Clathrin-associated endocytic adapters recruit cargoes to coated pits as a first step in endocytosis. We developed an unbiased quantitative proteomics approach to identify and quantify glycoprotein cargoes for an endocytic adapter, Dab2. Surface levels of integrins β1, α1, α2, and α3 but not α5 or αv chains were specifically increased on Dab2-deficient HeLa cells. Dab2 colocalizes with integrin β1 in coated pits that are dispersed over the cell surface, suggesting that it regulates bulk endocytosis of inactive integrins. Depletion of Dab2 inhibits cell migration and polarized movement of integrin β1 and vinculin to the leading edge. By manipulating intracellular and surface integrin β1 levels, we show that migration speed correlates with the intracellular integrin pool but not the surface level. Together, these results suggest that Dab2 internalizes integrins freely diffusing on the cell surface and that Dab2 regulates migration, perhaps by maintaining an internal pool of integrins that can be recycled to create new adhesions at the leading edge.
Introduction
Endocytosis and exocytosis move membrane proteins between the cell surface and intracellular compartments, allowing cells to adapt to changes in their environment. Endocytosis can be clathrin dependent or independent (Schmid, 1997). Receptors that undergo clathrin-dependent endocytosis contain endocytosis signals that bind to adapter proteins, including the well-characterized tetramer AP2 and monomeric phosphotyrosine-binding domain proteins Dab2, Numb, and autosomal recessive hypercholesterolemia (ARH; Traub, 2003). Multiple low affinity interactions between adapter proteins and clathrin then cooperate to assemble a clathrin-coated pit that invaginates and pinches off to form an intracellular vesicle.
Dab2 is an NPXY sequence–specific clathrin adapter that internalizes the low density lipoprotein receptor (LDLR) and related receptors (Keyel et al., 2006; Maurer and Cooper, 2006). It forms dynamic complexes with its cargoes and recruits clathrin (Morris and Cooper, 2001; Mishra et al., 2002; Keyel et al., 2006; Chetrit et al., 2009). Dab2 expression is strongly reduced in many different carcinomas, particularly ovarian and mammary tumors (Mok et al., 1994; Schwahn and Medina, 1998), and Dab2 loss allows carcinoma cells to resist anoikis (Sheng et al., 2000; Wang et al., 2001). Dab2 has also been reported to regulate the migration of various cell types (Hocevar et al., 2005; Orlandini et al., 2008). It is unclear whether the roles of Dab2 in cancer and migration stem from its function as an endocytic adapter or other mechanisms.
Integrins are cell surface receptors for various ECM components, with different combinations of integrin α and β subunits conferring ECM ligand specificity (Hynes, 1992). Integrins act as bistable switches, toggling between an inactive, unbound state and an active conformation simultaneously able to bind the ECM and the cytoskeleton (Carman and Springer, 2003). Binding to the ECM and cytoskeleton induces the clustering of active integrins into structures known as focal complexes or adhesions from which signals are generated to regulate cellular responses. However, unbound integrins are inactive and diffuse rapidly in the plane of the membrane (Duband et al., 1988).
Cell migration requires active focal adhesion disassembly and integrin recycling to allow new contacts to form near the front of the cell (Webb et al., 2004; Jones et al., 2006). After focal adhesion disassembly, integrins may diffuse or are actively recycled, via intracellular compartments, to sites of new adhesion assembly (Bretscher, 1996; Caswell and Norman, 2006). Intracellular integrin trafficking routes and their regulation are becoming understood (Lawson and Maxfield, 1995; Pierini et al., 2000; Laukaitis et al., 2001; Rappoport and Simon, 2003). Specifically, integrin recycling can occur through “short loop,” returning directly from early endosomes to the nearby cell surface, or “long loop,” passing via a perinuclear recycling compartment and then returning to the cell surface at distant sites, including the leading edge. In cancer and epithelial cells, the long-loop pathway is needed for migration on collagen-coated surfaces and invasion of the collagen matrix (Powelka et al., 2004; Roberts et al., 2004; Li et al., 2005; Jones et al., 2006). However, the molecules that internalize integrins and route them to the appropriate recycling pathway are less clear. Endocytosis of different integrins may be clathrin dependent or independent, depending on the cell type and environment (Altankov and Grinnell, 1993; Memmo and McKeown-Longo, 1998; Upla et al., 2004; Caswell and Norman, 2006). Importantly, dynamin-dependent integrin endocytosis may drive focal adhesion disassembly (Ezratty et al., 2005). However, cells in suspension also internalize integrins, suggesting that mechanisms for bulk turnover of inactive integrins exist (Bretscher, 1989).
In an unbiased screen for Dab2-modulated receptors that may explain the role for Dab2 in cancer and cell migration, we found that depletion of Dab2 slows the endocytosis of several but not all integrins by HeLa cells. Measurements of specific integrins revealed that Dab2 regulates the bulk of constitutive endocytosis of inactive integrin β1 by HeLa cells and human foreskin fibroblasts (HFFs). Dab2 and integrin β1 colocalize in clathrin-coated pits at many sites dispersed over the cell surface, not specifically at adhesion sites, suggesting that Dab2 may trap freely diffusing integrins in coated pits. Dab2-dependent endocytosis maintains the intracellular pool of integrin β1. Dab2 also regulates cell migration depending on its endocytic function. Our data suggest that Dab2-mediated bulk integrin endocytosis is important to maintain an intracellular pool of integrin available for recycling to the front of the cell, thus enabling cell migration.
Results
Surface levels of specific integrins increase when Dab2 is absent
We devised a proteomics approach to identify cargoes whose endocytosis depends on Dab2. We reasoned that the surface levels of receptors are determined by the balance between appearance at the surface, by exocytosis of newly synthesized and recycling receptors, and loss from the surface by shedding or endocytosis for recycling or destruction. Therefore, cargoes for Dab2 may be increased on the surface and decreased on intracellular membranes of Dab2-deficient cells.
We used SILAC (stable isotope labeling with amino acids in cell culture) labeling for quantification and identified cell surface–exposed proteins using the recently developed cell surface capture (CSC) technology (Fig. 1 A; Ong et al., 2002; Wollscheid et al., 2009). Dab2-deficient HeLa cells were generated by use of a retroviral vector carrying Dab2-specific short hairpin RNA (shRNA). A control HeLa cell line was generated in parallel using control shRNA (Fig. 1 B). Dab2-deficient cells were grown in SILAC media containing [13C6,15N4]l-arginine and [13C6]l-lysine, and control cells were grown in normal media (Ong et al., 2002). After five to six generations, the cells were harvested and mixed, and their surface glycoproteins were tagged with biocytin hydrazide (Wollscheid et al., 2009). Subsequently, the cells were lysed, and a microsomal pellet was prepared. Microsomal proteins were trypsinized, and tagged glycopeptides were affinity purified with streptavidin beads. N-glycosylated peptides were specifically eluted by using protein N-glycosidase F. Eluted N-glycosites (formerly N-glycosylated peptides) were identified and quantified using mass spectrometry–based proteomics. Of the ∼100–150 surface glycoproteins detected in two independent experiments, 57 were identified, and 41 were reliably quantified in both experiments (Tables S1 and S2). 13 glycoproteins were reproducibly increased on the surface of Dab2-deficient cells (i.e., their light/heavy isotope ratios decreased in both experiments). 5 of these 13 glycoproteins were integrins (Fig. 1 C). Surface levels of integrins β1, α1, α2, and α3 increased the most, and integrin α5 increased slightly. Integrin αv was not changed (Table S2). Because an effect of Dab2 on integrins may partly explain the down-regulation of Dab2 in cancer, we investigated the effect of Dab2 on integrins in detail.
Identification of Dab2 cargoes by CSC proteomics. (A) Outline of rationale for the CSC approach. Black dots represent biotin tags. (B) Western blot analysis shows that Dab2 levels are greatly decreased in a Dab2-deficient HeLa cell line. (C) Relative surface abundance changes of glycoproteins measured by SILAC-based CSC proteomics. Mean and SD of SILAC ratios (control/Dab2 deficient) for six integrins (pink squares) and mean SILAC ratios for 35 other proteins (blue diamonds) that were quantified in two experiments are shown. Glycoproteins increased in both experiments are in the lower left quadrant (yellow). con, control; LC, liquid chromatography; MS, mass spectrometry; PNGaseF, protein N-glycosidase F.
Identification of Dab2 cargoes by CSC proteomics. (A) Outline of rationale for the CSC approach. Black dots represent biotin tags. (B) Western blot analysis shows that Dab2 levels are greatly decreased in a Dab2-deficient HeLa cell line. (C) Relative surface abundance changes of glycoproteins measured by SILAC-based CSC proteomics. Mean and SD of SILAC ratios (control/Dab2 deficient) for six integrins (pink squares) and mean SILAC ratios for 35 other proteins (blue diamonds) that were quantified in two experiments are shown. Glycoproteins increased in both experiments are in the lower left quadrant (yellow). con, control; LC, liquid chromatography; MS, mass spectrometry; PNGaseF, protein N-glycosidase F.
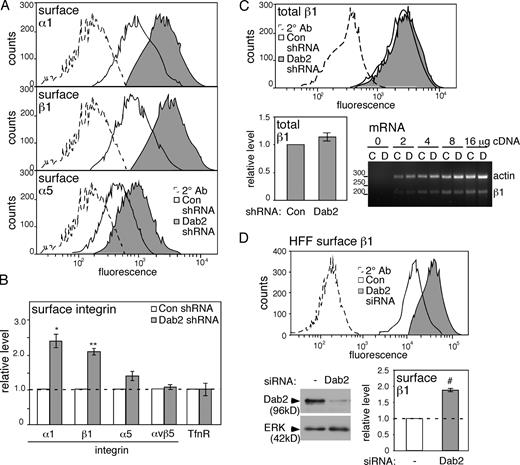
We measured surface integrin levels by staining fixed, nonpermeabilized Dab2-deficient and control cells with anti-integrin antibodies and fluorescent secondary antibodies, followed by FACS. HeLa cells express integrins α1β1, α3β1, α2β1, α5β1 (in approximate order of decreasing abundance), and αvβ5 (Riikonen et al., 1995). Consistent with the CSC proteomic results, removal of Dab2 caused ∼1.7–2.5-fold increases in surface levels of integrins β1 and α1, a small increase in α5, and no change in αvβ5 (Fig. 2, A and B). Because transferrin (Tfn) receptor (TfnR) endocytosis does not require Dab2 (Maurer and Cooper, 2006), we measured TfnR steady-state surface levels as a control. As expected, TfnR surface levels were unaltered by Dab2 depletion (Fig. 2 B). The observed effects were specific because surface integrin β1 was also increased when Dab2 was transiently depleted with siRNAs targeting different sequences in Dab2 (Fig. S1 A), and integrin levels were rescued by reexpressing Dab2 (see Fig. 6 C).
Steady-state surface levels of integrins α1 and β1 but not αv increase in Dab2-deficient cells. (A) Fixed, nonpermeabilized control and Dab2-deficient HeLa cells were analyzed by FACS. (B) Mean and standard error of fluorescent intensity from four independent experiments are shown. (C) FACS analysis of fixed, permeabilized HeLa cells shows that total integrin β1 only increases slightly on Dab2 removal. RT-PCR for integrin β1 and actin mRNA shows equal mRNA levels in control and Dab2-deficient cells. Mean and standard error from five independent experiments are shown. (D) HFFs treated with control or Dab2 siRNA. Western blot showing decreased Dab2 protein and FACS results showing increased surface integrin β1 (mean and standard error of two independent experiments). Data for total integrin β1 level in Dab2-deficient HFFs are shown in Fig. S1 B. (B and D) Dashed lines indicate the control levels. #, P < 0.05; *, P < 0.01; and **, P < 0.001 by t test. 2° Ab, secondary antibody control; con, control; ERK, extracellular signal-regulated kinase.
Steady-state surface levels of integrins α1 and β1 but not αv increase in Dab2-deficient cells. (A) Fixed, nonpermeabilized control and Dab2-deficient HeLa cells were analyzed by FACS. (B) Mean and standard error of fluorescent intensity from four independent experiments are shown. (C) FACS analysis of fixed, permeabilized HeLa cells shows that total integrin β1 only increases slightly on Dab2 removal. RT-PCR for integrin β1 and actin mRNA shows equal mRNA levels in control and Dab2-deficient cells. Mean and standard error from five independent experiments are shown. (D) HFFs treated with control or Dab2 siRNA. Western blot showing decreased Dab2 protein and FACS results showing increased surface integrin β1 (mean and standard error of two independent experiments). Data for total integrin β1 level in Dab2-deficient HFFs are shown in Fig. S1 B. (B and D) Dashed lines indicate the control levels. #, P < 0.05; *, P < 0.01; and **, P < 0.001 by t test. 2° Ab, secondary antibody control; con, control; ERK, extracellular signal-regulated kinase.
To determine whether the increase in steady-state surface integrin levels resulted from a change in overall abundance, cells were permeabilized before staining with integrin β1 antibody and analyzed by flow cytometry. Total integrin β1 levels were only slightly increased by Dab2 removal (Fig. 2 C). Moreover, integrin mRNA levels, measured by RT-PCR, were the same in control and Dab2-deficient cells (Fig. 2 C). These results suggest that most of the increase in surface integrin β1 is caused by altered traffic between the surface and intracellular pools. The effect of Dab2 β1 traffic was not restricted to HeLa cells because transient knockdown of Dab2 in HFFs also increased the surface but not total level (Fig. 2 D and Fig. S1 B).
To test whether other endocytic proteins regulate integrin surface levels, we compared the effects of removing Dab2, clathrin, AP2, ARH, or Numb. Numb was recently reported to regulate integrin endocytosis in HeLa cells (Nishimura and Kaibuchi, 2007). Removal of Dab2, AP2, or clathrin but not ARH increased the surface levels of integrins β1 and α1 to a similar extent (Fig. 3 A). Combined removal of Dab2 and AP2 caused little further increase in integrin β1 or α1, suggesting that Dab2 requires AP2 (and/or vice versa) to regulate integrin levels. Integrin α5 levels depended more on AP2 or clathrin than Dab2 (Fig. 3 A). In mirror image to Dab2, Numb removal had a greater effect on integrin α5 than integrin β1 or α1 (Fig. 3 B). These results suggest that clathrin and AP2 regulate β1, α1, and α5, but Dab2 and Numb regulate partly overlapping subsets with Dab2 dominant for β1 and α1 and Numb dominant for α5. Combined removal of Dab2 and Numb had a less than additive effect on surface levels of integrin β1 (Fig. 3 C), suggesting either that they are partially redundant or that maximum integrin levels have been reached. Neither Dab2 nor Numb affected TfnR levels.
Effect of ARH, AP2, clathrin, or Numb depletion on surface integrin levels. (A–C) HeLa cells were treated with various siRNAs and then fixed and analyzed by FACS with anti-integrin or anti-TfnR antibody. Mean values and standard errors from at least three independent experiments are shown. Dashed lines indicate the control levels. (A) ARH has no effect; Dab2 depletion increases surface α1 and β1; AP2 or clathrin depletion significantly increases surface α1, α5, and β1. (B) Numb depletion has a bigger effect on surface α5 than on α1 or β1. In contrast, Dab2 depletion has a bigger effect on surface α1 and β1 than on α5. The Dab2 data are the same as those shown in Fig. 2 B. (C) Effects of separate or combined removal of Dab2 and Numb. Combined removal of Dab2 and Numb caused little further increase in integrin β1. (A and C) Western blot analysis of HeLa total lysates demonstrates that target proteins ARH, Dab2, AP2, clathrin heavy chain (CHC), and Numb were greatly reduced by siRNA transfection. As a control, extracellular signal-regulated kinase (ERK) levels remained constant. (B and C) #, P < 0.05; *, P < 0.01; and **, P < 0.001 by t test.
Effect of ARH, AP2, clathrin, or Numb depletion on surface integrin levels. (A–C) HeLa cells were treated with various siRNAs and then fixed and analyzed by FACS with anti-integrin or anti-TfnR antibody. Mean values and standard errors from at least three independent experiments are shown. Dashed lines indicate the control levels. (A) ARH has no effect; Dab2 depletion increases surface α1 and β1; AP2 or clathrin depletion significantly increases surface α1, α5, and β1. (B) Numb depletion has a bigger effect on surface α5 than on α1 or β1. In contrast, Dab2 depletion has a bigger effect on surface α1 and β1 than on α5. The Dab2 data are the same as those shown in Fig. 2 B. (C) Effects of separate or combined removal of Dab2 and Numb. Combined removal of Dab2 and Numb caused little further increase in integrin β1. (A and C) Western blot analysis of HeLa total lysates demonstrates that target proteins ARH, Dab2, AP2, clathrin heavy chain (CHC), and Numb were greatly reduced by siRNA transfection. As a control, extracellular signal-regulated kinase (ERK) levels remained constant. (B and C) #, P < 0.05; *, P < 0.01; and **, P < 0.001 by t test.
Dab2 regulates integrin traffic
Control or Dab2-deficient HeLa cells were grown on collagen-coated coverslips, incubated with P5D2 anti–integrin β1 antibody at 4°C, and washed, and surface integrin β1 was detected with fluorescent secondary antibody. As predicted by CSC and FACS, Dab2-deficient HeLa cells bound ∼70–90% more P5D2 than control cells (Fig. 4 A, time 0; and Fig. S1 C). Most integrin β1 was spread over the cell surface as small puncta, as noted previously (Powelka et al., 2004). Focal adhesions were not detected, which is consistent with the ability of P5D2 to bind to integrin β1 and block adhesion (Dittel et al., 1993). Similar results were obtained with noninhibitory anti–integrin α1 antibody TS2/7 (Fig. S2 A). Parallel coverslips that had been incubated with P5D2 or integrin α1 antibody were warmed to 37°C and allowed to recycle antibody for various times. By 30 min (Fig. 4) or 2 h (Fig. S2 A), the amount of integrin β1 or α1 antibody on the surface of control cells had decreased by ∼50–55%, whereas that on the surface of Dab2-deficient cells had decreased by ∼15–20%, which is consistent with either decreased endocytosis or increased exocytosis in the absence of Dab2.
Dab2 regulates integrin β1 endocytosis. (A–C) Control (Con) and Dab2-deficient HeLa cells were incubated with anti–integrin β1 antibody (P5D2) for 30 min at 4°C, followed by warming at 37°C for 0, 15, or 30 min. Mean values and standard errors (∼20 cells/treatment) of pixel intensities were measured. (A) Surface antibody on fixed, nonpermeabilized cells was detected with fluorescent secondary antibody. 6-µm flattened z projections of the entire cell are shown. (B) Surface antibody was removed by acid stripping, and internalized antibody was detected. 6-µm flattened z projections of the entire cell are shown. (C) Internalized antibody colocalizes with EEA1 at 15 min and with Tfn at 30 min. Dab2-deficient cells internalize less integrin β1 antibody but the same amount of Tfn compared with control cells. (D–G) Control and Dab2-deficient HeLa cells were surface labeled with sulpho-NHS-SS-biotin at 4°C for 30 min and then warmed to 37°C in the absence (D and E) or presence (F and G) of 2 mM primaquine for the indicated times. Biotin was removed from surface receptors with MesNa treatment, and cells were lysed and incubated with an anti–integrin β1 antibody. Immunoprecipitates were analyzed by SDS-PAGE followed by Western blotting with peroxidase-conjugated streptavidin. (E and G) Quantification of three independent experiments. For each time point, mean values and standard errors are shown.
Dab2 regulates integrin β1 endocytosis. (A–C) Control (Con) and Dab2-deficient HeLa cells were incubated with anti–integrin β1 antibody (P5D2) for 30 min at 4°C, followed by warming at 37°C for 0, 15, or 30 min. Mean values and standard errors (∼20 cells/treatment) of pixel intensities were measured. (A) Surface antibody on fixed, nonpermeabilized cells was detected with fluorescent secondary antibody. 6-µm flattened z projections of the entire cell are shown. (B) Surface antibody was removed by acid stripping, and internalized antibody was detected. 6-µm flattened z projections of the entire cell are shown. (C) Internalized antibody colocalizes with EEA1 at 15 min and with Tfn at 30 min. Dab2-deficient cells internalize less integrin β1 antibody but the same amount of Tfn compared with control cells. (D–G) Control and Dab2-deficient HeLa cells were surface labeled with sulpho-NHS-SS-biotin at 4°C for 30 min and then warmed to 37°C in the absence (D and E) or presence (F and G) of 2 mM primaquine for the indicated times. Biotin was removed from surface receptors with MesNa treatment, and cells were lysed and incubated with an anti–integrin β1 antibody. Immunoprecipitates were analyzed by SDS-PAGE followed by Western blotting with peroxidase-conjugated streptavidin. (E and G) Quantification of three independent experiments. For each time point, mean values and standard errors are shown.
To examine the intracellular localization of integrin β1, cells were allowed to internalize P5D2 antibody for various times, cooled on ice, treated with low pH and high salt to remove surface antibody, fixed, permeabilized, and visualized with fluorescent secondary antibody (Fig. 4 B). In control HeLa cells, integrin β1 was initially detected in a dispersed population of tiny vesicles (Fig. 4 B, 15 min). It later moved into larger, perinuclear vesicles (Fig. 4 B, 30 min). As expected, only ∼35% as much P5D2 was detected inside Dab2-deficient cells than control cells, but both types of vesicles were detected. In both cell types, the dispersed tiny vesicles also stained with EEA1 (early endosome antigen 1), a marker of early endosomes (Fig. 4 C). To test whether the larger perinuclear vesicles may be recycling endosomes, we followed the traffic of Tfn (Powelka et al., 2004). Integrin β1 followed the same path as Tfn in both Dab2-deficient and control cells from dispersed tiny vesicles to larger perinuclear vesicles (Fig. 4 C). As expected from the lack of an effect of Dab2 on surface TfnR levels (Fig. 2 B), the quantity of internalized Tfn did not depend on Dab2 (Fig. 4 C). These results suggest that both control and Dab2-deficient cells internalize integrin β1 into early endosomes and then pass it into perinuclear recycling endosomes, but endocytosis is impaired in Dab2-deficient cells.
To monitor integrin β1 traffic more quantitatively, we chemically labeled surface proteins with a nonpermeable, reversible biotinylation reagent, sulpho-NHS-SS-biotin. After labeling and removal of excess reagent, we warmed the cells for various times before cooling again and stripping off surface biotin with a nonpermeable reducing agent (MesNa). The cells were then lysed, integrin β1 was immunoprecipitated, and the biotin label was detected by Western blotting (Fig. 4 D). Integrin β1 entered control HeLa cells at ∼1% min−1. This is slower than for serum-stimulated HeLa cells (Powelka et al., 2004), suggesting that we are measuring basal endocytosis. Internalization of integrin β1 was strongly inhibited in Dab2-deficient cells (Fig. 4, D and E). Even when reexport was inhibited using primaquine (Reid and Watts, 1990), control cells internalized integrin β1 more rapidly than Dab2-deficient cells (Fig. 4, F and G). These results show that integrin β1 endocytosis is inhibited when Dab2 is absent.
As an independent test of whether Dab2 regulates integrin traffic, we followed uptake of an integrin α1β1 ligand, collagen IV (Hynes, 1992). Cells were plated on fluorescent collagen IV for 2 h at 37°C and fixed, and collagen was visualized. Dab2-deficient cells internalized less collagen and removed less collagen from the surface than control cells (Fig. S2 B). These results suggest that Dab2 is needed for endocytosis of integrin–collagen complexes.
Dab2 colocalizes with integrin β1
Dab2 localizes to AP2-containing clathrin-coated pits distributed over the cell surface (Morris and Cooper, 2001). However, very little integrin β1 colocalized with Dab2 in these structures (unpublished data). Because coated pits only have a brief half-life (1–3 min; Puthenveedu and von Zastrow, 2006; Loerke et al., 2009) and only ∼1% of integrin β1 is internalized per minute, only a tiny fraction of the surface integrin may be in coated pits at any given time. To trap integrins in coated pits, we cooled cells to 4°C (Sorkin, 2004). Under these conditions, inactive integrin β1 labeled with antibody P5D2 extensively colocalized with Dab2 and clathrin (Fig. 5, A and B). Surprisingly, the Dab2-containing structures were not located close to focal adhesions, as visualized by staining fixed, permeabilized cells with antibodies to vinculin (Fig. 5 C). Rather, Dab2 colocalized with inactive integrin β1 at distant sites, predominantly on the dorsal surface of the cell (Fig. 5 A and Fig. S3 A). Thus, Dab2 is localized appropriately to mediate endocytosis of free but not engaged integrins.
Localization of integrin β1 and Dab2. (A) Confluent HeLa cells plated on collagen IV–coated coverslips were treated with anti–integrin β1 antibody for 1 h at 4°C and then fixed, permeabilized, and stained with anti-Dab2 or anticlathrin antibody. Single 0.2-µm sections at the ventral or dorsal surface are shown. (B) The number of Dab2 puncta that colocalize with integrin β1 (arrowheads in A) at the ventral and dorsal surfaces were counted. Mean values and standard errors (two independent experiments and ∼12 cells/treatment/experiment) are shown. (C) Confluent HeLa cells were fixed, permeabilized, and stained with anti-Dab2 antibodies. Antivinculin antibodies were used to visualize focal adhesions (arrowhead). Single 0.2-µm sections at the ventral surfaces of cells are shown. (A and C) The white boxes indicate the enlarged images shown in the insets.
Localization of integrin β1 and Dab2. (A) Confluent HeLa cells plated on collagen IV–coated coverslips were treated with anti–integrin β1 antibody for 1 h at 4°C and then fixed, permeabilized, and stained with anti-Dab2 or anticlathrin antibody. Single 0.2-µm sections at the ventral or dorsal surface are shown. (B) The number of Dab2 puncta that colocalize with integrin β1 (arrowheads in A) at the ventral and dorsal surfaces were counted. Mean values and standard errors (two independent experiments and ∼12 cells/treatment/experiment) are shown. (C) Confluent HeLa cells were fixed, permeabilized, and stained with anti-Dab2 antibodies. Antivinculin antibodies were used to visualize focal adhesions (arrowhead). Single 0.2-µm sections at the ventral surfaces of cells are shown. (A and C) The white boxes indicate the enlarged images shown in the insets.
In contrast to Dab2, Numb localizes to clathrin-coated structures that are near the leading edge of migrating cells (Nishimura and Kaibuchi, 2007) and the periphery of nonmigrating cells (Fig. S3 B). The different distributions of Numb- and Dab2-containing clathrin-coated pits are consistent with their partial redundancy for integrin endocytosis (Fig. 3, B and C).
Dab2-mediated endocytosis regulates cell migration
Because integrin recycling is needed for directed cell movement, we tested whether Dab2 regulates cell migration. Previous studies have shown that depleting Dab2 slows migration of human umbilical vein endothelial cells and NIH 3T3 cells, but the mechanism was not clear (Hocevar et al., 2005; Orlandini et al., 2008). Removing Dab2 inhibited migration on collagen IV, a ligand for the Dab2-regulated integrin α1β1 (Fig. 6 A). However, Dab2 had no effect on migration on vitronectin (Fig. 6 A), a ligand for the Dab2-independent integrin αvβ5 (Figs. 1 C and 2 B). This suggests that Dab2 may regulate migration by affecting integrin traffic.
Dab2-mediated migration requires the p96-specific exon. (A and D) Migration through filters coated with collagen IV (A and D) or vitronectin (A) toward 10% FBS was measured using a Boyden chamber. Cells passing through triplicate membranes were counted and averaged. The collagen IV results in A show mean and standard error of four independent experiments. *, P < 0.01. (B) Drawing of p96 and p67 forms of Dab2 showing known binding sites. (C and D) Surface integrin β1 levels (C) and migration (D) for Dab2-deficient HeLa cells reexpressing vector alone or T7-tagged mouse p96 or p67. Mean values and standard errors from two independent experiments are shown. Dashed lines indicate the control levels. (E) Western blots show Dab2 knockdown and reexpression of T7-tagged mouse p96 or p67. Con, control.
Dab2-mediated migration requires the p96-specific exon. (A and D) Migration through filters coated with collagen IV (A and D) or vitronectin (A) toward 10% FBS was measured using a Boyden chamber. Cells passing through triplicate membranes were counted and averaged. The collagen IV results in A show mean and standard error of four independent experiments. *, P < 0.01. (B) Drawing of p96 and p67 forms of Dab2 showing known binding sites. (C and D) Surface integrin β1 levels (C) and migration (D) for Dab2-deficient HeLa cells reexpressing vector alone or T7-tagged mouse p96 or p67. Mean values and standard errors from two independent experiments are shown. Dashed lines indicate the control levels. (E) Western blots show Dab2 knockdown and reexpression of T7-tagged mouse p96 or p67. Con, control.
Removal of Numb also inhibited migration, although to a lesser extent than removing Dab2 (Fig. S4). Removing both Dab2 and Numb caused a greater inhibition than removing either alone. These results showed an inverse correlation between cell surface integrin β1 level and migration speed (r = −0.988; Fig. S4), again suggesting that inhibiting integrin endocytosis slows migration.
If Dab2 stimulates migration by affecting endocytosis, a form of Dab2 that does not support endocytosis should not support migration. The p67 splice form of Dab2 lacks two clathrin-binding sites and one of two AP2-binding sites present in the other form, p96, and unlike p96, it does not associate with coated pits and is defective for LDLR endocytosis (Morris and Cooper, 2001; Mishra et al., 2002; Maurer and Cooper, 2006). T7 epitope–tagged Dab2 p96 or p67 was transiently expressed in Dab2-deficient HeLa cells. Reexpression of Dab2 p96 but not p67 rescued normal integrin levels and migration (Fig. 6, C and D). This suggests that Dab2 regulates migration via its effect on endocytosis. We reasoned that Dab2 may regulate migration by changing surface integrin levels, integrin signaling, or integrin recycling.
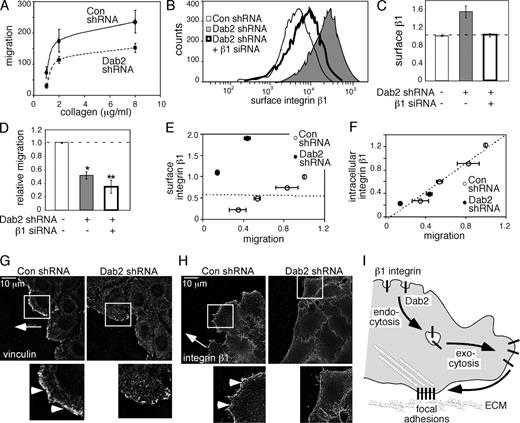
Cell-substrate adhesion affects migration in opposing ways: adhesion must be strong enough to provide traction but not so strong as to be an anchor (Palecek et al., 1997). We used two approaches to alter the adhesive forces of Dab2-deficient cells. First, we varied adhesion by use of different collagen concentrations. Decreasing or increasing collagen concentration decreased or increased the migration rate of both control and Dab2-deficient cells, but Dab2-deficient cells always migrated more slowly than the controls (Fig. 7 A). If lack of Dab2 had slowed cell migration by causing excess adhesion, we would have expected that their migration would increase on lower concentrations of collagen, but this was not observed. Second, we lowered total levels of integrin β1 in Dab2-deficient cells such that their surface integrin β1 levels were the same as those on control cells (Fig. 7, B and C). However, Dab2-deficient cells with normalized surface β1 integrin migrated even slower than Dab2-deficient cells with high surface β1 integrin (Fig. 7 D). In addition, lowering total integrin levels in control cells reduced their migration. Combining results from several experiments in which total integrin β1 levels were reduced by siRNA in either control or Dab2-deficient cells, we found no correlation between surface integrin levels and migration (r = −0.055; Fig. 7 E). Instead, there was an excellent correlation between migration rate and internal integrin levels (r = 0.977; Fig. 7 F). These results mean that the slow migration of Dab2-deficient cells is unlikely to be caused by excess adhesion and more likely caused by the altered intracellular pool.
Dab2-mediated endocytosis regulates cell migration. (A and D) Migration through filters coated with collagen IV toward 10% FBS was measured using a Boyden chamber. *, P < 0.01; **, P < 0.001. (A) Quantification of two independent experiments performed in triplicate. For each point, mean values and range are shown. (B and C) Integrin β1 levels were reduced in Dab2-deficient cells using β1-specific siRNA. The mean fluorescence intensity of surface integrin β1 was measured by flow cytometry. (B) FACS histogram from a representative experiment. (C) Means and standard errors from four independent experiments are shown. (D) Migration of control and Dab2-deficient cells with high and normalized β1 surface levels. Mean and standard error from four independent experiments are shown. (C and D) Dashed lines indicate the control levels. (E and F) Correlation between migration and surface integrin level (r = −0.055; E) or intracellular integrin pool (r = 0.977; F). Each point shows the mean and SD of triplicate migration measurements and a single surface integrin measurement on the same cells, normalized to a simultaneous control (control HeLa, no β1 siRNA migration = 1.0). Surface integrin β1 levels were measured by FACS. Intracellular integrin β1 levels were calculated from the measured surface level and the steady-state ratio of the intracellular and surface levels (Fig. 2 and Fig. S2 A). Control HeLa cells: surface β1 = 45%, intracellular β1 = 55%, intracellular/surface = 1.22; Dab2 shRNA cells: surface β1 = 83%, intracellular β1 = 17%, intracellular/surface = 0.20. Dashed lines are best fit to the data. (G and H) Scratch wound assay. Cells were stained with vinculin (G) or integrin β1 (H). Representative single 0.2-µm sections at the ventral surface of leading edge cells from one of two independent experiments are shown. The arrow indicates the direction of migration, and the white boxes indicate the enlarged images shown in the insets. Vinculin and integrin β1 (arrowheads) concentrated at the leading edge of control but not Dab2-deficient cells. (I) Model for the role of Dab2 in integrin endocytosis and cell migration. Dab2 mediates endocytosis of β1 integrins from coated pits scattered randomly over the cell surface and maintains an internal pool of integrins. This pool is important for efficient migration because it supplies integrins for recycling to the leading edge and thus allows the polarized formation of new focal complexes and focal adhesions. Con, control.
Dab2-mediated endocytosis regulates cell migration. (A and D) Migration through filters coated with collagen IV toward 10% FBS was measured using a Boyden chamber. *, P < 0.01; **, P < 0.001. (A) Quantification of two independent experiments performed in triplicate. For each point, mean values and range are shown. (B and C) Integrin β1 levels were reduced in Dab2-deficient cells using β1-specific siRNA. The mean fluorescence intensity of surface integrin β1 was measured by flow cytometry. (B) FACS histogram from a representative experiment. (C) Means and standard errors from four independent experiments are shown. (D) Migration of control and Dab2-deficient cells with high and normalized β1 surface levels. Mean and standard error from four independent experiments are shown. (C and D) Dashed lines indicate the control levels. (E and F) Correlation between migration and surface integrin level (r = −0.055; E) or intracellular integrin pool (r = 0.977; F). Each point shows the mean and SD of triplicate migration measurements and a single surface integrin measurement on the same cells, normalized to a simultaneous control (control HeLa, no β1 siRNA migration = 1.0). Surface integrin β1 levels were measured by FACS. Intracellular integrin β1 levels were calculated from the measured surface level and the steady-state ratio of the intracellular and surface levels (Fig. 2 and Fig. S2 A). Control HeLa cells: surface β1 = 45%, intracellular β1 = 55%, intracellular/surface = 1.22; Dab2 shRNA cells: surface β1 = 83%, intracellular β1 = 17%, intracellular/surface = 0.20. Dashed lines are best fit to the data. (G and H) Scratch wound assay. Cells were stained with vinculin (G) or integrin β1 (H). Representative single 0.2-µm sections at the ventral surface of leading edge cells from one of two independent experiments are shown. The arrow indicates the direction of migration, and the white boxes indicate the enlarged images shown in the insets. Vinculin and integrin β1 (arrowheads) concentrated at the leading edge of control but not Dab2-deficient cells. (I) Model for the role of Dab2 in integrin endocytosis and cell migration. Dab2 mediates endocytosis of β1 integrins from coated pits scattered randomly over the cell surface and maintains an internal pool of integrins. This pool is important for efficient migration because it supplies integrins for recycling to the leading edge and thus allows the polarized formation of new focal complexes and focal adhesions. Con, control.
Dab2 is reported to have signaling functions (Xu et al., 1998; Wang et al., 2001; Zhou et al., 2003). Dab2 absence may alter signaling from focal adhesions and thus affect focal adhesion assembly or disassembly. We measured the activities of Src and FAK using phosphoepitope antibodies. If anything, the activities of Src and FAK were increased in Dab2-deficient HeLa cells plated for 45 min on collagen IV (Fig. S5). This suggests that Dab2 is not required for focal adhesion signaling.
If Dab2-mediated integrin internalization is important for trafficking integrins to the leading edge, we predicted that Dab2-depleted migrating cells may fail to polarize their integrins toward the leading edge. We examined control and Dab2-deficient cells migrating into a scratch wound (Fig. 7, G and H). We found that migrating control cells are asymmetric, with an increased concentration of integrin β1 and the focal complex protein vinculin along the leading edge. However, Dab2-deficient cells are isotropic, with points of vinculin and integrin β1 scattered around the entire periphery. These results are consistent with a failure of polarized routing of integrin β1 to the leading edge, perhaps caused by reduced internalization.
Discussion
Discovery-driven quantitative analysis of cell surface glycoproteins and antibody analysis showed that Dab2 regulates the surface levels and endocytosis of specific integrins. In HeLa cells, Dab2 has the greatest effects on the surface levels of integrins β1, α1, α2, and α3 but not α5 or αv. In the case of integrin β1, the absence of Dab2 increases the surface level and reduces the intracellular pool, with no change in total level. The shift is caused by a decreased rate of endocytosis. Dab2 colocalizes with inactive integrin β1 at clathrin-coated pits that are evenly scattered over the cell surface, so Dab2 likely mediates endocytosis of freely diffusing integrin β1 that is not engaged with the actin cytoskeleton or the ECM. After endocytosis, integrin β1 is recycled via the perinuclear recycling compartment. In the absence of Dab2, integrin β1 and vinculin are not polarized to the leading edge, and cell migration is inhibited. Therefore, we propose that Dab2 promotes migration by maintaining an internal pool of integrin β1 that can be recycled to the surface to create new adhesion sites (Fig. 7 I).
Dab2 as an endocytic adapter for integrins
Integrins β1, α1, and α5 were up-regulated on the surface of cells lacking clathrin or AP2, but only integrins β1 and α1 were up-regulated when Dab2 was absent (Figs. 1 and 2 and Fig. S1 A). This suggests that Dab2 is selective. Because ARH and Numb are structurally similar to Dab2, we tested whether these endocytic adapters regulate integrin surface abundance. ARH had no detectable role, but Numb had a major influence on integrin α5 and small effects on integrins β1 and α1 (Fig. 3). This suggests that Dab2 and Numb may have partially separate but overlapping roles. Numb was previously shown to mediate endocytosis of a subpopulation of surface integrins β1 and β3 (Nishimura and Kaibuchi, 2007). Both Dab2 and Numb regulate cell migration (Fig. S4; Nishimura and Kaibuchi, 2007). Numb localizes near the front, ventral surface of migrating cells (Nishimura and Kaibuchi, 2007) and around the edge of stationary cells (Fig. S3 B), whereas Dab2 is found in most coated pits scattered over the cell surface (Fig. 5 A). This suggests that Numb may regulate the uptake of integrins β1 and α5 near the leading edge and that Dab2 may internalize integrins β1, α1, α2, and α3 elsewhere on the cell surface. The partial overlap between Dab2 and Numb in integrin traffic resembles the parallel effects of Dab2 and ARH in LDLR endocytosis (Keyel et al., 2006; Maurer and Cooper, 2006) and may explain why dab2 and numb mouse mutants do not show more pleiotropic phenotypes (Zhong et al., 2000; Morris et al., 2002; Maurer and Cooper, 2005).
How are integrins selected for endocytosis? Integrin β1 becomes trapped in Dab2-containing coated pits at low temperature (Fig. 5 A), suggesting a physical interaction. Integrin β chains contain one or more NPXY sequences related to the sequences in lipoprotein receptors that bind the Dab2 phosphotyrosine-binding domain (Morris and Cooper, 2001; Mishra et al., 2002). Indeed, Dab2 and integrin β1 have been coimmunoprecipitated from untreated or TGF-β–treated murine mammary epithelial cells (Prunier and Howe, 2005), suggesting direct binding. However, we and others (Chetrit et al., 2009) have been unable to coimmunoprecipitate Dab2 with integrin β1 from HeLa cells. Moreover, although Dab2 binds well to integrins β3 and β5 in vitro, it binds poorly to β1 (Calderwood et al., 2003). The importance of the NPXY sequence in integrin β chains for endocytosis is controversial. A conservative NPXF mutant integrin β1 appears to function and traffic normally in keratinocytes but not fibroblasts (Czuchra et al., 2006; Pellinen et al., 2008), whereas nonconservative mutations of the NPXY signals in αLβ2 and α5β1 have no effects on their endocytosis (Vignoud et al., 1994; Fabbri et al., 1999). Importantly, Bretscher (1992) noted that different integrin β1 dimers had different endocytic rates depending on their α chains. Therefore, it is possible that the integrin α chains rather than the β chains may be recognized for endocytosis. Indeed, the differential endocytosis of α chains by Dab2 and Numb implies that adapters may distinguish different αβ complexes based on α cytoplasmic domains. An alternative explanation, that Dab2 selects integrins for endocytosis only when they are disengaged from the cytoskeleton, is discussed next.
Focal adhesion disassembly and integrin endocytosis
Our evidence suggests that Dab2 mediates endocytosis of integrins that are not attached to the cytoskeleton. Altered integrin β1 levels and endocytosis were detected with an antibody that binds the inactive conformation (Dittel et al., 1993) and does not access focal adhesions (Fig. 4). Moreover, we detected integrin β1 in Dab2-containing pits even on the top of the cell (Fig. 5 A). In addition, Dab2 had a greater effect on surface levels of integrin α1β1 when cells were plated on plastic or fibronectin than collagen, the ligand for α1β1 (unpublished data). Integrins diffuse rapidly over the cell surface if they are not attached to the cytoskeleton (Duband et al., 1988). Dab2 can mediate uptake of ECM ligands (Fig. S2 B). Together, these results imply that Dab2 internalizes integrins that are disengaged from the cytoskeleton whether or not they are bound to ECM proteins.
A role for Dab2 in endocytosis of disengaged integrins is consistent with the possible binding of Dab2 to the NPXY sequences of integrin β chains. These sequences are known to be critical for binding the focal adhesion protein talin and thereby creating cytoskeletal attachments (Tadokoro et al., 2003). Talin release would be needed to expose the NPXY before Dab2 could bind. This would explain a selectivity of Dab2 for disengaged integrins.
In addition to this mechanism, it is clear that integrin endocytosis also occurs at sites of focal adhesion disassembly. In highly migratory cells, or in cells in which microtubules are disrupted and then allowed to repolymerize in synchrony, focal complexes are targeted by microtubules, which bring in the endocytic protein dynamin and stimulate focal adhesion disassembly (Kaverina et al., 1999; Ezratty et al., 2005). Recent evidence suggests that Dab2 may also be involved, targeting focal adhesions that are disassembling at the leading edge of migrating HT1080 cells (Chao and Kunz, 2009). However, in HeLa cells, there appears to be a division of labor: Numb is localized near focal adhesions at the periphery and leading edge (Nishimura and Kaibuchi, 2007), and it may be involved in the synchronized endocytosis of integrins from disassembling adhesion sites, whereas Dab2 mediates bulk endocytosis of disengaged integrins.
Regulation of migration
We and others have found that Dab2 removal inhibits migration of several types of cells (Hocevar et al., 2005; Orlandini et al., 2008; Chao and Kunz, 2009). The mechanism may vary in different cell types. One possible mechanism, based on overexpression experiments, is that Dab2–integrin complexes promote initial contacts between the cell and ECM (Chetrit et al., 2009). Alternatively, in HT1080 cells, Dab2 may stimulate focal adhesion disassembly (Chao and Kunz, 2009). However, in HeLa cells, Dab2 is not located near adhesion sites. Another possibility, which we favor, is that Dab2 regulates the dynamics of integrin–ECM contacts as an indirect effect of its role in bulk integrin endocytosis. Our finding that Dab2 regulates HeLa cell migration on collagen but not vitronectin is consistent with the specificity of Dab2 for endocytosis of collagen-binding but not vitronectin-binding integrins. Moreover, migration on collagen requires the endocytic activity of Dab2 (Fig. 6). A possible effect of Dab2 on adhesion is unlikely to be involved because Dab2 promoted migration regardless of collagen concentration (Fig. 7 A). The absence of Dab2 could also slow migration if the increased disengaged integrin on the surface slowed focal adhesion disassembly by a mass action effect. However, decreasing the total level of integrin β1 did not rescue the migration of Dab2-deficient cells (Fig. 7 D). Instead, these cells migrated even slower. These integrin-depleted cells have less internal β1 integrin available for exocytosis. Two lines of evidence suggest a model, illustrated in Fig. 7 I, in which Dab2 promotes migration by maintaining the intracellular integrin pool. First, there is a strong correlation between migration rate and the level of intracellular integrin (Fig. 7 F). Second, Dab2-deficient cells fail to polarize integrins and focal complex components in the direction of migration (Fig. 7, G and H). Therefore, we propose that Dab2 may stimulate migration by maintaining an internal store of integrin that can be recycled to the front of the cell and stabilize the leading edge (Lawson and Maxfield, 1995; Bretscher, 1996; Jones et al., 2006). Collectively, with the spatial separation between focal adhesion disassembly and integrin endocytosis, this means that endocytosis of disengaged integrins may play an indirect role in forming new adhesion contacts at the front of the cell. The model provides a rationale for how constitutive turnover of inactive cell surface integrin could impact the rapid dynamics of focal adhesion assembly and disassembly at the front of the cell.
Materials and methods
Cells and DNA constructs
HeLa cells and primary HFFs (gift from J. Roberts, Fred Hutchinson Cancer Research Center, Seattle, WA) were cultured in DME supplemented with 10% FBS and 1% penicillin-streptomycin. HeLa cells were transduced to stably express control or Dab2 shRNA. A pBabe puro vector with the histone H1 promoter cloned into the second long terminal repeat (Welcker et al., 2003) was modified by swapping in the hygromycin selection gene (gift from M. Maurer, Fred Hutchinson Cancer Research Center). Hairpins to target Dab2 or a control sequence were inserted downstream of the H1 promoter 5′-CAAAGGATGTGGGTCAACATT-3′. The control sequence targeted was 5′-TATGTCAAGTTGTATAGTTA-3′.
Retroviral particles were generated by cotransfection of HEK293T cells with constructs and packaging vector. HeLa cells were infected with retrovirus and, 48 h later, selected with 250 µg/ml hygromycin. Protein expression was detected by immunoblotting. For rescue experiments, vector alone (pCGT) or vector encoding T7-tagged mouse Dab2 (p96 or p67) was cotransfected with pGL1SuperC GFP expression vector into HeLa cells using Lipofectamine 2000 (Invitrogen).
Antibodies
The following integrin antibodies were provided by E. Wayner (Fred Hutchinson Cancer Research Center): inhibitory mouse anti–integrin β1 (P5D2-1), mouse anti–integrin α5 (P1D6-H9), and mouse anti–integrin αvβ5 (P1F6). Other antibodies included mouse anti–integrin α1 (TS2/7; Santa Cruz Biotechnology, Inc.), rabbit anti-Dab2 (Santa Cruz Biotechnology, Inc.), mouse anti-Dab2 (BD), mouse anticlathrin (X22; Abcam), mouse antiadaptin (clone AP.6; EMD), mouse anti-T7 (EMD), mouse anti-TfnR (Ab-1; Abcam), rabbit anti–phospho FAK (pY576; Invitrogen), rabbit anti-FAK (Santa Cruz Biotechnology, Inc.), rabbit anti–phospho Src (pY416; Cell Signaling Technology), rabbit anti-Numb (C29G11; Cell Signaling Technology), rabbit anti-ARH (gift from L. Traub, University of Pittsburgh, Pittsburgh, PA), rabbit anti-Fyn (FYN3; Santa Cruz Biotechnology, Inc.), mouse antivinculin (hVIN-1; Sigma-Aldrich), mouse anti-EEA1 (BD), rabbit anti-EEA1 (Thermo Fisher Scientific), and mouse anti–extracellular signal-regulated kinase (BD). The mouse hybridoma cell line (LP-016) expressing monoclonal Src antibody (LA074) was a gift from J. Meisenhelder (Salk Institute for Biological Studies, La Jolla, CA), and the 327 anti-Src mouse monoclonal antibody was provided by J. Brugge (Harvard Medical School, Boston, MA). Alexa Fluor–tagged secondary antibodies were purchased from Invitrogen.
CSC proteomics
The CSC method for suspension cells was previously described (Wollscheid et al., 2009). The following modifications were made for adherent cells and quantitative analysis (Ong et al., 2002). Control and Dab2-deficient HeLa cell lines were grown in normal and heavy lysine/arginine-supplemented media (Invitrogen), respectively, for five to six population doublings. When just confluent, cells were removed from 10 15-cm plates using 2 mM EDTA in PBS for 10 min at 37°C, harvested by centrifugation, and allowed to recover in suspension in the respective media at 37°C for 30 min. Equal cell numbers were then combined and washed once with cold PBS and once with cold labeling buffer (PBS containing 0.1% BSA and 20 mM Pipes, pH 6.7). All subsequent steps were performed at 0–4°C. Cells were oxidized with 1.2 mM NaIO4 in 40 ml of labeling buffer for 30 min, washed in cold PBS, and incubated in 25 mM biocytin hydrazide (Biotium, Inc.) in 10 ml of labeling buffer for 2 h. Cells were washed twice, resuspended in 20 ml of hypotonic buffer (10 mM Tris HCl, pH 7.5, and 0.5 mM MgCl2) for 10 min, and broken with 20 strokes of a tight Dounce homogenizer. A postnuclear supernatant was centrifuged in the SW41 rotor (Beckman Coulter) at 35,000 rpm for 1 h. Membrane pellets were resuspended and recentrifuged to remove cytoplasmic contamination. Subsequent steps of dissolving in Rapigest, digesting with Endo Lys-C and trypsin, binding to streptavidin beads, washing at high pH, and eluting bound N-glycosylated peptides with protein N-glycosidase F have been described previously (Wollscheid et al., 2009). Samples were analyzed using mass spectrometers (QTOF [Agilent Technologies]; Finnigan LTQ-FT [Thermo Fisher Scientific]). Peptides were identified by using the SEQUEST algorithm (Eng et al., 1994) in combination with Peptide Prophet (Nesvizhskii et al., 2003), searching for peptides containing aspartic acid instead of asparagine in NXS/T glycosylation signals. ASAP (automated statistical analysis of protein abundance) ratios were determined (Li et al., 2003) and curated manually.
Flow cytometry
Steady-state surface and total integrin levels were measured by flow cytometry. Cells were detached using 10 mM EDTA-PBS for 10 min at 37°C, washed with PBS, pelleted by centrifugation, and fixed in cold 4% paraformaldehyde-PBS for 20 min. Cells were incubated with anti-integrin or TfnR antibodies for 1 h at room temperature. To measure total integrin, cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min at 25°C before incubating with anti-integrin antibodies. Surface or total antibody was quantified by FACS analysis after staining with an Alexa Fluor 488 goat anti–mouse secondary antibody. Profiles were gated on intact cells, based on morphology, and mean fluorescent intensity was obtained. For rescue experiments, profiles were gated on GFP-positive cells, and surface antibody was quantified after staining with an Alexa Fluor 647 goat anti–mouse secondary antibody.
siRNA transfection
Knockdown experiments in HeLa cells and primary HFFs were performed as described previously (Maurer and Cooper, 2006). Cells were transfected with 50 pmol of a pool of four siRNA oligonucleotides specific for Dab2, clathrin, AP2 µ2, or ARH (Thermo Fisher Scientific) using Oligofectamine (Invitrogen) on days 1 and 3 and analyzed on day 5. Numb siRNA (Thermo Fisher Scientific) was given on days 1 and 3, and cells were analyzed on day 6. Total cell lysates were analyzed by immunoblotting to show that target protein levels were significantly reduced compared with a protein loading control (Fig. 3).
For rescue experiments, siRNA specific for human Dab2 (Thermo Fisher Scientific) was used to deplete cells of Dab2. Cells were transfected with T7-tagged mouse Dab2 on day 4 and analyzed on day 5. For partial knockdown of integrin β1, cells were transfected with 20 pmol of siRNA to integrin β1 (Santa Cruz Biotechnology, Inc.) on day 1 and analyzed on day 2.
RT-PCR
Integrin β1 RNA was measured as described previously (Laszlo and Cooper, 2009). Total RNA was extracted from 106 cells using TRIZOL reagent (Invitrogen). Total RNA was reverse transcribed using SuperScript II reverse transcription and random primers (Invitrogen). PCR was performed using Taq polymerase and the following primers: 5′-CCCTTGCACAAGTGAACAGA-3′ and 5′-ACATTCCTCCAGCCAATCAG-3′ (integrin β1) or 5′-GCGAGAAGATGACCCAGATCATGTT-3′ and 5′-GCTTCTCCTTAATGTCACGCACGAT-3′ (actin).
Antibody internalization assay
Antibody uptake was measured using a modification of a previous method (Roberts et al., 2001). Cells were plated on coverslips coated with 4 µg/ml collagen IV and then incubated with anti–integrin β1 antibody (P5D2) and/or Alexa Fluor 488–conjugated human Tfn diluted in assay media (DME, 10 mM Hepes, pH 7.4, and 0.1% BSA) for 30 min at 4°C. After washing off unbound antibody/Tfn with cold DME, cells were warmed to 37°C in DME with 10% FBS for 0, 15, or 30 min and fixed with cold 4% paraformaldehyde-PBS for 20 min. To visualize internalized receptors, surface-bound antibody/Tfn was removed by acid stripping for 5 min on ice (0.5 M NaCl and 0.2 M acetic acid) before fixing with cold 4% paraformaldehyde-PBS.
Surface biotinylation
Integrin recycling was measured as described previously (Roberts et al., 2001). Cells were washed twice with cold PBS before incubation with 0.2 mg/ml sulpho-NHS-SS-biotin (Thermo Fisher Scientific) in PBS for 30 min at 4°C. After surface labeling, cells were washed with cold PBS and transferred to DME with 10% FBS for 0, 15, or 30 min at 37°C to allow internalization. At the indicated times, cells were washed twice with cold PBS and then treated with 20 mM MesNa (50 mM Tris, pH 8.6, and 100 mM NaCl) for 15 min at 4°C to remove biotin. MesNa was quenched with 20 mM Iodoacetamide (50 mM Tris, pH 8.6, and 100 mM NaCl) for 10 min at 4°C. After two cold PBS washes, cells were lysed (75 mM Tris, pH 7.4, 200 mM NaCl, 15 mM NaF, 7.5 mM EDTA, 1.5% Triton X-100, 1 µg/ml aprotinin, 5 µg/ml leupeptin). Lysates were clarified by centrifugation at 15,000 g for 15 min. Supernatants were incubated with anti–integrin β1 antibody (P5D2), and immunoprecipitates were analyzed by SDS-PAGE.
Immunofluorescence
To visualize surface integrin, nonpermeabilized cells were fixed and stained with anti-integrin antibodies to extracellular epitopes. To detect intracellular proteins, cells were fixed and permeabilized with 0.1% Triton X-100 in PBS for 5 min at 25°C. Cells were blocked in a solution of 2% BSA, 5% normal goat serum, and PBS for 30 min at 25°C. Primary antibodies were diluted in blocking solution and added either for 3–4 h at 25°C or overnight at 4°C. Coverslips were rinsed three times in PBS before the addition of Alexa Fluor 488–, Alexa Fluor 568–, or Alexa Fluor 647–labeled secondary antibodies, diluted 1:1,000 (all from Invitrogen), for 1 h at 25°C. Secondary antibodies included goat anti–mouse, goat anti–mouse IgG1-isotype–specific and IgG2b-isotype–specific antibodies, and goat anti–rabbit. After several PBS rinses, coverslips were mounted in ProLong Gold solution (Invitrogen).
Cells were visualized using a 60× NA 1.42 oil objective on a microscope (DeltaVision IX70; Olympus). Images were recorded using fixed camera settings using a microscope (IX-HLSH100; Olympus). Images were acquired and deconvolved using SoftWoRx software (Applied Precision, LLC). Deconvolved images from single planes corresponding to the ventral surfaces of the cells or flattened z projections were analyzed using ImageJ (National Institutes of Health). Figures were assembled using Photoshop (Adobe) and Canvas (Deneba) softwares. Levels were adjusted equally for all images in a set.
Collagen uptake assay
Cells were detached using 10 mM EDTA-PBS for 10 min at 37°C, washed with PBS, pelleted by centrifugation, and plated on coverslips coated with 4 µg/ml Alexa Fluor 488 collagen IV at 37°C in DME containing 10% FBS. Cells were fixed with 4% paraformaldehyde-PBS after 2 h, permeabilized with 0.1% Triton X-100, and stained.
Cell migration
Boyden chamber assay.
Migration assays were performed in a 48-well micro chemotaxis chamber (Neuro Probe, Inc). Cells were detached using 10 mM EDTA-PBS for 10 min at 37°C, washed three times with DME, and resuspended in DME at 250,000 cells/ml. The lower wells of the chamber were loaded with 10% FBS in DME. An 8-µm pore diameter membrane (Neuro Probe, Inc.) coated with 4 µg/ml collagen IV or 2 µg/ml vitronectin separated the bottom and top chambers. Cells were added to the top wells. The chamber was incubated in a humidified atmosphere of 5% CO2 at 37°C for 12 h. The cells on the top side of the membrane were removed, and the migrated cells on the bottom side were stained with 0.1% crystal violet in 20% ethanol and counted by using a light microscope at a magnification of 40.
Scratch wound assay.
Dab2 shRNA and control cells were grown at equivalent density for 24 h on coverslips coated with 4 µg/ml collagen IV. A single scratch was made with a pipette tip, and cells were allowed to migrate into the wound for 10 h. Cells were stained with antibodies to integrin β1 and vinculin and visualized using a 40× oil objective on a microscope (DeltaVision; Carl Zeiss, Inc.).
Online supplemental material
Fig. S1 shows that steady-state surface levels of integrin β1 increase upon depletion of Dab2. Fig. S2 shows that Dab2 regulates integrin α1β1 endocytosis and collagen uptake. Fig. S3 shows that Dab2 and integrin colocalize over the entire dorsal surface, whereas Numb is enriched at the periphery of the cell on the ventral side. Fig. S4 shows that the combined removal of Dab2 and Numb caused a greater inhibition of migration than removing either alone. Fig. S5 shows that FAK and Src activation increases on Dab2 depletion. Table S1 shows isotope labeling ratios (ASAP; light/heavy) of 35 cell surface–labeled proteins, excluding integrins, that were quantified by SILAC and CSC. Table S2 shows surface integrin quantification by SILAC labeling and CSC of proteins from control cells labeled with light isotopes and Dab2-deficient cells labeled with heavy isotopes.
Acknowledgments
We gratefully acknowledge reagents, expert advice, and assistance from M. Maurer, J. Roberts, E. Wayner, H. Qian, C.J. McGlade, J. Ranish, D. Bausch-Fluck, and the Fred Hutchinson Cancer Research Center Flow Cytometry and Scientific Imaging resources. We are especially grateful to S. Parkhurst, E. Mulkearns, T. Parsons, D. Schlaepfer, L. Traub, V. Vasioukhin, and R. Walter for comments on an early draft of the manuscript.
This research was supported by National Institutes of Health grants R01-GM66257 (to J.A. Cooper), RO1-AI51344-01 (to J. Watts), and N01-HV-28179-22 (to B. Wollscheid) and the National Institutes of Health National Research Service Award GM078776 (to A. Teckchandani).
References
Abbreviations used in this paper: ARH, autosomal recessive hypercholesterolemia; CSC, cell surface capture; HFF, human foreskin fibroblast; LDLR, low density lipoprotein receptor; shRNA, short hairpin RNA; SILAC, stable isotope labeling with amino acids in cell culture; Tfn, transferrin; TfnR, Tfn receptor.
Author notes
B. Wollscheid's present address is National Center for Competence in Research Neuro Center for Proteomics, Institute of Molecular Systems Biology, Swiss Federal Institute of Technology Zurich and University of Zurich, 8093 Zurich, Switzerland.
C. Henderson's present address is Vaccine and Gene Therapy Institute, Oregon Health and Science University, Beaverton, OR 97006.