K+ channels exhibit strong selectivity for K+ ions over Na+ ions based on electrophysiology experiments that measure ions competing for passage through the channel. During this conduction process, multiple ions interact within the region of the channel called the selectivity filter. Ion selectivity may arise from an equilibrium preference for K+ ions within the selectivity filter or from a kinetic mechanism whereby Na+ ions are precluded from entering the selectivity filter. Here, we measure the equilibrium affinity and selectivity of K+ and Na+ ions binding to two different K+ channels, KcsA and MthK, using isothermal titration calorimetry. Both channels exhibit a large preference for K+ over Na+ ions at equilibrium, in line with electrophysiology recordings of reversal potentials and Ba2+ block experiments used to measure the selectivity of the external-most ion-binding sites. These results suggest that the high selectivity observed during ion conduction can originate from a strong equilibrium preference for K+ ions in the selectivity filter, and that K+ selectivity is an intrinsic property of the filter. We hypothesize that the equilibrium preference for K+ ions originates in part through the optimal spacing between sites to accommodate multiple K+ ions within the selectivity filter.
INTRODUCTION
Potassium (K+) channels mediate the near diffusion-limited flow of K+ ions across cellular membranes (Hille, 2001). The exquisite selectivity for K+ over Na+ is necessary to maintain cellular resting potential and to repolarize cells during an action potential. Mutagenesis experiments, nuclear magnetic resonance spectroscopy, and crystal structures of K+ channels show that a region within the channel, called the selectivity filter, is responsible for the high selectivity (Heginbotham et al., 1994; Doyle et al., 1998; LeMasurier et al., 2001; Zhou et al., 2001; Bhate et al., 2010; Ye et al., 2010; Derebe et al., 2011) (Fig. 1 A). This region of the channel is lined with backbone carbonyl atoms and hydroxyl side chains to create a queue of four K+ ion–binding sites that distinguish between K+ ions (r = 1.33 Å) and Na+ ions (r = 0.95 Å) (Doyle et al., 1998; Zhou and MacKinnon, 2003). In the filter, two K+ ions can distribute across these four sites spaced with water molecules between them in the so-called 1,3 and 2,4 configurations (Morais-Cabral et al., 2001; Zhou and MacKinnon, 2003) (Fig. 1 B). During K+ ion conduction, the near equipotent nature of these configurations was suggested to create a virtually barrier-less passage for K+ ions through the membrane (Aqvist and Luzhkov, 2000; Morais-Cabral et al., 2001).
K+ ion–binding sites within the KcsA K+ channel. (A) A cartoon representation of KcsA with two of the four subunits shown; the subunits closest to and furthest from the viewer are removed for clarity. The selectivity filter is shown as a stick representation, with the four K+ ion–binding sites indicated with green spheres. (B) The two K+ ions within the selectivity filter are separated by water molecules in either a 1,3 or 2,4 configuration, where the number indicates the sites occupied by K+ ions (S1–S4). Exchange between these two most populated states is thought to create a barrier-less passage for K+ ions through the membrane.
K+ ion–binding sites within the KcsA K+ channel. (A) A cartoon representation of KcsA with two of the four subunits shown; the subunits closest to and furthest from the viewer are removed for clarity. The selectivity filter is shown as a stick representation, with the four K+ ion–binding sites indicated with green spheres. (B) The two K+ ions within the selectivity filter are separated by water molecules in either a 1,3 or 2,4 configuration, where the number indicates the sites occupied by K+ ions (S1–S4). Exchange between these two most populated states is thought to create a barrier-less passage for K+ ions through the membrane.
The selectivity filter creates a more hostile environment for Na+ ions and practically precludes their transport across the membrane. Two general mechanisms have been proposed to explain how K+ channels accomplish this task (see Andersen, 2011, and references therein). In the first, a K+ channel is selective for K+ over Na+ ions at equilibrium, meaning that the energy wells would be deeper for K+ than Na+ ions. The strongest experimental support for this model comes from barium block experiments using electrophysiology, from crystal structures of the K+ channels and from thermal stability measurements on KcsA (Neyton and Miller, 1988a,b; Korn and Ikeda, 1995; Doyle et al., 1998; Ogielska and Aldrich, 1998; Zhou et al., 2001; Krishnan et al., 2005; Renart et al., 2010; Piasta et al., 2011). The second model postulates that K+ selectivity arises from a kinetic preference for K+ ions entering the selectivity filter over Na+ ions. This would be observed as different kon rates of the ions binding to the selectivity filter. Recent experimental support for this hypothesis comes from Na+ and Li+ block experiments, x-ray crystal structures, and molecular dynamic simulations (Thompson et al., 2009; Kim and Allen, 2011). Which of these two models best explains the selectivity observed in K+ channels?
In this study, we measured the equilibrium affinity of K+ ions interacting within the selectivity filter of K+ channels and determined that K+ channels are selective for K+ over Na+ ions at equilibrium. The equilibrium constants determined in this study provide a direct link between crystal structures and channel–ion interactions, and are qualitatively consistent with other studies measuring ion binding to K+ channels (Neyton and Miller, 1988a,b; Bhate et al., 2010; Piasta et al., 2011). The measured selectivity for K+ over Na+ ions is on the order found during ion conduction, suggesting that selectivity during ion conduction likely arises from an equilibrium preference for K+ ions within the selectivity filter. We hypothesize that K+ selectivity at equilibrium arises from the intrinsic selectivity of each K+ ion–binding site and the need to satisfy multiple linked ion-binding sites simultaneously within the filter.
MATERIALS AND METHODS
KcsA purification and preparation
Wild-type KcsA in pET28a expression vector was expressed in Escherichia coli BL21(DE3) cells. Cell pellets were resuspended and sonicated in 50 mM Tris, pH 7.8, 100 mM KCl, 10 µg/ml DNase, and 50 µg/ml lysozyme. n-decyl-β-d-maltopyranoside (DM; Affymetrix) was added to 40 mM, and KcsA was extracted for 3 h at room temperature. The soluble cell lysate was loaded onto Ni-NTA resin (QIAGEN), eluted, concentrated to 5–10 mg/ml, and dialyzed to remove imidazole. KcsA was cleaved with chymotrypsin (Sigma-Aldrich) for 2 h at room temperature and purified on a Superdex 200 column (GE Healthcare) equilibrated with 50 mM Tris, pH 7.5, 20 mM KCl, 100 mM NaCl, and 5 mM DM. The purified pore domain was concentrated and dialyzed against solutions containing the desired concentration of NaCl with 50 mM Tris, pH 7.8, and 10 mM DM. The concentration of protein for the isothermal titration calorimetry (ITC) experiments was determined by absorbance at 280 nm.
MthK purification and preparation
MthK was expressed and purified as described previously (Jiang et al., 2002). In brief, the channels were expressed in E. coli M15 cells by induction (at OD600 ≈ 0.8) with 0.4 mM IPTG at 37°C for 5 h. Expressed protein was extracted from cell lysate using 40 mM DM and purified on a Talon Co2+ affinity column (Takara Bio Inc.). The protein was cleaved with trypsin (Sigma-Aldrich) at room temperature for 5 h and was terminated using trypsin inhibitor from bovine pancreas (Sigma-Aldrich). The tetrameric pore was purified over a Superdex 200 column equilibrated with 50 mM Tris, pH 8.0, 10 mM KCl, 100 mM NaCl, and 5 mM DM. The purified protein was concentrated to 1 mg/ml and dialyzed against the desired buffer for the ITC experiments. Protein concentrations were determined by absorbance at 280 nm.
ITC measurement and fitting
Measurements of the heat exchange associated with K+ binding to both channels were acquired using a microcalorimeter (VP-ITC; GE Healthcare). All experiments were performed at a constant temperature of 25°C. All solutions were filtered and degassed before each experiment. For KcsA, the sample cell (1.3959 ml) was filled with protein solutions including 100–500 mM NaCl, 50 mM Tris, pH 7.8, and 10 mM DM, whereas the injector contained the same sodium buffer with 40 mM KCl. 25–30 injections were performed with 3 µl of ligand injected into sample cell each time. For MthK, the sample cell was filled with a solution containing 50 mM Tris, pH 7.8, 50–250 mM NaCl, 10 mM DM, and 10–20 µM MthK. The injection syringe was filled with a ligand solution containing 50 mM Tris, pH 7.8, 50–250 mM NaCl, 3–20 mM KCl, and 10 mM DM. 25–75 injections of 3 µl of ligand solution were titrated into the MthK protein solution. The data were fit to a one- or two-site sequential binding model in the Origin program. The affinities were reported as KD (or 1/K) in the text and figures. In the one-site model (used for KcsA), n was set to 1 and enthalpy (H) and association constant (K) were fit. In the two-site sequential mode (used for MthK), the enthalpy (H1 and H2) and association constant (K1 and K2) were fit. Binding isotherms with two identical ligands can sometimes have multiple solutions to the fraction-bound equations because of linked parameters and local or shallow minima. The isotherms were fit with different initial parameters and converged to values within 10% of each other, suggesting a single optimum within the searched parameter space; the parameters yielding the lowest χ2 were used in subsequent data analyses. The landscape around the optimal solution was explored with two types of perturbation analysis. In the first, one parameter was fixed and the remaining parameters refit such that χ2 was never >5% of the optimal value. This approach yielded variation of 1–23% from the optimal values. The second perturbation approach used a covariance matrix to extract the uncertainty of each fitted parameter and yielded a variance of 9–18% (Tellinghuisen, 2005; Freiburger et al., 2009). Collectively, these approaches demonstrate that the solutions obtained for MthK are sufficiently constrained by the experimental data. KcsA was also constrained by its experimental data: different initial parameters yielded exactly the same solutions, and both perturbation approaches yielded errors <3%.
Fitting the K+/Na+ competition ITC data
The apparent affinities (KD) for each K+ ion–binding event was fit individually to the following equation:
where and are the dissociation constants of the channel for K+ and Na+ binding to empty channels, respectively, and n is the Hill coefficient associated with Na+ binding. In effect, this equation describes the ITC-derived association constant K (because K = 1/KD) as a function of [Na+]. The fit values and their standard errors were determined using Prism (GraphPad Software). A perturbation approach was used to examine the landscape around the solution by fixing individual parameters and refitting the remaining variables. This yielded a variation of 2–6% and 0–6% for MthK (K1 and K2, respectively) and <1% variation for KcsA when χ2 was not allowed to vary by >5%. The equilibrium selectivity is .
Online supplemental material
Fig. S1 shows the MthK-binding isotherm from Fig. 3 fit to three different models and their corresponding residuals. Tables S1 and S2 contain the thermodynamic parameters obtained for KcsA and MthK at different concentrations of NaCl.
RESULTS
The goal of this study is to measure and explain the equilibrium affinities of K+ channels for K+ and Na+ ions. ITC is ideally suited to this purpose because of the ability to measure equilibrium ligand binding without the need to label the protein or ligand, and the measurement does not rely on ion conductance, which is required for electrophysiology-based experiments. We measure the net enthalpy associated with K+ ions binding to two different channels. These data are used to determine both the affinity of each binding event and its associated selectivity. Because the equilibrium constants are thermodynamic parameters, no information regarding the physical mechanism or the location of the binding sites in the selectivity filter can be obtained directly from these measurements. However, these results are used to constrain the possible physical mechanisms of selectivity derived from high resolution protein structures and models.
K+ ion affinity of the KcsA K+ channel
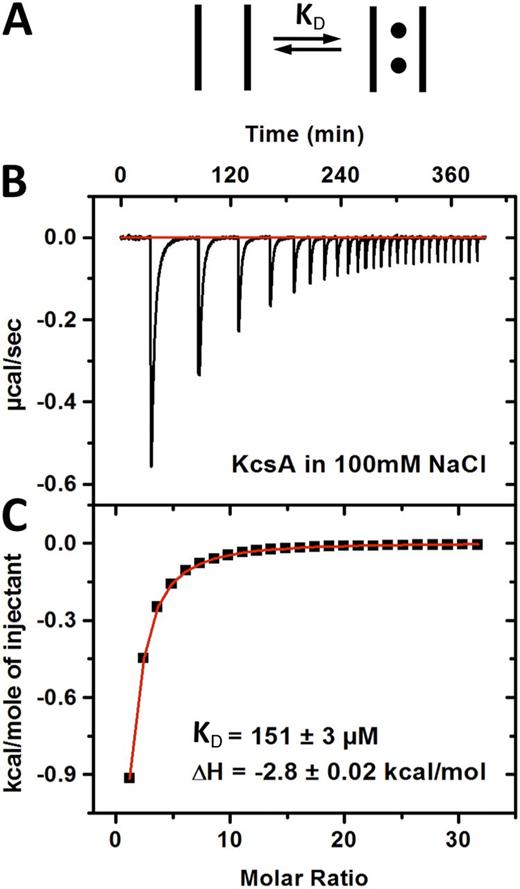
ITC was used to monitor K+ ion binding to the Streptomyces lividans K+ channel KcsA (Fig. 2 A). The thermogram (Fig. 2 B) shows the heat exchanged after equal injections of a KCl solution into a reaction cell containing the channel. The heat evolved is from the net enthalpy associated with K+ ions binding within the KcsA channel and from the enthalpy of diluting the concentrated KCl solution. The heat of dilution is constant throughout the experiment because in each case, the same amount of KCl is injected and the change in volume is small. The asymptotic nature of curve suggests that the final injections contain little to no K+ ion binding and that the heat observed at that point is primarily from diluting the concentrated KCl solution (Fig. 2 C).
K+ ion binding to KcsA K+ channel using ITC. (A) A generic model showing K+ ions binding to the channel. (B) Thermogram showing the heat exchange associated with K+ binding to the KcsA in 100 mM NaCl. (C) The ITC data were integrated and fit to a one-ion binding model to determine the affinity and enthalpy. The values shown are the parameters describing this experiment (see Table S1 for average values across multiple experiments).
K+ ion binding to KcsA K+ channel using ITC. (A) A generic model showing K+ ions binding to the channel. (B) Thermogram showing the heat exchange associated with K+ binding to the KcsA in 100 mM NaCl. (C) The ITC data were integrated and fit to a one-ion binding model to determine the affinity and enthalpy. The values shown are the parameters describing this experiment (see Table S1 for average values across multiple experiments).
In the analysis of this ITC data, an important assumption is made: ions bind in the selectivity filter and not elsewhere in the channel. Several experimental observations justify this assumption. First, high resolution x-ray crystal structures show ordered K+ ions within the selectivity filter and in the central cavity, limiting the number of likely places that ions would specifically interact (Zhou et al., 2001; Ye et al., 2010). Chemical shifts observed in both solution and solid-state nuclear magnetic resonance show changes in the chemical environment around atoms in the selectivity filter when K+ ions are titrated into the sample, suggesting that the highest affinity sites are within the selectivity filter and not the cavity (Chill et al., 2006; Bhate et al., 2010). Finally, previous ITC experiments with KcsA show that heat is only observed for ions known to conduct through the channel (K+, Rb+, Cs+, and Ba2+) and not with ions that do not readily conduct but can bind in the cavity (Li+, Na+, Mg2+, and Ca2+), demonstrating specificity for the interaction (Lockless et al., 2007).
The data in Fig. 2 C are fit to a one-ion binding model, as attempts to fit the data to more complicated models did not significantly improve the fit but added more parameters. The calculated affinity is 150 µM, which is similar to the 430-µM affinity we observed previously at a different temperature (20 instead of 25°C here) and in the A98G KcsA mutant channel instead of the wild-type channel used in this study (Lockless et al., 2007).
KcsA ion selectivity at equilibrium
A goal of this study is to measure the equilibrium affinity and selectivity of the KcsA channel for K+ and Na+ ions. The K+ affinity obtained from the data in Fig. 2 is an apparent affinity because K+ ions are competing with 100 mM Na+ ions in the buffer solution (Fig. 3 A). NaCl in the ITC buffer solution is necessary because the channel is unstable when small cations (such as Na+ or K+) are replaced by large organic cations (such as N-methyl-d-glucamine or glucosamine). Even if the channel were stable in the absence of K+ or Na+, the structural state of this or any K+ channel is not known, which could lead to erroneous interpretations of the data. Equally important, it is unclear how these measurements should be compared with permeability measurements that rely on a mixed Na+/K+ ion solution to determine ion channel selectivity.
K+ ions compete with Na+ ions within the KcsA selectivity filter. (A) A model of K+ ions displacing Na+ ions bound within the selectivity filter. (B) The apparent KDs vary as a function of [Na+] in the ITC chamber. The data are fit to a competition model to determine the affinity of K+ and Na+ ions. Each data point is determined from three or more independent experiments.
K+ ions compete with Na+ ions within the KcsA selectivity filter. (A) A model of K+ ions displacing Na+ ions bound within the selectivity filter. (B) The apparent KDs vary as a function of [Na+] in the ITC chamber. The data are fit to a competition model to determine the affinity of K+ and Na+ ions. Each data point is determined from three or more independent experiments.
The equilibrium K+ and Na+ ion–binding affinities were determined by measuring the change in K+-binding affinity (KD) as a function of [Na+] in the ITC buffer (Fig. 3 B). These data were fit to a competition model to determine , , and n (see Materials and methods for details). KcsA is highly selective for K+ over Na+, with a of 80 ± 2 µM and a of 60,000 ± 800 µM, for an equilibrium selectivity of 750 (K+:Na+). The affinity for K+ is very similar to the 30-µM affinity obtained through Ba2+ block experiments of the KcsA channel (Piasta et al., 2011). The Hill coefficient for Na+ ion displacement was n = 1.3, indicating a near-simple exchange of Na+ with K+ ions (Table 1).
Equilibrium affinity measurements obtained for ion binding to KcsA and MthK K+ channels
| Channel | Ion binding event | n | Selectivity | ||
| µM | µM | ||||
| KcsA | 80 ± 2 | 60,000 ± 800 | 1.3 ± 0.02 | 750 | |
| MthK | First | 10 ± 3 | 2,000 ± 200 | 2.0 ± 0.2 | 200 |
| Second | 80 ± 10 | 9,000 ± 6,000 | 2.2 ± 0.5 | 110 |
| Channel | Ion binding event | n | Selectivity | ||
| µM | µM | ||||
| KcsA | 80 ± 2 | 60,000 ± 800 | 1.3 ± 0.02 | 750 | |
| MthK | First | 10 ± 3 | 2,000 ± 200 | 2.0 ± 0.2 | 200 |
| Second | 80 ± 10 | 9,000 ± 6,000 | 2.2 ± 0.5 | 110 |
Ion binding to the MthK K+ channel
Although KcsA is a structural model for understanding ion selectivity in K+ channels, a linked conformational change associated with ion binding complicates the interpretation of the selectivity measurements. Crystal structures determined in Na+ or low concentration of K+ show the selectivity filter in a so-called collapsed conformation that cannot conduct ions (Zhou and MacKinnon, 2003; Zhou et al., 2001; Lockless et al., 2007; Cuello et al., 2010). Upon raising the concentration of K+, the channel adopts the conductive conformation that is thought to mediate the flow of ions across the membrane and is observed in all K+ channel structures solved in high K+ solutions to date (Zhou et al., 2001; Jiang et al., 2002, 2003; Kuo et al., 2003; Long et al., 2007; Tao et al., 2009; Ye et al., 2010; Whorton and MacKinnon, 2011; Brohawn et al., 2012; Miller and Long, 2012). This conformational change is proposed to contribute to the large selectivity observed in KcsA and could be the physical basis for the ion selectivity observed in our ITC experiments (Zhou and MacKinnon, 2003; Valiyaveetil et al., 2006). Is the conformational change necessary for KcsA’s high K+ ion selectivity? To test this, we measured the selectivity of the MthK channel from Methanobacterium thermoautotrophicum, whose selectivity filter is chemically identical to the conductive conformation of KcsA and remains in the same conformation with either K+ or Na+ in the filter (Ye et al., 2010) (Fig. 4).
K+ ion binding to MthK K+ channel using ITC. (A) A generic model showing two K+ ions binding to the channel. (B) Thermogram showing the heat exchange associated with K+ binding to MthK in 100 mM NaCl. (C) The ITC data were integrated and fit to a two-ion binding model to determine the affinities and enthalpies for each K+ ion–binding event. The values shown are the parameters describing this experiment (see Table S2 for average values across multiple experiments).
K+ ion binding to MthK K+ channel using ITC. (A) A generic model showing two K+ ions binding to the channel. (B) Thermogram showing the heat exchange associated with K+ binding to MthK in 100 mM NaCl. (C) The ITC data were integrated and fit to a two-ion binding model to determine the affinities and enthalpies for each K+ ion–binding event. The values shown are the parameters describing this experiment (see Table S2 for average values across multiple experiments).
The integrated heat changes upon K+ ion binding to MthK were initially fit to a one-ion binding model, but an inspection of the residuals to the fit suggested that a more complicated model is needed to account for the binding isotherm (Fig. S1, A and D). The addition of a second K+ ion–binding event with different affinities and enthalpies gave a significantly better fit both qualitatively and quantitatively (Fig. S1, C and F; P < 10−4, RSS = 72,347 for one-site vs. RSS = 2,193 for two-site models). Note that two K+ ions binding with identical affinities and enthalpies did not fit the data (Fig. S1, B and E), which is consistent with the “hook” observed at the beginning of the trace (Fig. 4 C); the nonmonotonic nature of the binding isotherm is indicative of multiple ligands binding with different binding constants (Freiburger et al., 2009; Krishnamoorthy and Mohanty, 2011). Measuring two K+ ion–binding events is not surprising. Both x-ray crystallography and molecular dynamics simulations suggest that two to three K+ ions bind within the conductive conformation of the KcsA selectivity filter, and the same would be expected for MthK, whose selectivity filter is identical to that of KcsA (Aqvist and Luzhkov, 2000; Bernèche and Roux, 2001; Zhou et al., 2001; Zhou and MacKinnon, 2003; Jensen et al., 2010; Kim and Allen, 2011). These ITC data alone cannot reveal which sites are occupied by each ion, but structural experiments suggest that at high K+ ion concentrations, K+ ions distribute nearly equally across all four sites of the filter, which are likely in rapid equilibrium with each other (Zhou et al., 2001; Zhou and MacKinnon, 2003; Ye et al., 2010).
The apparent affinity of K+ was measured in the presence of different Na+ ion concentrations to obtain the K+ and Na+ ion–binding dissociation constants (Fig. 5). The selectivity of the first ion-binding event is 200 (= 10 µM and = 2,000 µM), and the second is ∼110 (= 80 µM and = 9,000 µM) (Table 1). The lack of a ligand-linked conformational change in this channel demonstrates that the conductive conformation of K+ channels can be selective for K+ ions at equilibrium. Additionally, the difference in affinity of the first and second K+ ion binding suggests interactions between ions in the filter, which we discuss in the next section.
K+ ions compete with Na+ ions within the MthK selectivity filter. (A) A model of K+ ions displacing Na+ ions bound within the selectivity filter. (B) The apparent KDs (KD1 and KD2) vary as a function of [Na+] in the ITC chamber. The data are fit to a competition model to determine the affinity of K+ and Na+ for each binding event. Each data point is determined from three or more independent experiments.
K+ ions compete with Na+ ions within the MthK selectivity filter. (A) A model of K+ ions displacing Na+ ions bound within the selectivity filter. (B) The apparent KDs (KD1 and KD2) vary as a function of [Na+] in the ITC chamber. The data are fit to a competition model to determine the affinity of K+ and Na+ for each binding event. Each data point is determined from three or more independent experiments.
The Na+ dependence of K+ ion binding reveals a Hill coefficient of 2, but the physical meaning of this observation is unclear. The occupancy of Na+ ions in the selectivity filter is not known. Additionally, how Na+ is displaced by K+ ions is complicated because Na+ ions seem to prefer interacting in the plane of the oxygen atoms that typically form the oxygen cage around K+ ions, whereas K+ ions prefer residing within the cage itself (Zhou et al., 2001; Thompson et al., 2009; Ye et al., 2010). The simplest explanation for a Hill coefficient of 2 is that two (or more) Na+ ions dissociate for each K+ ion binding. However, this leads to the implausible conclusion that four total Na+ ions are bound to the filter when no K+ ions are present; only two to three positive charges are supported within the selectivity filter based on x-ray crystallography and Ba2+ block experiments (Neyton and Miller, 1988a,b; Zhou and MacKinnon, 2003; Piasta et al., 2011). An alternative explanation for our result is that the increased ionic strength of the solution reduces the apparent affinity for K+ ions by screening negative charges. A more complete model of K+ displacing Na+ from the selectivity filter will require correlating these ITC results with additional structural information on the occupancy and location of Na+ ions bound to MthK with different numbers of K+ ions in the selectivity filter.
DISCUSSION
In this study, we measure the equilibrium affinity of K+ and Na+ ions binding within the selectivity filter of two different K+ channels. Our main experimental observation is that both KcsA and MthK K+ channels have high equilibrium selectivity for K+ over Na+ ions. Although not entirely unexpected given the known selectivity of these K+ channels during ion conduction (LeMasurier et al., 2001; Ye et al., 2010; Piasta et al., 2011), it does provide a direct link between ion-binding experiments and crystal structures that allows us to propose a hypothesis relating to the physical origins of K+ channel selectivity at equilibrium.
Interactions between ions in the selectivity filter
The first K+ ion binds to the MthK channel with a greater affinity than the second (Table 1). Different ligand affinities can sometimes arise from a conformational change associated with ligand binding, such as is observed in KcsA, but MthK has the same conformation with either K+ or Na+ ions bound (Zhou et al., 2001; Zhou and MacKinnon, 2003; Ye et al., 2010). The close proximity (3–10 Å) of K+ ions in the selectivity filter of MthK leads us to speculate that the difference in affinities is from electrostatic repulsion between nearby ions within the filter. If so, then the interaction energy and the average distance between ions can be used to calculate the effective dielectric constant (εsf) encountered by the ions within the selectivity filter using Coulomb’s law. Given the interaction energy of ΔΔG = RT ln (80 µM/10 µM) = 1.2 ± 0.2 kcal/mol and spacing of 6.6 Å so that one water molecule separates two monovalent cations, the effective dielectric within the filter is εsf = 40 ± 7. This number must be tempered by the usual caveats that thermodynamic values do not reveal the actual mechanism of the process, and that it is possible that the protein itself has a role in mediating ion–ion interactions. However, the relatively high dielectric value suggests that the environment within the selectivity filter could be more like an aqueous solution (εw = 80) than a hydrocarbon one such as the lipid membrane (εm = 2). The large dielectric constant is not surprising; ion channels rapidly conduct ions across the membrane and could minimize energetic barriers to membrane crossing by making the internal protein environment encountered by the ion as similar to a hydrated ion as possible, both in terms of spacing between oxygen atoms and the dielectric environment (Parsegian, 1969; Doyle et al., 1998; Zhou et al., 2001).
K+ channels achieve the arduous task of conducting K+ ions at rates approaching 108 s−1 while maintaining 102–103 selectivity for K+ over Na+ ions. High ion selectivity suggests tight binding to the channel and long residence times. The rate of ions dissociating from channels (koff) set an upper limit on the conductance of ions. In the case of a 100-µM K+ affinity, similar to that observed for the second ion binding to MthK, koff would be 105 s−1 (KD = koff/kon; kon is likely near diffusion limited at 109 M−1 s−1). This koff rate is too small to fully account for the high ion conductance of many K+ channels. However, a rate approaching ion conductance can be obtained if a third ion is added to the selectivity filter during ion conduction, which undoubtedly happens at least some of the time. The third K+ ion would occupy a site adjacent to another K+ ion with a distance of 3.3 Å between the centers of each ion, and would feel larger charge–charge repulsion than the ions separated by water molecules (6.6 Å apart). The interaction between the ions would be ∼2.5 kcal/mol assuming a dielectric of 40, which puts the affinity of this third ion at ∼10 mM. The koff rate would then be ∼107 s−1 and within an order of magnitude of that expected for a 10-pA channel.
Equilibrium selectivity versus selective ion conduction
A channel that selectively conducts an ion does not need to selectively bind that same ion at equilibrium. For example, a proposed mechanism by which a nonselective channel at equilibrium could selectively conduct ions is when the kon rates of the competing ions are different (Bezanilla and Armstrong, 1972). The ions (say X+ and Y+) would conduct through the channel at a selective ratio proportional to their kon rates and concentrations (), but only if the channel binds one ion at a time. This mechanism is not what we observe for K+ channels. KcsA is ∼750-fold selective for K+ over Na+ ions at equilibrium, which is in line with the minimum 250-fold K+ over Na+ selectivity calculated from permeability studies (LeMasurier et al., 2001; Piasta et al., 2011). We measure an ∼100-fold selectivity of the second K+ ion binding to the MthK channel, which is also close to the 60-fold permeability of K+ over Na+ ions (Ye et al., 2010). Additionally, crystal structures of KcsA and MthK provide qualitative support that the channel is selective for K+ over Na+ at equilibrium (Morais-Cabral et al., 2001; Zhou and MacKinnon, 2003; Ye et al., 2010). These results demonstrate that K+ channels are both selective at equilibrium and during ion conduction.
The similarity between values for the equilibrium selectivity and K+ permeability determined from reversal potentials suggests that K+ channel selectivity can arise from equilibrium ion selectivity alone. A multi-ion mechanism such as that originally proposed by Hille and Schwarz (1978) suggests a straightforward way to convert an equilibrium preference for K+ ions into K+-selective conductance. The interactions between ions in the filter lead to the so-called anomalous mole fraction effect, whereby the total conductance of a channel in mixed ions is not determined solely by the ratio of the two ions in solution and their individual ion conductances. In the case where the ions have very different affinities, as we observe in this study, the higher affinity K+ ion effectively blocks conduction of the lower affinity Na+ ion by allowing the other sites in the channel to equilibrate before the “blocking” K+ ion dissociates into solution. This mechanism is built upon the assumption that multiple ions selectively bind within the selectivity filter, which has been observed using electrophysiology, crystal structures, and now ITC (Vestergaard-Bogind et al., 1985; Eisenman et al., 1986; Neyton and Miller, 1988a; Korn and Ikeda, 1995; Starkus et al., 1997; Ogielska and Aldrich, 1998; Morais-Cabral et al., 2001; Zhou et al., 2001; Zhou and MacKinnon, 2003).
Proposed role of the ion queue for equilibrium selectivity
The selectivity filter within K+ channels is formed by oxygen atoms, which are typical direct ligands for both Na+ and K+ ions in proteins (Gouaux and MacKinnon, 2005; Page and Di Cera, 2006). Extensive studies on synthetic K+-selective small molecules have demonstrated that one straightforward mechanism to achieve equilibrium cation selectivity is to build an oxygen-lined cavity that matches the size of the desired cation (Dietrich, 1985). This design principle presumably mimics the hydrated ion, thus creating a favorable environment for the ion to partition into. A Na+ ion–selective site would need a cavity with a radius of 0.95 Å, whereas the larger K+ ion would have a cavity closer to 1.33 Å. An inspection of a K+-binding site in K+ channels reveals optimal distances between oxygen and K+ ions in the center of the site and an optimal distance between oxygen and Na+ ions on the edge of the site (in the plane of the oxygen atoms). Consistent with this, crystal structures show that both K+ and Na+ ions are able to bind within the selectivity filter at different overlapping sites (Zhou et al., 2001; Lockless et al., 2007; Thompson et al., 2009; Ye et al., 2010).
The ITC-based selectivity measurements from this study show a high selectivity for K+ ions over Na+ ions at equilibrium. We propose that equilibrium selectivity arises not only from the interactions within individual ion-binding sites but also from the need to satisfy multiple binding sites simultaneously within the selectivity filter. Crystal structures of K+ channels bound to K+ ions show that ions distribute to the same sites that would be predicted from the synthetic small molecule studies (Zhou et al., 2001; Ye et al., 2010). This creates a densely packed selectivity filter with oxygen, water, and K+ ions forming an interconnected set of optimally spaced contacts. By the same chemical logic, the optimal site for any one Na+ ion would be predicted within the plane of oxygen atoms to form close-packing contacts with the oxygen atoms. However, the crystal structure of Na+ ions bound to K+ channels reveals a different arrangement of the ions. In MthK, a Na+ ion is observed at the uppermost site of the selectivity filter as would be predicted, but the density in the remaining sites is more consistent with that of water (r = 1.4 Å) or Na+ ions bound sub-optimally to K+-selective sites, which would likely result in a less favorable free energy of the overall system (Ye et al., 2010). Computational studies have also proposed that the oxygen cages within the selectivity filter create an unfavorable environment for Na+ ions (Ban et al., 2004; Noskov and Roux, 2006; Thompson et al., 2009; Egwolf and Roux, 2010; Kim and Allen, 2011). Collectively, these results support the “snug-fit” model such that the selectivity filter mimics the chemical properties and spacing of queued hydrated K+ ions to create an isoenergetic diffusion pathway through the channel (Doyle et al., 1998; Morais-Cabral et al., 2001; Zhou et al., 2001; Lockless et al., 2007).
Conclusion
In this study, we show that the KcsA and MthK K+ channels have a preference for K+ ions over Na+ ions in their selectivity filter at equilibrium. Although both ions can bind within the selectivity filter at different overlapping sites, the filter creates the most favorable sites for the larger K+ ions. In addition to the selectivity filter facilitating ion–ion repulsion between adjacent ions in the queue, we propose that the linked nature of the ion-binding sites sets the register of the bound ions within it, preferring multiple K+ ions to multiple Na+ ions in the lowest free energy state of the system.
Acknowledgments
We thank Drs. Champak Chatterjee, Rajul Jain, and Tom Muir for comments on the manuscript; the Department of Biochemistry and Biophysics (Texas A&M University) for extensive access to their ITC machine; Dr. Youxing Jiang for sharing the MthK construct and for discussions; Drs. Tony Mittermaier and Lee Freiburger for MatLab code and discussions; and members of the Lockless laboratory for helpful discussions.
The Welch Foundation (grant A-1742) and TAMU Startup Funds supported this work.
Author contributions: S. Liu, X. Bian, and S.W. Lockless designed the research; S. Liu measured KcsA ion binding; X. Bian measured MthK ion binding; S. Liu and S.W. Lockless developed the approach to analyze ion competition data; and S.W. Lockless wrote the manuscript with input from S. Liu and X. Bian.
Lawrence G. Palmer served as editor.
References
Author notes
S. Liu and X. Bian contributed equally to this paper.



![Figure 3. K+ ions compete with Na+ ions within the KcsA selectivity filter. (A) A model of K+ ions displacing Na+ ions bound within the selectivity filter. (B) The apparent KDs vary as a function of [Na+] in the ITC chamber. The data are fit to a competition model to determine the affinity of K+ and Na+ ions. Each data point is determined from three or more independent experiments.](https://cdn.rupress.org/rup/content_public/journal/jgp/140/6/10.1085_jgp.201210855/5/m_jgp_201210855_fig3.jpeg?Expires=1771796441&Signature=HXJHmuluZrbungsgO211Gl9G7~woiHnfoYYKnvovha8gP9QOCyRn8XhuTw3eOTeeEpIiMZeRny2mPlZ90Xgn5ipfMoXHAyYl2Nbr8hFNsmG37UegqM6bOswGrRDIhFE0sZ-svvkBnwOZf1ZIrAFyf7fNUMVk0gpKLZVv9VhxP7XtFQt5TOPme~3IKntzhDIb3TbvqMVCK2xy4MU9SudWrRW8~6q~cVsFadBxMWm8p4T35jF68rMJP97jnOoON~DxwWnZykPcSu9bRNnuv1m6IwY5m~FGHN5WmQQj8zo9uzOaWbCn0R7o4j2Www3q1ulgBHenSEFqSBYMPf1mgDcSfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. K+ ions compete with Na+ ions within the MthK selectivity filter. (A) A model of K+ ions displacing Na+ ions bound within the selectivity filter. (B) The apparent KDs (KD1 and KD2) vary as a function of [Na+] in the ITC chamber. The data are fit to a competition model to determine the affinity of K+ and Na+ for each binding event. Each data point is determined from three or more independent experiments.](https://cdn.rupress.org/rup/content_public/journal/jgp/140/6/10.1085_jgp.201210855/5/m_jgp_201210855_fig5.jpeg?Expires=1771796441&Signature=oCMD3dLnlTBFufw5pB7yrFyUDM3uMR~yJ5aQjVBRJ4NltMjVIei0aNKn170DrpRzbWug03b-alKbPA2C0ck6wHxUaHEcZidtV60F~XISNc7rX6udP3IxAQ1bvyWPGvEsWvHUooLzuMpuLvsdmmn0UGAi7AcvNP3T3sRh37cZgkFLNlkPyJxMFnDgYDUBpNF9ga-xAHVe5MTl1IOX8NXEXOOfmN0g73Q6LP7lyCXOsRALcZrXNesFwOoHqun5KroymJAKf6d2Bzgi3x6Ko67BLwcf1hgGN9OjAOe2Q8QMH1opgOg57XB36IXyBLkyjC7u4xIHsNJ81sfdYPLLAONYLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


