Mitochondria are dynamic organelles that play essential roles in cell growth and survival. Processes of fission and fusion are critical for the distribution, segregation, and maintenance of mitochondria and their genomes (mtDNA). While recent work has revealed the significance of mitochondrial organization for mtDNA maintenance, the impact of mtDNA perturbations on mitochondrial dynamics remains less understood. Here, we develop a tool to induce mitochondria-specific DNA damage using a mitochondrial-targeted base modifying bacterial toxin, DarT. Following damage, we observe dynamic reorganization of mitochondrial networks, likely driven by mitochondrial dysfunction. Changes in the organization are associated with the loss of mtDNA, independent of mitophagy. Unexpectedly, perturbation to exonuclease function of mtDNA replicative polymerase, Mip1, results in rapid loss of mtDNA. Our data suggest that, under damage, partitioning of defective mtDNA and organelle are de-coupled, with emphasis on mitochondrial segregation independent of its DNA. Together, our work underscores the importance of genome maintenance on mitochondrial function, which can act as a modulator of organelle organization and segregation.
Introduction
Mitochondrial networks are highly dynamic and undergo changes in shape and size throughout the cell cycle (Westermann, 2010). These dynamics are predominantly attributed to two processes of fission and fusion, carried out by evolutionarily conserved machineries (Westermann, 2008). In budding yeast, the fission of mitochondria is carried out by Dnm1, which is recruited to the outer membrane by adaptor proteins. Fusion of mitochondria occurs at the level of the inner membrane (carried out by Mgm1) as well as the outer membrane via Fzo1 (Scott and Youle, 2010; Shaw and Nunnari, 2002). Fission and fusion are required for the proper distribution and segregation of mitochondria during cell division and are also essential for moulding the organelle network in response to changing growth conditions (Detmer and Chan, 2007). Along with facilitating dynamic adaptation of mitochondrial function to the metabolic needs of the cell (Bagamery et al., 2020; Laporte et al., 2018), they play a crucial role in the quality control of the organelle (Detmer and Chan, 2007; Ni et al., 2015).
Apart from ensuring organelle health, fission and fusion are also important for maintaining mitochondrial genome integrity and its DNA copy numbers (Busch et al., 2014; Lewis et al., 2016; Lieber et al., 2019; Osman et al., 2015). All eukaryotic cells, with a few exceptions, contain multiple copies of mtDNA (chiefly encoding for part of the electron transport chain as well as mitochondrial translation RNAs and proteins) and are distributed throughout the organelle network (Brown et al., 2011; Chen and Butow, 2005; Osman et al., 2015). These DNA copies are packaged in the form of nucleoids (Farge et al., 2012), primarily by Abf2 in budding yeast (Chen and Butow, 2005). Studies have shown that mtDNA nucleoids are non-randomly distributed in the mitochondrial network and are present equidistant from each other (Osman et al., 2015). This organization appears to be essential for ensuring the segregation of accurate amounts of mtDNA during cell division (Jajoo et al., 2016). Indeed, lowered levels of mtDNA replication are observed when mitochondrial fusion is perturbed, leading to a decrease in mtDNA copy numbers (Silva Ramos et al., 2019). Mitochondrial fission is also linked to mtDNA replication and segregation in budding yeast, and it facilitates the distribution of mtDNA throughout the mitochondrial network (Lewis et al., 2016). Previous studies have also reported that deletions on mtDNA occur when pathways of fission and fusion are perturbed (Osman et al., 2015).
Accurate maintenance of mtDNA integrity is critical as mutations on mtDNA can have adverse effects on cell survival and are associated with several human pathologies (Hahn and Zuryn, 2019; Scheibye-Knudsen et al., 2015; Taylor and Turnbull, 2005). The close proximity of mtDNA to the electron transport chain makes it susceptible to damage via reactive species generated during oxidative phosphorylation (Alexeyev et al., 2013; Yakes and Van Houten, 1997). Even under physiological conditions, mtDNA accumulates mutations and deletions over time; these are also proposed to be major drivers of aging (Alexeyev et al., 2013; Richter, 1995; Wallace, 2010).
How do cells respond to mtDNA damage? There is evidence for the localization of certain DNA repair proteins to the mitochondria (such as those involved in base excision repair and recombination; Alexeyev et al., 2013; Boesch et al., 2011; Scheibye-Knudsen et al., 2015; Stevnsner et al., 2002). However, certain repair pathways seem to be absent from the mitochondria (for example, nucleotide excision repair; Clayton et al., 1974; Pascucci et al., 1997). Thus, while DNA repair pathways have been detected in the mitochondria, the importance and regulation of mtDNA damage repair has remained unclear. As mtDNA can directly affect the functionality of the organelle, it is possible that one layer of quality control is carried out by mechanisms that respond to mitochondrial dysfunction directly. Indeed, in response to organelle dysfunction, cells employ fission and fusion machineries to clear out any damaged mitochondria (Ni et al., 2015). For example, regulation of fusion can facilitate the complementation of mutations present in mitochondrial genomes (Nakada et al., 2001). Similarly, mitochondrial fission helps in the selective removal and degradation of mitochondria via PINK-Parkin mediated mitophagy or even mitocytosis (Jiao et al., 2021; Jin and Youle, 2012; Zhang et al., 2019). More recently, a role for mitochondrial reorganization at the level of the cristae has also been discovered to contribute to the segregation of defective mitochondrial domains, arising from mutant mtDNA copies (Jakubke et al., 2021). It is likely that these pathways contribute to different degrees of quality control, depending on the cell type and specific growth environments (Pickles et al., 2018). Although it is becoming increasingly evident that mitochondrial dynamics can regulate mtDNA synthesis, partitioning, and homeostasis, the influence of mtDNA damage on mitochondrial organization and segregation is not fully understood. A major challenge in studying mtDNA damage response and repair has been the ability to generate mtDNA-specific damage. In this context, tools to make mtDNA-specific DSBs have been invaluable, but are dependent on the presence of specific sequences on the mtDNA to allow for a targeted action of the enzyme. In most cases, generic damaging agents that can cause adducts or lesions on DNA (nuclear and mitochondrial) preclude the ability to disentangle mitochondria-specific effects.
To characterize the impact of mtDNA perturbations on organelle organization and function, we developed a novel system to generate inducible mtDNA-specific damage in Saccharomyces cerevisiae. We engineered a mitochondrially targeted bacterial toxin, mtDarT (which generates adducts on single-stranded DNA; Jankevicius et al., 2016), to probe the effect of mtDNA damage on mitochondrial dynamics in real-time. We find that mtDNA damage drives network fragmentation; based on our observations, we suggest that this is likely due to a lowering in fusion rates arising from mitochondrial dysfunction. We further implicate a critical role for the exonuclease function of the mtDNA polymerase in mtDNA maintenance under damage. Our study reveals an intricate connection between mtDNA integrity, mitochondrial function, and its role in regulating organelle organization and segregation.
Results
System to induce mitochondria-specific DNA damage
To perturb mtDNA integrity and observe the resultant mitochondrial dynamics in real-time, we developed an mtDNA-specific damage-inducing system in S. cerevisiae. For this, we utilized a bacterial toxin, DarT, that is part of a widely conserved DarT–DarG toxin-antitoxin system (Jankevicius et al., 2016). DarT functions via transferring an ADP-ribosyl group on single-stranded DNA using NAD+ as a substrate (Fig. 1 A) and has been shown to generate DNA damage-inducing adducts on the bacterial genome (likely via blocking replication progression; Lawarée et al., 2020). The adduct is attached to a thymidine within a “TNTC” motif (Jankevicius et al., 2016; Schuller et al., 2021), which is present abundantly on the mitochondrial genome.
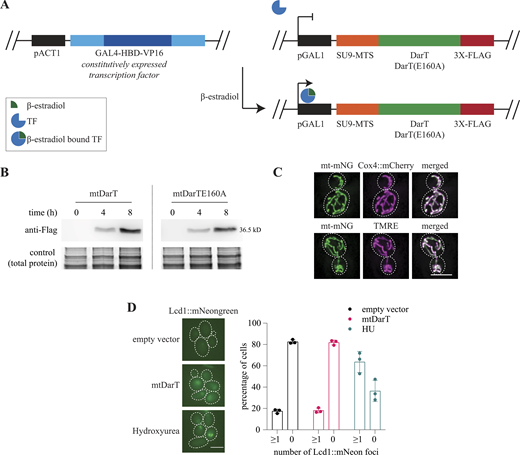
System to induce mitochondria-specific DNA damage. (A) Schematic representing the expression and localization of mtDarT on the addition of β-estradiol (left panel); diagrammatic representation of ADP-ribosylation of single-stranded DNA in presence of DarT and NAD+ (right panel). (B) Immunostaining for FLAG-tagged mtDarT/TE160A in cells expressing mitochondrially targeted mNeongreen. Intensity line profiles for mtDarT and mNeonGreen are shown at the bottom. Scale bar, 5 μm; white dotted lines mark cell outlines, here and in all other images. (C) Cell survival under increasing concentrations of β-estradiol on plates containing either dextrose or glycerol as a carbon source; cells were transformed with plasmids expressing mtDarT/TE160A or empty vector as a control. (D) Dot blot assay to assess ADP ribosylation on DNA, upon mtDarT expression. Both mitochondrial and nuclear DNA are probed. As a loading control, the same volumes of samples were spotted on a separate membrane and stained with 0.04% of methylene blue. Representative blot from two biological replicates. Source data are available for this figure: SourceData F1.
System to induce mitochondria-specific DNA damage. (A) Schematic representing the expression and localization of mtDarT on the addition of β-estradiol (left panel); diagrammatic representation of ADP-ribosylation of single-stranded DNA in presence of DarT and NAD+ (right panel). (B) Immunostaining for FLAG-tagged mtDarT/TE160A in cells expressing mitochondrially targeted mNeongreen. Intensity line profiles for mtDarT and mNeonGreen are shown at the bottom. Scale bar, 5 μm; white dotted lines mark cell outlines, here and in all other images. (C) Cell survival under increasing concentrations of β-estradiol on plates containing either dextrose or glycerol as a carbon source; cells were transformed with plasmids expressing mtDarT/TE160A or empty vector as a control. (D) Dot blot assay to assess ADP ribosylation on DNA, upon mtDarT expression. Both mitochondrial and nuclear DNA are probed. As a loading control, the same volumes of samples were spotted on a separate membrane and stained with 0.04% of methylene blue. Representative blot from two biological replicates. Source data are available for this figure: SourceData F1.
We constructed a mitochondrial matrix-targeted DarT (derived from Thermus aquaticus), mtDarT, via fusion of DarT to the first 69 amino acids of SU9 (subunit 9 of the F1-F0 ATPase; Horie et al., 2003). A 3XFlag-tag was added to the C-terminus of DarT to enable verification of expression as well as localization upon induction (Fig. S1 A). mtDarT was expressed from a β-estradiol inducible-promoter on a low copy plasmid (Louvion et al., 1993). This strategy was employed to separately express a catalytically dead variant of DarT (DarTE160A), which retains only negligible DarT activity (Jankevicius et al., 2016). We first confirmed the expression of mtDarT, as well as mtDarTE160A via Western blotting after 4 and 8 h of induction with 100 nM β-estradiol (Fig. S1 B). Immunostaining using an anti-flag antibody revealed that the proteins indeed localized to the mitochondria, marked using mitochondrially targeted mNeonGreen (mt-mNG; Fig. 1 B and Fig. S1 C).
System to induce mitochondria-specific DNA damage. (A) Schematic of β-estradiol inducible system. Transcription factor GAL4-HBD-VP16 is constitutively expressed under a pACT1 promoter, and is activated on binding of β-estradiol. The activated transcription factor binds to pGAL1 and initiates transcription of gene under this promoter. (B) Western blots of FLAG-tagged mtDarT/TE160A after 4 and 8 h of induction with 100 nM β-estradiol. (C) Representative cells expressing mt-mNeongreen and Cox4::mCherry (top panel), and cell expressing mt-mNeongreen stained with TMRE (bottom panel). (D) Representative cells expressing Lcd1::mNeongreen after 6 h of mtDarT induction or 2 h after hydroxyurea (100 mM) treatment. Percentage cells with or without Lcd1::mNeongreen foci is plotted. N > 600 cells from three biological replicates. Scale bars, 5 μm; white dotted lines mark cell outlines, in all figures. Source data are available for this figure: SourceData FS1.
System to induce mitochondria-specific DNA damage. (A) Schematic of β-estradiol inducible system. Transcription factor GAL4-HBD-VP16 is constitutively expressed under a pACT1 promoter, and is activated on binding of β-estradiol. The activated transcription factor binds to pGAL1 and initiates transcription of gene under this promoter. (B) Western blots of FLAG-tagged mtDarT/TE160A after 4 and 8 h of induction with 100 nM β-estradiol. (C) Representative cells expressing mt-mNeongreen and Cox4::mCherry (top panel), and cell expressing mt-mNeongreen stained with TMRE (bottom panel). (D) Representative cells expressing Lcd1::mNeongreen after 6 h of mtDarT induction or 2 h after hydroxyurea (100 mM) treatment. Percentage cells with or without Lcd1::mNeongreen foci is plotted. N > 600 cells from three biological replicates. Scale bars, 5 μm; white dotted lines mark cell outlines, in all figures. Source data are available for this figure: SourceData FS1.
We next assessed the impact of mtDarT on cell growth, utilizing the ability of yeast cells to show differential growth on fermentable/non-fermentable carbon sources, based on their mitochondrial activity (Ephrussi et al., 1955). Cells with impaired mitochondrial function were able to survive on media containing a fermentable carbon source (albeit forming smaller, “petite” colonies) due to the ability of these cells to carry out glycolysis. However, such cells are compromised for growth on non-fermentable carbon sources, where oxidative phosphorylation (and hence complete mitochondrial function) is essential. When compared with an empty vector control, we found that cells expressing mtDarT showed no associated loss in viability on plates supplemented with dextrose (fermentable carbon source; Fig. 1 C). These cells showed a significant loss of viability in case of plates supplemented with glycerol (non-fermentable carbon source; Fig. 1 C). Importantly, the growth defects observed were mtDarT-specific and required the DNA adduct-forming catalytic function of DarT (Fig. 1 C).
To further establish mitochondria-specific mtDarT effects, we carried out a dot-blot assay to probe for ADPr groups on both nuclear and mitochondrial DNA. We observed signal only for mtDNA, dependent on mtDarT expression (Fig. 1 D). In addition, we assessed the localization of Lcd1 (or Ddc2), which is reported to localize to the nucleus under nuclear DNA damage or replication stress (Lisby et al., 2004; Melo et al., 2001; Fig. S1 D, HU-treated cells). Upon mtDarT induction, we did not observe an increase in the number of cells with Lcd1 localizations (Fig. S1 D). Together, these results supported the idea that mtDarT had mitochondria-specific action.
mtDNA damage impacts mitochondrial organization and dynamics
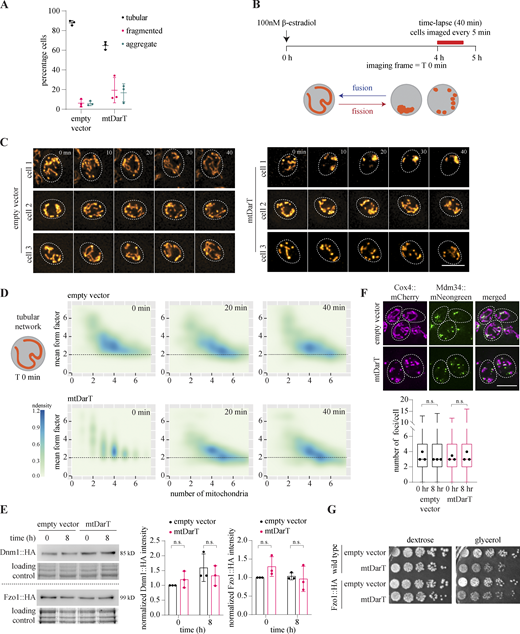
We next characterized the impact of mtDNA damage on mitochondrial organization and function in glycolysis replete conditions. For this, we followed mitochondrial dynamics in cells expressing Cox4-mCherry to track mitochondria in addition to mtDarT (or an empty vector control). We classified the observed mitochondrial organizations into three distinct categories: “tubular,” “fragmented,” and “aggregate” (representative examples are shown in Fig. 2 A). We found that the induction of mtDarT resulted in the fragmentation of the mitochondrial network (Fig. 2, A and B), with an increase in the percentage of cells with fragmented or aggregate mitochondria. Indeed, cells carrying mtDarT displayed some perturbation to the mitochondrial organization even before induction, likely due to leaky expression from the vector (Fig. S2 A).
mtDNA damage impacts mitochondrial organization and dynamics. (A) Representative cells showing three categories of mitochondrial morphologies observed (left panel); percentage of cells in each category in control (empty vector) or mtDarT cells after 8 h of induction (mean ± SD from three biological replicates, n > 150 in each replicate; right panel). (B) Cells expressing Cox4::mCherry imaged in widefield and SIM mode, with representative cells showing tubular and fragmented mitochondrial morphologies after 6 h of induction with 100 nM β-estradiol. (C) Two parameters: − mean form factor (MFF) and the number of mitochondria are used to quantify mitochondrial morphologies in the population. Details on the calculation of MFF are provided, along with a diagrammatic representation of possible mitochondrial organizations observed in the 2D density plots. (D) Density plots with mean form factor and number of mitochondria over time; n > 680 in each plot, pooled from three biological replicates. (E) Montage of cells with Cox4::mCherry, imaged every 5 min for 40 min, after 4 h of induction with β-estradiol; mitochondrial morphology at the start of the experiment is indicated. T0 refers to the frame at the start of the imaging (after the period of pre-induction) here and in Fig. 2 F. (F) Density plots with mean form factor and number of mitochondria over time, plotted for cells starting with MFF <2 at the beginning of time lapse; n = 84 for empty vector, n = 151 for mtDarT pooled from three biological replicates.
mtDNA damage impacts mitochondrial organization and dynamics. (A) Representative cells showing three categories of mitochondrial morphologies observed (left panel); percentage of cells in each category in control (empty vector) or mtDarT cells after 8 h of induction (mean ± SD from three biological replicates, n > 150 in each replicate; right panel). (B) Cells expressing Cox4::mCherry imaged in widefield and SIM mode, with representative cells showing tubular and fragmented mitochondrial morphologies after 6 h of induction with 100 nM β-estradiol. (C) Two parameters: − mean form factor (MFF) and the number of mitochondria are used to quantify mitochondrial morphologies in the population. Details on the calculation of MFF are provided, along with a diagrammatic representation of possible mitochondrial organizations observed in the 2D density plots. (D) Density plots with mean form factor and number of mitochondria over time; n > 680 in each plot, pooled from three biological replicates. (E) Montage of cells with Cox4::mCherry, imaged every 5 min for 40 min, after 4 h of induction with β-estradiol; mitochondrial morphology at the start of the experiment is indicated. T0 refers to the frame at the start of the imaging (after the period of pre-induction) here and in Fig. 2 F. (F) Density plots with mean form factor and number of mitochondria over time, plotted for cells starting with MFF <2 at the beginning of time lapse; n = 84 for empty vector, n = 151 for mtDarT pooled from three biological replicates.
mtDNA damage impacts mitochondrial organization and dynamics. (A) Percentage of cells in each category in control (empty vector) or mtDarT cells at t = 0 h (mean ± SD from three biological replicates, n > 150 in each replicate). (B) Schematic showing experimental design for time lapse experiments. (C) Montage of single cells expressing Cox4::mCherry tracked over time (mean form factor <2 at the beginning of the time lapse), imaged for 40 min after 4 h of induction. T0 refers to the frame at the start of the imaging (after the period of pre-induction) here and in Fig. S2 D. (D) Density plots with mean form factor and number of mitochondria over time, plotted for cells starting with MFF >2 at the beginning of time lapse; n = 362 for empty vector, n = 347 for mtDarT pooled from three biological replicates. (E) Dnm1::HA and Fzo1::HA Western blot with quantification from 3 biological replicates, normalized to empty vector at t = 0; error bars: SD. (F) Representative images of cells expressing Mdm34::mNeongreen and Cox4::mCherry after 8 h of induction. Number of Mdm34 foci per cell are plotted for cells pooled from three biological replicates; n > 680. (G) Cell survival on plates containing either dextrose or glycerol as a carbon source, to ensure C-terminal tagging of Fzo1 does not affect cell growth. Statistical significance in E and F was calculated using unpaired Student’s t test. (*** P < 0.0001, ** P < 0.005, * P < 0.05 in all cases). Source data are available for this figure: SourceData FS2.
mtDNA damage impacts mitochondrial organization and dynamics. (A) Percentage of cells in each category in control (empty vector) or mtDarT cells at t = 0 h (mean ± SD from three biological replicates, n > 150 in each replicate). (B) Schematic showing experimental design for time lapse experiments. (C) Montage of single cells expressing Cox4::mCherry tracked over time (mean form factor <2 at the beginning of the time lapse), imaged for 40 min after 4 h of induction. T0 refers to the frame at the start of the imaging (after the period of pre-induction) here and in Fig. S2 D. (D) Density plots with mean form factor and number of mitochondria over time, plotted for cells starting with MFF >2 at the beginning of time lapse; n = 362 for empty vector, n = 347 for mtDarT pooled from three biological replicates. (E) Dnm1::HA and Fzo1::HA Western blot with quantification from 3 biological replicates, normalized to empty vector at t = 0; error bars: SD. (F) Representative images of cells expressing Mdm34::mNeongreen and Cox4::mCherry after 8 h of induction. Number of Mdm34 foci per cell are plotted for cells pooled from three biological replicates; n > 680. (G) Cell survival on plates containing either dextrose or glycerol as a carbon source, to ensure C-terminal tagging of Fzo1 does not affect cell growth. Statistical significance in E and F was calculated using unpaired Student’s t test. (*** P < 0.0001, ** P < 0.005, * P < 0.05 in all cases). Source data are available for this figure: SourceData FS2.
We next followed the mtDarT-induced changes in mitochondrial organization over time via measuring two features (Fig. 2 C): (a) the mean form factor (MFF) of mitochondria per cell, an indicator of mitochondrial circularity (Chaudhry et al., 2020) and (b) the number of discrete mitochondria per cell. We plotted MFF and the number of mitochondria for all cells as 2D density plots, allowing us to visualize populations of cells having either tubular, fragmented, or aggregated mitochondria (Fig. 2 C). We observed that control cells harboring an empty vector largely populated MFF above 2 (predominantly tubular mitochondrial morphology) and maximum density represented an intermediate between a tubular network and a fragmented state (Fig. 2 D). This is as expected under unperturbed conditions, given the dynamic nature of the mitochondria throughout the cell cycle (Shaw and Nunnari, 2002). In contrast, upon mtDarT induction, mitochondria gradually transitioned from tubular (MFF >2) to fragmented/aggregate states, having MFF <2 (Fig. 2 D).
The organization of the mitochondrial network is regulated by a dedicated set of GTPases that control mitochondrial membrane fission and fusion (Westermann, 2008). Hence, to assess the contribution of changes in mitochondrial fission and/or fusion upon mtDNA damage, we followed mitochondrial dynamics via time-lapse microscopy. We imaged cells at 5 min intervals for 40 min after 4 h of damage exposure (Fig. 2 E; and Fig. S2, B and C) and plotted density plots as described above. The transition from tubular to fragmented states would reveal fission events, while transition in the opposite direction (toward a tubular network) would be indicative of fusion events.
To assess the impact of mtDNA damage on fission dynamics, we followed cells (control and damage) with tubular mitochondrial networks at the start of imaging (T0) after pre-induction. We found that the rates of mitochondrial fission (as assessed by the transition of cells with MFF > 2 to MFF < 2) were comparable between control and damage-treated cells (Fig. S2 D). In line with this observation, we did not find an increase in the levels of the fission protein Dnm1 (Bleazard et al., 1999; Fig. S2 E). Another mechanism for mitochondrial fission, independent of changes in the levels of Dnm1, is via ERMES contact sites (Lewis et al., 2016). An increase in the number of these contact sites (which can be imaged via tracking the number of Mdm34 localizations) could lead to the recruitment of Dnm1, thereby resulting in an increase in fission events. However, we did not observe a significant increase in the number of ERMES contact sites in cells facing damage (Fig. S2 F).
Unlike the observed fission dynamics, cells facing mtDarT-induced damage had significantly lower fusion events. When we followed individual cells over time, we found that mtDarT-expressing cells with fragmented or aggregate mitochondrial organization did not display dynamic reorganization toward a network morphology (Fig. 2 E and Fig. S2 C). Consistently, in comparison to the control, cells expressing mtDarT were compromised in transitioning from MFF < 2 to MFF > 2 (fragmented to tubular transition) and tended to maintain the density distribution observed at the start of the experiment (Fig. 2 F). The control cells on the other hand were able to dynamically transition from MFF < 2 to MFF > 2 within the 40-min imaging interval (Fig. 2, E and F). Importantly, the above-described effects of mtDarT were not cell size or cell cycle-stage specific (as assessed by the presence and size of the bud), further underscoring the direct impact of mtDNA damage on the mitochondrial organization (Fig. S3, A and B). Taken together, our data suggest that mtDNA damage drives a large-scale reorganization of the mitochondrial network, likely via an impact on mitochondrial fusion rates. Indeed, although we did not observe a significant change in the levels of the fusion protein, Fzo1 (Fig. S2, E and G), it is possible that other mechanisms contribute to the observed mitochondrial dynamics under damage (see Discussion).
mtDNA damage impacts mitochondrial organization and dynamics. (A) Cell area plotted as violin plots for all the cells in Fig. 2 and Fig. S2 D at t = 0 min. (B) Density plots with mean form factor and number of mitochondria over time, plotted for cells in Fig. 2 F, classified based on presence or absence of buds. T0 refers to the frame at the start of the imaging (after the period of pre-induction).
mtDNA damage impacts mitochondrial organization and dynamics. (A) Cell area plotted as violin plots for all the cells in Fig. 2 and Fig. S2 D at t = 0 min. (B) Density plots with mean form factor and number of mitochondria over time, plotted for cells in Fig. 2 F, classified based on presence or absence of buds. T0 refers to the frame at the start of the imaging (after the period of pre-induction).
mtDNA damage results in mitochondrial dysfunction
Given our observations on the impact of mtDNA damage on mitochondrial membrane fusion dynamics, we wondered whether mitochondrial function was perturbed in these cells. We thus measured two indicators of mitochondrial activity: (a) mitochondrial membrane potential (ψ) via imaging TMRE (a membrane potential–sensitive dye) and (b) oxygen consumption rate (OCR) using a Seahorse assay (Ludovico et al., 2001). We observed a decrease in membrane potential in cells expressing mtDarT (Fig. 3 A), similar to the impact of FCCP, a membrane decoupler that decreases ψ (Fig. S4 A). Similarly, mtDarT-treated cells also had lower OCR (Fig. S4 B). Together, these observations suggest that mitochondrial function in mtDarT-treated cells is compromised (we use the term “dysfunction” to encompass both these parameters of mitochondrial function). Importantly, a lowering in the membrane potential was observed even in mtDarT cells with reticular/tubular networks, suggesting that mitochondrial dysfunction upon mtDNA damage preceded changes in mitochondrial organization and dynamics (Fig. S4 D).
mtDNA damage results in mitochondrial dysfunction. (A) Representative images of cells expressing Cox4::GFP and stained with TMRE after 8 h of induction with β-estradiol (left panel). Total TMRE intensity normalized to cell area is plotted (n > 1,030 pooled from three biological replicates; right panel). (B) Schematic of experimental design for time-lapse experiment in Fig. 3, C and D. (C) Montage of cells expressing mt-mNeongreen stained with TMRE tracked over time, imaged every 5 min for 40 min. TMRE was imaged only at t = 0 of time-lapse. T0 refers to the frame at the start of the imaging (after the period of pre-induction). (D) Scatter plot with mean form factor and number of mitochondria over time, plotted for cells starting with MFF <2 at the beginning of time-lapse. Each data point represents one cell with color corresponding to TMRE intensity normalized to cell area at t = 0 min; n = 61 for empty vector, n = 91 for mtDarT pooled from three biological replicates. Statistical significance in A was calculated using Mann–Whitney U test. (*** P < 0.0001, ** P < 0.005, * P < 0.05 in all cases).
mtDNA damage results in mitochondrial dysfunction. (A) Representative images of cells expressing Cox4::GFP and stained with TMRE after 8 h of induction with β-estradiol (left panel). Total TMRE intensity normalized to cell area is plotted (n > 1,030 pooled from three biological replicates; right panel). (B) Schematic of experimental design for time-lapse experiment in Fig. 3, C and D. (C) Montage of cells expressing mt-mNeongreen stained with TMRE tracked over time, imaged every 5 min for 40 min. TMRE was imaged only at t = 0 of time-lapse. T0 refers to the frame at the start of the imaging (after the period of pre-induction). (D) Scatter plot with mean form factor and number of mitochondria over time, plotted for cells starting with MFF <2 at the beginning of time-lapse. Each data point represents one cell with color corresponding to TMRE intensity normalized to cell area at t = 0 min; n = 61 for empty vector, n = 91 for mtDarT pooled from three biological replicates. Statistical significance in A was calculated using Mann–Whitney U test. (*** P < 0.0001, ** P < 0.005, * P < 0.05 in all cases).
mtDNA damage results in mitochondrial dysfunction. (A) Representative images of cells expressing Cox4::GFP stained with TMRE after treatment with FCCP (2 μM) for 20 min or Oligomycin (0.5 μg/ml) for 4 h (left panel). Total TMRE intensity normalized to cell area is plotted; n > 650 pooled from three biological replicates for FCCP and Oligomycin and two biological replicates for empty vector (right panel). (B) Oxygen consumption rate (OCR) estimated using Seahorse assay after 4 h of induction. Mean and SD is plotted from three biological replicates. (C) Cox2 Western blot with quantification from three biological replicates, normalized to empty vector at t = 0; error bars: SD. (D) Representative images of cells expressing Cox4::GFP stained with TMRE after 4 h of induction with 100 nM β-estradiol. Examples show cells from three different morphological categories for both empty vector and mtDarT (left panel). Total TMRE intensity normalized to cell area is plotted for cells with three mitochondrial morphologies, after 4 h of induction (for empty vector, n = 215 for tubular, n = 35 for fragmented, n = 30 aggregate; for mtDarT, n = 258 for tubular, n = 79 for fragmented, n = 118 for aggregate; right panel). Cells were pooled from three biological replicates. Statistical significance in A and D was calculated using Mann–Whitney U test. Source data are available for this figure: SourceData FS4.
mtDNA damage results in mitochondrial dysfunction. (A) Representative images of cells expressing Cox4::GFP stained with TMRE after treatment with FCCP (2 μM) for 20 min or Oligomycin (0.5 μg/ml) for 4 h (left panel). Total TMRE intensity normalized to cell area is plotted; n > 650 pooled from three biological replicates for FCCP and Oligomycin and two biological replicates for empty vector (right panel). (B) Oxygen consumption rate (OCR) estimated using Seahorse assay after 4 h of induction. Mean and SD is plotted from three biological replicates. (C) Cox2 Western blot with quantification from three biological replicates, normalized to empty vector at t = 0; error bars: SD. (D) Representative images of cells expressing Cox4::GFP stained with TMRE after 4 h of induction with 100 nM β-estradiol. Examples show cells from three different morphological categories for both empty vector and mtDarT (left panel). Total TMRE intensity normalized to cell area is plotted for cells with three mitochondrial morphologies, after 4 h of induction (for empty vector, n = 215 for tubular, n = 35 for fragmented, n = 30 aggregate; for mtDarT, n = 258 for tubular, n = 79 for fragmented, n = 118 for aggregate; right panel). Cells were pooled from three biological replicates. Statistical significance in A and D was calculated using Mann–Whitney U test. Source data are available for this figure: SourceData FS4.
Specific lowering in mitochondrial fusion rates has been reported in cells with compromised membrane potential (and hence dysfunction; Duvezin-Caubet et al., 2006; Karbowski et al., 2004; Legros et al., 2004; Twig et al., 2008). We, thus, hypothesized that mtDNA damage-induced mitochondria with lowered membrane potential would display a lower probability of fusion when compared with mitochondria with unperturbed membrane potential. To assess this, we tracked mitochondrial dynamics (via mt-mNeongreen) in mtDarT-treated cells after a brief pulse of TMRE staining (Fig. 3, B and C). We then analyzed the mean form factor and number of mitochondria for cells specifically having MFF <2 (fragmented) along with their initial TMRE fluorescence intensities and asked whether these mitochondria were able to transition to a tubular state. We observed that mitochondria with higher membrane potential, but aggregate/fragmented organization (MFF <2), were able to fuse into tubular mitochondrial networks (MFF >2; Fig. 3, C and D). In contrast, fragmented mitochondria with lower membrane potential were unable to do so during the course of imaging (Fig. 3, C and D).
Our observations are consistent with the possibility that mtDNA damage results in mitochondrial dysfunction (reduced membrane potential and compromised OCR), which in turn affects mitochondrial dynamics via impairing fusion.
Changes in mitochondrial morphology accompany loss of mtDNA but not mitochondria
We next assessed the effects of mtDarT damage on mtDNA, and whether the observed mitochondrial dynamics contributed to the clearance of damaged DNA/dysfunctional mitochondria. Following a previously described method, we stained cells with SYBR Green I to visualize mtDNA nucleoids (Jajoo et al., 2016; Fig. 4 A). In the control sample without mtDNA damage, <2% of the cells had no detectable mtDNA foci (“ρ0” cells) at all time points of imaging (Fig. 4 B). In contrast, upon mtDarT induction, the percentage of ρ0 cells steadily increased, with ∼30% cells having no detectable foci after 8 h of damage induction (Fig. 4 B). Consistent with this, we also observed an overall decrease in the number of mtDNA foci per cell upon mtDNA damage (Fig. S5 A).
Changes in mitochondrial morphology accompany loss of mtDNA but not mitochondria. (A) Montage of cells expressing Cox4::mCherry stained with SYBR Green I to visualize mtDNA over time. (B) Percentage of cells over time without any mtDNA foci. Mean ± SD from three biological replicates; n > 100 in each biological replicate. (C) Cox4::mCherry and Tom20::mCherry Western blot with quantification from three biological replicates, normalized to an empty vector at t = 0; Error bars: SD. Statistical significance in C was calculated using unpaired Student’s t test. Source data are available for this figure: SourceData F4.
Changes in mitochondrial morphology accompany loss of mtDNA but not mitochondria. (A) Montage of cells expressing Cox4::mCherry stained with SYBR Green I to visualize mtDNA over time. (B) Percentage of cells over time without any mtDNA foci. Mean ± SD from three biological replicates; n > 100 in each biological replicate. (C) Cox4::mCherry and Tom20::mCherry Western blot with quantification from three biological replicates, normalized to an empty vector at t = 0; Error bars: SD. Statistical significance in C was calculated using unpaired Student’s t test. Source data are available for this figure: SourceData F4.
Changes in mitochondrial morphology accompany loss of mtDNA but not mitochondria. (A) Number of mtDNA foci per cell after 8 h of induction is plotted; n > 930 pooled from three biological replicates. (B) Representative cells imaged with structured illumination microscopy, along with segmentation output after Mitograph analysis (left panel). Mitochondrial volume was analyzed via estimation of number of voxels detected using Mitograph, each data point represents one cell; n = 12 for empty vector and n = 24 for mtDarT. (C) Representative images of cells expressing GFP::Atg8 under the endogenous ATG8 promoter treated with Rapamycin (20 nM) for 4 h and cells transformed with empty vector or mtDarT after 8 h of induction with 100 nM β-estradiol (left panel). Frequency distribution of number of GFP::Atg8 localization (right panel). N > 1,015 cells pooled from three biological replicates. (D) Representative cells showing GFP::Atg8 localizations associated or non-associated with mitochondria. Percentage localizations associated with mitochondria is plotted after 8 h of induction, or 4 h of rapamycin (20 nM) treatment. Data is pooled from two biological replicates. N > 90 localizations. (E) Vacuolar intensity of Idh1::GFP normalized to total cell intensity after 4 h of induction with 100 nM β-estradiol; n > 830 pooled from two biological replicates (right panel), with representative images of cells expressing Idh1::GFP and Vph1::mCherry (left panel). (F) Density plots with mean form factor and number of mitochondria over time for mip1exo− cells; n > 710 in each plot, pooled from three biological replicates. (G) Total TMRE intensity normalized to cell area for mip1exo− cells is plotted; n > 1,350 pooled from three biological replicates. Statistical significance in A, E, and G was calculated using Mann–Whitney U test. Statistical significance in B was calculated using unpaired Student’s t test.
Changes in mitochondrial morphology accompany loss of mtDNA but not mitochondria. (A) Number of mtDNA foci per cell after 8 h of induction is plotted; n > 930 pooled from three biological replicates. (B) Representative cells imaged with structured illumination microscopy, along with segmentation output after Mitograph analysis (left panel). Mitochondrial volume was analyzed via estimation of number of voxels detected using Mitograph, each data point represents one cell; n = 12 for empty vector and n = 24 for mtDarT. (C) Representative images of cells expressing GFP::Atg8 under the endogenous ATG8 promoter treated with Rapamycin (20 nM) for 4 h and cells transformed with empty vector or mtDarT after 8 h of induction with 100 nM β-estradiol (left panel). Frequency distribution of number of GFP::Atg8 localization (right panel). N > 1,015 cells pooled from three biological replicates. (D) Representative cells showing GFP::Atg8 localizations associated or non-associated with mitochondria. Percentage localizations associated with mitochondria is plotted after 8 h of induction, or 4 h of rapamycin (20 nM) treatment. Data is pooled from two biological replicates. N > 90 localizations. (E) Vacuolar intensity of Idh1::GFP normalized to total cell intensity after 4 h of induction with 100 nM β-estradiol; n > 830 pooled from two biological replicates (right panel), with representative images of cells expressing Idh1::GFP and Vph1::mCherry (left panel). (F) Density plots with mean form factor and number of mitochondria over time for mip1exo− cells; n > 710 in each plot, pooled from three biological replicates. (G) Total TMRE intensity normalized to cell area for mip1exo− cells is plotted; n > 1,350 pooled from three biological replicates. Statistical significance in A, E, and G was calculated using Mann–Whitney U test. Statistical significance in B was calculated using unpaired Student’s t test.
In some instances, mitochondrial dysfunction (and associated fragmentation) has been shown to occur in conjunction with mitophagy (Burman et al., 2017; Kleele et al., 2021). We thus asked whether loss of mtDNA under damage was a result of clearance of dysfunctional mitochondria. For this, we assessed the levels of two nuclear-encoded mitochondrial proteins, Cox4 and Tom20, via Western blot. We found that levels of these proteins did not change significantly under damage conditions (Fig. 4 C). This is in contrast to mitochondrially encoded Cox2 levels that decrease upon mtDarT expression (Fig. S4 C). We then measured mitochondrial volume (Harwig et al., 2018; Fig. S5 B) and found that here too no significant changes were observed over time. Consistent with the lack of change in mitochondrial content, we did not observe an increase in mitophagy-associated marker, Atg8 (Torggler et al., 2017) localizations (Fig. S5 C), or a significant increase in the number of Atg8 localizations associated with the mitochondria (Böckler and Westermann, 2014; Fig. S5 D). We also did not detect a significant difference in the amount of vacuolar localization of Idh1 (Kanki and Klionsky, 2008) upon mtDarT-induction (Fig. S5 E). This suggested that an mtDNA-specific mechanism might be responsible for mtDNA clearance in response to damage.
Exonuclease activity of the mtDNA polymerase, Mip1, is essential for mtDNA maintenance
In mammalian cells, the mtDNA replicative polymerase PolG can switch between synthesis and degradation functions to clear out defective mtDNA (Nissanka et al., 2018; Peeva et al., 2018). Evidence for such exonuclease function of the yeast homolog, Mip1, has also been reported in conditions of starvation or oxidative stress (Medeiros et al., 2018). We hence asked whether exonuclease activity could contribute to mtDNA clearance under mtDarT-induced damage. For this, we blocked the exonuclease activity of Mip1, via mutating the catalytic site (D171A, E173A) responsible for its degradative function (Foury and Vanderstraeten, 1992; Medeiros et al., 2018; Fig. 5 A) and assessed presence of mtDNA nucleoids via SYBR Green I staining. Surprisingly, upon mtDarT induction, we observed that Mip1 exonuclease deficient cells generated ρ0 cells significantly faster than the wild type. As shown earlier (Fig. 4 B), 30% of the wild-type cells were ρ0 after 8 h of mtDarT induction. mip1exo− cells already generated ρ0 cells at a low frequency (∼7%) in no damage conditions, highlighting that inability to clear damaged mtDNA (in this case likely due to replication errors) results in mtDNA loss in glucose replete growth environment. In line with this observation, induction of mtDNA damage resulted in 60% of the cells becoming ρ0 after 8 h of mtDarT induction (Fig. 5 B). Importantly, rapid mtDNA loss did not result in the production of cells devoid of mitochondria.
Exonuclease activity of the mtDNA polymerase, Mip1, is essential for mtDNA maintenance. (A) Schematic showing domain organization of Mip1, with amino acid mutations for mip1exo− marked in red (D171A, E173A). (B) Percentage of cells over time without any mtDNA foci (p0) are plotted. Mean ± SD from three biological replicates; n > 85 in each biological replicate. (C) Representative images of mip1exo− cells expressing Cox4::mCherry stained with SYBR Green I. Yellow arrows mark mitochondria devoid of mtDNA. (D and E) Fraction of mtDNA in the bud (number of mtDNA foci in bud/total number of mtDNA foci in the mother-bud pair) is plotted against fraction cell area (area of the bud/total area of the mother-bud pair) for both wild-type and mip1exo− cells, after 4 h (top panel) or 6 h of induction (bottom panel). Black line represents mean and shaded region represents SD (calculated after binning x-axis in bins of 0.05). R2 values were obtained via Pearson’s Correlation; n > 145 pooled from 4 biological replicates for mip1exo− and three biological replicates for wild type. Statistical significance in E was calculated using Fisher’s F test, applied on distributions of residual values of all data points (distance from linear fit curve). Comparisons were made to empty vector control of corresponding time point. (F) Schematic of experimental design to arrest cells, followed by SYBR staining at 0 and 4 h of induction. Flow cytometry profiles corresponding to the time point are shown at the bottom (left panel); percentage cells without mtDNA foci in wild type and mip1exo− cells expressing mtDarT (right panel). N > 670 cells from three biological replicates. Statistical significance in F was calculated using unpaired Student’s t test.
Exonuclease activity of the mtDNA polymerase, Mip1, is essential for mtDNA maintenance. (A) Schematic showing domain organization of Mip1, with amino acid mutations for mip1exo− marked in red (D171A, E173A). (B) Percentage of cells over time without any mtDNA foci (p0) are plotted. Mean ± SD from three biological replicates; n > 85 in each biological replicate. (C) Representative images of mip1exo− cells expressing Cox4::mCherry stained with SYBR Green I. Yellow arrows mark mitochondria devoid of mtDNA. (D and E) Fraction of mtDNA in the bud (number of mtDNA foci in bud/total number of mtDNA foci in the mother-bud pair) is plotted against fraction cell area (area of the bud/total area of the mother-bud pair) for both wild-type and mip1exo− cells, after 4 h (top panel) or 6 h of induction (bottom panel). Black line represents mean and shaded region represents SD (calculated after binning x-axis in bins of 0.05). R2 values were obtained via Pearson’s Correlation; n > 145 pooled from 4 biological replicates for mip1exo− and three biological replicates for wild type. Statistical significance in E was calculated using Fisher’s F test, applied on distributions of residual values of all data points (distance from linear fit curve). Comparisons were made to empty vector control of corresponding time point. (F) Schematic of experimental design to arrest cells, followed by SYBR staining at 0 and 4 h of induction. Flow cytometry profiles corresponding to the time point are shown at the bottom (left panel); percentage cells without mtDNA foci in wild type and mip1exo− cells expressing mtDarT (right panel). N > 670 cells from three biological replicates. Statistical significance in F was calculated using unpaired Student’s t test.
How do mip1exo− cells lose mtDNA in such a rapid manner under damage? We measured budding events of wild-type and mutant cells upon mtDNA damage and found them to be comparable during the course of the experiment (3 ± 1 budding events over 6 h, for both wild-type and mip1exo− cells expressing mtDarT), ruling out the possibility of faster division rates in the exonuclease mutant contributing to mtDNA loss over time. We next assessed mitochondrial dynamics in the mutant. As seen in wild-type cells, we found that mtDNA damage resulted in significant changes to mitochondrial organization in the case of exonuclease deficient cells as well (Fig. S5 F). Furthermore, in comparison to mtDarT-treated wild-type cells, we found that membrane potential was severely compromised in the mutant (Fig. S5 G), suggesting that these cells likely have significantly lowered mitochondrial membrane fusion events. A potential outcome of enhanced mitochondrial dysfunction is a defect in the segregation of mitochondria during division (McFaline-Figueroa et al., 2011). We thus wondered whether this could impact partitioning of the mtDarT-induced damaged mtDNA. Such partitioning defect would likely be more severe/pronounced in the mip1exo− condition, where defective mtDNA would accumulate due to lack of a clearance mechanism.
When we probed for mtDNA in these cells, we observed a significant proportion of cells with mitochondria devoid of mtDNA in mip1exo− background (Fig. 5 C). We asked whether mtDNA was differentially partitioned in these cells, resulting in the generation of mitochondria devoid of DNA as well. For this, we analyzed mother–bud pairs after SYBR Green I staining and quantified the fraction of mtDNA foci in the bud (Fig. 5 D). Consistent with previous reports (Jajoo et al., 2016; Osman et al., 2015), we found that the amount of mtDNA partitioned in the bud scaled with increasing bud area in control as well as wild-type cells after mtDarT induction (Fig. 5 E). However, unlike wild-type cells, there was significant heterogeneity in the amount of mtDNA in the bud (across cell areas) in the case of exonuclease deficient cells treated with mtDarT, suggesting imprecise mtDNA partitioning (Fig. 5 E), with several instances of buds receiving no mtDNA (ρ0 daughter cells; Fig. 5 E). We hence hypothesized that if such asymmetry indeed contributed to the rapid production of ρ0 cells in the exonuclease-deficient mutant, cells arrested for cell cycle progression would no longer generate ρ0 cells at a high frequency observed in the case of steady-state growth conditions. To assess this, we used bar1Δ strains treated with α-factor that would arrest cells in G1–S transition (Breeden, 1997). We confirmed the same using flow cytometry, as well as via assessing the morphology of the cells upon arrest (Fig. 5 F [left] and Rosebrock, 2017). In wild-type cells, we observed that loss of mtDNA occurred at a comparable frequency in arrest and non-arrest conditions. In contrast, in mip1exo− background, mtDNA loss was significantly lower under arrest (Fig. 5 F, right). Thus, asymmetric mitochondrial partitioning during cell division could significantly contribute to the rapid production of ρ0 cells in the exonuclease deficient mutant. Taking these observations together, our results suggest that mitochondrial dynamics could differentially impact damaged mtDNA segregation in conditions where defective mtDNA clearance mechanisms are compromised, likely facilitating organelle segregation independent of its DNA.
Discussion
In this study, we describe a system to generate mtDNA-specific damage in S. cerevisiae. Using a bacterial toxin DarT (Jankevicius et al., 2016), targeted to the mitochondria, we follow the impact of mtDNA damage on mitochondrial organization, segregation, and function. We find that mtDNA damage affects mitochondrial function via compromising membrane potential. This could be the causative force in mitochondrial reorganization by a reduction in mitochondrial membrane fusion events (Duvezin-Caubet et al., 2006; Karbowski et al., 2004; Legros et al., 2004; Twig et al., 2008), facilitating selective segregation of mitochondria without defective mtDNA (Fig. 6 and discussed below).
Summary schematic of key observations. mtDNA damage triggers mitochondrial dysfunction and associated changes in mitochondrial organization. Exonuclease function of replicative polymerase, Mip1, is required to prevent rapid loss of mtDNA under damage. For details, see discussion.
Summary schematic of key observations. mtDNA damage triggers mitochondrial dysfunction and associated changes in mitochondrial organization. Exonuclease function of replicative polymerase, Mip1, is required to prevent rapid loss of mtDNA under damage. For details, see discussion.
How cells sense and respond to mtDNA damage remains an outstanding and exciting question. Previous studies have detected the presence of specific DNA repair enzymes (including BER and recombination machinery) in the mitochondria (Alexeyev et al., 2013; Boesch et al., 2011; Scheibye-Knudsen et al., 2015; Stevnsner et al., 2002). While some of these pathways encode mitochondrially targeted isoforms (Swartzlander et al., 2010), many do not carry any known localization signal (Stuart et al., 2005; Thyagarajan et al., 1996). How they localize to the mitochondria remains unclear, as is the importance of specific repair pathways in mtDNA maintenance (Alexeyev et al., 2013; Scheibye-Knudsen et al., 2015). Indeed, there are certain types of damages (such as UV-induced pyrimidine dimers) for which active repair pathways have not been found in the mitochondria (Clayton et al., 1974; Pascucci et al., 1997). In such scenarios, where mtDNA damage becomes persistent, degradation of damaged mtDNA copies has been proposed to occur (Alexeyev et al., 2013; Shokolenko et al., 2013). Interestingly, this function is carried out by the exonuclease activity of the mtDNA polymerase (Mip1 or PolG) itself (Nissanka et al., 2018; Peeva et al., 2018).
Our work further underscores the central role of defective mtDNA clearance via exonuclease activity of the replicative polymerase under damage. We propose that the inability of cells to clear damaged mtDNA results in the rapid generation of ρ0 cells in glucose-replete conditions. Although in unperturbed conditions, mtDNA and mitochondrial segregation may be coordinated (Jajoo et al., 2016; Lewis et al., 2016; Osman et al., 2015), our observations support the role of selective segregation of damaged mtDNA in a manner that can result in the partitioning of mtDNA-less mitochondria in the daughter cells. Generation of cells devoid of mtDNA, without significant loss of mitochondria, suggests that cells can decouple mtDNA and mitochondrial partitioning and maintenance under conditions of damage, when complete mitochondrial function may not be essential (such as in glycolytic conditions). Indeed, while mitochondria cannot be made de novo (Westermann, 2014), ρ0 and ρ-cells in the environment could regain and complement lost mtDNA, albeit with different proficiencies (Ling et al., 2019; Perlman, 1976).
It is interesting to note that mtDNA damage triggers rapid changes in mitochondrial organization and segregation, both outcomes potentially stemming from mitochondrial dysfunction. It would be important to understand how mtDNA damage causes perturbations to mitochondrial function and how this in turn modulates rates of fusion. Although levels of Fzo1 did not change under mtDNA damage, it would be informative to understand what other mechanisms might regulate mitochondrial fusion to result in the observed dynamics in budding yeast. For example, mtDNA damage can perturb levels of mitochondrially encoded proteins, such as those part of the electron transport chain (Fig. S4 C), which requires a coordinated effort from nuclear and mitochondrial genomes (Fontanesi et al., 2006; Herrmann and Funes, 2005). This can adversely affect the maintenance of mitochondrial membrane potential. Separately, studies have reported a direct impact of lowered membrane potential on differential processing of Opa1 (a mitochondrial membrane fusion protein in mammals), affecting its association with the mitochondrial membrane (Song et al., 2007).
The change in the mitochondrial organization we report is consistent with observations in mammalian cells, where mitochondrial network fragmentation has been seen to occur in response to mtDNA damage (Ghosh et al., 2019). This suggests that mitochondrial reorganization may be a universal response to mtDNA perturbation as a fundamental quality control mechanism to deal with mitochondrial dysfunction and regulate mtDNA/mitochondrial segregation as well as clearance. Indeed in metazoans, mitochondrial fission has been proposed to facilitate selective removal and degradation of mitochondria via processes such as PINK-Parkin mediated mitophagy (Ni et al., 2015; Scheibye-Knudsen et al., 2015). While we cannot rule out the role of mitophagy in the mtDNA damage response in yeast, our results suggest that bulk mitophagy does not contribute significantly within the timescales of our present study and that quality control mechanisms likely act at the level of mtDNA and its segregation (Fig. S5 D). For example, previous reports have shown that budding yeast cells segregate mitochondria asymmetrically based on the activity of the organelle (McFaline-Figueroa et al., 2011; Vevea et al., 2014). Such asymmetry could also passively ensure selective segregation of intact mtDNA copies to daughter cells. Indeed, recent studies demonstrate selective removal or clearance of mutated mtDNA via sensing of local mitochondrial activity as well as downregulation of mitochondrial fusion (Hill et al., 2014; Jakubke et al., 2021; Lieber et al., 2019). It is tempting to speculate that in organisms like budding yeast, which divide asymmetrically, the inability of dysfunctional mitochondria to fuse with the rest of the mitochondrial network, along with selective segregation during division, is the predominant mitochondrial quality control mechanism even in response to mtDNA damage.
In sum, our study underscores the following: (a) the impact of perturbations to mtDNA integrity on mitochondrial dynamics and function; (b) the importance of Mip1 exonuclease activity in mtDNA maintenance under damage, and (c) the ability of cells to decouple mitochondrial and mtDNA partitioning under mtDNA damage, with an emphasis on mitochondrial maintenance even in the absence of mtDNA in glycolysis-replete conditions. Indeed, the mtDarT system developed here can provide a unique approach to enable future investigations on the mechanisms that govern mtDNA-specific damage repair and recovery across diverse growth conditions.
Materials and methods
Yeast strains and growth conditions
A prototrophic strain CEN.PK of S. cerevisiae was used as the background strain in all the experiments (van Dijken et al., 2000). Plasmids and strains used in the study are listed in Tables 1, 2, and 3. Yeast cultures were grown in rich YP media (containing 2% peptone, 1% yeast extract, and 2% dextrose or 3% glycerol as the carbon source) at 30°C with 200 rpm shaking with appropriate concentrations of antibiotics, as required. For all time-course and time-lapse experiments, cells were transformed with the plasmids, and the transformants were inoculated in YPDextrose containing NAT and allowed to grow overnight. These cultures were induced with β-estradiol at an OD ∼ 0.2. For induction, β-estradiol (working stock: 50 μM, prepared from a stock of 1 mM in 100% molecular biology grade ethanol) was added to a final concentration of 100 nM, unless mentioned otherwise. We performed our experiments in haploid budding yeast cells, and β-estradiol was maintained in the growth medium throughout.
Yeast transformations were done using the LiAc method. For plasmid transformations, cells were plated on YPDextrose plates containing appropriate concentrations of NAT and were allowed to grow for 48 h at 30°C. mip1exo− cells, transformed with plasmids, were plated on YPGlycerol plates containing appropriate concentrations of NAT and were allowed to grow for 3 d at 30°C.
mip1exo− mutant was generated as described earlier (Medeiros et al., 2018). Briefly, we first generated a mip1∆ strain which resulted in the formation of ρ0 cells. We then introduced a copy of the mip1 gene containing the mutations D171A and E173A along with a hygromycin selection cassette at the same locus. Successful transformants containing the mutated copy of the gene were then crossed with the wild-type strain of the opposite mating type to reintroduce mtDNA. The diploids were sporulated and tetrads were dissected to obtain ρ+ mip1exo− cells which were selected using hygromycin, and the mating type was confirmed using PCR primers specific for the mating type locus. mip1 gene was sequenced to confirm the presence of the mutations. The strain was maintained on YPGlycerol after dissection to ensure the selection of only ρ+ cells; experiments were carried out in YPDextrose.
Dot blot assay for ADP ribosylation
For the separation of mitochondrial and nuclear DNA, differential centrifugation followed by sucrose-gradient-based separation was employed to fractionate both organelles. Mitochondrial fractionation was done as described earlier in Gregg et al. (2009); Meisinger et al. (2006). DNA was isolated from the nuclear and mitochondrial fractions using a Qiagen DNeasy Blood and Tissue kit.
Isolated DNA was then used to perform a dot blot assay as described by Jia et al. (2017) and Luviano et al. (2018). Briefly, 180 ng of each DNA sample was denatured in 0.3 M NaOH for 10 min at 95°C. Immediately after denaturation, 5 μl (∼90 ng) of DNA was spotted on a nitrocellulose membrane. DNA was then crosslinked to the membrane using a UV-crosslinker at 1200J. The membrane was blocked using 5% blocking solution prepared in TBST for 1 h at RT, followed by incubation with 1:500 dilution of anti-pan-ADPr-binding reagent (reagent conjugated to Rabbit Fc is produced and purified from Escherichia coli, #MABE1016; Merck) in TBST for 1 h at RT. The membrane was then washed thrice with TBST for 10 min each and then probed with 1:5,000 dilution of HRP conjugated secondary antibody for 1 h at RT. After the incubation, the membrane was again washed thrice, 10 min each in TBST, and then developed using SuperSignal West PICO PLUS or FEMTO PLUS chemiluminescent substrate. As a loading control, the same volumes of samples were also spotted on another membrane, which after crosslinking, was stained with 0.04% of methylene blue prepared in 0.5 M sodium acetate.
mtDNA and mitochondrial staining
For visualizing mtDNA, a previously described protocol was used (Jajoo et al., 2016). Briefly, 1 ml of culture was pelleted and washed with 1× PBS. The supernatant was discarded and cells were resuspended in the residual buffer to give 50 μl cell suspension. Then 0.5 μl of undiluted SYBR Green I (Invitrogen) was added to the cell suspension and mixed with gentle tapping. Cells were immediately pelleted and the supernatant was discarded. The pellets were washed with 1 ml of 1× PBS. Cells were subsequently diluted to appropriate densities in 1× PBS before imaging.
For visualizing the mitochondrial membrane potential, cells were pelleted and resuspended in 10 ml of YPDextrose media (prewarmed to 30°C) containing 1.5 μM of TMRE. The cells were incubated in the dark for 20 min in a shaker incubator (30°C, 200 rpm). Subsequently, cells were pelleted down and washed with YPDextrose media in 2× the original volume of culture used for staining. The cell pellets were resuspended to appropriate density in YPDextrose before imaging. For time lapses, cells were stained with TMRE after 3.5 h of induction in presence of 100 nM β-estradiol. Following this, cells were washed and spotted on YPDextrose pads for imaging.
Fluorescence microscopy
Overnight cultures were allowed to grow till OD ∼ 0.2. At this OD, mtDarT expression was induced with 100 nM β-estradiol. For time course experiments, samples were spotted on 1% ultrapure agarose water pads, and for time-lapse experiments, samples were spotted on YPDextrose pads with 100 nM β-estradiol.
Microscopy was performed on a wide-field epifluorescence microscope (Eclipse Ti-2E; Nikon) with a 60× oil immersion objective (plan apochromat objective with NA 1.41) and illumination from pE4000 light source (CoolLED). The microscope was equipped with a motorized XYZ stage and focus was maintained using an infrared-based Perfect Focusing System (Nikon). Image acquisitions were done with Hamamatsu Orca Flash 4.0 camera using NIS-elements software (version 5.1). For time-lapse imaging, samples were placed inside an OkoLab incubation chamber maintained at 30°C and imaged at specific intervals for the indicated period of time.
For SIM imaging, coverslips were sequentially washed in methanol and 100% ethanol and flame-dried prior to sample preparation. Cells were fixed in 3.7% PFA (900 μl of cell suspension was added to 100 μl of 37% PFA) and then washed with 1× PBS. Cells were then spotted on 1% ultrapure agarose water pads and imaged. Samples were imaged with a Nikon 3D-SIM microscope equipped with an ANDOR iXon DU-897 EMCCD camera (512 × 512), 100× ApoTIRF 1.49 NA oil objective with Coherent Sapphire 561 nm solid-state laser as the light source. Image reconstruction was done using NIS elements software (Illumination modulation contrast – Auto, high-resolution noise suppression – 1×, out of focus blur suppression – 0.25). During acquisition, 800 ms exposure for Cox4-mCherry with 20% laser power, 150 EM gain, and 0.3 μm step size was used.
Seahorse assay
Basal respiration rate was estimated by performing seahorse flux assays using Agilent Seahorse XFe24 analyzer. A total of 1 ml of the XF Calibrant solution was aliquoted into each well of the plate and the sensor cartridge plate was hydrated overnight as per the manufacturer’s instructions. To adhere the cells to the bottom of the wells, the plate was first coated with poly-l-Lysine for 1 h at RT. Excess poly-l-lysine was removed and the plate was dried at 30°C. Cells grown in YPDextrose media containing an appropriate concentration of NAT were induced with 100 nM β-estradiol at OD ∼ 0.2. Cells were serially diluted 4 h after induction and then aliquoted into the wells such that the final number of cells in each well ∼3 × 105. The plate was centrifuged at 100 g for 2 min and incubated at 30°C for 30 min to allow the cells to adhere to the bottom of the wells. Simultaneously, the sensor cartridge was loaded with 1 mM of complex IV inhibitor sodium azide and equilibrated in the Seahorse XFe24 analyzer 1 h prior to the start of the assay. The plate was then loaded into the Seahorse XFe24 analyzer and the basal OCR was measured over time. A minimum of two measurements were taken with an intermittent 2-min mixing and waiting steps. This was followed by sodium azide injection to the sample wells, and three measurements were taken with intermittent 2-min mixing and waiting steps.
Arrest experiment
Cells transformed with empty vector or mtDarT plasmid were grown overnight in YPG media containing the appropriate concentration of NAT. Overnight cultures were allowed to reach the early log phase (OD ∼ 0.2–0.6), after which they were pelleted and resuspended in prewarmed YPD media (30°C) containing 20 ng/ml of alpha factor with NAT. The cultures were allowed to grow at 30°C for 2 h at 200 rpm. Simultaneously, separate cultures without alpha factor were also maintained. After 2 h, samples were collected for microscopy (SYBR staining) and flow cytometry. Samples were subsequently induced with 100 nM β-estradiol. Similarly, samples were collected after 4 h of induction for microscopy and flow cytometry. (Note: cells were pelleted and resuspended in fresh media containing alpha factor, 100 nM β-estradiol, and NAT 3 h after induction to ensure continuous arrest).
Image analysis
All image analyses were performed using MATLAB and Fiji (ImageJ; Schindelin et al., 2012). Mitochondrial analysis was done on 2D maximum projections of z-stacks, and deconvolution of the images were carried out using NIS-elements AR software (Richardson-Lucy algorithm with 60 iterations). Cell segmentation was performed using a MATLAB algorithm (Doncic et al., 2013). Cell outlines were extracted from .mat output files from the algorithm and then ROIs were obtained using Fiji. For the segmentation of mitochondria, a custom pipeline was set up in Fiji, briefly involving (a) background subtraction (rolling ball radius: 50 pixels); (b) sigma filter plus (radius = 1 pixel, use pixels within = 2 sigmas, minimum pixel fraction = 0.2); (c) enhanced local contrast (CLAHE; blocksize = 64, histogram = 256, maximum = 2); (d) tubeness filter (sigma = 1.000); (e) images converted to 8-bit; (f) thresholding (10,255; minimum threshold was adjusted between 8 and 15 depending on the quality of segmentation); (g) images converted to mask, despeckled, and outliers were removed (radius = 2, threshold = 50), and the mitochondrial descriptors (mean form factor, mtArea, and the number of mitochondria on per cell basis) were obtained using the mitochondrial analyzer (Chaudhry et al., 2020). Whole-cell TMRE intensity was analyzed from maximum projections using “Measure” in Fiji. For Fig. 2 A, cells were manually binned into three morphological categories, and the buds were excluded from the analysis. For detection of mtDNA foci after SYBR Green I staining, 3D maxima finder plugin (part of the 3D ImageJ Suite) in ImageJ was utilized. Since cells with less/without any mtDNA also show nuclear DNA staining, we manually scored these cells after segmentation to account for the detection of foci due to nuclear staining. For mtDNA analysis in mother versus bud, pairs without any mtDNA foci (in both cell types) were removed. Foci detection in Fig. S2 F was performed using a custom MATLAB script. Mitochondrial volume was analyzed using Mitograph and the volume was calculated from the number of voxels detected (Rafelski et al., 2012; Viana et al., 2015). Colocalization of GFP::Atg8 with Cox4::mCherry was scored manually as described in Böckler and Westermann (2014). Association of Atg8 foci with mitochondria was checked in the plane of z-stack where GFP::Atg8 intensity was maximum.
Western blotting
Transformants were inoculated in 100–120 ml of YPDextrose with appropriate concentrations of NAT. Cultures were induced with 100 nM β-estradiol at an OD ∼ 0.2. Cells were harvested and protein isolation was performed using the trichloroacetic acid (TCA) extraction method. Pellets were resuspended in 300 μl of 10% TCA and lysed by bead beating thrice. The precipitates were resuspended in 200 μl of SDS–glycerol buffer (7.3% SDS, 29.1% glycerol, and 83.3 mM Tris base) and boiled at 95°C for 10 min with frequent vortexing. The lysate was precipitated and the supernatant containing protein was obtained. Estimation of protein concentration was done using the BCA estimation method using the GBioSciences assay kit. The assay was performed in disposable cuvettes, where 5 μl was mixed with 1 ml solution A and 20 μl of solution B and incubated at 30°C for 30 min. The absorbance was recorded at 565 nm. Equal amounts of protein were then loaded on a polyacrylamide gel. For Tom20-mCherry, Cox4-mCherry and DarT-3XFlag blots, 10% gels were used. For Fzo1-HA and Dnm1-HA blots, 8% gels were used. For Cox2 blots, 12% gels were used. PVDF membranes were used for blotting, which were probed with anti-Flag (1:5,000; Rabbit IgG, #14793; CST), anti-mCherry (1:5,000; Mouse IgG, #SAB2702291; Sigma-Aldrich), anti-HA (1:5,000; Rabbit IgG, #3724; CST) antibodies, and anti-Cox2 (1:1,000; Mouse IgG, #459150; ThermoFisher Scientific). HRP-conjugated secondary antibodies were used (1:5,000; CST anti-Mouse IgG #7076 [source-horse], anti-Rabbit IgG #7074 [source-goat]) for Western blotting, and the blots were developed using SuperSignal West PICO PLUS or FEMTO PLUS chemiluminescent substrate. An equal amount of proteins was loaded on a separate gel and stained with Coomassie Brilliant Blue as the loading control.
Immunocytochemistry
Mid-log phase cells were harvested and fixed in 1 ml of 4% PFA for 30 min at room temperature. After washing the cells twice with a phosphate–sorbitol buffer (0.1 M KPO4 and 1× in 0.1 M KPO4/1.2 M sorbitol), the pellet was resuspended in 1 ml of 0.1 M KPO4/1.2 M sorbitol. Cells were then spheroplasted with 10 mg/ml Zymolyase for 10–30 min in the presence of β-mercaptoethanol. Spheroplasting was checked by imaging cells on the microscope. For imaging, slides/dishes were prepared by washing in chilled methanol and 95% ethanol. Slides were then coated with 0.1% poly-l-lysine for 5–10 min and then washed thrice with dH2O. A total of 15 μl of spheroplast suspension was placed in each well for 5–10 min at room temperature and then aspirated, and immediately plunged into ice-cold methanol for 5–6 min and then in cold acetone for 30 s. After air drying, 25 μl of PBS-BSA was used for blocking. Primary antibody probing was done following 30 min of blocking for 1 h. After five washes with PBS–BSA, secondary antibody was added and incubated for 1–2 h in a humid chamber in the dark. The wells were again washed with PBS–BSA five times (each wash for 10 min) and then twice with only PBS. Slides were mounted with mounting media (Slowfade DAPI reagent) and imaged.
Statistical analysis
All imaging, spotting assay, and seahorse flux experiments presented are representative of at least three biological replicates, except for data presented in Fig. 1 D; and Fig. S5, D and E, which are representative of two biological replicates. In Figs. 2, D and F; 3, A and D; 5 E; Figs. S2, D and F; S3, A and B; S4, A and D; S5, A and C; and S5, D–G, data were pooled from biological replicates. In Figs. 2 A; 4, B and C; 5, B and F; Figs. S1 D; S2, A and E; and S4 C, data points represent biological replicates, plotted with mean and SD. In Fig. 3 A; Figs. S4, A and D; and S5, A, E, and G, boxes represent the interquartile range (25th and 75th percentile) with whiskers. In Fig. S2 F, the box plot with interquartile range is plotted from pooled data from three biological replicates (data points represent median values from each biological replicate). In Fig. 3 A; Figs. S2 F; S4, A and D; and S5, A, E, and G, a two-tailed Mann–Whitney U test was applied (distributions were found to be non-parametric using the Shapiro–Wilk test applied in Graphpad Prism v9.3.0). In Fig. S5 B, distributions were found to be parametric using the Shapiro–Wilk test, and a two-tailed unpaired Student’s t test was applied. In Figs. 4 C and 5, E and F, distributions were assumed to be parametric (but not formally tested), and unpaired Student’s t test was applied. In Fig. 5 E, mean (black line) and SD (shaded region) were calculated by binning the x-axis (bin size = 0.05). R2 values were calculated for each distribution by applying Pearson’s correlation. Using these R2 values, we obtained residual values for each data point. To compare variances between datasets, Fisher’s F test was applied to distributions of these residual values (distance from the linear fit curve).
Online supplemental material
Fig. S1 shows additional characterization of mtDarT in budding yeast cells. Western blot analysis was used to check for expression of the constructs, and Lcd1::mNeon localization was to confirm that there are no off-target effects of mtDarT. Fig. S2 shows extended data for characterization of mitochondrial dynamics in the 40-min time lapse experiments. In addition to localization of an ERMES marker Mdm24::mNeongreen, Western blot analysis for Dnm1 and Fzo1 proteins under damage conditions is presented. In Fig. S3, the analysis of mitochondrial dynamics with respect to the cell cycle stage is presented. Fig. S4 shows seahorse flux analysis, Cox2 protein Western blot analysis, and morphological characterization in TMRE time course data. In Fig. S5, different parameters for assessment of bulk mitophagy are presented (GFP::Atg8 and Idh1::GFP localization) along with mitochondrial morphological characterization in mip1exo− cells. All strains used in the study are listed in Table S1. All the plasmids used in the study are listed in Table S2. All the oligonucleotides used in the study are listed in Table S3.
Data availability
All materials used in the present study are available upon request. All analysis methods can be found at https://github.com/badrinarayanan-lab/Dua-et-al-2022.
Acknowledgments
The authors are grateful to Dr. Ivan Ahel (University of Oxford, Oxford, UK) for sharing bacterial DarT and DarG constructs and Afroze Chimthanawala for assistance with SIM imaging. The authors thank Dr. Sunil Laxman and members of the AB and SL labs for helpful discussions and feedback on the manuscript.
This work was supported by fellowships from Tata Institute of Fundamental Research (N. Dua), Council of Scientific and Industrial Research (A. Seshadri) as well as grants from Human Frontier Science Program Career Development Award (00051/2017-C; A. Badrinarayanan) and intramural funding from National Centre for Biological Sciences-Tata Institute of Fundamental Research (A. Badrinarayanan).
The authors declare no competing financial interests.
Author contributions: N. Dua: conception of project, experimental design, generation of tools and reagents, execution of experiments, data analysis, writing of manuscript. A. Seshadri: experimental design, generation of tools and reagents, execution of experiments, data analysis, editing of manuscript. A. Badrinarayanan: conception of project, project supervision, experimental design, and writing of manuscript.
References
Supplementary data
lists strains used in present study.
lists oligos used in present study.