22q11.2 deletion syndrome has been primarily described as a disorder of T cell production secondary to thymic hypoplasia. However, there is great complexity in the clinical picture with infections, autoimmunity, and inflammation occurring. Emerging evidence suggests that qualitative T cell dysfunction occurs, and the goal of this study was to utilize single-cell RNA-seq to better define altered gene expression patterns that might inform on the processes associated with recurrent infections.
We utilized single-cell RNA-seq to define distinct populations in 22q11.2 deletion syndrome and controls as well as within a subcohort of patients with 22q11.2 deletion syndrome and recurrent infections.
The subcohort of patients with recurrent infections had a higher number of follicular helper T cells (Tfh) and a lower number of class-switched memory B cells (Figure 1). When we analyzed differentially expressed genes, we identified a strong signature of type I interferons across all cell types. Within the T cell compartment and particularly within the Tfh, we noted a strong senescence signature (Figure 2). Nearly every effect observed in T cells was most altered in the patients with recurrent infection. B cells had a less mature composition particularly in those with recurrent infections.
Subcohort of 22q11.2 DS patients with recurrent infections had a higher number of Tfh cells (1A), and a lower number of class-switched memory B cells (1B) compared to patients without recurrent infections.
Subcohort of 22q11.2 DS patients with recurrent infections had a higher number of Tfh cells (1A), and a lower number of class-switched memory B cells (1B) compared to patients without recurrent infections.
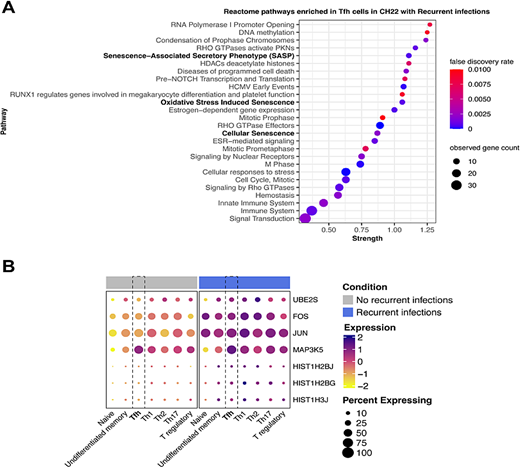
Differentially expressed modules within the Tfh cells. 2A) The Reactome modules are displayed comparing differentially expressed genes in Tfh in patient with recurrent infection vs. those without. We noted three senescence modules (bolded) and multiple RHO GTPase modules. 2B) We combined the three senescence modules and portrayed the expression according to whether the patients had a history of recurrent infections. In this dotplot, the highest expression levels were seen in the patients with recurrent infections across all T cell subsets.
Differentially expressed modules within the Tfh cells. 2A) The Reactome modules are displayed comparing differentially expressed genes in Tfh in patient with recurrent infection vs. those without. We noted three senescence modules (bolded) and multiple RHO GTPase modules. 2B) We combined the three senescence modules and portrayed the expression according to whether the patients had a history of recurrent infections. In this dotplot, the highest expression levels were seen in the patients with recurrent infections across all T cell subsets.
While the total T cell numbers can often normalize in patients with 22q11.2 deletion syndrome, the subcohort with recurrent infections had a lower number of class-switched memory B cells despite having a higher number of Tfh cells, suggesting a compensatory increase. Our data indicate significantly altered function as defined by differentially expressed genes and aligned with what is known about T cell senescence. This would be expected to impact T cell function and may account for ongoing symptoms, reduced B cell maturation, and possibly the risk of immune dysregulation.