Interleukin-7 (IL-7) is a cytokine required for T cell development and homeostasis. IL-7 signal transduction is mediated by STAT5 phosphorylation and results in cell proliferation and anti-apoptotic effects. While genetic deficiencies in any of the components of IL-7 receptor (IL-7Rα or γ-chain) result in severe combined immunodeficiency, the recently discovered autosomal recessive IL-7 cytokine deficiency presents with a less severe phenotype characterized by T cell lymphopenia, recurrent HPV diseases complicated by cutaneous squamous cell carcinomas, and other opportunistic infections. NT-I7 is a long-acting human recombinant IL-7, and we hypothesize that treatment of patients with autosomal recessive IL-7 deficiency with NT-I7 will result in T cell expansion, regression of HPV-related diseases, and prevention of HPV-related cancers and opportunistic infections.
Peripheral blood mononuclear cells (PBMCs) from 2 patients with IL-7 deficiency were studied. Flow cytometric assays were used to evaluate the integrity of the IL-7 signaling axis and its downstream effect on T cell survival and proliferation by measuring the expression of CD127 (IL-7Rα), STAT5-phosphorylation, and BCL-2 and Ki-67 expression in response to IL-7 stimulation.
Patients with IL-7 deficiency were found to have preserved expression of CD127 on CD4+ but not on CD8+ T cells. Accordingly, STAT5-phosphorylation in response to IL-7 stimulation was reduced in CD8+ T cells compared with healthy subjects. Nevertheless, prolonged ex vivo IL-7 stimulation increased BCL2 and Ki67 expression in both CD4+ and CD8+ T cells to a level comparable with that observed in healthy subjects. Furthermore, a preferential proliferation of CD31+ CD4+ naive T cells was noted. These ex vivo data supported the development of an investigational new drug expanded access clinical protocol using NT-I7, which was designed, approved, and launched. NT-I7 will be given up to 5 injections: 12 weeks apart for the first 3 doses, then every 24 weeks for the final 2 doses, allowing for dose escalation based on safety and clinical response.
The ex vivo anti-apoptotic and proliferative effect of IL-7 on T cells from patients with IL-7 deficiency raises the promising possibility that NT-I7 will have a similar effect in vivo in this as well as other inborn errors of immunity associated with impaired T cell homeostasis.
Baseline CD127 expression, phosphorylated STAT5 and BCL2 signal transduction with IL-7 stimulation, and T cell proliferation assays in IL-7–deficient patients as compared with healthy controls. (a) Expression of CD127 on CD4+ and CD8+ T cells. (b) Phosphorylated STAT5 percent in response to stimulation with IL-7 (1 ng/ml). (c) BCL2 expression after incubation of CD4+ and CD8+ T cells with IL-7. (d) CD4+ and CD8 + T cell proliferation in response to IL-7 incubation as measured by Ki67 expression. (e) Proliferation of naive CD4+ T cells in response to IL-7 incubation as measured by Ki67 expression.
Baseline CD127 expression, phosphorylated STAT5 and BCL2 signal transduction with IL-7 stimulation, and T cell proliferation assays in IL-7–deficient patients as compared with healthy controls. (a) Expression of CD127 on CD4+ and CD8+ T cells. (b) Phosphorylated STAT5 percent in response to stimulation with IL-7 (1 ng/ml). (c) BCL2 expression after incubation of CD4+ and CD8+ T cells with IL-7. (d) CD4+ and CD8 + T cell proliferation in response to IL-7 incubation as measured by Ki67 expression. (e) Proliferation of naive CD4+ T cells in response to IL-7 incubation as measured by Ki67 expression.
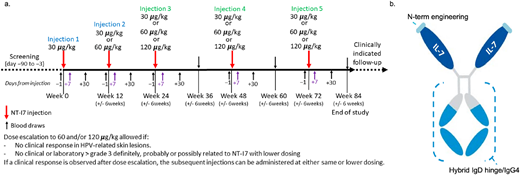
Timeline of expanded access protocol screening, NT-I7 dosing, and laboratory monitoring. (a) Timeline of expanded access protocol screening, NT-I7 dosing, and laboratory monitoring. (b) Structure of NT-I7 demonstrating N terminus human IL-7 fused to a hyFc long-acting platform that combines the hinge flexibility of IgD and recycling of IgG4 to minimize protein–protein interactions and increase serum half-life.
Timeline of expanded access protocol screening, NT-I7 dosing, and laboratory monitoring. (a) Timeline of expanded access protocol screening, NT-I7 dosing, and laboratory monitoring. (b) Structure of NT-I7 demonstrating N terminus human IL-7 fused to a hyFc long-acting platform that combines the hinge flexibility of IgD and recycling of IgG4 to minimize protein–protein interactions and increase serum half-life.