Interleukin (IL)-22–producing group 3 innate lymphoid cells (ILC3) promote mucosal healing and maintain barrier integrity, but how microbial signals are integrated to regulate mucosal protection offered by these cells remains unclear. Here, we show that in vivo depletion of CX3CR1+ mononuclear phagocytes (MNPs) resulted in more severe colitis and death after infection with Citrobacter rodentium. This phenotype was rescued by exogenous IL-22, which was endogenously produced by ILC3 in close spatial proximity to CX3CR1+ MNPs that were dependent on MyD88 signaling. CX3CR1+ MNPs from both mouse and human tissue produced more IL-23 and IL-1β than conventional CD103+ dendritic cells (cDCs) and were more efficient than cDCs in supporting IL-22 production in ILC3 in vitro and in vivo. Further, colonic ILC3 from patients with mild to moderate ulcerative colitis or Crohn’s disease had increased IL-22 production. IBD-associated SNP gene set analysis revealed enrichment for genes selectively expressed in human intestinal MNPs. The product of one of these, TL1A, potently enhanced IL-23– and IL-1β-induced production of IL-22 and GM-CSF by ILC3. Collectively, these results reveal a critical role for CX3CR1+ mononuclear phagocytes in integrating microbial signals to regulate colonic ILC3 function in IBD.
Mononuclear phagocytes (MNPs) are sentinels of the intestinal lamina propria, capable of responding to microbial products, and play a crucial role in orchestrating intestinal lymphocyte homeostasis. MNPs can be subdivided based on their expression of CD103 or CX3CR1, and each group has been ascribed critical functions in maintaining intestinal homeostasis (Bogunovic et al., 2009; Merad et al., 2013). CD103+ cells, which can be further subdivided based on the expression of CD11b, differentiate from a common DC precursor and are thought to be the conventional, migratory myeloid DCs (Varol et al., 2010). CD103+ CD11b− DCs require Irf8, Id2, and Batf3 for their development and are thought to play a critical role in cross-priming virus- and tumor-specific CTLs (Hildner et al., 2008; Merad et al., 2013). Loss of these cells, however, does not alter intestinal T cell homeostasis or lead to spontaneous inflammation (Edelson et al., 2010). CD103+CD11b+ DCs, in contrast, require Notch2 signaling, produce IL-23 in response to flagellin-induced TLR5 activation, resulting in IL-22 production by ILC3, and have additionally been proposed to support Th17 polarization (Lewis et al., 2011; Kinnebrew et al., 2012). These cells can produce retinoic acid, which promotes the expression of the gut-homing receptor CCR9 and synergizes with TGFβ to induce regulatory T cells (Sun et al., 2007). One recent study suggests that Notch2-dependent CD103+ CD11b+ DCs regulate protection from C. rodentium–induced colitis (Satpathy et al., 2013). However, specific depletion of CD103+ CD11b+ intestinal DCs revealed that these cells are not the MNP subset required for protection against C. rodentium or IL-22 production (Welty et al., 2013).
In contrast to CD103+ cDCs, CX3CR1+ MNPs differentiate from monocyte precursors (Varol et al., 2010). Although these cells were previously thought to be tissue-resident and to promote local Treg differentiation (Hadis et al., 2011), recent data from our group showed that they can up-regulate CCR7 and migrate to secondary lymphoid organs, suggesting a broader role in orchestrating immunity (Diehl et al., 2013). Notably, we observed that interaction with microbiota limits the migration of these cells to mesenteric LNs (MLNs; Diehl et al., 2013), and an increase in CX3CR1+ cells has been described in the lamina propria during mouse (Zigmond et al., 2012) and human colitis (Kamada et al., 2008). A recent study reported that fractalkine receptor (CX3CR1) expression supports innate cell–dependent clearance of C. rodentium infection (Manta et al., 2013), but a functional role for CX3CR1+ MNPs in regulating colitis-associated ILC3 remains unclear. To evaluate this question, we employed novel mouse models to enable selective depletion of CX3CR1+ MNPs in vivo. Our results reveal a critical role for CX3CR1+ MNPs from both mouse and human tissue in supporting IL-22 induction in ILC3 in vitro and in vivo. Moreover, we identify the ability of TL1A produced by MNPs to potently enhance IL-23– and IL-1β–induced production of IL-22 and GM-CSF by ILC3.
RESULTS
CX3CR1+ cells protect against C. rodentium–induced colitis
To investigate the role of the expanded population of CX3CR1+ cells in the intestinal lamina propria during colitis, we generated a mouse with the diphtheria toxin receptor (DTR) cDNA inserted into the Cx3cr1 locus (Diehl et al., 2013). Analysis of colonic lamina propria mononuclear cells (LPMCs) after infection of DT-treated mice revealed a reduction in the percentage of CD11c+ MHCII+ LPMCs (Fig. 1 A), which reflected a preferential loss of the CX3CR1+ CD11b+ CD14+ fraction of MNPs (Fig. 1 B; Tamoutounour et al., 2012), as well as CX3CR1+ monocytes in Cx3cr1DTR/+ mice compared with control mice. CD103+ CD11b+ cDCs were not depleted (Fig. 1 C). To induce colitis, mice were infected with C. rodentium, a mouse model for infectious colitis (Zheng et al., 2008; Sonnenberg et al., 2011; Qiu et al., 2012). DT-treated infected Cx3cr1DTR/+ mice, but not uninfected or control infected mice, lost more weight (Fig. 1 D), displayed more severe intestinal pathology (Fig. 1 E), and ultimately succumbed to infection (Fig. 1 F). Infected Cx3cr1DTR/+ mice also had increased bacterial burden in the spleen, consistent with the loss of barrier integrity (Fig. 1 G).
Intestinal CX3CR1+ cells protect mice from C. rodentium-induced colitis. (A and B) Depletion efficiency in the Cx3cr1DTR/GFP mice was assessed by flow cytometry of small intestinal lamina propria cells Cx3crDTR/GFP or littermate control mice after administration of DT to both groups daily for 2 d. (A) Surface staining for CD11b versus CX3CR1-GFP. (B) MHCII+ CD11c+ cells were assessed for expression of CX3CR1, CD103, and CD14. Results are representative of five independent experiments with a minimum of three animals per group. (C) Total number of MHCII+ CD11c+CD103+ (left) and MHCII+ CD11c+CX3CR1+ cells (right) per intestine as determined by flow cytometry analysis. n.s., P > 0.05; **, P ≤ 0.01. Two-tailed Student’s t test. Error bars represent the SEM. Results are representative of five independent experiments with a minimum of 3 animals per group. (D) Weight of DT-treated littermate WT (control) mice or Cx3cr1DTR/+ mice following infection with C. rodentium (n = 7 mice/group). DT was administered at days −2, −1, and 0 and every other day after infection. Data are representative of two independent experiments. (E) Representative colonic histology from littermate control mice or Cx3cr1DTR/+ mice (analyzed in D) infected with C. rodentium at day 7 after infection. <, areas of lymphocyte infiltration; *, areas of epithelial erosion. Bar, 100 µm. (F) Survival curves of DT-treated Cx3cr1DTR/+ and littermate control mice infected with C. rodentium or uninfected (n = 7 mice/group). Data are representative of three independent experiments. Animals were treated with DT as in D. (G) Bacterial CFUs of spleens from littermate WT (control, n = 5) mice or Cx3cr1DTR/+ mice (n = 5) infected with C. rodentium and treated with DT as above. *, P ≤ 0.05. Two-tailed Student’s t test. Error bars represent the SEM. One of two representative experiments is shown.
Intestinal CX3CR1+ cells protect mice from C. rodentium-induced colitis. (A and B) Depletion efficiency in the Cx3cr1DTR/GFP mice was assessed by flow cytometry of small intestinal lamina propria cells Cx3crDTR/GFP or littermate control mice after administration of DT to both groups daily for 2 d. (A) Surface staining for CD11b versus CX3CR1-GFP. (B) MHCII+ CD11c+ cells were assessed for expression of CX3CR1, CD103, and CD14. Results are representative of five independent experiments with a minimum of three animals per group. (C) Total number of MHCII+ CD11c+CD103+ (left) and MHCII+ CD11c+CX3CR1+ cells (right) per intestine as determined by flow cytometry analysis. n.s., P > 0.05; **, P ≤ 0.01. Two-tailed Student’s t test. Error bars represent the SEM. Results are representative of five independent experiments with a minimum of 3 animals per group. (D) Weight of DT-treated littermate WT (control) mice or Cx3cr1DTR/+ mice following infection with C. rodentium (n = 7 mice/group). DT was administered at days −2, −1, and 0 and every other day after infection. Data are representative of two independent experiments. (E) Representative colonic histology from littermate control mice or Cx3cr1DTR/+ mice (analyzed in D) infected with C. rodentium at day 7 after infection. <, areas of lymphocyte infiltration; *, areas of epithelial erosion. Bar, 100 µm. (F) Survival curves of DT-treated Cx3cr1DTR/+ and littermate control mice infected with C. rodentium or uninfected (n = 7 mice/group). Data are representative of three independent experiments. Animals were treated with DT as in D. (G) Bacterial CFUs of spleens from littermate WT (control, n = 5) mice or Cx3cr1DTR/+ mice (n = 5) infected with C. rodentium and treated with DT as above. *, P ≤ 0.05. Two-tailed Student’s t test. Error bars represent the SEM. One of two representative experiments is shown.
To examine potential involvement of signaling pathways for receptors of pathogen- or microbe-associated molecular patterns (PAMPs or MAMPs) in mediating this phenotype, mice with a conditional deletion of MyD88 in CD11c-expressing MNPs (CD11c-Cre/Myd88fl/fl) were infected with C. rodentium. Infection of CD11c-Cre/Myd88fl/fl mice, but not littermate controls, was lethal by 15 d after infection (Fig. 2 A), implicating PAMP/MAMP signaling as having a critical role in barrier protection mediated by CD11c-expressing MNPs. The C. rodentium colitis model depends on IL-22 for protection (Zheng et al., 2008). Thus, to test if exogenous IL-22 could rescue the susceptibility phenotype described above, CD11c-Cre/Myd88fl/fl (Fig. 2 A) and DT-treated CX3CR1-DTR (Fig. 2 B) mice were hydrodynamically injected with a plasmid encoding IL-22 (Qiu et al., 2012). The exogenous IL-22 rescued both lines of mice from colitis-induced death.
CX3CR1+ cells support colonic ILC3 production of IL-22. (A) Survival curves of C. rodentium–infected Myd88f/f littermate controls (n = 10, open circle) as compared with CD11c-cre/Myd88f/f mice without (n = 13, filled circle) or with (n = 5, open triangle) exogenous hydrodynamic delivery of an IL-22–producing plasmid. Results are a composite of two independent experiments. (B) Survival curves of DT-treated Cx3cr1DTR/+ mice infected with C. rodentium after hydrodynamic delivery of a plasmid expressing IL-22 (n = 8) or control vector (n = 9). DT was administered at days −2, −1, and 0 and every other day after infection. Results are a composite of three independent experiments. (C–E) Percentage (C and D) and total number (E) of colonic Lin− CD90.2+ ILCs producing IL-22 from DT-treated Cx3cr1DTR/+ (n = 10) and littermate control mice (n = 9) 7 d after C. rodentium infection and from uninfected mice (NT; n = 3). Results are a composite of two independent experiments. DT was administered at days −2, −1, and 0 and every other day after infection. Intracellular IL-22 was assayed by flow cytometry after 4-h culture. A representative flow cytometry plot from each group is shown in C. **, P ≤ 0.01. One way ANOVA with Bonferroni’s correction. Error bars represent the SEM. (F) Total number of ILC3 per colon in Cx3cr1DTR/+ (n = 9) or control (n = 8) mice administered DT. Error bars represent the SEM. Results are one of three representative experiments. (G) Total number of IL-17+ or IL-22+ colonic CD4+ T cells from DT-treated Cx3cr1DTR/+ (n = 10) and littermate control mice (n = 9) 7 d after C. rodentium infection and from uninfected mice (n = 3). Intracellular IL-22 and IL-17 was assayed by flow cytometry after 4-h culture. *, P ≤ 0.05. One way ANOVA with Bonferroni’s correction. Error bars represent the SEM. Results are a composite of two independent experiments. (H) Confocal immunofluorescence of colonic samples from Cx3Cr1GFP/+ mice stained for CD3 and RORγt. Bar, 10 µm. White arrows indicate sites of MNP and ILC3 juxtaposition.
CX3CR1+ cells support colonic ILC3 production of IL-22. (A) Survival curves of C. rodentium–infected Myd88f/f littermate controls (n = 10, open circle) as compared with CD11c-cre/Myd88f/f mice without (n = 13, filled circle) or with (n = 5, open triangle) exogenous hydrodynamic delivery of an IL-22–producing plasmid. Results are a composite of two independent experiments. (B) Survival curves of DT-treated Cx3cr1DTR/+ mice infected with C. rodentium after hydrodynamic delivery of a plasmid expressing IL-22 (n = 8) or control vector (n = 9). DT was administered at days −2, −1, and 0 and every other day after infection. Results are a composite of three independent experiments. (C–E) Percentage (C and D) and total number (E) of colonic Lin− CD90.2+ ILCs producing IL-22 from DT-treated Cx3cr1DTR/+ (n = 10) and littermate control mice (n = 9) 7 d after C. rodentium infection and from uninfected mice (NT; n = 3). Results are a composite of two independent experiments. DT was administered at days −2, −1, and 0 and every other day after infection. Intracellular IL-22 was assayed by flow cytometry after 4-h culture. A representative flow cytometry plot from each group is shown in C. **, P ≤ 0.01. One way ANOVA with Bonferroni’s correction. Error bars represent the SEM. (F) Total number of ILC3 per colon in Cx3cr1DTR/+ (n = 9) or control (n = 8) mice administered DT. Error bars represent the SEM. Results are one of three representative experiments. (G) Total number of IL-17+ or IL-22+ colonic CD4+ T cells from DT-treated Cx3cr1DTR/+ (n = 10) and littermate control mice (n = 9) 7 d after C. rodentium infection and from uninfected mice (n = 3). Intracellular IL-22 and IL-17 was assayed by flow cytometry after 4-h culture. *, P ≤ 0.05. One way ANOVA with Bonferroni’s correction. Error bars represent the SEM. Results are a composite of two independent experiments. (H) Confocal immunofluorescence of colonic samples from Cx3Cr1GFP/+ mice stained for CD3 and RORγt. Bar, 10 µm. White arrows indicate sites of MNP and ILC3 juxtaposition.
Colonic CX3CR1+ MNPs regulate ILC3 production of IL-22
High-dose infection with C. rodentium is controlled by ILC3, which represents the large majority of LPMCs producing IL-22 (Sonnenberg et al., 2011). At day 7 after infection, both the percentage and absolute number of IL-22+ colonic, lineage−, CD90hi, and RORγt+ ILCs (Fig. S1 shows gating strategy) from mice depleted for CX3CR1+ cells were reduced in comparison to ILCs from mice with intact CX3CR1+ cells (Fig. 2, C– E). Depletion of CX3CR1+ cells did not affect the absolute number of ILC3 (Fig. 2 F). Although T cells can also contribute to IL-22 production in low-dose C. rodentium infection (Basu et al., 2012), no statistically significant difference in the total IL-22+ (or IL-17+) T cells was noted in mice depleted for CX3CR1+ cells (Fig. 2 G). To assess the ability of colonic CX3CR1+ MNPs to interact with ILC3 within the colonic tissue, Cx3cr1GFP/+ mice were used to visualize CX3CR1+ MNPs in situ (Jung et al., 2000). Consistent with the ability of these cells to regulate ILC3 function, confocal microscopy revealed the spatial proximity of RORγt+ ILC3 cells with CX3CR1+ MNPs in the colonic lamina propria (Fig. 2 H).
To evaluate the ability of intestinal CX3CR1+ cells and cDCs to support ILC3 activation, CX3CR1+ (GFP+) cells and CD103+ CD11b+ DCs were sorted from the lamina propria of Cx3cr1GFP/+ mice (Fig. 3 A) and co-cultured with intestinal ILCs. TLR-stimulated CX3CR1+ cells were markedly more efficient than CD103+ CD11b+ DCs in supporting IL-22 production (Fig. 3, B and C). CX3CR1+ cells in the LP include Ly6Chi monocytes and Ly6Clo MHCIIhi MNPs (Fig. 3 D; Zigmond et al., 2012; Diehl et al., 2013). To evaluate the role of these distinct CX3CR1+ populations in supporting ILC3 function, we sorted CX3CR1+ monocytes and CX3CR1+ MNPs and co-cultured them with ILCs in the presence of TLR stimuli. TLR-stimulated MNPs were much more potent inducers of IL-22 than monocytes (Fig. 3, E and F). In an effort to confirm the functional potential of MNPs compared with monocytes in vivo, we selectively ablated CD11c-expressing CX3CR1+ MNPs. CD11c-cre mice were bred to mice engineered to express DTR only upon cre-mediated deletion of a LoxP-Stop cassette inserted into the Cx3cr1 locus (Diehl et al., 2013). Injection of DT resulted in a selective loss of Ly6Clo MHCIIhi MNPs, which express both intermediate and high levels of CX3CR1-GFP, and spared the Ly6Chi monocytes, which express intermediate levels of CX3CR1 (Fig. 4 A; Diehl et al., 2013). Similar to results with CX3CR1DTR/+ mice, in which both monocytes and MNPs were ablated, depletion of CX3CR1+ CD11c+ cells led to reduction in colitis-induced ILC3 production of IL-22 (Fig. 4 B).
TLR-stimulated CX3CR1+ MNPs are stronger inducers of ILC3 production of IL-22 than CD103+ CD11b+ DCs and monocytes. (A–C) CD103 or CX3CR1+ MHCII+ CD11c+ CD11b+ cells were isolated from the lamina propria of CX3CR1GFP/+ mice (sort strategy shown in A and co-cultured with Lin− RORγt-GFP+ ILCs with or without the indicated bacterial TLR ligands or IL-23. IL-22 was assessed by intracellular staining of CD90.2+ ILCs after 18 h. A representative flow cytometry plot is shown in B. (C) Percent IL-22+ ILCs is shown from seven independent experiments. **, P ≤ 0.01; *, P ≤ 0.05. One way ANOVA with Bonferroni’s correction. Error bars represent SEM. (D–F) Ly6C+ MHCIIlo (monocytes) and Ly6C− MHCIIhi (MNPs) were isolated from CX3CR1+ CD11b+ lamina propria cells (sort strategy is shown in D and co-cultured with intestinal ILCs with LPS or IL-23 as indicated. Intracellular cytokine staining for IL-22 is shown after 18 h (E). Supernatants were harvested after 18 h and IL-22 production quantitated by ELISA (F). Results are representative of two independent experiments performed in triplicate. ***, P ≤ 0.001. One-way ANOVA with Bonferroni correction. Error bars represent the SEM.
TLR-stimulated CX3CR1+ MNPs are stronger inducers of ILC3 production of IL-22 than CD103+ CD11b+ DCs and monocytes. (A–C) CD103 or CX3CR1+ MHCII+ CD11c+ CD11b+ cells were isolated from the lamina propria of CX3CR1GFP/+ mice (sort strategy shown in A and co-cultured with Lin− RORγt-GFP+ ILCs with or without the indicated bacterial TLR ligands or IL-23. IL-22 was assessed by intracellular staining of CD90.2+ ILCs after 18 h. A representative flow cytometry plot is shown in B. (C) Percent IL-22+ ILCs is shown from seven independent experiments. **, P ≤ 0.01; *, P ≤ 0.05. One way ANOVA with Bonferroni’s correction. Error bars represent SEM. (D–F) Ly6C+ MHCIIlo (monocytes) and Ly6C− MHCIIhi (MNPs) were isolated from CX3CR1+ CD11b+ lamina propria cells (sort strategy is shown in D and co-cultured with intestinal ILCs with LPS or IL-23 as indicated. Intracellular cytokine staining for IL-22 is shown after 18 h (E). Supernatants were harvested after 18 h and IL-22 production quantitated by ELISA (F). Results are representative of two independent experiments performed in triplicate. ***, P ≤ 0.001. One-way ANOVA with Bonferroni correction. Error bars represent the SEM.
CX3CR1+ MNP-derived IL-23 and IL-1β activate ILC3 to produce IL-22. (A) Phenotype analysis of colonic LPMCs from Cx3cr1STOP-DTR/GFP mice with or without CD11c-cre after DT injection for 2 d. (top) Selective depletion of CX3CR1hi MNPs. (bottom) Expression of Ly6C and MHCII on CX3CR1hi and CX3CR1int populations. (B) Expression of IL-22 in Lin− CD90.2+ colonic ILCs from Cx3cr1STOP-DTR/+ (Stop-DTR) or CD11c-Cre x Cx3cr1STOP-DTR/+ (Cre-DTR) mice at 7 d after C. rodentium infection. DT was administered at days −2, −1, and 0 and every other day postinfection. One representative intracellular cytokine flow cytometry plot is shown on the left and a composite graph (n = 6/group) on the right. *, P ≤ 0.05, two-tailed Student’s t test. Error bars represent the SEM. Results are a composite of two independent experiments. (C) Supernatants from APC-ILC co-cultures (Fig. 3, B and C) were harvested after 18 h and assayed for IL-23 by ELISA. Results are averages of three independent experiments and the SEM is shown. (D) CX3CR1+ MNPs or CD103+ CD11b+ DCs were sorted and incubated with media or CpG for 18 h and supernatants were assayed for IL-1β by ELISA. Results are the mean of two independent experiments performed in duplicate and the SEM is shown. *, P ≤ 0.05; **, P ≤ 0.01. (E) Lin− CD90.2hi ILCs from WT or Il1r−/− mice were co-cultured with sorted intestinal MNPs from WT or Il23p19−/− mice, with or without CpG, as indicated. IL-22 production by the ILCs was assessed after 18 h by ELISA. Data are combined from three independent experiments performed in duplicate. *, P ≤ 0.05; ***, P ≤ 0.001. One-way ANOVA with Bonferroni correction. Error bars represent the SEM.
CX3CR1+ MNP-derived IL-23 and IL-1β activate ILC3 to produce IL-22. (A) Phenotype analysis of colonic LPMCs from Cx3cr1STOP-DTR/GFP mice with or without CD11c-cre after DT injection for 2 d. (top) Selective depletion of CX3CR1hi MNPs. (bottom) Expression of Ly6C and MHCII on CX3CR1hi and CX3CR1int populations. (B) Expression of IL-22 in Lin− CD90.2+ colonic ILCs from Cx3cr1STOP-DTR/+ (Stop-DTR) or CD11c-Cre x Cx3cr1STOP-DTR/+ (Cre-DTR) mice at 7 d after C. rodentium infection. DT was administered at days −2, −1, and 0 and every other day postinfection. One representative intracellular cytokine flow cytometry plot is shown on the left and a composite graph (n = 6/group) on the right. *, P ≤ 0.05, two-tailed Student’s t test. Error bars represent the SEM. Results are a composite of two independent experiments. (C) Supernatants from APC-ILC co-cultures (Fig. 3, B and C) were harvested after 18 h and assayed for IL-23 by ELISA. Results are averages of three independent experiments and the SEM is shown. (D) CX3CR1+ MNPs or CD103+ CD11b+ DCs were sorted and incubated with media or CpG for 18 h and supernatants were assayed for IL-1β by ELISA. Results are the mean of two independent experiments performed in duplicate and the SEM is shown. *, P ≤ 0.05; **, P ≤ 0.01. (E) Lin− CD90.2hi ILCs from WT or Il1r−/− mice were co-cultured with sorted intestinal MNPs from WT or Il23p19−/− mice, with or without CpG, as indicated. IL-22 production by the ILCs was assessed after 18 h by ELISA. Data are combined from three independent experiments performed in duplicate. *, P ≤ 0.05; ***, P ≤ 0.001. One-way ANOVA with Bonferroni correction. Error bars represent the SEM.
Because both IL-23 and IL-1β regulate ILC3, we wished to determine the contribution of these cytokines to the observed regulation of IL-22 production by MNPs. LPS and CpG stimulation induced markedly increased IL-23 and IL-1β production by CX3CR1+ MNPs compared with CD103+ CD11b+ DCs in vitro (Fig. 4, C and D). To evaluate the role of MNP-derived IL-23 and IL-1β, co-cultures were performed with intestinal CD11c+ cells derived from WT or Il23p19−/− mice and ILCs from WT or Il1r−/− mice. IL-23–deficient MNPs and IL1R-deficient ILCs yielded significantly reduced production of IL-22 (Fig. 4 E), consistent with the importance of MNP-derived IL-1β and IL-23 in supporting IL-22 production. These data reveal a mechanistic role for IL-23 and IL-1β produced by colonic MNPs in supporting colitis-associated ILC3 secretion of IL-22.
Human intestinal ILC3 production of IL-22 is regulated by microbial stimulation of MNPs
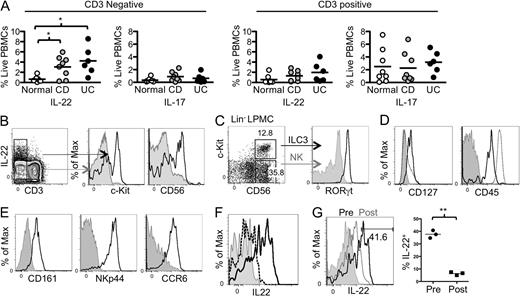
To evaluate the regulation of intestinal ILC3 in humans with IBD, we prepared LPMCs from descending colon biopsies of patients with endoscopically mild to moderate Crohn’s’ disease (CD, n = 8) or ulcerative colitis (UC, n = 6; Table S1) as well as age-matched non-IBD control patients undergoing routine screening colonoscopy (n = 8). Analysis of intracellular cytokine production revealed significantly increased IL-22 production in the CD3− fraction of colonic LPMCs from sites of colonic inflammation in both CD and UC compared with the non-IBD controls (Fig. 5 A and Fig. S2). In contrast, IL-22 production by T cells was not significantly different between the groups. Further characterization of the non–T cells producing IL-22 revealed that a large fraction of these cells expressed c-Kit and CD56, markers of ILCs (Cella et al., 2009; Fig. 5 B). Consistent with their being ILC3, these cells were lineage negative (Lin−; Fig. S3 A); expressed RORγt (Fig. 5 C); were CD45int CD127+ (Fig. 5 D); expressed CD161, NKp44, and CCR6 (Fig. 5 E), which are phenotypic surface markers of ILC3; and produced IL-22 in response to IL-23 stimulation (Fig. 5 F). As Myd88 deficiency abrogated ILC3 production of IL-22, we hypothesized that signals from the microbiota could induce ILC3 to produce IL-22. To investigate this in human tissue, we evaluated three patients who had a surgical diversion of the fecal stream (i.e., a diverting ostomy) as part of their therapy for IBD. Endoscopic biopsies were taken from a site proximal to the diversion (afferent limb), where the mucosa was exposed to intestinal microbiota in the fecal stream, and from mucosa distal to the diversion (efferent limb), that was unexposed to the fecal stream. ILCs were present at both mucosal locations, but not in PBMCs from the same donor (Fig. S3 B). In all three donors, ILCs from tissue exposed to bacteria in the fecal stream produced substantially more IL-22 compared with ILCs from unexposed tissue (postdiversion; Fig. 5 G).
Increased ILC3 production of IL-22 in mild to moderate IBD correlates with presence of fecal stream. (A) LPMCs isolated from descending colon biopsies from patients with endoscopically mild to moderate Crohn’s’ disease (n = 8, gray) or ulcerative colitis (n = 6, black; Table S1), as well as age-matched non-IBD control patients undergoing routine screening colonoscopy (n = 8, white), were stimulated ex vivo with PMA/ionomycin and evaluated by intracellular cytokine staining for expression of IL-17 and IL-22. The percentage of CD3+ or CD3− fraction expressing IL-17 or IL-22 is indicated. *, P ≤ 0.05, two-tailed Student’s t test. Black bars represent the geometric mean. (B) Expression of c-Kit and CD56 in electronically gated CD3− IL-22+ (black lines) and CD3− IL-22− (gray) LPMCs. (C) Expression of RORγt by c-Kit+CD56+ LPMCs. Lin− cells (CD14/CD19/CD3/CD11b/CD11c/TCRγδ−; Fig. S3 A) were stained with antibodies to surface markers c-Kit and CD56 and to intracellular RORγt. Lin− CD56+ c-Kit+ ILC3 (black line) were compared with Lin− CD56+ c-Kit− NK cells (gray) for RORγt expression. (D) Surface staining of Lin− c-Kit+ ILCs for the indicated markers (black line) compared with isotype control (gray) and all live LPMCs (dotted line) (E) LPMCs stained for intracellular IL-22 after stimulation with IL-23 for 3 h (solid line) or with control media (dotted line). Cells shown were gated on Lin− CD56+ c-Kit+. The isotype control is in gray. (F) CD11c+ MHCII+ human colonic APCs were electronically gated for expression of CD103 and CD14. One of three donors is shown. (G) Lamina propria cells from biopsy samples of tissue exposed (prediversion) or not exposed to the fecal stream (post-diversion) were cultured for 3 h and ILC3 production of IL-22 was assessed by flow cytometry. (left) Result from one representative donor. (right) Percentage of IL-22+ ILCs in afferent (Pre) and efferent (Post) limbs of three diverted patients. **, P ≤ 0.01, two-tailed Student’s t test. Black bars represent the geometric mean.
Increased ILC3 production of IL-22 in mild to moderate IBD correlates with presence of fecal stream. (A) LPMCs isolated from descending colon biopsies from patients with endoscopically mild to moderate Crohn’s’ disease (n = 8, gray) or ulcerative colitis (n = 6, black; Table S1), as well as age-matched non-IBD control patients undergoing routine screening colonoscopy (n = 8, white), were stimulated ex vivo with PMA/ionomycin and evaluated by intracellular cytokine staining for expression of IL-17 and IL-22. The percentage of CD3+ or CD3− fraction expressing IL-17 or IL-22 is indicated. *, P ≤ 0.05, two-tailed Student’s t test. Black bars represent the geometric mean. (B) Expression of c-Kit and CD56 in electronically gated CD3− IL-22+ (black lines) and CD3− IL-22− (gray) LPMCs. (C) Expression of RORγt by c-Kit+CD56+ LPMCs. Lin− cells (CD14/CD19/CD3/CD11b/CD11c/TCRγδ−; Fig. S3 A) were stained with antibodies to surface markers c-Kit and CD56 and to intracellular RORγt. Lin− CD56+ c-Kit+ ILC3 (black line) were compared with Lin− CD56+ c-Kit− NK cells (gray) for RORγt expression. (D) Surface staining of Lin− c-Kit+ ILCs for the indicated markers (black line) compared with isotype control (gray) and all live LPMCs (dotted line) (E) LPMCs stained for intracellular IL-22 after stimulation with IL-23 for 3 h (solid line) or with control media (dotted line). Cells shown were gated on Lin− CD56+ c-Kit+. The isotype control is in gray. (F) CD11c+ MHCII+ human colonic APCs were electronically gated for expression of CD103 and CD14. One of three donors is shown. (G) Lamina propria cells from biopsy samples of tissue exposed (prediversion) or not exposed to the fecal stream (post-diversion) were cultured for 3 h and ILC3 production of IL-22 was assessed by flow cytometry. (left) Result from one representative donor. (right) Percentage of IL-22+ ILCs in afferent (Pre) and efferent (Post) limbs of three diverted patients. **, P ≤ 0.01, two-tailed Student’s t test. Black bars represent the geometric mean.
Parallel subpopulations of CD11c+ MNPs present in the mouse intestine similarly exist in the human intestine (Fig. 6 A; Merad et al., 2013). To evaluate whether these distinct subpopulations of MNPs from human intestinal tissue functioned similarly, we examined the phenotypic properties of CD103+ DCs and CD14+ MNPs (which express CX3CR1; Kamada et al., 2008) within the CD11c+ MHCII+ fraction of LPMCs (Fig. 6 B). In contrast to CD103+ DCs, CD14+ MNPs expressed CD64 as well as higher levels of CD86. Consistent with the phenotypic characterization of these subsets, transcriptional analysis of these populations by RNA-seq revealed higher levels of CLEC9A, XCR1, and CD207 expression in the CD103+ cells, whereas MERTK, STAB1, and CX3CR1 were higher in the CD14+ cells (Fig. 6 C). We tested the potential of these subsets to induce IL-22 production by co-culturing TLR-stimulated CD14+ MNPs and CD103+ DCs from human intestinal resections with intestinal ILCs. Intracellular cytokine staining at 18 h revealed that the CD14+ MNP were more effective than the CD103+ DCs at stimulating IL-22 production by ILCs (Fig. 6 D). Neither cell population induced significant IL-17 or IFN-γ production by ILCs (Fig. 6, D and E).
Human ILC3 production of IL-22 is supported by IL-23 and IL-1β produced by TLR-stimulated CD14+ and CX3CR1+ MNPs. (A–C) HLA-DR+ CD11c+ cells from intestinal resection tissue were sorted into CD103+ DCs and CD14+ MNPs subpopulations and transcriptional profiles were assessed by RNA-seq. (A) Sorting strategy. (B) Each subset was examined for expression of the indicated cell surface markers. Isotype controls are shown in gray. One of three donors is shown. (C) Heatmap of relative expression of relevant MNP-related genes. Values represent the mean of two independent donors, and an asterisk denotes individual genes differentially expressed at an FDR = 0.01. (D and E) Induction of IL-22 in human ILCs in co-culture with CD14+ MNPs or CD103+ DCs in the presence of media alone, LPS, or flagellin, as indicated. c-Kit+ cells were examined for intracellular IL-22 production after 18-h culture. Data are representative of five independent experiments. (F) CD14+ MNPs or CD103+ DCs sorted from human intestine (as in A) were stimulated with the indicated TLR ligands for 18 h and qPCR or cytometric bead array analysis were used to quantitate IL-23p19 and IL-1β, respectively. Results are averaged from three independent donors, and technical replicates were performed in duplicate or triplicate, respectively. *, P ≤ 0.05. N.D., not detected. Two-tailed Student’s t test. Error bars represent the SEM. (G) Sorted human intestinal CD14+ MNPs and ILCs were left unstimulated or were co-cultured in the presence of LPS with or without neutralizing antibodies against IL-1β and IL-23. IL-22 ELISA was performed after 18h. Results are averaged from two independent donors performed in duplicate. *, P ≤ 0.05. Two-tailed Student’s t test. Error bars represent the SEM.
Human ILC3 production of IL-22 is supported by IL-23 and IL-1β produced by TLR-stimulated CD14+ and CX3CR1+ MNPs. (A–C) HLA-DR+ CD11c+ cells from intestinal resection tissue were sorted into CD103+ DCs and CD14+ MNPs subpopulations and transcriptional profiles were assessed by RNA-seq. (A) Sorting strategy. (B) Each subset was examined for expression of the indicated cell surface markers. Isotype controls are shown in gray. One of three donors is shown. (C) Heatmap of relative expression of relevant MNP-related genes. Values represent the mean of two independent donors, and an asterisk denotes individual genes differentially expressed at an FDR = 0.01. (D and E) Induction of IL-22 in human ILCs in co-culture with CD14+ MNPs or CD103+ DCs in the presence of media alone, LPS, or flagellin, as indicated. c-Kit+ cells were examined for intracellular IL-22 production after 18-h culture. Data are representative of five independent experiments. (F) CD14+ MNPs or CD103+ DCs sorted from human intestine (as in A) were stimulated with the indicated TLR ligands for 18 h and qPCR or cytometric bead array analysis were used to quantitate IL-23p19 and IL-1β, respectively. Results are averaged from three independent donors, and technical replicates were performed in duplicate or triplicate, respectively. *, P ≤ 0.05. N.D., not detected. Two-tailed Student’s t test. Error bars represent the SEM. (G) Sorted human intestinal CD14+ MNPs and ILCs were left unstimulated or were co-cultured in the presence of LPS with or without neutralizing antibodies against IL-1β and IL-23. IL-22 ELISA was performed after 18h. Results are averaged from two independent donors performed in duplicate. *, P ≤ 0.05. Two-tailed Student’s t test. Error bars represent the SEM.
Consistent with the importance of IL-23 and IL-1β in the mouse co-culture experiments, a higher level of IL23A expression was observed by RNA-seq in CD14+ MNPs compared with CD103+ DCs (Fig. 6 C). Stimulation of these human MNP subsets with LPS or flagellin revealed increased IL-23p19 mRNA and IL-1β protein produced by the CD14+ cells compared with the CD103+ cells (Fig. 6 F). In accord with this finding, in co-cultures of human intestinal ILC3 and CD14+ MNPs, IL-23 and IL-1β antibody blockade blunted IL-22 production (Fig. 6 G). These data reveal a mechanistic role for the expanded population of CD14+ MNPs (Kamada et al., 2008) in supporting colitis-associated IL-22 production by ILC3, through their secretion of IL-23 and IL-1β.
CX3CR1+ MNP-derived TL1A synergizes with IL-23 and IL-1β to induce IL-22
We hypothesized that MNP-derived factors in addition to IL-23 and IL-1β would contribute to the regulation of ILC3 function. The RNA-seq data from CD14+ human MNPs revealed a significant enrichment for genes associated with IBD in GWAS studies, suggesting that IBD-associated pathways are important in these cells (Fig. 7 A and Table S2). Notably, we identified TNF-like ligand 1A (TL1A, also designated TNFSF15) as a significant contributor to the IBD GWAS-derived gene set enrichment. Evaluation of Tnfsf15 transcript in sorted mouse colonic APC subsets by qPCR confirmed higher expression in CX3CR1+ MNPs (Fig. 7 B). DR3/TNFRSF25, the receptor for TL1A, is expressed both on T cells and ILC subsets, with the highest expression on ILC2 and ILC3 (Meylan et al., 2014). Ex vivo stimulation with mouse or human recombinant TL1A significantly enhanced the ability of IL-23 and IL-1β to induce IL-22 by both mouse (Fig. 7 C and Fig. S4) and human (Fig. 7 D) intestinal ILC3, respectively. TL1A and IL-1β also cooperated in inducing GM-CSF production by mouse ILC3 (Fig. 7 E). To assess the specificity of TL1A for DR3/TNFRSF25 on ILCs and its functional role in enhancing CX3CR1+ support of ILC3 activation, DR3 expression was specifically knocked down using siRNA nucleofection of sorted mouse intestinal ILC3. Efficiency of knock-down was confirmed by surface staining for DR3, comparing with a scrambled control siRNA (Fig. 7 F). Compared with the scramble control, cells targeted with siRNA for Tnfrsf25 had significantly reduced enhancement of IL-22 production upon treatment with recombinant TL1A (Fig. 7, G and H). The targeted ILCs also produced reduced amounts of IL-22 upon co-culture with LPS-stimulated CX3CR1+ MNPs. These data reveal a potent ability of TL1A to enhance IL-22 production via DR3/TNFRSF25 on ILC3 in both mouse and human.
CX3CR1+ MNP-derived TL1A synergizes with IL-23 and IL-1β to induce IL-22 in intestinal ILC3. (A) Gene-set enrichment analysis was used to determine whether the indicated disease-related SNP were differentially expressed between CD14+ MNPs and CD103+ DCs. Significance was estimated using the hypergeometric cumulative distribution, with a raw p-value cutoff of 0.05 for differential expression. Data were averaged from two independent donors. (B) B cells (CD3− CD19+), CX3CR1+ MNPs, CD103+ DCs, and Ly6C+ monocytes were sorted from the intestinal lamina propria of Cx3cr1GFP/+ and quantitative PCR for TL1A was performed. Relative quantitation was performed by ΔCt normalized to GAPDH expression. Data are from two biological replicates performed with two technical replicates. *, P ≤ 0.05. Two-tailed Student’s t test. (C–E) Sorted intestinal ILCs from mice (C and E) or cultured human intestinal ILCs (D) were stimulated with media alone, IL-1β, or IL-23 with or without TL1A as indicated for 18 h. Brefeldin was added to the cultures 4 h before intracellular cytokine staining for IL-22 (C and D) or GM-CSF (E). Data are representative of six independent experiments. (F–H) Sorted intestinal ILCs were transfected with siRNA targeting Tnfrsf25 or a scramble control. (F) Knockdown efficiency was assessed after 24 h by flow cytometry comparing scramble control (solid line) with Tnfrsf25 siRNA. One of two representative experiments is shown. ILCs were then cultured with media alone (-) or IL-23 and TL1A or co-cultured with CX3CR1+ MNPs with or without LPS as indicated for an additional 18 h. (G) IL-22 production was measured by intracellular flow cytometry. Brefeldin was added to the cultures 4 h before intracellular cytokine staining. Data are representative of two independent experiments. (H) IL-22 secretion by samples from G were assessed by ELISA, performed in duplicate, before addition of Brefeldin. *, P ≤ 0.05; **, P ≤ 0.01. Two-tailed Student’s t test. Error bars represent SEM.
CX3CR1+ MNP-derived TL1A synergizes with IL-23 and IL-1β to induce IL-22 in intestinal ILC3. (A) Gene-set enrichment analysis was used to determine whether the indicated disease-related SNP were differentially expressed between CD14+ MNPs and CD103+ DCs. Significance was estimated using the hypergeometric cumulative distribution, with a raw p-value cutoff of 0.05 for differential expression. Data were averaged from two independent donors. (B) B cells (CD3− CD19+), CX3CR1+ MNPs, CD103+ DCs, and Ly6C+ monocytes were sorted from the intestinal lamina propria of Cx3cr1GFP/+ and quantitative PCR for TL1A was performed. Relative quantitation was performed by ΔCt normalized to GAPDH expression. Data are from two biological replicates performed with two technical replicates. *, P ≤ 0.05. Two-tailed Student’s t test. (C–E) Sorted intestinal ILCs from mice (C and E) or cultured human intestinal ILCs (D) were stimulated with media alone, IL-1β, or IL-23 with or without TL1A as indicated for 18 h. Brefeldin was added to the cultures 4 h before intracellular cytokine staining for IL-22 (C and D) or GM-CSF (E). Data are representative of six independent experiments. (F–H) Sorted intestinal ILCs were transfected with siRNA targeting Tnfrsf25 or a scramble control. (F) Knockdown efficiency was assessed after 24 h by flow cytometry comparing scramble control (solid line) with Tnfrsf25 siRNA. One of two representative experiments is shown. ILCs were then cultured with media alone (-) or IL-23 and TL1A or co-cultured with CX3CR1+ MNPs with or without LPS as indicated for an additional 18 h. (G) IL-22 production was measured by intracellular flow cytometry. Brefeldin was added to the cultures 4 h before intracellular cytokine staining. Data are representative of two independent experiments. (H) IL-22 secretion by samples from G were assessed by ELISA, performed in duplicate, before addition of Brefeldin. *, P ≤ 0.05; **, P ≤ 0.01. Two-tailed Student’s t test. Error bars represent SEM.
DISCUSSION
Mononuclear phagocytes are spatially and functionally poised to integrate microbial signals from the luminal microbiota (Niess et al., 2005; Varol et al., 2010). Although MNPs were previously thought to remain in the tissue, we recently showed that CX3CR1+ MNPs can migrate to draining lymph nodes and initiate immune responses under conditions of dysbiosis (Diehl et al., 2013). In the context of inflammation, however, these CX3CR1+ MNPs expand within the lamina propria during chemical (Zigmond et al., 2012) or infectious colitis and in IBD patients (Kamada et al., 2008), and their function has remained obscure. Conventional DCs (cDCs), rather than the MNPs, have been postulated to regulate intestinal Th17 cell differentiation in response to microbiota (Denning et al., 2011; Lewis et al., 2011; Persson et al., 2013; Schlitzer et al., 2013; Goto et al., 2014), but there has been conflicting evidence as to which myeloid cell populations regulate IL-22 production by ILC3 cells (Kinnebrew et al., 2012; Manta et al., 2013; Satpathy et al., 2013).
Our data reveal that colonic CX3CR1+ MNPs regulate ILC3 production of IL-22 and likely play a critical role in promoting mucosal healing during colitis. The requirement for CX3CR1+ MNPs in regulating colitis-induced ILC3 shown here appears to contrast with recent work suggesting that Notch2-dependent CD103+ CD11b+ cDCs are required for the control of C. rodentium–induced colitis (Satpathy et al., 2013). These disparate results may reflect a coordinated contribution of multiple myeloid subsets in the immune response to C. rodentium (Schreiber et al., 2013), and our data do not exclude a contribution of other myeloid subsets. Alternatively, Notch2 may affect cellular subpopulations in addition to CD103+ CD11b+ cDCs. This latter hypothesis is supported by the report that an alternate selective depletion strategy for CD103+ CD11b+ cDCs, by expression of DT under the human Langerin promoter (Welty et al., 2013), did not result in increased susceptibility to C. rodentium. Some of the conflicting data may additionally reflect differential roles of inflammatory monocytes and MNPs in colitis. Inflammatory monocytes may exacerbate pathology, as antibody-mediated depletion of Ly6Chi monocytes during DSS treatment improved pathology in DSS colitis (Zigmond et al., 2012). Selective depletion of such monocytes may hence explain the intermediate results seen in the CCR2-deficient mice exposed to C. rodentium (Satpathy et al., 2013). In addition to supporting ILC3, MNPs may also directly promote epithelial barrier repair (Wynn et al., 2013). Although our data suggest a critical role for CX3CR1+ MNPs in regulating production of IL-22 by ILC3 during acute colitis, it will be important to examine the role for these myeloid subsets during chronic colitis and their impact on another major clinical endpoint in IBD—tumorigenesis (Huber et al., 2012; Kirchberger et al., 2013).
Our results also highlight a beneficial role for microbial signals in promoting intestinal homeostasis and driving ILC3 production of IL-22 through TLR/MyD88-dependent induction of IL-23 and IL-1β. At steady state, commensal-dependent signaling may negatively regulate ILC production of IL-22 via intestinal epithelial cell production of IL-25 (Sawa et al., 2011), but we and others (Satoh-Takayama et al., 2008) find that microbes support colitis-associated ILC production of IL-22, which promotes mucosal healing. Although intravenous delivery of flagellin can induce CD103+ CD11b+ cDCs to produce IL-23 that regulates ILC3 in the small intestine (Kinnebrew et al., 2012), we and others (Kamada et al., 2008) found that in vivo colonic inflammation and other bacterial-derived signals induced more robust production of IL-23 and IL-1β by MNPs, suggesting that the type of stimulation and/or colonic inflammation may confer specificity. Similar to our results in mice, we found marked reductions in IL-22 production by intestinal ILC3 from human tissue distal to a surgical diversion of the fecal stream. These findings may offer clinically relevant mechanistic insight into diverted IBD patients with persistent inflammatory disease or even non-IBD patients with de novo mucosal inflammation after diversion (e.g., diversion colitis; Harig et al., 1989). Although some evidence supports a role for luminal replacement of short chain fatty acids in diversion colitis (Harig et al., 1989; Vernia et al., 1995), further work is required to determine if particular metabolites or intestinal microbes may regulate ILC3 function to promote healing.
In light of their robust production of IL-22 and close proximity to the intestinal epithelial layer (Cella et al., 2009), ILC3 have been proposed to play an important role in mucosal healing and maintenance of barrier integrity, but their characterization in IBD patients has been limited. ILCs producing IL-17 or IFN-γ (classified as ILC1) have also been described in mouse models of colitis (Buonocore et al., 2010; Klose et al., 2013) and human IBD (Geremia et al., 2011; Bernink et al., 2013), supporting a potentially important role for ILCs in IBD pathogenesis. Here, we observed a specific increase in IL-22 production by ILCs from colonic biopsies of patients with mild to moderate CD and UC. Despite characteristically distinct phenotypes, both CD and UC share genetic susceptibility risk alleles within the IL-23 pathway (Duerr et al., 2006), which may underlie a common role for ILCs during colonic inflammation in both diseases. In contrast to previous studies, we did not observe any significant IL-17 production by CD3− cells from patients with CD (Geremia et al., 2011) or a reduction in ILC3 in CD patients (Bernink et al., 2013). This difference may reflect clinical differences in the patient cohorts and samples—colonoscopic biopsies in our cohort were taken from ambulatory patients with mild to moderate colonic CD and UC, in contrast to surgical resection of ileal tissue from medically refractory patients in the other studies. As such, mild to moderate colitis may be the ideal human model to evaluate ILC3-producing IL-22 promoting mucosal healing. The association of clinical phenotype with ILC phenotype and the potential plasticity of these ILCs in colitis remain to be evaluated.
Consistent with our results in mouse models, CX3CR1+ MNPs from human intestinal tissue were more potent than CD103+ DCs in supporting human intestinal ILC3 production of IL-22. In addition to the increased production of IL-23 and IL-1β by the CX3CR1+ subset described by us and others (Kamada et al., 2008), RNA seq analysis of these subsets revealed enrichment for transcripts from genes having IBD-associated SNPs, notably TL1A/TNFSF15. Polymorphisms in TNFSF15, the gene that encodes TL1A, are strongly associated with IBD, and expression of both TL1A and its receptors DR3 and DcDR3 is elevated in IBD patients (Kugathasan et al., 2008). TL1A has been shown to enhance Th17 differentiation and effector function (Pappu et al., 2008; Kamada et al., 2010); promote Treg expansion and ameliorate allergic asthma (Schreiber et al., 2010); and promote IL-13 production by ILC2s (Meylan et al., 2014). Interestingly, microbial stimulation and Fcγ-receptor engagement induces TL1A/TNFSF15 in monocytes and monocyte-derived mononuclear phagocytes (Shih et al., 2009). The data shown here reveal a potent ability of TL1A to enhance ILC3 production of IL-22 and suggest that the increased expression of TL1A by colonic CX3CR1+ MNPs enables their selective ability to potently support ILC3. Furthermore, the ability of TL1A to induce GM-CSF production by ILC3 is consistent with recent data suggesting that, even at steady state, CSF1R-dependent MNPs support ILC3 production of GM-CSF that, in turn, regulates oral tolerance (Mortha et al., 2014). Collectively, these data support a broader role for colonic CX3CR1+ MNPs in regulating mucosal homeostasis.
Mucosal ILC3 play a crucial role in barrier homeostasis, mucosal healing, and oral tolerance. CX3CR1+ MNPs are aptly poised to integrate microbial signals to regulate ILC3 and may serve as important targets for therapeutic manipulation. Further understanding of the contributions of discrete commensal bacterial species and of the mechanistic interactions between CX3CR1+ MNPs and ILC3 may provide novel strategies to promote intestinal healing. In light of the specific and potent regulation of ILC3 by colonic CX3CR1+ MNPs during colitis, diagnostics that correlate susceptible genotypes (Hueber et al., 2012) with clinical disease immunophenotype will provide insight into therapeutic strategies to manipulate colonic MNPs in the clinical management of IBD.
MATERIALS AND METHODS
Antibodies and flow cytometry.
Staining of human cells was performed with c-Kit-e450 (104D2), CD56-PECy5.5 (CMSSB), HLA-DR-PE (LN3), CD11c-FITC (3.9), CD3-e780 (UCHT1), CD19-PerCP5.5 (HIB19), CD103 PECy7 (B-Ly7), CD14 PE (61D3), CD86-PE (IT2.2), CD123 PE (6H6), CD11b PE (CBRM1/5), and RORγt (AFKJS-9) obtained from eBioscience; CD161-FITC, CD83-PE, CD11c PE (555392), CD127 FITC (M21), CCR6-biotin (11A9) were purchased from BD; BDCA-1-APC were obtained from Miltenyi Biotec; CD45-APC (4505) and CD64 (6404) were obtained from Invitrogen; NKp44-APC (p44-8.1) was purchased from R&D Systems; and BDCA-3 APC (M80) was purchased from BioLegend. Intracellular human cytokine staining was performed with IL-22 PE (IC7821P; R&D Systems), IL-17A-FITC (eBio64CAP17; eBioscience), or IFN-γ (45.B3; eBioscience). Staining of mouse cells was performed with CD90.2-e450 (53–2.1), CD3e (145-2C11), NKp46-e710 (29A1.4), RORγt-PE (B2D), CD11c-PECy7 (N418), MHCII A700 (M5/114.15.2), CD103 APC (2E7), CD14 PerCP5.5 (Sa2-8), CD11b e780 (M1/70), Ly6C PerCP5.5 (AL-21), and F4/80 PE (BM8) were from eBioscience. DR3-PE (4C12) was obtained from BioLegend. Intracellular mouse cytokine staining was performed with IL-22 APC (IL22JOP), IFN-γ PECy7 (XMG1.2), IL17A FITC (eBio17B7), all from eBioscience. Intracellular cytokine staining was performed according to the manufacturer’s protocol (Cytofix/Cytoperm buffer set; BD). Intranuclear staining for RORγt was performed according to manufacturer’s protocol (Intracellular Fixation and Permeabilization kit; eBioscience). Flow cytometry and analysis were performed with a LSR II (BD) and FlowJo software (Tree Star). Dead cells were excluded using the Live/Dead fixable aqua dead cell stain kit (Invitrogen).
Mice.
C57BL/6, Myd88flox, CD11c-cre (Caton et al., 2007), and Il1r−/− mice were purchased from The Jackson Laboratory. Myd88-deficient mice were obtained from S. Akira (Osaka University, Osaka, Japan; Adachi et al., 1998) and Il23p19−/− mice were obtained from D. Cua (Merck Research Laboratories, Palo Alto, CA; Cua et al., 2003). RORc(γt)GFP/+ (Eberl et al., 2004), Cx3cr1GFP/+ (Jung et al., 2000) and inducible CX3CR1-DTR mice (Diehl et al., 2013), were previously described. The latter mice were subsequently bred to a germline-expressing cre mouse to excise the transcriptional stop sequence and produce the Cx3cr1DTR/+ mice. All mice were kept in specific pathogen–free (SPF) conditions at the animal facility of the Skirball Institute. Mouse experiments were performed with at least three mice per group and multiple experiments were combined to assess statistically significant differences as noted. Littermates of the same genotype were randomly assigned to experimental groups. Animals were used between 8 and 16 wk of age. Males and females were used in approximately equal ratios. All animal experiments were performed in accordance with approved protocols for the NYU Institutional Animal Care and Usage Committee.
Preparation of LPMCs.
Endoscopic biopsies were obtained under IRB-approved protocols at Weill Cornell Medical College (1103011578 and Columbia University Medical Center (aaae5448) including patients >18 yr of age and able to give informed consent. IBD sample was defined based on endoscopic inflammation with history of ulcerative colitis or Crohn’s disease. Endoscopic score (mild, moderate, or severe) was based on Mayo Endoscopic subscore or SES-CD (1, 2, 3, respectively) at the site of biopsy (Pineton de Chambrun et al., 2010). All endoscopic biopsies were taken from the sigmoid or descending colon to reduce sampling variation. Study sample sizes for human biopsies were based on preliminary data and powered to achieve statistically significant differences in the production of IL-22 or IL-17. Surgical resections were obtained under an IRB-approved protocol at New York University Langone Medical Center. Mouse and human intestines were washed in PBS and 1 mM DTT twice with 30 mM EDTA, and then digested in collagenase 8 (Sigma-Aldrich) and DNase-containing media with 10% fetal bovine serum. Digested material was passed through a cell strainer and separated on a discontinuous 40%/80% Percoll gradient. LPMCs were cultured ex vivo in the presence of GolgiPlug (BD) for 4 h or stimulated with phorbol myristate acetate (PMA; 20 ng/ml) and ionomycin (1 µg/ml) or IL-23 (40 ng/ml; eBioscience) in the presence of GolgiPlug (BD) for 4 h before staining.
Intestinal ILC cultures.
Surgical resections were obtained from the NYU Biorepository (Rachel Brody). Lin− c-Kit+ CD45int ILCs were sorted and cultured in tissue culture media (RPMI 1640; Invitrogen) supplemented with 10% (vol/vol) heat-inactivated FBS (HyClone), 50 U penicillin-streptomycin (Invitrogen), 2 mM glutamine, and 50 µM β-mercaptoethanol), supplemented with IL-7 (50 ng/ml; PeproTech) and IL-2 (1,000 U/ml; PeproTech) for 8–10 d before stimulation. Lin−, CD90.2+, RORγt-GFP mouse ILC3 were sorted from LPMCs and resuspended in RPMI-based tissue culture media for stimulation directly ex vivo. Human and mouse ILCs were stimulated with human or mouse IL-23 (eBioscience; 40 ng/ml), IL-1β (eBioscience; 10 ng/ml), or TL1A (R&D Systems; 200 ng/ml) as indicated, respectively. After 18 h, supernatants were harvested for IL-22 ELISA (eBioscience) and Golgi Plug (BD) was added to cells for 4 h for subsequent intracellular cytokine staining.
Co-culture assay.
ILCs and APC populations were sorted on a FACSAria and co-cultured with 5 × 103 and 2.5 × 103 cells, respectively, in 96-well round-bottom plates in tissue culture media. TLR stimulation was performed with 1 µg/ml LPS (Escherichia coli; Sigma-Aldrich), 1 µM CpG 1668 (mouse), 1 µM CpG 2216 (human), or 1 µg/ml flagellin (Salmonella Typhi; InvivoGen). Cultures were incubated for 18 h. Supernatants were harvested for ELISA and remaining cells were incubated with Golgi Plug (BD) for 4 h and subsequently analyzed by flow cytometry.
siRNA transfection.
Sorted intestinal ILCs were cultured overnight in IL-7 (20 ng/ml) and SCF (20 ng/ml). After 24 h, 4 × 105 ILCs were transfected using AMAXA T cell nucleofection protocol. 300 pmol of Tnfrsf25 siRNA pool or scramble control (Thermo Fisher Scientific) was used per transfection. Cells were rested overnight and harvested at 24 h for experimental use. Knockdown efficiency was assessed at 24 h by DR3 surface staining.
Colitis models.
C. rodentium DBS100 (ATCC 51459; American Type Culture Collection) was harvested at log phase growth and 1010 CFU were delivered by gavage in PBS. 200 ng of DT was administered i.p. as indicated for depletion. Plasmid DNA expressing IL-22 or control plasmid (Qiu et al., 2012) were delivered i.v. at 5 µg DNA/mouse diluted in TransIT-EE Hydrodynamic Delivery Solution (Mirus) at 0.1 ml/g body weight. Immune cell functional analysis and histology were performed at day 7 after exposure. Spleens were harvested on day 21, homogenized, and plated at serial dilutions to determine CFU/spleen.
RNA-seq processing and gene set enrichment analysis.
Sequence reads were mapped to the human genome (version hg19) by Tophat (version 2.0.6), using Bowtie2 (version 2.0.2) and Samtools (version 0.1.18). Reads are deposited at Bioproject PRJNA219394. Reads mapped per transcript served as input to DESeq (version 1.12.0), an R package that calculates differential gene expression. To improve detection of APC lineage-dependent gene-expression changes and overcome donor-dependent variability, we used the following strategy: independently for each donor, differential gene expression, comparing CD103+ to CD14+ human myeloid cells, was estimated using the negative binomial distribution (“nbinomTest”), and then results from biological replicates were combined using Fisher’s method. Several gene set enrichment techniques (e.g., hypergeometric test and area under precision-recall curve) were used to test whether specific gene sets (Table S2) were significantly differentially regulated between the two lineages. The GWAS gene sets are derived from disease-associated SNPs from the NHGRI GWAS Catalog. False-discovery rates (FDRs) were calculated using the Benjamini-Hochberg procedure. The enrichment analyses were implemented in Matlab R2013a (8.1.0.604).
qPCR.
RNA from primary intestinal APCs stimulated as indicated was prepared with TRIzol (Invitrogen). RNA was reverse transcribed into cDNA (SuperScript III; Invitrogen) and QPCR was performed with a Roche LightCycler with SYBR Green Supermix (Bio-Rad Laboratories), 20 pmol forward and reverse primers, and 0.1 µg of cDNA from 5′-TGTTCCCCATATCCAGTGTGG-3′ and 5′-CTGGAGGCTGCGAAGGATTT-3′ for human IL23p19, 5′-ATGCTTCGGGCCATAACAGA-3′ and 5′-TGAAGGCCATCCCTAGGTCA-3′ for mouse TL1A; 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′ for human GAPDH; and 5′-AATGTGTCCGTCGTGGATCT-3′ and 5′-CATCGAAGGTGGAAGAGTGG-3′ for mouse GAPDH. The thermocycling program was 40 cycles at 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s, with an initial cycle of 95°C for 2 min. Relative levels of target gene were determined by using the delta Ct value compared with delta Ct (GAPDH).
Immunofluorescence.
Intestinal tissue was Swiss-rolled before fixing for 4 h in 4% paraformaldehyde. Tissue was incubated overnight in 30% sucrose before freezing in OCT. Tissue was cut into 5-µM sections. Tissue was blocked in PBS-XG (0.1% Triton X-100, 10% goat serum) before incubating overnight in primary antibody in PBS-XG. Tissue was washed and then incubated with secondary antibody for 1 h before DAPI staining. The following primary antibodies are from eBioscience: anti–human/mouse RORγt (clone AFKJS-9) and anti–mouse CD3e (clone 145-2C11). Secondary antibodies are from Jackson ImmunoResearch Laboratories (Cy3-AffiniPure goat anti–rat IgG and goat anti–Armenian hamster). Tissue was imaged using an LSM 710 confocal (Carl Zeiss) and images were processed using ImageJ.
Online supplemental material.
Fig. S1 shows the gating strategy for colonic ILC3. Fig. S2 shows increased production of IL-22 in ILCs from patients with IBD. Fig. S3 shows Lin− gating and surface phenotype for human intestinal ILC3. Fig. S4 shows that TL1A enhances IL-23 and IL-1β induction of IL-22 by intestinal ILC3s.
Acknowledgments
We thank Rachel Brody (NYU Biorepository), Fatiha Chabouni, Priyanka Patel, Ryan Warren and members of The Roberts Center for IBD for help with sample collection as well as the patients that participated in this study. We would like to thank Michael Cammer for assistance with microscopy.
This work was supported by the American Gastroenterological Association Research Foundation (R.S. Longman), National Institutes of Health K08 DK099381 (R.S. Longman), American Cancer Society (G.E. Diehl), NIH T32 CA009161 (G.E. Diehl), K99DK091508 (J.R. Huh), and the Howard Hughes Medical Institute (D.R. Littman).
The authors declare no competing financial interests.
Author contributions: R.S. Longman and G.E. Diehl designed and performed the experiments. R.S. Longman, G.E. Diehl and D.R. Littman planned experiments and wrote the manuscript with input from the co-authors. J.R. Huh, C. Galan, and D.A. Victorio helped plan and perform experiments. E.R. Miraldi and R. Bonneau performed data analysis. A. Swaminath and E.J. Scherl helped with sample collection and experimental design.
References
Author notes
R.S. Longman and G.E. Diehl contributed equally to this paper.
J.R. Huh’s present address is University of Massachusetts Medical School, Worcester, MA 01605.
A. Swaminath’s present address is Lennox Hill Hospital, New York, NY 10075.