Inborn errors of immunity (IEIs) comprise a group of heterogeneous diseases with a strong genetic background and a clear clinical predisposition to recurrent infections, multiple autoimmune conditions, hyperinflammation, severe/recalcitrant allergies, and a predisposition to neoplasms. Over the past 10 years, advances in genetic sequencing have allowed the recognized number of IEIs to expand to 565 in the latest International Union of Immunological Societies classification [1]. Epidemiological data from USIDNET in the USA found that 6 in 10,000 individuals may have an IEI with a frequency similar to that of rare diseases [2]. However, data from the LASID registry in Brazil estimated the frequency at 1 in 100,000 individuals [3]. Therefore, there is a need to improve the recognition and diagnosis of IEIs in our country. Moreover, there are several peculiarities in the genetic background of the Brazilian population, where unique findings are highly probable.
1. To establish a national multicenter study to conduct genetic sequencing for the diagnosis of IEIs in Brazil.
2. To extract robust clinical and epidemiological data on pediatric and adult IEIs in Brazil, in order to support public policies for this group of not-so-rare diseases.
3. To improve disease awareness and guarantee continual medical education by conducting periodic “active medical education” sessions with clinical case discussions and itinerant medical symposia in all five Brazilian macro regions.
4. To validate novel causative mutations using ex vivo and in vitro immunological functional assays for lymphocytes and phagocytes.
5. To guarantee adequate training for medical and other health professionals in the proper diagnosis and treatment of IEI patients and their families.
A novel and innovative Brazilian multicenter study is being conducted since November 2024 using the COBEII (National Network for Reference Centers for IEI) in Brazil. Clinical and laboratory data of patients with suspected IEIs of any age and gender have been included in an in-house platform for rare diseases registry (www.doencasraras.org). Genomic DNA was extracted from peripheral blood and sent to the central hub of the network located at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. The whole exome sequencing was performed using Illumina NovaSEQX and the analysis was done using the Emedgene Illumina.
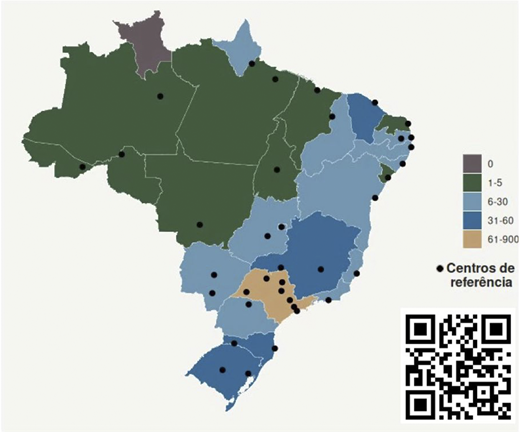
As shown in Figure 1, at least 53 reference centers from across Brazil joined the project so far. Most of these centers are located in the southeast, south, and northeast regions. As expected, there are no specialized centers in the northern and central regions of Brazil. Up to the last visit, 1,221 patient samples were sent to our center for genetic sequencing, and almost half of these have been entered into the online registry (n = 577). There were no gender discrepancies, with 43% (n = 253) of patients being male. The same pattern of patient frequency and geographical distribution was observed in Brazil (see Figure 1). Similar age distribution (< and > 16 years old) could be observed among the first individuals sequenced (n = 231; 41%). Sequencing and analysis started in July 2025 after the NovaSeq X was installed. At least 214 cases have already been sequenced, of which 50 have been analyzed. A definitive/conclusive diagnosis (genotype-related pathogenic/likely pathogenic variants) was found in 38% (n = 19) of cases, most of them were biallelic (recessive fashion), followed by X-linked, as observed in Figure 2. At least four (n = 4; 0,03%) of those analyzed died, all of them children under the age of 6. No novel mutation/gene was yet validated. Disease awareness and continuous medical education was conducted monthly by the CoBEII online meetings and by the Itinerant Symposium in Federal universities of Paraíba, Rio Grande do Norte, Santa Maria, Fronteira Sul, and São Carlos.
Geographical distribution of reference centers and patients with IEIs in Brazil. The black dots indicate the number of centers registered per state, as well as the frequency with which patients are sent for sequencing by state. The scale of individual distribution is on the right and only one state had more than 60 patients (orange: São Paulo). The QR code lists all the centers marked on the map.
Geographical distribution of reference centers and patients with IEIs in Brazil. The black dots indicate the number of centers registered per state, as well as the frequency with which patients are sent for sequencing by state. The scale of individual distribution is on the right and only one state had more than 60 patients (orange: São Paulo). The QR code lists all the centers marked on the map.
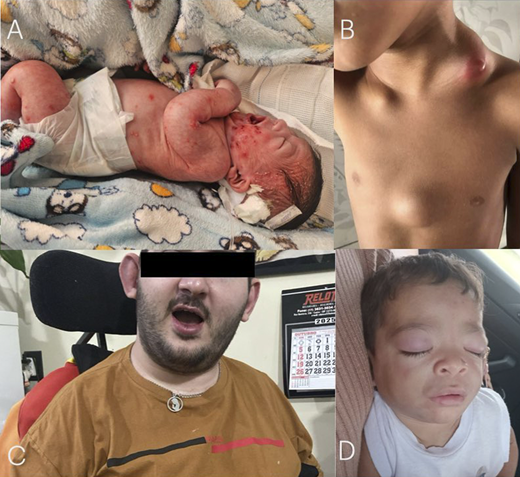
Remarkable diagnosis established by the initial analysis of the CNE3i project. A: Diffuse cutaneous pustulosis affecting a DIRA patient carrying the known homozygous c.213_227del; p.Asp72_Ile76del variant in the IL1RN gene. B: Diffuse and generalized paracoccidioidomycosis lymphadenopathy in a patient with a novel compound heterozygous variant in the DOCK8 gene (c.3209A>G; p.Asn1070Ser and c.2554G>A; p.Val852Met). C: An adult patient with a 24-year history of unexplained fever and severe neurological impairment, who carries a homozygous variant in the RNASEH2B gene (c.529G>A; p.Ala177Thr). D: Erythematous eyelash nodules in a patient with CANDLE syndrome harboring a novel homozygous mutation affecting the PSMB8 gene (c.280G>C; p.Ala94Pro).
Remarkable diagnosis established by the initial analysis of the CNE3i project. A: Diffuse cutaneous pustulosis affecting a DIRA patient carrying the known homozygous c.213_227del; p.Asp72_Ile76del variant in the IL1RN gene. B: Diffuse and generalized paracoccidioidomycosis lymphadenopathy in a patient with a novel compound heterozygous variant in the DOCK8 gene (c.3209A>G; p.Asn1070Ser and c.2554G>A; p.Val852Met). C: An adult patient with a 24-year history of unexplained fever and severe neurological impairment, who carries a homozygous variant in the RNASEH2B gene (c.529G>A; p.Ala177Thr). D: Erythematous eyelash nodules in a patient with CANDLE syndrome harboring a novel homozygous mutation affecting the PSMB8 gene (c.280G>C; p.Ala94Pro).
This work reports the initial findings resulting from a FINEP-funded project to construct a national center for inborn errors of immunity and immunodysregulation (CNE3i) in Brazil, which is open to the public. Although preliminary, the findings already demonstrate the project's significant impact on all regions of Brazil, with a representative number of individuals from across the country undergoing sequencing. Furthermore, we confirm the genetic variability of the Brazilian population due to the novel mutations identified in Figure 2. Finally, we identified another deficiency of the natural antagonist of interleukin-1 antagonist (DIRA) patient harboring the same mutation previously described by our group, suggesting that this variant may have a founder effect for DIRA patients in Brazil. There is a need for continuous medical education and disease awareness for IEIs in Brazil.