The evolution of ion channel clustering at nodes of Ranvier enabled the development of complex vertebrate nervous systems. At mammalian nodes, the K+ leak channels TRAAK and TREK-1 underlie membrane repolarization. Despite the molecular similarities between nodes and the axon initial segment (AIS), TRAAK and TREK-1 are reportedly node-specific, suggesting a unique clustering mechanism. However, we show that TRAAK and TREK-1 are enriched at both nodes and AIS through a common mechanism. We identified a motif near the C-terminus of TRAAK that is necessary and sufficient for its clustering. The motif first evolved among cartilaginous fish. Using AnkyrinG (AnkG) conditional knockout mice, CRISPR/Cas9-mediated disruption of AnkG, co-immunoprecipitation, and surface recruitment assays, we show that TRAAK forms a complex with AnkG and that AnkG is necessary for TRAAK’s AIS and nodal clustering. In contrast, TREK-1’s clustering requires TRAAK. Our results expand the repertoire of AIS and nodal ion channel clustering mechanisms and emphasize AnkG’s central role in assembling excitable domains.
Introduction
Clustered voltage-gated sodium (Nav) channels at nodes of Ranvier govern rapid action potential propagation and regeneration by driving membrane depolarization. Although voltage-gated potassium (Kv) channels drive membrane repolarization in dendrites, soma, and unmyelinated axons, Kv channels do not repolarize mammalian nodes (Binah and Palti, 1981; Chiu and Ritchie, 1980). Instead, repolarization relies primarily on the two-pore-domain K+ (K2P) leak channels, TRAAK and TREK-1 (Kanda et al., 2019). Both Nav and certain types of Kv channels are clustered at nodes and the axon initial segment (AIS) by the scaffolding protein AnkyrinG (AnkG) (Pan et al., 2006; Zhou et al., 1998). In contrast, TRAAK and TREK-1 were reported only at nodes and not the AIS (Brohawn et al., 2019; Kanda et al., 2019), suggesting that TRAAK and TREK-1 may have node-specific, ankyrin-independent clustering mechanisms.
Since AIS and nodes share a mostly common molecular organization and membrane protein clustering at these sites depends on ankyrin scaffolding proteins (Rasband and Peles, 2021), we considered a node-specific, ankyrin-independent ion channel clustering mechanism to be unlikely. Consistent with prior reports, we were unable to detect TRAAK or TREK-1 at AIS of cultured hippocampal neurons after fixation using 4% paraformaldehyde (PFA; Fig. 1 A). However, inspired by Konno et al. (2023), who reported that fixation using glyoxal greatly improves the immunoreactivity of ion channels and receptors embedded in the postsynaptic density, we tested a combination of glyoxal, acetic acid, and methanol (Glyox+MeOH) fixation in primary neurons. In contrast to fixation with 4% PFA alone, we found robust and specific AIS immunostaining for both TRAAK and TREK-1 after Glyox+MeOH fixation (Fig. 1 B); the TRAAK and TREK-1 immunoreactivity colocalized with AnkG. Furthermore, immunostaining of cortical brain sections after Glyox+MeOH fixation also yielded strong TRAAK and TREK-1 AIS labeling that colocalized with AnkG (Fig. 1 C). Consistent with previous reports, peripheral and central nerves fixed with Glyox+MeOH had strong and specific nodal immunostaining for both TRAAK and TREK-1 (Fig. 1, D and E). These results show the K+ leak channels TRAAK and TREK-1 are found at both the AIS and nodes of Ranvier, suggesting common mechanisms may underlie their clustering.
TRAAK and TREK-1 are found at the AIS and nodes of Ranvier. (A and B) Immunostaining of DIV 21 hippocampal neurons with antibodies against TRAAK (green), TREK-1 (green), AnkG (red), and MAP2 (magenta and shown only in the merged images). Neurons were fixed using either 4% PFA (A) or a combination of glyoxal, acetic acid, and methanol (B, Glyox+MeOH). Arrowheads indicate the AIS. Scale bars, 10 μm. (C) Immunostaining of cortex using antibodies against TRAAK (green), TREK-1 (green), and AnkG (magenta) in 3.5-mo-old wild-type mice. Tissues were fixed using Glyox+MeOH. Arrowheads indicate AIS. Scale bars, 20 μm. (D and E) Immunostaining of sciatic (D) and optic (E) nerves using antibodies against TRAAK (green) or TREK-1 (green), Caspr (red), and AnkG (magenta) in 3.5-mo-old wild-type mice. Tissues were fixed using Glyox+MeOH. Arrowheads indicate nodes of Ranvier. Scale bars, 5 μm.
TRAAK and TREK-1 are found at the AIS and nodes of Ranvier. (A and B) Immunostaining of DIV 21 hippocampal neurons with antibodies against TRAAK (green), TREK-1 (green), AnkG (red), and MAP2 (magenta and shown only in the merged images). Neurons were fixed using either 4% PFA (A) or a combination of glyoxal, acetic acid, and methanol (B, Glyox+MeOH). Arrowheads indicate the AIS. Scale bars, 10 μm. (C) Immunostaining of cortex using antibodies against TRAAK (green), TREK-1 (green), and AnkG (magenta) in 3.5-mo-old wild-type mice. Tissues were fixed using Glyox+MeOH. Arrowheads indicate AIS. Scale bars, 20 μm. (D and E) Immunostaining of sciatic (D) and optic (E) nerves using antibodies against TRAAK (green) or TREK-1 (green), Caspr (red), and AnkG (magenta) in 3.5-mo-old wild-type mice. Tissues were fixed using Glyox+MeOH. Arrowheads indicate nodes of Ranvier. Scale bars, 5 μm.
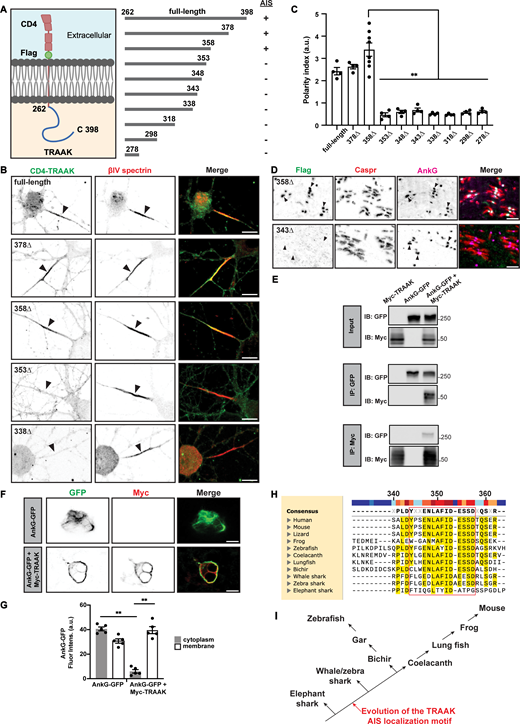
The C-terminus of TRAAK is predicted to be intrinsically unstructured (Brohawn et al., 2012). To identify the motif in TRAAK responsible for its AIS localization, we constructed a chimeric protein consisting of the transmembrane protein CD4 (for convenience and not normally found at the AIS), an extracellular Flag tag, and the entire cytoplasmic C-terminus of TRAAK including amino acids 262–398 (Fig. 2 A). We used AAV to transduce hippocampal neurons and found the chimera with the full C-terminus of TRAAK (full-length) was clustered at the AIS and colocalized with βIV spectrin (Fig. 2, A and B). We generated a series of progressively larger C-terminal truncations and found that truncations ending at amino acids 378 and 358 (378Δ and 358Δ, respectively) were enriched at the AIS (Fig. 2, A and B). However, truncations ending before or at amino acid 353 (353Δ) disrupted TRAAK clustering at the AIS, suggesting that the AIS localization motif for TRAAK is after, or includes, amino acid 353 (Fig. 2, A and B). We measured the polarity index for all the truncations; polarity index is defined as the ratio of AIS to dendritic immunofluorescence. Chimeras including the full-length, 378Δ, and 358Δ truncations had a polarity index >2, significantly higher than the 353Δ, 348Δ, 343Δ, 338Δ, 318Δ, 298Δ, and 278Δ truncations (Fig. 2 C). These results show that TRAAK has a C-terminal localization motif including amino acids 353–358 that is sufficient to target a CD4 chimera to the AIS.
TRAAK has a C-terminal AIS and node localization motif. (A) The CD4-Flag-TRAAK chimeras, truncations, and AIS localization. Created with https://www.Biorender.com. (B) Anti-CD4 surface staining of DIV 10 hippocampal neurons transduced with the indicated CD4-TRAAK C-terminal chimeras (green). AIS were labeled using anti-βIV spectrin (red) and indicated by arrowheads. Scale bar, 10 μm. (C) Polarity index (fluorescence intensity at AIS/fluorescence intensity at dendrites) for each CD4-TRAAK C-terminal chimera truncation. N = 4 cultures for each truncation (with the exception of 358Δ where N = 8), with 20–25 hippocampal neurons measured for each culture. Error bars indicate ± SEM. ****P < 0.0001; one-way ANOVA with multiple comparisons. (D) Mouse retinal ganglion cells were transduced by intravitreal injection with AAV to express 358Δ and 343Δ CD4-TRAAK chimeras. Optic nerves were then immunostained using antibodies against Flag (green, CD4-TRAAK chimeras), Caspr (red, paranodes), and AnkG (magenta, nodes and paranodes). Scale bar, 5 μm. (E) Immunoblots of total cell lysates (input) and immunoprecipitated protein complexes from Myc-TRAAK, AnkG-GFP, and AnkG-GFP + Myc-TRAAK transfections. Immunoblots and immunoprecipitation reactions were performed using antibodies against GFP and Myc. IP, immunoprecipitation; IB, immunoblot. (F) Immunostaining of HEK cells transfected with AnkG-GFP or AnkG-GFP + Myc-TRAAK; GFP (green) and Myc (red). Scale bar, 10 μm. (G) Quantification of AnkG-GFP fluorescence intensity at the cytoplasm and cell membrane. N = 4 cultures for each condition. n = 20–25 HEK cells for each culture. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons. (H) Sequence alignment of TRAAK AIS and node clustering motif across species. Highly conserved residues are highlighted in yellow and the minimal motif is boxed in red. Created with SnapGene. (I) Illustration of the phylogenetic tree of jawed vertebrates showing the acquisition of the TRAAK clustering motif in bony vertebrates. Source data are available for this figure: SourceData F2.
TRAAK has a C-terminal AIS and node localization motif. (A) The CD4-Flag-TRAAK chimeras, truncations, and AIS localization. Created with https://www.Biorender.com. (B) Anti-CD4 surface staining of DIV 10 hippocampal neurons transduced with the indicated CD4-TRAAK C-terminal chimeras (green). AIS were labeled using anti-βIV spectrin (red) and indicated by arrowheads. Scale bar, 10 μm. (C) Polarity index (fluorescence intensity at AIS/fluorescence intensity at dendrites) for each CD4-TRAAK C-terminal chimera truncation. N = 4 cultures for each truncation (with the exception of 358Δ where N = 8), with 20–25 hippocampal neurons measured for each culture. Error bars indicate ± SEM. ****P < 0.0001; one-way ANOVA with multiple comparisons. (D) Mouse retinal ganglion cells were transduced by intravitreal injection with AAV to express 358Δ and 343Δ CD4-TRAAK chimeras. Optic nerves were then immunostained using antibodies against Flag (green, CD4-TRAAK chimeras), Caspr (red, paranodes), and AnkG (magenta, nodes and paranodes). Scale bar, 5 μm. (E) Immunoblots of total cell lysates (input) and immunoprecipitated protein complexes from Myc-TRAAK, AnkG-GFP, and AnkG-GFP + Myc-TRAAK transfections. Immunoblots and immunoprecipitation reactions were performed using antibodies against GFP and Myc. IP, immunoprecipitation; IB, immunoblot. (F) Immunostaining of HEK cells transfected with AnkG-GFP or AnkG-GFP + Myc-TRAAK; GFP (green) and Myc (red). Scale bar, 10 μm. (G) Quantification of AnkG-GFP fluorescence intensity at the cytoplasm and cell membrane. N = 4 cultures for each condition. n = 20–25 HEK cells for each culture. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons. (H) Sequence alignment of TRAAK AIS and node clustering motif across species. Highly conserved residues are highlighted in yellow and the minimal motif is boxed in red. Created with SnapGene. (I) Illustration of the phylogenetic tree of jawed vertebrates showing the acquisition of the TRAAK clustering motif in bony vertebrates. Source data are available for this figure: SourceData F2.
To determine if the AIS localization motif is also sufficient for nodal clustering, we performed intravitreal injections of AAV in wild-type mice to express the 358Δ and 343Δ chimeras. Consistent with the results for AIS, we found that the 358Δ chimera was highly enriched at nodes of Ranvier where it colocalized with AnkG and was flanked by paranodal Caspr (Fig. 2 D). However, the 343Δ mutant either failed to traffic properly to nodes of Ranvier or failed to cluster at nodes.
Since AIS and nodal ion channels are clustered at these sites through AnkG, we coexpressed GFP-tagged AnkG and Myc-tagged TRAAK in HEK cells. After solubilization, we immunoprecipitated AnkG-GFP or Myc-TRAAK and found that each could coimmunoprecipitate the other (Fig. 2 E). To further establish that AnkG interacts with TRAAK, we performed a surface recruitment assay in HEK cells by coexpressing GFP-tagged AnkG with a Myc-tagged TRAAK. When AnkG was expressed alone, it was distributed diffusely throughout the cell’s cytoplasm. However, when AnkG was coexpressed with transmembrane TRAAK, AnkG was recruited to the cell membrane and colocalized with TRAAK (Fig. 2, F and G). These results support the conclusion that TRAAK forms a complex with AnkG.
Comparison of the C-terminus of TRAAK across multiple species showed that among bony vertebrates there is a highly conserved region from amino acids 344–358 (Fig. 2 H). This motif does not match other previously identified AIS or node localization motifs found in Kv7, Kv3.1b, or Nav channels (Garrido et al., 2003; Lemaillet et al., 2003; Pan et al., 2006; Stevens et al., 2021). Intriguingly, the AIS and nodal clustering motif appears in bony vertebrates, and some cartilaginous sharks, indicating the TRAAK localization motif evolved after nodes of Ranvier (Fig. 2 I).
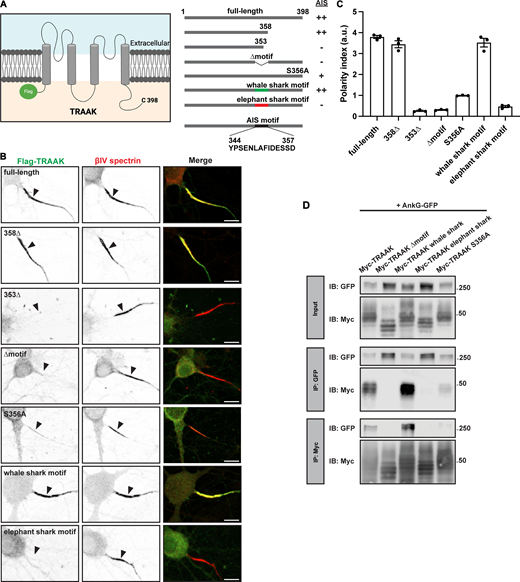
To further define the TRAAK AIS localization motif, we then generated the 358Δ and 353Δ C-terminal truncations in the TRAAK channel (Fig. 3 A). Consistent with the results observed using the CD4-TRAAK chimeras, the 358Δ mutant channel localized at the AIS, but the 353Δ mutant channel did not (Fig. 3 B). Measurement of the polarity index showed that the full-length and 358Δ channels were highly polarized at the AIS, but the 353Δ mutant channel was not (Fig. 3 C). An internal deletion of the TRAAK AIS localization motif including amino acids 344–357 (Δmotif; Fig. 3 A) also failed to cluster at the AIS and had a polarity index <1 (Fig. 3, B and C). Based on the evolutionary conservation of the TRAAK AIS localization motif (Fig. 2 H), we substituted the same region from whale shark or elephant shark, two cartilaginous fish. We found that the equivalent region in whale shark resulted in AIS clustering, but the elephant shark motif did not (Fig. 3 B). Similarly, TRAAK channels with the whale shark motif had a high polarity index while those with the elephant shark motif did not (Fig. 3 C).
The TRAAK motif is necessary for AIS localization and is evolutionarily conserved. (A) Schematic of the full-length Flag-TRAAK channels, truncations, deletions, and substitutions. AIS localization as measured in B is also indicated. Created with https://www.Biorender.com. (B) Anti-Flag staining of DIV 10 hippocampal neurons transduced with the indicated Flag-TRAAK constructs (green). AIS were labeled using anti-βIV spectrin (red) and indicated by arrowheads. Scale bar, 10 μm. (C) Polarity index (fluorescence intensity at AIS/fluorescence intensity at dendrites) for each Flag-TRAAK construct. N = 3 cultures for each construct, with 20–25 hippocampal neurons measured for each culture. Error bars indicate ± SEM. ****P < 0.0001; one-way ANOVA with multiple comparisons. (D) Immunoblots of total cell lysates (input) and immunoprecipitated protein complexes from Myc-TRAAK + AnkG-GFP, Myc-TRAAK Δmotif + AnkG-GFP, Myc-TRAAK whale shark motif + AnkG-GFP, Myc-TRAAK elephant shark motif + AnkG-GFP, and Myc-TRAAK S356A + AnkG-GFP transfections. Immunoblots and immunoprecipitation reactions were performed using antibodies against GFP and Myc. IP, immunoprecipitation; IB, immunoblot. Source data are available for this figure: SourceData F3.
The TRAAK motif is necessary for AIS localization and is evolutionarily conserved. (A) Schematic of the full-length Flag-TRAAK channels, truncations, deletions, and substitutions. AIS localization as measured in B is also indicated. Created with https://www.Biorender.com. (B) Anti-Flag staining of DIV 10 hippocampal neurons transduced with the indicated Flag-TRAAK constructs (green). AIS were labeled using anti-βIV spectrin (red) and indicated by arrowheads. Scale bar, 10 μm. (C) Polarity index (fluorescence intensity at AIS/fluorescence intensity at dendrites) for each Flag-TRAAK construct. N = 3 cultures for each construct, with 20–25 hippocampal neurons measured for each culture. Error bars indicate ± SEM. ****P < 0.0001; one-way ANOVA with multiple comparisons. (D) Immunoblots of total cell lysates (input) and immunoprecipitated protein complexes from Myc-TRAAK + AnkG-GFP, Myc-TRAAK Δmotif + AnkG-GFP, Myc-TRAAK whale shark motif + AnkG-GFP, Myc-TRAAK elephant shark motif + AnkG-GFP, and Myc-TRAAK S356A + AnkG-GFP transfections. Immunoblots and immunoprecipitation reactions were performed using antibodies against GFP and Myc. IP, immunoprecipitation; IB, immunoblot. Source data are available for this figure: SourceData F3.
To further define the interaction between AnkG and TRAAK, we performed coimmunoprecipitation experiments between AnkG270-GFP, and full-length TRAAK, 358Δ, and 353Δ mutant TRAAK channels, the Δmotif TRAAK channel, or the whale and elephant shark substitutions. Our results show that if a channel localized at the AIS, it coimmunoprecipitated with AnkG270-GFP (Fig. 3 D). Together, these results strongly support the conclusion that the evolutionarily conserved amino acids 344–357 constitute an AIS targeting motif in TRAAK that interacts with AnkG.
Since the affinity between AIS Na+ and Kv7 K+ channels is dramatically increased by phosphorylation of serines within the AnkG-binding domain (Bréchet et al., 2008), we considered whether serine-dependent phosphorylation of the TRAAK AIS localization motif might also modulate its AIS clustering and interaction with AnkG. We found that mutation of S356 to alanine (S356A) resulted in a dramatic reduction in the enrichment of the S356A TRAAK K+ channel at the AIS (Fig. 3 B) and its polarity index (Fig. 3 C). In addition, although AnkG270-GFP and S356A TRAAK could coimmunoprecipitate each other, the interaction was dramatically reduced compared with full-length, 358Δ TRAAK, or whale shark motif TRAAK (Fig. 3 D). These results suggest that serine phosphorylation at S356 regulates the interaction between AnkG and TRAAK.
A striking feature of AIS proteins is their detergent insolubility. This property reflects their strong association with the AIS cytoskeleton. To further establish that AIS TRAAK and TREK-1 associate with the AIS cytoskeleton, we treated hippocampal neurons with TX-100 before fixation. Whereas MAP2, a somatodendritic microtubule-associated protein is readily solubilized, AIS TRAAK and TREK-1 are highly resistant to detergent extraction (Fig. 4, A and B). The AIS periodic axonal cytoskeleton is comprised of rings of actin that are spaced at ∼190-nm intervals by tetramers of βIV and αII spectrin. This submembranous cytoskeleton is linked to membrane proteins through AnkG (Huang et al., 2017; Leterrier et al., 2015; Xu et al., 2013). To determine if the AIS K+ leak channel TRAAK associates with the AIS periodic cytoskeleton, we immunolabeled cultured hippocampal neurons using antibodies against TRAAK and AnkG. Super-resolution STED imaging and line scans revealed that these proteins are highly colocalized and have a periodic spacing of ∼190 nm (Fig. 4, C–E). These results suggest that TRAAK is strongly associated with the AIS membrane periodic cytoskeleton.
TRAAK clustering at the AIS and nodes requires AnkG. (A and B) Immunostaining of DIV 21 Control (A) and 21 Triton X-100 (TX-100) extracted hippocampal neurons (B) for TRAAK (green), TREK-1 (green), and AnkG (red). Arrowheads indicate the AIS. Scale bar, 10 μm. (C) STED imaging of TRAAK (magenta) and AnkG (cyan). (D) Fluorescence intensity for TRAAK (magenta) and AnkG (cyan) from a line scan along an AIS. (E) Distance between peak immunofluorescence for TRAAK and AnkG. N = 13; error bars indicate ± SEM. ns, not significant; one-way ANOVA with multiple comparison. (F and H) Immunostaining of DIV 21 cortical neurons transduced with AAV to express ctrl or AnkG 3X sgRNAs + smFP-HA and Cas9. Neurons were immunostained for TRAAK (F) or TRIM46 (H) (green), HA (red), and AnkG (magenta). Arrowheads indicate AIS. Scale bars, 10 μm. (G and I) Quantification of HA+ neurons with AIS TRAAK (G) or TRIM46 (I) immunostaining. N = 5 cultures for each condition, and n = 20–25 hippocampal neurons were counted for each culture. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons. (J) Immunostaining of control and Avil-Cre;Ank3f/f dorsal roots using antibodies against TRAAK (green), Caspr (red), and AnkG (magenta). Arrowheads indicate nodes of Ranvier. Scale bar, 5 μm. (K) Quantification of AnkG+ TRAAK+ nodes of Ranvier in ventral and dorsal roots of control and AnkG cKO (Avil-Cre;Ank3f/f) mice. N = 3 mice of each genotype. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons. (L) Optic nerves from Ank3f/f mice with and without intravitreal injection of AAV-Cre-tdT for deletion of AnkG immunostained for TRAAK (green), Caspr (red), and AnkG (magenta). Arrowheads indicate nodes of Ranvier. Scale bar, 5 μm. (M) Quantification of AnkG+ TRAAK+ nodes of Ranvier in optic nerve of Ank3f/f mice with and without intravitreal injection of AAV-Cre-tdT. N = 3 mice of each genotype. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons.
TRAAK clustering at the AIS and nodes requires AnkG. (A and B) Immunostaining of DIV 21 Control (A) and 21 Triton X-100 (TX-100) extracted hippocampal neurons (B) for TRAAK (green), TREK-1 (green), and AnkG (red). Arrowheads indicate the AIS. Scale bar, 10 μm. (C) STED imaging of TRAAK (magenta) and AnkG (cyan). (D) Fluorescence intensity for TRAAK (magenta) and AnkG (cyan) from a line scan along an AIS. (E) Distance between peak immunofluorescence for TRAAK and AnkG. N = 13; error bars indicate ± SEM. ns, not significant; one-way ANOVA with multiple comparison. (F and H) Immunostaining of DIV 21 cortical neurons transduced with AAV to express ctrl or AnkG 3X sgRNAs + smFP-HA and Cas9. Neurons were immunostained for TRAAK (F) or TRIM46 (H) (green), HA (red), and AnkG (magenta). Arrowheads indicate AIS. Scale bars, 10 μm. (G and I) Quantification of HA+ neurons with AIS TRAAK (G) or TRIM46 (I) immunostaining. N = 5 cultures for each condition, and n = 20–25 hippocampal neurons were counted for each culture. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons. (J) Immunostaining of control and Avil-Cre;Ank3f/f dorsal roots using antibodies against TRAAK (green), Caspr (red), and AnkG (magenta). Arrowheads indicate nodes of Ranvier. Scale bar, 5 μm. (K) Quantification of AnkG+ TRAAK+ nodes of Ranvier in ventral and dorsal roots of control and AnkG cKO (Avil-Cre;Ank3f/f) mice. N = 3 mice of each genotype. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons. (L) Optic nerves from Ank3f/f mice with and without intravitreal injection of AAV-Cre-tdT for deletion of AnkG immunostained for TRAAK (green), Caspr (red), and AnkG (magenta). Arrowheads indicate nodes of Ranvier. Scale bar, 5 μm. (M) Quantification of AnkG+ TRAAK+ nodes of Ranvier in optic nerve of Ank3f/f mice with and without intravitreal injection of AAV-Cre-tdT. N = 3 mice of each genotype. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons.
Since the clustering of AIS Kv7 and Nav channels requires AnkG (Garrido et al., 2003; Lemaillet et al., 2003; Pan et al., 2006), we next determined if AIS clustering of TRAAK also requires AnkG. We used AAV and CRISPR/Cas9-mediated genome editing to transduce DIV0 neurons and disrupt the Ank3 gene which encodes AnkG. At DIV 21, we labeled these neurons for AnkG and TRAAK; the 3X sgRNA AAV also expresses a spaghetti monster fluorescent protein with HA tags (smFP-HA). We found that control sgRNAs had no effect on AIS AnkG or AIS TRAAK, but HA+ neurons lacking AIS AnkG also had no clustered AIS TRAAK (Fig. 4, F and G). To confirm the identity of the axon in these experiments, we labeled neurons lacking AnkG with TRIM46, a marker of the AIS cytoskeleton that is retained in the proximal axon (van Beuningen et al., 2015). We found that TRIM46-labeled axons that lacked AIS AnkG had no clustered AIS TRAAK (Fig. 4, H and I). Thus, AIS clustering of TRAAK requires AnkG.
To determine if nodal TRAAK requires AnkG, we immunostained dorsal and ventral roots from Avil-Cre;Ank3f/f mice (AnkG cKO) using antibodies against TRAAK and AnkG. Nodes in dorsal roots of AnkG cKO mice lack AnkG since recombination occurs only in sensory neurons; ventral root nodes of Ranvier have normal levels of AnkG. In contrast to control mice, we found TRAAK and AnkG were absent from AnkG cKO dorsal root nodes (Fig. 4, J and K). To determine if AnkG is required for TRAAK clustering at CNS nodes, we performed intravitreal injections of AAV-Cre-tdTomato in Ank3f/f mice to remove AnkG from the CNS nodes of Ranvier. We found that CNS nodes lacking AnkG also lacked nodal TRAAK (Fig. 4, L and M), even though Nav channel clustering in AnkG cKO mice can be compensated for by AnkR in both the CNS and PNS (Ho et al., 2014). These results show that TRAAK clustering at nodes and AIS requires AnkG and that AnkR cannot compensate.
How is TREK-1 clustered at the AIS? Although we identified an AnkG-dependent localization motif in TRAAK, we were unable to identify any motif in TREK-1. Since TRAAK and TREK-1 can form heteromeric channels (Blin et al., 2016; Levitz et al., 2016), we considered whether TREK-1’s localization depends on TRAAK. We first validated our TREK-1 antibody. We used AAV and CRISPR/Cas9 to disrupt TREK-1 expression in DIV 1 primary cortical neurons; we generated one AAV with three sgRNAs targeting TREK-1 (TREK-1 3X sgRNA); the 3X sgRNA AAV also expresses a spaghetti monster fluorescent protein with HA tags (smFP-HA). We then immunostained these neurons at DIV 21 using antibodies against TREK-1, HA, and AnkG. Compared with neurons expressing control sgRNAs (Fig. 5 A), HA+ neurons with sgRNAs targeting TREK-1 had intact AnkG-labeled AIS but no AIS TREK-1 (Fig. 5, A and B). We next used AAV and CRISPR/Cas9 to disrupt TRAAK expression in DIV 1 primary cortical neurons; we generated two different AAVs with distinct sgRNAs targeting TRAAK (TRAAK 3X sgRNAs). We then immunostained these neurons at DIV 21 using antibodies against TRAAK, AnkG, HA, and TREK-1. Compared with neurons expressing control sgRNAs (Fig. 5, C and E), HA+ neurons with sgRNAs targeting TRAAK had intact AnkG-labeled AIS, but neither AIS TRAAK nor AIS TREK-1 (Fig. 5, D and E).
TREK-1 clustering at the AIS and nodes requires TRAAK. (A) Immunostaining of DIV 21 cortical neurons transduced with AAV to express control 3X sgRNAs+smFP-HA or TREK-1 3X sgRNAs + smFP-HA and Cas9. Neurons were immunostained for TREK-1 (green), HA (red), and AnkG (magenta). Arrowheads indicate AIS. Scale bars, 10 μm. (B) Quantification of HA+ AIS TREK-1+ neurons transduced with AAV to express control or TREK-1 sgRNAs and Cas9 (the Cas9 AAV expresses HA). N = 3 cultures for each condition. n = 100 cortical neurons measured culture. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons. (C and D) Immunostaining of DIV 21 cortical neurons transduced with AAV to express control 3X sgRNAs+smFP-HA (A) or TRAAK (B) 3X sgRNAs + smFP-HA and Cas9. Neurons were immunostained for TRAAK or TREK-1 (green), HA (red), and AnkG (magenta). Arrowheads indicate AIS. Scale bars, 10 μm. (E) Quantification of HA+ AIS TREK-1+ neurons transduced with AAV to express control or TRAAK sgRNAs and Cas9 (the Cas9 AAV expresses HA). N = 3–4 cultures for each condition. n = 20–25 hippocampal neurons measured culture. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons. (F) Immunostaining of Cas9 transgenic mouse optic nerves using antibodies against TRAAK (green), TREK-1 (green), Caspr (red), and AnkG (magenta). Arrowheads indicate nodes of Ranvier. Scale bar, 5 μm. (G) Immunostaining of optic nerves from Cas9 transgenic mice after intravitreal injection of AAV to express TRAAK sgRNAs. Optic nerves were immunostained using antibodies against TRAAK (green), TREK-1 (green), Caspr (red), and AnkG (magenta). Arrowheads indicate nodes of Ranvier. Scale bar, 5 μm. (H) Quantification of the number of TRAAK+ and TREK-1+ nodes of Ranvier. N = 3 mice for controls, and 4 mice for TRAAK sgRNA #1. n = 150–200 nodes of Ranvier measured for each mouse. Error bars indicate ± SEM. **P < 0.001; two-tailed t test. (I) Illustration of the interactions occurring between ion channels, ankyrins, and spectrins at nodes of Ranvier and the AIS.
TREK-1 clustering at the AIS and nodes requires TRAAK. (A) Immunostaining of DIV 21 cortical neurons transduced with AAV to express control 3X sgRNAs+smFP-HA or TREK-1 3X sgRNAs + smFP-HA and Cas9. Neurons were immunostained for TREK-1 (green), HA (red), and AnkG (magenta). Arrowheads indicate AIS. Scale bars, 10 μm. (B) Quantification of HA+ AIS TREK-1+ neurons transduced with AAV to express control or TREK-1 sgRNAs and Cas9 (the Cas9 AAV expresses HA). N = 3 cultures for each condition. n = 100 cortical neurons measured culture. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons. (C and D) Immunostaining of DIV 21 cortical neurons transduced with AAV to express control 3X sgRNAs+smFP-HA (A) or TRAAK (B) 3X sgRNAs + smFP-HA and Cas9. Neurons were immunostained for TRAAK or TREK-1 (green), HA (red), and AnkG (magenta). Arrowheads indicate AIS. Scale bars, 10 μm. (E) Quantification of HA+ AIS TREK-1+ neurons transduced with AAV to express control or TRAAK sgRNAs and Cas9 (the Cas9 AAV expresses HA). N = 3–4 cultures for each condition. n = 20–25 hippocampal neurons measured culture. Error bars indicate ± SEM. **P < 0.001; one-way ANOVA with multiple comparisons. (F) Immunostaining of Cas9 transgenic mouse optic nerves using antibodies against TRAAK (green), TREK-1 (green), Caspr (red), and AnkG (magenta). Arrowheads indicate nodes of Ranvier. Scale bar, 5 μm. (G) Immunostaining of optic nerves from Cas9 transgenic mice after intravitreal injection of AAV to express TRAAK sgRNAs. Optic nerves were immunostained using antibodies against TRAAK (green), TREK-1 (green), Caspr (red), and AnkG (magenta). Arrowheads indicate nodes of Ranvier. Scale bar, 5 μm. (H) Quantification of the number of TRAAK+ and TREK-1+ nodes of Ranvier. N = 3 mice for controls, and 4 mice for TRAAK sgRNA #1. n = 150–200 nodes of Ranvier measured for each mouse. Error bars indicate ± SEM. **P < 0.001; two-tailed t test. (I) Illustration of the interactions occurring between ion channels, ankyrins, and spectrins at nodes of Ranvier and the AIS.
To determine if nodal TREK-1 depends on TRAAK, we used 2–3 mo-old Cas9 transgenic mice that were intravitreally injected with AAV for expression of TRAAK 3XsgRNAs. 5 wk after injection, we collected optic nerves. Whereas control mice had clear TRAAK and TREK-1 clustering at CNS nodes (Fig. 5, F and H), mice transduced with AAV to express the TRAAK 3XsgRNAs had AnkG+ nodes that lacked both TRAAK and TREK-1 (Fig. 5, G and H). These results show that TREK-1 is clustered at the AIS and nodes of Ranvier through TRAAK.
The results reported here challenge previous conclusions regarding the subcellular localization of TRAAK and TREK-1 and extend our understanding of the key molecular determinants and interdependencies that govern the assembly and composition of the AIS and nodes of Ranvier. We report four main findings: (1) TRAAK and TREK-1 are highly enriched at both the AIS and nodes of Ranvier, (2) TRAAK has an evolutionarily conserved C-terminal AIS and nodal localization motif that is required for its interaction with AnkG, (3) TRAAK clustering at the AIS and nodes requires AnkG, and (4) TREK-1 clustering at the AIS and nodes requires TRAAK.
TRAAK and TREK-1 are closely related members of the two-pore-domain K+ (K2P) channel family (Enyedi and Czirják, 2010). Mice lacking TRAAK or TREK-1 have hypersensitivity to mechanical force, mechanical and temperature allodynia, and mechanical hyperalgesia during inflammation (Alloui et al., 2006; Noël et al., 2009), consistent with the fact that these channels underlie leak K+ currents and have both thermal and mechanical sensitivity (Kang et al., 2005; Noël et al., 2009). The recent discovery that TRAAK and TREK-1 repolarize the nodal membrane to facilitate rapid action potential propagation in mammalian myelinated axons led to renewed interest in these channels and their functions in the nervous system (Brohawn et al., 2019; Kanda et al., 2019). Although previous studies examined the functional importance of K2P channels at nodes of Ranvier (Kanda et al., 2019), our results showing these channels are also present at the AIS should prompt neurophysiologists to investigate their functions at the AIS and contributions to action potential initiation.
Both Nav and Kv7 channels found at the AIS and nodes of Ranvier have analogous AnkG-binding motifs that are thought to have evolved independently, with the Kv7 channel motif evolving after myelin and nodes of Ranvier (Hill et al., 2008). The similarity of the motif suggests that Nav and Kv7 channels may have overlapping binding sites on AnkG within its 24 ankyrin repeats (Xu and Cooper, 2015). However, Nav and Kv7 channels are found at nodes and the AIS at a ratio of ∼40:1 (Battefeld et al., 2014; Röper and Schwarz, 1989), suggesting differences in expression level, or posttranslational modifications modulating trafficking or binding affinity. For example, phosphorylation of the Nav channel Ankyrin-binding-motif can modulate the motif’s affinity for AnkG by 1,000-fold (Bréchet et al., 2008). In contrast, the AnkG-dependent motif we identified in TRAAK is entirely distinct and its potential interaction with AnkG may be very different compared with Nav and Kv7 channels. Like the Nav and Kv7 localization motif, the TRAAK motif has evolutionarily conserved tyrosine and serine residues that may be phosphorylated. Our results substituting the serine at amino acid 356 with a non-phosphorylatable alanine suggest that TRAAK’s affinity for AnkG may also depend on serine-dependent phosphorylation. Like the Kv7 AnkG-binding motif, TRAAK’s clustering motif evolved after myelin and nodes of Ranvier. Strong evolutionary pressure for efficient and rapid action potential propagation may have driven the acquisition of the AnkG-dependent motifs in these K+ channels (Hill et al., 2008).
TRAAK’s dependence on AnkG for its own and TREK-1’s nodal and AIS clustering expands our understanding of the central role played by AnkG in node and AIS assembly. Our results suggest that at least three different types of ion channels may bind simultaneously to AnkG: Nav, Kv, and K2P channels (Fig. 5 I). At the AIS and nodes, the ion channel/AnkG complex is further stabilized and retained through binding to the βIV/αII spectrin-based membrane periodic cytoskeleton. While our experiments support a direct interaction between AnkG and TRAAK, we cannot rule out an indirect interaction. Structural studies of Ankyrins in complex with membrane binding partners suggest that the unstructured nature of their ankyrin-interacting domains (as is found in TRAAK) allowed for diverse proteins to evolve motifs that associate with the peptide binding groove formed by ankyrin’s 24 ankyrin-repeat solenoid structure (Wang et al., 2014). Surprisingly, TRAAK cannot bind to AnkR. Although AnkG cKO mice have normal nodal Nav channel clustering due to compensation by AnkR and βI spectrin (Fig. 5 I; Ho et al., 2014), AnkR fails to cluster Kv7 channels and cannot compensate for the loss of AnkG at AIS (Liu et al., 2020; Wang et al., 2018). Instead, when AnkR is present at nodes, AnkR clusters Kv3.1b/3 K+ channels to nodes of Ranvier (Fig. 5 I). Thus, although Nav channels promiscuously bind all ankyrins, K+ channels are much more selective.
TREK-1 is also found at the AIS and nodes of Ranvier but does not have an AnkG-dependent motif. TREK-1 and TRAAK can form heterodimers (Levitz et al., 2016). We conclude that nodal and AIS K2P channels normally consist of heterodimers of TRAAK and TREK-1, although we cannot rule out the possibility of TRAAK homodimers at these sites. Other Kv channels that form heteromultimeric complexes have domains that interact with scaffolding proteins. For example, the Kv1 channels have C-terminal PDZ domain-binding motifs, and the AIS-enriched Kv7.2 and Kv7.3 channels each have AnkG-binding motifs. We are unaware of other heteromeric K+ channels where one subunit’s localization is dictated entirely by other subunits.
In conclusion, our studies identify the molecular mechanism whereby K2P channels are clustered at the AIS and nodes of Ranvier. Our results illustrate how multiple ion channels engage simultaneously with ankyrin scaffolding proteins to facilitate action potential initiation and rapid action potential propagation in mammalian myelinated axons.
Materials and methods
Animals
Timed pregnant Sprague–Dawley rats were obtained from Charles River Laboratories. Rats were euthanized for embryo collection at E18. Ank3 conditional-null mutant mice harbor an allele in which exons 23 and 24 of the Ank3 gene (encoding the AnkG protein) are flanked by loxP sites (Ank3f/f mice) and can be excised in the presence of Cre recombinase (Cat# JAX:029797), RRID: IMSR_JAX:029797). Adult ICR mice were used for intravitreal injection of AAV for overexpression of Flag-TRAAK. Transgenic Cas9 mice (Cat# JAX:027650, RRID: IMSR_JAX:027650) were used for intravitreal injection of AAV to perform knockout of TRAAK. Our studies used both male and female mice. All experimental procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee at Baylor College of Medicine protocol AN-4634.
Hippocampal neuron cultures
Primary cultures of hippocampal and cortical neurons were obtained from E18 Sprague–Dawley rat embryos. Hippocampi were dissected and dissociated. For imaging, neurons were plated onto Poly-D-Lysine (Sigma-Aldrich) and laminin-coated glass coverslips (Life Technologies) at a density of ∼1.25 × 104 cells/cm2. Hippocampal and cortical neurons were maintained in a Neurobasal medium (Life Technologies) containing 1% Glutamax (Life Technologies), 1% penicillin and streptomycin (Life Technologies), and 2% B27 supplement (Life Technologies) in an incubator at 37°C with 5% CO2. Half of the media was removed and replaced every 5 days.
Antibodies
The primary antibodies used here include mouse monoclonal antibodies against AnkG (1:200, UC Davis/NIH NeuroMab N106/36, RRID:AB_10673030), Caspr (1:200, UC Davis/NIH NeuroMab K65/35, RRID:AB_2083496), CD4 (1:500, Cat# sc-19541, RRID:AB_10554681; Santa Cruz Biotechnology), Flag-tag or DDDDK-tag (1:1,000, Cat# M185-3L, RRID:AB_11123930; MBL International Corporation), and Myc (1:2,000, Cat# M192, RRID:AB_11160947; MBL International Corporation). The rabbit polyclonal primary antibodies used include antibodies against βIV spectrin (1:200, RRID:AB_2315634; Rasband lab), GFP (1:200, Cat# A-11122, RRID:AB_221569; Invitrogen), and GFP (1:3,000, Cat# 50430-2-AP, RRID:AB_11042881; Proteintech). A rabbit polyclonal antibody against TRAAK (1:200) was a gift from Dr. Roderick MacKinnon (The Rockefeller University, New York, NY, USA). A rabbit polyclonal antibody against TREK-1 (1:200) was generated against the following sequence of human TREK-1: DPKSAAQNSKPRLSFSTKC. The antibodies were affinity-purified in the Rasband laboratory (RRID:AB_3101972). We used a chicken polyclonal primary antibody against MAP2 (1:4,000; Cat# CPCA-MAP2, RRID:AB_2138173; EnCor Biotechnology) and guinea pig polyclonal primary antibodies against AnkG (1:500; Cat# 386 005, RRID:AB_2725774; Synaptic Systems) and TRIM46 (1:1,000; Cat# 377 308, RRID:AB_2924929; Synaptic Systems). Secondary antibodies were purchased from Thermo Fisher Scientific and Jackson ImmunoResearch Laboratories and used at a concentration of 1:1,000.
Immunofluorescence labeling
Cultured rat primary hippocampal neurons were fixed in 4% paraformaldehyde (PFA, pH 7.2) for 15 min at 4°C. Fixed neurons were permeabilized and blocked with 10% normal goat serum in 0.1 M PB with 0.3% Triton X-100 (PBTGS) for 1 h at 4°C. Cells were then incubated in primary antibodies diluted in PBTGS for 1 h at 4°C. Cells were then washed three times using PBTGS for 5 min each. Fluorescent secondary antibodies were then diluted in PBTGS and added to cells for 1 h at 4°C. Cells were then washed once using PBTGS, 0.1 M PB, and finally 0.05 M PB for 5 min each. Coverslips were then mounted using Vectashield plus (Vector Labs) antifade mounting media.
For immunolabeling of cultured rat primary hippocampal and cortical neurons using the TRAAK and TREK-1 antibodies, neurons were fixed with a glyoxal solution (Glyox+MeOH; 3% glyoxal, 0.8% acetic acid, 20% methanol) with a pH of 5.0 for 10 min at 4°C. Fixed neurons were then postfixed with methanol prechilled to −20°C for 10 min. Neurons were then washed three times using PBS and blocked with PBSTg (0.2% fish skin gelatin in 0.1% TX-100 in PBS) for 30 min at RT. Neurons were then incubated in primary antibody diluted in PBSTg for 1 h at RT and then washed three times using PBS. Neurons were then incubated in secondary antibodies diluted in PBSTg for 1 h at RT and then washed three times using PBS. Coverslips were then mounted using Vectashield plus (Vector Labs) anti-fade mounting media. In some instances, cultured rat primary hippocampal neurons were first detergent-extracted before fixation as follows: unfixed neurons were treated with ice-cold PBS with 0.5% Triton X-100 for 10 min on ice.
The localization of CD4-TRAAK chimeras was evaluated by surface labeling. Neurons were fixed in 4% PFA (pH 7.2) for 15 min at 4°C and then blocked with 10% normal goat serum in 0.1 M PB (PBGS) for 30 min at 4°C. Cells were then incubated with anti-CD4 antibodies diluted in PBGS for 1 h at 4°C. Cells were then washed three times using PBGS for 5 min each. Cells were then permeabilized with PBTGS for 15 min at 4°C and then incubated with anti-βIV spectrin antibodies for 1 h at 4°C. Cells were then washed, and primary antibodies were labeled using secondary antibodies as described above.
For labeling of tissues, mice were euthanized and perfused with a glyoxal solution (Glyox+MeOH; 3% glyoxal, 0.8% acetic acid, 20% methanol). The brain, sciatic nerve, and optic nerve were dissected out and post-fixed overnight in Glyox+MeOH. Tissues were then immersed in 30% sucrose overnight at 4°C. Brain tissues were embedded in Tissue-Tek OCT (Cat# 4583; Sakura Finetek) mounting medium and frozen on dry ice. Brains were sectioned at 25 μm using a cryostat (Thermo Fisher Scientific; Cryostar NX70). Sciatic and optic nerves were frozen in OCT directly on the stage of the microtome (HM 450 Microtome; Thermo Fisher Scientific) and were sectioned at 10 μm. Sections were placed on 1% bovine gelatin precoated coverslips (Thermo Fisher Scientific). Sections were postfixed in methanol prechilled to −20°C for 10 min. Sections were then washed with 0.1 M PB at 4°C, permeabilized with 0.1 M PB with 0.3% Triton X-100 (PBST) at 4°C for 15 min, incubated with Image-iT FX Signal Enhancer (Cat# 11932S; Cell Signaling Technology) at room temperature for 30 min, washed with 0.1 M PB at 4°C, washed with PBST at 4°C, blocked with 2% normal goat serum in PBST (PBST-NGS) at room temperature for 1 h, incubated with primary antibodies in PBST-NGS at 4°C overnight, washed with PBST-NGS at 4°C, incubated with secondary antibodies in PBST at 20°C for 1 h, washed with PBST-NGS at 4°C, washed with 0.1 M PB at 4°C, and then mounted with Vectashield plus (Vector Labs) antifade mounting media.
For immunostaining of HEK293T cells and surface recruitment of AnkG by TRAAK, plasmids were cotransfected into HEK293T cells using Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. The following plasmids were used: hSyn-Myc-TRAAK and AnkG-GFP. After 48 h, transfected cells were fixed in 4% paraformaldehyde for 30 min at 4°C in PBS. After three washes with PBS, cells were permeabilized for 1 h with 0.3% Triton X-100 with 10% normal goat serum in PBS (PBTGS). After this, antibodies against Myc and GFP were added for 1 h at room temperature. After three washes with PBTGS, secondary antibodies were incubated for 1 h at room temperature and washed with PBS.
Plasmids
To generate CD4-TRAAK C-terminal or truncated chimeras, regions of the C-terminal cytoplasmic domain of TRAAK were PCR-amplified from full-length mouse TRAAK and then inserted into hSyn-CD4 vector using In-Fusion cloning reactions (Takara Bio). PCR primers were as follows.
CD4-Flag forward: 5′-GAGAAGGTACCGGATCCGCCACCATGAACCGGGGAGTCCCTTTTAGG-3′
CD4-Flag reverse: 5′-CAGTTTTTGCTCCATGTACCGGCACCTGACACAGAAGA-3′
CD4-TRAAK forward: 5′-CATGGACTACAAGGACGACGACGATAAAGTCGACATGTCCCGCCGAACTCGG-3′
CD4-TRAAK reverse: 5′-AGCTTGATATCGAATTCCTACACCGGCACGGCCTTGTCTCGGAGTCGCCC-3′
CD4-Flag-C378 reverse: 5′-TGATTATCGATAAGCTCTATGGGTTGGGTCGGCGGCGACCCCGAGGAGC-3′
CD4-Flag-C358 reverse: 5′-TGATTATCGATAAGCTCTACGTGTCTGAGGACTCGTCGATGAAGGCCAG-3′
CD4-Flag-C353 reverse: 5′-AGCTTGATATCGAATTCCTAGTCGATGAAGGCCAGATTCTCACTGGGGTA-3′
CD4-Flag-C348 reverse: 5′-AGCTTGATATCGAATTCCTAATTCTCACTGGGGTAATCCAGAGCTGAGGC-3′
CD4-Flag-C343 reverse: 5′-AGCTTGATATCGAATTCCTAATCCAGAGCTGAGGCCGTGGGCGGGGACGG-3′
CD4-Flag-C338 reverse: 5′-TGATTATCGATAAGCTCTACGTGGGCGGGGACGGAGTCTCAACCTTCTC-3′
CD4-Flag-C318 reverse: 5′-TGATTATCGATAAGCTCTATGGCTCAACAACAGCAGGCGGTGCCGGCAA-3′
CD4-Flag-C298 reverse: 5′-TGATTATCGATAAGCTCTATGGCGGCGGGGCGCTGGGCCCAGTTCGCTG-3′
CD4-Flag-C278 reverse: 5′-TGATTATCGATAAGCTCTAGCTAGCAGCCTGTGCCGTTAGGCCACCCAT-3′
To generate Flag-tagged full-length, truncated, shark motif swap, and phosphomutant TRAAK constructs, the relevant C-terminal regions of TRAAK were PCR-amplified from full-length (FL) mouse TRAAK and then inserted into hSyn-Flag vector for immunostaining experiments. PCR primers were as follows.
Flag-TRAAK FL forward: 5′-GAGAAGGTACCGGATCCGCCACCATGGACTACAAGGACGACGACGATAAA-3′
Flag-TRAAK FL reverse: 5′-AGCTTGATATCGAATTCCTACACCGGCACGGCCTTGTCTCGGAGTCGCCC-3′
Flag FL C358 forward: 5′-CAGACACGTAGGAATTCGATATCAAGCTTATCG-3′
Flag FL C358 reverse: 5′-ATTCCTACGTGTCTGAGGACTCGTCG-3′
Flag FL C353 forward: 5′-TCATCGACTAGGAATTCGATATCAAGCTTATCG-3′
Flag FL C353 reverse: 5′-ATTCCTAGTCGATGAAGGCCAGATTCTC-3′
Flag FL delta motif forward: 5′-CTCTGGATACGCAGAGTGAGCGTGGC-3′
Flag FL delta motif reverse: 5′-TCTGCGTATCCAGAGCTGAGGCCGTGG-3′
Flag FL whale shark forward: 5′-TCTGGCGTTTATTGATGCGGAAGAAAGCGATCAGAGTGAGCGTGGCTGTG-3′
Flag FL whale shark reverse: 5′-TCAATAAACGCCAGATCTTCGCCCAGAAAATCCAGAGCTGAGGCCGTG-3′
Flag FL elephant shark forward: 5′-GGTTGACGTACATTGATGCCACGCCTGGCCAGAGTGAGCGTGGCTGTG-3′
Flag FL elephant shark reverse: 5′-CAATGTACGTCAACCCCTGGATGGTGAAATCCAGAGCTGAGGCCGTG-3′
Flag FL S356A forward: 5′-CGAGTCCGCCGACACGCAGAGTGAGCGTG-3′
Flag FL S356A reverse: 5′-GTGTCGGCGGACTCGTCGATGAAGGCCAG-3′
The pcDNA3-Traak-Myc plasmid was linearized by restriction enzyme digestion with BbvCI and PpuMI. The different mutants listed above were PCR-amplified from the AAV plasmids using the following primers.
BbvCI-R 5′-CCGCCCACGGCCTCAGCTCT-3′
PpuMI-F 5′-GGAGTCGCCCAGGACCCCG-3′
The PCR products were gel-purified and then cloned into the linearized backbone by infusion cloning and confirmed by whole plasmid sequencing.
We constructed two different Kcnk4 (TRAAK) AAVs to express three sgRNAs (TRAAK 3X sgRNAs) and one Kcnk2 (TREK-1) AAV to express three sgRNAs (TREK-1 3x sgRNAs). We also constructed Ank3 (AnkG) AAVs to express three sgRNAs targeting Ank3. All 3X sgRNA constructs also expressed spaghetti monster fluorescent protein-HA (smFP-HA). The sequences are as follows.
Kcnk4 3X sgRNA #1: 1-1, 5′-GGGACCATCATCACTACCAT-3′; 1-2, 5′-GCCAGCAGCATCCCGAACAG-3′; 1-3, 5′-CTCCATGTAGGAGAACACGA-3′
Kcnk4 3X sgRNA #2: 2-1, 5′-AGAAAAAGAAGGCGCTGCCC-3′; 2-2, 5′-TCCTCTCTGCGCCGGGGCAT-3′ 2-3, 5′-GAAGATACAAAAGAGACGCC-3′
Kcnk2 3X sgRNA: 1, 5′-AACATCTCCCCACGAACTGA-3′; 2, 5′-CTCCAATCAAGTTAGTCACAT-3′; 3, 5′-GTCATATTCAAGCACATAGA-3′
Ank3 3X sgRNA: 1, 5′-GCTTTATGGTGGACGCGAGA-3′; 2, 5′-GCCAGTAGGCTGGTAGAAAT-3′; 3, 5′-GCGTGTCCAATGGGTACAAG-3′
Control 3X sgRNA: 1, 5′-CTGTCTTCATCATGGCCGAC-3′; 2, 5′-GTTCGCATTATCCGAACCAT-3′; 3, 5′-TAAGCGTCGCAAGAAGACGG-3′
Adeno-associated virus (AAV) production
For AAV use in vitro, small-scale cell lysates were produced using the AAVpro Purification Kit (All Serotypes) (Takara) with slight modifications. Briefly, HEK293T cells were triple-transfected with AAV plasmid, helper plasmid (Cat# 240071; Agilent Technologies), and serotype PHP.S plasmid (#103005; Addgene plasmid) with PEI Max (Cat# 24765; Polysciences). The medium was changed the next day following transfection and cells were incubated for 3 days after transfection. HEK cells were then collected and lysed with the AAV Extraction Solution A plus. The extracted solution was centrifuged at 10,000 × g for 10 min to remove debris and mixed with Extraction Solution B. This small-scale AAV solution was stored at −80°C until use.
For AAV use in vivo, HEK293T cells were subjected to a triple-transfection procedure involving the introduction of an AAV plasmid, a helped plasmid, and an AAV2-RC plasmid, with PEI MAX (Cat # 24765; Polysciences). One day following transfection, the culture medium was replaced and cells were incubated for another 2 days. HEK293T cells were then harvested and subjected to further AAV extraction and purification using the AAVpro Purification Kit Maxi (All Serotypes) (Cat# 6666; Takara).
Transfection of neurons
Plasmids were transfected into DIV12 hippocampal neurons using Lipofectamine 3000 (Thermo Fisher Scientific). Tube A containing 100 μl of Neurobasal medium (Life Technologies) and 1 μl of Lipofectamine 3000 was prepared. Tube B containing 100 μl of Neurobasal medium and 1 μg of respective DNA was prepared. Tubes were incubated for 5 min at room temperature. Tubes A and B were combined and gently vortexed. The tube was incubated for 20 min at room temperature. Following incubation, 200 μl of the DNA and Lipofectamine 3000 complex was added to respective wells. After 4 h, the DNA and Lipofectamine 3000 complex was aspirated, and cells were washed with Neurobasal medium once. The following plasmids were used: hSyn-Flag-TRAAK, hSyn Myc-TREK1, hSyn-TRAAK-Flag, and hSyn-TREK1-Myc. After 48 h, neurons were fixed in 4% paraformaldehyde for 30 min at 4°C in PBS. After three washes with PBS, cells were permeabilized for 1 h with 0.3% Triton X-100 with 10% normal goat serum in PBS (PBTGS). After this, antibodies against Myc or FLAG were added for 1 h at room temperature. After three washes with PBTGS, secondary antibodies were incubated for 1 h at room temperature and washed with PBS.
Viral transduction of neurons
For viral transduction of cultured neurons, 10 μl of AAV (TRAAK or TREK-1 AAV and their variants) and 10 μl of AAV-Cas9 were added into each well of a 12-well plate at 10–12 DIV. The medium was replaced 2 days after infection. For sgRNA-mediated KO of AnkG, TRAAK, and TREK-1 in cultured neurons, 10 μl of AAV (sgRNA) and 10 μl of AAV-Cas9 were added into a well of a 12-well plate at DIV 0. The medium was replaced 2 days after infection and neurons were fixed and stained at DIV 21. For transduction of neurons in vivo, 1–2 μl of AAV were injected intravitreally in adult ICR or Cas9 transgenic mice.
Immunoprecipitation
Plates were seeded with HEK293T cells to reach ∼70–80% confluency. The following day, cells were transfected with plasmids using Lipofectamine 3000 (L3000001; Thermo Fisher Scientific). The media was changed the following day. 2 days after transfection, cells were lysed using a supplemented lysis buffer (1% [vol/vol] Triton X-100, 20 mM Tris-HCl pH 8.0, 10 mM EDTA, 150 mM NaCl, 10 mM NaN3) on ice for 15 min. Cells were then collected into corresponding chilled tubes and rotated end-to-end at 20 RPM at 4°C for 10 min. Lysed cells were then centrifuged at 14,000 × g at 4°C for 10 min. Supernatants were then collected and 4 μl of rabbit anti-GFP antibody or 2 μl of mouse anti-Myc antibody was added; the lysate antibody mixture was then rotated end-to-end at 20 RPM at 4°C overnight. Antibodies were then immunoprecipitated using Protein A (Cat# 28951378; Cytiva) or Protein G (Cat# 28951379; Cytiva) magnetic sepharose beads. The beads were pretreated with 0.1% BSA before being added to the lysate antibody mixture. Beads were then added to the lysate antibody mixture and rotated at 20 RPM at 4°C for 1 h. After washing the beads with lysis buffer seven times, the supernatant was removed and sample buffer was added to the beads. The precipitates were boiled at 95°C for 5 min. Input and immunoprecipitated proteins were analyzed by immunoblot with an acquisition time of 30 s. Light-chain–specific HRP-conjugated secondary antibodies were used to avoid interference with TRAAK detection (∼50 kDa).
Microscopy and image acquisition
Immunofluorescence labeling was visualized, and images were captured using an AxioImager (Carl Zeiss) fitted with an apotome for optical sectioning and a digital camera (Prime BSI; Teledyne Photometrics). AxioVision (Carl Zeiss) acquisition software was used for the collection of images. 40X (0.95 NA) and 100X (1.4 NA) objectives were used. Super-resolution imaging was performed by Stimulated Emission Depletion (STED) microscope using a STEDyCON (Abberior) system fitted to a Nikon Eclipse Ti2 microscope. The periodicity of TRAAK and AnkG was measured in Fiji-ImageJ after generating a line scan across the AIS. The distance between peak fluorescence intensities (maximum to maximum) was then measured. All imaging was performed at room temperature as cells and tissues were previously fixed before immunostaining.
Image analysis
For measurements of AIS and dendritic fluorescence intensity, 20–25 neurons per coverslip were imaged. To calculate the polarity index (PI) for each neuron, the mean fluorescence intensities along segments of dendrites and AIS were measured using NIH FIJI and averaged. The PI was determined using the formula: PI = (Ia/Id), where Ia represents the average axon intensity and Id represents the average dendrite intensity. A PI value of 1 indicates a protein distribution without polarization, while PI values >1 or <1 indicate polarization toward AIS or dendrites, respectively. For surface recruitment analysis, 20–25 cells per coverslip were imaged and fluorescence intensity was measured using NIH FIJI. This was used to calculate the fluorescence intensity at the cytoplasm and the cell membrane. Images were exported to Fiji, Adobe Photoshop, and Adobe Illustrator for figure presentation. Fig. 2 A was created using BioRender and Fig. 2 G was created using SnapGene.
Statistics
Data were analyzed using GraphPad Prism and Microsoft Excel. Statistical analyses were performed by two-tailed t test for two-group comparisons and by one-way ANOVA for multiple group comparisons. Data distribution was assumed to be normal, but this was not formally tested. Graphs are presented as the mean ± SEM.
Data availability
All data are included in the manuscript and all materials are available upon request.
Acknowledgments
This work was supported by National Institutes of Health grant R35 NS122073 and by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
Author contributions: G. Escobedo Jr.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing, Y. Ogawa: Investigation, Methodology, Resources, X. Ding: Investigation, Validation, Visualization, Writing—review & editing, M.N. Rasband: Conceptualization, Funding acquisition, Project administration, Supervision, Writing—original draft, Writing—review & editing, Y. Wu: Investigation.
References
Author notes
Disclosures: The authors declare no competing interests exist.