Rho GTPases are central regulators of cell polarity and signaling. How Rho GTPases are directed to function in certain settings remains unclear. Here, we show the protein levels of the yeast Rho GTPase Cdc42p are regulated, which impacts a subset of its biological functions. Specifically, the active conformation of Cdc42p was ubiquitinated by the NEDD4 ubiquitin ligase Rsp5p and HSP40/HSP70 chaperones and turned over in the proteasome. A GTP-locked (Q61L) turnover-defective (TD) version, Cdc42pQ61L+TD, hyperactivated the MAPK pathway that regulates filamentous growth (fMAPK). Cdc42pQ61L+TD did not influence the activity of the mating pathway, which shares components with the fMAPK pathway. The fMAPK pathway adaptor, Bem4p, stabilized Cdc42p levels, which resulted in elevated fMAPK pathway signaling. Our results identify Cdc42p turnover regulation as being critical for the regulation of a MAPK pathway. The control of Rho GTPase levels by stabilization and turnover may be a general feature of signaling pathway regulation, which can result in the execution of a specific developmental program.
Introduction
Cells establish an axis of polarity to maintain cell shape and to orient growth and cell division. The polarity of the cell changes throughout the cell cycle and can be reorganized in response to extrinsic cues to impact biological processes, like cell motility (chemotaxis/chemotropism) and differentiation to specific cell types. Ras homology (Rho) GTPases are evolutionarily conserved regulators of cell polarity. These proteins control the organization of the cytoskeleton and regulate signal transduction pathways (Hall, 2005). Like other monomeric GTPases, Rho GTPases undergo a conformational change to the active (GTP-bound) conformation through GTP/GDP exchange, which is stimulated by guanine nucleotide exchange factors (GEFs). Rho GTPases are inactivated by GTPase-activating proteins (GAPs) that stimulate the intrinsic GTPase activity of the proteins. In the GTP-bound conformation, Rho GTPases interact with effector proteins that include formins that regulate the actin cytoskeleton (Evangelista et al., 1997; Sit and Manser, 2011) and p21-activated kinases (PAKs; Ha and Boggon, 2018; Rane and Minden, 2014; Tetley et al., 2017) that control signaling pathways like mitogen-activated protein kinase (MAPK) pathways (Coso et al., 1995; Van Aelst and D'Souza-Schorey, 1997). Rho GTPases interact with many other proteins and are also regulated by post-translational modifications (PTMs) that impact their localization, stability, and activity (Majolée et al., 2019). Collectively, the protein interactions and PTMs that occur on Rho GTPases are required for the precise execution of a biological response.
An interesting question surrounding Rho GTPases is to understand how they function in specific contexts. Cells typically contain multiple Rho GTPases, including CDC42, RAC, and RHO. Each one of these GTPases can execute multiple biological functions. For example, the well-understood yeast Cdc42p (Bi and Park, 2012; Kozminski et al., 2000), which is functionally and evolutionarily related to human CDC42 (the proteins are 81% identical), has multiple functions in the cell. Yeast Cdc42p is an essential protein that controls the establishment of cell polarity (Bi and Park, 2012; Irazoqui and Lew, 2004; Pringle et al., 1995), impacts endocytosis (Aguilar et al., 2006), and is a component of the exocyst (Adamo et al., 2001; Zhang et al., 2001), a complex which controls the delivery and fusion of vesicles at the plasma membrane (Munson and Novick, 2006). Cdc42p also regulates three MAPK pathways that induce different responses. In addition to Cdc42p, the MAPK pathways share other components, including the p21-activated kinase (PAK) Ste20p, and MAPKKK Ste11p. Pathway-specific components include scaffold proteins, which are known to drive activation of a specific pathway (Bardwell, 2005; Saito, 2010; Schwartz and Madhani, 2004) and pathway-specific transcription factors (Bao et al., 2004; Chou et al., 2004). For example, Cdc42p regulates the mating pathway, which allows haploid cells to sense and response to secreted pheromones (Alvaro and Thorner, 2016; Bardwell, 2005; Simon et al., 1995). Cdc42p also regulates the MAPK pathway that controls filamentous growth (fMAPK), which is a common growth mode that occurs in response to limiting nutrients (Peter et al., 1996; Truckses et al., 2006; Wu et al., 1998). Filamentous growth occurs in many fungal species, including pathogens and is controlled by analogous MAPK pathways that promote host-cell attachment, invasion into tissues and pathogenicity (Fisher et al., 2020; Gimeno et al., 1992; Jin et al., 2008; Latgé, 2010; Lorenz and Heitman, 1997; Mitchell, 1998). Cdc42p also controls the response to osmotic stress by regulation of the HOG pathway (Hohmann, 2015; Saito and Posas, 2012; Tatebayashi et al., 2006). In addition to these roles, Cdc42p also regulates aspects of lysosome/vacuole function (Jones et al., 2010; Müller et al., 2001) and sealing of the nuclear envelope during cell division (Lu and Drubin, 2020). How a small GTPase regulates all of these cellular processes remains at present unclear.
We hypothesized that PTMs of Cdc42p might selectively impact its functions in the cell. One type of PTM that occurs in mammalian Rho GTPases is the addition of the small protein ubiquitin (Goka and Lippman, 2015; Li et al., 2016; Oberoi-Khanuja and Rajalingam, 2012; Tian et al., 2011; Wang et al., 2003; Wei et al., 2013; Zhao et al., 2013a). Ubiquitin-dependent turnover of the active or GTP-bound conformation of Rho GTPases is critical for the control of effector processes such as cell migration and invasion (Castillo-Lluva et al., 2013; Murali et al., 2017; Torrino et al., 2011). We show here that the yeast Cdc42p protein is ubiquitinated and preferentially turned over in its GTP-bound conformation. This result was unexpected because yeast Cdc42p is widely considered to be a stable protein (Adamo et al., 2001; Atkins et al., 2013; Daniels et al., 2018; Freisinger et al., 2013; Kozminski et al., 2000; Ziman et al., 1993). Since this represents the first example of Rho GTPase turnover in yeast, we utilized this genetic system to define the regulation of Cdc42p turnover/stability. Cdc42p turnover required the ubiquitin ligase Rsp5p, a member of the NEDD4 family of E3 ligases, and the heat shock proteins (HSPs) Ydj1p and Ssa1p, which were required for degradation of the protein in the 26S proteasome. By investigating the role of lysine residues as sites for ubiquitination, we identified a version of GTP-bound Cdc42p that was defective for turnover. Turnover-defective GTP-bound Cdc42p preferentially induced the MAPK pathway that regulates filamentous growth among other Cdc42p pathways in the cell. We further show that an Smg GDS-type scaffold that selectively regulates the fMAPK pathway, called Bem4p, stabilized Cdc42p protein levels, which resulted in fMAPK pathway activation and filamentous growth. Our results demonstrate that regulating Rho GTPase levels by stabilization and turnover can induce a pathway-specific response. Regulating Rho GTPase levels might be a general mechanism for controlling pathway-specific responses in many settings.
Result
Yeast GTP-bound Cdc42p is ubiquitinated and turned over
To determine whether the yeast Cdc42p protein is ubiquitinated, cells were examined that contained an epitope-tagged and functional version of the protein, GFP-Cdc42p (Woods et al., 2016). The GFP-Cdc42p protein expressed from a plasmid was immunoprecipitated from cell extracts with anti-GFP antibodies and examined by immunoblot analysis. Immunoprecipitated GFP-Cdc42p cross-reacted with anti-ubiquitin antibodies, indicating that the protein can be ubiquitinated (Fig. 1 A). Multiple bands were detected in the co-immunoprecipitated fraction, which may indicate that Cdc42p is poly-ubiquitinated.
Cdc42p is ubiquitinated, and the GTP-bound conformation is rapidly degraded compared to wild type. (A) Lysates prepared from wild-type cells (PC538) with (+) and without (−) the pGFP-Cdc42p plasmid (PC6454) were examined by co-immunoprecipitation analysis. Total protein (Total) was assessed by Ponceau S staining. Input lysates and immunoprecipitated fractions were examined by anti-GFP and anti-ubiquitin (Ub) antibodies. Co-IP Ub/GFP refers to the relative band intensity of co-immunoprecipitated Ub compared to immunoprecipitated GFP protein. Numbers indicate the mean from multiple biological replicates (n ≥ 2). Representative blots are shown. Values varied <10% across trials. IP; immunoprecipitated. (B) Levels of GFP-Cdc42p (Cdc42, PC6454) and GFP-Cdc42pQ61L (Q61L, PC7458) proteins were examined in wild-type cells (PC538). Anti-GFP and anti-Pgk1p antibodies were used. Cdc42/Pgk1 ratio refers to relative levels of GFP-Cdc42p to Pgk1p. Numbers represent the mean from multiple biological replicates (n ≥ 2). Representative blots are shown. Values varied <20% across trials. (C) Wild-type cells expressing a plasmid containing GFP-Cdc42p (Cdc42, PC6454) were incubated in SD-URA media containing 25 µg/ml of CHX and analyzed by immunoblotting at the indicated time points. Top panel, HMW products correspond to a longer exposure of the anti-GFP blot. See B for details. (D) Same as in C except cells containing the plasmid GFP-Cdc42pQ61L (Q61L, PC7458). Immunoblot analysis of C and D were taken from the same blots (same exposure) to quantify and directly compare levels of GFP-Cdc42p. See B for details. (E) Levels of GFP-Cdc42p (Cdc42, PC6454, set to 1 for time 0) and GFP-Cdc42pQ61L (Q61L, PC7458). Error bars represent the standard deviation (SD) among biological replicates (n = 2). (F) Fluorescence microscopy of wild-type cells expressing GFP-Cdc42p (Cdc42, PC6454) or GFP-Cdc42pQ61L (Q61L, PC7458). Micrographs were taken at the same exposure. Scale bar, 5 µm. (G) Comparison of relative fluorescence intensity of GFP-Cdc42p in the same cells described in F. Error bars indicate SD among three biological replicates (n = 3); 50 cells were examined in each replicate. Data were analyzed using unpaired t test (two-sided). P refers to P value. (H) Levels of ubiquitinated GFP-Cdc42p (Cdc42, PC6454) and GFP-Cdc42pQ61L (Q61L, PC7458) in wild-type cells by co-immunoprecipitation analysis. Different exposures are shown for immunoprecipitated and co-immunoprecipitated blots based on the level of the GFP-Cdc42p protein. Values varied <15% across trials. See A for details. Source data are available for this figure: SourceData F1.
Cdc42p is ubiquitinated, and the GTP-bound conformation is rapidly degraded compared to wild type. (A) Lysates prepared from wild-type cells (PC538) with (+) and without (−) the pGFP-Cdc42p plasmid (PC6454) were examined by co-immunoprecipitation analysis. Total protein (Total) was assessed by Ponceau S staining. Input lysates and immunoprecipitated fractions were examined by anti-GFP and anti-ubiquitin (Ub) antibodies. Co-IP Ub/GFP refers to the relative band intensity of co-immunoprecipitated Ub compared to immunoprecipitated GFP protein. Numbers indicate the mean from multiple biological replicates (n ≥ 2). Representative blots are shown. Values varied <10% across trials. IP; immunoprecipitated. (B) Levels of GFP-Cdc42p (Cdc42, PC6454) and GFP-Cdc42pQ61L (Q61L, PC7458) proteins were examined in wild-type cells (PC538). Anti-GFP and anti-Pgk1p antibodies were used. Cdc42/Pgk1 ratio refers to relative levels of GFP-Cdc42p to Pgk1p. Numbers represent the mean from multiple biological replicates (n ≥ 2). Representative blots are shown. Values varied <20% across trials. (C) Wild-type cells expressing a plasmid containing GFP-Cdc42p (Cdc42, PC6454) were incubated in SD-URA media containing 25 µg/ml of CHX and analyzed by immunoblotting at the indicated time points. Top panel, HMW products correspond to a longer exposure of the anti-GFP blot. See B for details. (D) Same as in C except cells containing the plasmid GFP-Cdc42pQ61L (Q61L, PC7458). Immunoblot analysis of C and D were taken from the same blots (same exposure) to quantify and directly compare levels of GFP-Cdc42p. See B for details. (E) Levels of GFP-Cdc42p (Cdc42, PC6454, set to 1 for time 0) and GFP-Cdc42pQ61L (Q61L, PC7458). Error bars represent the standard deviation (SD) among biological replicates (n = 2). (F) Fluorescence microscopy of wild-type cells expressing GFP-Cdc42p (Cdc42, PC6454) or GFP-Cdc42pQ61L (Q61L, PC7458). Micrographs were taken at the same exposure. Scale bar, 5 µm. (G) Comparison of relative fluorescence intensity of GFP-Cdc42p in the same cells described in F. Error bars indicate SD among three biological replicates (n = 3); 50 cells were examined in each replicate. Data were analyzed using unpaired t test (two-sided). P refers to P value. (H) Levels of ubiquitinated GFP-Cdc42p (Cdc42, PC6454) and GFP-Cdc42pQ61L (Q61L, PC7458) in wild-type cells by co-immunoprecipitation analysis. Different exposures are shown for immunoprecipitated and co-immunoprecipitated blots based on the level of the GFP-Cdc42p protein. Values varied <15% across trials. See A for details. Source data are available for this figure: SourceData F1.
The ubiquitination of Cdc42p might promote the degradation/turnover of the protein, which for mammalian Rho GTPases can preferentially occur for the GTP-bound conformation. To test this possibility, the level of GFP-Cdc42p was compared to the level of GFP-Cdc42pQ61L, which mimics the GTP-bound conformation (Ziman et al., 1991). In yeast, Cdc42p is an essential protein, and cells expressing GFP-Cdc42pQ61L as the sole copy are not viable (Ziman et al., 1991). Therefore, as above, proteins were expressed from plasmids in strains containing a chromosomal copy of the CDC42 gene. Immunoblot analysis with anti-GFP antibodies showed that GFP-Cdc42pQ61L was present at lower steady-state levels in the cell than GFP-Cdc42p (Fig. 1 B). Another version of Cdc42p, GFP-Cdc42pG12V, which contains a change that mimics the GTP-bound conformation in several small GTPases (Smith et al., 2013), was also present at lower levels in the cell (Fig. S1 A). To further examine the turnover of Cdc42p, cells expressing GFP-Cdc42p and GFP-Cdc42pQ61L were treated with the protein synthesis inhibitor cycloheximide (CHX). In line with the idea that yeast Cdc42p is a stable protein, the levels of GFP-Cdc42p remained similar throughout the course of the experiment (Fig. 1 C). By comparison, the level of GFP-Cdc42pQ61L became reduced over time (Fig. 1, D and E), indicating that this version of the protein is turned over. Cells expressing GFP-Cdc42p did not accumulate high molecular weight (HMW) products (Fig. 1 C, upper panel) that were observed in cells expressing GFP-Cdc42pQ61L, which might represent ubiquitin conjugates (Fig. 1 D, upper panel).
GTP-locked versions of Cdc42p are present at low levels. Role of the WW domains of Rsp5p and the HSP40 Ydj1p in the degradation of Cdc42p.(A) Levels of GFP-Cdc42p (Cdc42, PC6454) and GFP-Cdc42pG12V (G12V, PC7668) were examined in wild-type cells (PC538) grown in SD-URA for 6 h. Cdc42/Pgk1 ratio refers to relative levels of GFP-Cdc42p to Pgk1p. Anti-GFP and anti-Pgk1p antibodies were used. (B) GFP-Cdc42p (PC6454) levels were examined in wild-type cells (WT, PC5801), and in the rsp5-ww2 (PC5802) and the rsp5-ww3 (PC5803) mutants in the S288c background. See A for details. (C) Levels of GFP-Cdc42p (Cdc42, PC6454) and GFP-Cdc42pQ61L (Q61L, PC7458) in wild-type cells (WT, PC5801) and in the rsp5-ww3 (PC5803) mutant. See A for details. (D) GFP-Cdc42p (PC6454) levels in wild-type cells (WT, PC986) and in the ydj1Δ mutant (PC7657) in the S288c background. See A for details. Source data are available for this figure: SourceData FS1.
GTP-locked versions of Cdc42p are present at low levels. Role of the WW domains of Rsp5p and the HSP40 Ydj1p in the degradation of Cdc42p.(A) Levels of GFP-Cdc42p (Cdc42, PC6454) and GFP-Cdc42pG12V (G12V, PC7668) were examined in wild-type cells (PC538) grown in SD-URA for 6 h. Cdc42/Pgk1 ratio refers to relative levels of GFP-Cdc42p to Pgk1p. Anti-GFP and anti-Pgk1p antibodies were used. (B) GFP-Cdc42p (PC6454) levels were examined in wild-type cells (WT, PC5801), and in the rsp5-ww2 (PC5802) and the rsp5-ww3 (PC5803) mutants in the S288c background. See A for details. (C) Levels of GFP-Cdc42p (Cdc42, PC6454) and GFP-Cdc42pQ61L (Q61L, PC7458) in wild-type cells (WT, PC5801) and in the rsp5-ww3 (PC5803) mutant. See A for details. (D) GFP-Cdc42p (PC6454) levels in wild-type cells (WT, PC986) and in the ydj1Δ mutant (PC7657) in the S288c background. See A for details. Source data are available for this figure: SourceData FS1.
Cdc42p localizes to the plasma membrane and internal compartments (Richman et al., 2002). By fluorescence microscopy, the level of GFP-Cdc42p was higher than GFP-Cdc42pQ61L (Fig. 1 F). GFP-Cdc42pQ61L was preferentially localized to the plasma membrane as previously reported (Woods et al., 2016). The difference in Cdc42p levels by fluorescence intensity (Fig. 1 G) was similar to the difference in band intensity by immunoblot analysis (Fig. 1 B). Co-immunoprecipitation analysis showed that GFP-Cdc42pQ61L was ubiquitinated at 35-fold higher levels than GFP-Cdc42p (Fig. 1 H). Therefore, the yeast Cdc42p protein can be ubiquitinated, which occurred preferentially for the GTP-bound conformation of the protein.
NEDD4 E3 ubiquitin ligase Rsp5p and HSP40/HSP70 chaperones promote turnover of GTP-bound Cdc42p in the 26S proteasome
E3 ubiquitin ligases covalently attach ubiquitin onto substrates (Buetow and Huang, 2016). A candidate approach was used to identify the ubiquitin ligase required for turnover of Cdc42p. Among several proteins tested was Rsp5p, a member of the NEDD4 (neuroprecursor cell expressed developmentally downregulated 4) family of E3 ubiquitin ligases (Ingham et al., 2004). Rsp5p is an essential protein in yeast (Huibregtse et al., 1995). Cells carrying a temperature-sensitive allele, rsp5-1, that contains a point mutation in the catalytic homologous to E6AP C-terminus (HECT) domain (Fisk and Yaffe, 1999; Wang et al., 1999), were defective for turnover of Cdc42p (Fig. 2 A). Compared to 30°C, where the levels of Cdc42p were stable (Fig. 1 C), Cdc42p degradation was accelerated at 37°C, which facilitated evaluation of turnover. The requirement of Rsp5p in Cdc42p turnover was confirmed with antibodies to the Cdc42p protein (Fig. 2 B), which was used interchangeably with anti-GFP antibodies that detect GFP-Cdc42p in the study. Turnover of GTP-bound Cdc42p, GFP-Cdc42pQ61L, also required Rsp5p (Fig. 2 C). Moreover, ubiquitin conjugates associated with GFP-Cdc42pQ61L were reduced in the rsp5-1 mutant (Fig. 2 D, 30°C), which supports the idea that Rsp5p is required for turnover of the active conformation of Cdc42p.
GTP-locked Cdc42p is turned over in a Ydj1p-, Ssa1p-, Rsp5p-, and proteasome-dependent manner. (A) Level of GFP-Cdc42p (PC6454) expressed in wild-type cells (WT, S288c background, PC3288) and rsp5-1 mutant (PC3290) grown for 4 h at 30°C and shifted to 37°C for the times indicated. Values varied <25% across trials. See Fig. 1 B for details. (B) Similar experiment as A except extracts were probed with anti-Cdc42p antibodies to detect GFP-Cdc42p. Cells were grown at 37°C for 2 h. See Fig. 1 B for details. (C) GFP-Cdc42pQ61L (PC7458) levels in same cells as in A grown at the permissive temperature, 30°C. See Fig. 1 B for details. (D) Levels of ubiquitinated GFP-Cdc42pQ61L in wild-type cells (WT, S288c background, PC3288) and in the rsp5-1 mutant (PC3290) grown at 30°C for 6 h. Colored circle refers to cells expressing the indicated versions of Cdc42p and Rsp5p. See Fig. 1 A for details. (E) GFP-Cdc42pQ61L (PC7458) levels in wild-type cells (WT, S288c background, PC986) and ydj1Δ mutant (PC7657). See Fig. 1 B for details. (F) GFP-Cdc42pQ61L (PC7458) levels in wild-type cells (WT, PC6016) and ssa1Δ mutant (PC7700). See Fig. 1 B for details. (G) Wild-type cells (WT, S288c background, PC5851) and the cim3-1 mutant (PC5852) expressing GFP-Cdc42p (PC6454) were grown at 37°C and analyzed by immunoblotting at the indicated time points. See Fig. 1 B for details. (H) Fluorescence microscopy of same cells as in G grown at 30 and 37°C for 2 h. Micrographs were taken at the same exposure, the elevated signal of GFP-Cdc42p observed in the cim3-1 mutant at 37°C might be due to prolonged exposure time. Scale bar, 5 µm. (I) Comparison of relative fluorescence intensity of cells described in G. Error bars indicate SD among three biological replicates (n = 3); 35 cells were examined in each experiment. Data were analyzed by one-way ANOVA, and P values (p) were calculated using Tukey’s multiple comparison test. (J) Levels of ubiquitinated GFP-Cdc42p in wild-type cells (WT, S288c background, PC5851) and in the cim3-1 (PC5852) mutant grown at 37°C for 2 h. Colored circle refers to cells expressing the indicated versions of Cdc42p and Cim3p. See Fig. 1 A for details. (K) The pdr5Δ mutant containing GFP-Cdc42pQ61L (Q61L, PC7458) was supplemented with 0.5% ethanol (CTL, control) or 75 μM MG132 and incubated for 2 h. Values varied <25% across trials. See Fig. 1 B for details. Source data are available for this figure: SourceData F2.
GTP-locked Cdc42p is turned over in a Ydj1p-, Ssa1p-, Rsp5p-, and proteasome-dependent manner. (A) Level of GFP-Cdc42p (PC6454) expressed in wild-type cells (WT, S288c background, PC3288) and rsp5-1 mutant (PC3290) grown for 4 h at 30°C and shifted to 37°C for the times indicated. Values varied <25% across trials. See Fig. 1 B for details. (B) Similar experiment as A except extracts were probed with anti-Cdc42p antibodies to detect GFP-Cdc42p. Cells were grown at 37°C for 2 h. See Fig. 1 B for details. (C) GFP-Cdc42pQ61L (PC7458) levels in same cells as in A grown at the permissive temperature, 30°C. See Fig. 1 B for details. (D) Levels of ubiquitinated GFP-Cdc42pQ61L in wild-type cells (WT, S288c background, PC3288) and in the rsp5-1 mutant (PC3290) grown at 30°C for 6 h. Colored circle refers to cells expressing the indicated versions of Cdc42p and Rsp5p. See Fig. 1 A for details. (E) GFP-Cdc42pQ61L (PC7458) levels in wild-type cells (WT, S288c background, PC986) and ydj1Δ mutant (PC7657). See Fig. 1 B for details. (F) GFP-Cdc42pQ61L (PC7458) levels in wild-type cells (WT, PC6016) and ssa1Δ mutant (PC7700). See Fig. 1 B for details. (G) Wild-type cells (WT, S288c background, PC5851) and the cim3-1 mutant (PC5852) expressing GFP-Cdc42p (PC6454) were grown at 37°C and analyzed by immunoblotting at the indicated time points. See Fig. 1 B for details. (H) Fluorescence microscopy of same cells as in G grown at 30 and 37°C for 2 h. Micrographs were taken at the same exposure, the elevated signal of GFP-Cdc42p observed in the cim3-1 mutant at 37°C might be due to prolonged exposure time. Scale bar, 5 µm. (I) Comparison of relative fluorescence intensity of cells described in G. Error bars indicate SD among three biological replicates (n = 3); 35 cells were examined in each experiment. Data were analyzed by one-way ANOVA, and P values (p) were calculated using Tukey’s multiple comparison test. (J) Levels of ubiquitinated GFP-Cdc42p in wild-type cells (WT, S288c background, PC5851) and in the cim3-1 (PC5852) mutant grown at 37°C for 2 h. Colored circle refers to cells expressing the indicated versions of Cdc42p and Cim3p. See Fig. 1 A for details. (K) The pdr5Δ mutant containing GFP-Cdc42pQ61L (Q61L, PC7458) was supplemented with 0.5% ethanol (CTL, control) or 75 μM MG132 and incubated for 2 h. Values varied <25% across trials. See Fig. 1 B for details. Source data are available for this figure: SourceData F2.
Rsp5p contains WW domains that recognize PPxY (PY) motifs in substrate proteins (Gajewska et al., 2001). Rsp5p lacking a functional WW3 domain, rsp5-WW3, was defective for turnover of GFP-Cdc42p (Fig. S1 B) and GFP-Cdc42pQ61L (Fig. S1 C) at 30°C. These observations indicate that Rsp5p impacts the stability of GTP-bound Cdc42 at 30°C and occurs in a PY-dependent manner. Cdc42p does not contain PY motifs and may be directed to Rsp5p by adaptor proteins. HSPs are evolutionarily conserved adaptors that promote protein folding and the degradation of proteins that cannot be folded properly (Balchin et al., 2016; Whitesell and Lindquist, 2005). Several members of the HSP family function as adaptors that regulate protein turnover in an Rsp5p-dependent manner (Fang et al., 2014). Ydj1p is a member of the DnaJ/HSP40 family of protein chaperones (Caplan and Douglas, 1991; Tsai and Douglas, 1996) that directs client proteins to Rsp5p for turnover (Fang et al., 2014). We found that Ydj1p was required for turnover of GFP-Cdc42pQ61L (Fig. 2 E) and turnover of wild-type Cdc42p (Fig. S1 D). HSP40 functions as a co-chaperone for HSP70 proteins (Glover and Lindquist, 1998; Hartl et al., 2011). One member of this family in yeast is Ssa1p, which along with Ydj1p monitors protein folding and targets proteins for degradation (Fan et al., 2005; Fernández-Fernández et al., 2017). Like Ydj1p, Ssa1p was required for turnover of GTP-locked Cdc42 (Fig. 2 F). These findings show that HSP adaptors mediate turnover of the active conformation of a Rho GTPase in yeast.
Proteins turned over by Ydj1p and Rsp5p are degraded by the 26S proteasome (Fang et al., 2014), a macromolecular complex that degrades ubiquitinated proteins outside of the secretory pathway (Guerriero et al., 2013; Lee et al., 2016). A temperature-sensitive mutant in the 26S proteasome, cim3-1 (also known as rpt6-1; Ghislain et al., 1993), was defective for turnover of GFP-Cdc42p, based on immunoblot analysis (Fig. 2 G) and measurement of the fluorescence intensity of the protein (Fig. 2, H and I). In addition, ubiquitinated GFP-Cdc42p accumulated in cim3-1 mutant (Fig. 2 J) indicating that Cdc42p is turned over by the proteasome. To study the role of the proteasome in the stability of GFP-Cdc42Q61L at 30°C, the proteasome inhibitor MG132 was used (Fenteany et al., 1995; Finley et al., 2012). Because this drug has low permeability, tests were performed in the pdr5Δ mutant, which exhibits reduced drug efflux (Fleming et al., 2002). We observed that GFP-Cdc42pQ61L was stabilized by addition of MG132 (Fig. 2 K), which indicates that the proteasome is required for degradation of the active species of Cdc42p. Therefore, the active species of Cdc42p is turned over in a Ydj1p-, Ssa1p-, and Rsp5p-dependent manner in the 26S proteasome.
A turnover-defective version of GTP-bound Cdc42p preferentially activates the MAP kinase pathway that controls filamentous growth
Cells that accumulate active Cdc42p may show phenotypes in Cdc42p-dependent processes. Cdc42p is a major regulator of polarity establishment (Bi and Park, 2012; Pringle et al., 1995) and exocytosis (Adamo et al., 2001), processes that are essential for viability in yeast. Cells lacking Ydj1p or Ssa1p did not show a growth defect (Fig. S2 A), indicating they are not involved in regulating these essential processes. Cdc42p also regulates MAP kinase pathways, which control differentiation to specific cell types (Fig. 3 A, mating and filamentous growth, fMAPK). During filamentous growth, haploid cells switch from axial to distal-unipolar budding (Cullen and Sprague, 2002; Taheri et al., 2000). Haploid cells lacking Ydj1p and Ssa1p showed distal-unipolar budding, even under nutrient-replete conditions that do not induce filamentous growth (Fig. 3 B, arrows). During filamentous growth, cells also exhibit an elongated cell shape that resulted from a delay in the cell cycle (Gimeno et al., 1992; Kron et al., 1994; Roberts and Fink, 1994). Elongated cells were also observed in the ydj1Δ and ssa1Δ mutants (Fig. 3 B; see Fig. S2, B and C, for more examples). These phenotypes were dependent on the transcription factor Tec1p (Fig. 3 B, tec1Δ; Fig. S2, D and E), which specifically regulates the fMAPK pathway (Fig. 3 A; Madhani et al., 1999). By comparison, cells lacking Ydj1p or Ssa1p showed a similar mating response as wild-type cells based on growth arrest and the formation of mating projections (Fig. 3 C) induced by exposure to the pheromone α-factor (Sprague et al., 1983). These results suggest that the loss of HSP40/HSP70 proteins selectively impacts one MAPK pathway over another. The rsp5-1 mutant also had an elongated morphology (Fig. S2 F) that was partially dependent on Tec1p, and accumulation of GTP-locked Cdc42p induced the formation of elongated cells (Fig. S2 G). Although the ydj1Δ, ssa1Δ, and rsp5-1 mutants showed similar phenotypes, there were some phenotypic differences, which may occur because the proteins have partially non-overlapping client proteins and targets.
Role of the HSP40 Ydj1p, the HSP70 Ssa1p, and Rsp5p in regulating fMAPK activity. (A) Serial dilutions of wild-type cells (WT, PC538), ydj1Δ (PC7616), and ssa1Δ (PC7621) mutants grown on YEPD plates for 2 d. (B) Fluorescence microscopy of wild-type cells (WT, PC986) and the ydj1Δ mutant (PC7657) in the S288c background expressing GFP-Cdc42p (PC6454). Scale bar, 5 µm. (C) Fluorescence microscopy of wild-type cells (WT, PC6016) and the ssa1Δ mutant (PC7700) expressing GFP-Cdc42p (PC6454). Scale bar, 5 µm. (D) Microscopic examination of wild-type cells (WT, PC538), ydj1Δ (PC7616), tec1Δ (PC6102), and the tec1Δ ydj1Δ double mutants (PC7820) grown on YEPD plates for 16 h. Scale bar, 10 µm. (E) Microscopic examination of wild-type cells (WT, PC538), ssa1Δ (PC7621), tec1Δ (PC6102), and the tec1Δ ssa1Δ double mutants (PC7819) grown on YEPD plates for 16 h. Scale bar, 10 µm. (F) Left: Microscopic examination of wild-type cells (WT, PC538), rsp5-1 (PC7543), tec1Δ (PC6102), and the tec1Δ rsp5-1 double mutants (PC7826) grown on YEPD plates for 16 h. Scale bar, 10 µm. Right: Quantification of cell length-to-width ratio of same cells, from three biological replicates (n = 3); >200 cells were examined in each replicate. Data were analyzed by one-way ANOVA, and P values (asterisk < 0.01) were calculated using Tukey’s multiple comparison test. (G) Left: Wild-type cells (WT, PC3288) and the rsp5-1 mutant (PC3290) expressing GFP-Cdc42p (Cdc42, PC6454) or GFP-Cdc42pQ61L (Q61L, PC7458) were examined by microscopy. Scale bar, 5 µm. Right: Quantification of cell length of same cells, n > 25. Data were analyzed by one-way ANOVA, and P values (asterisk < 0.01) were calculated using Tukey’s multiple comparison test.
Role of the HSP40 Ydj1p, the HSP70 Ssa1p, and Rsp5p in regulating fMAPK activity. (A) Serial dilutions of wild-type cells (WT, PC538), ydj1Δ (PC7616), and ssa1Δ (PC7621) mutants grown on YEPD plates for 2 d. (B) Fluorescence microscopy of wild-type cells (WT, PC986) and the ydj1Δ mutant (PC7657) in the S288c background expressing GFP-Cdc42p (PC6454). Scale bar, 5 µm. (C) Fluorescence microscopy of wild-type cells (WT, PC6016) and the ssa1Δ mutant (PC7700) expressing GFP-Cdc42p (PC6454). Scale bar, 5 µm. (D) Microscopic examination of wild-type cells (WT, PC538), ydj1Δ (PC7616), tec1Δ (PC6102), and the tec1Δ ydj1Δ double mutants (PC7820) grown on YEPD plates for 16 h. Scale bar, 10 µm. (E) Microscopic examination of wild-type cells (WT, PC538), ssa1Δ (PC7621), tec1Δ (PC6102), and the tec1Δ ssa1Δ double mutants (PC7819) grown on YEPD plates for 16 h. Scale bar, 10 µm. (F) Left: Microscopic examination of wild-type cells (WT, PC538), rsp5-1 (PC7543), tec1Δ (PC6102), and the tec1Δ rsp5-1 double mutants (PC7826) grown on YEPD plates for 16 h. Scale bar, 10 µm. Right: Quantification of cell length-to-width ratio of same cells, from three biological replicates (n = 3); >200 cells were examined in each replicate. Data were analyzed by one-way ANOVA, and P values (asterisk < 0.01) were calculated using Tukey’s multiple comparison test. (G) Left: Wild-type cells (WT, PC3288) and the rsp5-1 mutant (PC3290) expressing GFP-Cdc42p (Cdc42, PC6454) or GFP-Cdc42pQ61L (Q61L, PC7458) were examined by microscopy. Scale bar, 5 µm. Right: Quantification of cell length of same cells, n > 25. Data were analyzed by one-way ANOVA, and P values (asterisk < 0.01) were calculated using Tukey’s multiple comparison test.
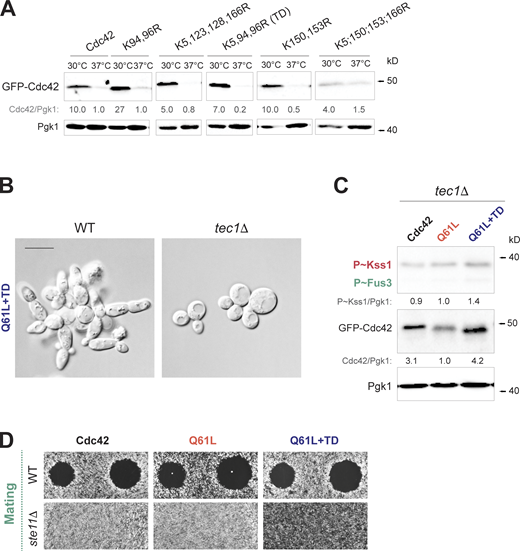
Lysine residues are required for GTP-locked Cdc42p turnover. Turnover of Cdc42p differentially impacts MAPK pathway signaling. (A) Two Cdc42p-depenedent MAP kinase pathways. fMAPK- (red) and mating- (green) specific components. Grey, common components. (B) Left: Microscopic examination of wild-type cells (WT, PC538), and the ydj1Δ (PC7616), ssa1Δ (PC7621), tec1Δ (PC6102), tec1Δ ydj1Δ (PC7820), and the tec1Δ ssa1Δ (PC7819) double mutants grown on YEPD plates for 16 h. Right: Elongated cells expressed as a percentage for the same strains. A cell was considered elongated with >1.40 of length/width ratio. Error bars represent the SD from three biological replicates (n = 3); 200 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk < 0.01, all samples were compared to WT) was calculated using Tukey’s multiple comparison test. (C) Top left: Halo formation in response to α-factor for wild-type cells (WT, PC6810), and the ydj1Δ (PC7619), ssa1Δ (PC7623), and ste11Δ (PC6604) mutants. Cells were grown for 16 h, spread on YEPD media, and α-factor was spotted at two concentrations on the surface, 2 and 6 µM, to study cell-cycle arrest. Bottom: Phenotypes of same strains grown for 3 h in SD-URA media supplemented with 6 µM of α-factor. Shmooing cells were counted and represented as a percentage on the graph at right. Error bars represent the SD from three biological replicates (n = 3); 200 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk < 0.01, all samples were compared to WT) was calculated using Tukey’s multiple comparison test. (D) Wild-type cells (PC538) expressing GFP-Cdc42pQ61L (No change, PC7458), GFP-Cdc42pQ61L,K94R,K96R (PC7662), GFP-Cdc42pQ61L,K150R,K153R (PC7664), GFP-Cdc42pQ61L,K183R,K184R,K186R,K187R (PC7665), pGFP-Cdc42pK5R,Q61L,K150R,K153R,K166R (PC7667), pGFP-Cdc42pK5R,Q61L,K94R,K96R,K123R,K128R,K150R,K153R,K166R,K183R,K184R,K186R,K187R (12KR, PC7666), pGFP-Cdc42pK5R,Q61L,K123R,K128R,K166R (PC7651), and pGFP-Cdc42pK5R,Q61L,K94R,K96R (Q61L+TD, PC7654) were grown in SD-URA for 6 h and analyzed by immunoblotting. Values varied <25% across trials. See Fig. 1 B for details. (E) Predicted structure of Cdc42p. Blue, lysines required for GTP-Cdc42p turnover; magenta, effector-binding domain or P-loop. (F) Wild-type cells (PC6810) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458) or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654) were grown for 6 h in SD-URA media. Numbers indicate relative P∼Kss1p, P∼Fus3p and GFP-Cdc42p levels compared to Pgk1p using Q61L as the control condition. Anti-phospho-p44/42, anti-GFP, and anti-Pgk1 antibodies were used. Representative blots are shown. Values varied <25% across trials. (G) Left: Wild-type cells (WT, PC538) and the tec1Δ mutant (PC6102) expressing the plasmids described in F were grown to mid-log phase and examined by microscopy. Cells that represent the most common phenotype are shown in the figure. Scale bar, 5 µm. Right: Elongated cells expressed as a percentage for the same strains shown on the left. A cell was considered elongated with >1.40 of length/width ratio. Error bars represent the SD from three biological replicates (n = 3); 200 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk < 0.01) was calculated using Tukey’s multiple comparison test. n.s., not significant. (H) Top left: Halo formation in response to α-factor of wild-type cells (WT, PC6810) and the ste11Δ mutant (PC6604) expressing the same plasmids as in F. Cells grown for 16 h in the selective SD-URA media were spread on SD-URA media, and α-factor was spotted at two concentrations on the surface, 2 and 6 µM, to study cell-cycle arrest. Bottom: Phenotypes of same cells expressing plasmids described in F grown for 2 h in SD-URA media supplemented with 6 µM of α-factor, images represent the most common phenotype. Shmooing cells were counted and represented as a percentage on the graph at right. Error bars represent the SD from three biological replicates (n = 3); 200 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk < 0.01) was calculated using Tukey’s multiple comparison test. Comparisons were made to WT cells expressing Cdc42 unless otherwise indicated. Source data are available for this figure: SourceData F3.
Lysine residues are required for GTP-locked Cdc42p turnover. Turnover of Cdc42p differentially impacts MAPK pathway signaling. (A) Two Cdc42p-depenedent MAP kinase pathways. fMAPK- (red) and mating- (green) specific components. Grey, common components. (B) Left: Microscopic examination of wild-type cells (WT, PC538), and the ydj1Δ (PC7616), ssa1Δ (PC7621), tec1Δ (PC6102), tec1Δ ydj1Δ (PC7820), and the tec1Δ ssa1Δ (PC7819) double mutants grown on YEPD plates for 16 h. Right: Elongated cells expressed as a percentage for the same strains. A cell was considered elongated with >1.40 of length/width ratio. Error bars represent the SD from three biological replicates (n = 3); 200 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk < 0.01, all samples were compared to WT) was calculated using Tukey’s multiple comparison test. (C) Top left: Halo formation in response to α-factor for wild-type cells (WT, PC6810), and the ydj1Δ (PC7619), ssa1Δ (PC7623), and ste11Δ (PC6604) mutants. Cells were grown for 16 h, spread on YEPD media, and α-factor was spotted at two concentrations on the surface, 2 and 6 µM, to study cell-cycle arrest. Bottom: Phenotypes of same strains grown for 3 h in SD-URA media supplemented with 6 µM of α-factor. Shmooing cells were counted and represented as a percentage on the graph at right. Error bars represent the SD from three biological replicates (n = 3); 200 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk < 0.01, all samples were compared to WT) was calculated using Tukey’s multiple comparison test. (D) Wild-type cells (PC538) expressing GFP-Cdc42pQ61L (No change, PC7458), GFP-Cdc42pQ61L,K94R,K96R (PC7662), GFP-Cdc42pQ61L,K150R,K153R (PC7664), GFP-Cdc42pQ61L,K183R,K184R,K186R,K187R (PC7665), pGFP-Cdc42pK5R,Q61L,K150R,K153R,K166R (PC7667), pGFP-Cdc42pK5R,Q61L,K94R,K96R,K123R,K128R,K150R,K153R,K166R,K183R,K184R,K186R,K187R (12KR, PC7666), pGFP-Cdc42pK5R,Q61L,K123R,K128R,K166R (PC7651), and pGFP-Cdc42pK5R,Q61L,K94R,K96R (Q61L+TD, PC7654) were grown in SD-URA for 6 h and analyzed by immunoblotting. Values varied <25% across trials. See Fig. 1 B for details. (E) Predicted structure of Cdc42p. Blue, lysines required for GTP-Cdc42p turnover; magenta, effector-binding domain or P-loop. (F) Wild-type cells (PC6810) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458) or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654) were grown for 6 h in SD-URA media. Numbers indicate relative P∼Kss1p, P∼Fus3p and GFP-Cdc42p levels compared to Pgk1p using Q61L as the control condition. Anti-phospho-p44/42, anti-GFP, and anti-Pgk1 antibodies were used. Representative blots are shown. Values varied <25% across trials. (G) Left: Wild-type cells (WT, PC538) and the tec1Δ mutant (PC6102) expressing the plasmids described in F were grown to mid-log phase and examined by microscopy. Cells that represent the most common phenotype are shown in the figure. Scale bar, 5 µm. Right: Elongated cells expressed as a percentage for the same strains shown on the left. A cell was considered elongated with >1.40 of length/width ratio. Error bars represent the SD from three biological replicates (n = 3); 200 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk < 0.01) was calculated using Tukey’s multiple comparison test. n.s., not significant. (H) Top left: Halo formation in response to α-factor of wild-type cells (WT, PC6810) and the ste11Δ mutant (PC6604) expressing the same plasmids as in F. Cells grown for 16 h in the selective SD-URA media were spread on SD-URA media, and α-factor was spotted at two concentrations on the surface, 2 and 6 µM, to study cell-cycle arrest. Bottom: Phenotypes of same cells expressing plasmids described in F grown for 2 h in SD-URA media supplemented with 6 µM of α-factor, images represent the most common phenotype. Shmooing cells were counted and represented as a percentage on the graph at right. Error bars represent the SD from three biological replicates (n = 3); 200 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk < 0.01) was calculated using Tukey’s multiple comparison test. Comparisons were made to WT cells expressing Cdc42 unless otherwise indicated. Source data are available for this figure: SourceData F3.
To better interpret the consequences of Cdc42p turnover on effector pathways, we turned to a cis-based approach by identifying sites on the Cdc42p protein that influence its abundance in the cell. We specifically sought to identify lysine residues in the Cdc42p protein that are required for turnover of the GTP-bound conformation. Lysine residues typically serve as sites for ubiquitination (Ciechanover et al., 1980). Cdc42p has 13 lysines, 12 of which are exposed on the surface of the protein. Site-directed mutagenesis was used to change lysines in Cdc42pQ61L to arginines in pairs or groups, because non-preferred lysines can be used as substrates for ubiquitination when the preferred lysine is absent. No pair of lysine substitutions led to elevated levels of the GFP-Cdc42pQ61L protein (Fig. 3 D, K94, 96R; K150, 153R; K183, 184, 186, 187R; K5, 150, 153, 166R; or K5, 123, 128, 166R), but a version lacking all 12 lysines stabilized the protein (Fig. 3 D, 12KR). Additional combinations showed that three lysine substitutions (K5R, K94R, K96R or turnover defective, TD) stabilized GFP-Cdc42pQ61L to the same degree as 12KR (Fig. 3 D, right panel). The K94 96R pair did not stabilize Cdc42pQ61L (Fig. 3 D), nor did K5 alone or in combination with other residues (Fig. 3 D, K5, 150, 153, 166R). Modeling the residues onto the three-dimensional structure of the Cdc42p protein, which was adapted from human Cdc42p, showed that K94 and K96 are located on the opposite side of the protein as the effector-binding domain, and were across from K5 (Fig. 3 E and Video 1). The residues presumably serve as sites for ubiquitination, and in fact an equivalent residue, K6, is a site for ubiquitination of RhoA in humans (Deglincerti et al., 2015). These residues were specific for the GTP-bound conformation and did not stabilize the wild-type version of the protein at 30°C or 37°C (Fig. S3 A).
Lysine residues of Cdc42p that were required for turnover of a GTP-locked version of the protein. The yeast Cdc42p protein sequence was overlaid onto the structure of human Cdc42p using the Expasy web server SWISS-MODEL (https://swissmodel.expasy.org; Nassar et al., 1998). Blue refers to K5, K94, and K96, and magenta to the effector-binding loop. Video displays at 25 frames/s intervals.
Lysine residues of Cdc42p that were required for turnover of a GTP-locked version of the protein. The yeast Cdc42p protein sequence was overlaid onto the structure of human Cdc42p using the Expasy web server SWISS-MODEL (https://swissmodel.expasy.org; Nassar et al., 1998). Blue refers to K5, K94, and K96, and magenta to the effector-binding loop. Video displays at 25 frames/s intervals.
Lysines residues impact Cdc42p stability. Stabilization of GTP-bound Cdc42p induces fMAPK activity in a Tec1p-dependent manner.(A) Levels of GFP-Cdc42p containing the indicated versions of Cdc42p. Wild-type cells (PC538) were grown in SD-URA at 30°C for 5 h (30°C) and shifted to 37°C for 2 h (37°C). See Fig. S1 A for details. (B) Wild-type cells (WT, PC538) and tec1Δ mutant (PC6102) expressing GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654). Scale bar, 10 µm. (C)tec1Δ cells (PC6102) expressing GFP-Cdc42p (PC6454, Cdc42), GFP-Cdc42pQ61L (PC7458, Q61L) or GFP-Cdc42pQ61L+TD (PC7654, Q61L+TD) were grown for 6 h in SD-URA media. See Fig. 3 F for details. (D) Halo formation in response to α-factor for wild-type cells (WT, PC6810) and the ste11Δ mutant (PC6604) expressing same plasmids as in C. Cells grown for 16 h in selective SD-URA media were spread on SD-URA media, and α-factor was spotted at two concentrations on the surface, 2 and 6 µM, to study cell-cycle arrest. Source data are available for this figure: SourceData FS3.
Lysines residues impact Cdc42p stability. Stabilization of GTP-bound Cdc42p induces fMAPK activity in a Tec1p-dependent manner.(A) Levels of GFP-Cdc42p containing the indicated versions of Cdc42p. Wild-type cells (PC538) were grown in SD-URA at 30°C for 5 h (30°C) and shifted to 37°C for 2 h (37°C). See Fig. S1 A for details. (B) Wild-type cells (WT, PC538) and tec1Δ mutant (PC6102) expressing GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654). Scale bar, 10 µm. (C)tec1Δ cells (PC6102) expressing GFP-Cdc42p (PC6454, Cdc42), GFP-Cdc42pQ61L (PC7458, Q61L) or GFP-Cdc42pQ61L+TD (PC7654, Q61L+TD) were grown for 6 h in SD-URA media. See Fig. 3 F for details. (D) Halo formation in response to α-factor for wild-type cells (WT, PC6810) and the ste11Δ mutant (PC6604) expressing same plasmids as in C. Cells grown for 16 h in selective SD-URA media were spread on SD-URA media, and α-factor was spotted at two concentrations on the surface, 2 and 6 µM, to study cell-cycle arrest. Source data are available for this figure: SourceData FS3.
We next tested whether turnover-defective versions of GTP-bound Cdc42p influences its functions in the cell. GFP-Cdc42pQ61L+TD did not induce a growth defect in wild-type cells (see below in Fig. S4 J, CTL plate) and therefore may not interfere with the essential functions of Cdc42p in polarity establishment and exocytosis. The phosphorylation of the MAP kinases for the fMAPK (Kss1p) and mating (Fus3p) pathways (Fig. 3 A) were examined by anti-phospho-p44/p42 antibodies, which provide a diagnostic readout of MAPK pathway activity. Cells expressing GFP-Cdc42p were compared to cells expressing GFP-Cdc42pQ61L and GFP-Cdc42pQ61L+TD. GFP-Cdc42pQ61L stimulated both the fMAPK and mating pathways, although it stimulated the fMAPK pathway to higher levels (Fig. 3 F, Q61L). This may be because Fus3p needs to be catalytically unlocked by the mating pathway scaffold Ste5p to be conformationally active (Good et al., 2009). GFP-Cdc42pQ61L+TD further stimulated the fMAPK pathway but had a minimal effect on mating (Fig. 3 F). Thus, accumulation of GTP-bound Cdc42p preferentially induced the fMAPK pathway.
Cells lacking the fMAPK scaffold Bem4p show reduced levels of Cdc42p. (A) Cdc42p levels in extracts prepared from wild-type cells (WT, PC538) or mutants lacking interacting proteins (see Table S1). See Fig. S1 A for details. (B) Levels of GFP-Cdc42p in wild-type cells (WT, PC538) and the gic1Δ gic2Δ double mutant (PC7044). See Fig. S1 A for details. (C) Wild-type cells (PC986) and cells lacking Bem4p (PC4351) in the S288c background examined by IB analysis. See Fig. S1 A for details. (D) Wild-type cells (WT, PC538) and bem4Δ mutant (PC3551) expressing GFP-Cdc42p (PC6454) analyzed by IB analysis. See Fig. S1 A for details. GFP mRNA levels were examined by RT-PCR, SD < 0.2 based on two independent trials. (E) Levels of ubiquitinated GFP-Cdc42pQ61L in wild-type cells (WT) and in the bem4Δ mutant. Numbers indicate relative band intensity of co-immunoprecipitated Ub compared to immunoprecipitated GFP protein. Colored circle refers to cells expressing the indicated versions of Cdc42p and Bem4p. See Fig. 1 A for details. IP; immunoprecipitated. (F) Levels of Bem4-HA and Cdc42p in wild-type cells (WT, PC538), and in the bem4Δ mutant (PC3551) containing versions of Bem4p including Bem4p (BEM4, PC2754), Bem4p16–99 (PC4805), Bem4p99–200 (PC4807), Bem4p200–300 (PC4809), Bem4p300–400 (PC4811). See Fig. S1 A for details. (G) Fluorescence microscopy of wild-type cells (WT, PC538) and the bem4Δ mutant (PC3551) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pC188S (C188S, PC7350), GFP-Cdc42pD57Y (D57Y, PC7455) and Cdc42pQ61L (Q61L, PC7458). Micrographs were taken at the same exposure. Scale bar, 5 µm. (H) Levels of GFP-Cdc42p in same cells described in G grown in SD-URA media for 6 h. Top panel (HMW) corresponds to a longer exposure of the anti-GFP blot. See Fig. S1 A for details. (I) Analysis of P∼Fus3p, GFP-Cdc42p, and Pgk1p levels in wild-type cells (PC6810) expressing GFP-Cdc42p (PC6454) grown in YEPD media supplemented with 12 µM of α-factor the time indicated. Numbers indicate relative protein P∼Fus3p and GFP-Cdc42p compared to Pgk1p to time 0 and were represented in Fig. S3 I. See Fig. 3 F for details. (J) Serial dilutions of the ste4Δ mutant (PC538), ste4Δ bem4Δ (PC3551), and ste4Δ ste11Δ (PC3862) double mutants expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458), or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654) were grown on SD-URA (CTL, control) and SD-URA-HIS (fMAPK reporter) media to evaluate the activity of the growth reporter FUS1-HIS3. Source data are available for this figure: SourceData FS4.
Cells lacking the fMAPK scaffold Bem4p show reduced levels of Cdc42p. (A) Cdc42p levels in extracts prepared from wild-type cells (WT, PC538) or mutants lacking interacting proteins (see Table S1). See Fig. S1 A for details. (B) Levels of GFP-Cdc42p in wild-type cells (WT, PC538) and the gic1Δ gic2Δ double mutant (PC7044). See Fig. S1 A for details. (C) Wild-type cells (PC986) and cells lacking Bem4p (PC4351) in the S288c background examined by IB analysis. See Fig. S1 A for details. (D) Wild-type cells (WT, PC538) and bem4Δ mutant (PC3551) expressing GFP-Cdc42p (PC6454) analyzed by IB analysis. See Fig. S1 A for details. GFP mRNA levels were examined by RT-PCR, SD < 0.2 based on two independent trials. (E) Levels of ubiquitinated GFP-Cdc42pQ61L in wild-type cells (WT) and in the bem4Δ mutant. Numbers indicate relative band intensity of co-immunoprecipitated Ub compared to immunoprecipitated GFP protein. Colored circle refers to cells expressing the indicated versions of Cdc42p and Bem4p. See Fig. 1 A for details. IP; immunoprecipitated. (F) Levels of Bem4-HA and Cdc42p in wild-type cells (WT, PC538), and in the bem4Δ mutant (PC3551) containing versions of Bem4p including Bem4p (BEM4, PC2754), Bem4p16–99 (PC4805), Bem4p99–200 (PC4807), Bem4p200–300 (PC4809), Bem4p300–400 (PC4811). See Fig. S1 A for details. (G) Fluorescence microscopy of wild-type cells (WT, PC538) and the bem4Δ mutant (PC3551) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pC188S (C188S, PC7350), GFP-Cdc42pD57Y (D57Y, PC7455) and Cdc42pQ61L (Q61L, PC7458). Micrographs were taken at the same exposure. Scale bar, 5 µm. (H) Levels of GFP-Cdc42p in same cells described in G grown in SD-URA media for 6 h. Top panel (HMW) corresponds to a longer exposure of the anti-GFP blot. See Fig. S1 A for details. (I) Analysis of P∼Fus3p, GFP-Cdc42p, and Pgk1p levels in wild-type cells (PC6810) expressing GFP-Cdc42p (PC6454) grown in YEPD media supplemented with 12 µM of α-factor the time indicated. Numbers indicate relative protein P∼Fus3p and GFP-Cdc42p compared to Pgk1p to time 0 and were represented in Fig. S3 I. See Fig. 3 F for details. (J) Serial dilutions of the ste4Δ mutant (PC538), ste4Δ bem4Δ (PC3551), and ste4Δ ste11Δ (PC3862) double mutants expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458), or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654) were grown on SD-URA (CTL, control) and SD-URA-HIS (fMAPK reporter) media to evaluate the activity of the growth reporter FUS1-HIS3. Source data are available for this figure: SourceData FS4.
Consistent with this result, microscopic examination showed that cells expressing GFP-Cdc42pQ61L+TD exhibited highly polarized growth, which resembled the elongated morphologies seen when cells undergo filamentous growth (Fig. 3 G). The filamentous morphologies required an intact fMAPK pathway and were absent in cells lacking the filamentation-specific transcription factor, Tec1p (Fig. 3 G and Fig. S3 B, tec1Δ). Tec1p was also required for the induction of P∼Kss1p seen in cells expressing GFP-Cdc42pQ61L+TD (Fig. S3 C), which induces positive feedback (Prabhakar et al., 2021). By comparison, GFP-Cdc42pQ61L+TD did not influence the growth arrest of cells exposed to mating pheromone measured by halo size and shmoo formation in response to α-factor (Fig. 3 H). Halo assays have the capacity to show elevated mating pathway activity (Dietzel and Kurjan, 1987). As expected, cells lacking Ste11p were defective for mating pathway activity. Similarly, the number of cells containing mating projections was similar between Cdc42pQ61L and Cdc42pQ61L+TD (Fig. 3 H), supporting the idea that the stabilization of GTP-bound Cdc42p does not have a major impact on the mating pathway. Some hyperpolarized phenotypes induced by Q61L and Q61L+TD might occur as a result of induction of the mating pathway. The fact that elongation was Tec1p dependent (Fig. 3 G) might indicate that hyperpolarization was mainly driven by induction of the fMAPK pathway. Taken together, these results identify a role for Cdc42p turnover in regulating MAPK pathway signaling. Therefore, stabilization of the active conformation of a Rho GTPase impacts a specific pathway.
Adaptor Bem4p stabilizes the Cdc42p protein
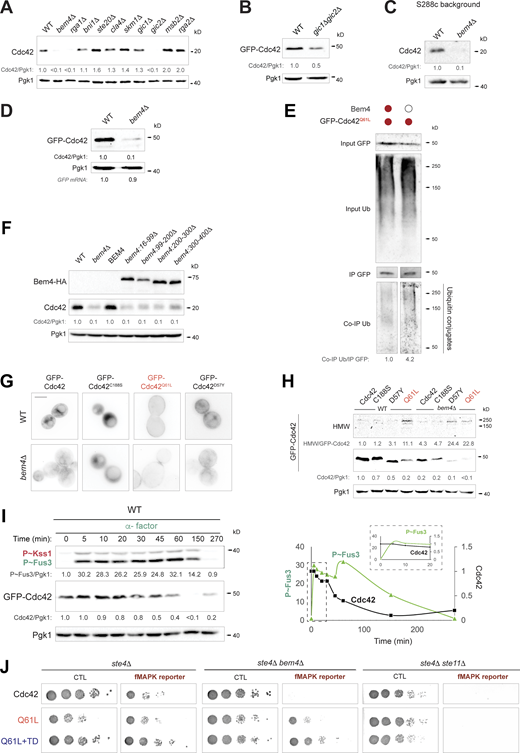
Cdc42p interacts with different proteins to execute an array of biological functions. Cells lacking a panel of Cdc42p-intercting proteins were examined by immunoblot analysis for changes in Cdc42p protein levels. Most mutants lacking Cdc42p-interacting proteins did not show a change in Cdc42p levels, but a few did, including Bem4p (Fig. 4 A; and Fig. S4, A and B), which we focused on here. Bem4p was originally identified as a high copy suppressor of conditional alleles of CDC42 (Mack et al., 1996) and RHO1 (Hirano et al., 1996). Bem4p interacts with Cdc42p and other Rho GTPases (including Rho 1p, Rho 2p, Rho 3p, and Rho 4p; Hirano et al., 1996; Hruby et al., 2011; Mack et al., 1996) and binds to Cdc42p in vivo and in vitro (Drees et al., 2001; Hirano et al., 1996; Mack et al., 1996; Pitoniak et al., 2015). Previous studies from our lab have shown that Bem4p regulates the fMAPK pathway but not other Cdc42p-dependent MAPK pathways (Fig. 3 A; Basu et al., 2020; Pitoniak et al., 2015). In addition, Bem4p does not influence Cdc42p activity (Hirano et al., 1996; Mack et al., 1996; Pitoniak et al., 2015). Thus, Bem4p may impact Cdc42p levels in a different way than by altering the GTP/GDP ratio of Cdc42p.
Role of the fMAPK scaffold Bem4p in stabilization of Cdc42p levels. (A) Cdc42p-interacting proteins. Colors indicate Cdc42p levels by immunoblot analysis in cells lacking the indicated protein. Red, lower levels; green, higher levels; grey, not tested. (B) Cdc42p levels in wild-type cells (WT, PC538), and the bem1Δ (PC6680), bem4Δ (PC3351), and ste11Δ (PC3862) mutants using antibodies to Cdc42p. See Fig. 1 B for details. CDC42 mRNA levels were detected by RT-PCR from two independent replicates, SD < 0.1 for all strain tested. (C) Wild-type cells (WT, PC538) and bem4Δ mutant (PC3551) expressing GFP-Cdc42p (PC6454) were grown for 4 h at 30°C and examined by fluorescence microscopy. Micrographs were taken at the same exposure time. Scale bar, 5 µm. (D) Relative fluorescence intensity of GFP-Cdc42p in wild-type cells and the bem4Δ mutant. Error bars indicate SD among three biological replicates (n = 3); 50 cells were examined in each replicate. Data were analyzed using unpaired t test (two-sided). P refers to P value. (E) Levels of ubiquitinated GFP-Cdc42p in wild-type cells and the bem4Δ mutant. Colored circle refers to cells expressing the indicated versions of Cdc42p and Bem4p. See Fig. 1 A for details. (F) Serial dilutions of wild-type cells (WT) and bem4Δ mutant (PC3551) expressing GFP-Cdc42p (PC6454) on SD-URA media. (G) Levels of GFP-Cdc42pQ61L (Q61L, PC7458) in wild-type cells (WT) and the bem4Δ mutant. See Fig. 1 B for details. (H) Localization of Gic2p-PBD-tdTomato in wild-type cells (WT, PC538) and the bem4Δ mutant (PC3551). Micrographs were taken at the same exposure. Scale bar, 5 µm. Source data are available for this figure: SourceData F4.
Role of the fMAPK scaffold Bem4p in stabilization of Cdc42p levels. (A) Cdc42p-interacting proteins. Colors indicate Cdc42p levels by immunoblot analysis in cells lacking the indicated protein. Red, lower levels; green, higher levels; grey, not tested. (B) Cdc42p levels in wild-type cells (WT, PC538), and the bem1Δ (PC6680), bem4Δ (PC3351), and ste11Δ (PC3862) mutants using antibodies to Cdc42p. See Fig. 1 B for details. CDC42 mRNA levels were detected by RT-PCR from two independent replicates, SD < 0.1 for all strain tested. (C) Wild-type cells (WT, PC538) and bem4Δ mutant (PC3551) expressing GFP-Cdc42p (PC6454) were grown for 4 h at 30°C and examined by fluorescence microscopy. Micrographs were taken at the same exposure time. Scale bar, 5 µm. (D) Relative fluorescence intensity of GFP-Cdc42p in wild-type cells and the bem4Δ mutant. Error bars indicate SD among three biological replicates (n = 3); 50 cells were examined in each replicate. Data were analyzed using unpaired t test (two-sided). P refers to P value. (E) Levels of ubiquitinated GFP-Cdc42p in wild-type cells and the bem4Δ mutant. Colored circle refers to cells expressing the indicated versions of Cdc42p and Bem4p. See Fig. 1 A for details. (F) Serial dilutions of wild-type cells (WT) and bem4Δ mutant (PC3551) expressing GFP-Cdc42p (PC6454) on SD-URA media. (G) Levels of GFP-Cdc42pQ61L (Q61L, PC7458) in wild-type cells (WT) and the bem4Δ mutant. See Fig. 1 B for details. (H) Localization of Gic2p-PBD-tdTomato in wild-type cells (WT, PC538) and the bem4Δ mutant (PC3551). Micrographs were taken at the same exposure. Scale bar, 5 µm. Source data are available for this figure: SourceData F4.
We confirmed that Cdc42p levels were reduced in cells lacking Bem4p, both in the filamentous background using anti-Cdc42p antibodies to detect endogenous Cdc42p levels (Fig. 4 B, Σ1278b) and in a commonly used laboratory strain (Fig. S4 C, S288c). By comparison, loss of another Bem-type adaptor and Cdc42p-interacting protein, Bem1p (Butty et al., 2002; Irazoqui et al., 2003; Park et al., 1997), did not impact Cdc42p levels (Fig. 4 B). Bem4p did not impact the expression of the CDC42 gene (Fig. 4 B, CDC42 mRNA), and Cdc42p levels were not altered in cells lacking an intact fMAPK pathway (Fig. 4 B, ste11Δ), which indicates that Bem4p stabilizes Cdc42p levels separate from its role in regulating the fMAPK pathway. Cells lacking Bem4p also showed reduced GFP-Cdc42p levels by fluorescence microscopy (Fig. 4, C and D; and Fig. S4 D), which explains a previously reported localization defect for Cdc42p in cells lacking Bem4p (Pitoniak et al., 2015). Moreover, cells lacking Bem4p showed elevated ubiquitination of Cdc42p (Fig. 4 E) and Cdc42pQ61L (Fig. S4 E, and see Fig. S4 H for stabilization of HMW products of Cdc42p by Bem4p), indicating that Bem4p inhibits ubiquitination and turnover of Cdc42p.
Cells lacking Bem4p did not exhibit a growth defect (Fig. 4 F), which indicates that cells produce more Cdc42p than is necessary for growth. Cells expressing the cdc42-1 allele also show reduced Cdc42p levels yet are viable at permissive temperatures (Adamo et al., 2001; Kozminski et al., 2000). The higher levels of Cdc42p in the cell may have a pathway-specific function. In the previous section, we showed that elevated levels of GTP-bound Cdc42p specifically induced the fMAPK pathway. By stabilizing GTP-bound Cdc42p, Bem4p may likewise promote activation of the fMAPK pathway. Bem4p stabilized GFP-Cdc42pQ61L by immunoblot analysis (Fig. 4 G). In addition, the levels of Gic2p-Td-Tomato (Okada et al., 2017), which provides a readout of Cdc42p activity, were reduced in cells lacking Bem4p (Fig. 4 H). Multiple domains of the Bem4p protein were required to stabilize Cdc42p (Fig. S4 F), and Bem4p stabilized GTP-locked versions of Cdc42p and versions that cannot bind to membranes (Cdc42pC188S) or that are locked in the GDP-bound state (Cdc42pD57Y; Fig. S4 G). Therefore, Bem4p may stabilize Cdc42p independent of its localization and activation states. To summarize, one way that Bem4p may regulate the fMAPK pathway is by stabilization of the GTP-bound conformation of Cdc42p.
Stabilization of Cdc42p by Bem4p impacts the activity of the fMAPK pathway
To further define how Cdc42p turnover regulation impacts MAP kinase signaling, the activity of the fMAPK pathway was examined over its activation cycle, which can be stimulated by growth in the non-preferred carbon source, galactose (Basu et al., 2020). Growth of cells in galactose led to phosphorylation of Kss1p from 160 to 480 min, which then declined over time (Fig. 5 A, P∼Kss1p, red line). In cells lacking Bem4p, P∼Kss1p levels did not accumulate (Fig. 5 B, P∼Kss1p, red line), which correlated with low levels of Cdc42p (Fig. 5 A, Cdc42p, right panel black line). This finding supports the idea that by stabilizing Cdc42p, Bem4p stimulates the fMAPK pathway. Interestingly, in wild-type cells, Cdc42p levels declined after the fMAPK pathway was activated (Fig. 5 A, Cdc42p, starting at 420 min). The reduction in Cdc42p levels may result in attenuation of the fMAPK pathway. Cdc42p levels also fell when cells were exposed to α factor, which induces the mating response (Fig. S4 I), reinforcing the idea that activation of Cdc42p leads to degradation of the GTP-bound form of the protein and attenuation of MAP kinase signaling.
High levels of Cdc42p induce fMAPK pathway activity. (A) Analysis of P∼Kss1p, Cdc42p, and Pgk1p levels in wild-type cells (WT, PC538) grown in YEPGAL (Gal) media for the times indicated. See Fig. 3 F for details. (B)bem4Δ cells (PC3551) were examined as indicated in A. See Fig. 3 F for details. (C) Wild-type cells (WT, PC6810) and the bem4Δ mutant (PC7179) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458) or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654) were grown for 6 h in SD-URA media. See Fig. 3 F for details. (D) Microscopic examination of cells described in C. Scale bar, 5 µm. Right: Elongated cells expressed as a percentage for the same strains shown on the left. A cell was considered elongated with >1.40 of length/width ratio. Error bars represent the SD detected from three biological replicates, (n = 3); 200 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk < 0.01) was calculated using Tukey’s multiple comparison test. Source data are available for this figure: SourceData F5.
High levels of Cdc42p induce fMAPK pathway activity. (A) Analysis of P∼Kss1p, Cdc42p, and Pgk1p levels in wild-type cells (WT, PC538) grown in YEPGAL (Gal) media for the times indicated. See Fig. 3 F for details. (B)bem4Δ cells (PC3551) were examined as indicated in A. See Fig. 3 F for details. (C) Wild-type cells (WT, PC6810) and the bem4Δ mutant (PC7179) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458) or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654) were grown for 6 h in SD-URA media. See Fig. 3 F for details. (D) Microscopic examination of cells described in C. Scale bar, 5 µm. Right: Elongated cells expressed as a percentage for the same strains shown on the left. A cell was considered elongated with >1.40 of length/width ratio. Error bars represent the SD detected from three biological replicates, (n = 3); 200 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk < 0.01) was calculated using Tukey’s multiple comparison test. Source data are available for this figure: SourceData F5.
In addition to Cdc42p, Bem4p binds to other proteins that regulate the fMAPK pathway, including the GEF, Cdc24p, and MAPKKK, Ste11p (Pitoniak et al., 2015). To determine the contribution of stabilizing Cdc42p by Bem4p on fMAPK pathway activity, cells that fail to turnover GTP-bound Cdc42p, GFP-Cdc42pQ61L+TD, were expressed in cells lacking Bem4p. In the bem4Δ mutant, GFP-Cdc42pQ61L+TD restored much of the fMAPK pathway activity seen in wild-type levels, based on P∼Kss1p levels (Fig. 5 C) and the activity of a growth reporter (Fig. S4 J). As expected, GFP-Cdc42pQ61L+TD did not bypass the signaling defect of cells lacking the MAPKKK Ste11p (Fig. S4 J). GFP-Cdc42pQ61L+TD partially restored the hyperpolarized morphologies to the bem4Δ mutant (Fig. 5 D). The suppression was higher than seen in the tec1Δ mutant but not to the degree seen in wild-type cells (Fig. 5 D). Because Cdc42pQ61L+TD did not fully suppress the signaling defects of cells lacking Bem4p, we suggest that Bem4p regulates the fMAPK pathway by stabilizing Cdc42p and by interaction with fMAPK pathway regulatory proteins characterized previously.
Stabilization of GTP-bound Cdc42p leads to intrinsic polarity establishment mediated by the fMAPK pathway
Wild-type cells expressing Cdc42pQ61L+TD also induced unusual morphologies, such as growth of cells at multiple sites (Fig. 6 A, WT). At first, we thought that this phenotype reflected a role for Cdc42p in regulating cell polarity; however, the phenotype was dependent on the fMAPK pathway, being reduced in cells lacking Ste11p and Tec1p (Fig. 6, A and B). We have previously shown that hyperactivation of the fMAPK pathway can induce growth at multiple sites (Prabhakar et al. 2020), which interferes with normal budding where cells grow at a single site through a mechanism known as singularity in budding (Caviston et al., 2002; Goryachev and Leda, 2017; Irazoqui et al., 2003; Slaughter et al., 2009; Wedlich-Soldner et al., 2003; Woods et al., 2016).
Turnover-defective GTP-locked Cdc42p causes multiple growth sites in a fMAPK-dependent manner. (A) Wild-type cells (WT, PC538), and the ste11Δ (PC3862), tec1Δ (PC6102), bni1Δ (PC7086), gic1Δ gic2Δ (PC7044), rsr1Δ (PC4256), and bem4Δ (PC3551) mutants expressing GFP-Cdc42pQ61L+TD (PC7654, Q61L+TD) were examined by fluorescence microscopy. Blue arrows indicate multiple growth projections. Scale bar, 5 µm. (B) Multiple growth sites expressed as percentage for the strains described in A. Error bars indicate SD from two biological replicates (n = 2); 300 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk, <0.01, all samples were compared to WT) was calculated using Tukey’s multiple comparison test. (C) Wild-type cells (PC538) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458), or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654). Cells were stained with Phalloidin-Atto 532 (F-actin, magenta) and fluorescent brightener #28 (CFW, calcofluor white, yellow, cell wall). Magenta arrows refer to multiple sites of actin polymerization. Scale bar, 5 µm. (D) Fluorescence microscopy of Cdc3p-mCherry cells (PC7365) expressing same plasmids as in C. Cdc3p-mCherry (red) is a septin ring marker. Red arrows refer to septin rings. Scale bar, 5 µm. (E) The cdc42Δ mutant (PC6684) harboring the pGFP-Cdc42 (URA3, PC6454) and pGFP-Cdc42 (LEU2-based plasmid, PC6457) or pGFP-Cdc42TD (TD, K5,94,96R; LEU2-based plasmid, PC7698) were examined for growth on SD-URA-LEU (top) and 5-FOA (middle) media. Bottom: Morphology of the cdc42Δ mutant harboring the pRS316-GFP-Cdc42 (URA3) and pRS315-GFP-Cdc42 (LEU2) or pRS315-GFP-Cdc42TD (TD, K5,94,96R; LEU2) cells after 2 d on 5-FOA media. Blue arrows indicate multiple buds. Scale bar, 5 µm.
Turnover-defective GTP-locked Cdc42p causes multiple growth sites in a fMAPK-dependent manner. (A) Wild-type cells (WT, PC538), and the ste11Δ (PC3862), tec1Δ (PC6102), bni1Δ (PC7086), gic1Δ gic2Δ (PC7044), rsr1Δ (PC4256), and bem4Δ (PC3551) mutants expressing GFP-Cdc42pQ61L+TD (PC7654, Q61L+TD) were examined by fluorescence microscopy. Blue arrows indicate multiple growth projections. Scale bar, 5 µm. (B) Multiple growth sites expressed as percentage for the strains described in A. Error bars indicate SD from two biological replicates (n = 2); 300 cells were analyzed in each replicate. Data were analyzed by one-way ANOVA, and the P value (asterisk, <0.01, all samples were compared to WT) was calculated using Tukey’s multiple comparison test. (C) Wild-type cells (PC538) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458), or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654). Cells were stained with Phalloidin-Atto 532 (F-actin, magenta) and fluorescent brightener #28 (CFW, calcofluor white, yellow, cell wall). Magenta arrows refer to multiple sites of actin polymerization. Scale bar, 5 µm. (D) Fluorescence microscopy of Cdc3p-mCherry cells (PC7365) expressing same plasmids as in C. Cdc3p-mCherry (red) is a septin ring marker. Red arrows refer to septin rings. Scale bar, 5 µm. (E) The cdc42Δ mutant (PC6684) harboring the pGFP-Cdc42 (URA3, PC6454) and pGFP-Cdc42 (LEU2-based plasmid, PC6457) or pGFP-Cdc42TD (TD, K5,94,96R; LEU2-based plasmid, PC7698) were examined for growth on SD-URA-LEU (top) and 5-FOA (middle) media. Bottom: Morphology of the cdc42Δ mutant harboring the pRS316-GFP-Cdc42 (URA3) and pRS315-GFP-Cdc42 (LEU2) or pRS315-GFP-Cdc42TD (TD, K5,94,96R; LEU2) cells after 2 d on 5-FOA media. Blue arrows indicate multiple buds. Scale bar, 5 µm.
Consistent with our previous work, the formation of multiple growth sites in cells expressing Cdc42pQ61L+TD required Bni1p (Fig. 6, A and B, bni1Δ), an effector of Cdc42p and member of the formin family of proteins (Evangelista et al., 1997). The phenotype was also dependent to a lesser degree on the Gic1p and Gic2p proteins (Fig. 6, A and B, gic1Δ gic2Δ), which control assembly of the septin ring that creates a barrier to restrict growth to the emerging bud (Brown et al., 1997; Chen et al., 1997; Daniels et al., 2018; Iwase et al., 2006; Okada et al., 2013). The phenotype was enhanced in cells lacking bud-site-selection proteins (Fig. 6, A and B, rsr1Δ), which direct growth at specific sites on the cell cortex (Chant and Pringle, 1995; Howell and Lew, 2012; Miller et al., 2020; Moran et al., 2019; Park et al., 2002). Rsr1p also delimits the formation of multi-budded cells induced by cell size (Chiou et al., 2021). Cdc42pQ61L+TD formed multiple buds in cells lacking Bem4p (Fig. 6, A and B, bem4Δ), to about half the levels seen in wild-type cells. This result supports the idea that Bem4p has multiple functions in regulating the fMAPK pathway. Therefore, our results fit with the idea that failure to turn over GTP-bound Cdc42p leads to hyper-activation of the fMAPK pathway.
These phenotypes were examined in more detail. Cell growth depends on polymerization of filamentous actin (F-actin), which normally occurs at a single site (Adamo et al., 2001). Immunofluorescence staining showed that F-actin was localized to multiple sites in cells expressing Cdc42pQ61L+TD (Fig. 6 C). Wild-type cells expressing GFP-Cdc42p showed <0.1% of cells with actin at multiple sites (n = 250), and cells expressing GFP-Cdc42pQ61L showed 0.9% of cells with actin at multiple sites (n = 152) compared to 15.8% of cells expressing GFP-Cdc42pQ61L+TD (n = 184). Cells expressing Cdc42pQ61L+TD also had multiple septin rings and multiple growth sites (Fig. 6 D and Fig. S5 A). Cells expressing GFP-Cdc42p had <0.1% of cells with multiple growth sites (n = 229), and cells expressing GFP-Cdc42pQ61L had 1.7% cells with multiple growth sites (n = 114) compared to 9.9% of cells expressing Cdc42pQ61L+TD (n = 429).
Turnover-defective GTP-bound Cdc42p (Cdc42p Q61L+TD ) affects fMAPK pathway-dependent polarity. (A) Confocal time-lapse analysis of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654). Arrows refer to two buds growing simultaneously. Scale bar, 5 µm. (B) Fluorescence microscopy of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458), or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654). Cdc3p-mCherry (red) is a septin ring marker. Red arrows refer to multiple septin rings. Scale bar, 5 µm. (C) Microscopic examination of wild-type cells (PC986, S288c background) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458), or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654). Scale bar, 5 µm. (D) Same strains as described in C were explored by microscopy.
Turnover-defective GTP-bound Cdc42p (Cdc42p Q61L+TD ) affects fMAPK pathway-dependent polarity. (A) Confocal time-lapse analysis of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654). Arrows refer to two buds growing simultaneously. Scale bar, 5 µm. (B) Fluorescence microscopy of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458), or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654). Cdc3p-mCherry (red) is a septin ring marker. Red arrows refer to multiple septin rings. Scale bar, 5 µm. (C) Microscopic examination of wild-type cells (PC986, S288c background) expressing GFP-Cdc42p (Cdc42, PC6454), GFP-Cdc42pQ61L (Q61L, PC7458), or GFP-Cdc42pQ61L+TD (Q61L+TD, PC7654). Scale bar, 5 µm. (D) Same strains as described in C were explored by microscopy.
Time-lapse microscopy showed that in some cells, multiple growth sites formed and extended at the same time (Fig. S5 B, 24% of cells with multiple buds), while in other cells, growth sites extended in sequence (Video 2, Cdc42p; Video 3, Cdc42pQ61L; Video 4, Cdc42pQ61l+TD, 76%). In some cells, growth sites initiated by GFP-Cdc42pQ61L+TD occurred outside the septin ring (Fig. 6 D, bottom panel). Multiple projections also formed in growing buds (Fig. 6 A, second example, right; Video 5). Here, actin cables extended to multiple sites (Fig. 6 C, bottom two panels), which led to the formation of multiple projections within the bud. The multibudding and hyperpolarized phenotypes were observed in multiple strain backgrounds (Fig. S5, C and D) like S288c, which although defective for filamentous growth has an activatable fMAPK pathway, which indicates that the phenotype is not strain-specific. Construction of a version of Cdc42p as the sole copy in cells that cannot be turned over (Cdc42pTD) was not viable, based on the inability of a cdc42Δ mutant to survive with the pCDC42TD plasmid compared to wild type, pCDC42 (Fig. 6 E), and also showed multiple growth sites (Fig. 6 E, bottom). Interestingly, although cells expressing TD (K5R + K94R K96R) were not viable, we previously showed that cells expressing K5A or the K94A K96A pair are viable (Basu et al., 2020). Therefore, failure to turnover Cdc42p leads to hyperactivation of the fMAPK pathway and consequently multiple rounds of polarity establishment.
Confocal time-lapse microscopy of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42p (PC6454) grown on SD-URA media. Cells were grown for 5 h at 30°C. Green refers to GFP-Cdc42p, and Cdc3p-mCherry (red) is a septin ring marker. Time interval, 10 min, at 1 frame/s intervals.
Confocal time-lapse microscopy of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42p (PC6454) grown on SD-URA media. Cells were grown for 5 h at 30°C. Green refers to GFP-Cdc42p, and Cdc3p-mCherry (red) is a septin ring marker. Time interval, 10 min, at 1 frame/s intervals.
Confocal time-lapse microscopy of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42pQ61L(PC7458) grown on SD-URA media. Cells were grown for 5 h at 30°C. Green refers to GFP-Cdc42p, and Cdc3p-mCherry (red) is a septin ring marker. Time interval, 10 min, at 1 frame/s intervals.
Confocal time-lapse microscopy of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42pQ61L(PC7458) grown on SD-URA media. Cells were grown for 5 h at 30°C. Green refers to GFP-Cdc42p, and Cdc3p-mCherry (red) is a septin ring marker. Time interval, 10 min, at 1 frame/s intervals.
Confocal time-lapse microscopy of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42pQ61L+TD(PC7654) grown on SD-URA media. Cells were grown for 5 h at 30°C. Green refers to GFP-Cdc42p, and Cdc3p-mCherry (red) is a septin ring marker. Time interval, 10 min, at 1 frame/s intervals.
Confocal time-lapse microscopy of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42pQ61L+TD(PC7654) grown on SD-URA media. Cells were grown for 5 h at 30°C. Green refers to GFP-Cdc42p, and Cdc3p-mCherry (red) is a septin ring marker. Time interval, 10 min, at 1 frame/s intervals.
Confocal time-lapse microscopy of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42pQ61L+TD(PC7654) grown on SD-URA media. Cells were grown for 5 h at 30°C. Green refers to GFP-Cdc42p, and Cdc3p-mCherry (red) is a septin ring marker. Time interval, 10 min, at 1 frame/s intervals.
Confocal time-lapse microscopy of Cdc3p-mCherry cells (PC7365) expressing GFP-Cdc42pQ61L+TD(PC7654) grown on SD-URA media. Cells were grown for 5 h at 30°C. Green refers to GFP-Cdc42p, and Cdc3p-mCherry (red) is a septin ring marker. Time interval, 10 min, at 1 frame/s intervals.
Discussion
We show here that the yeast Cdc42p protein, a highly studied and well-understood member of the Rho GTPase family, is ubiquitinated, and the levels of the protein are regulated by turnover by the ubiquitin-proteasome system. It was unexpected that Cdc42p protein levels are regulated, because Cdc42p has been widely shown to be a stable protein in yeast. In mammals, Rho GTPase ubiquitination and turnover are well characterized (Hodge and Ridley, 2016). As has been shown for several Rho GTPases in mammals (Castillo-Lluva et al., 2013; Murali et al., 2017; Torrino et al., 2011), we show that the GTP-bound conformation of Cdc42p is preferentially degraded, which attenuates some Cdc42p functions in the cell. Our findings indicate that Rho GTPase turnover is an evolutionarily conserved mechanism to regulate Rho GTPase activity and function.
Yeast Cdc42p is regulated by GEFs and GAPs, and the fact that it is also regulated by turnover adds an important aspect to the overall regulation of the protein (Fig. 7). We further show that GTP-Cdc42p turnover requires the NEDD4 E3 ubiquitin ligase Rsp5p. The NEDD4 family of ubiquitin ligases has not been previously connected to Rho GTPase regulation. The active conformation might be susceptible to turnover because that species of the protein is preferentially localized to the plasma membrane, which is a site where Rsp5p is known to function. NEDD4 ubiquitin ligase family is conserved throughout eukaryotes (Boase and Kumar, 2015), and may regulate the degradation of Rho GTPases to control aspects of signaling and polarity in many systems.
Regulatory mechanism of Cdc42p turnover, and its impact on fMAPK pathway activity. Cdc42p is known to be activated by GEFs and inactivated by GAPs. Here, we show that GTP-bound Cdc42p is ubiquitinated and turned over by a mechanism involving HSP40 (Ydj1p), HSP70 (Ssa1p), and the NEDD4 E3 ubiquitin ligase, Rsp5p. In the model, poly-ubiquitin conjugates of Cdc42p are degraded in the proteasome. Turnover of Cdc42p attenuates fMAPK pathway signaling (green block arrow). By comparison, the Smg GDS-type protein Bem4p stabilizes Cdc42p to promote fMAPK pathway activity. The Cdc42p effectors Bni1p, Gic1p, and Gic2p regulate fMAPK-dependent polarity establishment, and the bud-site selection protein Rsr1p may focus polarization at bud sites (Focus). Block green arrow (Gated) indicates that the mating pathway is not sensitive to Cdc42p levels, because the MAPK Fus3p is catalytically locked when cells are not mating. Cdc42p may be ubiquitinated in other contexts besides those described here. Created with BioRender.com.
Regulatory mechanism of Cdc42p turnover, and its impact on fMAPK pathway activity. Cdc42p is known to be activated by GEFs and inactivated by GAPs. Here, we show that GTP-bound Cdc42p is ubiquitinated and turned over by a mechanism involving HSP40 (Ydj1p), HSP70 (Ssa1p), and the NEDD4 E3 ubiquitin ligase, Rsp5p. In the model, poly-ubiquitin conjugates of Cdc42p are degraded in the proteasome. Turnover of Cdc42p attenuates fMAPK pathway signaling (green block arrow). By comparison, the Smg GDS-type protein Bem4p stabilizes Cdc42p to promote fMAPK pathway activity. The Cdc42p effectors Bni1p, Gic1p, and Gic2p regulate fMAPK-dependent polarity establishment, and the bud-site selection protein Rsr1p may focus polarization at bud sites (Focus). Block green arrow (Gated) indicates that the mating pathway is not sensitive to Cdc42p levels, because the MAPK Fus3p is catalytically locked when cells are not mating. Cdc42p may be ubiquitinated in other contexts besides those described here. Created with BioRender.com.
We also show that members of the HSP family of protein chaperones, including HSP40 and HSP70, regulate the turnover of Cdc42p with Rsp5p in the 26S proteasome. Rsp5p ubiquitinates most transmembrane proteins at the plasma membrane (Guiney et al., 2016; Rotin et al., 2000; Zhao et al., 2013b), including the mucin-type glycoprotein that regulates the fMAPK pathway, Msb2p (Adhikari et al., 2015b). However, Rsp5p also promotes endocytosis/degradation of the mating receptors Ste2p and Ste3p (Alvaro and Thorner, 2016; Hicke and Dunn, 2003), and β subunit, Ste4p, which are required for mating (Zhu et al., 2011). Therefore, we suggest that Rsp5p may function as a general protein whose specificity is dictated by adaptors, which for the fMAPK pathway are Ydj1p and Ssa1p. HSPs are global regulators of cell polarity and signal-dependent cell differentiation (Calderwood and Gong, 2016) and drivers of morphogenetic responses and phenotypic plasticity (Rutherford and Lindquist, 1998). The discovery that HSPs regulate the levels of Rho GTPases may have implications in the roles that chaperones play in cell polarity and signaling pathway regulation.
HSPs control the folding and turnover of many proteins, including proteins that become damaged or mis-folded. In yeast, Ydj1p controls turnover of mis-folded proteins with Rsp5p in the cytosol (Fang et al., 2014). It is intriguing that Ydj1p and Ssa1p promote degradation of the active conformation of Cdc42p. Conformational changes in Cdc42p induced by binding GTP may destabilize the protein and make it more accessible to Ydj1p/Ssa1p/Rsp5p. This possibility might suggest that the active forms of Rho GTPases are prone to turnover, perhaps by proteins that comprise quality-control pathways.
Our effort to define residues in the Cdc42p protein that are required for turnover uncovered lysines that mediate degradation of the GTP-locked version of the protein. Substitutions of these lysines for similarly charged residues created a version of GTP-locked Cdc42p that was defective for turnover. This version of the protein specifically hyperactivated the Cdc42p-dependent MAPK pathway (fMAPK) that controls filamentous growth among other Cdc42p-dependent pathways in the cell. The mating pathway, which shares components with the fMAPK pathway, may not be sensitive to GTP-Cdc42p levels because the MAP kinase Fus3p is conformationally closed until the mating pathway is activated (Good et al., 2009). Therefore, stabilizing the active species of Cdc42p has a pathway-specific consequence on a cell differentiation program. This discovery is important because it provides a connection between Rho GTPase turnover/stability and MAPK pathway regulation that may be conserved in many systems.
By examining Cdc42p levels in cells lacking a panel of Cdc42p-interacting proteins, we found that the fMAPK pathway adaptor Bem4p stabilized Cdc42p protein levels. Bem4p interacts with Cdc42p and may promote folding and/or protect the protein from accessibility by HSPs and Rsp5p. Bem4p interacts with several proteins to regulate the fMAPK pathway (Basu et al., 2020; Pitoniak et al., 2015), and by stabilizing Cdc42p, Bem4p may funnel GTP-bound Cdc42p into the fMAPK pathway (Fig. 5 F). Bem4p exhibits amino acid sequence homology with Smg GDS proteins (Pitoniak et al., 2015), which are evolutionarily conserved regulators of Rho GTPase function (Hamel et al., 2011; Jennings et al., 2018; Shimizu et al., 2018; Shimizu et al., 2017; Vikis et al., 2002; Vithalani et al., 1998). It may be interesting to determine whether these proteins have a general function in the stabilization of Rho GTPases. From this perspective, it will be interesting to explore other proteins that may function to stabilize Rho GTPases to influence their functions in different contexts.
Interestingly, cells lacking Bem4p show fivefold lower levels of Cdc42p in the cell yet do not show growth defects or defects in other Cdc42p-dependent MAPK pathways (Pitoniak et al., 2015). Likewise, elevated levels of GTP-locked Cdc42p do not show growth defects. These results together indicate that Cdc42p levels can vary widely without impacting many of the protein’s functions in the cell, including its essential functions in polarity establishment (Bi and Park, 2012; Ziman et al., 1991) and exocytosis (Adamo et al., 2001; Munson and Novick, 2006). Although GTP-locked versions of Cdc42p that cannot be turned over show polarity problems, these appear to be mediated through the fMAPK pathway. In this way, the fMAPK pathway may be sensitive to the levels of active Cdc42p in the cell. This may be because in comparison to polarity establishment, which is transient, the fMAPK pathway is active over long time scales. The fMAPK pathway is also sensitive to Cdc42p activation at bud sites by bud-site-selection proteins during bud emergence (Basu et al., 2016). Thus, the fMAPK pathway may be highly sensitive to Cdc42p activity as part of its activation mechanism. Intriguingly, in fungal pathogens, Cdc42p and other Rho GTPases contribute to many fungal attributes underlying virulence (Brand et al., 2014; Chen et al., 2019; Silva et al., 2019). HSPs have also been implicated in the regulation of fungal pathogenesis (Horianopoulos and Kronstad, 2021). It may be interesting to explore how the HSP40/70/Bem4p axis impacts Rho GTPase levels to regulate MAP kinase signaling during the pathogenic response.
Once activated, the fMAPK pathway is attenuated, in part, by ubiquitin-dependent turnover of the mucin-type sensor, Msb2p, by Rsp5p (Adhikari et al., 2015b). Msb2p is also proteolytically processed to activate the pathway by release of an auto-inhibitory domain (Vadaie et al., 2008). We show here that the stabilization of Cdc42p by Bem4p also promotes pathway signaling, and that Cdc42p is turned over, perhaps to attenuate pathway activity. Bem4p may actively protect Cdc42p from turnover in a regulated manner or constitutively promote stabilization of the GTPase. In this latter case, Bem4p might have evolved pathway-specific functions as a result of its ability to bind to and stabilize Cdc42p. Thus, a balance of proteolytic functions shapes the kinetics of the filamentation response. The regulation of Rho GTPase protein levels by stabilization and turnover may be a general way to direct Rho GTPases to function in pathway-specific contexts.
Materials and methods
Yeast strains, reagents, and media
Strains are listed in Table S1, plasmids are listed in Table S2, and primers are listed in Table S3. Yeast cultivation was performed in synthetic media (SD; 0.67% yeast nitrogen base without amino acids and 2% dextrose), supplemented with amino acids as required, yeast extract peptone dextrose media (YEPD; 1% bacto-yeast extract, 2% bacto-peptone, and 2% dextrose) and YEPGAL media (1% bacto-yeast extract, 2% bacto-peptone, and 2% galactose) have been described (Rose et al., 1990). Cells were grown at 30°C or unless otherwise indicated. pRS306-GFP-linker-CDC42 (pDLB3609) plasmid was provided by the Lew Lab (Woods et al., 2016). To construct pRS316-GFP-linker-CDC42P (pGFP-Cdc42; PC6454), GFP-linker-CDC42 (containing the CDC42 promoter) was subcloned with EcoRI and SalI into pRS316 (CEN/URA [Sikorski and Hieter, 1989]).
To insert point mutations in plasmids carrying CDC42 (pGFP-Cdc42; PC6454), GeneART Site-Directed Mutagenesis (SDM) Kit (Cat#A13282; Thermo Fisher Scientific) was used according to the manufacturer’s protocols using primers listed in Table S3. Nucleotides encoding lysine residues were changed in pairs or groups because non-preferred lysines can be ubiquitinated when a preferred lysine is absent (Kravtsova-Ivantsiv and Ciechanover, 2011). Some mutations were introduced by in vivo homologous recombination in yeast. Primers containing the desired point mutations and a flanking region for homologous recombination were amplified by PCR using pGFP-Cdc42 (PC6454) as a template. pGFP-Cdc42 was linearized by PshAI (Cat#R0593S; New England Biolabs) and co-transformed with the PCR product into an uracil auxotrophic strain (PC538). Plasmid rescue was performed from colonies arising on SD-URA semi-solid agar media. Nucleotide sequences were confirmed by Sanger Sequencing (Genewiz). Gene disruptions were performed by antibiotic resistance markers NAT, HYG, and KanMX6 using PCR-based approaches using published templates (Goldstein and McCusker, 1999; Longtine et al., 1998). Selection on 5-FOA (5-fluoroorotic acid) was used to force out the pGFP-Cdc42 plasmid (PC6454) and determine whether cells containing plasmids pGFP-Cdc42 (LEU2, PC6457) or pGFP-Cdc42TD (TD, K5,94,96R; LEU2, PC7698) were viable.
Assays to evaluate protein turnover of Cdc42p
CHX assays were performed as described in Adhikari et al. (2015a). 100 ml of wild-type cells (PC538) and cells expressing pGFP-Cdc42 (PC6454) or pGFP-Cdc42Q61L (PC7458) at 0.02 of O.D. were grown in SD or SD-URA media (to maintain selection) for 4 h. After 4 h, the medium was supplemented with 25 μg/ml of CHX, and 10 ml of samples were collected at 0, 15, 30, 45, 60, 90, and 120 min to generate cell extracts for immunoblot analysis. Experiments were performed in two independent biological replicates.
The proteasome inhibitor MG132 (Cat#474790, carbobenzoxy-Leu-Leu-leucinal; CAS133407-82-6; Calbiochem) was resuspended in ethanol (20 mg/ml) according to the manufacturer’s protocol. 20 ml of wild-type cells containing GFP-Cdc42p or GFP-Cdc42pQ61L at 0.02 O.D. were grown at 30°C for 4 h in SD-URA. At 4 h, media was supplemented with 0.5% ethanol (control) or 75 μM MG132, and cells were harvested after 2 h to generate extracts for immunoblot analysis. Experiments were performed in two independent replicates.
Co-immunoprecipitation analysis
For co-immunoprecipitation analysis, wild-type cells (PC538) were grown to mid-log phase in SD media or cells expressing the pGFP-Cdc42 or pGFP-Cdc42Q61L grown to mid-log phase in SD-URA media. Cells were harvested by centrifugation, washed with 1% phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4), and resuspended in 500 µl of IP buffer (50 mM Tris-Cl, pH 8, 1 mM EDTA, 100 mM NaCl, 1.5% NP-40, 1 mM PMSF, and 1× protease inhibitor cocktail). 200 µl of glass beads were added, and the cells were lysed by vortexing (FastPrep-24, MP Biomedicals) for 10 min at 4°C. After a 30 min incubation at 4°C, lysed cells were centrifuged at 4°C for 10 min at 14,000 rpm. The immunoprecipitation was performed as described in Elu et al. (2019). Briefly, clarified lysate was incubated with 25 µl GFP-Trap magnetic beads (ChromoTek GFP-Trap Magnetic Agarose), at 4°C for 2 h. Beads were separated by a magnet (Cat#1614916; Bio-Rad, Inc.) and washed twice with 500 µl of dilution buffer (10 mM Tris-Cl, pH 7.5, 150 mM NaCl, and 0.5 mM EDTA). Proteins were eluted from the beads by treatment at 95°C for 2 h in 2 × SDS-polyacrylamide–sample buffer (120 mM Tris-Cl, pH 6.8, 20% glycerol, 4% SDS, 0.04% bromophenol blue, and 10% BME). The supernatant was examined by immunoblot analysis. Ponceau S staining was used to determine total protein levels (Cat#P7170; Millpore Sigma).
Phosphoblot and immunoblot analysis
Wild-type cells or cells lacking indicated genes were grown to saturation in SD or YEPD media for 16 h and transferred fresh media (SD, YEPD, or YPED + 6 μM of α-factor) and grown for 4–6 h to mid-log phase or for the designated time points. Cells were harvested by centrifugation. Proteins extracts were prepared by mechanical disruption with beads followed by the trichloroacetic acid (TCA) precipitation method described in Basu et al. (2020). Protein precipitates were analyzed by SDS-PAGE and transferred to a nitrocellulose membrane (Cat#10600003, Amersham Protran Premium 0.45 μm NC; GE Healthcare Life sciences). Monoclonal mouse anti-GFP antibodies were used (Cat#11814460001, clones 7.1 and 13.1; Roche) at 1:1,000 dilution. Polyclonal rabbit phospho-p44/42 MAPK antibodies (Erk1/2, Cat#3102; Cell Signaling Technology) were used at 1:10,000 dilution. Mouse anti-Kss1p antibodies (yC-19, Cat#6775; Santa Cruz Biotechnology) were used at 1:10,000 dilution. Monoclonal mouse anti-ubiquitin antibodies were used at 1:5,000 dilution (P4G7, Cat#Sc-53509; Santa Cruz Biotechnology). Rabbit anti-Cdc42p antibodies (Kozminski et al., 2000) were used at 1:1,000 dilution and were generously provided by Dr. Keith Kozminski (University of Virginia, Charlottesville, VA). Monoclonal mouse anti-Pgk1p antibodies (22C5D8, Cat#459250; Invitrogen) were 1:1,000 dilution. Secondary anti-mouse IgG-HRP (Cat#1706516; Bio-Rad, Inc.) and goat anti-rabbit IgG-HRP (Cat#115-035-003; Jackson ImmnunoResearch Laboratories) were used. The nitrocellulose membrane was blocked with 5% non-fat dried milk or 5% BSA (for p44/42 antibody) for 1 h prior antibody detection. Primary incubations were performed at 4°C for 16 h and secondary at 20°C for 1 h. Immunoblots were visualized by Gel Doc XR Imaging System (Bio-Rad, Inc.), after addition of Chemiluminescent HRP substrate for chemiluminescent Westerns (Radiance Plus Substrate, Azure Biosystems).
Band intensity quantitation of P∼Fus3p, P∼Kss1p, Cdc42p, GFP-Cdc42p and ubiquitin was performed under non-saturated conditions and normalized to the housekeeping protein Pgk1p using the Image Lab Software (Bio-Rad, Inc.). Wild-type cells and control conditions were set to 1 and adjusted for other samples accordingly.
Fluorescence microscopy
The localization of Cdc42p was examined in strains containing the plasmid pGFP-Cdc42 (PC6454). In this plasmid, the expression of the GFP-tagged Cdc42p is controlled by its own promoter. In all experiments, cells were grown to mid log phase at 30°C in SD-URA media before examination, except in the case of the temperature sensitive mutants, rsp5-1 (PC3290) and cim3-1 (PC5852), which were grown for an additional 2 h at 37°C. Differential interference contrast (DIC), fluorescence microscopy using fluorescein isothiocyanate (FITC) and Rhodamine filter sets were used in an Axioplan 2 fluorescence microscope (Zeiss) with a Plan-Apochromat 100×/1.4 (oil) objective (N.A. 1.4; cover slip 0.17) with the Axiocam MRm camera (Zeiss). Images were analyzed using Axiovision 4.4 software (Zeiss). Actin staining from exponentially grown cells was performed as described (Amberg et al., 2006) using Phalloidin-Atto 532 (Cat#49429; Millpore Sigma). Briefly, cells were grown until 2 O.D. and fixed with 10% of formaldehyde for 1 h at 25°C. Cells were washed twice with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and1.8 mM KH2PO4, pH 7.4). 20 μl of Phalloidin-Atto 532 (6.6 μM) were added to 180 μl of cells and incubated for 1 h in the dark. Cells were washed twice with PBS and visualized by microscopy.
Images were analyzed in Adobe Photoshop and ImageJ (https://imagej.nih.gov). Fluorescence images were converted to grayscale and inverted using ImageJ. For the quantitative comparison of fluorescence intensity, the corrected total fluorescence (CTCF) was determined using the measure tool of the software ImageJ from images taken at the same exposure time. To calculate CTCF, the area selected multiplied by fluorescence background was subtracted to the integrated density (Keith R. Porter Imaging Facility, University of Maryland, Baltimore County; https://kpif.umbc.edu). Images taken with the same exposure time were compared, and for all analysis >4 frames containing >10 cells were used. For relative quantification the highest value of the control condition was set to 1 and the other values were calculated accordingly. Three biological replicates were performed, and >35 cells in each replicate were analyzed. To quantify elongated cells and cells showing multiple growth sites, wild-type cells and cells lacking specific genes and expressing indicated plasmids were grown for 16 h at 30°C. In all cases, at least two biological replicates were analyzed and >200 cells were observed by microscopy per replicate.
Modeling protein structure
The yeast Cdc42p protein sequence was overlaid onto the crystal structure of human Cdc42p using the Expasy web server SWISS-MODEL (https://swissmodel.expasy.org; Nassar et al., 1998).
fMAPK pathway reporters
The activity of the fMAPK pathway was tested using FUS1-HIS3 growth reporter (McCaffrey et al., 1987). Cells lacking an intact mating pathway (ste4) show basal activity of the fMAPK pathway by this reporter (Cullen et al., 2004). Wild-type cells (PC538) and control strains (ste11Δ, PC3862; tec1Δ PC6102) expressing indicated plasmids were grown on SD-URA media to maintain plasmid selection (SD-URA, control), and spotted onto media lacking histidine (SD-URA-HIS) to evaluate the activity of the FUS1-HIS3 reporter.
Mating pathway activity and the response to pheromone
Mating pathway activity was evaluated by phosphoblot analysis (Basu et al., 2020) which is described above, shmoo formation, and halo assays (Sprague et al., 1983). To evaluate the morphogenetic response to α-factor (shmoos), 500 μl of cells were grown for 16 h, were washed with water and resuspended in 5 ml SD-URA media containing 6 μM of α-factor. Cells were incubated for 2.5 h at 30°C and analyzed by microscopy. For halo assays, cells were grown for 16 h, and 200 μl of a 1:100 dilution were spread on SD-URA plates. After the plates were air dried, two concentrations of α-factor, 3 μl (1.8 μM) and 10 μl (6 μM) were applied to the surface. Plates were incubated at 30°C for 2 d and photographed.
Quantitative polymerase chain reaction (qPCR) analysis
qPCR analysis was performed as described (González et al., 2017). 0.02 O.D. of yeast cells, WT (PC538), bem1Δ (PC6680), bem4Δ (PC3351), and ste11Δ (PC6604) were inoculated in SD media. Samples were grown at 30°C and collected after 4 h. RNA extraction was performed by hot acid phenol-chloroform followed by a purification step with the RNeasy Mini Kit (Cat#79254). RNA stability was determined by agarose gel electrophoresis in 1.2% agarose Tris-Borate-EDTA (TBE, 89 mM Tris base, 89 mM boric acid, and 2 mM EDTA). For reverse transcription reactions, RNA concentration was adjusted to 60 ng/μl. Reverse transcription was performed iScript Reverse Transcriptase Supermix (Cat#1708840; BioRad). qPCR was performed using iTaq Universal SYBR Green Supermix (Cat#1725120; BioRad) following the manufacturer’s instructions. Reactions contained 10 μl samples (180 ng/μl cDNA, 0.2 μM each primer, and 5 μl SYBRGreen master mix). qPCR was performed using an BioRad thermocycler (CFX384 Real-Time System; Applied Biosystems). Relative gene expression was calculated using the 2−ΔCt formula, where Ct is defined as the cycle at which fluorescence was determined to be statistically significant above background; ΔCt is the difference in Ct of the CDC42 gene and housekeeping gene (ACT1). The primers used are listed in Table S3. Values represent the mean of at least two independent biological replicates and two technical replicates.
Confocal time-lapse microscopy
Experiments were performed based on (Prabhakar et al., 2020). Cells were grown at 30°C for 16 h on SD-URA. 5 μl of cells diluted to 0.02 O.D. were placed under 1% agarose (SD-URA media) pads using a 12 mm Nunc glass base dish (Cat#150680; Thermo Fisher Scientific). A wet cotton pad was placed around the agar to prevent dehydration. Cells were grown at 30°C for 2 h prior to imaging, and the incubator was set to 30°C unless otherwise indicated. Live-cell microscopy was performed with a Zeiss 170 confocal microscope equipped with a Plan-Apochromat 40×/1.4 Oil DIC M27 objective. During imaging Cdc3p-mCherry cells (PC7365) expressing different alleles, GFP-Cdc42p (PC6454), GFP-Cdc42pQ61L (PC7458), or GFP-Cdc42pQ61L+TD (PC7654) were grown at 30°C for 4 h, and images were taken in intervals of 10 min. For the detection of GFP-Cdc42p, a 488 nm laser (496 nm–548 nm filter), and for Cdc3p-mCherry, a 580 nm laser (589 nm–708 nm filter) were used. Images were taken with multiple Z-stacks (8–10) and a distance of 1 μm between each Z-stack. Exposure time was modified to every GFP- and mCherry-labeled protein to minimize bleaching and phototoxicity. Images were analyzed with ImageJ using the Z-project and template matching plugins.
Statistical analysis
Statistical evaluations were performed with the 2021.1 XLSTAT software (https://www.xlstat.com) and Prism 7 (GraphPad; https://www.graphpad.com/scientific-software/prism/). Unpaired t test (two-sided) was used to determine statistical significance and generate P values for comparison of two datasets. Data distribution was assumed to be normal but was not formally tested. One-way ANOVA test followed by Tukey’s multiple comparison test were used to compare more than two datasets. Tests were indicated for each experiment in the corresponding figure legend.
Online supplemental material
Fig. S1 shows the protein levels of Cdc42p in cells harboring a version of GTP-locked Cdc42p. The role of WW domains of Rsp5p and Ydj1p in regulating Cdc42p protein levels were also examined. Fig. S2 shows the role of Ydj1p (HSP40), Ssa1p (HSP70), and Rsp5p (NEDD4 E3) in regulating fMAPK pathway activity. Fig. S3 shows the role of lysines in the stability of Cdc42p and the impact of turnover of GTP-locked Cdc42p in regulating fMAPK pathway activity. Fig. S4 shows the role of Bem4p in regulating Cdc42p levels. Fig. S5 shows the role of GTP-locked Cdc42p in in regulating fMAPK pathway-dependent polarity. Table S1 indicates the strains used in the study. Table S2 indicates the plasmids used in the study. Table S3 indicates the primers used in the study. Video 1 shows the localization of lysine residues of Cdc42p required for turnover of GTP-locked Cdc42p. Video 2 is a time-lapse analysis of wild-type cells expressing Cdc3p-mCherry and GFP-Cdc42p. Video 3 is a time-lapse analysis of wild-type cells expressing Cdc3p-mCherry and GFP-Cdc42pQ61L. Video 4 is a time-lapse analysis of wild-type cells expressing Cdc3p-mCherry and GFP-Cdc42pQ61L+TD. Video 5 is a second example of a time-lapse analysis of wild-type cells expressing Cdc3p-mCherry and GFP-Cdc42pQ61L+TD.
Acknowledgments
Thanks to John Pringle (Stanford University, Stanford, CT), Charles Boone (University of Toronto, Toronto, Canada), Keith Kozminski (University of Virginia), Gerald Fink (Massachusetts Institute of Technology, Boston, MA), Erfei Bi (University of Pennsylvania, Philadelphia, PA), Daniel Lew (Duke University, Durham, NC), Thibault Mayor (University of British Columbia, Vancouver, Canada), Chris Burd (Yale University, New Haven, CT), David Pellman (Harvard Medical School, Boston, MA), and Scott Emr (Cornell University, Ithaca, NY) for sharing reagents. Thanks to Rick Cerione, Daniel Lew, Sophie Martin and Martí Aldea for providing helpful suggestions. Thanks to Aditi Prabhakar and Alan Seigel for assistance with confocal microscopy, and laboratory members for suggestions.
The work was supported by a grant from the National Institutes of Health (GM098629).
The authors declare no competing financial interests.
Author contributions: B. Gonzalez designed experiments, generated data, and wrote the paper. P.J. Cullen designed experiments and wrote the paper.
References
Supplementary data
shows plasmids used in the study
shows primers used in the study