Little is known about quality control of proteins that aberrantly or persistently engage the endoplasmic reticulum (ER)-localized translocon en route to membrane localization or the secretory pathway. Hrd1 and Doa10, the primary ubiquitin ligases that function in ER-associated degradation (ERAD) in yeast, target distinct subsets of misfolded or otherwise abnormal proteins based primarily on degradation signal (degron) location. We report the surprising observation that fusing Deg1, a cytoplasmic degron normally recognized by Doa10, to the Sec62 membrane protein rendered the protein a Hrd1 substrate. Hrd1-dependent degradation occurred when Deg1-Sec62 aberrantly engaged the Sec61 translocon channel and underwent topological rearrangement. Mutations that prevent translocon engagement caused a reversion to Doa10-dependent degradation. Similarly, a variant of apolipoprotein B, a protein known to be cotranslocationally targeted for proteasomal degradation, was also a Hrd1 substrate. Hrd1 therefore likely plays a general role in targeting proteins that persistently associate with and potentially obstruct the translocon.
Introduction
The ubiquitin–proteasome system accounts for a major portion of eukaryotic protein degradation. Proteins destined for proteolysis by this pathway are covalently modified with chains of the protein ubiquitin through the sequential action of ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s; Ciechanover et al., 1980; Scheffner et al., 1995). Ubiquitylated substrates are degraded by the 26S proteasome (Ravid and Hochstrasser, 2008). In the yeast Saccharomyces cerevisiae, the transmembrane (TM) RING domain proteins Hrd1 and Doa10 are the predominant ER-associated degradation (ERAD) E3s that target misfolded and regulatory proteins for proteolysis (Hampton et al., 1996; Plemper et al., 1999; Wilhovsky et al., 2000; Bays et al., 2001; Swanson et al., 2001). With a few exceptions, Hrd1 and Doa10 recognize unique substrate classes depending on degron disposition relative to the ER membrane (Vashist and Ng, 2004; Carvalho et al., 2006). Specifically, Hrd1 recognizes proteins with ER luminal or intramembrane degrons, called ERAD-L (lumen) and ERAD-M (membrane) substrates, respectively (Carvalho et al., 2006; Gauss et al., 2006; Sato et al., 2009). In contrast, Doa10 promotes degradation of proteins with cytoplasmically disposed degrons, ERAD-C (cytoplasm) substrates (Huyer et al., 2004; Ravid et al., 2006; Metzger et al., 2008). Hrd1 and Doa10 exhibit overlapping E2 requirements. Hrd1 mediates ubiquitin transfer from Ubc7 and, to a lesser extent, Ubc1 (Plemper et al., 1999; Bays et al., 2001), whereas Doa10 functions with Ubc6 and Ubc7 (Swanson et al., 2001).
The prototypical Doa10 substrate is the transcriptional repressor MATα2, the proteolytic control of which is critical for regulating the yeast-mating phenotype (Swanson et al., 2001; Laney and Hochstrasser, 2003). The N-terminal 67 residues of MATα2, Deg1, contain a degron for Doa10-dependent degradation (Hochstrasser and Varshavsky, 1990; Johnson et al., 1998; Swanson et al., 2001). Importantly, Deg1 confers Doa10-dependent instability when fused to the cytoplasmic N termini of heterologous soluble proteins (Hochstrasser and Varshavsky, 1990; Chen et al., 1993; Johnson et al., 1998; Swanson et al., 2001). Besides soluble Deg1 fusions, we have fused Deg1 to the ER-resident TM protein Vma12 to study Doa10-dependent ERAD (Ravid et al., 2006). Deg1 has also been fused to the topologically similar ER protein Sec62 (Mayer et al., 1998). Like Vma12, Sec62 has two TM segments, with both protein termini on the cytoplasmic side of the membrane (Deshaies and Schekman, 1990). Attachment of Deg1 destabilizes both Sec62 and Vma12 (Mayer et al., 1998; Ravid et al., 2006; Scott and Schekman, 2008). As expected for a Doa10 substrate, Deg1-Sec62 is stabilized in cells lacking both Ubc6 and Ubc7 (Mayer et al., 1998). Because eliminating Ubc7 inactivates both Doa10 and Hrd1 pathways, however, it remained formally possible that Deg1-Sec62 is a Hrd1 substrate. We show that this is indeed the case and that aberrant engagement of the translocon by Deg1-Sec62 is responsible for this E3 switch. This engagement triggers a topological change in Deg1-Sec62 that is closely associated with Hrd1-dependent degradation.
We extended our investigation to a known cotranslocationally degraded protein. Apolipoprotein B (apoB), the major protein component of low- and very-low-density lipoproteins, stalls in the translocon if it cannot associate with its lipid ligand and is subsequently degraded by the proteasome (Yeung et al., 1996; Fisher et al., 1997; Pariyarath et al., 2001). We found that, like Deg1-Sec62, an apoB variant expressed in yeast that is also associated with the translocon (Hrizo et al., 2007) is targeted by the Hrd1 ubiquitin ligase. These results suggest that Hrd1 may play a general role in the degradation of proteins that aberrantly or persistently engage the translocon.
Results
Deg1-Sec62 is a Hrd1 substrate
We used pulse-chase analysis to examine the metabolic stability of the ER-resident TM proteins Sec62 and Vma12 when the Deg1 degron was appended to their N termini (depicted in Fig. 1, A and B). Deg1 destabilized both proteins (Fig. 1, C and D). As previously demonstrated (Ravid et al., 2006), Deg1-Vma12 was strongly stabilized in cells lacking Doa10. It was also stabilized when either of the E2s that function with Doa10 (Ubc6 or Ubc7) were absent (Fig. 1 C). Unexpectedly, Deg1-Sec62 was only stabilized in the absence of Ubc7, which works with either Doa10 or Hrd1, but not in cells lacking Ubc6, which functions exclusively in Doa10-dependent ERAD (Fig. 1, D and E).
Deg1-Sec62 is a Hrd1 substrate. (A) Schematic diagram of Deg1 fusion proteins (with predicted topologies) used in this study. For each construct, the N-terminal Deg1 is followed, in sequence, by the Flag (F) epitope, the 2-TM ER protein (Sec62 or Vma12), and two copies of the Protein A (PrA) tag. For clarity, the fusion proteins are referred to as Deg1-Sec62 or Deg1-Vma12. Any additional alterations to protein sequence will be noted. (B) Linear representation of Deg1-Sec62, drawn to scale. Positions of boundaries between elements within the fusion protein and residue numbers mentioned in the text are highlighted. (C) Pulse-chase analysis of Deg1-Vma12 in the indicated yeast strains. (D and F) Pulse-chase analysis of Deg1-Sec62 in the indicated yeast strains. Molecular weight markers for these autoradiographs are not available; however, migration of the same protein can be seen in Fig. 4 B. (E and G) Quantification of autoradiographs in D and F. Data are representative of three experiments. The black line indicates that intervening lanes have been spliced out. (H) Pulse-chase analysis of Deg1*-Sec62 in the indicated yeast strains. Deg1 fusions in C, D, and F were precipitated with anti-Deg1 antibodies. Deg1*-Sec62 in H was precipitated with anti-Flag antibodies. Cycloheximide was included in the chase depicted in H. Deg1*, F18S/I22T double mutant.
Deg1-Sec62 is a Hrd1 substrate. (A) Schematic diagram of Deg1 fusion proteins (with predicted topologies) used in this study. For each construct, the N-terminal Deg1 is followed, in sequence, by the Flag (F) epitope, the 2-TM ER protein (Sec62 or Vma12), and two copies of the Protein A (PrA) tag. For clarity, the fusion proteins are referred to as Deg1-Sec62 or Deg1-Vma12. Any additional alterations to protein sequence will be noted. (B) Linear representation of Deg1-Sec62, drawn to scale. Positions of boundaries between elements within the fusion protein and residue numbers mentioned in the text are highlighted. (C) Pulse-chase analysis of Deg1-Vma12 in the indicated yeast strains. (D and F) Pulse-chase analysis of Deg1-Sec62 in the indicated yeast strains. Molecular weight markers for these autoradiographs are not available; however, migration of the same protein can be seen in Fig. 4 B. (E and G) Quantification of autoradiographs in D and F. Data are representative of three experiments. The black line indicates that intervening lanes have been spliced out. (H) Pulse-chase analysis of Deg1*-Sec62 in the indicated yeast strains. Deg1 fusions in C, D, and F were precipitated with anti-Deg1 antibodies. Deg1*-Sec62 in H was precipitated with anti-Flag antibodies. Cycloheximide was included in the chase depicted in H. Deg1*, F18S/I22T double mutant.
We directly tested which ERAD E3s were responsible for Deg1-Sec62 degradation. Surprisingly, deletion of DOA10 did not impair Deg1-Sec62 degradation, whereas the Deg1 fusion protein was strongly stabilized in the absence of Hrd1 (Fig. 1, F and G). The Hrd1 dependence for Deg1-Sec62 degradation was unanticipated because Deg1 typically targets proteins to which it is fused for Doa10-dependent proteolysis. Moreover, the predicted cytoplasmic disposition of Deg1 represents an apparent exception to the division of ERAD-L/M and ERAD-C substrates between Hrd1 and Doa10, respectively (Vashist and Ng, 2004; Carvalho et al., 2006).
Doa10-mediated degradation of Deg1-harboring proteins depends on the hydrophobic face of a predicted amphipathic helix within Deg1 (Johnson et al., 1998). When mutations in this helix that stabilize Deg1-containing Doa10 substrates were introduced into Deg1-Sec62, the resultant protein (Deg1*-Sec62) was still degraded in a Hrd1-dependent manner (Fig. 1 H), unlike Deg1*-Vma12, which is strongly stabilized (Ravid et al., 2006). Thus, the details underlying molecular recognition of Deg1-containing substrates differ between the two ERAD E3s.
Posttranslational modification (PTM) of Deg1-Sec62
Deg1-Sec62 migrated as multiple species by SDS-PAGE and became increasingly modified with time after its synthesis (Fig. 1, D and F). It was previously noted that Deg1-Sec62 is N-glycosylated, in contrast to wild-type (WT) Sec62, which is not typically posttranslationally modified (Kim et al., 2006; Scott and Schekman, 2008). Immediately after radiolabeling, Deg1-Sec62 migrated as two distinct species (Fig. 2 A, lane 1). After treatment with Endoglycosidase H (Endo H), which removes all N-glycans from yeast proteins, the slower-migrating band collapsed to the faster migrating species (lane 2). Mutational analysis revealed that N153 was the major site of N-glycosylation (with a possible minor contribution by N90; lanes 5–12), which is consistent with an earlier study (Scott and Schekman, 2008). After 60 min, the higher mass species appeared as multiple diffuse bands (lane 3). Endo H treatment only partially increased the mobility of these poorly resolved bands (lane 4), which indicates additional PTM.
N-glycosylation of Deg1-Sec62. (A) doa10Δ hrd1Δ yeast cells expressing Deg1-Sec62 or Deg1-Sec62 with the indicated consensus N-glycosylation sites mutated were pulse labeled for 10 min and lysed immediately or after 60 min in the presence of excess nonradioactive amino acids and cycloheximide. Deg1 fusion proteins were precipitated with anti-Flag antibodies and incubated in the presence or absence of Endo H. (B) Pulse-chase analysis of Deg1-Sec62-N90D/N153D in the indicated yeast strains. Deg1-Sec62 was precipitated with anti-Flag antibodies. Cycloheximide was included in the chase.
N-glycosylation of Deg1-Sec62. (A) doa10Δ hrd1Δ yeast cells expressing Deg1-Sec62 or Deg1-Sec62 with the indicated consensus N-glycosylation sites mutated were pulse labeled for 10 min and lysed immediately or after 60 min in the presence of excess nonradioactive amino acids and cycloheximide. Deg1 fusion proteins were precipitated with anti-Flag antibodies and incubated in the presence or absence of Endo H. (B) Pulse-chase analysis of Deg1-Sec62-N90D/N153D in the indicated yeast strains. Deg1-Sec62 was precipitated with anti-Flag antibodies. Cycloheximide was included in the chase.
Deg1-Sec62 is also modified by O-mannosylation, an ER-specific modification with a role in the ERAD of some Hrd1 substrates (Vashist et al., 2001; Hirayama et al., 2008). We performed pulse-chase analysis of doa10Δ hrd1Δ yeast expressing Deg1-Sec62-N90D/N153D cultured in the presence of a global inhibitor of O-mannosylation (Orchard et al., 2004). Under these conditions, the more slowly migrating species of Deg1-Sec62-N90D/N153D exhibited enhanced mobility but did not obtain the mobility of the fastest migrating, presumably unmodified species (Fig. S1 A). The full complement of PTMs decorating Deg1-Sec62 and the substrate residues that they modify remain to be characterized.
Neither N-glycosylation nor O-mannosylation was required for Hrd1-mediated degradation of Deg1-Sec62 (Fig. 2 B and Fig. S1 B). Preventing glycosylation also did not restore Doa10-mediated targeting of Deg1-Sec62 (Fig. 2 B and Fig. S1 B). Conversely, N-glycosylation at engineered sites in Deg1-Vma12 did not prevent its Doa10-dependent degradation (Fig. S2 A). Nonetheless, Deg1-Sec62 PTMs serve as a convenient indicator of a topological transition that, as subsequent experiments will demonstrate, plays an important role in the switch from Doa10 to Hrd1 dependency.
Topology of Deg1-Sec62
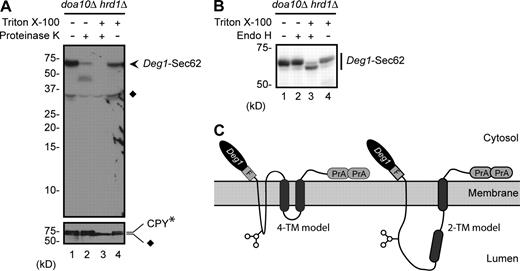
Strikingly, N-glycosylation of Deg1-Sec62 occurred on residues in the predicted cytoplasmic N-terminal domain (Fig. 1 B), whereas the glycosylation machinery resides in the ER lumen. Therefore, fusion of Deg1 to Sec62 must stimulate dislocation of at least a portion of the cytoplasmically disposed N-terminal domain into the ER lumen. As judged by immunoblot analysis, the majority of Deg1-Sec62 at steady state was posttranslationally modified, and therefore topologically altered (Fig. S1 C). Previous work indicated that both the Flag epitope immediately downstream of Deg1 and the C-terminal Protein A tag (Fig. 1, A and B) of Deg1-Sec62 remain on the cytoplasmic face of the ER membrane (Scott and Schekman, 2008). To confirm the cytoplasmic disposition of Deg1, ER-derived microsomal membranes were incubated with Proteinase K followed by anti-Deg1 immunoblotting (Fig. 3 A). The Deg1-dependent topological rearrangement that allows glycosylation did not protect Deg1 from protease exposure to the external (cytoplasmic) face of microsomal membranes in the absence of detergent (lane 2). Cytoplasmic exposure of Deg1 is particularly intriguing given that Doa10 is incapable of targeting Deg1-Sec62.
Membrane topology of Deg1-Sec62. (A) Intact microsomal membranes prepared from doa10Δ hrd1Δ cells expressing Deg1-Sec62 and ER luminal control protein CPY* were treated with 5 µg/ml Proteinase K (or mock-treated) in the presence or absence of 1% Triton X-100. Samples were separated by SDS-PAGE and detected by immunoblotting with antibodies against Deg1 (top) or CPY (bottom). Diamonds denote nonspecific bands. Partially protease-resistant, anti–Deg1-reactive species are seen between 37 and 50 kD when detergent is excluded (lane 2). Species of similar size and intensity are observed when Deg1-Vma12 is subjected to the same treatment (Fig. S3, lane 8). Thus, the Deg1 moiety of Deg1-Sec62 exhibits protease accessibility similar to that of Deg1-Vma12. (B) The same as in A, but microsomal preparations were incubated with Endo H instead of Proteinase K. Deg1-Sec62 was detected by immunoblotting with peroxidase anti-peroxidase antibody, which recognizes the Protein A epitope. (C) Models for topological rearrangement of Deg1-Sec62 with respect to the ER membrane. In each case, both N-terminal Deg1 and C-terminal Sec62 tail remain on the cytoplasmic face of the ER membrane. In the 4-TM model, the normally cytoplasmic sequence of Deg1-Sec62 downstream of Deg1 loops into the ER lumen, flanked by two novel membrane-spanning segments. In the 2-TM model, the first membrane-spanning segment of Deg1-Sec62 is significantly upstream of the first normally used Sec62 TM. The approximate position of the N-glycosylated N153 residue is indicated with a cartoon representation of the N-glycan.
Membrane topology of Deg1-Sec62. (A) Intact microsomal membranes prepared from doa10Δ hrd1Δ cells expressing Deg1-Sec62 and ER luminal control protein CPY* were treated with 5 µg/ml Proteinase K (or mock-treated) in the presence or absence of 1% Triton X-100. Samples were separated by SDS-PAGE and detected by immunoblotting with antibodies against Deg1 (top) or CPY (bottom). Diamonds denote nonspecific bands. Partially protease-resistant, anti–Deg1-reactive species are seen between 37 and 50 kD when detergent is excluded (lane 2). Species of similar size and intensity are observed when Deg1-Vma12 is subjected to the same treatment (Fig. S3, lane 8). Thus, the Deg1 moiety of Deg1-Sec62 exhibits protease accessibility similar to that of Deg1-Vma12. (B) The same as in A, but microsomal preparations were incubated with Endo H instead of Proteinase K. Deg1-Sec62 was detected by immunoblotting with peroxidase anti-peroxidase antibody, which recognizes the Protein A epitope. (C) Models for topological rearrangement of Deg1-Sec62 with respect to the ER membrane. In each case, both N-terminal Deg1 and C-terminal Sec62 tail remain on the cytoplasmic face of the ER membrane. In the 4-TM model, the normally cytoplasmic sequence of Deg1-Sec62 downstream of Deg1 loops into the ER lumen, flanked by two novel membrane-spanning segments. In the 2-TM model, the first membrane-spanning segment of Deg1-Sec62 is significantly upstream of the first normally used Sec62 TM. The approximate position of the N-glycosylated N153 residue is indicated with a cartoon representation of the N-glycan.
The susceptibility of the Deg1-Sec62 N terminus to proteolysis despite ER luminal access of N153 could be explained in two ways. Deg1 fusion to Sec62 might stimulate stable movement of a portion of the normally cytoplasmic N-terminal domain downstream of Deg1 into the ER lumen. Alternatively, Deg1 attachment may allow reversible movement of the entire N-terminal Deg1-Sec62 domain into and out of the ER lumen. Return of the N terminus to the cytoplasmic face of the ER would explain protease accessibility. Consistent with the first alternative, we found that microsomal Deg1-Sec62 was sensitive to Endo H only when detergent was added (Fig. 3 B, lane 3). Therefore, fusion of Deg1 to Sec62 triggers the stable movement of a portion of the N-terminal Sec62 tail into the ER lumen while Deg1 remains on the cytoplasmic side. These experiments are consistent with two models for the final topology of Deg1-Sec62, which differ in how much of the N-terminal domain is dislocated into the ER lumen (Fig. 3 C; see Discussion).
Impaired translocon binding to Deg1-Sec62 allows Doa10-dependent degradation
Based on genetic and physical interactions, Sec61 has been proposed to function as a retrotranslocation channel for Hrd1 substrates, although this remains controversial (de Virgilio et al., 1998; Willer et al., 2008; Schäfer and Wolf, 2009). Sec62 is a component of the Sec61 complex involved in posttranslational translocation (PTT; Deshaies et al., 1991; Ng et al., 1996). In a strain background that limits its degradation, Deg1-Sec62 complements sec62 mutants, which suggests that it associates with Sec61 in a manner similar to WT Sec62 (Mayer et al., 1998). We hypothesized that recognition of Deg1-Sec62 as a Hrd1 substrate is mediated by its incorporation into the translocon complex. To test this, we introduced a point mutation into Deg1-Sec62 (G127D, Deg1-sec62†) that partially impairs Sec62 association with Sec63, its interacting partner within the translocon complex (Deshaies and Schekman, 1990; Deshaies et al., 1991; Wittke et al., 2000), and assayed protein degradation.
Deg1-sec62† exhibited a shorter half-life than Deg1-Sec62 (Fig. 4 A, compare WT cells, top and bottom). Strikingly, this single point mutation directed a switch in the E3 dependence of degradation (Fig. 4 A, top). Unlike Deg1-Sec62, Deg1-sec62† was strongly (though incompletely) stabilized in doa10Δ cells and remained unstable in hrd1Δ cells. Full metabolic stabilization was not observed unless both E3s were absent, which is consistent with the incomplete ablation of sec62† interaction with Sec63 (Wittke et al., 2000).
Inhibiting Deg1-Sec62 association with the translocon delays its PTM and restores Doa10-dependent degradation. (A–D) Pulse-chase analysis of Deg1-Sec62 variants in the indicated yeast strains. Where noted, yeast harbor a plasmid encoding WT Sec63 or an empty vector. Deg1 fusions in A and D were precipitated with anti-Deg1 antibodies. Deg1 fusions in B and C were chased in the presence of cycloheximide and precipitated with anti-Flag antibodies. Molecular weight markers for the autoradiograph in A are not available; however, migration of the same protein can be seen in Fig. S1 D. sec62†, G127D of Deg1-Sec62, equivalent to G37D of untagged Sec62. sec63-201, 27-residue C-terminal truncation of Sec63. Deg1*, F18S/I22T double mutant.
Inhibiting Deg1-Sec62 association with the translocon delays its PTM and restores Doa10-dependent degradation. (A–D) Pulse-chase analysis of Deg1-Sec62 variants in the indicated yeast strains. Where noted, yeast harbor a plasmid encoding WT Sec63 or an empty vector. Deg1 fusions in A and D were precipitated with anti-Deg1 antibodies. Deg1 fusions in B and C were chased in the presence of cycloheximide and precipitated with anti-Flag antibodies. Molecular weight markers for the autoradiograph in A are not available; however, migration of the same protein can be seen in Fig. S1 D. sec62†, G127D of Deg1-Sec62, equivalent to G37D of untagged Sec62. sec63-201, 27-residue C-terminal truncation of Sec63. Deg1*, F18S/I22T double mutant.
The dramatically reduced Hrd1 dependence of Deg1-sec62† degradation coincided with significantly delayed glycosylation (which requires topological rearrangement of the Sec62 N-terminal domain), which suggested a mechanistic link between the two (Fig. 4 A, compare doa10Δ hrd1Δ cells, top and bottom panels; and Fig. S1 D). The remaining trace of posttranslationally modified Deg1-sec62† was stabilized in hrd1Δ cells (Fig. 4 A). As with the Deg1-sec62† mutant, the sec63-201 mutant, which interacts poorly with WT Sec62 (Ng and Walter, 1996; Wittke et al., 2000), caused an increase in the degradation rate, a switch in E3 specificity, and a delay in modification of Deg1-Sec62 (Fig. 4 B). Complementation of sec63-201 cells with WT SEC63 caused all these changes to be reverted (Fig. 4 C). When the sec62† mutation was introduced in the context of Deg1*, which is not recognized by Doa10, the protein was almost completely stabilized (Fig. 4 D). These data strongly suggest that persistent interaction of Deg1-Sec62 with the translocon stimulates both Deg1-Sec62 domain dislocation into the ER lumen and its switch to Hrd1-dependent degradation.
Topological rearrangement of Deg1-Sec62 via PTT
Most N-glycosylated proteins are modified while they are being translocated (Whitley et al., 1996; Popov et al., 1997; Scheper et al., 2003). Like WT Sec62, Deg1-Sec62 is likely cotranslationally translocated into the ER membrane. The gradual acquisition of N-glycosylation, especially when translocon binding is partially impaired (Fig. 4 and Fig. S1 D), suggested that the topological transition allowing such modification occurs after the initial “normal” membrane insertion, possibly through the Sec61 channel itself. We hypothesized that appending Deg1 to Sec62 triggers insertion of a portion of the cytoplasmic N-terminal domain through the Sec61 translocon via a PTT mechanism after initial cotranslational translocation of Deg1-Sec62 (Zimmermann et al., 2011). Moreover, we speculated that this insertion is critical for triggering Hrd1-dependent degradation and preventing Doa10 recognition of Deg1-Sec62.
To test these ideas, we analyzed Deg1-Sec62 E3 specificity and PTM kinetics in cells expressing a variant of Sec61—sec61-L7B(ala)—which harbors four mutations in a luminal loop and is predominantly defective for PTT (Trueman et al., 2011). Strikingly, in the context of sec61-L7B(ala), Deg1-Sec62 degradation reverted to Doa10 dependence (Fig. 5 A). A delay in PTM coincided with impaired degradation in doa10Δ cells expressing sec61-L7B(ala), likely signifying delayed movement of the Sec62 N-terminal domain into the ER lumen. The gradually appearing, posttranslationally modified species of Deg1-Sec62 were cleared in a Hrd1-dependent manner. A similar delay in PTM and reversion to Doa10-dependent degradation were observed in cells lacking the BiP co-chaperone Lhs1, which is required for efficient PTT of many proteins (Fig. 5 B; Hamilton et al., 1999; Tyson and Stirling, 2000; Steel et al., 2004). Passage of a portion of Deg1-Sec62 through the translocon is consistent with the reported formation of a transient disulfide linkage between a cysteine within the channel interior of Sec61 and a cysteine in the N-terminal domain of Deg1-Sec62 (but not WT Sec62; Scott and Schekman, 2008). Indeed, mutating either participating cysteine accelerated Deg1-Sec62 degradation and partially switched E3 dependence from Hrd1 to Doa10 (Fig. 5, C and D).
Deg1-Sec62 topological rearrangement and Hrd1 targeting depend on the PTT pathway. (A–D) Pulse-chase analysis of denoted Deg1-Sec62 variants in the indicated yeast strains. Where noted, yeast harbor a plasmid encoding a variant of Sec61. Cycloheximide was included in the chase. Deg1 fusion proteins were precipitated with anti-Flag antibodies.
Deg1-Sec62 topological rearrangement and Hrd1 targeting depend on the PTT pathway. (A–D) Pulse-chase analysis of denoted Deg1-Sec62 variants in the indicated yeast strains. Where noted, yeast harbor a plasmid encoding a variant of Sec61. Cycloheximide was included in the chase. Deg1 fusion proteins were precipitated with anti-Flag antibodies.
In summary, these data strongly suggest that topological rearrangement of Deg1-Sec62 takes place through the Sec61 translocon by a PTT mechanism and that this is necessary for Hrd1 recognition.
Degradation of Deg1-Sec62 differs from that of previously characterized ERAD-L and ERAD-M substrates
Deg1-Sec62 domain dislocation moves normally cytoplasmic residues of Sec62 to the ER lumen and membrane. We therefore determined if Deg1*-Sec62 degradation requires Hrd1 cofactors required for ERAD-L and ERAD-M substrate degradation. Deg1*-Sec62 was efficiently degraded in the absence of Yos9 and the Derlins (Der1 and Dfm1), proteins with roles in ERAD-L, and only marginally stabilized in the absence of Usa1, which functions in both ERAD-L and ERAD-M (Fig. 6 A; Knop et al., 1996; Buschhorn et al., 2004; Bhamidipati et al., 2005; Kim et al., 2005; Szathmary et al., 2005; Carvalho et al., 2006; Goder et al., 2008; Horn et al., 2009; Carroll and Hampton, 2010; Carvalho et al., 2010; Kanehara et al., 2010). Deg1*-Sec62 was significantly, but incompletely, stabilized in the absence of Hrd3, which is required for degradation of both ERAD-L and ERAD-M substrates (Hampton et al., 1996; Carvalho et al., 2006; Gauss et al., 2006). However, this effect may be indirect, as Hrd1 levels are markedly reduced in the absence of Hrd3 (Plemper et al., 1999). A control ERAD-L substrate, CPY*-HA, was significantly stabilized in these mutant strains (Fig. 6 B). Degradation requirements for Deg1*-Sec62 bear some resemblance to those for the ERAD-M substrate Hmg2. However, Hmg2 degradation displays a stronger dependence on Hrd3 (Wilhovsky et al., 2000) and potentially Usa1 (Horn et al., 2009; Carroll and Hampton, 2010) for its degradation. Together, these results suggest that recognition of Deg1-Sec62 occurs through a novel mechanism requiring the minimal ERAD machinery consisting of Hrd1, Ubc7, presumably Cue1 (which anchors Ubc7 to the ER membrane), and possibly Hrd3. Novel cofactors might also be involved.
Hrd1 cofactor requirements for Deg1-Sec62 degradation. (A) Pulse-chase analysis of Deg1*-Sec62 in the indicated yeast strains. The percentage of input protein remaining at each time-point is indicated below the autoradiograph. Cycloheximide was included in the chase. Deg1*-Sec62 was precipitated with anti-Flag antibodies. Deg1*, F18S/I22T double mutant. The black line indicates that intervening lanes have been spliced out. (B) Cycloheximide chase analysis of CPY*-HA in the indicated yeast strains. CPY*-HA was detected by anti-HA immunoblotting. Pgk1 serves as a loading control and was detected by anti-Pgk1 immunoblotting.
Hrd1 cofactor requirements for Deg1-Sec62 degradation. (A) Pulse-chase analysis of Deg1*-Sec62 in the indicated yeast strains. The percentage of input protein remaining at each time-point is indicated below the autoradiograph. Cycloheximide was included in the chase. Deg1*-Sec62 was precipitated with anti-Flag antibodies. Deg1*, F18S/I22T double mutant. The black line indicates that intervening lanes have been spliced out. (B) Cycloheximide chase analysis of CPY*-HA in the indicated yeast strains. CPY*-HA was detected by anti-HA immunoblotting. Pgk1 serves as a loading control and was detected by anti-Pgk1 immunoblotting.
Degradation of an apolipoprotein B derivative requires Hrd1
We hypothesized that the mechanism used by Hrd1 to target Deg1-Sec62 at the translocon may be related to that of a known cotranslocationally degraded protein with intriguing links to human health. Under conditions in which lipid binding of apoB (a major component of low-density and very-low-density lipoproteins) is impaired, apoB translocation is arrested and the protein remains associated with the translocon (Fisher et al., 1997; Pariyarath et al., 2001). Incompletely translocated apoB is redirected to proteasomal degradation in the cytoplasm (Yeung et al., 1996), perhaps via a mechanism similar to that of Deg1-Sec62 degradation. ApoB copurifies with several mammalian ERAD components, including HRD1, Derlin-1, and p97 (Fisher et al., 2008; Rutledge et al., 2009). Moreover, overexpression of the mammalian E3 gp78 (a HRD1 paralogue) stimulates degradation of apoB (Liang et al., 2003). Knockdown of gp78 expression decreases apoB ubiquitylation and enhances apoB secretion (Fisher et al., 2011). A model apoB derivative (apoB29) expressed in yeast copurifies with the translocon and is partially stabilized in ubc7Δ or doa10Δ hrd1Δ cells but not ubc6Δ cells, which is consistent with a wider role for Hrd1 in degradation of proteins persistently associated with the translocon (Hrizo et al., 2007). We therefore performed cycloheximide-chase/immunoblot analysis to test directly whether apoB29 degradation requires this E3. ApoB29 was in fact significantly stabilized in hrd1Δ cells (Fig. 7 A).
ApoB29 is a Hrd1 substrate. (A–C) Cycloheximide chase analysis of ApoB29-3HA, expressed under the control of the GAL1 promoter in the indicated yeast strains, which were grown for 5 h in SD medium containing 2% galactose. ApoB29-3HA was detected by anti-HA immunoblotting. Pgk1 serves as a loading control and was detected by anti-Pgk1 immunoblotting. The diamonds denote a nonspecific band. (A) As controls, hrd1Δ yeast harboring a plasmid with GAL1-driven ApoB29-3HA were grown in 2% glucose (repressing) medium, and hrd1Δ yeast harboring an empty vector were grown in 2% glucose (repressing) or 2% galactose (inducing) medium and processed similarly. (B) Lysates prepared immediately or 90 min after cycloheximide addition were incubated in the presence or absence of Endo H.
ApoB29 is a Hrd1 substrate. (A–C) Cycloheximide chase analysis of ApoB29-3HA, expressed under the control of the GAL1 promoter in the indicated yeast strains, which were grown for 5 h in SD medium containing 2% galactose. ApoB29-3HA was detected by anti-HA immunoblotting. Pgk1 serves as a loading control and was detected by anti-Pgk1 immunoblotting. The diamonds denote a nonspecific band. (A) As controls, hrd1Δ yeast harboring a plasmid with GAL1-driven ApoB29-3HA were grown in 2% glucose (repressing) medium, and hrd1Δ yeast harboring an empty vector were grown in 2% glucose (repressing) or 2% galactose (inducing) medium and processed similarly. (B) Lysates prepared immediately or 90 min after cycloheximide addition were incubated in the presence or absence of Endo H.
ApoB29 appeared as multiple bands when separated by SDS-PAGE, with a gradual conversion of lower to higher molecular weight species over time. This is most apparent under stabilizing conditions (i.e., in hrd1Δ cells). ApoB29 is a translocon-engaged protein with its N terminus exposed to the ER lumen. These slower migrating bands of apoB29 were sensitive to Endo H, which indicates N-glycosylation of apoB29 (Fig. 7 B). A complete collapse to the fastest migrating species was not observed, however, which suggests that apoB29, like Deg1-Sec62, acquires additional PTMs distinct from N-glycosylation. These additional PTMs became more prominent after inhibition of protein synthesis, as has previously been observed for other ERAD substrates (Wilhovsky et al., 2000; Sato and Hampton, 2006).
Finally, we analyzed the cofactor requirements for apoB29 degradation. In contrast to Deg1-Sec62, apoB29 was markedly stabilized in cells lacking Hrd3, Yos9, Usa1, or the Derlins (Fig. 7 C). Thus, although both proteins may be targeted by Hrd1 by virtue of their aberrant or prolonged translocon engagement, they differ with respect to the exact cofactors required for their degradation.
Discussion
Many proteins pass through the Sec61 translocon en route to their ultimate subcellular or extracellular destinations. Potentially, ERAD substrates may also pass through this channel in a retrograde fashion en route to proteasomal degradation. Aberrant, translocationally stalled proteins might limit flux through the channel in either direction, and eukaryotes may therefore have evolved mechanisms for eliminating such obstructions. Although Deg1-Sec62 (like many heavily studied model ERAD substrates) is an artificial protein, it may illuminate a previously unappreciated protein quality-control pathway mediated by the Hrd1 ubiquitin ligase and serve as a prototype for proteins that aberrantly engage or occupy the translocon. Indeed, we find that clearance of apoB, a protein previously reported to be cotranslocationally degraded, also depends on Hrd1.
Deg1-Sec62 inserts into the ER membrane in two distinct steps. Initially, the two TM segments of Deg1-Sec62 are probably cotranslationally translocated to yield the same topology as WT Sec62. In a second, Deg1-dependent step, a loop within the normally cytoplasmic N-terminal domain of Deg1-Sec62 penetrates the ER membrane. This rearrangement allows access of previously cytoplasmic residues to the N- and O-glycosylation machinery of the ER lumen. Although glycosylation is not required for Hrd1-mediated degradation, the switch in degradation dependence from Doa10 to Hrd1 tracks very closely with acquisition of these PTMs. Our data strongly suggest that the topological rearrangement across the ER membrane is the crucial event dictating E3 specificity (Fig. 8).
Model for Hrd1-dependent ERAD of Deg1-Sec62. (A) After its initial cotranslational translocation, Deg1-Sec62 aberrantly engages the Sec61 translocon, resulting in PTT of the normally cytoplasmic N-terminal Sec62 tail. After membrane penetration, Deg1-Sec62 becomes N-glycosylated. In this rearranged conformation, Doa10 does not recognize Deg1-Sec62 as an ERAD-C substrate. Deg1-Sec62 remains trapped in this orientation unless Hrd1 targets it for degradation, thereby restoring functionality to the Sec61 translocon. With the exception of the E2 Ubc7 (and presumably its membrane-anchoring binding partner Cue1; Biederer et al., 1997), and potentially Hrd3 (not depicted), well-characterized Hrd1 cofactors that function in ERAD-L and ERAD-M are dispensable for ERAD of Deg1-Sec62. The approximate position of N-glycosylated N153 residue is indicated with a cartoon representation of the N-glycan. (B) When translocon engagement is prevented by disrupting Deg1-Sec62 interaction with the translocon or impairing PTT, Deg1-Sec62 retains its original topology and is targeted by Doa10 as an ERAD-C substrate. Black circles, ubiquitin.
Model for Hrd1-dependent ERAD of Deg1-Sec62. (A) After its initial cotranslational translocation, Deg1-Sec62 aberrantly engages the Sec61 translocon, resulting in PTT of the normally cytoplasmic N-terminal Sec62 tail. After membrane penetration, Deg1-Sec62 becomes N-glycosylated. In this rearranged conformation, Doa10 does not recognize Deg1-Sec62 as an ERAD-C substrate. Deg1-Sec62 remains trapped in this orientation unless Hrd1 targets it for degradation, thereby restoring functionality to the Sec61 translocon. With the exception of the E2 Ubc7 (and presumably its membrane-anchoring binding partner Cue1; Biederer et al., 1997), and potentially Hrd3 (not depicted), well-characterized Hrd1 cofactors that function in ERAD-L and ERAD-M are dispensable for ERAD of Deg1-Sec62. The approximate position of N-glycosylated N153 residue is indicated with a cartoon representation of the N-glycan. (B) When translocon engagement is prevented by disrupting Deg1-Sec62 interaction with the translocon or impairing PTT, Deg1-Sec62 retains its original topology and is targeted by Doa10 as an ERAD-C substrate. Black circles, ubiquitin.
Other work supports the 4-TM model of rearranged topology (Fig. 3 C). Potential O-mannosylation sites (serines) were systematically introduced throughout Deg1-Sec62 (Scott and Schekman, 2008). We inferred that residues that became O-mannosylated reached the ER lumen; nonmodified serine substitutions were judged to be in the membrane-spanning or cytoplasmic segments. O-mannosylation of serines inserted at positions 132 and 170 confirmed that this span is present within the ER lumen (see Fig. 1 B). Substitutions at other positions, such as immediately upstream of the first known TM, did not result in O-mannosylation. The most straightforward interpretation of these data is the 4-TM model of Deg1-stimulated topological rearrangement (Fig. 3 C), although the failure to observe O-mannosylation of a particular serine does not exclude its luminal residence (Lommel and Strahl, 2009).
Topological rearrangement of Deg1-Sec62 likely occurs via Sec61-mediated PTT. Disrupting contacts of Deg1-Sec62 with the translocon or impairing PTT by Sec61 mutation prevents domain dislocation and significantly reverts degradation dependence to Doa10 (Figs. 4 and 5). Additionally, a cysteine found within the initially cytoplasmic N-terminal domain of Deg1-Sec62 forms a transient disulfide linkage with a cysteine on the interior of the Sec61 channel (Scott and Schekman, 2008). This bond appears to facilitate the altered topology of the Sec62 N-terminal domain, as mutation of either participating cysteine delays Deg1-Sec62 PTM and causes a partial switch to Doa10-dependent degradation (Fig. 5, C and D).
Fusion of Deg1 to Sec62 was not anticipated to trigger PTT of the normally cytoplasmic Sec62 N-terminal domain. MATα2, from which Deg1 is derived, is a soluble nuclear protein, and Deg1 fused to the isolated soluble Sec62 N-domain (residues 1–149) does not trigger membrane translocation (Scott and Schekman, 2008). Rather, Deg1-Sec621–149 is degraded in a Doa10-dependent manner, behaving like other characterized soluble Deg1 fusions (Fig. S2 B). Potentially, fusing any protein sequence to the Sec62 N terminus could promote the observed topological rearrangement. This is not the case, however, as Deg1 bearing a 20-residue internal deletion does not promote PTM (or degradation) when fused to Sec62 (Scott and Schekman, 2008). Deg1, by virtue of novel protein–protein and/or protein–membrane interactions stimulated by enforced proximity to the translocon, may conformationally alter the N-terminal portion of Sec62 or cause it to linger persistently near the opening of the Sec61 translocon channel. The translocon could respond by attempting to conduct the Sec62 N-terminal tail into the ER lumen. The disulfide linkage between Deg1-Sec62 and the Sec61 channel may strengthen the interaction of the Sec62 N-domain with a signal sequence-binding site within the channel. Consistent with this, the cysteine cross-link is not strictly necessary for topological rearrangement but significantly accelerates it (Fig. 5, C and D; and Fig. S1 D).
Lateral release of true TM segments from the translocon into the plane of the lipid bilayer depends strongly on the hydrophobic character of the TM helices (Shao and Hegde, 2011; Zimmermann et al., 2011). The N-terminal loop of Deg1-Sec62 does not include bona fide TM domains; thus, we speculate that the membrane-spanning polypeptide segments flanking the N-glycosylated luminal element resist lateral release from the translocon and persistently occupy the Sec61 channel. We propose that Hrd1 recognizes persistent translocon engagement by Deg1-Sec62 after the aberrant translocation event. In this respect, Deg1-Sec62 may resemble a translocationally stalled or arrested protein. Hrd1, by virtue of its reported association with the translocon (Schäfer and Wolf, 2009), may be ideally poised to target such incompletely translocated proteins for ubiquitin-mediated degradation. It is also possible that the Sec61-dependent topological change in Deg1-Sec62 generates a permanently misfolded TM protein or misassembled translocon complex that is recognized by Hrd1. Nevertheless, the cofactor requirements for Deg1-Sec62 differ from other well-characterized Hrd1 substrates (Fig. 8), which is consistent with a unique mechanism for recognition and degradation.
Deg1 has been found to destabilize other TM proteins in a partially Hrd1-dependent manner. Like Deg1-Sec62, both Deg1-Hmg1 and Deg1-Hmg2 fusion proteins are strongly stabilized in the absence of Ubc7. Deletion of HRD1 partially impairs turnover of these proteasome substrates (Wilhovsky et al., 2000). Whether these proteins are targeted in a mechanism similar to that targeting Deg1-Sec62 remains to be determined.
The factors preventing Doa10 from recognizing Deg1-Sec62 remain enigmatic. After topological rearrangement of Deg1-Sec62, Deg1 remains on the cytoplasmic face of the ER membrane (Fig. 3). However, this posttranslationally translocated form of the fusion protein is resistant to Doa10-mediated proteolysis. The translocon-dependent rearrangement might alter the position or conformation of Deg1 such that it is inaccessible to the Doa10 complex. Preventing interaction of Deg1-Sec62 with the translocon causes a strong reversion to Doa10-dependent degradation (Fig. 4). Therefore, Deg1-Sec62 is not inherently unrecognizable by Doa10. Interfering with events downstream of translocon binding (e.g., inhibiting PTT or preventing Deg1-Sec62–Sec61 disulfide formation) also causes a significant switch to Doa10-dependent degradation (Fig. 5). Thus, Doa10 is capable of recognizing Deg1-Sec62 even when conditions allow initial interaction with the translocon. In these cases, Doa10 might target Deg1-Sec62 that is in complex with the translocon but has not yet undergone PTT or might recognize Deg1-Sec62 that has transiently dissociated from Sec61.
The discovery that abnormal translocon engagement precedes Deg1-Sec62 degradation led us to hypothesize that cotranslocational proteolysis of apoB might occur by a similar mechanism. ApoB uses the translocon as a platform for progressive lipid binding. When lipid binding is inhibited, translocation into the ER lumen is slowed, and the protein is destroyed by the cytoplasmic proteasome (Fisher et al., 1997; Pariyarath et al., 2001). Earlier studies implied that degradation of a model variant of apoB occurred via the ERAD system in yeast (Hrizo et al., 2007). Our results directly demonstrate Hrd1 dependence for apoB29 degradation in yeast (Fig. 7 A). The cofactor requirements for Deg1-Sec62 and apoB Hrd1-dependent degradation, however, differed, which suggests that the details of E3-substrate recognition diverge for these substrates.
A previous study had led to the suggestion that Deg1-Sec62 glycosylation and disulfide formation with Sec61 occur during Sec61-dependent retrotranslocation of a Doa10 substrate (Scott and Schekman, 2008). Our results, however, strongly suggest that these events occur in the process of Hrd1 substrate generation. Given the general dependence of substrate retrotranslocation upon functional ubiquitin conjugation machinery (Biederer et al., 1997; de Virgilio et al., 1998), N-glycosylation of Deg1-Sec62 in doa10Δ hrd1Δ cells (Fig. 2 A) indicates that such modification happens upstream of substrate selection. The disulfide cross-link between Deg1-Sec62 and Sec61 was interpreted as representing a briefly stalled retrotranslocation intermediate, as preventing this disulfide from forming accelerated degradation. However, we found that the increased degradation rate of Deg1-Sec62 in the absence of the disulfide linkage is caused by a switch from Hrd1-dependent degradation to comparatively rapid Doa10-mediated proteolysis (Fig. 5, C and D). Therefore, although our data establish a role for Sec61 in the biogenesis of a Hrd1 substrate, they do not directly implicate Sec61 as a retrotranslocon for Deg1-Sec62, although this remains possible. It is tempting to speculate that, in a manner mirroring its membrane insertion, retrotranslocation of Deg1-Sec62 occurs in two steps: extraction of the aberrantly rearranged portion from the Sec61 channel followed by removal of the normal TM segments, potentially also through Sec61. Similarly, the precise mechanism by which apoB is extracted from the ER membrane remains to be elucidated. Translocationally stalled apoB might simply be extruded back into the cytoplasm via retrograde transport through the translocon in which it stalls, or it might be transferred to another retrotranslocating complex.
We speculate that Hrd1 may play an important role in removing proteins that persistently engage the translocon in a process provisionally called ERAD-T (for “translocon associated”). ERAD-T substrates may include proteins that aberrantly stall in the translocon either because of an abnormality in the translocating protein (such as may be the case for Deg1-Sec62) or because of malfunction in the translocation process itself. Such substrates may be difficult to identify experimentally, as they likely represent a small subpopulation of any given translocated gene product. Artificial translocon-occupying substrates, such as Deg1-Sec62, may serve as a model for understanding such mechanisms. Other cellular pathways may have co-opted this quality-control pathway to mediate the regulated destruction of otherwise normal proteins. The low-density and very-low-density lipoprotein biosynthesis pathway provides a likely example of a protein—apoB—whose regulated degradation utilizes a basic cellular quality control mechanism. Future studies will seek to understand the mechanisms by which Hrd1 recognizes this potentially distinct class of substrates that persistently engage the translocon.
Materials and methods
Yeast and bacterial methods
With the exception of cells prepared for cycloheximide chase/immunoblot analysis of apoB29, yeast were grown in synthetic defined (SD) medium that was prepared as described previously (Guthrie and Fink, 1991). For experiments involving apoB29, yeast cells were cultured in modified SD medium with 5 mg/ml of acid-hydrolyzed casein and 0.08 mg/ml tryptophan as the amino acid source. Standard techniques were used for genetic manipulation of yeast strains (Guthrie and Fink, 1991). To introduce gene deletions, selection markers were PCR-amplified from donor strains with flanking sequences containing homology upstream and downstream of target gene start and stop codons. PCR products were transformed into yeast to replace genes by homologous recombination. Gene disruptions were confirmed by PCR. Standard techniques were used for plasmid manipulation in Escherichia coli (Ausubel et al., 1989). Site-directed mutagenesis was used to introduce point mutations (Zheng et al., 2004). Yeast strains and plasmids used in this study are presented in Tables S1 and S2, respectively. Yeast strains and plasmids not constructed in our laboratory were provided by C. Andreasson (Stockholm University, Stockholm, Sweden), J. Brodsky (University of Pittsburgh, Pittsburgh, PA), E. Craig (University of Wisconsin, Madison, Madison, WI), R. Gilmore (University of Massachusetts Medical School, Boston, MA), Y. Jigami (National Institute for Advanced Industrial Science and Technology, Tsukuba, Japan), N. Johnsson (Ulm University, Ulm, Germany), D. Ng (National University of Singapore, Singapore), R. Schekman (University of California, Berkeley, Berkeley, CA), D. Scott (St. Jude Children’s Research Hospital, Memphis, TN), and T. Yoko-o (National Institute for Advanced Industrial Science and Technology, Tsukuba, Japan).
Pulse-chase analysis
Pulse-chase analysis was performed essentially as described previously (Chen et al., 1993). Yeast cells were labeled with ∼20 µCi TRAN35S-LABEL (MP Biomedicals) per 1 OD600 unit of cells at 30°C for 10 min in SD medium lacking methionine and cysteine. Chases with excess unlabeled Met and Cys were performed in the absence of cycloheximide, except where noted in the figure legends. Inclusion of 500 µg/ml cycloheximide had no detectable impact on degradation kinetics, modification kinetics, or E3 specificity. Immunoprecipitation of Deg1 fusion proteins was performed with anti-FLAG M2 affinity resin (Sigma-Aldrich) or sequential incubation with anti-Deg1 antibody (Hochstrasser and Varshavsky, 1990) and agarose cross-linked to recombinant Protein A (Repligen), as indicated in the figure legends. Immunoprecipitated proteins were separated by SDS-PAGE and analyzed by autoradiography, using a Storm 860 Phosphorimager system and ImageQuant 5.2 software (Molecular Dynamics).
Cycloheximide chase and standard immunoblot analyses
Cycloheximide was added to a final concentration of 250 µg/ml to logarithmically growing cells. For analysis of CPY*-HA, cells were lysed essentially as described by Kushnirov (2000). 2.5 OD600 units of cells were collected at each time point, pelleted by centrifugation, resuspended in 400 µl of 0.2 N NaOH, and incubated for 5 min at room temperature. Cells were pelleted by centrifugation and resuspended in sample loading buffer (Laemmli, 1970). Samples were boiled for 5 min before separation by SDS-PAGE and immunoblotting.
For analysis of apoB29, cells were lysed essentially as described by Loayza and Michaelis (1998). 2.5 OD600 units of cells were collected at each time point, pelleted by centrifugation, and resuspended in 1 ml of ice-cold water. Cells were lysed by the addition of NaOH (to 0.26 N final concentration) and β-mercaptoethanol (to 0.13 M final concentration) and allowed to incubate on ice for 15 min. TCA was added to 5% final concentration to precipitate proteins. Proteins were pelleted by centrifugation at 4°C. Pellets were washed with 2% TCA and resuspended in 50 µl TCA sample buffer (3.5% SDS, 0.5 M DTT, 80 mM Tris [not pH adjusted], 8 mM EDTA, 15% glycerol, and 0.1 mg/ml of bromophenol blue) before separation by SDS-PAGE and immunoblotting.
For analysis of Deg1-Sec621–149, 2.5 OD600 units of cells were collected at each time point, pelleted by centrifugation, resuspended in 100 µl of 0.2 N NaOH, and incubated for 5 min at room temperature. Cells were pelleted by centrifugation and resuspended in 30 µl of urea lysis buffer (1% NP-40, 1% sodium deoxycholate, 1% SDS, and 8 M urea). Laemmli sample buffer was added and samples were boiled for 5 min before separation by SDS-PAGE and immunoblotting.
Proteins were immunoblotted to polyvinylidene fluoride membranes (Millipore), which were blocked with 5% skim milk in TBS/T (20 ml Tris, pH 7.6, 136 mM NaCl, and 0.1% Tween 20) for 60 min at room temperature or overnight at 4°C. All antibody incubations were conducted for 45–90 min at room temperature in 1% skim milk in TBS/T followed by three 5-min washes in TBS/T. The following mouse monoclonal antibodies were used at the indicated dilutions: anti-FLAG M2 (Sigma-Aldrich) at 1:10,000, anti-HA 16B12 (Covance) at 1:2,000, anti-yeast Pgk1 (Molecular Probes) between 1:20,000 and 1:40,000, and anti–yeast CPY at 1:5,000. Anti-Deg1 (MATα2) rabbit polyclonal antibodies were used at a dilution of 1:5,000. The anti–yeast CPY antibody is a monoclonal mouse antibody raised against yeast CPY and was a gift of N. Segev (University of Illinois at Chicago, Chicago, IL). Anti-Deg1 antibodies are polyclonal rabbit anti–α2 antiserum raised against a full-length α2-β-galactosidase fusion protein (Hochstrasser and Varshavsky, 1990). Mouse primary antibody incubations were followed by incubation with peroxidase-coupled sheep anti–mouse IgG (GE Healthcare) at a dilution of 1:5,000; anti-Deg1 antibody incubation was followed by incubation with peroxidase-coupled donkey anti–rabbit IgG (GE Healthcare). The peroxidase anti-peroxidase (PAP) antibody (Sigma-Aldrich) was used to detect the Protein A epitope at a dilution of 1:8,000 and required no secondary antibody. Bound antibody was visualized using Enhanced Chemiluminescence (GE Healthcare).
Endo H treatment of cellular proteins
After pulse chase, Deg1-Sec62 variants were immunoprecipitated. After five washes with wash buffer (150 mM NaCl, 50 mM Hepes, pH 7.5, 5 mM EDTA, 1% Triton X-100, and 0.1% SDS), two additional equilibrating washes were performed with 1× NEBuffer 4 (New England Biolabs). The resin was resuspended in 50 µl of 1× NEBuffer 4 that had been supplemented with potassium acetate, pH 5.6, to an 80-mM final concentration. After adding 0.005–0.0075 U of Endo H (Roche) to the resin suspension, samples were incubated at 37°C for 2 h with gentle mixing every 20–30 min. Laemmli sample buffer was added, and samples were boiled for 8 min.
For treatment of whole cell lysates, lysates were prepared by glass-bead vortexing 3 × 5.0 m/s for 20 s (with 5 min on ice between pulses) using a FastPrep-24 bead beating machine (MP Biomedicals) in a buffer containing 110 mM Hepes, pH 7.4, (adjusted with KOH), 2 mM MgCl2, 0.1% Tween 20, and 1× Complete Protease Inhibitor Cocktail (Roche). Lysate volumes corresponding to 0.075 OD600 units of cells were incubated with 0.005 U of Endo H (or mock-treated with water) at 378 for 2 h. Laemmli sample buffer was added, and samples were boiled for 8 min.
Proteinase K and Endo H protection of microsomal proteins
Yeast microsomal membranes were prepared essentially as described previously (Kreft et al., 2006; Scott and Schekman, 2008). 15 OD600 units of cells were harvested by centrifugation, resuspended in 1 ml resuspension buffer (10 mM Tris, pH 9.4, and 10 mM DTT), and incubated at room temperature for 10 min. Cell pellets were harvested by centrifugation and washed with spheroplast buffer (1 M sorbitol, 20 mM sodium phosphate, pH 7.5, 150 mM NaCl, and 2 mM dithiothreitol). Cells were digested in 140 µg zymolyase 100T (MP Biomedicals)/10 OD600 units of cells in spheroplast buffer for 20 min at 30°C. Spheroplasts were harvested by centrifugation (5 min at 600 g at 4°C) and washed in spheroplast buffer containing 20 µg/ml pepstatin A and 1 mM EDTA (for samples destined for protease protection analysis) or spheroplast buffer containing 1× EDTA-free Complete Protease Inhibitor Cocktail (Roche) and 1 mM EDTA (for samples destined for Endo H protection analysis). Spheroplasts were centrifuged again (5 min at 600 g at 4°C) and resuspended in fractionation buffer (200 mM d-mannitol, 20 mM sodium phosphate, pH 7.5, and 150 mM NaCl) containing appropriate protease inhibitors and lysed by vortexing in the presence of glass beads for three 30-s pulses with 1 min on ice between pulses. Unbroken cells and cellular debris were centrifuged (5 min at 600 g at 4°C), and the supernatant was used as the microsomal preparation. For Proteinase K protection experiments, microsome preparations corresponding to 3.75 OD600 units were incubated with or without 1% Triton X-100 and treated with Proteinase K (Roche) in storage buffer (20 mM Tris, pH 7.5, 1 mM CaCl2, and 50% glycerol) at a final concentration of 5 µg/ml or mock-treated with storage buffer alone. Reactions were performed on ice for 30 min. Proteinase K activity was quenched with 10 mM PMSF. For Endo H protection experiments, microsome preparations corresponding to 3.75 OD600 units were supplemented with potassium acetate, pH 5.6, to a final concentration of 80 mM. Samples were incubated with or without 1% Triton X-100 and treated with 0.004 U Endo H or mock-treated with water. Reactions were performed at 37°C for 2 h with gentle mixing every 20–30 min. Reactions were terminated with ice-cold TCA added to a final concentration of 10%.
For both Proteinase K and Endo H protection experiments, proteins were precipitated with 10% ice-cold TCA. Proteins were pelleted by centrifugation at 4°C. Pellets were washed with 2% TCA and resuspended in 50 µl TCA sample buffer (Loayza and Michaelis, 1998). Acidic samples (which caused the bromophenol blue indicator to turn yellow) were neutralized, and samples were boiled for 8 min.
Inhibition of O-mannosylation
Yeast cells were cultured for 3 h at 30°C in the presence of 1 µM 5-[[3-(1-phenylethoxy)-4-(2-phenylethoxy)phenyl]methylene]-4-oxo-2-thioxo-3-thiazolidineacetic acid (“Compound 5a”; Biotrend Chemicals; Orchard et al., 2004), provided by S. Strahl (University of Heidelberg, Heidelberg, Germany), a global inhibitor of O-mannosylation in S. cerevisiae (Strahl, S., personal communication; Arroyo et al., 2011).
Online supplemental material
Fig. S1 shows PTM of Deg1-Sec62. Fig. S2 shows Doa10-dependent degradation of Deg1 fusion proteins. Fig. S3 shows membrane topology of Deg1-Sec62, Deg1-sec62†, and Deg1-Vma12. Table S1 shows yeast strains used in this study. Table S2 shows plasmids used in this study.
Acknowledgments
We thank Claes Andreasson, Jeffrey Brodsky, Elizabeth Craig, Reid Gilmore, Yoshifumi Jigami, Nils Johnsson, Davis Ng, Randy Schekman, Danny Scott, Nava Segev, and Takehiko Yoko-o for yeast strains, plasmids, and antibodies. We also thank Sabine Strahl for the O-mannosylation inhibitor and Davis Ng, Sabine Strahl, and David Andrews for technical advice. We thank Christopher Hickey, Robert Tomko, Dimitrios Zattas, and Christian Schlieker for critical reading of the manuscript.
This work was supported by National Institutes of Health (NIH) grant GM046904 to M. Hochstrasser and NIH National Research Service Award postdoctoral fellowship F32 GM088995 to E.M. Rubenstein.
References
Author notes
S.G. Kreft’s present address is Dept. of Biology, University of Konstanz, 78457 Konstanz, Germany.
W. Greenblatt’s present address is Harvard-MIT Division of Health Sciences and Technology, Harvard Medical School, Boston, MA 02115.