demonstrate a functional interaction between Top2 and Rad17, to the best of our knowledge: Rad17 promotes localization of the MRN complex to Top2-generated DNA ends on chromatin bridges. Mre11 is required for formation of Rad17 foci on DNA knots, in agreement with previous findings that Rad17 interaction with the MRN is required for Rad17 localization to double-strand DNA break sites in the DNA damage response (Wang et al., 2014). MDC1 also promotes MRN recruitment to sites of DNA damage (Melander et al., 2008; Spycher et al., 2008; Stucki et al., 2005); however, we did not detect MDC1 or γ-H2AX foci on the bridge DNA in control cells with intact chromatin bridges. Because stretching of chromosome bridges causes histone removal (Bennink et al., 2001; Maciejowski et al., 2015), the Rad17 axis may represent a more efficient mechanism for recruiting the MRN complex to DNA knots than the MDC1/γ-H2AX axis. Phosphorylation of Rad17 by ATM results in a direct interaction of Rad17 with Nbs1 to promote MRN recruitment to sites of double-strand DNA breaks in the DNA damage response (Wang et al., 2014); however, whether a similar mechanism promotes MRN localization to DNA knots requires further investigation. Overall, our results identify a novel crosstalk between DNA damage and cytokinesis machineries (Petsalaki and Zachos, 2020) and indicate that cells have evolved mechanisms to efficiently recycle proteins to maintain genome stability during different stages of the cell cycle.
The above findings show fundamental differences in the mechanisms for sensing chromatin bridges by the abscission checkpoint between budding yeast and higher eukaryotes. In budding yeast, chromatin bridges associated with replication stress, chromosome condensation, or decatenation defects stabilize the mitotic spindle to act as a platform for the Ipl1/Aurora homolog; in turn, Ipl1 acts as a sensor to monitor the presence of chromatin at the spindle midzone and activate the abscission delay (“NoCut”; Amaral et al., 2016; Mendoza et al., 2009; Norden et al., 2006). In human cells, on the other hand, we show that Top2α localizes to knotted DNA and forms abortive Top2ccs next to the midbody to activate the abscission checkpoint. These distinct mechanisms may be due to differences in the mechanics and regulation of cytokinesis between yeast and higher eucaryotes affecting the detection of chromatin bridges by Top2α or Ipl1/Aurora.
Our model can also provide a possible explanation why, in contrast to budding yeast (Mendoza et al., 2009), lagging chromosomes trapped inside the intercellular canal do not inhibit abscission in human cells (Janssen et al., 2011): because lagging chromosomes are not expected to exhibit knotted DNA, they are unable to recruit Top2α next to the midbody to activate an abscission delay. Consistently, dicentric chromosome bridges that are generated by deprotection of telomere ends are predicted to be properly condensed, replicated, and decatenated, and fail to induce an abscission delay. Because the abscission checkpoint cannot protect against breakage of dicentric chromosome bridges with potentially devastating consequences for genome integrity (Maciejowski et al., 2015, 2020; Umbreit et al., 2020), this situation can perhaps explain why cells go to great lengths, such as inactivating the DNA double-strand break repair, to avoid telomere fusions in mitosis (Orthwein et al., 2014) and can be cancer-relevant, especially in aging tissues where the frequency of dicentric bridges increases due to telomere attrition (Rudolph et al., 2001).
Our results also demonstrate novel functions for Top2α and Rad17 in maintaining chromosome stability through their roles in activating the abscission checkpoint. Because chromosomal instability is a hallmark of cancer, the identification of genes that safeguard chromosomal integrity is important to understand the etiology of cancer (Hanahan and Weinberg, 2011). Furthermore, because the abscission checkpoint protects anaphase bridges that arise from replication stress from breaking in cytokinesis, it will perhaps be important to examine whether drug-inhibition of Topoisomerase II catalytic activity can sensitize cancer cells to replication stress by relatively low doses of DNA polymerase inhibitors (Sadler et al., 2018). In conclusion, these findings are the first to describe a mechanism by which human cells detect chromatin bridges to activate the abscission checkpoint to maintain genome integrity.
Materials and methods
Antibodies
Mouse monoclonal antibodies against Topoisomerase IIα (G-6; sc-166934; used throughout this study except from Fig. S1 N), Topoisomerase IIβ (H-8; sc-25330), Mre11 (18; sc-135992), Rad50 (G-2; sc-74460), ATM (1B10; sc-135663), TDP2 (H-6; sc-377280), RFC3 (G10; sc-390293), RFC4 (C-9; sc-28301), RFC5 (F9; sc-376528), TopBP1 (B-7; sc-271043), FANCD2 (FI17; sc-20022), and phospho-ATM-Ser1981 (10H11.E12; sc-47739) were from Santa Cruz Biotechnology. Rabbit polyclonal antibodies against GFP (FL; sc-8334), Chk2 (H-300; sc-9064), Nbs1 (Nibrin; H-300; sc-11431), and Mklp1 (N-19; sc-867) were also from Santa Cruz Biotechnology. Mouse monoclonal anti-MDC1 antibody (P2B11; ab241048) and rabbit polyclonal anti-INCENP (ab12183), anti-Aurora B (ab2254; used in immunofluorescence), anti-Lamin A (ab26300), and anti-KIF4A (ab122227) antibodies were from Abcam. Mouse monoclonal anti-phospho-Histone H2A.X (S139; clone JBW301; γ-H2AX) and anti-PICH (04-1540; clone 142-26-3) antibodies were from Millipore. Mouse monoclonal antibodies against α-tubulin (DM1A) and actin (AC-40) were from Sigma-Aldrich. Rabbit polyclonal anti-phospho-Chk2-Thr68 (2661) and mouse monoclonal anti-Ubiquitin (P4D1, 3936) antibodies were obtained from Cell Signaling Technology, rabbit polyclonal anti-Cep55 (N2C3; GTX112190; used in immunofluorescence) antibody was from Genetex, and mouse monoclonal anti-AIM1 (Aurora B; 611082; used in Western blotting) antibody was from BD Biosciences. Mouse monoclonal anti-bromodeoxyuridine (anti-BrdU; clone BMC9318, MAB3424) antibody was from Merck-Millipore and rabbit polyclonal antibody to human Topoisomerase IIα (2011-1; used in Fig. S1 N) was from Topogen. Anti-pSer91 rabbit polyclonal antiserum against phosphorylated serine-91 of human INCENP was previously described (Petsalaki and Zachos, 2021b).
Secondary antibodies
For immunofluorescence, goat anti-rabbit IgG Alexa Fluor 633-conjugated (A21070) and goat anti-mouse IgG Alexa Fluor 633-conjugated (A21050) antibodies were from Thermo Fisher Scientific. Goat anti-rabbit IgG FITC (fluorescein)-conjugated (111-096-047), goat anti-mouse IgG FITC (fluorescein)-conjugated (115-096-072), goat anti-rabbit IgG Rhodamine (TRITC)-conjugated (111-025-046), and sheep anti-mouse IgG Rhodamine (TRITC)-conjugated (515-025-072) antibodies were from Jackson ImmunoResearch. For Western blotting, the horse anti-mouse IgG HRP-linked (7076) antibody was from Cell Signaling Technology, and goat anti-rabbit IgG (H+L)-HRP (170-6515) antibody was from Biorad.
Plasmids and cloning
The pT104.1(pEGFP-C3)/TopoIIα plasmid encoding human Topoisomerase IIα fused to GFP was created by William Beck and provided to us by Gary Gorbsky (Oklahoma Medical Research Foundation, Oklahoma City, OK, USA; Mo et al., 1998). For plasmids pT104.1(pEGFP-C3)/TopoIIα(WT)R, pT104.1(pEGFP-C3)/TopoIIα(Y805F)R, or pT104.1(pEGFP-C3)/TopoIIα(AEEA)R encoding, respectively, siRNA-resistant WT, Y805F, or 342-KKKK-345 to 342-AEEA-345 mutant Topoisomerase IIα (Top2α) fused to GFP; refer to the Mutagenesis section below. To generate plasmid pEGFPN1/GapR coding for GapR protein fused to GFP, GapR was amplified by PCR by using pKVS45/gapR plasmid as the template (a gift from Monica Guo; School of Medicine, Department of Microbiology, Seattle, WA, USA) and cloned into the pEFGP-N1 vector (Clontech) as a KpnI–BamHI fragment. To generate plasmid pEGFPN1/TDP2 coding for human TDP2 protein fused to GFP, TDP2 was amplified by PCR by using plasmid pCMV6-TDP2-Entry as the template (a gift from Yves Pommier and Shar-yin Naomi Huang; Laboratory of Molecular Pharmacology, Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA) and cloned into the pEFGP-N1 vector (Clontech) as a HindIII–BamHI fragment. To generate pT104.1(pEGFP-C3)/TopoIIα(Y805F)R/Rad17Δ plasmid coding for a chimeric GFP:Top2(Y805F)R:Rad17Δ protein, first the stop codon was removed from the pT104.1(pEGFP-C3)/TopoIIα(WT)R vector by site-directed mutagenesis. Then, the 1,710–2,010-nt sequence of human Rad17 was amplified by PCR by using FLAG-EGFP-RAD17/pcDNA3 plasmid as a template (a gift from Yasunori Fukumoto; School of Pharmaceutical Sciences, Chiba University, Chiba, Japan) and inserted into the pT104.1(pEGFP-C3)/TopoIIα(WT)R vector without stop codon as a SacII-SmaI fragment. Plasmid pEGFP-N1 coding for GFP under cytomegalovirus promoter was obtained from Takara Bio and plasmid pEGFP-vps4-K173Q encoding human Vps4 harboring the K173Q point mutation fused to EGFP into pEGFP-C1 vector (Clontech) was a gift from Wesley Sundquist (University of Utah, Salt Lake City, UT, USA; Morita et al., 2007). Plasmid pDEST686/GFP-ZNF451 encoding human ZNF451 protein fused to GFP was from Felipe Cortés-Ledesma (Spanish National Cancer Research Centre, Madrid, Spain). Plasmid pEGFPN1/INCENP(FB) encoding GFP-tagged human INCENP (amino acids 49–919) that is targeted to the FB by fusion with the Mklp1 (amino acids 456–858) fragment was previously described (Petsalaki and Zachos, 2021b). All plasmids were completely sequenced.
Mutagenesis
Point mutations were generated by using the Q5 site-directed mutagenesis kit (New England Biolabs). To generate the pT104.1/TopoIIα(WT)R plasmid encoding an siRNA-resistant form of WT Topoisomerase IIα fused to GFP, the pT104.1/TopoIIa plasmid was used to introduce A1173C, C1176T, A1179G, T1905A, C1908A, G1911A, T2691A, T2694A, and T2697A point mutations giving resistance to the three different individual Topoisomerase IIα siRNAs. To generate pT104.1/TopoIIα(Y805F)R plasmid, the pT104.1/TopoIIα(WT)R plasmid was used to introduce an A2414T point mutation. To generate pT104.1/TopoIIα(AEEA)R plasmid, the pT104.1/TopoIIα(WT)R plasmid was used to introduce A1024G, A1025C, A1027G, A1030G, A1036G, and A1037C point mutations. To remove the stop codon from the pT104.1(pEGFP-C3)/TopoIIα(WT)R plasmid, the T4594G, A4595C point mutations were introduced.
siRNA sequences and PNA probes
Human Topoisomerase IIα (a pool of three different siRNAs: 5′-GGAUCAACAUGCCAAUUGA-3′, 5′-GACAUCGUAUCCAGUUCAA-3′, 5′-CAAGGGUACUAUUGAAGAA-3′), Rad17 (a pool of three different siRNAs: 5′-GCAUGGUAUUCAAGUACAA-3′, 5′-GAAGAUAAUUCUGGUUGAA-3′, 5′-GUUGCCCUCUCAUUUAUCA-3′), and Mre11 (a pool of three different siRNAs: 5′-CAGAACAGAUGGCUAAUGA-3′, 5′-GUGAGGGAAUGGUCACUAA-3′, and 5′-GUAGGGAAUUCUUCUGUUA-3′) siRNAs were from Santa Cruz Biotechnology. Only the sense sequences of the siRNA duplexes are shown.
FITC-labeled C-rich telomere probe (tel probe; PN-TC011-005) and Cy3-labeled centromere probe against α-satellite DNA (cen probe; PN-CN050-005) were from Eurogentec.
Cell culture and treatments
Human colon carcinoma BE cells (diploid cells that contain an oncogenic Kras-G13D mutation as well as the BRAF-G463V oncogenic mutation; a gift from Simon Wilkinson and Christopher Marshall, Institute of Cancer Research, London, UK; Petsalaki and Zachos, 2021b), cervical carcinoma HeLa cells stably expressing LAP2b fused to RFP (a gift from Daniel Gerlich, Institute of Molecular Biotechnology, Vienna, Austria; Steigemann et al., 2009), or HeLa cells stably expressing α-tubulin fused to GFP (a gift from Jan-Michael Peters, Institute of Molecular Pathology, Vienna, Austria) were grown in DMEM (Biosera) containing 10% FBS (Biosera), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2. Telomerase-immortalized human RPE-1 (retinal pigment epithelial-1) cells (a gift from Titia de Lange; The Rockefeller University, New York, NY, USA) and John Maciejowski (Memorial Sloan Kettering, New York, NY, USA; Umbreit et al., 2020) were grown in DMEM/F12 (1:1) media (Biosera) supplemented with 10% TC-free FBS (Pan Biotech), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2.
Cells were treated with 1 mM HU (Sigma-Aldrich), 10 μM etoposide (Sigma-Aldrich), 50 ng/ml nocodazole (Sigma-Aldrich), 300 nM VX-680 (S1048; Selleckchem), 10 μM MG132 (474790; Calbiochem-Novagen), 10 μM MLN7243 (S8341; Selleckchem), 10 μM ICRF-193 (I4659; Sigma-Aldrich), or 0.1 μg/ml TC (Sigma-Aldrich), as appropriate. siRNA duplexes were transfected into BE cells 24 h before analysis using Lipofectamine 2000 (Invitrogen), with the exception of Top2α siRNAs that were transfected for 48 h before analysis. For expression of GFP proteins, plasmids were transfected into cells in the absence or presence of appropriate siRNA duplexes 24–48 h before analysis or further drug treatment using Lipofectamine 2000 (Invitrogen). All cell lines used exhibited consistent morphology and growth properties and were negative for mycoplasma contamination.
Enrichment of cells in cytokinesis
BE cells were treated with 50 ng/ml nocodazole (Sigma-Aldrich) for 16 h, washed twice with prewarmed (37°C) PBS, and released in prewarmed fresh medium for 2 h. Microscopic examination showed that ∼30% cells were at the midbody stage after this treatment.
Time-lapse imaging
HeLa LAP2b:RFP or HeLa tubulin:GFP cells were seeded onto Petri dishes with a 30-mm glass base (Greiner) and an inverted fluorescence microscope (Observer D1; Zeiss) was used. Fluorescence or phase-contrast images were taken by using a 63× Plan Neofluor 0.75 NA Ph2 dry objective (Zeiss). Imaging was performed at 37°C in 5% CO2 by using a Zeiss AxioCam MRm camera and Zeiss ZEN 2 acquisition software. Drugs were added to the medium immediately before filming as appropriate.
Indirect immunofluorescence microscopy and microscope image acquisition
For phospho-INCENP-Ser91 staining, cells were extracted in prewarmed (37°C) Phem buffer (60 mM Pipes, 25 mM Hepes, pH 7.0, 10 mM EGTA, and 4 mM MgSO4) supplemented with 0.5% CHAPS and 100 nM microcystin (Sigma-Aldrich) for 5 min at room temperature, fixed with prewarmed (37°C) 4% paraformaldehyde in Phem buffer for 10 min at room temperature, permeabilized in 0.5% Triton X-100 in Phem buffer for 2 min at room temperature, washed twice with PBS, and immunostained. For phospho-ATM-Ser1981 or total Chk2 staining, cells were fixed in ice-cold methanol for 5 min at −20°C, washed twice with PBS at room temperature, and immunostained. For all other fluorescence microscopy applications, cells were fixed in 4% paraformaldehyde in cytoskeleton buffer (1.1 M Na2HPO4, 0.4 M KH2PO4, 137 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM EGTA, 5 mM Pipes, and 5 mM glucose, pH 6.1) for 5 min at 37°C, permeabilized in 0.5% Triton X-100 in cytoskeleton buffer, washed twice with PBS at room temperature, and immunostained. FITC- or rhodamine-TRITC-conjugated (Jackson ImmunoResearch) or Alexa Fluor 633-conjugated (Thermo Fisher Scientific) secondary antibodies were used as appropriate. DNA was stained with 10 μg/ml DAPI (Biotium) and cells were mounted in Vectashield medium (Vector Laboratories). Actin patches were stained with Fluorescein Phalloidin (F432; Invitrogen, Thermo Fisher Scientific).
Images were collected by using a super-resolution SP8 LIGHTNING laser-scanning spectral confocal microscope (Leica Microsystems), LASX software (Leica Microsystems), and a 63× Apochromat 1.40 NA oil objective. The low-fluorescence immersion oil (11513859; Leica Microsystems) was used and imaging was performed at room temperature. Images were processed with the Lightning module and mean projections of image stacks were obtained by using the LASX software. To visualize proteins at midbodies without overexposing the nuclei in cytokinesis with chromatin bridges in photos showing higher magnifications of midbodies, the brightness and contrast were increased using the LASX software and images were re-exported using the snapshot tool.
Differential retention assay
Cells were extracted on ice with gentle agitation for 2 min with HPEM buffer (30 mM Hepes, 65 mM Pipes, 10 mM EGTA, and 2 mM MgCl2 at pH = 6.9) supplemented with 350 mM NaCl, 0.5% Triton X-100, and 1 mM PMSF, essentially as described (Agostinho et al., 2004). Cells were then fixed with 4% PFA in HPEM for 10 min, followed by permeabilization with 0.5% Triton in PBS for 2 min at room temperature, washed 3× with PBS, and immunostained.
FISH
Cells were fixed on slides with 4% PFA in cytoskeleton buffer for 10 min at room temperature and hybridized with the appropriate PNA probe, essentially as described (Cesare et al., 2015). Briefly, slides were dehydrated with a graded series of 70%, 90%, and 100% ethanol each time for 3 min and air dried for 10 min at room temperature. Cells were then hybridized with 0.5 μM PNA probe on hybridization buffer (70% formamide, 20 μl/ml salmon sperm DNA, and 10 mM Tris·HCl, pH 7.5) at 80°C for 5 min, followed by incubation for 2 h in the dark in a humidified chamber at room temperature. Slides were then washed once with PBS, twice with PNA wash A (70% formamide and 10 mM Tris·HCl, pH 7.5), twice with PNA wash B (50 mM Tris HCl, pH 7.5, 150 mM NaCl, and 0.8% Tween 20), counterstained with DAPI and visualized by immunofluorescence.
BrdU labeling and immunofluorescence
Cells were labeled with 10 μM 5-bromo-2′-Deoxyuridine (BrdU; 19-160; Merck-Millipore) at 37°C for 2 h. Cells were then fixed with 4% PFA in cytoskeleton buffer (1.1 M Na2HPO4, 0.4 M KH2PO4, 137 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM EGTA, 5 mM Pipes, and 5 mM glucose, pH 6.1) for 10 min, permeabilized with 0.5% Triton in PBS, washed three times in PBS, and incubated with 1 M HCl for 5 min, all at room temperature. Afterward, cells were stained with 1:100 anti-BrdU antibody (mouse monoclonal MAB3424; Merck-Millipore) and processed for immunofluorescence.
TUNEL assay and immunofluorescence
For TUNEL staining, the ab66108 in situ Direct DNA Fragmentation (TUNEL) Assay Kit (Abcam; Fig. 1; Fig. 7 B; Fig. S2 E, F, and I; and Fig. S5 M) or the ab66110 TUNEL Assay kit-BrdU-Red (Abcam; Fig S2 K and Fig. S4 I) were used. Cells were fixed on slides with 1% PFA in PBS for 15 min on ice followed by 70% ethanol in PBS for 30 min on ice. Cells were then washed three times with PBS at room temperature, stained with 100 μl per slide of the appropriate DNA labeling solution at 37°C for 1 h following the instructions of the manufacturer and processed for immunofluorescence.
Quantification of fluorescence signals
Fluorescence intensity signals at midbodies or DNA knots were quantified using the LASX polygon tool by analyzing an image area of 2 μm2 around each midbody or DNA knot. Fluorescence intensity values were normalized versus background values obtained by analyzing an identical area within the cell immediately adjacent to the midbody by subtracting the background-signal value from the midbody value (Petsalaki and Zachos, 2016, 2021b). After subtraction of the background, the average values at control or mutant midbodies were calculated and were then all divided with the average value at controls to obtain the relative intensity values plotted (i.e., relative to control = 1).
For weak versus strong DNA staining at the midbody, DNA-fluorescence intensity signals next to the midbodies from 86 spontaneous control bridges were quantified and the mean value was set to 1. DNA bridges exhibiting relative DNA-fluorescence intensity >1 were taken as strong, whereas those exhibiting relative DNA-fluorescence intensity <1 were classified as weak DNA signals. Afterward, the Top2α relative intensity at the midbodies in bridges with strong versus weak DNA signal (i.e., relative to strong = 1) was calculated as described in the previous paragraph.
To measure the length of DNA bridges, the LASX line tool was used. To analyze midbody thickness, the diameter of each microtubule bundle at the midbody was measured using the LASX line tool and the average value was calculated (Petsalaki and Zachos, 2021b).
Actin patch-fluorescence intensity signals were quantified using the LCS Lite polygon tool by analyzing an image area of 40 μm2 around the base of the DNA bridge, and intensity values were normalized versus background values obtained by analyzing an identical area within the cell near the base of the DNA bridge by subtracting the background-signal value from the actin-patch value (Dandoulaki et al., 2018). After background subtraction, the average values from control or mutant actin patches were calculated and were then divided with the control actin patches’ average value to obtain the relative intensity of the values plotted (i.e., relative to control = 1).
Broken bridges
Cells were fixed and stained for immunofluorescence microscopy as appropriate. The percentage of broken chromatin (DAPI), LAP2b:RFP, or Lamin A bridges was calculated as follows: (number of bridges that appear broken/total number of bridges) *100%. The entire bridge was photographed and a bridge was marked “broken” if a clear gap in DAPI, LAP2b:RFP, or Lamin A staining of >2 μm appeared in all image contrasts.
Western blotting
Cells were lysed in ice-cold, whole-cell extract buffer (20 mM Hepes, 5 mM EDTA, 10 mM EGTA, 0.4 M KCl, 0.4% Triton X-100, 10% glycerol, 5 mM NaF, 1 mM DTT, 5 μg/ml leupeptin, 50 μg/ml PMSF, 1 mM benzamidine, 5 μg/ml aprotinin, and 1 mM Na3VO4) for 30 min on ice. Lysates were cleared by centrifugation at 15,000 g for 10 min at 4°C. Samples were then analyzed by SDS-PAGE, transferred onto nitrocellulose membrane (Amersham Protran Premium 0.45 NC, Cat #10600003; GE Healthcare), and incubated with the appropriate antibodies. Secondary antibodies were detected by chemiluminescence (Clarity Western ECL Substrate, Cat #1705061; Biorad) and documented with the Sapphire Biomolecular Imager (Azure Biosystems).
Densitometry
Densitometric analysis of bands was performed using ImageJ (National Institutes of Health).
Statistical analysis and repetitions
For fluorescence intensities at midbodies, a minimum of 10 cells per experiment from two independent experiments were analyzed per treatment (n ≥ 20) and SD was calculated. For the frequency of broken chromatin (DAPI) bridges, LAP2b:RFP, or Lamin A bridges, at least 40 cells with bridges per experiment from three independent experiments were scored blindly and the SD was calculated (n > 120). For FISH (cen or tel positive), at least 20 cells with bridges per experiment from three independent experiments were scored blindly and the SD was calculated (n > 60). For DNA knots frequency, ∼30–35 cells with bridges per experiment from three independent experiments were scored blindly and the SD was calculated (n > 100). For actin-patches fluorescence, 25–35 cells per experiment from two independent experiments were examined (n > 55) and the SD was calculated. For γ-H2AX foci (Fig. S4 J), at least 250 cells per experiment from three independent experiments were scored blindly and the SD was calculated (n > 750). Statistically significant differences among three or more groups were determined by one-way ANOVA followed by two-tailed Students’ t test between two groups. No statistical method was used to predetermine the sample size. Western blots were done twice and representative gels are shown.
Online supplemental material
Fig. S1 shows the localization of FANCD2 and Top2β in BE cells with spontaneous chromatin bridges, the frequency of chromatin bridges exhibiting DNA knots, and the localization of GapR:GFP and Top2α on HU-induced chromatin bridges. Fig. S1 also shows the localization of Top2α using a polyclonal anti-Top2α antibody, localization of KIF4A, and localization of Top2α to the bridge DNA next to the midbody on relatively short versus long chromatin bridges or chromatin bridges with relatively strong versus weak DNA signal. Fig. S2 shows the localization of PICH and TopBP1 in BE cells with spontaneous chromatin bridges, localization of Top2α:DNA covalent complexes, and double-strand DNA breaks in HU-induced chromatin bridges and spontaneous Lamin A bridges. Fig. S2 also shows DNA knots, localization of Top2α:GFP, and double-strand DNA breaks (TUNEL) on intact chromatin bridges in HeLa cells. Fig. S3 shows actin patches in control or Top2α-depleted BE cells, localization of Mre11, ATM, or INCENP, and chromatin bridge breakage after Top2-inhibition by ICRF-193 in cytokinesis-enriched cells. Fig. S3 also shows localization of Mre11, ATM, or Aurora B to the bridge DNA or the midbody ring in cytokinesis with HU-induced chromatin bridges, and the localization of Top2α or Rad17 in normally segregating cells. Fig. S4 shows localization of phosphorylated ATM-S1981 in cytokinesis in normally segregating BE cells and kinetics of midbody disassembly in control cells or cells treated with ICRF-193 by live-cell imaging. Fig. S4 also shows double-strand DNA breaks on DNA knots in intact chromatin bridges in BE cells expressing WT, Y805F, or AEEA GFP:Top2, and the localization of γ-H2AX, MDC1, RFC3, or RFC5 on spontaneous chromatin bridges. Fig. S5 shows localization of RFC4, incorporation of BrdU, and localization of Rad17 to the bridge DNA on chromatin bridges of various lengths in BE cells. Fig. S5 also shows the localization of Mre11 to the bridge DNA, localization of Aurora B to the midbody, and chromatin bridge breakage in Rad17-depleted or control cells exhibiting HU-induced chromatin bridges. Finally, Fig. S5 shows FISH analysis and TUNEL staining of intact chromatin bridges in TC-, HU-, or TC+HU-induced RPE-1 cells. Video 1 shows a control HeLa LAP2b:RFP cell exhibiting a stable LAP2b:RFP bridge in cytokinesis. Video 2 shows a control HeLa LAP2b:RFP cell exhibiting a stable LAP2b-positive intercellular canal in cytokinesis. Video 3 shows a HeLa LAP2b:RFP cell exhibiting breakage of the LAP2b:RFP bridge in the presence of ICRF-193. Video 4 shows a HeLa LAP2b:RFP cell exhibiting breakage of the LAP2b-positive intercellular canal in the presence of ICRF-193. Video 5 shows a control HeLa tubulin:GFP cell exhibiting midbody disassembly in cytokinesis. Video 6 shows a HeLa tubulin:GFP cell exhibiting midbody disassembly in the presence of ICRF-193.
Data availability
All data are available in the published article and its online supplemental material.
Acknowledgments
We thank John Maciejowski for comments on the manuscript. We also thank Felipe Cortés-Ledesma, Yasunori Fukumoto, Daniel Gerlich, Gary Gorbsky, Monica Guo, Shar-yin Naomi Huang, Steve Jackson (University of Cambridge, Cambridge, UK), Titia de Lange, John Maciejowski, Jan-Michael Peters, Yves Pommier, Wesley Sundquist, and Ken-ichi Yano (Kumamoto University, Kumamoto, Japan) for generously sharing reagents.
Work in our laboratory was supported by Fondation Santé. This work was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “2nd Call for H.F.R.I. Research Projects to support Faculty Members and Researchers” (Project Number: 2486). E. Petsalaki and S. Balafouti were supported by the H.F.R.I. under the “2nd Call for H.F.R.I. Research Projects to support Post-Doctoral Researchers” (Project Number: 629).
Author contributions: E. Petsalaki, S. Balafouti, A. Kyriazi, and G. Zachos performed experiments and analyzed the results. G. Zachos and E. Petsalaki designed and supervised the study. G. Zachos wrote the manuscript and all authors made comments on the manuscript.
References
Author notes
Disclosures: The authors declare no competing interests exist.