Skip Nav Destination
Close Modal
Update search
- Title
- Author
- Author Affiliations
- Full Text
- Abstract
- Keyword
- DOI
- ISBN
- EISBN
- ISSN
- EISSN
- Issue
- Volume
- References
- Title
- Author
- Author Affiliations
- Full Text
- Abstract
- Keyword
- DOI
- ISBN
- EISBN
- ISSN
- EISSN
- Issue
- Volume
- References
- Title
- Author
- Author Affiliations
- Full Text
- Abstract
- Keyword
- DOI
- ISBN
- EISBN
- ISSN
- EISSN
- Issue
- Volume
- References
- Title
- Author
- Author Affiliations
- Full Text
- Abstract
- Keyword
- DOI
- ISBN
- EISBN
- ISSN
- EISSN
- Issue
- Volume
- References
- Title
- Author
- Author Affiliations
- Full Text
- Abstract
- Keyword
- DOI
- ISBN
- EISBN
- ISSN
- EISSN
- Issue
- Volume
- References
- Title
- Author
- Author Affiliations
- Full Text
- Abstract
- Keyword
- DOI
- ISBN
- EISBN
- ISSN
- EISSN
- Issue
- Volume
- References
NARROW
Format
Subjects
Journal
Article Type
Date
1-20 of 163
Follow your search
Access your saved searches in your account
Would you like to receive an alert when new items match your search?
1
Sort by
Journal Articles
In Special Collection:
Immune Cell Biology 2021
Alexander Leithner, Lukas M. Altenburger, Robert Hauschild, Frank P. Assen, Klemens Rottner, Theresia E.B. Stradal, Alba Diz-Muñoz, Jens V. Stein, Michael Sixt
Journal:
Journal of Cell Biology
J Cell Biol (2021) 220 (4): e202006081.
Published: 03 February 2021
Images
in Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse
> Journal of Cell Biology
Published: 03 February 2021
Figure 1. DC F-actin depolymerization affects immune synapse structure and T cell priming efficiency . (A) Bright field images of mature DCs treated with 1 µM MycB (right) or DMSO (left). Scale bar: 10 µm. (B) Flow cytometry profiles of DMSO More about this image found in DC F-actin depolymerization affects immune synapse structure and T cell pri...
Images
in Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse
> Journal of Cell Biology
Published: 03 February 2021
Figure S1. Further characterization of MycB treated DCs. (A) Flow cytometry experiment displaying relative mean fluorescence intensities (MFIs) for Cd11c (left) and MHC II (right) of DMSO- or 1 µM MycB–treated mature DCs, three biological More about this image found in Further characterization of MycB treated DCs. (A) Flow cytometry experimen...
Images
in Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse
> Journal of Cell Biology
Published: 03 February 2021
Figure S2. Characterization of the structure and dynamics of the DC and T cell actin cytoskeleton at the immunological synapse. (A) 3D projection of Lifeact-eGFP–expressing OT-II T cells (red), interacting with DMSO control (upper) or 1 µM More about this image found in Characterization of the structure and dynamics of the DC and T cell actin c...
Images
in Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse
> Journal of Cell Biology
Published: 03 February 2021
Figure 2. Dynamic F-actin foci at the DC immune synapse. (A) Schematic overview of PDMS confiner setup. (B) Left: Maximum intensity projection of Lifeact-eGFP–expressing mature WT DC interacting with TAMRA-stained T cells. Scale bar: 10 µm. More about this image found in Dynamic F-actin foci at the DC immune synapse. (A) Schematic overview of ...
Images
in Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse
> Journal of Cell Biology
Published: 03 February 2021
Figure S3. Flow cytometry analysis of immature and mature wt and hem1 −/− DCs. (A) Flow cytometry profiles of MHC II– and Cd11c-stained immature (left) or mature (right) WT or hem1−/− DCs. (B) Flow cytometry histograms of immature (light More about this image found in Flow cytometry analysis of immature and mature wt and hem1 −/− ...
Images
in Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse
> Journal of Cell Biology
Published: 03 February 2021
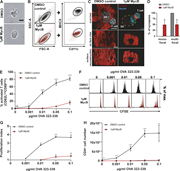
Figure 3. Hem1−/− DCs are impaired in T cell activation. (A) Percentage of activated T cells assessed by CD62L/CD69 surface expression at indicated OVA323-339 peptide concentrations, three biological replicates, mean ± SD. (B) Exemplary More about this image found in Hem1−/− DCs are impaired in T cell activation. (A) Percentage ...
Images
in Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse
> Journal of Cell Biology
Published: 03 February 2021
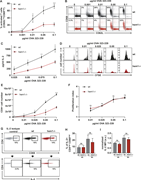
Figure 4. Hem1−/− DCs alter synapse structure and dynamics. (A) Fluorescence microscopy images of synapses formed between mature WT or hem1−/− DCs and T cells stained with phalloidin and DAPI. Scale bar: 5 µm. (B) Percentages of T cell More about this image found in Hem1−/− DCs alter synapse structure and dynamics. (A) Fluoresc...
Images
in Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse
> Journal of Cell Biology
Published: 03 February 2021
Figure S4. The dependency of T cell priming defects on the pERM-ICAM1-LFA-1 axis are specific to hem1−/− DCs. (A) Quantification of the number of T cells contacted by mature WT and hem1−/− DCs. Bars represent the median. Mann-Whitney test, three More about this image found in The dependency of T cell priming defects on the pERM-ICAM1-LFA-1 axis are s...
Images
in Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse
> Journal of Cell Biology
Published: 03 February 2021
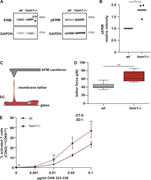
Figure 5. The hem1−/− DC–T cell priming defect is mediated by the pERM–ICAM1–LFA-1 axis. (A) Western blots for ERM, pERM, and GAPDH in mature WT and hem1−/− DCs, representative example of three biological replicates. (B) Relative intensity of More about this image found in The hem1−/− DC–T cell priming defect is mediated by the pERM–ICA...
Images
in Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse
> Journal of Cell Biology
Published: 03 February 2021
Figure 6. Hem1−/− DCs have T cell priming defects in vivo. (A) Schematic overview of experimental setup for two-photon intravital microscopy. (B) In vivo interaction times of mature WT or hem1−/− DCs and T cells, n = 45 cell–cell interactions More about this image found in Hem1−/− DCs have T cell priming defects in vivo. (A) Schematic...
1