Neuroblastoma (NB), the most frequent solid tumor of early childhood, is diagnosed as a disseminated disease in >60% of cases, and several lines of evidence support the resistance to apoptosis as a prerequisite for NB progression. We show that autocrine production of netrin-1, a multifunctional laminin-related molecule, conveys a selective advantage in tumor growth and dissemination in aggressive NB, as it blocks the proapoptotic activity of the UNC5H netrin-1 dependence receptors. We show that such netrin-1 up-regulation is a potential marker for poor prognosis in stage 4S and, more generally, in NB stage 4 diagnosed infants. Moreover, we propose that interference with the netrin-1 autocrine loop in malignant neuroblasts could represent an alternative therapeutic strategy, as disruption of this loop triggers in vitro NB cell death and inhibits NB metastasis in avian and mouse models.
Dependence receptors now number more than a dozen, including deleted in colorectal cancer (DCC) (1), UNC5H (2), Patched (3), some integrins (4), neogenin (5), p75NTR (6), RET (7), ALK (8), and TrkC (9). Although they have no structural homology (other than possibly in a domain referred to as the DART [dependence-associated receptor transmembrane] domain) (10), they all share the functional property of inducing cell death when disengaged from their trophic ligands, whereas the presence of their trophic ligands blocks this proapoptotic activity. Such receptors thus create cellular states of dependence on their respective ligands (11, 12).
The prototype dependence receptors are the netrin-1 receptors. Netrin-1, a diffusible laminin-related protein, has been shown to play a major role in the control of neuronal navigation during the development of the nervous system by interacting with its main receptors, DCC (13, 14, 15) and UNC5H (16, 17). However, DCC and UNC5H (i.e., UNC5H1, UNC5H2, UNC5H3, and UNC5H4) have been shown to belong to the dependence receptor family (1, 2). This dependence effect upon netrin-1 has been suggested to act as a mechanism for eliminating tumor cells that would develop in settings of ligand unavailability (for reviews see references 18, 19). Along this line, disruption of the proapoptotic signaling of these netrin-1 receptors in the gastrointestinal tracts of mice, by netrin-1 overexpression or by inactivation of UNC5H3, is associated with intestinal tumor progression (20, 21).
Thus, loss of the dependence receptors' proapoptotic activity represents a selective advantage for tumor cells. In this respect, DCC was proposed in the early 1990s to function as a tumor suppressor gene, whose expression is lost in the vast majority of human cancers (22, 23). This hypothesis also fits with the observation that UNC5H genes are down-regulated in most colorectal tumors, hence suggesting that loss of UNC5H genes represents a selective advantage for tumor development (21, 24, 25). We have analyzed expression of netrin-1 and its receptors in neuroblastoma (NB), the most frequent extracranial solid tumor of early childhood. The aggressive and metastatic stage 4 NB displays three distinct clinical patterns at presentation and dissemination sites based on patients' ages. Indeed, neonates and infants (<1 yr of age) with stage 4S and stage 4 without 4S features have an overall good prognosis, whereas stage 4 in children (>1 yr of age) shows a poor prognosis. We describe in this paper that, rather than the loss of netrin-1 receptor expression, a large fraction of aggressive NBs has evolved to select a gain of ligand expression that apparently represents a similar selective growth advantage. We therefore propose to use disruption of this selective advantage as an anticancer strategy in NB.
RESULTS
Netrin-1 is up-regulated in a large fraction of aggressive NB
We focused on stage 4 NB with a specific interest in comparing netrin-1 and its receptors' expression levels between the three distinct clinical patterns of stage 4, based on disease distribution and age of the patients (26). On the one hand, there are the neonates and infants (<1 yr of age) with stage 4S (2–5% of all NB) and the similarly young stage 4 without 4S features, hereafter termed [1yr−] stage 4, who make up 10% of the NB population. On the other hand, there are the stage 4 children (>1 yr of age), comprising 45% of all NBs, who will hereafter be termed [1yr +] stage 4. These three clinical aspects of stage 4 NB differ in their respective malignant behaviors and associated prognoses: good for stage 4S and [1yr −] stage 4 (5-yr event-free survival >80%), and dismal for [1yr +] stage 4 (5-yr event-free survival of ∼30%) despite intensive treatment including high-dose chemotherapy and hematopoietic stem cell transplantation (27, 28).
We first analyzed the expression of netrin-1 and its dependence receptors, DCC, UNC5H1, UNC5H2, UNC5H3, and UNC5H4, by quantitative RT-PCR (Q-RT-PCR) in a panel of 102 stage 4 NB tumors including 24 stage 4S and 12 [1yr−] stage 4. As shown in Fig. 1 A, netrin-1 is up-regulated in [1yr+] stage 4 as compared with both stage 4S (P < 0.05) and [1yr−] stage 4 (P < 0.01). Similar results were obtained when comparing netrin-1 protein level by immunohistochemistry (Fig. 1 B and quantification in Fig. S1 A). Interestingly, netrin-1 is detected mainly in tumor cells and is barely detected in stroma cells (Fig. 1 B and Fig. S1 B). Conversely, netrin-1 dependence receptor expression analysis showed that DCC was only weakly expressed in the different stage 4 NB (Fig. S1 C) as reported (29), whereas UNC5H1, UNC5H2, UNC5H3, and UNC5H4 expression showed no significant differences when comparing [1yr−] versus [1yr+] stage 4 (Fig. 1 C). However, we observed that the different UNC5H receptors are up-regulated specifically in stage 4S (mean increase in UNC5H expression in stage 4S vs. other stage 4 NBs: 2.98-fold, P < 0.007), suggesting UNC5H receptors as hallmarks of stage 4S NB. The UNC5H1 and UNC5H4, which show the highest messenger RNA (mRNA) expression, could also be seen at the protein level by immunohistochemistry (Fig. 1 D).
Netrin-1 up-regulation is detected in aggressive NB. (A) Netrin-1 mRNA levels in 102 stage 4S (n = 24), [1yr −] stage 4 (n = 12), and [1yr+] stage 4 (n= 66) NBs measured by Q-RT-PCR. HPRT housekeeping gene was used as a control. Mean netrin-1 mRNA expression value for each subgroup is indicated by an “m” value. Mean netrin-1 mRNA levels in stage 4S and [1yr−] stage 4 were, respectively, compared with the mean netrin-1 detected in [1yr+] stage 4. The data were statistically determined using Student's t test compared with levels of [1yr+] stage 4. *, P < 0.05; **, P < 0.01. Each sample was assessed in two independent experiments. (B) Representative netrin-1 immunohistochemistry on one [1yr+] stage 4 and one stage 4S tumor. Insets show control without primary antibody. Bars, 50 µm. T, tumor cells; S, stromal cells. Netrin-1 antibody specificity is further shown in Fig. 2 D and Fig. S1 B. Immunohistochemistry was performed on four [1yr+] stage 4 and four stage 4S tumors. (C) Mean UNC5H mRNA levels in the different stage 4 NBs. Q-RT-PCR using UNC5H1–4-specific primers was performed. Mean UNC5H1–4 mRNA levels in [1yr−] stage 4 and [1yr+] stage 4 were, respectively, compared with the mean UNC5H1–4 levels detected in stage 4S. Error bars indicate SEM. The data were statistically determined using Student's t test compared with levels of stage 4S. *, P < 0.05; **, P < 0.01. Samples were analyzed in duplicates for each gene. (D) Representative UNC5H1 and UNC5H4 immunohistochemistries on [1yr+] stage 4 and stage 4S tumors. Insets show control without primary antibody. Bars, 50 µm. Immunohistochemistry was performed on four stage 4 [1yr+] and four stage 4S tumors. (E) Netrin-1 up-regulation is a marker of poor prognosis in stage 4S NB. Overall survival in a panel of 24 infants diagnosed with stage 4S NB with primary tumors showing either netrin-1–low (gray) or netrin-1–high (black) levels. The data was statistically determined using the Kaplan-Meier method. P-value is indicated. (F) Netrin-1 up-regulation is a marker of poor prognosis in infants with NB. Data are presented as in E, with a panel of 30 infants bearing NB.
Netrin-1 up-regulation is detected in aggressive NB. (A) Netrin-1 mRNA levels in 102 stage 4S (n = 24), [1yr −] stage 4 (n = 12), and [1yr+] stage 4 (n= 66) NBs measured by Q-RT-PCR. HPRT housekeeping gene was used as a control. Mean netrin-1 mRNA expression value for each subgroup is indicated by an “m” value. Mean netrin-1 mRNA levels in stage 4S and [1yr−] stage 4 were, respectively, compared with the mean netrin-1 detected in [1yr+] stage 4. The data were statistically determined using Student's t test compared with levels of [1yr+] stage 4. *, P < 0.05; **, P < 0.01. Each sample was assessed in two independent experiments. (B) Representative netrin-1 immunohistochemistry on one [1yr+] stage 4 and one stage 4S tumor. Insets show control without primary antibody. Bars, 50 µm. T, tumor cells; S, stromal cells. Netrin-1 antibody specificity is further shown in Fig. 2 D and Fig. S1 B. Immunohistochemistry was performed on four [1yr+] stage 4 and four stage 4S tumors. (C) Mean UNC5H mRNA levels in the different stage 4 NBs. Q-RT-PCR using UNC5H1–4-specific primers was performed. Mean UNC5H1–4 mRNA levels in [1yr−] stage 4 and [1yr+] stage 4 were, respectively, compared with the mean UNC5H1–4 levels detected in stage 4S. Error bars indicate SEM. The data were statistically determined using Student's t test compared with levels of stage 4S. *, P < 0.05; **, P < 0.01. Samples were analyzed in duplicates for each gene. (D) Representative UNC5H1 and UNC5H4 immunohistochemistries on [1yr+] stage 4 and stage 4S tumors. Insets show control without primary antibody. Bars, 50 µm. Immunohistochemistry was performed on four stage 4 [1yr+] and four stage 4S tumors. (E) Netrin-1 up-regulation is a marker of poor prognosis in stage 4S NB. Overall survival in a panel of 24 infants diagnosed with stage 4S NB with primary tumors showing either netrin-1–low (gray) or netrin-1–high (black) levels. The data was statistically determined using the Kaplan-Meier method. P-value is indicated. (F) Netrin-1 up-regulation is a marker of poor prognosis in infants with NB. Data are presented as in E, with a panel of 30 infants bearing NB.
In an attempt to correlate netrin-1 up-regulation with the molecular signature of these tumors, we compared netrin-1 up-regulation and DCC/UNC5H1 levels to the profile of gene expression performed in a small panel of nine stage 4 NBs (30). We failed to detect any correlation between netrin-1 up-regulation or DCC/UNC5H1 levels with the molecular signature of apoptosis or invasion effectors (Fig. S1 D). Considering patients' outcomes, although 38% of [1yr+] stage 4 NBs have selected up-regulation of netrin-1, this event failed to be significantly associated with poor outcome in this aggressive form of the disease (unpublished data). Moreover no association between netrin-1 up-regulation and MYCN amplification (MNA) was detected (unpublished data). Thus, netrin-1 up-regulation may be considered as an additional component of the genetic complexity that these tumors display.
Despite a largely favorable prognosis among infants with stage 4 NB (i.e, stage 4S and [1yr−] stage 4) with no MNA, many succumb to the disease. Thus, we assessed whether netrin-1 expression may serve as a prognostic marker for the infants with stage 4 NB. As shown in Fig. 1 E, the overall survival of infants with stage 4S differed markedly based on whether the tumor displayed high levels of netrin-1 expression (netrin-1 high) or low-level expression (netrin-1 low), with the netrin-1 expression threshold being its median expression value in the 102 cases. Indeed, although 100% of the infants survived after 10 yr (including 1 MNA out of 17), when the NB 4S was netrin-1 low, the 5-yr overall survival was only 46% when the NB 4S was netrin-1 high (P = 0.0109). Furthermore, 43% of the non-MNA patients with high-level netrin-1 expression died. More generally, when a similar overall survival analysis was performed on all infants with stage 4 NB (i.e, stage 4S and [1yr−] stage 4), a similar dichotomy was observed. Indeed, 5-yr overall survival was found to be 90% for the netrin-1–low infants yet only 48% for netrin-1–high infants (P = 0.032; Fig. 1 F). These data suggest that netrin-1 is a potential prognostic marker for aggressiveness in stage 4 NB diagnosed in infants. Whether or not it constitutes an independent prognostic marker of stage 4 NB in neonates and infants deserves to be tested in a larger patient cohort. Nevertheless, these data indicate that a netrin-1 threshold may turn as an alternative determinant for the biological behavior of stage 4 NB in infants, potentially suggesting its involvement in a cell death process of very early childhood neuroblasts, reminiscent of that operating during nervous system development (31).
Netrin-1 high expression is not only detected in 38% of [1yr+] stage 4 and in poor outcome [1yr−] stage 4 primary NB tumors but also in a fraction of NB cell lines mainly derived from stage 4 tumor material (Fig. 2 A and Fig. S2 A). Two human NB cell lines, IMR32 (netrin-1 high) and CLB-Ge2 (netrin-1 low), were evaluated further. In spite of a marked difference in netrin-1 and DCC expression, the UNC5H levels are similar in IMR32 and CLB-Ge2 cells; UNC5H1, UNC5H3, and UNC5H4 show the highest expression (Fig. 2 B). Specifically, UNC5H1, UNC5H3, and UNC5H4 proteins could be detected at the plasma membrane by confocal analysis (Fig. 2 C). To test the hypothesis that the high netrin-1 mRNA levels detected in IMR32 cells are associated with an autocrine netrin-1 production, we next performed netrin-1 immunohistochemistry on IMR32 and CLB-Ge2 cells. As shown in Fig. 2 D, a netrin-1–specific membrane staining was detected in a homogeneous pattern in IMR32 cells, whereas no specific staining was detected for CLB-Ge2 cells. Confocal analysis further confirmed the presence of netrin-1 at the cell membrane (Fig. 2 E and Fig. S2 B). To further analyze whether netrin-1 is secreted from IMR32 cells, netrin-1 ELISA assay was used to detect netrin-1 in the conditioned medium. As shown in Fig. 2 F, 11.7 ng/ml netrin-1 was recovered from the conditioned medium of IMR32 cells, whereas no netrin-1 was detected from the conditioned medium of CLB-Ge2 cells. Thus, together these data suggest that the high netrin-1 content observed in aggressive NB could result from an autocrine expression of netrin-1 in NB cells.
Netrin-1 up-regulation is detected in NB cell lines. (A) Netrin-1 expression measured by Q-RT-PCR in a panel of 28 NB cell lines. HPRT housekeeping gene was used as a control. The netrin-1 level is indicated as follows: −, not detectable; −/+, barely detectable; + to ++++, moderate to very high expression. Mouse cell lines are in italics. Cell lines outlined and highlighted in grey are, respectively, netrin-1–low and netrin-1–high cell lines further used in the experiments. (B) Netrin-1 receptor expression in IMR32 and CLB-Ge2 cell lines. DCC/UNC5H Q-RT-PCR was performed on netrin-1–expressing (IMR32) or netrin-1–low (CLB-Ge2) cells using specific primers. Ratio of netrin-1 and netrin-1 receptor expression to the HPRT housekeeping gene is presented. (C) Confocal analysis of UNC5H1, UNC5H3, and UNC5H4 receptor immunostaining in human IMR32 cells. Left and right correspond to IMR32 cells transfected with scramble siRNA and specific siRNA UNC5H, respectively. A fluorescence intensity profile corresponding to the white dashed bar is presented under each panel. Bars, 10 µm. (D) Immunostaining on human IMR32 and CLB-Ge2 cell lines using netrin-1 antibody. Bottom insets show control without primary antibody. Top inset: antibody specificity was tested by adding human recombinant netrin-1. Bars, 50 µm. (E) Confocal analysis of netrin-1 immunostaining on IMR32 cells. A fluorescence intensity profile corresponding to the white dashed bar is presented below. Bar, 5 µm. (F) Quantification of netrin-1 protein secreted in IMR32 and CLB-Ge2 cells conditioned medium by sandwich ELISA assay. Quantification in ng/ml was made according to a dose curve done with recombinant human netrin-1. Data are means of three independent experiments. Error bars indicate SEM. *, P < 0.05 using a two-sided Mann-Whitney test compared with level in IMR32 cells. (G) Quantification of NTN1 and MYCN genomic DNA compared with control NAGK genomic DNA by PCR, using genomic DNA specific primers for each gene, in IMR32 and CLB-Ge2 cells. (H) Quantification of NTN1 promoter activity in IMR32 and CLB-Ge2 cells. Both cell lines were transfected with the vector pGL3-NetP-Luc encoding luciferase under the control of NTN1 promoter. Data presented are normalized on luciferase activity in cells transfected with pGL3 empty vector. Data are means of four independent experiments. Error bars indicate SEM. *, P < 0.05 using a two-sided Mann-Whitney test compared with levels in IMR32 cells.
Netrin-1 up-regulation is detected in NB cell lines. (A) Netrin-1 expression measured by Q-RT-PCR in a panel of 28 NB cell lines. HPRT housekeeping gene was used as a control. The netrin-1 level is indicated as follows: −, not detectable; −/+, barely detectable; + to ++++, moderate to very high expression. Mouse cell lines are in italics. Cell lines outlined and highlighted in grey are, respectively, netrin-1–low and netrin-1–high cell lines further used in the experiments. (B) Netrin-1 receptor expression in IMR32 and CLB-Ge2 cell lines. DCC/UNC5H Q-RT-PCR was performed on netrin-1–expressing (IMR32) or netrin-1–low (CLB-Ge2) cells using specific primers. Ratio of netrin-1 and netrin-1 receptor expression to the HPRT housekeeping gene is presented. (C) Confocal analysis of UNC5H1, UNC5H3, and UNC5H4 receptor immunostaining in human IMR32 cells. Left and right correspond to IMR32 cells transfected with scramble siRNA and specific siRNA UNC5H, respectively. A fluorescence intensity profile corresponding to the white dashed bar is presented under each panel. Bars, 10 µm. (D) Immunostaining on human IMR32 and CLB-Ge2 cell lines using netrin-1 antibody. Bottom insets show control without primary antibody. Top inset: antibody specificity was tested by adding human recombinant netrin-1. Bars, 50 µm. (E) Confocal analysis of netrin-1 immunostaining on IMR32 cells. A fluorescence intensity profile corresponding to the white dashed bar is presented below. Bar, 5 µm. (F) Quantification of netrin-1 protein secreted in IMR32 and CLB-Ge2 cells conditioned medium by sandwich ELISA assay. Quantification in ng/ml was made according to a dose curve done with recombinant human netrin-1. Data are means of three independent experiments. Error bars indicate SEM. *, P < 0.05 using a two-sided Mann-Whitney test compared with level in IMR32 cells. (G) Quantification of NTN1 and MYCN genomic DNA compared with control NAGK genomic DNA by PCR, using genomic DNA specific primers for each gene, in IMR32 and CLB-Ge2 cells. (H) Quantification of NTN1 promoter activity in IMR32 and CLB-Ge2 cells. Both cell lines were transfected with the vector pGL3-NetP-Luc encoding luciferase under the control of NTN1 promoter. Data presented are normalized on luciferase activity in cells transfected with pGL3 empty vector. Data are means of four independent experiments. Error bars indicate SEM. *, P < 0.05 using a two-sided Mann-Whitney test compared with levels in IMR32 cells.
As a first approach to apprehend the mechanisms leading to netrin-1 up-regulation in aggressive NB, we analyzed whether netrin-1 gene (NTN1) is amplified in IMR32 cells. As shown in Fig. 2 G, although MYCN was amplified both in IMR32 and CLB-Ge2 cells compared with the NAGK control gene, the NTN1 gene was not found to be amplified in these two cell lines. We then analyzed whether the increase in netrin-1 expression could be caused by a differential netrin-1 promoter activation. A luciferase reporter gene fused to netrin-1 promoter (32) was then transfected into IMR32 or CLB-Ge2 cells, and luciferase activity was reported to an internal control in each cell line. As shown in Fig. 2 H, netrin-1 promoter activity was 13.8-fold higher in IMR32 cells than in CLB-Ge2 cells, thus supporting the view that netrin-1 up-regulation in NB is related to a gain in netrin-1 promoter activation.
Netrin-1 up-regulation is a selective advantage for NB cell survival
To investigate whether the netrin-1 autocrine expression observed in IMR32 cells provides a selective advantage for survival, as would be expected from the dependence receptor theory, cell death was analyzed in response to the disruption of this autocrine loop. As a first approach, netrin-1 was down-regulated by RNA interference. As shown in Fig. 3 A, the addition of netrin-1 small interfering RNA (siRNA) to IMR32 cells was associated with a significant reduction in netrin-1 mRNA. This mRNA reduction was associated with a decrease of netrin-1 protein as observed by immunohistochemistry (Fig. 3 B). Although scramble siRNA failed to affect IMR32 cell survival, as measured by trypan blue exclusion, netrin-1 siRNA treatment was associated with IMR32 cell death (Fig. 3 C). In contrast, CLB-Ge2 cell survival was unaffected after netrin-1 siRNA treatment (Fig. 3 C). To determine whether this increase in cell death was in part caused by an increase in apoptotic cell death, caspase-3 activity was measured in response to netrin-1 siRNA treatment. As shown in Fig. 3 D, although significant apoptotic cell death was detected upon netrin-1 siRNA treatment in IMR32 cells, no such effect was observed in CLB-Ge2 cells. A similar proapoptotic effect of the netrin-1 siRNA was observed in CLB-VolMo cells, another netrin-1 high cell line (unpublished data).
Down-regulation of netrin-1 autocrine loop by siRNA triggers NB tumor cell death. (A) Analysis of netrin-1 expression using Q-RT-PCR in nontransfected (control) IMR32 cell line or 24 h after transfection with scramble siRNA (siRNA scr.) or with netrin-1 siRNA (siRNA net.). Data are means of three independent experiments. Error bars indicate SEM. *, P < 0.05 using a two-sided Mann-Whitney test compared with levels in control. (B) Immunostaining on IMR32 cell line using netrin-1 antibody in the absence of transfection (control) or 24 h after transfection with scramble siRNA or netrin-1 siRNA. Note that the general caspase inhibitor z-VAD-fmk was added to avoid cell death induced by netrin-1 siRNA. Insets show control without primary antibody. Bars, 50 µm. (C and D) Cell death induction in IMR32 and CLB-Ge2 cell lines was quantified in nontransfected cells (control) or after transfection with either scramble siRNA or netrin-1 siRNA using trypan blue exclusion assay (C) or relative caspase-3 activity assay (D). Data are means of four independent experiments. In C and D, error bars indicate SEM. *, P < 0.05 calculated using a two-sided Mann-Whitney test compared with level of control.
Down-regulation of netrin-1 autocrine loop by siRNA triggers NB tumor cell death. (A) Analysis of netrin-1 expression using Q-RT-PCR in nontransfected (control) IMR32 cell line or 24 h after transfection with scramble siRNA (siRNA scr.) or with netrin-1 siRNA (siRNA net.). Data are means of three independent experiments. Error bars indicate SEM. *, P < 0.05 using a two-sided Mann-Whitney test compared with levels in control. (B) Immunostaining on IMR32 cell line using netrin-1 antibody in the absence of transfection (control) or 24 h after transfection with scramble siRNA or netrin-1 siRNA. Note that the general caspase inhibitor z-VAD-fmk was added to avoid cell death induced by netrin-1 siRNA. Insets show control without primary antibody. Bars, 50 µm. (C and D) Cell death induction in IMR32 and CLB-Ge2 cell lines was quantified in nontransfected cells (control) or after transfection with either scramble siRNA or netrin-1 siRNA using trypan blue exclusion assay (C) or relative caspase-3 activity assay (D). Data are means of four independent experiments. In C and D, error bars indicate SEM. *, P < 0.05 calculated using a two-sided Mann-Whitney test compared with level of control.
Interference with netrin-1 triggers UNC5H-induced apoptosis in NB cells
As a second approach, we looked for a compound that could interfere with the netrin-1 ability to block DCC/UNC5H proapoptotic activity. We recently reported that the fifth fibronectin type III domain of DCC (DCC-5Fbn; Fig. 4 A), which is located in the DCC ectodomain, interacts with netrin-1 and blocks the ability of netrin-1 to trigger multimerization of DCC and UNC5H receptors. Because multimerization inhibits DCC or UNC5H-induced cell death (unpublished data), DCC-5Fbn antagonizes netrin-1 function, disrupting netrin-1–mediated inhibition of DCC/UNC5H proapoptotic activity. Thus, DCC-5Fbn acts as a trap for netrin-1 survival function. As shown in Fig. 4 (B–D), the addition of DCC-5Fbn, but not the unrelated protein IL3R, triggered IMR32 apoptotic cell death as measured by trypan blue exclusion (Fig. 4 B), caspase-3 activity assay (Fig. 4 C), and terminal deoxynucleotidyl transferase–mediated dUTP-biotin nick end labeling (TUNEL) staining (Fig. 4 D). This effect was specific for netrin-1 inhibition because DCC-5Fbn had no effect on CLB-Ge2 cells, and the addition of netrin-1 ultimately reversed the DCC-5Fbn–induced IMR32 apoptotic cell death (Fig. 4, B–D). Similar results were obtained with the CLB-VolMo cells (Fig. S2 D). To determine whether the ability of DCC-5Fbn to kill NB cells is restricted to established NB cell lines, a surgical biopsy from a tumor with high netrin-1 level (unpublished data) was semidissociated and further incubated with DCC-5Fbn. As shown in Fig. 4 E, DCC-5Fbn triggered cell death as measured by caspase-3 activation, demonstrating that in vitro, disruption of the netrin-1 autocrine loop is associated with NB cell death.
Disruption of netrin-1 autocrine loop by a decoy receptor fragment triggers NB tumor cell death. (A) Scheme representing netrin-1 and its receptors DCC and UNC5H and the fifth fibronectin type III domain of DCC (DCC-5Fbn) used to induce cell death. The downstream effector DAPK implicated in UNC5H-induced cell death is also represented. (B–D) Quantitative analysis of cell death in IMR32 and CLB-Ge2 cell lines treated with 1 µg/ml DCC-5Fbn, with or without addition of netrin-1 in excess (150 ng/ml) to reverse the effect of DCC-5Fbn. A negative control was also performed by adding an unrelated IL3R peptide produced in the same condition as DCC-5Fbn. Cell death was quantified by trypan blue exclusion assay (B) while apoptosis was monitored by measuring relative caspase-3 activity (C) or by TUNEL staining (D). Bars, 100 µm. In D, TUNEL staining was performed on three independent experiments. (E) Effect of DCC-5Fbn on fresh [1yr+] stage 4 NB. Tumoral cells were directly resuspended from the surgical punction and were plated for 24 h in presence of treatment. In B and C, data are means of six independent experiments. In E, data are means of two independent experiments. Error bars indicate SEM. **, P < 0.01 calculated using a two-sided Mann-Whitney test compared with level of control.
Disruption of netrin-1 autocrine loop by a decoy receptor fragment triggers NB tumor cell death. (A) Scheme representing netrin-1 and its receptors DCC and UNC5H and the fifth fibronectin type III domain of DCC (DCC-5Fbn) used to induce cell death. The downstream effector DAPK implicated in UNC5H-induced cell death is also represented. (B–D) Quantitative analysis of cell death in IMR32 and CLB-Ge2 cell lines treated with 1 µg/ml DCC-5Fbn, with or without addition of netrin-1 in excess (150 ng/ml) to reverse the effect of DCC-5Fbn. A negative control was also performed by adding an unrelated IL3R peptide produced in the same condition as DCC-5Fbn. Cell death was quantified by trypan blue exclusion assay (B) while apoptosis was monitored by measuring relative caspase-3 activity (C) or by TUNEL staining (D). Bars, 100 µm. In D, TUNEL staining was performed on three independent experiments. (E) Effect of DCC-5Fbn on fresh [1yr+] stage 4 NB. Tumoral cells were directly resuspended from the surgical punction and were plated for 24 h in presence of treatment. In B and C, data are means of six independent experiments. In E, data are means of two independent experiments. Error bars indicate SEM. **, P < 0.01 calculated using a two-sided Mann-Whitney test compared with level of control.
We next investigated whether netrin-1 autocrine expression in NB cells acts as a general cell survival factor, i.e., whether it has a trophic effect similar to that of neurotrophins, or whether it specifically inhibits death induced by netrin-1 dependence receptors. IMR32 cells were transfected with either a dominant-negative mutant for DCC (DN-DCC) or UNC5H (DN-UNC5H) proapoptotic activity. These dominant-negative mutants of dependence receptors actually encode the intracellular domain of these receptors mutated in their caspase cleavage sites, and these mutants have been shown both in vitro and in vivo to specifically block the proapoptotic activity of their wild-type counterparts (3, 9, 33). Cell death was then analyzed after netrin-1 inhibition by siRNA. Although DN-DCC expression failed to inhibit netrin-1 siRNA-induced IMR32 cell death, expression of DN-UNC5H rendered IMR32 cells resistant to netrin-1 siRNA (Fig. 5 A). To more formally exclude the role in this death process of DCC or of altered forms of DCC that are known to be expressed in IMR32 cells (Fig. S2 C) (34), DCC was down-regulated by a siRNA approach and cell death was induced via netrin-1 siRNA. As shown in Fig. 5 B, DCC siRNA had no effect on cell death per se and failed to inhibit netrin-1 siRNA-induced caspase-3 activation, strengthening the perception that in these cells DCC is not proapoptotic. Neogenin, a DCC homologue, has also been proposed to act as a receptor for netrin-1 (35, 36) even though this is still a matter of controversy (5, 37). We then investigated whether neogenin, which is expressed in IMR32 cells (Fig. S2 C), could be implicated in the IMR32 cell death observed here. Neogenin was down-regulated by a siRNA approach and, as shown in Fig. S3 A, this has no effect on netrin-1 siRNA-mediated IMR32 cell death. Thus, the netrin-1 autocrine loop probably blocks UNC5H-induced IMR32 cell death.
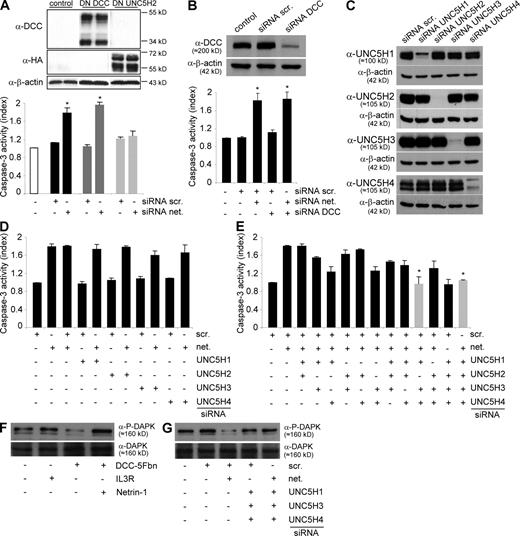
NB tumor cell death occurs via UNC5H/DAPK proapoptotic signaling. (A) Quantification of cell death in IMR32 cells transfected with either a dominant-negative mutant for DCC proapoptotic activity (DN-DCC) or a dominant-negative mutant for UNC5H proapoptotic activity (DN-UNC5H). Top: DN-DCC and DN-UNC5H proteins expression were analyzed by Western blot. Bottom: cell death was quantified by measuring relative caspase-3 activity after scramble or netrin-1 siRNA transfection. SEM are indicated. Data are means of three independent experiments. *, P < 0.05 calculated using a two-sided Mann-Whitney test compared with level of control. (B) Quantification of cell death in IMR32 cells transfected with either a scramble siRNA or a netrin-1 siRNA together or not with a DCC siRNA. Top: DCC siRNA efficiency was verified by Western blotting on HEK293T cells transfected with pCR-hDCC together with scramble or DCC siRNA. Bottom: cell death was quantified by measuring relative caspase-3 activity. Data are means of three independent experiments. SEM are indicated. *, P <0.05 calculated using a two-sided Mann-Whitney test compared with level of control. Similar results were obtained when neogenin or MYCN were down-regulated (Fig. S3 A). (C) Analysis of specificity and efficiency of each UNC5H siRNA by Western blot in HEK293T cells transfected with UNC5H1, H2, H3, or H4 encoding vector together with each UNC5H siRNA. (D and E) Quantification of cell death in IMR32 cells transfected with either a scramble siRNA or a netrin-1 siRNA together with various combinations of UNC5H siRNA, i.e, one UNC5H (D) or two or four UNC5H (E). Apoptosis was monitored by measuring relative caspase-3 activity. The use of combined UNC5H1, UNC5H2, UNC5H3, and UNC5H4 or UNC5H1, UNC5H3, and UNC5H4 siRNAs, leading to the absence of death induced by netrin-1 siRNA, are presented in gray. Data are means of three independent experiments. Error bars indicate SEM. *, P <0.05 calculated using a two-sided Mann-Whitney test compared with level of control. (F and G) Immunodetection of phosphorylated DAPK (P-DAPK) in IMR32 cells either treated with DCC-5Fbn (F) or transfected with netrin-1 siRNA alone or with UNC5H1, H3, and H4 siRNAs (G). In F and G, immunodetection was performed on three independent experiments.
NB tumor cell death occurs via UNC5H/DAPK proapoptotic signaling. (A) Quantification of cell death in IMR32 cells transfected with either a dominant-negative mutant for DCC proapoptotic activity (DN-DCC) or a dominant-negative mutant for UNC5H proapoptotic activity (DN-UNC5H). Top: DN-DCC and DN-UNC5H proteins expression were analyzed by Western blot. Bottom: cell death was quantified by measuring relative caspase-3 activity after scramble or netrin-1 siRNA transfection. SEM are indicated. Data are means of three independent experiments. *, P < 0.05 calculated using a two-sided Mann-Whitney test compared with level of control. (B) Quantification of cell death in IMR32 cells transfected with either a scramble siRNA or a netrin-1 siRNA together or not with a DCC siRNA. Top: DCC siRNA efficiency was verified by Western blotting on HEK293T cells transfected with pCR-hDCC together with scramble or DCC siRNA. Bottom: cell death was quantified by measuring relative caspase-3 activity. Data are means of three independent experiments. SEM are indicated. *, P <0.05 calculated using a two-sided Mann-Whitney test compared with level of control. Similar results were obtained when neogenin or MYCN were down-regulated (Fig. S3 A). (C) Analysis of specificity and efficiency of each UNC5H siRNA by Western blot in HEK293T cells transfected with UNC5H1, H2, H3, or H4 encoding vector together with each UNC5H siRNA. (D and E) Quantification of cell death in IMR32 cells transfected with either a scramble siRNA or a netrin-1 siRNA together with various combinations of UNC5H siRNA, i.e, one UNC5H (D) or two or four UNC5H (E). Apoptosis was monitored by measuring relative caspase-3 activity. The use of combined UNC5H1, UNC5H2, UNC5H3, and UNC5H4 or UNC5H1, UNC5H3, and UNC5H4 siRNAs, leading to the absence of death induced by netrin-1 siRNA, are presented in gray. Data are means of three independent experiments. Error bars indicate SEM. *, P <0.05 calculated using a two-sided Mann-Whitney test compared with level of control. (F and G) Immunodetection of phosphorylated DAPK (P-DAPK) in IMR32 cells either treated with DCC-5Fbn (F) or transfected with netrin-1 siRNA alone or with UNC5H1, H3, and H4 siRNAs (G). In F and G, immunodetection was performed on three independent experiments.
To more specifically address the identity of the UNC5H receptors involved in this cell death induction, we down-regulated the expression of each UNC5H receptor individually (UNC5H1, UNC5H2, UNC5H3, or UNC5H4) by a siRNA approach (Fig. 5 C and Fig. S3 B) while inducing cell death using netrin-1 siRNA (Fig. S3 C). None of the single UNC5H siRNAs was sufficient to inhibit netrin-1 siRNA-mediated IMR32 cell apoptosis, suggesting some redundancy in UNC5H-induced cell death (Fig. 5 D). However, when combinations of siRNAs were used, we observed that combined silencing of the four UNC5H receptors was sufficient to fully inhibit the death triggered by netrin-1 autocrine loop disruption (Fig. 5 D), whereas the same combination of siRNAs had no effect on CLB-Ge2 cell survival (Fig. S3 D). The respective importance of UNC5H receptors in netrin-1 siRNA-induced cell death was then assessed by the different combination of two or three siRNAs; the combination of UNC5H1, UNC5H3, and UNC5H4 siRNAs was the only one to fully block cell death (Fig. 5 E). Thus, in agreement with the level of UNC5H receptors expressed in IMR32 cells, it appears that disruption of the netrin-1 autocrine survival loop triggers UNC5H-induced cell death. Moreover, UNC5H1, UNC5H3, and UNC5H4 are the receptors involved in this proapoptotic effect.
Furthermore, in IMR32 cells, the proapoptotic serine threonine kinase DAP kinase (DAPK), which is shown to be required for UNC5H-induced cell death, exhibited a loss of its inhibitory autophosphorylation (38) upon DCC-5Fbn treatment (Fig. 5 F) or netrin-1 siRNA transfection (Fig. 5 G). Accordingly, autophosphorylation was restored by a treatment with excess netrin-1 or by a combination of UNC5H1, UNC5H3, and UNC5H4 siRNAs. Moreover, the transfection of the antiapoptotic protein BCL-2 was sufficient to inhibit netrin-1 siRNA-induced cell death but did not inhibit DAPK dephosphorylation, hence suggesting that DAPK activation is not a result of cell death but is specifically engaged by UNC5H after netrin-1 inhibition (unpublished data).
Interference with netrin-1 inhibits NB progression and dissemination
We next assessed whether in vivo modulation of netrin-1 could be used to limit/inhibit NB progression and dissemination. An elegant chicken model has been developed in which graft of NB cells in the chorioallantoic membrane (CAM) of 10-d-old chick embryos recapitulates both tumor growth at a primary site, i.e., within the CAM, and tumor invasion and dissemination at a secondary site, metastasis to the lung (Fig. 6 A). In a first approach, IMR32 or CLB-Ge2 cells were loaded in 10-d-old CAM and embryos were treated on days 11 and 14 with PBS or DCC-5Fbn. 17-d-old chicks were then analyzed for primary tumor growth and metastasis to the lung. As shown in Fig. 6 (B and C), specifically in CAMs grafted with IMR32 but not with CLB-Ge2 cells, DCC-5Fbn significantly reduced primary tumor size. This size reduction was associated with increased tumor apoptosis, as shown by an increased caspase-3 activity in the tumor lysate (Fig. 6 D). More importantly, DCC-5Fbn also reduced lung metastasis formation, as shown in Fig. 6 E. Similar results were obtained when CAM-grafted embryos were treated with netrin-1 siRNA (unpublished data). To next assess whether DCC-5Fbn could also induce the regression of metastatic lesions, IMR32 cells were CAM grafted and DCC-5Fbn (or PBS) treatment started after metastasis to the lung is known to occur, i.e., treatments were performed on days 14 and 15 because pulmonary metastases are routinely detectable at day 13. As shown in Fig. 6 F, pulmonary metastases were markedly reduced, suggesting that DCC-5Fbn not only inhibits tumor dissemination but also induces regression of metastatic lesions at the secondary site.
Disruption of netrin-1 autocrine loop inhibits NB progression and dissemination in a chick model. (A) Schematic representation of the experimental chick model. IMR32 or CLB-Ge2 cells were grafted in CAM at day 10 and DCC-5Fbn or PBS was injected on days 11 and 14. Tumors and lungs were harvested on day 17. (B–D) Effect of DCC-5Fbn on primary tumor growth and apoptosis. (B) Representative images of IMR32 (top) or CLB-Ge2 (bottom) primary tumors formed on CAM treated either with DCC-5Fbn (right) or PBS (left). Bars, 2 mm. (C) Quantitative analysis showing the mean primary tumor size. (D) Caspase-3 activity was determined in the primary tumor lysates from DCC-5Fbn/PBS-treated IMR32 or CLB-Ge2–grafted embryos. (E) Effect of DCC-5Fbn on lung metastasis. Percentage of embryos with lungs invaded by human IMR32 or CLB-Ge2 cells after two injections (days 11 and 14) of either DCC-5Fbn or PBS was performed as described in the Materials and methods. (F) Effect of DCC-5Fbn on lung metastasis regression. Quantification of lung metastasis in embryos CAM grafted with IMR32 cells and treated after metastasis formation (days 14 and 15) with DCC-5Fn or PBS. The number of embryos studied in each condition is indicated above the graphs and results are from three independent experiments. In C–F, errors bars indicate SEM. C–F: *, P < 0.05; **, P < 0.005 calculated using a two-sided Mann-Whitney test compared with level of control. E: **, P < 0.005 calculated using a Chi-squared test.
Disruption of netrin-1 autocrine loop inhibits NB progression and dissemination in a chick model. (A) Schematic representation of the experimental chick model. IMR32 or CLB-Ge2 cells were grafted in CAM at day 10 and DCC-5Fbn or PBS was injected on days 11 and 14. Tumors and lungs were harvested on day 17. (B–D) Effect of DCC-5Fbn on primary tumor growth and apoptosis. (B) Representative images of IMR32 (top) or CLB-Ge2 (bottom) primary tumors formed on CAM treated either with DCC-5Fbn (right) or PBS (left). Bars, 2 mm. (C) Quantitative analysis showing the mean primary tumor size. (D) Caspase-3 activity was determined in the primary tumor lysates from DCC-5Fbn/PBS-treated IMR32 or CLB-Ge2–grafted embryos. (E) Effect of DCC-5Fbn on lung metastasis. Percentage of embryos with lungs invaded by human IMR32 or CLB-Ge2 cells after two injections (days 11 and 14) of either DCC-5Fbn or PBS was performed as described in the Materials and methods. (F) Effect of DCC-5Fbn on lung metastasis regression. Quantification of lung metastasis in embryos CAM grafted with IMR32 cells and treated after metastasis formation (days 14 and 15) with DCC-5Fn or PBS. The number of embryos studied in each condition is indicated above the graphs and results are from three independent experiments. In C–F, errors bars indicate SEM. C–F: *, P < 0.05; **, P < 0.005 calculated using a two-sided Mann-Whitney test compared with level of control. E: **, P < 0.005 calculated using a Chi-squared test.
As a second in vivo approach, we used the IGR-N-91 model derived from a BM metastasis of human MYCN-amplified stage 4 NB. When subcutaneously xenografted into nude mice, IGR-N-91 cells gave rise to different tumor cell lines derived from the nude mouse primary tumor xenograft (PTX) or from disseminated metastatic foci into BM, blood, and myocardium (Myoc) of the animal (39). It is of interest that although the parental IGR-N-91 and the cell line derived from the PTX show no or very low netrin-1 expression, the different cell lines derived from secondary localizations showed a marked expression of netrin-1 both at the RNA level (Fig. 7 A) and at the protein level (Fig. 7 B). When cell death was investigated in these different cell lines upon treatment with DCC-5Fbn, a direct correlation was observed between the level of netrin-1 and cell susceptibility to DCC-5Fbn (Fig. 7 C). Specifically, although PTX cells failed to undergo cell death upon DCC-5Fbn treatment, this treatment triggered netrin-1–high Myoc cell death (Fig. 7 C). This observation supports the overall view that gaining netrin-1 dependence receptor resistance, via an autocrine netrin-1 expression in the case of the IGR-N-91 model, likely promotes NB tumor cell survival outside of the primary tumor site. To test whether this netrin-1 expression may then be used as a target to inhibit metastasis in vivo, Myoc and PTX cells were injected intravenously into nude mice and lung colonization was quantitated after daily intraperitoneal treatment with PBS or DCC-5Fbn. Although lung colonization was not reduced upon DCC-5Fbn treatment in PTX-injected mice (not depicted), a significant decrease in lung colonization was detected in DCC-5Fbn–treated Myoc-injected mice (Fig. 7 D). Thus, in both chick and mouse models, disruption of the netrin-1 autocrine loop inhibited or completely prevented the dissemination of netrin-1–expressing NB cells.
Disruption of netrin-1 autocrine loop inhibits NB dissemination in a mouse model. (A) Analysis of netrin-1 expression using Q-RT-PCR in IGR-N-91 cell line and the IGR-N-91–derived cell lines PTX, BM, Blood, and Myoc. Note that although PTX cells fail to express netrin-1, netrin-1 mRNA is highly expressed in Myoc cells. (B) Immunostaining on IGR-N-91 cells and the different derived cell lines PTX, BM, Blood, or Myoc using netrin-1 antibody. Bar, 50 µm. (C) Cell death was quantified in IGR-N-91, PTX, BM, Blood, or Myoc cell lines treated or not with DCC-5Fbn, with or without addition of netrin-1 in excess to reverse the effect of DCC-5Fbn. A negative control was also performed by adding an unrelated IL3R peptide produced in the same condition as DCC-5Fbn. Data are means of three independent experiments. Errors bars indicate SEM. *, P < 0.05, calculated using a two-sided Mann-Whitney test compared with level of control. (D) Quantification of lung colonization in PTX or Myoc cells in i.v injected mice treated with PBS or DCC-5Fbn for 22 d. Quantification was performed as described in the Materials and methods. Large bars indicate the median values for both groups. P-value was calculated using a two-sided Mann-Whitney test compared with level of control.
Disruption of netrin-1 autocrine loop inhibits NB dissemination in a mouse model. (A) Analysis of netrin-1 expression using Q-RT-PCR in IGR-N-91 cell line and the IGR-N-91–derived cell lines PTX, BM, Blood, and Myoc. Note that although PTX cells fail to express netrin-1, netrin-1 mRNA is highly expressed in Myoc cells. (B) Immunostaining on IGR-N-91 cells and the different derived cell lines PTX, BM, Blood, or Myoc using netrin-1 antibody. Bar, 50 µm. (C) Cell death was quantified in IGR-N-91, PTX, BM, Blood, or Myoc cell lines treated or not with DCC-5Fbn, with or without addition of netrin-1 in excess to reverse the effect of DCC-5Fbn. A negative control was also performed by adding an unrelated IL3R peptide produced in the same condition as DCC-5Fbn. Data are means of three independent experiments. Errors bars indicate SEM. *, P < 0.05, calculated using a two-sided Mann-Whitney test compared with level of control. (D) Quantification of lung colonization in PTX or Myoc cells in i.v injected mice treated with PBS or DCC-5Fbn for 22 d. Quantification was performed as described in the Materials and methods. Large bars indicate the median values for both groups. P-value was calculated using a two-sided Mann-Whitney test compared with level of control.
DISCUSSION
Together, the data obtained in the chick and mouse models described in the previous sections, in NB cell lines, and in the human pathology all support the view that a fraction of NB shows an autocrine production of netrin-1. This elevated netrin-1 level likely confers a selective advantage acquired by the cancer cell to escape netrin-1 dependence receptor–induced apoptosis and, consequently, to survive in settings of environmental absence or limitation of netrin-1. It is therefore interesting to note that not only NB but also other neoplasms associated with poor prognosis, for example, metastatic breast cancer and pancreatic cancer, also express high levels of netrin-1 (33, 40), suggesting that netrin-1 up-regulation may be a common feature for several aggressive cancers. From a mechanistic point of view, in a large fraction of NB, this autocrine expression of netrin-1 probably inhibits UNC5H-induced cell death. However, because DCC has been shown to display reduced expression in NB and because this reduction has been associated with NB aggressiveness in human NB (29), it is tempting to speculate that netrin-1 up-regulation can also, in some NB, inhibit DCC-induced apoptosis. Interestingly, this netrin-1 up-regulation appears to block a death signal involving the serine threonine DAPK, whose activity is regulated via its autophosphorylation. It is therefore of interest to note that DAPK was described to be a negative regulator of tumor progression and, more specifically, of metastasis (41). Thus, it can be suggested that a fraction of low netrin-1–expressing NB may have selected a loss of function of DAPK for survival. This would be compatible with the finding that metastasis in NB is associated with a loss of caspase-8, a selective advantage which provides survival to NB cells by inhibiting the proapoptotic signaling of the dependence receptor integrin α3β1 (42).
It is then interesting to wonder why such a large fraction of aggressive NBs have selected a gain of netrin-1 rather than a loss of the netrin-1 dependence receptor death pathways. A possible explanation is that netrin-1 expression not only confers a gain in survival, but may also lead to enhancement of nonapoptotic signaling mediated by netrin-1 receptors. Netrin-1 was indeed shown to bind a complex that includes some integrins (43). These integrins regulate cell migration and invasiveness and, thus, may play a role in cancer progression. Netrin-1 was also proposed to play a role in angiogenesis, although whether netrin-1 is proangiogenic or antiangiogenic is controversial (44–47) and this effect on angiogenesis may increase NB metastases development. It is also of interest to note that NB is a complex disease that originates from migrating neural crest cells. Netrin-1 up-regulation may then be implicated in the main function played by netrin-1 during nervous system development that is neuronal navigation. Along this line, netrin-1 and DCC have been shown to play an important role during neural crest cell migration (48), and it is then tempting to suggest that the gain in netrin-1 expression also promotes NB cell migration.
As different types of stage 4 NBs are distinguished, not only by the age of the children but also by the tissues in which metastases are found, one may wonder about the implication of netrin-1 produced in the normal tissues in which the tumor cells spread, and this would be interesting to explore. In particular, it would be interesting to evaluate whether, according to the classical “seed and soil” theory for metastasis (for review see reference 18), netrin-1 expression in specific tissues may favor metastasis development more specifically in these tissues. It is also intriguing to note that others have shown that in some particular cell lines, netrin-1 is able to promote apoptosis rather than inhibit apoptosis (49), so that the view of netrin-1 up-regulation being only a survival-selective advantage to block apoptosis via dependence receptors is probably only part of the overall regulatory mechanisms that links NB, netrin-1, and its receptors.
The observation that low levels of netrin-1 in NB correlate with good outcome is of clinical interest, in particular in NB diagnosed in neonates and infants. Indeed, low netrin-1 expression predicts long-term survival in infants (100% in 4S stage and 90% in infants in general) in a type of pathology in which therapeutic management is highly dependent on presentation and MNA (50, 51). This is particularly true with stage 4S infants who receive no (or minimal) treatment based on the lack of MNA, even though current statistics show that 1 in 10 of these infants will eventually die of NB. In this paper, we propose that determination of low netrin-1 level confirms a good prognosis for these infants without therapy, whereas the infants with high netrin-1 expression should be considered for more intensive treatment. Regarding infants or children with high netrin-1–expressing NB tumors, an alternative or supplementary targeted treatment based on disruption of the netrin-1 autocrine survival loop may improve standard high-dose chemotherapy regimen efficiency. We propose that a treatment based on inhibition of the interaction between netrin-1 and its dependence receptors, or inhibition of the ability of netrin-1 to multimerize its receptors, could potentially improve the survival of a large fraction of the patients suffering from aggressive NB. Moreover, it is interesting to note that no correlation between netrin-1 up-regulation and molecular signature of apoptosis and invasion was observed in NB tumors (Fig. S1 D), strengthening the case for netrin-1 as an original target for NB. Future preclinical and clinical studies should assess whether such therapeutic strategies, which could include small molecules (drugs), monoclonal antibodies, or the DCC-5Fbn recombinant protein presented in this paper, used alone or in combination with standard chemotherapy, could be of therapeutic benefit for infants and children with NB.
MATERIALS AND METHODS
Cell lines, transfection procedure, and reagents.
Human NB cell lines were obtained from the tumor banks at Centre Léon Bérard and at Institut Gustave Roussy. More specifically, IMR32 and CLB-Ge2 cell lines were cultured in RPMI 1640 GlutaMAX medium (Invitrogen) containing 10% FBS. IGR-N-91 cell line and its derivatives, as well as HEK293T cells, were cultured in DME medium (Invitrogen) containing 10% FBS. Cell lines were transfected using Lipofectamine 2000 reagent (Invitrogen) for siRNA or Lipofectamine Plus reagent (Invitrogen) for plasmids. Netrin-1 was obtained from Axxora and was used at a concentration of 150 ng/ml in all in vitro assays.
Human NB tumor samples and biological annotations.
According to parental consent, surgical human NB tumor material was immediately frozen. Material and annotations were obtained from the Biological Resources Centers of both national referent Institutions for NB treatment (Centre Léon Bérard and at Institut Gustave Roussy). Protocols using human material were approved by the local ethics Committees of Lyon University and Paris XI University. MYCN genomic content was assessed on histologically qualified tumors as previously described (52). For immunohistochemistries, 5-µm sections were prepared and frozen at −80°C.
Plasmid constructs, siRNA, and DCC-5Fbn production.
The dominant-negative mutant for UNC5H and DCC (pCR-UNC5H2-IC-D412N and pCR-DCC-IC-D1290N, respectively) and the plasmids encoding Neogenin (pCDNA3-Neogenin) and UNC5H1 (pCDNA3.1-UNC5H1-HA) have been previously described (1, 2, 5). The plasmids encoding UNC5H2 (pCDNA3.1-UNC5B-HA), UNC5H3 (pCDNA3-UNC5C-HA), and UNC5H4 (pCAG3-hU5H4-His) were gifts from H. Arakawa (National Cancer Institute, Tokyo, Japan), M. Tessier-Lavigne (Genentech, San Francisco, CA), K.L. Guan (University of Michigan, Ann Arbor, MI), and N. Yamamoto (Osaka University, Osaka, Japan). Human netrin-1–encoding plasmid (peak8-hNTN1-His) was obtained from D.E. Bredesen (The Buck Institute for Age Research, Novato, CA). Ps974-DCC-5Fbn allowing bacterial expression of the fifth fibronectin type III domain of DCC was obtained by inserting a Pst1–BamH1 DNA fragment generated by PCR using pDCC-CMV-S as a template. DCC-5Fbn production was performed using a standard procedure. In brief, BL21 cells were forced to express DCC-5Fbn in response to imidazole, and the BL21 lysate was subjected to affinity chromatography using FLAG-Sepharose (Sigma-Aldrich). A peptide corresponding to the ectodomain of IL3R was produced in the same conditions and used as a control. For cell culture use, netrin-1, DCC, and neogenin siRNAs (Santa Cruz Biotechnology, Inc.) were designed as a pool of three target-specific 20–25-nt siRNAs. UNC5H1, UNC5H2, UNCH3, and UNC5H4 siRNAs were designed by Sigma-Aldrich. MYCN siRNA was designed by Thermo Fisher Scientific.
Cell death assays.
2 × 105 cells were grown in serum-poor medium and were treated (or not) with 1 µg/ml DCC-5Fbn or transfected with siRNA using Lipofectamine 2000. Cell death was analyzed using trypan blue staining procedures as previously described (1). The extent of cell death is presented as the percentage of trypan blue–positive cells in the different cell populations. Apoptosis was monitored by measuring caspase-3 activity as described previously (1) using the Caspase 3/CPP32 Fluorimetric Assay kit (Gentaur). For detection of DNA fragmentation, treated cells were cytospun, and TUNEL was performed with 300 U/ml TUNEL enzyme and 6 µM biotinylated dUTP (Roche) as previously described (53).
Q-RT-PCR.
To assay netrin-1, DCC, and UNC5H receptor expression in NB samples, total RNA was extracted from histologically qualified tumor biopsies (>60% immature neuroblasts) using the NucleoSpin RNAII kit (Macherey-Nagel), and 200 ng were reverse transcribed using 1U Superscript II reverse transcription (Invitrogen), 1U RNase inhibitor (Roche), and 250 ng of random hexamer (Roche). Total RNA was extracted from mouse and human cell lines using the NucleoSpin RNAII kit and 1 µg was reverse transcribed using the iScript cDNA Synthesis kit (Bio-Rad Laboratories). Real-time Q-RT-PCR was performed on a LightCycler 2.0 apparatus (Roche) using the LightCycler FastStart DNA Master SYBER Green I kit (Roche). Reaction conditions for all optimal amplifications, as well as primer selection for murine and human netrin-1, DCC, and UNC5H1-4, were determined as already described. The ubiquitously expressed human HPRT genes showing the least variability in expression in NB was used as an internal control (54). The sequences of the primers are the following: NTN1, 5′-TGCAAGAAGGACTATGCCGTC-3' and 5′-GCTCGTGCCCTGCTTATACAC-3′; UNC5H1, 5′-CATCACCAAGGACACAAGGTTTGC-3′ and 5′-GGCTGGAAATTATCTTCTGCCGAA-3′; UNC5H2, 5′-GGGCTGGAGGATTACTGGTG-3′ and 5′-TGCAGGAGAACCTCATGGTC-3′; UNC5H3, 5′-GCAAATTGCTGGCTAAATATCAGGAA-3′ and 5′-GCTCCACTGTGTTCAGGCTAAATCTT-3′; UNC5H4, 5′-GGTGAACCCAGCCTCCAGTCAG-3′ and 5′-CTTCCACTGACATCACTTCCTCCC-3′; DCC, 5′-AGCCAATGGGAAAATTACTGCTTAC-3′ and 5′-AGGTTGAGATCCATGATTTGATGAG-3′; and HPRT, 5′-TGACACTGGCAAAACAATGCA-3′ and 5′-GGTCCTTTTCACCAGCAAGCT-3′.
Genomic DNA quantification.
Genomic DNA from IMR32 and CLB-Ge2 cells was extracted with the NucleoSpin Tissue kit (Macherey-Nagel). 50 ng of genomic DNA was used to perform quantitative PCR using primers specific to NTN1 and MYCN genomic sequences. Real-time quantitative PCR was performed on a LightCycler 2.0 apparatus using the Light Cycler FastStart DNA Master SYBER Green I kit. NAGK (the N-acetylglucosamine kinase gene), which is located on chromosome 2 similarly to the MYCN gene but separated from the MYCN amplicon, was used as an internal control gene to determine the gene dosage (55). For each pair of primers, genomic DNA amplification was assessed by polymerase activation at 95°C for 10 min, followed by 35 cycles at 95°C for 10 s, 65°C for 30 s, and 72°C for 10 s. The sequences of the primers are the following: NTN1, 5′-CTGTGTCCCCCACTTGTTCT-3′ and 5′-CCATGAACCCCACTGACTCT-3′; MYCN, 5′-GTGCTCTCCAATTCTCGCCT-3′ and 5′-GATGGCCTAGAGGAGGGCT-3′; and NAGK, 5′-TGGGCAGACACATCGTAGCA-3′ and 5′-CACCTTCACTCCCACCTCAAC-3′.
Immunohistochemistry and immunoblotting analysis.
105 cells were centrifugated on coverslips with a cytospinner (Shandon Cytospin 3; Thermo Fisher Scientific). Tumor slides and cells were fixed in 4% paraformaldehyde. The slides were then incubated at room temperature for 1 h with an antibody recognizing the human netrin-1 (1:150; R&D Systems), UNC5H1 (1:100; Abcam), UNC5H3 (1:100; R&D system), or UNC5H4 (1:100; Santa Cruz Biotechnology, Inc.). After rinsing in PBS, the slides were incubated with an Alexa 488 donkey anti–rat antibody (Invitrogen), an Alexa 488 donkey anti–rabbit antibody (Invitrogen), a Cy3 donkey anti–mouse antibody (Jackson ImmunoResearch Laboratories), or an Alexa 488 donkey anti–goat antibody (Invitrogen), respectively. For tumor slides, netrin-1 and UNC5H4 signals were amplified using biotinyl-tyramide (TSA; Thermo Fisher Scientific) and Alexa 488–streptavidin (Invitrogen). Nuclei were visualized with Hoechst staining. Densitometric value corresponding to netrin-1 signal was quantified with AxioVision Release 4.6 software. Immunoblots were performed as already described using anti–phospho-DAPK and anti-DAPK (Sigma-Aldrich) (38), anti-DCC (1:500; Santa Cruz Biotechnology, Inc.), anti-neogenin (1:500; Santa Cruz Biotechnology, Inc.), anti-HA (1:7,500; Sigma-Aldrich), anti-HIS (1:1,000; QIAGEN), anti-MYCN (1:1000; BD), or anti-β-actin (1:1,000; Millipore) antibodies.
Netrin-1 ELISA assay.
Detection of netrin-1 protein in IMR32 and CLB-Ge2 cell culture medium was performed using a modified ELISA assay. In brief, 96-well plates (Nunc-Immuno plate MaxiSorp; Thermo Fisher Scientific) were coated with 200 ng/well of purified recombinant extracellular domain of DCC (DCC-Ec-Fc). To minimize aspecific binding, each well was incubated with 100 µl of blocking solution, containing 5% (wt/vol) BSA (Sigma-Aldrich) in 0.05% PBS-Tween. 3 ml FBS free cell culture medium was added sequentially (300 µl/well) to coated 96-well plates and incubated for 1 h at 37°C. After three washes with 0.5% BSA/PBS, 100 µl of rat anti–netrin-1 antibody (diluted 1:500 in blocking solution) was added to each well and incubated for 30 min at 37°C. After extensive washing, each well was incubated with 100 µl HRP-conjugated goat anti–rat antibody (1:1,000; Jackson ImmunoResearch Laboratories) for 30 min at 37°C. After removal of unbound antibody by three washes in 0.5% BSA/PBS, the plates were incubated for 5 min at room temperature with ECL Western Blotting Substrate (Thermo Fisher Scientific). Luminescent signal was measured using a Luminoskan Ascent apparatus (Thermo Fisher Scientific).
Reporter assay.
105 cells were plated in 12-well plates and transfected with the firefly luciferase reporter under the control of the netrin-1 promoter (pGL3-NetP-Luc) or the pGL3 empty vector. All transfections were done in triplicate and the Dual-Luciferase Reporter Assay system (Promega) was performed 48 h after transfection according to the manufacturer's protocol, using the Luminoskan Ascent apparatus. As an internal control of transfection efficiency, the renilla luciferase-encoding plasmid (pRL-CMV; Promega) was cotransfected, and for each sample firefly luciferase activity was normalized to the renilla luciferase activity.
Chicken model for NB progression and dissemination.
107 NB cells suspended in 40 µl of complete medium were seeded on 10-d-old chick CAM. 10 µg DCC-5Fbn or the same PBS volume was injected in the tumor on days 11 and 14. For siRNA treatment, 4 µg of scramble or netrin-1 siRNA was injected under the same conditions as for DCC-5Fbn. On day 17, tumors were resected and the area was measured with AxioVision Release 4.6 software (Carl Zeiss, Inc.). To test the effect of DCC-5Fbn on metastasis regression, 3 µg DCC-5Fbn or PBS was injected on days 14 and 15 in a chorioallantoic vessel. To assess metastasis, lungs were harvested from the tumor-bearing embryos and genomic DNA was extracted with a NucleoSpin Tissue kit (Macherey-Nagel). Metastasis was quantified by PCR-based detection of the human Alu sequence using the primers 5′-ACGCCTGTAATCCCAGCACTT-3′ (sense) and 5′-TCGCCCAGGCTGGAGTGCA-3′ (antisense) with chick GAPDH-specific primers (sense, 5′-GAGGAAAGGTCGCCTGGTGGATCG-3′; antisense, 5′-GGTGAGGACAAGCAGTGAGGAACG-3′) as controls. For both couples of primers, metastasis was assessed by polymerase activation at 95°C for 2 min followed by 30 cycles at 95°C for 30 s, 63°C for 30 s, and 72°C for 30 s. Genomic DNA extracted from lungs of healthy chick embryos was used to determine the threshold between NB cell–invaded and –noninvaded lungs. To monitor apoptosis in primary tumors, primary tumors and surrounding CAM were resected and broken up in lysis buffer and caspase-3 activity was measured using the Caspase 3/CPP32 Fluorimetric Assay kit.
NB metastasis in nude mice.
7-wk-old (20–22 g body weight) female athymic nu/nu mice were obtained from Charles River Laboratories. The mice were housed in sterilized filter-topped cages and maintained in a pathogen-free animal facility. IGR-N-91–derived PTX and Myoc cell lines were implanted by i.v. injection of 106 cells in 130 µl of PBS into a tail vein (day 0). 20 µg DCC-5Fbn or PBS with equal volume was i.p. injected daily during 22 d. Lungs were harvested on day 23. Lung genomic DNA was extracted with the NucleoSpin Tissue kit, and quantification of human tumor cells in lungs was done by PCR-based detection of the human Alu sequence using the primers 5′-CACCTGTAATCCCAGCACTTT-3′ (sense) and 5′-CCCAGGCTGGAGTGCAGT-3′ (antisense), using 25 ng of genomic DNA as previously described (56). PCR was performed under the following conditions: 95°C for 2 min, 30 cycles at 95°C for 30 s, 65°C for 20 s, and 72°C for 20 s. Quantification of human DNA in mice lungs was based on a standard curve using human genomic DNA isolated from PTX and Myoc cell lines.
Online supplemental material.
Fig. S1 associates netrin-1 and DCC expression in NB tumors with their apoptosis and invasion molecular signatures obtained with microarrays. Fig. S2 presents netrin-1 mRNA and protein expression in NB cell lines and shows CLB-VolMo netrin-1–high cell line sensitivity to DCC-5Fbn decoy fragment. In Fig. S3, MYCN and neogenin implication in netrin-1 siRNA-induced cell death is studied, and specificity and efficiency of UNC5H siRNAs are presented at the mRNA, protein, and cellular levels.
Acknowledgments
We thank M.M. Coissieux, C. Guix, M.L. Puiffe, I. Iacono, and S. Bréjon for technical assistance. We thank J. Bouzas, J.G. Delcros, and P. Leissner for excellent support. We thank Dale E. Bredesen for precious advice and text correction.
This work was supported by the Ligue Contre le Cancer (P. Mehlen), the Agence Nationale de la Recherche (P. Mehlen), the Institut National du Cancer (J. Bénard, A. Puisieux, and P. Mehlen), the Société Francaise des Cancers de l'Enfant (J. Bénard), The French Health Minister (J. Bénard and D. Valteau-Couanet), The EU fundings Hermione (P. Mehlen) and APOSYS (P. Mehlen), the Centre National de la Recherche Scientifique, and the Centre Léon Bérard.
The authors have no conflicting financial interests.
References
Abbreviations used: CAM, chorioallantoic membrane; DAPK, DAP kinase; DCC, deleted in colorectal cancer; MNA, MYCN amplification; mRNA, messenger RNA; Myoc, myocardium; NB, neuroblastoma; PTX, primary tumor xenograft; Q-RT-PCR, quantitative RT-PCR; siRNA, small interfering RNA; TUNEL, terminal deoxynucleotidyl transferase–mediated dUTP-biotin nick end labeling.
Author notes
J. Bénard, A. Bernet, and P. Mehlen contributed equally to this paper.


![Figure 1. Netrin-1 up-regulation is detected in aggressive NB. (A) Netrin-1 mRNA levels in 102 stage 4S (n = 24), [1yr −] stage 4 (n = 12), and [1yr+] stage 4 (n= 66) NBs measured by Q-RT-PCR. HPRT housekeeping gene was used as a control. Mean netrin-1 mRNA expression value for each subgroup is indicated by an “m” value. Mean netrin-1 mRNA levels in stage 4S and [1yr−] stage 4 were, respectively, compared with the mean netrin-1 detected in [1yr+] stage 4. The data were statistically determined using Student's t test compared with levels of [1yr+] stage 4. *, P < 0.05; **, P < 0.01. Each sample was assessed in two independent experiments. (B) Representative netrin-1 immunohistochemistry on one [1yr+] stage 4 and one stage 4S tumor. Insets show control without primary antibody. Bars, 50 µm. T, tumor cells; S, stromal cells. Netrin-1 antibody specificity is further shown in Fig. 2 D and Fig. S1 B. Immunohistochemistry was performed on four [1yr+] stage 4 and four stage 4S tumors. (C) Mean UNC5H mRNA levels in the different stage 4 NBs. Q-RT-PCR using UNC5H1–4-specific primers was performed. Mean UNC5H1–4 mRNA levels in [1yr−] stage 4 and [1yr+] stage 4 were, respectively, compared with the mean UNC5H1–4 levels detected in stage 4S. Error bars indicate SEM. The data were statistically determined using Student's t test compared with levels of stage 4S. *, P < 0.05; **, P < 0.01. Samples were analyzed in duplicates for each gene. (D) Representative UNC5H1 and UNC5H4 immunohistochemistries on [1yr+] stage 4 and stage 4S tumors. Insets show control without primary antibody. Bars, 50 µm. Immunohistochemistry was performed on four stage 4 [1yr+] and four stage 4S tumors. (E) Netrin-1 up-regulation is a marker of poor prognosis in stage 4S NB. Overall survival in a panel of 24 infants diagnosed with stage 4S NB with primary tumors showing either netrin-1–low (gray) or netrin-1–high (black) levels. The data was statistically determined using the Kaplan-Meier method. P-value is indicated. (F) Netrin-1 up-regulation is a marker of poor prognosis in infants with NB. Data are presented as in E, with a panel of 30 infants bearing NB.](https://cdn.rupress.org/rup/content_public/journal/jem/206/4/10.1084_jem.20082299/7/m_jem_20082299_rgb_fig1.jpeg?Expires=1771339031&Signature=gOhZ87stwv4CXSWMeTLWiMCw84fp56-aQ1jsrkqtuqDf8~ZLQ4RhN008~KJX1piiDJpW7lWfrtq8ZQRfU-F4etFP35XdTKeV1sqV2G1pz2VoFyvvOGZR08ldgajsvzts0swZb1Er7Apq0JQF0zLowmTirRNFrrbS0wgPb63VH~h09HjyI3bdJJyCAtNmujDgWAv2T5OOuW2HdOJBIe8JEi80qaAmofUDubNFqS2mmWXAPTcdRQgEAcIim86zFYo2D~P3FfHNeRY1~3CZbVBv5tzUrlo9XWL-kIWIRjV3vSJFOO4nqzVwpkOlI3KqBCYao8Q0gFBSp8OOAp9BxDhXBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 4. Disruption of netrin-1 autocrine loop by a decoy receptor fragment triggers NB tumor cell death. (A) Scheme representing netrin-1 and its receptors DCC and UNC5H and the fifth fibronectin type III domain of DCC (DCC-5Fbn) used to induce cell death. The downstream effector DAPK implicated in UNC5H-induced cell death is also represented. (B–D) Quantitative analysis of cell death in IMR32 and CLB-Ge2 cell lines treated with 1 µg/ml DCC-5Fbn, with or without addition of netrin-1 in excess (150 ng/ml) to reverse the effect of DCC-5Fbn. A negative control was also performed by adding an unrelated IL3R peptide produced in the same condition as DCC-5Fbn. Cell death was quantified by trypan blue exclusion assay (B) while apoptosis was monitored by measuring relative caspase-3 activity (C) or by TUNEL staining (D). Bars, 100 µm. In D, TUNEL staining was performed on three independent experiments. (E) Effect of DCC-5Fbn on fresh [1yr+] stage 4 NB. Tumoral cells were directly resuspended from the surgical punction and were plated for 24 h in presence of treatment. In B and C, data are means of six independent experiments. In E, data are means of two independent experiments. Error bars indicate SEM. **, P < 0.01 calculated using a two-sided Mann-Whitney test compared with level of control.](https://cdn.rupress.org/rup/content_public/journal/jem/206/4/10.1084_jem.20082299/7/m_jem_20082299_rgb_fig4.jpeg?Expires=1771339031&Signature=lligK0pn3KH0NFcFUfEB1BkJPqyD6Kh5U6WD~SZ7mUld9PzfOczNSdBRz~GXI5Nxc1elwifqSNbZghrmJiUGhfAbs~bzswdYTTBmg7829Gld-he8Jw~ogbEVsdgFoSlAJOOZQxnsH33xNwmKAsymiGm~Ir9KGW9rZ2BsHlRFoBbkRI2CafxMka0P6CsKQzz5lRDHZn~jzRZWXqyb5cL0O4q2M2Et~oYq~YzVdayxXpzVwuF~w1N8Hf-5aBF164XeiUL~jP2MmDmW~-ZYQ6lBShhjnGp63Uvkjeh0tD5-psFLqWWalL5w76Qo87Kng2ozlO-MMQnuYh3Grmajx2cEnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)