The human homologue of Drosophila Toll (hToll) is a recently cloned receptor of the interleukin 1 receptor (IL-1R) superfamily, and has been implicated in the activation of adaptive immunity. Signaling by hToll is shown to occur through sequential recruitment of the adapter molecule MyD88 and the IL-1R–associated kinase. Tumor necrosis factor receptor–activated factor 6 (TRAF6) and the nuclear factor κB (NF-κB)–inducing kinase (NIK) are both involved in subsequent steps of NF-κB activation. Conversely, a dominant negative version of TRAF6 failed to block hToll-induced activation of stress-activated protein kinase/c-Jun NH2-terminal kinases, thus suggesting an early divergence of the two pathways.
Immune response to infection requires the production of cytokines and costimulatory molecules by antigen-presenting cells. Distinct cell-associated receptors on myelomonocytic cells, such as CD14, allow the recognition of pathogen-associated molecules and trigger natural immune response by inducing the production of inflammatory cytokines that subsequently signal to activate adaptive immunity (1).
The molecular mechanisms that control the initial induction of these signals upon infection have been explored recently. In particular, a novel transmembrane receptor homologous to the Drosophila Toll, human Toll (hToll, also called TLR4), has been cloned recently (2, 3). The Drosophila Toll protein controls the potent antifungal response in Drosophila adults (4). Analogously, hToll has been shown to signal activation of adaptive immunity in humans by inducing the expression of B7.1, IL-6, and IL-8; thus, it represents a key molecule for the switching from natural to acquired immunity. However, the biochemical transduction pathway triggered by hToll was ill-defined (2).
hToll is a type I orphan receptor with an extracellular portion containing leucine-rich repeats, and a cytoplasmic domain significantly similar to the intracellular portion of the IL-1R type I (IL-1RI) and the IL-1R accessory protein (IL-1RAcP) (2, 5, 6); these observations suggest that they may use an analogous molecular framework for signaling.
IL-1 triggers the activation of distinct transcription factors, including NF-κB and c-Jun/activator protein 1, that subsequently drive the transcriptional induction of several cytokine genes (7). The molecular events occurring from the IL-1R signaling complex to the induction of NF-κB activity have been characterized recently; in particular, the adapter protein MyD88 recruits two distinct putative Ser/ Thr kinases, namely IL-1R–associated kinase (IRAK) and IRAK-2, to the receptor complex (8–10). IRAK and IRAK-2 interact subsequently with the adapter molecule TNFR-activated factor (TRAF) 6, which bridges them to the protein kinase NF-κB–inducing kinase (NIK) (8, 11, 12). Finally, NIK activates the I-κB kinase complex (including IKKα and IKKβ) that directly phosphorylates I-κBα (13–17).
In this study, we identified and molecularly ordered the mediators of the hToll-induced NF-κB and stress-activated protein kinase (SAPK)/c-Jun NH2-terminal kinase (JNK) activation cascade.
Materials And Methods
Expression Vectors and Transfection.
TRAF6-Flag, ΔTRAF6-Flag (or TRAF6 298–522), ΔTRAF2–Flag (or TRAF2 87–501), MyD88-AU1, ΔMyD88-AU1 (or MyD88 152–296), IRAK, NIK(KK-AA), and HA-p46SAPKγ-pCDNA3 expression vectors have been described (8, 18). Expression vectors for NH2-terminal Flag-tagged hToll and ΔhToll (amino acids 1–666) were constructed by inserting PCR-generated cDNA fragments lacking the coding sequence for the signal peptide, into the mammalian expression vector pFlag-CMV-1 (Eastman Kodak Co., Rochester, NY). CD4/Toll has been described previously (2).
Human embryonic 293 or 293T cells were transiently transfected by the calcium phosphate method with the indicated plasmids. The total amount of DNA was kept constant by supplementation with an empty vector (pCDNA3; Invitrogen Corp., Carlsbad, CA).
Northern Blotting Analysis.
Coimmunoprecipitation Analysis.
24–36 h after transfection, cells were lysed in 0.5 ml buffer (1% NP-40, 150 mM NaCl, 50 mM Tris, 1 mM EDTA, and protease inhibitor cocktail). Cell lysates were adjusted to 0.7 M NaCl, and the indicated antibodies were added for 1–4 h at 4°C. Immune complexes were precipitated by the addition of protein G–Sepharose (Sigma Chemical Co., St. Louis, MO). After extensive washing (in lysis buffer with the addition of 0.1% SDS), the Sepharose beads were boiled in sample buffer, and eluted proteins were fractionated by SDS-PAGE. Subsequent immunoblotting was performed as described (8).
NF-κB Activation Assay.
Cells were transfected with endothelial leukocyte adhesion molecule–Luciferase reporter plasmid (NF-κB luc; 0.1 μg), pCMV-β-galactosidase (βGal; 0.2 μg), and the indicated expression vectors. Relative NF-κB activity was calculated by normalizing relative luciferase activity with βGal activity as described previously (8). Data shown are from one out of two to five independent experiments with similar qualitative results. Data from experiments performed in duplicate or triplicate are expressed as mean ± SE.
SAPK/JNK Activation Assay.
Cells were transfected with NF-κB luc (0.5 μg), HA-p46SAPKγ-pCDNA3 (2 μg), and the indicated expression vectors. 48 h after transfection, cells were lysed in RIPA buffer containing 0.5 mM dithiothreitol, 20 mM β-glycerophosphate, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 1 mM PMSF, leupeptin (20 μg/ml), and aprotinin (20 μg/ml). Lysates were cleared by centrifugation, and protein concentration was measured using a commercial Bradford protein assay (Promega Corp., Madison, WI). Equal amounts of each lysate (usually 500 μg) were incubated on ice with 2 μg antiserum to HA (rabbit polyclonal; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 2 h at 4°C. Immune complexes were collected by protein A–Sepharose for 20 min and washed four times with RIPA buffer. Precipitates were boiled in sample buffer and run onto an SDS-polyacrylamide gel. Finally, immunoblotting was performed to detect the active phosphorylated form of SAPK (pThr-183/pTyr-185 of hJNK1), or SAPK as a control, by using specific antibodies (anti-pJNK mouse monoclonal, and anti-HA; Santa Cruz Biotechnology, Inc.). An aliquot of the cell lysate was also analyzed for NF-κB activation as above.
Results And Discussion
The expression and eventual regulation of specific transcripts encoding for hToll were analyzed in distinct cell types that play a critical role in the natural immune response. In particular, human monocytes were separated from healthy donors and treated with the bacterial product LPS for different periods of time. As shown in Fig. 1 A, specific transcripts for hToll were present in these cells and were induced significantly after treatment with LPS. These observations suggest that modulation of a nonclonal receptor, namely hToll, after exposure to infectious agents may play a regulatory role in the natural immune response. Of note, PMN and dendritic cells also transcribed hToll mRNA at different levels (our unpublished observations).
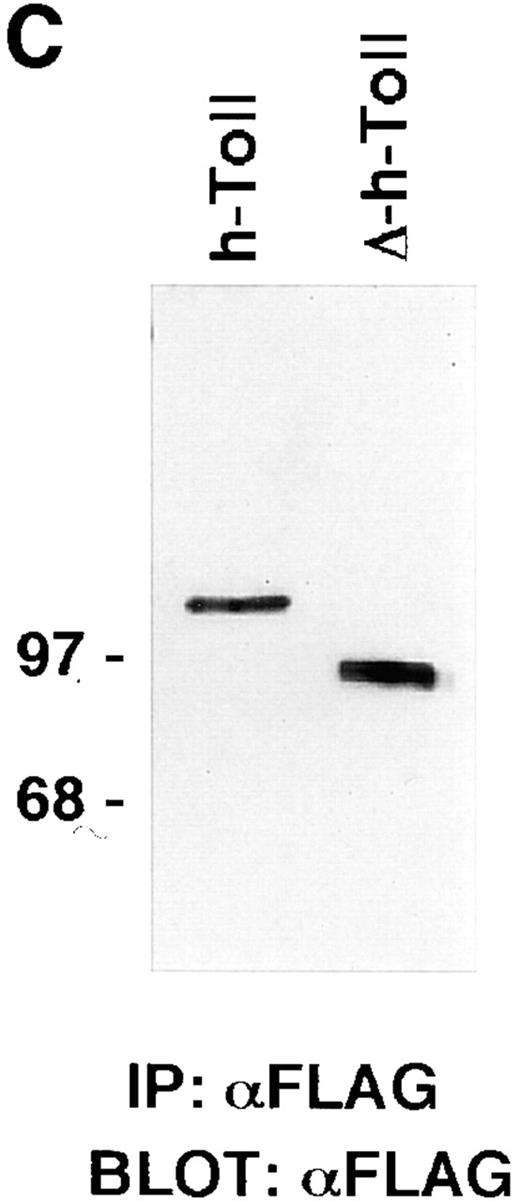
hToll expression and induction of NF-κB. (A) Human monocytes were treated with LPS (100 ng/ml) for different periods of time and analyzed for their hToll mRNA content by Northern blotting. Two distinct transcripts specific for hToll are detected and induced by LPS stimulation. (B) Ectopic expression of hToll-Flag and CD4/Toll but not the mutant version ΔhToll-Flag activate NF-κB in 293T cells in a dose-dependent manner, as measured by NF-κB reporter gene activity. (C) Equal amounts of hToll-Flag and ΔhToll-Flag are produced upon ectopic expression in 293T cells (3.2 μg of each expression construct were used for this experiment). IP, Immunoprecipitation. BLOT, Immunoblotting analysis.
hToll expression and induction of NF-κB. (A) Human monocytes were treated with LPS (100 ng/ml) for different periods of time and analyzed for their hToll mRNA content by Northern blotting. Two distinct transcripts specific for hToll are detected and induced by LPS stimulation. (B) Ectopic expression of hToll-Flag and CD4/Toll but not the mutant version ΔhToll-Flag activate NF-κB in 293T cells in a dose-dependent manner, as measured by NF-κB reporter gene activity. (C) Equal amounts of hToll-Flag and ΔhToll-Flag are produced upon ectopic expression in 293T cells (3.2 μg of each expression construct were used for this experiment). IP, Immunoprecipitation. BLOT, Immunoblotting analysis.
We next analyzed the hToll signaling pathway at the molecular level. A chimeric version of hToll in which the extra cellular portion was substituted with the corresponding region of CD4 (CD4/hToll) has been shown previously to induce NF-κB activation in Jurkat cells (2). We engineered distinct expression constructs encoding for Flag epitope– tagged hToll (hToll-Flag) or for a truncated version of hToll that lacks most of the cytoplasmic portion (ΔhToll-Flag). Ectopic expression of hToll-Flag but not ΔhToll-Flag induced NF-κB activation in human embryonic 293T cells at levels similar to the CD4/Toll chimeric protein that served as a positive control (Fig. 1, B and C). From these observations, it is apparent that either CD4-driven or ectopic expression–forced aggregation of the cytoplasmic portions of distinct hToll receptors is sufficient to trigger a signaling cascade that leads ultimately to activation of the transcription factor NF-κB. Given this, one could speculate that an as yet unidentified hToll ligand binds to hToll and induces its oligomerization and subsequent signaling cascade, in a manner similar to IL-1 and TNF with their cognate receptors.
hToll shares significant sequence similarity with distinct members of the IL-1R family, including IL-1RI, IL-1RAcP, and MyD88; of note, Phe 513 and Trp 514 in IL-1RI, which are conserved in all of these proteins, have been shown to be essential for IL-1RI to signal (Fig. 2,A) (20). Since we have shown recently a homophilic interaction to occur between the IL-1RAcP and MyD88 throughout their homologous domains (8), we asked whether hToll and the adapter protein MyD88 could interact. Upon coexpression, MyD88 and hToll formed an immunoprecipitable complex; in contrast, a mutant version of hToll, which lacks the region of homology to MyD88 and which was unable to induce NF-κB activation, failed to bind MyD88 (Fig. 2 B).
Functional and structural evidence of MyD88 recruitment to the hToll signaling complex. (A) Sequence alignment of human MyD88 (amino acids 152–296), hToll (667– 840), hIL-1RAcP (391–570), and hIL-1RI (381–569). Alignment was performed with Clustall software. Shading, Identical amino acids with a score <3. Boxes, Identical amino acids with a score = 0. Dots, Conserved amino acids that are essential for IL-1RI to signal (reference 20). (B) MyD88 associates with hToll but not with a truncated version of hToll (ΔhToll) lacking the cytoplasmic region sharing sequence similarity with MyD88. 293T cells were transfected with hToll-Flag, ΔhToll-Flag, or IL-1RAcP–Flag as a positive control together with AU1-tagged MyD88. The presence of MyD88 that coprecipitated with the receptors was detected by immunoblotting with a rabbit polyclonal antiserum to MyD88. (C) ΔMyD88 inhibits hToll-induced but not the unrelated TNFR-2–induced NF-κB activity. 1 μg of receptors and 1.5 μg of ΔMyD88 were transfected. Data are expressed as the percentage of relative receptor-induced NF-κB activity.
Functional and structural evidence of MyD88 recruitment to the hToll signaling complex. (A) Sequence alignment of human MyD88 (amino acids 152–296), hToll (667– 840), hIL-1RAcP (391–570), and hIL-1RI (381–569). Alignment was performed with Clustall software. Shading, Identical amino acids with a score <3. Boxes, Identical amino acids with a score = 0. Dots, Conserved amino acids that are essential for IL-1RI to signal (reference 20). (B) MyD88 associates with hToll but not with a truncated version of hToll (ΔhToll) lacking the cytoplasmic region sharing sequence similarity with MyD88. 293T cells were transfected with hToll-Flag, ΔhToll-Flag, or IL-1RAcP–Flag as a positive control together with AU1-tagged MyD88. The presence of MyD88 that coprecipitated with the receptors was detected by immunoblotting with a rabbit polyclonal antiserum to MyD88. (C) ΔMyD88 inhibits hToll-induced but not the unrelated TNFR-2–induced NF-κB activity. 1 μg of receptors and 1.5 μg of ΔMyD88 were transfected. Data are expressed as the percentage of relative receptor-induced NF-κB activity.
A mutant version of MyD88 (ΔMyD88), encoding only for the COOH-terminal IL-1R–like domain, abrogates IL-1RI/IL-1RAcP–induced NF-κB activation (8); given this, we analyzed whether ΔMyD88 could act as a dominant negative inhibitor of hToll-induced NF-κB activation. As predicted, ΔMyD88 specifically inhibited hToll-induced but not TNFR-2–induced NF-κB activity, lending functional credence to the interaction occurring between hToll and MyD88 (Fig. 2 C). From these observations, it is apparent that both IL-1R and hToll recruit the adapter protein MyD88 to their respective signaling complex.
IRAK and IRAK-2 are two additional proximal mediators of the IL-1R signaling complex; IRAK is recruited to the IL-1RAcP, whereas IRAK-2 preferentially binds IL-1RI (8, 10). Given this, we asked whether IRAK or IRAK-2 could interact with hToll. Upon ectopic expression, IRAK and hToll formed an immunoprecipitable complex. In contrast, IRAK-2 bound only weakly to hToll compared with IL-1RI (Fig. 3, A and B), thus suggesting that it may not represent a relevant hToll signal transducer.
NF-κB activation by hToll occurs through IRAK, TRAF6, and NIK. (A) IRAK is recruited to hToll but not to the inactive truncated version of hToll (ΔhToll). IL-1RAcP served as a positive control. Cells were transfected and analyzed as in Fig. 2 B. (B) IRAK-2 binds very weakly to hToll. IL-1RI served as a positive control. Cells were transfected and analyzed as in A. (C) ΔTRAF6 but not the unrelated ΔTRAF2 attenuates CD4/Toll-induced NF-κB activity. A dominant negative mutant version of the downstream molecule NIK [NIK(KK-AA)] also inhibits CD4/hToll-induced NF-κB activity.
NF-κB activation by hToll occurs through IRAK, TRAF6, and NIK. (A) IRAK is recruited to hToll but not to the inactive truncated version of hToll (ΔhToll). IL-1RAcP served as a positive control. Cells were transfected and analyzed as in Fig. 2 B. (B) IRAK-2 binds very weakly to hToll. IL-1RI served as a positive control. Cells were transfected and analyzed as in A. (C) ΔTRAF6 but not the unrelated ΔTRAF2 attenuates CD4/Toll-induced NF-κB activity. A dominant negative mutant version of the downstream molecule NIK [NIK(KK-AA)] also inhibits CD4/hToll-induced NF-κB activity.
NF-κB activation induced by a number of cytokine receptors is mediated by members of the TRAF adapter family. TRAF2, for example, plays a critical role in NF-κB activation mediated by TNFR-1 and TNFR-2 (21, 22). TRAF6 has been implicated in the IL-1 signaling pathway and has been shown to complex with IRAK and IRAK-2 downstream to the receptor signaling complex (8, 11). Therefore, we determined whether dominant negative versions of either could act to inhibit hToll-induced NF-κB activity. ΔTRAF6 but not ΔTRAF2 significantly impaired hToll-induced NF-κB activity, suggesting that TRAF6 may act as an additional downstream mediator of the hToll-induced NF-κB activation cascade (Fig. 3 C).
The protein kinase NIK has been shown to act as a general mediator of TRAF-induced NF-κB activation (12); once activated, NIK directly phosphorylates and activates the IKKα/β complex, which is responsible for I-κBα phosphorylation and subsequent NF-κB activation (13–17). Dominant negative versions of NIK, in which the critical lysine has been mutated to alanine [NIK 429–430 (KK-AA)], act to inhibit NF-κB activation induced by Fas, TNF, and IL-1 (12). Given this, we asked whether NIK(KK-AA) could inhibit hToll-induced NF-κB activation. NIK(KK-AA) abrogated NF-κB activity triggered by hToll ectopic expression (Fig. 3 C) as well as by TRAF6 overexpression (not shown).
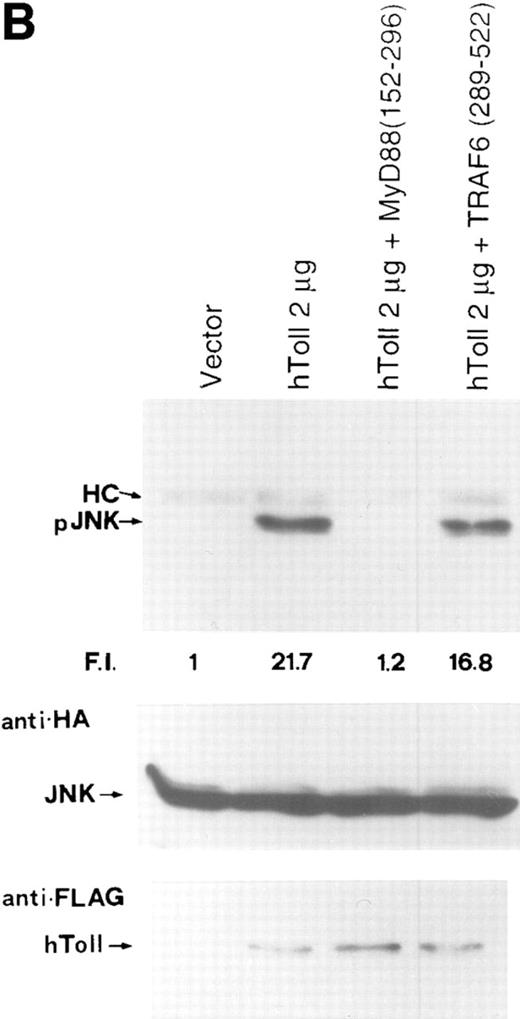
In addition to inducing activation of NF-κB, distinct inflammatory cytokines also induce SAPK, also known as JNK. The active phosphorylated form of SAPK binds to and phosphorylates the transcription factors c-Jun, activating transcription factor 2 (ATF2), and ternary complex factor (TCF)/Elk1 (23–25). In particular, activation of SAPK/ JNK by the TNFR-1 occurs through a TRAF2-dependent pathway, as a dominant negative version of TRAF2 acts to inhibit both NF-κB and c-Jun activation induced by TNF (18, 26, 27). In contrast, a dominant negative version of NIK, which abrogates TNF-induced NF-κB activation, fails to inhibit c-Jun phosphorylation, supporting a model wherein TNF-mediated NF-κB and c-Jun pathways bifurcate at TRAF2 (28, 29). Therefore, we analyzed whether hToll induced SAPK/JNK. Ectopic expression of increasing amounts of hToll-Flag but not ΔhToll-Flag resulted in activation of SAPKγ as indicated by specific phosphorylation at Thr 183 and Tyr 185 (Fig. 4,A). Overexpression of TRAF6 also activated SAPK as described previously (28). To identify mediators of hToll-mediated JNK activation, we cotransfected 293 cells with hToll and dominant negative versions of either MyD88 or TRAF6. Surprisingly, ΔMyD88 but not ΔTRA6 acted to inhibit hToll-triggered JNK phosphorylation (Fig. 4,B). Importantly, under the same experimental conditions, both ΔMyD88 and ΔTRAF6 abrogated hToll-induced NF-κB activation (96 and 90% inhibition, respectively). Additionally, ΔTRAF6 alone, as well as ΔMyD88, failed to activate JNK (data not shown). Collectively, these observations indicate that although ectopic expression of TRAF6 induced SAPK, a dominant negative version of TRAF6 failed to inhibit hToll-induced JNK phosphorylation. Given this, one could speculate that although TRAF6 overexpression is sufficient to activate JNK/ SAPK, endogenous TRAF6 does not provide a significant contribution to JNK/SAPK activation by hToll (Fig. 5).
Overview of hToll and TNFR-1 signaling pathways. The diagram shows that the bifurcation of NF-κB and JNK/SAPK activation by hToll or TNFR-1 occurs at different levels with respect to the specific TRAFs. TRADD, TNFR-1–associated death domain. RIP, Receptor-interacting protein.
Overview of hToll and TNFR-1 signaling pathways. The diagram shows that the bifurcation of NF-κB and JNK/SAPK activation by hToll or TNFR-1 occurs at different levels with respect to the specific TRAFs. TRADD, TNFR-1–associated death domain. RIP, Receptor-interacting protein.
hToll-induced SAPK activation requires MyD88 but not TRAF6. (A) hToll activates SAPK/JNK in a dose-dependent manner, as determined by the presence of the active phosphorylated form of JNK (pJNK). F.I., Fold induction calculated by normalizing pJNK with JNK. HC, IgG heavy chain. (B) MyD88 (152–296) (ΔMyD88) but not TRAF6 (289–522) (ΔTRAF6) abolishes hToll-induced SAPK/JNK phosphorylation. F.I., Fold induction calculated by normalizing pJNK with JNK. HC, IgG heavy chain.
hToll-induced SAPK activation requires MyD88 but not TRAF6. (A) hToll activates SAPK/JNK in a dose-dependent manner, as determined by the presence of the active phosphorylated form of JNK (pJNK). F.I., Fold induction calculated by normalizing pJNK with JNK. HC, IgG heavy chain. (B) MyD88 (152–296) (ΔMyD88) but not TRAF6 (289–522) (ΔTRAF6) abolishes hToll-induced SAPK/JNK phosphorylation. F.I., Fold induction calculated by normalizing pJNK with JNK. HC, IgG heavy chain.
Regardless, MyD88 appears to represent the most upstream mediator of the hToll-mediated signaling cascade, which ultimately activates NF-κB and c-Jun, thus driving transcriptional activation of several cytokines and costimulatory molecules. Therefore, it may represent a potentially useful therapeutic target for controlling the molecular switch from the innate to the adaptive immune response.
Acknowledgments
We wish to thank R. Medzhitov and C.A. Janeway for CD4/Toll cDNA, James Woodgett for HA-SAPKγ-pCDNA3, and Z. Cao for reagents and for critical reading of the manuscript.
References
M. Muzio is supported by Federazione Italiana Ricerca sul Cancro. This work was supported by European Community Special Project Biotechnology, Consiglio Nazionale Ricerche, and Associazione Italiana Ricerca sul Cancro.
Author notes
Address correspondence to Marta Muzio, Dept. Immunology and Cell Biology, Mario Negri Institute, via Eritrea 62, I-20157, Milano, Italy. Phone: 39-2-39014532; Fax: 39-2-3546277; E-mail: [email protected]






![Figure 3. NF-κB activation by hToll occurs through IRAK, TRAF6, and NIK. (A) IRAK is recruited to hToll but not to the inactive truncated version of hToll (ΔhToll). IL-1RAcP served as a positive control. Cells were transfected and analyzed as in Fig. 2 B. (B) IRAK-2 binds very weakly to hToll. IL-1RI served as a positive control. Cells were transfected and analyzed as in A. (C) ΔTRAF6 but not the unrelated ΔTRAF2 attenuates CD4/Toll-induced NF-κB activity. A dominant negative mutant version of the downstream molecule NIK [NIK(KK-AA)] also inhibits CD4/hToll-induced NF-κB activity.](https://cdn.rupress.org/rup/content_public/journal/jem/187/12/10.1084_jem.187.12.2097/5/m_jem980326_f3.jpeg?Expires=1771339817&Signature=F14jmBBWpXH~AbutNemJYaAiJ-gpjxpN5e4n8EiSMzwV7HPpuB3nCD0IuhgQTWSbLluyatZwwIAxZ-MkcpIwf1fncmLxHGJSyVvC35F~hCjvDZiCCxfWNf0A6zx-zVvRgyCIzxCS7ZHgFECNOJVPKm9vfMogJ841swxcCYbK-dju1douqjg~9yhTRAY0S24rECfyToD4sGXIuLBm50FaWuC7lNM5kv5kGXnCCzEG8QnKhaIrBpQ1LGRiQermSYYKHeUAYRmCPK0HfVkKmUDtS2Cr79mjfvg8WhlEU0DHfjozxXby26ElzL7C1RAvpPWvF4ChtNLx5R8BrOnUikCMWg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)