A subset of patients with systemic juvenile idiopathic arthritis (sJIA) develop sJIA-associated lung disease (sJIA-LD). Allogeneic hematopoietic stem cell transplant (HSCT) is a treatment option for patients with refractory sJIA and can ameliorate associated lung disease. Infusion of T-replete grafts into sJIA-LD patients who have a high background of preexisting systemic and pulmonary inflammation may contribute to risks of inflammatory complications or graft versus host disease following allogeneic HSCT. We hypothesized that a T cell–depleted approach would achieve durable engraftment while minimizing the risks of GVHD, pulmonary complications, and TRM.
We report our single-center retrospective analysis of 4 pediatric patients with sJIA-LD who underwent allogeneic HSCT with reduced intensity conditioning (RIC) with alemtuzumab (days 14-12), fludarabine (150 mg/2 over days -8 to -4), melphalan (140 mg/m2 on day -3), and thiotepa (200 mg/m2 on day -2) and received either a CD34+-selected (n = 3) or TCR-αβ–depleted (n = 1) peripheral blood stem cell product.
Patient and transplant characteristics are shown in Table 1. All patients had highly refractory disease and were heavily pretreated with a median of 8 (6-12) lines of sJIA-directed therapy. At the time of HSCT, three patients required respiratory support (supplemental O2 in n = 2, overnight BiPAP with supplemental O2 in n = 1).
All patients engrafted with >95% donor chimerism (range, 9-10 days post-HSCT). One patient experienced secondary graft failure on day +43 requiring a second HSCT with a haploidentical parental donor and post-transplant cyclophosphamide (Table 1). Second HSCT was complicated by grade 1 (skin) acute GVHD. None of the other patients developed acute or chronic GVHD or significant pulmonary complications. All had significant improvement in their pulmonary status and were able to discontinue their respiratory support (Figure 1). At a median follow up of 19.8 months (range, 6-36 months), all patients have 100% donor chimerism. None of the patients have had relapse of their sJIA. IL-18 levels declined post-HSCT to near normal levels in all patients (Table 1).
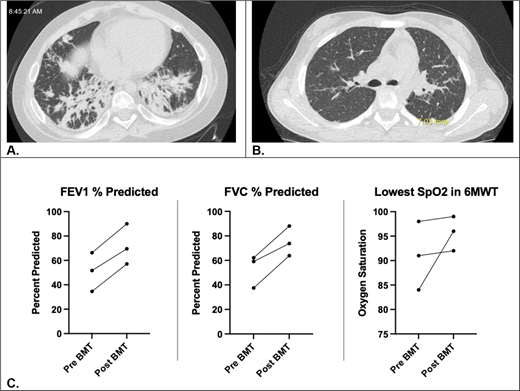
Progress of pulmonary disease pre- and post-HSCT. High-resolution CT chest images of patient #1 pre- (A) and 2.5 years post- (B) HSCT. (C) This shows percent predicted FEV1 and FVC and lowest SpO2 in 6-minute walk test for patients #1-3 (patient 4 unable to reliably perform spirometry).
Progress of pulmonary disease pre- and post-HSCT. High-resolution CT chest images of patient #1 pre- (A) and 2.5 years post- (B) HSCT. (C) This shows percent predicted FEV1 and FVC and lowest SpO2 in 6-minute walk test for patients #1-3 (patient 4 unable to reliably perform spirometry).
Our experience suggests that allogeneic HSCT with a T cell–depleted approach for patients with sJIA-LD offers durable engraftment with low risk of GVHD, pulmonary complications, and TRM.