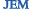IgM+ B cells (brown) develop in the mouse gut even when the spleen and gut lymphoid tissues are missing.
B cells are thought to mature in the bone marrow and spleen. As they mature, B cells express the immunoglobulin receptor IgM, which distinguishes them from their antigen-activated brethren. The activated cells are mostly found in gut lymphoid tissues, where they secrete antibodies.
The mouse gut, however, also contains IgM+ B cells, which increase in number during inflammation. These cells were assumed to be either antigen-inexperienced cells that were just passing through or antigen-activated immigrants that hadn't yet matured.
But Shimomura et al. now find that the gut IgM+ B cells did not express the same pattern of cellular receptors that mark B cells that mature elsewhere, suggesting that that these cells may be long-term gut residents. The authors found IgM+ gut cells in mice that lacked spleens, gut lymphoid tissues, or inflammation—conditions that deter activated B cells from entering and surviving in the gut.
The cells also differed from other B cells in their ability to survive in the absence of antigen. They only needed the cytokine BAFF, which helps B cells transit through developmental checkpoints. And although the IgM+ cells multiplied during inflammation, the expanded population included a diversity of clones instead of a narrow, antigen-specific repertoire.
The function of these cells is still unclear, but the study offers some clues. The cells expanded only as the inflammation was dying down, and in response to innate immune receptor activation, they secreted IL-12—a cytokine that tempers chronic gut inflammation. The authors propose that these IgM+ B cells, which can also produce the suppressive cytokine IL-10, may be the precursors of regulatory B cells previously associated with chronic gut inflammation.