Border cells are an in vivo model for collective cell migration. Here, we identify the gene cactin as essential for border cell cluster organization, delamination, and migration. In Cactin-depleted cells, the apical proteins aPKC and Crumbs (Crb) become abnormally concentrated, and overall cluster polarity is lost. Apically tethering excess aPKC is sufficient to cause delamination defects, and relocalizing apical aPKC partially rescues delamination. Cactin is conserved from yeast to humans and has been implicated in diverse processes. In border cells, Cactin’s evolutionarily conserved spliceosome function is required. Whole transcriptome analysis revealed alterations in isoform expression in Cactin-depleted cells. Mutations in two affected genes, Sec23 and Sec24CD, which traffic Crb to the apical cell surface, partially rescue border cell cluster organization and migration. Overexpression of Rab5 or Rab11, which promote Crb and aPKC recycling, similarly rescues. Thus, a general splicing factor is specifically required for coordination of cluster polarity and migration, and migrating border cells are particularly sensitive to splicing and cell polarity disruptions.
Introduction
Embryonic development requires extensive cell migrations (Scarpa and Mayor, 2016; Yang et al., 2020). Movements of tumor cells, endothelial cells, fibroblasts, and immune cells also contribute to tumor metastasis (Stuelten et al., 2018). Such movements can be collective and involve multiple cell types. For example, cancer-associated fibroblasts lead migrating tumor cell collectives (Labernadie et al., 2017), macrophages promote breast cancer dissemination (Goswami et al., 2005), and in at least some types of cancer, cells circulating collectively are more effective at seeding distant metastases than single cells (Aceto et al., 2014; Au et al., 2016; Cheung et al., 2016).
Border cells in the Drosophila ovary serve as a model for collective, cooperative cell migration that is amenable to genetic screening and live imaging (Montell et al., 2012). Border cells are a group of 6–10 cells derived from the follicular epithelium, which surrounds the developing germline during oogenesis in Drosophila (Fig. 1, A–D). A special pair of follicle cells named polar cells develops at each end of the egg chamber. These polar cells secrete a cytokine that activates Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling and motility in the neighboring cells (Silver and Montell, 2001), which then begin to extend and retract dynamic protrusions in between the adjacent germ cells, called nurse cells. Eventually the cells delaminate from the epithelium and squeeze between the 15 nurse cells, traveling ∼150 μm until they reach the anterior border of the oocyte. When they arrive, the border cells attach to the oocyte and inwardly migrating follicle cells, called centripetal cells, forming a continuous epithelium in a process termed neolamination (Miao et al., 2020). Border cells and centripetal cells construct an eggshell structure essential for sperm entry (Montell et al., 1992) and secrete a critical patterning signal (Savant-Bhonsale and Montell, 1993). Thus, if border cell migration fails, females are sterile.
While much is known concerning the chemical, physical, mechanical, and adhesive factors that steer the border cells (Montell et al., 2012; Dai et al., 2020) and the cytoskeletal determinants of their morphology (Chen et al., 2020; Majumder et al., 2012; Aranjuez et al., 2016; Murphy and Montell, 1996), less is known about the mechanisms that govern their initial delamination from the follicular epithelium and the coordination of individual cell polarization, morphology, and behavior. Border cells undergo morphological hallmarks of a partial epithelial to mesenchymal transition (EMT), though they do not appear to depend upon classic EMT transcription factors. Instead, JAK/STAT activity is necessary and sufficient to confer migratory behavior (Silver and Montell, 2001). Multiple additional transcription factors feed back to distinguish migratory from nonmigratory cell fates (Jang et al., 2009; Berez et al., 2020; Monahan and Starz-Gaiano, 2013; Manning et al., 2017; Starz-Gaiano et al., 2008).
Here, we report that RNAi knockdown of the gene cactin causes severe defects in border cell delamination. In cactin-RNAi follicle cells, the apical proteins aPKC and Crumbs (Crb) become highly concentrated apically. Individual Cactin-depleted border cells are polarized and mobile, but protrusion, cluster polarity, organization, and migration are severely diminished. Reduction of aPKC ameliorates the defects, and tethering excess aPKC to apical membranes is sufficient to impair delamination. Cactin was first identified in Drosophila as a protein that binds and inhibits Cactus, which is an inhibitor of Dorsal, the fly NF-κB homolog (Lin et al., 2000). In Caenorhabditis elegans, the Cactin homolog CACN-1 inhibits the mig-2/Rac pathway to regulate distal tip cell migration (Tannoury et al., 2010), and human Cactin is a component of the spliceosome complex C. Human Cactin interacts with spliceosome-associated factors DHX8 and SRRM2 to ensure efficient pre-RNA splicing and sister chromatid cohesion (Zanini et al., 2017). We found that the Cactin spliceosome function is required for border cell cluster polarization and coordination of individual cell behaviors to produce coherent, collective movement. We report global changes in mRNA isoform expression in Cactin knockdown cells. Mutations in two affected genes, Sec23 and Sec24CD, which promote Crb trafficking to the apical domain, dominantly suppress cactin-RNAi phenotypes. We conclude that the general splicing factor Cactin is required for specific features of coordinating collective cell polarity and migration. Moreover, border cells are particularly vulnerable to polarity disruptions, possibly due to their loss of cues from the basement membrane and neighboring cells as they delaminate from the follicular epithelium. These findings may shed light on how mutations affecting general factors can have cell-type specific effects, a phenomenon observed in numerous human diseases.
Results
Cactin is required for border cell delamination
To identify mechanisms of border cell delamination, we performed an RNAi screen of genes previously identified by mRNA expression profiling as enriched in border cells (Wang et al., 2006). Using c306-Gal4, which is expressed in border cells before and during migration (Fig. 1, A–D′), three UAS-cactin-RNAi lines showed strong delamination defects compared to controls (Fig. 1, E–H′; and Fig. S1, A–D). In control egg chambers, border cells are specified in stage 8 (Fig. 1, A and A′), then round up and extend and retract protrusions (Fig. 1, B and B′), migrate in between the nurse cells during stage 9 (Fig. 1, C and C′), and arrive at the oocyte by stage 10 (Fig. 1, D and D′). In Cactin-depleted border cells, however, no major forward-directed protrusions were observed (Fig. 1, E–G′). By stage 10, most cactin-RNAi-expressing clusters had failed to delaminate and remained at the egg chamber anterior (Fig. 1, H and H′).
Next, we asked which cell types required Cactin. The c306-Gal4 driver is expressed in both the outer, migratory border cells and the inner, nonmigratory polar cells, which the cluster carries to the oocyte (Fig. 1 I). To distinguish whether Cactin is required in border cells and/or polar cells, we expressed cactin-RNAi with fruitless-Gal4 (Fig. 1 J), which expresses in outer, migratory cells but not polar cells, and upd-Gal4 (Fig. 1 L), a polar cell driver. Fruitless-Gal4 caused a similar delamination defect as c306-Gal4 (Fig. 1 L), while upd-gal4 did not (Fig. 1 L). These results show that Cactin is required in migratory border cells.
We then generated UAS-cactin and UAS-cactin::RFP transgenic lines. Both UAS-cactin and UAS-cactin::RFP rescued cactin-RNAi delamination defects (Fig. 1, L–O). UAS-cactin fully rescued the delamination defect, while UAS-cactin::RFP reduced the incidence of delamination defects from 90% to ∼25% (Fig. 1 L), suggesting that RFP slightly impaired Cactin function. The rescue was not a consequence of titrating the Gal4 because the addition of UAS-mCherry-CAAX did not provide any rescue. Cactin::RFP protein localized predominantly to nuclei (Fig. 1 O; and Fig. S1, E and E′) and was substantially reduced in the presence of cactin-RNAi, confirming the effectiveness of the RNAi (Fig. S1, F–G). We conclude that Cactin is a predominantly nuclear protein required in migratory border cells during the delamination process.
Cactin functions independently of JAK/STAT or Toll signaling pathways in border cells
In stage 8, the polar cells secrete Upd, which activates STAT in neighboring cells (Fig. S2, A and A′) to specify them as migratory border cells. Fewer border cells form when JAK/STAT is reduced, and those that do form exhibit profound delamination and migration defects (Silver and Montell, 2001). To test whether Cactin knockdown might cause delamination defects by compromising JAK/STAT signaling, we combined the 10XSTAT-GFP activity reporter (Bach et al., 2007) with cactin-RNAi. We then measured GFP intensity in anterior follicle cells adjacent to the polar cells of stage 8 egg chambers (Fig. S2, A–B′). 10XSTAT-GFP intensity was ∼17% lower in cactin-RNAi–expressing cells (Fig. S2 C). This effect cannot explain the strong delamination defect caused by cactin-RNAi because a 50% reduction in STAT causes incomplete migration in only 10% of egg chambers and no delamination defect (Silver et al., 2005). Further, because JAK/STAT signaling alters border cell specification, border cell numbers are reduced when STAT activity is significantly impaired (Silver and Montell, 2001). We found no meaningful difference in border cell number between controls (3.9 ± 0.6, n = 9) and cactin-RNAi (3.5 ± 1.3, n = 10). We conclude that Cactin does not affect border cell delamination through the JAK/STAT pathway.
In Drosophila, Cactin was first identified as a Cactus binding protein, and Cactin overexpression enhanced cactus mutant phenotypes, which suggests Cactin is an inhibitor of Cactus (Lin et al., 2000). However, overexpression of Cactus did not cause a border cell delamination defect (Fig. S2, D–E′), and the Cactus protein level was unchanged in cactin-RNAi-expressing cells (Fig. S2, F and F′). Cactus is the fly ortholog of IkB, an inhibitor of NF-κB signaling. Thus, Cactin promotes NF-κB activity at least in some contexts. In Drosophila, Cactus plays a central role in Toll receptor signaling, which is required for dorsal/ventral patterning and the immune response (Valanne et al., 2011). To further test whether Cactin is related to Cactus in border cells, we performed an RNAi screen for components of the Drosophila Toll signaling pathway. We found that no RNAi line targeting the ligand Spätzle, the receptor Toll, the kinase Pelle, or myd88 caused a border cell delamination defect (Fig. S2 G). The only exceptions were two RNAi lines targeting the fly NF-κB homolog Dorsal (Fig. S2 G). By immunostaining, Dorsal is expressed in stalk and polar cells, but not detectably in border cells (Fig. S2, H–I′). Dorsal accumulates in the cytoplasm whereas it must translocate to the nucleus to function as a transcription factor. Dorsal expression and localization were unchanged in cactin-RNAi–expressing cells compared to control (Fig. S2, I–K′). Although it seems that Dorsal contributes to border cell migration perhaps by functioning in polar cells, together these results suggest Cactin function in border cells is independent of the Toll signaling pathway.
Cactin is required for border cell cluster polarization
To determine how border cell delamination is affected by cactin-RNAi, we carried out high-resolution fixed and live imaging to monitor border cell organization. In control egg chambers, four to eight migratory cells surround and carry two polar cells. The migratory cells round up and one or two cells extend a protrusion toward the oocyte. Eventually, one cell takes the lead while the other cells retract their protrusions (Fig. 2, A and B). Prior to migration, border cell and polar cell apical surfaces contact the germline, lateral surfaces adhere to each other, and basal surfaces adhere to the basement membrane that surrounds the egg chamber. As the cluster delaminates, protrusions extend from lateral surfaces and the cells retain a shared apicobasal polarization (Niewiadomska et al., 1999; Pinheiro and Montell, 2004; Wang et al., 2018). Additionally, as the cluster pulls away from the basement membrane and anterior follicle cells, it rotates ∼90° so that the apical surface ends up roughly orthogonal to the direction of migration (Fig. 2, A and B).
In Cactin-depleted clusters, no cell took the lead, no large protrusion was observed (Fig. 2, C and D), and the cluster frequently failed to detach (Fig. 2, C and D). Live imaging revealed that, in contrast to controls (Fig. 2 E and Video 1), even though individual border cells were highly mobile, the movements of individual cactin-RNAi–expressing cells were not coordinated, so the clusters failed to advance (Fig. 2 F and Video 1). Furthermore, the apical polar cell surfaces were randomly oriented, sometimes even toward the anterior tip of the egg chamber, opposite from the direction of migration (Fig. 2, C and D), which was never seen in controls. The fixed and live imaging data suggest that depletion of Cactin disrupts protrusion and coordination of individual cell behaviors.
In control egg chambers, the follicle cells invariably form a monolayer, which covers the germline (Fig. 3, A–B‴). However, in 20% of cactin-RNAi–expressing egg chambers (27/136 egg chambers), follicle cells formed more than one layer within the c306-Gal4 expression domain at the posterior of the egg chamber (Fig. 3, C–D‴), which is characteristic of disrupted apicobasal polarity (Cox et al., 2001). So, we generated cactin-RNAi clones in the outer follicle cells and stained for aPKC, an apical marker. Relative to control clones (Fig. 3, E and E′), aPKC expression was increased apically in cactin-RNAi–expressing cells (Fig. 3, F and F′). In the most extreme cases, Cactin knockdown cells exhibited an approximately twofold increase in apical aPKC without significantly affecting overall aPKC levels (Fig. 3, E–I). These cells also exhibited apical constriction, which resulted in apical concentration of Armadillo (Arm; Fig. 3, G–H′).
Even in the absence of apical constriction, aPKC was 1.4-fold more concentrated at the apical surfaces of Cactin-knockdown cells compared to control cells (Fig. 3, J–L), suggesting that the excess aPKC was not simply a consequence of apical constriction. Neither myosin (Sqh::mCherry; Fig. 3, J‴, K‴, and L) nor Arm (Fig. S3, A–C) was concentrated at apical junctions in the unconstricted cells. These results suggest that Cactin normally prevents excess aPKC accumulation at apical epithelial cell surfaces.
We then examined aPKC localization in border cells. Control border cell clusters retain coordinated apicobasal polarity during delamination and migration with aPKC concentrated on one side of the cluster, especially in polar cells, which have small apical surfaces (arrowhead in Fig. 3, M and M′). The lateral marker Dlg localizes in a distinct domain from aPKC (Fig. 3, M″ and N). In contrast, border cell clusters with Cactin knockdown showed excess aPKC compared to controls (Fig. 3, O and O′), while Cactin knockdown did not significantly alter Dlg (Fig. 3, O″ and P). A second apical marker, Crb, accumulated apically, similar to aPKC (Fig. 3, Q and R′), and showed a 12% increase in apical junctions of Cactin knockdown clusters compared to control (Fig. S3 C). One interpretation of the border cell phenotype is that individual border cells may be apically constricting similar to the most extreme follicle cell clones (Fig. 3, E–H′ and M–R′; and Fig. S3, D–E″). A previous study showed that there are normally two pools of aPKC in border cell clusters: one at apical junctions keeping the cluster collectively polarized and a second in protrusions, especially the lead cell protrusion (Wang et al., 2018). Our results suggest that cactin-RNAi disrupts this balance, leading to excess apical aPKC.
Nonmuscle Myosin-II binds actin microfilaments, which drive cell motility, and Myosin-II is required for communication between border cells and coordination of their collective direction sensing (Aranjuez et al., 2016; Mishra et al., 2019; Combedazou et al., 2017). In control border cells, the Myosin-II light chain tagged with mCherry (Sqh::mCherry) accumulates strongly in puncta at the apical polar cell surfaces and transiently at the base of the main border cell protrusion (Mishra et al., 2019) as well as in dynamic cortical flashes around the outside of the cluster (Aranjuez et al., 2016; Fig. S3 F and Video 2). In cactin-RNAi–expressing border cells, Sqh::mCherry was distributed in random and dynamic cortical flashes at the periphery of each individual border cell (Fig. S3, G–I and Video 2), suggesting the coordination of individual border cell polarity was disrupted. Thus, fixed and live imaging confirmed a defect in coordination of individual border cell polarities and overall cluster polarization. Border cells may be more sensitive than epithelial follicle cells to loss of polarity because they have fewer neighbors from which to receive polarity cues.
Reducing apical aPKC rescued delamination defects in cactin-RNAi
The endocytic recycling machinery is required to regulate the balance between the two pools of Crb and aPKC (Wang et al., 2018). Rab proteins are essential for intracellular trafficking and Rab5 and Rab11 in particular are known to function during border cell migration (Ramel et al., 2013). So, we expressed UAS-YFP-tagged Rab5, Rab7, and Rab11 with c306-Gal4 and UAS-cactin-RNAi. Strikingly, overexpression of YFP-Rab5 and YFP-Rab11 but not YFP-Rab7 partially rescued aPKC localization in cactin-RNAi egg chambers (Fig. 4, A–D′) and delamination defects (Fig. 4 N). We predicted that overexpression of Rab5 or Rab11 might enhance recycling of aPKC, thus partially reducing the excess apical accumulation of aPKC. Consistent with this idea, when we expressed cactin-RNAi in flies heterozygous for an aPKC null mutation, which reduced the aPKC level in border cells, including the apical junctions (Fig. 4, E–F′), the delamination defects were also similarly rescued (Fig. 4 N).
Consistent with the interpretation that Rab5 and Rab11 affect apicobasal polarity and aPKC localization, the expression of a dominant-negative form of Rab5 (Fig. S4, A–B′) or Rab11 (Fig. S4, C–D′) caused aPKC mislocalization and border cell delamination defects resembling those caused by cactin-RNAi. Combining Rab5-DN and cactin-RNAi caused significant egg chamber lethality and enhanced the follicle cell multilayering defect in those egg chambers that survived (Fig. S5, E–H′). One possibility is that Rab5 and Cactin affect polarity by independent mechanisms and thus produce additive effects. However, since the Cactin RNAi is not a null allele, the interpretation of this genetic interaction is not unambiguous. There is a clear correlation though between excess apical aPKC and border cell delamination defects.
We hypothesized that the excess accumulation of apical aPKC observed in cactin-RNAi–expressing cells might also reduce the available basolateral aPKC necessary for protrusions. This pool is difficult to detect by staining, so we asked whether forcing aPKC to basolateral membranes in cactin-RNAi–expressing clusters would rescue migration. To relocalize aPKC, we took the advantage of a nanobody-based Grab-FP system (Harmansa et al., 2017; and Fig. 4 G). Briefly, we combined endogenous EGFP-tagged aPKC with a basolateral anti-GFP nanobody (Grab-FP-basal), to relocalize EGFP-aPKC to basolateral membranes (Fig. 4, G–I′). Indeed, in cactin-RNAi–expressing clusters (Fig. 4, J and J′), relocalizing aPKC to basolateral membranes (Fig. 4, K and K′) partially rescued migration (Fig. 4 N). Conversely, combining GrabFP-Apical with EGFP-aPKC caused a high level of aPKC to accumulate apically and was sufficient to impair delamination (Fig. 4, L–N). These results suggest that the proper level and distribution of aPKC are required for border cell cluster polarization, organization, and delamination.
cactin-RNAi effects on border cell Rac activity
Cactin is conserved from yeast to humans and has been analyzed in multiple organisms. C. elegans Cactin was first identified in a genome-wide screen where it was shown to genetically interact with the Rac GTPase MIG-2 and to regulate distal tip cell migration (Cram et al., 2006). Since border cells also require spatiotemporally regulated Rac activity to migrate (Murphy and Montell, 1996; Geisbrecht and Montell, 2004; Wang et al., 2010), we investigated whether Rac activity is affected by cactin-RNAi. We combined an established Rac activity sensor (Wang et al., 2010; Cai et al., 2014) with cactin-RNAi and used live imaging to monitor Rac activity during delamination. In control border cell clusters, Rac activity is typically elevated in the leading cell as it extends a main protrusion (Mishra et al., 2019; and Fig. S4 I). In contrast, in cactin-RNAi, no leading cell protrusion was observed (Fig. S4 J) and the front/back bias of Rac Förster resonance energy transfer (FRET) was correspondingly absent (Fig. S4 K). Interestingly, the total FRET index in cactin-RNAi increased by 25% compared to control (Fig. S4 L), suggesting Rac activity is higher in Cactin-depleted border cells, consistent with the study in C. elegans (Tannoury et al., 2010). To test whether excess Rac activity contributes to the Cactin knockdown phenotypes, we assessed whether decreasing Rac activity might rescue delamination defects. There are three genes encoding Rac GTPases in Drosophila, which are largely functionally redundant in border cells (Geisbrecht and Montell, 2002). When we expressed cactin-RNAi in border cells heterozygous for Rac1, Rac2, and Mtl null mutations, no rescue was observed (Fig. S4 M). We also found that homozygous Rac2 and Mtl double mutants, though normally viable, died when combined with cactin-RNAi (Fig. S4 M), again showing that reduction in Rac does not ameliorate cactin-RNAi. These results suggest that although Cactin knockdown causes a small increase in Rac activity, this effect does not seem to account for the delamination defect. In further support of this interpretation, Rac overexpression causes defects that are distinct from cactin-RNAi in that cluster organization is normal.
Cactin regulates aPKC and Crb localizations via its spliceosome function
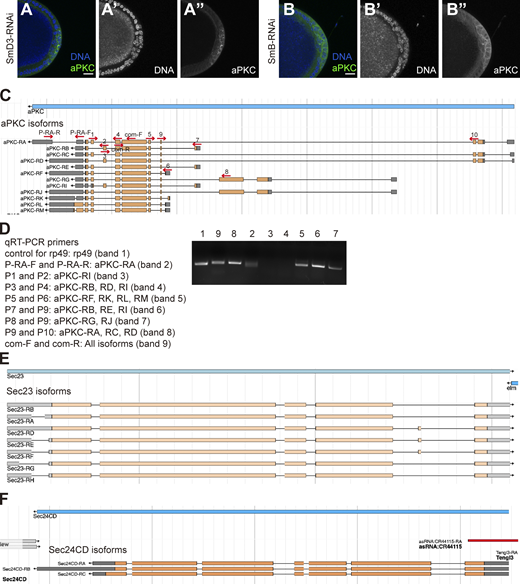
Although diverse functions have been ascribed to Cactin, its most conserved role appears to be as a component of the eukaryotic spliceosome (Baldwin et al., 2013; Zanini et al., 2017; Thakran et al., 2018; Fica et al., 2019; Cecchetelli et al., 2016; Doherty et al., 2014). However, its role in splicing has not yet been demonstrated in Drosophila or associated with cell migration. Specifically, Cactin is a component of the C complex, which carries out the second catalytic step of intron removal, after the B* complex (Matera and Wang, 2014). To test whether Cactin’s spliceosome function is required for border cell delamination, we performed an RNAi screen of other spliceosome complex C components in Drosophila (http://flybase.org/reports/FBgg0000536.html). We tested 103 lines corresponding to 70 genes. Of the 71 lines (targeting 56 genes) that were viable when expressed with c306-Gal4, so border cell migration could be evaluated, 18 (17 genes) caused >10% incomplete migration (Fig. 5, A–G). Importantly, these RNAi lines caused delamination and cluster morphology defects similar to the cactin-RNAi, with aPKC abnormally concentrated in apical junctions (Fig. 5, A–G). This phenotype has not been reported for other border cell mutants or knockdowns. Multiple layers of follicle cells were also observed (Fig. S5, A–B″). These results suggest that Cactin most likely regulates aPKC and Crb localization via its spliceosome function.
We then asked whether Cactin knockdown alters aPKC and Crb alternative splicing directly, as both aPKC and Crb are alternatively spliced (Kumichel et al., 2015; Xu et al., 2014; and Fig. S5 C). We performed quantitative RT-PCR (qRT-PCR) and compared both the overall levels of aPKC and Crb mRNA between control and cactin-RNAi (Fig. S5, C and D) as well as the proportions of different isoforms (Fig. 5, H–K). To our surprise, the isoform proportions were not significantly altered; rather the overall mRNA levels were increased. Rab5 and Rab11 mRNA levels were unchanged in cactin-RNAi, consistent with the idea that Cactin and the Rab GTPases regulate aPKC localization independently of one another (Fig. 5 L).
The Cactin knockdown phenotype was surprisingly specific considering that most genes are spliced and reduction of a core splicing factor is likely to impact many cellular functions. To assess whether Cactin regulates the splicing of a few relevant target mRNAs or is required more globally, we performed RNA sequencing (RNA-seq) on ovaries from control (UAS-GFP-RNAi) and UAS-cactin-RNAi and compared the mRNA levels and isoform profiles using CY2-Gal4 (Miao et al., 2020) to drive the RNAi lines in all follicle cells. We found 289 genes, which represent <10% of the roughly 8,000 expressed genes, whose isoform proportions changed significantly in cactin-RNAi (Table S1). This result shows that although Cactin functions as a general splicing factor, the knockdown does not affect all spliced mRNAs equally.
Gene ontology and pathway analyses did not reveal specificity in the set of affected genes (Table S2). However, among the 289 genes, we found four that are known to regulate aPKC and/or Crb apical localization in Drosophila: Glaikit (gkt), a member of the phospholipase D superfamily, localizes Crb to the apical membrane during Drosophila embryogenesis (Dunlop et al., 2004); Twins (tws), a Drosophila B-type protein phosphatase 2A subunit, forms a complex with aPKC and is required to maintain aPKC in apical junctions in larval neuroblasts (Chabu and Doe, 2009); Sec23 (also known as haunted) and Sec24CD (also known as ghost) promote transit of Crb from the ER to the Golgi and thus regulate the amount of Crb that reaches the apical plasma membrane (Kumichel et al., 2015). The overall mRNA levels of these genes did not show significant differences between control and cactin-RNAi (Fig. 6 A). However, specific isoforms of Sec23 and Sec24CD, Sec23-RD and Sec24CD-RA, showed higher expression in cactin-RNAi (Fig. 6 B; and Fig. S5, E and F). Cactin-RNAi cells also contained a higher proportion of these isoforms relative to all isoforms when compared to control cells (Fig. 6 C; and Fig. S5, E and F). Therefore, the overexpression of specific Sec23 and Sec24CD isoforms may cause excess apical Crb in cactin-RNAi–expressing cells, which would in turn lead to excess aPKC. This model predicts that reducing the expression of Sec 23 or Sec24CD might ameliorate the cactin-RNAi phenotypes. To test this possibility, we combined cactin-RNAi with heterozygotes containing one copy of a Sec23 or Sec24CD null mutant. Remarkably, Sec23 or Sec24CD null mutant heterozygotes partially rescued delamination defects in cactin-RNAi (Fig. 6, D and E) whereas neither gkt-RNAi nor tws null mutant heterozygotes had a significant effect. Thus, multiple independent genetic manipulations that reduce apical aPKC and/or Crb provided partial rescue of the cactin-RNAi cluster polarization and delamination defect (Fig. 6, F–I).
Discussion
Collective polarization of border cells requires Cactin
Collective cell migration is a fundamental cell behavior in normal development and cancer metastasis. While many studies have focused on motility and chemotaxis mechanisms, less is known about how cells detach collectively from an epithelium to initiate migration—the process of delamination. In this work, we used the Drosophila border cell system to study the regulation of delamination.
During development, epithelial cells like neural crest precursors undergo an EMT and individualize to become migratory (Piacentino et al., 2020). These cells downregulate E-cadherin and dismantle apical–basal polarity (Sauka-Spengler and Bronner-Fraser, 2008). However, some collectively migrating cells, including border cells, retain coordinated apicobasal polarity, even as they delaminate (Niewiadomska et al., 1999). Border cell cluster apicobasal polarity is required to maintain cluster cohesion during migration, and knockdown of the apical PAR3 or PAR6 proteins causes the cluster to split apart (Pinheiro and Montell, 2004). Border cell delamination is initiated when the outer, migratory cells begin to round up and one or two cells extend large protrusions between the anterior nurse cells. As one cell takes the lead, and the cluster moves out of the epithelium, the cells detach from the basement membrane that surrounds the egg chamber and from the anterior follicle cells that stay behind. Prior to delamination, all apical follicle cell surfaces contact the nurse cells, lateral surfaces contact neighboring follicle cells, and basal surfaces contact the basement membrane. As the border cell cluster delaminates, the cluster turns such that the shared apical surface becomes oriented approximately orthogonal to the direction of migration. In contrast, Cactin knockdown clusters did not carry out this coordinated, collective delamination. In cactin-RNAi-expressing clusters, individual border cells still rounded up and surrounded the polar cells, and individual cells moved rapidly within the cluster. However, the cells no longer moved cooperatively as a cluster and no cell extended a large, forward-directed protrusion or took the leading position. Instead, Crb and aPKC appeared overly concentrated at the border cell/polar cell interface and lacking from border cell/border cell junctions.
Prior work has shown that the lead cell usually communicates overall cluster polarization to the following cells (Montell et al., 2012). In the absence of shared apicobasal polarity, Cactin knockdown cells do not appear to be able to produce a lead cell protrusion or coordinate the directional motility of individual cells. The observation that restoring shared apicobasal polarity at least partially rescues delamination and migration suggests that this lack of coordinated polarity contributes significantly to the Cactin knockdown phenotype.
Within the follicular epithelium, clones of cactin-RNAi–expressing follicle cells exhibited excess Crb and aPKC and in extreme cases apical constriction. Crb is known to promote tracheal cell apical constriction during tracheal placode invagination, and overexpression of Crb leads to enlarged tracheal pits, where more cells initiate internalization, and to precocious and ectopic epidermal depressions (Letizia et al., 2011). A role for Crb and aPKC in apical constriction has also been observed during morphogenesis of the amnioserosa in the fly embryo (David et al., 2010). In contrast, border cell delamination is distinct from invagination and is inhibited by excess apical Crb and aPKC.
Consistent with the idea that excess apical border cell aPKC impairs border cell delamination, apical targeting of extra aPKC in border cells using the Grab-FP system was sufficient to impair delamination and migration. Moreover, multiple genetic manipulations that reduced apical aPKC and/or Crb in cactin-RNAi–expressing border cells rescued delamination and migration. For example, relocalizing aPKC to basolateral surfaces partially rescued the phenotype, as did expressing cactin-RNAi in flies heterozygous for mutations in aPKC or Sec23 or Sec24CD or overexpressing Rab5 or Rab11 (Fig. 6, F–I). Our work thus suggests that achieving the proper level and localization of aPKC is required to maintain coordinated cluster polarity, which is required for the delamination process.
Although Cactin-knockdown epithelial follicle cells show an increase in apical aPKC and Crb, the epithelium appears relatively normal and functions, whereas border cell delamination and migration are severely impaired. Border cells may be more sensitive to perturbations of apicobasal polarity because they have fewer polarity cues than cells within the epithelium. For example, border cells lack the basal cue from attachment to the basement membrane and lateral cues from connections to follicle cell neighbors.
Drosophila Cactin functions as a spliceosome C component
Cactin is conserved in organisms as diverse as fungi, plants, and animals, suggesting it serves a fundamental cell biological function. Consistent with that idea, in numerous organisms, Cactin has been found to physically associate with the spliceosome C complex, which catalyzes the second step of intron removal (Matera and Wang, 2014). Even though the fly studies described to date had not yet connected Cactin to the spliceosome, our data suggest that in flies too, Cactin serves as a spliceosome C component because knockdowns of multiple spliceosome C component proteins cause similar border cell delamination defects.
It is striking that knockdown of general splicing factors like Cactin causes a relatively specific defect in collective border cell polarization and migration. The RNA-seq analysis demonstrates widespread changes in abundance of numerous mRNA isoforms. These changes are likely due to a combination of direct effects on splicing and indirect effects. For example, the splicing of multiple transcription factors is dysregulated, which could lead to observed changes in mRNA abundances, including the observed increases in Sec23 and Sec24CD isoforms. Although many genes are affected upon Cactin knockdown, we were nevertheless able to show functional significance of those that regulate aPKC and/or Crumbs apical accumulation.
Mutations in general splicing factors also cause specific defects in humans and can lead to cell-type–specific diseases. For example, mutations in several genes encoding spliceosomal proteins cause autosomal dominant retinitis pigmentosa, indicating that human retinal cells are especially sensitive to splicing defects although the mechanistic basis for the sensitivity is not known (Matera and Wang, 2014). Photoreceptor cells have enormous apical domains with extensive invaginations—the rod and cone outer segments. The work described here shows that disrupted apicobasal polarization is one mechanism by which mutation of a general splicing factor can lead to a cell-type–specific defect in cells with specialized polarity requirements.
Materials and methods
Key resources
Key resources are listed in Table 1.
Fly husbandry
Files were kept at 25°C, 80% humidity on a 12-h light/dark cycle unless otherwise noted. For RNAi knockdown experiments, 2–4-d-old females were kept in 29°C for 2 d, then transferred to a vial with dry yeast and further kept in 29°C overnight before dissection. For clonal analyses (heat shock flpout clones), 2–4-d-old females were heat-shocked for 1 h at 37°C to induce clones, then transferred to a vial with dry yeast at 25°C for 3 d before dissection.
Detailed fly genotypes in each figure are listed in Table 2.
Generation of UAS-cactin and UAS-cactin-RFP transgenic lines
To generate UAS-cactin clones, the cactin-cDNA fragment was amplified from a DGRC cDNA (LP09118) and subcloned into a pUAST-attB vector using EcoRI and XbaI sites. To generate the UAS-cactin-RFP clone, the UAS-R-Inx2-RFP vector (Miao et al., 2020) was digested with EcoRI and BsrGI to remove the Inx2 cDNA fragment. The same cactin-cDNA fragment described above was subcloned to the vector with EcoRI and BsrgI sites. The clones were sequence-verified, and transgenic lines were established through ΦC-31 integrase mediated transformation (Bestgene). The attP2 site was used (Bloomington Drosophila Stock Center [BDSC]: 8622).
Immunostaining and imaging
Adult female ovaries were dissected in Schneider’s medium (Thermo Fisher Scientific) with 20% fetal bovine serum. Ovarioles were immediately fixed for 20 min in 4% paraformaldehyde at room temperature. After fixation, ovarioles were washed with PBS/0.1% Triton X-100 (PBST) four times (15 min each), and then incubated with primary antibodies overnight at 4°C. The following day, ovarioles were washed with PBST four times (10 min each) before incubation in the secondary antibody for 2 h at room temperature. After removal of secondary antibodies, samples were washed with PBST four times (10 min each) and then stored in Vectashield (Vector Laboratories) at 4°C before mounting. The following antibodies were used in this study: rat anti-E-cadherin (1:50, DCAD2; Developmental Studies Hybridoma Bank [DSHB]), mouse anti-Armadillo (1:75, N2.7A1; DSHB), mouse anti-Cactus (1:100; DSHB), mouse anti-Dorsal (1:100; DSHB), mouse anti-Crumbs (1:10, cq4; DSHB), mouse anti-Dlg (1:20; DSHB), rabbit anti-mCherry (1:500; Novus) rabbit anti-aPKC (1:200; Santa Cruz), rabbit anti-GFP (1:300; Lifetech), Hoechst (1:1,000), Alexa 488, 568, 633 (1:300; Lifetech), phalloidin 488, 568 (1:300; Lifetech). Images were taken on a Zeiss LSM 780 or 800 confocal microscope, using a 20 × 1.2 NA objective or 40 × 1.4 NA water objective. Z-stacks covering the egg chambers were taken with a 1-μm step size for border cell clusters.
Live imaging
For live imaging, ovaries were dissected in Schneider’s medium (Thermo Fisher Scientific) with 20% fetal bovine serum. Individual ovarioles were carefully pulled out and stage 10 or older egg chambers were removed. The egg chambers were collected in a 1.7-ml tube and washed with dissecting medium twice, then added 200 μl dissecting medium with insulin (200 μg/ml) and 1% low melt agarose. 90 μl medium with the egg chambers then was mounted on a 50-mm Lumox dish. Time-lapse imaging was performed using a 40 × 1.4 NA water immersion objective. The 1-μm-thick z-sections including the entire border cell cluster were collected at 1-min or 2-min intervals.
Quantification of border cell migration index
For quantification of border cell migration, stage 10B egg chambers were imaged at 20× magnification. Z-stacks projection images of LifeAct-GFP or anti-Arm were used to analyze the position of the border cell cluster.
Quantification of 10XSTAT-GFP intensity
From the SUM intensity Z-stack images of the anterior end of stage 8 egg chambers, the threshold was adjusted in the GFP channel (ImageJ function: Image > Adjust > Threshold) and background subtracted in FIJI. Then the three cells adjacent to the polar cells (based on the Arm channel) were selected and the GFP intensity was measured.
Quantification of aPKC, Crb, sqh-mCherry, Arm expression in cactin-RNAi clones
For quantification of staining level, 40× magnification SUM intensity projection images of anti-aPKC, Crb, sqh-mCherry and Arm channel were measured in FIJI. Threshold was adjusted for the channels (ImageJ function: Image > Adjust > Threshold) to subtract background. The apical and basal regions were selected manually. The mean intensity was used as the readout of expression. Except in Fig. 3 I, the total aPKC was equal to mean intensity × apical selected region.
qRT-PCR
For each genotype (Control: CY2-Gal4>UAS-GFP-RNAi; cactin-RNAi: CY2-Gal4>UAS-cactin-RNAi), 15 pairs of ovaries were dissected from 2–4-d-old females. Total RNA was extracted using the RNeasy kit (Qiagen). Turbo DNase (Thermo Fisher Scientific) was used to remove genomic DNA. Reverse transcription was carried out using the SuperScript III First-Strand Synthesis System (Thermo Fisher Scientific). qRT-PCR was carried out using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) on Bio-Rad CFX96 real-time PCR detection system. The data were collected using Bio-Rad CFX Manager software (Version 3.1, Bio-Rad). The primers used for qRT-PCR were listed in Table 1.
RNA-seq
For each genotype (Control: CY2-Gal4>UAS-GFP-RNAi; cactin-RNAi: CY2-Gal4>UAS-cactin-RNAi), three biological replicates were prepared. For each replicate, 20 pairs of ovaries were dissected from 2–4-d-old females. Total RNA extraction and sequencing steps were carried out using Genewiz.
Trimmed reads were mapped to the Drosophila genome by HISAT2. Drosophila reference genome indices were downloaded from http://daehwankimlab.github.io/hisat2/download/#d-melanogaster, and the genome_tran was used. Transcript assembly was permitted by stringtie (Drosophila_melanogaster.BDGP6.32.104.gtf). Estimated transcript abundances in this way were used for further differential gene and isoform analysis.
Differential gene expression analysis was performed with the DEseq2 R package
Isoform switch analysis was performed with isoformswitchanalysis R package (https://bioconductor.org/packages/devel/bioc/vignettes/IsoformSwitchAnalyzeR/inst/doc/IsoformSwitchAnalyzeR.html).
Gene ontology and pathway analyses were performed with DAVID bioinformatics Resources 6.8 (https://david.ncifcrf.gov/tools.jsp). 286 out of 289 genes were analyzed. Gene ontology of biological pathway, cellular component, and molecular function were analyzed.
Statistics and data presentation
Standard statistical tests were performed using Prism 8. Unpaired t test (two-tailed) was used for comparing two groups with similar variance as determined by the F test. Mann–Whitney nonparametric test (two-tailed) was used for comparing two groups with different variances. Ordinary one-way ANOVA, followed by Tukey’s multiple comparisons test, was used for comparing multiple groups with similar variance as determined by Brown–Forsythe test. All graphs were generated using Prism 8. All confocal images belonging to the same experiment were acquired using the exact same settings. For visualization purposes, brightness adjustments were applied using FIJI to the confocal images shown in the figure panels. All quantitative analyses were carried out on unadjusted raw images or sum intensity projections. All fly crosses were repeated at least twice and ovary dissections and staining were repeated at least three times. The exact sample size (n) is listed in each figure, representing biological replicates. Sample size was not predetermined by statistical methods, but we used prior knowledge to estimate minimum sample size. The experiments were not randomized. Investigators were not blinded.
Number of samples in each figure are listed in Table 3.
Online supplemental material
Fig. S1 shows multiple cactin-RNAi lines cause delamination defects. Fig. S2 shows Cactin functions independently of JAK/STAT or Toll signaling pathways in border cells. Fig. S3 shows excess apical Crb in Cactin knockdown cells. Fig. S4 shows that cactin-RNAi indirectly affects Rac activity in border cells. Fig. S5 shows Cactin regulates aPKC and Crb localizations indirectly via its spliceosome function. Table S1 shows the 289 genes whose isoform proportions changed significantly in cactin-RNAi. Table S2 shows the Gene ontology and pathway analyses of the 289 genes. Video 1 shows time-lapse videos of c306-Gal4>UAS-LifeAct-GFP/+ (control, left) and c306-Gal4>UAS-LifeAct-GFP, UAS-cactin-RNAi (right). Video 2 shows time-lapse videos of c306-Gal4, sqh-mCherry/+ (control, left) and c306-Gal4>UAS-cactin-RNAi/sqh-mCherry (right).
Acknowledgments
We thank D.J. Montell lab members for helpful discussions. We thank Jane Moon for technical support in Fig. S2 G. We thank Yang Hong (University of Pittsburgh, Pittsburgh, PA) for reagents and BDSC and Vienna Drosophila Resource Center for fly stocks.
The work was funded by National Institutes of Health grant R01 GM073164 to D.J. Montell.
The authors declare no competing financial interests.
Author contributions: G. Miao and D.J. Montell designed the experiments and coordinated the project. G. Miao performed the experiments, collected, and analyzed the data. L. Guo analyzed the RNA-seq data. G. Miao and D.J. Montell wrote the manuscript.