A growing number of yeast and mammalian plasma membrane proteins are reported to be modified with K63-linked ubiquitin (Ub) chains. However, the relative importance of this modification versus monoubiquitylation in endocytosis, Golgi to endosome traffic, and sorting into the multivesicular body (MVB) pathway remains unclear. In this study, we show that K63-linked ubiquitylation of the Gap1 permease is essential for its entry into the MVB pathway. Carboxypeptidase S also requires modification with a K63-Ub chain for correct MVB sorting. In contrast, monoubiquitylation of a single target lysine of Gap1 is a sufficient signal for its internalization from the cell surface, and Golgi to endosome transport of the permease requires neither its ubiquitylation nor the Ub-binding GAT (Gga and Tom1) domain of Gga (Golgi localizing, gamma-ear containing, ARF binding) adapter proteins, the latter being crucial for subsequent MVB sorting of the permease. Our data reveal that K63-linked Ub chains act as a specific signal for MVB sorting, providing further insight into the Ub code of membrane protein trafficking.
Introduction
Ubiquitin (Ub) is a highly conserved protein composed of 76 aa that can be covalently linked to a lysine residue of a target protein. Attachment of a single Ub monomer to one or several lysines of a protein (mono- or multimonoubiquitylation, respectively) is notably involved in endocytosis and the regulation of histones (Hicke, 2001). Ub itself possesses several lysines that can be used for the attachment of another Ub molecule, allowing substrates to be modified with different types of Ub chains (Peng et al., 2003; Pickart and Fushman, 2004). The consequences of this polyubiquitylation appear to depend on the length of the Ub chain and on the type of linkage used. The most abundant Ub chains in living cells are K48-linked chains, which constitute a signal for protein degradation by the 26-S proteasome (Thrower et al., 2000), and K63-linked chains, which function in a variety of cellular processes, including DNA repair (Spence et al., 1995), stress responses (Arnason and Ellison, 1994), signal transduction (Sun and Chen, 2004; Mukhopadhyay and Riezman, 2007), and intracellular trafficking of membrane proteins (Hicke, 1999; Mukhopadhyay and Riezman, 2007).
Among the protein cargoes of Saccharomyces cerevisiae used to investigate the roles of Ub and Ub chains in membrane trafficking is the general amino acid permease Gap1 (Haguenauer-Tsapis and André, 2004). Gap1 is highly active and stable at the plasma membrane in cells growing on poor nitrogen sources like proline. Upon addition of a favored nitrogen source like ammonium, it is ubiquitylated on its amino-terminal lysines 9 and 16 by the HECT (homologous to E6-associated protein carboxy terminus)-type Rsp5/Npi1 Ub ligase (Soetens et al., 2001), thus causing its internalization (Springael and André, 1998). After reaching the late endosome, Gap1 is sorted into vesicles that bud into the lumen of this compartment as it matures into a multivesicular body (MVB). Upon fusion of the MVB with the vacuole, internal vesicles are released in the vacuolar lumen where the permease is ultimately degraded (Nikko et al., 2003). Both K9 and K16 of Gap1 are modified with K63-linked Ub chains (Springael et al., 1999). Although monoubiquitylation is sufficient to trigger endocytic internalization of Gap1, K63-linked ubiquitylation appears necessary for this process to occur at a maximal rate (Springael et al., 1999). The control by nitrogen of Gap1 trafficking also affects the newly synthesized protein in the secretory pathway. On poor nitrogen media, Gap1 is sorted from the Golgi complex to the plasma membrane, whereas under nitrogen-rich conditions, the permease is targeted directly from the Golgi to the vacuolar degradation pathway without passing via the cell surface (Helliwell et al., 2001; Soetens et al., 2001). Polyubiquitylation of Gap1 is required for this direct sorting to the vacuolar lumen (Helliwell et al., 2001), and a recent study proposed that this modification is specifically involved in transport of the permease between the TGN and the late endosome (Risinger and Kaiser, 2008). The latter trafficking step involves the Golgi-localizing, gamma-ear–containing, ARF-binding (Gga) adapters (Bonifacino, 2004), which contain a Ub-binding Gga and Tom1 (GAT) domain proposed to mediate sorting of ubiquitylated Gap1 from the TGN to the late endosome (Scott et al., 2004).
Other yeast membrane proteins such as the uracil (Fur4; Galan and Haguenauer-Tsapis, 1997), ferrichrome (Arn1; Kim et al., 2007), and siderophore (Sit1; Erpapazoglou et al., 2008) transporters undergo K63-linked ubiquitylation, as reported also for a growing number of mammalian plasma membrane proteins (Traub and Lukacs, 2007) like the nerve growth factor receptor (tropomyosin-regulated kinase A; Geetha et al., 2005), major histocompatibility complex class I molecules (Duncan et al., 2006), and the EGF (Huang et al., 2006) or prolactin receptor (Varghese et al., 2008). Although several studies are consistent in demonstrating an important role of K63-linked ubiquitylation in targeting internalized proteins to the lysosome/vacuole (Huang et al., 2006; Barrière et al., 2007; Erpapazoglou et al., 2008), other data suggest a contribution of K63-linked Ub chains at the internalization step of endocytosis (Galan and Haguenauer-Tsapis, 1997; Springael et al., 1999; Geetha et al., 2005; Duncan et al., 2006; Hawryluk et al., 2006; Erpapazoglou et al., 2008). Thus, a unifying picture of the respective roles of K63 ubiquitylation versus monoubiquitylation in the different steps of down-regulation of membrane proteins is still lacking.
In this study, we have used the Gap1 permease and carboxypeptidase S (CPS) as model cargoes to better understand the respective roles of mono- and polyubiquitylation in different trafficking events in yeast. We show that K63-linked Ub chains act as a signal specifically required for protein sorting into the MVB pathway. Moreover, we show that the GAT domain of the Gga proteins is essential to MVB sorting of Gap1, not to its Golgi to endosome traffic. We also discuss a general model of the Ub code of yeast membrane protein trafficking, which may also apply to many proteins of higher organisms.
Results
Monoubiquitylation even on a single lysine is sufficient for Gap1 endocytic internalization
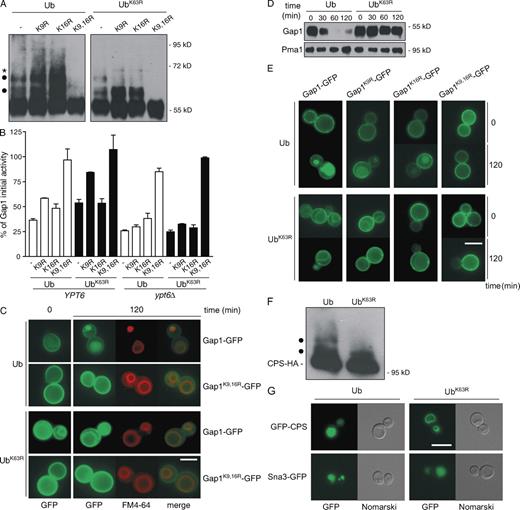
Previously, the role of K63-linked ubiquitylation in trafficking of the Gap1 permease was studied in doa4Δ mutant cells, in which the reduced internal Ub pool was compensated by overproducing either native Ub or a UbKR variant unable to form K29, K48, and/or K63 chains (Springael et al., 1999). Yet our recent observation that the doa4Δ mutation impairs MVB sorting of Gap1 even when Ub is overproduced (Nikko and André, 2007) means the doa4Δ strain is unsuited for testing a potential role of K63-linked ubiquitylation at a late step of endocytosis. As we have also obtained evidence that trafficking artifacts can result from the 10–20-fold Ub overproduction achieved in these experiments as compared with wild-type cells (Nikko and André, 2007), we decided to investigate the role of Gap1 K63 ubiquitylation in mutant cells lacking all four Ub-encoding genes and expressing normal levels of plasmid-encoded Ub or UbK63R as the unique Ub source (see Materials and methods; Spence et al., 1995). In agreement with previous results (Springael et al., 1999), the addition of ammonium to proline-grown cells triggered K63-linked ubiquitylation of K9 and K16 of Gap1: cells coexpressing UbK63R and the Gap1K9R or Gap1K16R variant were only monoubiquitylated, whereas two Ub moieties were found on Gap1K9R or Gap1K16R coexpressed with native Ub (K63-linked ubiquitylation) and on Gap1 coexpressed with UbK63R (double monoubiquitylation; Fig. 1 A). In the case of Gap1 in Ub-producing cells, we could hardly detect more than two bands above the main Gap1 signal; thus, the slowest migrating band likely corresponds to a mixture of Gap1 proteins modified with two Ub monomers or with a K63-linked di-Ub chain. We next examined how different Gap1 ubiquitylation patterns (mono-, dimono-, or short-chain K63 ubiquitylation) affect the Gap1 internalization rate by monitoring the loss of Gap1 activity after the addition of ammonium. This assay reflects the progressive removal of the permease from the plasma membrane (Springael and André, 1998). However, in some cases, the monitored inactivation is an underestimation of the actual internalization rate of the permease because endocytosed Gap1 may recycle to the cell surface as a result of a defect further downstream in the endocytic pathway, as occurs in several mutants defective in MVB sorting. In such situations, Gap1 recycling requires the Ypt6 GTPase involved in endosome to TGN transport (Nikko et al., 2003; Nikko and André, 2007). Thus, we performed our experiments with Ub- and UbK63R-expressing cells in which Gap1 recycling was either possible or prevented by deletion of the YPT6 gene. The results obtained in the absence of Ypt6 clearly show (Fig. 1 B) that Gap1 is internalized in these cells at a similar rate regardless of whether it is monoubiquitylated or K63 ubiquitylated and of whether one or two lysines are modified. In cells possessing an intact YPT6 gene (Fig. 1 B), internalization seemed generally less pronounced, this being particularly visible in cells expressing UbK63R as the sole Ub source. These results show that one or two Ub monomers or a short K63-Ub chain constitutes an equally efficient internalization signal for Gap1. Furthermore, internalized Gap1 tends to recycle to the cell surface via the TGN if its K63 ubiquitylation is impaired.
K63-linked ubiquitylation is required for MVB sorting of Gap1 and CPS. Cells expressing Ub or UbK63R as the sole source of Ub were grown in glucose- and proline-containing yeast nitrogen base medium. (A) gap1Δ cells were transformed with a centromeric vector carrying the GAP1 gene or its gap1K9R, gap1K16R, or gap1K9,16R allele. Cells were collected 5 min after the addition of 100 mM ammonium and were used to prepare total protein extracts, and the Gap1 ubiquitylation profile was examined by Western blotting using anti-Gap1 antibodies. Ubiquitylated forms of Gap1 corresponding to an additional mass of ∼7 kD are indicated with dots, whereas the molecular mass of the band marked with an asterisk suggests that it corresponds to a modified form of the upper ubiquitylated conjugate. In the case of cells expressing native Gap1 and Ub, more than two bands may be detected if Ub is overproduced or after a longer incubation in the presence of ammonium (Fig. S1). (B) gap1Δ or gap1Δ ypt6Δ cells were transformed with vectors as in A. Gap1 activity was measured before and 120 min after the addition of 100 mM ammonium. Graph bars represent the percentage of Gap1 initial activity remaining at time 120 min (mean of two independent experiments). Error bars represent standard deviation. (C and E) gap1Δ cells were transformed with a centromeric vector encoding Gap1-GFP, Gap1K9R-GFP, Gap1K16R-GFP, or Gap1K9,16R-GFP. Gap1-GFP localization was examined by fluorescence microscopy before and 120 min after the addition of 100 mM ammonium. The vacuolar membrane was labeled with the lipophilic marker FM4-64. (D) Total protein extracts were prepared from cells as in A and collected before and at different times after the addition of ammonium. Gap1 stability was analyzed by immunoblotting with anti-Gap1 antibodies. (F) The ubiquitylation profile of CPS was examined by Western blotting using anti-HA antibodies in total protein extracts prepared from CPS-HA3 cells. Ubiquitylated forms corresponding to an additional mass of ∼7 kD are indicated with dots. (G) Cells were transformed with a vector encoding GFP-CPS or Sna3-GFP. The localization of GFP-CPS and Sna3-GFP was examined by fluorescence microscopy. Bars, 5 µm.
K63-linked ubiquitylation is required for MVB sorting of Gap1 and CPS. Cells expressing Ub or UbK63R as the sole source of Ub were grown in glucose- and proline-containing yeast nitrogen base medium. (A) gap1Δ cells were transformed with a centromeric vector carrying the GAP1 gene or its gap1K9R, gap1K16R, or gap1K9,16R allele. Cells were collected 5 min after the addition of 100 mM ammonium and were used to prepare total protein extracts, and the Gap1 ubiquitylation profile was examined by Western blotting using anti-Gap1 antibodies. Ubiquitylated forms of Gap1 corresponding to an additional mass of ∼7 kD are indicated with dots, whereas the molecular mass of the band marked with an asterisk suggests that it corresponds to a modified form of the upper ubiquitylated conjugate. In the case of cells expressing native Gap1 and Ub, more than two bands may be detected if Ub is overproduced or after a longer incubation in the presence of ammonium (Fig. S1). (B) gap1Δ or gap1Δ ypt6Δ cells were transformed with vectors as in A. Gap1 activity was measured before and 120 min after the addition of 100 mM ammonium. Graph bars represent the percentage of Gap1 initial activity remaining at time 120 min (mean of two independent experiments). Error bars represent standard deviation. (C and E) gap1Δ cells were transformed with a centromeric vector encoding Gap1-GFP, Gap1K9R-GFP, Gap1K16R-GFP, or Gap1K9,16R-GFP. Gap1-GFP localization was examined by fluorescence microscopy before and 120 min after the addition of 100 mM ammonium. The vacuolar membrane was labeled with the lipophilic marker FM4-64. (D) Total protein extracts were prepared from cells as in A and collected before and at different times after the addition of ammonium. Gap1 stability was analyzed by immunoblotting with anti-Gap1 antibodies. (F) The ubiquitylation profile of CPS was examined by Western blotting using anti-HA antibodies in total protein extracts prepared from CPS-HA3 cells. Ubiquitylated forms corresponding to an additional mass of ∼7 kD are indicated with dots. (G) Cells were transformed with a vector encoding GFP-CPS or Sna3-GFP. The localization of GFP-CPS and Sna3-GFP was examined by fluorescence microscopy. Bars, 5 µm.
K63 polyubiquitylation is required for sorting of Gap1 and CPS in the MVB pathway
After internalization, Gap1 normally enters the MVB pathway and finally reaches the vacuolar lumen (Fig. 1 C) where it is degraded (Fig. 1 D), but, in cells expressing UbK63R, the permease was deviated to the limiting membrane of the vacuole (Fig. 1 C) and consistently failed to be degraded (Fig. 1 D). We also looked at the localization of the Gap1K9R and Gap1K16R variants and found that they were correctly delivered, at least in part, to the vacuolar lumen in Ub-expressing cells, whereas they failed to do so in UbK63R-expressing cells (Fig. 1 E). Thus, Gap1 monoubiquitylated on one or two lysines, undergoing endocytic internalization at a maximal rate (Fig. 1 B), fails to be sorted into the MVB pathway. For correct MVB sorting, internalized Gap1 must undergo K63-linked ubiquitylation on at least one of its target lysines. The permease lacking K63-Ub chains tends to be recycled to the plasma membrane via the TGN, whereas a residual amount of the protein accumulates at the vacuolar membrane. We have obtained another line of evidence supporting an essential role of K63-linked ubiquitylation in Gap1 MVB sorting. Overproduction of the Ubp2 deubiquitylating enzyme (DUB), which is known to act preferentially on K63-linked Ub chains (Kee et al., 2005, 2006), indeed prevented internalized Gap1 from being sorted into the MVB pathway, which is an effect that is not observed when other DUBs are overexpressed (Fig. S1).
To see whether K63-linked ubiquitylation might contribute to MVB sorting of cargoes other than Gap1, we examined the location of CPS in strains expressing UbK63R as the sole Ub source. This type II membrane protein is delivered from the TGN to the late endosome, where it enters the MVB pathway to reach the vacuolar lumen. CPS is ubiquitylated on its lysine 8 by the Rsp5/Npi1 Ub ligase (Katzmann et al., 2004), which is known to preferentially catalyze K63-linked polyubiquitylation (Kee et al., 2006). CPS appears to undergo K63-linked ubiquitylation because it was modified with only one Ub moiety in UbK63R-expressing cells (Fig. 1 F). Under these conditions, CPS failed to reach the vacuolar lumen and accumulated at the limiting membrane of the vacuole (Fig. 1 G). Thus, like for the Gap1 permease, K63-linked ubiquitylation is also required for MVB sorting of the biosynthetic cargo CPS. Interestingly, vacuolar delivery of the Sna3-GFP protein, which is also K63 ubiquitylated (Stawiecka-Mirota et al., 2007), was unaffected in these mutant cells (Fig. 1 G). This result indicates that some cargoes do not need to undergo K63-linked ubiquitylation to be sorted into the MVB pathway (see Discussion). It also shows that the MVB machinery is not profoundly altered in cells unable to perform K63-linked polyubiquitylation.
Golgi to endosome sorting of Gap1 does not require its ubiquitylation
We next tested whether K63-Ub chains are involved in other trafficking steps of the Gap1 permease. In the presence of a good nitrogen source, newly synthesized Gap1 is targeted directly from the Golgi to the vacuolar degradation pathway without passing via the plasma membrane. It was reported that polyubiquitylation of Gap1 is required for this direct sorting to the vacuole (Helliwell et al., 2001; Risinger and Kaiser, 2008). To determine whether Ub chains are involved in Golgi to endosome transport or only in subsequent MVB sorting of the permease, we designed a new experimental approach based on the use of a thermosensitive sec14-3 mutant strain, in which TGN to cell surface transport is defective (Bankaitis et al., 1989). Gap1 produced at the restrictive temperature in these cells accumulated in intracellular membranes (Fig. 2, A and B). Glutamine and ammonium were then added to the medium (causing repression of GAP1 gene transcription), and this led to vacuolar degradation of presynthesized Gap1 (Fig. 2 C and Fig. S2 A). In contrast, no degradation was observed when the same experiment was performed in sec7-1 mutant cells (Achstetter et al., 1988), in which Gap1 is blocked upstream from the TGN (Fig. 2 C and not depicted). Thus, in keeping with previous studies (Roberg et al., 1997; De Craene et al., 2001; Helliwell et al., 2001; Soetens et al., 2001), Gap1 present in the late Golgi is deviated to the vacuole for degradation if a favored nitrogen source is available. We also observed that the Gap1 permease blocked in the late Golgi is modified with short Ub chains on its lysines 9 and 16 in an Rsp5-dependent manner (Fig. S2 B). To determine whether this modification is required for exit of the permease from the Golgi, we examined the fate of the Gap1K9,16R permease preaccumulated in sec14-3 cells after the addition of glutamine and ammonium. If ubiquitylation of Gap1 is necessary for its exit from the Golgi, Gap1K9,16R should remain in this compartment. Remarkably, what we observed instead is an ammonium- and glutamine-dependent redirection of the Gap1K9,16R protein to the plasma membrane (Fig. 2 A). This delivery to the cell surface was confirmed by cofractionation of Gap1K9,16R with the plasma membrane marker Pma1 (Fig. 2 B) and by activity measurements (not depicted). We confirmed that good nitrogen sources do not have a general effect on the secretory block caused by the sec14-3 mutation because, under the same conditions, the Hxt1-GFP permease preaccumulated in the late Golgi remained intracellular (Fig. 2 A). Furthermore, this cell surface delivery necessitates the dynamin-like protein Vps1 (Fig. 2 B and Fig. S3 A) involved in Golgi to endosome vesicle transport. Thus, addition of ammonium and glutamine induces exit from the Golgi of the nonubiquitylable Gap1K9,16R permease via the Vps1 pathway, but, instead of being targeted to the vacuole, the protein is recycled to the cell surface. This cell surface delivery likely occurs from the late endosome because it was blocked by the vps23Δ class E mutation (Fig. S2 C) previously shown to trap Gap1 in this compartment (Nikko et al. 2003). We considered that the Gap1K9,16R permease present in the late Golgi could be ubiquitylated on lysines other than K9 and K16 and that this ubiquitylation promotes Golgi to endosome sorting. However, we could not detect any ubiquitylation of Gap1K9,16R accumulated in the sec14-3 vps1Δ mutant (Fig. S2 B). Furthermore, the same Vps1-dependent Golgi to plasma membrane delivery was observed with a Gap19KR-GFP variant protein (Fig. S3 A), in which all nine amino-terminal lysines are replaced with arginines (Lauwers et al., 2007), and plasma membrane delivery of Gap1K9,16R-GFP also occurs in sec14-3 cells carrying the npi1/rsp5 mutation (Fig. S3, B and C), which markedly reduces the level of the Rsp5/Npi1 Ub ligase and impairs Gap1 ubiquitylation (Hein et al., 1995). Thus, these results show that in the presence of a favored nitrogen source, Gap1 is modified with short Ub chains in the late Golgi and exits this compartment toward the late endosome via the classical Vps1-dependent pathway. However, ubiquitylation of the permease on its amino-terminal lysines is not required for this sorting step. This ubiquitylation only plays its role once the permease reaches the late endosome, most likely for subsequent entry into the MVB pathway. To determine whether this MVB sorting requires K63-linked ubiquitylation, Gap1 synthesis was induced in Ub- and UbK63R-expressing cells under conditions normally causing its direct sorting from the Golgi to the vacuolar lumen. The permease was targeted to the vacuolar lumen in Ub-expressing cells, as expected, but it was mis-sorted to the limiting membrane of the vacuole in UbK63R cells (Fig. S4 A). This indicates that although Gap1 ubiquitylation is not required for its transport from the Golgi to the late endosome, the permease reaching this compartment must exhibit K63-Ub chains to be efficiently sorted into the MVB pathway.
Gap1 exit from the Golgi is Ub independent and Vps1 dependent. Cells transformed with a centromeric vector encoding Gap1-GFP or Gap1K9,16R-GFP were grown at 24°C in glucose-, glutamine-, and ammonium (Am)-containing medium to repress GAP1 gene expression. Synthesis of the permease was then induced at the restrictive temperature by transferring the cells to proline medium at 37°C. After 90 min, each culture was divided in two, glutamine and ammonium were added to one of the flasks, and the cells were incubated for 1 h more at 37°C. (A) Localization of Gap1-GFP and Gap1K9,16R-GFP in sec14-3 cells was examined at different time points by fluorescence microscopy. GFP fluorescence is shown in the top rows, and Nomarski images are shown in the bottom rows of each pair of panels. (B) Localization of Gap1-GFP and Gap1K9,16R-GFP in sec14-3 and vps1Δ sec14-3 cells at time 150 min was examined by subcellular fractionation in a sucrose gradient. (C) The level of Gap1-GFP protein was analyzed by immunoblotting in total protein extracts prepared from sec14-3 and sec7-1 cells collected at different times with anti-GFP antibodies. Bar, 5 µm.
Gap1 exit from the Golgi is Ub independent and Vps1 dependent. Cells transformed with a centromeric vector encoding Gap1-GFP or Gap1K9,16R-GFP were grown at 24°C in glucose-, glutamine-, and ammonium (Am)-containing medium to repress GAP1 gene expression. Synthesis of the permease was then induced at the restrictive temperature by transferring the cells to proline medium at 37°C. After 90 min, each culture was divided in two, glutamine and ammonium were added to one of the flasks, and the cells were incubated for 1 h more at 37°C. (A) Localization of Gap1-GFP and Gap1K9,16R-GFP in sec14-3 cells was examined at different time points by fluorescence microscopy. GFP fluorescence is shown in the top rows, and Nomarski images are shown in the bottom rows of each pair of panels. (B) Localization of Gap1-GFP and Gap1K9,16R-GFP in sec14-3 and vps1Δ sec14-3 cells at time 150 min was examined by subcellular fractionation in a sucrose gradient. (C) The level of Gap1-GFP protein was analyzed by immunoblotting in total protein extracts prepared from sec14-3 and sec7-1 cells collected at different times with anti-GFP antibodies. Bar, 5 µm.
The role of Gga proteins in Golgi to endosome transport of Gap1 does not involve their Ub-binding GAT domain
It has been proposed that the Gga adapter proteins at the TGN bind to ubiquitylated Gap1 via their GAT domain and divert it into vesicles bound for the late endosome (Scott et al., 2004). Yet the aforementioned data clearly show that ubiquitylation of Gap1 is not required for this transport step. Thus, we reexamined the importance of the Gga proteins and of the GAT domain in the exit of Gap1 from the Golgi. Gap1-GFP preaccumulated in sec14-3 gga1Δ gga2Δ triple mutant cells was not degraded upon addition of glutamine and ammonium to the medium (Fig. 3 A), and it remained in intracellular membranes (Fig. 3 B). Interestingly, rerouting of Gap1K9,16R-GFP from the Golgi to the plasma membrane after the addition of glutamine and ammonium was also impaired in these cells (Fig. 3 B). This indicates that the Gga proteins are required for Gap1 exit from the Golgi, whether the protein is ubiquitylated or not. Accordingly, rerouting of Gap1K9,16R-GFP to the plasma membrane in the triple mutant cells could be restored upon expression of either a full-length Gga2 protein or even of a truncated Gga2 lacking the Ub-binding region of the GAT domain (Fig. 3 B; Scott et al., 2004). Thus, the exit of Gap1 from the Golgi toward the late endosome depends on the Gga proteins but not on their Ub-binding GAT domain. This suggests that in addition to Ub, Gap1 exhibits other signals that are alone sufficient for Gga-dependent exit from the Golgi.
The Gga proteins but not their Ub-binding GAT domain are required for Gap1 exit from the Golgi. sec14-3, gga1Δ gga2Δ, and sec14-3 gga1Δ gga2Δ cells were transformed with a centromeric vector encoding Gap1-GFP or Gap1K9,16R-GFP or the latter plasmid additionally carrying the GGA2 or gga2ΔGAT gene. Cells were treated as in Fig. 2. (A) Gap1-GFP stability was examined in total protein extracts prepared from cells collected before and at different times after the transfer to 37°C by immunoblotting with anti-GFP antibodies. (B) Gap1-GFP localization was examined by fluorescence microscopy before (not depicted) and at different times after the shift to 37°C. Am, ammonium. Bar, 5 µm.
The Gga proteins but not their Ub-binding GAT domain are required for Gap1 exit from the Golgi. sec14-3, gga1Δ gga2Δ, and sec14-3 gga1Δ gga2Δ cells were transformed with a centromeric vector encoding Gap1-GFP or Gap1K9,16R-GFP or the latter plasmid additionally carrying the GGA2 or gga2ΔGAT gene. Cells were treated as in Fig. 2. (A) Gap1-GFP stability was examined in total protein extracts prepared from cells collected before and at different times after the transfer to 37°C by immunoblotting with anti-GFP antibodies. (B) Gap1-GFP localization was examined by fluorescence microscopy before (not depicted) and at different times after the shift to 37°C. Am, ammonium. Bar, 5 µm.
The GAT domain of Ggas is required at a late step of Gap1 endocytosis
We also investigated the importance of the Gga proteins and the GAT domain in Gap1 endocytosis. In gga1Δ gga2Δ mutant cells, ammonium-induced loss of Gap1 activity occurred at a much slower rate than in wild-type cells (Fig. 4 A), and the permease was protected from degradation (Fig. 4 B). Strikingly, deletion of the YPT6 gene in gga1Δ gga2Δ cells restored a normal rate of Gap1 loss of activity (Fig. 4 A) without restoring permease degradation (Fig. 4 B). This indicates that the Gga proteins play an important role at a postinternalization step of Gap1 endocytosis and that lack of their function causes recycling of internalized Gap1 to the cell surface. Accordingly, yeast Ggas are localized not only at the TGN but also in late endosomes (Costaguta et al., 2001; Singer-Krüger et al., 2008), suggesting that they might play an additional role in this compartment. Interestingly, the defects exhibited by the gga1Δ gga2Δ double mutant could be complemented by expression of the full-length Gga2 protein but not by a Gga2 protein lacking the Ub-binding part of the GAT domain (Fig. 4). These results show that the GAT domain of the yeast Gga proteins is required for vacuolar targeting of Gap1 after its internalization. Finally, we examined the genetic epistasis relationship between the gga1Δ gga2Δ and vps27Δ mutant phenotypes and found that localization of Gap1-GFP in the triple gga1Δ gga2Δ vps27Δ strain was similar to that of a double gga1Δ gga2Δ mutant (Fig. S4 B). This indicates that the step of endocytosis where the Gga proteins and their GAT domain intervene lies upstream from the one that depends on Vps27, i.e., recognition of the ubiquitylated permease by the ESCRT-0 complex followed by its delivery to the other successive ESCRT (endosomal sorting complex required for transport) complexes of the MVB pathway (Babst, 2005). Thus, the Ggas likely recruit Gap1 at the late endosome for subsequent sorting into the MVB pathway. This recruitment could involve an interaction between the GAT domain of Ggas and the K63-linked Ub chain displayed by the permease. In support of this view, deletion of the GAT domain of Ggas and defective K63-linked ubiquitylation of Gap1 lead to the same phenotype; i.e., internalized Gap1 fails to be sorted into the MVB pathway and is mostly recycled back to the cell surface.
The GAT domain of the Gga proteins is required for a postinternalization event during Gap1 endocytosis. (A and B) Wild-type (wt), ypt6Δ, gga1Δ gga2Δ, or ypt6Δ gga1Δ gga2Δ cells were transformed with an empty centromeric vector or with a derived plasmid carrying the GGA2 or gga2ΔGAT gene. (A) Gap1 activity was measured before and at different times after the addition of ammonium. Error bars represent standard deviation. (B) Gap1 stability was analyzed in total protein extracts prepared from the same cells as in A collected before and 60 min after the addition of ammonium.
The GAT domain of the Gga proteins is required for a postinternalization event during Gap1 endocytosis. (A and B) Wild-type (wt), ypt6Δ, gga1Δ gga2Δ, or ypt6Δ gga1Δ gga2Δ cells were transformed with an empty centromeric vector or with a derived plasmid carrying the GGA2 or gga2ΔGAT gene. (A) Gap1 activity was measured before and at different times after the addition of ammonium. Error bars represent standard deviation. (B) Gap1 stability was analyzed in total protein extracts prepared from the same cells as in A collected before and 60 min after the addition of ammonium.
Discussion
The main conclusion of this study is that K63-linked ubiquitylation of two abundantly studied yeast membrane proteins, CPS and Gap1, is specifically required for their sorting into the MVB pathway. Whereas Golgi to endosome transport of CPS occurs whether this cargo is ubiquitylated or not (Katzmann et al., 2001), its K63-linked ubiquitylation is essential for entry into the MVB pathway. Similarly, Golgi to endosome sorting of Gap1 is independent of its ubiquitylation, even though the permease undergoes short-chain ubiquitylation in the Golgi. This modification is important only for subsequent MVB sorting of the protein. In the case of Gap1 present at the plasma membrane, monoubiquitylation of even a single lysine residue is sufficient to promote efficient internalization of the permease, whereas K63-linked ubiquitylation is required for subsequent entry into the MVB pathway. Our conclusions differ from those recently reported by Risinger and Kaiser (2008), according to which mono-Ub is a sufficient signal for Gap1 targeting to the vacuole. This conclusion is based on the study of bul1Δ bul2Δ and gap1K16R,E383D mutants, in which Gap1 is reported to be mono- but not polyubiquitylated and to be targeted to the vacuole. However, in our study, Gap1 fails to be monoubiquitylated in the bul mutant, whereas the Gap1K16R,E383D variant undergoes normal K63-linked ubiquitylation (Fig. S5).
The molecular mechanisms through which K63-linked ubiquitylation promotes MVB sorting of Gap1, CPS, and probably many other cargoes remain to be determined. It is possible that Ub-binding proteins of the MVB machinery preferentially handle cargoes exhibiting short K63-linked Ub chains. One such protein could be Vps27 because its mammalian homologue, Hrs, binds preferentially to these chains and negligibly to mono-Ub (Barrière et al., 2007). That a yeast Vps protein displays such properties would account for our observation that the Gap1K9R and Gap1K16R variants modified by a single K63-linked di-Ub chain are efficiently sorted into the MVB pathway, unlike Gap1-carrying Ub monomers attached to two different lysines (despite the fact that Gap1 likely associates into multimers; unpublished data). However, as Vps27 and other yeast Ub-binding proteins involved in endocytosis were reported to bind GST-Ub in vitro (Bilodeau et al., 2002; Scott et al., 2004), further experiments are required to determine whether the yeast MVB sorting machinery preferentially binds to cargoes modified with Ub chains. K63-linked Ub chains might also be recognized by protein components upstream from ESCRT-0, e.g., by the Gga proteins acting at the endosomal level. Our data indeed show that the progression of internalized Gap1 along the endocytic pathway requires the Gga proteins and their GAT domain, and genetic epistatic analysis suggests that they act upstream from Vps27. Interestingly, the human Gga proteins are known to function in endocytosis by concentrating ubiquitylated cargoes destined for the MVB pathway in specialized clathrin-coated regions of the endosomal membrane before their recognition by the ESCRT machinery (Puertollano and Bonifacino, 2004; Babst, 2005). Thus, the yeast Gga proteins might play a similar role by binding to ubiquitylated Gap1 via the GAT domain before handing the cargo over to the ESCRT-0 complex. Consistent with a role of Gga in recognition of K63-linked chain, deletion of the GAT domain of Gga proteins and lack of K63-linked ubiquitylation of Gap1 cause a similar phenotype, i.e., internalized Gap1 tends to recycle to the cell surface via the Golgi complex.
Cargo proteins exhibiting short K63-Ub chains could also be more efficiently sorted into the MVB pathway because they are less susceptible to complete deubiquitylation by specific DUBs that target endocytic cargoes (Clague and Urbe, 2006). This alternative model could also account for the previous observation that nonubiquitylable protein substrates artificially fused in frame to a Ub monomer are efficiently delivered to the vacuolar lumen even if the nonremovable fused Ub contains lysine to arginine substitutions, making it unable to form Ub chains (Reggiori and Pelham, 2001; Urbanowski and Piper, 2001). Unfortunately, we have not been able to investigate the trafficking of a Ub-Gap1 fusion protein because, in all of the constructions we have made, the presence of a Ub monomer blocked the exit of newly synthesized permease out of the ER.
Finally, we cannot exclude the notion that K63 polyubiquitylation of one or several components of the MVB machinery, in addition to cargoes, might be required for proper sorting of proteins to the vacuolar lumen. Mammalian ESCRT components are indeed known to be ubiquitylated, but further experiments are required to determine whether this is also true for yeast Vps class E proteins and whether such modification involves linkage via the lysine 63 of Ub.
Strikingly, several studies addressed the role of Ub in the vacuolar delivery of Sna3 (Reggiori and Pelham, 2001; McNatt et al., 2007; Oestreich et al., 2007; Stawiecka-Mirota et al., 2007; Watson and Bonifacino, 2007), and, in one of these, ubiquitylation of Sna3 was found to be necessary for its entry into the MVB pathway (Stawiecka-Mirota et al., 2007). Because our results show that the formation of K63-linked Ub chains is not required for this process, it seems that monoubiquitylation is a necessary and sufficient signal for MVB sorting of Sna3. It is noteworthy that vacuolar delivery of Sna3 occurs normally in the absence of some key elements that are absolutely required for other cargoes such as Gap1 and CPS, i.e., a catalytically active Doa4 DUB (Reggiori and Pelham, 2001; McNatt et al., 2007) or a full-length Bro1 protein able to recruit Doa4 at the late endosome (Richter et al., 2007; Nikko and André, 2007). Thus, Sna3 might be representative of a particular type of MVB cargo that does not require Doa4- and K63-linked ubiquitylation to reach the vacuolar lumen.
Our conclusions, corroborated by other observations (Kim et al., 2007; Blondel et al., 2004; Erpapazoglou et al., 2008), allow us to propose a coherent and unifying picture of the roles of Gga proteins and Ub in yeast membrane protein sorting in the secretory and endocytic pathways (Fig. 5). A growing number of plasma membrane proteins of animal cells are reported to undergo K63 polyubiquitylation (Traub and Lukacs, 2007) or short-chain K63 ubiquitylation (Kamsteeg et al., 2006). Thus, it will be interesting to determine whether the model proposed in this study for yeast proteins is applicable to those of more complex species.
Schematic model of the roles of Ub and the Gga proteins in intracellular trafficking of Gap1 and CPS. Upon addition of a favored nitrogen source or an excess of amino acids, the Gap1 permease present at the plasma membrane is K63 ubiquitylated by the Rsp5/Npi1 ligase, but monoubiquitylation of the permease on at least one lysine residue already constitutes a perfectly efficient internalization signal. K63 ubiquitylation of Gap1 is required only for further sorting into the MVB pathway. The short Ub chains are possibly recognized first by the Gga proteins (via the GAT domain) and then by Vps27 (via its Ub-interacting motif domain). If only monoubiquitylated, internalized Gap1 is partially mis-sorted to the vacuolar membrane, and the rest is recycled to the plasma membrane. Good nitrogen sources also promote K63 ubiquitylation of neosynthesized Gap1 present in the Golgi by Rsp5. Under these conditions, Gap1 exits this compartment and is sorted to the late endosome. However, this sorting step does not depend on Gap1 ubiquitylation. It requires the Gga proteins (but not their GAT domain), which likely recognize cis elements exposed by Gap1. Golgi to endosome transport of CPS is also independent of its ubiquitylation. At the late endosome level, K63-Ub chain formation is required for entry into the MVB pathway of both CPS and neosynthesized Gap1, as it is for endocytosed Gap1. This model might also be valid for many other yeast cargoes, including the Fur4, Arn1, and Sit1 permeases. All of these proteins indeed undergo Ub-independent, Gga-dependent sorting from the Golgi to the late endosome, whereas their ubiquitylation is only important for subsequent entry into the MVB pathway (Bilodeau et al., 2004; Blondel et al., 2004; Kim et al., 2007; Erpapazoglou et al., 2008). In some other cases, e.g., for the Tat2 permease and the Pma1 ATPase, cargo ubiquitylation was reported to be required for exit of the TGN (Beck et al., 1999; Helliwell et al., 2001; Umebayashi and Nakano, 2003; Pizzirusso and Chang, 2004). Yet, in these studies, vacuolar delivery was examined using experimental approaches that do not allow for distinguishing between the trafficking steps from the Golgi to the endosome and then from the endosome to the vacuole. In fact, these data are also consistent with a role of Ub only at the endosomal level, where ubiquitylation is absolutely required for MVB sorting of yeast plasma membrane proteins, whether the cargo reaches the late endosome by endocytosis or directly after exiting the Golgi (Reggiori and Pelham, 2001; Blondel et al., 2004; Stimpson et al., 2006; Kim et al., 2007; Stawiecka-Mirota et al., 2007; Erpapazoglou et al., 2008). If this ubiquitylation is defective, the cargo proteins either accumulate at the vacuolar membrane or are redirected to the plasma membrane. This direct late endosome to cell surface route taken by nonubiquitylable Gap1 variants might be equivalent to the alternative secretory pathway of S. cerevisiae involving a transit step through the late endosome (Gurunathan et al., 2002; Harsay and Schekman, 2002). Moreover, rerouting of the Arn1 and Sit1 transporters from the late endosome to the plasma membrane, without passing by the Golgi, has recently been observed under particular physiological conditions (Kim et al., 2007; Erpapazoglou et al., 2008).
Schematic model of the roles of Ub and the Gga proteins in intracellular trafficking of Gap1 and CPS. Upon addition of a favored nitrogen source or an excess of amino acids, the Gap1 permease present at the plasma membrane is K63 ubiquitylated by the Rsp5/Npi1 ligase, but monoubiquitylation of the permease on at least one lysine residue already constitutes a perfectly efficient internalization signal. K63 ubiquitylation of Gap1 is required only for further sorting into the MVB pathway. The short Ub chains are possibly recognized first by the Gga proteins (via the GAT domain) and then by Vps27 (via its Ub-interacting motif domain). If only monoubiquitylated, internalized Gap1 is partially mis-sorted to the vacuolar membrane, and the rest is recycled to the plasma membrane. Good nitrogen sources also promote K63 ubiquitylation of neosynthesized Gap1 present in the Golgi by Rsp5. Under these conditions, Gap1 exits this compartment and is sorted to the late endosome. However, this sorting step does not depend on Gap1 ubiquitylation. It requires the Gga proteins (but not their GAT domain), which likely recognize cis elements exposed by Gap1. Golgi to endosome transport of CPS is also independent of its ubiquitylation. At the late endosome level, K63-Ub chain formation is required for entry into the MVB pathway of both CPS and neosynthesized Gap1, as it is for endocytosed Gap1. This model might also be valid for many other yeast cargoes, including the Fur4, Arn1, and Sit1 permeases. All of these proteins indeed undergo Ub-independent, Gga-dependent sorting from the Golgi to the late endosome, whereas their ubiquitylation is only important for subsequent entry into the MVB pathway (Bilodeau et al., 2004; Blondel et al., 2004; Kim et al., 2007; Erpapazoglou et al., 2008). In some other cases, e.g., for the Tat2 permease and the Pma1 ATPase, cargo ubiquitylation was reported to be required for exit of the TGN (Beck et al., 1999; Helliwell et al., 2001; Umebayashi and Nakano, 2003; Pizzirusso and Chang, 2004). Yet, in these studies, vacuolar delivery was examined using experimental approaches that do not allow for distinguishing between the trafficking steps from the Golgi to the endosome and then from the endosome to the vacuole. In fact, these data are also consistent with a role of Ub only at the endosomal level, where ubiquitylation is absolutely required for MVB sorting of yeast plasma membrane proteins, whether the cargo reaches the late endosome by endocytosis or directly after exiting the Golgi (Reggiori and Pelham, 2001; Blondel et al., 2004; Stimpson et al., 2006; Kim et al., 2007; Stawiecka-Mirota et al., 2007; Erpapazoglou et al., 2008). If this ubiquitylation is defective, the cargo proteins either accumulate at the vacuolar membrane or are redirected to the plasma membrane. This direct late endosome to cell surface route taken by nonubiquitylable Gap1 variants might be equivalent to the alternative secretory pathway of S. cerevisiae involving a transit step through the late endosome (Gurunathan et al., 2002; Harsay and Schekman, 2002). Moreover, rerouting of the Arn1 and Sit1 transporters from the late endosome to the plasma membrane, without passing by the Golgi, has recently been observed under particular physiological conditions (Kim et al., 2007; Erpapazoglou et al., 2008).
Materials and methods
Strains, plasmids, and growth conditions
The S. cerevisiae strains and the plasmids used in this study are listed in Table S1. Cells were grown at 24°C or 29°C in minimal buffered medium, pH 6.1 (Jacobs et al., 1980), or in yeast nitrogen base medium (Difco) for the strains transformed with episomal plasmids carrying the Ub gene under the CUP1 promoter. In the latter case, excessive Ub overproduction was avoided by not adding any extra copper to the medium (Nikko and André, 2007). Carbon sources were 3% glucose or 0.3% glucose and 3% galactose. Nitrogen sources were added to the final concentrations of 0.1% for proline and glutamine and 20 or 100 mM for (NH4)2SO4. Gap1 vacuolar targeting was triggered by the addition of 20 or 100 mM ammonium or 5 mM phenylalanine, as indicated in the figure legends. Gene deletion, chromosomal tagging, and yeast cell transformations were performed as described previously (Lauwers et al., 2007). All plasmids were constructed by in vivo recombination and checked by sequencing.
Permease assays
Gap1 activity was determined by measuring the initial rate of uptake of 20 µM 14C-labeled citrulline, which is a specific substrate of the permease (Grenson et al., 1966).
Protein extracts and Western blotting
Proteins were immunodetected in total protein extracts (Hein et al., 1995) or membrane-enriched extracts (Springael and André, 1998). After transfer to a nitrocellulose membrane (Schleicher & Schüll), the proteins were probed with polyclonal antibodies raised against Gap1 or Pma1 (De Craene et al., 2001) or with monoclonal antibodies raised against GFP (Roche) or HA (Sigma-Aldrich). Primary antibodies were detected with horseradish peroxidase–conjugated anti–rabbit or anti–mouse IgG secondary antibody (GE Healthcare) followed by enhanced chemiluminescence (Roche).
Subcellular fractionation
Fractionation was performed as described previously (Lauwers et al., 2007). After ultracentrifugation, six fractions of equal volume were collected from the top of the sucrose gradient, and the distribution of Pma1 and Gap1 was analyzed by Western blotting.
Fluorescence microscopy
Labeling of the vacuolar membrane with FM4-64 was performed as described previously (Nikko et al., 2003). Living cells expressing a protein fused to GFPS65T were laid down on a thin layer of 1% agarose and viewed at room temperature with a microscope (Eclipse E600; Nikon) equipped with a 100× differential interference contrast NA 1.40 Plan-Apochromat objective (Nikon) and appropriate fluorescence light filter sets. Images were captured with a digital camera (DXM1200; Nikon) and ACT-1 acquisition software (Nikon) and processed with Photoshop CS (Adobe Systems).
Online supplemental material
Fig. S1 shows the negative effect of overproducing the Ubp2 DUB on Gap1 ubiquitylation and vacuolar sorting. Fig. S2 shows Rsp5-dependent ubiquitylation of Gap1 in the Golgi complex and the role of the Pep12, Pep4, and Vps23 proteins in Gap1 nitrogen-induced sorting from the Golgi to the vacuole. Fig. S3 shows Rsp5-independent, Vps1-dependent exit of the Gap1K9,16R and Gap19KR variants from the Golgi complex. Fig. S4 shows the role of K63-linked Ub chains in MVB sorting of neosynthesized Gap1 and the epistasis relationship between the gga1Δ gga2Δ and the vps27Δ mutations. Fig. S5 shows that, in our study, Gap1 ubiquitylation depends on the Bul1 and Bul2 proteins, whereas the Gap1K16R,E583D protein is ubiquitylated and down-regulated as the native permease. Table S1 shows the strains and plasmids used in this study.
Acknowledgments
We are grateful to Catherine Jauniaux for her excellent technical assistance and to Valérie Spinelli and Benjamin Laplace for their contribution to the Ubp2 and Gap1K16R,E582D experiments. We thank Scott Emr, Daniel Finley, Rosine Haguenauer-Tsapis, Jon Huibregtse, Hugh Pelham, and Peter Novick for generously providing plasmids and strains. We also thank members of the laboratory for fruitful discussions and comments.
This work was supported by a Fonds de le Recherche Scientifique Médicale grant (3.4.592.08.F) and an Actions de Recherche Concertée grant (04/09-307) of the Communauté Française de Belgique and by the Cibles grant (716760) of the Région Wallonne de Belgique. E. Lauwers is a Research Fellow of the National Fund for Scientific Research, Belgium.
References
Abbreviations used in this paper: CPS, carboxypeptidase S; DUB, deubiquitylating enzyme; ESCRT, endosomal sorting complex required for transport; GAT, Gga and Tom1; Gga, Golgi localizing, gamma-ear containing, ARF binding; MVB, multivesicular body; Ub, ubiquitin.
Author notes
C. Jacob's present address is Laboratoire ARN-RNP, structure-fonction-maturation, Enzymologie Moléculaire et Structurale, Centre National de la Recherche Scientifique Unité Mixte de Recherche 7214, Université Henri Poincare, 54506 Vandoeuvre-lès-Nancy, France.