Ca2+ import into the lumen of the trans-Golgi network (TGN) by the secretory pathway calcium ATPase1 (SPCA1) is required for the sorting of secretory cargo. How is Ca2+ retained in the lumen of the Golgi, and what is its role in cargo sorting? We show here that a soluble, lumenal Golgi resident protein, Cab45, is required for SPCA1-dependent Ca2+ import into the TGN; it binds secretory cargo in a Ca2+-dependent reaction and is required for its sorting at the TGN.
Introduction
Several proteins that simultaneously recognize soluble cargoes in the lumen of a secretory compartment and coat components on the cytoplasmic surface, thereby directing cargo into transport carriers during protein secretion, have been identified (Mellman and Nelson, 2008; Dancourt and Barlowe, 2010; Pfeffer, 2011). Examples of such cargo receptors include ERGIC-53 family members that recognize the high mannose oligosaccharide-containing secretory proteins in the lumen of the ER and Sec23/24 of the COPII coats through a diphenylalanine signal in their cytoplasmic tail (Nichols et al., 1998; Nyfeler et al., 2006; Kamiya et al., 2008). The ER exit site localized TANGO1, and binds collagen VII by its SH3-like domain in the ER lumen and Sec23/Sec24, on the cytoplasmic side, via its proline-rich domain (Saito et al., 2009). TANGO1-null mice die at birth due to defects in bone mineralization as they fail to sort and secrete collagens (Wilson et al., 2011). In the budding yeast Saccharomyces cerevisiae, α-factor is exported by binding its cognate receptor, Erv29p (Belden and Barlowe, 2001; Malkus et al., 2002; Otte and Barlowe, 2004). But how are proteins that cannot bind ERGIC53, TANGO1, and Erv29p sorted and exported form the ER?
Upon arrival in the Golgi, soluble proteins destined for retrieval to the ER are captured by the KDEL receptor, packaged into COPI vesicles, and exported from the Golgi (Munro and Pelham, 1987; Townsley et al., 1993; Majoul et al., 1998). Likewise, oligosaccharide chains of lysosomal hydrolases are recognized by Mannose 6-phosphate (M6P)-specific receptors and are collected into clathrin-coated vesicles that transport them to endosomes for subsequent delivery to lysosomes (Reitman and Kornfeld, 1981; Kornfeld and Mellman, 1989; Le Borgne and Hoflack, 1997). Although a yeast orthologue of M6PR, called MRL1, has been identified, its role in sorting of vacuolar hydrolases is unclear (Whyte and Munro, 2001). Sorting of vacuolar hydrolases in yeast is predominantly mediated by a sortilin-like protein, Vps10, which recognizes vacuolar targeting pro sequences (Cooper and Stevens, 1996).
A specific coat for proteins exported from the TGN and destined directly for secretion has not been identified. How are such secretory proteins sorted and packed into the respective transport carriers? We have previously shown that the actin filament severing protein ADF/cofilin and the transmembrane P type ATPase secretory pathway calcium ATPase 1 (SPCA1)-dependent Ca2+ entry into the lumen of the TGN is crucial for the sorting of secretory cargo in HeLa cells and in S. cerevisiae (von Blume et al., 2009, 2011; Curwin et al., 2012). We show here that a soluble Golgi resident protein named Cab45 (Scherer et al., 1996) is required for Ca2+ homeostasis and sorting of cargoes that are destined for secretion at the TGN in HeLa cells.
Results and discussion
Cab45 is a soluble, Ca2+-binding resident protein of the Golgi membranes, but the mechanism of its retention and its physiological function are not known (Scherer et al., 1996; Honoré and Vorum, 2000; Honoré, 2009). Is Ca2+ required for the retention of Cab45 in the Golgi membranes? We incubated HeLa cells in Ca2+-free medium for 1 h followed by 5 min of treatment with DMSO, the Ca2+ ionophore A23187, or A23187 with 10 mM Ca2+. The cells were then processed for immunofluorescence microscopy to localize Cab45 with an anti-Cab45–specific antibody, and the medium was Western blotted with anti-Cab45 antibody to monitor Cab45 secretion (Fig. 1, A and B). Cab45 was exported from the Golgi membranes in HeLa cells treated with A23187 (Fig. 1 A, middle) and released (secreted) into the medium (Fig. 1 B, lane 2). However, the addition of 10 mM Ca2+ to the medium prevented A23187-mediated secretion of Cab45 (Fig. 1 A, bottom; and Fig. 1 B, lane 3).
Ca2+ is required for retention of Cab45 in the Golgi apparatus. (A) HeLa cells were incubated in a Ca2+-free medium. After 1 h, cells were incubated for 5 min with A23187 with or without 10 mM CaCl2, after which the localization of Cab45 and TGN46 was visualized by immunofluorescence microscopy. Bars, 5 µm. (B) The experiment described in A was repeated; the cell lysates and the corresponding media were analyzed by Western blotting with an anti-Cab45 antibody (lane 1, DMSO; lane 2, 2.5 µM A23187; lane 3, 2.5 µM A23187 + 10 mM CaCl2). (C) Golgi membranes isolated from HeLa cells were incubated with DMSO or 25 µM BAPTA for 15 min. The membranes were subjected to five cycles of rapid freeze-thaw and finally centrifuged at 100,000 g. The pellets and the supernatants (sup) were analyzed by Western blotting with anti-Cab45 (top) and anti-TGN46 (bottom) antibodies, respectively. (D) Western blot bands from three independent experiments were quantified by densitometry using the ImageJ software. Bar graphs represent the densitometry values of external Cab45 (released from the Golgi membranes) normalized to the internal Cab45 values. Error bars show the mean ± SD densitometric values of three independent experiments. **, P < 0.01. (E) HeLa cells were transfected with SPCA1 siRNA. After 72 h at 37°C, the cells were incubated in Ca2+-free medium and treated with A23187 in the absence or presence of 10 mM CaCl2 and visualized by fluorescence microscopy as described in A. (F) The experiment was repeated as described in E; the cell lysates and the corresponding media were analyzed by Western blotting with an anti-Cab45 antibody (lane 1, DMSO; lane 2, 2.5 µM A23187; lane 3, 2.5 µM A23187 + 10 mM CaCl2).
Ca2+ is required for retention of Cab45 in the Golgi apparatus. (A) HeLa cells were incubated in a Ca2+-free medium. After 1 h, cells were incubated for 5 min with A23187 with or without 10 mM CaCl2, after which the localization of Cab45 and TGN46 was visualized by immunofluorescence microscopy. Bars, 5 µm. (B) The experiment described in A was repeated; the cell lysates and the corresponding media were analyzed by Western blotting with an anti-Cab45 antibody (lane 1, DMSO; lane 2, 2.5 µM A23187; lane 3, 2.5 µM A23187 + 10 mM CaCl2). (C) Golgi membranes isolated from HeLa cells were incubated with DMSO or 25 µM BAPTA for 15 min. The membranes were subjected to five cycles of rapid freeze-thaw and finally centrifuged at 100,000 g. The pellets and the supernatants (sup) were analyzed by Western blotting with anti-Cab45 (top) and anti-TGN46 (bottom) antibodies, respectively. (D) Western blot bands from three independent experiments were quantified by densitometry using the ImageJ software. Bar graphs represent the densitometry values of external Cab45 (released from the Golgi membranes) normalized to the internal Cab45 values. Error bars show the mean ± SD densitometric values of three independent experiments. **, P < 0.01. (E) HeLa cells were transfected with SPCA1 siRNA. After 72 h at 37°C, the cells were incubated in Ca2+-free medium and treated with A23187 in the absence or presence of 10 mM CaCl2 and visualized by fluorescence microscopy as described in A. (F) The experiment was repeated as described in E; the cell lysates and the corresponding media were analyzed by Western blotting with an anti-Cab45 antibody (lane 1, DMSO; lane 2, 2.5 µM A23187; lane 3, 2.5 µM A23187 + 10 mM CaCl2).
How is Cab45, without an obvious membrane attachment or spanning domain, retained in the Golgi membranes? Golgi membranes isolated from HeLa cells were incubated with either DMSO or 25 µM BAPTA (a Ca2+ chelator) for 15 min at 32°C and then subjected to five cycles of freeze-thaw (von Blume et al., 2009). After centrifugation, the membrane pellet and the supernatant were Western blotted with antibodies to Cab45 and the TGN-specific transmembrane protein TGN46 (Fig. 1 C). Treatment with BAPTA caused a sixfold increase in the amount of Cab45 in the supernatant (Fig. 1, C and D), which indicates the requirement of Ca2+ for its retention in the Golgi membranes.
SPCA1 is a major regulator of Ca2+ homeostasis at the TGN, and we have shown that this process is required for secretory cargo sorting (Sorin et al., 1997; Sepúlveda et al., 2009; Lissandron et al., 2010; Feng et al., 2010; von Blume et al., 2011). Is SPCA1 required for the retention of Cab45? HeLa cells were transfected with SPCA1 siRNA as described previously (von Blume et al., 2011). The cells were visualized by fluorescence microscopy, and cell lysates and respective media from a parallel experiment were Western blotted with anti-Cab45 antibody. SPCA1 knockdown by siRNA resulted in loss of 53% of total Cab45 from the Golgi membranes and its secretion into the medium (Fig. 1, E and F). In cells depleted of SPCA1, compared with wild-type (wt) cells, high Ca2+ was ineffective in alleviating the A23187-mediated loss and secretion of Cab45 (Fig. 1 E, bottom panels; and Fig. 1 F, lane 3). This suggests that Cab45 requires both SPCA1 and Ca2+ for its retention in the Golgi membranes.
Is Cab45 required for Ca2+ homeostasis at the TGN? We measured the Ca2+ concentration in the lumen of the TGN with a fluorescence resonance energy transfer (FRET)-based Ca2+ sensor (Go-D1cpv) that is targeted to the TGN (Lissandron et al., 2010; von Blume et al., 2011). HeLa cells were transfected with two different Cab45-specific siRNAs or a scrambled oligo (control); after 72 h, cells were lysed and equal amounts of total cell lysates were analyzed by Western blotting with an anti-Cab45 antibody (Fig. 2 A). Cab45-specific oligos reduced the levels of the cognate polypeptide by 75–80%, respectively, compared with control oligos (Fig. 2, A and B). HeLa cells transfected with a scrambled (control), SPCA1-specific, or Cab45-specific siRNA were subsequently transfected with Go-D1cpv to measure the concentration of Ca2+ in the TGN. Fluorescent signals reflecting TGN [Ca2+] are presented as ΔR/R0 (ΔR = change in the ratio of YFP/CFP emission intensity at any time, and R0 is the value obtained before addition of 2.2 mM Ca2+ to the bathing solution of cells that had been previously depleted of Ca2+). Compared with control cells, Ca2+ import into the TGN of SPCA1 knockdown cells, as described previously (von Blume et al., 2011), and Cab45 knockdown cells was greatly reduced (Fig. 2 C). These results reveal that knockdown of Cab45 affects Ca2+ concentration in the TGN.
Ca2+ homeostasis of the TGN requires Cab45. (A) Lysates of HeLa cells transfected with control or Cab45-specific siRNA (Oligo1 and Oligo2) were Western blotted with anti-Cab45 (left) and β-actin antibodies (right). (B) The knockdown efficiency of Cab45 from three different experiments was quantitated by densitometry (histograms). Bar graphs represent the mean ± SD of triplicate experiments (error bars). Compared datasets were statistically significant (**) when P < 0.01. (C) HeLa cells were transfected with scrambled (control), Cab45 (Oligo1), and SPCA1-specific siRNA. After 48 h, cells were transfected with the TGN-specific Ca2+ FRET sensor GoD1cpv. After 12 h, cells were depleted of Ca2+ by incubation in a Ca2+-free solution and 1 µM Ionomycin for 1 h at 4°C. Subsequently, TGN Ca2+ influx was measured by detecting FRET signals of YFP (520 nm) and CFP (480) laser lines using an inverted confocal microscope (TCS SP5; Leica). Images were taken at 400 Hz at 20°C. TGN [Ca2+] fluorescent signals were presented as ΔR/R0 (R0 is the value measured before of 2.2 mM Ca2+ addition).
Ca2+ homeostasis of the TGN requires Cab45. (A) Lysates of HeLa cells transfected with control or Cab45-specific siRNA (Oligo1 and Oligo2) were Western blotted with anti-Cab45 (left) and β-actin antibodies (right). (B) The knockdown efficiency of Cab45 from three different experiments was quantitated by densitometry (histograms). Bar graphs represent the mean ± SD of triplicate experiments (error bars). Compared datasets were statistically significant (**) when P < 0.01. (C) HeLa cells were transfected with scrambled (control), Cab45 (Oligo1), and SPCA1-specific siRNA. After 48 h, cells were transfected with the TGN-specific Ca2+ FRET sensor GoD1cpv. After 12 h, cells were depleted of Ca2+ by incubation in a Ca2+-free solution and 1 µM Ionomycin for 1 h at 4°C. Subsequently, TGN Ca2+ influx was measured by detecting FRET signals of YFP (520 nm) and CFP (480) laser lines using an inverted confocal microscope (TCS SP5; Leica). Images were taken at 400 Hz at 20°C. TGN [Ca2+] fluorescent signals were presented as ΔR/R0 (R0 is the value measured before of 2.2 mM Ca2+ addition).
Is Cab45 required for cargo sorting? HeLa cells stably expressing signal sequence HRP (ss-HRP) were transfected with siRNA specific for Cab45 (Oligo1), SPCA1, or a scrambled oligo (control). After 72 h, secretion of HRP was measured by chemiluminescence as described previously (Bard et al., 2006; Bossard et al., 2007). Knockdown of Cab45 or SPCA1 decreased the HRP activity in the medium by 60% and 55%, respectively, compared with control cells (Fig. 3 A). Does HRP accumulate in the Golgi membranes under these conditions? To test this, HeLa cells stably expressing ss-HRP were transfected with a scrambled (control), SPCA1-specific, or Cab45-specific siRNA for 72 h. The cells were then incubated at 20°C for 2 h in the presence of cycloheximide to arrest HRP in the TGN. After washing extensively to remove extracellular HRP, cells were placed in fresh medium and transferred to 32°C to restart cargo export from the TGN. The localization of HRP was then visualized by fluorescence microscopy. After the block at 20°C, HRP was localized at the TGN (Fig. 3 B, top). Upon shifting cells to 32°C for 1 h, HRP was exported from the TGN in control cells (lack of staining in the TGN) but still visible in the TGN of SPCA1 or Cab45 knockdown cells (Fig. 3, B and C). Therefore, like SPCA1, Cab45 is also required for the export of HRP from the TGN.
The siRNA knockdown of the Golgi isoform of Cab45 causes missorting of secretory cargo. (A) HeLa ss-HRP–expressing cells were transfected with scrambled (control), Cab45, or SPCA1 siRNA for 72 h. The cell culture supernatant from the respective cells was analyzed for HRP activity by chemiluminescence. Error bars show mean ± SD of external HRP activity normalized to internal HRP activity of three independent experiments. Datasets were statistically significant when P < 0.01 (**). (B) HeLa cells were transfected as described in A and incubated at 20°C for 2 h. Subsequently, the temperature was changed to 32°C, and the cellular distribution of HRP was monitored by fluorescence microscopy using an anti-HRP antibody. Bars, 5 µm. (C) HRP location was quantitated in 100 cells for each of the conditions described in B. Bar graphs represent data from at least three different experiments. (D) Schematic presentation of the mRNAs encoding full-length Cab45 (Cab45-G contained in the Golgi membrane) or Cab45 C (the cytoplasmic isoform), as reported previously (Lam et al., 2007). The exons are numbered 1–7; ATG, methionine start codon; STOP, stop codon; SS, cleavable amino-terminal signal sequence of Cab45G. The diagram is modified from Lam et al. (2007). (E) Cells expressing HA-Cab45-G or HA-Cab45-C were stained with an HA antibody and analyzed by confocal microscopy. Bars, 5 µm. (F) HeLa cells were transfected with control or Cab45 siRNA. After 48 h, cells were transfected with ss-HRP–Flag and siRNA-resistant HA-Cab45-G or HA-Cab45-C. Media from these cells was analyzed for HRP activity by chemiluminescence. Error bars indicate the mean ± SD of HRP activity in the medium normalized to HRP activity in cell lysates measured in at least three independent experiments. **, P < 0.01.
The siRNA knockdown of the Golgi isoform of Cab45 causes missorting of secretory cargo. (A) HeLa ss-HRP–expressing cells were transfected with scrambled (control), Cab45, or SPCA1 siRNA for 72 h. The cell culture supernatant from the respective cells was analyzed for HRP activity by chemiluminescence. Error bars show mean ± SD of external HRP activity normalized to internal HRP activity of three independent experiments. Datasets were statistically significant when P < 0.01 (**). (B) HeLa cells were transfected as described in A and incubated at 20°C for 2 h. Subsequently, the temperature was changed to 32°C, and the cellular distribution of HRP was monitored by fluorescence microscopy using an anti-HRP antibody. Bars, 5 µm. (C) HRP location was quantitated in 100 cells for each of the conditions described in B. Bar graphs represent data from at least three different experiments. (D) Schematic presentation of the mRNAs encoding full-length Cab45 (Cab45-G contained in the Golgi membrane) or Cab45 C (the cytoplasmic isoform), as reported previously (Lam et al., 2007). The exons are numbered 1–7; ATG, methionine start codon; STOP, stop codon; SS, cleavable amino-terminal signal sequence of Cab45G. The diagram is modified from Lam et al. (2007). (E) Cells expressing HA-Cab45-G or HA-Cab45-C were stained with an HA antibody and analyzed by confocal microscopy. Bars, 5 µm. (F) HeLa cells were transfected with control or Cab45 siRNA. After 48 h, cells were transfected with ss-HRP–Flag and siRNA-resistant HA-Cab45-G or HA-Cab45-C. Media from these cells was analyzed for HRP activity by chemiluminescence. Error bars indicate the mean ± SD of HRP activity in the medium normalized to HRP activity in cell lysates measured in at least three independent experiments. **, P < 0.01.
A cytoplasmic splice isoform of Cab45 (Cab45-C) interacts with Munc 18 and Syntaxin 13, and controls exocytosis (Lam et al., 2007). The siRNA oligos used in this study target only the second exon, which encodes the signal sequence required for the translocation of the Golgi-specific isoform of Cab45 (Cab45-G) into the ER (Fig. 3 D, modified diagram from Lam et al., 2007). Therefore, this oligo should only affect the Cab45 levels in the Golgi membrane pool of Cab45 (Cab45-G). However, to rule out the potential role of the cytoplasmic Cab45 (Cab45-C), we performed the following experiment. HeLa cells were transfected with siRNA that specifically targets the UTR of the second exon containing the signal sequence of Cab45-G (Fig. 3 D). After 48 h, cells were transfected with siRNA-resistant HA-Cab45-G or HA-Cab45-C, and Flag-ss-HRP. Cells were visualized to monitor the localization of the HA-tagged proteins. The siRNA-resistant HA-Cab45-G and HA-Cab45-C were not targeted by the Cab45 UTR-specific siRNA (Fig. 3 E). In parallel, the medium from cells was collected to measure secreted HRP by chemiluminescence. Cab45-G siRNA inhibited secretion of HRP, which was rescued by the expression of siRNA-resistant Cab45-G but not Cab45-C (Fig. 3 F). The exogenously expressed siRNA-resistant Cab45-G was localized to the Golgi membranes, whereas Cab45-C was found mainly in the nucleus and the cytoplasm (Fig. 3 E). The effects on HRP secretion are therefore caused by siRNA-dependent depletion of the Golgi membrane pool of Cab45; the cytoplasmic spliced variant of Cab45 has no role in this process.
We then tested the effect of Cab45 knockdown on the trafficking of another secretory cargo, cartilage oligomeric matrix protein (COMP), and a lysosomal hydrolase, Cathepsin D. Cathepsin D is synthesized as a polypeptide of 53 kD, which is subsequently transported to lysosomes and converted to a 47-kD intermediate and a 31-kD mature polypeptide. HeLa cells were transfected with a scrambled (control), Cab45, or SPCA1 siRNA for 72 h. Media and cell lysates were then split into two equal aliquots and analyzed by Western blotting with anti-Cathepsin D– and anti-COMP–specific antibodies, respectively. Analysis of medium from Cab45 or SPCA1 knockdown HeLa cells revealed the presence of the immature (47 kD) form of Cathepsin D (Fig. 4, A and B) and a reduction in the intracellular mature form of cathepsin D in the corresponding cell lysates (Fig. 4 A). COMP was secreted in the medium of cells transfected with control siRNA; it was, however, not detected in the medium and accumulated in cells transfected with Cab45 or SPCA1 siRNA (Fig. 4, C and D). Finally, ss-HRP–expressing HeLa cells that were depleted of SPCA1 or Cab45 by siRNA were transfected with Flag-Lysozyme C (LysC; another secretory protein) and incubated for an additional 24 h at 37°C. Cell lysates and media were then analyzed by Western blotting with an anti-Flag antibody. SPCA1 and Cab45 siRNA reduced the level of LysC in the medium by 50% compared with control cells, and caused its accumulation within the cells (Fig. 4, E and F). These findings reveal that Cab45 affects the export of secretory proteins (COMP and LysC) and the lysosomal hydrolase Cathepsin D from the TGN.
Cab45 knockdown by siRNA causes missorting of Cathepsin D, COMP, and LysC. (A and C) HeLa cells were stably transfected with scrambled (control), Cab45, or SPCA1 siRNA for 72 h as described in Fig. 3 A. Media and cell lysates from the respective cells were Western blotted with specific antibodies against Cathepsin D (A) or COMP (C). (B and D) Western blots from three independent experiments were quantified by densitometry using the ImageJ software. Bar graphs represent the densitometry values of external Cathepsin D and COMP normalized to internal Cathepsin D and COMP values, respectively. (E) The medium and the lysates of HeLa cells expressing Flag-LysC were analyzed by Western blotting with an anti-Flag antibody. (F) Western blots from three independent experiments were quantified by densitometry using the ImageJ software. Bar graphs represent the densitometric values of external Flag-LysC normalized to the internal Flag-LysC values. Error bars show mean ± SD of the densitometric values of three independent experiments.
Cab45 knockdown by siRNA causes missorting of Cathepsin D, COMP, and LysC. (A and C) HeLa cells were stably transfected with scrambled (control), Cab45, or SPCA1 siRNA for 72 h as described in Fig. 3 A. Media and cell lysates from the respective cells were Western blotted with specific antibodies against Cathepsin D (A) or COMP (C). (B and D) Western blots from three independent experiments were quantified by densitometry using the ImageJ software. Bar graphs represent the densitometry values of external Cathepsin D and COMP normalized to internal Cathepsin D and COMP values, respectively. (E) The medium and the lysates of HeLa cells expressing Flag-LysC were analyzed by Western blotting with an anti-Flag antibody. (F) Western blots from three independent experiments were quantified by densitometry using the ImageJ software. Bar graphs represent the densitometric values of external Flag-LysC normalized to the internal Flag-LysC values. Error bars show mean ± SD of the densitometric values of three independent experiments.
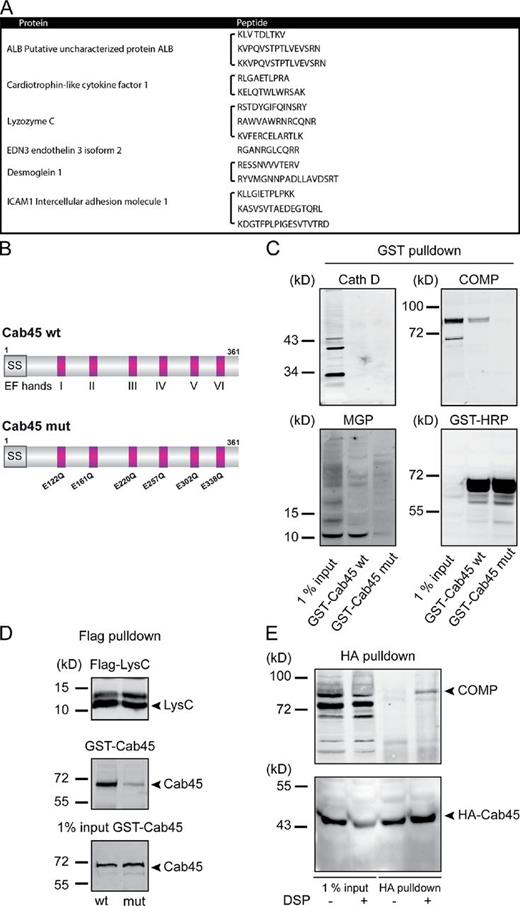
Does Cab45 bind secretory cargo? Purified recombinant full-length GST-fused Cab45 from Escherichia coli was conjugated to glutathione Sepharose beads. GST and Cab45-GST Sepharose beads were incubated with HeLa cell lysates. Afterward, proteins bound specifically to Cab45-GST or GST beads were released, analyzed by SDS-PAGE, excised from the gels, and sequenced by mass spectrometry. The pool of Cab45-binding proteins included secretory proteins: Albumin, Cardiotrophin-like cytokine factor, LysC, and EDN3 as well as two transmembrane proteins: Desmoglein1 and ICAM (Fig. 5 A and Table S1).
Cab45 binds secretory cargo. (A) GST and GST Cab45 expressed in E. coli were purified on GST beads. The respective preparations of beads were incubated with HeLa cell lysate for 4 h at 4°C, washed extensively, and finally incubated with SDS-PAGE sample buffer to release the bound proteins, which were then analyzed by SDS-PAGE. Gels were stained with Coomassie blue, and protein bands were excised and analyzed by mass spectrometry. The full list of identified proteins is shown in Table S1. This pool contained several proteins that are secreted or transported to the plasma membranes and are shown here. (B) Schematic presentation of Cab45 wt and the amino acids mutated in the EF hand domains of Cab45 mutant. (C) Recombinant purified GST-Cab45 wt or GST-Cab45 mutant bound to glutathione Sepharose beads were incubated with HeLa cell lysates. Beads were washed extensively and then incubated with SDS-PAGE sample buffer to elute the bound proteins, which were Western blotted with antibodies to Cathepsin D, COMP, MGP, and GST. (D) Cells expressing Flag-LysC were lysed and incubated with anti-Flag beads for 1 h at 4°C. Beads were then incubated with recombinant GST-Cab45 wt or GST Cab45 mutant. Bound proteins were eluted with SDS-PAGE sample buffer and Western blotted with anti-GST and anti-Flag antibodies. (E) HeLa cells stably expressing HA-Cab45 wt were incubated with PBS-DMSO or 1 mM PBS-DSP for 30 min at room temperature. The reaction was terminated and the HA-tagged Cab45 was immunoprecipitated and analyzed by Western blotting with antibodies against HA and COMP.
Cab45 binds secretory cargo. (A) GST and GST Cab45 expressed in E. coli were purified on GST beads. The respective preparations of beads were incubated with HeLa cell lysate for 4 h at 4°C, washed extensively, and finally incubated with SDS-PAGE sample buffer to release the bound proteins, which were then analyzed by SDS-PAGE. Gels were stained with Coomassie blue, and protein bands were excised and analyzed by mass spectrometry. The full list of identified proteins is shown in Table S1. This pool contained several proteins that are secreted or transported to the plasma membranes and are shown here. (B) Schematic presentation of Cab45 wt and the amino acids mutated in the EF hand domains of Cab45 mutant. (C) Recombinant purified GST-Cab45 wt or GST-Cab45 mutant bound to glutathione Sepharose beads were incubated with HeLa cell lysates. Beads were washed extensively and then incubated with SDS-PAGE sample buffer to elute the bound proteins, which were Western blotted with antibodies to Cathepsin D, COMP, MGP, and GST. (D) Cells expressing Flag-LysC were lysed and incubated with anti-Flag beads for 1 h at 4°C. Beads were then incubated with recombinant GST-Cab45 wt or GST Cab45 mutant. Bound proteins were eluted with SDS-PAGE sample buffer and Western blotted with anti-GST and anti-Flag antibodies. (E) HeLa cells stably expressing HA-Cab45 wt were incubated with PBS-DMSO or 1 mM PBS-DSP for 30 min at room temperature. The reaction was terminated and the HA-tagged Cab45 was immunoprecipitated and analyzed by Western blotting with antibodies against HA and COMP.
As shown in the previous paragraph, Cab45 is required for the LysC secretion (Fig. 4, E and F). The fact that LysC coprecipitated with Cab45 prompted us to test whether Cab45 bound other secretory cargoes and whether the binding was Ca2+ dependent.
Wt Cab45 and Cab45 with all Ca2+ binding sites of the EF hand domains mutated (Cab45 mutant, Fig. 5 B) were expressed in E. coli as GST fusion proteins and purified by adsorption on glutathione Sepharose beads. GST-Cab45 wt and GST-Cab45 mutant beads were incubated with equal amounts of lysates from HeLa cells. The beads were washed extensively, and bound proteins were released by treatment with SDS-PAGE sample buffer and analyzed by Western blotting with antibodies to Cathepsin D, COMP, matrix glia protein (MGP; another secretory cargo), and GST. GST-Cab45 wt binds endogenous COMP and MGP but not Cathepsin D (Fig. 5 C). The GST-Cab45 mutant did not bind COMP, MGP, or Cathepsin D (Fig. 5 C). In parallel, HeLa cells expressing Flag-LysC were lysed and Flag-LysC was affinity purified with Flag-agarose beads. The Flag-LysC beads were washed extensively and then incubated with recombinant GST-Cab45 wt or GST-Cab45 mutant. After washing, bound proteins were eluted by adding SDS-PAGE sample buffer and analyzed by Western blotting with anti-Flag and GST antibodies, respectively (Fig. 5 D). The data clearly indicate that Cab45 wt, but not Cab45 mutant, binds LysC. Thus, Ca2+ is required for the binding of Cab45 to the secretory cargoes.
We then tested the binding of Cab45 to COMP in intact cells by a different procedure. HeLa cells stably expressing HA-Cab45 were incubated briefly with DMSO or the membrane-permeant chemical cross-linker dithiobis(succinimidylpropinate) (DSP). Cells were lysed, the HA-tagged Cab45 was immunoprecipitated, and the immunoprecipitate was Western blotted with anti-HA– and COMP-specific antibodies, respectively. As shown in Fig. 5 E, in the presence of DSP, COMP was cross-linked to Cab45. These findings further support our hypothesis that Cab45 binds secretory cargo.
Collectively, our findings reveal that Ca2+ is required for the retention of Cab45 in the Golgi membranes; Cab45 binds secretory cargo in a Ca2+-dependent reaction and is required for their sorting at the TGN. The actin filament–ADF/Cofilin–SPCA1–Cab45–Ca2+ pathway is beginning to unravel the mechanism of a receptor- and coat protein–independent sorting and export of secretory cargo from the TGN.
Materials and methods
Antibodies and chemicals
We thank H. Gaisano (University of Toronto, Toronto, Canada) for the Cab45-b-pGEX1λT (cytosolic Cab45) and Y. Wakana (Tokyo College of Pharmacy, Tokyo, Japan) for the LysC construct. We also thank A. Hegele (Max Planck Institute for Biochemistry, Munich, Germany) for cloning and H. Reiterer (Max Planck Institute for Biochemistry) for viral HeLa cell transduction. A monoclonal antibody against HRP and a polyclonal antibody against COMP and MGP were from Abcam; monoclonal anti–β-actin and Flag M2 antibodies were from Sigma-Aldrich; anti–GST-HRP antibody was from Santa Cruz Biotechnology, Inc.; anti–rat HA clone 16E12 antibody was from Roche; anti–mouse HA was from Covance; sheep anti–human TGN46 was from AbD Serotec; monoclonal anti–Cathepsin D antibody was from Cell Signaling Technology; anti–Cab 45 monoclonal antibody was from BD; anti–GST Sepharose was from GE Healthcare; and the µMACs Epitope Tag Protein Isolation kit was from Miltenyi Biotech. Secondary antibodies for immunofluorescence microscopy and Western blot analysis were from Life Technologies. DSP was from Thermo Fisher Scientific. A23187 and BAPTA were from Sigma-Aldrich.
Cell culture transient and stable transfection and transduction
HeLa cells and HeLa cells stably expressing ss-HRP or HA-Cab45 were grown in DMEM (PAA Laboratories) supplemented with 10% FCS. The cells were cultivated at 37°C and 7% CO2. Cells were transfected with TransIT-HeLaMONSTER (Mirus Bio LLC) according to the manufacturer’s instructions. To create the stable HA-Cab45–expressing HeLa cell line, packaging vectors (HIV gag, pol, and rev), with the plasmid encoding VSV-G envelope and the pLPCX-HA-Cab45 vector, were transfected into 293T cells with the calcium phosphate method. The virus in the cell culture medium was harvested after 24 h by centrifugation at 68,000 g for 2 h, followed by another centrifugation (59,000 g for 2.5 h at 20°C). The pellet was resuspended in Hank’s buffer and HeLa cells were transduced with 1 × 105 IU (infectious units).
siRNA transfection
HeLa cells were transfected with 150 nM of scrambled (control), SPCA1 (von Blume et al., 2011), or Cab45 siRNA using HiPerfect (QIAGEN). Stealth RNA against Cab45 5′-GAGCCAGGACCTCACTTCCTCCTCT-3′ (Cab45 Oligo 1, UTR oligo), 5′-GGTCACGTGTCTTGGGACGAGTATA-3′ (Cab45 Oligo 2) and the negative control were purchased from Invitrogen.
Plasmids and recombinant protein production
The full-length wt Cab45 was generated by RT-PCR from HeLa cells total RNA. The cDNA was then amplified using the primers Cab45-F 5′-CGTGGATCCATGGTCTGGCCCTGGTG-3′ and Cab45-R 5′-GCGTCGCACGTGCTCCTCAAACTTAAGGCT-3′ and ligated into pGEX-4T1 with an N-terminal GST tag vector using BamHI and EcoRI restriction sites. The Cab45 mutant was generated by inserting point mutations in EF hand1 (E122Q), EF hand2 (E161Q), EF hand3 (E220Q), EF hand4 (E257Q), EF hand5 (E302Q), and EF hand6 (E338Q). The GST fusion proteins were produced in BL21 (DE3) cells and purified on glutathione Sepharose 4B.
For the HA-Cab45-G (Golgi construct), Cab45 was amplified by PCR using the primers HA-Cab45-F 5′-CGTGGATCCATGGTCTGGCCCTGGGTG-3′ and HA-Cab45- R 5′-GCAGAATTCTTAAGCGTAGTCTGGGACGTCGTATGGGTAAAACTCCTCGTGCACGCTGCG-3′.
PCR products were ligated into the pLPCX backbone using BglII and EcoRI restriction sites. Cab45-C (cytosolic construct) was amplified from the Cab45b-pGEX1λT template described previously (Lam et al., 2007) using the primers HA-Cab45-B-F 5′-CTCGGATCCATGCTCAGGTTCATGGTGAAGG-3′ and HA-Cab45-B-R 5′-GCAGAATTCTTAAGCGTAGTCTGGGACGTCGTATGGGTAAAACTCCTCGTGCACGCTGCG-3′. The insert was ligated into the pLPCX vector using BglII and EcoRI restriction sites. All constructs were verified by sequencing.
Flag pull down assay
HeLa cells expressing Flag-LysC were incubated at 20°C for 2 h to arrest secretory proteins in the TGN. Cells were then lysed with 0.5% Triton X-100, 50 mM Tris, and 150 mM NaCl, pH 7.4, supplemented with protease inhibitors. After centrifugation at 13,000 g, lysate was incubated with Flag agarose beads for 4 h at 4°C. After washing, beads were incubated with 1 mg/ml GST-Cab45 or GST-Cab45 mutant for an additional 1 h at 4°C. Proteins were released by boiling the samples in Laemmli buffer and analyzed by SDS-PAGE and/or Western blotting.
GST pull-down assay
HeLa cells were incubated at 20°C for 2 h to arrest secretory proteins in the TGN. Cells were then lysed with 1% Chaps in 50 mM Tris and 150 mM NaCl, pH 7.4, supplemented with protease inhibitors. After centrifugation at 13,000 g, the cell lysate was incubated with GST Sepharose beads for 4 h at 4°C. After washing, proteins were released by boiling the sample in Laemmli buffer and analyzed by SDS-PAGE and/or Western blotting.
In vivo cross-linking and HA pull down assay
HeLa cells stably transfected with HA-Cab45 were incubated at 20°C for 2 h to arrest secretory proteins at the TGN. Cells were then incubated with 1 mM of the membrane-permeant cross-linker DSP-PBS for 30 min at 20°C. The cross-linking reaction was stopped by adding 20 mM Tris, pH 7.5, for 15 min. Cells were lysed in PBS supplemented with 1% Chaps and protease inhibitors for 20 min at 4°C. After centrifugation, lysates were incubated with µMACS anti-HA Micro magnetic beads and incubated for 30 min at 4°C. HA-Cab45–associated proteins were eluted from the microbeads using the µMACS anti-HA isolation kit according to the manufacturer’s instructions.
Mass spectrometry
Proteins bound to GST-Cab45 were separated by 1D gel electrophoresis, and the bands were excised and trypsinized (Promega). Extracted peptides were analyzed by reversed phase HPLC (Agilent 1200 nano flow pump; Agilent Technologies) coupled to a mass spectrometer (Orbitrap XL; Thermo Fisher Scientific). Tandem mass spectrometry data were extracted using ProteomeDiscoverer 1.2.208 (Thermo Fisher Scientific) and queried against IPI Human using Mascot version 2.2 (Matrix Science LLC). Proteins were filtered using P > 0.05.
Preparation of Golgi membranes from HeLa cells
20 × 15 cm plates of confluent HeLa cells were harvested and pelleted. The cells were washed in breaking buffer (250 mM sucrose and 10 mM Tris, pH 7.4), diluted fivefold in breaking buffer, and homogenized in a Balch homogenizer 20 times. Samples were loaded on a sucrose gradient and centrifuged at 40,000 rpm in a SW28 rotor (Beckman Coulter) for 1.5 h at 4°C. The band corresponding to the Golgi membranes was collected using a 1-ml syringe with a 20/21G needle. Protein concentration was determined using a BCA assay (Thermo Fisher Scientific), and Golgi membranes were snap frozen in liquid nitrogen and stored at −80°C.
Cab45 release assay
Golgi membranes isolated from HeLa cells were diluted threefold in breaking buffer (250 mM sucrose and 10 mM Tris, pH 7.4). The membranes were pelleted by centrifugation at 100,000 g for 1 h and the membrane pellet was resuspended in 50 µl of breaking buffer. The membranes were then incubated with DMSO or BAPTA-AM (25 µM) and incubated for 15 min at 32°C. These samples were subjected to five cycles of freeze-thaw and finally centrifuged at 100,000 g for 1 h. The supernatant and the pellet were mixed with SDS-PAGE sample buffer and analyzed by Western blotting with anti-Cab45 and anti-TGN46 antibody, respectively.
ss-HRP transport assay
HeLa cells stably expressing ss-HRP were transfected with siRNA. 68 h after transfection, medium was changed and cells were incubated for an additional 4 h at 37°C. Then 50 µl of the medium was harvested and mixed with ECL reagent (Thermo Fisher Scientific). HRP activity was determined by luminescence detection applying a multi-label counter (WALLAC1420; PerkinElmer). For normalization, cells were lysed with 0.5% Triton X-100 in PBS and internal HRP activity was measured. For rescue experiments, cells were transfected with Cab45 siRNA1 (targeting exon 2 UTR). 48 h later, cells were transfected with either Flag ss-HRP or Flag ss-HRP and HA-Cab45-G or HA-Cab45-C.
Cathepsin D, COMP, and LysC sorting assays
Control or siRNA-treated cells were washed with serum-free medium five times. Then these cells were incubated in serum-free medium for 2 h. After that, media from control and siRNA-treated cells were collected and filtered using a 0.45 µm filter (EMD Millipore) and centrifuged at 5,000 g for 15 min. Then media were subjected to another round of centrifugation at 100,000 g for 2 h. Finally, media were concentrated applying a 3,000-D molecular mass cut-off spin column (EMD Millipore). In parallel, cells were lysed with 0.5% Triton X-100 in PBS and protease inhibitors. Cell lysates and concentrated media were then analyzed by Western blotting using anti-Cathepsin D, anti-COMP, or anti-Flag antibodies, respectively.
Immunofluorescence microscopy
To analyze fixed samples, a confocal microscope (SPE; Leica) equipped with a camera (IXON plus; Andor) was used (63× Plan-Apochromat NA 1.3 objective lens). To detect Alexa Fluor 594 (red channel), the 532 nm laser line was applied, and for Alexa Fluor 488 (green channel), the 488 nm laser line was applied. Images were taken using the Leica software and image analysis was performed with ImageJ (version 1.37).
Ca2+ imaging
The quantification of the [Ca2+] in the TGN was performed using a FRET Ca2+ sensor (Go-D1cpv) that is targeted to the TGN (Lissandron et al., 2010). First of all, internal Ca2+ was depleted from Go-D1cpv–expressing HeLa cells by incubation in Ca2+-free solution (140 mM NaCl, 5 mM KCl, 1.2 mM MgCl2, 5 mM glucose, 10 mM Hepes, and 0.5 mM EGTA) supplemented with 1 µM Ionomycin at 4°C for 1 h. After washing of the cells with 2% BSA in Ca2+-free solution, Ca2+ entry into the TGN was measured. To perform FRET analysis, an inverted confocal microscope (TCS SP5; Leica) using a 63× Plan-Apochromat NA 1.4 objective lens was used. YFP and CFP were excited applying the 428 nm laser line, and emissions were measured at 520 nm (YFP) and 480 nm (CFP). Images were captured at 400 Hz at 20°C using the Leica software. Fluorescent signals detecting TGN [Ca2+] were indicated as ΔR/R0, where R0 is the value obtained before addition of 2.2 mM Ca2+.
Statistical analysis
To determine statistical significance, unpaired Student’s t tests were performed applying the Graph Pad Prism software. Datasets were termed statistically significant when P-values were < 0.01 (**).
Online supplemental material
To identify interaction partners of Cab45, GST pull-downs were performed. GST and GST-Cab45 were purified in E. coli and incubated with HeLa cell lysates. Proteins bound specifically to Cab45-GST or GST beads were released, analyzed by SDS-PAGE, excised from the gels, and sequenced by mass spectrometry. Table S1 shows the list of putative interaction partners.
Acknowledgments
V. Malhotra is an Institució Catalana de Recerca i Estudis Avançats (ICREA) professor at the Center for Genomic Regulation, and the work in his laboratory is funded by grants from Plan Nacional (BFU2008-00414), Consolider (CSD2009-00016), Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) Grups de Recerca Emergents (SGR2009-1488; AGAUR-Catalan Government), and the European Research Council (268692). The group of J. von Blume is funded by an Emmy Noether fellowship (project BL 1186/1-1) of the Deutsche Forschungsgemeinschaft (DFG). M.A. Valverde is the recipient of an ICREA Academia Award and the work in his laboratory is supported by the Spanish ministry of Science and Innovation (SAF2012-38140), Fondo de Investigación Sanitaria (RD12/0042/0014), FEDER Funds, and Generalitat de Catalunya (SGR05-266). The project has received research funding from the European Union. This paper reflects only the author’s views. The Union is not liable for any use that may be made of the information contained therein. The mass spectrometry–based protein identification and proteomic analysis was carried out in the Joint UPF/CRG Proteomics Facility at Parc de Recerca Biomèdica de Barcelona, a member of ProteoRed network.



![Figure 2. Ca2+ homeostasis of the TGN requires Cab45. (A) Lysates of HeLa cells transfected with control or Cab45-specific siRNA (Oligo1 and Oligo2) were Western blotted with anti-Cab45 (left) and β-actin antibodies (right). (B) The knockdown efficiency of Cab45 from three different experiments was quantitated by densitometry (histograms). Bar graphs represent the mean ± SD of triplicate experiments (error bars). Compared datasets were statistically significant (**) when P < 0.01. (C) HeLa cells were transfected with scrambled (control), Cab45 (Oligo1), and SPCA1-specific siRNA. After 48 h, cells were transfected with the TGN-specific Ca2+ FRET sensor GoD1cpv. After 12 h, cells were depleted of Ca2+ by incubation in a Ca2+-free solution and 1 µM Ionomycin for 1 h at 4°C. Subsequently, TGN Ca2+ influx was measured by detecting FRET signals of YFP (520 nm) and CFP (480) laser lines using an inverted confocal microscope (TCS SP5; Leica). Images were taken at 400 Hz at 20°C. TGN [Ca2+] fluorescent signals were presented as ΔR/R0 (R0 is the value measured before of 2.2 mM Ca2+ addition).](https://cdn.rupress.org/rup/content_public/journal/jcb/199/7/10.1083_jcb.201207180/4/m_jcb_201207180_fig2.jpeg?Expires=1767661354&Signature=FC6H4e7oAipwC67AXn-PqFbO7FFx358yPXFhzqMOA6Nv7jttVvB615SgA3V-dIMFHneTQ~MnNcAN0VGd41iHzM8PlasWg0EcWON6YXMs5cH6TQWWOOz0GdjOcCPYRAcOJl7hom3PlRQ5xjn4H8-UW~2j-QNWmaKLICU0ZpvHxOGwssJzc2u9L7SzuY0fgrbnEb-PMLWrPytba9EZFLXFx0~-dvN6sBrG0oYPq2ym4PzWiwdss8BxIc7COeyKcgpiuB53dVvrm7kJcwAtl9IepFKhFyah4MNTsre0PecEL0UwOnEcIzizDln3~34c~gCvyRz8VMrGmvIrJxsZklam9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)