Text and Interview by Hema Bashyam
Robert Ménard is stalking the malaria parasite Plasmodium from the moment it invades the skin to find a way to stop its infection cycle.
The most elusive period in Plasmodium's life cycle spans from the moment a human feels the sting of an infected mosquito to the time the victim starts to shiver—a sign that the parasite has infected the red blood cells. During this interval, the bugs are few in number, and they dash to the liver at high speeds.
At the Pasteur Institute in Paris, Robert Ménard spies on the parasites' journey in real-time to figure out how they move and gain access to their various cellular targets. How pathogens sneak into cells is a question that has interested Ménard since the start of his research career. As a Ph.D. student, he identified the proteins that Shigella, the bacterium that causes dysentery, uses to penetrate the gut epithelium (1, 2). Later, as a postdoctoral fellow, Ménard discovered that Plasmodium uses a protein called circumsporozoite to both develop inside the mosquito midgut and to grab on to mammalian liver cells (3).
After starting his own lab in 2001, Ménard developed techniques to track Plasmodium during its odyssey within infected mosquitoes and mice. His discoveries have challenged many presumptions about Plasmodium's early behavior within the host.
Ménard's movies show that injected parasites don't just glide to the liver as previously thought. Some make a detour to lymph nodes—one of the training centers of the immune system (4), while others remain at the bite site (5). Those that make it to the liver shield themselves from macrophages and dendritic cells by clustering within vacuole-like structures called merosomes that eventually bud out into the blood (6). Ménard is now combining genetic tools with his movie-making skills to identify host and parasite genes that direct the parasites' early itinerary within the host.
MOVING INTO MALARIA
Did you grow up wanting to make movies on disease-causing bugs? No, I actually wanted to be a doctor and initially went to medical school to study neurology. But then I went to Asia for two years in the middle of my medical studies to get some field experience. I spent some time working in rural clinics near Pune in India and in a small biology lab in Jakarta, and somewhere along the way, I guess I got bitten by the infectious disease bug.
Not literally, I hope. What happened after you got back to France? Unfortunately, in France it's difficult to do both medical studies and research at the same time, because we don't have the same sort of M.D./Ph.D. programs that you have in the US. After I finished the M.D. part, I wanted to switch to research, but the problem was that I was no scientist. So I ended up as a graduate student in Phillipe Sansonetti's lab at the Pasteur and worked on bacterial dysentery, an infectious disease problem worldwide.
How did your interest in malaria come about? It definitely wasn't a predetermined choice but has more to do with the chaotic nature of life (mine, at least). I wanted to move to New York for personal reasons. Victor Nussenzweig's lab at New York University and his work on malaria was especially attractive for postdoctoral work because I thought malaria was a wonderful opening into the world of parasitology. And although I wouldn't be in that world as a medical doctor, I could still contribute my research expertise to an important global health problem.
MUTATING PARASITES
How did your work in the Nussenzweig lab contribute to the field?
Sporozoites (green) crawl through a mosquito's gut (red arrowhead) toward the salivary gland (white arrowhead).
Why focus on this protein? The entire field was interested in this protein as a vaccine target because it is essential for the parasite's infectivity in mammals and is expressed at a stage when the parasite has not yet infected the red blood cell. So stopping the parasite at this stage of the infection might potentially prevent the triggering of the disease. Because this protein covered the sporozoite surface, we expected to get a mutant sporozoite that lacked this protein. But what we got instead was a parasite that could not form sporozoites at all. That finding led to us showing that this protein was also required for the parasite's development in the mosquito.
And what did you learn about the parasite's motility? We did in vitro work looking at sporozoites slithering around on glass slides to study their motility. Using gene targeting, we found that the parasite relies on a transmembrane protein called TRAP to move with its characteristic gliding motion—a form of locomotion where there's no change in cell shape. But we didn't know anything about sporozoite motility inside the mammalian host.
REAL-TIME SURVEILLANCE
And you moved back to Paris to pursue this question? When my postdoc ended, it was the perfect time for me to go back to the Pasteur, which to me is one of the temples of infectious disease research. At the time, the director, Philippe Kourilsky, was steering the institute toward giving more responsibility to young people and allowing them to head labs. I started putting together a project using time-lapse microscopy techniques and molecular and genetic approaches that I had learned in Victor's lab to dissect the preerythrocytic stage of the parasite life cycle.
Until quite recently, this stage was believed to be very rapid and simple: the sporozites were thought to zip from the skin to the liver, differentiate and multiply inside the hepatocytes, and then escape into the blood. This was thought to be the starting point for all the action, as it was presumed that there was little host–pathogen interaction during the parasites' trip to the liver and that the bugs were invisible to the immune system.
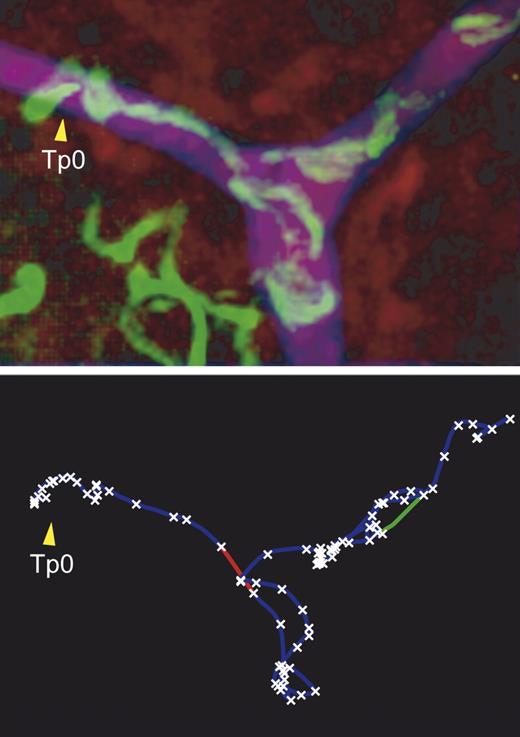
Real-time tracking of sporozoites (green, top) inside blood vessels (purple) reveals a trajectory (bottom) marked by stops (crosses) and high speed moves (lines).
But you've now shown that this phase is actually very complex. We've observed that the sporozoites that enter the skin have many fates and the liver is only one of many potential destinations. We've found that some of the sporozoites lose their motility, stay in the skin, and might differentiate there. Their presence in the skin triggers a great deal of inflammation, but we don't yet understand all the stages of this immune response.
Some of the bugs move as fast as they do in vitro—we've estimated that they move at two to four microns per second—and reach the lymphatic system and infect the draining lymph nodes. The lymph node parasites, however, don't actually develop into the form that can infect erythrocytes.
How does the parasite get away with growing right under the immune system's nose, so to speak? We don't yet know what the bugs are doing inside the lymph nodes. Some of them do interact with the immune cells and some get degraded within hours of the bite, suggesting that the immune system might be attacking them. But the other possibility is that the partially developed parasites are desensitizing the body to their presence and escaping destruction by inducing immune tolerance.
The parasites that complete their maturation in the liver also have a way of leaving the infected cell unnoticed. They enter the liver blood vessels within merosomes, which have budded out of the hepatocytes. The parasites also manipulate host cell membranes by preventing the exposure of danger signals that would attract liver macrophages to the infected cells. I think it's one of the most sophisticated forms of immune evasion. It's much more than just one pathogen gene product modifying a set of genes of the host cell. It's a mechanism that directs a new fate for the host cell.
COUNTER-MEASURES
Do your findings have implications for malaria vaccine development? Ruth Nussenzweig discovered decades ago that injecting irradiated sporozoites, which reach the liver but fail to multiply, induces an immune response that completely wipes out the bugs. This approach is not practical, as it would require producing and maintaining doses of vaccines made of live parasites that can only be grown within mosquitoes. But it tells us that it is possible to arrest this phase of infection.
Our studies suggest that there are several target sites for interfering with the parasite during this stage. We still need to figure out what the parasites in the lymph node and the skin are doing to the immune system—inducing protective immunity or tolerance. But if we find that they are priming the immune system, we might be able to direct all injected parasites to that site by genetically modifying them. To that end, we're currently generating mutants that get stuck in the skin (7).