Lysosome-related organelles (LROs) are specialized lysosomes with cell type–specific roles in organismal homeostasis. Dysregulation of LROs leads to many human disorders, but the mechanisms underlying their biogenesis are not fully understood. Here, we identify a group of LYSMD proteins as evolutionarily conserved regulators of LROs. In Caenorhabditis elegans, mutations of LMD-2, a LysM domain–containing protein, reduce the levels of the Rab32 GTPase ortholog GLO-1 on intestine-specific LROs, the gut granules, leading to their abnormal enlargement and defective biogenesis. LMD-2 interacts with GLO-3, a subunit of GLO-1 guanine nucleotide exchange factor (GEF), thereby promoting GLO-1 activation. Mammalian homologs of LMD-2, LYSMD1, and LYSMD2 can functionally replace LMD-2 in C. elegans. In mammals, LYSMD1/2 physically interact with the HPS1 subunit of BLOC-3, the GEF of Rab32/38, thus promoting Rab32 activation. Inactivation of both LYSMD1 and LYSMD2 reduces Rab32 activation, causing melanosome enlargement and decreased melanin production in mouse melanoma cells. These findings provide important mechanistic insights into LRO biogenesis and functions.
Introduction
Lysosome-related organelles (LROs) are cell type–specific organelles with unique contents, structures, and functions. Examples of LROs include melanosomes in pigment cells, dense and alpha granules in platelets, Weibel-Palade bodies in endothelial cells, and lytic granules in cytotoxic T cells. They share certain features with endosomes and lysosomes, as they are derived from endosomal and secretory systems to meet the specific needs of diverse cell types (Bowman et al., 2019; Delevoye et al., 2019). Defective biogenesis or dysfunction of LROs leads to a wide variety of human disorders such as Hermansky-Pudlak syndrome (HPS), which is characterized by albinism and excessive bleeding, and Chediak-Higashi syndrome, which manifests as congenital immunodeficiency, bleeding, albinism, and progressive neurodegeneration (Bowman et al., 2019; Li et al., 2022; Talbert et al., 2023).
The biogenesis of LROs is best exemplified with melanosomes. Genetic studies in mice and humans revealed that loss-of-function mutations in any of the subunits of four protein complexes—adaptor protein 3 (AP-3), biogenesis of LRO complexes (BLOC) −1, −2, and −3—lead to defective melanosome biogenesis (Bowman et al., 2019; Bultema et al., 2012; Delevoye et al., 2019; Ma et al., 2021). Mechanistically, the AP-3 complex is responsible for sorting of endosomal cargos (TYR, OCA2, etc.) to be targeted to maturing melanosomes. AP-3 also delivers cargo from the Golgi apparatus to melanosomes (Bowman et al., 2019; Ma et al., 2021; Sitaram and Marks, 2012). BLOC-1, together with AP-3, is engaged in formation of endosomal tubules for cargo transport to melanosomes (Di Pietro et al., 2006). BLOC-1 interacts with the kinesin motor KIF13A and/or the WASH complex (Delevoye et al., 2016; Ryder et al., 2013). BLOC-2 functions to direct BLOC-1–dependent endosomal tubules to maturing melanosomes (Bultema et al., 2012; Delevoye et al., 2014; Dennis et al., 2015; Di Pietro et al., 2006). BLOC-3, which consists of HPS1 and HPS4 subunits, acts as the major guanine exchange factor (GEF) of Rab32/38 (Gerondopoulos et al., 2012; Martina et al., 2003). Rab32 and Rab38 may function redundantly to regulate biogenesis of melanosomes by coordinating the traffic in and out of melanosomes (Bowman et al., 2019; Ohbayashi et al., 2017). In addition, homotypic fusion and vacuole protein sorting (HOPS) complex and soluble N-ethylmaleimide–sensitive fusion attachment protein receptor (SNARE) proteins are required for biogenesis of melanosomes (Bowman et al., 2019; Delevoye et al., 2019; Jani et al., 2016; Le et al., 2021). Thus, the coordinated actions of these protein complexes and Rabs are essential for appropriate formation and function of melanosomes.
In the model organism Caenorhabditis elegans, gut granules are intestine-specific LROs that coexist with endosomes and lysosomes (Hermann et al., 2005). C. elegans gut granules/LROs are storage sites of metals (e.g., zinc, etc.) and other materials, which function in lipid transport, metabolism, signaling, and detoxification (Ardelli and Prichard, 2013; Chun et al., 2017; Folick et al., 2015; O’Rourke and Ruvkun, 2013; Panda et al., 2017; Raposo et al., 2007; Roh et al., 2012). The regulators of gut granule/LRO biogenesis include AP-3, BLOC-1, and HOPS complexes and are essentially similar to those involved in LRO biogenesis in mammals (Bowman et al., 2019; Bultema et al., 2012; Setty et al., 2007). The Rab32/38 homolog GLO-1 functions as the major GTPase for gut granule/LRO biogenesis (Hermann et al., 2005; Morris et al., 2018; Noda et al., 2023). GLO-3 and CCZ-1, which are weak C. elegans homologs of the BLOC-3 subunits HPS1 and HPS4, are also required. GLO-3 and CCZ-1 form a complex and function as the GEF of GLO-1 (Delevoye et al., 2014; Morris et al., 2018). These features make C. elegans an excellent model to identify novel regulators and dissect the underlying mechanisms of LRO biogenesis (Hermann et al., 2005). In this study, we performed unbiased genetic screening and identified a previously unknown factor, LMD-2, as an important regulator of gut granule/LRO biogenesis in C. elegans. We further revealed that mammalian homologs of LMD-2, LYSMD1, and LYSMD2 are essential for melanosome maturation and melanin production. Thus, LYSMD proteins promote the activation of Rab32-family GTPases in both C. elegans and mammalian cells. Our findings provide important mechanistic insights into the regulatory mechanisms of LRO biogenesis and functions.
Results
Loss of lmd-2 leads to formation of abnormal LROs in intestinal cells
C. elegans intestine contains a variety of intracellular organelles that are visible under differential interference contrast (DIC) optics, which provides an ideal system to study diverse endocytic organelles. To identify novel regulators that are engaged in endocytic homeostasis, we performed ethyl methanesulfonate (EMS) mutagenesis of C. elegans and screened for mutants that exhibit altered morphologies of organelles in adult intestine cells under DIC optics. A mutant named yq114, which contained greatly enlarged vacuoles in adult intestine, was identified (Fig. 1 A). A time-course examination indicated that yq114 mutants displayed enlarged intestinal vacuoles from the fourfold embryonic stage to adult stages (Fig. 1 A and Fig. S1 A). These vacuoles, which were not observed in wild-type (WT) animals, are distinct from the conventional button-like gut granules in the intestine under DIC optics (Fig. 1 A and Fig. S1 A). Genetic mapping and sequencing revealed that the yq114 mutation caused a g.764 C > T mutation in the lmd-2 gene and consequently a Q99stop premature termination mutation in the encoded protein (Fig. 1 B). LMD-2 is orthologous to the human LysM domain–containing proteins LYSMD1 and LYSMD2 (Fig. 1 B and Fig. S1 B), whose functions are not understood. Transgenic expression of LMD-2 fused with mCherry (mCh::LMD-2) under the control of its own promoter rescued the intestinal vacuoles in lmd-2(yq114) mutants (Fig. 1 C). tm6851, a deletion mutant of lmd-2, also exhibited similar enlargement of intestinal vacuoles (Fig. 1, A and B; and Fig. S1, A and B). Together, these results suggest that loss of lmd-2 causes abnormal enlargement of intestinal vacuoles.
Loss of lmd-2 leads to the enlargement of intestinal vesicles in C. elegans. (A) Representative DIC images of intestinal cells in WT, yq114, and tm6851 mutants at the indicated developmental stages. The boxed region is magnified (embryos and L4, 3×; L1, 2×; adult, 2.5×) at the bottom left in each image. Scale bars, 5 μm. Quantification of the diameters of intestinal vesicles containing autofluorescence materials (see also Fig. S1 A) is shown in the bar chart (upper right). 100 intestinal vesicles in 10 L4-stage animals were examined for each strain (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, **P < 0.01). (B) Schematic depiction of the lmd-2 gene (top) and comparison of the LMD-2 protein with human LYSMD1 and LYSMD2 proteins (bottom). The yq114 and tm6851 mutations in the lmd-2 gene and the LysM domain in the proteins are indicated. (C) Rescue of enlarged intestinal vacuoles in lmd-2(yq114) by transgenic Plmd-2mCh::LMD-2. Representative DIC and fluorescence images (left). Scale bars, 5 μm. Quantification (right) of diameters of 100 gut granules in L2 animals are shown for the indicated genotypes (mean ± SEM, n = 100 gut granules, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (D–F) Labeling of intestinal vesicle in WT and lmd-2(yq114) animals by early endosome markers (D) and late endosome/lysosome markers (E). The boxed region is magnified (4×) at the bottom right in each image. Scale bars, 5 μm. Quantification of organelle sizes in D and E is shown in F. 100 intestinal vesicles in 10 L4-stage animals were examined for each strain (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (G) Labeling (left) and quantification (right) of gut granules in WT and lmd-2(yq114) animals with LRO markers. The boxed region is magnified (2×) at the bottom right in each image. Scale bars, 5 μm. 100 intestinal vesicles in 10 L4-stage were examined for each marker (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001).
Loss of lmd-2 leads to the enlargement of intestinal vesicles in C. elegans. (A) Representative DIC images of intestinal cells in WT, yq114, and tm6851 mutants at the indicated developmental stages. The boxed region is magnified (embryos and L4, 3×; L1, 2×; adult, 2.5×) at the bottom left in each image. Scale bars, 5 μm. Quantification of the diameters of intestinal vesicles containing autofluorescence materials (see also Fig. S1 A) is shown in the bar chart (upper right). 100 intestinal vesicles in 10 L4-stage animals were examined for each strain (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, **P < 0.01). (B) Schematic depiction of the lmd-2 gene (top) and comparison of the LMD-2 protein with human LYSMD1 and LYSMD2 proteins (bottom). The yq114 and tm6851 mutations in the lmd-2 gene and the LysM domain in the proteins are indicated. (C) Rescue of enlarged intestinal vacuoles in lmd-2(yq114) by transgenic Plmd-2mCh::LMD-2. Representative DIC and fluorescence images (left). Scale bars, 5 μm. Quantification (right) of diameters of 100 gut granules in L2 animals are shown for the indicated genotypes (mean ± SEM, n = 100 gut granules, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (D–F) Labeling of intestinal vesicle in WT and lmd-2(yq114) animals by early endosome markers (D) and late endosome/lysosome markers (E). The boxed region is magnified (4×) at the bottom right in each image. Scale bars, 5 μm. Quantification of organelle sizes in D and E is shown in F. 100 intestinal vesicles in 10 L4-stage animals were examined for each strain (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (G) Labeling (left) and quantification (right) of gut granules in WT and lmd-2(yq114) animals with LRO markers. The boxed region is magnified (2×) at the bottom right in each image. Scale bars, 5 μm. 100 intestinal vesicles in 10 L4-stage were examined for each marker (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001).
Characterization of intestinal vesicles in lmd-2 mutants and LMD-2 protein. (A) Representative DIC and autofluorescence images shown in Fig. 1 A of intestinal cells of WT and mutant animals of indicated development stages. Arrowheads indicate intestinal vesicles with autofluorescence that were analyzed for diameters and shown in the right panel of Fig. 1 A. Scale bars, 5 μm. (B) Alignment of amino acid sequences of C. elegans LMD-2 and human LYSMD1 and LYSMD2. Identical amino acid residues are shaded in black. LysM domains are framed with red boxes.
Characterization of intestinal vesicles in lmd-2 mutants and LMD-2 protein. (A) Representative DIC and autofluorescence images shown in Fig. 1 A of intestinal cells of WT and mutant animals of indicated development stages. Arrowheads indicate intestinal vesicles with autofluorescence that were analyzed for diameters and shown in the right panel of Fig. 1 A. Scale bars, 5 μm. (B) Alignment of amino acid sequences of C. elegans LMD-2 and human LYSMD1 and LYSMD2. Identical amino acid residues are shaded in black. LysM domains are framed with red boxes.
To determine the identity of the abnormal vacuoles in lmd-2 mutants, we introduced into lmd-2(yq114) animals transgenic arrays expressing GFP-tagged markers of different intracellular organelles. The early endosome markers (GFP::RAB-5 and GFP::2xFYVE), but not the recycling endosome markers (GFP::RAB-10 and GFP::RAB-11.1), labeled the enlarged intestinal vacuoles at discernible levels in lmd-2(yq114) animals, with a few GFP::RAB-5 and GFP::2xFYVE puncta positioned near the LROs (Fig. 1, D and F; and Fig. S2, A and B). Intriguingly, the enlarged vacuoles were positive for GFP::RAB-7, a characteristic small GTPase on late endosomes/lysosomes, but not the lysosomal cystine transporter CTNS-1 fused with GFP (CTNS-1::GFP) (Town et al., 1998) (Fig. 1, E and F; and Fig. S2 C). Similarly, we examined whether the enlarged intestinal vacuoles could be labeled by proteins that mark gut granules/LROs. GLO-1 is the C. elegans homolog of Rab32/38, the specific Rab GTPases on LROs in mammals. GLO-1 with a C-terminal GFP tag (GLO-1::GFP), which was expressed by the hjIs9 integrated array, localizes to LROs (Hermann et al., 2005; Zhang et al., 2010). In lmd-2(yq114) animals, the enlarged vacuoles were positive for labeling by GLO-1::GFP (Fig. 1 G and Fig. S2 D). Several other GFP-tagged LRO proteins, including the zinc transporter CDF-2 (CDF-2::GFP) (Davis et al., 2009), the membrane carboxylesterase CEST-2.2 (CEST-2.2::GFP) (Le et al., 2020; Piazzesi et al., 2022), and the ATPase-coupled transmembrane transporter PGP-2 (GFP::PGP-2) (Schroeder et al., 2007), also labeled the abnormal vacuoles in lmd-2 mutants (Fig. 1 G and Fig. S2, D and E). These results suggest that the enlarged intestinal vacuoles in lmd-2 mutants are either endosomes or LROs, or both. To distinguish between these possibilities, we investigated the requirement for key regulators of endosomal trafficking and LRO biogenesis in formation of these abnormal intestinal vacuoles. Consistent with the partial labeling of lmd-2(yq114) vacuoles by GFP::RAB-5 (Fig. 1 D), RNAi knockdown of rab-5 significantly reduced the sizes of the vacuoles labeled by GLO-1::GFP (Fig. 2 A). This suggests that these abnormal vacuoles were likely derived from early endosomes (Marks et al., 2013; Setty et al., 2007). In contrast, RNAi of rab-7 or sand-1, which encodes a RAB-7 GEF component, did not affect lmd-2(yq114) vacuoles (Fig. 2 A). RNAi of rab-10 or rab-11.1 had no obvious effect on the enlarged vacuoles in lmd-2(yq114) mutants either. These data suggest that the enlarged intestinal vacuoles did not result from late endosomes or recycling endosomes (Fig. 2 A).
Characterization of enlarged gut granules in lmd-2(yq114) mutants. (A) Labeling of gut granules in WT and lmd-2(yq114) animals by the recycling endosome markers GFP::RAB-10 and GFP::Rab-11.1. Fluorescence and DIC images are shown. The boxed region is magnified at the bottom right in each image. Scale bars, 5 μm. (B–D) Fluorescence images shown in Fig. 1, D, E, and G are shown with corresponding DIC images. The boxed region is magnified at the bottom right in each image. Scale bars, 5 μm. (E) Representative images of CEST-2.2::GFP labeling of gut granules indicated with autofluorescence (blue) in WT and lmd-2(yq114) mutants. Scale bars, 5 μm.
Characterization of enlarged gut granules in lmd-2(yq114) mutants. (A) Labeling of gut granules in WT and lmd-2(yq114) animals by the recycling endosome markers GFP::RAB-10 and GFP::Rab-11.1. Fluorescence and DIC images are shown. The boxed region is magnified at the bottom right in each image. Scale bars, 5 μm. (B–D) Fluorescence images shown in Fig. 1, D, E, and G are shown with corresponding DIC images. The boxed region is magnified at the bottom right in each image. Scale bars, 5 μm. (E) Representative images of CEST-2.2::GFP labeling of gut granules indicated with autofluorescence (blue) in WT and lmd-2(yq114) mutants. Scale bars, 5 μm.
LMD-2 functions in the GLO-dependent LRO biogenesis pathway. (A) Images (left) and quantification (right) of lmd-2(yq114) LROs (labeled with GLO-1::GFP) treated with RNAi to knock down different endosomal regulators. Scale bars, 5 μm. 100 intestinal vesicles in 10 L4-stage were examined for RNAi treatment (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (B and C) Images (B) and quantification (C) of LROs (labeled with PGP-2::GFP) in WT, lmd-2(yq114) single mutants, glo-1(zu437) single mutants, and double mutants of lmd-2(yq114) with glo-1(zu437). Scale bars, 5 μm. 100 intestinal vesicles in 10 L4-stage were examined for each mutant (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001). (D) Images (left) and quantification (right) of LROs (labeled with GLO-1::GFP) in WT, lmd-2(yq114), sand-1(ok1963), ccz-1(ok2182), and glo-3(kx94) single mutants and double mutants of lmd-2 with sand-1, ccz-1, and glo-3. Scale bars, 5 μm. 100 intestinal vesicles in 10 L4-stage were examined for each mutant (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001; ns: not significant). (E) Rescue of enlarged LROs (labeled with GLO-1::GFP) by transgenic Plmd-2mCh::LMD-2. Representative fluorescence images (left). Scale bars, 5 μm. For quantification (right), 100 intestinal vesicles in 10 L4-stage were examined (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001). (F) Emission spectrum analysis of LRO autofluorescence in WT and lmd-2(tm6851) animals. Representative images of LROs with emission at 405 nm (blue), 488 nm (green), and 561 nm (red) are shown on the left. Scale bars, 5 μm. Consecutive scanning profiles of autofluorescence are shown on the right. (G) Representative images (left) and quantification (right) of staining by acridine orange, LysoTracker Red, FluoZin-3, and Nile red in WT and lmd-2(tm6851) intestine. Scale bars, 5 μm. For quantification, 30 LROs were examined (mean ± SEM, n = 30 LROs, unpaired two-tailed t test, ***P < 0.001).
LMD-2 functions in the GLO-dependent LRO biogenesis pathway. (A) Images (left) and quantification (right) of lmd-2(yq114) LROs (labeled with GLO-1::GFP) treated with RNAi to knock down different endosomal regulators. Scale bars, 5 μm. 100 intestinal vesicles in 10 L4-stage were examined for RNAi treatment (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (B and C) Images (B) and quantification (C) of LROs (labeled with PGP-2::GFP) in WT, lmd-2(yq114) single mutants, glo-1(zu437) single mutants, and double mutants of lmd-2(yq114) with glo-1(zu437). Scale bars, 5 μm. 100 intestinal vesicles in 10 L4-stage were examined for each mutant (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001). (D) Images (left) and quantification (right) of LROs (labeled with GLO-1::GFP) in WT, lmd-2(yq114), sand-1(ok1963), ccz-1(ok2182), and glo-3(kx94) single mutants and double mutants of lmd-2 with sand-1, ccz-1, and glo-3. Scale bars, 5 μm. 100 intestinal vesicles in 10 L4-stage were examined for each mutant (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001; ns: not significant). (E) Rescue of enlarged LROs (labeled with GLO-1::GFP) by transgenic Plmd-2mCh::LMD-2. Representative fluorescence images (left). Scale bars, 5 μm. For quantification (right), 100 intestinal vesicles in 10 L4-stage were examined (mean ± SEM, n = 100 intestinal vesicles, unpaired two-tailed t test, ***P < 0.001). (F) Emission spectrum analysis of LRO autofluorescence in WT and lmd-2(tm6851) animals. Representative images of LROs with emission at 405 nm (blue), 488 nm (green), and 561 nm (red) are shown on the left. Scale bars, 5 μm. Consecutive scanning profiles of autofluorescence are shown on the right. (G) Representative images (left) and quantification (right) of staining by acridine orange, LysoTracker Red, FluoZin-3, and Nile red in WT and lmd-2(tm6851) intestine. Scale bars, 5 μm. For quantification, 30 LROs were examined (mean ± SEM, n = 30 LROs, unpaired two-tailed t test, ***P < 0.001).
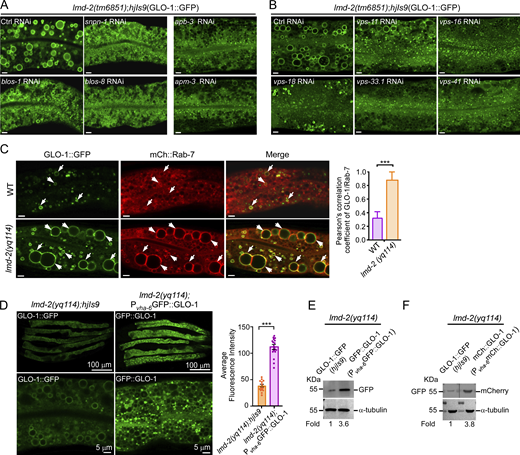
We next investigated the requirement for the Rab32/38-family GTPase GLO-1 and its regulators in the enlargement of intestinal vacuoles in lmd-2 mutants. Compared with lmd-2 single mutants, neither glo-1(zu437) single mutants nor lmd-2(yq114);glo-1(zu437) double mutants exhibited enlarged vacuoles labeled with PGP-2::GFP (Fig. 2, B and C), suggesting that GLO-1 is required for the vacuole enlargement in lmd-2 mutants. Similarly, no enlargement of GLO-1::GFP-labeled vacuoles was observed in ccz-1(ok2182) or glo-3(kx94) single mutants or their double mutants with lmd-2(yq114) (Fig. 2 D). Because CCZ-1 and GLO-3 act in a complex as the GEF of GLO-1 (Morris et al., 2018), these findings suggest that the enlarged intestinal vacuoles in lmd-2 mutants are abnormal LROs, the formation of which is dependent on GLO-1 and its regulators. Supporting this conclusion, RNAi inactivation of genes that encode proteins essential for LRO biogenesis, including the APB-3 and APM-3 subunits of the AP-3 complex, the SNPN-1, BLOS-1, and BLOS-8 subunits of the BLOC-1, and the VPS-11, -16, -18, -33.1 and -41 of the HOPS complex, inhibited the abnormal enlargement of LROs in lmd-2 mutants (Fig. S3, A and B).
Engagement of BLOC-3, AP-3, HOPS, RAB-7, and GLO-1 in formation of abnormal LROs in lmd-2 mutants. (A) Representative images of LROs (labeled with GLO-1::GFP) in lmd-2(tm6851) mutants treated with RNAi to knock down BLOC-1 subunits (snpn-1, blos-1, and blos-8) and AP-3 subunits (apb-3 and apm-3). Scale bars, 5 μm. (B) Representative images of LROs (labeled with GLO-1::GFP) in lmd-2(tm6851) mutants treated with RNAi to knock down HOPS subunits. Scale bars, 5 μm. (C) Representative images (left) and quantification (right) of colocalization of GLO-1::GFP with mCh::RAB-7 in WT and lmd-2(yq114) mutants. Arrows indicate the vesicles with GLO-1::GFP and mCh::Rab-7 colocalization. Scale bars, 5 μm. 20 LROs were chosen to analyze the Pearson’s correlation coefficient of GLO-1/Rab-7 (mean ± SEM, n = 20 LROs, unpaired two-tailed t test, ***P < 0.001). (D) Left: Comparison of fluorescence intensity between GLO-1::GFP (hjIs9) and GFP::GLO-1(Pvha-6GFP::GLO-1) in lmd-2(yq114) mutants (top row), scale bars, 100 μm, and the rescue of enlarged LROs by GFP::GLO-1 in lmd-2(yq114) mutants (bottom row), scale bars, 5 μm. Right: Quantification of fluorescence intensity of GLO-1::GFP (hjIs9) and GFP::GLO-1(Pvha-6GFP::GLO-1) in lmd-2(yq114) mutants. Fluorescence intensity was measured in 15 animals for each group (mean ± SEM, n = 15 animals, unpaired two-tailed t test, ***P < 0.001). (E) Western blotting of GLO-1::GFP (hjIs9) and GFP::GLO-1(Pvha-6GFP::GLO-1) in lmd-2 mutants. (F) Western blotting of GLO-1::GFP (hjIs9) and mCh::GLO-1(Pvha-6mCh::GLO-1) in lmd-2 mutants. Total lysates from lmd-2(yq114); hjIs9 and lmd-2(yq114); Pvha-6mCh:: GLO-1 animals were resolved on the same membrane. The membrane was cut into halves and botted with GFP antibody and mCh antibody, respectively (top row). In E and F, protein levels were normalized to that of α-tubulin, and the fold change is indicated. Source data are available for this figure: SourceData FS3.
Engagement of BLOC-3, AP-3, HOPS, RAB-7, and GLO-1 in formation of abnormal LROs in lmd-2 mutants. (A) Representative images of LROs (labeled with GLO-1::GFP) in lmd-2(tm6851) mutants treated with RNAi to knock down BLOC-1 subunits (snpn-1, blos-1, and blos-8) and AP-3 subunits (apb-3 and apm-3). Scale bars, 5 μm. (B) Representative images of LROs (labeled with GLO-1::GFP) in lmd-2(tm6851) mutants treated with RNAi to knock down HOPS subunits. Scale bars, 5 μm. (C) Representative images (left) and quantification (right) of colocalization of GLO-1::GFP with mCh::RAB-7 in WT and lmd-2(yq114) mutants. Arrows indicate the vesicles with GLO-1::GFP and mCh::Rab-7 colocalization. Scale bars, 5 μm. 20 LROs were chosen to analyze the Pearson’s correlation coefficient of GLO-1/Rab-7 (mean ± SEM, n = 20 LROs, unpaired two-tailed t test, ***P < 0.001). (D) Left: Comparison of fluorescence intensity between GLO-1::GFP (hjIs9) and GFP::GLO-1(Pvha-6GFP::GLO-1) in lmd-2(yq114) mutants (top row), scale bars, 100 μm, and the rescue of enlarged LROs by GFP::GLO-1 in lmd-2(yq114) mutants (bottom row), scale bars, 5 μm. Right: Quantification of fluorescence intensity of GLO-1::GFP (hjIs9) and GFP::GLO-1(Pvha-6GFP::GLO-1) in lmd-2(yq114) mutants. Fluorescence intensity was measured in 15 animals for each group (mean ± SEM, n = 15 animals, unpaired two-tailed t test, ***P < 0.001). (E) Western blotting of GLO-1::GFP (hjIs9) and GFP::GLO-1(Pvha-6GFP::GLO-1) in lmd-2 mutants. (F) Western blotting of GLO-1::GFP (hjIs9) and mCh::GLO-1(Pvha-6mCh::GLO-1) in lmd-2 mutants. Total lysates from lmd-2(yq114); hjIs9 and lmd-2(yq114); Pvha-6mCh:: GLO-1 animals were resolved on the same membrane. The membrane was cut into halves and botted with GFP antibody and mCh antibody, respectively (top row). In E and F, protein levels were normalized to that of α-tubulin, and the fold change is indicated. Source data are available for this figure: SourceData FS3.
In contrast to ccz-1(ok2182) deletion, deletion of sand-1 did not suppress the formation of enlarged LROs in lmd-2(yq114);sand-1(ok1963) double mutants (Fig. 2 D), which is consistent with the effect of sand-1 RNAi (Fig. 2 A). Thus, although SAND-1 and CCZ-1 form a complex to promote RAB-7 activation in the endosome–lysosome pathway, SAND-1 and RAB-7 do not act with LMD-2 in the LRO biogenesis pathway. This is consistent with previous findings that both RAB-7 and SAND-1 do not have a significant role in LRO biogenesis (Delahaye et al., 2014). Thus, the presence of RAB-7 on the enlarged LROs (Fig. 1 E) probably resulted from a mislocalization of RAB-7 to LROs in the absence of lmd-2. In agreement with this assumption, we found that the colocalization of RAB-7 with GLO-1 was significantly increased in lmd-2(yq114) mutants compared with that in WT animals (Fig. S3 C). Supporting the conclusion that LMD-2 regulates LRO biogenesis, transgenic expression of mCh::LMD-2, which is driven by lmd-2 promoter and fully rescues the enlargement of LROs in lmd-2 mutants, colocalized with GLO-1::GFP-labeled LROs (Fig. 1 C and Fig. 2 E).
The contents of C. elegans gut granules are autofluorescent; therefore, we examined the autofluorescence of the enlarged LROs in lmd-2 mutants by performing emission spectrum scanning. Compared with WT animals, lmd-2(tm6851) mutants had strongly decreased emission fluorescence signals at 405, 488, and 561 nm (Fig. 2 F). Moreover, the enlarged LROs in lmd-2(tm6851) intestine were stained much less intensely by the dyes LysoTracker Red, FluoZin-3, Nile red, and acridine orange than in WT animals (Fig. 2 G). These results suggest that the abnormal LROs in lmd-2 mutants are defective in formation.
LMD-2 regulates LRO biogenesis through GLO-1/Rab32
GLO-1 is the major GTPase that controls gut granule/LRO biogenesis in C. elegans (Morris et al., 2018). Following the above genetic analyses, we tested whether reinforced expression of GLO-1 was able to rescue the defective LROs in lmd-2 mutants. Although expression of GLO-1::GFP by the integrated hjIs9 array did not obviously affect the size of lmd-2(yq114) LROs (Fig. 1 G), reinforced expression of GLO-1 with N-terminally tagged GFP (GFP::GLO-1) or mCherry (mCh::GLO-1) driven by the intestine-specific vha-6 promoter, which exhibited much higher protein expression levels, strongly suppressed the enlargement of LROs in lmd-2(yq114) mutants (Fig. 3 A and Fig. S3, D–F). Consistent with this, the enlargement LROs (labeled with CDF-2::GFP) in lmd-2(yq114) animals was strongly suppressed by similar expression of the constitutively active mCh::GLO-1(Q71L) and mCh::GLO-1(D132A), but not the constitutively inactive GLO-1(T25N) (Morris et al., 2018) (Fig. 3 A). In addition, reinforced expression of mCh-tagged components of the GLO-1 GEF complex, GLO-3 and CCZ-1, did not rescue the defective LROs in lmd-2 mutants (Fig. 3 B). These results suggest that LMD-2 is essential for the functional efficiency of the GLO-1–GEF complex, underscoring its significance in regulating LRO biogenesis through GLO-1.
LMD-2 acts through GLO-1. (A and B) Rescuing effect on lmd-2(yq114) LROs (labeled with CDF-2::GFP) by mCh::GLO-1(WT), mCh::GLO-1(Q71L) (active form), mCh::GLO-1(D132A) (constitutively active form), mCh::GLO-1(T25N) (inactive form) (A), and GLO-3::mCh and CCZ-1::mCh (B). Representative fluorescence images (left). Scale bars, 5 μm. For quantifications (right), 100 total LROs were examined for each group (mean ± SEM, n = 100 LROs, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (C) Western blotting of knock-in (KI) GFPKI::GLO-1 in WT and lmd-2(yq114) mutants. (D) Loss of LMD-2 reduces GLO-1 levels on LROs. Left: GFPKI::GLO-1 fluorescence images of LROs in WT and lmd-2(yq114) mutants. White and yellow arrowheads indicate representative vesicles with diameter (d) >2 μm and d ≤2 μm, respectively. Scale bars, 5 μm. Middle: Quantification of GFPKI::GLO-1 around the entire perimeter (360°) of each LRO. Right: Quantification of average GFPKI::GLO-1 intensity on individual LROs. 30 LROs were examined in WT animals (mean ± SEM, n = 30, unpaired two-tailed t test, **P < 0.01, ***P < 0.001). In lmd-2(yq114) animals, 30 LROs with d ≤2 μm and 30 LROs with d >2 μm were examined (mean ± SEM, n = 30 LROs, unpaired two-tailed t test, **P < 0.01, ***P < 0.001). (E) Fluorescence images of CDF-2::GFP (left). Scale bars, 5 μm. For quantification of CDF-2::GFP around the entire perimeter (360°) of each LRO (middle), and quantification of average CDF-2::GFP intensity on individual LROs (right) in WT and lmd-2(yq114) animals, 30 LROs in WT animals, and 30 LROs with d ≤ 2 μm and 30 LROs with d > 2 μm in lmd-2 mutants were examined for CDF-2::GFP intensity as in D (mean ± SEM, n = 30 LROs, unpaired two-tailed t test, ns: not significant). (F) Rescue of GFPKI::GLO-1–labeled defective LROs in lmd-2(yq114) mutants by transgenic expression of mCh::LMD-2 (Plmd-2mCh::LMD-2). Scale bars, 5 μm. Quantification of GFPKI::GLO-1 intensity on LROs is shown on the right (mean ± SEM, n = 30 LROs, unpaired two-tailed t test). Source data are available for this figure: SourceData F3.
LMD-2 acts through GLO-1. (A and B) Rescuing effect on lmd-2(yq114) LROs (labeled with CDF-2::GFP) by mCh::GLO-1(WT), mCh::GLO-1(Q71L) (active form), mCh::GLO-1(D132A) (constitutively active form), mCh::GLO-1(T25N) (inactive form) (A), and GLO-3::mCh and CCZ-1::mCh (B). Representative fluorescence images (left). Scale bars, 5 μm. For quantifications (right), 100 total LROs were examined for each group (mean ± SEM, n = 100 LROs, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (C) Western blotting of knock-in (KI) GFPKI::GLO-1 in WT and lmd-2(yq114) mutants. (D) Loss of LMD-2 reduces GLO-1 levels on LROs. Left: GFPKI::GLO-1 fluorescence images of LROs in WT and lmd-2(yq114) mutants. White and yellow arrowheads indicate representative vesicles with diameter (d) >2 μm and d ≤2 μm, respectively. Scale bars, 5 μm. Middle: Quantification of GFPKI::GLO-1 around the entire perimeter (360°) of each LRO. Right: Quantification of average GFPKI::GLO-1 intensity on individual LROs. 30 LROs were examined in WT animals (mean ± SEM, n = 30, unpaired two-tailed t test, **P < 0.01, ***P < 0.001). In lmd-2(yq114) animals, 30 LROs with d ≤2 μm and 30 LROs with d >2 μm were examined (mean ± SEM, n = 30 LROs, unpaired two-tailed t test, **P < 0.01, ***P < 0.001). (E) Fluorescence images of CDF-2::GFP (left). Scale bars, 5 μm. For quantification of CDF-2::GFP around the entire perimeter (360°) of each LRO (middle), and quantification of average CDF-2::GFP intensity on individual LROs (right) in WT and lmd-2(yq114) animals, 30 LROs in WT animals, and 30 LROs with d ≤ 2 μm and 30 LROs with d > 2 μm in lmd-2 mutants were examined for CDF-2::GFP intensity as in D (mean ± SEM, n = 30 LROs, unpaired two-tailed t test, ns: not significant). (F) Rescue of GFPKI::GLO-1–labeled defective LROs in lmd-2(yq114) mutants by transgenic expression of mCh::LMD-2 (Plmd-2mCh::LMD-2). Scale bars, 5 μm. Quantification of GFPKI::GLO-1 intensity on LROs is shown on the right (mean ± SEM, n = 30 LROs, unpaired two-tailed t test). Source data are available for this figure: SourceData F3.
We next investigated how loss of lmd-2 affects GLO-1 by examining the endogenous GLO-1 levels on LROs. To do so, we knocked in GFP at the N-terminus of GLO-1 (GFPKI::GLO-1) and measured the fluorescence intensity on LROs. While the total level of endogenous GLO-1 was not changed between WT and lmd-2(yq114) animals, GLO-1 intensities on LROs of varying sizes in lmd-2(yq114) mutants were greatly reduced, as measured by quantifying the fluorescence intensities around the entire perimeter (360°) of individual organelles of varying sizes and by quantifying the average GFPKI::GLO-1 fluorescence intensity of individual organelles (Fig. 3 D). In contrast, no obvious difference in CDF-2::GFP intensities on LROs was detected between lmd-2 mutants and WT animals (Fig. 3 E). Because membrane-localized GTPase represents the active GTP-bound form, these results suggest that loss of lmd-2 probably impaired GLO-1 activation on LROs. Consistent with this, transgenic mCh::LMD-2 fully rescued the reduction of endogenous GFP::GLO-1 levels on LROs as well as LRO enlargement in lmd-2 mutants (Fig. 3 F).
LMD-2 promotes the GEF activity of GLO-3–CCZ-1 for GLO-1 activation
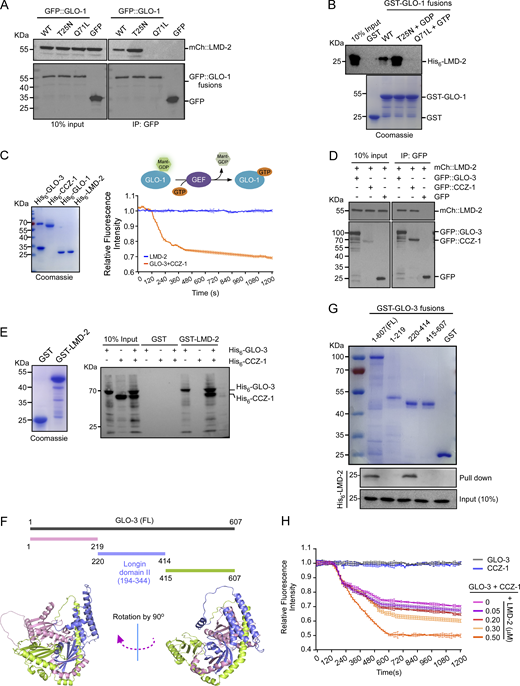
To understand how LMD-2 is engaged in GLO-1 activation, we examined if LMD-2 interacts with and activates GLO-1 directly. Co-immunoprecipitation (IP) assays in animals co-expressing mCh::LMD-2 with different forms (WT, inactive, and active) of GLO-1 tagged with GFP at the N-terminus indicated that LMD-2 was co-precipitated with WT and inactive (T25N), but not the active (Q71L), forms of GLO-1 (Fig. 4 A). Consistent with this, GST-fused GLO-1(WT) and GLO-1(T25N), but not GLO-1(Q71L), directly interacted with His6–LMD-2 in in vitro GST pull-down assays (Fig. 4 B). We thus tested if LMD-2 possesses GEF activity toward GLO-1. In in vitro GEF assays, however, His6-LMD-2 did not display pronounced GEF activities toward His6–GLO-1 compared with that of the GLO-3–CCZ-1 complex (Fig. 4 C). Thus, LMD-2 on its own does not act as a GEF to activate GLO-1.
LMD-2 interacts with the GEF complex of GLO-1 and promotes its activity. (A) Co-IP, using GFP antibody, of mCh::LMD-2 with GFP-tagged GLO-1(WT), GLO-1(T25N), and GLO-1(Q71L) from lysates of worms co-expressing mCh::LMD-2 with the indicated GFP::GLO-1 proteins. Precipitated proteins were detected with antibodies against GFP and mCh. (B) Purified His6–LMD-2 was incubated with GST–GLO-1(WT), GST–GLO-1(T25N), and GST–GLO-1(Q71L) bound on glutathione Sepharose beads in the absence or presence of GDP or GTP. After extensive washing, bound proteins were detected with His6 antibody (top). Input GST-fused proteins were stained with Coomassie blue (bottom). (C) LMD-2 possesses no GEF activity for GLO-1 activation. Purified proteins used in GEF assays are shown on the left. The schematic depicts the GEF assay (top right). The relative Mant-GDP fluorescence intensity reflects the GEF activity (bottom right). The value of each time point in the GEF assay was from three independent experiments (mean ± SD, n = 3 independent experiments). (D) Co-IP of mCh::LMD-2 with GFP::GLO-3 and GFP::CCZ-1 from lysates of worms co-expressing mCh::LMD-2 with GFP::GLO-3 or GFP::CCZ-1. IPs were performed with GFP antibody and precipitated proteins were detected with antibodies against GFP and mCh. (E) LMD-2 interacts with GLO-3 to form a tripartite complex with GLO-3 and CCZ-1. Purified His6–GLO-3 and His6–CCZ-1 were incubated with GST–LMD-2 (left) bound on glutathione Sepharose beads. After extensive washing, bound proteins were eluted and detected with His6 antibody (right). (F) Schematic representation of GLO-3 truncations (top) and the structure of GLO-3 predicted by using AlphaFold 3 (https://alphafoldserver.com) (bottom). In the bottom images, the Login domain II shared by mammalian HPS1 and C. elegans GLO-3 is shown in purple. The left image is rotated clockwise by 90° to yield the right image. (G) Purified full-length (FL) and truncated GLO-3 proteins fused with GST were bound on glutathione Sepharose beads (top) and incubated with His6–LMD-2. After extensive washing, bound proteins were eluted and detected with His6 antibody (bottom). (H) LMD-2 promotes GLO-3–CCZ-1 GEF activity for GLO-1 activation. The GEF activity of GLO-3–CCZ-1 complex on GLO-1 was examined as in C with increasing concentrations of LMD-2. The value of each time point in the GEF assay was from three independent experiments (mean ± SD, n = 3 independent experiments). Source data are available for this figure: SourceData F4.
LMD-2 interacts with the GEF complex of GLO-1 and promotes its activity. (A) Co-IP, using GFP antibody, of mCh::LMD-2 with GFP-tagged GLO-1(WT), GLO-1(T25N), and GLO-1(Q71L) from lysates of worms co-expressing mCh::LMD-2 with the indicated GFP::GLO-1 proteins. Precipitated proteins were detected with antibodies against GFP and mCh. (B) Purified His6–LMD-2 was incubated with GST–GLO-1(WT), GST–GLO-1(T25N), and GST–GLO-1(Q71L) bound on glutathione Sepharose beads in the absence or presence of GDP or GTP. After extensive washing, bound proteins were detected with His6 antibody (top). Input GST-fused proteins were stained with Coomassie blue (bottom). (C) LMD-2 possesses no GEF activity for GLO-1 activation. Purified proteins used in GEF assays are shown on the left. The schematic depicts the GEF assay (top right). The relative Mant-GDP fluorescence intensity reflects the GEF activity (bottom right). The value of each time point in the GEF assay was from three independent experiments (mean ± SD, n = 3 independent experiments). (D) Co-IP of mCh::LMD-2 with GFP::GLO-3 and GFP::CCZ-1 from lysates of worms co-expressing mCh::LMD-2 with GFP::GLO-3 or GFP::CCZ-1. IPs were performed with GFP antibody and precipitated proteins were detected with antibodies against GFP and mCh. (E) LMD-2 interacts with GLO-3 to form a tripartite complex with GLO-3 and CCZ-1. Purified His6–GLO-3 and His6–CCZ-1 were incubated with GST–LMD-2 (left) bound on glutathione Sepharose beads. After extensive washing, bound proteins were eluted and detected with His6 antibody (right). (F) Schematic representation of GLO-3 truncations (top) and the structure of GLO-3 predicted by using AlphaFold 3 (https://alphafoldserver.com) (bottom). In the bottom images, the Login domain II shared by mammalian HPS1 and C. elegans GLO-3 is shown in purple. The left image is rotated clockwise by 90° to yield the right image. (G) Purified full-length (FL) and truncated GLO-3 proteins fused with GST were bound on glutathione Sepharose beads (top) and incubated with His6–LMD-2. After extensive washing, bound proteins were eluted and detected with His6 antibody (bottom). (H) LMD-2 promotes GLO-3–CCZ-1 GEF activity for GLO-1 activation. The GEF activity of GLO-3–CCZ-1 complex on GLO-1 was examined as in C with increasing concentrations of LMD-2. The value of each time point in the GEF assay was from three independent experiments (mean ± SD, n = 3 independent experiments). Source data are available for this figure: SourceData F4.
We then investigated whether LMD-2 functions together with the GLO-3–CCZ-1 complex, the GEF of GLO-1. In co-IP assays, mCh::LMD-2 was co-immunoprecipitated with GFP::GLO-3 and GFP::CCZ-1 (Fig. 4 D). In GST pull-down assays, GST–LMD-2 pulled down His6–GLO-3 but not His6–CCZ-1. In the presence of His6–GLO-3, however, His6–CCZ-1 was pulled down by GST–LMD-2 (Fig. 4 E). These findings suggest that LMD-2 and GLO-3–CCZ-1 form a tripartite complex by directly interacting with GLO-3. Further GST pull-down assays revealed that LMD-2 binds to the middle region (aa 220–414) of GLO-3, which shares a conserved Longin II domain with mammalian HPS1 (Fig. 4, F and G; and Fig. S4) (Kinch and Grishin, 2006; Sanchez-Pulido and Ponting, 2020). Longin domains are engaged in membrane fusion, vesicle tethering and budding, and regulation of Rab GTPases (Daste et al., 2015). We thus assessed the GEF activity of GLO-3–CCZ-1 toward GLO-1 in the absence or presence of LMD-2. Like LMD-2, neither His6–GLO-3 nor His6–CCZ-1 alone had obvious GEF activities in vitro (Fig. 4 H). When both proteins were present, the GEF activities were detected (Fig. 4, C and H). Importantly, the GEF activities of the GLO-3–CCZ-1 complex toward GLO-1 were significantly enhanced by LMD-2 in a concentration-dependent manner (Fig. 4 H). Collectively, these results suggest that LMD-2 promotes GLO-1 activation through the GLO-3–CCZ-1 complex. Notably, the subcellular localization of either GLO-3 or CCZ-1 was not obviously changed in lmd-2 mutants compared with WT (Fig. 3 B and Fig. S5, A and B). Thus, LMD-2 probably fulfills the GLO-1 GEF-promoting function by directly interacting with the GLO-3–CCZ-1 complex (Fig. 4, E–H).
Alignment of amino acid sequences of C. elegans GLO-3 and human HPS1. Identical amino acid residues are shaded in black. Longin domains are framed with red and blue boxes.
Alignment of amino acid sequences of C. elegans GLO-3 and human HPS1. Identical amino acid residues are shaded in black. Longin domains are framed with red and blue boxes.
Loss of lmd-2 does not affect the subcellular localization of GLO-3 and CCZ-1. (A and B) Representative images (left) and quantification (right) of colocalization of GLO-1::GFP with GLO-3::mCh (A) and CCZ-1::mCh (B) in WT and lmd-2 mutants. Scale bars, 5 μm. For quantification, 20 LROs were analyzed for the Pearson’s correlation coefficient (mean ± SEM, n = 20 LROs, unpaired two-tailed t test, ns: not significant).
Loss of lmd-2 does not affect the subcellular localization of GLO-3 and CCZ-1. (A and B) Representative images (left) and quantification (right) of colocalization of GLO-1::GFP with GLO-3::mCh (A) and CCZ-1::mCh (B) in WT and lmd-2 mutants. Scale bars, 5 μm. For quantification, 20 LROs were analyzed for the Pearson’s correlation coefficient (mean ± SEM, n = 20 LROs, unpaired two-tailed t test, ns: not significant).
LMD-2 and mammalian LYSDM1/2 share evolutionarily conserved functions in LRO biogenesis
In mammals, LYSMD1 and LYSMD2 share similar LysM domains that are homologous to C. elegans LMD-2 (Fig. 1 B and Fig. S1 B). In addition, the major Rabs and GEF components required for LRO biogenesis appear to be similar between C. elegans and humans (Fig. 5 A). To determine whether LYSMD1/2 have similar functions to LMD-2, we heterologously expressed LYSMD1 or LYSMD2 in lmd-2 mutants. mCh-fused LYSMD1 or LYSMD2 strongly reduced the sizes of GFPKI::GLO-1–labeled LROs in lmd-2(yq114) mutants, suggesting that mammalian LYSMD1/2 and worm LMD-2 regulate biogenesis of LROs in an evolutionarily conserved manner (Fig. 5 B). In addition, we tested if heterologous expression of Rab32 can rescue the defective LROs in lmd-2 mutants. mCh-tagged WT Rab32 and constitutively active Rab32(Q85L), but not inactive Rab32(T39N), significantly suppressed the enlargement of LROs in lmd-2(yq114) mutants (Fig. 5 C). The similar rescuing effect of LYSM1/2 and Rab32 on defective LROs in lmd-2 mutants suggests the possibility that LYSMD1/2 also regulate LRO biogenesis through Rab32 in mammalian cells.
Overexpression of LYSMD1/2 or Rab32 (WT and active form) rescues the defective LROs in C. elegans lmd-2 mutants. (A) List of Rabs, GEFs, and LYSMD proteins required for LRO biogenesis in C. elegans and humans. (B) Representative images (left) and quantification (right) of LROs in WT, lmd-2(yq114), and lmd-2(yq114) animals overexpressing mCh::LYSMD1 or mCh::LYSMD2. LROs are labeled with GFPKI::GLO-1. Scale bars, 5 μm. Diameters of 100 total LROs were quantified for each group (mean ± SEM, n = 100 LROs, unpaired two-tailed t test, ***P < 0.001). (C) Representative images (left) and quantification (right) of LROs in WT, lmd-2(yq114), and lmd-2(yq114) animals overexpressing mCh::Rab32(WT), mCh::Rab32(Q85L), and mCh::Rab32(T25N). LROs are labeled with CDF-2::GFP and their diameters are quantified. Scale bars, 5 μm. 100 total LROs were examined for each group (mean ± SEM, n = 100 LROs, unpaired two-tailed t test, ***P < 0.001, ns: not significant).
Overexpression of LYSMD1/2 or Rab32 (WT and active form) rescues the defective LROs in C. elegans lmd-2 mutants. (A) List of Rabs, GEFs, and LYSMD proteins required for LRO biogenesis in C. elegans and humans. (B) Representative images (left) and quantification (right) of LROs in WT, lmd-2(yq114), and lmd-2(yq114) animals overexpressing mCh::LYSMD1 or mCh::LYSMD2. LROs are labeled with GFPKI::GLO-1. Scale bars, 5 μm. Diameters of 100 total LROs were quantified for each group (mean ± SEM, n = 100 LROs, unpaired two-tailed t test, ***P < 0.001). (C) Representative images (left) and quantification (right) of LROs in WT, lmd-2(yq114), and lmd-2(yq114) animals overexpressing mCh::Rab32(WT), mCh::Rab32(Q85L), and mCh::Rab32(T25N). LROs are labeled with CDF-2::GFP and their diameters are quantified. Scale bars, 5 μm. 100 total LROs were examined for each group (mean ± SEM, n = 100 LROs, unpaired two-tailed t test, ***P < 0.001, ns: not significant).
LYSMD1/2 interact with the BLOC-3 complex and promote its GEF activity for Rab32
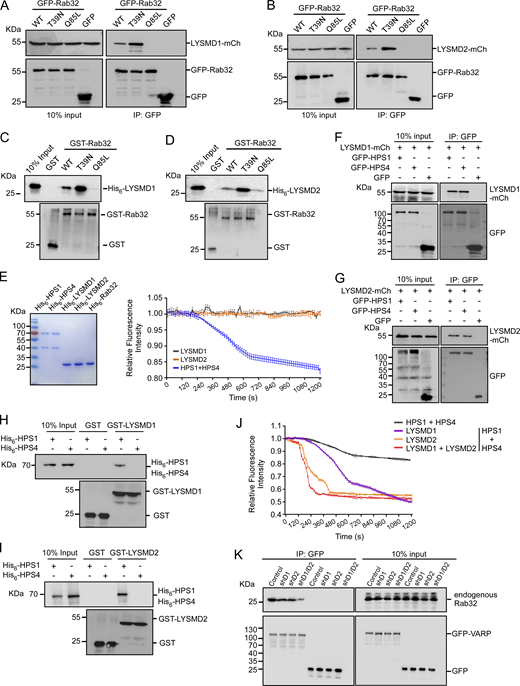
We next examined whether LYSDM1/2 interact with Rab32. In co-IP assays, LYSMD1-mCh and LYSMD2-mCh were co-precipitated with GFP-tagged Rab32(WT) and the inactive Rab32(T39N), but not the active Rab32(Q85L) (Fig. 6, A and B). In vitro, His6-tagged LYSMD1 and LYSMD2 were directly pulled down by GST-Rab32(WT) and GST-Rab32(T39N), but barely by GST-Rab32(Q85L) (Fig. 6, C and D). In both co-IP and GST pull-down assays, the inactive Rab32(T39N) had stronger interaction with LYSMD1/2, similar to the interaction of GLO-1(T25N) with LMD-2 in C. elegans (Fig. 4 A). Nevertheless, neither LYSMD1 nor LYSMD2 displayed obvious GEF activity for Rab32 in GEF assays (Fig. 6 E). Thus, LYSMD1/2 on their own cannot activate Rab32.
LYSMD1/2 interact with Rab32 and the BLOC-1 complex and promote the GEF activity of BLOC-1 to activate Rab32. (A and B) Co-IP, using GFP antibody, of mCh-tagged LYSMD1 (A) and LYSMD2 (B) with GFP-Rab32(WT), -Rab32(T39N), and -Rab32(Q85L) from lysates of HeLa cells coexpressing LYSMD1/2-mCh with the indicated GFP-Rab32 proteins. Precipitated proteins were detected with antibodies against GFP and mCh. (C and D) His6-LYSMD1 (C) and His6-LYSMD2 (D) were incubated with GST-Rab32 proteins bound on glutathione Sepharose beads. After extensive washing, bound proteins were eluted and detected with His6 and GST antibodies. (E) LYSMD1/2 possess no GEF activity for Rab32 activation. Purified proteins used in GEF assays are shown on the left, and relative Mant-GDP fluorescence intensity reflecting the GEF activity is shown on the right. The value of each time point in the GEF assay was from three independent experiments (mean ± SD, n = 3 independent experiments). (F and G) Co-IP of mCh-tagged LYSMD1 (F) and LYSMD2 (G) with GFP-HPS1 and GFP-HPS4 from lysates of HeLa cells co-expressing the corresponding proteins. IPs were performed with GFP antibody and precipitated proteins were detected with mCh and GFP antibodies. (H and I) His6-HPS1 and His6-HPS4 were incubated with GST-LYSMD1 (H) or GST-LYSMD2 (I) bound on glutathione Sepharose beads. After extensive washing, bound proteins were eluted and detected with His6 and GST antibodies. (J) LYSMD1 and LYSMD2 promote the GEF activity of the BLOC-3 (HPS1–HPS4) complex to activate Rab32. The GEF activities of the indicated protein groups were examined as in Fig. 4 C. The value of each time point in the GEF assay was from three independent experiments (mean ± SD, n = 3 independent experiments). (K) Co-IP of Rab32 with GFP-VARP from lysates of GFP-VARP–expressing B16F10 cells with shRNA knockdown of Lysmd1, Lysmd2, or both. IPs were performed with GFP antibody and precipitated proteins were detected with antibodies against Rab32 and GFP. Source data are available for this figure: SourceData F6.
LYSMD1/2 interact with Rab32 and the BLOC-1 complex and promote the GEF activity of BLOC-1 to activate Rab32. (A and B) Co-IP, using GFP antibody, of mCh-tagged LYSMD1 (A) and LYSMD2 (B) with GFP-Rab32(WT), -Rab32(T39N), and -Rab32(Q85L) from lysates of HeLa cells coexpressing LYSMD1/2-mCh with the indicated GFP-Rab32 proteins. Precipitated proteins were detected with antibodies against GFP and mCh. (C and D) His6-LYSMD1 (C) and His6-LYSMD2 (D) were incubated with GST-Rab32 proteins bound on glutathione Sepharose beads. After extensive washing, bound proteins were eluted and detected with His6 and GST antibodies. (E) LYSMD1/2 possess no GEF activity for Rab32 activation. Purified proteins used in GEF assays are shown on the left, and relative Mant-GDP fluorescence intensity reflecting the GEF activity is shown on the right. The value of each time point in the GEF assay was from three independent experiments (mean ± SD, n = 3 independent experiments). (F and G) Co-IP of mCh-tagged LYSMD1 (F) and LYSMD2 (G) with GFP-HPS1 and GFP-HPS4 from lysates of HeLa cells co-expressing the corresponding proteins. IPs were performed with GFP antibody and precipitated proteins were detected with mCh and GFP antibodies. (H and I) His6-HPS1 and His6-HPS4 were incubated with GST-LYSMD1 (H) or GST-LYSMD2 (I) bound on glutathione Sepharose beads. After extensive washing, bound proteins were eluted and detected with His6 and GST antibodies. (J) LYSMD1 and LYSMD2 promote the GEF activity of the BLOC-3 (HPS1–HPS4) complex to activate Rab32. The GEF activities of the indicated protein groups were examined as in Fig. 4 C. The value of each time point in the GEF assay was from three independent experiments (mean ± SD, n = 3 independent experiments). (K) Co-IP of Rab32 with GFP-VARP from lysates of GFP-VARP–expressing B16F10 cells with shRNA knockdown of Lysmd1, Lysmd2, or both. IPs were performed with GFP antibody and precipitated proteins were detected with antibodies against Rab32 and GFP. Source data are available for this figure: SourceData F6.
We next investigated whether LYSMD1/2 act through HPS1 and HPS4, the counterparts of C. elegans GLO-3 and CCZ-1 (Fig. 5 A), to regulate Rab32 activation. First, we examined the interaction of LYSMD1/2 with HPS1 and HPS4. In co-IP assays, both GFP-HPS1 and GFP-HPS4 interacted with mCh-tagged LYSMD1 and LYSMD2 (Fig. 6, F and G). However, GST-LYSMD1 and GST-LYSMD2 only interacted with His6-HPS1 in pull-down assays (Fig. 6, H and I). These findings suggest that LYSMD1 and LYSMD2 directly interact with the HPS1 subunit of the GEF complex of Rab32, similar to the interaction of LMD-2 with the GEF complex (GLO-3–CCZ-1) of GLO-1. We then examined whether LYSMD1 and LYSMD2 can enhance the GEF activities of the HPS1–HPS4 complex toward Rab32. In vitro, His6-LYSMD1 and His6-LYSMD2 alone significantly enhanced the GEF activity of the HPS1–HPS4 complex. Interestingly, when both proteins were present, the enhancing effect on HPS1–HPS4 GEF activity toward Rab32 was not further increased compared with LYSMD2 alone (Fig. 6 J). This suggests that LYSMD1 and LYSMD2 probably act redundantly to promote Rab32 activation through the BLOC-3 complex. Supporting this conclusion, co-IP assays revealed that the interaction of endogenous Rab32 with ectopically expressed GFP-fused VARP, an effector of Rab32/38 (Tamura et al., 2009; Wang et al., 2008), was significantly decreased in mouse melanoma B16F10 cells when both Lysmd1 and Lysmd2 were knocked down by shRNA compared with single shRNA treatment (Fig. 6 K).
LYSMD1/2 are required for melanosome maturation and melanin production
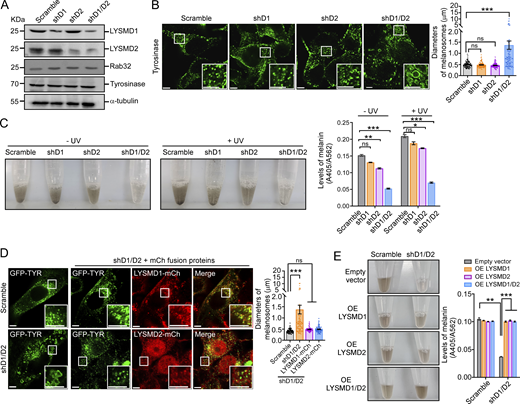
We went on to investigate the requirement for LYSMD1/2 in LRO biogenesis by performing shRNA of Lysmd1 or Lysmd2, or both, to examine the effect on melanosomes in mouse B16F10 cells. Knocking down Lysmd1 or Lysmd2 alone did not obviously change the sizes of melanosomes marked with immunostaining of tyrosinase (Fig. 7, A and B). When both Lysmd1 and Lysmd2 were knocked down, however, the sizes of melanosomes were significantly increased (Fig. 7, A and B), similar to the LRO enlargement in C. elegans lmd-2 mutants. In line with this, the production of melanin, regardless of UV treatment, was reduced in cells treated with shRNA targeting Lysmd1 or Lysmd2 and was further decreased when both genes were knocked down (Fig. 7 C). Moreover, overexpression of LYSMD1-mCh or LYSMD2-mCh, which colocalized with melanosomes, mostly rescued the enlargement of melanosomes in B16F10 cells treated with shRNA against both Lysmd1 and Lysmd2 (Fig. 7 D). Accordingly, overexpression of either LYSMD1 or LYSMD2, or both, rescued the defects in melanin production in cells with shRNA knockdown of both genes (Fig. 7 E). In addition, we overexpressed mCh-tagged Rab32(WT), Rab32(Q85L), and Rab32(T39N) in B16F10 cells with shRNA knockdown of Lysmd1/2. Rab32(WT) and Rab32(Q85L), but not Rab32(T39N), strongly suppressed the enlargement of melanosomes induced by Lysmd1/2 double shRNA (Fig. 8 A). Moreover, Rab32(WT) and Rab32(Q85L), but not Rab32(T39N), significantly increased the production of melanin in Lysmd1/2 double shRNA-treated cells (Fig. 8 B). Overexpression of mCh-tagged HPS1 or HPS4, however, did not have similar rescuing effects on melanosome enlargement and defective melanin production in cells with double knockdown of Lysmd1/2 (Fig. 8, C and D). Taken together, these findings suggest that LYSMD1 and LYSMD2 act redundantly through Rab32 to promote melanosome biogenesis and functions (Fig. 8 E).
Loss of Lysmd1/2 causes abnormal enlargement of melanosomes and reduces melanin production in mouse B16F10 cells. (A) Western blotting of LYSMD1, LYSMD2, Rab32, and Tyrosinase in B16F10 cells with shRNA knockdown of Lysmd1 (shD1), Lysmd1 (shD2), or both (shD1/D2). (B) Representative images (left) and quantification (right) of melanosomes stained with tyrosinase antibody in B16F10 cells with the indicated shRNA treatment. The boxed region is magnified (3×) at the bottom right in each image. Scale bars, 5 μm. Diameters of 80 melanosomes were measured in each group (mean ± SEM, n = 80 melanosomes, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (C) Representative images (left) and quantification (right) of melanin production in shRNA-treated B16F10 cells with or without UV irradiation. Melanin production was quantified by calculating A405/A562 (mean ± SEM, n = 4 independent experiments, unpaired two-tailed t test, **P < 0.01, ***P < 0.001, ns: not significant). (D) Reinforced expression of LYSMD1, LYSMD2, or both rescues melanosome enlargement in B16F10 cells treated with shD1/D2. Representative images are shown on the left and quantification of melanosome diameters is shown on the right. The boxed region is magnified (3×) at the bottom right in each image. Scale bars, 5 μm. Diameters of 80 melanosomes were measured in each group (mean ± SEM, n = 80 melanosomes, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (E) Reinforced expression of LYSMD1, LYSMD2, or both rescues defective melanin production in B16F10 cells treated with shD1/D2. Representative images (left) and quantification of melanin production (right) are shown. Melanin production was quantified by calculating A405/A562 (mean ± SEM, n = 4 independent experiments, unpaired two-tailed t test, **P < 0.01, ***P < 0.001). OE, overexpressing. Source data are available for this figure: SourceData F7.
Loss of Lysmd1/2 causes abnormal enlargement of melanosomes and reduces melanin production in mouse B16F10 cells. (A) Western blotting of LYSMD1, LYSMD2, Rab32, and Tyrosinase in B16F10 cells with shRNA knockdown of Lysmd1 (shD1), Lysmd1 (shD2), or both (shD1/D2). (B) Representative images (left) and quantification (right) of melanosomes stained with tyrosinase antibody in B16F10 cells with the indicated shRNA treatment. The boxed region is magnified (3×) at the bottom right in each image. Scale bars, 5 μm. Diameters of 80 melanosomes were measured in each group (mean ± SEM, n = 80 melanosomes, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (C) Representative images (left) and quantification (right) of melanin production in shRNA-treated B16F10 cells with or without UV irradiation. Melanin production was quantified by calculating A405/A562 (mean ± SEM, n = 4 independent experiments, unpaired two-tailed t test, **P < 0.01, ***P < 0.001, ns: not significant). (D) Reinforced expression of LYSMD1, LYSMD2, or both rescues melanosome enlargement in B16F10 cells treated with shD1/D2. Representative images are shown on the left and quantification of melanosome diameters is shown on the right. The boxed region is magnified (3×) at the bottom right in each image. Scale bars, 5 μm. Diameters of 80 melanosomes were measured in each group (mean ± SEM, n = 80 melanosomes, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (E) Reinforced expression of LYSMD1, LYSMD2, or both rescues defective melanin production in B16F10 cells treated with shD1/D2. Representative images (left) and quantification of melanin production (right) are shown. Melanin production was quantified by calculating A405/A562 (mean ± SEM, n = 4 independent experiments, unpaired two-tailed t test, **P < 0.01, ***P < 0.001). OE, overexpressing. Source data are available for this figure: SourceData F7.
Reinforced expression of active Rab32 suppresses the defects in melanosome biogenesis and melanin production in B16F10 cells with shD1/D2. (A) Overexpression of mCh-Rab32(WT) and mCh-Rab32(Q85L), but not mCh-Rab32(T39N), suppresses melanosome enlargement in cells treated with shD1/D2. Melanosomes were labeled with GFP-Tyrosinase. Representative images (left) and quantification of the diameters of melanosomes (right) are shown. The boxed region is magnified (3×) at the bottom right in each image. For each form of Rab32, the corresponding merged images (GFP-TYR+mCh-Rab32) are magnified (6×) and shown in the right column. Scale bars, 5 μm. Diameters of 80 melanosomes were measured in each group (mean ± SEM, n = 80 melanosomes, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (B) Overexpression of mCh-Rab32 (WT) and mCh-Rab32 (Q85L), but not mCh-Rab32 (T39N), restores melanin production in B16F10 cells treated with shD1/D2. Representative images (left) and melanin production was quantified by calculating A405/A562 (right) (mean ± SEM, n = 4 independent experiments, unpaired two-tailed t test, ***P < 0.001, ns: not significant). EV, empty vector. (C and D) Overexpression of mCherry-HPS1 or mCherry-HPS4 does not rescue melanosome enlargement (C) or melanin production (D) in B16F0 cells with shD1/D2. Representative fluorescence images are shown in C. Scale bars, 5 μm. (D) Representative images of melanin (left) and melanin production was quantified by calculating A405/A562 (right) (mean ± SEM, n = 4 independent experiments, unpaired two-tailed t test, ***P < 0.001; ns: not significant). (E) Graphic summary of the roles of the LMD-2–(GLO-3–CCZ-1)–GLO-1 axis in promoting LRO biogenesis in C. elegans and the LYSMD1/2–(HPS1–HPS4)–Rab32 axis in promoting melanosome biogenesis in mammals.
Reinforced expression of active Rab32 suppresses the defects in melanosome biogenesis and melanin production in B16F10 cells with shD1/D2. (A) Overexpression of mCh-Rab32(WT) and mCh-Rab32(Q85L), but not mCh-Rab32(T39N), suppresses melanosome enlargement in cells treated with shD1/D2. Melanosomes were labeled with GFP-Tyrosinase. Representative images (left) and quantification of the diameters of melanosomes (right) are shown. The boxed region is magnified (3×) at the bottom right in each image. For each form of Rab32, the corresponding merged images (GFP-TYR+mCh-Rab32) are magnified (6×) and shown in the right column. Scale bars, 5 μm. Diameters of 80 melanosomes were measured in each group (mean ± SEM, n = 80 melanosomes, unpaired two-tailed t test, ***P < 0.001, ns: not significant). (B) Overexpression of mCh-Rab32 (WT) and mCh-Rab32 (Q85L), but not mCh-Rab32 (T39N), restores melanin production in B16F10 cells treated with shD1/D2. Representative images (left) and melanin production was quantified by calculating A405/A562 (right) (mean ± SEM, n = 4 independent experiments, unpaired two-tailed t test, ***P < 0.001, ns: not significant). EV, empty vector. (C and D) Overexpression of mCherry-HPS1 or mCherry-HPS4 does not rescue melanosome enlargement (C) or melanin production (D) in B16F0 cells with shD1/D2. Representative fluorescence images are shown in C. Scale bars, 5 μm. (D) Representative images of melanin (left) and melanin production was quantified by calculating A405/A562 (right) (mean ± SEM, n = 4 independent experiments, unpaired two-tailed t test, ***P < 0.001; ns: not significant). (E) Graphic summary of the roles of the LMD-2–(GLO-3–CCZ-1)–GLO-1 axis in promoting LRO biogenesis in C. elegans and the LYSMD1/2–(HPS1–HPS4)–Rab32 axis in promoting melanosome biogenesis in mammals.
Discussion
Combining genetic and cell biological studies, we here identified LMD-2 and LYSMD1/2 as evolutionarily conserved regulators of LRO biogenesis. Our findings reveal that, in C. elegans, loss of lmd-2 leads to abnormal enlargement of gut granules/LROs, which are defective in maturation and functions. Further genetic analyses revealed that lmd-2 acts upstream of glo-3, ccz-1, and glo-1. Mechanistically, LMD-2 does not activate the GLO-1 GTPase on its own. Instead, it interacts with GLO-3, thereby forming a tripartite complex with GLO-3 and CCZ-1, the GEF of GLO-1. LMD-2 thus promotes the GEF activity of the GLO-3–CCZ-1 complex to activate GLO-1. In line with this, while lmd-2 mutants exhibited reduced levels of GLO-1 on gut granules/LROs, reinforced expression of WT and constitutively active GLO-1 ameliorated the defective LRO phenotype caused by lmd-2 mutation. Importantly, the conservation of the LMD-2–GLO-1 axis in regulating LRO biogenesis and functions is evidenced by the findings that heterologous expression of LYSMD1/2, the mammalian homologs of LMD-2, or Rab32 (WT and active forms), the homolog of GLO-1, mostly rescued the defective LROs in lmd-2 mutants. LYSMD1 and LYSMD2 probably act redundantly as shRNA knockdown of either of them did not obviously cause defects in melanosomes in mouse melanoma B16F10 cells, but knocking down both led to enlargement of melanosomes and reduction of melanin production, which is similar to LRO enlargement and dysfunction in C. elegans lmd-2 mutants. In addition, reinforced expression of either LYSMD1 or LYSMD2 was sufficient to rescue the defective melanosomes in cells with double shRNA knockdown of these two genes. Consistent with this, LYSMD1 and LYSMD2 individually interact with the HPS1, the subunit of the BLOC-3 complex, thereby enhancing the GEF activity to activate Rab32. Thus, like C. elegans LMD-2, mammalian LYSMD1/2 act through Rab32 to promote melanosome biogenesis. Supporting this conclusion, reinforced expression of Rab32 and its active form, Rab32(Q85L), strongly rescued the defective melanosomes and melanin production in B16F10 cells with shRNA knockdown of Lysmd1 and Lysmd2. Altogether, these findings establish an evolutionarily conserved regulatory axis in LRO biogenesis (Fig. 8 E).
Lysosomes and LROs divert from early endosomes. While late endosomes/lysosomes are characterized by the Rab7 GTPase, LROs are characterized by Rab32/Rab38. In C. elegans lmd-2 mutants, the enlarged immature LROs are positive for both RAB-7 and GLO-1/Rab32, suggesting that LMD-2 plays an essential role in determining the identity of LROs. The requirement for LMD-2 to exclude RAB-7 on LROs suggests that LMD-2 probably negatively regulates RAB-7. In reduction-of-function mutants of glo-3, RAB-7 also mislocalizes to gut granules/LROs in a SAND-1/MON1-dependent manner. Given that GLO-3 replaces SAND-1/MON1 to form a complex with CCZ-1, it is possible that in worms with reduced GLO-3 levels, the residual SAND-1–CCZ-1 complex can still activate RAB-7 on LROs (Morris et al., 2018). Because LMD-2 forms a complex with GLO-3 and CCZ-1, it is possible that LMD-2 acts together with GLO-3 to compete against SAND-1 for binding with CCZ-1. Another possibility is that, in the absence of LMD-2, the reduced levels of GLO-1 allow RAB-7 to exist on LROs because Rabs usually act in a cascade to regulate organelle biogenesis (Morris et al., 2018; Stenmark, 2009).
It was reported that BLOC-3 and Rab38 act together to control the recycling of VAMP7, a v-SNARE that mediates the fusion of endosomal tubules with maturing melanosomes (Dennis et al., 2016). Because LMD-2 and LYSMD1/2 interact with GLO-3 and HPS1, respectively, to promote the activation of GLO-1 and Rab32/38, we reason that GLO-1, like Rab32/38, is essential for the recycling of a fusogen-like VAMP7 out of gut granules/LROs in C. elegans. This is supported by the fact that gut granules/LROs in C. elegans lmd-2 mutants and melanosomes in shLysmd1/2 melanoma cells are similarly enlarged. In addition, as the positive regulators of Rab32/38, LYSMD1/2 are probably involved in many physiological/pathological processes engaging Rab32/38, such as resistance to bacteria, phagocytosis, and inflammation (Solano-Collado et al., 2018; Spanò and Galán, 2012; Spanò et al., 2016). Further studies using gene-edited mice will help to establish the physiological and pathological roles of these LYSMD proteins.
Materials and methods
C. elegans genetics
C. elegans culture and genetic analysis were performed with standard procedures. The Bristol WT strain was used as WT. The lmd-2(yq114) mutant was obtained by EMS mutagenesis. The lmd-2(tm6851) deletion mutant was provided by S. Mitani (Tokyo Women’s Medical University, Tokyo, Japan). The following mutants used in this study were provided by the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA): sand-1(ok1963), glo-3(kx94); ccz-1(ok2182), glo-1(zu437), rab-7(ok511). A list of all strains used in this study is provided in Table S1.
Expression vectors, antibodies, and reagents
EMS mutagenesis and gene cloning
To screen for mutants with abnormal vacuoles in the intestine, synchronized L4-stage WT animals were first treated with 50 mM EMS solution for 4 h at 25°C. They were then cultured on NGM (nematode growth media) plates for production of progeny at 20°C. F2 progeny at 24–48 h post-L4 stage were observed under DIC optics. The yq114 mutant was obtained from a screen of 12,000 haploid genomes. yq114 was mapped to the genetic position between −1.27 and −0.85 on Linkage Group I using single nucleotide polymorphism mapping, and the mutation was determined by genome sequencing.
RNA interference
L4 animals were cultured on NGM plates containing 1 mM IPTG and seeded with E. coli HT115 expressing double-stranded RNA of genes of interest. The progeny at L4 stage were examined for LRO defects.
CRISPR/Cas9-mediated knock-in assays
The GFP::GLO-1 knock-in strain was generated by using CRISPR/Cas9-mediated assays. Briefly, a specific sgRNA (single guide RNA) target sequence (5′-GATCATAAGCTGAGCTGCTA-3′) in the glo-1gene was introduced into the pPD162-Peft-3CAS9-PU6sgRNA vector that co-expresses the Cas9 enzyme and sgRNA. dpy-10 was used as the positive selection marker as reported previously (Paix et al., 2015). F1 worms with dumpy or roller phenotypes were examined for recombination by using PCR and sequenced to determine the correct occurrence of homology-directed repair. The oligos used for genotyping were N-terminal sense 5′-GATTTTGGATGTATAGACCCA-3′ and C-terminal anti-sense 5′-CTTGAAGAAGATGGTACGCT-3′.
FluoZin-3, LysoTracker red, acridine orange, and Nile red staining
L4 hermaphrodites were grown for 12–24 h in the dark on bacteria-seeded NGM plates containing 3 μM FluoZin-3 acetoxymethyl ester (Molecular Probes), or 2 μM LysoTracker RED DND-99 (Invitrogen), or 10 μM Nile red (Invitrogen), or 100 μg/ml acridine orange. The animals were then transferred to similar NGM plates without dyes for a further 30-min culture to reduce the dyes in the intestinal lumen, and imaged under a fluorescence microscope.
Western blotting and IPs
For western blotting, worms were collected and washed by M9 three times and then ground in a homogenizer in worm lysis buffer 25 mM Tris-HCl, pH 7.5, 50 mM NaCl, 0.1% NP-40, 1 mM PMSF (phenyl methane sulfonyl fluoride), and 1% glycerol, Complete Protease Inhibitor Cocktail (Roche). The lysates were cleared by centrifugation at 12,000 rpm for 10 min at 4°C. Mammalian cells were lysed in ice-cold radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 1 mM PMSF) containing Complete Protease Inhibitor Cocktail (Roche). Equal protein loads were resolved on SDS-PAGE, blotted to nitrocellulose membrane, and probed with antibodies. Proteins were detected with ECL chemiluminescent detection reagents (Thermo Fisher Scientific) and membranes were imaged with a Smartchem machine (Sage Creation).
For GFP-based IPs, worms expressing GFP-tagged proteins were lysed in worm lysis buffer by gentle grinding. The supernatants were collected by centrifugation at 12,000 g for 10 min at 4°C. Mammalian cells were lysed in ice-cold IP buffer (25 mM Tris-HCl, pH 7.5, 50 mM NaCl, 0.1% NP-40, 1 mM PMSF, and 1% glycerol, Complete Protease Inhibitor Cocktail) and spun down to collect the supernatants. The supernatants were incubated with GFP-trap beads (ChromoTek) overnight at 4°C. The beads were then centrifuged and washed three times with wash buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% NP-40, and 1 mM PMSF). Precipitated proteins were resolved by SDS-PAGE and detected by western blotting.
Protein purification and GST pull-down
The ORFs of genes of interests were cloned into pET-21a (+) or pGEX-4T-1 (Table S2) and transformed into the E. coli Rosetta (DE3) strain for expression. His6-tagged proteins and GST-fused proteins were purified with nickel Sepharose beads (GE Health Care) and glutathione Sepharose beads (GE Health Care), respectively, following the supplier’s instructions.
For GST pull-down, His6-tagged proteins were incubated with GST-fusion proteins immobilized on glutathione Sepharose beads at 4°C overnight. The beads were washed extensively and bound proteins were eluted and examined with western blotting.
GEF assays
GEF assays were performed in 96-well plates essentially as described (Kanie and Jackson, 2018; Tan and Sun, 2021). Briefly, His6–GLO-1 (1 μM) or His6-Rab32 (1 μM) were firstly incubated with 2 μM Mant-GDP (N-Methylanthraniloyl (Mant)-guanine nucleotide) on ice for 30 min to form GTPase–Mant-GDP complexes in a reaction buffer containing 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.5 mM MgCl2. Then His6–GLO-3/His6–CCZ-1 or His6-HPS1/His6-HPS4 (at a concentration of 10 μM) were added to the preformed GTPase–Mant-GDP complex in a total reaction volume of 200 μl. GTP–GDP exchange was triggered by adding 0.1 mM GTP to the reaction following baseline stabilization. Fluorescence excitation of samples was at 366 nm and detection was at 443 nm with an interval of 3 s. The reduction of Mant-GDP fluorescence with time is used to indicate nucleotide exchange.
To assess the effect of LMD-2 on GLO-3/CCZ-1 complex and of LYSMD1/2 on HPS1/HPS4 complex, His6-LMD2 or His6-LYSMD1/2 at varying concentrations were preincubated with His6–GLO-3/His6–CCZ-1 or His6-HPS1/His6-HPS4, respectively, at 4°C overnight and then added to the preformed GTPase–Mant-GDP complex to measure the GEF activity as described above.
Cell culture and transfection
HeLa and B16F10 cells were cultured at 37°C with 5% CO2 in DMEM (Gibco) supplemented with 10% FBS (Biological Industries), 100 U/ml penicillin, and 100 mg/ml streptomycin (HyClone). HeLa cells were transfected with Lipo2000 (Invitrogen), and B16F10 cells were transfected with ExFect Transfection Reagent (Vazyme) following the suppliers’ instructions. The B16F10 cell line was a gift from Dr. Likun Wang (Institute of Biophysics, Chinese Academy of Science, Beijing, China).
shRNA
To knock down Lysmd1/2 by shRNA, pLKO.1-Lysmd1-puro and pLKO.1-Lysmd2-puro vectors were constructed using the following oligos: Lysmd1 5′-CAGAGACCTGTTCAATGGTTT-3′; Lysmd2 5′-GAGAAGCCTTTGTTGTTTAAT-3′. Cells were grown in 6-well plates and transfected with either 1 μg of individual shRNA plasmids (pLKO.1-Lysmd1-puro or pLKO.1-Lysmd2-puro) for single gene knockdown or 0.5 μg of each plasmid for simultaneous knockdown of both genes. As a control, cells were transfected with 1 μg of scramble shRNA. All transfections were performed using Lipofectamine 2000 (Invitrogen). 24 h following the transfection, puromycin (2 μg/ml; Sigma-Aldrich) was added to the culture medium for an additional 48 h to select transfected cells. The surviving cells were collected and seeded in new 6-well plates for further analysis.
Immunostaining
B16F10 cells cultured on confocal dishes (Cellvis) were fixed in 4% PFA for 15 min followed by permeabilization with 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 10 min. After washing with PBS, cells were incubated in blocking buffer (5% BSA, 0.05% Triton X-100 in PBS) for 1 h at room temperature (RT) and then incubated with anti-Tyrosinase antibody (1:500) in blocking buffer at 4°C overnight. After extensive washing, cells were incubated with FITC-conjugated secondary antibody for 1 h at RT. Cells were extensively washed with PBS and imaged with an alpha Plan-Apochromat ×100/1.46 oil objective using Zeiss LSM880 confocal microscope at RT.
Microscopy and image analysis
All images were captured at RT with an inverted confocal microscope system (LSM880; Zeiss) coupled with a camera using a 100× 1.42 NA oil objective. Excitation was achieved using 405-, 488-, and 560-nm lasers. Images were processed with Zen software (Blue edition; Zeiss). The spectral fingerprinting function of the Zeiss LSM880 was used to distinguish GFP/mCherry signals from the autofluorescence generated by LROs/gut granules (Shi and Grant, 2015).
Melanin measurement
B16F10 cells (5 × 105 cells/well) were seeded and grown in 6-well plates at 37°C with 95% humidified air and 5% CO2. Cells were lysed with RIPA buffer, and the protein concentration of the cell suspension was measured with the bicinchoninic acid assay (with absorbance at 596 nm, A596). The cell suspension was then incubated with NaOH (1 N) at 60°C for 1 h and measured for absorbance at 405 nm (A405). Melanin levels were normalized with A405/A596. For UV treatment, cells in a 6-well plate were irradiated with 0.125 J/cm2 UVB for 1 min, then returned to 37°C and cultured for a further 24 h. Melanin production was then measured as described above.
Statistical analysis
Prism (GraphPad Software 8.0) was used to generate curves or bar graphs. Error bars represent the standard error of the mean (SEM) or standard deviation (SD), as stated in the figure legends. Comparison of two groups of samples was performed with the two-tailed unpaired t test. *P < 0.05, **P < 0.01, ***P < 0.001. P > 0.05 was considered not significant (ns). The intensity of the bands in the western blot was quantified using ImageJ software.
Online supplemental material
Fig. S1 characterizes intestinal vesicles in lmd-2 mutants and LMD-2 protein. Fig. S2 characterizes the enlarged gut granules in lmd-2(yq114) mutants. Fig. S3 shows the engagement of BLOC-3, AP-3, HOPS, RAB-7, and GLO-1 in formation of abnormal LROs in lmd-2 mutants. Fig. S4 shows the alignment of amino acid sequences of C. elegans GLO-3 and human HPS1. Fig. S5 shows the colocalization of GLO-1 with GLO-3 and CCZ-1 in WT and lmd-2 mutants. Table S1 shows the C. elegans strains used in this study. Table S2 shows the information on expression vectors used in this study. Table S3 shows the antibodies and reagents used in this study.
Data availability
All data are included in the manuscript or supplements and are available from the corresponding authors on reasonable request.
Acknowledgments
We thank Dr. I. Hanson for proofreading the manuscript.
This research is supported by grants 32293201 and 92354303 (to C. Yang) from the National Science Foundation of China, 2021YFA1300302 (to C. Yang) from the National Basic Research Program of China, 31960143 (to J. Li) and 32070743 (to M. Yang) from the National Science Foundation of China, and 202001BB050077 (to C. Yang) and 202101AS070002 (to M. Yang) from Yunnan Province Science and Technology Department. C. Yang is supported by the Program of Yunnan Province Leading Talents in Science and Technology (202105AB160003).
Author contributions: J. Li: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing, Q. Yin: Data curation, Formal analysis, Investigation, Methodology, Visualization, N. Xuan: Data curation, Formal analysis, Investigation, Methodology, Q. Gan: Conceptualization, Investigation, Methodology, C. Liu: Formal analysis, Investigation, Methodology, Q. Zhang: Data curation, Formal analysis, Investigation, Methodology, Visualization, M. Yang: Funding acquisition, Supervision, C. Yang: Conceptualization, Funding acquisition, Project administration, Supervision, Writing—original draft, Writing—review & editing.
References
Author notes
J. Li and Q. Yin contributed equally to this paper.
Disclosures: The authors declare no competing interests exist.